Non-thermal bremsstrahlung as the dominant hard X-ray continuum emission from the supernova
平衡磷酸烯醇式丙酮酸节点通量强化筑波链霉菌合成他克莫司

2018年第37卷第6期 CHEMICAL INDUSTRY AND ENGINEERING PROGRESS·2347·化 工 进展平衡磷酸烯醇式丙酮酸节点通量强化筑波链霉菌合成他克莫司吕蒙蒙1,2,刘蛟1,2,刘欢欢1,2,陈红1,2,王成1,2,闻建平1,2(1天津大学化工学院系统生物工程教育部重点实验室,天津 300072;2天津大学天津化学化工协同创新中心,天津 300072)摘要:他克莫司(FK506)是最重要的免疫抑制剂之一,然而前体代谢供应不足制约着工业化生产。
通过优化平衡磷酸烯醇式丙酮酸(PEP )节点支路通量可提高FK506产量。
本文首先在S. tsukubaensis D852中过表达基因fkb O (编码分支酸水合还原酶)和 fkb L (编码赖氨酸环化酶)得到S. tsukubaensis -OL1,FK506的产量仅从158.7mg/L 提高到163.9mg/L 。
随后调节PEP 节点支路回补途径和莽草酸途径通量强化FK506的合成:先分别将不同菌株中编码磷酸烯醇式丙酮酸羧化酶(PPC )和3-脱氧-D-阿拉伯糖基-heptulosonate-7-磷酸合酶(DAHPS )的基因在S. tsukubaensis -OL1中过表达,FK506的产量分别提高40%(ppc ,S. tsukubaensis )和47%(dah P ,S. roseosporus );然后采用4个不同强度的组成型启动子(P ermE *,P sco 4503,P sco 3410 and P sco 5768)平衡ppc 和dah P 的表达水平获得9株工程菌,最终使FK506的产量由163.9mg/L 显著提高到350.3mg/L 。
这个结果说明优化平衡PEP 节点竞争支路通量是提高FK506产量的有效策略。
关键词:他克莫司;回补途径;莽草酸途径;组成型启动子;筑波链霉菌中图分类号:Q591 文献标志码:A 文章编号:1000–6613(2018)06–2347–07 DOI :10.16085/j.issn.1000-6613.2017-1482Balancing carbon flux rebalancing around phosphoenolpyruvate node forenhancement of FK506 production in Streptomyces tsukubaensisLÜ Mengmeng 1,2,LIU Jiao 1,2,LIU Huanhuan 1,2,CHEN Hong 1,2,WANG Cheng 1,2,WEN Jianping 1,2(1Key Laboratory of System Bioengineering (Tianjin University ),Ministry of Education ,Tianjin 300072,China ;2SynBio Research Platform ,Collaborative Innovation Center of Chemical Science and Engineering (Tianjin),School ofChemical Engineering and Technology ,Tianjin University ,Tianjin 300072,China )Abstract :Tacrolimus (FK506),as one of the widely used immunosuppressants produced by Streptomyces species ,has drawn much attention on clinic application. However ,the low FK506 fermentation titer restricts its industrial production ,which is mainly due to the insufficient precursor metabolism of the producing strain. In this work ,balancing carbon flux rebalancing around phosphoenolpyruvate (PEP )node for enhancement of FK506 production were carried on. Firstly ,the genes fkb O and fkb L were overexpressed in S. tsukubaensis D852,achieving S. tsukubaensis -OL1,of which FK506 production changed only slightly from 158.7mg/L to 163.9mg/L. Then ,two precursor metabolic pathways ,the anaplerotic and shikimate pathways emanating from PEP node ,were fine-tuned for eliminating the inefficient supply of precursors of DHCHC and pipecolate. The genes encoding PPC and DAHPS were cloned from various species and expressed in S. tsukubaensis -OL1,自然科学基金项目(21376171)。
聚氨酯灌封胶MSDS 英文

Safety Data Sheetaccording to Regulation (EC) No 1907/20061.1. Product identifier1.2. Relevant identified uses of the substance or mixture and uses advised againstUse of the substance/mixtureDi- / poly-isocyanate component for the production of polyurethanes1.3. Details of the supplier of the safety data sheetCompany name:Street:Place:Post-office box:Telephone:e-mail:e-mail (Contact person):Internet:1.4. Emergency telephonenumber:2.1. Classification of the substance or mixtureRegulation (EC) No. 1272/2008Hazard categories:Carcinogenicity: Carc. 2Acute toxicity: Acute Tox. 4Skin corrosion/irritation: Skin Irrit. 2Serious eye damage/eye irritation: Eye Irrit. 2Respiratory or skin sensitisation: Resp. Sens. 1Respiratory or skin sensitisation: Skin Sens. 1Specific target organ toxicity - single exposure: STOT SE 3Specific target organ toxicity - repeated exposure: STOT RE 2Hazard Statements:Harmful if inhaled.Causes skin irritation.Causes serious eye irritation.May cause allergy or asthma symptoms or breathing difficulties if inhaled.May cause an allergic skin reaction.Suspected of causing cancer.May cause respiratory irritation.May cause damage to organs through prolonged or repeated exposure.2.2. Label elementsRegulation (EC) No. 1272/2008Hazard components for labellingFormaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI) Signal word:DangerPictograms:Hazard statementsH315Causes skin irritation.H317May cause an allergic skin reaction.H319Causes serious eye irritation.H332Harmful if inhaled.H334May cause allergy or asthma symptoms or breathing difficulties if inhaled.H335May cause respiratory irritation.H351Suspected of causing cancer.H373May cause damage to organs through prolonged or repeated exposure.Precautionary statementsP201Obtain special instructions before use.P260Do not breathe dust/fume/gas/mist/vapours/spray.P280Wear protective gloves/protective clothing/eye protection/face protection.P284In case of inadequate ventilation wear respiratory protection.P304+P340IF INHALED: Remove person to fresh air and keep comfortable for breathing.P305+P351+P338IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, ifpresent and easy to do. Continue rinsing.P308+P313IF exposed or concerned: Get medical advice/attention.Special labelling of certain mixturesEUH204Contains isocyanates. May produce an allergic reaction.Additional advice on labellingClassification according to directive 67/548/EEC or 1999/45/EC:Classification according EC regulation 1272/2008 (CLP):2.3. Other hazardsPeople who suffer from asthma, allergies, chronic or recurring respiratory illnesses must not be deployed in processes, which use this substance. Symptoms of the respiratory tract can still occur several hours afteroverexposure. Dust, fumes and aerosols are the main respiratory hazard.3.1. SubstancesChemical characterizationdiphenylmethane-diisocyanate, isomers and homologuesFull text of H and EUH statements: see section 16.Further InformationThis product contains no substances of very high concern in concentrations where an information obligation applies (REACH Regulation (EC) No. 1907/2006, Article 59).4.1. Description of first aid measuresGeneral informationRemove contaminated clothing immediatley and dispose off safely.After inhalationRemove casualty to fresh air and keep warm and at rest. Get medical advice/attention if you feel unwell.After contact with skinIn case of skin contact, wash immediately with large quantities of water/polyethylene glycol 400 (Roticlean).If skin irritation occurs: Get medical advice/attention.After contact with eyesIn case of contact with eyes, rinse immediately with plenty of flowing water for 10 to 15 minutes holding eyelids apart. Subsequently consult an ophthalmologist.After ingestionDo NOT induce vomiting. Get immediate medical advice/attention.4.2. Most important symptoms and effects, both acute and delayedEye contact.Irritating to eyes.Irritant effect on the respiratory tract:Respiratory or skin sensitisation: May cause sensitisation especially in sensitive humans.inhalation.Inhalation of dust may cause irritation of the respiratory system.May cause sensitization by inhalation.Coughing. Asthmatic complaints.skin contact.Causes skin irritation. May cause sensitization by skin contact.erythema (redness)ingestion.gastro-intestinal ailment.4.3. Indication of any immediate medical attention and special treatment neededIn case of respiratory tract irritation, consult a physician.Treat symptomatically.5.1. Extinguishing mediaSuitable extinguishing mediaCarbon dioxide (CO2). Foam. Extinguishing powder.For larger fires: Water spray.Unsuitable extinguishing mediaHigh power water jet.5.2. Special hazards arising from the substance or mixtureIn case of fire may be liberated: Carbon dioxide (CO2). Carbon monoxide Nitrogen oxides (NOx). Isocyanates.Possible in traces: Hydrocyanic acid (hydrocyanic acid).In case of fire and/or explosion do not breathe fumes.Heating causes rise in pressure with risk of bursting. Use water spray jet to protect personnel and to coolendangered containers.Move undamaged containers from immediate hazard area if it can be done safely.5.3. Advice for firefightersIn case of fire: Wear a self-contained breathing apparatus and chemical protective clothing.Additional informationDo not allow water used to extinguish fire to enter drains or waterways. Do not allow to enter into soil/subsoil.6.1. Personal precautions, protective equipment and emergency proceduresUse personal protective equipment as required. (See section 8) Provide adequate ventilation. Evacuate area.Do not breathe gas/fumes/vapour/spray.6.2. Environmental precautionsDo not allow to enter into surface water or drains. Do not allow to enter into soil/subsoil.6.3. Methods and material for containment and cleaning upTake up mechanically. Cover residue with moist, liquid-binding material (eg sawdust, chemical binder based oncalcium silicate hydrate, sand). After approx. 1 hour pick up in waste container, do not close (CO2development!). Keep moist and leave in a secure place outdoors for several days. Delivery to an approvedwaste disposal company.The leakage area can be decontaminated with the following recommended decontaminantDecontamination solution 1: 8-10% sodium carbonate and 2% of liquid soap in waterDecontamination solution 2: Liquid/yellow soap (potassium soap with ~15% anionic tenside): 20ml; Water: 700ml; Polyethylenglycol (PEG 400): 350ml6.4. Reference to other sectionsSafe handling: see section 7Personal protection equipment: see section 8Disposal: see section 137.1. Precautions for safe handlingAdvice on safe handlingProvide adequate ventilation as well as local exhaustion at critical locations.At workplaces or parts of installations where isocyanate aerosols and / or vapors can be produced in higher concentrations (eg pressure relief, mold venting, blowing of mixing heads with compressed air), it is necessary to prevent the occupational hygiene limit values being exceeded by air extraction. The air must be moved away from the people. The effectiveness of the equipment must be checked periodically. Air limit values mentioned in section 8 must be controlled.The personal protective measures described in chapter 8 must be observed. Avoid contact with skin and eyes and inhalation of vapors.Keep away from food and beverages. Wash hands before breaks and at the end of work. Keep work clothes separate. Take off dirty, soaked clothes immediately. Decontaminate, destroy and dispose of contaminated protective clothing (see section 13).7.2. Conditions for safe storage, including any incompatibilitiesRequirements for storage rooms and vesselsKeep container tightly closed and dry.Hints on joint storageKeep away from food, drink and animal feedingstuffs.Do not store together with: Water. Alcohol. amines. strong alkalis.Do not mix with acids.Further information on storage conditionsRecommended storage temperature +15 - +25°C7.3. Specific end use(s)Di- / poly-isocyanate component for the production of polyurethanes8.1. Control parametersDNEL/DMEL valuesPNEC valuesAdditional advice on limit valuesTo date, no national critical limit values exist.8.2. Exposure controlsAppropriate engineering controlsUse in closed process, no likelihood of exposure.If local exhaust ventilation is not possible or not sufficient, the entire working area must be ventilated bytechnical means.Protective and hygiene measuresKeep away from food, drink and animal feedingstuffs. Wash hands before breaks and after work. Protect skin by using skin protective cream. Separate storage of work clothes. Decontaminate, destroy and dispose of contaminated protective clothing (see section 13).Safety precautions for handling freshly molded polyurethane parts: see section 16Wear eye/face protection.Eye/face protectionSuitable materials for protective gloves (DIN EN 374-3): Polychloroprene (CR): Thickness >= 0.50 mm; Breakthrough time >= 480 min. Nitrile rubber (NBR): thickness >= 0.35 mm; Breakthrough time >= 480 min. Butyl rubber (IIR): thickness >= 0.50 mm; Breakthrough time >= 480 min. Fluororubber (FKM): Thickness >= 0.40 mm; Breakthrough time >= 480 min. Recommendation: Dispose of contaminated glovesThe selection of a suitable glove not only depends on the material but also on other quality features and varies from manufacturer to manufacturer. Since the product is a preparation of several substances, the resistance of glove materials is not predictable and must therefore be checked before use. Always get advice from the glove supplier.Hand protectionWear suitable protective clothing.Skin protectionAt insufficiently ventilated workplaces and at spray-processing respiratory protection required. Recommended are fresh air mask or for short-term work combination filter A2-P2.Respiratory protectionbrownliquid Physical state:Colour:9.1. Information on basic physical and chemical propertiesearthy, mustyOdour:Test methodChanges in the physical state No data availableMelting point:>300 °C Initial boiling point and boiling range:217,5 °C EG A9Flash point:Flammabilitynot applicable Solid:not applicableGas:not determinedExplosive propertiesNo data available Lower explosion limits:No data availableUpper explosion limits:>600 °C Ignition temperature:Auto-ignition temperaturenot applicable Solid:not applicableGas:not determinedOxidizing properties<0,00001 hPa Vapour pressure: (at 20 °C)Vapour pressure: (at 50 °C)<0,0005 hPa Density (at 22 °C):1,20 - 1,24 g/cm³Water solubility: (at 15 °C)Immisciblenot determined Partition coefficient:OECD (TG) 117Viscosity / dynamic: (at 25 °C)100 - 170 mPa·s Vapour density:not determinedEvaporation rate:not determined9.2. Other informationFor products with a very low vapor pressure, the apparent vapor pressure may exceed the vapor pressure of the pure product due to conditions of manufacturing, storage or transportation, e.g. by solved gases like nitrogen or carbon dioxide.10.1. ReactivityNo data available10.2. Chemical stability>200 °C: Polymerization.Formation of: Carbon dioxide (CO2).10.3. Possibility of hazardous reactionsExothermic reactions with: amines., Alcohol.Avoid contact with water. Formation of: Carbon dioxide (CO2).Due to gaseous decomposition products, overpressure can occur in tightly sealed containers.10.4. Conditions to avoidNo data available10.5. Incompatible materialsWater. Alcohol. amines. strong alkalis. Do not mix with acids.10.6. Hazardous decomposition productsNo hazardous decomposition products when properly stored and handled.11.1. Information on toxicological effectsAcute toxicityHarmful if inhaled.Below are the available toxicological data on componentsFormaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)Harmful if inhaled. The product causes irritation of eyes, skin and mucous membranes May cause sensitization by inhalation and skin contact.Irritation and corrosivityCauses skin irritation.Causes serious eye irritation.Primary skin irritation:Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)Species: RabbitResult: mild irritant.Method: OECD 404Primary mucous membrane irritation:Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)Species: RabbitResult: Not an irritant.Method: OECD 405 (Investigation on a comparable product.)Sensitising effectsContains isocyanates. May produce an allergic reaction.May cause allergy or asthma symptoms or breathing difficulties if inhaled. (Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)) May cause an allergic skin reaction. (Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI))Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI):skin sensitisation test according to Magnusson/Kligman (maximisation test)Species: Guinea-pig.Results: negative.Method: OECD 406.skin sensitisation test (local lymph node assay (LLNA))Species: Mouse.Results: positive.Method: OECD 429 (Investigation on a comparable product.)Sensitisation to the respiratory tractSpecies: RatResults: May cause sensitization by inhalation.Carcinogenic/mutagenic/toxic effects for reproductionSuspected of causing cancer. (Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI))Germ cell mutagenicity: Based on available data, the classification criteria are not met.Reproductive toxicity: Based on available data, the classification criteria are not met.Carcinogenicity:Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)Species: RatApplication Route: inhalation.dosages: 0 - 0,2 - 1 - 6 mg/m³Test substance: AerosolExposure time: 2 aFrequency of treatment: 6 hours a day, 5 days a weekMethod: OECD 453.Occurrence of tumors in the highest dose groupReproductive toxicity:/fertility:No data availableReproductive toxicity:/teratogenicityFormaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)NOAEL: (teratogenicity) : 12 mg/m³NOAEL: (maternally): 4 mg/m³NOAEL: (developmental toxicity): 4 mg/m³Species: RatApplication Route: inhalation.dosages: 0 - 1 - 4 - 12 mg/m³Frequency of treatment: 6 hours / day (exposure duration: 10 days (day 6 - 15 p.c.))Length of test: 20 dTest substance: AerosolMethod: OECD 414.NOAEL: (developmental toxicity) : 4 mg/m³Did not show teratogenic effects in animal experiments.Type judging:Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)Carcinogenicity: May cause cancer by inhalation. On the basis of these data, material is classified as carcinogenic.Mutagenicity: In vivo and in vitro tests showed no mutagenic effectsTeratogenicity: Did not show teratogenic effects in animal experiments.Reproductive toxicity: If the available database is used, the classification criteria are not met.STOT-single exposureMay cause respiratory irritation. (Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI))STOT-repeated exposureMay cause damage to organs through prolonged or repeated exposure. (Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI))Subacute, subchronic and long-term toxicity:Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)NOAEL: 0,2 mg/m³LOAEL: 1 mg/m³Application Route: inhalation.Species: Ratdosages: 0 - 0,2 - 1 - 6 mg/m³Exposure time: 2 aFrequency of treatment: 6 hours a day, 5 days a weekTarget organ: Lungs, nasal cavityTest substance: AerosolMethod: OECD 453.Findings: Irritation of the nasal cavities and lungs.Investigation on a comparable product.Assessment STOT - repeated exposure:Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)Exposure route: inhalation.Target organ: Respiratory SystemMay cause damage to organs (or state all organs affected, if known) through prolonged or repeated exposure (state route of exposure if it is conclusively proven that no other routes of exposure cause the hazard).Aspiration hazardBased on available data, the classification criteria are not met.Additional information on testsGenotoxicity in vitro:Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)Test type: Salmonella / microsome test (Ames test)Test system: Salmonella typhimuriumMetabolic activation: with / withoutResult: negative.Method: OECD 471 (Ames test). (Investigation on a comparable product.)Genotoxicity in vivo:Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)Test Type: Micronucleus TestSpecies: RatApplication Route: inhalation. (Exposure duration: 3x1h / day over 3 weeks)Result: negative.Method: OECD 474 (Investigation on a comparable product.)Practical experienceOther observationsIrritating to respiratory system.May cause sensitization by inhalation.Further informationSpecial properties / effects: In case of overexposure, there is a risk of a concentration-dependent irritant effect on eyes, nose, throat and respiratory tract. Delayed onset of symptoms and development of hypersensitivity (difficulty in breathing, cough, asthma) are possible. In hypersensitive individuals reactions can be triggered even at very low isocyanate concentrations, even below the occupational exposure limit. After prolongedcontact with the skin, tanning and irritation effects are possible.12.1. ToxicityDo not allow to enter into surface water or drains. Do not allow to enter into soil/subsoil.Below are the available ecotoxicological data on componentsFormaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)Acute aquatic toxicity: If the available database is used, the classification criteria are not met.There is no evidence of chronic aquatic toxicityThe substance is classified as uncritical to soil organismsIn biological sewage treatment plants, there is no risk of impairing the cleaning performance due to the low12.2. Persistence and degradabilityFormaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)Not potentially biodegradableStability in water:12.3. Bioaccumulative potentialFormaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)Does not accumulate appreciably in organisms.The substance hydrolyzes rapidly in waterPartition coefficient n-octanol/waterLog Pow CAS NoChemical nameFormaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)32055-14-44,51 BCFChemical nameCAS NoSpeciesSourceBCF200Cyprinus carpio OECD 305E32055-14-4Formaldehyde, oligomeric reactionproducts with aniline and phosgene(oligomeric MDI)12.4. Mobility in soilNo data available12.5. Results of PBT and vPvB assessmentFormaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI): This substance doesnot meet the criteria for classification as PBT or vPvB.12.6. Other adverse effectsThe product reacts with water at the interface under formation of carbon dioxide to a solid, high-melting andinsoluble reaction product (polyurea). This reaction is greatly promoted by surfactants (eg, liquid soaps) orwater-soluble solvents. Polyurea is according to previous experience inert and non-degradable.13.1. Waste treatment methodsAdvice on disposalDisposal under consideration of all applicable international, national and local laws, ordinances and statutes After final product withdrawal, all residues must be removed from containers (drip-free, powderfree orpaste-free). Once the product residues adhering to the walls of the containers have been rendered harmless, the product and hazard labels must be invalidated. These containers can be returned for recycling to the appropriate centres set up within the framework of the existing takeback scheme of the chemical industry. Containers must be recycled in compliance with national legislation and environmental regulations. Ensure all waste water is collected and treated via a waste water treatment plant.Contaminated packagingLand transport (ADR/RID)14.1. UN number:No dangerous good in sense of this transport regulation.No dangerous good in sense of this transport regulation.14.2. UN proper shipping name:No dangerous good in sense of this transport regulation.14.3. Transport hazard class(es):14.4. Packing group:No dangerous good in sense of this transport regulation.Inland waterways transport (ADN)14.1. UN number:No dangerous good in sense of this transport regulation.14.2. UN proper shipping name:No dangerous good in sense of this transport regulation.14.3. Transport hazard class(es):No dangerous good in sense of this transport regulation.14.4. Packing group:No dangerous good in sense of this transport regulation.Marine transport (IMDG)14.1. UN number:No dangerous good in sense of this transport regulation.No dangerous good in sense of this transport regulation.14.2. UN proper shipping name:14.3. Transport hazard class(es):No dangerous good in sense of this transport regulation.14.4. Packing group:No dangerous good in sense of this transport regulation.Air transport (ICAO-TI/IATA-DGR)14.1. UN number:No dangerous good in sense of this transport regulation.No dangerous good in sense of this transport regulation.14.2. UN proper shipping name:No dangerous good in sense of this transport regulation.14.3. Transport hazard class(es):No dangerous good in sense of this transport regulation.14.4. Packing group:14.5. Environmental hazardsnoENVIRONMENTALLY HAZARDOUS:14.6. Special precautions for userIrritating to eyes and skin.Sensitive to cold from +5 °C Heat sensitive from +40 °C Protect from moisture.Keep away from food, drink and animal feedingstuffs.14.7. Transport in bulk according to Annex II of Marpol and the IBC CodeNo dangerous good in sense of this transport regulation.15.1. Safety, health and environmental regulations/legislation specific for the substance or mixtureEU regulatory informationRestrictions on use (REACH, annex XVII):Entry 56: Formaldehyde, oligomeric reaction products with aniline and phosgene (oligomeric MDI)National regulatory informationObserve restrictions to employment for juvenils according to the 'juvenile work protection guideline' (94/33/EC). Observe employment restrictions under the Maternity Protection Directive (92/85/EEC) for expectant or nursing mothers.Employment restrictions:1 - slightly water contaminatingWater contaminating class (D):Causes allergic hypersensitivity reactions.Skin resorption/Sensitization:Additional informationPlease note the leaflet of BG Chemie M 044 "Polyurethane Production and Processing / Isocyanates".ChangesThis data sheet contains changes from the previous version in section(s): 1,2,3,4,6,7,8,9,10,11,12,13,14,15,16.H315Causes skin irritation.H317May cause an allergic skin reaction.H319Causes serious eye irritation.H332Harmful if inhaled.H334May cause allergy or asthma symptoms or breathing difficulties if inhaled.H335May cause respiratory irritation.H351Suspected of causing cancer.H373May cause damage to organs through prolonged or repeated exposure.EUH204Contains isocyanates. May produce an allergic reaction.Relevant H and EUH statements (number and full text)Safety precautions for handling freshly molded polyurethane parts: Depending on the production parameters, any uncovered surfaces of freshly molded polyurethane parts using this raw material may contain traces ofsubstances (e. g. starting and reaction products, catalysts, release agents) with hazardous characteristics. Skin contact with traces of these substances must be avoided. Therefore, during demolding or other handling of fresh molded parts, protective gloves tested according to DIN-EN 374 (e.g. nitrile rubber >= 1.3 mm thick, breakthrough time >= 480 min, or according to recommendations from glove makers thinner gloves that need to be changed in compliance with breakthrough times more frequently) must be used. Depending on formulation and processing conditions, the requirements may be different from handling of the pure substances. Closed protective clothing is required for the protection of other areas of skin.The above information describes exclusively the safety requirements of the product and is based on ourpresent-day knowledge. The information is intended to give you advice about the safe handling of the product named in this safety data sheet, for storage, processing, transport and disposal. The information cannot be transferred to other products. In the case of mixing the product with other products or in the case ofprocessing, the information on this safety data sheet is not necessarily valid for the new made-up material.Further Information。
2010-26-EU-欧盟新排放指令

DIRECTIVESCOMMISSION DIRECTIVE 2010/26/EUof 31 March 2010amending Directive 97/68/EC of the European Parliament and of the Council on the approximation of the laws of the Member States relating to measures against the emission of gaseous and particulate pollutants from internal combustion engines to be installed in non-road mobile machinery(Text with EEA relevance)THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union,Having regard to Directive 97/68/EC of 16 December 1997 of the European Parliament and of the Council on the approxi mation of the laws of the Member States relating to measures against the emission of gaseous and particulate pollutants from internal combustion engines to be installed in non-road mobile machinery ( 1 ), and in particular Articles 14 and 14a thereof, Whereas:(1) Article 14a of Directive 97/68/EC sets out the criteria and the procedure for extending the period referred to in Article 9a(7) of that Directive. Studies carried out in accordance with Article 14a of Directive 97/68/EC show that there are substantial technical difficulties to comply with stage II requirements for professional use, multi- positional, hand-held mobile machinery in which engines of classes SH:2 and SH:3 are installed. It is therefore necessary to extend the period referred to in Article 9a(7) until 31 July 2013. (2) Since the amendment of Directive 97/68/EC in 2004, technical progress has been made in the design of diesel engines with a view to make them compliant with the exhaust emission limits for stages IIIB and IV. Electronically controlled engines, largely replacing me- chanically controlled fuel injection and control systems, have been developed. Therefore, the current general type- approval requirements in Annex I to Directive 97/68/EC should be adapted accordingly and general type-approval requirements for stages IIIB and IV should be introduced. (3) Annex II to Directive 97/68/EC specifies the technical details of the information documents that need to be submitted by the manufacturer to the type-approval authority with the application for engine type-approval. The details specified regarding the additional anti- pollution devices are generic and should be adapted to the specific after-treatment systems that need to be used to ensure that engines comply with exhaust emission limit stages IIIB and IV. More detailed information on the after-treatment devices installed on the engines should be submitted to enable type-approval authorities to assess the engine’s capability to comply with stages IIIB and IV.(4) Annex III to Directive 97/68/EC sets out the methodtesting the engines and determining their level of emissions of gaseous and particulate pollutants. The type-approval testing procedure of engines to demon strate compliance with the exhaust emission limits of stage IIIB and IV should ensure that the simultaneous compliance with the gaseous (carbon monoxide, hydro carbons, oxides of nitrogen) and the particulate emission limits is demonstrated. The non-road steady cycle (NRSC) and non-road transient cycle (NRTC) should be adapted accordingly. (5) Point 1.3.2 of Annex III to Directive 97/68/EC foreseesthe modification of the symbols (section 2.18 of Annex I), the test sequence (Annex III) and calculation equations (Appendix III to Annex III), prior to the introduction of the cold/hot composite test sequence. The type approval procedure to demonstrate compliance with the exhaust emission limits of stage IIIB and IV requires the intro duction of a detailed description of the cold start cycle. (6) Section 3.7.1 of Annex III to Directive 97/68/EC sets out the test cycle for the different equipment specifications. The test cycle under point 3.7.1.1 (specification A) needs to be adapted to clarify which engine speed needs to be used in the type approval calculation method. It is also necessary to adapt the reference to the updated version of the international testing standard ISO 8178-4:2007.( 1 ) OJ L 59, 27.2.1998, p. 1.(7) Section 4.5 of Annex III to Directive 97/68/EC outlines the emissions test run. This section needs to be adapted to take account of the cold start cycle. (8) Appendix 3 of Annex III to Directive 97/68/EC sets out the criteria for the data evaluation and calculation of the gaseous emissions and the particulate emissions, for both the NRSC test and the NRTC test set out in Annex III. The type approval of engines in accordance with stage IIIB and IV requires the adaptation of the calculation method for the NRTC test. (9) Annex XIII to Directive 97/68/EC sets out the provisions for engines placed on the market under a ‘flexible scheme’. To ensure a smooth implementation of stage IIIB, an increased use of this flexibility scheme may be needed. Therefore, the adaptation to technical progress to enable the introduction of stage IIIB compliant engines needs to be accompanied by measures to avoid that the use of the flexibility scheme may be hampered by notifi cation requirements which are no longer adapted to the introduction of such engines. The measures should aim at simplifying the notification requirements and the reporting obligations, and at making them more focused and tailored to the need for market surveillance authorities to respond to the increased use of the flexi bility scheme that will result from the introduction of stage IIIB. (10) Since Directive 97/68/EC provides for the type-approval of stage IIIB engines (category L) as from 1 January 2010 it is necessary to provide for the possibility to grant type approval from that date. (11) For reasons of legal certainty this Directive should enter into force as a matter of urgency. (12) The measures provided for in this Directive are in accordance with the opinion of the Committee estab lished in Article 15(1) of Directive 97/68/EC, HAS ADOPTED THIS DIRECTIVE: Article 1 Amendments to Directive 97/68/EC Directive 97/68/EC is amended as follows: 1. in Article 9a(7), the following subparagraph is added: ‘Notwithstanding the first subparagraph, an extension of the derogation period is granted until 31 July 2013, within the category of top handle machines, for professional use, multi- positional, hand-held hedge trimmers and top handle tree service chainsaws in which engines of classes SH:2 and SH:3 are installed.’;2. Annex I is amended in accordance with Annex I to this Directive;3. Annex II is amended in accordance with Annex II to this Directive;4. Annex III is amended in accordance with Annex III to this Directive;5. Annex V is amended in accordance to Annex IV to this Directive;6. Annex XIII is amended in accordance with Annex V to this Directive.Article 2Transitional provisionWith effect from the day following the publication of this Directive in the Official Journal, Member States may grant type-approval in respect of electronically controlled engines which comply with the requirements laid down in Annexes I, II, III, V and XIII to Directive 97/68/EC, as amended by this Directive.Article 3Transposition1. Member States shall bring into force the laws, regulations and administrative provisions necessary to comply with the Directive within 12 months after the publication of the Directive. They shall forthwith communicate to the Commission the text of those provisions.They shall apply those provisions from 31 March 2011.When Member States adopt those provisions, they shall contain a reference to this Directive or be accompanied by such a reference on the occasion of their official publication. Member States shall determine how such reference is to be made.2. Member States shall communicate to the Commission the text of the main provisions of national law which they adopt in the field covered by this Directive.Article 4Entry into forceThis Directive shall enter into force on the day following its publication in the Official Journal of the European Union .Article 5AddresseesThis Directive is addressed to the Member States. Done at Brussels, 31 March 2010. For the Commission The President José Manuel BARROSOANNEX IThe following section 8 is added to Annex I to Directive 97/68/EC:IIIBIVSTAGESANDFOR‘8. TYPEAPPROVALREQUIREMENTS8.1. This section shall apply to the type-approval of electronically controlled engines, which uses electronic control todetermine both the quantity and timing of injecting fuel (hereafter “engine”). This section shall apply irrespective of the technology applied to such engines to comply with the emission limit values set out in sections 4.1.2.5 and 4.1.2.6 of this Annex.8.2. DefinitionsFor the purpose of this section, the following definitions shall apply:8.2.1. “emission control strategy” means a combination of an emission control system with one base emission controlstrategy and with one set of auxiliary emission control strategies, incorporated into the overall design of an engine or non-road mobile machinery into which the engine is installed.8.2.2. “reagent” means any consumable or non-recoverable medium required and used for the effective operation of theexhaust after-treatment system.8.3. Generalrequirements8.3.1. Requirements for base emission control strategy8.3.1.1. The base emission control strategy, activated throughout the speed and torque operating range of the engine,shall be designed as to enable the engine to comply with the provisions of this Directive8.3.1.2. Any base emission control strategy that can distinguish engine operation between a standardised type approvaltest and other operating conditions and subsequently reduce the level of emission control when not operating under conditions substantially included in the type approval procedure is prohibited.8.3.2. Requirements for auxiliary emission control strategy8.3.2.1. An auxiliary emission control strategy may be used by an engine or a non-road mobile machine, provided thatthe auxiliary emission control strategy, when activated, modifies the base emission control strategy in response toa specific set of ambient and/or operating conditions but does not permanently reduce the effectiveness of theemission control system:(a) where the auxiliary emission control strategy is activated during the type approval test, sections 8.3.2.2 and8.3.2.3 shall not apply;(b) where the auxiliary emission control strategy is not activated during the type approval test, it must bedemonstrated that the auxiliary emission control strategy is active only for as long as required for thepurposes identified in section 8.3.2.3.8.3.2.2. The control conditions applicable to this section are all of the following:(a) an altitude not exceeding 1 000 metres (or equivalent atmospheric pressure of 90 kPa);(b) an ambient temperature within the range 275 K to 303 K (2 °C to 30 °C);(c) the engine coolant temperature above 343 K (70 °C).Where the auxiliary emission control strategy is activated when the engine is operating within the control conditions set out in points (a), (b) and (c), the strategy shall only be activated exceptionally.8.3.2.3. An auxiliary emission control strategy may be activated in particular for the following purposes:(a) by onboard signals, for protecting the engine (including air-handling device protection) and/or non-roadmobile machine into which the engine is installed from damage;(b) for operational safety and strategies;(c) for prevention of excessive emissions, during cold start or warming-up, during shut-down;(d) if used to trade-off the control of one regulated pollutant under specific ambient or operating conditions, formaintaining control of all other regulated pollutants, within the emission limit values that are appropriate forthe engine concerned. The purpose is to compensate for naturally occurring phenomena in a manner thatprovides acceptable control of all emission constituents.8.3.2.4. The manufacturer shall demonstrate to the technical service at the time of the type-approval test that theoperation of any auxiliary emission strategy complies with the provisions of section 8.3.2. The demonstration shall consist of an evaluation of the documentation referred to in section 8.3.3.8.3.2.5. Any operation of an auxiliary emission control strategy not compliant with section 8.3.2 is prohibited.8.3.3. Documentation requirements8.3.3.1. The manufacturer shall provide an information folder accompanying the application for type-approval at thetime of submission to the technical service, which ensures access to any element of design and emission control strategy and the means by which the auxiliary strategy directly or indirectly controls the output variables. The information folder shall be made available in two parts:(a) the documentation package, annexed to the application for type-approval, shall include a full overview of theemission control strategy. Evidence shall be provided that all outputs permitted by a matrix, obtained fromthe range of control of the individual unit inputs, have been identified. This evidence shall be attached to theinformation folder as referred to in Annex II;(b) the additional material, presented to the technical service but not annexed to the application for type-approval, shall include all the modified parameters by any auxiliary emission control strategy and theboundary conditions under which this strategy operates and in particular:(i) a description of the control logic and of timing strategies and switch points, during all modes ofoperation for the fuel and other essential systems, resulting in effective emissions control (such asexhaust gas recirculation system (EGR) or reagent dosing);(ii) a justification for the use of any auxiliary emission control strategy applied to the engine, accompanied by material and test data, demonstrating the effect on exhaust emissions. This justification may be basedon test data, sound engineering analysis, or a combination of both;(iii) a detailed description of algorithms or sensors (where applicable) used for identifying, analysing, or diagnosing incorrect operation of the NO x control system;(iv) the tolerance used to satisfy the requirements in section 8.4.7.2, regardless of the used means.8.3.3.2. The additional material referred to in point (b) of section 8.3.3.1 shall be treated as strictly confidential. It shallbe made available to the type-approval authority on request. The type-approval authority shall treat this material as confidential.ofoperationNO x control measures8.4. Requirementstoensurecorrect8.4.1. The manufacturer shall provide information that fully describes the functional operational characteristics of theNO x control measures using the documents set out in section 2 of Appendix 1 to Annex II and in section 2 of Appendix 3 to Annex II.8.4.2. If the emission control system requires a reagent, the characteristics of that reagent, including the type of reagent,information on concentration when the reagent is in solution, operational temperature conditions and reference to international standards for composition and quality must be specified by the manufacturer, in section 2.2.1.13 of Appendix 1 and in section 2.2.1.13 of Appendix 3 to Annex II.8.4.3. The engine emission control strategy shall be operational under all environmental conditions regularly pertainingin the territory of the Community, especially at low ambient temperatures.8.4.4. The manufacturer shall demonstrate that the emission of ammonia during the applicable emission test cycle ofthe type approval procedure, when a reagent is used, does not exceed a mean value of 25 ppm.8.4.5. If separate reagent containers are installed on or connected to a non-road mobile machine, means for taking asample of the reagent inside the containers must be included. The sampling point must be easily accessible without requiring the use of any specialised tool or device.8.4.6. Use and maintenance requirements8.4.6.1. The type approval shall be made conditional, in accordance with Article 4(3), upon providing to each operator ofnon-road mobile machinery written instructions comprising the following:(a) detailed warnings, explaining possible malfunctions generated by incorrect operation, use or maintenance ofthe installed engine, accompanied by respective rectification measures;(b) detailed warnings on the incorrect use of the machine resulting in possible malfunctions of the engine,accompanied by respective rectification measures;(c) information on the correct use of the reagent, accompanied by an instruction on refilling the reagentbetween normal maintenance intervals;(d) a clear warning, that the type-approval certificate, issued for the type of engine concerned, is valid only whenall of the following conditions are met:(i) the engine is operated, used and maintained in accordance with the instructions provided;(ii) prompt action has been taken for rectifying incorrect operation, use or maintenance in accordance with the rectification measures indicated by the warnings referred to in point (a) and (b);(iii) no deliberate misuse of the engine has taken place, in particular deactivating or not maintaining an EGR or reagent dosing system.The instructions shall be written in a clear and non-technical manner using the same language as is used in the operator’s manual on non-road mobile machinery or engine.8.4.7. Reagent control (where applicable)8.4.7.1. The type approval shall be made conditional, in accordance with the provisions of section 3 of Article 4, uponproviding indicators or other appropriate means, according to the configuration of the non-road mobile machinery, informing the operator on:(a) the amount of reagent remaining in the reagent storage container and by an additional specific signal, whenthe remaining reagent is less than 10 % of the full container’s capacity;(b) when the reagent container becomes empty, or almost empty;(c) when the reagent in the storage tank does not comply with the characteristics declared and recorded insection 2.2.1.13 of Appendix 1 and section 2.2.1.13 of Appendix 3 to Annex II, according to the installedmeans of assessment.(d) when the dosing activity of the reagent is interrupted, in cases other than those executed by the engine ECUor the dosing controller, reacting to engine operating conditions where the dosing is not required, providedthat these operating conditions are made available to the type approval authority.8.4.7.2. By the choice of the manufacturer the requirements of reagent compliance with the declared characteristics andthe associated NO x emission tolerance shall be satisfied by one of the following means:(a) direct means, such as the use of a reagent quality sensor.(b) indirect means, such as the use of a NO x sensor in the exhaust to evaluate reagent effectiveness.(c) any other means, provided that its efficacy is at least equal to the one resulting by the use of the means ofpoints (a) or (b) and the main requirements of this section are maintained.’ANNEX IIAnnex II to Directive 97/68/EC is amended as follows:1. Section 2 of Appendix 1 is replaced by the following:POLLUTIONAIRAGAINSTTAKEN‘2. MEASURESyes/no(*)............................................................................................................gases:recyclingcrankcase2.1. Deviceforcoverednotbyheading)ifanother(ifanti-pollutiondevices2.2. Additionalandany,(*)yes/noconverter:2.2.1. Catalytic.......................................................................................................................................................................................2.2.1.1. Make(s):........................................................................................................................................................................................2.2.1.2. Type(s):converterselements................................................................................................................andcatalytic2.2.1.3. Numberofconverter(s):...............................................................................................thecatalyticofandvolume2.2.1.4. Dimensions-........................................................................................................................................................action:ofcatalytic2.2.1.5. Typeprecious........................................................................................................................................metals:of2.2.1.6. Totalchargeconcentration:...........................................................................................................................................................2.2.1.7. Relative.....................................................................................................................................material):and2.2.1.8. Substrate(structure...............................................................................................................................................................................2.2.1.9. Celldensity:2.2.1.10. Type of casing for the catalytic converter(s): .................................................................................................................2.2.1.11. Location of the catalytic converter(s) (place(s) and maximum/minimum distance(s) from engine): ............2.2.1.12. Normal operating range (K): ................................................................................................................................................2.2.1.13. Consumable reagent (where appropriate): .......................................................................................................................2.2.1.13.1. Type and concentration of reagent needed for catalytic action: .............................................................................2.2.1.13.2. Normal operational temperature range of reagent: ......................................................................................................2.2.1.13.3. International standard (where appropriate): ....................................................................................................................2.2.1.14. NO x sensor: yes/no (*)(*)yes/nosensor:2.2.2. Oxygen.......................................................................................................................................................................................2.2.2.1. Make(s):............................................................................................................................................................................................2.2.2.2. Type:.....................................................................................................................................................................................2.2.2.3. Location:(*)yes/noinjection:2.2.3. Airetc.):.........................................................................................................................................pump,2.2.3.1. Type(pulseair,air(*)yes/no2.2.4. EGR:etc.):pressure,........................................................................2.2.4.1. Characteristicspressure/low(cooled/uncooled,high(*)yes/no2.2.5. Particulatetrap:particulate.........................................................................................................thetrap:capacityof2.2.5.1. Dimensionsandparticulatetrap:.........................................................................................................................theandof2.2.5.2. Typedesignengine):..................................................................fromdistance(s)2.2.5.3. Locationand(place(s)maximum/minimumdescriptionand/ordrawing:regeneration,............................................................................ofor2.2.5.4. Methodsystempressure(kPa)and..................................................................................range:2.2.5.5. Normal(K)operatingtemperature(*)yes/nosystems:2.2.6. Otheroperation:...................................................................................................................................................and2.2.6.1. Description___________(*) Strike out what does not apply.’2. Section 2 of Appendix 3 is replaced by the following:POLLUTIONAGAINSTAIRTAKEN‘2. MEASURESyes/no(*)............................................................................................................gases:crankcase2.1. Deviceforrecyclingcoverednotbyheading)ifanotherany,anti-pollutiondevices(ifand2.2. Additional(*)yes/noconverter:2.2.1. Catalytic.......................................................................................................................................................................................2.2.1.1. Make(s):........................................................................................................................................................................................2.2.1.2. Type(s):and................................................................................................................converterselementscatalyticof2.2.1.3. Numberconverter(s):...............................................................................................thecatalyticofandvolume2.2.1.4. Dimensions-........................................................................................................................................................action:ofcatalytic2.2.1.5. Typeprecious........................................................................................................................................metals:of2.2.1.6. Totalchargeconcentration:...........................................................................................................................................................2.2.1.7. Relative.....................................................................................................................................material):and2.2.1.8. Substrate(structure...............................................................................................................................................................................2.2.1.9. Celldensity:2.2.1.10. Type of casing for the catalytic converter(s): .................................................................................................................2.2.1.11. Location of the catalytic converter(s) (place(s) and maximum/minimum distance(s) from engine): ............2.2.1.12. Normal operating range (K) .................................................................................................................................................2.2.1.13. Consumable reagent (where appropriate): .......................................................................................................................2.2.1.13.1. Type and concentration of reagent needed for catalytic action: .............................................................................2.2.1.13.2. Normal operational temperature range of reagent: ......................................................................................................2.2.1.13.3. International standard (where appropriate): ....................................................................................................................2.2.1.14. NO x sensor: yes/no (*)yes/no(*)sensor:2.2.2. Oxygen.......................................................................................................................................................................................2.2.2.1. Make(s):............................................................................................................................................................................................2.2.2.2. Type:.....................................................................................................................................................................................2.2.2.3. Location:(*)yes/noinjection:2.2.3. Airetc.):.........................................................................................................................................pump,2.2.3.1. Type(pulseair,air(*)yes/no2.2.4. EGR:etc.):pressure,........................................................................2.2.4.1. Characteristicspressure/low(cooled/uncooled,high(*)yes/no2.2.5. Particulatetrap:particulate.........................................................................................................thetrap:capacityof2.2.5.1. Dimensionsandparticulatetrap:.........................................................................................................................theandof2.2.5.2. Typedesignengine):..................................................................fromdistance(s)2.2.5.3. Locationand(place(s)maximum/minimumdescriptionand/ordrawing:regeneration,............................................................................ofor2.2.5.4. Methodsystempressure(kPa)and..................................................................................range:2.2.5.5. Normal(K)operatingtemperature(*)yes/nosystems:2.2.6. Otheroperation:...................................................................................................................................................and2.2.6.1. Description___________(*) Strike out what does not apply.’。
萨特利斯斯泰迪姆生物技术公司Biostat RM TX产品说明书

Engineered for Life –BIOSTAT ® RM TX with Flexsafe® RM TX for Culturing Consistent Quality Cellular ProductsThe fight against cancer has taken a dra-matic step forward in recent years with the development of cellular immunotherapies such as CAR-T cells. To produce these cells to a consistent quality, manufacturers face issues including: maintaining product sterility, protecting the cell product from adverse stress or environment and maxi-mizing cell yield with efficient processing. These can be achieved with gentle expan-sion and harvesting techniques, in process controls and the use of bioanalytics to ensure lot-to-lot consistency, characterize the cellular product, as well as utilizing rapid and robust lot release testing methods.Our Competence in Cell and Gene TherapySartorius Stedim Biotech is a global solution provider to the biologics industry and is well positioned to support regenerative medicine companies with our tried and trusted technologies for applications in this sector. Utilizing our strong expertise in single-use technology and biopharmaceutical automation, Sartorius supports the development, analysis and manufacture of various types of regenerative medicines, including cellular immunotherapies.Sartorius provides a wide range of plat-forms to address the unique challenges around the production of both allogeneic and autologous cells.Solutions for Cellular ImmunotherapiesBIOSTAT ® RM TX bioreactor system and Flexsafe® RM TX bags –The Ideal Combination for Your CellsThe BIOSTAT ® RM TX and Flexsafe® RM TX bag combination provides you with an automated, wave-mixed and closed environment suitable for optimal growth of cell products in working volumes up to 5 L.The BIOSTAT ® RM TX system consists ofan automated control unit (based on our well-established BIOSTAT ® B) and a rocking platform, for gently agitating a single-use Flexsafe® RM TX bag. Enabling the ex vivo expansion of patient-specific T cells or other types of immune cells, the BIOSTAT ® RM TX is suitable for process development, as well as for the expansion of relevant cell numbers. Fed-batch, perfusion processes or a combination of culture modes are all possible with this system. You Can Rely on:– P roven industry leading Flexsafe® RM TX film that supports consistent cell growth – C losed system for minimal contamination risk– U nique gravity harvesting for maximizing cell recovery– A dvanced, automated system for walk-away monitoring and control of the cell culture including online biomass– P roven rocking motion platform for optimal cell growthBIOSTAT ® RM TX and Flexsafe® RM TX bags are for research use or further manufacturing use only – not for use in therapeutic or diagnostic procedures. They are not CE marked for in vitro diagnostic use nor are they medical devices. Drug manufacturers and clinicians are responsible for obtaining the appropriate IND | BLA | NDA approvals for clinicalapplications.The Right Solution for Your NeedsAt Sartorius, we help you develop a scalable, cost-effective process and combine this with the security of our worldwide supply chain and manufacturing capabilities. Product development in close cooperation with external industry partners guarantees the reliability of your equipment. Our expertise and experience allow us to provide you with a proven product portfolio to support early stage process development and establish scalable processes.Minimize Impact of Single-Use Material Flexsafe ® RM TX bags were introduced in June 2014 and have been used with con-tinuing success ever since by major global biopharma and cell therapy customers. The complete control of our raw materials, the extrusion process and the bag assembly, provides lot-to-lot product consistency. In collaboration with our resin and film suppliers, we have optimized the resin and minimized the additives in our Flexsafe ® film technology* ensuring excellent, reproducible batch-to-batch culture performance of even the most sensitive cells.**Protect Your Cell Product– A perfusion membrane (PES, 1.2 µm) is fixed at the bottom of the bag, forming a compartment for removal of cell free media during the perfusion process – for minimal loss or damage to the cells – S ingle-use sensors for pH, DO and viable biomass enable sophisticated process control with reduced sampling need – 100% integrity tested, gamma-irradiat-able and fully validated Sartopore ® Air sterile filters continuously protect the culture from contamination– I ndustry standard tubing option(DEHP free PVC) for seamless connection to up- & downstream processes* I ndependent labs have confirmed that Flexsafe ® bags are free of cytotoxic leachables. No bDtBPP is identified in WFI extracts of Flexsafe ® bags ** F enge et al. 2014. Consistently Superior Cell Growth: Achieved with New Polyethylene Film Formulation. Bioprocess International, Volume 12 Suppl 5.Zero Slipping Agents & Nontoxic Mechanical Antiblocking k N o risk of interference due to these agents*Optimize Cell GrowthRobust & Consistent Manufacturing Our BIOSTAT ® B control unit is ideal for walk-away automated analysis and control of high cell density perfusion cultures. Benefit from:– A dvanced control and monitoring of gas mixture and flow rate, filling volume and substrate addition; parallel activation of multiple controllers provides maximum flexibility– O n-line viable biomass analysis with culture volumes greater than 500 mL – U p to 4 internal pumps can be integrated into control loops for ease of operation without the need to constantly change the pumps' function.– E asy connection to industry standard Distributed Control (DCS) or Supervisory Control and Data Acquisition (SCADA) systems such as BioPAT ® MFCS, Siemens PCS 7 or Emerson DeltaV™.Straightforward integration into existing automation and single-use infrastructure for data and process consistency throughout– C omplete qualification of the system for GMP use to support regulatory complianceMaximize Cell YieldConventional harvesting procedures mostly use pumping which can reduce the number of live cells and affect cell viability. Since cell yield is critical for autologous cell therapies, we have designed our Flexsafe ® RM TX bags with a special port for hands-free gravity harvesting. This unique gravity harvesting concept in combination with the Flexsafe ® RM TX Harvest Device allows the safe recovery of as many cells as possible by reducing shear stress on these delicate cells and minimizes contaminationrisks from manual handling.* Patent pending.Viable biomass sensor (BioPAT ® ViaMass) integrated in the Flexsafe ® RM bag – connection to the hard-ware componentEfficient Cell ProcessingFlexsafe ® RM TX Harvest Device for hands-free gravity harvest of the cell culture with maximum recovery.BIOSTAT ® RM TXThe BIOSTAT ® RM TX system in combination with Flexsafe® RM TX bags support theculturing of consistent quality cells and is perfect for small volume autologousprocesses with multi-parallel scale out needs. Using this system, one Flexsafe® RM TX bagcan be controlled and monitored via the BIOSTAT ® B control unit. For scale-out, twoFlexsafe® RM TX bags and two separate rocking platforms can be attached to a twinBIOSTAT ® B control unit.Single | Twin ConfigurationOne controller can run up to twoBIOSTAT ® RM TX completely independentlyto save valuable lab space.12” Touch ScreenEasy-to-use and reliable operation of yourBIOSTAT ® RM TX system due to intuitivedesign of human-machine interface andadvanced touch-screen technology – evenwhile wearing laboratory gloves. IntegratedContains aeration, pumps and temperaturecontrol modules for various applicationneeds. The BioPAT® MFCS multi fermentercontrol system ensures reliable datamanagement and automation.Temperature ControlTogether with the control towerthe integrated temperature controlis optimized for small working volumesand perfusion membrane bags.Easy to UseTwo flap door magnetic lid concept forconvenient access to bag and filters.Handles allow for easy transport.Status LED – full control via DCU tower.Load CellsThe integrated precise load cells are idealfor small volume perfusion processes.The BioPAT® MFCS multi fermentercontrol system or third party SCADAsystem integration (DeltaV™) ensuresreliable data management andautomation.Protects operator & tubing frommechanical hazards of moving tray.Flexsafe ®RM TX BagsDifferent sizes of Flexsafe ® RM bags: 1 L, 2 L and 10 L total volume can be used with the BIOSTAT ® RM TX rocking platform, providing a working volume of up to 5 L. The Flexsafe ® RM TX bag has been intelligently designed with features including a special port for gravity harvesting and an internal cell retention membrane, making it ideal for perfusion culture of cellular products such as CAR-T cells.Vent FiltersSartopore ® Air filters are 100% integrity tested before gamma irradiation for improved process safety.BioPAT ®ViaMassIntegrated sensor for online biomass determination and reduced sampling need.*Perfusion MembraneIntegrated 1.2 membrane for secure cell retention during perfusion processes. No fouling and reduced shear as wave constantly flushes over the bottom fixed filter*.FilmIndustry leading proprietary Flexsafe ® film for optimal cell growth of most sensitive cells.Special harvest port for hands-freegravity harvesting. PVC tubing for seamless connectability to upstream and down-stream processes.Single use sensors for advanced process control. No cell accumulation as sensors are inserted from top into the liquid and constantly flushed.* perfusion bag design protected by patents US 9 017 997 B2 and EP 2 268 788 B1Cellular Immunotherapy ProcessesSartorius provides a wide range of single-use technologies. Our portfolio supports viral vector transduction, cell expansion and downstream processing steps including harvest, wash and concentration of cells.AnalyticsSartorius provides various analytical technologies that monitor and control your product during the entire manufacturing process.BIOSTAT STR Flexsafe ® 2D Bags– S ingle-use Flexsafe ® bags for media storage coupled with Flexsafe ® pre-designed solutions for sterile filtration, storage and transfer of media and buffers– P roven integrity to enhance process and product safety by reducing risks of contamination of valuable cell products BioPAT ® MFCS– W orld standard for supervisory process control with GAMP category 4 software package BIOSTAT STR ®– S calable, single-use bioreactor family based on stirred-tank design – W ide range of sizes (12.5 L to 2000 L working volume) and process regimes for flexible manufacturing kSep ® Centrifuge– C losed seal-less single-use fluidized bed centrifugation platform– T he opposing centrifugal and fluid flow mechanism provides low shear force which is ideal for wash & harvest of sensitive cells Biowelder ® TC– A utomated welder for sterile connection of dry or liquid filled thermoplastic tubing to support a functionally closed processProcessiQue ® Screener PLUS Platform Virus Counter ® 3100BioPAT ® Trace*Alternative:Sartorius Transfer SetsMicrosart® ATMP Mycoplasmaand Bacteria KitsCharacterization & CellBanking Services BioPAT® ViaMassService Level Agreement: All-Inclusive Coverage for Maximum Process SecurityOur Comprehensive Service Level Agreement offers the highest level of protection for your critical process equipment. Experience our worry-free contract support including our quickest reaction times and full cost coverage, in addition to the planned preventa-tive maintenance. Benefit from our technical helpdesk response within 4 hours and on-site response within 48 hours.We are working closely with customers to fully understand their needs, so we can help them address these during the early phase of their process development.We apply innovative design approaches to new product developments and test early so there is the opportunity to influence and adjust the scope.We hear what our customers tell us and are committed to serve their needs in the best possible way from start to end of the manufacturing process.Technical helpdesk response within 4 hours and on-site response within 48 hoursReaction Time Commitment:Sartorius as Your Partner for Cell and Gene Therapy ManufacturingTechnical Specifications BIOSTAT ® RM TXPower Supply (Country Specific) | Frequency | Electricity Consumption | Protection Class Rocker platform 230 V | 50 Hz | 1.3 A | IP23or120 V | 60 Hz | 2.5 A | IP23Control tower 230 V | 50 Hz | 10 A | IP21or120 V | 60 Hz | 12 A | IP21Load cells Integrated in rocker Gas Supply via BIOSTAT B Tower Inlet pressure (barg)1.5Connection hose coupling, externalHose barb for tubing with 6 mm IDGas Specification According to ISO 8573-1: dry, free of oil and dust Particle size: < 0.1 mm •Max. amount 0.1 mg/m3 (class 1) •Condensate: dew point < 3°C (class 4)•Oil < 0.01 mg/m3 (class 1) •Germs (class 0)•Operative EnvironmentAmbient temperature of between 5 – 40°CRelative humidity [%]< 80% for temperatures up to 31 °C (87.8 °F),decreasing linearly < 50% at 40 °C (104 °F)Facility and Utility RequirementsTotal Volume1 L2 L 10 L Working volume [L]*0.1 – 0.50.2 – 1 1 – 5Basic Bags for cultivations under constant conditions •••Optical Bags with SU pH & DO sensors••Perfusion Membrane Bags with SU pH & DO ••Integrated Viamass Sensor*••Flexsafe RM TX Design**•Applicable Bag Sizes and Designs* B ags with sensors might require higher minimum working volumes depending on rocking rate and angle. We recommend using 20 % of the total volume as the minimum working volume. ** i ncl. Sartopore Air Midisart vent filters, harvest port for gravity harvest, Press-In Plugs, PVC or C-Flex tubingTemperature Module Temperature controlHeating only – electrical heating plates Temperature control rangeambient temperature + 5°C to 40°C (min. set point 15°C , min. controllable temp = ambient temp. + 15°C)Temperature measurement 2°C to 50°C Temperature control accuracy (excl. measurement error)±0.2°CHeating capacity1 × 120 W (24 VDC)Over temperature protection •Gassing Module Control Tower 4-Gas mix (O 2, N 2, CO 2, air) with headspace outlet MFC– flow rates – accuracymax. 40.003 lpm – 5 lpm ± 1% full scale Advanced DO controller •Sensors & Measurement Temperature probe Pt 100•– temperature range Pt 1000 – 99°C – display resolution 0.1°C– amplifiers 1 (single) | 2 (twin)pH single use•– measurement range 6.5 – 8.5– display resolution 0.1 pH– amplifiers1 (single) |2 (twin)– recalibration function •DO single-use•– measurement range 0 – 250%– display resolution 0.1%– amplifiers1 (single) |2 (twin)– recalibration function•Process ControlDimensions W + D + H Weight MaterialBIOSTAT ® B control Tower Single | Twin 410 × 520 × 810 mm 16 × 20 × 32 in 40 | 55 kg 88 | 121 lbs Stainless steel AISI 304BIOSTAT ® RM TX Rocker complete 439 × 602 × 561 mm 17 × 24 × 22 in 35 kg 77 lbs Stainless steel, ABS Bag holder TX 430 × 602 × 86 mm 17 × 24 × 3.4 in 5.5 kg 12.1 lbs Stainless steel, ABS Lid TX430 × 602 × 495 mm 17 × 24 × 20 in 2.5 kg 5.5 lbsABS Lab-cart (optional)800 × 800 × 900 mm32 × 32 × 36 in 88 kg 194 lbsStainless steelSystem CharacteristicsSensors & MeasurementSingle-use viable biomass(BioPAT® ViaMass)Optional Integrated load cells•Media weight control range0 to 5 kg- Scale, absolute accuracy Static:± (10 + 0% of load) gDynamic:± (25 + 0% of load) g - Scale, relative accuracy Static:±3 gDynamic:±5 g*Resolution (DCU) 1 gExternal signal input max. 20 – 10 V or 4 – 20 mA Pump Module | Built-in PumpsWatson Marlow 114, fast load pump headFixed Speed for Base Addition / pHControl– Speed 5 rpmFlow rate (tubing wall thickness 1.6 mm)ID: 0.5 mm: 0 – 0.1 ml/min ID: 0.8 mm: 0.05 – 2.4 ml/min ID: 1.6 mm: 0.01 – 0.7 ml/min ID: 2.4 mm: 0.03 – 1.5 ml/min ID: 3.2 mm: 0.05 – 2.4 ml/min ID: 4.8 mm: 0.09 – 4.3 ml/minSpeed Controlled for Feed Addition– Speed 5 – 150 rpmFlow rate (tubing wall thickness 1.6 mm)ID: 0.5 mm: 0.1 – 3 ml/min ID: 0.8 mm: 0.2 – 6 ml/min ID: 1.6 mm: 0.7 – 21 ml/min ID: 2.4 mm: 1.45 – 43.5 ml/min ID: 3.2 mm: 2.35 – 70.5 ml/min ID: 4.8 mm: 4.25 – 127.5 ml/min* D ynamic weight measurement (while rocking) can be influenced by cables and tubing and interferences caused by the same.The BIOSTAT ® RM TX system is designed to communicate with industrial SCADA or DCS systems (e.g. DeltaV) through the Modbus TCP/IP protocol.Temperature Module Max. total volume (L)10 Max. working volume (L)5Rocking speed control range [rpm] 2 – 42 rpm ±1Rocking angle control range (°) 2 – 12 ± 0.3Clamping rails for bag fixation •Sensor clamps for secure fixation of glass fiber cables •Filter heater(2 variants: for std. Hepa filter or for Midisart Sartopore Air)•Safety measurement and shut-off 30 mbar Additional safety valve gasses (mbar)100 mbarWater inlet pressure reduction value 1.5 bar, integrated pressure control Different user level log in (•)Logbook function(•)Lab-cart for BIOSTAT ® B Control TowerSeparately available on requestTechnical DataCommunicationSales and Service Contacts For further contacts, visit EuropeGermanySartorius Stedim Biotech GmbH August-Spindler-Strasse 11 37079 GoettingenPhone +49.551.308.0Sartorius Stedim Systems GmbH Robert-Bosch-Strasse 5 – 7 34302 GuxhagenPhone +49.5665.407.0FranceSartorius Stedim FMT S.A.S.ZI des PaludsAvenue de Jouques – CS 91051 13781 Aubagne Cedex Phone +33.442.845600 Sartorius Stedim France SASZI des PaludsAvenue de Jouques – CS 71058 13781 Aubagne Cedex Phone +33.442.845600 AustriaSartorius Stedim Austria GmbH Modecenterstrasse 221030 ViennaPhone +43.1.7965763.18BelgiumSartorius Stedim Belgium N.V. Rue Colonel Bourg 1051030 BruxellesPhone +32.2.756.06.80HungarySartorius Stedim Hungária Kft. Kagyló u. 52092 BudakesziPhone +36.23.457.227ItalySartorius Stedim Italy S.r.l.Via dell’Antella, 76/A50012 Antella-Bagno a Ripoli (FI)Phone +39.055.63.40.41NetherlandsSartorius Stedim Netherlands B.V.Phone +31.30.60.25.080****************************************PolandSartorius Stedim Poland Sp. z o.o.ul. Wrzesinska 7062-025 KostrzynPhone +48.61.647.38.40Russian FederationLLC “Sartorius Stedim RUS”Vasilyevsky Island5th line 70, Lit. A199178 St. PetersburgPhone +7.812.327.53.27SpainSartorius Stedim Spain, S.A.U.Avda. de la Industria, 32Edificio PAYMA28108 Alcobendas (Madrid)Phone +34.913.586.098SwitzerlandSartorius Stedim Switzerland AGRingstrasse 24 a8317 TagelswangenPhone +41.52.354.36.36U.K.Sartorius Stedim UK Ltd.Longmead Business CentreBlenheim Road, EpsomSurrey KT19 9 QQPhone +44.1372.737159UkraineLLC “Sartorius Stedim RUS”Post Box 440 “B”01001 Kiev, UkrainePhone +380.44.411.4918AmericasUSASartorius Stedim North America Inc.5 Orville Drive, Suite 200Bohemia, NY 11716Toll-Free +1.800.368.7178ArgentinaSartorius Argentina S.A.Int. A. Ávalos 4251B1605ECS MunroBuenos AiresPhone +54.11.4721.0505BrazilSartorius do Brasil LtdaAvenida Senador Vergueiro 2962São Bernardo do CampoCEP 09600-000 - SP- BrasilPhone +55.11.4362.8900MexicoSartorius de México, S.A. de C.V.Libramiento Norte de Tepotzotlan s/n,Colonia Barrio Tlacateco,Municipio de Tepotzotlan,Estado de México,C.P. 54605Phone +52.55.5562.1102**********************Asia|PacificAustraliaSartorius Stedim Australia Pty. Ltd.Unit 5, 7-11 Rodeo DriveDandenong South Vic 3175Phone +61.3.8762.1800ChinaSartorius Stedim (Shanghai)Trading Co., Ltd.3rd Floor, North Wing, Tower 1No. 4560 Jinke RoadZhangjiang Hi-Tech ParkPudong DistrictShanghai 201210, P.R. ChinaPhone +86.21.6878.2300Sartorius Stedim (Shanghai)Trading Co., Ltd.Beijing Branch OfficeNo. 33 Yu’an RoadAirport Industrial Park Zone BShunyi District, Beijing 101300Phone +86.10.8042.6501Sartorius Stedim (Shanghai)Trading Co., Ltd.Guangzhou Branch OfficeRoom 1105Xing Guang Ying Jing BuildingNo. 119, Shui Yin RoadYue Xiu District, Guangzhou 510075Phone +86.20.3836.4193IndiaSartorius Stedim India Pvt. Ltd.#69/2-69/3, NH 48, JakkasandraNelamangala Tq562 123 Bangalore, IndiaPhone +91.80.4350.5250JapanSartorius Stedim Japan K.K.4th Fl., Daiwa Shinagawa North Bldg.8-11, Kita-Shinagawa 1-chomeShinagawa-ku, Tokyo, 140-0001 JapanPhone +81.3.4331.4300MalaysiaSartorius Stedim Malaysia Sdn. Bhd.Lot L3-E-3B, Enterprise 4Technology Park MalaysiaBukit Jalil57000 Kuala Lumpur, MalaysiaPhone +60.3.8996.0622SingaporeSartorius Stedim Singapore Pte. Ltd.10 Science Park RdThe Alpha #02-13/14Singapore Science Park IISingapore 117684Phone +65.6872.3966South KoreaSartorius Korea Biotech Co., Ltd.8th Floor, Solid Space B/D,PanGyoYeok-Ro 220, BunDang-GuSeongNam-Si, GyeongGi-Do, 463-400Phone +82.31.622.5700 S p e c i f icationssubjecttochangewithoutnotice.CopyrightSartoriusStedimBiotechGmbH.PrintedintheEUonpaperbleachedwithoutchlorine.Version1/219/1。
制作非牛顿流体的英语作文

制作非牛顿流体的英语作文英文回答:Non-Newtonian fluids are fluids that do not follow the traditional Newtonian model of viscosity. Newtonian fluids, such as water, have a constant viscosity that is independent of the shear rate. In contrast, non-Newtonian fluids exhibit a change in viscosity when subjected to an applied force. This unique property gives rise to a wide range of fascinating behaviors, from the ability to flow like a liquid under low shear to acting like a solid under high shear.Non-Newtonian fluids are classified into several types based on their flow behavior. Some common types include:Shear-thickening fluids: These fluids increase in viscosity when subjected to higher shear rates. Examples of shear-thickening fluids include cornstarch suspensions and ketchup.Shear-thinning fluids: These fluids decrease in viscosity when subjected to higher shear rates. Examples of shear-thinning fluids include honey and blood.Thixotropic fluids: These fluids exhibit time-dependent changes in viscosity. When at rest, they behave like a gel, but when subjected to shear, they graduallythin out. Examples of thixotropic fluids include mayonnaise and toothpastes.Rheopectic fluids: These fluids exhibit the opposite behavior of thixotropic fluids, becoming thicker with time. Examples of rheopectic fluids are yogurt and custard.Non-Newtonian fluids have numerous applications in various fields. For instance, shear-thickening fluids are used as body armor and in anti-vibration devices. Shear-thinning fluids find applications in paints, lubricants, and cosmetics. Thixotropic fluids are commonly used in food products, adhesives, and paints.中文回答:非牛顿流体是不遵循牛顿粘性模型的流体。
抹香鲸呼吸 英语作文

抹香鲸呼吸英语作文Title: The Breath of the Sperm Whale: A Unique Physiology of Respiration.The sperm whale, a majestic creature of the deep seas,is renowned for its immense size, powerful jaws, and captivating social behaviors. However, it is its unique respiratory system that truly sets it apart from other cetaceans. This essay delves into the fascinating world of sperm whale respiration, exploring the anatomy, physiology, and adaptations that allow this remarkable mammal to thrive in its aquatic habitat.The sperm whale's respiratory system is remarkable for its efficiency and adaptability. Unlike terrestrial mammals, which rely on lungs to extract oxygen from the air, the sperm whale has evolved to extract oxygen from water. This adaptation is crucial for survival in the deep sea, whereair pockets are scarce and water is the primary source of oxygen.The sperm whale's respiratory system begins with its nostrils, which are located on the top of its head in apair of slits known as "blowholes." When the whale needs to breathe, it rises to the surface, opening its blowholes to allow air to flow into its lungs. This process is remarkable for its efficiency; the whale can inhale and exhale in just a few seconds, ensuring that it spends as little time as possible at the surface, where it is vulnerable to predators.Once air enters the lungs, the sperm whale'srespiratory system takes on an even more remarkable feat. Unlike most mammals, which have alveoli in their lungs to increase the surface area for oxygen exchange, the sperm whale's lungs are filled with spongy tissue. This tissue is rich in blood vessels, allowing for efficient oxygen and carbon dioxide exchange even in the absence of alveoli. This unique anatomy allows the whale to extract oxygen from water even while diving deep, where oxygen levels are significantly lower than at the surface.In addition to its anatomical adaptations, the sperm whale's respiratory system is also physiologically flexible. The whale can adjust its breathing rate and depth according to its needs, allowing it to conserve energy while cruising at depth or to increase oxygen intake during periods ofhigh activity. This flexibility is crucial for survival in the demanding environment of the deep sea.Moreover, the sperm whale's respiratory system is intricately connected to its social and reproductive behaviors. Pods of sperm whales often synchronize their breathing patterns, rising to the surface together to breathe and then descending back into the depths. This synchronized breathing is thought to enhance social bonding within the pod and may also serve as a communication mechanism, allowing whales to stay in touch even in the dark, deep sea.In conclusion, the sperm whale's respiratory system isa marvel of evolutionary adaptation and physiological efficiency. Its unique anatomy and physiology allow it to thrive in the oxygen-poor environment of the deep sea,extracting oxygen from water and adjusting its breathing patterns to meet the demands of its lifestyle. This remarkable system is not only crucial for the whale's survival but also serves as a fascinating window into the world of cetacean biology and evolution. As we continue to explore the mysteries of the deep sea, the sperm whale's respiratory system remains a fascinating and essential component of our understanding of this remarkable mammal and its remarkable habitat.。
AS 1171-1998

AS1171—1998Australian Standard™Non-destructive testing—Magnetic particle testingof ferromagnetic products, components and structures Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., .Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., .This Australian Standard was prepared by Committee MT/7,Non-destructiveTesting of Metals and Materials.It was approved on behalf of the Council ofStandards Australia on29May1998and published on5September1998.The following interests are represented on Committee MT/7:Australasian Railway AssociationAustralian Aerospace Non-destructive Testing CommitteeAustralian Institute for Non-Destructive TestingAustralian Nuclear Science and Technology OrganisationAustralian Pipeline Industry AssociationBureau of Steel Manufacturers of AustraliaElectricity Supply Association of AustraliaIndustrial Research Limited New ZealandInstitution of Engineers AustraliaMetal Trades Industry Association of AustraliaNational Association of Testing Authorities,AustraliaNew Zealand Non-destructive Testing AssociationSociety of Automotive Engineers,AustralasiaVictorian WorkCover AuthorityWelding Technology Institute of AustraliaWorkCover New South WalesReview of Australian Standards.To keep abreast of progress in industry,Australian Standards aresubject to periodic review and are kept up to date by the issue of amendments or new editions asnecessary.It is important therefore that Standards users ensure that they are in possession of the latestedition,and any amendments thereto.Full details of all Australian Standards and related publications will be found in the Standards AustraliaCatalogue of Publications;this information is supplemented each month by the magazine‘TheAustralian Standard’,which subscribing members receive,and which gives details of new publications,new editions and amendments,and of withdrawn Standards.Suggestions for improvements to Australian Standards,addressed to the head office of StandardsAustralia,are welcomed.Notification of any inaccuracy or ambiguity found in an Australian Standardshould be made without delay in order that the matter may be investigated and appropriate action taken.This Standard was issued in draft form for comment as DR96526.Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., .AS1171—1998Australian Standard™Non-destructive testing—Magnetic particle testingof ferromagnetic products,structurescomponents andPublished by Standards Australia(Standards Association of Australia)1The Crescent,Homebush,NSW2140AS1171—19982PREFACEThis Standard was prepared by the Joint Standards Australia/Standards New ZealandCommittee MT/7,Non-destructive Testing of Metals and Materials,to supersedeAS1171—1976,Methods for magnetic particle testing of ferromagnetic products andcomponents.This Standard is the result of a consensus among Australian and New Zealandrepresentatives on the Joint Committee to produce it as an Australian Standard.The objective of this revision is to upgrade the requirements for the magnetic particletesting of ferromagnetic materials for the detection of surface and near-surfacediscontinuities.In preparing this Standard,the Committee took cognizance of BS6072:1981,Method formagnetic particle flaw detection,and ASTM E1444—94a,Practice for magnetic particleexamination.This Standard now refers to ASTM E1444for the requirements formagnetic particle testing media instead of AS2085—1977,Magnetic particle testingmedia.Currently there are no International(ISO)Standards that cover the general practice ofmagnetic particle testing.The terms‘normative’and‘informative’have been used in this Standard to define theapplication of the appendix to which they apply.A‘normative’appendix is an integralpart of a Standard,whereas an‘informative’appendix is only for information and guidance.Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., .Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., .3AS1171—1998CONTENTSPageSECTION1SCOPE AND GENERAL1.1SCOPE (4)1.2REFERENCED DOCUMENTS (4)1.3DEFINITIONS (4)1.4PRINCIPLE OF TEST METHOD (5)1.5WRITTEN PROCEDURE REQUIREMENTS (5)1.6SAFETY (5)1.7TESTING PERSONNEL (6)SECTION2EQUIPMENT AND MATERIALS2.1GENERAL (7)2.2REQUIREMENTS FOR MAGNETIZING,DEMAGNETIZINGAND AUXILIARY EQUIPMENT (7)2.3REQUIREMENTS FOR MAGNETIC POWDERS AND INKS (8)SECTION3METHODS OF TEST3.1SCOPE (9)3.2PREPARATION OF TEST SURFACE (9)3.3MAGNETIZATION (9)3.4MAGNETIZING METHODS (10)3.5INSPECTION (15)3.6MARKING THE LOCATION OF DISCONTINUITIES (15)3.7DEMAGNETIZATION (16)3.8POST-TEST CLEANING (16)SECTION4PROCESS CONTROL PROCEDURES AND REQUIREMENTS4.1GENERAL (23)4.2CONTROL PROCEDURES (23)4.3PROCESS CONTROL RECORDS (25)SECTION5TEST RECORDS AND REPORTS5.1TEST RECORD (26)5.2TEST REPORT (26)APPENDICESA PURCHASING GUIDELINES (27)B PREPARATION AND USE OF STANDARD TEST PIECES FORCHECKING TEST EQUIPMENT AND SYSTEM OPERATION (28)C REQUIREMENTS FOR CONDUCTING MAGNETIC PARTICLEEXAMINATIONS ON PAINTED SURFACES (31)D GENERAL INFORMATION ON TEST METHODS FOR DETERMININGFLUX DENSITY AND MAGNETIZING CURRENT LEVELS (32)E CURRENT WAVEFORMS AND CONVERSION FACTORS (36)F GUIDANCE ON THE USE OF COIL MAGNETIZING METHODSEMPLOYING PARALLEL CONDUCTORS (37)Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., . AS1171—19984STANDARDS AUSTRALIAAustralian StandardNon-destructive testing—Magnetic particle testingof ferromagnetic products,components and structuresS E C T I O N1S C O P E A N D G E N E R A L1.1SCOPE This Standard specifies requirements for magnetic particle testing for thedetection of surface and near-surface discontinuities in all types of ferromagneticproducts,components and structures.It also specifies requirements for magnetic particletesting process control.Requirements for materials(media)are specified inASTM E1444.NOTES:1Advice and recommendations on information to be supplied by the purchaser at the time of enquiry and order are given in Appendix A.2This Standard does not indicate the method to be used for the testing of any particular product.The method and the appropriate acceptance/rejection criteria should be specified inthe relevant product Standard or application code.3The unit symbols used in this Standard are defined in AS1000.1.2REFERENCED DOCUMENTS The following documents are referred to in thisStandard:AS1000The International System of Units(SI)and its application1239Steel—Schedule of tool steel compositions1442Carbon steels and carbon-manganese steels—Hot-rolled bars and semifinishedproducts1929Non-destructive testing—Glossary of terms2536Surface texture3000Electrical installations—Buildings,structures and premises(known as the SAAWiring Rules)3669Non-destructive testing—Qualification and registration of personnel—Aerospace3998Non-destructive testing—Qualification and certification of personnel—GeneralengineeringAS/NZS3100Approval and test specification—General requirements for electrical equipmentASTME1444Practice for magnetic particle examinationBS5044Specification for contrast aid paints used in magnetic particle flaw detection1.3DEFINITIONS For the purpose of this Standard,the definitions given in AS1929apply.NOTE:The term‘product’in this Standard is taken to include component,test piece,workpiece or structure.Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., .5AS1171—19981.4PRINCIPLE OF TEST METHOD Ferromagnetic materials are magnetized,usingelectrical currents or permanent magnets,to a level where the magnetic field becomesdistorted by surface and near-surface discontinuities,causing local flux leakage fields.Finely divided particles of ferro-or ferri-magnetic materials,applied as a powder or in acarrier fluid,are attracted to these flux leakage fields and accumulate to indicate thepresence of discontinuities.1.5WRITTEN PROCEDURE REQUIREMENTS All magnetic particle inspectionsshall be performed to a specific written procedure that implements the requirements ofthis Standard for the components under test.A master written procedure may be utilizedto cover the requirements common to a variety of similar components.As a minimum,thefollowing information,where relevant,shall be included in individual procedures,amaster procedure,or a combination thereof:(a)Company name and address.(b)Description and identity of the component.(c)Written procedure identification number and date of issue.(d)Material specification or type.(e)A sketch of the component showing its main dimensions and the area to be tested.(f)Surface condition at the time of testing,including the thickness and uniformity ofany coating present.(g)Purpose of the test.(h)The manufacturing or overhaul stage at which the component is to be tested.(i)Magnetizing technique to be used,including waveform and current value.(j)The method of application of the indicating medium.(k)Technique,i.e.whether continuous or residual.(l)Equipment to be used.(m)Distance between contact areas,or coil dimensions.(n)Detecting media to be used.(o)Contrast aids to be used.(p)Viewing conditions.(q)Demagnetization procedure.(r)Method of reporting results.(s)Acceptance/rejection criteria,if applicable.(t)Minimum qualification of test operator.(u)Identity of person responsible for the procedure.(v)Reporting requirements.1.6SAFETY1.6.1General As magnetic particle testing methods may require the use of toxic,flammable and volatile materials,safety precautions shall be observed and testing shall becarried out in well-ventilated areas remote from heat and naked flame.NOTE:Safety data sheets are available on request from suppliers of consumable materials andshould be consulted to determine the hazards to personnel.AS1171—199861.6.2Fire hazards As the current flow methods described in this Standard require theuse of high levels of current,it is important to ensure that any occurrence of overheatingand arcing will not ignite flammable vapours that may be present when a carrier fluidcontaining magnetic particles is used.CAUTION:ENSURE THAT ALL ELECTRICAL EQUIPMENT IS PROPERLYMAINTAINED AND THAT FIRE SAFETY PRECAUTIONS ARE OBSERVED.KEEPMATERIALS AND AUXILIARY EQUIPMENT AWAY FROM HEAT AND NAKEDFLAMES.THE USE OF HIGH INTENSITY LAMPS OF BOTH BLACK AND WHITE LIGHT CANIGNITE EXPLOSIVE GAS MIXTURES.ENSURE HAZARDOUS AREAS ARE WELLVENTILATED.1.6.3Electrical safety Electrical equipment used shall comply with the requirementsof AS/NZS3100and shall be wired to comply with the requirements of AS3000.NOTE:Magnetic fields resulting from the use of high current may affect the functioning ofheart pacemakers.1.6.4Toxic materials Magnetic particle testing media shall be used with caution andalways in accordance with the manufacturer’s printed instructions.WARNING:PROVIDE ADEQUATE VENTILATION TO ENSURE THE REMOVALOF VAPOURS AND AIRBORNE PARTICLES GENERATED WHERE TOXICMATERIALS ARE USED IN CONFINED SPACES.Testing personnel shall use appropriate protective equipment to prevent skin contact andeye exposure to toxic liquids and the inhalation of fumes.1.6.5The use of black lights A black light should never be used when a filter glass iscracked or broken,as ultraviolet radiation harmful to the eyes may be emitted.1.7TESTING PERSONNEL The effectiveness of magnetic particle testing dependson the technical competence of the personnel performing the tests and on their ability tointerpret indications.The responsibility for the nomination of acceptance/rejection criteriadoes not lie with the testing authority.Personnel who perform testing to this Standard shall have appropriate qualifications in thespecific area of test and shall meet the visual acuity requirements of a relevant nationalStandard.NOTES:1The Australian Standards for qualification of personnel who perform non-destructive testing are AS3669and anizations responsible for personnel certification are theAustralian Institute for Non-Destructive Testing(AINDT)and the Certification Board forInspection Personnel(CBIP),New Zealand.2The accreditation of testing laboratories is carried out in Australia by the National Association of Testing Authorities(NATA),and in New Zealand,by International Accreditation New Zealand(IANZ).Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., .Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., .7AS1171—1998S E C T I O N2E Q U I P M E N T A N D M A T E R I A L S2.1GENERAL The test equipment shall be capable of inducing magnetic flux in theproduct under test,in accordance with the requirements of the applicable test procedure.The magnetic field employed for this purpose may be generated from any source that iscapable of producing the required flux density at the surface under test.2.2REQUIREMENTS FOR MAGNETIZING,DEMAGNETIZING ANDAUXILIARY EQUIPMENT2.2.1Bench-type magnetizing equipment(incorporating a reservoir containingmagnetic ink)Bench-type current flow,magnetizing,demagnetizing and associatedequipment shall—(a)when designed either for continuous or stepped current adjustment,be capable ofproducing current values that are within±10%of the current setting or within±50A,whichever is the greater,over the range from zero to the maximum currentvalue;(b)include a calibrated ammeter to indicate the current output;(c)be capable of meeting the expected current demand;(d)have,indicated on or near the ammeter,the nominal waveform of the magnetizingcurrent together with the current parameter,e.g.peak,r.m.s.,mean,or mean of theconducting half cycle;and(e)have a means to ensure that current-carrying contacts make firm electrical contactwith the test part to prevent arcing or mechanical damage.2.2.2Permanent magnets and d.c.electromagnets Unless otherwise specified,permanent magnets and d.c.electromagnets(d.c.yokes)shall be capable of lifting not lessthan18kg of mild steel at a pole spacing of between75mm and300mm.2.2.3 a.c.electromagnets Unless otherwise specified, a.c.electromagnets(a.c.yokes)shall be capable of lifting not less than4.5kg of mild steel at a pole spacing of between75mm and300mm.NOTES:1As the strength of magnetization resulting from the use of d.c.electromagnets and especially permanent magnets is relatively low,this type of equipment should only be used whereother methods of magnetization are not available or are unsuitable.2The lifting test pieces applicable to Clauses2.2.2and 2.2.3are manufactured from a rectangular bar with one face at least30mm wide.The steel should be similar in chemicalcomposition to grade1020of AS1442and be in the annealed condition.2.2.4Auxiliary equipment2.2.4.1Light sources for viewing The following light sources are required for theinspection of work pieces during testing:(a)For white light viewing when using non-fluorescent magnetic particles—unlesssuitable natural light is available,an artificial light source capable of illuminating atest area to a level of not less than1000lx.(b)For black light viewing when using fluorescent magnetic particles—a black lightsource capable of giving an irradiance of not less than10W/m2at a distance of380mm.NOTE:For black light viewing it may be necessary to provide an inspection booth torestrict the ambient white light illuminance(see Clause3.5.3).AS1171—199882.2.4.2Standard test pieces Test pieces that are used for checking test equipment,orthat contain known discontinuities,shall be prepared in accordance with Appendix B.2.2.4.3Magnetic field strength meters and field indicators Magnetic field strengthmeters and field indicators are required to measure the flux density near a magnetizedcomponent and to prove the efficacy of demagnetizing procedures.2.3REQUIREMENTS FOR MAGNETIC POWDERS AND INKS2.3.1Composition The composition and properties of magnetic powders and inks shallbe in accordance with the requirements of ASTM E1444.2.3.2Concentration of inks The percentage volume of magnetic particles in wetsuspension shall be in the following ranges:(a)Fluorescent particles..........0.1%to0.5%(preferred range0.15%to0.25%).(b)Non-fluorescent particles............................... 1.0%to3.0%.2.3.3Labelling The following information shall be legibly and durably marked oneach container of magnetic powder or ink,or on a label fixed securely to each container:(a)The name of the manufacturer.(b)A description of the contents.(c)The contents by mass or by volume.(d)The batch number.(e)Information for the user.NOTE:Manufacturers making a statement of compliance with this Australian Standard on aproduct,packaging or promotional material related to that product are advised to ensure that such compliance is capable of being verified.Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., .Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., .9AS1171—1998 S E C T I O N3M E T H O D S O F T E S T3.1SCOPE This Section gives details of magnetizing methods that are designed toensure that discontinuities oriented in any direction in the test component can be revealed.3.2PREPARATION OF TEST SURFACE All areas to be tested shall be free fromany foreign matter which could interfere with the interpretation of the test results,e.g.scale,dirt,grease,loose or flaking paint.The processes of surface preparation andcleaning shall not be detrimental to the product,its dimensional tolerances and surfacefinish,or to the testing media.To improve contrast when using methods employing dry powder or magnetic inks,theproduct prior to test may be painted with a strippable lacquer,a titanium-based whitepaint or some other suitable material,provided that the coating chosen is compatible withthe test medium.Care shall be taken to ensure that the thickness of the contrast aid doesnot adversely affect the sensitivity of the test.Where a current flow method(see Clause3.4.3and3.4.4)is being used,any paint orother non-conductive coatings present on the contact areas of the test component shall beremoved to prevent arcing.Where magnetic particle testing is to be carried out on surfaces that have a non-magneticcoating,pre-qualification testing shall be undertaken to ensure the efficacy of the method.If the surface being tested has a non-magnetic coating that cannot be removed,thethickness and uniformity of the coating and its likely effect on the test results shall bereported.Paint shall be removed from the area under test unless a degree of cracking inthe test object is permitted by the specification.Requirements and advice on conductingmagnetic particle inspections on painted surfaces are given in Appendix C.NOTE:Information on contrast aid paints is given in BS5044.3.3MAGNETIZATION3.3.1General During magnetization,the flux density and flux direction within the testpart shall be such that the leakage flux from all discontinuities that require detection iscapable of attracting and holding enough magnetic particles from the indicating mediumto ensure that the discontinuities are detectable.NOTES:1Whereas a.c.magnetization should be used for the detection of surface discontinuities,d.c.magnetization is the preferred method for the detection of sub-surface discontinuities.2Information on test methods for determining flux density and magnetizing current levels is contained in Appendix D.3.3.2Magnetic field directions Discontinuities are difficult to detect by the magneticparticle method when they make an angle of less than45°to the direction of magnetization.To ensure their detection,regardless of their orientation,each part shall bemagnetized in at least two directions at right angles to each other.Depending on partgeometry,this may consist of—(a)circular magnetization in two or more directions;(b)both circular and longitudinal magnetization;or(c)longitudinal magnetization in two or more directions.Exceptions,necessitated by part geometry,size,or other factors,require specific approvalof the contracting agency.Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., .AS1171—1998103.3.3Peak current The effective current value used in magnetic particle testing is thepeak current.Appendix E gives wave forms and correction factors for sinusoidal-basedwaves to be applied should a test meter be calibrated in a convention other than peakvalue.NOTE:Most magnetic particle machines using solid state controls employ non-sinusoidal waveforms in their magnetizing current circuits.3.3.4Application of indicating media To achieve the greatest test sensitivity,magnetic powder or ink should be applied immediately prior to magnetization,or duringthe magnetization of the product under test.Application should cease before the source ofmagnetization is removed.The time of magnetization shall comply with the requirements of the product Standard orthe written procedure,and shall be long enough to allow indications produced by the testto resolve,but shall be not less than0.5s.NOTE:A good practice is to apply two successive magnetizing pulses of at least0.5s duration,such that the second follows the first in rapid succession.3.4MAGNETIZING METHODS3.4.1General The methods commonly employed to magnetize the work piece aretabulated in Figure3.1and listed as follows:(a)Magnetic flow methods These methods employ magnetic flow through the workpiece,and require the use of either of the following equipment:(i)Permanent magnets of various suitable configurations.(ii)Electromagnetic yokes(either a.c.or d.c.).(b)Current flow methods These methods employ the passage of current through thework piece and require the use of either of the following equipment:(i)Contact heads.(ii)Prods or clamps.(c)Coil methods Coil methods employ magnetizing current flowing around the workpiece by means of insulated coils,and comprise the following techniques:(i)Low fill factor techniques.(ii)High fill factor techniques.(iii)Flat,spiral,astride and adjacent coil techniques.(d)Threading bar and threading cable methods These methods employ current flowthrough bars or flexible cables that are threaded through the work piece,for testinghollow products and holes.(e)Induced current methods These methods employ induced current and are used formagnetizing ring-shaped components.Examples of these magnetizing methods are shown in Figures3.2to3.7.3.4.2Magnetic flow methods3.4.2.1Application Magnetic flow methods are suitable for the general location ofdiscontinuities in work pieces and employ an electromagnet or a permanent magnet.Theyare suitable for detecting discontinuities oriented transverse to a line joining the poles ofthe magnet(see Figures3.2(a)and(b)).Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., .11AS1171—19983.4.2.2Procedure The following procedure shall be used to detect discontinuitieswhose orientation is not known:(a)Test with the magnet positioned in one direction,then retest in this same directionas many times as necessary,until the whole test area is covered.NOTE:When using permanent magnets or d.c.or a.c.electromagnets,ensure that the widthof the inspected area is not greater than50mm either side of the axis of the yoke polepieces.(b)During each test,apply magnetic media,in accordance with Clause3.3.4.(c)Inspect the work piece during and after each magnetization stage.(d)Repeat Steps(a),(b)and(c)using a test direction at right angles to the originaldirection.NOTE:For complicated shapes,the use of two or more of these methods may be required.FIGURE 3.1METHODS OF MAGNETIZATION FOR FERROMAGNETIC PRODUCTS3.4.3Current flow through work piece using contact heads method3.4.3.1Application The current flow method is suitable for the detection of discontinuities where the plane or axis of the discontinuity is essentially parallel to thedirection of current flow.3.4.3.2Procedure The following test procedure shall be used:(a)Clamp the work piece firmly between the contact pads of the testing machine andpass a magnetizing current through the work piece taking precautions to preventburning or arcing of the work piece(see Figure3.3(a)).The value of current should be between12A/mm and32A/mm of part diameter(normally up to20A/mm).NOTE:The diameter of the part is taken as the greatest distance between any two points onthe outside circumference,at the same cross-section.(b)Apply magnetic ink or powder in accordance with Clause3.3.4.(c)Inspect the work piece using the appropriate method given in Clause3.5.Copyrighted material licensed to qingchuan niu on 24-Nov-2009 for licensee's use only. No further reproduction or networking is permitted. Distributed by Thomson Scientific, Inc., .AS1171—1998123.4.4Current flow through work piece using prods or clamps method3.4.4.1Application This method is suitable for the detection of discontinuities in workpieces where the plane or axis of each discontinuity is essentially parallel to the directionof current flow.3.4.4.2Procedure The following procedure shall be used:(a)Position the prods on the surface and pass a magnetizing current through the workpiece(see Figure3.3(b)).The current spread and relative intensity are complexwhen using prods,especially if the component has changes of form.Takeprecautions to prevent burning or arcing.NOTE:When inspecting flat surfaces and those with radii of curvature greater than half theprod spacing,and provided that the peak current value is not less than7.5A/mm of prodspacing,the inspected area is a circle inscribed between the prods.(b)When testing large areas,ensure that the circles inscribed between the prods over-lap each other.This is shown in Figure3.4(a),which designates successive prodpositions as CF1—CF1,CF2—CF2,and so on.(c)Apply magnetic media in accordance with Clause3.3.4.(d)Inspect the work piece using the appropriate method(see Clause3.5).(e)Repeat the test with the test pattern rotated through90°to ensure thatdiscontinuities oriented in all directions are detected.NOTES:1Multiplication factors to convert indicated current values to peak current values are given in Appendix E.2As an alternative to the procedure in Step(a)for testing flat surfaces and surfaces with radii of curvature greater than half the prod spacing,an elliptical inspection area inscribedbetween the prods with the minor axis equal to one half of the prod spacing can be used(see Figure3.4(b)),provided that the peak current value is at least 4.70A/mm of prodspacing.For the inspection of a narrow region similar to the prod width,the peak current shouldbe not less than3.75A/mm of prod spacing.These methods of assessment do not apply to the inspection of surfaces having a radius ofcurvature between the prods of less than half the prod spacing,as measured along thesurface.In such cases,the effective area shall be determined and an appropriate searchpattern following the general principles illustrated in Figure3.4(b)shall be used.3.4.5Coil methods3.4.5.1Application Coil methods are suitable for testing hollow and solid products tolocate transverse discontinuities(parallel to the direction of the coil windings).Eitherhigh fill factor or low fill factor coils may be employed to magnetize the product.High fill factor coils comprising parallel conductors may be rigid or flexible.Flexiblecoils are particularly useful for in situ testing of structural steelwork,off-shore structures,nozzles in boilers,pressure vessels,shafts,gears and pipework.Parallel conductors havethe ability to examine large areas with the application of one magnetization and are usedeffectively for the detection of longitudinally oriented defects in tubular structures.Examples of a coil with a low fill factor and a coil with a high fill factor are given inFigure3.5(a)and(b)respectively.Examples of astride coils,an adjacent coil and a flatcoil are given in Figure3.5(c),(d)and(e)respectively.NOTE:Guidance on the use of these procedures is given in Appendix F.。
超声增强的输送的物料进入并通过皮肤翻译

超声增强的输送的物料进入并通过皮肤翻译Ultrasound-enhanced delivery of materials into and through the skinA method for enhancing the permeability of the skin or other biological membrane to a material such as a drug is disclosed. In the method, the drug is delivered in conjunction with ultrasound having a frequency of above about 10 MHz. The method may also be used in conjunction with chemical permeation enhancers and/or with iontophoresis.图片(11)权利要求(21)We claim:1. A method for enhancing the rate of permeation of a drug medium into a selected intact area of an individual's body surface, which method comprises:(a) applying ultrasound having a frequency of above 10 MHz to said selected area, at an intensity and for a period of timeeffective to enhance the permeability of said selected area;(b) contacting the selected area with the drug medium; and(c) effecting passage of said drug medium into and through said selected area by means of iontophoresis.2. The method of claim 1, wherein said ultrasound frequency is in the range of about 15 MHz to 50 MHz.3. The method of claim 2, wherein said ultrasound frequency is in the range of about 15 to 25 MHz.4. The method of claim 1, wherein said period of time is in the range of about 5 to 45 minutes.5. The method of claim 4, wherein said period of time is in the range of about 5 to 30 minutes.6. The method of claim 1, wherein said period of time is less than about 10 minutes.7. The method of claim 1, wherein said intensity of said ultrasound is less than about 5.0W/cm.sup.2.8. The method of claim 7, wherein said intensity of said ultrasound is in the range of about 0.01 to 5.0 W/cm.sup.2.9. The method of claim 8, wherein said intensity of said ultrasound is in the range of about 0.05 to 3.0 W/cm.sup.2.10. The method of claim 1, wherein said area of the stratum corneum is in the range of about 1 to 100 cm.sup.2.11. The method of claim 10, wherein said area of the stratum corneum is in the range of about 5 to 100 cm.sup.2.12. The method of claim 11, wherein said area of the stratum corneum is in the range of about 10 to 50 cm.sup.2.13. The method of claim 1 wherein said drug medium comprises a drug and a coupling agent effective to transfer said ultrasound to the body from an ultrasound source.14. The method of claim 13 wherein said coupling agent is a polymer or a gel.15. The method of claim 13 wherein said coupling agent is selected from the group consisting of glycerin, water, and propylene glycol.16. The method of claim 1 wherein said drug medium further comprises a chemical permeation enhancer.17. The method of claim 1, wherein steps (a) and (b) are carried out approximately simultaneously.18. The method of claim 1, wherein step (b) is carried out before step (a).19. The method of claim 1, wherein step (a) is carried out before step (b).20. The method of claim 1, wherein the ultrasound is applied continuously.21. The method of claim 1, wherein the ultrasound is pulsed.说明This application is a division of application Ser. No. 07/844,732 filed Mar. 2, 1992, now U.S. Pat. No. 5,231,975 which is a divisional of application Ser. No. 07/484,560, now U.S. Pat. No. 5,115,805, filed Feb. 23, 1990.TECHNICAL FIELDThis invention relates generally to the field of drug delivery. More particularly, the invention relates to a method of enhancing the rate of permeation of topically, transmucosally or transdermally applied materials using high frequency ultrasound.BACKGROUNDThe delivery of drugs through the skin ("transdermal drug delivery" or "TDD") provides many advantages; primarily, such a means of delivery is a comfortable, convenient and non-invasiveway of administering drugs. The variable rates of absorption and metabolism encountered in oral treatment are avoided, and other inherent inconveniences--e.g., gastrointestinal irritation and the like--are eliminated as well. Transdermal drug delivery also makes possible a high degree of control over blood concentrations of any particular drug.Skin is a structurally complex, relatively impermeable membrane. Molecules moving from the environment into and through intact skin must first penetrate the stratum corneum and any material on its surface. They must then penetrate the viable epidermis, the papillary dermis, and the capillary walls into the blood stream or lymph channels. To be so absorbed, molecules must overcome a different resistance to penetration in each type of tissue. Transport across the skin membrane is thus a complex phenomenon. However, it is the stratum corneum, a layer approximately 5-15 micrometers thick over most of the body, which presents the primary barrier to absorption of topical compositions or transdermally administered drugs. It is believed to be the high degree of keratinization within its cells as well as their dense packing and cementation by ordered, semicrystalline lipids which create in many cases a substantially impermeable barrier to drug penetration. Applicability of transdermal drug delivery is thus presently limited, because the skin is such an excellent barrier to the ingress of topically applied materials. For example, many of the new peptides and proteins now produced as a result of the biotechnology revolution cannot be delivered across the skin in sufficient quantities due to their naturally low rates of skin permeability.Various methods have been used to increase skin permeability, and in particular to increase the permeability of thestratum corneum (i.e., so as to achieve enhanced penetration, through the skin, of the drug to be administered transdermally). The primary focus has been on the use of chemical enhancers, i.e., wherein drug is coadministered with a penetration enhancing agent (or "permeation enhancer"). While such compounds are effective in increasing the rate at which drug is delivered through the skin, there are drawbacks with many permeation enhancers which limit their use. For example, many permeation enhancers are associated with deleterious effects on the skin (e.g., irritation). In addition, control of drug delivery with chemical enhancement can be quite difficult.Iontophoresis has also been used to increase the permeability of skin to drugs, and involves (1) the application of an external electric field, and (2) topical delivery of an ionized form of drug (or of a neutral drug carried with the water flux associated with ion transport, i.e., via "electroosmosis"). While permeation enhancement via iontophoresis has, as with chemical enhancers, been effective, there are problems with control of drug delivery and the degree of irreversible skin damage induced by the transmembrane passage of current.The presently disclosed and claimed method involves the use of ultrasound to decrease the barrier function of the stratum corneum and thus increase the rate at which a drug may be delivered through the skin. "Ultrasound" is defined as mechanical pressure waves with frequencies above 20,000 Hz (see, e.g., H. Lutz et al., Manual of Ultrasound: 1. Basic Physical and Technical Principles (Berlin: Springer-Verlag, 1984)).As discussed by P. Tyle et al. in Pharmaceutical Research 6(5):355-361 (1989), drug penetration achieved via "sonophoresis" (the movement of drugs through skin under theinfluence of an ultrasonic perturbation; see D. M. Skauen and G. M. Zentner, Int. J. Pharmaceutics 20:235-245 (1984)), is believed to result from thermal, mechanical and chemical alteration of biological tissues by the applied ultrasonic waves. Unlike iontophoresis, the risk of skin damage appears to be low.Applications of ultrasound to drug delivery have been discussed in the literature. See, for example: P. Tyle et al., supra (which provides an overview of sonophoresis); S. Miyazaki et al., J. Pharm. Pharmacol. 40:716-717 (1988) (controlled release of insulin from a polymer implant using ultrasound); J. Kost et al., Proceed. Intern. Symp. Control. Rel. Bioact. Mater.16(141):294-295 (1989) (overview of the effect of ultrasound on the permeability of human skin and synthetic membranes); H. Benson et al., Physical Therapy 69(2):113-118 (1989) (effect of ultrasound on the percutaneous absorption of benzydamine); E. Novak, Arch. Phys. Medicine & Rehab. 45:231-232 (1964) (enhanced penetration of lidocaine through intact skin using ultrasound); J. E. Griffin et al., Amer. J. Phys. Medicine 44(1):20-25 (1965) (ultrasonic penetration of cortisol into pig tissue); J. E. Griffin et al., J. Amer. Phys. Therapy Assoc.46:18-26 (1966) (overview of the use of ultrasonic energy in drug therapy); J. E. Griffin et al., Phys. Therapy 47(7):594-601 (1967) (ultrasonic penetration of hydrocortisone); J. E. Griffin et al., Phys. Therapy 48(12):1336-1344 (1968) (ultrasonic penetration of cortisol into pig tissue); J. E. Griffin et al., Amer. J. Phys. Medicine 51(2):62-72 (1972) (same); J. C. McElnay, Int. J. Pharmaceutics 40:105-110 (1987) (the effect of ultrasound on the percutaneous absorption of fluocinolone acetonide); and C. Escoffier et al., Bioeng. Skin 2:87-94 (1986) (in vitro study of the velocity of ultrasound in skin).In addition to the aforementioned art, U.S. Pat. Nos. 4,767,402 and 4,780,212 to Kost et al. relate specifically to the use of specific frequencies of ultrasound to enhance the rate of permeation of a drug through human skin or through a synthetic membrane.While the application of ultrasound in conjunction with drug delivery is thus known, results have for the most part been disappointing, i.e., enhancement of skin permeability has been relatively low.SUMMARY OF THE INVENTIONThe present invention provides a novel method for enhancing the rate of permeation of a given material through a selected intact area of an individual's body surface. The method comprises contacting the selected intact area with the material and applying ultrasound to the contacted area. The ultrasound preferably has a frequency of above about 10 MHz, and is continued at an intensity and for a period of time sufficient to enhance the rate of permeation of the material into and through the body surface. The ultrasound can also be used to pretreat the selected area of the body surface in preparation for drug delivery, or for diagnostic purposes, i.e., to enable non-invasive sampling of physiologic material beneath the skin or body surface.In addition to enhancing the rate of permeation of a material, the present invention involves increasing the permeability of a biological membrane such as the stratum corneum by applying ultrasound having a frequency of above about 10 MHz to the membrane at an intensity and for a period of time sufficient to give rise to increased permeability of the membrane. Once the permeability of the membrane has been increased, it is possible to apply a material thereto and obtain an increased rate of flowof the material through the membrane.It is accordingly a primary object of the invention to address the aforementioned deficiencies of the prior art by providing a method of enhancing the permeability of biological membranes and thus allow for an increased rate of delivery of material therethrough.It is another object of the invention to provide such a method which is effective with or without chemical permeation enhancers.It is still another object of the invention to minimize lag time in such a method and provide a relatively short total treatment time.It is yet another object of the invention to provide such a method in which drug delivery is effected using ultrasound.It is a further object of the invention to enable sampling of tissue beneath the skin or other body surface by application of high frequency (>10 MHz) ultrasound thereto.A further feature of the invention is that it preferably involves ultrasound of a frequency greater than about 10 MHz.Additional objects, advantages and novel features of the invention will be set forth in part in the description which follows, and in part will become apparent to those skilled in the art upon examination of the following, or may be learned by practice of the invention.BRIEF DESCRIPTION OF THE DRAWINGSFIGS. 1A, 1B and 1C are theoretical plots of energy dissipation within the skin barrier versus frequency of applied ultrasound.FIGS. 2, 3 and 4 are graphic representations of the amount of salicylic acid recovered from the stratum corneum after ultrasound treatment at different frequencies.FIGS. 5 and 6 represent the results of experiments similar to those summarized in FIGS. 2, 3 and 4, but with a shorter treatment time.FIGS. 7, 8, 9 and 10 are plots of enhancement versus "tape-strip number," as described in the Example.FIG. 11 illustrates the effect of ultrasound on the systemic availability of salicylic acid following topical application.DETAILED DESCRIPTION OF PREFERRED EMBODIMENTSBefore the present method of enhancing the rate of permeation of a material through a biological membrane and enhancing the permeability of membranes using ultrasound are disclosed and described, it is to be understood that this invention is not limited to the particular process steps and materials disclosed herein as such process steps and materials may, of course, vary. It is alto to be understood that the terminology used herein is used for purpose of describing particular embodiments only and is not intended to be limiting since the scope of the present invention will be limited only by the appended claims.It must be noted that as used in this specification and the appended claims, the singular forms "a", "an" and "the" include plural reference unless the context clearly dictates otherwise. Thus, for example, reference to "a drug" includes mixtures of drugs and their pharmaceutically acceptable salts, reference to "an ultrasound device" includes one or more ultrasound devices of the type necessary for carrying out the present invention, and reference to "the method of administration" includes one or more different methods of administration known to those skilled in the art or which will become known to those skilled in the art upon reading this disclosure.In one aspect of the invention a method is provided forenhancing the permeation of a given material such as a drug, pharmacologically active agent, or diagnostic agent into and/or through a biological membrane on an individual's body surface, which method comprises: (a) contacting the membrane with the chosen material in a pharmacologically acceptable carrier medium; and (b) applying ultrasound of an intensity and for a treatment time effective to produce delivery of the material through the membrane. The material is preferably a drug and it is preferable to obtain a desired blood level of the drug in the individual. The ultrasound is of a frequency and intensity effective to increase the permeability of the selected area to the applied drug over that which would be obtained without ultrasound. The ultrasound preferably has a frequency of more than 10 MHz, and may be applied either continuously or pulsed, preferably continuously. The ultrasound may be applied to the skin either before or after application of the drug medium so long as administration of the ultrasound and the drug medium is relatively simultaneous, i.e., the ultrasound is applied within about 6, more preferably within about 4, most preferably within about 2 minutes of drug application.The invention is useful for achieving transdermal permeation of pharmacologically active agents which otherwise would be quite difficult to deliver through the skin or other body surface. For example, proteinaceous drugs and other high molecular weight pharmacologically active agents are ideal candidates for transdermal, transmucosal or topical delivery using the presently disclosed method. In an alternative embodiment, agents useful for diagnostic purposes may also be delivered into and/or through the body surface using the present method.The invention is also useful as a non-invasive diagnostictechnique, i.e., in enabling the sampling of physiologic material from beneath the skin or other body surface and into a collection (and/or evaluation) chamber.The present invention will employ, unless otherwise indicated, conventional pharmaceutical methodology and more specifically conventional methodology used in connection with transdermal delivery of pharmaceutically active compounds and enhancers.In describing the present invention, the following terminology will be used in accordance with the definitions set out below.A "biological membrane" is intended to mean a membrane material present within a living organism which separates one area of the organism from another and, more specifically, which separates the organism from its outer environment. Skin and mucous membranes are thus included."Penetration enhancement" or "permeation enhancement" as used herein relates to an increase in the permeability of skin to a material such as a pharmacologically active agent, i.e., so as to increase the rate at which the material permeates into and through the skin. The present invention involves enhancement of permeation through the use of ultrasound, and, in particular, through the use of ultrasound having a frequency of greater than 10 MHz."Transdermal" (or "percutaneous") shall mean passage of a material into and through the skin to achieve effective therapeutic blood levels or deep tissue therapeutic levels. While the invention is described herein primarily in terms of "transdermal" administration, it will be appreciated by those skilled in the art that the presently disclosed and claimed methodalso encompasses the "transmucosal" and "topical" administration of drugs using ultrasound. "Transmucosal" is intended to mean passage of any given material through a mucosal membrane of a living organism and more specifically shall refer to the passage of a materialfrom the outside environment of the organism, through a mucous membrane and into the organism. "Transmucosal" administration thus includes delivery of drugs through either nasal or buccal tissue. By "topical" administration is meant local administration of a topical pharmacologically active agent to the skin as in, for example, the treatment of various skin disorders or the administration of a local anaesthetic. "Topical" delivery can involve penetration of a drug into the skin but not through it, i.e., topical administration does not involve actual passage of a drug into the bloodstream."Carriers" or "vehicles" as used herein refer to carrier materials without pharmacological activity which are suitable for administration with other pharmaceutically active materials, and include any such materials known in the art, e.g., any liquid, gel, solvent, liquid diluent, solubilizer, or the like, which is nontoxic and which does not interact with the drug to be administered in a deleterious manner. Examples of suitable carriers for use herein include water, mineral oil, silicone, inorganic gels, aqueous emulsions, liquid sugars, waxes, petroleum jelly, and a variety of other oils and polymeric materials.By the term "pharmacologically active agent" or "drug" as used herein is meant any chemical material or compound suitable for transdermal or transmucosal administration which can either (1) have a prophylactic effect on the organism and prevent an undesired biological effect such as preventing aninfection, (2) alleviates a condition caused by a disease such as alleviating pain caused as a result of a disease, or (3) either alleviates or completely eliminates the disease from the organism. The effect of the agent may be local, such as providing for a local anaesthetic effect or it may be systemic. Such substances include the broad classes of compounds normally delivered through body surfaces and membranes, including skin. In general, this includes: anti-infectives such as antibiotics and antiviral agents; analgesics and analgesic combinations; anorexics; antihelminthics; antiarthritics; antiasthmatic agents; anticonvulsants; antidepressants; antidiabetic agents; antidiarrheals; antihistamines; antiinflammatory agents; antimigraine preparations; antinauseants; antineoplastics; antiparkinsonism drugs; antipruritics; antipsychotics; antipyretics; antispasmodics; anticholinergics; sympathomimetics; xanthine derivatives; cardiovascular preparations including potassium and calcium channel blockers, beta-blockers, and antiarrhythmics; antihypertensives; diuretics; vasodilators including general coronary, peripheral and cerebral; central nervous system stimulants; cough and cold preparations, including decongestants; hormones such as estradiol and other steroids, including corticosteroids; hypnotics; immunosuppressives; muscle relaxants; parasympatholytics; psychostimulants; sedatives; and tranquilizers. By the method of the present invention, both ionized and nonionzed drugs may be delivered, as can drugs of either high or low molecular weight.Proteinaceous and polypeptide drugs represent a preferred class of drugs for use in conjunction with the presently disclosed and claimed invention. Such drugs cannot generally be administered orally in that they Are often destroyed in the G.I.tract or metabolized in the liver. Further, due to the high molecular weight of most polypeptide drugs, conventional transdermal delivery systems are not generally effective. It is also desirable to use the methodof the invention in conjunction with drugs to which the permeability of the skin is relatively low, or which give rise to a long lag-time (application of ultrasound as described herein has been found to significantly reduce the lag-time involved with the transdermal administration of most drugs).By a "therapeutically effective" amount of a pharmacologically active agent is meant a nontoxic but sufficient amount of a compound to provide the desired therapeutic effect. The desired therapeutic effect may be a prophylactic effect, in preventing a disease, an effect which alleviates a system of the disease, or a curative effect which either eliminates or aids in the elimination of the disease.As noted above, the present invention is a method for enhancing the rate of permeation of a drug through an intact area of an individual's body surface, preferably the human skin. The method involves transdermal administration of a selected drug in conjunction with ultrasound. Ultrasound causes thermal, mechanical and chemical alterations of biological tissue, thereby enhancing the rate of permeation of a given material therethrough.While not wishing to be bound by theory, applicants propose that the use of higher frequency ultrasound as disclosed herein specifically enhances the permeation of the drug through the outer layer of skin, i.e., the stratum corneum, by causing momentary and reversible perturbations within (and thus short-term, reversible reduction in the barrier function of) the layer ofthe stratum corneum. It will be appreciated by those skilled in the art of transdermal drug delivery that a number of factors related to the present method will vary with the drug to be administered, the disease or injury to be treated, the age of the selected individual, the location of the skin to which the drug is applied, and the like.As noted above, "ultrasound" is ultrasonic radiation of a frequency above 20,000 Hz. As may be deduced from the literature cited above, ultrasound used for most medical purposes typically employs frequencies ranging from 1.6 to about 10 MHz. The present invention, by contrast, employs ultrasound frequencies of greater than about 10 MHz, preferably in the range of about 15 to 50 MHz, most preferably in the range of about 15 to 25 MHz. It should be emphasized that these ranges are intended to be merely illustrative of the preferred embodiment; in some cases higher or lower frequencies may be used.The ultrasound may be pulsed or continuous, but is preferably continuous when lower frequencies are used. At very high frequencies, pulsed application will generally be preferred so as to enable dissipation of generated heat.The preferred intensity of the applied ultrasound is less than about 5.0 W/cm.sup.2, more preferably is in the range of about 0.01 to 5.0 W/cm.sup.2, and most preferably is in the range of 0.05 to 3.0 W/cm.sup.2. The total treatment time, i.e., the period over which drug and ultrasound are administered, will vary depending on the drug administered, the disease or injury treated, etc., but will generally be on the order of about 30 seconds to 60 minutes, preferably 5 to 45 minutes, more preferably 5 to 30 minutes, and most preferably 5 to 10minutes. It should be noted that the aforementioned ranges represent suggested, or preferred, treatment times, but are not in any way intended to be limiting. Longer or shorter times may be possible and in some cases desirable. Virtually any type of device may be used to administer the ultrasound, providing that the device is callable of producing the higher frequency ultrasonic waves required by the present method. A device will typically have a power source such as a small battery, a transducer, a reservoir in which the drug medium is housed (and which may or may not be refillable), and a means to attach the system to the desired skin site.As ultrasound does not transmit well in air, a liquid medium is generally needed to efficiently and rapidly transmit ultrasound between the ultrasound applicator and the skin. As explained by P. Tyle et al., cited above, the selected drug medium should contain a "coupling" or "contacting" agent typically used in conjunction with ultrasound. The coupling agent should have an absorption coefficient similar to that of water, and furthermore be nonstaining, nonirritating to the skin, and slow drying. It is clearly preferred that the coupling agent retain a paste or gel consistency during the time period of ultrasound administration so that contact is maintained between the ultrasound source and the skin. Examples of preferred coupling agents are mixtures of mineral oil and glycerine and propylene glycol, oil/water emulsions, and a water-based gel. A solid-state, non-crystalline polymeric film having the above-mentioned characteristics may also be used. The drug medium may also contain a carrier or vehicle, as defined alone.A transdermal patch as well known in the art may be used in conjunction with the present invention, i.e., to deliver the drugmedium to the skin. The "patch", however, must have the properties of the coupling agent as described in the preceding paragraph so as to enable transmission of the ultrasound from the applicator, through the patch, to the skin.As noted earlier in this section, virtually any chemical material or compound suitable for transdermal, transmucosal or topical administration may be administered using the present method. Again, the present invention is particularly useful to enhance delivery of proteinaceous and other high molecular weight drugs.The method of the invention is preferably carried out as follows. The drug medium, i.e., containing the selected drug or drugs in conjunction with the coupling agent and optionally a carrier or vehicle material, is applied to an area of intact body surface. Ultrasound preferably having a frequency greater than about 10 MHz may be applied before or after application of the drug medium, but is preferably applied immediately before application of the drug so as to "pretreat" the skin prior to drug administration.It should also be pointed out that the present method may be used in conjunction with a chemical permeation enhancer as known in the art, wherein the ultrasound enables the use of much lower concentrations of permeation enhancer--thus minimizing skin irritation and other problems frequently associated with such compounds--than would be possible in the absence of ultrasound. The permeation enhancer may be incorporated into the drug medium or it maybe applied in a conventional transdermal patch after pretreatment of the body surface with ultrasound.The present invention may also be used in conjunction with。
开启片剂完整性的窗户(中英文对照)
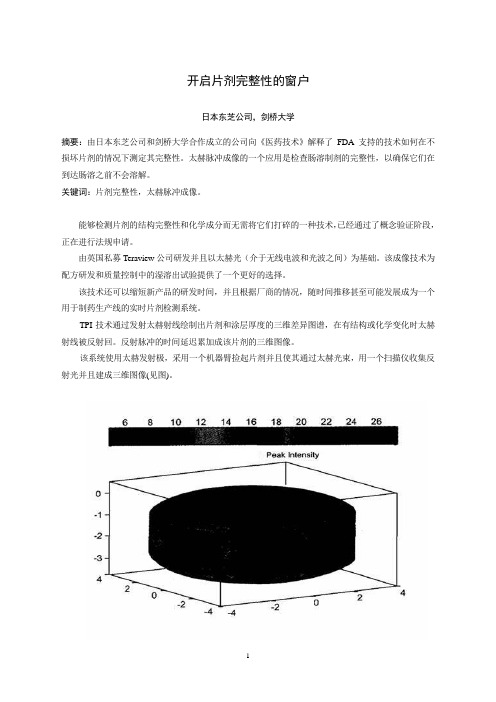
开启片剂完整性的窗户日本东芝公司,剑桥大学摘要:由日本东芝公司和剑桥大学合作成立的公司向《医药技术》解释了FDA支持的技术如何在不损坏片剂的情况下测定其完整性。
太赫脉冲成像的一个应用是检查肠溶制剂的完整性,以确保它们在到达肠溶之前不会溶解。
关键词:片剂完整性,太赫脉冲成像。
能够检测片剂的结构完整性和化学成分而无需将它们打碎的一种技术,已经通过了概念验证阶段,正在进行法规申请。
由英国私募Teraview公司研发并且以太赫光(介于无线电波和光波之间)为基础。
该成像技术为配方研发和质量控制中的湿溶出试验提供了一个更好的选择。
该技术还可以缩短新产品的研发时间,并且根据厂商的情况,随时间推移甚至可能发展成为一个用于制药生产线的实时片剂检测系统。
TPI技术通过发射太赫射线绘制出片剂和涂层厚度的三维差异图谱,在有结构或化学变化时太赫射线被反射回。
反射脉冲的时间延迟累加成该片剂的三维图像。
该系统使用太赫发射极,采用一个机器臂捡起片剂并且使其通过太赫光束,用一个扫描仪收集反射光并且建成三维图像(见图)。
技术研发太赫技术发源于二十世纪九十年代中期13本东芝公司位于英国的东芝欧洲研究中心,该中心与剑桥大学的物理学系有着密切的联系。
日本东芝公司当时正在研究新一代的半导体,研究的副产品是发现了这些半导体实际上是太赫光非常好的发射源和检测器。
二十世纪九十年代后期,日本东芝公司授权研究小组寻求该技术可能的应用,包括成像和化学传感光谱学,并与葛兰素史克和辉瑞以及其它公司建立了关系,以探讨其在制药业的应用。
虽然早期的结果表明该技术有前景,但日本东芝公司却不愿深入研究下去,原因是此应用与日本东芝公司在消费电子行业的任何业务兴趣都没有交叉。
这一决定的结果是研究中心的首席执行官DonArnone和剑桥桥大学物理学系的教授Michael Pepper先生于2001年成立了Teraview公司一作为研究中心的子公司。
TPI imaga 2000是第一个商品化太赫成像系统,该系统经优化用于成品片剂及其核心完整性和性能的无破坏检测。
《物理化学》的中英文翻译

《物理化学》的中英文翻译第一篇:《物理化学》的中英文翻译复习《物理化学》过程中,顺便整理了专业名词的翻译,大家凑合着,依我看,简单的会考汉译英,复杂的会考英译汉。
不管怎么样,中文英文背过最好。
如果有错误,赶紧的,说。
1多相系统 heterogeneous system2自由度degree of freedom3相律 phase rule4独立组分数 number of independent component5凝聚系统 condensed system6三相点 triple point7超临界流体 supercritical fluid8超临界流体萃取supercritical fluid extraction9超临界流体色谱supercritical fluid chromatography10泡点 bubbling point11露点dew point12杠杆规则 level rule13连结线 tie line14部分蒸馏(分馏)fractional distillation15缔合分子 associated molecule16最低恒沸点 minimum azeotropic point17最低恒沸混合物low-boiling azeotrope18无水乙醇(绝对乙醇)absolute ethyl alcohol19最高恒沸点maximum azeotropic point20会溶点 consolute point21共轭层 conjugate layer22烟碱 nicotine23蒸汽蒸馏 steam distillation24步冷曲线 cooling curve25热分析法 thermal analysis26低共熔点 eutectic point27低共熔混合物eutectic mixture28异成分熔点 incongruent melting point29转熔温度 peritectic tempreture30固溶体 solid solution31退火 annealing32淬火 quenching33区域熔炼 zone melting34分凝系数 fractional coagulation coefficient35褶点 plait point36等温会溶点 isothermal consolute point37双节点溶解度曲线 binodal solubility cueve38一(二)级相变first(second)order phase transition39超流体 super fluid40顺磁体 paramagnetic substance41铁磁体 ferromagnetic substance第二篇:中英文翻译蓄电池 battery 充电 converter 转换器 charger开关电器Switch electric 按钮开关Button to switch 电源电器Power electric 插头插座 Plug sockets第三篇:中英文翻译Fundamentals This chapter describes the fundamentals of today’s wireless communications.First a detailed description of the radio channel and its modeling are presented, followed by the introduction of the principle of OFDM multi-carrier transmission.In addition, a general overview of the spread spectrum technique, especially DS-CDMA, is given and examples of potential applications for OFDM and DS-CDMA areanalyzed.This introduction is essential for a better understanding of the idea behind the combination of OFDM with the spread spectrum technique, which is briefly introduced in the last part of this chapter.1.1 Radio Channel Characteristics Understanding the characteristics of the communications medium is crucial for the appropriate selection of transmission system architecture, dimensioning of its components, and optimizing system parameters, especially since mobile radio channels are considered to be the most difficult channels, since they suffer from many imperfections like multipath fading, interference, Doppler shift, and shadowing.The choice of system components is totally different if, for instance, multipath propagation with long echoes dominates the radio propagation.Therefore, an accurate channel model describing the behavior of radio wave propagation in different environments such as mobile/fixed and indoor/outdoor is needed.This may allow one, through simulations, to estimate and validate the performance of a given transmission scheme in its several design phases.1.1.1 Understanding Radio Channels In mobile radio channels(see Figure 1-1), the transmitted signal suffers from different effects, which are characterized as follows: Multipath propagation occurs as a consequence of reflections, scattering, and diffraction of the transmitted electromagnetic wave at natural and man-made objects.Thus, at the receiver antenna, a multitude of waves arrives from many different directions with different delays, attenuations, and phases.The superposition of these waves results in amplitude and phase variations of the composite received signal.Doppler spread is caused by moving objects in the mobile radio channel.Changes in the phases and amplitudes of the arriving waves occur which lead to time-variant multipathpropagation.Even small movements on the order of the wavelength may result in a totally different wave superposition.The varying signal strength due to time-variant multipath propagation is referred to as fast fading.Shadowing is caused by obstruction of the transmitted waves by, e.g., hills, buildings, walls, and trees, which results in more or less strong attenuation of the signal pared to fast fading, longer distances have to be covered to significantly change the shadowing constellation.The varying signal strength due to shadowing is called slow fading and can be described by a log-normal distribution [36].Path loss indicates how the mean signal power decays with distance between transmitter and receiver.In free space, the mean signal power decreases with the square of the distance between base station(BS)and terminal station(TS).In a mobile radio channel, where often no line of sight(LOS)path exists, signal power decreases with a power higher than two and is typically in the order of three to five.Variations of the received power due to shadowing and path loss can be efficiently counteracted by power control.In the following, the mobile radio channel is described with respect to its fast fading characteristic.1.1.2 Channel Modeling The mobile radio channel can be characterized by the time-variant channel impulse response h(τ , t)or by the time-variant channel transfer function H(f, t), which is the Fourier transform of h(τ, t).The channel impulse response represents the response of the channel at time t due to an impulse applied at time t −τ.The mobile radio channel is assumed to be a wide-sense stationary random process, i.e., the channel has a fading statistic that remains constant over short periods of time or small spatial distances.In environments with multipath propagation, the channel impulseresponse is composed of a large number of scattered impulses received over Np different paths,Whereand ap, fD,p, ϕp, and τp are the amplitude, the Doppler frequency, the phase, and the propagation delay, respectively, associated with path p, p = 0,..., Np −1.The assigned channel transfer function isThe delays are measured relative to the first detectable path at the receiver.The Doppler Frequencydepends on the velocity v of the terminal station, the speed of light c, the carrier frequency fc, and the angle of incidence αp of a wave assigned to path p.A channel impulse response with corresponding channel transfer function is illustrated in Figure 1-2.The delay power density spectrum ρ(τ)that characterizes the frequency selectivity of the mobile radio channel gives the average power of the channel output as a function of the delay τ.The mean delay τ , the root mean square(RMS)de lay spread τRMS and the maximum delay τmax are characteristic parameters of the delay power density spectrum.The mean delay isWhereFigure 1-2 Time-variant channel impulse response and channel transfer function with frequency-selective fading is the power of path p.The RMS delay spread is defined as Similarly, the Doppler power density spectrum S(fD)can be defined that characterizes the time variance of the mobile radio channel and gives the average power of the channel output as a function of the Doppler frequency fD.The frequency dispersive properties of multipath channels are most commonly quantified by the maximum occurring Doppler frequency fDmax and the Doppler spread fDspread.The Doppler spread is the bandwidth of theDoppler power density spectrum and can take on values up to two times |fDmax|, i.e.,1.1.3Channel Fade Statistics The statistics of the fading process characterize the channel and are of importance for channel model parameter specifications.A simple and often used approach is obtained from the assumption that there is a large number of scatterers in the channel that contribute to the signal at the receiver side.The application of the central limit theorem leads to a complex-valued Gaussian process for the channel impulse response.In the absence of line of sight(LOS)or a dominant component, the process is zero-mean.The magnitude of the corresponding channel transfer functionis a random variable, for brevity denoted by a, with a Rayleigh distribution given byWhereis the average power.The phase is uniformly distributed in the interval [0, 2π].In the case that the multipath channel contains a LOS or dominant component in addition to the randomly moving scatterers, the channel impulse response can no longer be modeled as zero-mean.Under the assumption of a complex-valued Gaussian process for the channel impulse response, the magnitude a of the channel transfer function has a Rice distribution given byThe Rice factor KRice is determined by the ratio of the power of the dominant path to thepower of the scattered paths.I0 is the zero-order modified Bessel function of first kind.The phase is uniformly distributed in the interval [0, 2π].1.1.4Inter-Symbol(ISI)and Inter-Channel Interference(ICI)The delay spread can cause inter-symbol interference(ISI)when adjacent data symbols overlap and interfere with each other due to differentdelays on different propagation paths.The number of interfering symbols in a single-carrier modulated system is given by For high data rate applications with very short symbol duration Td < τmax, the effect of ISI and, with that, the receiver complexity can increase significantly.The effect of ISI can be counteracted by different measures such as time or frequency domain equalization.In spread spectrum systems, rake receivers with several arms are used to reduce the effect of ISI by exploiting the multipath diversity such that individual arms are adapted to different propagation paths.If the duration of the transmitted symbol is significantly larger than the maximum delay Td τmax, the channel produces a negligible amount of ISI.This effect is exploited with multi-carrier transmission where the duration per transmitted symbol increases with the number of sub-carriers Nc and, hence, the amount of ISI decreases.The number of interfering symbols in a multi-carrier modulated system is given byResidual ISI can be eliminated by the use of a guard interval(see Section 1.2).The maximum Doppler spread in mobile radio applications using single-carrier modulation is typically much less than the distance between adjacent channels, such that the effect of interference on adjacent channels due to Doppler spread is not a problem for single-carrier modulated systems.For multi-carrier modulated systems, the sub-channel spacing Fs can become quite small, such that Doppler effects can cause significant ICI.As long as all sub-carriers are affected by a common Doppler shift fD, this Doppler shift can be compensated for in the receiver and ICI can be avoided.However, if Doppler spread in the order of several percent of the sub-carrier spacing occurs, ICI may degrade the system performance significantly.T oavoid performance degradations due to ICI or more complex receivers with ICI equalization, the sub-carrier spacing Fs should be chosen assuch that the effects due to Doppler spread can be neglected(see Chapter 4).This approach corresponds with the philosophy of OFDM described in Section 1.2 and is followed in current OFDM-based wireless standards.Nevertheless, if a multi-carrier system design is chosen such that the Doppler spread is in the order of the sub-carrier spacing or higher, a rake receiver in the frequency domain can be used [22].With the frequency domain rake receiver each branch of the rake resolves a different Doppler frequency.1.1.5Examples of Discrete Multipath Channel Models Various discrete multipath channel models for indoor and outdoor cellular systems with different cell sizes have been specified.These channel models define the statistics of the 5 discrete propagation paths.An overview of widely used discrete multipath channel models is given in the following.COST 207 [8]: The COST 207 channel models specify four outdoor macro cell propagation scenarios by continuous, exponentially decreasing delay power density spectra.Implementations of these power density spectra by discrete taps are given by using up to 12 taps.Examples for settings with 6 taps are listed in Table 1-1.In this table for several propagation environments the corresponding path delay and power profiles are given.Hilly terrain causes the longest echoes.The classical Doppler spectrum with uniformly distributed angles of arrival of the paths can be used for all taps for simplicity.Optionally, different Doppler spectra are defined for the individual taps in [8].The COST 207 channel models are based on channel measurements with a bandwidth of 8–10 MHz in the 900-MHz band used for 2Gsystems such as GSM.COST 231 [9] and COST 259 [10]: These COST actions which are the continuation of COST 207 extend the channel characterization to DCS 1800, DECT, HIPERLAN and UMTS channels, taking into account macro, micro, and pico cell scenarios.Channel models with spatial resolution have been defined in COST 259.The spatial component is introduced by the definition of several clusters with local scatterers, which are located in a circle around the base station.Three types of channel models are defined.The macro cell type has cell sizes from 500 m up to 5000 m and a carrier frequency of 900 MHz or 1.8 GHz.The micro cell type is defined for cell sizes of about 300 m and a carrier frequency of 1.2 GHz or 5 GHz.The pico cell type represents an indoor channel model with cell sizes smaller than 100 m in industrial buildings and in the order of 10 m in an office.The carrier frequency is 2.5 GHz or 24 GHz.COST 273: The COST 273 action additionally takes multi-antenna channel models into account, which are not covered by the previous COST actions.CODIT [7]: These channel models define typical outdoor and indoor propagation scenarios for macro, micro, and pico cells.The fading characteristics of the various propagation environments are specified by the parameters of the Nakagami-m distribution.Every environment is defined in terms of a number of scatterers which can take on values up to 20.Some channel models consider also the angular distribution of the scatterers.They have been developed for the investigation of 3G system proposals.Macro cell channel type models have been developed for carrier frequencies around 900 MHz with 7 MHz bandwidth.The micro and pico cell channel type models have been developed for carrier frequencies between 1.8 GHz and 2 GHz.The bandwidths of the measurements are in the range of 10–100 MHz for macro cells and around 100 MHz for pico cells.JTC [28]: The JTC channel models define indoor and outdoor scenarios by specifying 3 to 10 discrete taps per scenario.The channel models are designed to be applicable for wideband digital mobile radio systems anticipated as candidates for the PCS(Personal Communications Systems)common air interface at carrier frequencies of about 2 GHz.UMTS/UTRA [18][44]: Test propagation scenarios have been defined for UMTS and UTRA system proposals which are developed for frequencies around 2 GHz.The modeling of the multipath propagation corresponds to that used by the COST 207 channel models.HIPERLAN/2 [33]: Five typical indoor propagation scenarios for wireless LANs in the 5 GHz frequency band have been defined.Each scenario is described by 18discrete taps of the delay power density spectrum.The time variance of the channel(Doppler spread)is modeled by a classical Jake’s spectrum with a maximum terminal speed of 3 m/h.Further channel models exist which are, for instance, given in [16].1.1.6Multi-Carrier Channel Modeling Multi-carrier systems can either be simulated in the time domain or, more computationally efficient, in the frequency domain.Preconditions for the frequency domain implementation are the absence of ISI and ICI, the frequency nonselective fading per sub-carrier, and the time-invariance during one OFDM symbol.A proper system design approximately fulfills these preconditions.The discrete channel transfer function adapted to multi-carrier signals results inwhere the continuous channel transfer function H(f, t)is sampled in time at OFDM symbol rate s and in frequency at sub-carrier spacing Fs.The durations is the total OFDM symbol duration including the guardinterval.Finally, a symbol transmitted onsub-channel n of the OFDM symbol i is multiplied by the resulting fading amplitude an,i and rotated by a random phase ϕn,i.The advantage of the frequency domain channel model is that the IFFT and FFT operation for OFDM and inverse OFDM can be avoided and the fading operation results in one complex-valued multiplication per sub-carrier.The discrete multipath channel models introduced in Section 1.1.5 can directly be applied to(1.16).A further simplification of the channel modeling for multi-carrier systems is given by using the so-called uncorrelated fading channel models.1.1.6.1Uncorrelated Fading Channel Models for Multi-Carrier Systems These channel models are based on the assumption that the fading on adjacent data symbols after inverse OFDM and de-interleaving can be considered as uncorrelated [29].This assumption holds when, e.g., a frequency and time interleaver with sufficient interleaving depth is applied.The fading amplitude an,i is chosen from a distribution p(a)according to the considered cell type and the random phase ϕn,I is uniformly distributed in the interval [0,2π].The resulting complex-valued channel fading coefficient is thus generated independently for each sub-carrier and OFDM symbol.For a propagation scenario in a macro cell without LOS, the fading amplitude an,i is generated by a Rayleigh distribution and the channel model is referred to as an uncorrelated Rayleigh fading channel.For smaller cells where often a dominant propagation component occurs, the fading amplitude is chosen from a Rice distribution.The advantages of the uncorrelated fading channel models for multi-carrier systems are their simple implementation in the frequency domain and the simple reproducibility of the simulation results.1.1.7Diversity The coherence bandwidth of amobile radio channel is the bandwidth over which the signal propagation characteristics are correlated and it can be approximated byThe channel is frequency-selective if the signal bandwidth B is larger than the coherence bandwidth.On the other hand, if B is smaller than , the channel is frequency nonselective or flat.The coherence bandwidth of the channel is of importance for evaluating the performance of spreading and frequency interleaving techniques that try to exploit the inherent frequency diversity Df of the mobile radio channel.In the case of multi-carrier transmission, frequency diversity is exploited if the separation of sub-carriers transmitting the same information exceeds the coherence bandwidth.The maximum achievable frequency diversity Df is given by the ratio between the signal bandwidth B and the coherence bandwidth,The coherence time of the channel is the duration over which the channel characteristics can be considered as time-invariant and can be approximated byIf the duration of the transmitted symbol is larger than the coherence time, the channel is time-selective.On the other hand, if the symbol duration is smaller than , the channel is time nonselective during one symbol duration.The coherence time of the channel is of importance for evaluating the performance of coding and interleaving techniques that try to exploit the inherent time diversity DO of the mobile radio channel.Time diversity can be exploited if the separation between time slots carrying the same information exceeds the coherence time.A number of Ns successive time slots create a time frame of duration Tfr.The maximum time diversity Dt achievable in one time frame is given by the ratio between the duration of a timeframe and the coherence time, A system exploiting frequency and time diversity can achieve the overall diversityThe system design should allow one to optimally exploit the available diversity DO.For instance, in systems with multi-carrier transmission the same information should be transmitted on different sub-carriers and in different time slots, achieving uncorrelated faded replicas of the information in both dimensions.Uncoded multi-carrier systems with flat fading per sub-channel and time-invariance during one symbol cannot exploit diversity and have a poor performance in time and frequency selective fading channels.Additional methods have to be applied to exploit diversity.One approach is the use of data spreading where each data symbol is spread by a spreading code of length L.This, in combination with interleaving, can achieve performance results which are given forby the closed-form solution for the BER for diversity reception in Rayleigh fading channels according to [40] Whererepresents the combinatory function,and σ2 is the variance of the noise.As soon as the interleaving is not perfect or the diversity offered by the channel is smaller than the spreading code length L, or MCCDMA with multiple access interference is applied,(1.22)is a lower bound.For L = 1, the performance of an OFDM system without forward error correction(FEC)is obtained, 9which cannot exploit any diversity.The BER according to(1.22)of an OFDM(OFDMA, MC-TDMA)system and a multi-carrier spread spectrum(MC-SS)system with different spreading code lengths L is shown in Figure 1-3.No other diversity techniques are applied.QPSK modulation is used for symbol mapping.The mobile radio channel is modeled as uncorrelatedRayleigh fading channel(see Section 1.1.6).As these curves show, for large values of L, the performance of MC-SS systems approaches that of an AWGN channel.Another form of achieving diversity in OFDM systems is channel coding by FEC, where the information of each data bit is spread over several code bits.Additional to the diversity gain in fading channels, a coding gain can be obtained due to the selection of appropriate coding and decoding algorithms.中文翻译 1基本原理这章描述今日的基本面的无线通信。
电芬顿法英文

电芬顿法英文Electrochemical Fenton Process: A Promising Approach for Wastewater TreatmentThe rapid industrialization and urbanization have led to the generation of a vast array of pollutants, posing a significant threat to the environment and human health. Among the various pollutants, organic contaminants have become a major concern due to their persistence, toxicity, and potential for bioaccumulation. Conventional wastewater treatment methods often struggle to effectively remove these recalcitrant organic compounds, necessitating the development of more efficient and sustainable treatment technologies.One such promising approach is the electrochemical Fenton process, which combines the principles of electrochemistry and the Fenton reaction to achieve the degradation of organic pollutants. The Fenton reaction, named after its discoverer Henry John Horstman Fenton, involves the generation of highly reactive hydroxyl radicals (•OH) through the reaction between hydrogen peroxide (H2O2) and ferrous ions (Fe2+). These hydroxyl radicals are potent oxidizing agents capable of breaking down a wide range of organiccompounds into less harmful or even harmless substances.The electrochemical Fenton process takes the Fenton reaction a step further by integrating an electrochemical system. In this approach, the ferrous ions required for the Fenton reaction are generated in situ through the electrochemical oxidation of an iron or steel electrode. This eliminates the need for the external addition of ferrous salts, which can lead to the generation of unwanted sludge. Additionally, the electrochemical system allows for the in situ production of hydrogen peroxide, further enhancing the efficiency of the Fenton reaction.The electrochemical Fenton process offers several advantages over traditional wastewater treatment methods. Firstly, it is highly effective in the degradation of a wide range of organic pollutants, including dyes, pesticides, pharmaceuticals, and industrial chemicals. The hydroxyl radicals generated during the process are capable of breaking down complex organic molecules into simpler, less harmful compounds, ultimately leading to the mineralization of the pollutants.Secondly, the electrochemical Fenton process is a relatively simple and cost-effective technology. The in situ generation of the required reagents, such as ferrous ions and hydrogen peroxide, eliminates the need for the external addition of costly chemicals, reducing theoverall operational costs. Additionally, the process can be easily integrated into existing wastewater treatment systems, making it a versatile and adaptable solution.Furthermore, the electrochemical Fenton process is considered an environmentally friendly technology. Unlike some conventional treatment methods that may generate hazardous sludge or byproducts, the electrochemical Fenton process typically produces only innocuous end products, such as carbon dioxide and water, minimizing the environmental impact.The implementation of the electrochemical Fenton process in wastewater treatment has been the subject of extensive research and development. Numerous studies have demonstrated the effectiveness of this technology in treating a wide range of organic pollutants, including dyes, pesticides, pharmaceuticals, and industrial chemicals. The process has been successfully applied at both laboratory and pilot scales, showcasing its potential for large-scale industrial applications.One of the key factors in the successful implementation of the electrochemical Fenton process is the optimization of various operating parameters, such as pH, current density, and the concentration of reactants. Researchers have explored different electrode materials, reactor configurations, and processmodifications to enhance the efficiency and performance of the system.Additionally, the integration of the electrochemical Fenton process with other treatment technologies, such as adsorption, membrane filtration, or biological treatment, has been investigated to further improve the overall treatment efficiency and expand the range of pollutants that can be effectively removed.As the global demand for sustainable and efficient wastewater treatment solutions continues to grow, the electrochemical Fenton process emerges as a promising technology that can contribute to addressing the pressing environmental challenges. With its ability to effectively degrade a wide range of organic contaminants, its cost-effectiveness, and its environmental friendliness, the electrochemical Fenton process holds great potential for widespread adoption in the field of wastewater treatment.。
赛龙智能防雾加热器温度传感器 说明书

The Thermistor for Smart DewHeater Controllers is required for automatic “smart control” of a non-Celestron heating band or heating strip with the Celestron Smart DewHeater Controllers. The thermistor allows the controller to detect how much heat the heating band is applying to the telescope. Along with the controller’s integrated environmental sensor, the thermistor helps determine how much power the heating band needs to keep the optics free from dew. This system provides the most effi cient power usage for dew prevention, greatly extending battery life. If you choose not to use the thermistor, you can only set the heating band power manually. If you are using a Celestron Dew Heater Ring, this thermistor assembly is not required, as the ring already has a built-in thermistor.If you are using another manufacturer’s heating band or strip, it needs an RCA-type plug to connect to the controller’s heater output ports. Connect the heating band to the telescope and connect its plug to one of the controller’s dew heater ports. Then, place the tip of the probe thermistor under the heating band (Figure 1). We suggest using tape to secure it fi rmly in place.THERMISTOR FOR SMART DEWHEATER CONTROLLERS# 94037INSTRUCTION MANUAL uired for automaticUALFig. 1: Place the thermistor under the heating band so that it is secure.Fig. 2: Connect the thermistor’s plug to the thermistor port corresponding to the dew heater port you are using on the controller0821SPECIFICATIONS: Thermistor plug: 2.5mm AudioFCC NOTE: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates, uses, and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to radio communications. However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one or more of the following measures:• R eorient or relocate the receiving antenna.• Increase the separation between the equipment and receiver.• Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.• Consult the dealer or an experienced radio/TV technician for help.This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This device may not cause harmful interference, and (2) this device must accept any interference received, including interference that may cause undesired operation.Please note that changes or modifications not expressly approved by the party responsible for compliance could void the user’s authority to operate the equipment.Product design and specifications are subject to change without prior notification. This product is designed and intended for use by those 14 years of age and older.© 2021 Celestron • All rights reserved/pages/technical-supportTelephone: 1(800) 421-96492835 Columbia Street • Torrance, CA 90503 U.S.A.Connect the thermistor’s plug to the thermistor port corresponding to the dew heater port you are using on the controller (Figure 2). Once the thermistor is connected, the controller will automatically adjust the power to the heating band based on the data from the thermistor and the controller’s environmental sensor.Each dew heater port on the controller can deliver a maximum of 84W power (7A max current), which should be more than enough for most dew heating bands or strips. For more information about automatic “smart control” of a dew heater, refer to the instruction manual included with your Celestron Smart DewHeater Controller.。
SEISCO商用无限热水器使用说明书

SEISCOEndless Hot Water�Copyright © 2006 Microtherm, Inc.223 West Airtex, Houston, TX. 77090, Ph:888-296-9293, Fx:281-876-3338, 5-Year Limited Warranty*Features & BenefitsFor Additional Features See Inside PageChamber and PartsHigh Efficiency Compact Design Provides Unlimited Hot Water -Commercial models provideunlimited hot water while operating within their designed gallons per minute range, immediately heating water when a hot water outlet is opened. Seisco models install inside closets, or on almost any wall. Water is only heated on de-mand, virtually eliminating standby heat loss and providing over 99% efficiency.Microprocessor Control -On-board computer logiccontrols temperature through a modified proportional,integral, derivative (PID) control scheme. The control also provides self-diagnostics, signal the need for maintenance in case of a water leak inside the heater, incorporates an optional switch for local on/off power interuption, and intel-ligently controls power distribution to the heating elements while continuously monitoring critical functions in order to shut down the water heater if a failure occurs.PowerShare™ Technology -Patented power dis-tribution control technology utilizes computer algorithms and electronic triacs with patented cooling technology to pulse power on and off to all heating elements resulting in uniform temperature modulation between 1-100% of the element’s range. PowerShare along with constantly flowing water across heating elements ensure that heat-ing elements operate at the lowest possible temperature. When the elements are turned off, low sheath temperature prevents boiling minerals out of the water causing buildup on the heating elements, prolonging element life. Power-Share also eliminates electrical disturbances in the home from the water heater, such as flickering lights.Thermistor Temperature Sensing -Five thermis-tors continuously monitor inlet/outlet water temperature and the water temperature in each heating element cham-ber. Outlet water temperature is factory set to 120°F and is field adjustable between 90-145°F. In addition, water flow is sensed using the same thermistors, eliminating the need for troublesome flow switches.Heating Elements -Industry standard 1 ½” hexhead, direct immersion, heating elements utilizePowerShare technology to help eliminate scale build-up and prolong element life far beyond that of tank type water heaters. If an element fails, a replacement can be purchased from virtually any plumbing distributor.Redundant Safety Features -Two water levelsensors are located at the top of the water heater’s chambers to prevent powering on the heating elements if sufficient water is not detected inside the water heater. In addition, two safety cutoff switches, one manual and one automatic reset, shut down power to the heating elements should an unsafe condition occur.Built-in Leak Detector and Alarm -A switchlocated inside the water heater’s cover along thebottom of the mounting plate activates an alarm when contacted by water.Code Approvals -UL/CUL 499/64, HUD MobileHome, ISRAEL, NOM, and NEC. Hydrostatically tested to 300 psi.*Limited Warranty-See written warranty for details.Two & Four Chamber Models ShownSEISCOEndless Hot Water�Copyright © 2006 Microtherm, Inc. Do You Know?223 West Airtex, Houston, TX. 77090, Ph:888-296-9293, Fx:281-876-3338, 934567821181213141516171110Cold Water InletHot Water OutletHeating ElementsMicroprocessorThermistor Temperature SensorsSafety Cutoff SwitchesTemperature Control KnobOff Peak Control ConnectionWater Leak DetectorCleanout PlatesLED Indicator LightSpeakerService ButtonPower LugsTriacs3/4” Conduit ConnectionsNon-Ferrous Water PassagesHeavy Gauge Steel MountingPanelFour Chamber Design-Inside View... the majority of stan-dard tank type commercialelectric water heaters usesurface mounted thermostatsfor temperature sensing...Seisco models use thermis-tors immersed in the waterfor more accurate tempera-ture control.... most standard tank typecommercial electric waterheaters are limited to 12KWmaximum input, one 6KWelement located in the topof th tank, and one 6KWelment located in the bottomof the tank. At maximuminput, their recovery is reallynot equivalent to 12KW/hourbecause the top heating ele-ment turns off as soon as thetop 1/3 of the tank reachesthe set point of the upperthermostat...Seisco modelsalways perform up to 100%of their rated KW input.... most tank type commer-cial electric water heatershave a 3-year warranty...Seisco models include a 5-year warranty.... most tank type commer-cial electric water heatershave standby heat loss of ap-proximately 2-3% per hour...Seisco virtually eliminatesheat loss, conserving fueland saving money on waterheating.181414149817161615151515110105551347121165653333214SEISCOEndless Hot Water�Do You Know?223 West Airtex, Houston, TX. 77090, Ph:888-296-9293, Fx:281-876-3338, MICROAdditional Features & BenefitsService Button/LED Light/Speaker-While on the phone with a Microtherm technician, the service agent pushes a service button in order to activate an audible diagnostic sequence. The service agent can also diagnose a problem through observing an LED that indicates service issues through a series of flashes.Rust and Corrosion Resistant Engineering Composite Material- All water passages are constructed of a high temperature resistant DuPont® resin. Grades of the same resin family, but with lower properties, are so strong that they are used as the “header” for automobile radiators. Ability to resist extreme temperature changes far above those found inside Seisco models and superior chemical resistance make DuPont’s engineering composite material the ideal material for Seisco’s commercial tankless electric water heaters. Continuous Venting Design-As water is heated, dissolved gases are released. Tiny bypass air passages at the top of each water chamber allow air and dissolved gases to exit unnoticed through the hot water outlet as hot water is being used.Optional Demand Side Management Connection- Allows connec-tion of an on/off control to the water heater (specify this option when ordering). Cleanout Plates-Should sediment such as sand or silt from the water supply accumulate inside the water chambers, removable plates sealed with reusable gaskets allow access for cleaning without disconnecting wires or removing the heating module and control board.¾” Non-Ferrous Inlet/Outlet Connections-Factory installed ¾” MNPT thermoplastic nipples allow easy plumbing connections and eliminate the need for dielectric fittingsHeavy Gauge Steel Mounting Panel-Modular water passages, the control board, and other components are assembled and then mounted to a heavy gauge steel back panel that includes four tabs with rubber feet for mounting the unit to the wall. A decorative plastic cover encloses all components.¾” Electrical Conduit Connection-Standard ¾” electrical conduit connections allow fast wiring of the water heater.... large commercial elec-tric tank type water heaters sequentially step heating elements on at 100% of full power to avoid flickering lights and electrical distur-bances... Seisco models, even when installed in multiples, use PowerShare technology to modulate power to the heating elements from 1-100% of full power, elimina-teing electrical disturbances. ... due to space constraints in most commercial instal-lations, it is usually improb-able that more than four commercial electric tank type water heates can be plumbed together... Seisco’s compact design allows more units to be manifolded together in the same amount of space occupied by just one commercial electric tank type water heater.... the cost of professionally replacing a heating element in a commercial electric tank type water heater can easily exceed $150... Seisco reduces the cost of repairs by prolonging element life and eliminating a large tank to drain as part of the service call.... Seisco models can be used with one or more manifolded commercial storage tank(s) to provide hot water for a large hot water demand over a short periof of time.Copyright © 2006 Microtherm, Inc.SEISCOEndless Hot Water�Copyright © 2006 Microtherm, Inc.223 West Airtex, Houston, TX. 77090, Ph:888-296-9293, Fx:281-876-3338, Suggested Written SpecificationCommercial electric water heater(s) shall be Seisco tankless water heater model _______ as manufactured by Microtherm, or an approved equal and shall have a 5-year limited warranty* when installed in a commercial application. Heater(s) shall have a rated input of _____ KW at ____ Volts. Electrical control of the heater must be accomplished using an integrated microprocessor that employs a proportional/integral/derivative (PID) control scheme using thermistors for sensing temperature and flow. Heating elements must use PowerShare technology to simultaneously vary KW input across all heating elements from 1% to maximum input. Stepping individual elements on and off at full power is not acceptable. Flow switches that restrict the flow of water in any way are not acceptable. (Optioinal-Heater(s) shall include an electrical connection for demand side control). Heater(s) shall include dual water level sensors, high limit control via the microprocessor, and dual high limit cutoff switches, one manualthe status of all monitored functions. Heater(s) shall have a built-in water leak detector. All water passages must be constructed of a high temperature resistant DuPont ® resin. Clean-cut access for removing sediment must be provided without removing plumbing connections or exposing electrical components of the water heater. Heater(s) must be UL/CUL 499, CSA, NSF , HUD Mobile Home, ISRAEL, NOM, and NEC approved.CA 12/05 R1*Limited Warranty-See written warranty for details.*Limited Warranty-See written warranty for details.Recovery (gpm & gph) @ Temperature Rise (F)*Depth: Not Shown On Drawing **Optional: 1-60 amp. breaker, ***Optional: 2-60 amp. breakers. All models are 99+% efficient.Written specifications subject to change without notice.。
非平衡热动力学 书

非平衡热动力学书英文回答:Non-equilibrium thermodynamics is a branch of thermodynamics that deals with systems that are not in thermodynamic equilibrium. In contrast to equilibrium thermodynamics, which focuses on systems in a state of balance, non-equilibrium thermodynamics studies the behavior of systems that are undergoing processes and are far from equilibrium.In non-equilibrium thermodynamics, the laws of thermodynamics still apply, but they are modified to account for the deviations from equilibrium. These modifications are necessary because non-equilibrium systems are characterized by the presence of gradients, such as temperature, pressure, and concentration gradients, which drive the flow of energy and matter.One of the key concepts in non-equilibriumthermodynamics is entropy production. Entropy is a measure of the disorder or randomness in a system, and in equilibrium, it reaches its maximum value. However, in non-equilibrium systems, entropy can be produced or consumed. This production of entropy is related to the irreversible processes that occur in non-equilibrium systems.To illustrate this concept, let's consider a cup of hot coffee placed in a room at a lower temperature. Initially, the coffee is in a non-equilibrium state because there is a temperature gradient between the coffee and the room. As time passes, heat will flow from the hot coffee to the colder room until they reach thermal equilibrium. During this process, entropy is being produced due to the irreversible heat transfer. Once equilibrium is reached, there is no more entropy production.Another important aspect of non-equilibrium thermodynamics is the concept of steady-state. A system is said to be in a steady-state when its macroscopic properties, such as temperature and pressure, remain constant over time, even though there may be continuousflow of energy or matter through the system. A common example of a steady-state system is a heat exchanger, where hot and cold fluids flow through separate channels but maintain a constant temperature difference.中文回答:非平衡热力学是热力学的一个分支,研究的是不处于热力学平衡状态的系统。
驱蚊手环纳米技术如何讲解功能作文

驱蚊手环纳米技术如何讲解功能作文英文回答:The mosquito repellent wristband, based on nanotechnology, is a revolutionary product that offers a convenient and effective solution to keep mosquitoes at bay. This innovative device utilizes the principles of nanotechnology to release a repellent substance that repels mosquitoes.One of the key functions of the mosquito repellent wristband is its ability to emit a scent that mosquitoesfind repulsive. The nanotechnology used in the wristband allows for the controlled release of this scent, ensuring that it remains effective for an extended period of time. This means that wearers can enjoy long-lasting protection against mosquito bites without the need for constant reapplication of repellent sprays or lotions.In addition to its repellent properties, the mosquitorepellent wristband also offers a convenient and non-intrusive way to protect oneself from mosquito-borne diseases. Unlike traditional methods such as mosquito nets or sprays, the wristband can be worn on the wrist like a regular accessory, allowing for freedom of movement and comfort. This makes it an ideal solution for outdoor activities such as camping, hiking, or simply enjoying a picnic in the park.Furthermore, the mosquito repellent wristband is environmentally friendly. Traditional mosquito repellents often contain harmful chemicals that can have negative effects on the environment. However, the nanotechnology used in the wristband allows for the use of natural and biodegradable repellent substances, minimizing the impact on the environment.中文回答:驱蚊手环是一种基于纳米技术的革命性产品,它提供了一种方便有效的解决方案,可以让我们远离蚊虫的困扰。
磷酸铁锂材料烧结湿度控制

磷酸铁锂材料烧结湿度控制英文回答:The control of sintering humidity for lithium iron phosphate materials is crucial for achieving high-quality sintered products. Sintering is a process in which powdered materials are heated to a temperature below their melting point, causing the particles to bond together and form a solid mass. The sintering humidity refers to the moisture content in the environment during the sintering process.Maintaining the appropriate humidity level is important because it can significantly affect the sintering process and the properties of the final product. If the humidity is too high, it can lead to the formation of undesirable defects such as cracks and voids in the sintered material. On the other hand, if the humidity is too low, it canresult in poor densification and inadequate bonding between particles.One method to control the sintering humidity is by adjusting the moisture content of the raw materials. This can be achieved by pre-drying the powdered materials before the sintering process. By removing excess moisture from the raw materials, the sintering process can be carried out under controlled humidity conditions. This ensures that the moisture content is within the desired range, promoting proper bonding and densification of the particles.Another approach is to control the humidity of the sintering atmosphere. This can be done by introducing a controlled amount of moisture into the sintering furnace or by using a humidifier. By maintaining a specific humidity level in the sintering environment, the moisture content of the raw materials can be regulated, leading to improved sintering results.It is worth noting that the optimal sintering humidity may vary depending on the specific lithium iron phosphate material and the desired properties of the final product. Therefore, it is important to conduct experiments and optimize the sintering conditions for each specific case.In conclusion, the control of sintering humidity is crucial for achieving high-quality sintered products of lithium iron phosphate materials. By adjusting the moisture content of the raw materials and controlling the humidity of the sintering atmosphere, the sintering process can be optimized to ensure proper bonding and densification of the particles.中文回答:磷酸铁锂材料的烧结湿度控制对于获得高质量的烧结产品至关重要。
MURPHYMATIC

A MURPHYMATIC ®panel offers a convenient service to users of pumps. Motor controls are in a NEMA 3 type weatherproof enclosure. This panel is used primarily to control transfer pumps, disposal pumps or pipeline pumps.In the panel at the right, the first SWICHGAGE ®shown is an OPLHFC liquid level control that senses fluid level in water storage tanks. This SWICHGAGE ®automatically stops and starts an electric motor according to pre-adjusted levels.The second SWICHGAGE ®on the panel at the right is an OPLFC that provides both high and low pressure control of the injection pumps in addition to the stop-start function, and the third SWICHGAGE ®is an OPLHFC which normally is required on waterflood installations to control the storage tank supply pump, which maintains adequate level in the tank.Some panels are equipped with a “time delay after restart” when electrical power has been interrupted by wind, storm, etc.Field hook-up time is reduced to a minimum by combining the prewiring of Murphy SWICHGAGE ®control instruments and Motor Controls at the factory.Various functions available, consult MP selector chart MP-6691-A.continued on reverse sideMP-6691B Revised 08-93Catalog Section 45(00-02-0031)MURPHYMATIC ®Pump Panels• For electric motor powered pumps • Combination motor controller and limit switch®The MURPHYMATIC ®Pump Panel for electric motor powered pumps combination motor controller and limit switch package conveniently arranged in a weather-proofenclosure. It can be custom designed to almost any user specifications.The “direct dialing ” SWICHGAGE ®s give continuous indications of line pressure and tank level during operation.026610FEATURES:A.Indicating Lights –indicate when pumps are operating or on stand-by.B.TATTLETALE ®Indicators –indicate cause when emergency shutdown occurs.C.Control Functions Module. Various Murphy transformer relay assemblymodules are available which reduce line voltages down to a 24 V control circuit.(See Bulletin MP-6691-A.)D.OPLHFC Tank Level SWICH-GAGE ®–signals transformer relay assembly to start and stop, or shutdown motor at pre-set adjustable tank levels.Provides visible indication of tank level at all times.E.OPLCFC pressure SWICH-GAGE ®–Normally used to moni-tor pump discharge pressure. Operates in conjunction with transformer relay assembly to control pump motor on changes in discharge pressure.Pulsation Dampener –reduce pointer flutter and eliminate excessive wear on gauge movement.F.Additional space is provided to incorporate supply pump transformer relay and SWICHGAGE ®.G.Magnetic Motor Starter –Dual voltage holding coil, three ambient compensated overload relays and heaters.Silver-Cadmium Oxide Contacts –Have excellent conductivity plus resistance to arc erosion and welding which assure long contact life and minimum replacement cost.Simplified Construction –All mounting screws are front accessible. All components can be removed with a standard screwdriver.H.Adjustable time delay-relay available to lockout shutdown devices during automatic startup.J.Heavy Duty H-O-A Switch allows for automatic or manual operation. Pushbutton provides for manual reset after emergency shutdown.K.Fusible disconnect with interlock handle. Door cannot be accidentally opened without disconnecting circuit. Power can be connected with door open by following instructions that accompany unit. Fuses included.L.Lighting Arrester –Absorbs excessive voltage as the result of lightning, giving reasonable protection against high voltage damage to control panel components. (Is not designed for electric motor protection.)STANDARD MP PANEL COMPONENTS INCLUDE:*•NEMA-3 weatherproof enclosure.•Three phase disconnect with safety interlock handle. Fused panel only.•Three dual element line fuses.•Breaker panel includes motor circuit protector with safety interlock handle. No fuses.•Magnetic motor starter and three ambient compensated overload relays with standard trip heaters.•Heavy Duty H-O-A Selector Switch.•Heavy Duty Manual Start Push Button.•External Overload Reset.See bulletin MP-6691-A for ordering information or contact our sales/engineering department for special applications.*Before modifications such as Skirt, Control Circuit and SWICHGAGE ®Instruments.Printed in U.S.A.In order to consistently bring you the highest quality, full featured products, we reserve the right to change our specifications and designs at any time.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ar Xi v:a st ro -p h /0202210v 1 11 F e b 2002NON-THERMAL BREMSSTRAHLUNG AS THE DOMINANT HARD X-RAY CONTINUUMMECHANISM FOR THE SUPERNOV A REMNANT MSH14-63(RCW 86)Jacco Vink 1,2,Johan A.M.Bleeker,Jelle S.Kaastra,Kurt van der Heyden 3,Andrew Rasmussen 1,John Dickel 41Columbia Astrophysics Laboratory,Columbia University,MC 5247,550W 120th street,New York,NY 10027,USA2Chandra fellow3SRON National Institute for Space Research,Sorbonnelaan 2,NL-3584CA,Utrecht,The Netherlands 4Astronomy Department,University of Illinois,1002West Green Street,Urbana,IL 61801,USAAbstractWe present an analysis of the X-ray emission of the su-pernova remnant MSH14-63,which was partially covered by four observations with XMM-Newton.The detection of Fe K emission at 6.4keV,and the lack of spatial corre-lation between hard X-ray and radio emission is evidence against a dominant X-ray synchrotron component.We ar-gue that the hard X-ray continuum is best explained by non-thermal bremsstrahlung from a supra-thermal tail to an otherwise cool electron gas.The existence of low elec-tron temperatures,required to explain the absence of line emission,is supported by low temperatures found in other parts of the remnant,which are as low as 0.2keV in some regions.Key words:ISM:individual (G315.4-2.3)–supernova rem-nants –X-rays:ISMProc.Symposium ‘New Visions of the X-ray Universe in the XMM-Newton and Chandra Era’26–30November 2001,Figure1.Mosaic of exposure cor-rected EPIC PN and MOS images covering the SW,SE and NW of MSH14-63.The color coding is as follows.Red:0.54-1.0keV;green: 1.0-1.95keV;blue:1.95-6.8keV. The labels are referred to in the text and in Fig.2.Thefigure on the right shows the1.95-6.8keV X-ray emis-sion with radio emission as observed by ATCA(Dickel et al.2000)over-layed in contours.Unfortunately,these consist of observations taken with the thinfilter,possibly resulting in a background overes-timation at low photon energies.We therefore used addi-tional off-centerfields of the publicly available observation of G21.5-0.9,a small supernova remnant,which was ob-served with the mediumfilter.In practice we only used these data to estimate the background for the soft X-ray emission from the Southeast of MSH14-63.3.Data analysisFig.1shows a mosaic of the EPIC observations.It il-lustrates the dramatic change in morphology going from energies of0.5-1keV to those>1keV,in more detail than previously observed by ASCA and BeppoSAX(Vink et al.1997;Bocchino et al.2000).As shown in Fig.2a, the spectra of the soft X-ray regions are dominated by emission lines of O VII,O VIII,Fe XVII and Ne IX,with some spatial variation in relative strength of the line emis-sion.This is illustrated with better resolution by the RGS spectrum of the narrow,bright shell in the Northwest of MSH14-63(Fig.2c).The presence of O VII is especially indicative of a low ionization,which is the result of a low electron tempera-ture,and/or a small ionization time1.A lower limit to the electron temperature is obtained by assuming collisional ionization equilibrium(CIE).This gives temperatures for the spectra in Fig.2between0.10keV and0.17keV,see Table1.2More realistic non-equilibrium ionization(NEI) models result in higher temperature estimates and some-what better spectralfits(Table1).Although there are still discrepancies between the NEI models and the data,possi-bly as a result of sharp density and temperature gradients and projection effects,they indicate that at least some regions have electron temperatures as low as0.2keV.These temperatures are lower than indicated by the ASCA and the BeppoSAX data,but in accordance withLy alphaOVII He alphaFe XVII 2p−3sLy betaNe X Fe XVII 2p−3dNe IX He alphaOVIII Ly alphaOVIII Figure 2.a)Three EPIC PN spectra arbitrary normalized to show a sequence of increasingionizationfrom toptobottom.Thelabels refer to the regions indicated in Fig.1.b)Part of the EPIC-PN spectra from the total hard X-ray emitting region in the Southeast,showing Fe K emission around 6.4keV,indicating underionized iron.c)The first order RGS spectrum of the Northwestern rim,with labels identifying the line emission (cf.Fig.2a).the ROSAT PSPC data of the Northwestern rim (Bocchino et al.2000).The electron temperatures are also consistent with shock velocities of 310-605km/s,inferred from the width of the H αand H βlines,which,under the assump-tion of full electron-ion equilibration (Ghavamian 1999;Ghavamian et al.2001),translates into shock velocities of 310-605km/s,and post shock gas temperatures of 0.11-0.42keV.Spectra from regions dominated by hard X-ray emis-sion are characterized by a dominant continuum and very faint line emission.The spectrum from “hard region 1”,which is one of the brightest hard X-ray emitting regions,shows more prominent line emission than from “hard re-gion 2”.It is not clear whether the more prominent line emission is something intrinsic to this bright X-ray knot,or that this is caused by a superposition of a soft X-ray emitting region,as this part of MSH14-63has a compli-cated morphology.Good heuristic fits to the hard X-ray spectra consist of a power law continuum and a thermal components,but currently the spectral quality of this very diffuse component is not good enough to determine for instance whether the emission lines come from a plasma out of ionization-equilibrium.Clearly,the appearance of the hard spectra in itself can be explained by X-ray syn-chrotron emission,with the line emission belonging to the thermal plasma,which is superimposed on the hard synchrotron continuum,but the XMM-Newton data show that there are two problems with this interpretation.The first problem is that the hard X-ray emitting re-gion in the Southeast as a whole shows evidence for Fe K shell emission at 6.4keV (Fig.2).This confirms the ASCA detection of Fe K emission,but for a different region,and with a lower equivalent width of 0.24keV.Model calcula-tions show that for a solar iron abundance the measured equivalent width is consistent with electron temperatures in excess of ∼3keV,and/or a power law electron distri-bution with an electron index <∼3.3Therefore,only iftions,and the bremsstrahlung gaunt factors proposed by Haug(1997).The Lotz formula was used for Fe K shell ionization cross sections,and an approximate fluorescence yield of 0.3was assumed (see e.g.Mewe (1999)).than for SN1006,where the X-ray synchrotron emission seems confined to a region close to the shock front,and is highly limb brightened(Koyama et al.1995).4.Summary and ConclusionsWe have presented spatially resolved XMM-Newton spec-troscopy of the supernova remnant MSH14-63,which shows that the hard X-ray emission is not correlated with the radio emission.Fe K emission at6.4keV is also present. Both observations contradict the idea that the hard X-ray emission is dominated by synchrotron radiation.The Fe K emission indicates the presence of electrons with energies in excess of the ionization threshold(>∼7keV), but these electrons should also give rise to bremsstrahlung.A non-thermal electron distribution seems likely,as a ther-mal distribution requires electron temperatures kT e>3keV, which is at odds with both the measured shock velocities (Ghavamian1999)and the electron temperatures of the regions dominated by soft X-ray emission.Not discussed so far is the lack of line emission from O VII,which requires that the energetic electrons are part of an otherwise very cool electron distribution(kT e<30 eV).Although we presented evidence for very low temper-atures this clearly deserves future attention.Moreover,as most electrons have low energies it means that the elec-tron density is probably much higher than indicated by the X-ray data.Low temperatures may be the result of in-sufficient electron-ion temperature equilibration.Evidence for weak electron-ion equilibration was obtained from UV spectroscopy of SN1006,indicating T e∼0.05T i,where T i is the ion temperature(Laming et al.1996).Recent cal-culations of ionization fractions for non-thermal electron distributions show that the ion fractions are quite similar to those of the equivalent temperature of the Maxwellian part of the distribution,provided that the plasma is not in ionization equilibrium,as is usually the case in supernova remnants(Porquet et al.2001).Clearly,more model cal-culations are needed to assess the effects of non-thermal electron distributions on the line emission.The evidence for non-thermal bremsstrahlung,raises the question whether other remnants may not have a sim-ilar X-ray component.This may be true,but in general it is hard to judge from hard X-ray tails alone whether the emission is non-thermal bremsstrahlung or X-ray syn-chrotron emission.The situation in MSH14-63is more for-tunate,as the overall electron temperature is relatively cool,which causes the energetic electrons to betray them-selves by causing Fe Kfluorescence emission.Moreover, it is possible that we observe MSH14-63during a special period in its evolution,as non-thermal electron distribu-tions can only exist for a time scale comparable to the electron self-equilibration time scale(τee∼108/n e s,see Itoh1984).This suggests that the extreme properties of the X-ray emission from this remnant may be related to a recent interaction of the blast wave with the steep density Table1.Summary of spectralfits to the spectra of Fig.2a. The degrees of freedom were47for CIE models(i.e.no en-try in column3)and46for NEI models.Elemental abun-dances were assumed to be bels refer to Fig.1.Soft10.088+0.03−0.01-114+136−1049.0±0.11400.14±0.0111.6±0.23.3+1.5−1.16.9±0.2106 Soft20.15±0.01-1.2±0.36.7±0.21590.37±0.1010.3±0.20.06+0.08−0.035.1±0.560 Soft30.17±0.1-1.6±0.66.9±0.11051.1±0.69.9±0.10.02+0.02−0.014.2±0.593。
