2010版中国药典英文
GMP(2010年修订版)中的中英文术语

ICH(International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use)人用药物注册技术要求国际协调会。
Q10:药品质量管理体系(PQS),基于QMS(质量管理体系)而来,最合适的指南应该是ICH的Q10。
Q9:质量风险管理。
Q8:药物开发。
自动化生产规范GAMP5(Good Automated Manufacturing Practice)由ISPE(International Society For Pharmaceutical Engineering国际制药工程协会。
创立于1980年,是致力于培训制药领域专家并提升制药行业水准的世界最大的非盈利性组织之一)的GAMP论坛(GAMP Forum)组织编写发布的关于制药企业计算机化系统的综合性指南。
纠正和预防措施CAPA(Corrective Action & Preventive Action)超出标准的可疑结果OOS(out of specification)质量管理 QM(Quality Management)质量保证 QA(Quality Assurance)质量控制 QC(Quality Control)生产管理 PM(Production Maanagement)工程设备管理 EM (Enginccr Equipment Managemant)销售管理 MS (Maragement Of Sales)行政人员管理AP (Administration and Personael Managemeht)工艺验证 PV (Process Validation)设备验证 EV (Equipment Validation)工艺规程 PP (Process Procedure)质量标准 QS (Quality Management)物料管理 MM (Materid Management)原料管理 RM (Raw Material Management)辅料管理 EM (Excipient Management)质量标准 QS (Quality Standard)增补和修订A.A.A Addition and Amendments空调器AC Air Conditioner药物不良反应ADR Adverse Drug Reaction食品与药品官员协会(美国)AFDO Association of Food and Drug Officials 接受ACC Accept合格质量标准AQL Acceptable Quality Level简化的新药申请ADNA Abbreviated New Drug Application物料清单BOM Bill of Material原料药BPC Bulk pharmaceutical Chemiclls生物制品评价与研究中心CBER Center for Biologics Evaluation Research 菌落形成单位CFU Colony Forming Unet药品管理档案DMF Drug Master File药物评价与研究中心CDER Cemter for Drug Evaluation amd Research企业识别(形象)CI Corporate Identity (Image)在线清洗CIP Cleaning in Place消费者安全调查员CSI Consumer Safety Insepctor在线清洗程序CLP Cleaning Line Procedure缺陷作用水平DAL Defect Action Level管制药品管理DEA Drug Enforcement Adminestration文件系统DS Documentation Systim食品与药品管理局(美国)FDA Food and Drug Administration关贸总协会GATT General Agreemernt on Tariffs and Trade药品生质量管理规范GMP Good Manufacturing Practice药品临床实验管理规范GCP Good Clinical Practice实验室管理规范GLP Good Laboratory Practice药品商业质量规范GSP Good Supply Practice药品零业质量管理规范GRP Good RaTAIL Practice药材生产管理规范GAP Good Agriculture Practice验证管理规范GVP Good Validation Prctice药品使用规范GUP Good Use Practice国际标准化组织ISO Intematonal Organization for Standardization 谅解备忘录MOU Memorandum of Understanding生产记录用表格PF Porduction File非处方药品OTC Over the Counter (Drug)产品许可申请PLA Product License Application质量管理程序QMP Quality Management Procedure国家药品监督管理局SDA State Drug Administration标准管理程序SMP Standard Managmert Procedure标准操作程序SOP Standard Operating Procedure全面质量管理TQC Tatal Quality Control美国药典USA Uneted States Pharmacopeia生产管理中的术语系统: system起始原料:starting material物料:material物料平衡:reconcilination批: batch or lot批号:batch number (lot number)批档案:batch records文件: doocument标准操作规程:standard operating processsing工艺规程:master for processing纯化水: puritied water工艺用水:water for processing蒸馏水:distilled water去离子水:deionized water注射用水:water for injection无菌:sterile灭菌:sterlization(sterilise)无菌制剂:sterile product ?(preparation,dosage from) 非无菌制剂:non-sterile product状态标志:status mark (labet)中间产品:intermediate product制造:manufacture带包装品:bulk product成品:finished product活性药物组分:active pharmaceutical ingredient理论产量:theoretical yield质量管理中的术语待验:quarantine控制点:control point质量保证:quality assurance质量控制:quality control质量管理:quality management质量体系:quality system质量监督:quality surveillance规格标准:specification生产过程中控制:in-process control返工:reprocessing退货:retured product拒收:rejected交叉污染:cross contamination放行:released质量要求:quality requirement质量管理体系:quality management system可追溯性:traceability合格(符合):conformity不合格:nonconformity预防措施:preventive action质量手册:quality manual计量确认:metrological confirmation验证术语:验证:validation空调净化系统:HVAC( heating ventilation and air conditioning) 起泡点实验:bubbling point挑战性试验:challenge test最差状况:worst case不合格限:edge-of-failure严整方案:validation protocol在线清洗:CIP(cleaning in place)在线灭菌:SIP(sterilization in place)预确认:pre qualification安装确认:IQ(instalation qualification)运行确认:OQ(operational qualitification)性能确认:PQ(performance qualificantion产品验证:PV(product validation)工艺验证:process validation前验证:prospective validation同步验证:concurrent validation回顾性验证:retrospective认证:certification其他术语:管理体系:management system组织结构:organizational structure指南:guideline销售许可证:marketing authorization计算机系统:computerized system生物反应器:biogenerator生物试剂:biological agents细胞库系统:cell bank system主细胞库:master cell bank工作细胞库:working cell bank细胞培养:cell culture种子库:seed lot主种子库:master seed lot工作种子库:working seed lot外源生物体:exotic organism放射性药品:radio pharmaceutical原植物(植物药)crude plant(vegetable drug)药用植物:medicinal plant草药品:herbal pharmaceuticalOn the Road推荐阅读:•CAPA(Corrective Action & Preventive Action)纠正2010-08-09 19:25:00•oos和偏差2010-07-09 15:27:01•车间标识中英对照2010-08-16 15:37:56•GMP文件管理常用的英文缩写2009-06-08 11:23:40。
2010版中国药典英文2012

一、药材来源
1.植(动)物来源的药材的来源部分的一般译法为 植 动 物来源的药材的来源部分的一般译法为 物来源的药材的来源部分的一般译法为: 药材英文名称is 药用部位(名词单数 名词单数) 药材英文名称 the 药用部位 名词单数 of 植(动)物拉丁学名 动 物拉丁学名 [属名和种 变种 加词为斜体字 科名 属名和种(变种 加词为斜体字](科名 属名和种 变种)加词为斜体字 科名). 例 : Pummelo Peel is the dried unripe or almost ripe exocarp of Citrus grandis “Tomentosa” or Citrus grandis (L.) Osbeck (Fam. Rutaceae). 本品为芸香科植物化州柚Citrus grandi“Tomentosa”或柚 本品为芸香科植物化州柚 或柚 Citrus grandis(L.)Osbeck的未成熟或近成熟的干燥外层果皮。 的未成熟或近成熟的干燥外层果皮。 的未成熟或近成熟的干燥外层果皮
二、理化鉴别
1.化学鉴别 化学鉴别 Shake 0. 5 g of the powder with 5 ml of ethanol for 5 minutes and filter. Evaporate the filtrate to dryness, add dropwise antimony trichloride saturation solution in chloroform and evaporate again to dryness. A violet-red colour is produced. 取本品粉末0.5g,加乙醇 , 加乙醇5ml,振摇 分 , 滤过 , 滤 分钟, 取本品粉末 , 振摇5分钟 滤过, 液蒸干, 滴加三氯化锑饱和的三氯甲烷溶液, 再蒸干, 液蒸干 , 滴加三氯化锑饱和的三氯甲烷溶液 , 再蒸干 , 即显紫红色。 即显紫红色。
2010版GMP英文版

SDA Order #79Order by Ministry of Health of the People’s Republic of ChinaPublished on February 12, 2011No. 79Good Manufacturing Practice for Pharmaceutical Products (Amended in 2010) has passed by Affairs Meeting on October 19, 2010. This Regulation is now published and shall be effective from March 1, 2011.Director Zhu CHENJanuary 17, 2011Chapter 1 General ProvisionsArticle 1 In order to standardize good manufacturing for pharmaceutical products, this Regulation is enacted in accordance with the “Drug Administration Law of the People’s Republic of China” and “The Regulation on the Implementation of Drug Administration Law of the People’s Republic of China”.Article 2 A p harmaceutical enterprise shall establish pharmaceutical goods’ quality control system. The system shall contain all factors which may affect the quality of pharmaceutical goods, including all organized and planned activities ensuring pharmaceutical goods’ quality in accordance with intending purpose.Article 3 This Regulation is part of quality control system, is basic requirement for manufacturing and quality control of pharmaceutical products. This Regulation aims to reduce the risks in pharmaceutical g oods’ manufacturing process at its maximum, such as pollution,cross pollution and confusion, mistake, ensure for continuous stably manufacturing pharmaceutical goods in accordance with intending purpose and registered requirements.Article 4 The enterprise shall obey this Regulation strictly, insist on honesty and keep faith, prohibit any ostensible and spurious activities.Chapter 2 Quality ControlSection 1 PrincipleArticle 5 The enterprise shall establish quality target in accordance with pharmaceutical goods’ quality control requirements, carry out all requirements related to safety, effective and quality control into the process of pharmaceutical goods’ manufacturing, control and products’ discharging, storag e, delivering, ensure all pharmaceutical goods are produced in accordance with intending purpose and registered requirements.Article 6 Senior administrator in enterprise shall ensure the achievement of intending quality target. Personnel in different levels and provider, dealer shall participate in and take each responsibility.Article 7 The enterprise shall equip adequate personnel, workshop, establishment and equipment in accordance with requirements, and provide essential condition for achieving quality target.Section 2 Quality GuaranteeArticle 8 Quality guarantee is a part of quality control system. The enterprise must establish quality guarantee system, and establish integrate document system at the same time, in order to ensure the system’s e ffective running.Article 9 Quality guarantee system shall ensure the following:I. Represent the requirements of this Regulation in pharmaceutical goods’ design anddevelopment.II. In accordance with the requirements of this Regulation in manufacturing management and quality control activities;III. Specific management responsibility;IV. Exact stocked and used raw material and wrapper;V. Effective control in semifinished product;VI. Implement of confirmation and validation;VII. Manufacture, examine, inspect and double examined according to rules strictly; VIII. Each batch of products shall only discharge after quality authorizing person’s approval;IX. Applicable measures to ensure pharmaceutical goods’ quality during the process of storage, delivering and all succedent operation process;X. According to self-examine rules, examine and evaluate the validity and applicability of the quality guarantee system quality.Article 10 Basic requirements of pharmaceutical goods’ manufacture quality management: I. Frame manufacturing technique, systemic review and demonstrate it couldcontinuous stably manufacturing products in accordance with requirements;II. Manufacturing technique and its important changes shall be validated;III. Equip all required resources, include, but not limited the following:1. Hold applicable qualification and the eligible trained personnel;2. Adequate workshop and space;3. Applicable equipment and maintain guarantee;4. Accurate raw material, wrapper and label;5. Approved technique rules and operate rules;6. Applicable storage and freight condition.IV. Use accurate and easy understand language to frame operate rules;V. The operate person could accurate operate according to operate rules after training;VI. The whole manufacture process shall be recorded. The windage shall be researched and be recorded;VII. Batch record and delivering record shall be traced back to the whole history of the batch of products, and the records shall be saved appropriately and be easy consult; VIII. Reduce the quality risk during the pharmaceutical goods’ delivering process;IX. Establish pharmaceutical goods’recall system, and ensure any batch delivered and sold products could be recalled;X. Survey the reasons leading to pharmaceutical goods’complaints and quality objections, take measures to prevent similar quality objections.Section 3 Quality ControlArticle 11 Quality control includes corresponding organization, document system and sampling, test and so on, to ensure material or products finish necessary examination before delivering, and to verify its quality is in accordance with the requirements.Article 12 Basic requirements of quality control:I. Equip applicable establishment, equipment, instrument and trained personnel to effective and reliable finish all related quality control activities;II. Have approved operate rules, which used to sampling, examine, inspect raw material, wrapper, semifinished product, bulk product and finished product and products’stability, monitor environment when necessary, to ensure the products is in accordance with the requirements of this Regulation;III. Authorized person shall sampling to raw material, wrapper, semifinished product, bulk product and finished product according to stated methods;IV. Inspect methods shall be confirmed and validated;V. Sampling, check, inspect shall be recorded, the windage shall be researched and be recorded;VI. Material, semifinished product, bulk product and finished product shall be checked and inspected according to quality standard and be recorded;VII. Material and packaged finished product shall have enough reserved samples so that necessary check or inspect shall be taken; except the finished product with too large package container, the reserved sample s’package shall be the same with the final package of the finished product.Section 4 Quality Risk ManagementArticle 13 Quality risk management is evaluate, control, communicate, audit system process to quality risk during the whole product life period, via the manner of foresee or review.Article 14 Quality risk shall evaluate according to science knowledge and experience in order to ensure products’ quality.Article 15 The method, measure, form take during the quality risk management process and the documents formed in the said process shall accommodate to the level of the existent risk.Chapter 3 Organization and PersonnelSection 1 PrincipleArticle 16 An enterprise shall establish management organization which accommodate to the pharmaceutical goods’ product and have its organization framework chart.The enterprise shall set up independent quality management department, which carries out the responsibilities of quality guarantee and quality control. The quality management department could set up quality guarantee department and quality control department respectively.Article 17 Quality management department shall take part in all activities relating to quality, and take responsibility to audit all documents relating to this Regulation. The personnel inquality management department shall not relegate his responsibility to the personnel in other department.Article 18 The enterprise shall be staffed by an appropriate number of management and technical personnel with appropriate qualification (including education background, training and practice experience), and the responsibilities of each department and each station shall be clarified. Station’s responsibility shall not be missed and cross responsibility shall be prescribed specifically. Responsibility taken by each person shall not be overfull.Every person shall clear and understand his own responsibilities, be familiar with the requirements related to his responsibilities, and accept necessary training, including pre-job training and on-job training.Article 19 Generally, one shall not relegate his responsibility to other person. If the responsibilities do need to be relegated, the one should relegate his responsibility to the designated person who has equivalent qualification.Section 2 Important PersonArticle 20 The important person shall be the full-time person of the enterprise, at least including the director of the enterprise, director of manufacturing management, director of quality management and authorized person of quality.Director of quality management and director of manufacturing management shall be independent of each other. Director of quality management and authorized person of quality shall not be independent of each other. Operation proceduress shall be established so that authorized person of quality could take his responsibility independently, with no interference from director of enterprise and other person.Article 21 Director of enterpriseDirector of enterprise is the main responsible person of pharmaceutical goods’ quality, who comprehensive responsible to the daily management of the enterprise. In order to ensure theenterprise complete quality target and manufacture pharmaceutical goods according to this Regulation, the director of enterprise shall take responsible for providing necessary resources, reasonable plan, organize and correspond to ensure the quality management department could take its responsibility independently.Article 22 Director of manufacturing managementI. Qualification:Director of manufacturing management shall at least have pharmacology or related specialty undergraduate education background (or secondary professional technical title or licensed pharmacist qualification), have at least three years’ pharmaceutical goods’ manufacturing and quality management experience, including at least one year’s pharmaceutical goods’manufacturing management experience, have taken part in professional knowledge training related to manufacturing products.II. Main responsibility:1. Manufacture and storage the pharmaceutical goods according to approvedtechnology procedure in order to ensure the quality of the pharmaceutical goods;2. Ensure every operation proceduress related to manufacturing operation areperformed strictly;3. Ensure batch production record and batch package record are audited by designatedperson and submitted to quality management department;4. Ensure the maintenance of workshop and equipment in order to preserve its goodworking condition;5. Ensure all kind of necessary validation work is completed;6. Ensure person related to manufacturing have been trained by pre-job training andon-job training, adjust training content according to actual demands.Article 23 Director of quality managementI. Qualification:Director of quality management shall at least have pharmacology or related specialty undergraduate education background (or secondary professional technical title or licensedpharmacist qualification), have at least five years’ pharmaceutical goods’ manufacturing and quality management experience, including at least one year’s pharmaceutical goods’ quality management experience, have taken part in professional knowledge training related to manufacturing products.II. Main responsibility:1. Ensure the raw material, wrapper, semifinished product, bulk product and finishedproduct are in accordance with the registered approved requirements and quality standard;2. Ensure the products are audited to batch record before delivering;3. Ensure necessary inspection is finished;4. Approve quality standard, sampling method, inspection method and other operationproceduress of quality management;5. Audit and approve all changes related to quality;6. Ensure all important windage and exceed criterion inspection results have beenresearched and been dealt with in time;7. Approve and supervise consigned inspection;8. Supervise the maintenance of workshop and equipment in order to maintain its goodworking condition;9. Ensure to finish every necessary confirmation and validation work, checking andapproving confirmation or validation scheme and report;10. Ensure to finish self-check;11. Evaluate and approve material supplier;12. Ensure all complaints related to product quality have been researched, and have beendealt with in time and accurately;13. Ensure to finish products’persistent stability review plan, provide the data ofpersistent stability review;14. Ensure to finish product quality review analysis;15. Ensure quality control and quality guarantee person have been trained by pre-jobtraining and on-job training, adjust training content according to actual demands.Article 24 Director of manufacturing management and director of quality management often have the following common responsibility:I. Audit and approve the documents of products’technology procedure, operation proceduress;II. Supervise the sanitation condition of factory;III. Ensure the key equipment have been confirmed;IV. Ensure to finish the validation of production technology;V. Ensure all related person in enterprise been trained by pre-job training and on-job training, adjust training content according to actual demands;VI. Approve and supervise consigned manufacture;VII. Ensure and monitor the storage condition of material and goods;VIII. Save the record;IX. Supervise the implement condition of this Regulation;X. Monitor the factors influence the quality of the products.Article 25 Authorized person of qualityI. Qualification:Authorized person of quality shall at least have pharmacology or related specialty undergraduate education background (or secondary professional technical title or licensed pharmacist qualification), have at least five years’ pharmaceutical goods’ manufacturing and quality management experience, have the experience of manufacturing process control and quality check work.Authorized person of quality shall have necessary professional theory knowledge, have taken part in the train about product delivering, and could take his responsibility independently.II. Main responsibility:1. Take part in the establishment of enterprise quality system, interior self-check,exterior quality audit, validate and pharmaceutical goods’bad reaction report, product recall and other quality management activities;2. Take the responsibility of product delivering, to ensure the manufacturing, checkingof every batch of delivered products are all in accordance with corresponding code, pharmaceutical goods’ registered requirements and quality standard;3. Before delivering the products, authorized person of quality must issue productdelivering audit record according to the said item 2 and bring it into batch record.Section 3 TrainingArticle 26 The enterprise shall designate department or person to take responsible for training management work, and shall have the training scheme or plan audited or approved by director of manufacture management or director of quality management. The training record shall be preserved.Article 27 All personnel related to pharmaceutical goods’manufacturing, quality shall be trained, the training content shall accommodate to the post. Except the training of theory and practice of this Regulation, responsibility, skill training about the related code, relevant post shall also be trained, and actual effect shall be periodic evaluated.Article 28 The working person in high risk operating area (such as: manufacture area of high activity, high toxic, infective, high sensitive material) shall take expert training.Section 4 Personnel SanitationArticle 29 All personnel shall take sanitation requirements training, the enterprise shall establish personnel sanitation operation proceduress, so that to reduce the pollution risk to pharmaceutical goods taken by person at its maximum.Article 30 Personnel sanitation operation procedures shall include the content related to health, sanitation practice and personnel dress. Personnel in manufacturing area and quality control area shall correctly understand related personnel sanitation operation procedures. Theenterprise shall take measures to ensure the implement of personnel sanitation operation procedures.Article 31 The enterprise shall manage personnel’s health and establish health file. The manufacture personnel contact pharmaceutical goods directly shall receive physical check, and take at least one physical check per year.Article 32 The enterprise shall take appropriate measure to avoid the person have wound in body surface, have infection disease or other person may pollute pharmaceutical goods to take the manufacture work which directly contact pharmaceutical goods.Article 33 Visiting person and untrained person shall not enter manufacture area and quality control area, if the persons need to enter in special conditions, the items of their individual sanitation, clothes changing and so on shall be instructed.Article 34 Any person enters into manufacturing area shall change clothes according to prescription. The material, style and dressing method of work clothes shall be accommodate to the work engaged and the level of air clean degree.Article 35 The person enters into clean manufacturing area shall not make up and adorn with accouterment.Article 36 Manufacturing area, storage area shall forbid smoking and having meals, forbid to store food, beverage, cigarette and individual pharmaceutical goods and other non-manufacturing goods.Article 37 Operation person shall avoid to touch the surface of pharmaceutical goods, wrapper material contact pharmaceutical goods directly and equipment by hand.Chapter 4 Workshop and EstablishmentSection 1 PrincipleArticle 38 The workshop location’s choice, design, overall arrangement, construct, reconstruct and maintenance must in accordance with the pharmaceutical goods’manufacturing requirements, which may avoid pollution, cross pollution, confusion and mistake at its maximum, and may convenient for clean, operate and maintenance.Article 39 The workshop’s location shall consider according to the workshop and manufacturing safety measures. The environment of the workshop shall reduce the pollution risk to material or products at its maximum.Article 40 The enterprise shall have tidy manufacturing environment. The floor, road surface of the workshop and transport shall not pollute the pharmaceutical goods’manufacturing. The overall arrangement of the manufacturing area, administration area, living area and assistant area shall be reasonable arranged and do not interfere each other. Stream of people and material in factory area and workshop shall reasonable.Article 41 The workshop shall take appropriate maintenance, and ensure the maintenance activity may not influence the quality of pharmaceutical goods. Clean or take necessary sterilization to workshop according to detailed written operation procedures.Article 42 The workshop shall have appropriate illumination, temperature, humidity and aeration, to ensure the quality of produced and reserved products and the performance of related equipment shall not be influenced directly or indirectly.Article 43 The design and installation of workshop, establishment shall effective prevent insect or other animal to enter in. Necessary measures shall be taken to prevent of using raticide, insecticide, smoke fumigant and so on which may pollute equipment, material, goods.Article 44 Necessary measures shall be taken to prevent unauthorized person to enter in. Manufacturing, storage and quality control area shall not be the direct gate for staffs not in this area.Article 45 Complete drawing of constructed or reconstructed workshop, public service, fixed pipeline shall be saved.Section 2 Manufacturing AreaArticle 46 In order to reduce pollution and cross pollution risk, workshop, manufacturing establishment and equipment shall reasonable designed, overall arranged and used according to the characteristic, technique process and corresponding clean level, and in accordance with the following requirements:I. Comprehensive considering the pharmaceutical goods’ characteristic, technique andintending purpose and other factors, to confirm feasibility of using workshop, manufacturing establishment and equipment together, and hing related evaluating report;II. Special and independent workshop, manufacturing establishment and equipment must be used when manufacturing special character pharmaceutical goods, such as high sensitive pharmaceutical goods (i.e. Penicillin) or biologics goods (i.e. bcg vaccine or other pharmaceutical goods prepared by active microorganism). The operating area with large quantity of dust forpenicillin species pharmaceutical goods shall keep comparatively negative pressure, the exhaust gas discharged to outdoor shall carry cleanse management and make it be up to the mustard. Air outlet shall be away from air inlet of other air cleanse system;III. Special establishment (i.e. independent air cleanse system) and equipment must be used when manufacturing β-lactam structure species pharmaceutical goods, sexhormone species prophylactic pharmaceutical goods, and shall be detached with other pharmaceutical goods’ manufacturing area strictly;IV. Special establishment (i.e. independent air cleanse system) and equipment shall be used when manufacturing some hormone species, cell toxicity species, high active chemical pharmaceutical goods; under special circumstance, if necessary validation shall be take before taking special protection measures, the above said pharmaceutical goods preparation could use the same establishment and equipment via manufacturing modes in different phases;V. Ventilation of air cleanse system in the above said item II, III, IV shall take cleanse management;VI. Pharmaceutical goods’ m anufacturing workshop shall not used for manufacturing non-officinal used products which may have bad influence to pharmaceutical goods’ quality.Article 47 Manufacturing area and storage area shall have enough space to ensure equipment, material, semifinished product, bulk product and finished product could storage in order, to prevent the confusion, cross pollution of different products or material, to prevent missing or mistake during the operation of manufacturing or quality control.Article 48 Air condition cleanse system shall be equipped according to pharmaceutical goods’variety, manufacturing operation’s requirement and exterior environment condition which let the manufacturing area can effective aeration, and have temperature, humidity control and atmosphere cleanse filtration, to ensure the manufacturing environment of pharmaceutical goods is in accordance with the requirements.The pressure difference between clean area and non-clean area, clean areas in different level shall not lower than 10Pascal. When necessary, appropriate pressure difference grads shall also be kept in different functional areas (operation area) with same clean level.Exposure working procedure area for oral liquid and solid preparation, chamber use pharmaceutical goods (including rectal pharmaceutical goods), surface external use pharmaceutical goods and other non-asepsis preparation manufacturing and exposureworking procedure area for wrapper material directly contact pharmaceutical goods shall equip according to the requirements of “Asepsis Pharmaceutical Goods” D level clean area in Annex. The enterprise could take appropriate microorganism monitor measures according to product s’ standard and characteristic.Article 49 Inner surface of clean area (wall, floor, sunshade) shall be slick, non-crack, narrow joint, non granule fell off, prevent stored dust, convenient for effective clean, and take sterilization when necessary.Article 50 The design and installation of every kind of pipeline, lighting establishment, place with a draught and other public establishment shall prevent having the part of not easy to clean, and shall try best to maintain outside the manufacturing area.Article 51 Sewerage shall have appropriate size and install the device of reversing flow. Try best to prevent drain in clear channel, if it is inevitably, the clear channel shall be flat so that it is convenient to clean and sterilize.Article 52 Quantify of preparation’s raw material often carry on in the metage room special designed.Article 53 Operation rooms which produce dust (such as sampling, metage, mix, package of dry material or products rooms and other operation rooms) shall keep comparatively negative pressure or take special measure, so as to prevent the diffusion of powder dust, avoid cross pollution and easy to keep clean.Article 54 Pharmaceutical goods’packaging workshop or area shall be reasonable designed and arranged, to prevent confusion or cross pollution. If there are several packaging lines in one area, isolation measures shall be taken.Article 55 Manufacturing area shall have appropriate light, the light in contact manufacturing area shall satisfy the operation requirements.Article 56 Manufacturing area could set middle control area. However, middle control operation shall not bring quality risk to pharmaceutical goods.Section 3 Storage AreaArticle 57 Storage area shall have enough space, which ensure the under-examined, qualified, disqualified, withdrew or recalled raw material, wrapper, semifinished product, bulk product and finished product and all kinds of material and products could store in order.Article 58 The design and construct of storage area shall ensure good storage condition, and have aeration and lighting establishment. Storage area shall satisfy material or products’storage condition (such as temperature, humidity, avoid of light) and safety storage requirements, and check and monitor shall be taken.Article 59 High active material or product and printing package material shall storage in safe area.Article 60 Receiving, extending and delivering area shall protect material, product out of influence of weather outside (such as rain, snow). The arrangement and establishment of receiving area shall ensure taking necessary cleaning to the received material’s outside packaging.Article 61 If the under-examined material is stored in separate isolate area, the under-examine area shall have distinct mark, and only authorized person could in and out. Disqualified, withdrew or recalled material or product shall be stored isolated.If other method is used for replacing physics isolation, this method shall have equal security.Article 62 Separate material sampling area is often set up. Air cleaning level of sampling area is the same with the manufacturing requirements. If sample is taken in other area or use other method, pollution or cross pollution shall prevent.Section 4 Quality Control AreaArticle 63 Quality control laboratory is often divided from manufacturing area. Biology examine, microorganism and radioactivity isotope laboratory shall also divided from each other.Article 64 The design of laboratory shall ensure for applying it’s intending purpose, and prevent confusion and cross pollution, enough area shall have for disposing samples, reserved samples and samples for reviewing stability and keeping the records.Article 65 When necessary, set a special instrument room to prevent static, shaking, humid or other outside factors’ disturbance to instrument with high sensitivity.Article 66 The laboratory disposing special goods such as biology samples or radioactivity samples shall in accordance with the related requirements of the country.Article 67 Experiment animal room shall be strictly divided from other area. Its design, construct shall in accordance with the related requirements of the country and have independent air disposing establishment and animal’s special channel.Section 5 Assistant AreaArticle 68 Retiring room shall not take bad influence to manufacturing area, storage area and quality control area.。
2010年药典第三部

2010年药典第三部英文回答:In 2010, the third edition of the Pharmacopoeia was published, which set forth the requirements and standardsfor pharmaceutical substances and preparations. Thisedition aimed to ensure the quality, safety, and efficacyof medicines used in the healthcare industry. It included guidelines for the identification, testing, and control of active pharmaceutical ingredients, as well as theformulation and labeling of finished products.The Pharmacopoeia serves as a vital reference for healthcare professionals, including pharmacists, physicians, and regulatory authorities. It provides them with comprehensive information on the quality and standards of medicines, allowing them to make informed decisions regarding patient care. For example, if a physician prescribes a particular medication, they can consult the Pharmacopoeia to ensure that the product meets the requiredspecifications and is safe for use.One of the key features of the 2010 edition is the inclusion of monographs, which provide detaileddescriptions and specifications for individual drugs ordrug classes. These monographs cover a wide range of topics, including the physical and chemical properties of the drug, the recommended dosage forms and strengths, and the analytical methods for testing its quality. By followingthe monographs, pharmaceutical manufacturers can ensurethat their products are consistent with the established standards.Moreover, the Pharmacopoeia also addresses the issue of impurities in pharmaceutical substances. It sets limits for impurities, such as residual solvents, heavy metals, and microbial contaminants, to ensure that medicines are free from potentially harmful substances. These limits are based on scientific evidence and are designed to protect patient safety. For example, if a medication contains excessive levels of a particular impurity, it may pose a risk to the patient's health. The Pharmacopoeia helps to prevent suchsituations by providing guidelines for testing and controlling impurities.In addition to the standards for pharmaceutical substances, the Pharmacopoeia also includes guidelines for the formulation and labeling of finished products. These guidelines ensure that medicines are formulated in a way that maximizes their stability, efficacy, and safety. For example, the Pharmacopoeia may specify the acceptable range of pH for a particular oral solution to ensure itsstability over time. It may also provide labeling requirements, such as the mandatory inclusion of certain warnings or precautions on the product packaging.中文回答:2010年,第三版药典出版,规定了药物物质和制剂的要求和标准。
中国药典2010年版
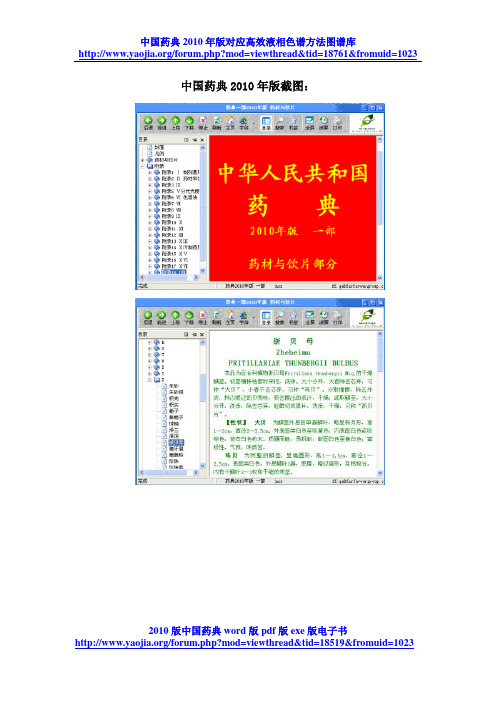
年版截图:中国药典20102010版中国药典word版pdf版exe版电子书2010版中国药典word版pdf版exe版电子书中国药典2010年版一部正文一枝黄花(p3)YizhihuanghuaSOLIDAGINIS HERBA本品为菊科植物一枝黄花Solidago decurrens Lour.的干燥全草。
秋季花果期采挖,除去泥沙,晒干。
【性状】本品长30~lOOcm。
根茎短粗,簇生淡黄色细根。
茎圆柱形,直径0.2~0.5cm;表面黄绿色、灰棕色或暗紫红色,有棱线,上部被毛;质脆,易折断,断面纤维性,有髓。
单叶互生,多皱缩、破碎,完整叶片展平后呈卵形或披针形,长1~9cm,宽0.3~1.5cm;先端稍尖或钝,全缘或有不规则的疏锯齿,基部下延成柄。
头状花序直径约0.7cm,排成总状,偶有黄色舌状花残留,多皱缩扭曲,苞片3层,卵状披针形。
瘦果细小,冠毛黄白色。
气微香,味微苦辛。
【鉴别】 (1)叶表面观:上表皮细胞多角形,垂周壁略呈念珠状增厚。
下表皮细胞垂周壁波状弯曲,气孔不定式,略下陷。
非腺毛有两类:表皮非腺毛由3个细胞组成,壁薄,顶端1个细胞常萎缩成鼠尾状,较小;叶缘非腺毛睫毛状由3~7个细胞组成,壁稍厚,长180~500μm。
(2)取本品粉末2g,加石油醚(60~90℃)50ml,超声处理30分钟,放冷,滤过,弃去石油醚液,药渣挥干溶剂,加70%乙醇30ml,加热回流1小时,放冷,滤过,滤液蒸干,残渣加甲醇1ml使溶解,作为供试品溶液。
另取一枝黄花对照药材2 g,同法制成对照药材溶液。
再取芦丁对照品,加甲醇制成每1ml含0.5mg的溶液,作为对照品溶液。
照薄层色谱法(附录ⅥB)试验,吸取供试品溶液5~10μl、对照药材溶液和对照品溶液各5μl,分别点于同一以含4%磷酸氢二钠溶液制备的硅胶G薄层板上,以乙酸乙酯-甲醇-甲酸-水(8:1:2010版中国药典word版pdf版exe版电子书1:1)为展示剂,展开,取出,晾干,喷以3%三氯化铝乙醇溶液,晾干,置紫外光灯(365nm)下检视,供试品色谱中,在与对照药材色谱和对照品色谱相应的位置上,显相同颜色的荧光斑点。
中国药典2010版[1]
![中国药典2010版[1]](https://img.taocdn.com/s3/m/2eed273de2bd960590c67714.png)
计量---计量单位
长度:米,分米,厘米,毫米,微米,纳米
体积:升,毫升,微升 质量:千克,克,毫克,微克,纳克 压力:兆帕,千帕,帕 密度:千克每立方米,克每立方厘米
波数:负一次方厘米
动力粘度:帕秒
试药、试液、指示剂
试药:除另有规定外,选用不同等级并符合 国家有关行政主管部门规定的试剂标准
编排
乙酰唑胺
Acetazolamide
《中国药品通用名称》
按WHO编订的国际非专利 药品名称命名
C4H6N4O3S2,222.25
N-[5-(氨磺酰基)-1,3,4噻二唑-2-基]乙酰胺。 中国化学会《有机化学命名
原则》IUPAC
项目与要求
溶解度
极易溶解 :系指溶质1g(ml)能在溶剂不到1ml 易溶:系指溶质1g(ml)能在溶剂1~不到10ml 溶解:系指溶质1g(ml)能在溶剂10~不到30ml 略溶:系指溶质1g(ml)能在溶剂30~不到100ml 微溶:系指溶质 1g(ml) 能在溶剂 100 ~ 不到 1000ml 中溶 解; 极 微 溶 解 : 系 指 溶 质 1g(ml) 能 在 溶 剂 1000~ 不 到 10000ml 几乎不溶或不溶:系指溶质1g(ml)在溶剂10000ml中不能 完全溶解
主要国外药典标准
(四)《日本药局方》(日本薬局方)
Japanese Pharmacopoeia(简称JP)。
1、目前为第16版。
2、由一部和二部组成,共一册。第一部主要收载原 料药及其基础制剂。第二部主要收集生药、家庭药制 剂和制剂原料。
3、日本厚生省专门出版一本关于抗生素质量标准的 法典《日本抗生物质基准解说》, 简称“日抗基”。
2010版药典

2010版药典介绍2010版药典(Pharmacopoeia of the People’s Republic of China, 2010 Edition)是中华人民共和国的一项法定药典,由中国食品药品监督管理局主管,于2010年首次正式发布。
药典是规定和标准化药物质量的权威性文献,对于保证药品的质量和安全至关重要。
每个国家都有自己的药典,以提供关于药物标准、药物检测及质量控制的指导。
药典发展历程自从2000年第一版药典发布以来,每隔十年,中国都会出版一次新版的药典,以适应药物和医疗行业的发展需求。
2010版药典在追求高质量药品的同时,也考虑到了国际药品质量要求的变化。
它包含了中国传统药材和西药等各类药品的质量标准。
药典的内容2010版药典详细描述了每种药品的质量标准,包括药物成分、理化性质、检测方法、用法用量和质量控制等。
药典的目的是确保药物的质量、有效性和安全性,从而保护患者的健康。
药典中还包含了一些药物鉴别和检测的基本原则和指导,以及药品生产和质量控制的规范要求。
药典在保障药品质量中的作用药典是确保药品质量的重要工具。
它为药物生产企业提供了明确的质量标准,帮助企业进行药品质量的控制和管理。
同时,药典还为药物检验机构提供了参考标准和检验方法,确保对药品质量进行准确和有效的检测。
此外,药典还为医疗机构提供了选择合格药品的参考依据,保证患者用药的安全性和有效性。
药典的重要性药典的制定和实施对于维护药品质量、保障患者用药安全具有重要意义。
药典是一个国家药品质量管理体系的重要组成部分,发挥着规范药品生产和流通环节的功能。
它对于推动药品质量的提升、促进药物研发和创新以及保护公众的健康起着至关重要的作用。
结论2010版药典是中华人民共和国发布的一项法定药典,是保障药品质量和保护患者用药安全的重要工具。
药典规定了药品的质量标准,对于药物的生产、检验和使用提供了指导。
药典的制定和实施对于提高药品质量、推动医药行业发展、保护公众健康具有重要意义。
中国药典2010年版修订简介

- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
2.药材的采收与产地加工部分的一般译法为: 药材的采收与产地加工部分的一般译法为: 用The drug作主语,单数。用被动语态,不用主动语态。“采 drug作主语 单数。用被动语态,不用主动语态。 作主语, collected” 不用“ picked” up” 收”用“to be collected”(不用“to be picked”,“to be dug up” 除去杂质” 名词单数) 等 ) 。 “ 除去杂质 ” 等 ( 名词单数 ) 用 “ removed from foreign matter” 洗净” matter”,“洗净”用“washed clean”,“干燥”用“dried”, “晒 clean” 干燥” dried” sun” 干 ” 为 “ dried in the sun” , “ 阴 干 ” 为 “ dried in the shade” 低温干燥” shade”,“低温干燥”为“dried at a lower temperature”。“栽培 temperature” 变种” cultivar” 泥砂” soil” 须根. 细根” 变种 ” 为 “ cultivar” 。 “ 泥砂 ” 为 “ soil” 。 “ 须根 . 细根 ” : root”用于单子叶植物、根茎上的不定根, rootlet” “fibrous root”用于单子叶植物、根茎上的不定根,“rootlet” 用于双子叶植物主根上的细小侧根、支根。 用于双子叶植物主根上的细小侧根、支根。 例1 The drug is collected in autumn, removed from foreign matter, washed clean, and dried in the sun. sun. 例 2 The drug is collected at flowering to fruiting stage, removed from thick stem, cut into section, and dried. dried. ( 注 : 中国药典 2005年版一部英文版药用部位采用名词单数 , 如 中国药典2005 年版一部英文版药用部位采用名词单数 年版一部英文版药用部位采用名词单数, stem, leaf, fibrous root, rootlet等). rootlet等
滤液用水饱和的正丁醇提取二次, 滤液用水饱和的正丁醇提取二次 , 合并正丁醇 提取液,蒸干,残渣加无水乙醇1ml使溶解 使溶解, 提取液,蒸干,残渣加无水乙醇1ml使溶解, 加适量氧化铝在水浴上拌匀,干燥, 加适量氧化铝在水浴上拌匀,干燥,装入一预 先装填好的中性氧化铝小柱( 200目 先装填好的中性氧化铝小柱 ( 200 目 , 1g , 内 10-15mm) 18小柱上 用乙酸乙酯小柱上, 径10-15mm)或C18小柱上,用乙酸乙酯-甲醇 30ml预洗 再用乙酸乙酯-甲醇( 预洗, (3:1)30ml预洗,再用乙酸乙酯-甲醇(1: 1)30ml洗脱,收集洗脱液,残渣加乙醇0.5ml 30ml洗脱 收集洗脱液,残渣加乙醇0 洗脱, 使溶解,作为供试品溶液。 使溶解,作为供试品溶液。
二、理化鉴别
1.化学鉴别 Shake 0. 5 g of the powder with 5 ml of ethanol for 5 minutes and filter. Evaporate the filtrate to dryness, filter. add dropwise antimony trichloride saturation solution in chloroform and evaporate again to dryness. A dryness. violetviolet-red colour is produced. produced. 取本品粉末0 取本品粉末0.5g,加乙醇5ml,振摇5分钟,滤过,滤 加乙醇5ml,振摇5分钟,滤过, 液蒸干, 滴加三氯化锑饱和的三氯甲烷溶液, 液蒸干 , 滴加三氯化锑饱和的三氯甲烷溶液 , 再蒸 干,即显紫红色。 即显紫红色。
Citrus grandis “Tomentosa” or Citrus grandis (L.) Osbeck Tomentosa” (L.
(Fam. Rutaceae). (Fam. Rutaceae). 本 品 为 芸 香 科 植 物 化 州 柚 Citrus grandi“Tomentosa” 或 柚 grandi“Tomentosa” Citrus grandis(L.)Osbeck的未成熟或近成熟的干燥外层果皮。 grandis(L.)Osbeck的未成熟或近成熟的干燥外层果皮 的未成熟或近成熟的干燥外层果皮。
(2)薄层鉴别基本的四个步骤,即供试品溶液制备; (2)薄层鉴别基本的四个步骤 即供试品溶液制备; 薄层鉴别基本的四个步骤, 对照品溶液制备;点样、展开、显色;结果判 对照品溶液制备;点样、展开、显色; 断。
a. 供试品溶液制备 常见以下几种情况: 常见以下几种情况:
超声处理提取 To 9g, cut into pieces, add 20ml of ether, 20ml ultrasonicate for 20 minutes, and filter. filter. Evaporate the filtrate to dryness, and dissolve the residue in 0.5ml of n-hexane as the test solution. 取本品9 solution. 取本品9g,切碎,加乙醚20ml,超声 切碎,加乙醚20ml, 处理20 分钟 滤过, 滤液挥干, 残渣加0 分钟, 处理 20分钟 , 滤过 , 滤液挥干 , 残渣加 0.5ml 正己烷溶解,作为供试品溶液。 艾附暖宫丸) 正己烷溶解,作为供试品溶液。(艾附暖宫丸)
2.薄层鉴别 2.薄层鉴别 (1)经常用到需统一的词汇和短语: 经常用到需统一的词汇和短语: a.超声处理 用ultrasonicate a.超声处理 b.提取 用extract b.提取 c.温浸 用 warm macerate c.温浸 d.水浴加热 用heat on a water bath d.水浴加热 e.对照品(对照品溶液) 用 “对照品名”加“CRS” e.对照品(对照品溶液) 对照品名” CRS” 对照品 表示(reference solution); 对照药材(对照药材 solution) 对照药材( 表示( 溶液) drug( solution) 溶液) 用reference drug(reference drug solution); 供试品溶液 用test solution
f.薄层板 : 硅胶G薄层板... 硅胶G薄层板... using silica gel G as the coating substance 用1%氢氧化钠溶液制备的硅胶G薄层板... 氢氧化钠溶液制备的硅胶G薄层板... using silica gel G mixed with 1% solution of sodium hydroxide as the coating substance 含 4% 醋酸钠的羧甲基纤维素钠溶液为黏合剂的硅胶 G 醋酸钠的羧甲基纤维素钠溶液为黏合剂的硅胶G 薄层板上... 薄层板上... using silica gel G mixed with sodium carboxymethylcellulose containing 4% solution of sodium acetate as the coating substance. substance.
加热回流提取 Heat under reflux 2g, cut into pieces, with pieces, 20ml of ethanol for 1 hour, cool and filter, 20ml use the filtrate as the test solution. 取本品2g, solution. 取本品2 剪碎,加乙醇20ml,加热回流1 小时,放冷, 剪碎,加乙醇20ml,加热回流1 小时,放冷, 滤过,滤液作为供试品溶液。 滤过,滤液作为供试品溶液。
萃取、 萃取、过柱纯化 Extract the filtrate with two 15-ml quantities of n15butanol saturated with water, combine the n-butanol extracts, and evaporate to dryness. Dissolve the dryness. residue in 1 ml of dehydrated ethanol, add a quantity of alumina, stir well on a water bath, dry, and apply to a small (10-15mm in diameter) 或 Sepack C18 column 10-15mm packed with neutral alumina (200 mesh, 1g, 10~15mm 10~15mm in diameter ), pre-elute with 30ml of a mixture of pre30ml ethyl acetate and methanol (3:1). Elute with 30 ml of a mixture of ethyl acetate and methanol (1:1), and collect the eluate. Evaporate to dryness and dissolve eluate. the residue in 0.5 ml of ethanol as the test solution. solution.
g. 斑点 用spot(s), 条斑 用 band(s) spot(s), h. 分别置日光及紫外光灯(365nm)下检视,日 分别置日光及紫外光灯(365nm)下检视 下检视, 光下显…色的斑点;紫外光灯下显… 光下显…色的斑点;紫外光灯下显…色的 斑点 用 … spot in daylight and … spot under ultraviolet light at 365 nm respectivte 0.5 g of the pills in 20 ml of methanol for 1 hour, and filter. Evaporate 5 ml of the filtrate to dryness, dissolve the residue in 10 ml of water, add 1 ml of hydrochloric acid, heat on a water bath for 30 minutes, and cool immediately. 取本品0.5 g,加甲醇20ml浸泡 小时,滤过。 取本品0.5 g,加甲醇20ml浸泡1 小时,滤过。取5ml 浸泡1 滤液蒸干,残渣加10ml水溶解 水溶解, 1ml盐酸 盐酸, 滤液蒸干,残渣加10ml水溶解,加1ml盐酸,水浴加 热30分钟,立即冷却。 30分钟 立即冷却。 分钟,
