USP重金属检查法
(完整版)USP231重金属

USP<231>重金属本检验用以证实供试品中与硫离子作用显色的金属杂质含量,在规定检验条件下,不超过个论中规定的供试品重金属限度,以铅百分含量(重量比)计,与用标准铅溶液配制的对照进行目测比较(参看分光光度法和光散射法<851>中操作步骤部分的视觉比较)测定。
[注意:与本检验起反应的代表性物质为铅、汞、铋、砷、锑、锡、镉、银、铜和钼。
]除个论另有规定外,用方法I测定重金属含量。
方法I用于规定条件下可生成无色的、透明的制品的物质。
方法II适用于在方法I规定条件下无法生成无色透明制品的物质,或由于自身复杂特性,会对硫离子生成的金属沉淀造成干扰的物质,以及不挥发油和挥发油。
方法III湿消化法,仅在方法I和方法II均不适用时使用。
专用试剂硝酸铅贮备液:取硝酸铅159.8 mg,加已加硝酸1 mL的水100 mL,溶解,加水稀释至1000 mL。
于不含可溶铅盐的玻璃容器中配制和贮存该溶液。
标准铅溶液:使用当天配制。
取硝酸铅贮备液10.0 mL,加水稀释至100.0 mL。
每mL标准铅溶液相当于10 µg的铅。
以每g供试品100µL标准铅溶液为基准制备的参比溶液相当于每一百万份供试品中含有一份铅。
方法IpH 3.5醋酸盐缓冲液:取醋酸铵25.0 g,加水25 mL,溶解,加6 mol/L盐酸38.0 mL。
如有需要,用6 mol/L氨水或6 mol/L盐酸调节pH 为3.5,用水稀释至100 mL。
混匀。
标准溶液:用移液管取标准铅溶液2 mL(20 µg的铅),置50 mL比色管中,用水稀释至25 mL。
使用pH计或窄范围pH试纸作外部指示剂,用1mol/L醋酸或6mol/L氨水调节pH至3.0-4.0,用水稀释至40 mL,混匀。
供试溶液:取如个论所述制备的供试液25 mL,置50 mL比色管中;或当个论另有规定时,使用个论中规定体积量的酸溶解,并用水稀释至25 mL。
USP 231中文

USP<231 >重金属-方法1+2此次试验是提供阐明被硫化物染色的重金属物质含量,在特定情况下,在测试物质中铅含量(重量)不超过标准规定的限值。
其方法为通过与标准配制的标准铅溶液目视比色。
注1:在此次试验具有明显回应(显色)的物质为铅,汞,砷,锡,锑,镉,银,铜与钼。
注2:在可见光与紫外光目视比色的方法见<851>除非另有个别标准文献中规定,一般采用方法1测定重金属含量。
方法1用于在特定测试条件下产生透明,无色的指示剂的物质。
方法2用于在方法1规定的试验条件下不产生透明,无色的指示剂的物质,或者由于其复合性质,通过硫化物离子或固定和挥发性物质干扰金属沉淀的物质油。
方法3是一个湿度消化的方法,仅在方法1与方法2都不适用的情况下使用。
1 特定试剂制备1.1硝酸铅储备液的配制159.8mg硝酸铅溶于含有1ml硝酸的100ml纯化水中,然后稀释到1000ml,稀释液为纯化水。
配制与存放使用玻璃容器,远离可溶性铅盐。
1.2铅标准溶液现用现配,稀释10ml硝酸铅储备液加水至100ml。
铅标准溶液浓度为10ug/ml.方法12-方法1所需试剂2.1 Ph3.5乙酸盐缓冲液25.0g的乙酸铵溶于25ml水中,加入38ml6mol/L的盐酸。
若有需要则使用6mol/L氨水或者6mol/L的盐酸调节ph至3.5.加水稀释至100ml.2.2配制标准液在50ml比色管中加入2ml铅标准溶液(10ug/ml)加水稀释至25ml.使用ph 计或者ph试纸作为外部检测指示器,用1mol/L的醋酸或者6mol/L氨水调节ph至3.0-4.0,然后加水稀释至40ml,混匀。
2.3试验液配制在50ml比色管中加入25ml测试液,(或使用指定体积的酸,在个别专着中规定的情况下,溶解并用水稀释至25 mL,待测物质的量(g),按公式2.0 /(1000L)计算,其中L为重金属限值,%。
)使用ph计或者ph试纸作为外部检测指示器,用1mol/L的醋酸或者6mol/L氨水调节ph至3.0-4.0,然后加水稀释至40ml,混匀。
美国药典重金属检测方法-中文

铑
10
40
0.0001
钌
10
40
0.0002
铬
25
100
0.002
钼
25
100
0.0002
镍
25
100
0.002
钒
25
100
0.005
铜
250
1000
0.002
镁
250
1000
0.001
样品制备
范围广泛的各种样品都可以用 USP<232>/<233> 进行分 析,所以提供适合所有样品类型的详细样品处理方法并不现 实。有些药物样品可以直接分析(不用溶解),而其他样品 可以用水性溶剂(如水或稀酸)或适当的有机溶剂(如 2-丁 氧乙醇 : 水(25 : 75)[3],DMSO 或 DGME)简单稀释或 溶解进行制备。用水性或有机溶剂进行简单稀释或溶解的 方法必须考虑样品的化学稳定性,并且对于有机溶剂溶解, 还要考虑样品中组分化合物的不同挥发性。对许多 API 来 说,用有机溶剂稀释是首选方法,这种情况下有必要采取有 助于稳定分析物的方法,以避免因较高或较低挥发性(与校 正标准品相比)成分的存在而造成的回收率波动 [7]。
USP<232> 包括一个涉及元素形态的章节,指出 As 和 Hg 的某些形态值得关注,因为其毒性比其它形态要大得 多。As 的 PDE 是指无机 As,如果总 As 浓度超出限度, 必须用一种能够对不同 As 形态进行分离和定量的方法对样 品进行重新分析。这样做的原因是无机 As 比常见的有机形 式(如,砷甜菜碱)毒性大得多,因此形态分析必须能够分 离其不同化学形态,确定无机 As(亚砷酸盐(三价 As)和 砷酸盐(四价 As))的总量低于限量。同样,Hg 限量也是 指无机 Hg(Hg2+),虽然甲基汞(MeHg)是毒性更大的 形态,但通常认为药物中不可能存在 MeHg。但如果样品来 自于可能含有相当量甲基汞的原料(如,鱼组织),也必须 对其进行特别的分离和测定。
USP232-233重金属检测方法-中文

铑
10
40
0.0001
钌
10
40
0.0002
铬
25
100
0.002
钼
25
100
0.0002
镍
25
100
0.002
钒
25
100
0.005
铜
250
1000
0.002
镁
250
1000
0.001
样品制备
范围广泛的各种样品都可以用 USP<232>/<233> 进行分 析,所以提供适合所有样品类型的详细样品处理方法并不现 实。有些药物样品可以直接分析(不用溶解),而其他样品 可以用水性溶剂(如水或稀酸)或适当的有机溶剂(如 2-丁 氧乙醇 : 水(25 : 75)[3],DMSO 或 DGME)简单稀释或 溶解进行制备。用水性或有机溶剂进行简单稀释或溶解的 方法必须考虑样品的化学稳定性,并且对于有机溶剂溶解, 还要考虑样品中组分化合物的不同挥发性。对许多 API 来 说,用有机溶剂稀释是首选方法,这种情况下有必要采取有 助于稳定分析物的方法,以避免因较高或较低挥发性(与校 正标准品相比)成分的存在而造成的回收率波动 [7]。
元素 镉 铅 无机砷 无机汞 铱 锇 钯 铂 铑 钌 铬 钼 镍 钒 铜 镁
每日剂量 PDE(µg/日) 5 10 15 15 100 100 100 100 100 100 250 250 250 250 2500 2500
虽然 USP<232> 中制定的 PDE 限度用任何 USP<233> (ICP-OES 或 ICP-MS)中参考的仪器技术都可以测定 [6], 但许多新药中使用越来越复杂而珍贵的原料药,可能只包 含非常少的量。对这些毫克级样品进行大比例稀释制备, 意味着使用检测限尽可能低的仪器非常重要。低检测限和 宽动态范围线性校正(安捷伦 7700 系列达到 9 个数量级) 是 ICP-MS 最非常可贵的特性。低检测限对于 USP<232> 要求必须以最低限量进行控制的潜在毒性痕量元素(特别是 As、Cd、Hg 和 Pb)尤为重要。
USP 231重金属

USP<231>重金属本检验用以证实供试品中与硫离子作用显色的金属杂质含量,在规定检验条件下,不超过个论中规定的供试品重金属限度,以铅百分含量(重量比)计,与用标准铅溶液配制的对照进行目测比较(参看分光光度法和光散射法<851>中操作步骤部分的视觉比较)测定。
[注意:与本检验起反应的代表性物质为铅、汞、铋、砷、锑、锡、镉、银、铜和钼。
]除个论另有规定外,用方法I测定重金属含量。
方法I用于规定条件下可生成无色的、透明的制品的物质。
方法II适用于在方法I规定条件下无法生成无色透明制品的物质,或由于自身复杂特性,会对硫离子生成的金属沉淀造成干扰的物质,以及不挥发油和挥发油。
方法III湿消化法,仅在方法I和方法II均不适用时使用。
专用试剂硝酸铅贮备液:取硝酸铅159.8 mg,加已加硝酸1 mL的水100 mL,溶解,加水稀释至1000 mL。
于不含可溶铅盐的玻璃容器中配制和贮存该溶液。
标准铅溶液:使用当天配制。
取硝酸铅贮备液10.0 mL,加水稀释至100.0 mL。
每mL标准铅溶液相当于10 µg的铅。
以每g供试品100µL标准铅溶液为基准制备的参比溶液相当于每一百万份供试品中含有一份铅。
方法IpH 3.5醋酸盐缓冲液:取醋酸铵25.0 g,加水25 mL,溶解,加6 mol/L盐酸38.0 mL。
如有需要,用6 mol/L氨水或6 mol/L盐酸调节pH 为3.5,用水稀释至100 mL。
混匀。
标准溶液:用移液管取标准铅溶液2 mL(20 µg的铅),置50 mL比色管中,用水稀释至25 mL。
使用pH计或窄范围pH试纸作外部指示剂,用1mol/L醋酸或6mol/L氨水调节pH至3.0-4.0,用水稀释至40 mL,混匀。
供试溶液:取如个论所述制备的供试液25 mL,置50 mL比色管中;或当个论另有规定时,使用个论中规定体积量的酸溶解,并用水稀释至25 mL。
五重金属检查法(1)重金属概念_工业上_比重5以上的金属药典

过程:第一步同古蔡氏法生成AsH3 第二步将生成的AsH3经导管
导入盛有Ag-DDC吡淀溶液中,与之 作用使Ag-DDC中银还原为红色胶态 银溶液,同法处理标准砷溶液后比 较(目视或于510nm处测定吸收 度)。
优点:①灵敏度高 0.5μg As/30ml;②重现性好,仪器测定; ③消除锑干扰
(3)干扰物的排除: ①硫化物的排除:S2-、S032-、
S2032- H+ H2S、SO2 与溴化汞试纸作用 生成HgS色斑或Hg。排除方法先加浓 HNO3。
②Fe3+:供试品为铁盐,可消耗还 原剂,影响测定条件,并能氧化砷化 氢,干扰测定:排除方法可先加酸性 氯化亚锡SnC12,使Fe3+ Fe2+除去干 扰,如枸橼酸铁铵中砷盐的检查。
Fe3++Vc Fe2++去氢抗坏血酸
②大量的Fe3+(如枸橼酸铁胺):加入浓
HCI Fe3++HCI—HFeCl62-(六氯合铁离子) 黄色,用乙醚提取到无色,剩余少量Fe3+ ,
在氨碱性溶液中,加掩蔽剂 KCN
K3[Fe(CN)6],(Na2S为显色剂)(第三 法)。
③药物本身能够与显色剂生成不溶性硫化
3.讨论:
(1)试剂的作用:
①KI还原剂As5+-As3+,五价砷在酸性溶液
也能被金属Zn还原为砷化氢,但生成砷化氢
速度较慢,故加KI还原As5+为三价砷。
②SnC12:还原剂As5+-As3+;除去KI被氧化 生成的I2,生成的I-与锌离子形成稳定配位 离子,有利于生成砷化氢反应,4I-+Zn2+-
CH3CSNH2 H2O pH3.5 CH3CONH2 H2S
重金属检查法(USP和EP)
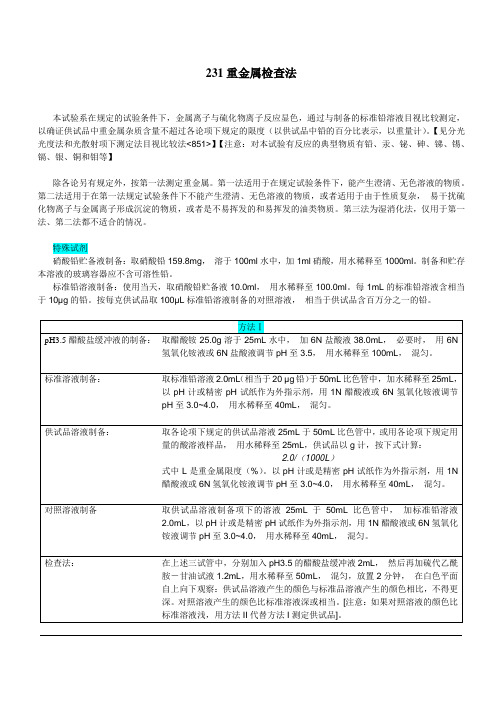
231重金属检查法本试验系在规定的试验条件下,金属离子与硫化物离子反应显色,通过与制备的标准铅溶液目视比较测定,以确证供试品中重金属杂质含量不超过各论项下规定的限度(以供试品中铅的百分比表示,以重量计)。
【见分光光度法和光散射项下测定法目视比较法<851>】【注意:对本试验有反应的典型物质有铅、汞、铋、砷、锑、锡、镉、银、铜和钼等】除各论另有规定外,按第一法测定重金属。
第一法适用于在规定试验条件下,能产生澄清、无色溶液的物质。
第二法适用于在第一法规定试验条件下不能产生澄清、无色溶液的物质,或者适用于由于性质复杂,易干扰硫化物离子与金属离子形成沉淀的物质,或者是不易挥发的和易挥发的油类物质。
第三法为湿消化法,仅用于第一法、第二法都不适合的情况。
特殊试剂硝酸铅贮备液制备:取硝酸铅159.8mg,溶于100ml水中,加1ml硝酸,用水稀释至1000ml。
制备和贮存本溶液的玻璃容器应不含可溶性铅。
标准铅溶液制备:使用当天,取硝酸铅贮备液10.0ml,用水稀释至100.0ml。
每1mL的标准铅溶液含相当于10µg的铅。
按每克供试品取100µL标准铅溶液制备的对照溶液,相当于供试品含百万分之一的铅。
在上述二试管中,分别加入pH3.5的醋酸盐缓冲液2mL,然后再加硫代乙酰胺—甘油试液1.2mL,用水稀释至50mL,混匀,放置2分钟,在白色平面自上向下观察:供试品溶液产生的颜色与标准品溶液产生的颜色相比,不得更深。
EP 版的重金属分析方法重金属方法A供试溶液:12ml待测水溶液,2ml pH为3.5的缓冲溶液,混合后加1.2ml的硫代乙酰胺试液,立即混合。
对照溶液:10ml的标准铅溶液(1ppm or 2ppm Pb),2ml pH为3.5的缓冲溶液,2ml的待测液,混合后加1.2ml的硫代乙酰胺试液,立即混合。
空白溶液:10ml的水,2ml pH为3.5的缓冲溶液,2ml的测试溶液。
美国药典USP32-重金属测试

<231> 重金属本试验系在规定的试验条件下,金属离子与硫化物离子反应显色,通过制备的标准铅溶液目视比较测定,以确证供试品中重金属杂质含量不超过各论项下规定的限度(以供试品中铅的百分比表示,以重量计)。
(见分光光度法和光散射项下测定法目视比较法<851>)[ 注意:对本试验有响应的典型物质有铅、汞、铋、砷、锑、锡、镉、银、铜和钼等]。
除各论另有规定外,按第一法测定重金属。
第一法适用于在规定试验条件下,能产生澄清、无色溶液的物质。
第二法适用于在第一法规定试验条件下不能产生澄清、无色溶液的物质,或者适用于由于性质复杂,易干扰硫化物离子与金属离子形成沉淀的物质,或者是不易挥发的和易挥发的油类物质。
第三法为湿消化法,仅用于第一法、第二法都不适合的情况。
特殊试剂特殊试剂特殊试剂特殊试剂硝酸铅贮备液—取硝酸铅159.8mg,溶于100ml水中,加1ml硝酸,用水稀释至1000ml。
制备和贮存本溶液的玻璃容器应不含可溶性铅。
标准铅溶液—使用当天,取硝酸铅贮备液10.0ml,用水稀释至100.0ml。
每1ml的标准铅溶液含相当于10µg的铅。
按每克供试品取100µl标准铅溶液制备的对照溶液,相当于供试品含百万分之一的铅。
方法方法方法方法IIII pH3.5醋酸盐缓冲液—取醋酸铵25.0g溶于25ml水中,加6N盐酸液38.0ml,必要时,用6N氢氧化铵液或6N盐酸液调节pH至3.5,用水稀释至100ml,混匀。
标准溶液准备—精密量取标准铅溶液2ml,(相当于20µg的Pb),置50ml比色管中,加水稀释至25ml,以精密pH试纸作为外指示剂,用1N醋酸液或6N 氢氧化铵液调节pH至3.0~4.0,用水稀释至40ml,混匀。
供试品溶液制备—取各论项下规定的供试品溶液25ml,置50ml比色管中,或用各论项下规定用量的酸溶解样品,再用水稀释至25ml,供试品以g计,按下式计算: 2.0/(1000L)式中L是重金属限度(%)。
USP 金属检查法 科学探讨及发展趋势 Darrell R. Abernethy MD PhD USP首席科学官.ppt

重金属污染存在的证据
• 1989 – 2008期间健康关怀产品中出现高浓有毒金属元素 / 非金属的报道*
• 元素 健康产品
报道年份
•砷 •
注射用玻璃安培 婴儿稻米产品,阿育吠陀草药
2006 2004 , 2008
• 镉 药用植物 ,尼日利亚草药
1989, 2005, 2006
•铅
药物,阿育吠陀草药
> 10000 > 10000
有毒
低毒性
无毒性
不同形式汞的吸收差异
汞蒸气 一价汞 二价汞 二甲基汞
Hg0 Hg1+ Hg2+ MeHg
80% 通过肝脏 7-15% 通过胃肠道 95%通过胃肠道
新问题
• 在低剂量重金属暴露下敏感亚人群不断提高的顾虑 • 药品/草药产品及其原料从发展中国家进口 • 新产品存在未知属性和潜在污染(如草药的纳米材料) • 健康关怀产品需要一种快速花费少效果好的筛选系统
问题是什么?
• 哪些重金属? • 风险评估和限度设定 • 风险评估的修正因素 • 测定
现行法规文件—USP/EP
• USP 通则 <231> “重金属” • USP 通则 <730> “等离子体光谱化学”
– USP-NF 29 <730>, p. 2700, 2006, 2007
• EP 01/2008:20408 “重金属”
1989, 2004, 2007
•汞
阿育吠陀草药 /草药
2004, 2007
•硒
尼日利亚草药 , 营养补充剂
2006, 2008
• * 有限的审核公开文献,使用Google 和PubMed
美国食品供应中有毒元素的主要饮食来源*
231 重金属USP35

231 重金属本实验用于证明在规定检验条件下,与硫化物离子作用显色的金属杂质的含量不得超过个论中规定的重金属限度供试品中铅的百分比(重量比),与标准铅溶液配制的对照组进行视觉比较(参见分光光度法和光散射法(851)中规程部分的视觉比较)测定。
【标注:与本检验起反应的代表性物质有铅、汞、砷、锑、铋、镉、锡、银、铜、钼】除另有规定外,法一测定重金属的含量。
法一适用于规定条件下的澄清、无色制剂。
法二用于法一规定条件下的非澄清、无色的制剂或者用于具有复杂的性质,干扰降解的金属硫化物离子、或为固体和挥发性油的物质。
法三,湿法消解法,适用于法一、法二都不能用的情况下。
专用试剂【硝酸铅原液】取硝酸铅159.8mg至已加入1ml硝酸的100ml水中,溶解,加水稀释至1000ml。
在不含可溶性铅盐的玻璃容器中配备和储存该液。
【标准铅溶液】当天使用,取10ml硝酸铅原液,用水稀释至100ml.每毫升标准铅溶液相当于10ug铅。
对照液为用100ul的标准铅溶液溶解1g供试品。
(相当于供试品中铅含量的百万分之一)法一【PH 3.5 的醋酸盐缓释剂】用25ml水溶解25.0g醋酸铵,加入38.0ml的6N 盐酸。
必要的话,可用6N 氨水或6N 盐酸调节PH 至3.5,加水稀释至100ml,摇匀。
【标准溶液】往50ml的比色管加入2ml的标准铅溶液(20ug铅),加水稀释至25ml。
使用pH计或短范围pH试纸作为外指示剂,用1N 醋酸或 6N 氢氧化铵调节PH 至3.0-4.0,加水稀释至40ml,摇匀。
【供试溶液】往50ml的比色管加入25ml该品种项下规定方法制成的供试品溶液,或当个论有规定时,使用指定体积的酸溶解按公式计算的供试品的数量(单位g)并用水稀释至25ml。
2.0/(1000L)公式中, L 是重金属的限度,作为一个百分数。
使用pH计或短范围pH试纸作为外指示剂,用1N 醋酸或 6N 氢氧化铵调节PH 至3.0-4.0,加水稀释至40ml,摇匀。
USP35-ROI和重金属检查方法

USP35 重金属检查和灼烧残渣方法231 HEAVY METALS (重点看Method Ⅱ)This test is provided to demonstrate that the content of metallic impurities that are colored by sulfide ion, under the specified test conditions, does not exceed the Heavy metals limit specified in the individual monograph in terms of the percentage (by weight)of lead in the test substance, as determined by concomitant visual comparison (see Visual Comparison in the section Procedure under Spectrophotometry and Light-Scattering <851>)with a control prepared from a Standard Lead Solution.[NOTE—Substances that typically will respond to this test are lead,mercury,bismuth,arsenic,antimony,tin,cadmium,silver,copper,and molybdenum.] Determine the amount of heavy metals by Method I, unless otherwise specified in the individual monograph. Method I is used for substances that yield clear, colorless preparations under the specified test conditions. Method II is used for substances that do not yield clear, colorless preparations under the test conditions specified for Method I, or for substances that, by virtue of their complex nature, interfere with the precipitation of metals by sulfide ion, or for fixed and volatile oils. Method III, a wet-digestion method, is used only in those cases where neither Method I nor Method II can be utilized.Special ReagentsLead Nitrate Stock Solution— Dissolve 159.8mg of lead nitrate in 100mLof water to which has been added 1mLof nitric acid, then dilute with water to 1000mL.Prepare and store this solution in glass containers free from soluble lead salts.Standard Lead Solution— On the day of use, dilute 10.0mLof Lead Nitrate Stock Solution with water to 100.0mL.Each m L of Standard Lead Solution contains the equivalent of 10µg of lead. A comparison solution prepared on the basis of 100µLof Standard Lead Solution per g of substance being tested contains the equivalent of 1part of lead per million parts of substance being tested.Method IpH3.5 Acetate Buffer— Dissolve 25.0 g of ammonium acetate in 25mL of water, and add 38.0mLof 6N hydrochloric acid. Adjust, if necessary, with 6N ammonium hydroxide or 6N hydrochloric acid to a pH of 3.5, dilute with water to 100mL, and mix.Standard Preparation— Into a 50-mLcolor-comparison tube pipet 2mL of Standard Lead Solution(20µg of Pb ),and dilute with water to 25mL.Adjust with 1N acetic acid or 6N ammonium hydroxide to a pH between 3.0and 4.0,using short-range pH indicator paper as external indicator, dilute with water to 40mL,and mix.Test Preparation— Into a 50-mLcolor-comparison tube place 25mLof the solution prepared for the test as directed in the individual monograph; or, using the designated volume of acid where specified in the individual monograph, dissolve in and dilute with water to 25mLthe quantity, in g, of the substance to be tested, as calculated bythe formula:2.0/(1000L),in which L is the Heavy metals limit, in percentage. Adjust with 1N acetic acid or 6N ammonium hydroxide to a pH between 3.0 and 4.0, using short-range pH indicator paper as external indicator, dilute with water to 40mL, and mix.Monitor Preparation— Into a third 50-mLcolor-comparison tube place 25mLof a solution prepared as directed for Test Preparation, and add 2.0mLof Standard Lead Solution. Adjust with 1N acetic acid or 6N ammonium hydroxide to a pH between 3.0and 4.0, using short-range pH indicator paper as external indicator, dilute with water to 40mL, and mix.Procedure— To each of the three tubes containing the Standard Preparation, the Test Preparation, and the Monitor Preparation, add 2mLof pH3.5Acetate Buffer, then add 1.2mLof thioacetamide–glycerin base TS, dilute with water to 50mL,mix,allow to stand for 2minutes,and view downward over a white surface *:the color of the solution from the Test Preparation is not darker than that of the solution from the Standard Preparation, and the intensity of the color of the Monitor Preparation is equal to or greater than that of the Standard Preparation.[NOTE—If the color of the Monitor Preparation is lighter than that of the Standard Preparation, use Method II instead of Method I for the substance being tested.]Method IIpH 3.5 Acetate Buffer— Prepare as directed under Method I.Standard Preparation— Prepare as directed under Method I.Test Preparation— Use a quantity, in g, of the substance to be tested as calculated by the formula:2.0/(1000L),in which L is the Heavy metals limit, in percentage. Transfer the weighed quantity of the substance to a suitable crucible, add sufficient sulfuric acid to wet the substance, and carefully ignite at a low temperature until thoroughly charred. (The crucible may be loosely covered with a suitable lid during the charring.) Add to the carbonized mass 2mLof nitric acid and 5drops of sulfuric acid, and heat cautiously until white fumes no longer are evolved. Ignite, preferably in a muffle furnace, at 500℃ to 600℃, until the carbon is completely burned off. Cool, add 4mLof 6N hydrochloric acid, cover, digest on a steam bath for 15minutes, uncover, and slowly evaporate on a steam bath to dryness. Moisten the residue with 1drop of hydrochloric acid, add 10mLof hot water, and digest for 2minutes.Add 6N ammonium hydroxide dropwise, until the solution is just alkaline to litmus paper, dilute with water to 25mL,and adjust with 1Nacetic acid to a pH between 3.0 and 4.0,using short-range pH indicator paper as external indicator. Filter if necessary, rinse the crucible and the filter with 10mLof water, combine the filtrate and rinsing in a 50-mLcolor-comparison tube, dilute with water to 40mL, and mix.Procedure— To each of the tubes containing the Standard Preparation and the Test Preparation, add 2mLof pH3.5 Acetate Buffer, then add 1.2mLof thioacetamide–glycerin base TS, dilute with water to 50mL, mix,allow to stand for2minutes,and view downward over a white surface*:the color of the solution from the Test Preparation is not darker than that of the solution from the Standard Preparation. Method IIIpH3.5 Acetate Buffer— Prepare as directed under Method I.Standard Preparation— Transfer a mixture of 8mLof sulfuric acid and 10mLof nitric acid to a clean,dry,100-mL Kjeldahl flask, and add a further volume of nitric acid equal to the incremental volume of nitric acid added to the Test Preparation. Heat the solution to the production of dense, white fumes, cool, cautiously add 10mLof water and, if hydrogen peroxide was used in treating the Test Preparation, add a volume of 30percent hydrogen peroxide equal to that used for the substance being tested, and boil gently to the production of dense, white fumes. Again cool, cautiously add 5mLof water, mix, and boil gently to the production of dense, white fumes and to a volumeof 2-3mL.Cool, dilute cautiously with a few mL of water, add 2.0mLof Standard Lead Solution(20µg of Pb),and mix. Transfer to a 50-mLcolor-comparison tube, rinse the flask with water, adding the rinsing to the tube until the volume is 25mL, and mix.Test Preparation—If the substance is a solid— Transfer the quantity of the test substance specified in the individual monograph to a clean, dry, 100-mL Kjeldahl flask. [NOTE—A300-mLflask may be used if the reaction foams excessively.]Clamp the flask at an angle of 45 , and add a sufficient quantity of a mixture of 8mLof sulfuric acid and 10mLof nitric acid to moisten the substance thoroughly. Warm gently until the reaction commences, allow the reaction to subside, and add additional portions of the same acid mixture, heating after each addition, until a total of 18mLof the acid mixture has been added. Increase the amount of heat, and boil gently until the solution darkens. Cool, add 2mLof nitric acid, and heat again until the solution darkens. Continue the heating, followed by addition of nitric acid until no further darkening occurs, then heat strongly to the production of dense, white fumes. Cool, cautiously add 5mLof water, boil gently to the production of dense, white fumes, and continue heating until the volume is reduced to a few mL. Cool, cautiously add 5mLof water, and examine the color of the solution. If the color is yellow, cautiously add 1mLof 30percent hydrogen peroxide, and again evaporate to the production of dense, white fumes and a volume of 2- 3mL.If the solution is still yellow in color, repeat the addition of 5mLof water and the peroxide treatment. Cool, dilute cautiously with a few mL of water, and rinse into a 50-mLcolor-comparison tube, taking care that the combined volume does not exceed 25mL.If the substance is a liquid— Transfer the quantity of the test substance specified in the individual monograph to a clean, dry, 100-mL Kjeldahl flask.[NOTE—A300-mLflask may be used if the reaction foams excessively.]Clamp the flask at an angle of 45, and cautiously add a few mL of a mixture of 8mLof sulfuric acid and 10mLof nitric acid. Warm gently until the reaction commences, allow the reaction to subside, and proceed as directed under If the substance is a solid, beginning with “add additional portions of the same acid mixture.”Procedure— Treat the Test Preparation and the Standard Preparation as follows: Adjust the solution to a pH between 3.0and 4.0, using short-range pH indicator paperas external indicator, with ammonium hydroxide (a dilute ammonia solution may be used, if desired, as the specified range is approached), dilute with water to 40mL, and mix.To each tube add 2mL of pH3.5 Acetate Buffer, then add 1.2mL of thioacetamide-glycerin base TS, dilute with water to 50mL,mix, allow to stand for 2minutes, and view downward over a white surface*:the color of the Test Preparation is not darker than that of the Standard Preparation.。
(完整版)USP231重金属

USP<231>重金属本检验用以证实供试品中与硫离子作用显色的金属杂质含量,在规定检验条件下,不超过个论中规定的供试品重金属限度,以铅百分含量(重量比)计,与用标准铅溶液配制的对照进行目测比较(参看分光光度法和光散射法<851>中操作步骤部分的视觉比较)测定。
[注意:与本检验起反应的代表性物质为铅、汞、铋、砷、锑、锡、镉、银、铜和钼。
]除个论另有规定外,用方法I测定重金属含量。
方法I用于规定条件下可生成无色的、透明的制品的物质。
方法II适用于在方法I规定条件下无法生成无色透明制品的物质,或由于自身复杂特性,会对硫离子生成的金属沉淀造成干扰的物质,以及不挥发油和挥发油。
方法III湿消化法,仅在方法I和方法II均不适用时使用。
专用试剂硝酸铅贮备液:取硝酸铅159.8 mg,加已加硝酸1 mL的水100 mL,溶解,加水稀释至1000 mL。
于不含可溶铅盐的玻璃容器中配制和贮存该溶液。
标准铅溶液:使用当天配制。
取硝酸铅贮备液10.0 mL,加水稀释至100.0 mL。
每mL标准铅溶液相当于10 µg的铅。
以每g供试品100µL标准铅溶液为基准制备的参比溶液相当于每一百万份供试品中含有一份铅。
方法IpH 3.5醋酸盐缓冲液:取醋酸铵25.0 g,加水25 mL,溶解,加6 mol/L盐酸38.0 mL。
如有需要,用6 mol/L氨水或6 mol/L盐酸调节pH 为3.5,用水稀释至100 mL。
混匀。
标准溶液:用移液管取标准铅溶液2 mL(20 µg的铅),置50 mL比色管中,用水稀释至25 mL。
使用pH计或窄范围pH试纸作外部指示剂,用1mol/L醋酸或6mol/L氨水调节pH至3.0-4.0,用水稀释至40 mL,混匀。
供试溶液:取如个论所述制备的供试液25 mL,置50 mL比色管中;或当个论另有规定时,使用个论中规定体积量的酸溶解,并用水稀释至25 mL。
USP重金属检查法
标准操作规程Stan dard Operat ing Procedure1. 简述1.1重金属是指在规定实验条件下能与硫代乙酰胺或硫化钠作用显色的金属盐类杂质1. 2硫化钠或硫代乙酰胺在弱酸性条件下水解产生硫化氢,与供试品中重金属在规定实验条件下所显颜色,与一定量的标准铅溶液在同样操作条件下所显的颜色比较。
1. 3由于实验条件不同。
分为三种检查方法:第一法适用于在规定条件下能生成澄清无色溶液的供试品。
第二法适用于在规定条件下不能生成澄清无色溶液的供试品;第三法适用于那些不能用一法和二法的样品。
2仪器2. 1仪器设备50ml纳氏比色管2. 2试剂和溶液a)硫代乙酰胺试液:称取硫代乙酰胺4g加水溶解,用水稀释至100ml,摇匀。
b)甘油基准试液:称取200g甘油加水至总重量为235g,然后加142.5ml1N氢氧化钠溶液和47.5ml的水。
临用前用0.2ml硫代乙酰胺试液和1ml甘油基准试液混合,在沸水浴中加热20秒钟,立即使用•c)硝酸铅贮备液:称取硝酸铅0.1598g,置1000ml量瓶中,加硝酸1ml与水100ml溶解后,用水稀释至1000ml摇匀,作为贮备液。
标准铅溶液临用前,精密量取贮备液10ml,置100ml量瓶中,加水稀释至刻度,摇匀,即得(每1ml相当于10 g的Pb)。
d)PH3.5醋酸盐缓冲液:取醋酸铵25.0g,加水25ml溶解后,加38.0ml6N盐酸,如有必要用6N氨水或6N盐酸调PH至3.5 ,用水稀释至100ml,摇匀。
第一法:标准溶液的制备取50ml比色管,用移液管移取2.0ml标准铅溶液(20 gPb),用水稀释到25ml,用1N醋酸或6N氨水溶液调PH至3.0~4.0之间,用窄范围的精密pH试纸作指示,然后加水稀释至40ml,摇匀。
供试品溶液的制备另取一支50ml比色管,加入按该品种项下规定的方法制成的供试液25ml ;或加入供试品的量以g计,按公式2.0/1000L计算,其中L 为重金属的限度(%),用该品种项下规定的酸的体积溶解,加水溶解并稀释至25ml,供试品溶液用1N醋酸或6N氨水溶液调PH至3.0〜4.0之间,用窄范围的精密pH试纸作指示,然后加水稀释至40ml,摇匀。
USP重金属检查法
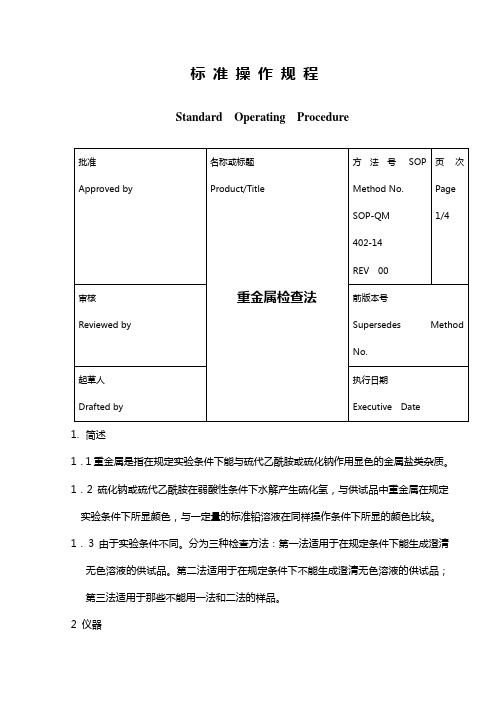
标准操作规程Standard Operating Procedure1.简述1.1重金属是指在规定实验条件下能与硫代乙酰胺或硫化钠作用显色的金属盐类杂质。
1.2硫化钠或硫代乙酰胺在弱酸性条件下水解产生硫化氢,与供试品中重金属在规定实验条件下所显颜色,与一定量的标准铅溶液在同样操作条件下所显的颜色比较。
1.3由于实验条件不同。
分为三种检查方法:第一法适用于在规定条件下能生成澄清无色溶液的供试品。
第二法适用于在规定条件下不能生成澄清无色溶液的供试品;第三法适用于那些不能用一法和二法的样品。
2 仪器2.1仪器设备50ml纳氏比色管2.2试剂和溶液a)硫代乙酰胺试液: 称取硫代乙酰胺4g加水溶解,用水稀释至100ml,摇匀。
b)甘油基准试液: 称取200g甘油加水至总重量为235g,然后加142.5ml1N氢氧化钠溶液和47.5ml的水。
临用前用0.2ml硫代乙酰胺试液和1ml甘油基准试液混合,在沸水浴中加热20秒钟,立即使用.c)硝酸铅贮备液:称取硝酸铅0.1598g,置1000ml量瓶中,加硝酸1ml与水100ml溶解后,用水稀释至1000ml 摇匀,作为贮备液。
标准铅溶液临用前,精密量取贮备液10ml,置100ml量瓶中,加水稀释至SOP-QM 402-14 页号Page:2/4刻度,摇匀,即得(每1ml相当于10μg的Pb)。
d)PH3.5醋酸盐缓冲液: 取醋酸铵25.0g,加水25ml溶解后,加38.0ml6N盐酸,如有必要用6N氨水或6N盐酸调PH至3.5 ,用水稀释至100ml,摇匀。
第一法:标准溶液的制备取50ml比色管,用移液管移取2.0ml标准铅溶液(20 μgPb),用水稀释到25ml,用1N醋酸或6N氨水溶液调PH至3.0~4.0之间,用窄围的精密pH试纸作指示,然后加水稀释至40ml,摇匀。
供试品溶液的制备另取一支50ml比色管,加入按该品种项下规定的方法制成的供试液25ml;或加入供试品的量以g计, 按公式2.0/1000L计算,其中L为重金属的限度(%),用该品种项下规定的酸的体积溶解,加水溶解并稀释至25ml,供试品溶液用1N醋酸或6N氨水溶液调PH至3.0~4.0之间,用窄围的精密pH试纸作指示,然后加水稀释至40ml,摇匀。
USP方法与EP方法对比实验

USP方法与EP方法对比实验USP方法与EP方法是两种在药品行业中常用的药品质量控制方法。
它们在检测、分析和评估药品的质量、安全性和有效性方面都起着重要作用。
在本文中,我们将对USP和EP方法进行比较,以了解它们之间的异同以及在不同情境下的应用。
首先,我们来了解一下USP方法。
USP(United States Pharmacopeia)方法是由美国药典委员会制定的一套药品质量控制标准。
它包含了药物检验的各个方面,如药物成分分析、纯度检验、微生物检查、重金属和有害物质的检测等。
USP方法主要侧重于药品的物质性质和纯度,以确保药品符合严格的质量标准。
EP方法,即欧洲药典法,是由欧洲药典委员会制定的一套药品质量标准。
EP方法和USP方法在很大程度上是相互兼容的,它们都依赖国际统一的药物检验方法和标准。
EP方法的目标是提供一套欧洲范围内适用的质量控制标准,以确保药品在欧洲市场的质量和安全性。
在比较USP方法和EP方法时,我们可以从以下几个方面进行比较:1.适用范围:USP方法主要适用于美国市场,而EP方法适用于欧洲市场。
虽然它们在一些方面可能存在差异,但在大多数情况下,二者是相互兼容的。
2.法规依据:USP方法是美国食品药品监督管理局(FDA)认可的标准,而EP方法是欧洲药品管理局(EMA)认可的标准。
这意味着在相应市场中,药品必须符合相应的标准才能得到批准和上市。
3.检测方法:USP方法和EP方法在很多方面都有相似之处,比如药物成分的定量方法、杂质检测的方法等。
然而,有时它们在一些细节上可能有一些差异,比如使用的试剂、仪器的差异等。
4.质量要求:USP方法和EP方法都要求药品符合一系列的纯度、效力和内容要求。
然而,在特定的情况下,它们可能会对一些特殊要求给出不同的答案。
因此,在制定和实施药品质量控制方法时,必须针对特定市场的要求进行调整。
5.更新频率:USP方法和EP方法都需要不断更新以适应不断变化的药品和药物检验技术。
USP重金属检查法
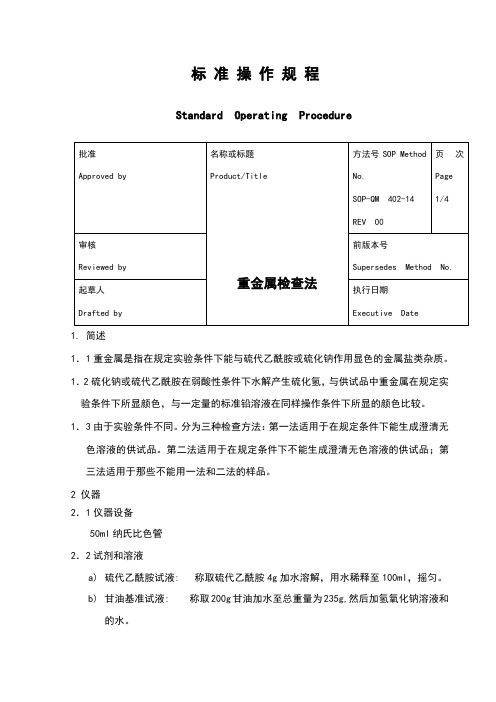
标准操作规程Standard Operating Procedure1.简述1.1重金属是指在规定实验条件下能与硫代乙酰胺或硫化钠作用显色的金属盐类杂质。
1.2硫化钠或硫代乙酰胺在弱酸性条件下水解产生硫化氢,与供试品中重金属在规定实验条件下所显颜色,与一定量的标准铅溶液在同样操作条件下所显的颜色比较。
1.3由于实验条件不同。
分为三种检查方法:第一法适用于在规定条件下能生成澄清无色溶液的供试品。
第二法适用于在规定条件下不能生成澄清无色溶液的供试品;第三法适用于那些不能用一法和二法的样品。
2 仪器2.1仪器设备50ml纳氏比色管2.2试剂和溶液a)硫代乙酰胺试液: 称取硫代乙酰胺4g加水溶解,用水稀释至100ml,摇匀。
b)甘油基准试液: 称取200g甘油加水至总重量为235g,然后加氢氧化钠溶液和的水。
临用前用硫代乙酰胺试液和1ml甘油基准试液混合,在沸水浴中加热20秒钟,立即使用.c)硝酸铅贮备液:称取硝酸铅0.1598g,置1000ml量瓶中,加硝酸1ml与水100ml溶解后,用水稀释至1000ml 摇匀,作为贮备液。
标准铅溶液临用前,精密量取贮备液10ml,置100ml量瓶中,加水稀释至刻SOP-QM 402-14 页号 Page:2/4度,摇匀,即得(每1ml相当于10g的Pb)。
d)醋酸盐缓冲液: 取醋酸铵25.0g,加水25ml溶解后,加盐酸,如有必要用6N氨水或6N盐酸调PH至,用水稀释至100ml,摇匀。
第一法:标准溶液的制备取50ml比色管,用移液管移取标准铅溶液(20 gPb),用水稀释到25ml,用1N醋酸或6N氨水溶液调PH至~之间,用窄范围的精密pH试纸作指示,然后加水稀释至40ml,摇匀。
供试品溶液的制备另取一支50ml比色管,加入按该品种项下规定的方法制成的供试液25ml;或加入供试品的量以g计, 按公式1000L计算,其中L为重金属的限度(%),用该品种项下规定的酸的体积溶解,加水溶解并稀释至25ml,供试品溶液用1N醋酸或6N氨水溶液调PH至~之间,用窄范围的精密pH试纸作指示,然后加水稀释至40ml,摇匀。
USP35-233 重金属杂质elemental impurity

5634〈232〉 Elemental Impurities—Limits / Chemical TestsSecond Supplement to USP 35–NF 30Table 1. Elemental Impurities for Drug Products (Continued)Individual components may need to be limited at levels dif-ferent from those in the table depending on monograph-Paren-Inhala-specific mitigating factors.]Oral teral tional LVP Daily Daily Daily Compo-Table 2. Default Concentration Limits for Drug Substances andDose Dose Dose nent ExcipientsPDE a PDE PDE LimitElement (µg/day)(µg/day)(µg/day)(µg/g)Concentra-Concentra-Concentra-Nickel 50050 1.5 5.0tion Limits tion Limits tion Limits Vanadium 1001030 1.0(µg/g) for (µg/g) for (µg/g) for Oral Drug Parenteral Inhalational Copper 10001007025Products Drug Prod-Drug Prod-a PDE = Permissible daily exposure based on a 50-kg person.with a ucts with a ucts with a b See Speciation section.Maximum Maximum Maximum *Not a safety concern.Daily Dose Daily Dose Daily Dose Element of ≤10 g/dayof ≤10 g/dayof ≤10 g/dayOptions for Demonstrating ComplianceCadmium 2.50.250.15Lead0.50.50.5Inorganic DRUG PRODUCT ANALYSIS OPTIONarsenic 0.150.150.15Inorganic The results obtained from the analysis of a typical dosage mercury 1.50.150.15unit, scaled to a maximum daily dose, are compared to the Iridium 10 1.00.15Daily Dose PDE .Osmium 10 1.00.15Daily Dose PDE ≥ measured value (µg/g) × maximum dailyPalladium 10 1.00.15dose (g/day)Platinum 10 1.00.15Rhodium 10 1.00.15The measured amount of each impurity is NMT the Daily Ruthenium 10010 1.5Dose PDE , unless otherwise stated in the individual monograph.Chromium ** 2.5Molybdenum 10 1.025Nickel 50 5.00.15SUMMATION OPTIONVanadium 1001030Copper 100107Separately add the amounts of each elemental impurity (in µg/g) present in each of the components of the drug *Not a safety concern.product using the following equation:ANALYTICAL TESTINGDaily Dose PDE ≥ [ΣM 1(C M × W M )] × D DIf, by validated processes and supply-chain control, manu-wherefacturers can demonstrate the absence of impurities, then M = each ingredient used to manufacture a dosage unit further testing is not needed. When testing is done to C M = element concentration in component (drug sub-demonstrate compliance, proceed as directed in general stance or excipient) (µg/g)chapter Elemental Impurities—Procedures 〈233〉, and mini-W M = weight of component in a dosage unit (g/dosage mally include As, Cd, Pd, and Hg in the Target Element eval-unit)uation.s 2S (USP35)D D = number of units in the maximum daily dose (unit/day)The result of the summation of each impurity is NMT the Daily Dose PDE , unless otherwise stated in the individual monograph. Before products can be evaluated using this option, the manufacturer must validate that additional ele-mental impurities cannot be inadvertently added through Add the following:the manufacturing process.DRUG SUBSTANCE AND EXCIPIENTSs〈233〉 ELEMENTAL IMPURITIES—The presence of elemental impurities in drug substances PROCEDURESand excipients must be controlled and, where present, re-ported. The acceptable levels for these impurities depend on the material’s ultimate use. Therefore, drug product manu-facturers must determine the acceptable level of elemental impurities in the drug substances and excipients used to INTRODUCTIONproduce their products.This chapter describes two analytical procedures (Proce-The values provided in Table 2 represent concentration dures 1 and 2) for the evaluation of the levels of the ele-limits for components (drug substances and excipients) of mental impurities. The chapter also describes criteria for ac-drug products dosed at a maximum daily dose of ≤10 g/ceptable alternative procedures. Alternative procedures that day. These values serve as default concentration limits to aid meet the validation requirements described herein may be discussions between drug product manufacturers and the considered equivalent to Procedures 1 and 2 for the pur-suppliers of the components of their drug products. [N OTE —poses of this test. In addition, system standardization andSecond Supplement to USP 35–NF 30Chemical Tests / 〈233〉 Elemental Impurities—Procedures5635suitability evaluation using applicable reference materials COMPENDIAL PROCEDURES 1 AND 2 should be performed on the day of analysis. The require-ment for an elemental impurity test is specified in GeneralNotices and Requirements or in the individual monograph. Bymeans of verification studies, analysts will confirm that the Procedure and Detection Technique analytical procedures described herein, as well as alternativeanalytical procedures, are suitable for use on specified Procedure 1 can be used for elemental impurities generally material.amenable to detection by inductively coupled plas-ma–atomic (optical) emission spectroscopy (ICP–AES orICP–OES). Procedure 2 can be used for elemental impurities Speciation generally amenable to detection by ICP–MS. Before initialuse, the analyst should verify that the procedure is appropri-The determination of the oxidation state, organic complex ate for the instrument and sample used (procedural verifica-or combination is termed speciation. Analytical procedures tion) by meeting the Alternative Procedure Validation require-for speciation are not included in this chapter but examples ments below.may be found elsewhere in the USP–NF and in the literature.Sample PreparationDefinitionsForms of sample preparation include Neat, Direct Aqueous Concentrated Acid:Concentrated ultra-pure nitric, sulfu-Solution, Direct Organic Solution, and Indirect Solution. The ric, hydrochloric, or hydrofluoric acids or Aqua Regia.selection of the appropriate sample preparation depends onthe material under test and is the responsibility of the ana-Aqua Regia:Aqua regia is a mixture of concentrated hy-lyst. When a sample preparation is not indicated in the drochloric and nitric acids, typically at ratios of 3:1 or 4:1,monograph, an analyst may use any of the following appro-respectively.priately verified preparation procedures. In cases where spik-Matched Matrix:Solutions having the same solvent com-ing of a material under test is necessary to provide an ac-position as the Sample solution. In the case of an aqueous ceptable signal intensity, the blank should be spiked with solution, Matched Matrix would indicate that the same acids,the same Target Elements, and where possible, using the acid concentrations, and mercury stabilizer are used in both same spiking solution. Standard solutions may contain mul-preparations.tiple Target Elements. [NOTE—All liquid samples should be Target Elements:Elements with the potential of being weighed.]present in the material under test. Include As, Cd, Pd, and Neat:Used for liquids or alternative procedures that allows Hg in the target element evaluation when testing is done to the examination of unsolvated samples.demonstrate compliance. Target elements should also in-Direct Aqueous Solution:Used when the sample is solu-clude any elements that may be added through materialble in an aqueous solvent.processing or storage, and any elements whose presencemay interfere with the operation of the analytical proce-Direct Organic Solution:Used where the sample is solu-dures.ble in an organic solvent.Target Limit or Target Concentration:The acceptance Indirect Solution:Used when a material is not directly sol-value for the elemental impurity being evaluated. Exceeding uble in aqueous or organic solvents. Digest the sample us-the target limit indicates that a material under test exceeds ing a closed-vessel digestion procedure, similar to the proce-the acceptable value. The determination of compliance is dure provided below. The sample preparation scheme addressed in other chapters. [N OTE—When applying this should yield sufficient sample to allow quantification of each chapter to Elemental Impurities—Limits 〈232〉 and Elemental element at the limit specified in the corresponding mono-Contaminants in Dietary Supplements 〈2232〉,1Target Limits graph or chapter.can be approximated by dividing the Daily Dose PDEs by the Closed Vessel Digestion:This sample-preparation proce-maximum daily dose for the Drug Product Analysis Option in dure is designed for samples that must be digested in a〈232〉 or the Daily Serving PDE divided by the maximum Concentrated Acid using a closed-vessel digestion apparatus. daily serving size in 〈2232〉]Closed-vessel digestion minimizes the loss of volatile impuri-J:The concentration (w/w) of the element(s) of interest at ties. The choice of a Concentrated Acid depends on the sam-the Target Limit, appropriately diluted to the working range ple matrix. The use of any of the Concentrated Acids may be of the instrument. For example, if the target elements are appropriate, but each introduces inherent safety risks. There-Pb and As for an analysis of an oral solid drug product with fore, appropriate safety precautions should be used at alla daily dose of 10g/day using an inductively coupled plas-times. [N OTE—Weights and volumes provided may be ad-ma–mass spectrometry (ICP-MS). The target limit for these justed to meet the requirements of the digestion apparatus elements would be 0.5 µg/g and 0.15 µg/g (see Table 2 in used.]chapter 〈232〉). However, in this case, the linear dynamic An example procedure that has been shown to have range of the ICP-MS is known to extend from 0.01 ng/mL broad applicability is the following. Dehydrate and predigest to 0.1 µg/mL for these elements. Therefore, a dilution factor0.5 g of primary sample in 5 mL of freshly prepared Concen-of at least 1:10 is required to ensure that the analysis occurs trated Acid. Allow to sit loosely covered for 30 minutes in a in the linear dynamic range of the instrument. J would thus fume hood. Add an additional 10 mL of Concentrated Acid, equal 0.05 µg/mL and 0.015 µg/mL for Pb and As, respec-and digest, using a closed vessel technique, until digestion tively, when the dilution factor is added.or extraction is complete. Repeat if necessary by adding anadditional 5 mL of Concentrated Acid. [N OTE—Where closed Appropriate Reference Materials:Where Appropriate Ref-vessel digestion is necessary, follow the manufacturer’s rec-erence Materials are specified in the chapter, certified refer-ommended procedures to ensure safe use.]ence materials (CRM) from a national metrology institute(NMI), or reference materials that are traceable to the CRM Reagents:All reagents used for the preparation of sample of a NMI should be used. An example of a NMI in the and standard solutions should be free of elemental impuri-United States is the National Institute of Standards and ties, in accordance with Plasma Spectrochemistry 〈730〉. Technology.1This dietary supplement chapter is still under revision and will appear onlinein PF 38(3) [May–June 2012].5636〈233〉 Elemental Impurities—Procedures / Chemical Tests Second Supplement to USP 35–NF 30system well (60 seconds) before introducing the Sample in Procedure 1: ICP-AESorder to minimize carryover.]Analysis:Analyze according to the manufacturer’s sugges-Standardization solution 1:2J of the Target Element(s) intions for program and m/z. Calculate and report resultsa Matched Matrixbased on the original sample size. [N OTE—Appropriate Standardization solution 2:0.5J of the Target Element(s)measures must be taken to correct for matrix-induced inter-in a Matched Matrix ferences (e.g., argon chloride interference with arsenic Sample stock solution:Proceed as directed in Sample determinations.]Preparation above. Allow the sample to cool, if necessary.For mercury determination, add an appropriate stabilizer.ALTERNATE PROCEDURE VALIDATION Sample solution:Dilute the Sample Stock Solution with anappropriate solvent to obtain a final concentration of theIf a specified compendial procedure does not meet the Target Elements at NMT 2J.needs of a specific application, an alternative procedure may Blank:Matched Matrixbe used (see General Notices 6.30). Alternative procedures Elemental spectrometric system must be validated and must be acceptable and therefore (See Plasma Spectrochemistry 〈730〉.)equivalent to the compendial procedures for the purposes Mode:ICP of the test. The principles of validation are provided in gen-Detector:Optical detection system eral chapter Validation of Compendial Procedures 〈1225〉. Thelevel of validation necessary to ensure that an alternative Rinse:Diluent usedprocedure is acceptable depends on whether a limit test or Standardization:Standardization solution 1, Standardi- a quantitative determination is necessary. The requirementszation solution 2, and Blank for validation of an elemental impurities procedure for either System suitability type of determination are described below. Where this infor-Sample:Standardization solution 1mation differs from that presented in Validation of Com-pendial Procedures 〈1225〉, the parameters and acceptance Suitability requirementscriteria presented in this chapter take precedence. Any alter-Drift:Compare results obtained from Standardizationnative procedure that has been validated and meets the ac-solution 1 before and after the analyis of the Sampleceptance criteria that follow is considered to be equivalent solutions.to the compendial procedures for the purposes of this test.Suitability criteria:NMT 20% for each Target Element.[N OTE—If samples are high in mineral content, rinse systemwell (60 seconds) before introducing the Sample in order to LIMIT PROCEDURESminimize carryover.]The following section defines the validation parameters Analysis:Analyze according to the manufacturer’s sugges-for the acceptability of alternative limit procedures. Meeting tions for program and wavelength. Calculate and report re-these requirements must be demonstrated experimentally sults on the basis of the original sample size. [N OTE—Appro-using an appropriate system suitability procedure and refer-priate measures must be taken to correct for matrix-inducedence material. Meeting these requirements demonstrates interferences (e.g., Wavelength overlaps).]that the procedure is equivalent to the compendial proce-dure as a limit procedure for the Target Element.Procedure 2: ICP-MS The suitability of the method must be determined byconducting studies with material or mixture under test sup-plemented with known concentrations of each Target Ele-Standardization solution 1:2J of the Target Element(s) inment of interest at the appropriate acceptance limit concen-a Matched Matrixtration. The material or mixture under test must be spiked Standardization solution 2:0.5J of the Target Element(s)before any sample preparation steps are performed.in a Matched MatrixSample stock solution:Proceed as directed for SamplePreparation above. Allow the sample to cool, if necessary. DetectabilityFor mercury determination, add an appropriate stabilizer.Sample solution:Dilute the Sample stock solution with an Standard solution: A preparation of reference materials for appropriate solvent to obtain a final concentration of the the Target Element(s) at the Target Concentrations.Target Elements at NMT 2J.Spiked sample solution 1:Prepare a solution of sample Blank:Matched Matrix under test, spiked with appropriate reference materials forthe Target Elements at the Target Concentration, solubilized Elemental spectrometric systemor digested as described in Sample Preparation.(See Plasma Spectrochemistry 〈730〉.)Spiked sample solution 2:Prepare a solution of the sam-Mode:ICP. [N OTE—An instrument with a cooled sprayple under test, spiked with appropriate reference materials chamber is recommended. (A collision cell or reaction cellat 80% of the Target Concentration for the Target Elements, may also be beneficial.)]solubilized or digested as described in Sample Preparation.Detector:Mass spectrometerUnspiked sample solution: A sample of material under Rinse:Diluent usedtest, solubilized or digested in the same manner as the Sam-Standardization:Standardization solution 1, Standardi-ple solutions.zation solution 2, and BlankAcceptance criteriaSystem suitabilityNon-instrumental procedures:Spiked sample solution 1 Sample:Standardization solution 1provides a signal or intensity equivalent to or greater thanSuitability requirements that of the Standard Solution. Spiked sample solution 2 must Drift:Compare results obtained from Standardization provide a signal or intensity less than that of the Spiked solution 1 before and after the analysis of the Sample sample solution 1. [N OTE—The signal from each Spiked sam-solutions.ple solution is NLT the Unspiked sample solutiondetermination.]Suitability criteria:Drift NMT 20% for each Target Ele-ment. [N OTE—If samples are high in mineral content, rinse Instrumental procedures:The average value of thethree replicate measurements of Spiked sample solution 1 isSecond Supplement to USP 35–NF 30Physical Tests / 〈616〉 Bulk Density and Tapped Density of Powders 5637within (±15%) of the average value obtained for the repli-RUGGEDNESScate measurements of the Standard solution . The average value of the replicate measurements of Spiked sample solu-Perform the Repeatability analysis over three independent tion 2 must provide a signal intensity or value less than that events using the following events or combinations thereof:of the Standard solution . [N OTE —Correct the values obtained 1.on different days, orfor each of the spiked solutions using the Unspiked sample 2.with different instrumentation, or solution .]3.with different analysts.Acceptance criteriaRelative standard deviation:NMT 25% for each Target Precision for Instrumental MethodsElement(Repeatability)[N OTE —Non-instrumental precision is demonstrated by Specificitymeeting the Detectability requirement above.]Sample solutions:Six independent samples of the mate-The procedure must be able to unequivocally assess (see rial under test, spiked with appropriate reference materials Validation of Compendial Procedures 〈1225〉) each Target Ele-for the Target Elements at the Target Concentration .ment in the presence of components that may be expected Acceptance criteriato be present, including other Target Elements , and matrix components.Relative standard deviation:NMT 20% for each Target Element .Limit of Quantitation, Range, and LinearitySpecificityDemonstrated by meeting the Accuracy requirement.s 2S (USP35)The procedure must be able to unequivocally assess (see Validation of Compendial Procedures 〈1225〉) each Target Ele-ment in the presence of components that may be expected to be present, including other Target Elements , and matrix components.Physical Tests and QUANTITATIVE PROCEDURESDeterminationsThe following section defines the validation parameters for the acceptability of alternative quantitative procedures.Meeting these requirements must be demonstrated experi-mentally, using an appropriate system suitability procedure 〈616〉 BULK DENSITY AND and reference materials. Meeting these requirements dem-onstrates that the procedure is equivalent to the compendial TAPPED DENSITY OF POWDERSprocedure for the purpose of quantifying the Target Elements .AccuracyChange to read:Standard solutions:Prepare solutions containing the Tar-get Elements at concentrations ranging from 50% to 150%of J , using appropriate reference materials.BULK DENSITYTest samples:Prepare samples of the material under test spiked with appropriate reference materials before any sam-This general chapter has been harmonized with the corre-ple preparation steps (digestion or solubilization) at concen-sponding texts of the European Pharmacopoeia and/or the trations ranging from 50% to 150% of J for each Target Japanese Pharmacopoeia . 3The portion that is not harmo-Element .nized is marked with symbols (33) to specify this fact.3Acceptance criteriaThe bulk density of a powder is the ratio of the mass of Spike recovery:70%–150% for the mean of three rep-an untapped powder sample and its volume including the licate preparations at each concentrationcontribution of the interparticulate void volume. Hence, the bulk density depends on both the density of powder parti-cles and the spatial arrangement of particles in the powder Precisionbed. The bulk density is expressed in grams per mL (g/mL)although the international unit is kilograms per cubic meter (1g/mL = 1000kg/m 3) because the measurements aremade using cylinders. It may also be expressed in grams per REPEATABILITYcubic centimeter (g/cm 3). The bulking properties of a pow-der are dependent upon the preparation, treatment, and Test samples:Six independent samples of material understorage of the sample, i.e., how it was handled. The parti-test (taken from the same lot) spiked with appropriate refer-cles can be packed to have a range of bulk densities; how-ence materials for the Target Element(s) at the indicated ever, the slightest disturbance of the powder bed may result level.in a changed bulk density. Thus, the bulk density of a pow-Acceptance criteriader is often very difficult to measure with good reproducibil-ity and, in reporting the results, it is essential to specify how Relative standard deviation:NMT 20% for each Target the determination was made. The bulk density of a powder Elementis determined by measuring the volume of a known weight of powder sample, that may have been passed through a s sieve s 2S (USP35), into a graduated cylinder (Method I ), or by。
重金属四国药典比较

对照溶液:4ml 250g/l的硫酸镁溶液(稀硫酸溶解硫酸镁),规定量的标准铅溶液(10ppmPb)。
按供试溶液的制备方法,加热灼烧,加盐酸,加酚酞试液,加氨水及冰醋酸等,并用水稀释至20ml。
取10ml的该溶液,加2ml待测液,2ml pH3.5的缓冲溶液,混合。
加1.2ml的硫代乙酰胺试液,立即混合。
监控液:按照供试溶液方法制备,只是在称量样品后需加入10ppm的铅溶液,取该液10ml,加入2ml供试液,加2ml pH3.5的缓冲溶液,混合。
加1.2ml硫代乙酰胺试液,立即混合。
空白溶液:10ml的水,加2ml待测液,2ml pH3.5的缓冲溶液,混合。
加1.2ml硫代乙酰胺试液,立即混合。
如果对照液与空白溶液比较,不显示浅棕色,或者监控溶液所显的颜色浅于对照液的颜色,那么该检测结果无效。
方法D供试溶液:在坩埚内,充分的混合规定量的待测物质和0.5克的氧化镁,灼烧退去暗红色,直至出现同质的白色或灰白色物质。
如果灼烧30分钟后仍有颜色取出冷却,用玻璃棒混和,重复进行灼烧。
如有必要,重复此项操作。
在800℃加热约1小时。
分别制备两份残渣,各加5mL等体积的盐酸和水的混和溶液。
加0.1ml 酚酞试液,然后滴加浓氨水直至出现粉红色。
冷却,加冰醋酸直到溶液褪去颜色,再多加0.5ml 冰醋酸。
如有必要,过滤并冲洗过滤器。
加水稀释至20ml。
对照溶液(标准):按供试溶液的制备方法,用规定量的铅标准溶液(10ppm Pb)代替待测物质并在100-105℃烘箱中干燥。
取10ml的该溶液,加2ml待测液。
监测溶液:按供试溶液的制备方法,向待测物质中加入配制对照溶液规定量的铅标准溶液(10ppm Pb)并在100-105℃烘箱中干燥。
取10ml的该溶液,加2ml待测液。
空白溶液:10ml的水和2ml待测液混合。
向12ml每种溶液中,加入2ml pH为3.5的缓冲溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
标准操作规程Standard Operating Procedure1.简述1.1重金属是指在规定实验条件下能与硫代乙酰胺或硫化钠作用显色的金属盐类杂质。
1.2硫化钠或硫代乙酰胺在弱酸性条件下水解产生硫化氢,与供试品中重金属在规定实验条件下所显颜色,与一定量的标准铅溶液在同样操作条件下所显的颜色比较。
1.3由于实验条件不同。
分为三种检查方法:第一法适用于在规定条件下能生成澄清无色溶液的供试品。
第二法适用于在规定条件下不能生成澄清无色溶液的供试品;第三法适用于那些不能用一法和二法的样品。
2 仪器2.1仪器设备50ml纳氏比色管2.2试剂和溶液a)硫代乙酰胺试液: 称取硫代乙酰胺4g加水溶解,用水稀释至100ml,摇匀。
b)甘油基准试液: 称取200g甘油加水至总重量为235g,然后加氢氧化钠溶液和的水。
临用前用硫代乙酰胺试液和1ml甘油基准试液混合,在沸水浴中加热20秒钟,立即使用.c)硝酸铅贮备液:称取硝酸铅0.1598g,置1000ml量瓶中,加硝酸1ml与水100ml溶解后,用水稀释至1000ml 摇匀,作为贮备液。
标准铅溶液临用前,精密量取贮备液10ml,置100ml量瓶中,加水稀释至刻SOP-QM 402-14 页号 Page:2/4度,摇匀,即得(每1ml相当于10g的Pb)。
d)醋酸盐缓冲液: 取醋酸铵25.0g,加水25ml溶解后,加盐酸,如有必要用6N氨水或6N盐酸调PH至,用水稀释至100ml,摇匀。
第一法:标准溶液的制备取50ml比色管,用移液管移取标准铅溶液(20 gPb),用水稀释到25ml,用1N醋酸或6N氨水溶液调PH至~之间,用窄范围的精密pH试纸作指示,然后加水稀释至40ml,摇匀。
供试品溶液的制备另取一支50ml比色管,加入按该品种项下规定的方法制成的供试液25ml;或加入供试品的量以g计, 按公式1000L计算,其中L为重金属的限度(%),用该品种项下规定的酸的体积溶解,加水溶解并稀释至25ml,供试品溶液用1N醋酸或6N氨水溶液调PH至~之间,用窄范围的精密pH试纸作指示,然后加水稀释至40ml,摇匀。
对照溶液的制备取第三只比色管,加入与上述供试品溶液方法同样制备的任意25ml,加入标准铅溶液,用1N醋酸或6N氨水溶液调PH至~之间,用窄范围的精密pH试纸作指示,然后加水稀释至40ml,摇匀。
操作方法在上述三只比色管中分别加入2ml醋酸缓冲溶液(), 然后加硫代乙酰胺_甘油基准试剂,加水稀释到50ml,摇匀,放置2分钟,同置白色背景下,至上向下透视.供试品溶液的颜色不得深于标准溶液的颜色;对照溶液的颜色相同于或较深于标准溶液的颜色( 注:如对照液的颜色浅于标准品溶液,则以第二法代替第一法)。
第二法:醋酸盐缓冲液照第一法配制标准溶液的制备用移液管取4ml标准铅溶液到适宜的试管中,并加入10ml6N盐酸。
照方法一配制供试品溶液的制备称取一定量的样品(以g计算) ,按公式1000L1000L计算,其中L 为重金属的限度(%),置适宜的坩埚中,加适量的硫酸预以湿润,在低温小心炽灼,直至全部炭化,(炭化过程坩埚盖可不盖严),炭化物中加2ml硝酸和5滴硫酸,小SOP-QM 402-14 页号 Page:3/4心加热,直至不再挥发白色烟雾,于500-600℃炽灼(最好在高温炉中)直至炭完全灰化(不得超过2小时),如果未完全炭化,则可将残渣冷却后加入几滴硫酸使脱水,然后重新炽灼。
放冷,加5ml 4ml6N盐酸,加盖,在蒸气浴上蒸10分钟15分钟,放冷,等量转移到一个试管中。
再用5ml6N盐酸少量多次冲洗坩锅,并将洗液转移到试管中。
去盖,在蒸气浴上缓缓蒸发至干。
残渣加1滴盐酸湿润之,再加10ml热水,蒸煮2分钟,滴加6N氢氧化铵,直至溶液对石蕊试纸刚呈碱性。
用水稀释至25ml 。
用1N醋酸溶液调PH至~之间,用窄范围的PH精密试纸作外指示剂,如需要,予以过滤,用10ml水淋洗坩埚和滤器,合并滤液和洗液于50ml比色管中,加水稀释至40ml,摇匀。
对照液的制备——用移液管取4ml标准铅溶液至与上述相同的坩锅中,溶液中含有被测物的量相当于供试液含量的10%。
在蒸气浴上蒸发至干。
在与供试液相同的条件下,在同一高温炉中同时炽灼燃烧。
放冷,加5ml6N盐酸,加盖,在蒸气浴上蒸10分钟。
放冷,等量转移到一个试管中。
再用5ml 6N盐酸少量多次冲洗坩锅,并将洗液转移到试管中。
3.3.5操作方法分别在标准溶液、供试液、对照液的试管中小心地逐滴加入氢氧化铵,调PH到9。
放冷,逐滴加入冰醋酸调PH到8,再加入过量的5ml冰醋酸。
用PH计或窄范围的PH精密试纸作外指示剂,测定PH,如有必要,用1N醋酸溶液或6N氢氧化铵调PH至-之间。
如有必要,过滤,用数ml水冲洗,合并滤液和洗液到50ml到比色管中,加水稀释至40ml。
加入2ml醋酸缓冲溶液(), 然后加硫代乙酰胺_甘油基准试剂,加水稀释到50ml,摇匀,放置2分钟,同置白色背景下,至上向下透视,供试品溶液的颜色不得深于标准溶液的颜色,对照品溶液的颜色相同于或深于标准溶液。
[注——如对照品溶液的颜色浅于标准溶液,则用第三法检查供试品。
]第三法标准品溶液的配制取8ml硫酸和10ml硝酸的混合液,置一干燥洁净的100ml凯氏烧瓶中,再加适量硝酸 ,使相当于供试品中增加的硝酸量,加热使产生白色烟雾,放冷,小心加入10ml水,如处理供试品溶液用过氧化氢,则加入相当于供试液中耗用量的30%过氧化氢,缓缓煮沸至产生白色浓烟雾。
再放冷,小心加入5ml水,混合,再缓缓煮沸至产生白色浓烟雾,并使体积剩2~3ml。
放冷,以数SOP-QM 402-14 页号 Page:4/4 ml水小心稀释,加标准铅溶液,摇匀,移入50ml比色管中,用水淋洗烧瓶,洗液并入比色管中,直至达25ml,摇匀。
供试品溶液的制备按公式1000L计算样品数量,其中L为重金属的限度(%)如为固体样品称取一定量的样品(以g计算), 置一干燥洁净的100ml凯氏烧瓶中[注—如反应时会产生大量的泡沫,则需用300ml的烧瓶]。
钳住烧瓶使成450角,加足量8ml硫酸和10ml硝酸的混合液,使样品完全湿润,加酸时先缓缓加热至反应开始,每次加酸后再加热至反应开始,待反应平息,分次补充加入同样的酸混合液,每次加酸后再加热 ,直至总量18ml混合酸加完,加热至微沸至溶液变黑,放冷,加入2ml硝酸,继续加热使溶液呈黑色。
继续加热,再加硝酸,直至不变黑,然后激烈加热使产生白色浓烟雾。
放冷,小心加入5ml水,缓缓加热至产生白色浓烟雾,再继续加热直至体积仅剩数ml,放冷,小心加入5ml水.观察颜色如呈黄色,可小心加入30%过氧化氢1ml,再蒸发至产生白色浓烟雾, 并使体积仅剩2~3ml.若溶液仍呈黄色,可重复加水5ml并以过氧化氢处理,放冷, 以数ml水小心稀释,淋洗烧瓶,洗液并入比色管中不得过25ml,摇匀.如为液体样品取规定的样品, 置一干燥洁净的100ml凯氏烧瓶中[注—如反应时会产生大量的泡沫,则需用300ml的烧瓶]。
钳住烧瓶使成450角,小心加入数ml的8ml硫酸和10ml硝酸混合液,缓缓加热至反应开始,待反应平息,按固体样品项下自”分次补充加入同样的酸混合液”起同样处理.对照溶液的制备——按固体样品项下供试液的制备方法,取等量的样品,同法操作至“放冷, 以数ml水小心稀释”。
加入标准铅溶液(20 µgPb),摇匀。
转移至50ml比色管中,用水淋洗烧瓶,将洗液并入比色管中至体积达到25ml,混合摇匀。
操作法供试品溶液、标准品溶液及对照溶液均按下列规定进行处理,用氢氧化铵调节~,可用窄PH精密试纸作外指示剂,当接近此特定的范围时可用稀氨液.然后用水稀释至40ml,摇匀.在上述比色管中分别加入2ml醋酸缓冲溶液(), 然后加硫代乙酰胺_甘油基准试剂,加水稀释到50ml,摇匀,放置2分钟5分钟,同置白色背景下,至上向下透视,供试品溶液的颜色不得深于标准溶液的颜色,标准品溶液的颜色相同于或深于标准品溶液的颜色。
附上我的翻译231重金属重金属检查法是指在规定实验条件下检测能与硫离子作用显色的金属杂质的量。
通过目视比较标准铅溶液对照品(见分光光度法和色差计法操作项下的目视比色法851),以供试品中铅的百分数(按重量)计算,不能超过各论中规定的重金属限度。
[注意——此法代表性的金属杂质有铅、汞、铋、砷、锑、锡、镉、银、铜、钼]除另有规定外,测定重金属的含量采用第一法。
第一法适用于在规定条件下能生成澄清无色溶液的物质。
第二法适用于在第一法规定条件下不能生成澄清无色溶液的物质,或由于是络合物,其硫离子能与重金属发生沉淀反应,或是不挥发油和挥发油。
第三法是湿法,适用于那些不能用第一法和第二法的物质。
特殊试剂硝酸铅贮备液——称取硝酸铅,加1ml硝酸与100ml水溶解后,加水稀释至1000ml。
制备此溶液并贮存在无可溶性铅盐的玻璃容器中。
标准铅溶液——在使用当天,量取硝酸铅贮备液,加水稀释至。
每1ml标准铅溶液相当于10µg的铅。
取100ul的标准铅溶液制成对照溶液,如与1g的供试品同法制成的溶液颜色相同,那1g供试品就相当于含有百万分之一的铅。
第一法的醋酸盐缓冲液——取醋酸铵25.0g,加水25ml溶解后,加6N盐酸。
如有必要用6N氨水或6N盐酸调PH至,用水稀释至100ml,混合摇匀。
标准溶液的制备——取一支50ml比色管,用移液管移取标准铅溶液(20µgPb),用水稀释至25ml。
用PH计或窄范围的精密PH试纸指示,用1N醋酸或6N氨水溶液调PH至~之间,然后加水稀释至40ml,混合摇匀。
供试品溶液的制备——取一支50ml比色管,加入按该品种项下规定的方法制成的供试液25ml;或加入以g计,按公式(1000L) (其中L为重金属的限度,以百分比计)算出的供试品的量,用该品种项下规定的酸的体积溶解,并加水稀释至25ml。
用PH计或窄范围的精密PH试纸指示,用1N醋酸或6N氨水溶液调PH至~之间,然后加水稀释至40ml,混合摇匀。
对照溶液的制备——取第三支比色管,加入与上述供试液同法制备的任意溶液25ml,加入标准铅溶液。
用PH计或窄范围的精密PH试纸指示,用1N 醋酸或6N氨水溶液调PH至~之间,然后加水稀释至40ml,混合摇匀。
操作方法——取上述含有标准溶液、供试品溶液、对照溶液的三支比色管,分别加入2ml 的醋酸缓冲溶液, 然后加硫代乙酰胺_甘油基准试剂,加水稀释到50ml,摇匀,放置2分钟,同置白色背景下,至上向下透视.供试品溶液的颜色不得深于标准溶液的颜色;对照溶液的颜色相同于或较深于标准溶液的颜色[ 注:如对照液的颜色浅于标准品溶液,则以第二法代替第一法]。
