高分子材料与工程专业英语
高分子材料工程专业英语课文翻译 (2)

高分子材料工程专业英语课文翻译Polymer Science and Polymer Engineering are closely related andoften used interchangeably. Polymer Science is concerned with the chemistry and physics of polymers, while Polymer Engineering teaches students how to design and manufacture polymer products. No matter which field you choose, there is constant innovation and new developments in the field of Polymer Science and Engineering.高分子科学和高分子工程密切相关,常常互换使用。
高分子科学研究聚合物的化学和物理学,而高分子工程则教授学生如何设计和制造聚合物产品。
无论您选择哪个领域,高分子科学和工程的领域中都不断有创新和新发展。
Polymers are large molecules that are made up of repeating units called monomers. These molecules are characterized by their high molecular weight, which gives them unique properties such as strength, elasticity, and durability. There are many types of polymers, including plastics, rubbers, and fibers.聚合物是由称为单体的重复单位组成的大分子。
(完整版)高分子材料工程专业英语第二版课文翻译(基本全了

A 高分子化学和高分子物理UNIT 1 What are Polymer?第一单元什么是高聚物?What are polymers? For one thing, they are complex and giant molecules and are different from low molecular weight compounds like, say, common salt. To contrast the difference, the molecular weight of common salt is only 58.5, while that of a polymer can be as high as several hundred thousand, even more than thousand thousands. These big molecules or ‘macro-molecules’ are made up of much smaller molecules, can be of one or more chemical compounds. To illustrate, imagine that a set of rings has the same size and is made of the same material. When these things are interlinked, the chain formed can be considered as representing a polymer from molecules of the same compound. Alternatively, individual rings could be of different sizes and materials, and interlinked to represent a polymer from molecules of different compounds.什么是高聚物?首先,他们是合成物和大分子,而且不同于低分子化合物,譬如说普通的盐。
高分子材料工程专业英语课文翻译

Polymer Materials Engineering Professional EnglishText TranslationIntroductionAs an interdisciplinary field incorporating elements of both chemistry and engineering, Polymer Materials Engineering seeks to synthesize, process, and analyze polymers and polymer-based materialsfor a variety of industrial applications. Materials in this field can range from thermoplastics to thermosets, from elastomers to composites, and from gels to liquid crystals. The study of Polymer Materials Engineering is crucial for industries such as manufacturing, automotive, aerospace, healthcare, and electronics.To master Polymer Materials Engineering, one must not only have a solid foundation in engineering, chemistry, and physics, but also be proficient in technical English. Therefore, reading and translating English texts related to Polymer Materials Engineering is a vital skill for students and professionals in this field.In this article, we will provide a translation of an English text related to Polymer Materials Engineering, with the m of improving readers’ understanding and usage of specialized vocabulary in this field.Text TranslationOriginal English text:Rightly or wrongly, a connection often is made between themechanical performance of a polymeric material and its degree of crystallinity. The inference, however, can be incorrect as many other factors affect the mechanical response of polymer materials. Simply stated, crystalline regions are usually stronger and stiffer than amorphous regions. Generally, the degree of crystallinity that yields optimum properties depends on the polymer type and on the application.Translated text:通常我们会认为高分子材料的力学性能与其结晶度相关联,这种推论并不总是正确的。
高分子材料与工程

高分子材料与工程Polymer Materials & Engineering专业代码:080407 学制:4年培养目标:培养适应国家社会经济与科技发展的需求,具有良好思想素质、人文社科素养和职业道德,具有安全与环保意识和国际化视野,系统掌握高分子材料科学与工程专业的基本原理、专业技能、研究方法等知识,具备科学创新思维、不断学习和适应发展的能力,能够在材料、化工、机械等领域从事与高分子材料相关的科学研究、技术开发、工程设计、生产及经营管理等方面工作的高素质、“三创型”(创新、创造和创业)高素质专门人才。
经过本科阶段的培养以及毕业之后在工作岗位的进一步学习和锻炼,本专业学生在毕业5年左右应具备以下能力:(1)能够在与本专业相关的工业、学术等领域成功开展工作,适应独立和团队工作环境;(2)能够运用所学的大材料学科和高分子材料学科的知识来分析和解决复杂的高分子材料工程问题;(3)能够从应用目标出发对高分子材料进行成本、工艺、环保、性能和效益的综合评估及材料选用,并具有对其中涉及的相关伦理、技术进行分析和管理的能力;(4)具有良好的职业道德和“三实一新”(基础扎实、工作踏实、作风朴实、勇于创新)的优秀品质,能够通过终身学习适应职业发展,在高分子材料科学与工程领域具有不断提升的职场竞争力和全球化视野。
毕业要求:№1.工程知识:能够将数学、自然科学、工程基础和高分子材料专业知识用于解决高分子材料与工程领域复杂工程问题。
№1.1能将数学、自然科学、工程科学的语言工具用于高分子材料与工程领域复杂工程问题的表述。
№1.2能针对高分子科学与工程领域复杂工程问题具体的对象建立数学模型并求解。
№1.3能够将高分子专业相关知识和数学模型方法用于推演、分析高分子科学与工程领域复杂工程问题。
№1.4能够将高分子专业相关知识和数学方法用于高分子科学与工程领域复杂工程问题解决方案的比较与综合。
№2.问题分析:能够应用数学、自然科学及高分子材料工程的基本原理和技术方法,识别、表达、并通过文献研究分析高分子材料与工程领域复杂工程问题,以获得有效结论。
高分子材料与工程专业英语答案

高分子材料与工程专业英语答案【篇一:高分子材料专业英语第二版部分答案2】t all polymers are built up from bonding together a single kindof repeating unit. at the other extreme ,protein molecules are polyamides in which n amino acide repeat units are bonded together. although we might still call n the degree of polymerization in this case, it is less usefull,since an aminoacid unit might be any one of some 20-odd molecules that are found in proteins. in this case the molecular weightitself,rather than the degree of the polymerization ,is generally used to describe the molecule. when the actual content of individual amino acids is known,it is their sequence that is of special interest to biochemists and molecular biologists.并不是所有的聚合物都是由一个重复单元链接在一起而形成的。
在另一个极端的情形中,蛋白质分子是由n个氨基酸重复单元链接在一起形成的聚酰胺。
尽管在这个例子中,我们也许仍然把n称为聚合度,但是没有意义,因为一个氨基酸单元也许是在蛋白质中找到的20多个分子中的任意一个。
高分子材料工程专业英语词汇及部分课文翻译

专业英语词汇accordion 手风琴activation 活化(作用)addition polymer 加成聚合物,加聚物aggravate 加重,恶化agitation 搅拌agrochemical 农药,化肥Alfin catalyst 醇(碱金属)烯催化剂align 排列成行aliphatic 脂肪(族)的alkali metal 碱金属allyl 烯丙基aluminum alkyl 烷基铝amidation 酰胺化(作用)amino 氨基,氨基的amorphous 无定型的,非晶体的anionic 阴(负)离子的antioxidant 抗氧剂antistatic agent 抗静电剂aromatic 芳香(族)的arrangement (空间)排布,排列atactic 无规立构的attraction 引力,吸引backbone 主链,骨干behavior 性能,行为biological 生物(学)的biomedical 生物医学的bond dissociation energy 键断裂能boundary 界限,范围brittle 脆的,易碎的butadiene 丁二烯butyllithium 丁基锂calendering 压延成型calendering 压延carboxyl 羧基carrier 载体catalyst 催化剂,触媒categorization 分类(法)category 种类,类型cation 正[阳]离子cationic 阳(正)离子的centrifuge 离心chain reaction 连锁反应chain termination 链终止char 炭characterize 表征成为…的特征chilled water 冷冻水chlorine 氯(气)coating 涂覆cocatalyst 助催化剂coil 线团coiling 线团状的colligative 依数性colloid 胶体commence 开始,着手common salt 食盐complex 络合物compliance 柔量condensation polymer 缩合聚合物,缩聚物conductive material 导电材料conformation 构象consistency 稠度,粘稠度contaminant 污物contour 外形,轮廓controlled release 控制释放controversy 争论,争议conversion 转化率conversion 转化copolymer 共聚物copolymerization 共聚(合)corrosion inhibitor 缓释剂countercurrent 逆流crosslinking 交联crystal 基体,结晶crystalline 晶体,晶态,结晶的,晶态的crystalline 结晶的crystallinity 结晶性,结晶度crystallite 微晶decomposition 分解defect 缺陷deformability 变形性,变形能力deformation 形变deformation 变形degree of polymerization 聚合度dehydrogenate 使脱氢density 密度depolymerization 解聚deposit 堆积物,沉积depropagation 降解dewater 脱水diacid 二(元)酸diamine 二(元)胺dibasic 二元的dieforming 口模成型diffraction 衍射diffuse 扩散dimension 尺寸dimensional stability 尺寸稳定性dimer 二聚物(体)diol 二(元)醇diolefin 二烯烃disintegrate 分解,分散,分离dislocation 错位,位错dispersant 分散剂dissociate 离解dissolution 溶解dissolve 使…溶解distort 使…变形,扭曲double bond 双键dough (生)面团,揉好的面drug 药品,药物elastic modulus 弹性模量elastomer 弹性体eliminate 消除,打开,除去elongation 伸长率,延伸率entanglement 缠结,纠缠entropy 熵equilibrium 平衡esterification 酯化(作用)evacuate 撤出extrusion 注射成型extrusion 挤出fiber 纤维flame retardant 阻燃剂flexible 柔软的flocculating agent 絮凝剂folded-chain lamella theory 折叠链片晶理论formulation 配方fractionation 分级fragment 碎屑,碎片fringed-micelle theory 缨状微束理论functional group 官能团functional polymer 功能聚合物functionalized polymer 功能聚合物gel 凝胶glass transition temperature 玻璃化温度glassy 玻璃(态)的glassy 玻璃态的glassy state 玻璃态globule 小球,液滴,颗粒growing chain 生长链,活性链gyration 旋转,回旋hardness 硬度heat transfer 热传递heterogeneous 不均匀的,非均匀的hydocy acid 羧基酸hydrogen 氢(气)hydrogen bonding 氢键hydrostatic 流体静力学hydroxyl 烃基hypothetical 假定的,理想的,有前提的ideal 理想的,概念的imagine 想象,推测imbed 嵌入,埋入,包埋imperfect 不完全的improve 增进,改善impurity 杂质indispensable 不了或缺的infrared spectroscopy 红外光谱法ingredient 成分initiation (链)引发initiator 引发剂inorganic polymer 无机聚合物interaction 相互作用interchain 链间的interlink 把…相互连接起来连接intermittent 间歇式的intermolecular (作用于)分子间的intrinsic 固有的ion 离子ion exchange resin 离子交换树脂ionic 离子的ionic polymerization 离子型聚合irradiation 照射,辐射irregularity 不规则性,不均匀的isobutylene 异丁烯isocyanate 异氰酸酯isopropylate 异丙醇金属,异丙氧化金属isotactic 等规立构的isotropic 各项同性的kinetic chain length 动力学链长kinetics 动力学latent 潜在的light scattering 光散射line 衬里,贴面liquid crystal 液晶macromelecule 大分子,高分子matrix 基体,母体,基质,矩阵mean-aquare end-to-end distance 均方末端距mechanical property 力学性能,机械性能mechanism 机理medium 介质中等的,中间的minimise 最小化minimum 最小值,最小的mo(u)lding 模型mobility 流动性mobilize 运动,流动model 模型modify 改性molecular weight 分子量molecular weight distribution 分子量分布molten 熔化的monofunctional 单官能度的monomer 单体morphology 形态(学)moulding 模塑成型neutral 中性的nonelastic 非弹性的nuclear magnetic resonance 核磁共振nuclear track detector 核径迹探测器number average molecular weight 数均分子量occluded 夹杂(带)的olefinic 烯烃的optimum 最佳的,最佳值[点,状态] orient 定向,取向orientation 定向oxonium 氧鎓羊packing 堆砌parameter 参数parison 型柸pattern 花纹,图样式样peculiarity 特性pendant group 侧基performance 性能,特征permeability 渗透性pharmaceutical 药品,药物,药物的,医药的phenyl sodium 苯基钠phenyllithium 苯基锂phosgene 光气,碳酰氯photosensitizer 光敏剂plastics 塑料platelet 片晶polyamide 聚酰胺polybutene 聚丁烯polycondensation 缩(合)聚(合)polydisperse 多分散的polydispersity 多分散性polyesterification 聚酯化(作用)polyethylene 聚乙烯polyfunctional 多官能度的polymer 聚合物【体】,高聚物polymeric 聚合(物)的polypropylene 聚苯烯polystyrene 聚苯乙烯polyvinyl alcohol 聚乙烯醇polyvinylchloride 聚氯乙烯porosity 多孔性,孔隙率positive 正的,阳(性)的powdery 粉状的processing 加工,成型purity 纯度pyrolysis 热解radical 自由基radical polymerization 自由基聚合radius 半径random coil 无规线团random decomposition 无规降解reactent 反应物,试剂reactive 反应性的,活性的reactivity 反应性,活性reactivity ratio 竞聚率real 真是的release 解除,松开repeating unit 重复单元retract 收缩rubber 橡胶rubbery 橡胶态的rupture 断裂saturation 饱和scalp 筛子,筛分seal 密封secondary shaping operation 二次成型sedimentation 沉降(法)segment 链段segment 链段semicrystalline 半晶settle 沉淀,澄清shaping 成型side reaction 副作用simultaneously 同时,同步single bond 单键slastic parameter 弹性指数slurry 淤浆solar energy 太阳能solubility 溶解度solvent 溶剂spacer group 隔离基团sprinkle 喷洒squeeze 挤压srereoregularity 立构规整性【度】stability 稳定性stabilizer 稳定剂statistical 统计的step-growth polymerization 逐步聚合stereoregular 有规立构的,立构规整性的stoichiometric 当量的,化学计算量的strength 强度stretch 拉直,拉长stripping tower 脱单塔subdivide 细分区分substitution 取代,代替surfactant 表面活性剂swell 溶胀swollen 溶胀的synthesis 合成synthesize 合成synthetic 合成的tacky (表面)发粘的,粘连性tanker 油轮,槽车tensile strength 抗张强度terminate (链)终止tertiary 三元的,叔(特)的tetrahydrofuran 四氢呋喃texture 结构,组织thermoforming 热成型thermondynamically 热力学地thermoplastic 热塑性的thermoset 热固性的three-dimensionally ordered 三维有序的titanium tetrachloride 四氯化钛titanium trichloride 三氯化铁torsion 转矩transfer (链)转移,(热)传递triethyloxonium-borofluoride 三乙基硼氟酸羊trimer 三聚物(体)triphenylenthyl potassium 三苯甲基钾ultracentrifugation 超速离心(分离)ultrasonic 超声波uncross-linked 非交联的uniaxial 单轴的unsaturated 不饱和的unzippering 开链urethane 氨基甲酸酯variation 变化,改变vinyl 乙烯基(的)vinyl chloride 氯乙烯vinyl ether 乙烯基醚viscoelastic 黏弹性的viscoelastic state 黏弹态viscofluid state 黏流态viscosity 黏度viscosity average molecular weight 黏均分子量viscous 粘稠的vulcanization 硫化weight average molecular weight 重均分子量X-ray x射线x光yield 产率Young's modulus 杨氏模量课文翻译第一单元什么是高聚物?什么是高聚物?首先,他们是合成物和大分子,而且不同于低分子化合物,譬如说普通的盐。
史上最全——高分子材料与工程专业英语词汇大全
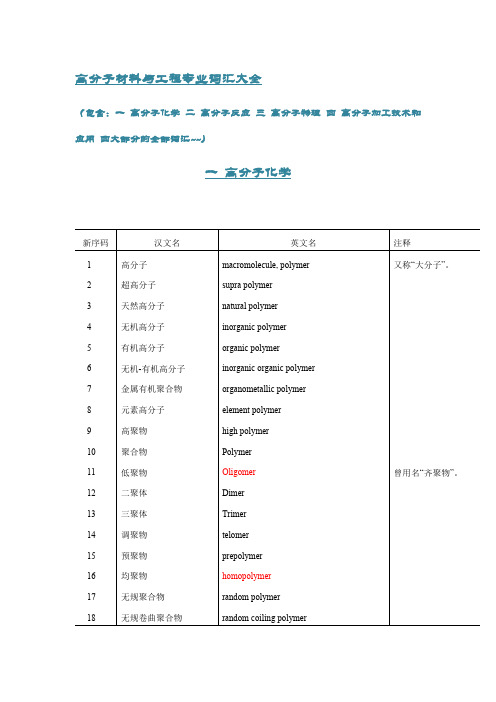
高分子材料与工程专业词汇大全(包含:一高分子化学二高分子反应三高分子物理四高分子加工技术和应用四大部分的全部词汇~~)一高分子化学新序码汉文名英文名注释1高分子macromolecule, polymer又称“大分子”。
2超高分子supra polymer3天然高分子natural polymer4无机高分子inorganic polymer5有机高分子organic polymer6无机-有机高分子inorganic organic polymer7金属有机聚合物organometallic polymer8元素高分子element polymer9高聚物high polymer10聚合物Polymer11低聚物Oligomer曾用名“齐聚物”。
12二聚体Dimer13三聚体Trimer14调聚物telomer15预聚物prepolymer16均聚物homopolymer17无规聚合物random polymer18无规卷曲聚合物random coiling polymer19头-头聚合物head-to-head polymer20头-尾聚合物head-to-tail polymer21尾-尾聚合物tail-to-tail polymer22反式有规聚合物transtactic polymer23顺式有规聚合物cistactic polymer24规整聚合物regular polymer25非规整聚合物irregular polymer26无规立构聚合物atactic polymer27全同立构聚合物isotactic polymer又称“等规聚合物”。
28间同立构聚合物syndiotactic polymer又称“间规聚合物”。
29杂同立构聚合物heterotactic polymer又称“异规聚合物”。
30有规立构聚合物stereoregular polymer, tactic polymer 又称“有规聚合物”。
高分子材料工程专业英语.pdf

在实验中,聚合速率应该根据这个系统的密度、折光率、粘度、或光吸收等许 多性质决定的。测量密度是这些方法中最精确和最灵敏的(之一)。相比单体, 聚合物的密度要增加20%~25%。在实际测定中,聚合系统的体积将以带出反应 中到膨胀计中的方式进行测量。这个带有特殊构造的毛细管容器对于细小的体 积变化可以有一个高度精确的测量。对于膨胀计法,是非常容易做到检测一个 非常小的聚合的。
聚乙烯 23. anionic
阴离子的 28. termination 终止
4.molecule
5.polymerization
分子
聚合反应
9. compound 10.molecular weight
化合物
分子量
14.characteristic 15. sodium chloride
特征
氯化钠
19. polystyrene 20.polyvinylchloride
Basic Writing II
10. Other Important Remarks About Polymer Science, Summary, Q & A
1.polymer
聚合物 6. gas 气体 11. synthesis 合成 16. product
产物 21. radical
自由基 26. initiation 引发
(2)对于离子聚合来说,不存在通过再结合反应而进行的强迫链终止,因为生长链 之间不能发生反应。链终止反应仅仅通过杂质而发生,或者说通过加入某些像水、 醇、酸、胺或氧这样的化合物进行加成而发生,且一般来说(链终止反应)可通过 这样的化合物来进行,这种化合物可以和活性聚合物离子进行反应,形成中性聚合 物或没有聚合活性的离子型聚合物。如果引发剂仅仅部分地离解,引发反应即为一 个平衡反应,在出现平衡反应的场合,在一个方向上进行链引发反应,而在另一个 方向上则发生链终止反应。
高分子材料与工程专业外语

NahNyHd4rHoPgOen4:phosphate ammonium
sodium
水合盐:结晶水读做water或hydrate
如AlCl36H2O: aluminum chloride 6-water
或
aluminum
chloride
hexahydrate
A12lK-(wSaOte4)r 212H2O aluminium potassium sulphate
二。2
其它的前缀还有 ortho-正 meta- 偏 thio-硫代 举例:
H2SO4 sulfuric acid
H2SO3 sulfurous acid
HNO3 nitric acid
HNO2 nitrous acid
HPO3 metaphosphoric acid S2O32- thiosulfate ion
cover
1. 氧化物的命名
直呼其名,即读其元素名称。 如CO: carbon monoxide ; Al2O3: aluminium oxide
N2O4 :Dinitrogen tetroxide (tetra-,mono-后缀中的a, o在后一o之前省去
CO2; SO; SO2 ;MgO ;Na2O ; P2O3
非最低价的二元化合物还要加前缀, 如O22-: peroxide O2- : superoxide acetonitrile
举例: NaF, AlCl3, Mg2N3,
Ag2S, CaC2, Fe(OH)2,H2O2
NaF: sodium fluoride AlCl3: aluminium chloride
cover
S-block Element
史上最全高分子材料和工程专业英语词汇大全
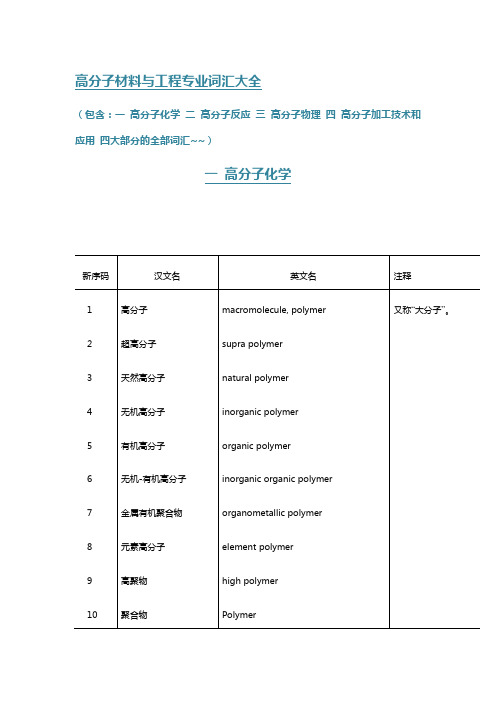
注释 又称“大分子”。
11
低聚物
12
二聚体
13
三聚体
14
调聚物
15
预聚物
16
均聚物
17
无规聚合物
18
无规卷曲聚合物
19
头-头聚合物
20
头-尾聚合物
21
尾-尾聚合物
22
反式有规聚合物
23
顺式有规聚合物
24
规整聚合物
25
非规整聚合物
26
无规立构聚合物
27
全同立构聚合物
Oligomer Dimer Trimer telomer prepolymer homopolymer random polymer random coiling polymer head-to-head polymer head-to-tail polymer tail-to-tail polymer transtactic polymer cistactic polymer regular polymer irregular polymer atactic polymer isotactic polymer
曾用名“齐聚物”。 又称“等规聚合物”。
28
间同立构聚合物
syndiotactic polymer
又称“间规聚合物”。
29
杂同立构聚合物
heterotactic polymer
又称“异规聚合物”。
30
有规立构聚合物
stereoregular polymer, tactic polymer 又称“有规聚合物”。
amphiphilic block copolymer
diblock copolymer
高分子材料工程专业英语

高分子材料工程专业英语Polymer materials engineering is a specialized field that focuses on the design, synthesis, and application of polymers. These high-performance materials are integral to various industries, including automotive, aerospace, and medical sectors.The study of polymer materials engineering involves understanding the molecular structure and properties of polymers, which are large molecules composed of repeating structural units. This knowledge is crucial for developing materials with tailored characteristics to meet specific industrial needs.In the lab, students of polymer materials engineering engage in hands-on research, synthesizing new polymers and testing their mechanical, thermal, and chemical properties. This practical experience is essential for mastering the artof material innovation.One of the key applications of polymer materials is inthe development of composites, which combine the strengths of different materials to create a product with superior performance. These composites are lighter and stronger,making them ideal for use in high-performance vehicles and structures.Environmental sustainability is another critical aspectof polymer materials engineering. Researchers are constantly exploring ways to develop biodegradable and recyclable polymers to reduce the environmental impact of material production and disposal.The future of polymer materials engineering is promising, with ongoing advancements in nanotechnology and material science opening up new possibilities for innovation. Graduates in this field can expect to be at the forefront of creating the next generation of materials that will shape our world.。
高分子材料与工程专业英语课文

UNIT 22 Mechanical Properties of Polymers聚合物的力学性能The mechanical properties of polymers are of interest in all applications where polymers are used as structural materials. Mechanical behavior involves the deformation of a material under the influence of applied forces.聚合物的力学性能感兴趣的所有应用中聚合物被用作结构材料。
机械行为涉及材料形变的影响下,施加的力。
The most important and most characteristic mechanical properties are called moduli. A modulus is the ratio between the applied stress and the corresponding deformation. The re-ciprocals of the moduli are called compliances. The nature of the modulus depends on the na-ture of the deformation. The three most important elementary modes of deformation and the moduli (and compliances) derived from them are given in Table 22.1, where the definitions of the elastic parameters are also given. ® Other very important, but more complicated, de-formations are bending and torsion. From the bending or flexural deformation the tensile modulus can be derived. The torsion is determined by the rigidity.最重要和最具特色的机械特性被称为模。
2020年高分子材料与工程专业英语翻译精编版

A 高分子化学和高分子物理UNIT 1 What are Polymer?第一单元什么是高聚物?What are polymers? For one thing, they are complex and giant molecules and are different from low molecular weight compounds like, say, common salt. To contrast the difference, the molecular weight of common salt is only 58.5, while that of a polymer can be as high as several hundred thousand, even more than thousand thousands. These big molecules or ‘macro-molecules’are made up of much smaller molecules, can be of one or more chemical compounds. To illustrate, imagine that a set of rings has the same size and is made of the same material. When these things are interlinked, the chain formed can be considered as representing a polymer from molecules of the same compound. Alternatively, individual rings could be of different sizes and materials, and interlinked to represent a polymer from molecules of different compounds.什么是高聚物?首先,他们是合成物和大分子,而且不同于低分子化合物,譬如说普通的盐。
高分子材料与工程专业英语课文

UNIT 12 Bulk PolymerizationBulk polymerization traditionally has been defined as the formation of polymer from pure, undiluted monomers. Incidental amounts of solvents and small amounts of catalysts, promoters, and chain-transfer agents may also be present according to the classical definition. This definition, however, serves little practical purpose. It includes a wide variety of polymers and polymerization schemes that have little in common, particularly from the viewpoint of reactor design. The modern gas-phase process for polyethylene satisfies the classical definition, yet is a far cry from the methyl methacrylate and styrene polymerization which remain single-phase throughout the polymerization and are more typically thought of as being bulk. ®A common feature of most bulk polymerization and other processes not traditionally classified as such is the need to process fluids of very high viscosity. The high viscosity results from the presence of, dissolved polymer in a continuous liquid phase. Signific ant concentrations of a high molecular-weight polymer typically increase fluid viscosities by 104 or more compared to the unreacted monomers. This suggests classifying a polymerization as bulk whenever a substantial concentration of polymer occurs in the continuous phase. Although this definition encompasses a wide variety of polymerization mechanisms, it leads to unifying concepts in reactor design. The design engineer must confront the polymer in its most intractable form, i. e. , as a high viscosity solution or polymer melt.The revised definition makes no sharp distinction between bulk and solution polymerizations and thus reflects industrial practice. Several so-called bulk processes for polystyrene and ABS® use 5%~15% solvent as a processing aid and chain-transfer agent. Few successful processes have used the very large amounts of solvent needed to avoid high viscosities in the continuous phase, although this approach is sometimes used for laboratory preparations.Bulk polymerizations often exhibit a second, discontinuous phase. They frequently exhibit high exothermicity, but this is more characteristic of the reaction mechanism than of bulk polymerization as such. Bulk polymerizations of the free-radical variety are most common, although several commercially important condensation processes satisfy the revised definition of a bulk polymerization.In all bulk polymerizations, highly viscous polymer solutions and melts are handled. This fact tends to govern the process design and to a lesser extent, the process economics. Suitably robust equipment has been developed for the various processing steps, including stirred-tank and tubular reactors, flash devolatilizers, extruder reactors, and extruder devolatilizers. Equipment costs are high based on working volume, but the volumetric efficiency of bulk polymerizations is also high. If a polymer can be made in bulk, manufacturing economics will most likely favor this approach. ®It is tempting to suggest that polymer processes will gradually evolve toward bulk.® Recently, the suspension process for impact polystyrene has been supplanted by the bulk process, and the emulsion process for ABS may similarly be replaced. However, the modern gas-phase process for polyethylene appears to represent an opposite trend. It seems that polymerization technology tends to eliminate solvents and suspending fluids other than the monomers themselves. When the monomer is a solvent for the polymer, bulk processes as described in this article are chosen. When the monomer is not a solvent, suspension and slurry processes like those for polyethylene and polypropylene are employed. Hence, it is worthwhile avoiding a highly viscouscontinuous phase, but not at the price of introducing extraneous material. ®Reading MaterialsPolymerization ViscostitiesViscosity in itself is a nebulous term when describing the polymer or polymer solution in most polymerizations. Some polymerizations are carried out in water with small beads being formed and suspended in the water. The "viscosity" of such a system could actually mean the viscos.ty of the water, the viscosity of the slurry present with the beads in the water, the impeller viscosity, the process viscosity, the bulk viscosity, or the viscosity at the heat transfer surface.In the bulk polymerization method, knowledge of viscosity is of vital importance. Bulk polymerizations typically operate between 100 000 and 500 000 cP (100 and 500Pa • s) bulk viscosity. An accurate determination of the bulk viscosity is extremely important in addition to the rheology associated with the particular polymer. Because bulk polymerizations are generally high viscosity in nature, the corresponding mixing Reynolds number® is very low, normally less than 100. This is in the laminar region. Power is proportional to N2D3u in the laminar range; so the actual horsepower which the mixer will draw is proportional to viscosity. Because of this, it is a requirement that viscosity vs. shear rate data be known. For example, assume two separate companies manufacturing bulk polystyrene have presented viscosity data to mixer vendors. Both companies have stated that the bulk viscosity of this material is 300 000 cP (300Pa • s). Company A furnished only this information to the mixer suppliers. Company B furnished the bulk viscosity information in addition to the viscosity vs. shear rate data. Because the mixer manufacturer could determine the proper viscosity to load the impeller from the information that customer B furnished, the mixer recommended was a 25-hp design (18. 5 kW). Company A received a quote for a 50-hp mixer (37kW). Both mixers were for tanks of the same size and shape and operating at the same speed. Naturally the quotation for customer A will be at a higher price and a higher operating cost than for customer B. However, both mixers will accomplish the required results.About 85% of all high-viscosity materials are pseudoplastic and viscoelastic in nature. Bulk polystyrene, polyesters, and polyelectrolytes are pseudoplastic in nature. Most materials have a slope of — 0. 2 to — 0. 6 when viscosity is plotted vs. shear rate. By reviewing these data and comparing the viscosity-vs. -shear rate information with the known shear rate constant of close-clearance impellers, the impeller viscosity can be determined. The shear rate constant for anchors and helical impellers is 30.As indicated earlier, helical impellers and anchors are typically used in bulk polymerizations. However, neither of these two devices can operate effectively without viscous drag at the wall of the vessel. Without some drag the material in the tank will turn as one entire mass, and almost no mixing will occur. Therefore, in bulk polymerizations it is important to be sure that inlet pipes of low-viscosity material and reflux lines are directed toward a point at the liquid level one-half of the distance from the mixer shaft to the tank wall. This will allow incorporation of the low-viscosity material and prevent its migration to the tank walls where it could act as a lubricating layer, thereby reducing the agitation. It is also important to optimize the temperature differential A T between the bulk fluid and the heat transfer surface. Normally bulk viscosity applications only require tank jackets to obtain temperature control. A very high jackettemperature could reduce the viscosity of the material at the tank wall to a point where it acts as a lubricating boundary layer. Too cold a temperature at the tank wall could increase the viscosity dramatically to a point where the mixer is not designed to handle it. In this case a totally stagnant boundary layer at the wall could occur and product quality could be affected. Further more, damage could result to the mixer, drive motor, and vessel.Viscosities for solution polymerizations are normally 25 000 and 500 000 cP (25 and 500 Pa • s) bulk viscosity. The same problems exist with the term viscosity in this type of polymerization as in bulk polymerizations. An exact knowledge of the bulk viscosity and viscosity at the impeller are important. In lower viscosity materials, where open impellers are used , the importance of the viscosity determination is slightly reduced because the mixing Reynolds numbers are normally in the transition region where horsepower is not proportional to viscosity. Therefore, a minor change in the viscosity will have little effect on the horsepower drawn by the mixer.Most solution polymerizations use tank jackets as heat transfer media; however, some solution polymerizations require additional surface area. Again, the A T optimization is important in solution polymerizations.UNIT 14 Styrene-Butadiene CopolymerThe synthetic rubber industry, based on the free-radical emulsion process, was created almost overnight during World War I . Styrene-butadiene (GR-S) rubber created at that time gives such good tire treads that natural rubber has never regained this market. ®The GR-S Standard recipe isThis mixture is heated with stirring and at 50°C gives conversions of 5% — 6% per hour. Polymerization is terminated at 70%~75% conversion by addition of a "short-stop", such as hydroquinone (approximately 0. 1 part), to quench radicals and prevent excessive branching and microgel formation. Unreacted butadiene is removed by flash distillation, and styrene by steam-stripping in a column. After addition of an antioxidant, such as iV-phenyl-β-naphthylamine (PBNA) (1.25 parts), the latex is coagulated by the addition of brine, followed by dilute sulfuric acid or aluminum sulfate. The coagulated crumb is washed, dried, and baled for shipment.This procedure is still the basis for emulsion polymerization today. An important improvement is continuous processing illustrated in Fig. 14.1; computer modeling has also been described.In the continuous process, styrene, butadiene, soap, initiator, and activator (an auxiliary initiating agent) are pumped continuously from storage tanks through a series of agitated reactors at such a rate that the desired degree of conversion is reached at the last reactor.® Shortstop is added, the latex warmed with steam, and the unreacted butadiene flashed off. Excess styrene is steam-stripped, and the latex finished as shown in Fig. 14.1.SBR prepared from the original GR-S recipe is often called hot rubber, cold rubber is made at 5'C by using a more active initiator system. Typical recipes are given in Table 14.1. At 5*C , 60% conversion to polymer occurs in 12~15h.Cold SBR tire treads are superior to those of hot SBR. Polymers with abnormally high molecular weight (and consequently too tough to process by ordinary factory equipment) can beprocessed after the addition of up to 50 parts of petroleum-base oils per hundred parts of rubber (phr) . These oil extenders make the rubbers more processible at lower cost and with little sacrifice in properties; they are usually emulsified and blended with the latex before coagulation.Recent trends have been toward products designed for specific uses. The color of SBR, which is important in many nontire uses, has been improved by the use of lighter-colored soaps, shortstops, antioxidants, and extending oils. For example, dithiocarbamates are substituted for hydroquinone as shortstop ; the latter is used on hot SBR where dark color is not objectionable. A shortstop such as sodium dimethyldithiocarbamate is more effective in terminating radicals and destroying peroxides at the lower temperatures employed for the cold rubbers.Free-radical dissociative initiators that function by dissociation of a molecule or ion into two radical species are normally limited to inorganic persulfates in the case of butadiene polymerization.The other important class of free-radical initiators, redox systems, contain two or more components that react to produce free radicals. Dodecyl mercaptan added to control molecular weight also appears to aid free-radical formation by reaction with persulfate. The commercial importance of such chain-transfer agents or modifiers cannot be overemphasized. ® Without molecular weight control the rubbers would be too tough to process.Reading MaterialsSteady-State Multilicity in Continuous Emulsion Polymerization The phenomenon of multiple steady states is seen in emulsion polymerization. Fig. 14. 2 is a plot of steady-state monomer conversion as a function of reactor residence time for methyl methacrylate emulsion polymerization in a CSTR. A region of multiplicity is indicated by the fact that the upper and lower branches of the curve overlap between residence times of 30 and 50 minutes. The dotted line is an estimate of the shape of the unstable middle branch which is experimentally unobservable. The dashed lines indicate experimental instances of ignition and extinction. At 50 minutes residence time the system has been observed to move from the lower steady state of 54% conversion to the upper steady state at approximately 80% with no discernible change in operating conditions (ignition). Extinction has been observed when the residence time is changed from 30 minutes to 20 minutes on the upper branch, resulting in a drop in conversion from the upper to the lower steady-state values. The phenomenon of multiple steady states arises in emulsion polymerization for much the same reason as it appears in solution polymerization: the autocatalytic nature of the polymerization (due to the gel effect), combined with the mass balance, results in the possibility of steady-state multiplicity.Steady-state multiplicity can be an operational problem for a number of reasons. If one wishes to operate at an intermediate level of monomer conversion (perhaps to minimize viscosity or prevent excessive chain branching), one may be forced to operate in the unstable region, relying on closed-loop control to stabilize the operating point. This is tricky at best. Additionally, the steady state (upper or lower) to which the system goes on start-up will depend on how the start-up is effected. A careful start-up policy may be needed to assure that the system arrives at the desired steady state. In general, a conservative start-up, with the temperature and initiator concentration brought to steady-state values slowly will result in operation on the lower branch, while aggressive start-up (high temperature and/or high initiator concentration during start-up) will result in steady-state operation on the upper branch. Finally, large upsets in the process may causeignition or extinction. This may lead to loss of temperature control in the case of ignition, or loss of reactor productivity in the case of extinction. A system designed to operate at the upper steady state will be operating way below design product yield at the lower steady state. Additionally, the product quality (MWD, CCD, etc. ) will be different for the two operating points. The polymerisation reactor designer should be aware of the potential for multiplicity, and, if possible, design the system to operate outside this region.CSTR polymerization reactors can also be subject to oscillatory behavior. A nonisothermal CSTR free radical solution polymerisation can exhibit damped oscillatory approach to a steady state, unstable (growing) oscillations upon disturbance, and stable (limit cycle) oscillations in which the system never reaches steady state,and never goes unstable, but continues to oscillate with a fixed period and amplitude. However, these Phenomena are more commonly observed in emulsion polymemation.High-volume products such as styrene-butadiene rubber (SBR) often are produced by continuous emulsion polymerization. This is most often done in a train of 5~15 CSTRs in series. Sustained oscillations (limit cycles) in conversion, particle number, and free emulsifier concentration gave been reported, under isothermal conditions in continuous emulsion polymerization systems. This limit cycle behavior leaves its mark on the product in the form of disturbances in the molecular weight distribution and particle size distribution which cannot be blended away. Fig. 14. 3 shows evidence of a sustained oscillation (limit cycle) during emulsion polymerization of methyl methacrylate in a single CSTR. Com parison of the monomer conversion and surface tension data graphically illustrates the mechanism of oscillation. It will be noted that the surface tension oscillates with the same period as the conversion (6—7 residence times). This can be explained with the classical micellar initiation mechanism (or with homogeneous nucleation) . Beginning at a time of about 300 minutes, the conversion rises rapidly as new particles form and old particles grow. As the particle surface area increases , additional surfactant is adsorbed on the particles. Meanwhile micelles dissociate to keep the aqueous phase saturated. Once all of the micelles have dissociated, it is no longer possible to maintain the aqueous phase at saturation, and the surface tension begins to rise. This is observed at about 320 minutes. At the point at which micelles are no longer present, micellar initiation stops and the rate of polymerization slows. Eventually, since particles are washing out while no new particles are being formed, the conversion begins to fall. Since the total particle surface area is decreasing at this point, and since surfactant is continually being introduced with the feed, the surface tension falls as the aqueous phase reapproaches saturation. As the aqueous phase becomes saturted initiation begins again. Saturation of aqueous phase may be observed by noting the point at which the surface tension reaches its CMC value. As new micelles are formed they adsorb free radicals, become polymer particles, and begin to grow and adsorb surfactant. The cycle then repeats.Modeling studies show that while the instability arises above the CMC (and is promoted by large values of initiator concentration and residence time, and low surfactant concentration), it is the on/off nature of the nucleation mechanism which governs the nature of oscillations in monomer conversion. The surface tension oscillation leads the conversion oscillation by approximately one residence time. This is consistent with the above explanation since changes in surfactant concentration are quite rapid while changes in the number of particles and rate of reaction require a finite growth time to appear as changes in the monomer conversion. Dampedoscillations at start-up have been noted for a large number of monomer systems.Damped oscillations will result in lost productivity since the product during these transients may be off quality. Unstable oscillations will, of course, preclude continued operation. Limit cycle oscillations, while not unstable, will result in a product having a quality (MWD, CCD, etc. ) which varies with time in a cyclic fashion. In most cases this is undesirable. As in the case of multiplicity, the polymerisation reactor designer must be aware of the potential for oscillatory phenomena, and should attempt to specify operating conditions at which these phenomena do not exist. In emulsion polymerizations • oscillations (both damped and sustained) are undesirable since the product is not of a consistent quality. and oscillations in free surfactant concentration may induce coagulation and reactor fouling. Several methods of eliminating oscillations in emulsion polymerization have been suggested. Poehlein has used a plug flow reactor upstream of a CSTR train. All polymer particles are nucleated in the PFR. Since PFR kinetics are essentially those of a batch reactor (and such oscillations do not occur in batch reactors), no oscillations occur. The CSTRs, then, are used to grow the existing particles. By segregating particle nucleation from particle growth, oscillations are eliminated. Another approach has been taken by Penlidis and others. This involves using a small CSTR as a seeder reactor. AH polymer particles are formed in the seeder. A portion of the monomer and water is bypassed around the seeder in such a way as to dilute out any remaining micelles in the reactor immediately following the seeder. Once again, nucleation and growth have been segregated, and oscillations are eliminated.UNIT 22 Mechanical Properties of PolymersThe mechanical properties of polymers are of interest in all applications where polymers are used as structural materials. Mechanical behavior involves the deformation of a material under the influence of applied forces.The most important and most characteristic mechanical properties are called moduli. A modulus is the ratio between the applied stress and the corresponding deformation. The re-ciprocals of the moduli are called compliances. The nature of the modulus depends on the na-ture of the deformation. The three most important elementary modes of deformation and the moduli (and compliances) derived from them are given in Table 22.1, where the definitions of the elastic parameters are also given. ® Other very important, but more complicated, de-formations are bending and torsion. From the bending or flexural deformation the tensile modulus can be derived. The torsion is determined by the rigidity.Cross-linked elastomers are a special case. Due to the cross-links this polymer class shows hardly any flow behavior. The kinetic theory of rubber elasticity was developed by Kuhn , Guth, James, Mark, Flory, Gee and Treloar. It leads, for Y oung's modulus at low strains, to the following equation sE=3RTp/Mcrl = 3zcrlRT/V=3C0The paragraphs above dealt with purely elastic deformations, i. e. deformations in which the strain was assumed to be a time-independent function of the stress. In reality, materials are never purely elastic: under certain circumstances they have nonelastic properties. This is especially true of polymers, which may show nonelastic deformation under circum-stances in which metals may be regarded as purely elastic. ® It is customary to use the ex-pression viscoelastic deformations that are not purely elastic. Literally the term viscoelastic means thecombinations of viscous and elastic properties. As the stress-strain relationship in viscous deformations is time-dependent, viscoelastic phenomena always involve the change of properties with time. Measurement of the response in deformation of a viscoelastic material to periodic forces, for instance during forced vjbration, shows that stress and strain are not in phase; the strain lags behind the stress by a phase angle 8, the loss angle. So the moduli of the materials, the complex moduli, include the storage moduli which determine the amount of recoverable energy stored as elastic energy, and the loss moduli which determine the dissipation of energy as heat when the material is deformed.UNIT 23 Thermal Properties of PolymerThe heat stability is closely related to the transition and decomposition temperature, i. e. to intrinsic properties. By heat stability is exclusively understood the stability (or re-tention) of properties (weight, strength, insulating capacity, etc. ) under the influence of heat. The melting point or the decomposition temperature invariably form the upper limit; the "use temperature" may be appreciably lower.The way in which a polymer degrades under the influence of thermal energy in an inert atmosphere is determined, on the one hand, by the chemical structure of the polymer itself, on the other hand, by the presence of traces of unstable structures.Thermal degradation does not occur until the temperature is so high that primary chemi-cal bonds are separated. For many polymers thermal degradation is characterized by the breaking of the weakest bond and is consequently determined by a bond dissociation energy. Since the change in entropy is of the same order of magnitude in almost all dissociation reac-tions, it may be assumed that also the activation entropy will be approximately the same. This means that, in principle, the bond dissociation energy determines the phenomenon. So it may be expected that the temperature at which the same degree of conversion is reached will be virtually proportional to this bond dissociation energy. ®The process of thermal decomposition or pyrolysis is characterized by a number of ex-perimental indices, such as the temperature of initial decomposition, the temperature of half decomposition, the temperature of the maximum rate of decomposition, and the average en-ergy of activation. The heat resistance of a polymer may be characterized by its "initial" and "half" decomposition.There are two types of thermal decomposition: chain depolymerization and random de-composition. The former is the successive release of monomer units from a chain end or at a weak link, which is essentially the reverse of chain polymerization; ® it is often called deprop-agation or unzippering. This depolymerization begins at the ceiling temperature. Random degradation occurs by chain rupture at random points along the chain, giving a disperse mix-ture of fragments which are usually large compared with the monomer unit. The two types of thermal degradation may occur separately or in combination; the latter case is rather nor-mal. Chain depolymerization is often the dominant degradation process in vinyl polymers, whereas the degradation of condensation polymers is mainly due to random chain rupture.The overall mechanism of thermal decomposition of polymers has been studied by Wolfs et al. The basic mechanism of pyrolysis is sketched in Fig. 23.1.In the first stage of pyrolysis (<550°C) a disproportionation takes place. Part of thede-composing materials is enriched in hydrogen and evaporated as tor and primary gas, the rest forming the primary char. In the second phase (>550°C) the primary char is further decom-posed, i.e. mainly dehydrogenated, forming the secondary gas and final char. During the disproportionation reaction, hydrogen atoms of the aliphatic parts of the structural units are "shifted" to saturate" part of the aromatic radicals. The hydrogen shift during dispropor-tionation is highly influenced by the nature of the structural groups.Reading MaterialsRequirements for Heat ResistanceHeat resistance is the capacity of a material to retain useful properties for a stated period of time at elevated temperatures (≥230°C) under defined conditions, such as pressure or vacuum, mechanical load, radiation, and chemical or electrical influences at temperatures ranging from cryogenic to above 500°C. Both reversible and irreversible changes can occur. In a reversible change, for example, as a polymer under load approaches the glass-transition temperature Tg, deformation occurs without change in chemical structure. Reversible changes occur primarily as & function of Tg, which for the purposes of this article, i. e. , for high temperature structural polymers, must be above 230°C. The maximum-use temperature for an amorphous or semicrystalline structural resin usually depends on Tg rather than the crystalline melt temperature Tm. A semicrystalline polymer can exhibit substantial loss of mechanical properties near the Tg, depending upon the degree of crystallinity. The Tm is usually so high that in its vicinity chemical degradation occurs. Irreversible changes alter the chemical structure. For example, exceeding the thermal stability results in bond breaking.The chemical factors which influence heat resistance include primary bond strength, secondary or van der Waals bonding forces, hydrogen bonding, resonance stabilization, mechanism of bond cleavage, molecular symmetry (structure regularity), rigid intrachain structure, and cross-Unking and branching. The physical factors include molecular weight and molecular weight distribution, close packing (crystallinity ), molecular (dipolar) interac-tions, and purity.The primary bond strength is the single most important influence contributing to heat resistance. The bond dissociation energy of a carbon-carbon single bond is ~ 350 kj/mol (83.6 kcal/mol), and that of a carbon-carbon double bond is ~610kJ/mol (145.8 kcal/ mol). In aromatic systems, the latter is even higher. Known as resonance stabilization, this phenomenon adds 164~287kJ/mol (39. 2—86. 6 kcal/mol). As a result, aromatic and hete-rocyclic rings are widely used in thermally stable polymers.Secondary or van der Waals bonding forces provide additional strength and thermal stability. Dipole-dipole interaction and H bonding contribute 25 ~ 41 kj/mol (6.0 ~ 9.8 kcal/mol)toward molecular stability and affect the cohesion energy density* which influences the stiffness , Tg, melting point , and solubility. Thus , beat-resistant polymers often contain polar groups, e.g. , —CO—, —S02—, that participate in strong intermolecular associa-tion. Polymers containing electron-withdrawing groups, e. g., —CO—, as connecting groups are generally more stable than those containing electron-donating groups, e. g. . —O—.The mechanism of bond cleavage also influences thermal stability. In polysiloxanes, for example, the energy of the silicon-oxygen single bond is ~445kJ/mol (106. 4kcal/mol), and that of the silicon-carbon single bond ~328kJ/mol (78. 4kcal/mol). Although the Si—C bond would be。
高分子材料工程专业英语词汇及部分课文翻译

专业英语词汇accordion手风琴activation活化(作用)additionpolymer加成聚合物,加聚物aggravate加重,恶化agitation搅拌agrochemical农药,化肥Alfincatalyst醇(碱金属)烯催化剂align排列成行aliphatic脂肪(族)的alkalimetal碱金属allyl烯丙基aluminumalkyl烷基铝amidation酰胺化(作用)amino氨基,氨基的amorphous无定型的,非晶体的anionic阴(负)离子的antioxidant抗氧剂antistaticagent抗静电剂aromatic芳香(族)的arrangement(空间)排布,排列atactic无规立构的attraction引力,吸引backbone 主链,骨干behavior性能,行为biological生物(学)的biomedical生物医学的bonddissociationenergy键断裂能boundary界限,范围brittle脆的,易碎的butadiene丁二烯butyllithium丁基锂calendering压延成型calendering压延carboxyl羧基carrier载体catalyst催化剂,触媒categorization分类(法)category种类,类型cation正[阳]离子cationic阳(正)离子的centrifuge离心chainreaction连锁反应chaintermination链终止char 炭characterize表征成为…的特征chilledwater冷冻水chlorine氯(气)coating涂覆cocatalyst 助催化剂coil线团coiling线团状的colligative依数性colloid胶体commence开始,着手commonsalt食盐complex络合物compliance柔量condensationpolymer缩合聚合物,缩聚物conductivematerial导电材料conformation构象consistency稠度,粘稠度contaminant污物contour外形,轮廓controlledrelease控制释放controversy争论,争议conversion转化率conversion转化copolymer 共聚物copolymerization共聚(合)corrosioninhibitor缓释剂countercurrent逆流crosslinking 交联crystal基体,结晶crystalline晶体,晶态,结晶的,晶态的crystalline结晶的crystallinity结晶性,结晶度crystallite微晶decomposition分解defect缺陷deformability变形性,变形能力deformation形变deformation变形degreeofpolymerization聚合度dehydrogenate使脱氢density密度depolymerization解聚deposit堆积物,沉积depropagation降解dewater脱水diacid二(元)酸diamine二(元)胺dibasic二元的dieforming口模成型diffraction衍射diffuse 扩散dimension尺寸dimensionalstability尺寸稳定性dimer二聚物(体)diol二(元)醇diolefin 二烯烃disintegrate分解,分散,分离dislocation错位,位错dispersant分散剂dissociate离解dissolution溶解dissolve使…溶解distort使…变形,扭曲doublebond双键dough(生)面团,揉好的面drug药品,药物elasticmodulus弹性模量elastomer弹性体eliminate消除,打开,除去elongation伸长率,延伸率entanglement缠结,纠缠entropy熵equilibrium平衡esterification酯化(作用)evacuate 撤出extrusion注射成型extrusion挤出fiber纤维flameretardant阻燃剂flexible柔软的flocculatingagent絮凝剂folded-chainlamellatheory折叠链片晶理论formulation配方fractionation分级fragment碎屑,碎片fringed-micelletheory缨状微束理论functionalgroup官能团functionalpolymer功能聚合物functionalizedpolymer功能聚合物gel凝胶glasstransitiontemperature玻璃化温度glassy玻璃(态)的glassy玻璃态的glassystate玻璃态globule小球,液滴,颗粒growingchain生长链,活性链gyration旋转,回旋hardness硬度heattransfer热传递heterogeneous不均匀的,非均匀的hydocyacid羧基酸hydrogen氢(气)hydrogenbonding氢键hydrostatic流体静力学hydroxyl烃基hypothetical假定的,理想的,有前提的ideal理想的,概念的imagine想象,推测imbed嵌入,埋入,包埋imperfect不完全的improve增进,改善impurity杂质indispensable 不了或缺的infraredspectroscopy红外光谱法ingredient成分initiation(链)引发initiator引发剂inorganicpolymer无机聚合物interaction相互作用interchain链间的interlink把…相互连接起来连接intermittent间歇式的intermolecular(作用于)分子间的intrinsic固有的ion离子ionexchangeresin离子交换树脂ionic离子的ionicpolymerization离子型聚合irradiation照射,辐射irregularity不规则性,不均匀的isobutylene异丁烯isocyanate异氰酸酯isopropylate异丙醇金属,异丙氧化金属isotactic等规立构的isotropic各项同性的kineticchainlength动力学链长kinetics动力学latent 潜在的lightscattering光散射line衬里,贴面liquidcrystal液晶macromelecule大分子,高分子matrix基体,母体,基质,矩阵mean-aquareend-to-enddistance 均方末端距mechanicalproperty力学性能,机械性能mechanism机理medium介质中等的,中间的minimise最小化minimum最小值,最小的mo(u)lding模型mobility流动性mobilize运动,流动model模型modify改性molecularweight分子量molecularweightdistribution分子量分布molten熔化的monofunctional单官能度的monomer单体morphology形态(学)moulding模塑成型neutral 中性的nonelastic非弹性的nuclearmagneticresonance核磁共振nucleartrackdetector核径迹探测器numberaveragemolecularweight数均分子量occluded夹杂(带)的olefinic烯烃的optimum最佳的,最佳值[点,状态]orient定向,取向orientation定向oxonium氧鎓羊packing 堆砌parameter参数parison型柸pattern花纹,图样式样peculiarity特性pendantgroup侧基performance性能,特征permeability 渗透性pharmaceutical药品,药物,药物的,医药的phenylsodium苯基钠phenyllithium苯基锂phosgene光气,碳酰氯photosensitizer光敏剂plastics塑料platelet片晶polyamide聚酰胺polybutene聚丁烯polycondensation缩(合)聚(合)polydisperse多分散的polydispersity多分散性polyesterification聚酯化(作用)polyethylene聚乙烯polyfunctional多官能度的polymer聚合物【体】,高聚物polymeric聚合(物)的polypropylene聚苯烯polystyrene聚苯乙烯polyvinylalcohol聚乙烯醇polyvinylchloride聚氯乙烯porosity多孔性,孔隙率positive正的,阳(性)的powdery粉状的processing加工,成型purity纯度pyrolysis热解radical自由基radicalpolymerization自由基聚合radius半径randomcoil无规线团randomdecomposition无规降解reactent反应物,试剂reactive反应性的,活性的reactivity反应性,活性reactivityratio竞聚率real真是的release 解除,松开repeatingunit重复单元retract收缩rubber橡胶rubbery橡胶态的rupture断裂saturation饱和scalp筛子,筛分seal密封secondaryshapingoperation二次成型sedimentation沉降(法)segment链段segment链段semicrystalline半晶settle沉淀,澄清shaping成型sidereaction副作用simultaneously同时,同步singlebond单键slasticparameter弹性指数slurry淤浆solarenergy太阳能solubility溶解度solvent溶剂spacergroup隔离基团sprinkle喷洒squeeze挤压srereoregularity立构规整性【度】stability稳定性stabilizer稳定剂statistical统计的step-growthpolymerization逐步聚合stereoregular有规立构的,立构规整性的stoichiometric当量的,化学计算量的strength强度stretch拉直,拉长strippingtower脱单塔subdivide细分区分substitution取代,代替surfactant 表面活性剂swell溶胀swollen溶胀的synthesis合成synthesize合成synthetic合成的tacky(表面)发粘的,粘连性tanker油轮,槽车tensilestrength抗张强度terminate(链)终止tertiary三元的,叔(特)的tetrahydrofuran四氢呋喃texture结构,组织thermoforming热成型thermondynamically热力学地thermoplastic热塑性的thermoset热固性的three-dimensionallyordered三维有序的titaniumtetrachloride四氯化钛titaniumtrichloride三氯化铁torsion转矩transfer(链)转移,(热)传递triethyloxonium-borofluoride三乙基硼氟酸羊trimer三聚物(体)triphenylenthylpotassium三苯甲基钾ultracentrifugation超速离心(分离)ultrasonic超声波uncross-linked非交联的uniaxial单轴的unsaturated不饱和的unzippering开链urethane氨基甲酸酯variation变化,改变vinyl乙烯基(的)vinylchloride氯乙烯vinylether乙烯基醚viscoelastic黏弹性的viscoelasticstate黏弹态viscofluidstate黏流态viscosity黏度viscosityaveragemolecularweight黏均分子量viscous粘稠的vulcanization硫化weightaveragemolecularweight重均分子量X-rayx射线x光yield产率Young'smodulus杨氏模量课文翻译第一单元什么是高聚物什么是高聚物首先,他们是合成物和大分子,而且不同于低分子化合物,譬如说普通的盐。
高分子材料与工程专业英语词汇

05. 高分子化学05.1高分子物质05.2coiling type polymer05.2 聚合与高分子化学反应3 平均官能度average functionality4 双官能[基]单体bifunctional monomer5 三官能[基]单体trifunctional monomer6 乙烯基单体vinyl monomer1,1-亚乙烯基单体,vinylidene monomer7偏[二]取代乙烯单体1,2-亚乙烯基单体,vinylene monomer81,2-二取代乙烯单体9 双烯单体,二烯单体diene monomer10 极性单体polar monomer11 非极性单体non polar monomer12 共轭单体conjugated monomer13 非共轭单体non conjugated monomer14 活化单体activated monomer15 官能单体functional monomer16 大分子单体macromer, macromonomer17 环状单体cyclic monomer18 共聚单体comonomer19 聚合[反应]polymerization20 均聚反应homopolymerization低聚反应,oligomerization21齐聚反应(曾用名)22 调聚反应telomerization23 自发聚合spontaneous polymerization24 预聚合prepolymerization25 后聚合post polymerization26 再聚合repolymerization27 铸塑聚合, 浇铸聚合cast polymerization28 链[式]聚合chain polymerization29 烯类聚合,乙烯基聚合vinyl polymerization30 双烯[类]聚合diene polymerization31 加[成]聚[合]addition polymerization32自由基聚合,游离基聚合(曾用名) free radical polymerization, radical polymerization33控制自由基聚合,可控自由基聚合controlled radical polymerization,CRP34 活性自由基聚合living radical polymerization35 原子转移自由基聚合atom transfer radical polymerization,ATRP36 反向原子转移自由基聚合reverse atom transfer radical polymerization,RATRP37可逆加成断裂链转移reversible addition fragmentation chaintransfer,RAFT38 氮氧[自由基]调控聚合nitroxide mediated polymerization39 稳定自由基聚合stable free radical polymerization,FRP40 自由基异构化聚合free radical isomerization polymerization41 自由基开环聚合radical ring opening polymerization42 氧化还原聚合redox polymerization43无活性端聚合,死端聚合(曾用名)dead end polymerization44 光[致]聚合photo polymerization45 光引发聚合light initiated polymerization46 光敏聚合photosensitized polymerization47 四中心聚合four center polymerization48 电荷转移聚合charge transfer polymerization49 辐射引发聚合radiation initiated polymerization50 热聚合thermal polymerization51 电解聚合electrolytic polymerization52 等离子体聚合plasma polymerization53 易位聚合metathesis polymerization54 开环易位聚合ring opening metathesis polymerization,ROMP55 精密聚合precision polymerization56 环化聚合cyclopolymerization57 拓扑化学聚合topochemical polymerization58 平衡聚合equilibrium polymerization59 离子[型]聚合ionic polymerization60 辐射离子聚合radiation ion polymerization61 离子对聚合ion pair polymerization62正离子聚合,阳离子聚合cationic polymerization63 碳正离子聚合carbenium ion polymerization,carbocationicpolymerization64 假正离子聚合pseudo cationic polymerization65 假正离子活[性]聚合pseudo cationic living polymerization66 活性正离子聚合living cationic polymerization67负离子聚合,阴离子聚合anionic polymerization68 碳负离子聚合carbanionic polymerization69 活性负离子聚合living anionic polymerization70 负离子环化聚合anionic cyclopolymerization71 负离子电化学聚合anionic electrochemical polymerization72 负离子异构化聚合anionic isomerization polymerization73 烯丙基聚合allylic polymerization74 活[性]聚合living polymerization75 两性离子聚合zwitterion polymerization76 齐格勒-纳塔聚合Ziegler Natta polymerization77 配位聚合coordination polymerization78 配位离子聚合coordinated ionic polymerization79 配位负离子聚合coordinated anionic polymerization80 配位正离子聚合coordinated cationic polymerization81 插入聚合insertion polymerization82定向聚合,立构规整聚合stereoregular polymerization, stereospecific polymerization83 有规立构聚合tactic polymerization84 全同立构聚合isospecific polymerization85 不对称诱导聚合asymmetric induction polymerization86 不对称选择性聚合asymmetric selective polymerization87 不对称立体选择性聚合asymmetric stereoselective polymerization88 对映[体]不对称聚合enantioasymmetric polymerization89 对映[体]对称聚合enantiosymmetric polymerization90 异构化聚合isomerization polymerization91 氢转移聚合hydrogen transfer polymerization92 基团转移聚合group transfer polymerization,GTP93 消除聚合elimination polymerization94 模板聚合matrix polymerization,templatepolymerization95 插层聚合intercalation polymerization96 无催化聚合uncatalyzed polymerization97 开环聚合ring opening polymerization98 活性开环聚合living ring opening polymerization99 不死的聚合immortal polymerization100 酶聚合作用enzymatic polymerization聚加成反应,polyaddition101逐步加成聚合(曾用名)102 偶联聚合coupling polymerization103 序列聚合sequential polymerization104 闪发聚合,俗称暴聚flash polymerization105 氧化聚合oxidative polymerization106 氧化偶联聚合oxidative coupling polymerization107 逐步[增长]聚合step growth polymerization缩聚反应condensation polymerization,108polycondensation酯交换型聚合transesterification type polymerization, 109ester exchange polycondensation110 自催化缩聚autocatalytic polycondensation111 均相聚合homogeneous polymerization112 非均相聚合heterogeneous polymerization113 相转化聚合phase inversion polymerization114 本体聚合bulk polymerization, mass polymerization 115 固相聚合solid phase polymerization气相聚合gaseous polymerization,116gas phase polymerization117 吸附聚合adsorption polymerization118 溶液聚合solution polymerization119 沉淀聚合precipitation polymerization120 淤浆聚合slurry polymerization121 悬浮聚合suspension polymerization122 反相悬浮聚合reversed phase suspension polymerization 123 珠状聚合bead polymerization, pearl polymerization 124 分散聚合dispersion polymerization125 反相分散聚合inverse dispersion polymerization126 种子聚合seeding polymerization127 乳液聚合emulsion polymerization128 无乳化剂乳液聚合emulsifier free emulsion polymerization 129 反相乳液聚合inverse emulsion polymerization130 微乳液聚合micro emulsion polymerization131 连续聚合continuous polymerization132 半连续聚合semicontinuous polymerization133 分批聚合,间歇聚合batch polymerization134 原位聚合in situ polymerization135 均相缩聚homopolycondensation136 活化缩聚activated polycondensation137 熔融缩聚melt phase polycondensation138 固相缩聚solid phase polycondensation139 体型缩聚three dimensional polycondensation140 界面聚合interfacial polymerization141 界面缩聚interfacial polycondensation142 环加成聚合cycloaddition polymerization143 环烯聚合cycloalkene polymerization144 环硅氧烷聚合cyclosiloxane polymerization145 引发剂initiator146 引发剂活性activity of initiator147 聚合催化剂polymerization catalyst148 自由基引发剂radical initiator149 偶氮[类]引发剂azo type initiator150 2,2′偶氮二异丁腈2,2'- azobisisobutyronitrile, AIBN151 过氧化苯甲酰benzoyl peroxide, BPO152 过硫酸盐引发剂persulphate initiator153 复合引发体系complex initiation system154 氧化还原引发剂redox initiator电荷转移复合物,charge transfer complex, CTC155电荷转移络合物156 聚合加速剂,聚合促进剂polymerization accelerator157 光敏引发剂photoinitiator158 双官能引发剂bifunctional initiator,difunctional initiator 159 三官能引发剂trifunctional initiator160 大分子引发剂macroinitiator161 引发-转移剂initiator transfer agent, inifer162 引发-转移-终止剂initiator transfer agent terminator, iniferter 163 光引发转移终止剂photoiniferter164 热引发转移终止剂thermoiniferter165 正离子催化剂cationic catalyst166 正离子引发剂cationic initiator167 负离子引发剂ionioic initiator168 共引发剂coinitiator169 烷基锂引发剂alkyllithium initiator170 负离子自由基引发剂anion radical initiator171 烯醇钠引发剂alfin initiator172 齐格勒-纳塔催化剂Ziegler Natta catalyst173 过渡金属催化剂transition metal catalyst174 双组分催化剂bicomponent catalyst175 后过渡金属催化剂late transition metal catalyst 176 金属络合物催化剂metal complex catalyst 177 [二]茂金属催化剂metallocene catalyst178 甲基铝氧烷methylaluminoxane, MAO179μ氧桥双金属烷氧化物催化剂bimetallic μ-oxo alkoxides catalyst180 双金属催化剂bimetallic catalyst 181 桥基茂金属bridged metallocene182限定几何构型茂金属催化剂constrained geometry metallocene catalyst183 均相茂金属催化剂homogeneous metallocene catalyst 184 链引发chain initiation185 热引发thermal initiation186 染料敏化光引发dye sensitized phtoinitiation187 电荷转移引发charge transfer initiation188 诱导期induction period189 引发剂效率initiator efficiency190 诱导分解induced decomposition191 再引发reinitiation192 链增长chain growth, chain propagation193 增长链端propagating chain end194 活性种reactive species195 活性中心active center196 持续自由基persistent radical197 聚合最高温度ceilling temperature of polymerization 198 链终止chain termination199 双分子终止bimolecular termination200 初级自由基终止primary radical termination201 扩散控制终止diffusion controlled termination202 歧化终止disproportionation termination203 偶合终止coupling termination204 单分子终止unimolecular termination205 自发终止spontaneous termination206 终止剂terminator207 链终止剂chain terminating agent208 假终止pseudotermination209 自发终止self termination210 自由基捕获剂radical scavenger211 旋转光闸法rotating sector method212 自由基寿命free radical lifetime213 凝胶效应gel effect214 自动加速效应autoacceleration effect215 链转移chain transfer216 链转移剂chain transfer agent217 尾咬转移backbitting transfer218 退化链转移degradation (degradative) chain transfer 219 加成断裂链转移[反应]addition fragmentation chain transfer 220 链转移常数chain transfer constant①缓聚作用retardation221②延迟作用222 阻聚作用inhibition223 缓聚剂retarder224 缓聚剂,阻滞剂retarding agent225 阻聚剂inhibitor226 封端[反应]end capping227 端基terminal group228 聚合动力学polymerization kinetics229 聚合热力学polymerization thermodynamics 230 聚合热heat of polymerization231 共聚合[反应]copolymerization232 二元共聚合binary copolymerization233 三元共聚合ternary copolymerization234 竞聚率reactivity ratio235 自由基共聚合radical copolymerization236 离子共聚合ionic copolymerization237 无规共聚合random copolymerization238 理想共聚合ideal copolymerization239 交替共聚合alternating copolymerization 240 恒[组]分共聚合azeotropic copolymerization 241 接枝共聚合graft copolymerization242 嵌段共聚合block copolymerization243 开环共聚合ring opening copolymerization 244 共聚合方程copolymerization equation245 共缩聚copolycondensation246 逐步共聚合step copolymerization247 同种增长homopropagation248 自增长self propagation249 交叉增长cross propagation250 前末端基效应penultimate effect251 交叉终止cross termination252 Q值Q value253 e值e value254 Q,e概念Q, e scheme255 序列长度分布sequence length distribution 256 侧基反应reaction of pendant group257 扩链剂,链增长剂chain extender258 交联crosslinking259 化学交联chemical crosslinking260 自交联self crosslinking261 光交联photocrosslinking262 交联度degree of crosslinking263 硫化vulcanization264 固化curing265 硫[黄]硫化sulfur vulcanization266 促进硫化accelerated sulfur vulcanization 267 过氧化物交联peroxide crosslinking268 无规交联random crosslinking269 交联密度crosslinking density270 交联指数crosslinking index271 解聚depolymerization272 ①降解②退化degradation273 链断裂chain breaking274 解聚酶depolymerase275 细菌降解bacterial degradation276 生物降解biodegradation277 化学降解chemical degradation278 辐射降解radiation degradation05.3 高分子物理化学与高分子物理17三单元组triad18四单元组tetrad19五单元组pentad20无规线团random coil21自由连接链freely-jointed chain22自由旋转链freely-rotating chain23蠕虫状链worm-like chain24柔性链flexible chain25链柔性chain flexibility26刚性链rigid chain27棒状链rodlike chain28链刚性chain rigidity29聚集aggregation30聚集体aggregate31凝聚、聚集coalescence32链缠结chain entanglement33凝聚缠结cohesional entanglement34物理缠结physical entanglement35拓扑缠结topological entanglement36凝聚相condensed phase37凝聚态condensed state38凝聚过程condensing process39临界聚集浓度critical aggregation concentration 40线团-球粒转换coil-globule transition41受限链confined chain42受限态confined state43物理交联physical crosslinking44统计线团statistical coil45等效链equivalent chain46统计链段statistical segment47链段chain segment48链构象chain conformation49无规线团模型random coil model50无规行走模型random walk model51自避随机行走模型self avoiding walk model52卷曲构象coiled conformation53高斯链Gaussian chain54无扰尺寸unperturbed dimension55扰动尺寸perturbed dimension56热力学等效球thermodynamically equivalent sphere 57近程分子内相互作用short-range intramolecular interaction 58远程分子内相互作用long-range intramolecular interaction 59链间相互作用interchain interaction60链间距interchain spacing61长程有序long range order62近程有序short range order63回转半径radius of gyration64末端间矢量end-to-end vector65链末端chain end66末端距end-to-end distance67无扰末端距unperturbed end-to-end distance68均方根末端距root-mean-square end-to-end distance 69伸直长度contour length70相关长度persistence length71主链;链骨架chain backbone72支链branch chain73链支化chain branching74短支链short-chain branch75长支链long-chain branch76支化系数branching index77支化密度branching density78支化度degree of branching79交联度degree of crosslinking80网络network81网络密度network density82溶胀swelling83平衡溶胀equilibrium swelling84分子组装,分子组合molecular assembly85自组装self assembly86微凝胶microgel87凝胶点gel point88可逆[性]凝胶reversible gel89溶胶-凝胶转化sol-gel transformation90临界胶束浓度critical micelle concentration,CMC91组成非均一性constitutional heterogenity, compositionalheterogenity92摩尔质量平均molar mass average 又称“分子量平均”93数均分子量number-average molecular weight,number-average molar mass94重均分子量weight-average molecular weight,weight-average molar mass95Z均分子量Z(Zaverage)-average molecular weight,Z-molar mass96黏均分子量viscosity-average molecular weight,viscosity-average molar mass97表观摩尔质量apparent molar mass98表观分子量apparent molecular weight99聚合度degree of polymerization100动力学链长kinetic chain length101单分散性monodispersity102临界分子量critical molecular weight103分子量分布molecular weight distribution,MWD104多分散性指数polydispersity index,PID105平均聚合度average degree of polymerization106质量分布函数mass distribution function107数量分布函数number distribution function108重量分布函数weight distribution function109舒尔茨-齐姆分布Schulz-Zimm distribution110最概然分布most probable distribution 曾用名“最可几分布”111对数正态分布logarithmic normal distribution 又称“对数正则分布”112聚合物溶液polymer solution113聚合物-溶剂相互作用polymer-solvent interaction114溶剂热力学性质thermodynamic quality of solvent115均方末端距mean square end to end distance116均方旋转半径mean square radius of gyration117θ温度theta temperature118θ态theta state119θ溶剂theta solvent120良溶剂good solvent121不良溶剂poor solvent122位力系数Virial coefficient 曾用名“维里系数”123排除体积excluded volume124溶胀因子expansion factor125溶胀度degree of swelling126弗洛里-哈金斯理论Flory-Huggins theory127哈金斯公式Huggins equation128哈金斯系数Huggins coefficient129χ(相互作用)参数χ-parameter130溶度参数solubility parameter131摩擦系数frictional coefficient132流体力学等效球hydrodynamically equivalent sphere133流体力学体积hydrodynamic volume134珠-棒模型bead-rod model135球-簧链模型ball-spring [chain] model136流动双折射flow birefringence, streaming birefringence 137动态光散射dynamic light scattering138小角激光光散射low angle laser light scattering139沉降平衡sedimentation equilibrium140沉降系数sedimentation coefficient141沉降速度法sedimentation velocity method142沉降平衡法sedimentation equilibrium method143相对黏度relative viscosity144相对黏度增量relative viscosity increment145黏度比viscosity ratio146黏数viscosity number147[乌氏]稀释黏度计[Ubbelohde] dilution viscometer148毛细管黏度计capillary viscometer149落球黏度计ball viscometer150落球黏度ball viscosity151本体黏度bulk viscosity152比浓黏度reduced viscosity153比浓对数黏度inherent viscosity, logarithmic viscositynumber154特性黏数intrinsic viscosity, limiting viscosity number 155黏度函数viscosity function156零切变速率黏度zero shear viscosity157端基分析analysis of end group158蒸气压渗透法vapor pressure osmometry, VPO159辐射的相干弹性散射coherent elastic scattering of radiation160折光指数增量refractive index increment161瑞利比Rayleigh ratio162超瑞利比excess Rayleigh ratio163粒子散射函数particle scattering function164粒子散射因子particle scattering factor165齐姆图Zimm plot166散射的非对称性dissymmetry of scattering167解偏振作用depolarization168分级fractionation169沉淀分级precipitation fractionation170萃取分级extraction fractionation171色谱分级chromatographic fractionation172柱分级column fractionation173洗脱分级,淋洗分级elution fractionation174热分级thermal fractionation175凝胶色谱法gel chromatography176摩尔质量排除极限molar mass exclusion limit177溶剂梯度洗脱色谱法solvent gradient [elution] chromatography 178分子量排除极限molecular weight exclusion limit179洗脱体积elution volume180普适标定universal calibration181加宽函数spreading function182链轴chain axis183等同周期identity period184链重复距离chain repeating distance185晶体折叠周期crystalline fold period186构象重复单元conformational repeating unit187几何等效geometrical equivalence188螺旋链helix chain189构型无序configurational disorder190链取向无序chain orientational disorder191构象无序conformational disorder192锯齿链zigzag chain193双[股]螺旋double stranded helix194[分子]链大尺度取向global chain orientation195结晶聚合物crystalline polymer196半结晶聚合物semi-crystalline polymer197高分子晶体polymer crystal198高分子微晶polymer crystallite199结晶度degree of crystallinity, crystallinity200高分子[异质]同晶现象macromolecular isomorphism201聚合物形态学morphology of polymer202片晶lamella, lamellar crystal203轴晶axialite204树枝[状]晶体dendrite205纤维晶fibrous crystal206串晶结构shish-kebab structure 207球晶spherulite208折叠链folded chain209链折叠chain folding210折叠表面fold surface211折叠面fold plane212折叠微区fold domain213相邻再入模型adjacent re-entry model 214接线板模型switchboard model215缨状微束模型fringed-micelle model 216折叠链晶体folded-chain crystal 217平行链晶体parallel-chain crystal 218伸展链晶体extended-chain crystal 219球状链晶体globular-chain crystal 220长周期long period221近程结构short-range structure 222远程结构long-range structure 223成核作用nucleation224分子成核作用molecular nucleation 225阿夫拉米方程Avrami equation226主结晶primary crystallization 227后期结晶secondary crystallization 228外延结晶,附生结晶epitaxial crystallizationepitaxial growth229外延晶体生长,附生晶体生长230织构texture231液晶态liquid crystal state232溶致性液晶lyotopic liquid crystal233热致性液晶thermotropic liquid crystal234热致性介晶thermotropic mesomorphism235近晶相液晶smectic liquid crystal236近晶中介相smectic mesophase237近晶相smectic phase238条带织构banded texture239环带球晶ringed spherulite240向列相nematic phase241盘状相discotic phase242解取向disorientation243分聚segregation244非晶相amorphous phase 曾用名“无定形相”245非晶区amorphous region246非晶态amorphous state247非晶取向amorphous orientation248链段运动segmental motion249亚稳态metastable state250相分离phase separation251亚稳相分离spinodal decomposition252bimodal decomposition253微相microphase254界面相boundary phase255相容性compatibility256混容性miscibility257不相容性incompatibility258不混容性immiscibility259增容作用compatiibilizationlower critical solution temperature, LCST 260最低临界共溶(溶解)温度upper critical solution temperature , UCST 261最高临界共溶(溶解)温度262浓度猝灭concentration quenching263激基缔合物荧光excimer fluorescence264激基复合物荧光exciplex fluorescence265激光共聚焦荧光显微镜laser confocal fluorescence microscopy 266单轴取向uniaxial orientation267双轴取向biaxial orientation, biorientation268取向度degree of orientation269橡胶态rubber state270玻璃态glassy state271高弹态elastomeric state272黏流态viscous flow state273伸长elongation274高弹形变high elastic deformation275回缩性,弹性复原nerviness276拉伸比draw ratio, extension ratio277泊松比Poisson's ratio278杨氏模量Young's modulus279本体模量bulk modulus280剪切模量shear modulus281法向应力normal stress282剪切应力shear stress283剪切应变shear strain284屈服yielding285颈缩现象necking 又称“细颈现象”286屈服应力yield stress287屈服应变yield strain288脆性断裂brittle fracture289脆性开裂brittle cracking290脆-韧转变brittle ductile transition291脆化温度brittleness(brittle) temperature292延性破裂ductile fracture293冲击强度impact strength294拉伸强度tensile strength 又称“断裂强度,breaking strength”295极限拉伸强度ultimate tensile strength296抗撕强度tearing strength 又称“抗扯强度”297弯曲强度flexural strength, bending strength298弯曲模量bending modulus299弯曲应变bending strain300弯曲应力bending stress301收缩开裂shrinkage crack302剪切强度shear strength303剥离强度peeling strength304疲劳强度fatigue strength, fatigue resistance305挠曲deflection306压缩强度compressive strength307压缩永久变形compression set308压缩变形compressive deformation309压痕硬度indentation hardness310洛氏硬度Rockwell hardness311布氏硬度Brinell hardness312抗刮性scrath resistance313断裂力学fracture mechanics314力学破坏mechanical failure315应力强度因子stress intensity factor316断裂伸长elongation at break317屈服强度yield strength318断裂韧性fracture toughness319弹性形变elastic deformation320弹性滞后elastic hysteresis321弹性elasticity322弹性模量modulus of elasticity323弹性回复elastic recovery324不可回复形变irrecoverable deformation325裂缝crack 俗称“龟裂”326银纹craze327形变;变形deformation328永久变形deformation set329剩余变形residual deformation330剩余伸长residual stretch331回弹,回弹性resilience332延迟形变retarded deformation333延迟弹性retarded elasticity334可逆形变reversible deformation335应力开裂stress cracking336应力-应变曲线stress strain curve337拉伸应变stretching strain338拉伸应力弛豫tensile stress relaxation339热历史thermal history340热收缩thermoshrinking341扭辫分析torsional braid analysis,TBA 342应力致白stress whitening343应变能strain energy344应变张量strain tensor345剩余应力residual stress346应变硬化strain hardening347应变软化strain softening348电流变液electrorheological fluid349假塑性pseudoplastic350拉胀性auxiticity351牛顿流体Newtonian fluid352非牛顿流体non-Newtonian fluid353宾汉姆流体Bingham fluid354冷流cold flow355牛顿剪切黏度Newtonian shear viscosity 356剪切黏度shear viscosity357表观剪切黏度apparent shear viscosity358剪切变稀shear thinning359触变性thixotropy360塑性形变plastic deformation361塑性流动plastic flow362体积弛豫volume relaxation363拉伸黏度extensional viscosity364黏弹性viscoelasticity365线性黏弹性linear viscoelasticity366非线性黏弹性non-linear viscoelasticity367蠕变creep368弛豫[作用] relaxation 又称“松弛”369弛豫模量relaxation modulus370蠕变柔量creep compliance371热畸变温度heat distortion temperature372弛豫谱relaxation spectrum373推迟[时间]谱retardation [time] spectrum374弛豫时间relaxation time375推迟时间retardation time376动态力学行为dynamic mechanical behavior377动态黏弹性dynamic viscoelasticity378热-机械曲线thermo-mechanical curve379动态转变dynamic transition380储能模量storage modulus381损耗模量loss modulus382复数模量complex modulus383复数柔量complex compliance384动态黏度dynamic viscosity385复数黏度complex viscosity386复数介电常数complex dielectric permittivity387介电损耗因子dielectric dissipation factor388介电损耗常数dielectric loss constant389介电弛豫时间dielectric relaxation time390玻璃化转变glass transition391玻璃化转变温度glass-transition temperature05.4 高分子加工技术和应用。
高分子材料与工程专业英语词汇[1]
![高分子材料与工程专业英语词汇[1]](https://img.taocdn.com/s3/m/9aa0e19cdd88d0d233d46a56.png)
Xiong Maolin, CMSE, BUCT
I
高 分 子 材 料 与 工 程 专 业 英 语 词 汇 表
graft copolymer 接枝共聚物 statistical copolymer 无规嵌段共聚物 random copolymer 无规共聚物 backbone [ ] n.主链 semicrystalline [ ]adj.半结晶的 ] adj.无定形的 amorphous [ morphology [ ] n.形态 phase [ ] n.相 living polymerization 活性聚合 compatibilizer n.增容剂 blend [ ] n.,v.共混(物) terpolymer [ ] n.三元共聚物 ] n.合金 alloy [ miscible [ ] adj.易混合的,可(溶)混的 ] n.粒子 particle [ cylinder [ ] n.圆柱体 lamella [ ] n.薄层, 薄片 synergistic adj.协同的 molecular architecture 分子构造 ] adj.线性的 linear [ short branched adj.短支化的 long branched adj.长支化的 ladder shaped adj.梯形的 star shaped adj.星形的 hyperbranched adj.超支化的 chain segment 链段 ] n.,v.蠕变 creep [ loading [ ] n.载荷 crystallinity n.结晶度 rheological [ ] adj.流变学的 stability [ ] n.稳定性 crystalline [ ] adj.结晶的 amorphous [ ] adj.无定形的 ] v.缠结 entangle [ strength [ ] n.强度 ] n.韧性 toughness [ configuration [ ] n.构型 conformation [ ] n.构象 regularity [ ] n.规整性 compactness [ ] n.紧密度[性] flexibility [ ] n.柔顺性 liquid-crystal polymer n.液晶聚合物 ] v.取向 orient [ extrusion [ ] n.挤出 injection molding 注射模塑成型 granular [ ] adj.粒状的 hopper [ ] n.漏斗 barrel [ ] n.机筒 screw [ : ] n.螺杆 ] n.顶杆 ram [ mold [ ] n.模具 eject [ ] v.顶出 cavity [ ] n.型腔 hydraulic [ ] adj.液压传动的 polyphenylene oxide 聚苯醚 compression molding 挤压模塑成型 ] v.固化 cure [ flash [ ] n.溢料 ] n.脱模 knockout [ pin [ ] n.销钉 overflow [ ] n.溢料口 runner [ ]n.流道,浇口 transfer molding 传递模塑成型 blow molding 吹塑成型 split mold 对开模具 ] n.(玻璃、塑料等)型坯 parison [ annulus [ ] n.环形套筒 extrusion [ ] n.挤出 die [ ] n.模具 thermoforming [ ] n.热成型 ] v.下垂 sag [ calendering n.压延 ] v.塑炼 plasticate [ vulcanize [ ] v.硫化 vulcanization [ ] n.硫化 casting [ ] n.浇铸 catalyze [ ] v.催化 ] n.固化,凝固 solidification [ urethane n.尿烷,氨基甲酸乙酯 reaction injection molding 反应注射模塑成型 reactant [ ] n.反应物 polyurethane [ ] n.聚氨酯 sintering [ ] n.烧结 coalescence [ ] n.融合 foam molding 发泡模塑成型 hardness [ ] n.硬度 flexural strength 抗挠(弯)强度 impact [ ] n., vt 冲击
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
第二单元链式聚合反应Staudinger第一个发现一例现象,许多烯烃和不饱和烯烃通过打开双键可以形成链式大分子。
二烯烃以同样的方式聚合,然而,仅限于两个双键中的一个。
这类反应是通过单体分子首先加成到引发剂自由基或引发剂离子上而进行的,靠这些反应活性中心由引发剂转移到被加成的单体上。
以同样的方式,借助于链式反应,单体分子一个接一个地被加成(每秒2000~20000个单体)直到活性中心通过不同的反应类型而终止。
聚合反应是链式反应的原因有两种:因为反应动力学和因为作为反应产物它是一种链式分子。
链分子的长度与动力学链长成正比。
链式反应可以概括为以下过程(R·相当与引发剂自由基):略借助于聚合度估算的分子链长,在一个大范围内可以通过选择适宜的反应条件被改变。
通常,通过大量地制备和利用聚合物,聚合度在1000~5000范围内,但在许多情况下可低于500、高于10000。
这不应该把所有聚合物材料的分子量理解为由500,或1000,或5000个单体单元组成。
在几乎所有的事例中,聚合物材料由不同聚合度的聚合物分子的混合物组成。
聚合反应,链式反应,依照与众所周知的氯(气)-氢(气)反应和光气的分解机理进行。
双键活化过程的引发剂反应,可以通过热、辐射、超声波或引发剂产生。
用自由基型或离子型引发剂引发链式反应可以很清楚地进行观察。
这些是高能态的化合物,它们能够加成不饱和化合物(单体)并保持自由基或离子活性中心以致单体可以以同样的方式进一步加成。
对于增长反应的各个步骤,每一步仅需要相当少的活化能,因此通过一步简单的活化反应(即引发反应)即可将许多烯类单体分子转化成聚合物,这正如连锁反应这个术语的内涵那样。
因为少量的引发剂引发形成大量的聚合物原料(1:1000~1:10000),从表面上看聚合反应很可能是催化反应。
由于这个原因,通常把聚合反应的引发剂看作是聚合反应的引发剂,但是,严格地讲它们不是真正意义上的催化剂,因为聚合反应的催化剂进入到反应内部而成为一部分,同时可以在反应产物,既聚合物的末端发现。
此外离子引发剂和自由基引发剂有的是金属络合物引发剂(例如,通过四氯化钛或三氯化钛与烷基铝的反应可以得到),Z引发剂在聚合反应中起到了重要作用,它们催化活动的机理还不是十分清楚。
第三单元逐步聚合许多不同的化学反应通过逐步聚合可用于合成聚合材料。
这些反应包括酯化、酰胺化、氨基甲酸酯、芳香族取代物的形成等。
通过反应聚合反应在两种不同的官能团,如,羟基和羧基,或异氰酸酯和羟基之间。
所有的逐步聚合反应根据所使用单体的类型可分为两类。
第一类涉及两种不同的官能团单体,每一种单体仅具有一种官能团。
一种多官能团单体每个分子有两个或多个官能团。
第二类涉及含有两类官能团的单种单体。
聚酰胺的合成说明了聚合反应的两个官能团。
因此聚酰胺可以由二元胺和二元酸的反应或氨基酸之间的反应得到。
nH2N-R-NH2+nHO2C-R’-CO2H→H-(-NH-R-NHCO-R’-CO-)n-OH+(2n-1)H2O (3.1)or from the reaction of amino acids with themselvesnH2R-CO2H→ H-(-NH-R-CO-)n-OH+(n-1)H20 (3.2)两种官能团之间的反应一般来说可以通过下列反应式表示反应式略反应(3.1)说明前一种形式,而反应(3.2)具有后一种形式。
聚酯化,是否在二元酸和二元醇或羟基酸分子间进行,是逐步聚合反应过程的一个例子。
酯化反应出现在单体本体中两个单体分子相碰撞的位置,且酯一旦形成,依靠酯上仍有活性的羟基或羧基还可以进一步进行反应。
酯化的结果是单体分子很快地被消耗掉,而分子量却没有多少增加。
图3.1说明了这个现象。
例如,假定图3.1中的每一个方格代表一个羟基酸分子。
(b)中的二聚体分子,消耗二分之一的单体分子聚合物种类的聚合度(DP)是2。
(c)中当三聚体和更多的二聚体形成,大于80%的单体分子已反应,但DP仅仅还是2.5。
(d)中当所有的单体反应完,DP是4。
但形成的每一种聚合物分子还有反应活性的端基;因此,聚合反应将以逐步的方式继续进行,其每一步酯化反应的反应速率和反应机理均与初始单体的酯化作用相同。
因此,分子量缓慢增加直至高水平的单体转化率,而且分子量将继续增加直到粘度的增加使其难以除去酯化反应的水或难以找到相互反应的端基。
在A-A+B-B的聚合反应中也可以看到,精确的当量平衡是获得高分子量所必需的。
假如存在一些但官能团杂质,由于链的端基失活,反应将使分子量减少。
同样,在A-B类的缩聚反应中高纯度的单体是必要的,而且可以归结高收率的反应仅是形成聚合物的实际反应,因为副反应会破坏当量平衡。
第四单元离子聚合反应离子聚合反应,与自由基聚合反应相似,也有链反应的机理。
但是,离子聚合的动力学明显地不同于自由基聚合反应。
(1)离子聚合的引发反应仅需要很小的活化能。
因此,聚合反应的速率仅对温度有较少的依赖性。
在许多情况下离子聚合猛烈地发生甚至低于50℃(例如,苯乙烯的阴离子聚合反应在-70℃在四氢呋喃中,或异丁烯的阳离子聚合在-100℃在液态乙烯中)。
(2)对于离子聚合来说,不存在通过再结合反应而进行的强迫链终止,因为生长链之间不能发生链终止。
链终止反应仅仅通过杂质而发生,或者说通过和某些像水、醇、酸、胺或氧这样的化合物进行加成而发生,且一般来说(链终止反应)可通过这样的化合物来进行,这种化合物在中性聚合物或没有聚合活性的离子型聚合物生成的过程中可以和活性聚合物离子进行反应。
如果引发剂仅仅部分地离解,引发反应即为一个平衡反应,在出现平衡反应的场合,在一个方向上进行链引发反应,而在另一个方向上则发生链终止反应。
通常离子聚合反应能通过酸性或碱性化合物被引发。
对于阳离子聚合反应来说,BF3,AlCl3,TiCl4和SnCl4与水、或乙醇,或叔烊盐的络合物提供了部分活性。
正离子是产生链引发的化合物。
例如:(反应略)然而,BF3也可以与HCl、H2SO4和KHSO4引发阳离子聚合反应。
阴离子聚合反应的引发剂是碱金属和它们的有机金属化合物,例如苯基锂、丁基锂和三苯甲基锂,它们在不同的溶剂中或多或少地强烈分解。
所谓的Alfin催化剂就是属于这一类,这类催化剂是异丙醇钠、烯丙基钠和氯化钠的混合物。
BF3为引发剂(异丁烯为单体),证明仅在痕量水或乙醇的存在下聚合反应是可以进行的。
如果消除痕量的水,单纯的BF3不会引发聚合反应。
按照上述反应为了能形成BF3-络合物和引发剂离子水或乙醇是必需的。
但是不应将水或乙醇描述成“助催化剂”。
正与自由基聚合反应一样,通过离子聚合反应也能制备共聚物,例如,苯乙烯-丁二烯阴离子共聚物,或异丁烯-苯乙烯阳离子共聚物,或异丁烯-乙烯基醚共聚物,等等。
正如对自由基型聚合已经详细描述过那样,人们可以用所谓的竞聚率r1和r2来表征每单体对。
然而,这两个参数的实际意义不同于那些用于自由基共聚合反应的参数。
第五单元活性自由基聚合的研究进展活性聚合的传统方法是基于离子,配位或基团转移机理。
理论上活性聚合的机理只包括引发和增长反应步骤。
在紫合反应初期所有的链都被引发,然后增长反应器续下去直到所有的单体都该消框购尽。
最近开发了一种叫做话性自由基聚合的活性聚合新技术。
第一个活性自由基聚合的证实及目前对这一过程的解释或定义,应该归功于Szwaren。
到目前为止,一些活性自由基聚合过程,包括原子转移自由基联合,可逆加成-断裂链转移聚合,硝基氧介导等聚合过程一个接一个被报道。
活性自由基聚合的机理不仅完全不用于普通自由基聚合机理,也不同于传统的活性聚合机理。
活性自由基聚合依赖于向体系中引入一种可以和增长自由基进行可逆终止的试剂,形成体眠种:这种特殊的可逆引发-终止反应对于获得分子链活性来说具有决定性的重要意义。
可逆引发终止活性中心的浓度能够得以控制,这样就可以来选择适宜的反应条件,使得在整个聚反应过程中(只要没有平行反应)所有的分子链都能够以相同的速度增长。
这样就可以合成具有可控组成,结构和分子量分布的聚合物。
这些还可以提供得狭窄分布末端功能化聚合物,高纯嵌段共聚物,星型及更复杂结构高分子的合成方法。
活性自由基骏合是Ostu和他的同事于1982年率先开展的。
1985年,Solomon 等对氮氧化物稳定自由基聚合的研究使活性自由基聚合进一步发展。
这种方法首先在专利文献和会议论文中报道,但是直到1993年Georges等把这种方法应用在窄分子量分布聚苯乙烯之后,才得以广泛认知。
NMP的领域已经得到很大的延展,出现了新的更多样化的方法。
最引人注目的方法是原子转移自由基聚合和可逆加成断裂聚合。
到2000年,这个领域的论文已经占所有自由基聚合领域论文的三分之一。
如图5.1所示。
自然地,纸的数量的迅速增长在领域,因为1995在这个区域应该是几乎完全可归展的发展。
第六单元聚合物的分子量及其分布聚合物的分子量在其合成和应用方面有着最重要的作用,这种有趣又有用的力学性能仅与高分子材料相关,它是其超高分子量的-个结果。
大多数重要的力学性能都依据于分子星并且有很大的不同。
因此,直到最小相对分子质量增加到大约5000-10000以后,聚合物的强度才开始显出来。
超过这个尺寸后,当其分子量增加时,聚合物的机械性能也有一个迅速的提高;这种影响在更大的分子量的时候趋于平缓。
在大多数情况下,对于某特定的应用来说,某种聚合物存在着某一分子量范围,在这个范围之内其性能是最好的。
当人们谈到聚合物分子量的时候,他所指的是和低分子化合物的分子量完全不同的一回事。
聚合物在分子量上区别于小分子化合物是因为它们是多分散的和不均匀的。
即使-个高分子在合成中没有污物和杂质,在广义上它依旧不是一个纯物质。
高分子即使在其最纯的形式,也是不同分子量分子的混合物。
高分子多分散性的原因在于聚合过程中的统计差异里,当人们讨论聚合物的分子量时,实际上指的是它的平均分子量。
在一个聚合物中平均分子量和不同分子量的确切分布的目的是为了完全描绘它的特性。
分子量的控制和分子量的分布(MWD)经常被用来获取和提高聚合物产品中的某些所需的物理性质。
多种方法可用于实验测量聚合物样品的平均分子量。
这些包括基于依数性,光散射,粘度,超速离心分散和沉淀法。
不同的方法将获得不同的平均分子量。
(对同-聚合物)得到了不同平均分子量,因为所测得的性质对试样中不同尺寸的聚合物分子的偏差。
一些方法是偏向于较大的聚合物分子,而另一些方法是偏向于较小尺寸的分子。
结果是,平均分子量的获得是趋于较大或较小尺寸的分子。
最重要的平均分子量的确定方法是数均分子量Mn,重均分子量Mw和粘均分子量Mv。
一个聚合物样品除了不同的平均分子量,它通常还要知道分子量的确切分布。
