《白藜芦醇》-附录
白藜芦醇

Residue on ignition 0.5%
Heavy metal 10ppm max
Pesticides 2ppm max
Total Plate <1000CFU/g
Yeast&Mold <100CFU/g
Salmonella Negative
【药理作用】
白藜芦醇抗菌抗病毒、抗肿瘤、抗肝炎、抑制血小板凝集和血栓素B2的产生。白藜芦醇能够阻止低密度脂蛋白的氧化,具有潜在的防治心血管疾病、防癌、抗病毒及免疫调节作用,白藜芦醇作用主要表现为它的抗氧化特性。
1、抗菌作用研究表明,桑椹受到桑椹霜霉菌侵染后,离坏死果实较近且没有受到侵染的部位,白藜芦醇的含量很高,能有效地抑制坏死区的扩展。
【产品名称】白藜芦醇 Resveratrol 虎杖提取物
【产品来源】主要来源于蓼科植物虎杖Polygonum cuspidatum Sieb.et Zucc.的根茎提取物。
【化学名】芪三酚(3,4',5-三羟基-二苯乙)(3,',5-Trihydroxystlbene)
【分子式】C14H12O3
3. lower Blood Cholesterol and glycerin trilaurate
4. defer senility, so it is usually used in cosmetics.
Storage Store in cool & dry place. Keep away from strong light and heat.
Specifications Resveratrol ≥50%, 98% Polydatin≥98%
白藜芦醇说明书

R5010 Page 1 of 207/11/97 - CKVRESVERATROLSigma Prod. No. R5010CAS NUMBER: 501-36-0SYNONYM: trans-3,4',-5-trihydroxystilebene; 3,4',5-stilbenetriol;trans-resveratrol; (E)-5-(p-hydroxystyryl)resorcinol 1PHYSICAL DESCRIPTION:Appearance: White powder with yellow castMolecular formula: C 14H 12O 3Molecular weight: 228.2Absorbance: E mM (218 nm) = 21.5 (ethanol)2E mM (306 nm) = 30.0 (ethanol)2E mM (320 nm) = 29.1 (ethanol)2Comparison spectra for cis- and trans-resveratrol have been published.6STORAGE / STABILITY AS SUPPLIED:If stored at -20E C the product should be stable at least two years.2SOLUBILITY / SOLUTION STABILITY:Resveratrol is soluble in EtOH at 50 mg/mL, in DMSO at least 16 mg/mL and is only very slightly soluble (warming required) in water at 3 mg/100 mL.2A solution of 5 mM in 96% ethanol (by volume) was stable for at least 12 weeks at 4E C when protected from light. Irradiation for 5-10 minutes at 254 nm and intensity of 990 µW/cm 2 of a trans-resveratrol solution in 0.2 M phosphoric acid-acetonitrile (4:1, v/v) to give a new peak which was shown to be the cis isomer in an amount identical to the decrease in the trans isomer.3GENERAL REMARKS:Resveratrol, a hydroxylated stilbene found in grapes, wines, mulberries and other food products was purified and shown to have cancer chemopreventive activity in assays representing three major stages of carcinogenesis.4(Chemoprevention is the prevention of cancer by ingestion of chemical agents that reduce the risk of carcinogenesis.) In vitro tests, resveratrol inhibited free-radical formation in leukemia cells treated with a phorbol ester, inhibited mutations in another cell line and stimulated quinone reductase (which detoxifies carcinogens).4GENERAL REMARKS: (continued)Resveratrol reduced both the number of skin tumors that developed per mouse and the number of mice developing tumors in skin tests on mice.4,5 Orally administered resveratrol lowered lipid levels in the liver of rats and in humans. Siemann and Creasy analyzed selected wines for resveratrol and the factors possibly affecting its concentration.6 They reported an absorption spectra for trans- and cis-resveratrol.6 Grape flavinoids (including resveratrol) have been reported to help thin the blood, to detoxify bad LDL cholesterol, strengthen blood vessels, boost immunity, fight allergies and to inhibit cancer.7 Resveratrol has been correlated with inhibition of platelet aggregation.8 Research by Williams et al. in press (1977) indicates that trans-resveratrol has anti-estrogenic properties which may be involved in the reduction of breast cancer risk.9QUALITY CONTROL SYSTEMS:Gas Chromatography -Derivative: TMSCapillary column: 30 m x 0.32 mm ID with 0.25 µm particlesTemperature (E C): injector 280 detector 280 Flow rate: 25 cm/s HeColumn temp program: 260 to 280E C @ 4 deg/minSolvent: Sigma Sil A 10 mg/mL Volume Injected 1.0 µLRetention time: approximately 5 minThin Layer Chromatography -System: Silica gel platesSolvent: 30 parts n-butanol/20 parts pyridine/20 parts water/6 parts glacial acetic acidDetection: iodine vapor or methanolic sulfuric acid spraySample dissolved at 50 mg/mL in acetone, spotted from 0.5 to 250 µgR f approximately 0.7REFERENCES:1. Merck Index, 12th ed., #8326 (1996).2. Sigma quality control.3. Goldberg, D.M. et al., J. Chromatogr., vol. A 708, 89-98 (1995).4. Jang, M. et al, Science, 275, 218 (1997).5. Chemical and Engineering News, January 13, 1997, p. 20.6. Siemann, E.H. and Creasy, L.L., Amer. J. Enol. Vitic, 43, 49 (1992).7. USA Weekend, Jan 31 - Feb 2 (1997).8. Chung, M. et al., Planta Med., 58, 274 (1992).9. Williams, R.L. and Elliot, M. Virginia Academy of Science Abstracts, May 1997 (in press); cited inWine Institute Newsflash: Health & Social Issues, Vol. 2 (no. 12), May 1997.Sigma warrants that its products conform to the information contained in this and other Sigma-Aldrich publications. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see reverse side of the invoice or packing slip.R5010Page 2 of 207/11/97 - CKV。
白藜芦醇

【产品名称】白藜芦醇【别名】虎杖甙元【化学名】 (E)-5-[2-(4-羟苯基)-乙烯基]-1,3-苯二酚;3,5,4''-三羟基芪;芪三酚;Trans-3-4' -trihydroxystilbene,【分子式】 C14H12O3【分子量】 228.25【物理性质】无味、白色晶体粉末;难溶于水,易溶于乙醇,丙酮等有机溶剂。
【功能主治】白藜芦醇是一种天然的抗氧化剂,可降低血液粘稠度,抑制血小板凝结和血管舒张,保持血液畅通,可预防癌症的发生及发展,具有抗动脉粥样硬化和冠心病,缺血性心脏病,高血脂的防治作用。
抑制肿瘤的作用还具有雌激素样作用,可用于治疗乳腺癌等疾病。
通过药理研究证实,白藜芦醇具有抗菌、抗炎、抗过敏、抗血栓作用;明显的抗氧化、抗自由基的作用,而自由基是引起衰老、癌症、关节炎等疾病的起因;对冠心病、高血脂的防治作用;抗癌、抗诱变的作用,从而抑制肿瘤的产生。
千年之交,中国食品工业协会公布,我国食品与药物化学家从葡萄酒中分离并检测出可降低冠心病、动脉硬化症以及抑制肿瘤发生的功效成分白藜芦醇。
一时,白藜芦醇不仅是业界研究的热点问题,也引起人们普遍的关注。
通过药理研究证实,白藜芦醇具有抗菌、抗炎、抗过敏、抗血栓作用;明显的抗氧化、抗自由基的作用,而自由基是引起衰老、癌症、关节炎等疾病的起因;对冠心病、高血脂的防治作用;抗癌、抗诱变的作用,从而抑制肿瘤的产生。
令人感兴趣的是,在我国许多传统的中药如虎杖、首乌等都含有白藜芦醇,而作为治疗药物使用。
根据检测数据表明,我国优质干红葡萄酒中,白藜芦醇的含量每升已达到5—10毫克,从保健医疗角度看来,已达到了治疗的剂量。
白藜芦醇是葡萄生长过程中,为防止灰色霉菌感染导致葡萄腐烂,而产生的一种植物抗毒素。
它主要存在于葡萄果皮之中,果肉与果汁中含量甚少。
专家在检测时发现,白藜芦醇在葡萄中的含量,每升仅为几微克的级别,达不到治疗的效果。
在葡萄酒发酵生产过程中,由于葡萄汁酶的作用,而使酒中白藜芦醇的数量骤然增加。
白藜芦醇质量标准

白藜芦醇质量标准
一、分子式与分子量
白藜芦醇(Resveratrol)的分子式为C14H12O3,分子量为
228.24。
二、质量标准(HPLC)
本质量标准采用高效液相色谱法(HPLC)作为主要分析方法,以确保白藜芦醇的纯度与准确性。
三、最低含量要求
按照此质量标准,白藜芦醇的纯度应不低于98.0%。
所有批次产品的含量均应在此标准之上。
四、有害物质残留限量
重金属(以Pb计):不得过百万分之十。
砷盐:不得过百万分之二。
农药残留:应符合国家相关农药残留限量标准。
微生物限度:应符合国家药品微生物限度标准。
五、原料选用要求
原料应为符合质量标准的天然植物提取物,严禁使用任何形式的合成或半合成物质作为原料。
六、生产过程控制
生产过程应严格按照GMP标准进行,确保产品的纯净度与稳定性。
所有工艺环节应有明确的操作规范和质量监控措施。
七、质量检测要求
每一批次的产品都应进行质量检测,包括外观、色泽、气味、纯度等方面的检验。
所有检测结果应符合本质量标准的要求。
八、定性与定量检测
定性检测:采用适当的化学或物理方法,如紫外-可见光谱、红外光谱等,对白藜芦醇进行定性鉴别。
定量检测:采用HPLC等方法,对白藜芦醇的含量进行精确测定,确保产品含量符合最低含量要求。
本质量标准旨在为白藜芦醇的生产、质量控制和市场监管提供明确的技术依据,确保产品质量和患者用药安全。
所有相关企业和个人均应严格遵守本质量标准,以确保产品的质量和安全。
白藜芦醇

白藜芦醇
产品名称:白藜芦醇trans-3,4,5-Trihydroxystilbene3,4',5-三羟基芪;虎杖甙元;茋三酚;芪三酚; 3,4',5-三羟基茋;3,4',5-三羟基二苯乙烯; 3,4,5-三羟基反式(E)-3,5,4'-三羟基芪[1]
提取来源:蓼科Polygonaceae植物虎杖Polygonum cuspidatum Sieb. et Zucc.的干燥根茎和根,葡萄科植物葡萄Vitis vinifera果实的皮和籽,豆科Fabaceae植物花生Arachis hypogaea的种子等
化学名:5-(4-hydroxystyryl)benzene-1,3-diol
产品规格: 98%白藜芦醇
检测方法:HPLC
CAS号:501-36-0
理化性质:无色针状结晶,易溶于,氯仿、甲醇、乙醇、等
外观: 白色精细粉末
白藜芦醇的功效:
★退烧与止痛作用
★抗癌、抗突变作用
★心血管保护作用
★预防心脏和肝脏损伤
★抗血栓功能
★提升免疫系统活性
★抗氧化、抗自由基作用
★减肥降脂作用
★抗炎、抗菌作用
★延年益寿
包装方式:25公斤/纸板桶或铝箔袋
存储条件:本品应密封遮光,贮存在干燥、阴凉、通风良好的地方, 有效期:两年。
白藜芦醇——一种神奇的物质

谱 上 充斥 着 富含 脂肪 的 不健 康 食物— — 奶酪 、奶
糖尿 病 天 地 ・ 床 刊 2 O 年 5 第3 第 5 D a e e o 1 ,M y 2 0 , o 3 0 5 临 O9 月 卷 期 i b t s w r d a 0 9 v l ,N .
热 点 评 述
白藜 芦 醇
格 贝 尔
一
种 神 Байду номын сангаас 的 物 质
研 究 发 现 ,有 一 种 叫 白藜芦 醇 的物 质可 能 具 有 降低 血糖 甚 至延 长 寿命 的 的作 用。 而红 葡萄 酒
代 谢机 制。一 些 科学 家 认 为 , 白藜 芦 醇 在体 内分 解太 快 ,小 剂 量 起效 甚 微 。研 究者 们 正设 法 延 长
白藜芦 醇 在体 内 的存 留时 间 , 以期人 类 小剂 量 应 用该 物质 即可获得理 想效果 。 如果 相 关研究 得 到 了经得起 考验 的积 极效 果 , 2型糖 尿病患者 将是第 一批 获益 的群 体 。科学 家们 正在 研 究一 种 被 称 为 S RT5 l 缓慢 代谢 型 白藜 0 的
即使 “ 兰 西悖 论 ” 最 终被 推 翻 , 白藜芦 醇 法 依 然 会散 发 出迷 人 的光 芒 。一 些 研究 提 示 , 白藜 芦 醇 具有 抗 凝作 用 。还有 证据 表 明, 白藜芦 醇具 有 松 弛血 管 、抗氧 化 剂及 降低 胆 固醇 和 甘油 三 酯
白藜芦醇(Resveratrol):线粒体的保护神,打下长寿的基础

白藜芦醇(Resveratrol):线粒体的保护神,打下长寿的基础知道吗,尽你所想地吃,尽你所想地喝红酒,你也能活到120岁?唯一的条件是,你必须每天喝下1500瓶红酒。
但这会杀了你,在你获得永生之前!来自哈佛大学的大卫·辛克莱( David Sinclair)和他的团队发现,葡萄里含有一种叫作“白藜芦醇”(resveratrol)的红色素。
在对老鼠的活体实验中,这种色素能通过保护老鼠的线体来延长其寿命。
尽管这一发现具有重要意义,但还是有些人存在这样的误区:无论你吃了什么,这种“神奇的药丸”都能让你延年益寿。
不过,辛克菜还是抓住了某种要点,某种能将白藜芦醇、衰老和线粒体联系起来的要点,某种对大脑健康和一切慢性疾病产生影响的要点。
其实,辛克莱找对了方向,只是单个的“神奇药丸”往往于事无补。
我们应该聚焦于系统。
白藜芦醇,这种新型“药物”自吹自擂道:吃你想吃亦能益寿延年,无需锻炼仍可拥有运动员的体魄。
但是,白藜芦醇可不是什么药物。
让人们兴奋的,不过是对这样一种构想的确认:一种分子(管他来自药物还是植物),包治百病。
白藜芦醇主要取自葡萄、花生、浆果和一种叫作“虎杖”的中草药(很多中草药配方的常见成分)。
作为一种天然的植物抗病分子,白藜芦醇在植物营养素家族里应该归为“多酚”类。
植物营养素是一种有益的植物化合物,其家族很是庞大。
单靠一剂白藜芦醇就能治疗百病,这个听上去是不是不真实?真正的秘密为何?三个字:线粒体。
近期,有两项研究(其中一项的研究人是辛克莱)为这一领域带来了光明,它们不仅揭示了白藜芦醇的工作原理及其对氧化压力的影响,还指出线粒体的手中握有解开身体和大脑健康、减肥和长寿的钥匙。
第一项研究由辛克莱和他的同事发起,其结果发表在《自然》杂志上。
他们给一组老鼠喂食高脂肪(含60%的热量)的食物。
一段时期后,这些老鼠都变得肥胖不已,还得了糖尿病和脂肪肝,并且很早就死去了。
他们给另一组老鼠也喂了同样的食物,但是添加丁白藜芦醇补充剂量。
白藜芦醇-修改版

第一章:白藜芦醇简介二十一世纪长寿瑰宝——白藜芦醇什么是白藜芦醇?白藜芦醇的结构与特性白藜芦醇在天然植物中的分布与含量红葡萄与白藜芦醇第二章:白藜芦醇对人体的好处是什么?标题:白藜芦醇为什么可以抗癌白藜芦醇防治肝癌白藜芦醇防治肺癌白藜芦醇防治胃癌白藜芦醇防治结直肠癌白藜芦醇防治宫颈癌白藜芦醇防治乳腺癌白藜芦醇防治前列腺癌白藜芦醇防治白血病白藜芦醇防治皮肤癌标题:白藜芦醇如何防治心脑心血管系统疾病的白藜芦醇防治冠心病(抗动脉粥样硬化)白藜芦醇防治高血脂白藜芦醇防治心力衰竭标题:我们为什么会变老?白藜芦醇延缓机体衰老白藜芦醇抵抗肌肤老化白藜芦醇的美白功效标题:白藜芦醇是如何保护神经系统的白藜芦醇防治脑中风的研究白藜芦醇防治老年痴呆症的研究标题:白藜芦醇与肝病防治白藜芦醇防治乙型肝炎白藜芦醇防治脂肪肝白藜芦醇防治酒精肝白藜芦醇防治肝纤维化标题:白藜芦醇的抑菌、抗病毒作用白藜芦醇的抗病毒作用白藜芦醇的抗炎作用第三章:白藜芦醇的应用前景第四章:白藜芦醇升级时代第五章:红葡萄浓缩胶囊主要针对人群第六章:红葡萄浓缩胶囊真的有说的那么管用吗?第七章:一般使用红葡萄浓缩胶囊多久有效果?第八章:如何判断自身是否适用红葡萄浓缩胶囊?第九章:膳食营养第一章:白藜芦醇简介什么是白藜芦醇?白藜芦醇是多酚类化合物,主要来源于葡萄(红葡萄酒)、虎杖、花生、桑椹等植物。
白藜芦醇是一种生物性很强的天然多酚类物质,又称为芪三酚,是肿瘤的化学预防剂,也是对降低血小板聚集,预防和治疗动脉粥样硬化、心脑血管疾病的化学预防剂。
白藜芦醇的实验研究已经证实具有对心血管疾病和癌症的有益作用。
法国著名流行病学家塞尔吉·雷诺德教授注意到,法国人酷爱美食,平素爱吃牛油、奶油、肉泥酱、鹅肝酱、奶酪等高热量食物,而且法国人的频繁饮酒是出了名的,即使是病人也不会有所收敛。
按理这些都是不利于心脏健康的,但法国人的冠心病发病率却非常低,大约只有美国人的40%。
白藜芦醇产品介绍[1][1]
![白藜芦醇产品介绍[1][1]](https://img.taocdn.com/s3/m/e2eedcaefd0a79563c1e7247.png)
白藜芦醇产品介绍一、课题的提出源于白藜芦醇的发现白黎芦醇的发现缘于医学上的一种反常现象,即“法国怪圈”。
法国人的饮食中动物性脂肪含量高,胆固醇摄入量大,且吸烟嗜酒,其人均葡萄酒饮用量居世界首位,由此似乎可以认为由于饮食和生活方式的因素,法国人应该是一个具有健康危险的群体。
但出人意料的是,比起那些正常饮食中不包括葡萄酒的人来说,进餐中饮葡萄酒的法国人的心血管疾病的发病率及死亡率较低。
经研究发现其标准人群(35到64岁)中,冠心病的死亡率男性约为英国的l/2,为美国的l/4,女性约为英国的1/3,为美国的1/4。
法国怪圈引出的结论是法国人英国人和美国人的饮食结构基本相同,不同的是:英国人爱喝烈性蒸馏酒;美国人爱喝啤酒;法国人则钟情于葡萄酒。
显然,差别和起作用的就是葡萄酒。
而葡萄酒中含有白藜芦醇权威医学解释:法国人经常喝红葡萄酒,是红葡萄酒中的多酚类物质起到了保健作用。
1989年WHO证实:葡萄酒中所含的白藜芦醇导致了“法国悖论”现象。
二、白藜芦醇的生物活性1.抗肿瘤活性白藜芦醇对癌症的起始、增殖、发展三个主要阶段均有抑制乃至逆转作用。
在起始阶段,能减少自由基形成,诱导Ⅱ期药代酶增多,有拮抗二噁英作用;在增殖阶段,能抑制环氧合酶COX,抑制过氧化氢酶CAT,抑制癌细胞增殖;在发展阶段,诱导癌细胞分化和凋亡。
2.心血管保护作用白藜芦醇可以增加脉压,促进毛细血管开放,减少休克时白细胞的激活和黏附,扩张微血管,改善微循环,增强心功能,具有治疗休克作用。
3.抗氧化、抗自由基作用白藜芦醇具有抗氧化,清除自由基及影响花生四烯代谢的药理作用。
白藜芦醇浓度达到1.3μg/mL时能明显抑制大鼠红细胞自然氧化溶血和由H2O2引起的氧化溶血,对小鼠心肝肾的过氧化脂质产生有明显抑制作用。
Miura等证明白黎芦醇的多酚氢氧基结构具有抗氧化性,能显著抑制肝微粒体、脑线粒体及突触体氧化损伤过程中DNA 的生成。
4.保肝作用白藜芦醇对脂质过氧化物有很强的抑制作用,能降低血清和肝脏的脂质,减少脂质过氧化物在体内的积累,从而有保护肝脏免受损坏的作用。
白藜芦醇
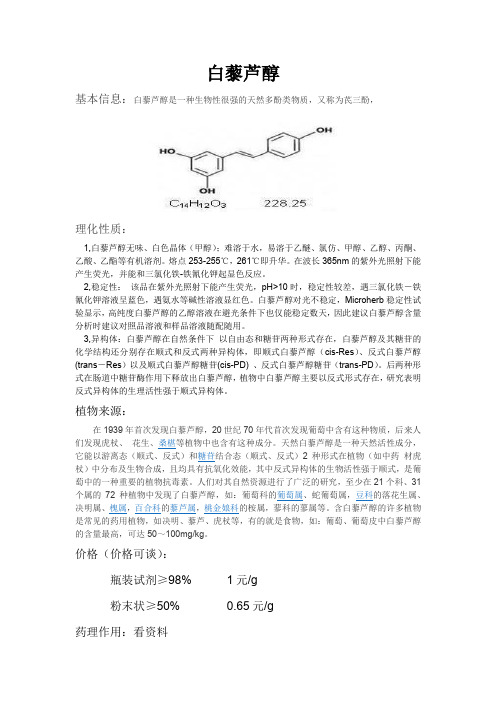
白藜芦醇
基本信息:白藜芦醇是一种生物性很强的天然多酚类物质,又称为芪三酚,
理化性质:
1,白藜芦醇无味、白色晶体(甲醇);难溶于水,易溶于乙醚、氯仿、甲醇、乙醇、丙酮、乙酸、乙酯等有机溶剂。
熔点253-255℃,261℃即升华。
在波长365nm的紫外光照射下能产生荧光,并能和三氯化铁-铁氰化钾起显色反应。
2,稳定性:该品在紫外光照射下能产生荧光,pH>10时,稳定性较差,遇三氯化铁-铁氰化钾溶液呈蓝色,遇氨水等碱性溶液显红色。
白藜芦醇对光不稳定,Microherb稳定性试验显示,高纯度白藜芦醇的乙醇溶液在避光条件下也仅能稳定数天,因此建议白藜芦醇含量分析时建议对照品溶液和样品溶液随配随用。
3,异构体:白藜芦醇在自然条件下以自由态和糖苷两种形式存在,白藜芦醇及其糖苷的化学结构还分别存在顺式和反式两种异构体,即顺式白藜芦醇(cis-Res)、反式白藜芦醇(trans-Res)以及顺式白藜芦醇糖苷(cis-PD) 、反式白藜芦醇糖苷(trans-PD)。
后两种形式在肠道中糖苷酶作用下释放出白藜芦醇,植物中白藜芦醇主要以反式形式存在,研究表明反式异构体的生理活性强于顺式异构体。
植物来源:
价格(价格可谈):
瓶装试剂≥98% 1元/g
粉末状≥50% 0.65元/g
药理作用:看资料。
白藜芦醇

Design,synthesis,and biological evaluation of resveratrol analogues asaromatase and quinone reductase 2inhibitors for chemoprevention of cancerBin Sun a, ,Juma Hoshino a ,Katie Jermihov c ,Laura Marler b ,John M.Pezzuto b ,Andrew D.Mesecar c ,Mark Cushman a,*aDepartment of Medicinal Chemistry and Molecular Pharmacology,School of Pharmacy and Pharmaceutical Sciences,and The Purdue Center for Cancer Research,Purdue University,West Lafayette,IN 47907,United States bCollege of Pharmacy,University of Hawaii at Hilo,Hilo,HI 96720,United States cCenter for Pharmaceutical Biotechnology and Department of Medicinal Chemistry and Pharmacognosy,College of Pharmacy,The University of Illinois at Chicago,Chicago,IL 60612,United Statesa r t i c l e i n f o Article history:Received 5March 2010Revised 11May 2010Accepted 14May 2010Available online 24May 2010Keywords:Resveratrol analogues AromataseQuinone reductase 2a b s t r a c trationalize docking,molec-ular mechanics energy minimization,and computer graphics molecular modeling,and the information was utilized to design several very potent inhibitors,including compounds 82(IC 5070nM)and 84(IC 5036nM).The aromatase inhibitory activities of these compounds are much more potent than that for the lead compound resveratrol,which has an IC 50of 80l M.In addition to aromatase inhibitory activ-ity,compounds 32and 44also displayed potent QR2inhibitory activity (IC 501.7l M and 0.27l M,respec-tively)and the high-resolution X-ray structures of QR2in complex with these two compounds provide insight into their mechanism of QR2inhibition.The aromatase and quinone reductase inhibitors resulting from these studies have potential value in the treatment and prevention of cancer.Ó2010Elsevier Ltd.All rights reserved.1.IntroductionResveratrol (3,5,40-trihydroxystilbene)(Fig.1)was first isolated from the roots of the white hellebore lily Veratrum grandiflorum O.Loes in 1940.1This compound is a naturally occurring phytoalexin produced by a wide range of plants (at least 72plant species)in re-sponse to environmental stress or pathogenic attack.Since the dis-covery of its cardioprotective activity in 1992,resveratrol research has steadily accelerated.This increased attention eventually re-sulted in the discovery of its cancer chemopreventive properties.2Resveratrol was found to be capable of interfering with all three steps of carcinogenesis (initiation,promotion,and progression).2Resveratrol has been reported to exert a variety of biological activities.Some of these include antioxidant,anti-inflammatory,anti-infective,anti-ischemic,cardioprotective,neuroprotective,anti-aging (prolongs lifespan),anti-obesity,anti-viral,and cancer chemopreventive effects.3These effects are mediated through sev-eral biological receptors,including cyclooxygenase (COX),lipooxy-genase (LOX),nuclear factor kappa-light-chain-enhancer ofactivated B cells (NF-kB),quinone reductase 1(QR1),quinone reduc-tase 2(QR2),ornithine decarboxylase (ODC),and aromatase.In the United States,breast cancer is the most commonly diag-nosed type of cancer in women and is the second leading cause of death from cancer in women.Approximately 180,000new cases of breast cancer were detected among women in the United States in 2008.4About two-thirds premenopausal and three-fourths post-menopausal breast cancer patients have estrogen-dependent cancer.5In hormone-dependent breast cancer,estrogens play a critical role in stimulating breast cancer cell proliferation.6High levels of estrogens promote the progression of breast cancer.Two main strat-egies have been devised by medicinal chemists to control or block the pathological activity of estrogens.7The first strategy involves0968-0896/$-see front matter Ó2010Elsevier Ltd.All rights reserved.doi:10.1016/j.bmc.2010.05.042*Corresponding author.Tel.:+17654941465;fax:+17654946790.E-mail addresses:cushman@ ,cushman@ (M.Cushman).On leave from Shandong University,China.the design and synthesis of estrogen receptor antagonists,which has yielded useful anticancer and chemopreventive drugs such as tamoxifen and raloxifene.8,9Aromatase,a pivotal enzyme responsi-ble for the conversion of androgens to estrogens,is‘the other’attrac-tive biological target for the development of new agents for the treatment of breast cancer.10–14As the enzyme responsible for the final step of the estrogen biosynthetic pathway,selective inhibition of aromatase will not interfere with the production of other steroids in the pathway(e.g.,adrenal corticoids).15,16Therefore,aromatase is a useful therapeutic target in the treatment or prevention of estro-gen-dependent breast cancers.17Quinone reductase2(QR2)is a cytosolic FAD-dependentflavo-enzyme that catalyzes the reduction of quinones by reduced N-al-kyl-and N-ribosylnicotinamides.18Recent studies have indicated that QR2may transform certain quinone substrates into more highly reactive species that are capable of causing increased cellu-lar damage.19–21Therefore,it is hypothesized that inhibition of QR2in certain cases may lead to protection of cells against these reactive species.18Several QR2inhibitors have been reported in the literature22and the X-ray crystal structure of QR2in complex with resveratrol has been determined.23,24These observations motivated the present study.Both aromatase and QR2have been targeted for discovery and identification of chemopreventive agents.24An array of resveratrol analogues have been synthesized in our laboratory in an attempt to discover new inhibitors of these two enzymes.The trans-stilbene JH-2-29(Fig.1,Scheme7),with a para-amino group,was found to exhibit versatile biological activities including nitric oxide syn-thase inhibition,aromatase inhibition(IC5022l M),and inhibition of TNF-a-induced NF-j B activity,and was therefore selected as a lead compound for further optimization.In order to improve the potency and selectivity of JH-2-29,a limited number of methoxy-lated compounds were synthesized and observed empirically to enhance aromatase inhibitory activity.In particular,compound 32(Fig.2)was found to possess significant aromatase inhibitory activity,with an IC50value of0.59l M,which is comparable to the clinically useful aromatase inhibitor2-aminoglutethimide (IC500.27l M)(Fig.2).18Molecular modeling was used to investi-gate the mechanism of action,and a variety of structurally related stilbenes were designed and synthesized based on the results.Sev-eral of these synthetic derivatives were found to inhibit aromatase activity with IC50values in the sub-micromolar range.In addition, two compounds,32and44,were discovered to exhibit potent QR2 inhibitory activity,and X-ray crystallographic analysis revealed these two compounds bind to QR2in an orientation similar to that of resveratrol,but both compounds demonstrate greater inhibitory potency than resveratrol.2.Results and discussion2.1.Chemistrytriphenylphosphonium bromide,and the following Heck reaction between appropriately substituted vinylbenzenes11–22and iodo-benzenes23–31as shown in Scheme1and Table1.The coupling constants of the vinylic protons of the trans-stilbenes were about 16Hz.The double bonds of the analogues32,37,41,and49were re-duced by catalytic hydrogenation to provide the corresponding derivatives60,61,62,and63(Scheme2).Dinitro analogues50 and52were reduced with stannous chloride to provide the respec-tive polyamines64and65in satisfactory yields(Scheme3).The ace-tate groups of compounds39and55were hydrolyzed by sodium methoxide in methanol to yield phenol derivatives66and67 (Scheme4).The tert-butyldimethylsilyl(TBDMS)-protected vinyl-benzene69(Scheme5)reacted with4-iodoaniline in a standard Heck reaction,but the yield was very low.The method of Angela et al.was used and the TBDMS group of intermediate compound 69was cleaved by tetrabutylammoniumfluoride(TBAF)to afford the diphenol70,which was acetylated to produce71.Heck reaction between compound71and4-iodoaniline or4-iodonitrobenzene led to the respective trans-stilbenes72and73.Acetate hydrolysis yielded the desired compounds74and75.25The synthetic route to imidazole analogue82began with the preparation of phosphonium salt78from aldehyde5,and ketone80from commercially available compound79(Scheme6).The building blocks78and80were then combined by the Wittig reaction in the presence of potassium car-bonate and18-crown-6,26,27and the resulting E and Z isomers were separated and purified by chromatography to provide the nitro ana-logue82.Reduction by stannous chloride resulted in the amine derivative84(Scheme6).The double bond conformation of com-pound82was confirmed by2D1H–1H NOESY spectroscopy(Fig.3).2.2.MechanismThe aromatase inhibitory activity of the resveratrol analogue32 (IC500.59l M)in comparison with resveratrol itself(IC5080l M) led to an investigation of the mechanism of action of compound 32.Recently,Ghosh et al.solved the crystal structure of human aromatase in complex with androgen.28Compound32was docked into the binding site of Ghosh’s structure(PDB code3eqm)using the GOLD(Genetic Optimization for Ligand Docking)program,29–31 and the energy minimized.In the resulting hypothetical structure (Fig.4),the amine group on ring A hydrogen bonds with the car-bonyl group of Ile305and carboxyl group of Asp309,which plays a critical role in catalysis.28Ring A is involved in a p–p interaction with the indole ring of Trp224,and a CH–p interaction with the methyl group of Thr310.Ring B forms a CH–p interaction with the methyl group of Val370.The methoxyl group on ring B is in-volved in a potential hydrogen bond with the backbone NH of Met374.All of the residues mentioned above are included in the catalytic cleft of aromatase.28The docking and energy minimization results provide a good foundation for design of new analogues.In order to minimize the possible cytotoxicity contributed by the aniline part of compound 32,the amino group on ring A was replaced by hydrogen bond-donating groups,such as hydroxy and aminomethyl groups,which may form hydrogen bonds with Asp309and Ile305(Fig.5).As shown in Figure4,the backbone carbonyl group of residue Leu477,a potential hydrogen bond acceptor located near ring B, suggested the addition of hydrogen bond-donating groups on ring B.Met374,working as a hydrogen bond donor,also suggests incor-poration of hydrogen bond acceptors on ring B.In order to probe the function of the stilbene double bond,compounds with single bonds were also synthesized.Many aromatase inhibitors have imidazole or triazole groups that can coordinate to the iron atom,32–36and these were therefore added to the stilbene frame-work and the resulting structures were modeled in complex with aromatase.B.Sun et al./Bioorg.Med.Chem.18(2010)5352–536653532.3.Aromatase inhibitory activityAn aromatase inhibition assay was performed on synthetic ana-logues of compound JH-2-29,as described in the Section 4.The inhibition percentage at a concentration of 20l g/mL and the IC 50values of the more active compounds are reported in Table 2.Most of the resveratrol analogues with an amino group on the para posi-tion of ring A (32,33,35,36,37,44,45,46,48,51,64,65,72,and 74)consistently exhibited significant activity,with IC 50values in the 0.59–14.51l M range.Substitution of the para -amino group with nitro,halogen,hydroxy,nitrile,acetyl,and aminomethylgroups made the resulting compounds inactive (compounds 38,39,40,41,43,52,53,55,56,57,58,59,66,67,and 75).Changing the position of amino group on ring A also led to reduced activity (compounds 34,42,and 54).Presumably,altering the position of the amino group is expected to influence its ability to simulta-neously hydrogen bond with the Asp309carboxylate and the back-bone carbonyl oxygen of Ile305(Fig.4).Double bond reduction impacted the activity positively (37vs 61,49vs 63,and 41vs 62)or negatively (32vs 60)to some extent.Within the series,the position of amino group on ring A was of greater importance than that of methoxy groups (ring B),and a para -amino group was better than an ortho-or meta -amino group.Compound 84was the most potent aromatase inhibitor,with an IC 50value of 36nM.As expected,molecular modeling indicated that one of the imidazole nitrogens coordinates with the iron atom of heme,which plays a critical role in catalysis (Fig.6).28K M and V max values for those analogues demonstrated to inhibit aromatase can be found in Table 3.V max values for each inhibitor are quite similar to each other and to the V max of the control (34.8pmol/min/mg protein),indicating that these resveratrol ana-logues are competitive inhibitors of CYP19.2.4.Quinone reductase 2inhibitory activity and X-ray structure analysisQR2inhibition assays were performed on analogues of syn-thetic compound JH-2-29,as described in the Section -pounds 32and 44exhibited potent QR2inhibitory activity with IC 50values of 1.7±0.06l M and 0.27±0.01l M,respectively.Both of these substances are more potent than resveratrol,which has an IC 50value of 5.1l M in the same assay.To gain insight into the binding mode of these two inhibitors,the X-ray crystal structures of both compounds 32and 44in com-plex with human QR2were plete X-ray data sets were collected and processed,and the final structural models were refined to 1.74Åand 1.55Åfor compound 32and compound 44complexes,respectively.As revealed by the strong and well-de-fined electron density shown in Figure 7,both compounds 32and 44bind to QR2in an orientation similar to that of resveratrol.However,these two compounds interact directly with the side chain of Thr71,whereas resveratrol binds to this residue indirectly through a water molecule.The direct hydrogen bond interaction between the amino group and the hydroxyl group of residue Thr71is most likely responsible for enhancing the QR2inhibitory activity of compounds 32and 44relative to resveratrol.The3-Table 1Structures of compounds 32–59Compd R 1R 2R 3R 4R 5R 6R 732H OCH 3H OCH 3H NH 2H 33OCH 3H OCH 3H H NH 2H 34OCH 3H OCH 3H NH 2H H 35OCH 3H H OCH 3H NH 2H 36OCH 3OCH 3H H H NH 2H 37H OCH 3OCH 3H H NH 2H 38H OCH 3OCH 3H H CN H 39H OCH 3OCH 3H H OAc H 40H OCH 3OCH 3H H F H 41H OCH 3OCH 3OCH 3H NO 2H 42H OCH 3OCH 3OCH 3NH 2HH 43H OCH 3OCH 3OCH 3H CH 2NH 2H 44H OCH 3OCH 3OCH 3H NH 2H 45OCH 3OCH 3OCH 3H H NH 2H 46OCH 3OCH 3H OCH 3H NH 2H 47H –OCH 2O–H NH 2H H 48H –OCH 2O–H H NH 2H 49H H NO 2H NO 2H H 50H NO 2H H H NH 2H 51H OCH 3H H H NH 2H 52H H NO 2H NO 2H NO 253H OCH 3H OCH 3H NO 2H 54H OCH 3H OCH 3NH 2H H 55H OCH 3H OCH 3H OAc H 56H OCH 3H OCH 3H CH 2NH 2H 57H OCH 3H OCH 3H F H 58H OCH 3H OCH 3H Cl H 59HOCH 3HOCH 3HCNH5354 B.Sun et al./Bioorg.Med.Chem.18(2010)5352–5366methoxy groups on ring B of compounds 32and 44interact with the amide NH 2group of residue Asn161,and there is one more hydrogen bond interaction between this side chain and the 4-methoxy group of compound 44,which may contribute to the higher potency of compound 44relative to 32.The X-ray structures of QR2in complex with compounds 32and 44also indicate that these resveratrol analogues sit parallel to the plane of the isoallox-azine ring of the bound cofactor FAD,occupying a position that is similar to resveratrol in the QR2-resveratrol complex.223.ConclusionsA focused set of 45resveratrol analogues have been designed,synthesized,and tested in an effort to maximize their aromatase and QR2inhibitory pounds 82and 84exhibit excel-lent aromatase inhibitory activity,with IC 50values 70nM and 36nM,respectively.From the preliminary structure–activity rela-tionships and molecular modeling results,it appears that the para -amino group on the trans -stilbene benzene ring is essential for aromatase inhibitory activity,and the introduction of an imidazole moiety improves the activity pounds 32and 44also displayed potent QR2inhibitory activity,and their crystal struc-tures in complex with QR2have been determined.They provide a detailed description of the enzyme active site,and can be utilized in the further modification for discovery of new QR2inhibitors.4.Experimental 4.1.General proceduresMelting points were determined in capillary tubes using a Mel-Temp apparatus and are not corrected.Infrared spectra were ob-tained as films on salt plates using CHCl 3as the solvent except where otherwise specified,using a Perkin–Elmer Spectrum One FT-IR spectrometer,and are baseline-corrected.1H NMR spectra were obtained at 300MHz (1H)and 75MHz (13C)or 500MHz (1H)and 125MHz (13C),using Bruker ARX300and Bruker Avance 500(TXI 5mm probe)spectrometers,respectively.Mass spectral analyses were performed at the Purdue University Campus-Wide Mass Spectrometry Center.Analytical thin-layer chromatography was performed on Baker-flex silica gel IB2-F plastic-backed TLC plates.Flash chromatography was performed with 230–400mesh silica gel.Unless otherwise stated,chemicals and solvents were of reagent grade and used as obtained from commercial sources without further purification.Dichloromethane was distilled from CaH 2and THF from sodium prior to use.4.1.1.General procedure 1(GP 1)for the preparation of aromatic alkenes from aromatic aldehydesMethyl triphenylphosphonium bromide (4.3g,12.05mmol)and sodium amide (0.47g,12.05mmol)in dry diethyletherB.Sun et al./Bioorg.Med.Chem.18(2010)5352–53665355(15mL)were stirred at room temperature under argon for 10h.A solution of benzaldehyde (6mmol)in diethyl ether (10mL)was added to the reaction mixture dropwise under argon in 30min at À10°C.After 10min,the reaction mixture was warmed up to room temperature and stirred for 2h.The reaction mixture was then fil-tered and the solvent was removed in vacuo to yield yellow oil that was purified by silica gel column chromatography,eluting with hexane–dichloromethane mixture,to yield the pure product in good yield.4.1.2.General procedure 2(GP 2)for the Heck reactionsIn a round-bottomed flask,iodobenzene (2.21mmol)was added to a mixture of tetrabutylammonium bromide (1.1g,3.33mmol),potassium acetate (350mg,3.57mmol),palladium acetate (25mg,0.11mmol)and aromatic alkenes (2.44mmol)stirred in DMF (20mL)at room temperature under argon.The reaction mixture was warmed up to 80°C for 3h and then cooled to room tempera-ture.Diethyl ether (25mL)was added and the organic layer was washed with water (3Â20mL)and then dried over anhydrous sodium sulfate.The solution was concentrated and the residuewas purified by silica gel column chromatography,eluting with hex-ane–dichloromethane mixture,to yield the pure product as a solid in moderate yield.4.1.3.General procedure 3(GP 3)for the hydrolysis of acetyl groupsNaOMe (2mg,0.04mmol)was added to a solution of (E )-(sty-ryl)phenyl acetate (0.5mmol)in ethanol (15mL).The reaction mixture was stirred for 10h and the solvent was evaporated in va-cuo.The residue was purified by silica gel column chromatography,eluting with a solution of 1%methanol in dichloromethane,to yield the pure product in good yield.4.1.4.General procedure 4(GP 4)for thecatalytic hydrogenation of nitro groups and stilbenesStilbene compound (0.35mmol)was dissolved in ethyl acetate (15mL)and stirred with palladium on carbon (10%,10mg)under H 2at room temperature for 24h.The mixture was filtered,and the solvent was removed in vacuo to provide a solid that was recrystal-lized from dichloromethane and hexane to yield the pure product in good yield.4.2.Syntheses4.2.1.1,2-Dimethoxy-3-vinylbenzene (11)37This compound was prepared in 97%yield by following GP 1.Colorless oil.1H NMR (300MHz,CDCl 3)d 7.14–6.99(m,3H),6.82(dd,J =10.8Hz,J =1.2Hz,1H),5.77(d,J =17.7Hz,1H),5.30(d,J =11.1Hz,1H),3.84(s,3H),3.81(s,3H).5356 B.Sun et al./Bioorg.Med.Chem.18(2010)5352–53664.2.2.2,4-Dimethoxy-1-vinylbenzene(12)38This compound was prepared in82%yield by following GP1. Colorless oil.1H NMR(300MHz,CDCl3)d7.41(d,J=8.4Hz,1H), 7.04–6.94(m,1H),6.51–6.45(m,2H),5.65(d,J=17.7Hz,1H), 5.17(d,J=11.4Hz,1H),3.83(s,3H),3.81(s,3H).4.2.3.1,4-Dimethoxy-2-vinylbenzene(13)39This compound was prepared in93%yield by following GP1. Colorless oil.1H NMR(300MHz,CDCl3)d7.12–7.01(m,2H),6.80 (s,2H),5.75(d,J=17.7Hz,1H),5.29(d,J=8.4Hz,1H),3.80(s, 3H),3.79(s,3H).4.2.4.1,2-Dimethoxy-4-vinylbenzene(14)39This compound was prepared in87%yield by following GP1. Colorless oil.1H NMR(300MHz,CDCl3)d6.95–6.93(m,2H),6.90 (d,J=1.8Hz,1H),6.69(dd,J=17.4Hz,J=10.6Hz,1H),5.59(d, J=17.4Hz,1H),5.13(d,J=10.6Hz,1H),3.88(s,3H),3.85(s,3H).4.2.5.1,3-Dimethoxy-5-vinylbenzene(15)40This compound was prepared in93%yield by following GP1. Colorless oil.1H NMR(300MHz,CDCl3)d6.59–6.54(m,3H),5.62 (d,J=17.4Hz,1H),5.16(d,J=10.8Hz,1H),3.82–3.81(m,9H). 4.2.6.1,2,3-Trimethoxy-4-vinylbenzene(16)41This compound was prepared in58%yield by following GP1. Colorless oil.1H NMR(300MHz,CDCl3)d7.18(d,J=8.7Hz,1H), 6.95–6.86(m,1H), 6.65(d,J=8.7Hz,1H), 5.63(dd,J=18.0, 1.2Hz,1H),5.17(dd,J=18.0,1.2Hz,1H),3.86(s,6H),3.84(s, 3H);13C NMR(75MHz,CDCl3)d153.2,151.4,142.2,130.9, 124.7,120.5,113.0,107.5,61.1,60.8,55.9.4.2.7.1,2,5-Trimethoxy-3-vinylbenzene(17)42This compound was prepared in42%yield by following GP1. White solid:mp54–55°C.1H NMR(300MHz,CDCl3)d7.02–6.92 (m,2H), 6.48(s,1H), 5.57(dd,J=17.7, 1.2Hz,1H), 5.13(dd, J=14.1,1.2Hz,1H),3.88(s,3H),3.85(s,3H),3.81(s,3H);13C NMR(75MHz,CDCl3)d151.3,149.5,143.2,130.8,118.4,112.0, 109.3,97.6,56.6,56.4,56.0.4.2.8.5-Vinylbenzo[d][1,3]dioxole(18)43This compound was prepared in60%yield by following GP1. Colorless oil.1H NMR(300MHz,CDCl3)d6.99(s,1H),6.85(d, J=8.1Hz,1H), 6.78(d,J=8.1Hz,1H), 6.64(dd,J=16.8Hz, J=10.8Hz,1H), 5.95(s,2H), 5.60(d,J=16.8Hz,1H), 5.15(d, J=10.8Hz,1H);13C NMR(75MHz,CDCl3)d147.9,147.3,136.3, 132.0,120.9,111.8,108.0,105.3,100.9.4.2.9.1,2,3-Trimethoxy-5-vinylbenzene(19)40This compound was prepared in85%yield by following GP1. Colorless oil.1H NMR(300MHz,CDCl3)d6.59–6.54(m,3H),5.62 (d,J=17.8Hz,1H),5.16(d,J=10.8Hz,1H),3.82–3.81(m,9H). 4.2.10.1-Methoxy-3-vinylbenzene(20)44This compound was prepared in73%yield by following GP1. Colorless oil.1H NMR(300MHz,CDCl3)d6.95–6.93(m,2H),6.90 (d,J=1.8Hz,1H),6.69(dd,J=17.4Hz,J=10.6Hz,1H),5.59(d, J=17.4Hz,1H),5.13(d,J=10.6Hz,1H),3.88(s,3H),3.85(s,3H).4.2.11.3,5-Dimethoxy-40-amino-trans-stilbene(32)This compound was prepared in64%yield by following GP2. Yellow solid:mp90–91°C.IR(film)3369,1595,1516,1456, Figure3.Region of a2D1H–1H NOESY spectrum of82.The red circle indicates the NOE signal of H8and H17/21.1203,1151,1066,962cm À1;1H NMR (300MHz,CDCl 3)d 7.31(d,J =8.4Hz,2H),6.99(d,J =16.2Hz,1H),6.83(d,J =16.2Hz,1H),6.65(d,J =8.4Hz,2H),6.62(d,J =2.1Hz,2H),6.34(t,J =2.1Hz,1H),3.81(s,6H),3.75(br,2H);13C NMR (75MHz,CDCl 3)d 160.8,146.2,139.9,129.1,127.7,124.9,115.1,104.0,99.2,55.3;CIMS 256(MH +);HRMS calcd for C 16H 17O 2N m/z 255.1259,found:m/z 255.1261(M +).4.2.12.2,4-Dimethoxy-40-amino-trans -stilbene (33)This compound was prepared in 59%yield by following GP 2.Yellow solid:mp 97–98°C.IR (film)3372,1609,1577,1517,1464,1286,1207,968cm À1;1H NMR (300MHz,CDCl 3)d 7.48(d,J =8.4Hz,1H),7.34(d,J =8.4Hz,2H),7.22(d,J =16.5Hz,1H),6.93(d,J =16.5Hz,1H), 6.66(d,J =8.4Hz,2H), 6.49–6.46(m,2H),3.85(s,3H),3.82(s,3H);13C NMR (75MHz,CDCl 3)d 159.9,157.6,145.3,129.1,127.4,127.0,126.6,120.0,119.6,115.3,104.8,98.4,55.4,55.3;CIMS m/z 256(MH +);HRMS calcd for C 16H 18O 2N m/z 256.1338,found:m/z 256.1344(MH +).4.2.13.2,4-Dimethoxy-30-amino-trans -stilbene (34)This compound was prepared in 42%yield by following GP 2.Yellow solid:mp 69–70°C.IR (film)3369,1598,1577,1504,1456,1287,1158,971cm À1;1H NMR (300MHz,CDCl 3)d 7.49(d,J =8.4Hz,1H),7.35(d,J =16.5Hz,1H),7.11(t,J =7.8Hz,1H),6.94–6.86(m,3H), 6.57(dd,J =2.1Hz,8.1Hz,1H), 6.50(dd,J =2.4Hz,8.7Hz,1H),6.46(d,J =2.4Hz,1H),3.85(s,3H),3.82(s,3H), 3.77(br,2H);13C NMR (75MHz,CDCl 3)d 160.4,157.9,146.1,139.3,129.4,127.1,127.0,123.0,119.5,117.4,114.2,112.8,104.9,98.4,55.4,55.3;ESIMS m/z 256(MH +);HRMS calcd for C 16H 18O 2N m/z 256.1338,found:m/z 256.1340(MH +).4.2.14.2,5-Dimethoxy-40-amino-trans -stilbene (35)45This compound was prepared in 35%yield by following GP 2.Yellow solid:mp 91–92°C.IR (film)3370,1601,1516,1493,1282,1215,1046,969cm À1;1H NMR (300MHz,CDCl 3)d 7.36(d,J =8.4Hz,2H),7.27(d,J =16.2Hz,1H),7.11(d,J =2.7Hz,1H),6.99(d,J =16.2Hz,1H),6.81(d,J =8.7Hz,1H),6.76(d,J =2.7Hz,1H),6.71(d,J =8.4Hz,2H),4.15(br,2H),3.82(s,3H),3.80(s,3H);13C NMR (75MHz,CDCl 3)d 153.7,151.1,146.0,129.3,128.3,127.8,119.4,115.1,112.8,112.2,111.1,56.2,55.7;CIMS m/z 256(MH +).4.2.15.2,3-Dimethoxy-40-amino-trans -stilbene (36)This compound was prepared in 75%yield by following GP 2.Yellow solid:mp 97–98°C.IR (film)3367,2923,1539,1515,1343,961cm À1;1H NMR (300MHz,CDCl 3)d 7.37(d,J =8.1Hz,2H),7.29(d,J =16.8Hz,1H),7.21(dd,J =1.2Hz,7.8Hz,1H),7.07–7.02(m,2H),6.82–6.77(m,3H),3.88(s,3H),3.84(s,3H);13C NMR (75MHz,CDCl 3)d 152.8,146.3,146.1,131.9,129.8,128.0,127.7,123.9,118.8,117.3,115.0,110.4,60.8,55.6;ESIMS m/z 256(MH +);HRMS calcd for C 16H 17O 2N m/z 255.1259,found:m/z 255.1257(M +).4.2.16.(3,4-Dimethoxy-40-amino-trans -stilbene (37)46This compound was prepared in 75%yield by following GP 2.Yellow solid:mp 158–159°C.IR (film)3368,1622,1604,1516,Figure 4.(A)Ribbon diagram illustrating the hypothetical complex structure human aromatase with compound 32bound to the active site,and the hydrogen bonds are labeled as yellow broken lines.(B)Potential interactions between compound 32and Ile305,Asp309,Thr310,Trp224,Val370,Met374in human aromatase active site.The hydrogen bonds are labeled as yellow broken lines.H 3COOH NH 2Hydrogen Bond Donor and AcceptorOAcChem.18(2010)5352–53661465,1137,1023,965cmÀ1;1H NMR(300MHz,CDCl3)d7.32(d,8.4Hz,2H),7.04(d,J=1.8Hz,1H),7.00(dd,J=1.8Hz,8.1Hz, 1H),6.88(s,1H),6.83(d,J=8.1Hz,1H),6.67(d,J=8.4Hz,2H), 3.92(s,3H),3.87(s,3H),3.75(br,2H);13C NMR(75MHz,CDCl3) d149.0,148.3,145.8,131.0,128.1,127.4,126.8,124.8,119.2, 115.2,111.1,108.3,55.8,55.7;CIMS m/z256(MH+).4.2.17.3,4-Dimethoxy-40-cyano-trans-stilbene(38)This compound was prepared in61%yield by following GP2. Yellow solid:mp102–103°C.IR(film)3398,2223,1595,1515, 1463,1267,1139,964cmÀ1;1H NMR(300MHz,CDCl3)d7.60(d, J=8.4Hz,2H),7.53(d,J=8.4Hz,2H),7.14(d,J=16.5Hz,1H), 7.06(d,J=6.3Hz,2H),6.93(d,J=16.5Hz,1H),6.86(d,J=9.0Hz, 1H),3.93(s,3H),3.90(s,3H);13C NMR(75MHz,CDCl3)d149.6, 149.1,142.0,132.4,132.1,129.2,126.5,124.6,120.6,119.0, 111.1,110.0,108.8,55.9;CIMS m/z266(MH+);HRMS calcd for C17H15O2N m/z265.1103,found:m/z265.1100(M+).4.2.18.3,4-Dimethoxy-40-acetoxy-trans-stilbene(39)47This compound was prepared in59%yield by following GP2. Yellow solid:mp125–126°C.IR(film)3368,2927,1760,1515, 1420,1196,964cmÀ1;1H NMR(300MHz,CDCl3)d7.47(d,J=8.4Hz,2H),7.07–6.97(m,5H),6.93(d,J=16.5Hz,1H),6.83 (d,J=7.8Hz,1H),3.93(s,3H),3.89(s,3H),2.29(s,3H);13C NMR (75MHz,CDCl3)d169.5,149.7,149.1,135.3,130.2,128.7,127.1, 125.7,121.7,119.9,111.1,108.6,55.9,55.8,21.1;CIMS m/z299 (MH+).4.2.19.3,4-Dimethoxy-40-fluoro-trans-stilbene(40)This compound was prepared in57%yield by following GP2. Yellow solid:mp117–118°C.IR(film)3368,1600,1515,1461, 1225,1139,1022,964cmÀ1;1H NMR(300MHz,CDCl3)d7.45 (dd,J=5.7Hz,8.4Hz,2H),7.07–7.02(m,4H),6.97(d,J=16.5Hz, 1H),6.91(d,J=16.5Hz,1H),6.86(d,J=7.8Hz,1H),3.95(s,3H), 3.90(s,3H);13C NMR(75MHz,CDCl3)d148.9,133.6,130.2, 128.2,127.7,127.5,125.5,119.7,115.6,115.3,111.1,108.5,55.8, 55.7;CIMS m/z259(MH+);HRMS calcd for C16H15O2F m/z 258.1056,found:m/z258.1051(M+).Table2Figure6.Potential interactions between compound84and Ile305,Asp309,Thr310,Trp224,Val370,Met374and heme in human aromatase active site.The hydrogenbonds are labeled as yellow broken lines.The IC50values are listed in Table2.B.Sun et al./Bioorg.Med.Chem.18(2010)5352–53665359。
「白藜芦醇神奇的胞生素」台湾专家翁明家著

「白藜芦醇神奇的胞生素」台湾专家翁明家著美释国际2017-12-26 11:10:44这两天在拜读翁明家先生的研究著作《白藜芦醇-神奇的胞生素》一书,具世界各国和生医学家均好奇与兴趣的不断投入研究发展,频发现新成果。
几度並引起全球性轰动报导,尤多专门访问或专辑节目推崇其《该生病而不生病该老化却还年轻》之神乎奇迹。
白藜芦醇是近年最早被发现:可抑制癌症干细胞又兼可增生活化修补滋润调节正常干细胞最典型的干细胞增生活化元素、癌症干细胞抑生元素,简直是智慧型巧夺天工,无异是(秦始皇梦寐以求的东西找到了),是继维生素,抗生素之后第三波医药革命崛起的干细胞增生活化元素(简称胞生素)明日之星!白藜芦醇的作用机制随着年代,越多发现对人体的帮助,从过去三年多来原本可对付29种癌症、肿瘤,而迄今发展增可对付37种癌症、肿瘤!另新增助女受孕、帮助男性不育改善,及可对付B肝、高血压、镇痛、伤口愈合、恢复骨骼肌损伤、脊髓损伤保护,及能抑制忧郁症、防止毛发脱色,缓解移植性排斥,帮助红斑性狼疮、懒惰变勤快及预防酒醉解酒功能、预发食物过敏,以及养颜与扩大医美者效果年限,並被荣选为太空食品等多种作用,功能作用越来越多获得证实奇妙!白藜芦醇被誉为21世纪最具有抗老化及抗氧化的有效成份。
不仅解开“法国逆论”该生病而不生病、该老化却很年轻之迷,更因而引起世界各个国家研发机构的研究热潮,迭有令人兴奋的新研究成果报告,更获得全球各大电视媒体、知名医学权威期刊与新闻媒体争相报导其特殊疗效,也是自抗生素长期研究以来,医学界中最大的突破!关于『白藜芦醇』的详细介绍白藜芦醇是一种天然的抗氧化剂,可降低血液粘稠度,抑制血小板凝结和血管舒张,保持血液畅通,可预防癌症的发生及发展,具有抗动脉粥样硬化和冠心病,缺血性心脏病,高血脂的防治作用,抑制肿瘤的作用还具有雌激素样作用,可用于治疗乳腺癌等疾病。
20世纪90年代,我国科技工作者对白藜芦醇的研究不断深入,并揭示其药理作用:抑制血小板非正常凝聚,预防心肌硬塞、脑栓塞,对缺氧心脏有保护作用,对肥胖者可以起一个控制与减肥作用,对烧伤或失血性休克引起的心输出量下降有效恢复,并能够扩张动脉血管及改善微循环。
白藜芦醇的功效与作用

白藜芦醇的功效与作用白藜芦醇(Resveratrol)是一种天然的化合物,主要存在于葡萄皮、红酒、花生等食物中。
它被广泛研究并被认为具有许多重要的功效和作用,特别是在预防和治疗慢性疾病方面。
本文将详细介绍白藜芦醇的功效与作用。
一、抗氧化作用白藜芦醇具有强烈的抗氧化作用,能够中和自由基并减少氧化应激的损害。
氧化应激是许多慢性疾病的发生机制之一,包括心血管疾病、癌症、糖尿病、神经退行性疾病等。
大量的研究表明,白藜芦醇具有抗氧化作用可以减少氧化应激对身体的损害,从而预防和治疗慢性疾病。
二、保护心血管健康白藜芦醇对心血管系统具有多重保护作用。
首先,它可以改善血管功能,扩张血管,增加血管弹性,降低血压。
其次,白藜芦醇可以降低胆固醇,并抑制氧化低密度脂蛋白(LDL-C),防止动脉粥样硬化的形成。
此外,白藜芦醇还能够抑制血小板凝聚,减少血栓形成的风险。
因此,白藜芦醇被认为是一种有效的保护心血管健康的物质。
三、抗癌作用白藜芦醇被广泛研究并被认为具有抗癌作用。
研究发现,白藜芦醇可以抑制肿瘤细胞的生长和增殖,并促使癌细胞凋亡。
它还可以阻断癌细胞的侵袭和转移,降低癌症的复发和转移风险。
此外,白藜芦醇还可以通过调节各种信号通路,影响癌细胞的增殖和转录,从而抑制癌细胞的发生和发展。
这些研究结果表明,白藜芦醇可能成为预防和治疗癌症的有效药物。
四、抗糖尿病作用白藜芦醇对糖尿病具有一定的保护作用。
糖尿病是一种常见的慢性代谢性疾病,其主要特征是血糖水平升高和胰岛功能异常。
研究发现,白藜芦醇可以降低血糖和胰岛素水平,并改善胰岛素的敏感性。
此外,白藜芦醇还可以减少胰岛素抵抗和肥胖的发生,从而预防和减轻糖尿病的发展。
五、保护神经系统白藜芦醇对神经系统具有保护作用。
神经退行性疾病如阿尔茨海默病、帕金森病等是造成老年人患病和死亡的重要原因之一。
研究发现,白藜芦醇具有抗氧化和抗炎作用,可以延缓神经退行性疾病的发展。
此外,白藜芦醇还可以增加神经干细胞的生成和存活,促进神经再生,提高记忆和学习能力。
白藜芦醇的发现

白藜芦醇的发现白藜芦醇(Resveratrol)是一种天然存在于一些植物中的化合物,最早于1940年被发现。
直到近年来,白藜芦醇因其多种保健功效而受到了广泛关注。
本文将以白藜芦醇的发现为主题,探讨其研究历程和相关的健康益处。
白藜芦醇的发现可以追溯到上世纪40年代。
当时,一位名叫Michio Takaoka的日本科学家从日本一种传统中草药中首次分离出了白藜芦醇。
然而,当时的科学界并没有对这一发现给予足够的重视,白藜芦醇的潜力也没有被深入研究。
直到1992年,法国一位科学家Jean-Michel Baur再次将白藜芦醇带入科学界的视野。
他发现白藜芦醇是一种强效的抗氧化剂,具有抗菌、抗炎和抗肿瘤的活性。
这一发现引起了广泛的兴趣,并且成为了科学界的研究热点。
随着研究的深入,科学家们发现白藜芦醇不仅存在于中草药中,还存在于葡萄、葡萄酒、花生、蓝莓等一些食物中。
尤其是在葡萄酒中的含量较高,这也解释了为什么法国人喝红酒的心脏病发病率较低。
白藜芦醇的抗氧化特性是其受关注的主要原因之一。
它可以清除体内自由基,减少氧化应激对细胞的损伤,从而具有延缓衰老、抗癌等作用。
研究还发现,白藜芦醇对心脑血管疾病、糖尿病、肥胖等疾病也有一定的预防和治疗作用。
白藜芦醇的健康益处主要通过激活SIRT1基因来实现。
SIRT1是一种重要的蛋白质,可以调节细胞的代谢和生命活力。
白藜芦醇可以通过激活SIRT1基因来促进脂肪代谢,抑制脂肪细胞的分化和增殖,从而起到减肥和防止肥胖的作用。
此外,白藜芦醇还可以通过抑制血小板的凝聚和血管收缩,降低血液粘稠度,防止血栓的形成,从而起到预防心脑血管疾病的作用。
白藜芦醇还有一些其他的保健功效。
例如,它可以调节免疫系统,增强机体的抵抗力;它还可以改善睡眠质量,缓解焦虑和抑郁症状;此外,白藜芦醇还对防止肝损伤、改善肝功能有一定的作用。
虽然白藜芦醇具有许多健康益处,但并不意味着我们可以随意大量摄入。
目前还没有确切的摄入量建议,但一般来说,适量摄入葡萄酒、葡萄等富含白藜芦醇的食物是安全的。
白藜芦醇(1)
白藜芦醇
• 自由基清除能力是维生素E50倍 • 是维生素C20倍 • 可被人体100%吸收,服用20分钟,血 液就能检测到,并在体内维持长达72 小时
白藜芦醇对糖尿病的作用
是因为糖尿病患者体内缺少胰岛素,使糖的 代谢无法正常进行,人体也就无法得到所需 的热能。 在这种情况下,人体就只有将体内贮存的 脂肪和蛋白质转化为能量,供给人体所需。 但脂肪和蛋白质转化为能量必须在钾的帮助 下才顺利进行,因而钾被大量消耗。由于人 体中钾的含量是有限的,结果导致人体大量 缺钾而引起多种并发症。
食物中残留大量的: 农 药 化 肥 激 素 色 素 防腐剂 洗洁精 ······
▲改变人体荷尔蒙,使女性分泌紊乱引致乳癌, 男性少精,精子成活率低。
▲通过食物链累积,经由孕妇 母体传至胎儿,影响胎儿发育, 由母乳传至婴儿。
毒素
身体有较强 的自净能力
并不可怕,可怕的是
毒素的积累毒垢来自抗氧化之王—白藜芦醇• 有助于预防多种与自由基有关的疾病,包括癌症,心脏 病,过早衰老和关节炎 • 通过防止应激反应和吸烟引起的血小板疑集来减少心脏 病和中风的发生 • 增强免疫系统能力来抵御致癌物质 • 降低感冒的次数和缩短持续时间 • 具有抗突变的功能从而减少致癌因子的形成 • 具有抗炎功能,因而可以预防包括关节炎和肿胀在内的 炎症 • 缓解花粉保护动脉血管内壁 • 病和其他过敏症 • 增强动脉、静脉和毛细血管弹性
白藜芦醇对糖尿病有奇效的原因:
白藜芦醇中葡聚糖成量很大,可迅 速将激活人体胰岛器官细胞体帮助 分泌胰岛素。同时白藜芦醇中含 (超强生物活性生长因子EGF),能 迅速帮助糖尿病患者分解血糖,平 稳血糖。
第一处溃烂
第二处溃烂
感谢您的观看!
祝大家身体健康,延年益寿!
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
《白藜芦醇》台湾版附录一自然治愈力普遍会产生的好转反应不要误会为副作用大多数人均以为医生及所有药品一定会医好所有疾病,这并不是一定正确的想法,所以坊间就有一种“医生缘,主人福”的说法,特别是一些慢性病,其真正能治疗这些疾病的是人类与生俱来的天生抵抗力及恒久的健康饮食补给。
当疾病的实力超过天生抵抗力时,身体产生不适,即称之为生病。
而天然抵抗力超过了疾病时,就是恢复健康,因此维持医食同源恢复健康有两种方法可循:1、维持天生抵抗力原状,食用药物让疾病的实力低于天生抵抗力之下,借此使病情较好的方法属西医之原理。
例如,对肝脏病患投以肝脏药物,使肝脏病势下降于天生抵抗力之下,肝脏就能治疗,但毕竟只是属于特定的肝脏药物,为对症下药,对胃或肾脏等其它组织恶化的病势超过天生抵抗力时,此种现象就成为“副作用”,这是病患最不欲乐见的现象。
2、病势维持原状,而另一方面食用具有作用的[健康保健食品]促使天生抵抗力增强,使其超过病势的方法,如能使天生抵抗力达到8,则病势7的肝脏病,病势6的肠胃病以及病势5的肾脏病(即凡病势在8以下的疾病)均会产生天生抵抗力而自然痊愈,这是东方医学的基本想法,也是自然治愈力(具有伤口愈合、接合骨头的自我再生机能,以及击败病原体和癌细胞的自我防卫机能。
)这种现象不受一种药只能针对一种特定疗效,它可能在天生抵抗力范围以下,同时自然治愈范围以下的所有疾病。
这也是既不是中医,也不是民俗疗法,而含欧美西方国家数千年来所共同一直使用的“自然医学”共同道理。
事有轻重缓急,对于急性疾病,自然顾不了有无副作用问题,先保命看医生救命要紧,若是慢性病或一般健康保健,那就不是西药能一气呵成或可把药当食物随便饮食的,而健康保健食品就属最恰当的把“药”当成食物良方,所以西方的医学之父希波克拉底说:可治病的是食物,利用食物来治病的医生“让食物成为你的药物。
”“我们身体里面的那股自然力量才是真正治疗疾病的人”。
Victor G. Rocine博士在能治病的食物理论说:“如果我们吃得不对,那么就没有医生可以救我们。
如果我们吃得正确的话,也就不需要任何医生了!”Roger J. Williams博士在对抗疾病的营养素理论说:“人体对于外来有机物与病毒的防御机制就在于身体组织制造抗体、干扰素与相关物质的能力,甚至像是抗生素之类的物质,身体的组织又是从何处获得这些建造物质呢?当然从食物当中!”Jethro Kloss博士在重返伊甸园书中说:“当食物为人体所吸收时,就能够提供养分、修护力、生命力与热能给人体,但如果在食物准备的过程中把赋予生命力的营养素去除的话,那么食物不仅无法提供生命力给人体,反而会妨碍人体的正常功能并造成失调现象!”附录二医食同源中国传统医学以“医食同源、药食同行”为基本观念,指出医药的来源,原本和食物如出一辙,西方医学虽在药物力主立竿见影,但今日对于慢性病治愈仅治标多,就不如中草药讲求的治本功能。
观医学宗师仍然主张“自然的自愈力”,希波克拉底说:“人类拥有神所赐予的自愈力”,“神所恩赐的自愈力,是借由医师或药物发挥出来。
医师不可以认为是自己治愈疾病而沾沾自喜。
”换言之,不论任何方法,克服疾病,就是要有天生抵抗力使其发挥自然的自愈力。
许多人都已知道要治疗生活习惯病(慢性病),则提高自愈力和免疫力的方法十分有效,要达成这种目标,则医食同源为最直接有效的方法,亦是治本为主的好方法!什么叫做“医食同源”?相传在三千多年前,商汤的宰相伊尹就用调味食材作为药用。
在《吕氏春秋-本味篇》中记载,有一天,商汤和伊尹讨论起烹调方法,伊尹即用引用“阳樸之薑,招摇之桂”为例,指出薑和桂既是调味食品,也是常用药,“药食同源”之说也由此而来,成了中国数千年来的独特饮食方法,我国历代医家都很重视运用食物调治疾病,隋唐名医孙思邈(世尊称药王)明确提出:“夫为医者当须先洞晓病源,知其所犯,以食治之,食疗不愈,然后命药。
”可见高明的医师治疗慢性病,首先应重视食疗,其次才是药物治疗(药有时是毒,药有时有副作用),同时预防重于治疗,食疗先于药疗,所以医食同源,近代营养学更发挥得淋漓尽致!附录三慢性病只能慢慢痊愈很多人平时不注重身体的“经营保养”,一旦进入衰老期,毛病就多了!慢性病产生了,更惨的是癌症也不幸发生在自己身上,一到有病,大都是“平时不烧香,临时抱佛脚”,看医生或吃药都想“一针见效”、“药到病除”或“根深剔除”,“妙手回春”,虽然这是普世的心理,但是一些非病菌、病毒的慢性病或癌症,不是用抗生素或止痛药,一般短期内很难有立即痊愈的疗效。
一个人从很健康的身体,一直演变到疾病的发生(指慢性病或癌症而言),至少需要5到15年以上,而演变到癌症的发生则至少需20年以上。
当人体有了种种不舒服的自觉症状时,则表示人体的脏器或组织已有生病现象,有了自觉症状至疾病形成还需5到10年的时间,往往都已病入膏肓或癌症末期了。
既然疾病的形成需15年以上,而痊愈的时间至今没有非常短暂的几天就烟消云散。
中医积几千年的经验,对生病与医病作了一句非常恰当的诠释,即“病来如山倒,病去如抽丝”,是真正观察入微与经验定论的写照,急也急不得,好像是宿命,至今仍未客服突破。
“病愈的时间”与人体“新陈代谢的时间”有极密切的关系。
构成人体最基本的单位是细胞,细胞因有原子、分子和电子而产生了生命现象。
人体细胞也在这种生命现象中不断的摄取养分,同时也必须不断的排出废物或毒素,这种现象我们称之为“新陈代谢”。
新陈代谢也是一种汰旧换新的现象,大自然有其自然的规律,而人体这小自然也有其自然法则,现在让我们来看一看人体新陈代谢的时间表:皮肤新陈代谢时间:四至六个月肌肉新陈代谢时间:二至三年筋的新陈代谢时间:三至五年骨的新陈代谢时间:需七年以上人体新陈代谢的时间表也是人体生命自然而且不变的法则,譬如胎儿必须在母体内怀孕十个月左右才会诞生,女性经期为二十八天,如果提前或延后则表示属于特殊和异常。
这种定律法则的时间也就是病愈的时间。
假如不给予人体细胞最佳生存条件或最充分的营养,使细胞可正常的完成种种新陈代谢工作时,永远也无法脱胎换骨、重获新生。
营养素群的组合可提供给人体细胞最佳的养分,欲使疾病早日痊愈,就看自己怎么安排设定了,欲使疾病痊愈的时间愈为缩短,则必须使人体更大量排毒,欲使人体更大量排毒,人体所需的营养素也必须加量,但人体愈大量排毒时,人体也必然会更加痛苦,这也属好转反应的现象之一,当人体内毒素渐渐排除时,疾病也将随之逐渐痊愈。
同时应注意到,病愈严重或年龄愈大者须慢慢改善,愈年轻者愈可加速改善;除此之外,欲求人体早日健康,平时仍需多喝水,多吃蔬菜和水果,适当的做运动。
一些老一辈的人都应该有这种经验或见闻,当一个人被打伤时,给予服用一剂青草配方之后,以前潜伏于人体内的旧伤将被引发出来,此表示当时没有被治愈,而只是被抑制而已。
这种被抑制的内伤,犹豫伤处淤血没排除,将渐渐变成毒素,最后必将演变成种种疾病或绝症。
当旧伤被引发出来后,绝对不要害怕,必须继续服用,一直到新伤、旧伤都完全不痛后,才表示人体的内伤都已痊愈。
因此不必惊慌失措,或是“人在福中不知福”,做了错误抉择到医院打针、吃药、把治本的疾病又抑制回去,得到的只是治标,但治本的功夫可说是前功尽弃。
人们已经知道“人体本身就是最好的修理工厂”,当人体觉得已有能力自我改善时,人体亦将自我斟酌改从何处着手,譬如人体认为需从肝脏先改变时,人就会觉得很困,甚至连走路都想睡,这种情形出现时,则表示以前没睡好或经常熬夜,也等于补充过去睡眠的不足,当肝脏机能已稍有改善,人体又将选择另一脏器或组织来改善,至人体的所有脏器和组织完全正常为止。
四附录瞑眩反应/好转反应医学之父希波克拉底又说:“让我发烧,我便能治愈任何的疾病。
”治疗危机的胜利勇士I-Lindh Iar博士也说:“让我经历瞑眩反应,我就能治愈任何的疾病。
”瞑眩反应是中国医学的说法,中医常言:“不起瞑眩,病不除。
”《尚书》记载:“若药弗瞑眩,厥疾弗疗”。
说明当我们服用了某些对身体异常而有益的物质后,其有效成分可以改善身体不适、变化体质,并排除体内的毒素或重金属废物,在整个服用排毒过程中,有时会出现身体各种症状及反应,即称之为“瞑眩反应”,亦称为“好转反应”。
这种变化现象通常快则一两天,慢到三、四个月才呈现,会因人而异及个体差别,与个人的病症、病史、环境、体质、基因差有关,约有3%的人会“无任何反应”或者“或许有反应,但并无特别感觉”。
瞑眩反应很容易与“病情恶化”发生混淆,多数人都很难分辨其分际,因而有害怕、紧张、担心的情形,唯数千年中医经验传承以来,以及西方自然医学见证,及近代健康保健食品改善人体临床验证结果,均有类似过程。
一般来说,如果因为服食“某种有益物质”,不是有药物过敏、交互作用的物质,而出现“更严重的反应”,时间很短,只要继续服用,这些不适反应就会自行消失(此即好转反应)。
要是经一段长时间后,不舒服的现象不但没有消失,而且越来越严重,那就要赶紧就医,一探究竟,不宜再服用或减量服用。
“好转反应”大约包括几种现象产生,特别是慢性病患者尤甚!(一)弛缓反应这是最常见的一种好转反应症状,特别是慢性病患者最为明显,这可能是患者的脏器功能正处在恢复期,而暂时失去平衡所致。
其主要症状是疲劳、嗜睡、四肢无力。
此时,千万不要心生疑虑而停止服用。
当然,如果非常想睡,或四肢无力持续好几个星期,就有必要告知主治医师。
(二)过敏反应这种反应现象约占整个好转反应的2%,那是好转前的反弹,而且是以重症至轻症的顺序连续反应。
一般而言,神经痛、风湿症需要较长时间,如出现便秘、下痢、盗汗、肿痛(含旧伤复疾痛)都是暂时性的,不必担心。
(三)排泄反应也可说是身体的解毒作用。
蓄积于体内的废物、酸性物质开始被分解,从皮肤等处被排出来时所出现的反应。
症状是发疹、皮肤出现变化、流眼屎、尿色产生变化等。
(四)康复反应此为恢复反应。
是血液循环恢复时,常常会发生的一种反应,症状有发烧、痛楚、恶心、呕吐、腹痛、倦怠感等。
很可能是以往流通不好,现在开始畅通的证据;虽然如此,仍需接受医师的诊断。
如果症状持续时间过久或太过激烈,就有必要减少摄取量。
