材料导论英语
材料导论 Chapter_8__Phase___transformation

Chapter 8 Phase transformation1.Make sure you understand language and concepts:•Annealing退火•Austenitizing奥氏体化•Bainite贝氏体•Coarse pearlite粗珠光体•Fine pearlite细珠光体•Full annealing完全退火•Isothermal transformation diagram等温转变图•Martensite马氏体•Normalizing正火•Nucleation形核率•Phase transformation 相变•Precipitation hardening沉淀强化(age hardening时效强化)•Precipitation heat treatment 沉淀热处理•Solution heat treatment固溶处理•Spheroidite球状珠光体•Spheroidizing球化•Supercooling过冷•Superheating过热•Tempered martensite回火马氏体•Thermally activated transformation热激活转变•Transformation rate转变速率2.you should be able to do the following:1. Briefly describe the microstructure for each of the following microconstituents thatare found in steel alloys: fine pearlite, coarse pearlite, spheroidite, bainite, martensite, and tempered martensite.2. Cite the general mechanical characteristics for each of the followingmicroconstituents: fine pearlite, coarse pearlite, spheroidite, bainite, martensite, and tempered martensite. Now, in terms of microstructure (or crystal structure), briefly explain these behaviors.3. Given the isothermal transformation (or continuous cooling transformation)diagram for some iron-carbon alloy, design a heat treatment that will produce a specified microstructure.4. Using a phase diagram, describe and explain the two heat treatments that are usedto precipitation-harden a metal alloy.。
材料导论中英文讲稿 (4)

6.2.2 Porosity and DensityHello, everybody, in this Section, we are going to talk about porosity and density.译文:在这一节,我们将讨论气孔率和密度。
When referring to a solid material such as a part made from copper or stainless steel, the word density takes into consideration the microstructures that contains no porosity. By which term we do not mean the voids or vacancies in the atomic structure. In speaking of a solid material we mean the material’s theoretical density or mass density, it is the mass of a material divided by its volume.我们通常说到密度,指的是理论密度,表示结构中不包含气孔。
通过质量除以体积得到。
译文:当提到一种固体材料,尤其是由铜或不锈钢制成的材料时,密度这个词通常会考虑到一个没有气孔率的微观结构,在这个词中,我们指的不是原子结构中的孔洞或空位。
我们通常说到密度,指的是理论密度,表示结构中不包含气孔。
通过质量除以体积得到。
There are two factors influence the density of material. Atomic weight is a major factor in determining the density of the materials. Low-atomic-weight elements have low densities. 影响材料密度的因素有两个。
材料导论中英文讲稿 (18)

Module 2 Unit Cells and Space LatticesIn 2.3 section -the Structure of Crystalline Solids, you are going to learn what are unit cell and space lattice, the seven crystal systems, and the significance of allotropy and polymorphism.The study of crystalline structure is called Crystallography. How can these ordered structures be portrayed? Firstly, we need an axis system; secondly, atoms, ions, molecules can be considered as solid spheres of varying sizes; thirdly, these components’ spatial arrangement can be represented by points at various distances from each other in space.How can we see these crystalline structures? There are many ways. One method is x-ray diffraction.This Figure shows the X-ray diffraction for a single crystal of magnesium. Magnesium has a hexagonal close-packed crystal structure. The diffraction spot pattern in this Figure reflects the hexagonal type symmetry of the magnesium single crystal.译文:在2.3节晶体结构中,你将学习晶胞和空间点阵、七种晶系以及同素异构和多晶态的重要性。
材料导论中英文讲稿 (66)

Family of Materials 材料家族Today, we are introducing the Family of Materials。
We are living in a world of materials. With so many materials, how can we understand them? The method used here is to consider all materials as members of a big family. The family is composed of four basic groups, which are metals, ceramics, polymers and composites. Each of the groups posses common characteristics.译文:今天我们来介绍材料家族。
我们生活在材料的世界里。
对于身边这么多的材料,我们如何了解它们呢?今天我们这里介绍的是把所有材料看成一个大家庭。
这个大家庭包括四类材料,它们是金属材料,陶瓷材料,聚合物材料和复合材料。
每一类材料都有他们的共性,我们将在下面逐一介绍.Metals金属材料Metals comprise about three-fourths of the elements that we use, they are the earliest materials used by man. Simply, metallic are broken into subgroups of ferrous and nonferrous metals. Ferrous is a Latin-based word meaning “iron”,including iron, steel, stainless steel. Metal elements other than iron are called non-ferrous metal, include aluminum, copper, magnesium, nickel, titanium,and precious metals, such as gold, silver, palladium,platinium,etc. In nature, few metals find service in their pure form, but in alloy form. So,the word “Metal Alloys” consist of one or more metallic elements, and nonmetallic elements in relatively small amounts, such as carbon, nitrogen, and oxygen. We will introduce the metallic materials in chapter 4.译文:金属元素在元素周期表里占了四分之三,是最早被人类制造和利用的材料。
材料导论 Chapter_3_____Structure_of___Solids

Chapter 3 Structure of Solids1.Make sure you understand language and concepts:Allotropy 同素异形现象,同素异构现象Amorphous 无定形的,非晶态的Anisotropy 各向异性Atomic packing factor (APF) 原子密堆因素Body-centered cubic (BCC) 体心立方Coordination number 配位数Crystal structure 晶体结构Crystalline 晶体的Face-centered cubic (FCC) 面心立方Grain 晶粒Grain boundary 晶界Hexagonal close-packed (HCP) 密排六方Isotropic 各向同性的Lattice parameter 晶格参数Non-crystalline 非晶的Polycrystalline 多晶的Polymorphism 多晶型现象,同质异构现象Single crystal 单晶Unit cell 晶胞,单胞2. You should be able to do the following1. Describe the difference in atomic/molecular structure between crystalline and noncrystalline materials.2. Draw unit cells for face-centered cubic, body-centered cubic, and hexagonal close-packed crystal structures.3. Compute the densities for metals having face-centered cubic and body-centered cubic crystal structures given their unit cell dimensions.4. Distinguish between single crystals and polycrystalline materials.5. Define isotropy and anisotropy with respect to material properties.。
材料导论中英文讲稿 (39)

Module 4 (Metallic Materials金属材料)Video7 Corroison of metals and its prevention金属的腐蚀与防护Hello everyone! Today, let’s talk about corrosion of metals and its prevention.译文:大家好!今天,我们来谈谈金属的腐蚀和防护。
Without any protection, most metals will oxidize (or corrode). Metallic corrosion causes serious economical loss. The newest data show that, the estimated cost of corrosion in China is about 3.34% of GDP every year.译文:在没有任何保护的状态下,多数金属会发生氧化(或者腐蚀)。
金属腐蚀可造成严重的经济损失。
最新的数据显示,中国每年由于腐蚀造成的经济损失估计约占GDP的3.34%。
Corrosion is a natural process, which converts a refined metal to a more chemically stable form, such as its oxide, hydroxide, or sulfide. It is the gradual destruction of metals by chemical or electrochemical reaction with their environment. Remove the environment, and corrosion will stop. Corrosion engineering is the field dedicated to controlling and stopping corrosion.译文:腐蚀是一个自然的过程,它使经过冶炼后得到的金属转变为更具化学稳定性的形式,比如金属的氧化物、氢氧化物或者硫化物。
材料导论中英文讲稿 (36)

Module 4 (Metallic Materials金属材料)Video 3 Processing of Metal Alloys金属的制备Hello, every one. Today, let’s talk about processing of Metal Alloys.译文:大家好!今天我们来学习金属的制备。
Metal fabrication techniques are normally preceded by refining, alloying and often heat treating processes that produce alloys with the desired characteristics. The techniques include various metal-forming methods金属成型方法, casting铸造, powder metallurgy粉末冶金, welding焊接, and machining机加工.译文:金属制备技术包含精炼、合金化以及热处理。
金属制备技术包括:不同的成型方法、铸造、粉末冶金、焊接以及机加工。
Forming operations are those, in which the shape of a metal piece is changed by plastic deformation. Common techniques include: forging锻造, rolling轧制, extrusion 挤压, and drawing拉拔.译文:通过塑性形变改变金属的形状称之为成型工艺。
包括:锻造、轧制、挤压和拉拔。
When deformation is carried out above the recrystallization temperature, it is termed hot working热加工. The material remains relatively soft and ductile during deformation. Deformation below its recrystallization temperature is called cold working冷加工. Cold working produces an increase in strength, meanwhile a decrease in ductility, because the metal strain hardens.译文:当成型在重结晶温度以上进行,称为热加工。
材料导论中英文讲稿 (24)

Video 2 Elastic deformation and plastic deformationFrom now on, we will learn concepts shown in the stress-strain diagram one by one. First, we start with the concepts of elastic deformation and plastic deformation.译文:从现在开始,我们将要逐一学习应力-应变曲线上的概念。
首先,我们学习弹性形变和塑性形变。
We know a material will deform if it experiences some external force. Some deformation is temporary which means the material can go back to its original shape after the external force is released.Other deformation is permanent. In this case the changed shape cannot be recovered. For instance, when a tennis bat hits a tennis ball, the net bends and the ball is spring back. After that, the bat net goes back to the initial shape. That is called plastic deformation.In other case, if you bend a steel spoon, then it cannot go back to straight shape automatically. This is called plastic deformation.译文:我们知道材料在外力的作用下会发生变形。
材料导论中英文讲稿 (42)

Video 2: How large is a polymer ?Now, we have known that polymer is a large molecule composed of many, many repeating units.Then, can we exactly know how many repeating units are contained in a polymer chain? Simply, we can count the number of repeating units linked together in a polymer chain. This number is called, known as the degree of polymerization (DP). DP indicates the length or size of a polymer molecule.我们已知道聚合物是由许多重复单元组成的大分子。
我们能否准确知道聚合物分子链中有多少重复单元呢? 简单地说,我们可以计算聚合物链中重复单元的数目,重复单元的数目称为聚合 度. 聚合度表示聚合物分子链的长度,即聚合物分子的大小。
Another way of quantifying the molecular length or size of a polymer is the use of the term molecular weight (Mw). Molecular weight is the result, product of degree of polymerization times molecular weight of a repeating unit.(product -乘积)For example, for PVC, Mw of Repeat Unit is 62, calculated as 2 12+3 1+1 35 = 62. If we consider a commercial grade PVC, deal with a DP of 1000.Consequently, the molecular weight of this type of PVC is 62,000.另一种计算聚合物分子大小、分子链长度的方法是计算分子量。
材料科学与工程导论(双语)复习用.docx

材料科学与工程导论1 Polymerization (聚合作用)is the process by which small molecules (分子) consisting of one unit or a few units are chemically joined to create these giant molecules. Those small molecule units are called _____A) polymers B) monomers (单体) C) oligomers D)elastomerscable.A)For automobile bumper, the best choice of polymer is Natural Rubber.B)For mineral water bottle, the best choice of polymer is Low-Density (密度) Polyethylene (聚乙烯)C)For insulating cable, the best choice of polymer is polystyrene (聚苯乙烯).D)For mineral water bottle^ the best choice of polymer is Polyethylene Terephthalate.3)In general, for a given type of thermoplastic (热塑性塑胶)the tensile (可延展的) strength, creep resistance impact toughness, wear resistance, and melting temperature all increase with _______A) degree of polymerization B)density of branching C)tacticity D) crystallinity4)ABS, composed of acrylonitrile, butadiene, and styrene (苯乙烯),is one of the most common polymer materials. Styrene and acrylonitrile form a liner copolymer (异量分子聚合物)(SNA) that serves as a matrix. Styrene and butadiene also form a liner copolymer, BS rubber, which acts as the __________ material.D or B?A)fire retardant B) filler C) cross-linking D)plasticizer5)Epoxies (环氧树月旨)are thermosetting polymers formed from molecules containinga tight C-O-C ring. During polymerization, the C-O-C rings are opened and the bonds are rearranged to join the molecules. If epoxy is used as an adhesive (粘齐ll) for a variety of applications, which kind of adhesives does it belong to?A)Chemically Reactive AdhesivesB)Evaporation or Diffusion AdhesivesC)Hot-Melt Adhesives D) Pressure-Sensitive Adhesives6)What area the major advantages associated with plastic compared to ceramics(陶瓷),glasses, and metallic materials?A)lightweight and corrosion(铁锈,腐蚀卜resistantB)high strength and high-temperature resistantC)high-temperature resistant and insulatingD)high stiffness and corrosion-resistant7)Which one is not a thermoset polymer?A) Polyethylene B) Natural Rubber C) Epoxy D) Phenolic8)Depending on the degree of cross-linking, the polyurethanes behave as thermosetting polymers, thermoplastics (热塑性塑胶),or elastomers. These polymers find application as fibers, coatings, and foams for furniture, mattress, and insulation. Why polyurethanes are versatile(为什么聚氨酯是通用的)?A) for their polar repeat unit B) for their linear structureC) for their multi-functional monomer D) for their tactictity structure9) Liquid Crystalline(透明的)Polymers are polymers which behave as _____A) liquid B) thermoplastic C) thernoset D) oriented (取向)rods10) The glass temperature (Tg) is typically about ______ t imes the absolute melting temperature (Tm).A) 0.2 to 0.3 B) 0.5 to 0.7 C) 0.8 to 1.0 D) 1.0 to 2.011) Most thermoplastic exhibit a non-Newtonian and visicoelastic behavio 匸 The stressand train are not linearly related for most parts of the stress-strain curve. The viscoelastic behavior means when an external (夕卜部的)force is applied to a B) both elastic and plastic (塑性) C) only plastic D) neither elastic nor plastic12) Compare the tensile strength of LDPE, HDPE, PVE, PP, and arrange them in sequence (顺序)from high strength to low tensile strength•A) LOPE>HDPE>PVE>PP B) LDPE>HDPE>PP>PVCC) PVC>PP>HDPE>LDPE D) PVC>HDPE>PP>LDPE13) Degree of polymerization is usually used to characterize _______A) cross-linking network B) thermosetting polymerC) polymer degradation D) thermoplastic polymer14) Silicones (硅)are important elastomer based on chains composed of silicon and oxygen atoms. The silicone rubbers provide high-temperature resistance, permitting use of the elastomer at temperature as high as ____________ °C. Low molecular weight silicones form liquids and are known as silicon oils.A) 100 B) 150 C) 200 D) 30015) There are a lot of thermoplastic processing methods, typical forming processincludes: extrusion, blow molding, injection molding, thermoforming, calendaring and spinning ・ If we want to produce sping water bottles, which processing way is best choice?A) extrusion 挤ill 成型 B) blow molding 吹塑成型 C) injection molding 注射成型D) thermoforming 热成型16) The recycling of thermoplastics is relatively easy and practiced widely. Many of the everyday plastic products you encounter (bags, soda bottles, yogurt containers, etc.) have numbers stamped on them. For PRT products, the number is 1. For HOPE and LDPE, the numbers are __________ ,respectively. Other plastics are marked number 7,A) 3 and 4 B) 4 and 5 C) 2 and 4 D) 2 and 317) The temperature above which a polymer burns, chars, or decompose. Which team is appropriately used in describing the remperature ・A) Tg B)Td C) Tm D) HDT18) Which forming process is not a best choice for thermosetting polymers.A) Compression molding B)transfer moldingC) reaction injection D) spinning19) Elastomers are thermoplastics or lightly cross-linked thermosets that exhibitthermoplastic _ A) only elasticdeformation occurs.greater than ______ elastic deformation.A) 0.2% B)2% C) 20% D)200°/o20)Thermoplastic elastomers combine feature of both thermoplastics and elastomers. At high temperature, these polymers behave as __________ and are plastically into shapes, at how temperature, they behave as ________A) thermoplastics; elastomers B) thermoplastics; thermosetting polymersC) elastomers; thermoplastic D) elastomers; thermosetting polymers21 Define (a) a thermoplastic, (b) thermosetting plastics, (c) elastomers, and (d) thermoplastic elastomers.Thermoplastics :热后可塑性物质是由一些单体聚合成长链组成,它们代表性的特性是可塑性,延展性。
材料导论习题(英文版)

1) …… toughness, wear resistance, and melting temperature all increase with ________.A) Degree of polymerizationB) Density of branchingC) TacticityD) Crystallinity2) Choose the type of polymer material you might select for the following applications: an automobile bumper, a mineral water bottle, and an insulation cable.A) For automobile bumper, the best choice of polymer is Natural Rubber.B) For mineral water bottle, the best choice of polymer is Low-Density Polyethylene.C) For insulation cable, the best choice of polymer is Polystyrene.D) For mineral water bottle, the best choice of polymer is Polyethylene Terephthalate.3) Polymerization is the process by which small molecules consisting of one unit or a few unitsare chemically joined to create these giant molecules. Those small molecule units are called _______.A) PolymersB) MonomersC) OligomersD) Elastomers4) Epoxies are thermosetting polymers formed from molecules containing a tight C-O-C ring.During polymerization, the C-O-C rings are opened and the bonds are rearranged to join the molecules. If epoxy is used as an adhesive for a variety of applications, which kind of adhesives does it belong to?A) Chemically Reactive AdhesivesB) Evaporation or Diffusion AdhesivesC) Hot-Melt AdhesivesD) Pressure-Sensitive Adhesives5) Which one is not a thermosetpolymer?A) PolyethyleneB) Natural RubberC) EpoxyD) Phenolic6) Most thermoplastics exhibit a non-Newtonian and viscoelastic behavior. The stress and strainare not linearly related for most parts of the stress-strain curve. The viscoelastic behavior means when an external force applied to a thermoplastic, ________.A) Only elasticB) Both elastic and plasticC) Only plasticD) Neither elastic nor plastic7) Compare the tensile strength of LDPE, HDPE, PVC, PP, and arrange them in sequence fromhigh strength to low tensile strength.A) LDPE>HDPE>PVC>PPB) LDPE>HDPE>PP>PVCC) PVC>PP>HDPE>LDPED) PVC>HDPE>PP>LDPE8) There are a lot of thermoplastic processing methods; typical forming processes includeextrusion, blow molding, injection molding, calendaring, and spinning. If we want to produce spring water bottles, which processing way is best choice?A) ExtrusionB) Blow moldingC) Injection moldingD) Thermoforming9) The recycling of thermoplastics is relatively easy and practiced widely. Many of the everydayplastic products you encounter (bags, soda bottles, yogurt containers, etc.) have numbers stamped on them. For PET products, the number is 1. For HDPE and LDPE, the numbers are ________, respectively. Other plastic are marked number 7.A) 3 and 4B) 4 and 5C) 2 and 4D) 2 and 310) The temperature above which a polymer burns, chars, or decomposes. Which term isappropriately used in describing the temperature?A) T gB) T dC) T mD) HDT11) If a unit cell has axes a=b=c and all angle between axes equal 90°, which crystal system itbelong to?A) CubicB) TetragonalC) OrthorhombicD) Hexagonal12) If a unit cell has axes a≠b≠c and all angles between axes equal 90°, the volume of the unitcell is ________A) a3B) a2cC) abcD) 0.866a2c。
材料科学导论-introduction(绪论)

石器时代(StoneAge Nhomakorabea:石斧、凿、刀、铲、箭头、 纺轮、钵等(西安半坡遗址)
石斧
青铜器时代(Bronze
巨型司母戊鼎 (河南安阳晚商遗址)
Age):
湖北江陵楚墓出土越王勾践宝剑
铁器时代(Iron Age)
中国古代铁器的金相组织 湖南长沙砂子塘战国凹形铁锄
古代科技名著:“考工记”(先秦)、“梦溪笔谈”(宋代沈 括)、 “天工开物”(明代宋应星)
金属材料(Metallic Materials):钢铁、铝、铜、钛合金; 陶瓷材料(Ceramics):AlO、SiC、SiN、SiO、TiN 或称为无机非金属材料(Inorganic Materials) ; 高分子材料(High Polymers):纤维、蛋白质、聚乙烯; Matel-Matrix 复合材料(Composites): Composties Polymer-Matrix
陶瓷材料-陶瓷学 材料科学导论 突出材料共性教学 高分子材料-高分子物理 金属材料-金属学
材料科学导论是研究材料的成分、组织结构与性能之间关系
效能
合成/制备
性质
结构/成分
明代后:封建统治、帝国主义侵略束缚了材料的发展 停滞状态 解放后:材料科学受到重视和发展,被列为现代技术三大支柱之一。 一整套材料体系 门类全齐 数量 质量 钢铁突破亿吨大关 世界第一 原子弹、氢弹、人造卫星、火箭
宝钢高炉
长征三号运载火箭在发射架上的图片
材料分类(Classification of Materials)
材料科学导论
The Fundamentals(Elements,Principles )of Materials Science
材料导论中英文讲稿 (53)

Video 13: Thermoplastic ElastomersToday, we are going to talk about thermoplastic elastomers. 热塑性弹性体。
译文:今天,让我们来学习热塑性弹性体。
In the previous class, we have already learned the molecular structures and properties of natural rubbers and synthetic rubbers. The main chemical composition of natural rubber is polyisoprene, but there are still some problems of natural rubbers.译文:在前面的课程里,我们已经学习了天然橡胶和合成橡胶的分子结构和性能。
天然橡胶的主要化学成份是聚异戊二烯。
然而,天然橡胶依然存在着一些问题。
Vulcanization is an effective method to improve the mechanical property of natural rubbers. And we have already understood how vulcanization process improve the mechanical property of natural rubbers. Crosslinked chain structure is the key point. Therefore, most of conventional rubbers are thermosetting elastomers.译文:硫化可以有效的提高天然橡胶的机械性能。
我们也已经解了硫化是如何提高天然橡胶机械性能的。
交联的链结构是关键。
因此,大部分的传统橡胶材料都是热固性弹性体。
材料导论中英文讲稿 (10)
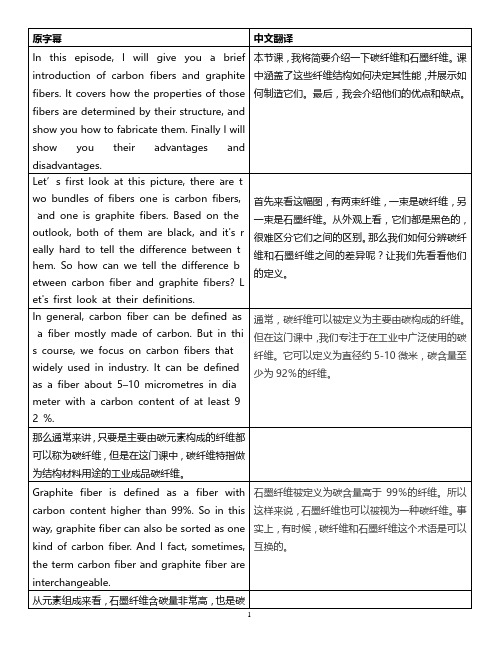
原字幕 中文翻译In this episode, I will give you a brief introduction of carbon fibers and graphite fibers. It covers how the properties of those fibers are determined by their structure, and show you how to fabricate them. Finally I will show you their advantages and disadvantages.本节课,我将简要介绍一下碳纤维和石墨纤维。
课中涵盖了这些纤维结构如何决定其性能,并展示如何制造它们。
最后,我会介绍他们的优点和缺点。
Let’s first look at this picture, there are t wo bundles of fibers one is carbon fibers, and one is graphite fibers. Based on the outlook, both of them are black, and it's r eally hard to tell the difference between t hem. So how can we tell the difference b etween carbon fiber and graphite fibers? L et's first look at their definitions.首先来看这幅图,有两束纤维,一束是碳纤维,另一束是石墨纤维。
从外观上看,它们都是黑色的,很难区分它们之间的区别。
那么我们如何分辨碳纤维和石墨纤维之间的差异呢?让我们先看看他们的定义。
In general, carbon fiber can be defined as a fiber mostly made of carbon. But in thi s course, we focus on carbon fibers that widely used in industry. It can be defined as a fiber about 5–10 micrometres in dia meter with a carbon content of at least 92 %.通常,碳纤维可以被定义为主要由碳构成的纤维。
材料导论中英文讲稿 (30)

Video 8. HardnessHardness. If I ask you what is the hardest material around the world? What is your answer? I believe most people will say ‘diamond’. Why you think diamond is hard? Because you can use diamond to scratch other material without any damage.Here comes the definition of hardness. It is a measure of a material’s resistance to penetration抗穿刺(local plastic deformation) or scratching抗划伤. 硬度就是衡量一个材料抗穿刺、抗划伤的能力。
Also, hard material has good resistance to wire or abrasion. 越坚硬的材料耐磨性也越强。
So, that is the reason why you use diamond to make cutting tools. 一些刀具或钻头往往用钻石类坚硬的材料制备。
译文:硬度。
如果我问你们什么是世界上最硬的材料?你会怎么回答?我相信大多数人会说:“钻石”。
为什么你们认为钻石最坚硬?因为你们可以用钻石划伤其他材料,而自身不受损坏。
硬度的定义是:硬度是衡量材料对穿刺或划伤的抵抗能力。
越坚硬的材料耐磨性越强,因此一些道具或钻头往往用钻石这种坚硬材料制备。
Here comes a measuring method to hardness. How can you quantitatively measure hardness? 你怎么来测量硬度呢?Based on the definition, we know hardness means the resistance to penetration or scratching. Therefore, the measuring methods are also corresponding to these two types of properties. You can either measure the degree of penetration, using some specifically selected indenters. There are four different measurements for hardness, where are called brinell hardness, Vickers hardness, Rockwell hardness and knoop hardness. You can also measure the resistance of scratching which is called Mohs hardness and most people are familiar with that (measuring method). There is another measuring method that is called rebounding method. It is called Scleroscope test.译文:这里给出一个测量硬度的方法。
材料导论词汇

Chapter 1 IntroductionRead the textbook (P3)and answer the following question:What is the phenomenon? And why?You should be able to do the following:1. List six different property classifications of materials that determine theirapplicability.2. Cite the four components that are involved in the design, production, andutilization of materials, and briefly describe the interrelationships between these components.3. Cite three criteria that are important in the materials selection process.4. (a) List the three primary classifications of solid materials, and then citethe distinctive chemical feature of each.(b) Note the other three types of materials and, for each, its distinctive feature(s).Chapter 2 Interatomic Bonding1 Make sure you understand concepts:“ Covalent bond 共价键A primary interatomic bond that is formed by the sharing of electrons between neighboring atoms.“Dipole (electric)偶极A pair of equal yet opposite electrical charges that are separated by a small distance.“Hydrogen bond氢键A strong secondary interatomic bond that exists between a bound hydrogen atom (its unscreened proton) and the lectrons of adjacent atoms.“ Ionic bond离子键A coulombic interatomic bond that exists between two djacent and oppositely charged ions.“Metallic bond金属键A primary interatomic bond involving the nondirectional sharing of nonlocalized valence electrons (‘‘sea of electrons’’)that are mutually shared by all the atoms in the metallic solid.“Polar molecule极性分子A molecule in which there exists a permanent electric dipole moment by virtue of the asymmetrical distribution of positively and negatively charged regions.“Primary bonding主价键Interatomic bonds that are relatively strong and for which bonding energies are relatively large. Primary bonding types are ionic, covalent, and metallic.“ Secondary bonding次价键Interatomic and intermolecular bonds that are relatively weak and for which bonding energies are relatively small. Normally atomic or molecular dipoles are involved. Secondary bonding types are van der Waals and hydrogen.“Van der Waals bond范德华键A secondary interatomic bond between adjacent molecular dipoles, which may be permanent or induced.“Valence electron价电子The electrons in the outermost occupied electron shell, which participate in interatomic bonding.2 you should be able to do the following:(1) Briefly describe formation and characteristics of ionic, covalent, metallic, hydrogen, and van der Waals bonds.(2) Note what materials exhibit each of these bonding types.Chapter 3 Structure of Solids1.Make sure you understand language and concepts:Allotropy 同素异形现象,同素异构现象The possibility of existence of two or more different crystal structures for a substance (generally an elemental solid). Amorphous无定形的,非晶态的Having a noncrystalline structure.Anisotropy各向异性Exhibiting different values of a property in different crystallographic directions.Atomic packing factor (APF) 原子密堆因素Body-centered cubic (BCC)体心立方Coordination number配位数The number of atomic or ionic nearest neighbors. Crystal structure晶体结构For crystalline materials, the manner in which atoms or ions are arrayed in space. It is defined in terms of the unit cell geometry and the atom positions within the unit cell.Crystalline晶体的The state of a solid material characterized by a periodic and repeating three-dimensional array of atoms, ions, or molecules.Face-centered cubic (FCC)面心立方Grain晶粒An individual crystal in a polycrystalline metal or ceramic.Grain boundary晶界The interface separating two adjoining grains having different crystallographic orientations.Hexagonal close-packed (HCP)密排六方Isotropic各向同性的Having identical values of a property in all crystallographic directions.Lattice parameter晶格参数The combination of unit cell edge lengths and interaxial angles that defines the unit cell geometry.Non-crystalline非晶的The solid state wherein there is no long-range atomic order. Sometimes the terms amorphous, glassy, and vitreous are used synonymously. Polycrystalline多晶的Referring to crystalline materials that are composed of more than one crystal or grain.Polymorphism多晶型现象,同质异构现象The ability of a solid material to exist in more than one form or crystal structure.Single crystal单晶A crystalline solid for which the periodic and repeated atomic pattern extends throughout its entirety without interruption.Unit cell晶胞,单胞The basic structural unit of a crystal structure. It is generally defined in terms of atom (or ion) positions within a parallelepiped volume.2. You should be able to do the following1. Describe the difference in atomic/molecular structure between crystalline and noncrystalline materials.2. Draw unit cells for face-centered cubic, body-centered cubic, and hexagonal close-packed crystal structures.3. Compute the densities for metals having face-centered cubic and body-centered cubic crystal structures given their unit cell dimensions.4. Distinguish between single crystals and polycrystalline materials.5. Define isotropy and anisotropy with respect to material properties.Chapter 4 Imperfections in solids1.Make sure you understand language and conceptsAlloy 合金A metallic substance that is composed of two or more elements.Atom percent 原子百分数Concentration specification on the basis of the number of moles (or atoms) of a particular element relative to the total number of moles (or atoms) of all elements within an alloy.Atomic vibration原子振动The vibration of an atom about its normal position in a substance. Boltzmann‘s constant波尔兹曼常数Burgers vector柏氏矢量A vector that denotes the magnitude and direction of lattice distortion associated with a dislocation.Composition成分The relative content of a particular element or constituent (i) within an alloy, usually expressed in weight percent or atom percent.Defect structure缺陷构造Relating to the kinds and concentrations of vacancies and interstitials in a ceramic compound.Dislocation line位错线The line that extends along the end of the extra half-plane of atoms for an edge dislocation, and along the center of the spiral of a screw dislocation.Edge dislocation刃位错A linear crystalline defect associated with the lattice distortion produced in the vicinity of the end of an extra halfplane of atoms within a crystal. The Burgers vector is perpendicular to the dislocation line.Electroneutrality电中性The state of having exactly the same numbers of positive and negative electrical charges (ionic and electronic), that is, of being electrically neutral.Frenkel defect弗兰克缺陷In an ionic solid, a cation–vacancy and cation–interstitial pair.Grain size晶粒尺寸The average grain diameter as determined from a random cross section. Imperfection缺陷,非理想性A deviation from perfection; normally applied to crystalline materials wherein there is a deviation from atomic/molecular order and/or continuity.Interstitial solid solution间隙式固溶体A solid solution wherein relatively small solute atoms occupy interstitial positions between the solvent or host atoms.Microscopy显微镜法The investigation of microstructural elements using some type of microscope.Microstructure显微结构,微观结构The structural features of an alloy (e.g., grain and phase structure) that are subject to observation under a microscope.Mixed dislocation混合位错A dislocation that has both edge and screw components. Photomicrograph显微照片Thephotograph made with a microscope, which records a microstructural image.Point defect点缺陷A crystalline defect associated with one or, at most, several atomic sites. Scanning electron microscope(SEM)扫描电子显微镜Scanning probe microscope (SPM)扫描探针显微镜Schottky defect肖脱基缺陷In an ionic solid, a defect consisting of a cation–vacancy and anion–vacancy pair.Screw dislocation螺旋位错A linear crystalline defect associated with the lattice distortion created when normally parallel planes are joined together to form a helical ramp. The Burgers vector is parallel to the dislocation line.Self-interstitial自间隙的A host atom or ion that is positioned on an interstitial lattice site.Solid solution固溶体A homogeneous crystalline phase that contains two or more chemical species. Both substitutional and interstitial solid solutions are possible.Solute溶质One component or element of a solution present in aminor concentration. It is dissolved in the solvent.Solvent溶剂The component of a solution present in the greatest amount. It is the component that dissolves a solute.Stoichiometry化学计量学For ionic compounds, the state of having exactly the ratio of cations to anions specified by the chemical formula.Substitutional solid solution置换固溶体A solid solution wherein the solute atoms replace or substitute for the host atoms.Transmission electron microscope (TEM)透射电子显微镜Vacancy空位A normally occupied lattice site from which an atom or ion is missing.Weight percent重量百分数Concentration specification on the basis of weight (or mass) of a particular element relative to the total alloy weight (or mass).2. You should be able to do the following1. Describe both vacancy and self-interstitial crystalline defects.2. Calculate the equilibrium number of vacancies in a material at some specified temperature, given the relevant constants.3. Name the two types of solid solutions, and provide a brief written definition and/or schematic sketch of each.4. Name and describe eight different ionic point defects that are found In ceramic compounds.5. Given the masses and atomic weights of two or more elements in a metal alloy, calculate the weight percent and atomic percent for each element.6. For each of edge, screw, and mixed dislocations:(a) describe and make a drawing of the dislocation;(b) note the location of the dislocation line; and(c) indicate the direction along which the dislocation line extends.7. Describe the atomic structure within the vicinity of (a) a grain boundary, and(b) a twin boundary.Chapter 5 mechanical properties1.M ake sure you understand language and conceptsAnelasticity滞弹性Anelastic deformation. Time-dependent elastic (nonpermanent) deformation. Charpy test简支梁试验One of two tests (see also Izod test) that may be used to measure the impact energy or notch toughness of a standard notched specimen. An impact blow is imparted to the specimen by means of a weighted pendulum.Ductility延展性A measure of a material’s ability to undergo appreciable plastic deformation before fracture; it may be expressed as percent elongation (%EL) or percent reduction in area (%RA) from a tensile test.Elastic deformation弹性变形Deformation that is nonpermanent, that is, totally recovered upon release of an applied stress.Elastic recovery弹性恢复Nonpermanent deformation that is recovered or regained upon the release of a mechanical stress.Engineering strain工程应变The change in gauge length of a specimen (in the direction of an applied stress) divided by its original gauge length.Engineering stress工程应力The instantaneous load applied to a specimen divided by its cross-sectional area before any deformation.Hardness硬度The measure of a material’s resistance to deformation by surface indentation or by abrasion.Impact energy冲击能A measure of the energy absorbed during the fracture of a specimen of standard dimensions and geometry when subjected to very rapid (impact) loading. Charpy and Izod impact tests are used to measure this parameter, which is important in assessing the ductileto-brittle transition behavior of a material.Izod test悬臂梁试验One of two tests (see also Charpy test) that may be used to measure the impact energy of a standard notched specimen. An impact blow is imparted to the specimen by a weighted pendulum.Modulus of elasticity弹性模量The ratio of stress to strain when deformation is totally elastic; also a measure of the stiffness of a material.Plastic deformation塑性变形Deformation that is permanent or nonrecoverable after release of the applied load. It is accompanied by permanent atomic displacements.Poisson’s ratio 泊松比For elastic deformation, the negative ratio of lateral and axial strains that result from an applied axial stress.Shear剪切A force applied so as to cause or tend to cause two adjacent parts of the same body to slide relative to each other, in a direction parallel to their plane of contact.Tensile strength拉伸强度The maximum engineering stress, in tension,that may be sustained without fracture.Often termed ultimate (tensile) strength.Toughness韧性A measure of the amount of energy absorbed by a material as it fractures. Toughness is indicated by the total area under the material’s tensile str ess–strain curve.Yielding屈服The onset of plastic deformation.Yield strength屈服强度The stress required to produce a very slight yet specified amount of plastic strain; a strain offset of 0.002 is commonly used.2.you should be able to do the following1. Define engineering stress and engineering strain.2. State Hooke's law, and note the conditions under which it is valid.3. Define Poisson's ratio.4. Given an engineering stress-strain diagram, determine (a) the modulus of elasticity, (b) the yield strength (0.002 strain offset), and (c) the tensile strength, and (d) estimate the percent elongation.5.For the tensile deformation of a ductile cylindricai specimen, describe changes in specimen profile to the point of fracture.6. Compute ductility in terms of both percent elongation and percent reduction of area fora material that is loaded in tension to fracture.7. State the reasons why hardness tests are performed more frequently than any other mechanical test .Name the two most common hardness-testing techniques.8. Compute the working stress for a ductile materiai.Chapter 6 Deformation and Strengthening Mechanisms 1.Make sure you understand language and concepts:Cold working冷加工The plastic deformation of a metal at a temperature below that at which it recrystallizes.Critical resolved shear stress临界分切应力That shear stress, resolved within a slip plane and direction, which is required to initiate slip.Dislocation density位错密度The total dislocation length per unit volume of material; alternately, the number of dislocations that intersect a unit area of a random surface section.Grain growth晶粒长大The increase in average grain size of a polycrystalline material; for most materials, an elevated-temperature heat treatment is necessary. Lattice strain晶格应变,晶格畸变Slight displacements of atoms relative to their normal lattice positions, normally imposed by crystalline defects such as dislocations, and interstitial and impurity atoms.Recovery回复The relief of some of the internal strain energy of a previously cold-worked metal, usually by heat treatment.Recrystallization再结晶The formation of a new set of strain-free grains within a previously cold-worked material; normally an annealing heat treatment is necessary. Recrystallization temperature再结晶温度For a particular alloy, the minimum temperature at which complete recrystallization will occur within approximately one hour.Resolved shear stress分切应力An applied tensile or compressive stress resolved into a shear component along a specific plane and direction within that lane.Slip滑移Plastic deformation as the result of dislocation motion; also, the shear displacement of two adjacent planes of atoms.Slip system滑移系The combination of a crystallographic plane and, within that plane, a crystallographic direction along which slip (i.e., dislocation motion) occurs. Strain hardening形变硬化The increase in hardness and strength of a ductile metal as it is plastically deformed below its recrystallization temperature.Solid-solution strengthening固溶强化Hardening and strengthening of metals that result from alloying in which a solid solution is formed. The presence of impurity atoms restricts dislocation mobility.2.you should be able to do the following1. Describe edge and screw dislocation motion from an atomic perspective.2. Describe how plastic deformation occurs by the motion of edge and screwdislocations in response to applied shear stresses.3. Define slip system and cite one example.4. Describe how the grain structure of a polycrystalline metal is altered whenit is plastically deformed.5. Explain how grain boundaries impede dislocation motion and why a metalhaving small grains is stronger than one having large grains.6. Describe and explain solid-solution strengthening for substitutionalimpurity atoms in terms of lattice strain interactions with dislocations.7. Describe and explain the phenomenon of strain hardening (or cold working)in terms of dislocations and strain field interactions.8. Describe recrystallization in terms of both the alteration of microstructureand mechanical characteristics of the material.9. Describe the phenomenon of grain growth from both macroscopic andatomic perspectives.Chapter 7 Phase diagrame1.Make sure you understand language and concepts:Austenite奥氏体Face-centered cubic iron; also iron and steel alloys that have the FCC crystal structure.Cementite渗碳体Iron carbide (Fe3C).Component组元A chemical constituent (element or compound) of an alloy, which may be used to specify its composition.Equilibrium平衡The state of a system where the phase characteristics remain constant over indefinite time periods. At equilibrium the free energy is a minimum.Eutectic phase共晶相One of the two phases found in the eutectic structure.Eutectic reaction共晶反应 A reaction wherein, upon cooling, a liquid phase transforms isothermally and reversibly into two intimately mixed solid phases.Eutectic structure共晶组织A two-phase microstructure resulting from the solidification of a liquid having the eutectic composition; the phases exist as lamellae that alternate with one another. Eutectoid reaction共析反应A reaction wherein, upon cooling, one solid phase transforms isothermally and reversibly into two new solid phases that are intimately mixed.Ferrite铁素体Ceramic oxide materials composed of both divalent and trivalent cations (e.g., Fe2 and Fe3 ), some of which are errimagnetic.Hypereutectoid alloy过共析合金For an alloy system displaying a eutectoid, an alloy for which the concentration of solute is greater than the eutectoid composition.Hypoeutectoid alloy亚共析合金For an alloy system displaying a eutectoid, an alloy for which the concentration of solute is less than the eutectoid composition.Intermetallic compound金属间化合物A compound of two metals that has a distinct chemical formula. On a phase diagram it appears as an intermediate phase that exists over a very narrowrange of compositions.Invariant point恒定点、三相平衡点A point on a binary phase diagram at which three phases are in equilibrium.Isomorphous匀晶的Having the same structure. In the phase diagram sense, isomorphicity means having the same crystal structure or complete solid solubility for all compositions.Metastable 亚稳的Nonequilibrium state that may persist for a very long time.Microstructure显微组织The structural features of an alloy (e.g., grain and phase structure) that are subject to observation under a microscope.Pearlite珠光体A two-phase microstructure found in some steels and cast irons; it results from the transformation of austenite of eutectoid composition and consists of alternating layers (or lamellae) of α-ferrite and cementite.Peritectic reaction包晶反应A reaction wherein, upon cooling, a solid and a liquid phase transform isothermally and reversibly to a solid phase having a different composition.Phase相 A homogeneous portion of a system that has uniform physical and chemical characteristics.Phase diagram相图A graphical representation of the relationships between environmental constraints (e.g., temperature and sometimes pressure), composition, and regions of phase stability, ordinarily under conditions of equilibrium.Phase equilibrium相平衡Proeutectoid cementite先共析渗碳体Primary cementite that exists in addition to pearlite for hypereutectoid steels.Proeutectoid ferrite先共析铁素体Primary ferrite that exists in addition to pearlite for hypoeutectoid steels.Solubility limit溶解度极The maximum concentration of solute that may be added without forming a new phase.2.you should be able to do the following1. (a) Schematically sketch simple isomorphous and eutectic phase diagrams.(b) On these diagrams label the various phase regions.(c) Label liquidus, solidus, and solvus lines.2. Given a binary phase diagram, the composition of an alloy, its temperature, and assuming that the alloy is at equilibrium, determine:(a) what phase(s) is (are) present;(b) the composition(s) of the phase(s).3. For some given binary phase diagram, do the following:(a) locate the temperatures and compositions of all eutectic, eutectoid, peritectic, and congruent phase transformations; and(b) write reaction for all these transformations for either heating or cooling.4. Given the composition of an iron-carbon alloy containing between 0.022 wt% C and 2.14 wt% C, be able to(a) specify whether the alloy is hypoeutectoid or hypereutectoid;(b) name the proeutectoid phase; and(c) make a schematic diagram of the microstructure at a temperature just below the eutectoid.Chapter 8 Phase transformation1.Make sure you understand language and concepts:Annealing退火A generic term used to denote a heat treatment wherein the microstructure and, consequently, the properties of amaterial are altered. ‘‘Annealing’’ frequently refers to a heat treatment whereby a previously cold-worked metal is softened by allowing it to recrystallize. Austenitizing奥氏体化Forming austenite by heating a ferrous alloy above its upper critical temperature—to within the austenite phase region from the phase diagram.Bainite贝氏体An austenitic transformation product found in some steels and cast irons. It forms at temperatures between those at which pearlite and martensite transformations occur. The microstructure consists of α-ferrite and a fine dispersion of cementite.Coarse pearlite粗珠光体Pearlite for which the alternating ferrite and cementite layers are relatively thick.Fine pearlite细珠光体Pearlite for which the alternating ferrite and cementite layers are relatively thin.Full annealing完全退火For ferrous alloys, austenitizing, followed by cooling slowly to room temperature.Isothermal transformation diagram等温转变图A plot of temperature versus the logarithm of time for a steel alloy of definite composition. Used to determine when transformations begin and end for an isothermal (constant-temperature) heat treatment of a previously austenitized alloy. Martensite马氏体A metastable iron phase supersaturated in carbon that is the product of a diffusionless (athermal) transformation from austenite.Normalizing正火For ferrous alloys, austenitizing above the upper critical temperature, then cooling in air. The objective of this heat treatment is to enhance toughness by refining the grain size.Nucleation形核率The initial stage in a phase transformation. It is evidenced by the formation of small particles (nuclei) of the new phase, which are capable of growing.Phase transformation 相变A change in the number and/or character of the phases that constitute the microstructure of an alloy.Precipitation hardening沉淀强化(age hardening时效强化)Hardening and strengthening of a metal alloy by extremely small and uniformly dispersed particles that precipitate from a supersaturated solid solution; sometimes also called age hardening.Precipitation heat treatment 沉淀热处理A heat treatment used to precipitate a new phase from a supersaturated solid solution. For precipitation hardening, it is termed artificial aging.Solution heat treatment固溶处理The process used to form a solid solution by dissolving precipitate particles. Often, the solid solution is supersaturated and metastable at ambient conditions as a result of rapid cooling from an elevated temperature.Spheroidite球状珠光体Microstructure found in steel alloys consisting of spherelike cementite particles within an α-ferrite matrix. It is produced by an appropriate elevated-temperature heat treatment of pearlite, bainite, or martensite, and is relatively soft.Spheroidizing球化For steels, a heat treatment carried out at a temperature just below the eutectoid in which the spheroidite microstructure is produced.Supercooling过冷Cooling to below a phase transition temperature without the occurrence of thetransformation.Superheating过热Heating to above a phase transition temperature without the occurrence of the transformation.Tempered martensite回火马氏体The microstructural product resulting from a tempering heat treatment of a martensitic steel. The microstructure consists of extremely small and uniformly dispersed cementite particles embedded within a continuous α-ferrite matrix. Toughness and ductility are enhanced significantly by tempering.Thermally activated transformation热激活转变A reaction that depends on atomic thermal fluctuations; the atoms having energies greater than an activation energy will spontaneously react or transform. The rate of this type of transformation depends on temperature according to Equation 10.3.Transformation rate转变速率The reciprocal of the time necessary for a reaction to proceed halfway to its completion.2.you should be able to do the following:1. Briefly describe the microstructure for each of the following microconstituents thatare found in steel alloys: fine pearlite, coarse pearlite, spheroidite, bainite, martensite, and tempered martensite.2. Cite the general mechanical characteristics for each of the followingmicroconstituents: fine pearlite, coarse pearlite, spheroidite, bainite, martensite, and tempered martensite. Now, in terms of microstructure (or crystal structure), briefly explain these behaviors.3. Given the isothermal transformation (or continuous cooling transformation)diagram for some iron-carbon alloy, design a heat treatment that will produce a specified microstructure.4. Using a phase diagram, describe and explain the two heat treatments that are usedto precipitation-harden a metal alloy.Chapter 9 Types and Applications of Materials 1、Make sure you understand language and concepts:Alloy steel 合金钢A ferrous (or ironbased) alloy that contains appreciable concentrations of alloying elements (other than C and residual amounts of Mn, Si, S, and P). These alloying elements are usually added to improve mechanical and corrosion resistance properties.Cast iron铸铁Generically, a ferrous alloy, the carbon content of which is greater than the maximum solubility in austenite at the eutectic temperature. Most commercial cast irons contain between 3.0 and 4.5 wt%C, and between 1 and 3 wt%Si.Malleable cast iron可锻铸铁White cast iron that has been heat treated to convert the cementite into graphite clusters; a relatively ductile cast iron.Nonferrous alloy 非铁合金,有色金属合金Ametal alloy for which iron is not the prime constituent.Ductile (nodular) iron 球墨铸铁 A cast iron that is alloyed with silicon and a small concentration of magnesium and/or cerium and in which the free graphite exists in nodular form. Sometimes called nodular iron.Ferrous alloy 铁合金A metal alloy for which iron is the prime constituent.Gray cast iron 灰铸铁A cast iron alloyed with silicon in which the graphite exists in the form of flakes. A fractured surface appears gray.Plain carbon steel普通碳素钢A ferrous alloy in which carbon is the prime alloying element. Stainless steel不锈钢 A steel alloy that is highly resistant to corrosion in a variety of environments. The predominant alloying element is chromium, which must be present in a concentration of at least 11 wt%; other alloy additions, to include nickel and molybdenum, are also possible.White cast iron白口铸铁A low-silicon and very brittle cast iron, in which the carbon is in combined form as cementite; a fractured surface appears white.2、you should be able to do the following:1. Name four different types of steels and, for each, cite compositional differences, distinctive properties, and typical uses.2. Name the four cast iron types and, for each, describe its microstructure and note its general mechanical characteristics.3. Name seven different types of nonferrous alloys and, for each, cite its distinctive physical and mechanical characteristics; in addition, list at least three typical applications.11/ 11。
材料科学导论英文阅读

Chapter 1 An Overview第一章概述1.1 Introduction1.1介绍Materials are the matter of the universe. These substances have properties that make them useful in structures, machines, devices, products, and systems. The term properties describe behavior of materials when subjected to some external force or condition. For example, the tensile strength of a metal is a measure of the material's resistance to a pulling force. The Family of Materials consists of four main groups of materials: Metals (e.g., steel), Polymers (e.g., plastics), Ceramics (e.g., porcelain), and Composites (e.g., glass-reinforced plastics). The materials in each group have similar properties and/or structures, as will be described later.材料是宇宙的物质。
这些物质的特性使其有用的结构、机器设备、产品和系统。
这个术语属性描述材料的行为时,受到一些外部力量或状态。
例如,抗拉强度的金属是测量的材料抵抗了拉力。
这个家庭的材料由四个主要群体的材料:金属(如钢)、高分子材料(例如:塑料)、陶瓷(如瓷),复合材料(例如,增强塑料)。
材料导论中英文讲稿 (67)

In this video, I will talk about another naturally occurring polymer which closely related to our daily life. It is called starch.在这个视频中,我将讨论另一种与我们日常生活密切相关的天然聚合物。
它被称为淀粉。
也就是我们生活中最常见到的食物-淀粉。
Starch is produced by most green plants as energy storage. It is the most common carbohydrate in human diets and is contained in large amounts in staple foods like potatoes, wheat, corn, and rice. Starch is a homopolysachride whose monomer is alpha-glucose.淀粉由大多数绿色植物产生并作为能量储存。
它是人类饮食中最常见的碳水化合物,马铃薯,小麦,玉米和大米等主食中含有大量淀粉。
淀粉是均聚物,其单体是α-葡萄糖。
请大家和我一起回忆一下上节课讲的纤维素,纤维素和淀粉的重复单元都是D-glucose。
It consists of two types of linkages: The alpha-1-4 linkages result in a linear structure, while alpha-1-6 linkages lead to a branched structure. Generally, the more highly branched starch fractions have been called “amylopectin” while the straight-chain starch fractions have been termed “amylose“.淀粉由两种类型的连接组成:α-1-4连接的线性结构,和α-1-6连接的支化结构。
材料导论中英文讲稿 (7)

6.5 GlassHello, everybody, we are going to talking about glass.译文:大家好,我们要讨论的是玻璃。
The glasses are a familiar group of ceramics; containers, lenses, and fiberglass represent typical applications. They are noncrystalline silicates containing other oxides, notably CaO, Na2O, K2O, and Al2O3, which influence the glass properties. A typical soda-lime glass consists of approximately 70 wt% SiO2, the balance being mainly Na2O (soda) and CaO (lime).译文:这种玻璃是一种熟悉的陶瓷制品,常应用于容器,透镜和玻璃纤维。
它们是含有其他氧化物的非晶态的硅酸盐,尤其是CaO、Na2O、K2O和Al2O3,它们影响着玻璃的性能。
典型的钠钙玻璃大约有70%左右的二氧化硅,用于平衡的是氧化钠(苏打)和氧化钙(石灰)。
The base raw material of glass is the very pure, white silica sand found in abundant supply in the world. Although there are approximately 750 different glasses and glass ceramics, most can fit into six groups: soda-lime, lead-alkali, borosilicate, aluminosilicate, 96% silica, and fused silica. 玻璃可以归为六类:钠钙玻璃、钠铅玻璃、硼硅玻璃、铝硅玻璃、高硅玻璃和熔融石英玻璃。
材料导论中英文讲稿 (13)

Property
Angular l momentum
角量子数
integers from 0 to n-1
Orbital轨道 shape
Subshells 支壳层 Sublevel 亚(能)级 Orbital (s, p, d, f)
Subshells or Sublevels ( l = 0 ~ n-1 ) s
Nucleus
Electron
Orbit
Energy Levels Introduction to Materials
12
Valence Electrons 价电子: Electrons in the outermost 最外面的 ring or shell from the nucleus
VValaelnenceceEElelcetcrtornon
② Neutrons 中子: uncharged particles in the nucleus with a mass nearly equal to the proton’s mass.
③ Nucleus 核: Almost all the mass of an atom
The atomic mass (A) 原子质量 = Total Mass of Atom ≈ mass of protons + mass of neutrons
give off
alpha particles, nucleons, electrons,
gamma rays 11
(2) Electrons
• Electrons: Negatively charged, Form a cloud around the nucleus.
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
A) only elastic B) both elastic and plastic (塑性)
C) only plastic D) neither elastic nor plastic
l) Compare the tensile strength of LDPE, HDPE, PVE, PP, and arrange them in sequence(顺序) from high strength to low tensile strength.
A) polymers B) monomers (单体) C) oligomers D)elastomers
b Choose the type of polymer material you might select for the following applications: an automobile bumper (保险杠), a mineral water bottle, and an insulating (绝热的) cable.
A) degree of polymerization B)density of branching C)tacticity D) crystallinity
d) ABS, composed of acrylonitrile, butadiene, and styrene (苯乙烯), is one of the most common polymer materials. Styrene and acrylonitrile form a liner copolymer (异量分子聚合物) (SNA) that serves as a matrix. Styrene and butadiene also form a liner copolymer, BS rubber, which acts as the ______ material.
A) For automobile bumper, the best choice of polymer is Natural Rubber.
B) For mineral water bottle, the best choice of polymer is Low-Density(密度) Polyethylene (聚乙烯)
g) Which one is not a thermoset polymer?
A) Polyethylene B) Natural Rubber C) Epoxy D) Phenolic
h) Depending on the degree of cross-linking, the polyurethanes behave as thermosetting polymers, thermoplastics (热塑性塑胶), or elastomers. These polymers find application as fibers, coatings, and foams for furniture, mattress, and insulation. Why polyurethanes are versatile?
A) Tg B) Td C) Tm D) HDT
a Polymerization (聚合作用) is the process by which small molecules (分子) consisting of one unit or a few units are chemically joined to create these giant molecules. Those small molecule units are called ______
A) LOPE>HDPE>PVE>PP B) LDPE>HDPE>PP>PVC
C) PVC>PP>HDPE>LDPE D) PVC>HDPE>PP>LDPE
m) Degree of polymerization is usually used to characterize ________
A) extrusion B) blow molding C) injection molding D) thermoforming
16) The recycling of thermoplastics is relatively easy and practiced widely. Many of the everyday plastic products you encounter (bags, soda bottles, yogurt containers, etc.) have numbers stamped on them. For PRT products, the number is 1. For HDPE and LDPE, the numbers are_______, respectively. Other plastics are marked number 7,
A) 100 B) 150 C) 200 D) 300
15) There are a lot of thermoplastic processing methods, typical forming process includes: extrusion, blow molding, injection molding, thermoforming, calendaring and spinning. If we want to produce sping water bottles, which processing way is best choice?
A) lightweight and corrosion(铁锈,腐蚀)-resistant
B) high strength and high-temperature resistant
C) high-temperature resistant and insulating
D) high stiffness and corrosion-resistant
A) Chemically Reactive Adhesives
B) Evaporation or Diffusion Adhesives
C) Hot-Melt Adhesives D) Pressure-Sensitive Adhesives
f) What area the major advantages associated with plastic compared to ceramics, glasses, and metallic materials?
A) liquid B) thermoplastic C) thernoset D) oriented(取向) rods
l) The glass temperature (Tg) is typically about ______ times the absolute melting temperature (Tm).
A) 3 and 4 B) 4 and 5 C) 2 and 4 D) 2 and 3
17) The temperature above which a polymer burns, chars, or decompose. Which team is appropriately used in describing the remperature.
c) In general, for a given type of thermoplastic (热塑性塑胶) the tensile (可延展的) strength, creep resistance impact toughness, wear resistance, and melting temperature all increase with______
A) cross-linking network B) thermosetting polymer
C) polymer degradation D) thermoplastic polymer
14) Silicones(硅) are important elastomer based on chains composed of silicon and oxygen atoms. The silicone rubbers provide high-temperature resistance, permitting use of the elastomer at temperature as high as _____℃. Low molecular weight silicones form liquids and are known as silicon oils.
C) For insulating cable, the best choice of polymer is polystyrene (聚苯乙烯).
D) For mineral water bottle, the best choice of polymer is Polyethylene Terephthalate.
A) 0.2 to 0.3 B) 0.5 to 0.7 C) 0.8 to 1.0 D) 1.0 to 2.0
i) Most thermoplastic exhibit a non-Newtonian and visicoelastic behavior. The stress and train are not linearly related for most parts of the stress-strain curve. The viscoelastic behavior means when an external (外部的) force is applied to a thermoplastic _______ deformation occurs.
