肾脏病理图片详解
24张肾脏病理图片

肾脏病理正常肾小球,显微这是光镜下的一个正常的肾小球。
肾小球血管袢薄而清晰。
内皮细胞和系膜细胞数目正常。
周围的肾小管也正常。
健康状态良好。
正常肾小球,显微,PAS染色这是正常肾小球,用PAS染色以突出基底膜。
肾小球血管袢薄而清晰。
正常肾小球,图示图示为一个正常肾小球。
注意血管袢和系膜的关系。
15%的肾小球滤过发生在系膜。
其它的通过有孔的上皮细胞滤过。
正常的阴离子屏障阻止象白蛋白这样的蛋白分子从内膜通过。
正常系膜含2~4个系膜细胞。
它具有巨噬细胞的功能。
微小病变,电镜这是微小病变。
它的特点是:上皮细胞(足细胞)的足突消失,正常的离子屏障丧失,表现为选择性白蛋白漏出,及随后的蛋白尿。
光镜下,微小病变的肾小球是正常的。
在这张电镜照片的下半部的毛细血管袢中有2个高电子密度的RBC。
内皮出现孔隙,但基底膜正常。
然而,上皮细胞表面的足突消失(呈融合状)。
局灶性节段性肾小球硬化,显微这是局灶性节段性肾小球硬化。
在肾小球的中央有一片胶原硬化区。
和微小病变相比,局灶性节段性肾小球硬化的病人更容易发生非选择性蛋白尿、血尿、发展为慢性肾衰,皮质激素的治疗效果不好。
局灶性节段性肾小球硬化,显微,三色法染色这是局灶性节段性肾小球硬化病人。
三色法染色显示了蓝色的胶原沉积。
成人和儿童肾病综合征有1/6是局灶性节段性肾小球硬化。
链球菌感染后肾小球肾炎,低倍镜小球充满细胞,毛细血管袢分辨不清。
这是增生性肾小球肾炎的一种,也称作链球菌感染后肾小球肾炎。
链球菌感染后肾小球肾炎,高倍镜链球菌感染后的细胞大量增生是因为在毛细血管袢内及周围有内皮细胞、上皮细胞、系膜细胞和中性粒细胞的数量增多造成的。
可在A组β溶血性链球菌的某一亚群感染的数周后发生此病。
病人抗O滴度特征性地升高。
链球菌感染后肾小球肾炎,免疫颗粒沉积,免疫荧光显微镜链球菌感染后肾小球肾炎是由免疫介导的。
因为免疫沉积过程具有局限性,免疫沉积物呈颗粒或团块状分布于毛细血管袢。
链球菌感染后肾小球肾炎,电镜电镜下,链球菌感染后肾小球肾炎的电子致密的免疫沉积物主要位于上皮下,这里看到在基底膜右侧一个大的上皮下驼峰。
肾脏病理图片详解

肾脏病理图片详解狼疮(Lupus)是一种可以累及全身脏器的自身免疫性疾病。
累及到肾脏就会出现以血尿、蛋白尿等为表现的狼疮性肾炎(lupus nephritis, LN)。
2003年国际肾脏病学会(ISN)和肾脏病理学会(PRS)结合多年的临床和病理经验修订了狼疮肾炎的病理组织学分类,针对以往病理学分类的不足,发表了新的分型标准,来看下图:I型的病理是轻微系膜型(图上的“微小病变”属笔误~),如1楼的墙壁轻微有些裂缝。
II型是系膜增生性狼疮肾炎,如图上2楼是“2”片“增生”的叶子“系”着“膜”。
III型是局灶性狼疮肾炎,“局灶”是指受累肾小球50%。
图中可见破坏的墙壁面积大于50%,“4”根立柱全部裸露,灰尘“弥漫”。
V型是膜性狼疮肾炎,可以理解为类似水立方的“五”边形“膜”。
VI型是严重硬化型狼疮肾炎,超过90%的肾小球呈现球性硬化,不再有活动性病变这是最基本的狼疮肾炎I到VI型的图说,值得注意的是III型和IV 型主要是受累程度上的差别,分水岭是受累肾小球是否达到50%。
III、VI型病变要区分活动性病变(A)、慢性病变(C)还是活动性病变和慢性病变混合(A/C),根据病变活动程度决定治疗方案。
VI型病变还要区分是VI-S(弥漫性节段性)还是IV-G(弥漫性球性),目前的循证医学证据表明这两种亚型之间存在症状、治疗和预后方面的差异。
附:狼疮性肾炎的病理学分型(ISN/RPS, 2003)─────────────────────────────────────Ⅰ型,轻微系膜型LN (ClassⅠ,Minimal mesangia l LN)光镜下肾小球正常,但荧光(和/或电镜)显示免疫复合物存在Ⅱ型,系膜增生性LN (Class Ⅱ,Meangial proliferstive LN)光镜下可见单纯系膜细胞不同程度的增生或伴有系膜基质增宽及系膜区免疫复合物沉积荧光和电镜下可有少量的上皮下或内皮下免疫复合物伴同沉积Ⅲ型,局灶性LN (Class Ⅲ,Focal LN)活动性或非活动性病变,呈局灶性(受累肾小球少于全部的50%)及节段性或球性的肾小球毛细血管内增生、膜增生和中重度系膜增生,或有新月体性形成,典型的局灶性的内皮下免疫复合物沉积,伴有或无系膜病变Ⅲ(A):活动性病变:局灶增生性LNⅢ(A/C):活动性和慢性病变:局灶增生和硬化性LNⅢ(C):慢性非活动性病变伴有肾小球硬化:局灶硬化性LN●应注明活动性和硬化性病变的肾小球的比例●应注明肾小管萎缩、肾间质细胞浸润和纤维化、肾血管硬化和其他血管病变的严重程度(轻度、中度和重度)和比例Ⅳ型,弥漫性LN (Class Ⅳ,Diffuse LN)活动性或非活动性病变,呈弥漫性(受累肾小球超过去全部的50%)节段性或球性的肾小球毛细血管内增生、膜增生和中重度系膜增生,或呈新月体性GN,典型的弥漫性内皮下免疫复合物沉积,伴有或无系膜病变。
肾脏解剖结构图(共7张PPT)
肾蒂内结构的排列关系
• 自前向后
肾静脉 肾门(renal hilum):肾的内侧缘中部的凹陷称肾门,为肾的血管、神经、淋巴管及肾盂出入之门户。
上端
下端
肾门(renal hilum):肾的内侧缘中部的凹陷称肾门,为肾的血管、神经、淋巴管及肾盂出入之门户。
上端
下端
上端
下端
平L2下缘
上端
下端
上端 左肾 平T11下缘 右肾 平T12上缘
下端
平L2下缘 平L3上缘
第12肋斜过 左肾后面中部 右肾后面上部
肾区:在腰背部 ,肾门(平L1) 的体表投影点在 竖脊肌外缘与第 12肋的夹角处, 称肾区。
左肾 平T11下缘 平L2下缘
位于脊柱两侧,腹膜后间隙内。
,腹膜后间隙 位于脊柱两侧,腹膜后间隙内。
上端
下端
肾门(renal hilum):肾的内侧缘中部的凹陷称肾门,为肾的血管、神经、淋巴管及肾盂出入之门户。
上端 上端
内。 下端
下端
位于脊柱两侧,腹膜后间隙内。
上端
下端
• 左高右低。 左肾 平T11下缘
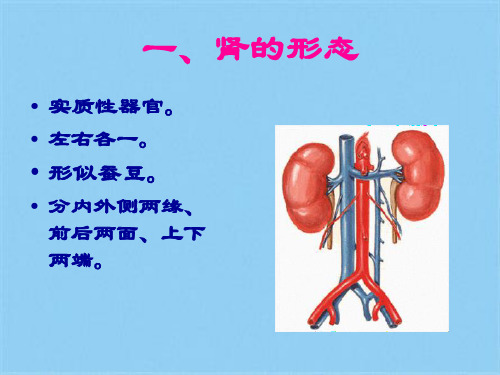
肾动脉 分内外侧两缘、前后两面、上下两端。
肾窦(renal sinus):由肾门深入肾实质的凹陷称肾窦,内含肾动脉分支、肾静脉属支、肾小盏、肾大盏、肾盂和脂肪组织等。
上端
下端
肾门(renal hilum):肾的内侧缘中部的凹陷称肾门,为肾的血管、神经、淋巴管及肾盂出入之门户。
肾盂 上端
下端
右肾 平T12上缘 平L3上缘
一、肾的形态
肾脏病理图谱(原创)

FROM:http://library.m /WebPath/REN AHTML/REN ALIDX.htm lMade by chenxh9905, copyright is reserved.Renal Pathology Index Normal and Incidental Findings1.Normal adult kidney, gross2.Normal adult kidney, cross section, gross3.Simple renal cyst, gross4.Double ureters, gross5.Horseshoe kidney, grossObstructive and Vascular diseases6.GIF animation of urinary tract lithiasis7.Hydronephrosis from obstruction by calculus, gross8.Staghorn calculus, gross9.Hydronephrosis, severe, gross10.Hydronephrosis with calculus at ureteropelvic junction, gross11.Ureteropelvic junction obstruction, gross12.Hydroureter and hydronephrosis, gross13.Atrophy of one kidney, gross14.Renal vein thrombosis, gross15.Acute renal infarction, gross16.Acute renal infarction, gross17.Acute renal infarction, microscopic18.Massive renal infarction, grossInfectious and Inflammatory Diseases19.Acute cystitis of bladder, gross20.Bladder hypertrophy and calculus with obstruction from nodular hyperplasia ofprostate, gross21.Ureteritis cystica, gross22.Renal abscess, gross23.Renal microabscesses, gross24.Renal microabscesses, gross25.Acute pyelonephritis, medium power microscopic26.Acute pyelonephritis, high power microscopic27.Renal papillary necrosis, gross28.Candida pyelonephritis, high power microscopic, PAS stain29.Chronic pyelonephritis, low power microscopic30.Chronic pyelonephritis, high power microscopic31.Xanthogranulomatous pyelonephritis, gross32.Xanthogranulomatous pyelonephritis, microscopicInterstitial Diseases33.Acute tubular necrosis, diagram34.Acute tubular necrosis with ischemia, microscopic35.Acute tubular necrosis with ethylene glycol poisoning, microscopic36.Drug-induced acute interstitial nephritis, microscopic37.Urate nephropathy, gross38.Uric acid crystals, polarized, microscopic39.Wegener granulomatosis with vasculitis, low power microscopic40.Wegener granulomatosis with vasculitis, high power microscopic41.Polyarteritis nodosa with vasculitis, microscopic42.Hyaline thrombus in glomerulus with thrombotic thrombocytopenic purpura (TTP),microscopic43.Hemolytic-uremic syndrome with fibrin thrombi in glomerulus, microscopic44.Benign nephrosclerosis, gross45.Malignant nephrosclerosis, gross46.Malignant nephrosclerosis with fibrinoid necrosis, microscopic47.Hyperplastic arteriolitis with hypertension, microscopic48.Nodular glomerulosclerosis, microscopic49.Nodular glomerulosclerosis and hyaline arteriolosclerosis, microscopic, PAS stain50.Diffuse glomerulosclerosis, microscopic, PAS stain51.End stage renal disease, gross52.End stage renal disease, microscopic53.Diabetic kidneys, renal transplant with chronic rejection, gross54.Acute transplant rejection, gross55.Acute tubulointerstitial cellular rejection, microscopic56.Chronic vascular rejection of kidney, microscopicCystic Diseases57.Normal term infant kidneys, external, gross58.Normal fetal kidneys, cut surface, gross59.Recessive polycystic kidney disease, gross60.Recessive polycystic kidney disease, cut surface, gross61.Recessive polycystic kidney disease, microscopic62.Congenital hepatic fibrosis in recessive polycystic kidney disease, microscopic63.Multicystic renal dysplasia, gross64.Multicystic renal dysplasia, grossparison of recessive polycystic renal disease and multicystic renal dysplasia,gross66.Multicystic renal dysplasia, microscopic67.Dominant polycystic kidney disease, gross68.Dominant polycystic kidney disease, gross69.Dominant polycystic kidney disease, gross70.Polycystic change with chronic renal dialysis, gross71.Renal cell carcinoma arising in dialysis-induced cystic change, gross72.Multiple simple renal cysts, grossNeoplasms73.Urothelial carcinoma of bladder, gross74.Urothelial carcinoma of bladder, gross75.Urothelial carcinoma of renal pelvis, gross76.Urothelial carcinoma of renal pelvis, gross77.Urothelial carcinoma, low power microscopic78.Urothelial carcinoma, high power microscopic79.Urothelial carcinoma in situ, high power microscopic80.Renal cell carcinoma, gross81.Renal cell carcinoma, gross82.Renal cell carcinoma, gross83.Renal cell carcinoma with renal vein invasion, gross84.Renal cell carcinoma, microscopic85.Metastases to kidney, gross86.Wilms tumor of kidney, gross87.Wilms tumor of kidney, low power microscopic88.Wilms tumor of kidney, high power microscopic89.Angiomyolipoma of kidney, gross90.Angiomyolipoma of kidney, microscopic91.Medullary fibroma (renomedullary interstitial cell tumor) of kidney, grossGlomerulonephritis92.Normal glomerulus, microscopic93.Normal glomerulus, microscopic, PAS stain94.Normal glomerulus, diagram95.Minimal change disease (MCD), electron micrograph96.Focal segmental glomerulosclerosis (FSGS), microscopic97.Focal segmental glomerulosclerosis (FSGS), microscopic, Trichrome stain98.Post-streptococcal glomerulonephritis, low power microscopic99.Post-streptococcal glomerulonephritis, high power microscopic100.Post-streptococcal glomerulonephritis, granular immune deposits, immunofluorescence microscopy101.Post-streptococcal glomerulonephritis, electron micrograph102.Membranous glomerulonephritis, microscopic103.Membranous glomerulonephritis, microscopic, Jones silver stain104.Membranous glomerulonephritis, immune deposits, immunofluorescence microscopy105.Membranous glomerulonephritis, electron micrograph106.Rapidly progressive glomerulonephritis with crescents, microscopic107.Rapidly progressive glomerulonephritis with crescents, microscopic108.Rapidly progressive glomerulonephritis with crescents, fluorescence microscopy109.Goodpasture syndrome with antiglomerular basement membrane antibody, immunofluorescence microscopy110.IgA nephropathy (Berger disease), microscopic111.IgA nephropathy (Berger disease), immunofluorescence microscopy112.Membranoproliferative glomerulonephritis, microscopic113.Membranoproliferative glomerulonephritis, microscopic, silver stain114.Membranoproliferative glomerulonephritis, type I, electron micrograph115.Membranoproliferative glomerulonephritis, type II, electron micrograph116.Membranoproliferative glomerulonephritis, type II, immunofluorescence microscopy117.Lupus nephritis, high power microscopic118.Hereditary nephritis (Alport syndrome), microscopic119.Chronic glomerulonephritis, gross1.Normal adult kidney, grossHere is a normal adult kidney. The capsule has been removed and a pattern of fetal lobulations still persists, as it sometimes does. The hilum at the mid left contains some adipose tissue. At the lower right is a smooth-surfaced, small, clear fluid-filled simple renal cyst. Such cysts occur either singly or scattered around the renal parenchyma and are not uncommon in adults.2.Normal adult kidney, cross section,grossIn cross section, this normal adult kidney demonstrates the lighter outer cortex and the darker medulla, with the renal pyramids into which the collecting ducts coalesce and drain into the calyces and central pelvis.3.Simple renal cyst, grossHere is a much larger simple renal cyst of the upper pole. Other smaller cysts are also scattered around the kidney. The ureter exits south on the left. Such a large renal cyst would be seen on a radiographic imaging procedure, but could probably be distinguished from a neoplasm by its uniform fluid density and thin wall. Such simple cysts are unlikely to compromise renal function.4.Double ureters, grossDouble ureters are seen exiting from each kidney and extending to the bladder that has been opened. A small segment of aorta is seen between the normal, smooth-surfaced kidneys. A partial or complete duplication of one or both ureters occurs in about 1 in 150 persons. There is a potential for obstructive problems due to the abnormal flow of urine and the entrance of two ureters into the bladder in close proximity, but most of the time this is an incidental finding (except to a urologist).5.Horseshoe kidney, grossHere is a "horseshoe" kidney. This is a congenital anomaly that most often occurs in association with other anomalies or syndromes with specific genetic defects such as trisomy 18. However, it can also occur as an isolated anomaly. The possible problem here is that the ureters take an abnormal course across the "bridge" of renal tissue and this can lead to partial obstruction with hydronephrosis.The passage of a calculus (stone) through the urinary tract is diagrammed here. Calculi form when there is increased excretion of solutes such as calcium and when urine alkalinity, acidity, stasis, and/or concentration are favorable. The most common varieties of calculi are:FrequencyType of StoneCalcium oxalate (or75%phosphate)Magnesium ammonium12%phosphate (struvite, or"triple phosphate")Uric acid6%Cystine1%Other6%6.GIF animation of urinary tract lithiasisStones containing calcium are far more frequent than other types, and about half the time occur when there is hypercalciuria. Only about 10% of the time do they appear as a consequence of hypercalcemia. The struvite stones are also known as "infection" stones because bacteria suchas Proteus that split urea to ammonia favor their formation. Uric acid stones may be seen inassociation with gout, but often are not, and may just reflect increased precipitation of urates in an acid urine. Rare cystine stones also form in acid urine.Urinary tract calculi are usually unilateral and about 1 to 3 mm in size. Their passage is marked by intense abdominal or back or flank pain. This pain can be paroxysmal, known as renal or ureteral "colic". Hematuria may also be present. Larger stones that cannot pass may produce hydronephrosis or hydroureter.7.Hydronephrosis from obstruction by calculus, grossThere was a large renal calculus (stone) that obstructed the calyces of the lower pole of this kidney, leading to a focal hydronephrosis (dilation of the collecting system). The stasis from the obstruction and dilation led to infection. The infection with inflammation is characterized by the pale yellowish-tan areas next to the dilated calyces with hyperemic mucosal surfaces. The upper pole is normal and shows good corticomedullary demarcations.8.Staghorn calculus, grossSometimes a very large calculus nearly fills the calyceal system, with extensions into calyces that give the appearance of a stag's (deer) horns. Hence, the name "staghorn calculus". Seen here is a horn-like stone extending into a dilated calyx, with nearly unrecognizable overlying renal cortex from severe hydronephrosis and pyelonephritis. Nephrectomy may be performed because the kidney is non-functional and serves only as a source for infection.9.Hydronephrosis, severe, grossHere is a kidney with much more advanced hydronephrosis in which there is only a thin rim of remaining renal cortex. Such a kidney is non-functional and a source for ongoing infection. If this process is unilateral, then the problem originates from the ureteral orifice up to the pelvis. In this case, a large "staghorn" calculus (so named because the prominent projections of the stone into the calyces resemble deer antlers) was present that filled up the pelvis and calyceal system. If this process were bilateral, then the problem would originate in the bladder trigone or urethra (or the prostate around the urethra) or some process (such as a large neoplasm) that could impinge on both ureters.10.Hydronephrosis with calculus at ureteropelvic junction, grossThe arrow points to the culprit in this case of hydronephrosis--a ureteral calculus caught at the ureteropelvic junction. This kidney demonstrates marked hydronephrosis with nearly complete loss of cortex. Such a kidney would be non-functional. If the other kidney had sufficient function, then renal failure will not ensue. There is sufficient renal reserve capacity that it is possible to survive with half of a normal kidney.There is scarring of this kidney from chronic obstruction and pyelonephritis. The renal pelvis is markedly dilated, but the ureter is not, indicating that the point of obstruction is the ureteropelvic junction.A long-standing obstruction (probably congenital) at the ureteral orifice through which the metal probe passes led to the marked hydroureter and hydronephrosis seen here. In the intravenous urogram below, note the dilation of the right ureter, compared to the normal left ureter. This patient had vesicoureteral reflux. Such obstructive processes increase the risk for urinary tract infection.There is one relatively normal-sized kidney with a granular surface and a few scattered, shallow cortical scars. The other kidney shows atrophy because of renal arterial occlusion. Such a situation can lead to hypertension (Goldblatt kidney).The white arrow marks a renal vein thrombus. There are a host of etiologies for renal vein thrombosis, including trauma, compression by neoplasms, renal vein invasion by renal cell carcinoma, and nephrotic syndrome with membranous glomerulonephritis.This is an acute renal infarction. Note the wedge shape of this zone of coagulative necrosis resulting from loss of blood supply with resultant tissue ischemia that produces the pale infarct. The small amount of blood supply from the capsule supplies the immediate subcortical zone. The remaining cortex is congested, as is the medulla.This acute renal infarction is pale, typical of coagulative necrosis. It is roughly wedge-shaped. Renal infarctions usually result from embolization of cardiac valvular vegetations or a portion of cardiac mural thrombus. Sometimes a renal arterial vasculitis can lead to infarction.This is the microscopic appearance of an acute renal infarct. At the far right is normal kidney, then to the left of that hyperemic kidney that is dying, then to the left of that pale pink infarcted kidney in which both tubules and glomeruli are dead.Acute renal arterial obstruction in this case led to massive renal infarction in which the entire cortex is pale yellow, with medullary hemorrhage.This bladder at autopsy has been opened to reveal areas of hyperemia of the mucosa. This is acute cystitis.The markedly enlarged prostate seen here has not only large lateral lobes, but a very large median lobe as well that obstructs the prostatic urethra and led to chronic urinary tract obstruction. As a result, the bladder became both enlarged and hypertrophied as it had to work against the obstruction with every episode of urination. That is why the surface of the bladder appears trabeculated. Note also that a yellowish-brown calculus formed in the bladder.The small bumps seen here over the ureteral mucosa are called ureteritis cystica and represent cystic areas of glandular metaplasia resulting from inflammation. They are more commonly seen in the bladder, where they are called cystitis cystica.In the lower pole of this kidney is a 1 cm pale yellow abscess. Infections can reach the kidney either by ascending up the urinary tract (from a bladder infection, for example) or by hematogenous spread with sepsis. This lone abscess was probably hematogenous in origin.The surfaces of both kidneys demonstrate multiple microabscesses from hematogenous spread of a bacterial infection. The microabscesses have yellow centers and prominent hyperemic borders.The cut surface of the kidney reveals many small yellowish microabscesses in both cortex and medulla. This type of pyelonephritis is most typical for hematogenous dissemination of infection to the kidney, rather than the more typical ascending urinary tract infection.This is an ascending bacterial infection leading to acute pyelonephritis. Numerous PMN's are seen filling renal tubules across the center and right of this picture.At high magnification, many neutrophils are seen in the tubules and interstitium in a case of acute pyelonephritis. The neutrophils can collect in the distal tubules and be passed in urine as WBC casts.The pale white areas involving some or all of many renal papillae are areas of papillary necrosis. This is an uncommon but severe complication of acute pyelonephritis, particularly in persons with diabetes mellitus. Papillary necrosis may also accompany analgesic nephropathy.[Image courtesy of Dr. John Nicholls, Hong Kong University]This PAS stain of a renal papilla with a portion of transitional lining epithelium at the lower right demonstrates many budding cells and pseudohyphae of Candida albicans. Fungal urinary tract infections are much less common than bacterial infections, but both are likely to be ascending infections, having originated in the lower urinary tract, typically bladder.The large collection of chronic inflammatory cells here is in a patient with a history of multiple recurrent urinary tract infections. This is chronic pyelonephritis.Both lymphocytes and plasma cells are seen in this case of chronic pyelonephritis. It is not uncommon to see lymphocytes accompany just about any chronic renal disease: glomerulonephritis, nephrosclerosis, pyelonephritis. However, the plasma cells are most characteristic for chronic pyelonephritis.Sometimes long-standing renal infection may be localized and form a mass-like lesion. This is a disease known as xanthogranulomatous pyelonephritis. It is uncommon, but may mimic a neoplasm.The microscopic appearance of xanthogranulomatous pyelonephritis shows many pale to foamy macrophages from breakdown of renal parenchyma with ongoing inflammation.These diagrams illustrate acute tubular necrosis. The distribution of the areas of necrosis is more segmental with ischemic injuries, while toxic injuries result in more diffuse proximal tubular injury.The epithelium of the tubules seen here is ragged from undergoing necrosis with acute tubular necrosis (ATN) from ischemia. In this case, heart failure with hypotension precipitated the ATN.The tubular vacuolization and tubular dilation here is a result of the toxic effect of ethylene glycol poisoning. This is representative of acute tubular necrosis (ATN), which has many causes. ATN resulting from toxins usually has diffuse tubular involvement, whereas ATN resulting from ischemia (as in profound hypotension from cardiac failure) has patchy tubular involvement.In this case of drug-induced interstitial nephritis, there are some scattered eosinophils, along with neutrophils and mononuclear cells in the inflamed interstitium. The classic example of this occurs occasionally with methicillin, but can occur with a variety of drugs such as thiazide diuretics and the H2 blocker cimetidine. The immune mechanism may be either type I or type IV hypersensitivity.This is chronic urate nephropathy with pale yellowish tan tophaceous deposits in the medulla. There is also an acute urate nephropathy that can occur with a "lysis" syndrome reulting from massive cellular necrosis of leukemia or lymphoma cells with chemotherapy. The metabolic breakdown of the cell nuclei yields large amounts of urate which, when excreted, plug renal tubules.Chronic urate nephropathy leads to deposition of uric acid crystals in the interstitium, forming tophi with surrounding foreign body inflammation, mononuclear cell infiltrates, and fibrosis. The long, needle-shaped crystals form the pale mass shown here at high magnification. Patients with hyperuricemia may also have nephrolithiasis with uric acid stones.This is a renal biopsy at low magnification in which there is a focal lesion centered around a blood vessel. Thus, a vasculitis is present. The one glomerulus at the lower center appears normal. An adequate renal biopsy should contain at least 6 glomeruli so that there is less chancethat focal lesions will be missed. Renal biopsies are often performed with ultrasound guidance.At high power, the vasculitis is seen to involve a renal artery branch. This is a necrotizing granulomatous vasculitis. In this case, the anti-neutrophil cytoplasmic autoantibody (ANCA) serology was positive and a diagnosis of Wegener granulomatosis was made. This patient also had pulmonary involvement with this disease.Here is a vasculitis of a renal arterial branch. Lymphocytes are scattered in and around the vessel. This happens to be the classic form of polyarteritis nodosa (PAN), a systemic vasculitisthat most often affects the kidneys. The ANCA serology is often negative.A small platelet-fibrin thrombus is seen in a glomerular capillary above the arrow. This occurred in a patient with thrombotic thrombocytopenic purpura (TTP). This rare coagulopathy mainly affects kidneys, heart, and brain with small arteriolar thrombi. Acute renal failure can occur. The classic pentad of fever, acute renal failure, neurologic changes, thrombocytopenia, and microangiopathic hemolytic anemia is often present. TTP results from a congenital or more often acquired defect in the ADAMTS-13 metalloproteinase enzyme that cleaves vonWillebrand factor (vWF) multimers, and the large multimers lead to abnormal platelet aggregation. TTP overlaps with hemolytic uremic syndrome (HUS) that may be precipitated by verotoxins from such organisms as E. coli (type O157:H7) that cause endothelial injury.Very similar to thrombotic thrombocytopenic purpura (TTP) is hemolytic-uremic syndrome (HUS). The two conditions may be difficult to tell apart. HUS can be a leading cause for acute renal failure in children. Ingestion of foods, such as poorly cooked ground beef, introduces a verotoxin-producing E. coli infection into the GI tract. Such strains are often identified by serotyping, typically type O157:H7. A bloody diarrhea is followed in a few days by renal failure caused by endothelial injury from the toxin, leading to the characteristic fibrin thrombi in glomerular and interstitial capillaries. Most patients recover in a few weeks with supportive dialysis.Here is an example of renal vascular disease known as benign nephrosclerosis. The smaller arteries in the kidney have become thickened and narrowed. Hyaline arteriolosclerosis with hypertension or diabetes mellitus is usually present. This leads to patchy ischemic atrophy with focal loss of parenchyma that gives the surface of the kidney the characteristic granular appearance as seen here.In malignant nephrosclerosis, the kidney demonstrates focal small hemorrhages. This is often due to an accelerated phase of essential hypertension in which blood pressures are very high (such as 300/150 mm Hg).Malignant hypertension leads to fibrinoid necrosis of small renal arteries as shown here. The damage to the arteries leads to formation of pink fibrin--hence the term "fibrinoid".Thickening of the arterial wall with malignant hypertension also is associated with a hyperplastic arteriolosclerosis (hyperplastic arteriolitis). This arteriole has an "onion skin" appearance.This is nodular glomerulosclerosis (the Kimmelstiel-Wilson lesion) of diabetes mellitus. Nodules of pink hyaline material form in regions of glomerular capillary loops in the glomerulus. This is due to a marked increase in mesangial matrix from damage as a result of non-enzymatic glycosylation of proteins.This is a PAS stain of nodular glomerulosclerosis(Kimmelstiel-Wilson disease) in a patient with long-standing diabetes mellitus. Note also the markedly thickened arteriole at the lower right which is typical for the hyaline arteriolosclerosis that is seen in diabetic kidneys as well.This PAS stain demonstrates diffuse glomerulosclerosis associated with long-standing diabetes mellitus. There is an increase in mesangial matrix, a slight increase in mesangial cellularity, and capillary basement membrane thickening. These changes gradually advance until the entire glomerulus is sclerotic.The end result of many renal diseases -- whether they are renal vascular diseases, glomerulonephritis, or chronic pyelonephritis--is end stage renal disease (ESRD). In ESRD, the kidneys are small bilaterally, as shown here. This condition is associated with chronic renal failure, and the patient's blood urea nitrogen (BUN) and serum creatinine continue to increase. Chronic renal failure can be treated by dialysis or by kidney transplantation, as shown here.The microscopic appearance of the "end stage kidney" is similar regardless of cause, which is why a biopsy in a patient with chronic renal failure yields little useful information. The cortex is fibrotic, the glomeruli are sclerotic, there are scattered chronic inflammatory cell infiltrates, and the arteries are thickened. Tubules are often dilated and filled with pink casts and give an appearance of "thyroidization."In this case, severe atherosclerosis in a patient with diabetes mellitus led to severe aortic atherosclerosis with renal arterial stenosis as well as nephrosclerosis and nodular glomerulosclerosis of the kidneys. The end stage renal disease was treated with renal transplantation. The transplant kidney is placed in the pelvis because this is technically easier and there is usually no point in trying to remove the native kidneys. In this case, the patient developed chronic rejection and that is why focal hemorrhages are seen in the kidney that is slightly swollen. A radiographic study would show decreased renal blood flow in the transplant kidney.This kidney was removed because of acute transplant rejection. Note the swollen and hemorrhagic appearance of this entire kidney.Seen here is acute tubulointerstitial cellular rejection of a renal transplant. This can occur days to months to years following transplantation. Both CD4 and CD8 lymphocytes participate in this rejection reaction. The immunosuppressive drugs such as cyclosporine given to counteract the rejection may lead to nephrotoxicity with similar changes as well.This is chronic vascular rejection of a renal transplant, which has a poor prognosis. Note the thickened arteries with intimal fibrosis and chronic inflammation. These changes gradually occur over months in affected patients.These are normal term infant kidneys. Note the presence of fetal lobulations and the smooth cortical surfaces with some attached adipose tissue.These fetal kidneys (from a gestation estimated at 25 weeks in the second trimester) demonstrate a normal cut surface. Note the pelvis and the calyces. Note the well-defined corticomedullary junctions.This infant died soon after premature birth at 23 weeks gestation from pulmonary hypoplasia as aresult of oligohydramnios. The oligohydramnios resulted from markedly diminished fetal urine output as a consequence of polycystic kidney disease. Note the bilaterally enlarged kidneys that nearly fill the abdomen below the liver. The histologic appearance in this case, coupled with the gross appearance., was consistent with autosomal recessive polycystic kidney disease (ARPKD).Here is a cut section of a kidney with autosomal recessive polycystic kidney disease (ARPKD). Note that the cysts are fairly small but uniformly distributed throughout the parenchyma so that the disease is usually symmetrical in appearance, with both kidneys markedly enlarged. The recurrence risk for this disease is, of course, 25% because of the autosomal recessive inheritance pattern. Affected babies usually do not survive long. This disorder is linked to an abnormal fibrocystin protein produced by the PKHD1 gene.。
肾脏解剖结构图

肾蒂内结构的排列关系
• 自前向后 肾门(renal hilum):肾的内侧缘中部的凹陷称肾门,为肾的血管、神经、淋巴管及肾盂出入之门户。
肾窦(renal sinus):由肾门深入肾实质的凹陷称肾窦,内含肾动脉分支、肾静脉属支、肾小盏、肾大盏、肾盂和脂肪组织等。 肾窦(renal sinus):由肾门深入肾实质的凹陷称肾窦,内含肾动脉分支、肾静脉属支、肾小盏、肾大盏、肾盂和脂肪组织等。
肾窦(renal sinus):由肾门深入肾实质的凹陷称肾窦,内含肾动脉分支、肾静脉属支、肾小盏、肾大盏、肾盂和脂肪组织等。 肾窦(renal sinus):由肾门深入肾实质的凹陷称肾窦,内含肾动脉分支、肾静脉属支、肾小盏、肾大盏、肾盂和脂肪组织等。
肾蒂(renal pedicle):出入肾门的结构被 肾门(renal hilum):肾的内侧缘中部的凹陷称肾门,为肾的血管、神经、淋巴管及肾盂出入之门户。
肾蒂(renal pedicle):出入肾门的结构被结缔组织包绕称肾蒂。 肾窦(renal sinus):由肾门深入肾实质的凹陷称肾窦,内含肾动脉分支、肾静脉属支、肾小盏、肾大盏、肾盂和脂肪组织等。
肾窦(renal sinus):由肾门深入肾实质 肾蒂(renal pedicle):出入肾门的结构被结缔组织包绕称肾蒂。
肾动脉 肾窦(renal sinus):由肾门深入肾实质的凹陷称肾窦,内含肾动脉分支、肾静脉属支、肾小盏、肾大盏、肾盂和脂肪组织等。
肾门(renal hilum):肾的内侧缘中部的凹陷称肾门,为肾的血管、神经、淋巴管及肾盂出入之门户。 肾门(renal hilum):肾的内侧缘中部的凹陷称肾门,为肾的血管、神经、淋巴管及肾盂出入之门户。
肾窦(renal sinus):由肾门深入肾实质的凹陷称肾窦,内含肾动脉分支、肾静脉属支、肾小盏、肾大盏、肾盂和脂肪组织等。 肾门(renal hilum):肾的内侧缘中部的凹陷称肾门,为肾的血管、神经、淋巴管及肾盂出入之门户。
肾内病理图解:局灶节段性肾小球硬化

肾内病理图解:局灶节段性肾小球硬化局灶节段性肾小球硬化--Focal Segmental Glomerulosclerosis (FSGS),是成人肾病综合征常见的病理类型之一,光镜下典型的表现为多少不等的球性硬化的肾小球背景下,可见局灶分布的节段性硬化的肾小球。
2004 年国际肾脏病理学会将其分为 5 型,也就是近 10 年以来一直使用的哥伦比亚分型,分别是:门部(门周)型、顶端型、细胞型、塌陷型、非特殊型。
各型 FSGS 的特点门部/门周型 FSGS:在血管极处(门部/门周)出现节段性硬化和透明样变,常有球囊粘连、小动脉透明样变等表现,原则上诊断该类型的 FSGS,需硬化部位在门部的肾小球应>肾活检标本 50% 肾小球。
顶端型 FSGS:在顶端处(近端肾小管处)出现球囊粘连,并有部分毛细血管袢疝入近端肾小管内,伴出现泡沫细胞、常有足细胞肥大、增生,偶有透明样变。
细胞型FSGS:一般表现为毛细血管内细胞增生/增多,常伴有泡沫细胞及多形核细胞浸润,以及足细胞肥大、增生等,球囊粘连和透明样变不常见。
塌陷型 FSGS:一般表现为毛细血管袢固缩、塌陷,毛细血管腔闭塞,伴随足细胞的大量肥大和增生,足细胞足突融合非常广泛。
非特殊型 FSGS:不能归入以上 4 个类型的 FSGS,即称为非特殊型,包括既往的外周型和系膜增生型,只要能看见一个节段性硬化,即可诊断。
病理表现多样,可有节段性硬化、透明样,足细胞肥大、增生等表现。
FSGS 的病理特点1. 光镜:肾小球出现节段性硬化或球囊粘连;2. 免疫荧光:常有 IgM 和或 C3 在硬化部位沉积,亦可无特殊;3. 电镜:无电子致密物沉积,脏层上皮细胞(足细胞)的足突弥漫性融合;鉴别诊断1. 继发性 FSGS:如 IgA 肾病、系统性红斑狼疮、乙肝相关性肾炎、ANCA 相关性血管炎肾损害等,均可以表现为FSGS,需结合免疫荧光和临床资料进行鉴别;2. 适应不良性 FSGS:即我们常说的肥胖相关性 FSGS、高血压肾损害引起的 FSGS,原则上从以下三个方面进行鉴别:(1)临床病史,包括是否出现肾病综合征,适应性 FSGS 的蛋白尿多< 3.5 g/24 h,即使出现大量蛋白尿,血白蛋白亦多不低于30 g/L,而原发性 FSGS 多为肾病综合征表现;(2)继发性 FSGS 病理类型多为门部型 FSGS;(3)电镜下足细胞足突融合范围,适应性(继发性)FSGS 多<50%,而原发性 FSGS 多>70%,多超过 90%。
肾内病理图解:膜性肾病

肾内病理图解:膜性肾病膜性肾病(Membranous Glomerulonephritis/Nephropathy,MN),是肾病综合征常见的病理类型之一,它是一个病理诊断,指肾小球脏层上皮细胞下免疫复合物的沉积,从而导致肾小球基底膜出现一系列的病变,典型的表现是肾小球基底膜弥漫性不均质的增厚和「钉突」生成,传统的病理分期,可以把膜性肾病分为5 期,即I、II、III、IV、V 期。
膜性肾病的病理特点1. 光镜:肾小球毛细血管基底膜弥漫性「钉突」形成或不均质增厚;2. 免疫荧光:IgG 和 C3 在肾小球毛细血管壁颗粒状沉积;3. 电镜:肾小球脏层上皮细胞(足细胞)下电子致密物沉积,足细胞足突弥漫性融合。
鉴别诊断1. 继发性膜性肾病如乙肝、丙肝病毒相关性肾炎、狼疮性肾炎等;可通过临床资料及实验室检查进行鉴别,特别是近年来研究发现的PLA2R(血清筛查PLA2R 抗体、肾组织筛查 Ig(G1、G2、G3、G4)及 PLA2R 等,在鉴别原发/继发膜性肾病上,带来了很大方便。
病理上原发/继发的鉴别可以参考以下病理表现光镜下:在肾小球基底膜病变的同时,是否伴随其他病变,如系膜细胞中-重度增生、内皮细胞增生、节段性坏死等;免疫荧光:是否表现为「满堂亮」,即IgA、IgG、IgM、C3、C4、C1q、Fib 均呈强阳性,特别是 C1q 强阳性,高度提示继发性疾病;电镜下:除了肾小球脏层上皮细胞下电子致密物沉积外,是否有系膜区或其他区域的电子致密物沉积。
2. 血液系统疾病如浆细胞病、单克隆球蛋白沉积相关性肾损害、淀粉样变、淋巴瘤等,上述疾病的早期病变常可单纯的表现为肾小球基底膜增厚,特别是对于年龄>50 岁的病人,最好能够常规加做刚果红染色或肾组织Kappa、Lamda 轻链免疫荧光/免疫组化,避免漏诊,此外亦需结合临床资料进行鉴别。
3. I 期膜性肾病需与微小病变、早期糖尿病肾病鉴别;III 期膜性肾病需与膜增生性肾炎鉴别。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
狼疮(Lupus)是一种可以累及全身脏器的自身免疫性疾病。
累及到肾脏就会出现以血尿、蛋白尿等为表现的狼疮性肾炎(lupus nephritis, LN)。
2003年国际肾脏病学会(ISN)和肾脏病理学会(PRS)结合多年的临床和病理经验修订了狼疮肾炎的病理组织学分类,针对以往病理学分类的不足,发表了新的分型标准,来看下图:I型的病理是轻微系膜型(图上的“微小病变”属笔误~),如1楼的墙壁轻微有些裂缝。
II型是系膜增生性狼疮肾炎,如图上2楼是“2”片“增生”的叶子“系”着“膜”。
III型是局灶性狼疮肾炎,“局灶”是指受累肾小球50%。
图中可见破坏的墙壁面积大于50%,“4”根立柱全部裸露,灰尘“弥漫”。
V型是膜性狼疮肾炎,可以理解为类似水立方的“五”边形“膜”。
VI型是严重硬化型狼疮肾炎,超过90%的肾小球呈现球性硬化,不再有活动性病变这是最基本的狼疮肾炎I到VI型的图说,值得注意的是III型和IV型主要是受累程度上的差别,分水岭是受累肾小球是否达到50%。
III、VI型病变要区分活动性病变(A)、慢性病变(C)还是活动性病变和慢性病变混合(A/C),根据病变活动程度决定治疗方案。
VI型病变还要区分是VI-S(弥漫性节段性)还是IV-G (弥漫性球性),目前的循证医学证据表明这两种亚型之间存在症状、治疗和预后方面的差异。
附:狼疮性肾炎的病理学分型(ISN/RPS, 2003)─────────────────────────────────────Ⅰ型,轻微系膜型LN (ClassⅠ,Minimal mesangial LN)光镜下肾小球正常,但荧光(和/或电镜)显示免疫复合物存在Ⅱ型,系膜增生性LN (Class Ⅱ,Meangial proliferstive LN)光镜下可见单纯系膜细胞不同程度的增生或伴有系膜基质增宽及系膜区免疫复合物沉积荧光和电镜下可有少量的上皮下或内皮下免疫复合物伴同沉积Ⅲ型,局灶性LN (Class Ⅲ,Focal LN)活动性或非活动性病变,呈局灶性(受累肾小球少于全部的50%)及节段性或球性的肾小球毛细血管内增生、膜增生和中重度系膜增生,或有新月体性形成,典型的局灶性的内皮下免疫复合物沉积,伴有或无系膜病变Ⅲ(A):活动性病变:局灶增生性LNⅢ(A/C):活动性和慢性病变:局灶增生和硬化性LNⅢ(C):慢性非活动性病变伴有肾小球硬化:局灶硬化性LN●应注明活动性和硬化性病变的肾小球的比例●应注明肾小管萎缩、肾间质细胞浸润和纤维化、肾血管硬化和其他血管病变的严重程度(轻度、中度和重度)和比例Ⅳ型,弥漫性LN (Class Ⅳ,Diffuse LN)活动性或非活动性病变,呈弥漫性(受累肾小球超过去全部的50%)节段性或球性的肾小球毛细血管内增生、膜增生和中重度系膜增生,或呈新月体性GN,典型的弥漫性内皮下免疫复合物沉积,伴有或无系膜病变。
又分两种亚型:(Ⅳ-S)LN:即受累肾小球超过50%,并呈节段性病变,(Ⅳ-G)LN:即受累肾小球超过50%,并呈球性病变出现弥漫性白金耳样病变时,即使轻度或无细胞增生的LN,也归入Ⅳ型弥漫性LNⅣ-S(A):活动性病变:弥漫性节段性增生性LNⅣ-G(A):活动性病变:弥漫性球性增生性LNⅣ-S(A/C):活动性和慢性病变:弥漫性节段性增生和硬化性LNⅣ-G(A/C):活动性和慢性病变:弥漫性球性增生和硬化性LNⅣ-S(C):慢性非活动性病变伴有硬化:弥漫性节段性硬化性LNⅣ-G(C):慢性非活动性病变伴有硬化:弥漫性球性硬化性LN●应注明活动性和硬化性病变的肾小球的比例●应注明肾小管萎缩、肾间质细胞浸润和纤维化、肾血管硬化和其他血管病变的严重程度(轻度、中度和重度)和比例Ⅴ型,膜性LN (Class Ⅴ,Membranous LN)肾小球基底膜弥漫增厚,可见球性或节段性上皮下免疫复合物沉积,伴有或无系膜病变。
Ⅴ型膜性LN可合并Ⅲ型或Ⅳ型病变,则应作出复合性诊断,如Ⅲ+Ⅴ,Ⅳ+Ⅴ等,并可进展为Ⅵ型硬化型LNⅥ型,严重硬化型LN (Class Ⅵ, Advanced sclerosing LN)超过90%的肾小球呈现球性硬化,不再有活动性病变临床执业医师考试:2011年《泌尿系统疾病》重难点肾小球肾炎!临床执业医师考试:2011年《泌尿系统疾病》重难点肾小球肾炎学慧网提示:2011年临床执业医师考试病理学《泌尿系统疾病》肾小球肾炎重难点汇总如下所示......第八单元泌尿系统疾病八、泌尿系统疾病1.肾小球肾炎(1)各型病理变化(2)临床病理联系2.慢性肾盂肾炎病理变化及临床病理联系第一节肾小球肾炎试试看,这些问题能回答吗?1.大红肾、蚤肾是 1.继发性颗粒性固缩肾是?2.大白肾是 2.原发性颗粒性固缩肾是?1.成人肾病综合征最常见的原因 1.新月体形成的是?2.多见于儿童的是 2.虫蚀状空隙形成的是?3.多见于幼儿的是 3.双轨征形成的是4.驼峰征是?5.钉状突起形成的是?本单元复习思路备考策略:巧记、牢记一个重要表格!最重要的基础知识:肾小球的组织学结构!最重要的基础知识肾小球的组织学结构特别注意:两个”膜”!基膜:毛细血管内皮下系膜:毛细血管之间的细胞肾小球肾炎病理学——2011改良版(TANG)根据《病理学》,人民卫生出版社,第七版类型病理特点临床病理联系特别强调(重要!!)光镜免疫荧光电镜1.急性弥漫性增生性肾小球肾炎弥漫性系膜细胞和内皮细胞增生GBM和系膜区颗粒状IgG和C3沉积上皮下驼峰状沉积物急性肾炎综合征大红肾/蚤咬肾多见于儿童2.急进抗GBM新月体线性IgG和C3 无沉积物快速进新月体肾病局灶性节段性增生或弥漫系膜增宽系膜区IgA和C3沉积系膜区沉积物反复发作的血尿、蛋白尿4.慢性肾小球肾炎肾小球玻璃样变性、纤维化、硬化因肾炎起始类型而异因肾炎起始类型而异慢性肾炎综合征慢性肾衰继发性颗粒性固缩肾(前后对比:原发性颗粒性固缩肾见于:高血压)骆驼是个急脾气,月亮一出就行进。
急性弥漫性肾小球肾炎--驼峰状沉积物急进性肾小球肾炎--新月体系膜增生3毫克,膜性肾炎钉突成。
3毫克=3mg,就是:系膜增生性肾小球肾炎沉积物是C3,IgM, IgG. 膜性肾小球病有钉突形成。
系膜插入基膜厚,双轨就是膜增生。
系膜增生,插入,基膜增厚双轨征诊断:膜增生性肾小球肾炎既然双轨就分型,只有C3是2型。
膜增生性肾小球肾炎分2型2型只有C3沉积局灶性节段性肾小球硬化驼峰征电镜下,链球菌感染后肾小球肾炎的电子致密的免疫沉积物主要位于脏层上皮下,图示基底膜右侧一个大的脏层上皮下驼峰状沉积物。
膜性肾小球肾炎,肾小球的银染显示呈黑色的基底膜及与之垂直排列的钉突。
肾小球内由增生的壁层上皮细胞组成的新月体。
如箭头所示,电镜下左下方的系膜细胞基质插入内皮细胞和基底膜之间,呈现双层基底膜现象。
这是I型膜增生性肾小球病。
引起肾病综合症的5个肾炎1.膜性(成人):基膜增厚2.系膜增生性:系膜增生3.膜增生性:1+2(双轨征)4.微小病变性(幼儿):仅足细胞突起消失而已5.局灶性阶段性肾小球硬化:足细胞剥脱试试看,这些问题能回答吗?1.大红肾、蚤肾是 1.继发性颗粒性固缩肾是?2.大白肾是 2.原发性颗粒性固缩肾是?1.成人肾病综合征最常见的原因 1.新月体形成的是?2.多见于儿童的是 2.虫蚀状空隙形成的是?3.多见于幼儿的是 3.双轨征形成的是4.驼峰征是?5.钉状突起形成的是二、慢性肾盂肾炎为肾小管间质的慢性炎症,病变特点:慢性间质性炎症、纤维化和瘢痕形成。
(一)病理变化1.肉眼一侧或双侧肾体积变小,出现不规则的瘢痕。
如为双侧,则两侧改变不对称。
【对比记忆】慢性肾小球肾炎:病变弥漫,颗粒状,分布均匀,两侧对称。
2.镜下病变特点是局灶性淋巴细胞、浆细胞浸润,间质纤维化。
肾小球萎缩、纤维化或玻璃样变性。
部分肾小管萎缩,部分肾小管扩张。
(二)临床病理联系1.多尿、夜尿——肾小管改变明显→尿浓缩功能下降而致。
2.高血压——肾组织纤维化和小血管硬化→肾缺血→肾素分泌增加而致。
3.氮质血症、尿毒症——晚期肾组织严重破坏而致。
常考相关的知识点大红肾急性肾小球肾炎大白肾膜性肾小球病(膜性肾病)的早期蚤咬肾急性肾小球肾炎原发性颗粒性固缩肾高血压肾病继发性颗粒性固缩肾慢性肾小球肾炎动脉粥样硬化性固缩肾动脉粥样硬化疤痕肾慢性肾盂肾炎【实战演习】1.急性弥漫性增生性肾小球肾炎特点是A.弥漫性系膜细胞和基质增生B.基底膜和上皮细胞间有驼峰状或小丘状致密物质沉积C.弥漫性肾小球内大量新月体或环状体形成D.电镜下呈弥漫性肾小球脏层上皮细胞足突消失E.是肾病综合征的重要原因『正确答案』B2.慢性肾盂肾炎是A.肾小管和肾间质的慢性炎B.一种肾小球免疫复合物性肾炎C.一种以增生为主的炎症D.一种以变质为主的炎症E.肾小球肾炎的一种特殊类型『正确答案』A3.毛细血管基底膜可形成钉状突起,见于A.弥漫性毛细血管内增生性肾小球肾炎B.弥漫性膜增生性肾小球肾炎C.弥漫性膜性肾小球肾炎,D.轻微病变性肾小球肾炎E.弥漫性新月体性肾小球肾炎『正确答案』C4.弥漫性毛细血管内增生性肾小球肾炎最主要的病变是A.肾小球毛细血管扩张充血及血栓形成B.毛细血管内血栓形成及基底膜增厚C.中性粒细胞漫润及肾球囊上皮细胞增生D.毛细血管壁纤维素样坏死E.毛细血管内皮细胞及系膜细胞增生『正确答案』E5.弥漫性膜增生性肾小球肾炎时,增生的细胞主要是A.肾小球脏层细胞和中性粒细胞B.肾小球壁层细胞和系膜细胞C.肾小球系膜细胞和基质D.肾小球毛细血管基底膜增厚和系膜细胞增生E.肾小球各种细胞均有较明显增生『正确答案』D6.慢性肾小球肾炎的肾脏表现为A.大红肾B.颗粒性固缩肾C.大白肾D.蚤咬肾E.大瘢痕性固缩肾『正确答案』B7.肉眼观察肾体积明显缩小,质地变硬,表面有大的不规则瘢疤凹陷,该病变性质最可能是A.晚期肾小球肾炎B.局灶性节段性肾小球肾炎C.轻微病变性肾小球肾炎D.良性高血压病引起的萎缩肾E.慢性肾盂肾炎『正确答案』E8.肾炎时,毛细血管管壁增厚呈车轨状或分层状见于A.急性弥漫性增生性肾小球肾炎B.弥漫性系膜增生性肾小球肾炎C.弥漫性膜增生性肾小球肾炎D.慢性肾小球肾炎E.新月体性肾小球肾炎『正确答案』C9.原发性肾小球疾病的病理分型不包括A.轻微肾小球病变B.局灶性节段性病变C.肾病综合征D.膜性肾病E.系膜增生性肾炎『正确答案』C(10~11题共用备选答案)A.弥漫性毛细血管内增生性肾小球肾炎B.弥漫性系膜增生性肾小球肾炎C.弥漫性新月体性肾小球肾炎D.弥漫性膜性增生性肾小球肾炎E.E.轻微病变性肾小球肾炎10.大红肾见于『正确答案』A11.毛细血管壁增厚呈车轨状或分层状见于『正确答案』D。
