NBMA N-butoxy methyl acrylamide正丁氧基甲基丙烯酰胺
有机化学中常见缩写

Ac Acetyl 乙酰基DMAP 4-dimethylaminopyridine 4-二甲氨基吡啶acac Acetylacetonate 乙酰丙酮基DME dimethoxyethane 二甲醚AIBN Azo-bis-isobutryonitrile 2,2'-二偶氮异丁腈DMF N,N'-dimethylformamide 二甲基甲酰胺aq. Aqueous 水溶液dppf bis (diphenylphosphino)ferrocene 双(二苯基膦基)二茂铁9-BBN 9-borabicyclo[3.3.1]nonane 9-硼二环[3.3.1]壬烷dppp 1,3-bis (diphenylphosphino)propane 1,3-双(二苯基膦基)丙烷BINAP (2R,3S)-2,2’-bis (diphenylphosphino)-1,1’-binaphthyl(2R,3S)-2.2'-二苯膦-1.1'-联萘亦简称为联二萘磷BINAP是日本名古屋大学的Noyori(2001年诺贝尔奖)发展的一类不对称合成催化剂dvb Divinylbenzene 二乙烯苯Bn Benzyl 苄基e- Electrolysis 电解BOC t-butoxycarbonyl 叔丁氧羰基(常用于氨基酸氨基的保护)%ee % enantiomeric excess 对映体过量百分比(不对称合成术语)%de % diasteromeric excess 非对映体过量百分比(不对称合成术语)Bpy (Bipy) 2,2’-bipyridyl 2,2'-联吡啶EDA (en) ethylenediamine 乙二胺Bu n-butyl 正丁基EDTA Ethylenediaminetetraacetic acid 乙二胺四乙酸二钠Bz Benzoyl 苯甲酰基EE 1-ethoxyethyl 乙氧基乙基c- Cyclo 环-Et Ethyl 乙基FMN Flavin mononucleotide 黄素单核苷酸CAN Ceric ammonium nitrate 硝酸铈铵Cat. Catalytic 催化Fp flash point 闪点CBz Carbobenzyloxy 苄氧羰基FVP Flash vacuum pyrolysis 闪式真实热解法h hours 小时Min Minute 分钟hv Irradiation with light 光照COT 1,3,5-cyclooctatrienyl 1,3,5-环辛四烯1,5-HD 1,5-hexadienyl 1,5-己二烯Cp Cyclopentadienyl 环戊二烯基HMPA Hexamethylphosphoramide 六甲基磷酸三胺CSA 10-camphorsulfonic acid 樟脑磺酸HMPT Hexamethylphosphorus triamide 六甲基磷酰胺CTAB Cetyltrimethylammonium bromide 十六烷基三甲基溴化铵(相转移催化剂)iPr isopropyl 异丙基Cy Cyclohexyl 环己基LAH Lithium aluminum hydride 氢化铝锂(LiAlH4)LDA Lithium diisopropylamide 二异丙基氨基锂(有机中最重要一种大体积强碱)2 有机化学合成常见缩写dba Dibenzylidene acetone 苄叉丙酮LHMDS Lithium hexamethyldisilazideDBE 1,2-dibromoethane 1,2- 二溴乙烷LTBA Lithium tri-tert-butoxyaluminum hydrideDBN 1,8-diazabicyclo[5.4.0]undec-7-ene 二环[5.4.0]-1,8-二氮-7-壬烯mCPBA meta-cholorperoxybenzoic acid 间氯过苯酸DBU 1,5-diazabicyclo[4.3.0]non-5-ene 二环[4.3.0]-1,5-二氮-5-十一烯Me Methyl 甲基DCC 1,3-dicyclohexylcarbodiimide 1,3-二环己基碳化二亚胺MEM b-methoxyethoxymethyl 甲氧基乙氧基甲基-DCE 1,2-dichloroethane 1,2-二氯乙烷Mes Mesityl 均三甲苯基(也就是1,3,5-三甲基苯基)不知对不对?DDQ 2,3-dichloro-5,6-dicyano-1,4-benzoquinone 2,3-二氯-5,6-二氰-1,4-苯醌MOM methoxymethyl 甲氧甲基DEA Diethylamine 二乙胺Ms Methanesulfonyl 甲基磺酰基(保护羟基用)TBDMS, TBS t-butyldimethylsilyl 叔丁基二甲基硅烷基(羟基保护基)DEAD Diethyl azodicarboxylate 偶氮二甲酸二乙酯MS Molecular sieves (3 or 4 )分子筛Dibal-H Diisobutylaluminum hydride 二异丁基氢化铝MTM Methylthiomethyl 二甲硫醚diphos (dppe) 1,2-bis (diphenylphosphino)ethane 1,2-双(二苯基膦)乙烷Naphth Naphthyl 萘基diphos-4 (dppb) 1,4-bis (diphenylphosphino)butane 1,2-双(二苯基膦)丁烷NBD Norbornadiene 二环庚二烯(别名:降冰片二烯)NBS N-Bromosuccinimide N-溴代丁二酰亚胺别名:N-溴代琥珀酰亚胺NCS N-chlorosuccinimide N-氯代丁二酰亚胺. 别名:N-氯代琥珀酰亚胺TBAF Tetrabutylammonium fluoride 氟化四丁基铵TASF Tris(diethylamino)sulfonium difluorotrimethyl silicateNi(R) Raney Nickel 雷尼镍(氢活性催化还原剂)NMO N-methyl morpholine-n-oxide N-甲基氧化吗啉TBHP t-butylhydroperoxide 过氧叔丁醇PCC Pyridinium chlorochromate 吡啶氯铬酸盐PDC Pyridinium dichromate 是什么东西?t-Bu Tert-butyl 叔丁基TEBA Triethylbenzylammonium 三乙基苄基胺PEG Polyethylene glycol 聚乙二醇TEMPO Tetramethylpiperdinyloxy free radicalPh Phenyl 苯基PhH Benzene 苯TFA Trifluoroacetic acid 三氟乙酸TFAA Trifluoroacetic anhydride 三氟乙酸酐PhMe Toluene 甲苯(亦称toluol;methylbenzene)Tol Tolyl 甲苯基Tf or OTf TriflatePhth Phthaloyl 邻苯二甲酰3楼THF Tetrahydrofuran 四氢呋喃Pip Piperidyl 哌啶基THP Tetrahydropyranyl 四氢吡喃基TMEDA Tetramethylethylenediamine 四甲基乙二胺Py Pyridine 吡啶TMP 2,2,6,6-tetramethylpiperidine 2,2,6,6-四甲基哌啶quant. quantitative yield 定量产率(对否?)TMS Trimethylsilyl 三甲基硅烷基Red-Al [(MeOCH2CH2O)AlH2]Na 直接看分子式就是了sBu sec-butyl 仲丁基Tr Trityl 三苯基sBuLi sec-butyllithium 仲丁基锂TRIS TriisopropylphenylsulfonylSiamyl DiisoamylTs (Tos) Tosyl (p-toluenesulfonyl) 对甲苯磺酰基谢谢观星人,我们合贴吧……(所有的都翻译了)%%de 非对映体过量百分比(不对称合成术语)%ee 对映体过量百分比(不对称合成术语)AA/MMA 丙烯腈/甲基丙烯酸甲酯共聚物AA 丙烯酸AAS 丙烯酸酯-丙烯酸酯-苯乙烯共聚物ABFN 偶氮(二)甲酰胺ABN 偶氮(二)异丁腈ABPS 壬基苯氧基丙烷磺酸钠Ac 乙酰基acac 乙酰丙酮基AIBN 2,2'-二偶氮异丁腈aq. 水溶液BBAA 正丁醛苯胺缩合物BAC 碱式氯化铝BACN 新型阻燃剂BAD 双水杨酸双酚A酯BAL 2,3-巯(基)丙醇9-BBN 9-硼二环[3.3.1]壬烷BBP 邻苯二甲酸丁苄酯BBS N-叔丁基-乙-苯并噻唑次磺酰胺BC 叶酸BCD β-环糊精BCG 苯顺二醇BCNU 氯化亚硝脲BD 丁二烯BE 丙烯酸乳胶外墙涂料BEE 苯偶姻乙醚BFRM 硼纤维增强塑料BG 丁二醇BGE 反应性稀释剂BHA 特丁基-4羟基茴香醚BHT 二丁基羟基甲苯BINAP (2R,3S)-2.2'-二苯膦-1.1'-联萘,亦简称为联二萘磷,BINAP是日本名古屋大学的Noyori(2 001年诺贝尔奖)发展的一类不对称合成催化剂BL 丁内酯BLE 丙酮-二苯胺高温缩合物BLP 粉末涂料流平剂BMA 甲基丙烯酸丁酯BMC 团状模塑料BMU 氨基树脂皮革鞣剂BN 氮化硼Bn 苄基BNE 新型环氧树脂BNS β-萘磺酸甲醛低缩合物BOA 己二酸辛苄酯BOC 叔丁氧羰基(常用于氨基酸氨基的保护)BOP 邻苯二甲酰丁辛酯BOPP 双轴向聚丙烯BP 苯甲醇BPA 双酚ABPBG 邻苯二甲酸丁(乙醇酸乙酯)酯BPF 双酚FBPMC 2-仲丁基苯基-N-甲基氨基酸酯BPO 过氧化苯甲酰BPP 过氧化特戊酸特丁酯BPPD 过氧化二碳酸二苯氧化酯BPS 4,4’-硫代双(6-特丁基-3-甲基苯酚)BPTP 聚对苯二甲酸丁二醇酯Bpy 2,2'-联吡啶BR 丁二烯橡胶BRN 青红光硫化黑BROC 二溴(代)甲酚环氧丙基醚BS 丁二烯-苯乙烯共聚物BS-1S 新型密封胶BSH 苯磺酰肼BSU N,N’-双(三甲基硅烷)脲BT 聚丁烯-1热塑性塑料BTA 苯并三唑BTX 苯-甲苯-二甲苯混合物Bu 正丁基BX 渗透剂BXA 己二酸二丁基二甘酯BZ 二正丁基二硫代氨基甲酸锌Bz 苯甲酰基Cc- 环-CA 醋酸纤维素CAB 醋酸-丁酸纤维素CAM 甲基碳酰胺CAN 硝酸铈铵CAN 醋酸-硝酸纤维素CAP 醋酸-丙酸纤维素Cat. 催化CBA 化学发泡剂CBz 苄氧羰基CDP 磷酸甲酚二苯酯CF 甲醛-甲酚树脂,碳纤维CFE 氯氟乙烯CFM 碳纤维密封填料CFRP 碳纤维增强塑料CLF 含氯纤维CMC 羧甲基纤维素CMCNa 羧甲基纤维素钠CMD 代尼尔纤维CMS 羧甲基淀粉COT 1,3,5-环辛四烯Cp 环戊二烯基CSA 樟脑磺酸CTAB 十六烷基三甲基溴化铵(相转移催化剂)Cy 环己基DDABCO 1,4-二氮杂双环[2.2.2]辛烷DAF 富马酸二烯丙酯DAIP 间苯二甲酸二烯丙酯DAM 马来酸二烯丙酯DAP 间苯二甲酸二烯丙酯DATBP 四溴邻苯二甲酸二烯丙酯DBA 己二酸二丁酯dba 苄叉丙酮DBE 1,2-?二溴乙烷DBEP 邻苯二甲酸二丁氧乙酯DBN 二环[5.4.0]-1,8-二氮-7-壬烯DBP 邻苯二甲酸二丁酯DBR 二苯甲酰间苯二酚DBS 癸二酸二癸酯DBU 二环[4.3.0]-1,5-二氮-5-十一烯DCC 1,3-二环己基碳化二亚胺DCCA 二氯异氰脲酸DCCK 二氯异氰脲酸钾DCCNa 二氯异氰脲酸钠DCE 1,2-二氯乙烷DCHP 邻苯二甲酸二环乙酯DCPD 过氧化二碳酸二环乙酯DDA 己二酸二癸酯DDP 邻苯二甲酸二癸酯DDQ 2,3-二氯-5,6-二氰-1,4-苯醌DEA 二乙胺DEAD 偶氮二甲酸二乙酯DEAE 二乙胺基乙基纤维素DEP 邻苯二甲酸二乙酯DETA 二乙撑三胺DFA 薄膜胶粘剂DHA 己二酸二己酯DHP 邻苯二甲酸二己酯DHS 癸二酸二己酯DIBA 己二酸二异丁酯Dibal-H 二异丁基氢化铝DIDA 己二酸二异癸酯DIDG 戊二酸二异癸酯DIDP 邻苯二甲酸二异癸酯DINA 己二酸二异壬酯DINP 邻苯二甲酸二异壬酯DINZ 壬二酸二异壬酯DIOA 己酸二异辛酯diphos(dppe) 1,2-双(二苯基膦)乙烷diphos-4(dppb) 1,2-双(二苯基膦)丁烷DMAP 4-二甲氨基吡啶DME 二甲醚DMF 二甲基甲酰胺dppf 双(二苯基膦基)二茂铁dppp 1,3-双(二苯基膦基)丙烷dvb 二乙烯苯Ee- 电解E/EA 乙烯/丙烯酸乙酯共聚物E/P 乙烯/丙烯共聚物E/P/D 乙烯/丙烯/二烯三元共聚物E/TEE 乙烯/四氟乙烯共聚物E/VAC 乙烯/醋酸乙烯酯共聚物E/VAL 乙烯/乙烯醇共聚物EAA 乙烯-丙烯酸共聚物EAK 乙基戊丙酮EBM 挤出吹塑模塑EC 乙基纤维素ECB 乙烯共聚物和沥青的共混物ECD 环氧氯丙烷橡胶ECTEE 聚(乙烯-三氟氯乙烯)ED-3 环氧酯EDA 乙二胺EDC 二氯乙烷EDTA 乙二胺四乙酸二钠EDTA 乙二胺四醋酸EE 乙氧基乙基EEA 乙烯-醋酸丙烯共聚物EG 乙二醇2-EH 异辛醇EO 环氧乙烷EOT 聚乙烯硫醚EP 环氧树脂EPI 环氧氯丙烷EPM 乙烯-丙烯共聚物EPOR 三元乙丙橡胶EPR 乙丙橡胶EPS 可发性聚苯乙烯EPSAN 乙烯-丙烯-苯乙烯-丙烯腈共聚物EPT 乙烯丙烯三元共聚物EPVC 乳液法聚氯乙烯Et 乙基EU 聚醚型聚氨酯EVA 乙烯-醋酸乙烯共聚物EVE 乙烯基乙基醚EXP 醋酸乙烯-乙烯-丙烯酸酯三元共聚乳液FF/VAL 乙烯/乙烯醇共聚物F-23 四氟乙烯-偏氯乙烯共聚物F-30 三氟氯乙烯-乙烯共聚物F-40 四氟氯乙烯-乙烯共聚物FDY 丙纶全牵伸丝FEP 全氟(乙烯-丙烯)共聚物FMN 黄素单核苷酸FNG 耐水硅胶Fp 闪点或茂基二羰基铁FPM 氟橡胶FRA 纤维增强丙烯酸酯FRC 阻燃粘胶纤维FRP 纤维增强塑料FRPA-101 玻璃纤维增强聚癸二酸癸胺(玻璃纤维增强尼龙1010树脂)FRPA-610 玻璃纤维增强聚癸二酰乙二胺(玻璃纤维增强尼龙610树脂)FVP 闪式真实热解法FWA 荧光增白剂精品文档GGF 玻璃纤维GFRP 玻璃纤维增强塑料GFRTP 玻璃纤维增强热塑性塑料促进剂GOF 石英光纤GPS 通用聚苯乙烯GR-1 异丁橡胶GR-N 丁腈橡胶GR-S 丁苯橡胶GRTP 玻璃纤维增强热塑性塑料GUV 紫外光固化硅橡胶涂料GX 邻二甲苯GY 厌氧胶Hh 小时H 乌洛托品1,5-HD 1,5-己二烯HDI 六甲撑二异氰酸酯HDPE 低压聚乙烯(高密度)HEDP 1-羟基乙叉-1,1-二膦酸HFP 六氟丙烯HIPS 高抗冲聚苯乙烯HLA 天然聚合物透明质胶HLD 树脂性氯丁胶HM 高甲氧基果胶HMC 高强度模塑料5楼HMF 非干性密封胶HMPA 六甲基磷酸三胺HMPT 六甲基磷酰胺HOPP 均聚聚丙烯HPC 羟丙基纤维素HPMC 羟丙基甲基纤维素HPMCP 羟丙基甲基纤维素邻苯二甲酸酯HPT 六甲基磷酸三酰胺HS 六苯乙烯HTPS 高冲击聚苯乙烯hv 光照IIEN 互贯网络弹性体IHPN 互贯网络均聚物IIR 异丁烯-异戊二烯橡胶IO 离子聚合物IPA 异丙醇IPN 互贯网络聚合物iPr 异丙基IR 异戊二烯橡胶IVE 异丁基乙烯基醚JJSF 聚乙烯醇缩醛胶JZ 塑胶粘合剂KKSG 空分硅胶LLAH 氢化铝锂(LiAlH4)LAS 十二烷基苯磺酸钠LCM 液态固化剂LDA 二异丙基氨基锂(有机中最重要一种大体积强碱)LDJ 低毒胶粘剂LDN 氯丁胶粘剂LDPE 高压聚乙烯(低密度)LDR 氯丁橡胶LF 脲LGP 液化石油气LHMDS 六甲基叠氮乙硅锂LHPC 低替代度羟丙基纤维素LIM 液体侵渍模塑LIPN 乳胶互贯网络聚合物LJ 接体型氯丁橡胶LLDPE 线性低密度聚乙烯LM 低甲氧基果胶LMG 液态甲烷气LMWPE 低分子量聚乙稀LN 液态氮LRM 液态反应模塑LRMR 增强液体反应模塑LSR 羧基氯丁乳胶LTBA 氢化三叔丁氧基铝锂MMA 丙烯酸甲酯MAA 甲基丙烯酸MABS 甲基丙烯酸甲酯-丙烯腈-丁二烯-苯乙烯共聚物MAL 甲基丙烯醛MBS 甲基丙烯酸甲酯-丁二烯-苯乙烯共聚物MBTE 甲基叔丁基醚MC 甲基纤维素MCA 三聚氰胺氰脲酸盐MCPA-6 改性聚己内酰胺(铸型尼龙6)mCPBA 间氯过苯酸MCR 改性氯丁冷粘鞋用胶MDI 二苯甲烷二异氰酸酯(甲撑二苯基二异氰酸酯)MDI 3,3’-二甲基-4,4’-二氨基二苯甲烷MDPE 中压聚乙烯(高密度)Me 甲基Me MethylMEK 丁酮(甲乙酮)MEKP 过氧化甲乙酮MEM 甲氧基乙氧基甲基-MES 脂肪酸甲酯磺酸盐Mes 均三甲苯基(也就是1,3,5-三甲基苯基)MF 三聚氰胺-甲醛树脂M-HIPS 改性高冲聚苯乙烯MIBK 甲基异丁基酮Min 分钟MMA 甲基丙烯酸甲酯MMF 甲基甲酰胺MNA 甲基丙烯腈MOM 甲氧甲基MPEG 乙醇酸乙酯MPF 三聚氨胺-酚醛树脂MPK 甲基丙基甲酮M-PP 改性聚丙烯MPPO 改性聚苯醚MPS 改性聚苯乙烯Ms 甲基磺酰基(保护羟基用)MS 分子筛MS 苯乙烯-甲基丙烯酸甲酯树脂MSO 石油醚MTBE 甲基叔丁基醚MTM 甲硫基甲基MTT 氯丁胶新型交联剂MWR 旋转模塑MXD-10/6 醇溶三元共聚尼龙MXDP 间苯二甲基二胺NNaphth 萘基NBD 二环庚二烯(别名:降冰片二烯)NBR 丁腈橡胶NBS N-溴代丁二酰亚胺?别名:N-溴代琥珀酰亚胺NCS N-氯代丁二酰亚胺.?别名:N-氯代琥珀酰亚胺NDI 二异氰酸萘酯NDOP 邻苯二甲酸正癸辛酯NHDP 邻苯二甲酸己正癸酯NHTM 偏苯三酸正己酯Ni(R) 雷尼镍(氢活性催化还原剂)NINS 癸二酸二异辛酯NLS 正硬脂酸铅NMO N-甲基氧化吗啉NMP N-甲基吡咯烷酮NODA 己二酸正辛正癸酯NODP 邻苯二甲酸正辛正癸酯NPE 壬基酚聚氧乙烯醚NR 天然橡胶OOBP 邻苯二甲酸辛苄酯ODA 己二酸异辛癸酯ODPP 磷酸辛二苯酯OIDD 邻苯二甲酸正辛异癸酯精品文档OPP 定向聚丙烯(薄膜)OPS 定向聚苯乙烯(薄膜)OPVC 正向聚氯乙烯OT 气熔胶PPA 聚酰胺(尼龙)PA-1010 聚癸二酸癸二胺(尼龙1010)PA-11 聚十一酰胺(尼龙11)PA-12 聚十二酰胺(尼龙12)PA-6 聚己内酰胺(尼龙6)PA-610 聚癸二酰乙二胺(尼龙610)PA-612 聚十二烷二酰乙二胺(尼龙612)PA-66 聚己二酸己二胺(尼龙66)PA-8 聚辛酰胺(尼龙8)PA-9 聚9-氨基壬酸(尼龙9)PAA 聚丙烯酸PAAS 水质稳定剂PABM 聚氨基双马来酰亚胺PAC 聚氯化铝PAEK 聚芳基醚酮PAI 聚酰胺-酰亚胺6楼PAM 聚丙烯酰胺PAMBA 抗血纤溶芳酸PAMS 聚α-甲基苯乙烯PAN 聚丙烯腈PAP 对氨基苯酚PAPA 聚壬二酐PAPI 多亚甲基多苯基异氰酸酯PAR 聚芳酯(双酚A型)PAR 聚芳酰胺PAS 聚芳砜(聚芳基硫醚)PB 聚丁二烯-〔1,3]PBAN 聚(丁二烯-丙烯腈)PBI 聚苯并咪唑PBMA 聚甲基丙烯酸正丁酯PBN 聚萘二酸丁醇酯PBS 聚(丁二烯-苯乙烯)PBT 聚对苯二甲酸丁二酯PC 聚碳酸酯PC/ABS 聚碳酸酯/ABS树脂共混合金PC/PBT 聚碳酸酯/聚对苯二甲酸丁二醇酯弹性体共混合金PCC 吡啶氯铬酸盐PCD 聚羰二酰亚胺PCDT 聚(1,4-环己烯二亚甲基对苯二甲酸酯)PCE 四氯乙烯PCMX 对氯间二甲酚PCT 聚己内酰胺PCT 聚对苯二甲酸环己烷对二甲醇酯PCTEE 聚三氟氯乙烯PD 二羟基聚醚PDAIP 聚间苯二甲酸二烯丙酯PDAP 聚对苯二甲酸二烯丙酯PDC 重铬酸吡啶PDMS 聚二甲基硅氧烷PEG 聚乙二醇Ph 苯基PhH 苯PhMe 甲苯Phth 邻苯二甲酰Pip 哌啶基Pr n-丙基Py 吡啶Qquant. 定量产率RRE 橡胶粘合剂Red-Al [(MeOCH2CH2O)AlH2]NaRF 间苯二酚-甲醛树脂RFL 间苯二酚-甲醛乳胶RP 增强塑料RP/C 增强复合材料RX 橡胶软化剂SS/MS 苯乙烯-α-甲基苯乙烯共聚物SAN 苯乙烯-丙烯腈共聚物SAS 仲烷基磺酸钠SB 苯乙烯-丁二烯共聚物SBR 丁苯橡胶SBS 苯乙烯-丁二烯-苯乙烯嵌段共聚物sBu 仲丁基sBuLi 仲丁基锂SC 硅橡胶气调织物膜SDDC N,N-二甲基硫代氨基甲酸钠SE 磺乙基纤维素SGA 丙烯酸酯胶SI 聚硅氧烷Siamyl 二异戊基SIS 苯乙烯-异戊二烯-苯乙烯嵌段共聚物SIS/SEBS 苯乙烯-乙烯-丁二烯-苯乙烯共聚物SM 苯乙烯SMA 苯乙烯-顺丁烯二酸酐共聚物SPP 间规聚苯乙烯SPVC 悬浮法聚氯乙烯SR 合成橡胶ST 矿物纤维TTAC 三聚氰酸三烯丙酯TAME 甲基叔戊基醚TAP 磷酸三烯丙酯TASF 三(二乙胺基)二氟三甲基锍硅酸盐TBAF 氟化四丁基铵TBDMS,?TBS 叔丁基二甲基硅烷基(羟基保护基)TBE 四溴乙烷TBHP 过氧叔丁醇TBP 磷酸三丁酯t-Bu 叔丁基TCA 三醋酸纤维素TCCA 三氯异氰脲酸TCF 磷酸三甲酚酯TCPP 磷酸三氯丙酯TDI 甲苯二异氰酸酯TEA 三乙胺TEAE 三乙氨基乙基纤维素TEBA 三乙基苄基胺TEDA 三乙二胺TEFC 三氟氯乙烯TEMPO 四甲基氧代胡椒联苯自由基TEP 磷酸三乙酯Tf?or?OTf 三氟甲磺酸TFA 三氟乙酸TFAA 三氟乙酸酐TFE 四氟乙烯THF 四氢呋喃THF 四氢呋喃THP 四氢吡喃基TLCP 热散液晶聚酯TMEDA 四甲基乙二胺TMP 三羟甲基丙烷TMP 2,2,6,6-四甲基哌啶TMPD 三甲基戊二醇TMS 三甲基硅烷基TMTD 二硫化四甲基秋兰姆(硫化促进剂TT)TNP 三壬基苯基亚磷酸酯Tol 甲苯基TPA 对苯二甲酸TPE 磷酸三苯酯TPS 韧性聚苯乙烯TPU 热塑性聚氨酯树脂Tr 三苯基TR 聚硫橡胶TRIS 三异丙基乙磺酰TRPP 纤维增强聚丙烯TR-RFT 纤维增强聚对苯二甲酸丁二醇酯TRTP 纤维增强热塑性塑料Ts?(Tos) 对甲苯磺酰基UU 脲UF 脲甲醛树脂UHMWPE 超高分子量聚乙烯UP 不饱和聚酯VVAC 醋酸乙烯酯VAE 乙烯-醋酸乙烯共聚物VAM 醋酸乙烯VAMA 醋酸乙烯-顺丁烯二酐共聚物VC 氯乙烯VC/CDC 氯乙烯/偏二氯乙烯共聚物VC/E 氯乙烯/乙烯共聚物VC/E/MA 氯乙烯/乙烯/丙烯酸甲酯共聚物VC/E/VAC 氯乙烯/乙烯/醋酸乙烯酯共聚物VC/MA 氯乙烯/丙烯酸甲酯共聚物VC/MMA 氯乙烯/甲基丙烯酸甲酯共聚物VC/OA 氯乙烯/丙烯酸辛酯共聚物VC/VAC 氯乙烯/醋酸乙烯酯共聚物VCM 氯乙烯(单体)VCP 氯乙烯-丙烯共聚物VCS 丙烯腈-氯化聚乙烯-苯乙烯共聚物VDC 偏二氯乙烯VPC 硫化聚乙烯VTPS 特种橡胶偶联剂WWF 新型橡塑填料WP 织物涂层胶WRS 聚苯乙烯球形细粒XXF 二甲苯-甲醛树脂XMC 复合材料YYH 改性氯丁胶YM 聚丙烯酸酯压敏胶乳YWG 液相色谱无定型微粒硅胶ZZE 玉米纤维ZH 溶剂型氯化天然橡胶胶粘剂ZN 粉状脲醛树脂胶。
有机化学缩写-名称-结构大全

有机化学缩写-名称-结构大全DINZ 壬二酸二异壬酯DIOA 己酸二异辛酯diphos(dppe) 1,2-双(二苯基膦)乙烷diphos-4(dppb) 1,2-双(二苯基膦)丁烷DMAP 4-二甲氨基吡啶DME 二甲醚DMF 二甲基甲酰胺dppf 双(二苯基膦基)二茂铁dppp 1,3-双(二苯基膦基)丙烷dvb 二乙烯苯Ee- 电解E/EA 乙烯/丙烯酸乙酯共聚物E/P 乙烯/丙烯共聚物E/P/D 乙烯/丙烯/二烯三元共聚物E/TEE 乙烯/四氟乙烯共聚物E/V AC 乙烯/醋酸乙烯酯共聚物E/V AL 乙烯/乙烯醇共聚物EAA 乙烯-丙烯酸共聚物EAK 乙基戊丙酮EBM 挤出吹塑模塑EC 乙基纤维素ECB 乙烯共聚物和沥青的共混物ECD 环氧氯丙烷橡胶ECTEE 聚(乙烯-三氟氯乙烯)ED-3 环氧酯EDA 乙二胺EDC 二氯乙烷EDTA 乙二胺四乙酸二钠EDTA 乙二胺四醋酸EE 乙氧基乙基EEA 乙烯-醋酸丙烯共聚物EG 乙二醇2-EH 异辛醇EO 环氧乙烷EOT 聚乙烯硫醚EP 环氧树脂EPI 环氧氯丙烷EPM 乙烯-丙烯共聚物EPOR 三元乙丙橡胶EPR 乙丙橡胶EPS 可发性聚苯乙烯EPSAN 乙烯-丙烯-苯乙烯-丙烯腈共聚物EPT 乙烯丙烯三元共聚物EPVC 乳液法聚氯乙烯Et 乙基EU 聚醚型聚氨酯EV A 乙烯-醋酸乙烯共聚物EVE 乙烯基乙基醚EXP 醋酸乙烯-乙烯-丙烯酸酯三元共聚乳液FF/V AL 乙烯/乙烯醇共聚物F-23 四氟乙烯-偏氯乙烯共聚物F-30 三氟氯乙烯-乙烯共聚物F-40 四氟氯乙烯-乙烯共聚物FDY 丙纶全牵伸丝FEP 全氟(乙烯-丙烯)共聚物FMN 黄素单核苷酸FNG 耐水硅胶Fp 闪点或茂基二羰基铁FPM 氟橡胶FRA 纤维增强丙烯酸酯FRC 阻燃粘胶纤维FRP 纤维增强塑料FRPA-101 玻璃纤维增强聚癸二酸癸胺(玻璃纤维增强尼龙1010树脂)FRPA-610 玻璃纤维增强聚癸二酰乙二胺(玻璃纤维增强尼龙610树脂)FVP 闪式真实热解法FWA 荧光增白剂GGF 玻璃纤维GFRP 玻璃纤维增强塑料GFRTP 玻璃纤维增强热塑性塑料促进剂GOF 石英光纤GPS 通用聚苯乙烯GR-1 异丁橡胶GR-N 丁腈橡胶GR-S 丁苯橡胶GRTP 玻璃纤维增强热塑性塑料GUV 紫外光固化硅橡胶涂料GX 邻二甲苯GY 厌氧胶Hh 小时H 乌洛托品1,5-HD 1,5-己二烯HDI 六甲撑二异氰酸酯HDPE 低压聚乙烯(高密度)HEDP 1-羟基乙叉-1,1-二膦酸HFP 六氟丙烯HIPS 高抗冲聚苯乙烯HLA 天然聚合物透明质胶HLD 树脂性氯丁胶HM 高甲氧基果胶HMC 高强度模塑料HMF 非干性密封胶HMPA 六甲基磷酸三胺HMPT 六甲基磷酰胺HOPP 均聚聚丙烯HPC 羟丙基纤维素HPMC 羟丙基甲基纤维素HPMCP 羟丙基甲基纤维素邻苯二甲酸酯HPT 六甲基磷酸三酰胺HS 六苯乙烯HTPS 高冲击聚苯乙烯hv 光照IIEN 互贯网络弹性体IHPN 互贯网络均聚物IIR 异丁烯-异戊二烯橡胶IO 离子聚合物IPA 异丙醇IPN 互贯网络聚合物iPr 异丙基IR 异戊二烯橡胶IVE 异丁基乙烯基醚JJSF 聚乙烯醇缩醛胶JZ 塑胶粘合剂KKSG 空分硅胶LLAH 氢化铝锂(LiAlH4)LAS 十二烷基苯磺酸钠LCM 液态固化剂LDA 二异丙基氨基锂(有机中最重要一种大体积强碱)LDJ 低毒胶粘剂LDN 氯丁胶粘剂LDPE 高压聚乙烯(低密度)LDR 氯丁橡胶LF 脲LGP 液化石油气LHMDS 六甲基叠氮乙硅锂LHPC 低替代度羟丙基纤维素LIM 液体侵渍模塑LIPN 乳胶互贯网络聚合物LJ 接体型氯丁橡胶LLDPE 线性低密度聚乙烯LM 低甲氧基果胶LMG 液态甲烷气LMWPE 低分子量聚乙稀LN 液态氮LRM 液态反应模塑LRMR 增强液体反应模塑LSR 羧基氯丁乳胶LTBA 氢化三叔丁氧基铝锂MMA 丙烯酸甲酯MAA 甲基丙烯酸MABS 甲基丙烯酸甲酯-丙烯腈-丁二烯-苯乙烯共聚物MAL 甲基丙烯醛MBS 甲基丙烯酸甲酯-丁二烯-苯乙烯共聚物MBTE 甲基叔丁基醚MC 甲基纤维素MCA 三聚氰胺氰脲酸盐1 有机化学合成常见缩写Ac Acetyl乙酰基DMAP 4-dimethylaminopyridine4-二甲氨基吡啶acac Acetylacetonate乙酰丙酮基DME dimethoxyethane二甲醚AIBN Azo-bis-isobutryonitrile2,2'-二偶氮异丁腈DMF N,N'-dimethylformamide二甲基甲酰胺aq. Aqueous水溶液dppf bis (diphenylphosphino)ferrocene双(二苯基膦基)二茂铁9-BBN 9-borabicyclo[3.3.1]nonane9-硼二环[3.3.1]壬烷dppp 1,3-bis (diphenylphosphino)propane1,3-双(二苯基膦基)丙烷BINAP (2R,3S)-2,2’-bis (diphenylphosphino)-1,1’-binaphthyl(2R,3S)-2.2'-二苯膦-1.1'-联萘亦简称为联二萘磷BINAP是日本名古屋大学的Noyori(2001年诺贝尔奖)发展的一类不对称合成催化剂dvb Divinylbenzene二乙烯苯Bn Benzyl苄基e- Electrolysis 电解BOC t-butoxycarbonyl 叔丁氧羰基(常用于氨基酸氨基的保护)%ee % enantiomeric excess 对映体过量百分比(不对称合成术语)%de % diasteromeric excess 非对映体过量百分比(不对称合成术语)Bpy (Bipy) 2,2’-bipyridyl 2,2'-联吡啶EDA (en) ethylenediamine乙二胺Bu n-butyl正丁基EDTA Ethylenediaminetetraacetic acid 乙二胺四乙酸二钠Bz Benzoyl苯甲酰基EE 1-ethoxyethyl乙氧基乙基c- Cyclo环-Et Ethyl乙基FMN Flavin mononucleotide黄素单核苷酸CAN Ceric ammonium nitrate硝酸铈铵Cat. Catalytic催化Fp flash point闪点CBz Carbobenzyloxy苄氧羰基FVP Flash vacuum pyrolysis闪式真实热解法h hours小时Min Minute分钟hv Irradiation with light 光照COT 1,3,5-cyclooctatrienyl1,3,5-环辛四烯1,5-HD 1,5-hexadienyl1,5-己二烯Cp Cyclopentadienyl环戊二烯基HMPA Hexamethylphosphoramide六甲基磷酸三胺CSA 10-camphorsulfonic acid樟脑磺酸HMPT Hexamethylphosphorus triamide六甲基磷酰胺CTAB Cetyltrimethylammonium bromide 十六烷基三甲基溴化铵(相转移催化剂)iPr isopropyl异丙基Cy Cyclohexyl环己基LAH Lithium aluminum hydride氢化铝锂(LiAlH4)LDA Lithium diisopropylamide 二异丙基氨基锂(有机中最重要一种大体积强碱)2 有机化学合成常见缩写dba Dibenzylidene acetoneLHMDS Lithium hexamethyldiDBE 1,2-dibromoethane溴乙烷LTBA Lithium tri-tert-butoxyaDBN 1,8-diazabicyclo[5.4[5.4.0]-1,8-二氮-7-壬烯mCPBA meta-cholorperoxybenzoic acid 间氯过苯酸DBU 1,5-diazabicyclo[4.3.0]non-5-ene 二环[4.3.0]-1,5-二氮-5-十一烯Me Methyl 甲基DCC 1,3-dicyclohexylcarbodiimide 1,3-二环己基碳化二亚胺MEM b-methoxyethoxymethyl 甲氧基乙氧基甲基-DCE 1,2-dichloroethane 1,2-二氯乙烷Mes Mesityl 均三甲苯基(也就是1,3,5-三甲基苯基)不知对不对DDQ 2,3-dichloro-5,6-dicyano-1,4-benzoquinone 2,3-二氯-5,6-二氰-1,4-苯醌MOM methoxymethyl 甲氧甲基DEA Diethylamine 二乙胺Ms Methanesulfonyl 甲基磺酰基(保护羟基用)TBDMS, TBS t-butyldimethylsilyl 叔丁基二甲基硅烷基(羟基保护基)DEAD Diethyl azodicarboxylate 偶氮二甲酸二乙酯MS Molecular sieves (3 or 4 )分子筛Dibal-H Diisobutylaluminum hydride 二异丁基氢化铝MTM Methylthiomethyl 二甲硫醚diphos (dppe) 1,2-bis (diphenylphosphino)ethane 1,2-双(二苯基膦)乙烷Naphth Naphthyl 萘基diphos-4 (dppb) 1,4-bis (diphenylphosphino)butane 1,2-双(二苯基膦)丁烷NBD Norbornadiene 二环庚二烯(别名:降冰片二烯)NBS N-Bromosuccinimide N-溴代丁二酰亚胺别名:N-溴代琥珀酰亚胺NCS N-chlorosuccinimide N-氯代丁二酰亚胺. 别名:N-氯代琥珀酰亚胺TBAF Tetrabutylammonium fluoride 氟化四丁基铵TASF Tris(diethylamino)sulfonium difluorotrimethyl silicateNi(R) Raney Nickel 雷尼镍(氢活性催化还原剂)NMO N-methyl morpholine-n-oxide N-甲基氧化吗啉TBHP t-butylhydroperoxide 过氧叔丁醇PCC Pyridinium chlorochromate 吡啶氯铬酸盐PDC Pyridinium dichromate 是什么东西?t-Bu Tert-butyl 叔丁基TEBA Triethylbenzylammonium 三乙基苄基胺PEG Polyethylene glycol 聚乙二醇TEMPO Tetramethylpiperdinyloxy free radicalPh Phenyl 苯基PhH Benzene 苯TFA Trifluoroacetic acid 三氟乙酸TFAA Trifluoroacetic anhydride 三氟乙酸酐PhMe Toluene 甲苯(亦称toluol;methylbenzene)Tol Tolyl 甲苯基Tf or OTf TriflatePhth Phthaloyl 邻苯二甲酰THF Tetrahydrofuran 四氢呋喃Pip Piperidyl 哌啶基THP Tetrahydropyranyl 四氢吡喃基TMEDA Tetramethylethylenediamine 四甲基乙二胺Py Pyridine 吡啶TMP 2,2,6,6-tetramethylpiperidine 2,2,6,6-四甲基哌啶quant. quantitative yield 定量产率(对否?)TMS Trimethylsilyl 三甲基硅烷基Red-Al [(MeOCH2CH2O)AlH2]Na 直接看分子式就是了sBu sec-butyl 仲丁基Tr Trityl 三苯基sBuLi sec-butyllithium 仲丁基锂TRIS TriisopropylphenylsulfonylSiamyl DiisoamylTs (Tos) Tosyl (p-toluenesulfonyl) 对甲苯磺酰基%%de 非对映体过量百分比(不对称合成术语)%ee 对映体过量百分比(不对称合成术语)AA/MMA 丙烯腈/甲基丙烯酸甲酯共聚物AA 丙烯酸AAS 丙烯酸酯-丙烯酸酯-苯乙烯共聚物ABFN 偶氮(二)甲酰胺ABN 偶氮(二)异丁腈ABPS 壬基苯氧基丙烷磺酸钠Ac 乙酰基acac 乙酰丙酮基AIBN 2,2'-二偶氮异丁腈aq. 水溶液BBAA 正丁醛苯胺缩合物BAC 碱式氯化铝BACN 新型阻燃剂BAD 双水杨酸双酚A酯BAL 2,3-巯(基)丙醇9-BBN 9-硼二环[3.3.1]壬烷BBP 邻苯二甲酸丁苄酯BBS N-叔丁基-乙-苯并噻唑次磺酰胺BC 叶酸BCD β-环糊精BCG 苯顺二醇BCNU 氯化亚硝脲BD 丁二烯BE 丙烯酸乳胶外墙涂料BEE 苯偶姻乙醚BFRM 硼纤维增强塑料BG 丁二醇BGE 反应性稀释剂BHA 特丁基-4羟基茴香醚BHT 二丁基羟基甲苯BINAP (2R,3S)-2.2'-二苯膦-1.1'-联萘,亦简称为联二萘磷,BINAP是日本名古屋大学的Noyori(2001年诺贝尔奖)发展的一类不对称合成催化剂BL 丁内酯BLE 丙酮-二苯胺高温缩合物BLP 粉末涂料流平剂BMA 甲基丙烯酸丁酯BMC 团状模塑料BMU 氨基树脂皮革鞣剂BN 氮化硼Bn 苄基BNE 新型环氧树脂BNS β-萘磺酸甲醛低缩合物BOA 己二酸辛苄酯BOC 叔丁氧羰基(常用于氨基酸氨基的保护)BOP 邻苯二甲酰丁辛酯BOPP 双轴向聚丙烯BP 苯甲醇BPA 双酚ABPBG 邻苯二甲酸丁(乙醇酸乙酯)酯BPF 双酚FBPMC 2-仲丁基苯基-N-甲基氨基酸酯BPO 过氧化苯甲酰BPP 过氧化特戊酸特丁酯BPPD 过氧化二碳酸二苯氧化酯BPS 4,4’-硫代双(6-特丁基-3-甲基苯酚)BPTP 聚对苯二甲酸丁二醇酯Bpy 2,2'-联吡啶BR 丁二烯橡胶BRN 青红光硫化黑BROC 二溴(代)甲酚环氧丙基醚BS 丁二烯-苯乙烯共聚物BS-1S 新型密封胶BSH 苯磺酰肼BSU N,N’-双(三甲基硅烷)脲BT 聚丁烯-1热塑性塑料BTA 苯并三唑BTX 苯-甲苯-二甲苯混合物Bu 正丁基BX 渗透剂BXA 己二酸二丁基二甘酯BZ 二正丁基二硫代氨基甲酸锌Bz 苯甲酰基Cc- 环-CA 醋酸纤维素CAB 醋酸-丁酸纤维素CAM 甲基碳酰胺CAN 硝酸铈铵CAN 醋酸-硝酸纤维素CAP 醋酸-丙酸纤维素Cat. 催化CBA 化学发泡剂CBz 苄氧羰基CDP 磷酸甲酚二苯酯CF 甲醛-甲酚树脂,碳纤维CFE 氯氟乙烯CFM 碳纤维密封填料CFRP 碳纤维增强塑料CLF 含氯纤维CMC 羧甲基纤维素CMCNa 羧甲基纤维素钠CMD 代尼尔纤维CMS 羧甲基淀粉COT 1,3,5-环辛四烯Cp 环戊二烯基CSA 樟脑磺酸CTAB 十六烷基三甲基溴化铵(相转移催化剂)Cy 环己基DDABCO 1,4-二氮杂双环[2.2.2]辛烷DAF 富马酸二烯丙酯DAIP 间苯二甲酸二烯丙酯DAM 马来酸二烯丙酯DAP 间苯二甲酸二烯丙酯DA TBP 四溴邻苯二甲酸二烯丙酯DBA 己二酸二丁酯dba 苄叉丙酮DBE 1,2-?二溴乙烷DBEP 邻苯二甲酸二丁氧乙酯DBN 二环[5.4.0]-1,8-二氮-7-壬烯DBP 邻苯二甲酸二丁酯DBR 二苯甲酰间苯二酚DBS 癸二酸二癸酯DBU 二环[4.3.0]-1,5-二氮-5-十一烯DCC 1,3-二环己基碳化二亚胺DCCA 二氯异氰脲酸DCCK 二氯异氰脲酸钾DCCNa 二氯异氰脲酸钠DCE 1,2-二氯乙烷DCHP 邻苯二甲酸二环乙酯DCPD 过氧化二碳酸二环乙酯DDA 己二酸二癸酯DDP 邻苯二甲酸二癸酯DDQ 2,3-二氯-5,6-二氰-1,4-苯醌DEA 二乙胺DEAD 偶氮二甲酸二乙酯DEAE 二乙胺基乙基纤维素DEP 邻苯二甲酸二乙酯DETA 二乙撑三胺DFA 薄膜胶粘剂DHA 己二酸二己酯DHP 邻苯二甲酸二己酯DHS 癸二酸二己酯DIBA 己二酸二异丁酯Dibal-H 二异丁基氢化铝DIDA 己二酸二异癸酯DIDG 戊二酸二异癸酯DIDP 邻苯二甲酸二异癸酯DINA 己二酸二异壬酯DINP 邻苯二甲酸二异壬酯MCPA-6 改性聚己内酰胺(铸型尼龙6)mCPBA 间氯过苯酸MCR 改性氯丁冷粘鞋用胶MDI 二苯甲烷二异氰酸酯(甲撑二苯基二异氰酸酯)MDI 3,3’-二甲基-4,4’-二氨基二苯甲烷MDPE 中压聚乙烯(高密度)Me 甲基Me MethylMEK 丁酮(甲乙酮)MEKP 过氧化甲乙酮MEM 甲氧基乙氧基甲基-MES 脂肪酸甲酯磺酸盐Mes 均三甲苯基(也就是1,3,5-三甲基苯基)MF 三聚氰胺-甲醛树脂M-HIPS 改性高冲聚苯乙烯MIBK 甲基异丁基酮Min 分钟MMA 甲基丙烯酸甲酯MMF 甲基甲酰胺MNA 甲基丙烯腈MOM 甲氧甲基MPEG 乙醇酸乙酯MPF 三聚氨胺-酚醛树脂MPK 甲基丙基甲酮M-PP 改性聚丙烯MPPO 改性聚苯醚MPS 改性聚苯乙烯Ms 甲基磺酰基(保护羟基用)MS 分子筛MS 苯乙烯-甲基丙烯酸甲酯树脂MSO 石油醚MTBE 甲基叔丁基醚MTM 甲硫基甲基MTT 氯丁胶新型交联剂MWR 旋转模塑MXD-10/6 醇溶三元共聚尼龙MXDP 间苯二甲基二胺NNaphth 萘基NBD 二环庚二烯(别名:降冰片二烯)NBR 丁腈橡胶NBS N-溴代丁二酰亚胺?别名:N-溴代琥珀酰亚胺NCS N-氯代丁二酰亚胺.?别名:N-氯代琥珀酰亚胺NDI 二异氰酸萘酯NDOP 邻苯二甲酸正癸辛酯NHDP 邻苯二甲酸己正癸酯NHTM 偏苯三酸正己酯Ni(R) 雷尼镍(氢活性催化还原剂)NINS 癸二酸二异辛酯NLS 正硬脂酸铅NMO N-甲基氧化吗啉NMP N-甲基吡咯烷酮NODA 己二酸正辛正癸酯NODP 邻苯二甲酸正辛正癸酯NPE 壬基酚聚氧乙烯醚NR 天然橡胶OOBP 邻苯二甲酸辛苄酯ODA 己二酸异辛癸酯ODPP 磷酸辛二苯酯OIDD 邻苯二甲酸正辛异癸酯OPP 定向聚丙烯(薄膜)OPS 定向聚苯乙烯(薄膜)OPVC 正向聚氯乙烯OT 气熔胶PPA 聚酰胺(尼龙)PA-1010 聚癸二酸癸二胺(尼龙1010)PA-11 聚十一酰胺(尼龙11)PA-12 聚十二酰胺(尼龙12)PA-6 聚己内酰胺(尼龙6)PA-610 聚癸二酰乙二胺(尼龙610)PA-612 聚十二烷二酰乙二胺(尼龙612)PA-66 聚己二酸己二胺(尼龙66)PA-8 聚辛酰胺(尼龙8)PA-9 聚9-氨基壬酸(尼龙9)PAA 聚丙烯酸PAAS 水质稳定剂PABM 聚氨基双马来酰亚胺PAC 聚氯化铝PAEK 聚芳基醚酮PAI 聚酰胺-酰亚胺PAM 聚丙烯酰胺PAMBA 抗血纤溶芳酸PAMS 聚α-甲基苯乙烯PAN 聚丙烯腈PAP 对氨基苯酚PAPA 聚壬二酐PAPI 多亚甲基多苯基异氰酸酯PAR 聚芳酯(双酚A型)PAR 聚芳酰胺PAS 聚芳砜(聚芳基硫醚)PB 聚丁二烯-〔1,3]PBAN 聚(丁二烯-丙烯腈)PBI 聚苯并咪唑PBMA 聚甲基丙烯酸正丁酯PBN 聚萘二酸丁醇酯PBS 聚(丁二烯-苯乙烯)PBT 聚对苯二甲酸丁二酯PC 聚碳酸酯PC/ABS 聚碳酸酯/ABS树脂共混合金PC/PBT 聚碳酸酯/聚对苯二甲酸丁二醇酯弹性体共混合金PCC 吡啶氯铬酸盐PCD 聚羰二酰亚胺PCDT 聚(1,4-环己烯二亚甲基对苯二甲酸酯)PCE 四氯乙烯PCMX 对氯间二甲酚PCT 聚己内酰胺PCT 聚对苯二甲酸环己烷对二甲醇酯PCTEE 聚三氟氯乙烯PD 二羟基聚醚PDAIP 聚间苯二甲酸二烯丙酯PDAP 聚对苯二甲酸二烯丙酯PDC 重铬酸吡啶PDMS 聚二甲基硅氧烷PEG 聚乙二醇Ph 苯基PhH 苯PhMe 甲苯Phth 邻苯二甲酰Pip 哌啶基Pr n-丙基Py 吡啶Qquant. 定量产率RRE 橡胶粘合剂Red-Al [(MeOCH2CH2O)AlH2]NaRF 间苯二酚-甲醛树脂RFL 间苯二酚-甲醛乳胶RP 增强塑料RP/C 增强复合材料RX 橡胶软化剂SS/MS 苯乙烯-α-甲基苯乙烯共聚物SAN 苯乙烯-丙烯腈共聚物SAS 仲烷基磺酸钠SB 苯乙烯-丁二烯共聚物SBR 丁苯橡胶SBS 苯乙烯-丁二烯-苯乙烯嵌段共聚物sBu 仲丁基sBuLi 仲丁基锂SC 硅橡胶气调织物膜SDDC N,N-二甲基硫代氨基甲酸钠SE 磺乙基纤维素SGA 丙烯酸酯胶SI 聚硅氧烷Siamyl 二异戊基SIS 苯乙烯-异戊二烯-苯乙烯嵌段共聚物SIS/SEBS 苯乙烯-乙烯-丁二烯-苯乙烯共聚物SM 苯乙烯SMA 苯乙烯-顺丁烯二酸酐共聚物SPP 间规聚苯乙烯SPVC 悬浮法聚氯乙烯SR 合成橡胶ST 矿物纤维TTAC 三聚氰酸三烯丙酯TAME 甲基叔戊基醚TAP 磷酸三烯丙酯TASF 三(二乙胺基)二氟三甲基锍硅酸盐TBAF 氟化四丁基铵TBDMS,?TBS 叔丁基二甲基硅烷基(羟基保护基)TBE 四溴乙烷TBHP 过氧叔丁醇TBP 磷酸三丁酯t-Bu 叔丁基TCA 三醋酸纤维素TCCA 三氯异氰脲酸TCEF 磷酸三氯乙酯TCF 磷酸三甲酚酯TCPP 磷酸三氯丙酯TDI 甲苯二异氰酸酯TEA 三乙胺TEAE 三乙氨基乙基纤维素TEBA 三乙基苄基胺TEDA 三乙二胺TEFC 三氟氯乙烯TEMPO 四甲基氧代胡椒联苯自由基TEP 磷酸三乙酯Tf?or?OTf 三氟甲磺酸TFA 三氟乙酸TFAA 三氟乙酸酐TFE 四氟乙烯THF 四氢呋喃THF 四氢呋喃THP 四氢吡喃基TLCP 热散液晶聚酯TMEDA 四甲基乙二胺TMP 三羟甲基丙烷TMP 2,2,6,6-四甲基哌啶TMPD 三甲基戊二醇TMS 三甲基硅烷基TMTD 二硫化四甲基秋兰姆(硫化促进剂TT)TNP 三壬基苯基亚磷酸酯Tol 甲苯基TPA 对苯二甲酸TPE 磷酸三苯酯TPS 韧性聚苯乙烯TPU 热塑性聚氨酯树脂Tr 三苯基TR 聚硫橡胶TRIS 三异丙基乙磺酰TRPP 纤维增强聚丙烯TR-RFT 纤维增强聚对苯二甲酸丁二醇酯TRTP 纤维增强热塑性塑料Ts?(Tos) 对甲苯磺酰基TTP 磷酸二甲苯酯UU 脲UF 脲甲醛树脂UHMWPE 超高分子量聚乙烯UP 不饱和聚酯VV AC 醋酸乙烯酯V AE 乙烯-醋酸乙烯共聚物V AM 醋酸乙烯V AMA 醋酸乙烯-顺丁烯二酐共聚物VC 氯乙烯VC/CDC 氯乙烯/偏二氯乙烯共聚物VC/E 氯乙烯/乙烯共聚物VC/E/MA 氯乙烯/乙烯/丙烯酸甲酯共聚物VC/E/V AC 氯乙烯/乙烯/醋酸乙烯酯共聚物VC/MA 氯乙烯/丙烯酸甲酯共聚物VC/MMA 氯乙烯/甲基丙烯酸甲酯共聚物VC/OA 氯乙烯/丙烯酸辛酯共聚物VC/V AC 氯乙烯/醋酸乙烯酯共聚物VCM 氯乙烯(单体)VCP 氯乙烯-丙烯共聚物VCS 丙烯腈-氯化聚乙烯-苯乙烯共聚物VDC 偏二氯乙烯VPC 硫化聚乙烯VTPS 特种橡胶偶联剂WWF 新型橡塑填料WP 织物涂层胶WRS 聚苯乙烯球形细粒XXF 二甲苯-甲醛树脂XMC 复合材料YYH 改性氯丁胶YM 聚丙烯酸酯压敏胶乳YWG 液相色谱无定型微粒硅胶ZZE 玉米纤维ZH 溶剂型氯化天然橡胶胶粘剂ZN 粉状脲醛树脂胶IDTr3-(Imidazole-1-ylmethyl)-4’,4”-dim ethoxytriphenylmethylIETr4,4’-Dimethoxy-3”-[N-(imidazolyle thyl)carbamoyl]tritylIm Imidazole-1-yl or 1-imidazolyl iMds2,6-Dimethoxy-4-methylbenzenesul fonylINADEQUATE Incredible Natural Aboudance DoublE QUAntum transfer ExperimentINDO Incomplete neglect of differential overlapIP Ionization potentialIpaoc 1-Isopropylallyloxycarbonyl Ipc IsopinocamphenylIPCC Isopropenyl chlorocarbonate orIPCF Isopropenyl chloroformate IPDMS IsopropyldimethylsilylIR InfraredJ Coupling constant (in NMR)k KiloKAPA Potassium 3-aminopropylamideKHMDS Potassium bis(trimethylsilyl)amide or potassium hexamrthyldisilaneL Triphenylphosphine ligand or liter LCAO Linear combination ofatomic orbitalsLDBB 4,4’-Di-tert-butyybiphenylylideLev LevulinoylLevS 4,4-(Ethylenedithio)pentanoylLevS Levulinoyldithioacetal esterLFER Linear free energyrelationshipLICA LithiumisopropylcyclohexylamideLHMDS Lithiumbis(trimethylsilyl)amide or lithiumhexamrthyldisilaneLTA Lead tetraacetateLTMP Lithium2,2,6,6-tetramethylpiperidideLUMO Lowest unoccupiedmolecular orbital有机合成常见缩写中英文对照版Ac Acetyl 乙酰基DMAP 4-dimethylaminopyridine 4-二甲氨基吡啶acac Acetylacetonate 乙酰丙酮基DME dimethoxyethane 二甲醚AIBN Azo-bis-isobutryonitrile 2,2'-二偶氮异丁腈DMF N,N'-dimethylformamide 二甲基甲酰胺aq. Aqueous 水溶液dppf bis (diphenylphosphino)ferrocene 双(二苯基膦基)二茂铁9-BBN 9-borabicyclo[3.3.1]nonane 9-硼二环[3.3.1]壬烷dppp 1,3-bis (diphenylphosphino)propane 1,3-双(二苯基膦基)丙烷BINAP (2R,3S)-2,2’-bis (diphenylphosphino)-1,1’-binaphthyl(2R,3S)-2.2'-二苯膦-1.1'-联萘亦简称为联二萘磷BINAP是日本名古屋大学的Noyori(2001年诺贝尔奖)发展的一类不对称合成催化剂dvb Divinylbenzene 二乙烯苯Bn Benzyl 苄基e- Electrolysis 电解BOC t-butoxycarbonyl 叔丁氧羰基(常用于氨基酸氨基的保护)%ee % enantiomeric excess 对映体过量百分比(不对称合成术语)%de % diasteromeric excess 非对映体过量百分比(不对称合成术语)Bpy (Bipy) 2,2’-bipyridyl 2,2'-联吡啶EDA (en) ethylenediamine 乙二胺Bu n-butyl 正丁基EDTA Ethylenediaminetetraacetic acid 乙二胺四乙酸二钠Bz Benzoyl 苯甲酰基EE 1-ethoxyethyl 乙氧基乙基c- Cyclo 环-Et Ethyl 乙基FMN Flavin mononucleotide 黄素单核苷酸CAN Ceric ammonium nitrate 硝酸铈铵Cat. Catalytic 催化Fp flash point 闪点CBz Carbobenzyloxy 苄氧羰基FVP Flash vacuum pyrolysis 闪式真实热解法h hours 小时Min Minute 分钟hv Irradiation with light 光照COT 1,3,5-cyclooctatrienyl 1,3,5-环辛四烯1,5-HD 1,5-hexadienyl 1,5-己二烯Cp Cyclopentadienyl 环戊二烯基HMPA Hexamethylphosphoramide 六甲基磷酸三胺CSA 10-camphorsulfonic acid 樟脑磺酸HMPT Hexamethylphosphorus triamide 六甲基磷酰胺CTAB Cetyltrimethylammonium bromide 十六烷基三甲基溴化铵(相转移催化剂)iPr isopropyl 异丙基Cy Cyclohexyl 环己基LAH Lithium aluminum hydride 氢化铝锂(LiAlH4)LDA Lithium diisopropylamide 二异丙基氨基锂(有机中最重要一种大体积强碱)dba Dibenzylidene acetone 苄叉丙酮LHMDS Lithium hexamethyldisilazideDBE 1,2-dibromoethane 1,2- 二溴乙烷LTBA Lithium tri-tert-butoxyaluminum hydrideDBN 1,8-diazabicyclo[5.4.0]undec-7-ene 二环[5.4.0]-1,8-二氮-7-壬烯mCPBA meta-cholorperoxybenzoic acid 间氯过苯酸DBU 1,5-diazabicyclo[4.3.0]non-5-ene 二环[4.3.0]-1,5-二氮-5-十一烯Me Methyl 甲基DCC 1,3-dicyclohexylcarbodiimide 1,3-二环己基碳化二亚胺MEM b-methoxyethoxymethyl 甲氧基乙氧基甲基-DCE 1,2-dichloroethane 1,2-二氯乙烷Mes Mesityl 均三甲苯基(也就是1,3,5-三甲基苯基)不知对不对DDQ 2,3-dichloro-5,6-dicyano-1,4-benzoquinone 2,3-二氯-5,6-二氰-1,4-苯醌MOM methoxymethyl 甲氧甲基DEA Diethylamine 二乙胺Ms Methanesulfonyl 甲基磺酰基(保护羟基用)TBDMS, TBS t-butyldimethylsilyl 叔丁基二甲基硅烷基(羟基保护基)DEAD Diethyl azodicarboxylate 偶氮二甲酸二乙酯MS Molecular sieves (3 or 4 )分子筛Dibal-H Diisobutylaluminum hydride 二异丁基氢化铝MTM Methylthiomethyl 二甲硫醚diphos (dppe) 1,2-bis (diphenylphosphino)ethane 1,2-双(二苯基膦)乙烷Naphth Naphthyl 萘基diphos-4 (dppb) 1,4-bis (diphenylphosphino)butane 1,2-双(二苯基膦)丁烷NBD Norbornadiene 二环庚二烯(别名:降冰片二烯)NBS N-Bromosuccinimide N-溴代丁二酰亚胺别名:N-溴代琥珀酰亚胺NCS N-chlorosuccinimide N-氯代丁二酰亚胺. 别名:N-氯代琥珀酰亚胺TBAF Tetrabutylammonium fluoride 氟化四丁基铵TASF Tris(diethylamino)sulfonium difluorotrimethyl silicateNi(R) Raney Nickel 雷尼镍(氢活性催化还原剂)NMO N-methyl morpholine-n-oxide N-甲基氧化吗啉TBHP t-butylhydroperoxide 过氧叔丁醇PCC Pyridinium chlorochromate 吡啶氯铬酸盐PDC Pyridinium dichromate 是什么东西?t-Bu Tert-butyl 叔丁基TEBA Triethylbenzylammonium 三乙基苄基胺PEG Polyethylene glycol 聚乙二醇TEMPO Tetramethylpiperdinyloxy free radicalPh Phenyl 苯基PhH Benzene 苯TFA Trifluoroacetic acid 三氟乙酸TFAA Trifluoroacetic anhydride 三氟乙酸酐PhMe Toluene 甲苯(亦称toluol;methylbenzene )Tol Tolyl 甲苯基Tf or OTf TriflatePhth Phthaloyl 邻苯二甲酰THF Tetrahydrofuran 四氢呋喃Pip Piperidyl 哌啶基THP Tetrahydropyranyl 四氢吡喃基TMEDA Tetramethylethylenediamine 四甲基乙二胺Py Pyridine 吡啶TMP 2,2,6,6-tetramethylpiperidine 2,2,6,6-四甲基哌啶quant. quantitative yield 定量产率(对否?)TMS Trimethylsilyl 三甲基硅烷基Red-Al [(MeOCH2CH2O)AlH2]Na 直接看分子式就是了sBu sec-butyl 仲丁基Tr Trityl 三苯基sBuLi sec-butyllithium 仲丁基锂TRIS TriisopropylphenylsulfonylSiamyl DiisoamylTs (Tos) Tosyl (p-toluenesulfonyl) 对甲苯磺酰基有机化学合成常见缩写%%de 非对映体过量百分比(不对称合成术语)%ee 对映体过量百分比(不对称合成术语)AA/MMA 丙烯腈/甲基丙烯酸甲酯共聚物AA 丙烯酸AAS 丙烯酸酯-丙烯酸酯-苯乙烯共聚物ABFN 偶氮(二)甲酰胺ABN 偶氮(二)异丁腈ABPS 壬基苯氧基丙烷磺酸钠Ac 乙酰基acac 乙酰丙酮基AIBN 2,2'-二偶氮异丁腈aq. 水溶液BBAA 正丁醛苯胺缩合物BAC 碱式氯化铝BACN 新型阻燃剂BAD 双水杨酸双酚A酯BAL 2,3-巯(基)丙醇9-BBN 9-硼二环壬烷BBP 邻苯二甲酸丁苄酯BBS N-叔丁基-乙-苯并噻唑次磺酰胺BC 叶酸BCD β-环糊精BCG 苯顺二醇BCNU 氯化亚硝脲BD 丁二烯BE 丙烯酸乳胶外墙涂料BEE 苯偶姻乙醚BFRM 硼纤维增强塑料BG 丁二醇BGE 反应性稀释剂BHA 特丁基-4羟基茴香醚BHT 二丁基羟基甲苯BINAP (2R,3S)-2.2'-二苯膦-1.1'-联萘,亦简称为联二萘磷,BINAP是日本名古屋大学的Noyori(2001年诺贝尔奖)发展的一类不对称合成催化剂BL 丁内酯BLE 丙酮-二苯胺高温缩合物BLP 粉末涂料流平剂BMA 甲基丙烯酸丁酯BMC 团状模塑料BMU 氨基树脂皮革鞣剂BN 氮化硼Bn 苄基BNE 新型环氧树脂BNS β-萘磺酸甲醛低缩合物BOA 己二酸辛苄酯BOC 叔丁氧羰基(常用于氨基酸氨基的保护)BOP 邻苯二甲酰丁辛酯BOPP 双轴向聚丙烯BP 苯甲醇BPA 双酚ABPBG 邻苯二甲酸丁(乙醇酸乙酯)酯BPF 双酚FBPMC 2-仲丁基苯基-N-甲基氨基酸酯BPO 过氧化苯甲酰BPP 过氧化特戊酸特丁酯BPPD 过氧化二碳酸二苯氧化酯BPS 4,4’-硫代双(6-特丁基-3-甲基苯酚)BPTP 聚对苯二甲酸丁二醇酯Bpy 2,2'-联吡啶BR 丁二烯橡胶BRN 青红光硫化黑BROC 二溴(代)甲酚环氧丙基醚BS 丁二烯-苯乙烯共聚物BS-1S 新型密封胶BSH 苯磺酰肼BSU N,N’-双(三甲基硅烷)脲BT 聚丁烯-1热塑性塑料BTA 苯并三唑BTX 苯-甲苯-二甲苯混合物Bu 正丁基BX 渗透剂BXA 己二酸二丁基二甘酯BZ 二正丁基二硫代氨基甲酸锌Bz 苯甲酰基Cc- 环-CA 醋酸纤维素CAB 醋酸-丁酸纤维素CAM 甲基碳酰胺CAN 硝酸铈铵CAN 醋酸-硝酸纤维素CAP 醋酸-丙酸纤维素Cat. 催化CBA 化学发泡剂CBz 苄氧羰基CDP 磷酸甲酚二苯酯CF 甲醛-甲酚树脂,碳纤维CFE 氯氟乙烯CFM 碳纤维密封填料CFRP 碳纤维增强塑料CLF 含氯纤维CMC 羧甲基纤维素CMCNa 羧甲基纤维素钠CMD 代尼尔纤维CMS 羧甲基淀粉COT 1,3,5-环辛四烯Cp 环戊二烯基CSA 樟脑磺酸CTAB 十六烷基三甲基溴化铵(相转移催化剂)Cy 环己基DDABCO 1,4-二氮杂双环辛烷DAF 富马酸二烯丙酯DAIP 间苯二甲酸二烯丙酯DAM 马来酸二烯丙酯DAP 间苯二甲酸二烯丙酯DATBP 四溴邻苯二甲酸二烯丙酯DBA 己二酸二丁酯dba 苄叉丙酮DBE 1,2-?二溴乙烷DBEP 邻苯二甲酸二丁氧乙酯DBN 二环-1,8-二氮-7-壬烯DBP 邻苯二甲酸二丁酯DBR 二苯甲酰间苯二酚DBS 癸二酸二癸酯DBU 二环-1,5-二氮-5-十一烯DCC 1,3-二环己基碳化二亚胺DCCA 二氯异氰脲酸DCCK 二氯异氰脲酸钾DCCNa 二氯异氰脲酸钠DCE 1,2-二氯乙烷DCHP 邻苯二甲酸二环乙酯DCPD 过氧化二碳酸二环乙酯DDA 己二酸二癸酯DDP 邻苯二甲酸二癸酯DDQ 2,3-二氯-5,6-二氰-1,4-苯醌DEA 二乙胺DEAD 偶氮二甲酸二乙酯DEAE 二乙胺基乙基纤维素DEP 邻苯二甲酸二乙酯DETA 二乙撑三胺DFA 薄膜胶粘剂DHA 己二酸二己酯DHP 邻苯二甲酸二己酯DHS 癸二酸二己酯DIBA 己二酸二异丁酯Dibal-H 二异丁基氢化铝DIDA 己二酸二异癸酯DIDG 戊二酸二异癸酯DIDP 邻苯二甲酸二异癸酯DINA 己二酸二异壬酯DINP 邻苯二甲酸二异壬酯DINZ 壬二酸二异壬酯DIOA 己酸二异辛酯diphos(dppe) 1,2-双(二苯基膦)乙烷diphos-4(dppb) 1,2-双(二苯基膦)丁烷DMAP 4-二甲氨基吡啶DME 二甲醚DMF 二甲基甲酰胺dppf 双(二苯基膦基)二茂铁dppp 1,3-双(二苯基膦基)丙烷dvb 二乙烯苯Ee- 电解E/EA 乙烯/丙烯酸乙酯共聚物E/P 乙烯/丙烯共聚物E/P/D 乙烯/丙烯/二烯三元共聚物E/TEE 乙烯/四氟乙烯共聚物E/VAC 乙烯/醋酸乙烯酯共聚物E/VAL 乙烯/乙烯醇共聚物EAA 乙烯-丙烯酸共聚物EAK 乙基戊丙酮EBM 挤出吹塑模塑EC 乙基纤维素ECB 乙烯共聚物和沥青的共混物ECD 环氧氯丙烷橡胶ECTEE 聚(乙烯-三氟氯乙烯)ED-3 环氧酯EDA 乙二胺EDC 二氯乙烷EDTA 乙二胺四乙酸二钠EDTA 乙二胺四醋酸EE 乙氧基乙基EEA 乙烯-醋酸丙烯共聚物EG 乙二醇2-EH 异辛醇EO 环氧乙烷EOT 聚乙烯硫醚EP 环氧树脂EPI 环氧氯丙烷EPM 乙烯-丙烯共聚物EPOR 三元乙丙橡胶EPR 乙丙橡胶EPS 可发性聚苯乙烯EPSAN 乙烯-丙烯-苯乙烯-丙烯腈共聚物EPT 乙烯丙烯三元共聚物EPVC 乳液法聚氯乙烯Et 乙基EU 聚醚型聚氨酯EVA 乙烯-醋酸乙烯共聚物EVE 乙烯基乙基醚EXP 醋酸乙烯-乙烯-丙烯酸酯三元共聚乳液FF/VAL 乙烯/乙烯醇共聚物F-23 四氟乙烯-偏氯乙烯共聚物F-30 三氟氯乙烯-乙烯共聚物F-40 四氟氯乙烯-乙烯共聚物FDY 丙纶全牵伸丝FEP 全氟(乙烯-丙烯)共聚物FMN 黄素单核苷酸FNG 耐水硅胶Fp 闪点或茂基二羰基铁FPM 氟橡胶FRA 纤维增强丙烯酸酯FRC 阻燃粘胶纤维FRP 纤维增强塑料FRPA-101 玻璃纤维增强聚癸二酸癸胺(玻璃纤维增强尼龙1010树脂)FRPA-610 玻璃纤维增强聚癸二酰乙二胺(玻璃纤维增强尼龙610树脂)FVP 闪式真实热解法FWA 荧光增白剂GGF 玻璃纤维GFRP 玻璃纤维增强塑料GFRTP 玻璃纤维增强热塑性塑料促进剂GOF 石英光纤GPS 通用聚苯乙烯GR-1 异丁橡胶GR-N 丁腈橡胶GR-S 丁苯橡胶GRTP 玻璃纤维增强热塑性塑料GUV 紫外光固化硅橡胶涂料GX 邻二甲苯GY 厌氧胶Hh 小时H 乌洛托品1,5-HD 1,5-己二烯HDI 六甲撑二异氰酸酯HDPE 低压聚乙烯(高密度)HEDP 1-羟基乙叉-1,1-二膦酸HFP 六氟丙烯HIPS 高抗冲聚苯乙烯HLA 天然聚合物透明质胶HLD 树脂性氯丁胶HM 高甲氧基果胶HMC 高强度模塑料HMF 非干性密封胶HMPA 六甲基磷酸三胺HMPT 六甲基磷酰胺HOPP 均聚聚丙烯HPC 羟丙基纤维素HPMC 羟丙基甲基纤维素HPMCP 羟丙基甲基纤维素邻苯二甲酸酯HPT 六甲基磷酸三酰胺HS 六苯乙烯HTPS 高冲击聚苯乙烯hv 光照IIEN 互贯网络弹性体。
丁基甲氧基二苯甲酰基甲烷 别名

丁基甲氧基二苯甲酰基甲烷别名下载提示:该文档是本店铺精心编制而成的,希望大家下载后,能够帮助大家解决实际问题。
文档下载后可定制修改,请根据实际需要进行调整和使用,谢谢!本店铺为大家提供各种类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by this editor. I hope that after you download it, it can help you solve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you! In addition, this shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts, other materials and so on, want to know different data formats and writing methods, please pay attention!丁基甲氧基二苯甲酰基甲烷的别名及其应用1. 引言丁基甲氧基二苯甲酰基甲烷,是一种在化学领域备受关注的化合物,具有广泛的应用前景。
丁基甲氧基二苯甲酰基甲烷_稳定剂_解释说明

丁基甲氧基二苯甲酰基甲烷稳定剂解释说明1. 引言1.1 概述稳定剂是一种重要的化学品,它在各个领域中起着极其关键的作用。
稳定剂的主要功能是提高化学反应的稳定性和延长产品的寿命。
丁基甲氧基二苯甲酰基甲烷(简称BDFM)是一种常用于稳定剂中的成分。
本文将对丁基甲氧基二苯甲酰基甲烷及其在稳定剂中的作用进行详细说明。
1.2 文章结构本文将分为五个部分进行讨论。
第二部分将介绍稳定剂的定义、作用以及在化学工业中的应用。
第三部分将详细探讨丁基甲氧基二苯甲酰基甲烷的合成方法,包括材料、设备、步骤以及反应机理等方面内容。
第四部分将涉及到丁基甲氧基二苯甲酰基甲烷稳定剂性能评价方法,包括测试方法选择、评价结果分析以及影响因素探讨等方面内容。
最后,第五部分将总结全文,并展望丁基甲氧基二苯甲酰基甲烷稳定剂的潜在应用前景,并提出后续研究的方向建议。
1.3 目的本文旨在对丁基甲氧基二苯甲酰基甲烷稳定剂进行详细解释和说明。
首先介绍稳定剂概念,探讨其作用和在化学工业中的应用。
接着深入探讨丁基甲氧基二苯甲酰基甲烷的合成方法,包括材料、设备、步骤以及反应机理等方面内容。
然后介绍评价丁基甲氧基二苯甲酰基甲烷稳定剂性能的方法,并分析评价结果以及影响因素。
最后总结全文,并展望丁基甲氧基二苯甲酰基甲烷稳定剂的潜在应用前景,并提出后续研究的方向建议。
通过本文的阐述,读者将更加深入了解丁基甲氧基二苯甲酰基甲烷稳定剂及其在化学工业中的重要作用。
2. 稳定剂的定义与作用2.1 稳定剂的概念稳定剂是一种在化学反应或储存过程中添加的物质,其主要作用是提高物质的稳定性并延长其使用寿命。
稳定剂能够抑制或减少不必要的化学反应,从而防止物质发生分解、腐败、劣变等现象。
2.2 稳定剂在化学工业中的应用稳定剂广泛应用于化学工业中,特别是在聚合物材料生产和储存过程中的应用更为突出。
在聚合物材料加工过程中,会受到温度、光照、氧气等外界条件的影响,容易引起分子链断裂、交联和退色等问题。
丙烯酸单体特征及特性
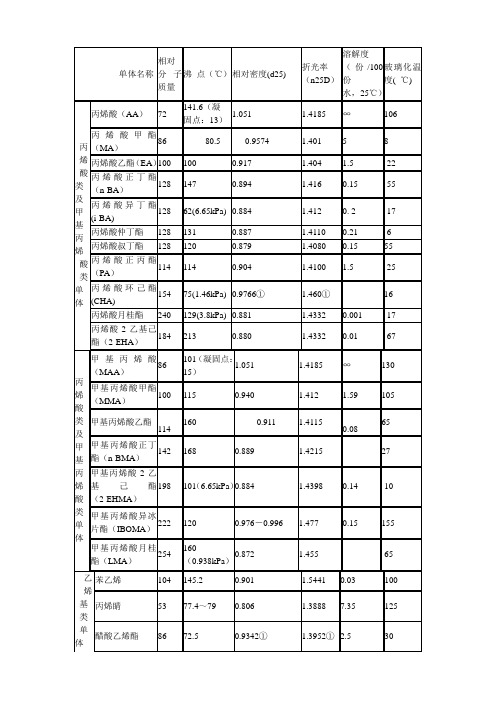
55
甲基丙烯酸-2-羟基丙酯(2-HPMA)
144.1
96(1.33kPa)
1.027
1.446
13.4
26
甲基丙烯酸三氟乙酯
168
107
1.181
1.359
0.04
82
甲基丙烯酸
缩水甘油酯(GMA)
142
189
1.073
1.4494
2.04
46
N-羟甲基丙烯酰胺
101
熔点:74~75
1.10
∞
153
55
丙烯酸正丙酯(PA)
114
114
0.904
1.4100
1.5
-25
丙烯酸环己酯(CHA)
154
75(1.46kPa)
0.9766①
1.460①
16
丙烯酸月桂酯
240
129(3.8kPa)
0.881
1.4332
0.001
-17
丙烯酸-2-乙基己酯(2-EHA)
184
213
0.880
1.4332
0.01
N-丁氧基甲基丙烯酰胺
157
125
0.96
0.001
二乙烯基苯
130.18
199.5
0.93
乙烯基三甲氧基硅烷
148
123
0.960
1.3920
γ-甲基丙烯酰氧基丙基三甲氧基硅烷
248
255
1.045
1.4295
单体名称
功能
甲基丙烯酸甲酯
甲基丙烯酸乙酯
苯乙烯
丙烯睛
提高硬度,称之为硬单体。
丙烯酸乙酯
甲基丙烯酸正丁酯(异丁酸正丁酯)的理化性质及危险特性表

①操作注意事项:密闭操作,注意通风。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴直接式防毒面具(半面罩),戴化学安全防护眼镜,穿防静电工作服,戴橡胶耐油手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。防止蒸气泄漏到工作场所空气中。避免与氧化剂、酸类、碱类接触。搬运时要轻装轻卸,防止包装及容器损坏。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。
甲基丙烯酸正丁酯(异丁酸正丁酯)的理化性质及危险特性表
标识
别名:
异丁酸正丁酯
危险货物编号:
33601
英文名:
n-butyl methacrylate
UN编号:
2227
CAS号:
97-88-1
分子式:
C8H14O
外观与性状:
无色、具有甜味和酯气味的液体。
主要用途:
用于有机合成,制造塑料、光学玻璃的粘结剂,纺织、皮革及造纸用助剂。
②储存注意事项:通常商品加有阻聚剂。储存于阴凉、通风的库房。远离火种、热源。库温不宜超过30℃。包装要求密封,不可与空气接触。应与氧化剂、酸类、碱类分开存放,切忌混储。不宜大量储存或久存。采用防爆型照明、通风设施。禁止使用易产生火花的机械设备和工具。储区应备有泄漏应急处理设备和合适的收容材料。
③运输注意事项:运输时运输车辆应配备相应品种和数量的消防器材及泄漏应急处理设备。夏季最好早晚运输。运输时所用的槽(罐)车应有接地链,槽内可设孔隔板以减少震荡产生静电。严禁与氧化剂、酸类、碱类、食用化学品等混装混运。运输途中应防曝晒、雨淋,防高温。中途停留时应远离火种、热源、高温区。装运该物品的车辆排气管必须配备阻火装置,禁止使用易产生火花的机械设备和工具装卸。公路运输时要按规定路线行驶,勿在居民区和人口稠密区停留。铁路运输时要禁止溜放。严禁用木船、水泥船散装运输。
化学名称缩写

APAO非晶性α-烯烃
APHA美国公共卫生事业协会
APR芳烃石油树脂
APS氨基丙基三乙氧基硅烷、过硫酸铵
A-PVA无规聚乙烯醇
AR丙烯酸酯橡胶、分析纯
AS澳大利亚标准
ASC胶黏剂与密封剂委员会
ASTM美国材料试验学会
ATBN端氨基液体丁腈橡胶
ATH氢氧化铝(三水合氧化铝)
ATO三氧化二锑
PIB聚异丁烯
PM丙二醇甲醚
PMA聚马来酸酐、丙二醇甲醚醋酸酯PMAA聚甲基丙烯酸
PMDA均苯四甲酸二酐
PMMA聚甲基丙烯酸甲酯
PMP丙二醇甲醚丙酸酯
PMS聚α-甲基苯乙烯
PN波兰国家标准
PNA苯基-β-萘胺
PNBR粉末丁腈橡胶
POE聚氧化乙烯
POP对辛基苯酚
PPA多聚磷酸
PPD六氢吡啶、对苯二胺
PPESK聚芳醚砜酮
EAL乙醇
EB水性环氧丙烯酸酯
EC乙基纤维素
ECH环氧氯丙烷
EDA乙二胺
EDTA乙二胺四乙酸
EEP 3-乙氧基丙酸乙酯
EEW环氧当量
EG乙二醇
EGDA二丙烯酸乙二醇酯
EGDE乙二醇二缩水甘油醚(669稀释剂)EGDMA双甲基丙烯酸乙二醇酯
2-EI 2-乙基咪唑
Em乳化剂
EMA甲基丙烯酸乙酯
EMI-2,4 2-乙基-4-甲基咪唑
BHT 2,6-二叔丁基对甲酚(264)
BIIR溴化丁基橡胶
Bis A双酚A
Bis F双酚F
Bis S双酚S
γ-BL γ-丁内酯
BMA甲基丙烯酸丁酯
BMI双马来酰来胺
BN安息香
BOA已二酸苄基辛基酯
有机化学缩写-名称-结构大全

有机化学缩写-名称-结构大全有机化学缩写-名称-结构大全DINZ 壬二酸二异壬酯DIOA 己酸二异辛酯diphos(dppe) 1,2-双(二苯基膦)乙烷diphos-4(dppb) 1,2-双(二苯基膦)丁烷DMAP 4-二甲氨基吡啶DME 二甲醚DMF 二甲基甲酰胺dppf 双(二苯基膦基)二茂铁dppp 1,3-双(二苯基膦基)丙烷dvb 二乙烯苯Ee- 电解E/EA 乙烯/丙烯酸乙酯共聚物E/P 乙烯/丙烯共聚物E/P/D 乙烯/丙烯/二烯三元共聚物E/TEE 乙烯/四氟乙烯共聚物E/V AC 乙烯/醋酸乙烯酯共聚物E/V AL 乙烯/乙烯醇共聚物EAA 乙烯-丙烯酸共聚物EAK 乙基戊丙酮EBM 挤出吹塑模塑EC 乙基纤维素ECB 乙烯共聚物和沥青的共混物ECD 环氧氯丙烷橡胶ECTEE 聚(乙烯-三氟氯乙烯)ED-3 环氧酯EDA 乙二胺EDC 二氯乙烷EDTA 乙二胺四乙酸二钠EDTA 乙二胺四醋酸EE 乙氧基乙基EEA 乙烯-醋酸丙烯共聚物EG 乙二醇2-EH 异辛醇EO 环氧乙烷EOT 聚乙烯硫醚EP 环氧树脂EPI 环氧氯丙烷EPM 乙烯-丙烯共聚物EPOR 三元乙丙橡胶EPR 乙丙橡胶EPS 可发性聚苯乙烯EPSAN 乙烯-丙烯-苯乙烯-丙烯腈共聚物EPT 乙烯丙烯三元共聚物EPVC 乳液法聚氯乙烯Et 乙基EU 聚醚型聚氨酯EV A 乙烯-醋酸乙烯共聚物EVE 乙烯基乙基醚EXP 醋酸乙烯-乙烯-丙烯酸酯三元共聚乳液FF/V AL 乙烯/乙烯醇共聚物F-23 四氟乙烯-偏氯乙烯共聚物F-30 三氟氯乙烯-乙烯共聚物F-40 四氟氯乙烯-乙烯共聚物FDY 丙纶全牵伸丝FEP 全氟(乙烯-丙烯)共聚物FMN 黄素单核苷酸FNG 耐水硅胶Fp 闪点或茂基二羰基铁FPM 氟橡胶FRA 纤维增强丙烯酸酯FRC 阻燃粘胶纤维FRP 纤维增强塑料FRPA-101 玻璃纤维增强聚癸二酸癸胺(玻璃纤维增强尼龙1010树脂)FRPA-610 玻璃纤维增强聚癸二酰乙二胺(玻璃纤维增强尼龙610树脂)FVP 闪式真实热解法FWA 荧光增白剂GGF 玻璃纤维GFRP 玻璃纤维增强塑料GFRTP 玻璃纤维增强热塑性塑料促进剂GOF 石英光纤GPS 通用聚苯乙烯GR-1 异丁橡胶GR-N 丁腈橡胶GR-S 丁苯橡胶GRTP 玻璃纤维增强热塑性塑料GUV 紫外光固化硅橡胶涂料GX 邻二甲苯GY 厌氧胶Hh 小时H 乌洛托品1,5-HD 1,5-己二烯HDI 六甲撑二异氰酸酯HDPE 低压聚乙烯(高密度)HEDP 1-羟基乙叉-1,1-二膦酸HFP 六氟丙烯HIPS 高抗冲聚苯乙烯HLA 天然聚合物透明质胶HLD 树脂性氯丁胶HM 高甲氧基果胶HMC 高强度模塑料HMF 非干性密封胶HMPA 六甲基磷酸三胺HMPT 六甲基磷酰胺HOPP 均聚聚丙烯HPC 羟丙基纤维素HPMC 羟丙基甲基纤维素HPMCP 羟丙基甲基纤维素邻苯二甲酸酯HPT 六甲基磷酸三酰胺HS 六苯乙烯HTPS 高冲击聚苯乙烯hv 光照IIEN 互贯网络弹性体IHPN 互贯网络均聚物IIR 异丁烯-异戊二烯橡胶IO 离子聚合物IPA 异丙醇IPN 互贯网络聚合物iPr 异丙基IR 异戊二烯橡胶IVE 异丁基乙烯基醚JJSF 聚乙烯醇缩醛胶JZ 塑胶粘合剂KKSG 空分硅胶LLAH 氢化铝锂(LiAlH4)LAS 十二烷基苯磺酸钠LCM 液态固化剂LDA 二异丙基氨基锂(有机中最重要一种大体积强碱)LDJ 低毒胶粘剂LDN 氯丁胶粘剂LDPE 高压聚乙烯(低密度)LDR 氯丁橡胶LF 脲LGP 液化石油气LHMDS 六甲基叠氮乙硅锂LHPC 低替代度羟丙基纤维素LIM 液体侵渍模塑LIPN 乳胶互贯网络聚合物LJ 接体型氯丁橡胶LLDPE 线性低密度聚乙烯LM 低甲氧基果胶LMG 液态甲烷气LMWPE 低分子量聚乙稀LN 液态氮LRM 液态反应模塑LRMR 增强液体反应模塑LSR 羧基氯丁乳胶LTBA 氢化三叔丁氧基铝锂MMA 丙烯酸甲酯MAA 甲基丙烯酸MABS 甲基丙烯酸甲酯-丙烯腈-丁二烯-苯乙烯共聚物MAL 甲基丙烯醛MBS 甲基丙烯酸甲酯-丁二烯-苯乙烯共聚物MBTE 甲基叔丁基醚MC 甲基纤维素MCA 三聚氰胺氰脲酸盐1 有机化学合成常见缩写Ac Acetyl乙酰基DMAP 4-dimethylaminopyridine4-二甲氨基吡啶acac Acetylacetonate乙酰丙酮基DME dimethoxyethane二甲醚AIBN Azo-bis-isobutryonitrile2,2'-二偶氮异丁腈DMF N,N'-dimethylformamide二甲基甲酰胺aq. Aqueous水溶液dppf bis (diphenylphosphino)ferrocene双(二苯基膦基)二茂铁9-BBN 9-borabicyclo[3.3.1]nonane9-硼二环[3.3.1]壬烷dppp 1,3-bis (diphenylphosphino)propane1,3-双(二苯基膦基)丙烷BINAP (2R,3S)-2,2’-bis (diphenylphosphino)-1,1’-binaphthyl(2R,3S)-2.2'-二苯膦-1.1'-联萘亦简称为联二萘磷BINAP是日本名古屋大学的Noyori(2001年诺贝尔奖)发展的一类不对称合成催化剂dvb Divinylbenzene二乙烯苯Bn Benzyl苄基e- Electrolysis 电解BOC t-butoxycarbonyl 叔丁氧羰基(常用于氨基酸氨基的保护)%ee % enantiomeric excess 对映体过量百分比(不对称合成术语)%de % diasteromeric excess 非对映体过量百分比(不对称合成术语)Bpy (Bipy) 2,2’-bipyridyl 2,2'-联吡啶EDA (en) ethylenediamine乙二胺Bu n-butyl正丁基EDTA Ethylenediaminetetraacetic acid 乙二胺四乙酸二钠Bz Benzoyl苯甲酰基EE 1-ethoxyethyl乙氧基乙基c- Cyclo环-Et Ethyl乙基FMN Flavin mononucleotide黄素单核苷酸CAN Ceric ammonium nitrateCat. Catalytic催化Fp flash point闪点CBz Carbobenzyloxy苄氧羰基FVP Flash vacuum pyrolysis闪式真实热解法h hours小时Min Minute分钟hv Irradiation with light 光照COT 1,3,5-cyclooctatrienyl1,3,5-环辛四烯1,5-HD 1,5-hexadienyl1,5-己二烯Cp Cyclopentadienyl环戊二烯基HMPA Hexamethylphosphoramide六甲基磷酸三胺CSA 10-camphorsulfonic acid樟脑磺酸HMPT Hexamethylphosphorus triamide六甲基磷酰胺CTAB Cetyltrimethylammonium bromide 十六烷基三甲基溴化铵(相转移催化剂)iPr isopropylCy Cyclohexyl环己基LAH Lithium aluminum hydride氢化铝锂(LiAlH4)LDA Lithium diisopropylamide 二异丙基氨基锂(有机中最重要一种大体积强碱)2 有机化学合成常见缩写dba Dibenzylidene aceton苄叉丙酮LHMDS LithiumhexamethyldisilazideDBE 1,2-dibromoethan1,2- 二溴乙烷LTBA Lithium tri-tert-butoxyaluminum hydrideDBN1,8-diazabicyclo[5.4.0]undec-7 -ene 二环[5.4.0]-1,8-二氮-7-壬烯mCPBAmeta-cholorperoxybenzoic acid间氯过苯酸DBU1,5-diazabicyclo[4.3.0]non-5-e ne 二环[4.3.0]-1,5-二氮-5-十一烯Me Methyl甲基DCC1,3-dicyclohexylcarbodiimide1,3-二环己基碳化二亚胺b-methoxyethoxymethyl甲氧基乙氧基甲基-DCE 1,2-dichloroethane1,2-二氯乙烷Mes Mesityl 均三甲苯基(也就是1,3,5-三甲基苯基)不知对不对?DDQ2,3-dichloro-5,6-dicyano-1,4-b enzoquinone 2,3-二氯-5,6-二氰-1,4-苯醌MOM methoxymethyl甲氧甲基DEA Diethylamine 二乙胺Ms Methanesulfonyl甲基磺酰基(保护羟基用)TBDMS, TBS t-butyldimethylsilyl叔丁基二甲基硅烷基(羟基保护基)DEAD Diethyl azodicarboxylate偶氮二甲酸二乙酯MS Molecular sieves (3 or 4 )分子筛Dibal-H Diisobutylaluminum hydride二异丁基氢化铝MTM Methylthiomethyl二甲硫醚diphos (dppe) 1,2-bis (diphenylphosphino)ethane 1,2-双(二苯基膦)乙烷Naphth Naphthyl萘基diphos-4 (dppb) 1,4-bis (diphenylphosphino)butane 1,2-双(二苯基膦)丁烷NBD Norbornadiene二环庚二烯(别名:降冰片二烯)NBS N-Bromosuccinimide N-溴代丁二酰亚胺别名:N-溴代琥珀酰亚胺NCS N-chlorosuccinimide N-氯代丁二酰亚胺. 别名:N-氯代琥珀酰亚胺TBAF Tetrabutylammonium fluoride氟化四丁基铵TASFTris(diethylamino)sulfonium difluorotrimethyl silicateNi(R) Raney Nickel雷尼镍(氢活性催化还原剂)NMO N-methyl morpholine-n-oxideN-甲基氧化吗啉TBHP t-butylhydroperoxide过氧叔丁醇PCC Pyridinium chlorochromate吡啶氯铬酸盐PDC Pyridinium dichromate是什么东西?t-Bu Tert-butyl叔丁基TEBA Triethylbenzylammonium三乙基苄基胺PEG Polyethylene glycol聚乙二醇TEMPO Tetramethylpiperdinyloxy free radicalPh Phenyl苯基PhH Benzene苯TFA Trifluoroacetic acid三氟乙酸TFAA Trifluoroacetic anhydride三氟乙酸酐PhMe Toluene甲苯(亦称toluol;methylbenzene)Tol Tolyl甲苯基Tf or OTf TriflatePhth Phthaloyl邻苯二甲酰THF Tetrahydrofuran四氢呋喃Pip Piperidyl哌啶基THP Tetrahydropyranyl四氢吡喃基TMEDA Tetramethylethylenediamine 四甲基乙二胺Py Pyridine吡啶TMP2,2,6,6-tetramethylpiperidine2,2,6,6-四甲基哌啶quant. quantitative yield定量产率(对否?)TMS Trimethylsilyl三甲基硅烷基Red-Al[(MeOCH2CH2O)AlH2]Na直接看分子式就是了sBu sec-butyl仲丁基Tr Trityl三苯基sBuLi sec-butyllithium仲丁基锂TRIS Triisopropylphenylsulfonyl Siamyl DiisoamylTs (Tos) Tosyl (p-toluenesulfonyl)对甲苯磺酰基%%de 非对映体过量百分比(不对称合成术语)%ee 对映体过量百分比(不对称合成术语)AA/MMA 丙烯腈/甲基丙烯酸甲酯共聚物AA 丙烯酸AAS 丙烯酸酯-丙烯酸酯-苯乙烯共聚物ABFN 偶氮(二)甲酰胺ABN 偶氮(二)异丁腈ABPS 壬基苯氧基丙烷磺酸钠Ac 乙酰基acac 乙酰丙酮基AIBN 2,2'-二偶氮异丁腈aq. 水溶液BBAA 正丁醛苯胺缩合物BAC 碱式氯化铝BACN 新型阻燃剂BAD 双水杨酸双酚A酯BAL 2,3-巯(基)丙醇9-BBN 9-硼二环[3.3.1]壬烷BBP 邻苯二甲酸丁苄酯BBS N-叔丁基-乙-苯并噻唑次磺酰胺BC 叶酸BCD β-环糊精BCG 苯顺二醇BCNU 氯化亚硝脲BD 丁二烯BE 丙烯酸乳胶外墙涂料BEE 苯偶姻乙醚BFRM 硼纤维增强塑料BG 丁二醇BGE 反应性稀释剂BHA 特丁基-4羟基茴香醚BHT 二丁基羟基甲苯BINAP (2R,3S)-2.2'-二苯膦-1.1'-联萘,亦简称为联二萘磷,BINAP是日本名古屋大学的Noyori(2001年诺贝尔奖)发展的一类不对称合成催化剂BL 丁内酯BLE 丙酮-二苯胺高温缩合物BLP 粉末涂料流平剂BMA 甲基丙烯酸丁酯BMC 团状模塑料BMU 氨基树脂皮革鞣剂BN 氮化硼Bn 苄基BNE 新型环氧树脂BNS β-萘磺酸甲醛低缩合物BOA 己二酸辛苄酯BOC 叔丁氧羰基(常用于氨基酸氨基的保护)BOP 邻苯二甲酰丁辛酯BOPP 双轴向聚丙烯BP 苯甲醇BPA 双酚ABPBG 邻苯二甲酸丁(乙醇酸乙酯)酯BPF 双酚FBPMC 2-仲丁基苯基-N-甲基氨基酸酯BPO 过氧化苯甲酰BPP 过氧化特戊酸特丁酯BPPD 过氧化二碳酸二苯氧化酯BPS 4,4’-硫代双(6-特丁基-3-甲基苯酚)BPTP 聚对苯二甲酸丁二醇酯Bpy 2,2'-联吡啶BR 丁二烯橡胶BRN 青红光硫化黑BROC 二溴(代)甲酚环氧丙基醚BS 丁二烯-苯乙烯共聚物BS-1S 新型密封胶BSH 苯磺酰肼BSU N,N’-双(三甲基硅烷)脲BT 聚丁烯-1热塑性塑料BTA 苯并三唑BTX 苯-甲苯-二甲苯混合物Bu 正丁基BX 渗透剂BXA 己二酸二丁基二甘酯BZ 二正丁基二硫代氨基甲酸锌Bz 苯甲酰基Cc- 环-CA 醋酸纤维素CAB 醋酸-丁酸纤维素CAM 甲基碳酰胺CAN 硝酸铈铵CAN 醋酸-硝酸纤维素CAP 醋酸-丙酸纤维素Cat. 催化CBA 化学发泡剂CBz 苄氧羰基CDP 磷酸甲酚二苯酯CF 甲醛-甲酚树脂,碳纤维CFE 氯氟乙烯CFM 碳纤维密封填料CFRP 碳纤维增强塑料CLF 含氯纤维CMC 羧甲基纤维素CMCNa 羧甲基纤维素钠CMD 代尼尔纤维CMS 羧甲基淀粉COT 1,3,5-环辛四烯Cp 环戊二烯基CSA 樟脑磺酸CTAB 十六烷基三甲基溴化铵(相转移催化剂)Cy 环己基DDABCO 1,4-二氮杂双环[2.2.2]辛烷DAF 富马酸二烯丙酯DAIP 间苯二甲酸二烯丙酯DAM 马来酸二烯丙酯DAP 间苯二甲酸二烯丙酯DA TBP 四溴邻苯二甲酸二烯丙酯DBA 己二酸二丁酯dba 苄叉丙酮DBE 1,2-?二溴乙烷DBEP 邻苯二甲酸二丁氧乙酯DBN 二环[5.4.0]-1,8-二氮-7-壬烯DBP 邻苯二甲酸二丁酯DBR 二苯甲酰间苯二酚DBS 癸二酸二癸酯DBU 二环[4.3.0]-1,5-二氮-5-十一烯DCC 1,3-二环己基碳化二亚胺DCCA 二氯异氰脲酸DCCK 二氯异氰脲酸钾DCCNa 二氯异氰脲酸钠DCE 1,2-二氯乙烷DCHP 邻苯二甲酸二环乙酯DCPD 过氧化二碳酸二环乙酯DDA 己二酸二癸酯DDP 邻苯二甲酸二癸酯DDQ 2,3-二氯-5,6-二氰-1,4-苯醌DEA 二乙胺DEAD 偶氮二甲酸二乙酯DEAE 二乙胺基乙基纤维素DEP 邻苯二甲酸二乙酯DETA 二乙撑三胺DFA 薄膜胶粘剂DHA 己二酸二己酯DHP 邻苯二甲酸二己酯DHS 癸二酸二己酯DIBA 己二酸二异丁酯Dibal-H 二异丁基氢化铝DIDA 己二酸二异癸酯DIDG 戊二酸二异癸酯DIDP 邻苯二甲酸二异癸酯DINA 己二酸二异壬酯DINP 邻苯二甲酸二异壬酯MCPA-6 改性聚己内酰胺(铸型尼龙6)mCPBA 间氯过苯酸MCR 改性氯丁冷粘鞋用胶MDI 二苯甲烷二异氰酸酯(甲撑二苯基二异氰酸酯)MDI 3,3’-二甲基-4,4’-二氨基二苯甲烷MDPE 中压聚乙烯(高密度)Me 甲基Me MethylMEK 丁酮(甲乙酮)MEKP 过氧化甲乙酮MEM 甲氧基乙氧基甲基-MES 脂肪酸甲酯磺酸盐Mes 均三甲苯基(也就是1,3,5-三甲基苯基)MF 三聚氰胺-甲醛树脂M-HIPS 改性高冲聚苯乙烯MIBK 甲基异丁基酮Min 分钟MMA 甲基丙烯酸甲酯MMF 甲基甲酰胺MNA 甲基丙烯腈MOM 甲氧甲基MPEG 乙醇酸乙酯MPF 三聚氨胺-酚醛树脂MPK 甲基丙基甲酮M-PP 改性聚丙烯MPPO 改性聚苯醚MPS 改性聚苯乙烯Ms 甲基磺酰基(保护羟基用)MS 分子筛MS 苯乙烯-甲基丙烯酸甲酯树脂MSO 石油醚MTBE 甲基叔丁基醚MTM 甲硫基甲基MTT 氯丁胶新型交联剂MWR 旋转模塑MXD-10/6 醇溶三元共聚尼龙MXDP 间苯二甲基二胺NNaphth 萘基NBD 二环庚二烯(别名:降冰片二烯)NBR 丁腈橡胶NBS N-溴代丁二酰亚胺?别名:N-溴代琥珀酰亚胺NCS N-氯代丁二酰亚胺.?别名:N-氯代琥珀酰亚胺NDI 二异氰酸萘酯NDOP 邻苯二甲酸正癸辛酯NHDP 邻苯二甲酸己正癸酯NHTM 偏苯三酸正己酯Ni(R) 雷尼镍(氢活性催化还原剂)NINS 癸二酸二异辛酯NLS 正硬脂酸铅NMO N-甲基氧化吗啉NMP N-甲基吡咯烷酮NODA 己二酸正辛正癸酯NODP 邻苯二甲酸正辛正癸酯NPE 壬基酚聚氧乙烯醚NR 天然橡胶OOBP 邻苯二甲酸辛苄酯ODA 己二酸异辛癸酯ODPP 磷酸辛二苯酯OIDD 邻苯二甲酸正辛异癸酯OPP 定向聚丙烯(薄膜)OPS 定向聚苯乙烯(薄膜)OPVC 正向聚氯乙烯OT 气熔胶PPA 聚酰胺(尼龙)PA-1010 聚癸二酸癸二胺(尼龙1010)PA-11 聚十一酰胺(尼龙11)PA-12 聚十二酰胺(尼龙12)PA-6 聚己内酰胺(尼龙6)PA-610 聚癸二酰乙二胺(尼龙610)PA-612 聚十二烷二酰乙二胺(尼龙612)PA-66 聚己二酸己二胺(尼龙66)PA-8 聚辛酰胺(尼龙8)PA-9 聚9-氨基壬酸(尼龙9)PAA 聚丙烯酸PAAS 水质稳定剂PABM 聚氨基双马来酰亚胺PAC 聚氯化铝PAEK 聚芳基醚酮PAI 聚酰胺-酰亚胺PAM 聚丙烯酰胺PAMBA 抗血纤溶芳酸PAMS 聚α-甲基苯乙烯PAN 聚丙烯腈PAP 对氨基苯酚PAPA 聚壬二酐PAPI 多亚甲基多苯基异氰酸酯PAR 聚芳酯(双酚A型)PAR 聚芳酰胺PAS 聚芳砜(聚芳基硫醚)PB 聚丁二烯-〔1,3] PBAN 聚(丁二烯-丙烯腈)PBI 聚苯并咪唑PBMA 聚甲基丙烯酸正丁酯PBN 聚萘二酸丁醇酯PBS 聚(丁二烯-苯乙烯)PBT 聚对苯二甲酸丁二酯PC 聚碳酸酯PC/ABS 聚碳酸酯/ABS树脂共混合金PC/PBT 聚碳酸酯/聚对苯二甲酸丁二醇酯弹性体共混合金PCC 吡啶氯铬酸盐PCD 聚羰二酰亚胺PCDT 聚(1,4-环己烯二亚甲基对苯二甲酸酯)PCE 四氯乙烯PCMX 对氯间二甲酚PCT 聚己内酰胺PCT 聚对苯二甲酸环己烷对二甲醇酯PCTEE 聚三氟氯乙烯PD 二羟基聚醚PDAIP 聚间苯二甲酸二烯丙酯PDAP 聚对苯二甲酸二烯丙酯PDC 重铬酸吡啶PDMS 聚二甲基硅氧烷PEG 聚乙二醇Ph 苯基PhH 苯PhMe 甲苯Phth 邻苯二甲酰Pip 哌啶基Pr n-丙基Py 吡啶Qquant. 定量产率RRE 橡胶粘合剂Red-Al [(MeOCH2CH2O)AlH2]NaRF 间苯二酚-甲醛树脂RFL 间苯二酚-甲醛乳胶RP 增强塑料RP/C 增强复合材料RX 橡胶软化剂SS/MS 苯乙烯-α-甲基苯乙烯共聚物SAN 苯乙烯-丙烯腈共聚物SAS 仲烷基磺酸钠SB 苯乙烯-丁二烯共聚物SBR 丁苯橡胶SBS 苯乙烯-丁二烯-苯乙烯嵌段共聚物sBu 仲丁基sBuLi 仲丁基锂SC 硅橡胶气调织物膜SDDC N,N-二甲基硫代氨基甲酸钠SE 磺乙基纤维素SGA 丙烯酸酯胶SI 聚硅氧烷Siamyl 二异戊基SIS 苯乙烯-异戊二烯-苯乙烯嵌段共聚物SIS/SEBS 苯乙烯-乙烯-丁二烯-苯乙烯共聚物SM 苯乙烯SMA 苯乙烯-顺丁烯二酸酐共聚物SPP 间规聚苯乙烯SPVC 悬浮法聚氯乙烯SR 合成橡胶ST 矿物纤维TTAC 三聚氰酸三烯丙酯TAME 甲基叔戊基醚TAP 磷酸三烯丙酯TASF 三(二乙胺基)二氟三甲基锍硅酸盐TBAF 氟化四丁基铵TBDMS,?TBS 叔丁基二甲基硅烷基(羟基保护基)TBE 四溴乙烷TBHP 过氧叔丁醇TBP 磷酸三丁酯t-Bu 叔丁基TCA 三醋酸纤维素TCCA 三氯异氰脲酸TCEF 磷酸三氯乙酯TCF 磷酸三甲酚酯TCPP 磷酸三氯丙酯TDI 甲苯二异氰酸酯TEA 三乙胺TEAE 三乙氨基乙基纤维素TEBA 三乙基苄基胺TEDA 三乙二胺TEFC 三氟氯乙烯TEMPO 四甲基氧代胡椒联苯自由基TEP 磷酸三乙酯Tf?or?OTf 三氟甲磺酸。
甲基丙烯酸(正)丁酯

2、甲基丙烯酸(正)丁酯(抑制了的)(n-Butyl methacrylate (inhibited))2.1标识别名:甲基败脂酸丁酯,异丁烯酸(正丁酯),Methacrylic acid n-butyl ester相对分子量:142.2 分子式:CH2C(CH3)2 COO(CH2)3CH3 2.2危规分类及编号按GB13690归类为第三类“易燃液体”危规分类及编号:GB13690 3.3类“高闪点液体”危规号:33601 UN No. 2227 IMDG CODE 3316页,3.3类。
2.3规格、用途规格:工业级,纯度≥98.5%;试剂级,化学纯,含量97.5%。
用途:有机合成,塑料、光学玻璃粘结剂、纺织、皮革及造纸的乳化剂。
2.4物化性质:无色具有甜味的酯气味的液体。
相对密度0.936。
凝固点<-50℃。
沸点160℃。
折射率1.424。
蒸气压653Pa(20℃),蒸气相对密度4.9。
不溶于水。
能与醇、醚混溶。
受热、光、电离辐射和催化剂作用易聚合。
商品一般加有阻聚剂,用量为0.01%~0.05%(对苯二酚)。
2.5 危险特性易燃。
闪点:52℃(o.c.)。
自燃点259℃。
蒸气能与空气形成爆炸性混合物。
遇明火、高热有引起着火、爆炸危险。
爆炸极限2%~8%。
在受热、光和紫外线的作用下易发生聚合,粘度逐渐增加,严重时可使整个容器的商品发生不规则爆炸性聚合。
低毒。
对皮肤、黏膜有中等刺激作用。
小鼠腹腔LD50:1490mg/kg;大鼠吸入>5113mg/m3,8小时致死;大鼠经口LD100:17900mg/kg(10~36分钟致死);兔经皮LD50:710mL/kg。
2.6 应急措施消防方法:用二氧化碳、干粉、泡沫、1211灭火。
用水冷却火场中的容器。
消防人员必须穿戴防毒面具。
大火时,必须在有防护措施的地方进行施救。
急救:应使吸入蒸气的患者脱离污染区,安置休息并保暖。
眼睛受刺激用水冲洗,严重者就医诊治。
[大分子]论ATRP大分子引发剂的合成及应用
![[大分子]论ATRP大分子引发剂的合成及应用](https://img.taocdn.com/s3/m/b6313690b307e87101f696e3.png)
论ATRP 大分子引发剂的合成及应用1 引言原子转移自由基聚合( atom transfer radicalpolymerization,ATRP) 是一种强大且灵活的合成技术,由于其具有分子量可控、分子量分布窄、聚合物端基易修饰及分子设计能力强,因此被称为精确可控大分子结构的合成方法。
如今ATRP 技术已成功应用于接枝、嵌段、梳状、星型、超支化和端基官能团聚合物的制备,且具有较高的链端保真度和精确的结构可控性,也有研究者将其应用于无机、生物材料表面修饰。
ATRP 技术适用于多种单体的可控聚合,且操作方便,其核心是引发剂的使用。
传统的ATRP 是以简单的有机卤化物为引发剂、过渡金属配合物为卤原子载体,通过氧化还原反应,在活性种与休眠种之间建立可逆的动态平衡,从而实现对聚合反应加以控制,随着技术的成熟和研究的深入,大分子引发体系成为研究的热点。
本文在介绍小分子引发剂的基础上重点介绍了大分子引发剂的合成方法及在ATRP 表面修饰中的应用。
2 小分子引发剂目前,制备活性可控聚合物的研究多集中于小分子有机卤化物作为引发剂,其所有位上含有诱导或共轭基团的卤代烷、芳基磺酰卤类引发剂都能引发ATRP 聚合,如苄基卤化物,-溴代酯,-卤代腈,-卤代酰胺,芳基磺酰氯和芳基磺酰溴类等。
可见,ATRP 的基本原理其实是通过一个交替的促活-失活可逆反应使得体系中的游离基浓度处于极低,迫使不可逆终止反应被降到最低程度,从而实现活性/可控自由基聚合。
有机卤代烷RX 的反应活性取决于烷基上的基团和可转移卤素基团的结构,不同结构烷基卤化物的活化速率常数。
由此可见,(1) 卤代烷的反应活性一般为I Cl,3 2 1,与键断裂所需要的键解离能一致;(2) -溴苯乙酸乙酯是活性最高的引发剂,其活性比苯乙基溴(PEB)高10 000倍,比溴丙酸甲酯(MBrP)高100 000倍;(3)-氰基、-苯基或酯基的存在有使活性基稳定性增强的作用,其中-氰基的增强程度大于-苯基或酯基。
单体中英对照
富马酸二异丁酯
I-dibutyl fumalate
(CH3)2CHCH2OOCCH=CHCOOCH2CH(CH3)2
2-磷酸乙甲基丙烯酸酯
2-Methacryloxyethyl phosphate
CH2=C(H2PO4)COOCH(CH3)CH3
DMAEMA
甲基丙烯酸2甲氨乙基酯
Dimethyl aminoehtyl methacrylate
EA
丙烯酸乙酯
Ethyl acrylate
CH2=CHCOOC2H5
IBA
丙烯酸异丁酯
I-Butyl acrylate
CH2=CHCOOCH2CH(CH3)2
IBMA
甲基丙烯酸异丁酯
I-Butyl methacrylate
CH2=C(CH3)COOCH2CH(CH3)2
TBMA
甲基丙烯酸叔丁酯
t-Butyl methacrylate
甲酸
Formic acid
无水TMA
偏苯三酸酐
Trimellic anhydride
安息香酸
苯甲酸,安息香酸
Benzoic acid
(C6H5O)3P
TPP-T
三苯基膦
Trithenyl phospine
P(OC6H5)3
TPP-N
磷酸三苯酯
Trithenyl phospate
PH3P
F-4100
BHT
丁羟基甲苯
Butylhydroxy toluene
C15H23OH
MEHQ
氢醌单甲醚(对羟基苯甲醚)
Phenol P-methoxy
TOLUENE 19.5%,BUAC19.5%聚酰氨树脂61%
啶氧菌酯组成结构、作用功能详解

啶氧菌酯(picoxystrobin)是由Zeneca Agrochemicals(现为Syngenta AG)公司开发的甲氧基丙烯酸酯类药剂,具有预防和治疗的功效,在某些作物中具有比嘧菌酯更好的治疗活性。
先正达首先于2001 年在欧洲引入啶氧菌酯,商品名为Acanto,用于防治小麦、大麦和燕麦作物田间实验也证明,啶氧菌酯相对于肟菌酯和醚菌酯,具有一定的谷类作物的增产作用。
01作用机理啶氧菌酯是线粒体呼吸抑制剂,即通过在细胞色素b和C1间电子转移抑制线粒体的呼吸。
防治对14-脱甲基化酶抑制剂、苯甲酰胺类、三羧酰胺类和苯并咪唑类产生抗性的菌株有效。
啶氧菌酯一旦被叶片吸收,就会在木质部中移动,随水流在运输系统中流动;它也在叶片表面的气相中流动并随着从气相中吸收进入叶片后又在木质部中流动。
正是由于啶氧菌酯的内吸活性和熏蒸活性,因而施药后,有效成分能有效再分配及充分传递,因此啶氧菌酯比商品化的嘧菌酯和肟菌酯有更好的治疗活性。
02靶标作物啶氧菌酯为广谱杀菌剂,主要应用于小麦和大麦上的相关病害防治,可有效防治小麦上由壳针孢菌引起的叶枯病、颖枯病、褐锈病、黄斑病和白粉病,大麦上的网斑病、云斑病、褐锈病和白粉病,燕麦上的冠锈病和叶斑病,以及黑麦上的褐锈病和云斑病等,用药量通常为250 g/hm2。
其主要应用作物包括:果树、谷物等。
03国内登记情况啶氧菌酯1988年2月9日申请中国专利,专利号为CN1020391B,专利已于2008年到期。
国外关于啶氧菌酯的合成文献报道主要有以下2条路线:据欧盟委员会介绍,他们在评估期间确定了以下有关啶氧菌酯的风险:不能排除其代谢物IN- H8612 的致畸变和细胞遗传毒性风险;代谢物IN- QDY63 对水生生物、蚯蚓以及以蚯蚓为食的哺乳动物具有高风险。
此外,尚有一些风险是评估无法确定的。
相对于甲氧基丙烯酸酯类的嘧菌酯和吡唑醚菌酯,以及肟菌酯。
啶氧菌酯并不具有显著的优势。
更不用提甲氧基丙烯酸酯类杀菌剂或多或少都有的增产效果。
化学英文缩写

化学术语缩写Ac Acetyl 乙酰基DMAP 4-dimethylaminopyridine 4-二甲氨基吡啶acac Acetylacetonate 乙酰丙酮基DME dimethoxyethane 二甲醚AIBN Azo-bis-isobutryonitrile 2,2'-二偶氮异丁腈DMF N,N'-dimethylformamide 二甲基甲酰胺aq. Aqueous 水溶液dppf bis (diphenylphosphino)ferrocene 双(二苯基膦基)二茂铁9-BBN 9-borabicyclo[3.3.1]nonane 9-硼二环[3.3.1]壬烷dppp 1,3-bis (diphenylphosphino)propane 1,3-双(二苯基膦基)丙烷BINAP (2R,3S)-2,2’-bis (diphenylphosphino)-1,1’-binaphthyl(2R,3S)-2.2'-二苯膦-1.1'-联萘亦简称为联二萘磷BINAP是日本名古屋大学的Noyori(2001年诺贝尔奖)发展的一类不对称合成催化剂dvb Divinylbenzene 二乙烯苯Bn Benzyl 苄基e- Electrolysis 电解BOC t-butoxycarbonyl 叔丁氧羰基(常用于氨基酸氨基的保护)%ee % enantiomeric excess 对映体过量百分比(不对称合成术语)%de % diasteromeric excess 非对映体过量百分比(不对称合成术语)Bpy (Bipy) 2,2’-bipyridyl 2,2'-联吡啶EDA (en) ethylenediamine 乙二胺Bu n-butyl 正丁基EDTA Ethylenediaminetetraacetic acid 乙二胺四乙酸二钠Bz Benzoyl 苯甲酰基EE 1-ethoxyethyl 乙氧基乙基c- Cyclo 环-Et Ethyl 乙基FMN Flavin mononucleotide 黄素单核苷酸CAN Ceric ammonium nitrate 硝酸铈铵Cat. Catalytic 催化Fp flash point 闪点CBz Carbobenzyloxy 苄氧羰基FVP Flash vacuum pyrolysis 闪式真实热解法h hours 小时Min Minute 分钟hv Irradiation with light 光照COT 1,3,5-cyclooctatrienyl 1,3,5-环辛四烯1,5-HD 1,5-hexadienyl 1,5-己二烯Cp Cyclopentadienyl 环戊二烯基HMPA Hexamethylphosphoramide 六甲基磷酸三胺CSA 10-camphorsulfonic acid 樟脑磺酸HMPT Hexamethylphosphorus triamide 六甲基磷酰胺CTAB Cetyltrimethylammonium bromide 十六烷基三甲基溴化铵(相转移催化剂)iPr isopropyl 异丙基Cy Cyclohexyl 环己基LAH Lithium aluminum hydride 氢化铝锂(LiAlH4)LDA Lithium diisopropylamide 二异丙基氨基锂(有机中最重要一种大体积强碱)dba Dibenzylidene acetone 苄叉丙酮LHMDS Lithium hexamethyldisilazideDBE 1,2-dibromoethane 1,2- 二溴乙烷LTBA Lithium tri-tert-butoxyaluminum hydrideDBN 1,8-diazabicyclo[5.4.0]undec-7-ene 二环[5.4.0]-1,8-二氮-7-壬烯mCPBA meta-cholorperoxybenzoic acid 间氯过苯酸DBU 1,5-diazabicyclo[4.3.0]non-5-ene 二环[4.3.0]-1,5-二氮-5-十一烯Me Methyl 甲基DCC 1,3-dicyclohexylcarbodiimide 1,3-二环己基碳化二亚胺MEM b-methoxyethoxymethyl 甲氧基乙氧基甲基-DCE 1,2-dichloroethane 1,2-二氯乙烷Mes Mesityl 均三甲苯基(也就是1,3,5-三甲基苯基)不知对不对?DDQ 2,3-dichloro-5,6-dicyano-1,4-benzoquinone 2,3-二氯-5,6-二氰-1,4-苯醌MOM methoxymethyl 甲氧甲基DEA Diethylamine 二乙胺Ms Methanesulfonyl 甲基磺酰基(保护羟基用)TBDMS, TBS t-butyldimethylsilyl 叔丁基二甲基硅烷基(羟基保护基)DEAD Diethyl azodicarboxylate 偶氮二甲酸二乙酯MS Molecular sieves (3 or 4 )分子筛Dibal-H Diisobutylaluminum hydride 二异丁基氢化铝MTM Methylthiomethyl 二甲硫醚diphos (dppe) 1,2-bis (diphenylphosphino)ethane 1,2-双(二苯基膦)乙烷Naphth Naphthyl 萘基diphos-4 (dppb) 1,4-bis (diphenylphosphino)butane 1,2-双(二苯基膦)丁烷NBD Norbornadiene 二环庚二烯(别名:降冰片二烯)NBS N-Bromosuccinimide N-溴代丁二酰亚胺别名:N-溴代琥珀酰亚胺NCS N-chlorosuccinimide N-氯代丁二酰亚胺. 别名:N-氯代琥珀酰亚胺TBAF Tetrabutylammonium fluoride 氟化四丁基铵TASF Tris(diethylamino)sulfonium difluorotrimethyl silicateNi(R) Raney Nickel 雷尼镍(氢活性催化还原剂)NMO N-methyl morpholine-n-oxide N-甲基氧化吗啉TBHP t-butylhydroperoxide 过氧叔丁醇PCC Pyridinium chlorochromate 吡啶氯铬酸盐PDC Pyridinium dichromate 是什么东西?t-Bu Tert-butyl 叔丁基TEBA Triethylbenzylammonium 三乙基苄基胺PEG Polyethylene glycol 聚乙二醇TEMPO Tetramethylpiperdinyloxy free radicalPh Phenyl 苯基PhH Benzene 苯TFA Trifluoroacetic acid 三氟乙酸TFAA Trifluoroacetic anhydride 三氟乙酸酐PhMe Toluene 甲苯(亦称toluol;methylbenzene )Tol Tolyl 甲苯基Tf or OTf TriflatePhth Phthaloyl 邻苯二甲酰THF Tetrahydrofuran 四氢呋喃Pip Piperidyl 哌啶基THP Tetrahydropyranyl 四氢吡喃基TMEDA Tetramethylethylenediamine 四甲基乙二胺Py Pyridine 吡啶TMP 2,2,6,6-tetramethylpiperidine 2,2,6,6-四甲基哌啶quant. quantitative yield 定量产率(对否?)TMS Trimethylsilyl 三甲基硅烷基Red-Al [(MeOCH2CH2O)AlH2]Na 直接看分子式就是了sBu sec-butyl 仲丁基Tr Trityl 三苯基sBuLi sec-butyllithium 仲丁基锂TRIS TriisopropylphenylsulfonylSiamyl DiisoamylTs (Tos) Tosyl (p-toluenesulfonyl) 对甲苯磺酰基。
3-甲氧基3-甲基丁酯

3-甲氧基-3-甲基丁酯实际上指的是两种化学结构相似但官能团不同的化合物:
1.3-甲氧基-3-甲基丁醇(3-Methoxy-3-methylbutanol),CAS号为
56539-66-3,其分子式为C6H14O2,分子量为118.174 g/mol。
根据您提供的信息,该化合物的物理性质如下:
o熔点:约-50℃
o沸点:164.2±8.0℃(在760mmHg条件下)
o密度:0.9±0.1 g/cm³
这种醇类化合物常作为溶剂或化工中间体使用,在香精香料工业中可能用于配制香味成分。
2.3-甲氧基-3-甲基醋酸丁酯(3-Methoxy-3-methylbutyl acetate),CAS
号为103429-90-9,分子式为C8H16O3,分子量为160.21 g/mol。
它
的特性包括:
o密度:0.96 g/cm³
o沸点:74°C/15mmHg
o闪点:73°C
o蒸汽压:2.58-21.3 (未给出具体温度范围)
这是一种酯类化合物,通常作为低毒性的溶剂应用在涂料、油墨、胶黏剂以及某些化学合成过程中,由于它与水部分互溶且能溶解多种有机物质,因此在许多配方中具有良好的溶解性能和较低的安全风险。
两者的主要区别在于一个为醇(包含羟基-OH),另一个为酯(包含酯基-COO)。
有机化学英文缩写

Ac acetyl 乙酰基acac acetylacetonate 乙酰基丙酮化物AIBN 2,2'-azobisisoblltyronitrile 偶氮二异丁腈Ar aryl 芳基的BBN borabicyclo[3.3.1]nonane 硼双环[3.3.1]壬烷BCME dis(chloromethyl)ether 双氯甲醚BHT butylated hydroxytoluene (2,6-di-t-butyl -p-cresol)别名抗氧化剂264 2,6-二叔丁基-4-甲基苯BINAL-H 2,2'-dihydroxy-1,1'-binaphthyl-lithium aluminum hydride 手性烷氧基联萘酚氢化铝锂BINAP 2,2' - bis(diphenylphosphino)-1,1' -binaphthyl双二苯基磷酰联萘BINOL 1,l'-bi-2,2'-naphthol 1,1'-联-2,2'-萘酚bipy 2,2' –bipyridyl 2,2'-联吡啶BMS borane-dimethyl sulfìde 硼烷吡啶Bn benzyl 苯甲基Boc t-butoxycarbonyl叔丁氧羰基BOM benzyloxymethyl苄氧甲基bp boiling point 沸点Bs brosyl (4-bromobenzenesulfonyl) 4-溴苯磺酰基BSA N, O-bis( trimethylsilyl )acetamide N,O-双三甲硅基乙酰胺Bu n-butyl 正丁基Bz benzoyl 苯甲酰CAN cerium(lV) ammonium nitrate 硝酸铈(Ⅳ)铵Cbz benzyloxycarbonyl 苄氧羰基CDI N,N-carbonyldiimidazole N,N'-羰基二咪唑CHIRAPHOS 2,3-bis(diphenylphosphino)butane 2,3-双(二苯基膦)丁烷Chx =Cy 环己基cod cyclooctadiene 环辛二烯cot cyclooctatetraene环辛四烯Cp cyclopentadienyl 环戊二烯基CRA complex reducing agent 复合还原试剂CSA 10-camphorsulfonic acid 10-樟脑磺酸CSI chlorosulfonyl isocyanate 氯磺酰异氰酸酯Cy cyclohexyl 环己基d density 密度DABCO 1,4-diazabicyclo[2.2.2]octane 1,4-重氮二环[2.2.2]辛烷DAST N,N'-diethylaminosulfur trifluoride二乙胺基三氟化硫dba dibenzylideneacetone二亚苄叉丙酮DBAD di-t-butyl azodicarboxylate偶氮二甲酸二叔丁酯DBN 1,5-diazabicyclo[4.3.0]non-5-ene 1,5-二氮杂二环[4,3,0]壬烯-5DBU 1 ,8-diazabicyclo[5.4.0]undec-7-ene 1,8-二氮杂二环-双环(5,4,0)-7-十一烯DCC N,N-dicyclohexylcarbodiimide N,N'二环己基碳二亚胺DCME dichloromethyl methyl ether二氯甲基甲醚DDO dimethyldioxirane双十二烷基二硫代乙二酰胺(又称钯试剂)DDQ 2,3-dichloro-5,6-dicyano-1,4-benzoquinone 2,3-二氯-5,6-二氰-1,4-苯醌de diastereomeric excess 非对映体过量DEAD diethyl azodicarboxylate偶氮二甲酸二乙酯DET diethyl tartrate酒石酸二乙酯DIBAL diisobutylaluminum hydride二异丁基氢化铝DIEA =DIPEA 二异丙基乙胺DIOP 2,3-O-isopropylidene-2,3-dihydroxy-1,4- bis-(diphenylphosphino)butane异丙烯-2,3-二羟-1,4-双二丙基膦丁烷DIPEA diisopropylethylamine二异丙基乙基胺diphos =dppe 1,2-双(二苯基磷酰)乙烷DIPT diisopropyl tartrate 二异丙基酒石酸盐DMA dimethylacetamid 二甲基乙酰胺DMAD dimethyl acetylenedicarboxylate 丁炔二酸二甲酯,别名:催泪瓦斯DMAP 4-(dimethylamino)pyridine 4-二甲基氨基吡啶DME 1,2-dimethoxyethane乙二醇二甲醚(二甲氧基乙烷)DMF dimethylformamide 二甲基甲酰胺dmg dimethylglyoximato 丁二酮肟(与Ni2+形成鲜红色螯合物)DMPU N,N' -dimethylpropyleneurea N,N-二甲基丙烯基脲DMS dimethyl sulfide 二甲基硫DMSO dimethyl sulfoxide 二甲基亚砜DMTSF dimethyl(methylthio)sulfonium tetrafluoroborate 二甲基(甲硫代)锍四氟硼酸盐dppb l ,4-bis(diphenylphosphino)butane 1,4-双(二苯基膦)丁烷dppe 1,2-bis(diphenylphosphino)ethane 1,2-双(二苯基磷)乙烷dppf l ,l'-bis(diphenylphosphino)ferrocene l , l'-双(二苯基磷)二茂铁dppp 1,3-bis(diphenylphosphino)propane 1,2-双(二苯基磷)丙烷DTBP di-t-butyl peroxide二叔丁基过氧化物EDA ethyl diazoacetate 重氮乙酸乙酯EDC l-ethyl-3-(3-dimethylaminopropyl)-carbodiimide 1-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐EDCI = EDCee enantiomeric excess对映体过量EE l-ethoxyethyl 乙氧基乙基Et ethyl 乙基ETSA ethyltrimethylsilylacetate (三甲基硅基)醋酸乙酯EWG electron withdrawing group 吸电基团Fc ferrocenyl 二茂铁基Fmoc 9-fluorenylmethoxycarbonyl 9-芴甲氧羰酰基fp ftash point 闪点Hex n-hexyl 正己基HMDS hexamethyldisilazane六甲基二硅胺烷HMPA hexamethylphosphoric triamide六甲基膦酸三酰胺HOBt 1-hydroxybenzotriazole 1-羟基苯并三唑HOBT =HOBtHOSu N-hydroxysuccinimide N-羟基琥珀酰亚胺Im imidazole (imidazolyl) 咪唑Ipc isopinocampheyl 异松蒎基IR infrared 红外KHDMS potassium hexamethyldisilazide 六甲基二硅胺钾LAH lithium aluminum hydride 氢化铝锂LD50 dose that is lethal to 50% of test subjects 致死量为受试者的50%LDA lithium diisopropylamide 二异丙基氨基锂LDMAN lithium1-(dimethylamino)naphthalenide ? 1-(二甲氨基)萘锂LHMDS(LiHMDS)lithium hexamethyldisilazide 六甲基叠氮乙硅锂, 六甲基二硅氨基锂LICA lithiuim isopropylcyclohexylamide 异丙基环己氨基锂LiTMP(LTMP) lithium2,2,6,6-tetramethylpiperidide 2,2,6,6-四甲基哌啶锂哌啶(氮杂环己烷)LTA lead tetraacetate 四乙酸铅lut 2,6-lutidine 二甲基吡啶MCPBA(m-CPBA) m-chloroperbenzoic acid 间氯过苯酸MA maleic anhydride 顺丁烯二酸酐MAD methyl aluminum bis(2,6-di-t-butyl-4-methylphenoxide) ?MAT methyl aluminum bis(2,4,6-tri-t-butylphenoxide) ?Me methyl 甲基MEK methyl ethyl ketone 甲基乙基酮MEM 2-methoxyethoxymethyl (2-甲氧基乙氧基)甲基-MIC methyl isocyanate 甲基异氰酸酯MMPP magnesium monoperoxyphthalate 单过氧邻苯二甲酸镁MOM methoxymethyl 甲氧甲基MoOPH oxodiperoxomolybdenum(pyridine)-(hexamethylphosphoric triamide)mp melting point 熔点MPM methoxy(phenylthio)methyl 甲氧基(苯硫基)甲基,Ms methanesulfonyl (mesyl) 甲基磺酰基(保护羟基用)MS mass spectrometry 质谱MS Molecular sieves 分子筛MTEE (MTBE) methyl t-butyl ether 甲基叔丁基醚MTM methylthiomethyl 二甲硫醚MVK methyl vinyl ketone 甲基乙烯基酮n refractive index 折射率NaHDMS sodium hexamethyldisilazide 六甲基二硅胺钠Naph(Np) naphthyl 萘基NBA N-bromoacetamide N-溴乙酰胺NBD norbornadiene(bicyclo[2.2.1]hepta-2,5-diene) 二环庚二烯(别名:降冰片二烯)NBS N-bromosuccinimide N-溴代丁二酰亚胺(别名:N-溴代琥珀酰亚胺)NCS N-chlorosuccinimide N-氯代丁二酰亚胺. (别名:N-氯代琥珀酰亚胺)NIS N-iodosuccinimide N-碘代丁二酰亚胺(别名:N-碘代琥珀酰亚胺)NMO N-methylmorpholine N-oxide N-甲基氧化吗啉NMP N-methyl-2-pyrrolidone N-甲基-2-吡咯烷酮NMR nuclear magnetic resonance 核磁共振NORPHOS 5,6-bis(diphenylphosphino)-2-norbornene ?5,6-双(二苯基磷)-2-降冰片烯PCC pyridinium chlorochromate 吡啶氯铬酸盐PDC pyridinium dichromate 二氯吡啶酯Pent n-pentyl 正戊基Ph phenyl 苯基Phen 1,10-phenanthroline 1,10-菲罗啉Phth phthaloyl 邻苯二甲酰基Piv pivaloyl 新戊酰基PMB p-methoxybenzyl 对甲氧苄基;对甲氧苯甲基PMDTAPPA polyphosphoric acid 多聚磷酸PPE Polyphenylene Ether 聚苯醚PPTS pyridinium p-toluenesulfonate吡啶对甲苯磺酸Pr propyl丙基PTC phase-transfer catalysis (phase-transfer catalyst)相转移催化(相转移催化剂)PTSA(or TsOH) p-toluenesulfonic acid对甲苯磺酸Py (pyr) pyridine (or pyridyl)吡啶(或吡啶)PAMPrt room temperature 室温salen 双水杨酰胺乙基钴SAMP (S)-1-amino-2-(methoxymethyl)pyrrolidine(s)-1 -氨基- 2-(甲氧甲基)吡咯烷SET single electron transfer单电子转移Sia siamyl (s-isoamyl or 1,2-dimethylpropyl)TASF tris(diethylamino)sulfonium difluorotrimethylsilicateTBAB tetra-n-butylammonium bromide四丁基溴化铵TBAF tetra-n-butylammonium fluoride四丁基氟化TBADTBAI tetra-n-butylammonium iodide四丁基碘化TBAPTBDMS(TBS) t-butyldimethylsilyl二甲基硅烷TBDPS(BPS) t-butyldiphenylsilylTBHP t-butyl hydroperoxide叔丁基氢TBS t-butyldimethylsilyl二甲基硅烷TCNE tetracyanoethylene四氰基乙烯TCNQ 7,7,8,8-tetracyano-para-quinodimethaneTEA triethylamine三乙胺TEAB tetratehylammonium bromideTEBAC triethylbenzylammonium chloride三乙基氯化铵TEMPO 2,2,6,6-tetramethylpipedinyloxyTES triethylsilyl三乙基硅烷Tf trifluoromethanesulfonyl三氟甲基TFA trifluoroacetic acid三氟乙酸TFAA trifluoroacetic anhydride三氟乙酸酐THF tetrahydrofuran四氢呋喃THP 2-tetrahydropyranyl2 -吡喃ThxTIPS triisopropylsilylTMAO (TMANO) trimethylamine N-oxide三甲胺氮氧化物TMEDA N,N,N',N-tetramethyl- -hexaacetic acidTMG 1,1,3,3-tetramethylguanidineTMS tetramethylsilane四甲基Tol p-tolyl对甲苯TPAP tetra-n-propylammonium perruthenateTBHPTPP thiamine pyrophosphate5,10,15,20 -四苯基卟啉Tr triphenylmethyl (trityl)三苯(三苯甲基)Ts p-toluenesulfonyl (tosyl)对甲苯磺酰(磺酰)TTN thallium(III)-trinitrate硝酸铊(Ⅲ)UHP urea-hydrogen peroxide complex尿素过氧化氢复合Z benzyloxycarbonyl苄氧羰基。
官能团缩写

DMAP 4-dimethylaminopyridine 4-二甲氨基吡啶acac Acetylacetonate 乙酰丙酮基DME dimethoxyethane 二甲醚AIBN Azo-bis-isobutryonitrile 2,2'-二偶氮异丁腈DMF N,N'-dimethylformamide 二甲基甲酰胺aq. Aqueous 水溶液dppf bis (diphenylphosphino)ferrocene 双(二苯基膦基)二茂铁9-BBN 9-borabicyclo[3.3.1]nonane 9-硼二环[3.3.1]壬烷dppp 1,3-bis (diphenylphosphino)propane 1,3-双(二苯基膦基)丙烷BINAP (2R,3S)-2,2’-bis (diphenylphosphino)-1,1’-binaphthyl (2R,3S)-2.2'-二苯膦-1.1'-联萘亦简称为联二萘磷BINAP是日本名古屋大学的Noyori(2001年诺贝尔奖)发展的一类不对称合成催化剂dvb Divinylbenzene 二乙烯苯Bn Benzyl 苄基e- Electrolysis 电解BOC t-butoxycarbonyl 叔丁氧羰基(常用于氨基酸氨基的保护)%ee % enantiomeric excess 对映体过量百分比(不对称合成术语)%de % diasteromeric excess 非对映体过量百分比(不对称合成术语)Bpy (Bipy) 2,2’-bipyridyl 2,2'-联吡啶EDA (en) ethylenediamine 乙二胺Bu n-butyl 正丁基EDTA Ethylenediaminetetraacetic acid 乙二胺四乙酸二钠Bz Benzoyl 苯甲酰基EE 1-ethoxyethyl 乙氧基乙基c- Cyclo 环-FMN Flavin mononucleotide 黄素单核苷酸CAN Ceric ammonium nitrate 硝酸铈铵Cat. Catalytic 催化Fp flash point 闪点CBz Carbobenzyloxy 苄氧羰基FVP Flash vacuum pyrolysis 闪式真实热解法COT 1,3,5-cyclooctatrienyl 1,3,5-环辛四烯1,5-HD 1,5-hexadienyl 1,5-己二烯Cp Cyclopentadienyl 环戊二烯基HMPA Hexamethylphosphoramide 六甲基磷酸三胺CSA 10-camphorsulfonic acid 樟脑磺酸HMPT Hexamethylphosphorus triamide 六甲基磷酰胺CTAB Cetyltrimethylammonium bromide 十六烷基三甲基溴化铵(相转移催化剂)Cy Cyclohexyl 环己基LAH Lithium aluminum hydride 氢化铝锂(LiAlH4)LDA Lithium diisopropylamide 二异丙基氨基锂(有机中最重要一种大体积强碱) dba Dibenzylidene acetone 苄叉丙酮LHMDS Lithium hexamethyldisilazide DBE 1,2-dibromoethane1,2- 二溴乙烷LTBA Lithium tri-tert-butoxyaluminum hydride DBN 1,8-diazabicyclo[5.4.0]undec-7-ene 二环[5.4.0]-1,8-二氮-7-壬烯mCPBA meta-cholorperoxybenzoic acid 间氯过苯酸DBU 1,5-diazabicyclo[4.3.0]non-5-ene 二环[4.3.0]-1,5-二氮-5-十一烯Me Methyl 甲基DCC 1,3-dicyclohexylcarbodiimide 1,3-二环己基碳化二亚胺MEM b-methoxyethoxymethyl 甲氧基乙氧基甲基-DCE 1,2-dichloroethane 1,2-二氯乙烷Mes Mesityl 均三甲苯基(也就是1,3,5-三甲基苯基)不知对不对 ?DDQ 2,3-dichloro-5,6-dicyano-1,4-benzoquinone 2,3-二氯-5,6-二氰-1,4-苯醌MOM methoxymethyl 甲氧甲基DEA Diethylamine 二乙胺Ms Methanesulfonyl 甲基磺酰基(保护羟基用)TBDMS, TBS t-butyldimethylsilyl 叔丁基二甲基硅烷基(羟基保护基)DEAD Diethyl azodicarboxylate 偶氮二甲酸二乙酯MS Molecular sieves (3 or 4 )分子筛Dibal-H Diisobutylaluminum hydride 二异丁基氢化铝MTM Methylthiomethyl 二甲硫醚diphos (dppe) 1,2-bis (diphenylphosphino)ethane 1,2-双(二苯基膦)乙烷Naphth Naphthyl 萘基diphos-4 (dppb) 1,4-bis (diphenylphosphino)butane 1,2-双(二苯基膦)丁烷NBD Norbornadiene 二环庚二烯(别名:降冰片二烯)NBS N-Bromosuccinimide N-溴代丁二酰亚胺别名:N-溴代琥珀酰亚胺NCS N-chlorosuccinimide N-氯代丁二酰亚胺. 别名:N-氯代琥珀酰亚胺TBAF Tetrabutylammonium fluoride 氟化四丁基铵TASF Tris(diethylamino)sulfonium difluorotrimethyl silicate Ni(R) Raney Nickel 雷尼镍(氢活性催化还原剂)NMO N-methyl morpholine-n-oxide N-甲基氧化吗啉TBHP t-butylhydroperoxide 过氧叔丁醇PCC Pyridinium chlorochromate 吡啶氯铬酸盐PDC Pyridinium dichromate 是什么东西?TEBA Triethylbenzylammonium 三乙基苄基胺PEG Polyethylene glycol 聚乙二醇TEMPO Tetramethylpiperdinyloxy free radical TFA Trifluoroacetic acid三氟乙酸TFAA Trifluoroacetic anhydride 三氟乙酸酐Tol Tolyl 甲苯基 Tf or OTf TriflatePhth Phthaloyl 邻苯二甲酰THF Tetrahydrofuran 四氢呋喃Pip Piperidyl 哌啶基THP Tetrahydropyranyl 四氢吡喃基TMEDA Tetramethylethylenediamine 四甲基乙二胺TMP 2,2,6,6-tetramethylpiperidine 2,2,6,6-四甲基哌啶TMS Trimethylsilyl 三甲基硅烷基Red-Al [(MeOCH2CH2O)AlH2]Na 直接看分子式就是了sBu sec-butyl 仲丁基Tr Trityl 三苯基sBuLi sec-butyllithium 仲丁基锂TRIS Triisopropylphenylsulfonyl Siamyl Diisoamyl Ts (Tos) Tosyl (p-toluenesulfonyl) 对甲苯磺酰基(二)Ac Acetyl 乙酰基DMAP 4-dimethylaminopyridine 4-二甲氨基吡啶acac Acetylacetonate 乙酰丙酮基DME dimethoxyethane 二甲醚AIBN Azo-bis-isobutryonitrile 2,2'-二偶氮异丁腈DMF N,N'-dimethylformamide 二甲基甲酰胺aq. Aqueous 水溶液dppf bis (diphenylphosphino)ferrocene 双(二苯基膦基)二茂铁9-BBN 9-borabicyclo[3.3.1]nonane 9-硼二环[3.3.1]壬烷dppp 1,3-bis (diphenylphosphino)propane 1,3-双(二苯基膦基)丙烷BINAP (2R,3S)-2,2’-bis (diphenylphosphino)-1,1’-binaphthyl (2R,3S)-2.2'-二苯膦-1.1'-联萘亦简称为联二萘磷dvb Divinylbenzene 二乙烯苯Bn Benzyl 苄基e- Electrolysis 电解BOC t-butoxycarbonyl 叔丁氧羰基%ee % enantiomeric excess 对映体过量百分比(不对称合成术语) %de % diasteromeric excess 非对映体过量百分比(不对称合成术语)Bpy (Bipy) 2,2’-bipyridyl 2,2'-联吡啶EDTA (en) ethylenediamine 乙二胺Bu n-butyl 正丁基EDTA Ethylenediaminetetraacetic acid 乙二胺四乙酸二钠Bz Benzoyl 苯甲酰基EE 1-ethoxyethyl 乙氧基乙基c- Cyclo 环-Et Ethyl 乙基FMN Flavin mononucleotide 黄素单核苷酸CAN Ceric ammonium nitrate 硝酸铈铵Cat. Catalytic 催化Fp flash point 闪点CBz Carbobenzyloxy 苄氧羰基FVP Flash vacuum pyrolysis 闪式真实热解法hv Irradiation with light 光照COT 1,3,5-cyclooctatrienyl 1,3,5-环辛四烯1,5-HD 1,5-hexadienyl 1,5-己二烯Cp Cyclopentadienyl 环戊二烯基HMPA Hexamethylphosphoramide 六甲基磷酸三胺CSA 10-camphorsulfonic acid 樟脑磺酸HMPT Hexamethylphosphorus triamide 六甲基磷酰胺CTAB Cetyltrimethylammonium bromide 十六烷基三甲基溴化铵(相转移催化剂) iPr isopropyl 异丙基Cy Cyclohexyl 环己基LAH Lithium aluminum hydride 氢化铝锂(LiAlH4) LDA Lithium diisopropylamide 二异丙基氨基锂(有机中最重要一种大体积强碱)dba Dibenzylidene acetone 苄叉丙酮DBE 1,2-dibromoethane 1,2- 二溴乙烷DBN 1,8-diazabicyclo[5.4.0]undec-7-ene 二环[5.4.0]-1,8-二氮-7-壬烯mCPBA meta-cholorperoxybenzoic acid 间氯过苯酸DBU 1,5-diazabicyclo[4.3.0]non-5-ene 二环[4.3.0]-1,5-二氮-5-十一烯Me Methyl 甲基DCC 1,3-dicyclohexylcarbodiimide 1,3-二环己基碳化二亚胺MEM b-methoxyethoxymethyl 甲氧基乙氧基甲基-DCE 1,2-dichloroethane 1,2-二氯乙烷DDQ 2,3-dichloro-5,6-dicyano-1,4-benzoquinone 2,3-二氯-5,6-二氰-1,4-苯醌 MOMmethoxymethyl 甲氧甲基DEADiethylamine 二乙胺Ms Methanesulfonyl 甲基磺酰基(保护羟基用)TBS t-butyldimethylsilyl 叔丁基二甲基硅烷基(羟基保护基)DEAD Diethyl azodicarboxylate 偶氮二甲酸二乙酯MS Molecular sieves (3 or 4 )分子筛Dibal-H Diisobutylaluminum hydride 二异丁基氢化铝MTM Methylthiomethyl 二甲硫醚diphos (dppe) 1,2-bis (diphenylphosphino)ethane 1,2-双(二苯基膦)乙烷Naphth Naphthyl 萘基NBD Norbornadiene 二环庚二烯(别名:降冰片二烯)NBS N-Bromosuccinimide N-溴代丁二酰亚胺别名:N-溴代琥珀酰亚胺NCS N-chlorosuccinimide N-氯代丁二酰亚胺. 别名:N-氯代琥珀酰亚胺TBAF Tetrabutylammonium fluoride 氟化四丁基铵Ni(R) Raney Nickel 雷尼镍(氢活性催化还原剂)NMO N-methyl morpholine-n-oxide N-甲基氧化吗啉TBHP t-butylhydroperoxide 过氧叔丁醇PCC Pyridinium chlorochromate 吡啶氯铬酸盐t-Bu Tert-butyl 叔丁基TEBA Triethylbenzylammonium 三乙基苄基胺PEG Polyethylene glycol 聚乙二醇Ph Phenyl 苯基PhH Benzene 苯TFA Trifluoroacetic acid 三氟乙酸TFAA Trifluoroacetic anhydride 三氟乙酸酐PhMe Toluene 甲苯(亦称methylbenzene)Tol Tolyl 甲苯基Phth Phthaloyl 邻苯二甲酰THF Tetrahydrofuran 四氢呋喃Pip Piperidyl 哌啶基THP Tetrahydropyranyl 四氢吡喃基TMEDA Tetramethylethylenediamine 四甲基乙二胺Py Pyridine 吡啶TMP 2,2,6,6-tetramethylpiperidine 2,2,6,6-四甲基哌啶quant. quantitative yield 定量产率(对否?)TMS Trimethylsilyl 三甲基硅烷基sBusec-butyl 仲丁基 TrTrityl 三苯基sBuLi sec-butyllithium 仲丁基锂Ts (Tos) Tosyl (p-toluenesulfonyl) 对甲苯磺酰基。
甲氧基甲基胺盐酸盐

甲氧基甲基胺盐酸盐甲氧基甲基胺盐酸盐是一种重要的有机化合物,广泛应用于医药、农药、染料、化妆品等领域。
本文将从化学结构、制备方法、应用领域等方面介绍甲氧基甲基胺盐酸盐的相关知识。
一、化学结构甲氧基甲基胺盐酸盐的化学式为CH3ONH3Cl,分子量为81.54g/mol。
其分子结构中含有一个氮原子和一个甲氧基基团,是一种弱碱性化合物。
二、制备方法甲氧基甲基胺盐酸盐的制备方法有多种,以下列举其中两种常用的方法:1. 氨水法将甲醇和氨水按一定比例混合,加入盐酸中反应,反应产物即为甲氧基甲基胺盐酸盐。
反应方程式如下:CH3OH + NH3 + HCl → CH3ONH3Cl + H2O2. 甲醛缩合法将甲醛和甲胺按一定比例混合,加入盐酸中反应,反应产物即为甲氧基甲基胺盐酸盐。
反应方程式如下:HCHO + CH3NH2 + HCl → CH3ONH3Cl + H2O三、应用领域1. 医药领域甲氧基甲基胺盐酸盐是一种常用的局部麻醉药物,常用于皮肤表面的麻醉,如皮肤切割、缝合等。
其作用机理是通过阻断神经传导,使病人感觉不到疼痛。
此外,甲氧基甲基胺盐酸盐还可用于治疗肌肉痉挛、神经性疼痛等症状。
2. 农药领域甲氧基甲基胺盐酸盐是一种有效的农药成分,可用于防治多种农作物的害虫和草甸杂草。
其作用机理是通过干扰害虫的神经系统,使其无法正常活动,从而达到防治的目的。
3. 染料领域甲氧基甲基胺盐酸盐是一种常用的染料中间体,可用于制备多种有机染料。
其作用机理是通过与染料分子中的亲电基团发生反应,形成稳定的染料结构。
4. 化妆品领域甲氧基甲基胺盐酸盐可用于制备多种化妆品,如口红、眼影等。
其作用机理是通过增加化妆品的黏度和粘稠度,使其更易于涂抹和使用。
四、注意事项甲氧基甲基胺盐酸盐是一种有毒化合物,应注意避免接触和吸入。
在制备和使用过程中,应采取必要的安全措施,如佩戴防护手套、呼吸器等。
同时,应储存于阴凉、干燥的环境中,避免与氧气、酸、碱等物质接触,以免发生不必要的化学反应。
甲基丙烯酸丁酯97-88-1

1 化学品及企业标识1.1 产品标识符化学品俗名或商品名:甲基丙烯酸丁酯CAS No.:97-88-1别名:异丁烯酸正丁酯;甲基丙烯酸正丁酯;丁基-2-甲基-2-丙烯酸酯;2-甲基丙烯酸正丁酯;正丁基甲基丙烯酸酯;1.2 鉴别的其他方法无数据资料1.3 有关的确定了的物质或混合物的用途和建议不适合的用途仅供科研用途,不作为药物、家庭备用药或其它用途。
2 危险性概述2.1 GHS分类物理性危害:FlammableLiquids:Flam.Liq.3健康危害急性毒性(经皮):AcuteTox.2严重损伤/刺激眼睛:EyeIrrit.2皮肤腐蚀/刺激:SkinCorr.1A特异性靶器官毒性(单一接触):STOTSE3环境危害急性水生毒性:AquaticAcute12.2 GHS 标记要素,包括预防性的陈述危害类型GHS02:易燃物; GHS07:感叹号;信号词 【警告】危险申明H226 易燃液体和蒸气。
H315 引起皮肤过敏。
H317 可能会导致皮肤过敏反应。
H319 造成了严重的眼睛发炎。
H335 可能引起呼吸道发炎。
警告申明P261 避免吸入粉尘/烟/气体/烟雾/蒸汽/喷雾。
P280 戴防护手套/防护服/护眼/防护面具。
P305+P351+P338 如进入眼睛:用水小心清洗几分钟。
如果可以做到,摘掉隐形眼镜,继续冲洗。
RSHazard symbol(s) XiR-phrase(s) R10;R43;R38S-phrase(s) 无数据资料2.3 其它危害物-无3 成分/组成信息3.1 物质分子式 - C8H14O2分子量 - 142.24 急救措施4.1 必要的急救措施描述一般的建议请教医生。
出示此安全技术说明书给到现场的医生看。
如果吸入如果吸入,请将患者移到新鲜空气处。
如果停止了呼吸,给于人工呼吸。
请教医生。
在皮肤接触的情况下用肥皂和大量的水冲洗。
请教医生。
在眼睛接触的情况下用大量水彻底冲洗至少15分钟并请教医生。
矿产

矿产资源开发利用方案编写内容要求及审查大纲
矿产资源开发利用方案编写内容要求及《矿产资源开发利用方案》审查大纲一、概述
㈠矿区位置、隶属关系和企业性质。
如为改扩建矿山, 应说明矿山现状、
特点及存在的主要问题。
㈡编制依据
(1简述项目前期工作进展情况及与有关方面对项目的意向性协议情况。
(2 列出开发利用方案编制所依据的主要基础性资料的名称。
如经储量管理部门认定的矿区地质勘探报告、选矿试验报告、加工利用试验报告、工程地质初评资料、矿区水文资料和供水资料等。
对改、扩建矿山应有生产实际资料, 如矿山总平面现状图、矿床开拓系统图、采场现状图和主要采选设备清单等。
二、矿产品需求现状和预测
㈠该矿产在国内需求情况和市场供应情况
1、矿产品现状及加工利用趋向。
2、国内近、远期的需求量及主要销向预测。
㈡产品价格分析
1、国内矿产品价格现状。
2、矿产品价格稳定性及变化趋势。
三、矿产资源概况
㈠矿区总体概况
1、矿区总体规划情况。
2、矿区矿产资源概况。
3、该设计与矿区总体开发的关系。
㈡该设计项目的资源概况
1、矿床地质及构造特征。
2、矿床开采技术条件及水文地质条件。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
矿产资源开发利用方案编写内容要求及审查大纲
矿产资源开发利用方案编写内容要求及《矿产资源开发利用方案》审查大纲一、概述
㈠矿区位置、隶属关系和企业性质。
如为改扩建矿山, 应说明矿山现状、
特点及存在的主要问题。
㈡编制依据
(1简述项目前期工作进展情况及与有关方面对项目的意向性协议情况。
(2 列出开发利用方案编制所依据的主要基础性资料的名称。
如经储量管理部门认定的矿区地质勘探报告、选矿试验报告、加工利用试验报告、工程地质初评资料、矿区水文资料和供水资料等。
对改、扩建矿山应有生产实际资料, 如矿山总平面现状图、矿床开拓系统图、采场现状图和主要采选设备清单等。
二、矿产品需求现状和预测
㈠该矿产在国内需求情况和市场供应情况
1、矿产品现状及加工利用趋向。
2、国内近、远期的需求量及主要销向预测。
㈡产品价格分析
1、国内矿产品价格现状。
2、矿产品价格稳定性及变化趋势。
三、矿产资源概况
㈠矿区总体概况
1、矿区总体规划情况。
2、矿区矿产资源概况。
3、该设计与矿区总体开发的关系。
㈡该设计项目的资源概况
1、矿床地质及构造特征。
2、矿床开采技术条件及水文地质条件。
