化学专业英语之含氧有机化合物
马永祥《化学专业英语》课文翻译 第14课

化学专业英语马永祥,兰州大学出版社课文翻译14. 含氧化合物醇和酚在醇的取代物和连结体的命名中,羟基作为主要基团,是通过后缀“醇”来表示,省略母体化合物名称末尾“e”(如果有的话),例如:甲醇,2-丙醇,三苯甲醇等。
下面是一些被保留的俗名的例子:烯丙醇,叔丁醇,苄醇,乙二醇,芳香醇等。
苯和其它芳香碳环族的羟基衍生物是通过在碳氢化合物名称加后缀“醇”“二醇”等命名的,“ol”前末端的“e”要省略,例如:1,2,4-苯三醇。
以下是一些芳香族羟基化合物仍被保留的俗名。
例如:苯酚,甲酚,荼酚,邻苯二酚,间苯二酚,对苯二酚等等。
基团RO-是通过在R基名称后加后缀“氧基”而命名,例如:戊氧基,烯丙氧基,苄氧基等,仅有下列含氧基团名称的缩写是不符合这一被推荐规则的:甲氧基,乙氧基,丙氧基,丁氧基,苯氧基,异丙氧基。
除去部分成环体系,二价基团-o-x-o-通过给双二价基团-x-名称附加“二氧基”而命名,例如:乙撑二氧基,丙撑二氧基。
盐醇或酚衍生的阴离子的命名是将醇或酚名称末尾的“-ol”变成“-olate”。
这一方法适用于取代基,官能团和俗名。
例如:甲醇钾,苯酚钠。
醚化合物R1-O-R2总称为“醚”,可以通过取代基或官能团来命名。
不对称醚取代物命名法是将基团R1O-的名称作为前缀加在相应第二个基团R2前面而形成。
较高级的组分被选作母体化合物。
醚的官能团命名法则是在基团R1和R2名称后面加“醚”字即可。
例如:1-异丙氧基丙烷,甲乙醚,二乙醚,乙基乙烯基醚。
醛醛是含有连结在C原子上的(C=O)H基团的化合物,命名是通过后缀“-al”“-aldehyde”或“carbaldehyde”或者是通过加前缀“formyl-”(当作为一个碳链的末端基团存在时代表-(C=O)H),或者与俗名有关,加前缀“oxo”(代表=0),无支链的非环的单醛或二醛的名字是通过给含有相同碳原子数的烃化合物的名字加后缀“-al”(对单醛)或“dial”(对双醛)形成。
有机化学专业英语

烷基Alkyl [ˈælkil]芳基aryl [ˈæril]甲基methyl [ˈmeθil]亚甲基methylene[ˈmeθili:n]乙基ethyl [ˈeθil,ˈeθəl]丙基propyl [ˈprəupil]异丙基isopropyl [ˌaisəuˈprəupil]丁基butyl [ˈbju:til]戊基pentyl [ˈpentil]己基hexyl [ˈheksil]庚基heptyl [ˈheptil]辛基octyl [ˈɔktəl]壬基nonyl [ˈnɔnil]奎基decyl ['desəl][di:'s i l]叔丁基tert-butyl异丁基iso-butyl环戊基cyclopentyl []环己基cyclohexyl []甲氧基methoxyl ['metɒksɪl]乙氧基ethoxyl [eˈθɔksil]丁氧基butoxyl酰基acyl[ˈæsil]甲酰基formyl [ˈfɔ:mil]乙酰基acetyl [ˈæsitil]乙烯基vinyl [ˈvaɪnəl]或ethenyl丁烯基butenyl [ˈbjutənil]己烯基hexenyl庚烯基heptenyl [ˈheptəˌnil]烯丙基allyl [ˈælil]乙炔基ethinyl [eˈθainil] 或alkynyl硝基nitro [ˈnaitrəu]亚硝基nitroso [naiˈtrəusəu]氨基amino [əˈmi:nəʊ, ˈæməˌnəʊ]二氨基diamino亚氨基imino [ˈiminəu,iˈmi:nəu]重氮基diazo [daiˈæzəu]苯基phenyl [ˈfenəl,ˈfi:nəl,ˈfi:nil]苄基benzyl [ˈbenzil]或phenmethyl [ˌfinˈmeθil]苯乙基phenethyl [fenˈeθəl]乙氧苯基ethoxyphenyl苯胺基anilino [ˈænili:n]1羰基carbonyl [ˈkɑ:bənil]羧基carboxyl [kɑ:ˈbɔksil]联苯基biphenyl [baiˈfenl]甲酰基formyl [ˈfɔ:mil]苯酰,苯甲酰benzoyl ['benzəʊɪl]脒基guanyl [il]羟基hydroxyl [haiˈdrɔksil]烷氧基alkoxy [ælˈkɔksi]或alkoxyl group 芳基aryl group二芳基diaryl group [daiˈæril]吡啶基pyridyl[ˈpiridil]三苯甲基trityl['traɪtl]二苯甲基benzhydryl [benaɪd'raɪl]氨基甲酰基carbamoyl[kɑ:'bæməɪl]三甲基硅基trimethylsilyl炔丙基propargyl [prəʊ'pɑ:dʒɪl]丙酮基(乙酰甲基)acetonyl['æsɪtənɪl]正n,normal异iso邻位ortho-[ˈɔ:θəu]间位meta-['mɛtə]对位para-[ˈpɑ:rə]伯Primary ['praimәri]仲Secondary ['sekәndәri]叔Tertiary ['tә:ʃәri] tert-季碳quaternary [kwəˈtə:nəri] carbon一,单mono-二di-,双bis ,bi(化学中只有碳酸氢根才用bi,如bicarbonate [baiˈkɑ:bənit])三tri-,tris四tetra-四quadric-五penta-五quinque-六hexa-七hepta-七septi八octa-九nona-十deca-['dɛkə]十一undeca ,hendeca-十二dodeca-十三trideca-十四tetradeca十五pentadeca-十六hexadeca-2十七heptadeca-顺式,cis-同,共syn反式trans有机化合物类名Aliphatic compound 脂肪族化合物[]Hydrocarbon 碳氢化合物[ˌhaɪdrəˈk ɑ:bən] Alkane 烷[]Wax 蜡[]Paraffin wax 石蜡arene 芳烃[]Alkene 烯[]Alkyne 炔[ˈælkain]Acetylide 炔化物[] Active hydrogen compounds 活泼氢化合物acid [ˈæsid]Carbon acid 碳氢酸Super acid 超酸Diene 双烯[ˈdaii:n]Triene 三烯[ˈtraii:n]Allene 丙二烯[ˈæli:n]Propylene丙烯[] cumulene 累积多烯[] Enyne 烯炔[eˈni:n]Diyne 二炔Alkyl halide 卤代烷[ˈælkil ˈhælaid]Alcohol 醇[]Homoallylic alcohol 高烯丙醇Ether 醚[ˈi:θə]Ester 酯[ˈestə]Ketone 酮Aldehyde 醛[ˈældihaid]Epoxide 环氧化物[eˈpɔksaid]Sulfone 砜[ˈsʌlf əun]Sulfoxide 亚砜Sulfonic acid 磺酸Carboxylic acid 羧酸Cellosolve 溶纤剂Crown ether 冠醚Nitro compound 硝基化合物Amine 胺[] Quaternaryammonium compound 季铵化合物[] []Amine oxide 氧化胺Diazoalkane 重氮烷[daɪ,æzəʊ'ælkeɪn] Mercaptan 硫醇[] Aldehyde hydrate 醛水合物Ketone hydrate 酮水合物Hemiacetal 半缩醛[ˌhemiˈæsitæl]3Acetal 缩醛acetal [化]乙缩醛, 乙缩醛二乙醇[ˈæsitæl] Ketal 缩酮[ˈki:tæl]thiazole噻唑[ˈθaiəˌzəul]Dithiane 二噻烷[daiˈθaiən]Aminal 缩醛胺;动物imine 亚胺[]Aldimine 醛亚胺Oxime 肟[]nitroso compound 亚硝基化合物aldoxime 醛肟,乙醛肟[ælˈdɔksi:m] Hydrazone 腙[ˈhaidrəˌzəun]Azine 嗪[ˈæzi:n]Semicarbazone 缩氯基脲Cyanohydrin 羟腈,氰醇[ˌsaiənəuˈhaidrin] Pinacol 频哪醇Enol 烯醇[ˈi:nɔl]Enol ether 烯醇醚Enol ester 烯醇酯[ˈi:nɔl][ˈestə] Enamine 烯胺[iˈnæmin]Ynamine 炔胺Mannich base 曼尼希碱orthoester 原酸酯Acyl halide 酰卤[ˈæsil]Acyl fluoride 酰氟[]Acyl chloride 酰氯Acyl bromide 酰溴Acyl iodide 酰碘[ˈaiədaid]Carbobenzoxy chloride 苄氧甲酰氯Acyl tosylate 酰基对甲苯磺酸酐Ketene 乙烯酮[ˈki:ti:n]Peracid 过酸Perester 过酸酯Acyl peroxide 酰基过氧化物Nitrile 腈[ˈnaitrail]acetonitrile 乙腈[ˌæsitəuˈnaitril]或methyl cyanide [ˈsaɪəˌnaɪd]Nitrile oxide 氧化腈Isonitrile 异腈,异氰化物Amide 酰胺[ˈæmaid]Imide 二酰亚胺[ˈimaid]N-bromo compound N-溴化物Hydrazide 酰肼[]Azide 叠氮化物[ˈæzaid,ˈeizid]Acyl azide 酰基叠氮[ˈæsil][ˈæzaid,ˈeizid] Amidine 脒[ˈæmiˌdi:n]Keto ester 酮酸酯Acyl cyanide 酰腈[ˈæsil][ˈsaɪəˌnaɪd] Carbon suboxide 二氧化三碳Glycidic acid 环氧丙酸Carbammic acid 氨基甲酸Carbamate 氨基甲酸酯[ˈkɑ:bəmeit]Urea 脲,尿素[] Cyanamide 氨腈[saiˈænəmaid] Carbodiimide 碳二亚胺[,kɑ:bədai'imaid] Allophanate 脲基甲酸酯Thioester 硫代酸酯[ˌθaiəuˈestə]Thiol acid 硫羰酸[ˈθaiəu]Lactone 内酯[ˈlæktəun]Lactol 内半缩醛[ˈlæktəl]4Macrolide 大环内酯[ˈmækrəlaid] Amino acid 氨基酸Zwitterion两性离子[ˈtsvitəraiən]Inner salt 内盐Betaine 甜菜碱[ˈbi:təi:n]Lactam 内酰胺[ˈlæktæm]Hydantoin 或glycolylurea 乙内酰脲[haiˈdæntəwin]Hydration水合,水合作用[haɪ'dreʃən] Peptide 肽[ˈpepˌtaɪd]Glycol 乙二醇[]Aldol 羟醛[ˈældəul]Acyloin 偶姻,酮醇[əˈsiləuin]acyloin condensation 酮醇缩合Carbohydrate 碳水化合物Aldose 醛糖[ˈældəus]Ketose 酮糖[ˈki:təus]Furanose 呋喃糖[ˈfjuərəˌnəus] Pyranose 吡喃糖[ˈpaiərənəus] Glycoside 糖苷[ˈɡlaikəˌsaid]Glucoside 葡[萄]糖苷Aglycon 苷元[əˈɡlaikɔn]Saccharide 糖类[ˈsækəraid] Oligosaccharide 寡糖[ˌɔliɡəuˈsækəraid] Polysaccharide 多糖[pɔliˈsækəraid] Alditol 糖醇[ˈælditɔl]Osazone 脎[ˈəusəˌzəun]Alicyclic compound 脂环化合物[æliˈsiklik] Cycloalkane 环烷Cycloalkene 环烯Spirane 螺烷[ˈspaiərein]Cage compound 笼型化合物Propellane 螺桨烷Rotazane 轮烷Catenane 索烃[ˈkætnein]Fused ring 稠环[fju:zd riŋ]化学专业英语词汇常用前后缀-acetal 醛缩醇acetal- 乙酰acid 酸-al 醛alcohol 醇-aldehyde 醛alkali- 碱allyl 丙烯基 'alkoxy- 烷氧基Methoxy甲氧基的-amide 酰胺 []amino- 氨基的[əˈmi:nəʊ, ˈæməˌnəʊ]-amidine 脒[ˈæmiˌdi:n]-amine 胺-ane 烷anhydride 酐[ænˈhaidraid]anilino- 苯胺基[ˈænili:n]aquo- 含水aqueous水的,水成的[ˈeikwiəs] -ase 酶-ate 含氧酸的盐、酯-atriyne 三炔azo- 偶氮[ˈæzəu]azoxy-氧化偶氮-N=N(O)-hydrazo-氢化偶氮-NH-NH-5benzene 苯[ˈbenˌzi:n, benˈzi:n] bi- 在盐类前表示酸式盐bis- 双-borane 硼烷[ˈbəurein]bromo- 溴butyl 丁基 .-carbinol 甲醇carbonyl 羰基-caboxylic acid 羧酸centi- 10-2chloro- 氯代cis- 顺式condensed 缩合的、冷凝的cyclo- 环deca- 十deci 10-1di二-dine 啶dodeca- 十二-ene 烯epi- 表epoxy- 环氧 []-ester 酯-ether 醚ethoxy- 乙氧基[] ethyl 乙基fluoro-或fluor- 氟代-form 仿-glycol 二醇hemi- 半hendeca- 十一hepta- 七heptadeca- 十七hexa- 六hexadeca- 十六-hydrin 醇hydro- 氢或水hydroxyl 羟基hypo- 低级的,次-ic 酸的,高价金属-ide 无氧酸的盐,酰替胺,酐-il 偶酰-imine 亚胺 /iodine 碘[]iodo- 碘代iso- 异,等,同-ite 亚酸盐keto- 酮ketone 酮-lactone 内酯mega- 106meta- 间,偏methoxy- 甲氧基methyl 甲基micro- 10-6milli- 10-3mono- ( mon-) 一,单nano- 10-9nitro- 硝基nitroso- 亚硝基nona- 九nonadeca- 十九octa- 八octadeca- 十八6-oic 酸的-ol 醇9 a$ f! Q, H: [5 n& G-one 酮ortho- 邻,正,原-ous 亚酸的,低价金属oxa- 氧杂-oxide 氧化合物-oxime 肟 []oxo- 酮 []oxy- 氧化 []-oyl 酰para- 对位,仲penta- 五pentadeca- 十五per- 高,过petro- 石油phenol 苯酚[ˈfi:nəl]phenyl 苯基 []pico- 10-12poly- 聚,多quadri- 四quinque- 五semi- 半septi- 七sesqui 一个半sulfa- 磺胺 []sym- 对称syn- 顺式,同,共ter- 三 -tetra- 四tetradeca- 十四tetrakis- 四个thio- 硫代[]trans- 反式,超,跨tri- 三trans- 反式,超,跨tri- 三trideca- 十三tris- 三个undeca- 十一 .Alkylation 烷基化C- alkylation C-烷基化O- alkylation O-烷基化N-alkylation N-烷基化Silylation 硅烷[基]化Exhaustive methylation 彻底甲基化Seco alkylation 断裂烷基化Demethylation 脱甲基化Ethylation 乙基化Arylation 芳基化Acylation 酰化Formylation 甲酰化Carbalkoxylation 烷氧羰基化Carboamidation 氨羰基化Carboxylation 羧基化Amination 氨基化Bisamination 双氨基化Cine substitution 移位取代Transamination 氨基交换Hydroxylation 羟基化acyloxyation 酰氧基化7Decarboxylative nitration 脱羧卤化Allylic halogenation 烯丙型卤化Dehalogenation 脱卤Nitration 硝化Decarboxylative nitration 脱羧硝化Nitrosation 亚硝化Sulfonation 磺化Chlorosulfonation 氯磺酰化Desulfonation 脱磺酸基Sulfenylation 亚磺酰化Sulfonylation 磺酰化Chlorosulfenation 氯亚磺酰化Chlorocarbonylation 氯羰基化Diazotization 重氮化[daiˌæzətaiˈzeiʃən] Diazo transfer 重氮基转移Coupling reaction 偶联反应uni- 单,一unsym- 不对称的,偏位-yl 基-ylene 撑(二价基,价在不同原子上)-yne 炔Diazonium coupling 重氮偶联[ˌdaiəˈzəuniəm]Cross-coupling reaction 交叉偶联反应1,4-addition 1,4-加成C-C Pi-bond C-C π键Conjugate addition 共轭加成[ˈkɔndʒəˌgeɪt] Dimerization 二聚Trimefization 三聚Additive dimerization 加成二聚Sulfurize 使硫化[]sulfurization 硫化Selenylation 硒化Hydroboration 硼氢化[ˈhaidrəuˌbɔ:ˈreiʃən] Oxyamination 羟氨基化Insertion 插入carbonylation 羧基化Hydroformylation 加氢甲酰基化Hydroacylation 加氢酰化Oxo process 羰基合成Decarbonylation 脱羰[di:ˌkɑ:bənəˈleiʃən] Hydrocarboxylation 氢羧基化Homologization 同系化Cyanoethylation 氰乙基化Decyanoethylation 脱氰乙基Ring closure 环合,闭环Diene synthesis 双烯合成Dienophile 亲双烯体Endo addition 内型加成Exo addition 外型加成Diels-Alder reaction 第尔斯-尔德反应Retro Diels-Alder reaction 逆第尔斯-阿尔德反应Ene synthesis 单烯合成Anionic cycloaddition 负离子环加成Dipolar addition 偶极加成Dehydrohalogenation 脱卤化氢Deamination 脱氨基Pyrolytic elimination 热解消除Elimination-addition 消除-加成Decarboxylation 脱羧Decarboxamidation 脱酰胺8Decyanation 脱氰基Alkylolysis,alkyl cleavage 烷基裂解Acylolysis,acyl cleavage 酰基裂解Flash pyrolysis 闪热裂Fragmentation 碎裂Chiletropic reaction 螯键反应Chelation 螯环化Esterification 酯化Transesterification 酯交换Saponification 皂化Alcoholysis 醇解Ethanolysis 乙醇解Cyanomethylation 氰甲基化Aminomethylation 氨甲基化Hydroxymethylation 羟甲基化Hydroxyalkylation 羟烷基化Cholromethylation 氯甲基化Haloalkylation 卤烷基化Transacetalation 缩醛交换Enolization 烯醇化Haloform reaction 卤仿反应Condensation 缩合Aldol condensation 羟醛缩合Cross aldol condensation 交叉羟醛缩合Retrograde aldol condensation 逆羟醛缩合Acyloin condensation 偶姻缩合Cyclization 环化Annulation,annelation 增环反应Spiroannulation 螺增环Autoxidation 自氧化Allylic hydroperoxylation 烯丙型氢过氧化Epoxidation 环氧化Oxonolysis 臭氧解Electrochemical oxidation 电化学氧化Oxidative decarboxylation 氧化脱羧Aromatization 芳构化Catalytic hydrogenation 催化氢化Heterogeneous hydrogenation 多相氢化Homogeneous hydrogenation 均相氢化Catalytic dehydrogenation 催化脱氢Transfer hydrogenation 转移氢化Hydrogenolysis 氢解Dissolving metal reduction 溶解金属还原Single electron transfer 单电子转移Bimolecular reduction 双分子还原Electrochemical reduction 电化学还原Reductive alkylation 还原烷基化Reductive acylation 还原酰化Reductive dimerization 还原二聚Deoxygenation 脱氧Desulfurization 脱硫Deselenization 脱硒Mitallation 金属化Lithiation 锂化Hydrometallation 氢金属化Mercuration 汞化Oxymercuration 羟汞化Aminomercuration 氨汞化Abstraction 夺取[反应]Internal abstraction 内夺取[反应] Rearrangement 重排Prototropic rearrangement 质了转移重排Double bond migration 双键移位Allylic migration 烯丙型重排Allylic migration 烯丙型迁移Ring contraction 环缩小[反应]Ring expansion,ring enlargement 扩环[反应] -ketol rearrangement -酮醇重排Pinacol rearrangement 频哪醇重排Retropinacol rearrangement 逆频哪醇重排Semipinacol rearrangement 半频哪醇重排Benzilic rearrangement 二苯乙醇酸重排Acyl rearrangement 酰基重排Migratory aptitude 迁移倾向Transannular insertion 跨环插入Transannular rearrangement 跨环重排Migration 迁移Prototropy 质子转移Cationotropic rearrangement 正离子转移重排Anionotropy 负离子转移Anionotropic rearrangement 负离子转移重Sigmatropic rearrangement -迁移重排Homosigmatropic rearrangement 同迁移重排Electrophilic rearrangement 亲电重排Photosensitization 光敏化Forbidden transition 禁阻跃迁photooxidation 光氧化Photoisomerization 光异构化Photochemical rearrangement 光化学重排Angular methyl group 角甲基Alkylidene group 亚烷基[ælˈkiləˌdi:n]Methylene 亚甲基[ˈmeθili:n]Allyl group 烯丙基Allylic 烯丙型[的] [ˈæləˌlik]Phenyl group 苯基[ˈfenəl,ˈfi:nəl,ˈfi:nil]Aryl group 芳基Benzyl group 苄基Benzylic 苄型[的]Activating group 活化基团Chromophore 生色团[ˈkrəuməfɔ:] Auxochrome 助色团[ˈɔ:ksəkrəum] Magnetically anisotropic group 磁各向异性基团[əˌnaisəuˈtrɔpik]Smally ring 小环Common ring 普通环Medium ring 中环[ˈmi:djəm]Large ring 大环Bridged-ring system 桥环体系Spiro compound 螺环化合物Helical molecule 螺旋型分子Octahedral compound 八面体化合物Conjugation 共轭[]Conjugated-system 共轭体系Acyl cation 酰[基]正离子Benzylic cation 苄[基]正离子[ˈkætaiən] Arenium ion 芳[基]正离子或aryl cation Ketyl radical 羰自由基Radical ion 自由基离子[ˈaiən]Radical cation 自由基正离子Radical anion 自由基负离子[ˈænaiən] Isomerism 异构[现象]Acid form 酸式Fluxional structure 循变结构Stereochemistry 立体化学Optical activity 光学活性,旋光性Dextro isomer 右旋异构体[]Laevo isomer 左旋异构体[] Tetrahedral configuration 四面体构型Stereoisomerism 立体异构[现象] Asymmetric atom 不对称原子Asymmetric carbon 不对称碳Pseudoasymmetric carbon 假不对称碳10Phantom atom 虚拟原子[]Homotopic 等位[的]Heterotopic 异位[的]Enantiotopic 对映[异构体]的Diastereotopic 非对映异构体[的] [ˌdaiəstiəri əˈtɔpik]Configuration 构型[kənˌfiɡjuˈreiʃən] Absolute configuration 绝对构型Chirality 手性Chiral 手性[的] [英] [ˈtʃirəl][美] [ˈkaɪrəl] Chiral center 手性中心Chiral molecule 手性分子Achiral 非手性[的] [ei'kairəl]Fischer projection 费歇尔投影式Neoman projection 纽曼投影式D-L system of nomenclature D-L命名体系R-S syytem of nomenclature R-S命名体系Cahn-Ingold-Prelon sequence 顺序规则Symmetry factor 对称因素Plane of symmetry 对称面Mirror symmetry 镜面对称Enantiomer 对映[异构]体[]Diastereomer 非对映[异构]体[]Epimer 差向异构体[] Anomer 端基[差向]异构体Erythro configuration 赤型构型Erythro isomer 赤型异构体Threo configuration 苏型构型Threo isomer 苏型异构体Trigonal carbon 三角型碳Cis-trans isomerism 顺反异构E isomer E异构体Z isomer Z异构体Endo isomer 内型异构体Exo isomer 外型异构体Prochirality 前手性Pro-R group 前R基团Pro-S proup 前S基团Re face Re 面Si face Si 面Racemic mixture 外消旋混合物[]Racemic compound 外消旋化合物Racemic solid solution 外消旋固体溶液Meso compound 内消旋化合物Quasi recemate 准外消旋体[ˈkwɑ:zi(:),ˈkweis ai]Conformation 构象Conformational 构象的Torsion angle 扭转角Rotamer 旋转异构体Anti conformation 反式构象Bisecting conformation 等分构象Anti periplanar conformation 反叠构象Synperiplanar conformation 顺叠构象Synclinal conformation 反错构象Synclinal conformation 顺错构象Eclipsed conformation 重叠构象Gauche conformation, skew con-formation 邻位交叉构象Staggered conformation 对位交叉构象11Steric effect 空间效应[ˈstiərik,ˈsterik] Steric hindrance 位阻Atropismer 阻转异构体Puckered ring 折叠环Conformational inversion 构象反转Chair conformation 椅型构象Boat conformation 船型构象Twist conformation 扭型构象Skew boat conformation 扭船型构象Half-chair conformation 半椅型构象Pseudorotation 假旋转Envelope conformation 信封[型]构象Axial bond 直[立]键[ˈæksi:əl] Equatorial bond 平[伏]键[ˌi:kwəˈtɔ:ri:əl, -ˈtəʊr-, ˌekwə-] Cisoid conformation 顺向构象Transoid conformation 反向构象Retention of configuration 构型保持Regioselectivity 区域选择性Regiospecificity 区域专一性Stereocelectivity 立体选择性Stereospecificty 立体专一性Conformer 构象异构体Conformational effect 构象效应Cram’s rube 克拉姆规则Prelog’rule普雷洛格规则Stereochemical orientation 立体[化学]取向Conformational transmission 构象传递Homolog 同系物Ipso position 本位Ortho position 邻位Meta position 间位Para position 对位Amphi position 远位Peri position 近位Trigonal hybridization 三角杂化Molecular orbiral method 分子轨道法Valence bond method 价键法Delocalezed bond 离域键Cross conjugation 交叉共轭Vinylog 插烯物Mesomeric effect 中介效应Resonance 共振[ˈrezənəns]Resonance effect 共振效应Hyperconjugation 超共轭Isovalent hyperconjugation 等价超共轭No-bond resonance 无键共振Aromaticity 芳香性Aromatic sexter 芳香六隅Huckel’rule休克尔规则Paramagnetic ring current 顺磁环电流Diamagnetic ring cruuent 抗磁环电流Homoaromaticity 同芳香性Antiaromaticity 反芳香性Alternant hydrocarbon 交替烃Non-alternant hydrocarbon 非交替烷Pericyclic reaction 周环反应Electrocyclic rearrangement 电环[化]重排Conrotatory 顺旋Disroatatory 对旋Cycloaddition 环加成Symmetry forbidden-reaction 对称禁阻反应Synfacial reaction 同面反应Antarafacial reaction 异面反应Mobius system 默比乌斯体系Leois structure 路易斯结构Coordinate-covalent bond 配位共价键Banana bond 香蕉键Pauling electronegativity scale 鲍林电负性标度Polarizability 可极化性Inductive effect 诱导效应Field effect 场效应Electrical effect 电场效应tautomerism 互变异构Tautomerization 互变异构化Keto-enol tautomerism 酮-烯醇互变异构Phenol-keto tautomerism 酚-酮互变异构Imine-enamine atutomerism 亚胺-烯胺互变异构Ring-chain tautomerism 环-链互变异构Valence tautomerism 价互变异构Ambident 两可[的]Solvent effect 溶剂效应Acid-base catalyxed reaction 酸性溶剂Basic solvent 碱性溶剂Dielectric constant 介电常数Solvated electron 溶剂化电子Acid-base catalyzed reaction 酸碱催化反应Conjugate base 共轭酸Conjugate base 共轭碱Therm odynamic acidity 热力学酸度Kinetic acidity 动力学酸度Electron donof-acceptor complex,EDAcomplex 电子给[体]受体络合物Host 主体Guest 客体Primary isotope effect 一级同位素效应Secondary isotope effect 二级同位数效应Inverse isotope effect 逆同位素效应Kinetic control 动力学控制Thermodynamic control 热力学控制Substrate 底物Intermediate 中间体Reactive intermediate 活泼中间体Microscopic reversibility 微观可逆性Hammond postulate 哈蒙德假说Linear free energy 线性自由能Non-bonded interaction 非键相互作用Torsional effect 扭转效应Pitzer strain 皮策张力Restricted rotation 阻碍旋转Eclipsing effect 重叠效应Eclipsing strain 重叠张力Small-angle strain 小角张力Large angle strain 大角张力Transannular interaction 跨环相互作用Transannular strain 跨环张力I strain 内张力F strain 前张力B strain 后张力Anomeric effect 端基异构效应Walden inversion 瓦尔登反转Racemization 外消旋化Isoinversion 等反转Isoracemization 等消旋Homochiral 纯手性[的]Mechanism 机理Unimolecular nucleophilic 单分子亲核取代Bimolecular nucleophilic sub-stitution 双分子亲核取代Bimolecular nucleophilic substi-tution(with allyl ic rearrange-ment) 双分子亲核取代(含烯丙型重排)Internal nucleophilic substiru-tion 分子内亲核取代Aromatic nucleophilic substitu-tion 芳香亲核取代Unimolecular electrophilic sub-stitution 单分子亲电取代Bimolecular electrophilic substi-tution 双分子亲电取代Nucleophile-assisted unimolecu-lar electrophilic substitution 亲核体协助单分子亲电取代Unimolecular elimination 单分子消除Bimolecular elimination 双分子消除Unimolecular elimination through the conjugate base 单分子共轭碱消除Bimolecular elimination through the conjugate b ase 双分子共轭碱消除Bimolecular elimination with for-mation of a car bonyl group 双分子羰基形成消除Unimolecular acid-catalyzed acyl-oxygen cleava ge 单分子酸催化酰氧断裂Bimolecular base-catalyzed acyl-oxygen cleavag e 双分子碱催化酰氧断裂Unimolecular acid-catalyzed alkyl-oxygen cleav age 单分子酸催化烷氧断裂Bimllecular base-catalyzed alkyl-oxygen cleavag e 双分子碱催化烷氧断裂π-allyl complex mechanism π烯丙型络合机理Borderline mechanism 边理机理Homolysis 均裂Heterolysis 异裂Heterolytic michanism 异裂机理Counrer[gegen]ion 反荷离子Ion pair 离子对Carbocation 碳正离子[ˌkɑ:bəˈkeiʃən] Nonclassical carbocation 非经典碳正离子Carbanion 碳负离子[ˈkɑ:bənaiən]Masked carbanion 掩蔽碳负离子Carbenoid 卡宾体Carbene 卡宾[]Nitrene 氮宾[a] Carbine 碳炔[] Electrophilic addition 亲电加成Electrophile 亲电体Diaxial addition 双直键加成Markovnikov’s rube 马尔科夫尼科规则Anti-Markovnikov addition 反马氏加成Michael addition 迈克尔加成Substitution 取代Electrophilic substitution 亲电取代Addition-elimination mechanism 加成消除机理[ˈmekənizəm]Electrophilic aromatic substitu-tion 亲电芳香取代Electron transfer 电子转移Electron-donating group 给电子基团Electron-Withdrawing group 吸电子基团Deactivating group 钝化基团Orinentation 取向Ortho-para directing group 邻对位定位基Meta directing group 间位定位基Ortho effect 邻位效应Partial rate factor 分速度系数Nucleophilic reaction 亲核反应Internal return 内返Nucleophilicity 亲核体Nucleophilicity 亲核性α-effect α-效应Backside attack 背面进攻Inversion 反转Umbrella effect 伞效应Push-pull effect 推拉效应Leaving group 离去基团Electrofuge 离电体Nucleofuge 离核体14Phase-transfer catalysis 相转移催化Neighboring group participation 邻基基参与Neighboring proup assistance,anchimeric assista nce 邻助作用Neighboring group effect 邻基效应Apofacial reaction 反面反应Briddgehead displacement 桥头取代Aryl action 芳正离子Benzyne 苯炔Zaitsev rule 札依采夫规则Anti-Zaitsev orientation 反札依采夫定向Hofmann’s rule 霍夫曼规则Bredt rule 布雷特规则Initiation 引发Anionic cleavage 负离子裂解Partial bond fixation 键[的]部分固定化exothermic发热的,放出热量的[ˌeksəuˈθə:mik]各种反应类型Halogenations reaction卤化反应Hydrogenation reaction氢化反应Alkylation reaction烷基化(烃化)反应Hydrocarbylation 烃基化反应Oxidation and reduction reaction氧化还原反Reductive amination还原胺化反应Cross-coupling reaction交叉耦合反应Cycloaddition reaction环加成反应Rearrangement reaction重排反应Acylation reaction酰化反应Acetylization reaction乙酰化反应Amide reaction酰胺反应Sulfonylation磺酰化反应Nitration reaction硝化反应Esterification酯化反应Anhydride reaction酸酐反应Oximation reaction肟化反应Coupled(或coupling)reaction偶联反应1,3-dipolar cycloaddition 1,3-偶极环加成Pericyclic reaction周环反应Hydrolysis reaction 水解反应Ester hydrolysis 酯水解反应Hydrolytic-polymeric reaction水解聚合反应Dehydrogenation reaction脱氢反应Dehydrohalogenation reaction 脱卤化氢反应Dehydration reaction脱水反应Decarboxylation reaction脱羧反应Addition reaction加成反应Substitution reaction取代反应Cracking reaction 裂化反应Elimination reaction消除反应And metal response reaction与活泼金属反应Phase transfer catalytic reaction相转移催化反应Acid-base catalyzed reaction酸碱催化反应Polymerization reaction聚合反应Polycondensation reaction缩聚反应Condensation reaction 缩合反应Silver mirror reaction银镜反应Nucleophilic reaction亲核反应Electrophilic reaction亲电反应Nucleophilic cycloaddition reaction亲核环加成反应Nucleophilic substitution亲核取代反应Electrophilic substitution亲电取代反应Unimolecular electrophilic substitution单分子亲电取代反应Bimolecular electrophilic substitution双分子亲电取代反应Unimolecular elimination reaction单分子消除反应Bimolecular elimination reaction双分子消除反应Unimolecular nucleophilic substitution单分子亲核取代Bimolecular nucleophilic substitution双分子亲核取代反应Internal nucleophilic substitution分子内亲核取代Aromatic nucleophilic substitution reaction芳香亲核取代反应活化剂的中英文名称Bis(2-ethylhexyl) sebacate (癸二酸二仲辛酯;癸二酸二2-乙基己酯)Zinc stearate (硬脂酸锌)Suberic acid (辛二酸)Adipic acid, (己二酸)Hexanedioic acid, (己二酸)Sebacic acid, dibutyl ester (癸二酸二丁酯)Abietic acid (松香酸)Lactic acid (乳酸)Poly(ethylene glycol) (聚乙二醇)Glycerol stearate (硬脂酸甘油酯)Imidazoline (咪唑啉,间二氮杂环戊烯)β-Pinene (β-蒎烯,β-松油二环烯)Adipic acid (脂肪酸)Butyl acetate (乙酸丁酯)Ethylene glycol butyl ether (乙二醇丁醚)Sebacic acid, (癸二酸)Decanedioic acid, (癸二酸)Ethylene glycol ethyl ether (乙二醇乙醚)2-Butenedioic acid (E)-, (2-丁烯二酸)Succinic acid, (琥珀酸,丁二酸)Ethylene glycol methyl ether (乙二醇甲醚)Acetyl acetate (乙酸乙酰脂)1H-Benzotriazole (1-H-笨并三唑)α-Pinene (α-蒎烯,α-松香二环烯)Salicylic acid (水杨酸)Iso-Propanol (异丙醇) Ethanol (乙醇)Lysine (赖氨酸)Glutamic acid (谷氨酸,2-氨基戊二酸) Glyceroyl, (甘油酰)N,N,N',N'-Tetrakis-(2-hydroxypropyl)-ethylene-diamine(N,N,N,N-四(2-羟基丙基)乙烯二氨)Isoleucine, (异亮氨酸)Decamethylenedicarboxylic acid, disalicyloylhy drazide (Tris(2,3-dibromopropyl)isocyanurate(3(2,3-2溴丙基)异氰尿酸盐)3-(N-Salicyloyl)amino-1,2,4-triazole (3-(N-水杨酰)氨-1,2,4-三唑)Isocyanuric acid (异氰尿酸)Salicylamide (水杨酰胺)Polyethylene glycol (聚乙二醇)Diethylene glycol diethyl ether (二甘醇二乙醚,(一缩)二乙二醇二乙醚)Butyl carbitol (丁基卡必醇)Ethyl carbitol (乙基卡必醇)Methyl carbitol (甲基卡必醇)Ethylene glycol monobutyl ether (乙二醇单丁醚)Glutaric acid (戊二酸,谷酸)Succinic acid (琥珀酸,丁二酸)Citric acid (柠檬酸)Salicylic acid (水杨酸)Lactic acid (2-羟基丙酸,乳酸)Glycerin monostearate (甘油一硬脂酸)Pentaerythritol (季戊四醇)tetrakis[β-(3,5-di-tert-butyl-4-hydroxy-phenyl)pr opionate] 四[β-(3,5-二叔丁基-4-羟基-苯基)丙酸酯Dioctyl sebacate (癸二酸二辛酯)N-Methyl pyrrolidone, (N-甲基吡咯烷酮)Diethylene glycol ethyl ether (二甘醇乙醚)Propylene glycol (丙二醇)Octanedioic acid (辛二酸)Oleamide (油酸酰胺)[olamine 乙醇胺]2-Mercapto benzothiazole (2-巯基-苯并噻唑)Nonanedioic acid, (壬二酸)cis-9-Octadecenoic acid, (顺式-9-十八炭烯酸,油酸)Sebacic acid, uses (癸二酸)12-Hydroxy stearic acid (十二羟基硬脂酸)Phthalic acid (苯二甲酸)1,1,3-Tris(2-methyl-4-hydroxy-5-tert-butylpheny l)butane(1,1,3-三(2-甲基-4-羟基-5-叔丁基苯基)丁烷)1,3,5-Trimethyl-2,4,6-tris(3,5-di-tert-butyl-4-hyd roxybenzyl)benzene(1,3,5-三甲基-2,4,6-三(3,5-二叔丁基-4-羟基苯基)苯)1-Methyl-2-pyrrolidone, (1-甲基-2-吡咯烷酮)Carbonic acid, (碳酸)Phthalic acid, (苯二甲酸)Malic acid (苹果酸,羟基丁二酸)2,3-Dibromo-2-butene-1,4-diol (2,3-二溴-2-丙烯-1,4-二醇)Cetylpyridinium bromide (溴代十六烷基吡啶)Pentanedioic acid (戊二酸) pentanediol (戊二醇)pentanoic acid ( 戊酸) pentanol (戊醇) Butanedioic acid, (丁二酸)1,2-Dibromoethylbenzene (1,2-二溴乙基苯)Salicylic acid, (水杨酸)Stearic acid (硬脂酸)Pentanedioic acid (戊二酸)Maleic acid, (马来酸,失水苹果酸)Phthalic acid, (苯二甲酸)Tartaric acid, (酒石酸)Acetic acid, (乙酸)Polyoxyethylene octylphenol ether (聚氧乙烯辛基酚醚,聚氧化亚乙基辛基分醚)Ethanedioic acid, (乙二酸)Polyethylene glycol (聚乙二醇)Diethylene glycol butyl ether (二甘醇丁醚)Diethylene glycol monoethyl ether (二甘醇单乙醚)Ethylene glycol monobutyl ether (乙二醇单丁醚)Pentaerythritol, (季戊四醇)Diglycol, (二甘醇,一缩二乙二醇)Hexylene glycol (己二醇)Ethylene glycol, (乙二醇)Glycerol, (甘油,丙三醇)Cyclobutanediamine (环丁烷二胺)Dibromobutenediol (二溴丁二醇)Cyclohexanediamine (环己烷二胺)Succinamide (琥珀酰胺,丁二酸胺)Ethylenediamine, (乙二胺)Triethanolamine, (三乙醇胺)5-Aminoisophthalic acid (5-氨基间苯二甲酸)p-tert-Butylbenzoic acid (对叔丁基苯甲酸)Propionic acid, (丙酸)Benzoic acid, (安息香酸, 苯(甲)酸)Salicylamide (水杨酰胺)Aniline, (苯胺)Palmitic acid, (棕榈酸, 十六酸, 软脂酸)Glutamic acid, (谷氨酸) Glutaric acid (谷酸,戊二酸)Glycine, (甘氨酸,氨基乙酸)Malic acid (苹果酸,羟基丁二酸)Adipic acid, (己二酸)Diethanolamine (二乙醇胺)Triethylamine, (三乙胺)Malic acid (苹果酸)Oxalic acid, (草酸)Oleic acid, (油酸)Glutaric acid (谷氨酸)Sorbic acid (山梨酸) sorbic alcohol (山梨醇)=sorbit Reactant 反应物nProduct 产物nCatalyst 催化剂catalytic agent,catalyzer Degree Celsius摄氏度Sodium Borohydride硼氢化钠Lithium Aluminum Hydride (LAH)氢化铝锂Lithiumtrit-ButoxyaluminohydrideLiAlH(O t-C4H9)3叔丁氧基氢化铝锂Diisobutylaluminum Hydride AlH[CH2CH(CH3)2]2二异丙基氢化铝Diborane B2H6硼烷Reactive Metals 活泼金属如 Na, or Li, or K ,Mg or Al or Zn or FePotassium carbonate碳酸钾Sodium carbonate 碳酸钠Sodium bicarbonate 碳酸氢钠[]Sodium chloride氯化钠Sodium acetate 乙酸钠Sodium cyanide[ˈsaɪəˌnaɪd]氰化钠Sodium methoxide或Sodium methylate甲醇钠,甲氧基钠Sodium ethoxide[ˈsəʊdi:əm i:ˈθɔksaid]或Sodium ethylate[ˈsəʊdi:əm ˈeθileit]乙醇钠Sodium sulfate 硫酸钠Magnesium sulfate [mægˈni:zi:əm ]硫酸镁Acetic acid乙酸Formic acid甲酸Ammonium format甲酸铵Formamide[fɔ:ˈmæmid]或formyl amide 甲酰胺formaldehyde 甲醛[f ɔ:ˈmældəˌha ɪd]Methyl iodide碘甲烷Potassium iodide碘化钾[]Potassium chloride氯化钾Potassium cyanide[ˈsaɪəˌnaɪd]氰化钾Dimethyl sulfate硫酸二甲酯Palladium –carbon 钯碳Pd/C [pəˈleidiəm] Palladium chloride氯化钯Palladium diacetate醋酸钯Chloroacetone 氯丙酮Tetrabutyl ammonium bromide四丁基溴化铵Tetrabutyl ammonium fluoride四丁基氟化铵容器类:量杯measuring cup烧杯beaker 不锈钢杯stainless-steel beaker量筒measuring flask/measuring cylinder 量筒graduated flask/measuring cylinder坩埚crucible 坩埚钳crucible clamp 坩埚crucible pot, melting pot试管test tube 试管架test tube holder漏斗funnel 分液漏斗separatory funnel烧瓶flask 锥形瓶conical flask塞子stopper洗瓶plastic wash bottle滴定管burette玻璃活塞stopcock冷凝器condenser试剂瓶reagent bottles玻棒glass rod 搅拌棒stirring rod蒸馏烧瓶distilling flask碘量瓶iodine flask表面皿watch glass蒸发皿evaporating dish容量瓶volumetric flask/measuring flask移液管(one-mark) pipette刻度移液管graduated pipettes20称量瓶weighing bottle吸液管pipette滤管filter天平balance/scale分析天平analytical balance台秤platform balance游码crossbeams and sliding weights酒精灯alcohol burner酒精喷灯blast alcohol burner搅拌装置stirring device洗耳球rubber suction bulb研磨钵mortar 研磨棒pestle 玛瑙研钵agate mortar瓷器porcelain白细口瓶flint glass solution bottle with stopper 滴瓶dropping bottle 小滴管dropper蒸馏装置distilling apparatus蒸发器evaporator试验用器材:升降台lab jack铁架台iron support万能夹extension clamp蝴蝶夹double-buret clamp双顶丝clamp regular holder止水夹flatjaw pinchcock圆形漏斗架cast-iron ring移液管架pipet rack试管架tube rack沸石boiling stone橡胶管rubber tubing药匙lab spoon镊子forceps坩埚钳crucible tong剪刀scissor 打孔器stopper borer石棉网asbestos-free wire gauze电炉丝wire coil for heater脱脂棉absorbent cottonphph试纸universal ph indicator paper滤纸filter paper称量纸weighing paper擦镜纸wiper for lens秒表stopwatch量杯glass graduates with scale白滴定管(酸)flint glass burette with glass stopcock棕色滴定管(酸)brown glass burette with glass stopcock白滴定管(碱)flint glass burette for alkali棕色滴定管(碱)brown glass burette for alkali 比重瓶specific gravity bottle水银温度计mercury-filled thermometerph计ph meter折光仪refractometer真空泵vacuum pump冷、热浴bath离心机centrifuge口罩respirator防毒面具respirator、gasmask磁力搅拌器magnetic stirrer电动搅拌器power basic stirrer烘箱oven闪点仪flash point tester马弗炉furnace电炉heater微波炉电热套heating mantleBunsen burner 本生灯product 化学反应产物apparatus 设备。
【有机化学专业英语词汇】

有机化学专业英语词汇(精)acetal 醛缩醇-ene 烯acetal- 乙酰epi- 表acid 酸epoxy- 环氧-al 醛-ester 酯alcohol 醇-ether 醚-aldehyde 醛ethoxy- 乙氧基alkali- 碱ethyl 乙基allyl 烯丙基[propenyl(丙烯基)]alkoxy- 烷氧基fluoro- 氟代-amide 酰胺form 仿amino- 氨基的-amidine 脒-glycol 二醇-amine 胺-ane 烷hemi- 半anhydride 酐hendeca- 十一anilino- 苯胺基hepta- 七aquo- 含水的heptadeca-十七-ase 酶hexa- 六-ate 含氧酸的盐、酯hexadeca- 十六-atriyne 三炔-hydrin 醇azo- 偶氮hydro- 氢或水hydroxyl 羟基benzene苯hypo- 低级的,次bi- 在盐类前表示酸式盐hyper- 高级的,高bis- 双-borane 硼烷-ic 酸的,高价金属bromo- 溴-ide 无氧酸的盐,酰替胺,酐butyl 丁基-il 偶酰-imine 亚胺-carbinol 甲醇iodine 碘carbonyl 羰基iodo- 碘代-carboxylic acid 羧酸-2centi- 10chloro- 氯代i so- 异,等,同-ite 亚酸盐cis- 顺式keto- 酮condensed缩合的、冷凝的ketone 酮cyclo- 环-lactone 内酯deca- 十-1 deci 106 mega- 10-dine 啶meta- 间,偏dodeca- 十二methoxy- 甲氧基methyl 甲基1-6micro- 10-3milli- 10ter- 三mono- ( mon-) 一,单tetra- 四-9 nano- 10 tetradeca- 十四tetrakis- 四个nitro- 硝基thio- 硫代nitroso- 亚硝基trans- 反式,超,跨nona- 九thio- 硫代nonadeca-十九tri- 三trideca- 十三octa- 八tris- 三个octadeca- 十八-oic 酸的undeca- 十一-ol 醇uni- 单,一-one 酮unsym- 不对称的,偏位ortho- 邻,正,原-ous 亚酸的,低价金属-yl 基oxa- 氧杂-ylene 撑(二价基,价在不同原子上) -oxide 氧化合物-yne 炔-oxime 肟oxo- 酮organic compounds 有机化合物oxy- 氧化compounds of carbon 碳化合物-oyl 酰hydrocarbons and their derivatives碳氢化合物及其衍生物para- 对位,仲organic chemistry 有机化学penta- 五structure of molecule 分子结构pentadeca-十五chemical bond 化学键per- 高,过covalent bond 共价键petro- 石油hybrid orbital 杂化轨道phenol 苯酚bond length 键长phenyl 苯基-12 pico- 10 bond angle 键角bond energy键能poly- 聚,多polarity 极性dissociation energy 离解能quadri- 四constitution 构造quinque- 五contiguration 构型conformation 构象semi- 半stereochemistry立体化学septi- 七tetrahedral正四面体sesqui 一个半cis-顺sulfa- 磺胺trans-反sym- 对称isomerism 同分异构现象syn- 顺式,同,共isomer异构体stereoisomer立体异构2constitutional isomer 构造异构Common ring 普通环structural formula 结构式Medium rimg 中环octet八隅体Large ring 大环perspective 透视式Bridged-ring system 桥环体系eclipsed conformation重叠式构象Spiro compound 螺环化合物staggered conformation交叉式构象Helical molecule 螺旋型分子newman projection纽曼投影式Octahedral compound 八面体化合物functional group 官能团Conjugation 共轭chain compoud 链状化合物Conjugated-system 共轭体系carbocyclic compound碳环化合物Acyl cation 酰[基]正离子heterocyclic compound杂环化合物Benzylic cation 苄[基]正离子dipole-dipole interactions 偶极-偶极相Arenirm ion 芳[基]正离子互作用Ketyl radical 羰自由基van derWaals forces范德华力Radical ion 自由基离子hydrogenbonding 氢键Radical cation 自由基正离子dipolemoment 偶极矩Radical anion 自由基负离子electronegativity 电负性Isomerism 异构[现象]physical property物理性质Aci form 酸式melting point 熔点Fluxional structure 循变结构boiling point 沸点Stereochemistry 立体化学reaction mechanism反应机理Optical activity 光学活性homolysis 均裂Dextro isomer 右旋异构体free redical自由基Laevo isomer 左旋异构体heterolysis异裂Tetrahedral configuration 四面体构型ionic type 离子型Stereoisomerism 立体异构[现象] electrophilic reagent亲电试剂Asymmetric atom 不对称原子electrophilic reaction 亲电反应Asymmetric carbon 不对称碳nucleophilic reagent亲核试剂Pseudoasymmetric carbon假不对称碳nucleophilic reaction 亲核反应Phantom atom 虚拟原子英文名汉文名Homotopic 等位[的] Angular methyl group 角甲基Heterotopic 异位[的] Alkylidene group 亚烷基Enantiotopic 对映异位[的] Allyl group 烯丙基Diastereotopic 非对映异位[的] Allylic 烯丙型[的]Configuration 构型Phenyl group 苯基Absolute configuration 绝对构型Aryl group 芳基Chirality 手性Benzyl group 苄基Chiral 手性[的] Benzylic 苄型[的] Chiral center 手性中心Activating group 活化基团Chiral molecule 手性分子Chromophore 生色团Achiral 非手性[的] Auxochrome 助色团Fischer projection 费歇尔投影式Magnetically anisotropic group 磁各向Neoman projection 纽曼投影式异性基团D-L system of nomenclature D-L 命名Smally ring 小环体系3R-S syytem of nomenclature R-S命名体Staggered conformation 对位交叉构象系Steric effect 空间效应Cahn-Ingold-Prelon sequence顺序规则Steric hindrance 位阻Symmetry factor 对称因素Atropismer 阻转异构体Plane of symmetry 对称面Puckered ring 折叠环Mirror symmetry 镜面对称Conformational inversion 构象反转Enantiomer 对映[异构]体Chair conformation 椅型构象Diastereomer 非对映[异构]体Boat conformation 船型构象Epimer 差向异构体Twist conformation 扭型构象Anomer 端基[差向]异构体Skew boat conformation 扭船型构象Erythro configuration 赤型构型Half-chair conformation 半椅型构象Erythro isomer 赤型异构体Pseudorotation 假旋转Threo configuration 苏型构型Envelope conformation 信封[型]构象Threo isomer 苏型异构体Axial bond 直[立]键Trigonal carbon 三角型碳Equatorial bond 平[伏]键Cis-trans isomerism 顺反异构Cisoid conformation 顺向构象E isomer E异构体Transoid conformation 反向构象Z isomer Z 异构体Retention of configuration 构型保持Endo isomer 内型异构体Regioselectivity 区域选择性Exo isomer 外型异构体Regiospecificity 区域专一性Prochirality 前手性Stereocelectivity 立体选择性Pro-R group 前R 基团Stereospecificty 立体专一性Pro-S proup 前S基团Conformer 构象异构体Re face Re面Conformational effect 构象效应Si face Si面Cram’s rube克拉姆规则Racemic mixture 外消旋混合物Prelog ’ru普l e 雷洛格规则Racemic compound 外消旋化合物Stereochemical orientation 立体[化学] Racemic solid solution 外消旋固体溶取向液Conformational transmission 构象传递Meso compound 内消旋化合物Homolog 同系物Quasi recemate准外消旋体Ipso position 本位Conformation 构象Ortho position 邻位Conformational 构象分析Meta position 间位Torsion angle 扭转角Para position 对位Rotamer 旋转异构体Amphi position 远位Anti conformation 反式构象Peri position 近位Bisecting conformation 等分构象Trigonal hybridization 三角杂化Anti periplanar conformation 反叠构象Molecular orbiral method 分子轨道法Synperiplanar conformation 顺叠构象Valence bond method 价键法Synclinal conformation 反错构象Delocalezed bond 离域键Synclinal conformation 顺错构象Cross conjugation 交叉共轭Eclipsed conformation 重叠构象Vinylog 插烯物Gauche conformation, skew Mesomeric effect 中介效应con-formation 邻位交叉构象Resonance共振4Resonance effect共振效应Ambident 两可[的] Hyperconjugation 超共轭Solvent effect 溶剂效应Isovalent hyperconjugation 等价超共轭Acid-base catalyxed reaction 酸性溶剂No-bond resonance无键共振Basic solvent 碱性溶剂Aromaticity 芳香性Dielectric constant 介电常数Aromatic sexter 芳香六隅Solvated electron 溶剂化电子Huckel ’rule休克尔规则Acid-base catalyzed reaction酸碱催化Paramagnetic ring current 顺磁环电流反应Diamagnetic ring cruuent 抗磁环电流Conjugate base共轭酸Homoaromaticity 同芳香性Conjugate base共轭碱Antiaromaticity 反芳香性Therm odynamic acidity 热力学酸度Alternant hydrocarbon 交替烃Kinetic acidity 动力学酸度Non-alternant hydrocarbon 非交替烷Electron donof-acceptorPericyclic reaction 周环反应complex,EDAcomplex 电子给[体]受体Electrocyclic rearrangement 电环[化]络合物重排Host 主体Conrotatory 顺旋Guest 客体Disroatatory 对旋Primary isotope effect 一级同位素效应Cycloaddition 环加成Secondary isotope effect二级同位数效Symmetry forbidden-reaction 对称禁阻应反应Inverse isotope effect 逆同位素效应Synfacial reaction 同面反应Kinetic control 动力学控制Antarafacial reaction 异面反应Thermodynamic control 热力学控制Mobius system 默比乌斯体系Substrate 底物Leois structure 路易斯结构Intermediate 中间体Coordinate-covalent bond 配位共价键Reactive intermediate 活泼中间体Banana bond 香蕉键Microscopic reversibility 微观可逆性Pauling electronegativity scale 鲍林电Hammond postulate 哈蒙德假说负性标度Linear free energy 线性自由能Polarizability 可极化性Non-bonded interaction 非键相互作用Inductive effect 诱导效应Torsional effect 扭转效应Field effect 场效应Pitzer strain 皮策张力Electrical effect 电场效应Restricted rotation 阻碍旋转tautomerism 互变异构Eclipsing effect 重叠效应Tautomerization 互变异构化Eclipsing strain 重叠张力Keto-enol tautomerism 酮-烯醇互变异Small-angle strain 小角张力构Large angle strain 大角张力Phenol-keto tautomerism 酚-酮互变异Transannular interaction 跨环相互作用构Transannular strain 跨环张力Imine-enamine atutomerism 亚胺-烯胺I strain 内张力互变异构 F strain 前张力Ring-chain tautomerism 环-链互变异 B strain 后张力构Anomeric effect 端基异构效应Valence tautomerism 价互变异构Walden inversion 瓦尔登反转5π-allyl complex mechanism 烯丙π型络Racemization 外消旋化Isoinversion 等反转合机理Isoracemization 等消旋Borderline mechanism 边理机理Homochiral 纯手性[的] Homolysis 均裂Mechanism 机理Heterolysis 异裂Unimolecular nucleophilic 单分子亲核Heterolytic michanism 异裂机理取代Counrer[gegen]ion 反荷离子Bimolecular nucleophilic sub-stitution Ion pair 离子对双分子亲核取代Carbocation 碳正离子Bimolecular nucleophilic Nonclassical carbocation 非经典碳正substi-tution(with allylic rearrange-ment)离子双分子亲核取代(含烯丙型重排)Carbanion 碳负离子Internal nucleophilic substiru-tion 分子Masked carbanion 掩蔽碳负离子内亲核取代Carbenoid 卡宾体Aromatic nucleophilic substitu-tion 芳Carbene 卡宾香亲核取代Nitrene 氮宾Unimolecular electrophilic sub-stitution Carbine 碳炔单分子亲电取代Electrophilic addition 亲电加成Bimolecular electrophilic substi-tution Electrophile 亲电体双分子亲电取代Diaxial addition 双直键加成Nucleophile-assisted unimolecu-lar Markovnikov ’s rub马e尔科夫尼科规electrophilic substitution 亲核体协助单则分子亲电取代Anti-Markovnikov addition 反马氏加Unimolecular elimination 单分子消除成Bimolecular elimination 双分子消除Michael addition 迈克尔加成Unimolecular elimination through the Substitution 取代conjugate base单分子共轭碱消除Electrophilic substitution 亲电取代Bimolecular elimination through the Addition-elimination mechanism 加成conjugate base双分子共轭碱消除消除机理Bimolecular elimination with for-mation Electrophilic aromatic substitu-tion 亲of a carbonyl group 双分子羰基形成消电芳香取代除Electron transfer 电子转移Unimolecular acid-catalyzed Electron-donating group 给电子基团acyl-oxygen cleavage 单分子酸催化酰Electron-Withdrawing group 吸电子基氧断裂团Bimolecular base-catalyzed acyl-oxygen Deactivating group 钝化基团cleavage 双分子碱催化酰氧断裂Orinentation 取向Unimolecular acid-catalyzed Ortho-para directing group 邻对位定位alkyl-oxygen cleavage 单分子酸催化基烷氧断裂Meta directing group 间位定位基Bimllecular base-catalyzed al- Ortho effect 邻位效应kyl-oxygen cleavage 双分子碱催化烷Partial rate factor 分速度系数氧断裂Nucleophilic reaction 亲核反应Internal return 内返6Nucleophilicity 亲核体Carboxylation 羧基化Nucleophilicity 亲核性Amination 氨基化α-effect -效α应Bisamination 双氨基化Backside attack 背面进攻Cine substitution 移位取代Inversion 反转Transamination 氨基交换Umbrella effect 伞效应Hydroxylation 羟基化Push-pull effect 推拉效应acyloxyation 酰氧基化Leaving group 离去基团Decarboxylative nitration 脱羧卤化Electrofuge 离电体Allylic halogenation 烯丙型卤化Nucleofuge 离核体Dehalogenation 脱卤Phase-transfer catalysis相转移催化Nitration 硝化Neighboring group participation 邻基Decarboxylative nitration 脱羧硝化基参与Nitrosation 亚硝化Neighboring proup Sulfonation 磺化assistance,anchimeric assistance邻助Chlorosulfonation 氯磺酰化作用Desulfonation 脱磺酸基Neighboring group effect 邻基效应Sulfenylation 亚磺酰化Apofacial reaction 反面反应Sulfonylation 磺酰化Briddgehead displacement桥头取代Chlorosulfenation 氯亚磺酰化Aryl action 芳正离子Chlorocarbonylation 氯羰基化Benzyne 苯炔Diazotization 重氮化Zaitsev rule 札依采夫规则Diazo transfer 重氮基转移Anti-Zaitsev orientation 反札依采夫定Coupling reaction 偶联反应向Diazonium coupling 重氮偶联Hofmann’s rule霍夫曼规则Cross-coupling reaction 交叉偶联反应Bredt rule 布雷特规则1,4-addition 1,4-加成Initiation 引发Conjugate addition 共轭加成Anionic cleavage 负离子裂解Dimerization 二聚Partial bond fixation 键[的]部分固定化Trimefization 三聚02.3 有机化学反应Additive dimerization 加成二聚Alkylation 烷基化sulfurization 硫化C- alkylation C-烷基化Selenylation 硒化O- alkylation O-烷基化Hydroboration 硼氢化N-alkylation N- 烷基化Oxyamination 羟氨基化Silylation 硅烷[基]化Insertion 插入Exhaustive methylation 彻底甲基化carbonylation 羧基化Seco alkylation 断裂烷基化Hydroformylation 加氢甲酰基化Demethylation 脱甲基化Hydroacylation 加氢酰化Ethylation 乙基化Oxo process 羰基合成Arylation芳基化Decarbonylation 脱羰Acylation 酰化Hydrocarboxylation 氢羧基化Formylation 甲酰化Homologization 同系化Carbalkoxylation 烷氧羰基化Cyanoethylation 氰乙基化Carboamidation 氨羰基化Decyanoethylation 脱氰乙基7Ring clsure 环合Cross aldol condensation交叉羟醛缩Diene synthesis 双烯合成合Dienophile 亲双烯体Retrograde aldol condensation逆羟醛Endo addition 内型加成缩合Exo addition 外型加成Acyloin condensation 偶姻缩合Diels-Alder reaction 第尔斯-尔德反应Cyclization 环化Retro Diels-Alder reaction 逆第尔斯- Annulation,annelation 增环反应阿尔德反应Spiroannulation 螺增环Ene synthesis单烯合成Autoxidation 自氧化Anionic cycloaddition 负离子环加成Allylic hydroperoxylation 烯丙型氢过Dipolar addition 偶极加成氧化- elimination -消除Epoxidation 环氧化- elimination -消除Oxonolysis 臭氧解- elimination -消除Electrochemical oxidation 电化学氧化-elimination -消除Oxidative decarboxylation 氧化脱羧Dehydrohalogenation 脱卤化氢Aromatization 芳构化Deamination 脱氨基Catalytic hydrogenation 催化氢化Pyrolytic elimination 热解消除Heterogeneous hydrogenation多相氢Elimination-addition 消除-加成化Decarboxylation 脱羧Homogeneous hydrogenation均相氢化Decarboxamidation 脱酰胺Catalytic dehydrogenation 催化脱氢Decyanation 脱氰基Transfer hydrogenation 转移氢化Alkylolysis,alkyl cleavage 烷基裂解Hydrogenolysis 氢解Acylolysis,acyl cleavage 酰基裂解Dissolving metal reduction 溶解金属还Flash pyrolysis 闪热裂原Fragmentation 碎裂Single electron transfer 单电子转移Chiletropic reaction 螯键反应Bimolecular reduction 双分子还原Chelation 螯环化Electrochemical reduction 电化学还原Esterification 酯化Reductive alkylation 还原烷基化Transesterification 酯交换Reductive acylation 还原酰化Saponification 皂化Reductive dimerization 还原二聚Alcoholysis 醇解Deoxygenation 脱氧Ethanolysis 乙醇解Desulfurization 脱硫Cyanomethylation 氰甲基化Deselenization 脱硒Aminomethylation 氨甲基化Mitallation 金属化Hydroxymethylation 羟甲基化Lithiation 锂化Hydroxyalkylation 羟烷基化Hydrometallation 氢金属化Cholromethylation 氯甲基化Mercuration 汞化Haloalkylation 卤烷基化Oxymercuration 羟汞化Transacetalation 缩醛交换Aminomercuration 氨汞化Enolization 烯醇化Abstraction 夺取[反应]Haloform reaction 卤仿反应Internal abstraction 内夺取[反应] Condensation 缩合Rearrangement重排Aldol condensation 羟醛缩合8Prototropic rearrangement质了转移重Alkyen 炔排Acetylide 炔化物Double bond migration 双键移位Active hydrogen compounds 活泼氢化Allylic migration 烯丙型重排合物Allylic migration 烯丙型迁移Carbon acid 碳氢酸Ring contraction 环缩小[反应] Super acid 超酸Ring expansion,ring enlargement扩环Diene 双烯[反应] Triene 三烯-ketol rearrangement 酮-醇重排Allene 丙二烯Pinacol rearrangement频哪醇重排Ccumulene 累积多烯Retropinacol rearrangement逆频哪醇Enyne 烯炔重排Diyne 二炔Semipinacol rearrangement半频哪醇Alkyl halide 卤代烷重排Alcohol 醇Benzilic rearrangement 二苯乙醇酸重Homoallylic alcohol 高烯丙醇排Ether 醚Acyl rearrangement 酰基重排Epoxide 环氧化物Migratory aptitude 迁移倾向Cellosolve 溶纤剂Transannular insertion 跨环插入Crown ether 冠醚Transannular rearrangement跨环重排Netro compound 硝基化合物Migration 迁移Amine 胺Prototropy 质子转移Quaternaryammonium com-pound 季铵Cationotropic rearrangement正离子转化合物移重排Amine oxide 氧化胺Anionotropy 负离子转移Diazoalkane 重氮烷Anionotropic rearrangement 负离子转Mercaptan 硫醇移重排Sulfonic acid 磺酸Sigmatropic rearrangement 迁-移重排Sulfoxide 亚砜Homosigmatropic rearrangement同迁Sulfone 砜移重排Aldehyde 醛Electrophilic rearrangement 亲电重排Detone 酮Photosensitization 光敏化Aldehyde hydrate 醛水合物Forbidden transition 禁阻跃迁Ketone hydrate 酮水合物photooxidation 光氧化Hemiacetal 半缩醛Photoisomerization 光异构化Acetal 缩醛Photochemical rearrangement光化学Ketal 缩酮重排Dithiane 二噻烷2.4 有机化合物类名Aminal 缩醛胺Aliphatic compound 脂肪族化合物imine 亚胺Hpdrocarbon 碳氢化合物Aldimine 醛亚胺Alkane 烷Oxime 肟Wax 蜡Aldimine 醛肟Paraffin wax 石蜡Oxime 亚硝基化合物Alkene 烯aldoxime 硝酮9Hydrazone 腙Thioester 硫代酸酯Azine 嗪Thiol acid 硫羰酸Semicarbazone缩氯基脲Lactone 内酯Cyanohydrin 羟腈Lactol 内半缩醛Pinacol 频哪醇Macrolide 大环内酯Enol 烯醇Amino acid 氨基酸Enol ether 烯醇醚Zwitterions 两性离子Enol ester 烯醇酯Inner salt 内盐Enamine 烯胺Betaine 甜菜碱Ynamine 炔胺Lactam 内酰胺Mannich base 曼尼希碱Hydantion 乙内酰脲Carboxylic acid 羧酸Peptide 肽Ester 酯Glycol 二醇orthoester 原酸酯Aldol羟醛Acyl halide 酰卤Acyloin 偶姻Acyl fluoride 酰氟Carbohydrate 碳水化合物Acyl chloride 酰氯Aldose 醛糖Acyl rtomide 酰溴Ketose 酮糖Acyl iodide 酰碘Furanose 呋喃糖Carbobenzoxy chloride 苄氧甲酰氯Pyranose 吡喃糖Acyl tosylate 酰基对甲苯磺酸酐Glycoside 糖苷Ketene 乙烯酮Glucoside 葡[萄]糖苷Peracid 过酸Aglycon 苷元Perester过酸酯Saccharide 糖类Acyl peroxide 酰基过氧化物Oligosaccharide 寡糖Nitrile 腈Polysaccharide 多糖Nitrile oxide 氧化腈Alditol 糖醇Isonitrile 异腈Osazone 脎Amide 酰胺Alicyclic compound 脂环化合物Imide 二酰亚胺Cycloalkene 环烷N-bromo compound N-溴化物Spirane 环烯Hydrazide 酰肼Cage compound螺烷Acyl azide 酰叠氮Propellane 笼型化合物Amidine 脒Rotazane 螺桨烷Keto ester 酮酸酯Catenane 轮烷Acyl cyanide 酰腈Rused ring 索烃Carbon suboxide 二氧化三碳Glycidic acid 环氧丙酸Carbammic acid 氨基甲酸Carbamate 氨基甲酸酯Urea 脲Cyanamide 氨腈Carbodiimide 碳二亚胺Allophanate 脲基甲酸酯10。
专业英语(无机化合物命名 )

(5) Acids derived from binary compounds 由二元化合物得到得酸 For binary compounds with hydrogen as the positive ion, place the term hydro at the front of the stem of the negative ion, the letter ic at the end of the stem, and add the word acid. 以H作为阳离子,在成酸元素之前加hydro, 成酸元素之后改 ic , 再加acid. Eg: HCl: hydrochloric acid H2S: hydrosulfuric adic
不同氧化度的二元化合物
- 过氧化物: peroxide [pəˈr ɔkˈsad] O22ɪ
H2O2: hydrogen peroxide •超氧化物: superoxide [ˈsju:p ˈ ə ɔksaid] O2-
KO2: Potassium superoxide
[pəˈtæsi əm]
(4) Exceptions that use the ide endings These polyatomic ions take the ending ide even though more than two elements are present: -Hydroxide (OH-) -Cyanides(CN-) ['saiənaid] -Ammonium (NH4+) Eg: NH4I : Ammonium iodide Ca(OH)2: Calcium hydroxide KCmon Transition Elememts
有机化学专业英语词汇

有机化学专业英语词汇organic compounds 有机化合物compounds of carbon 碳化合物hydrocarbons and their derivatives 碳氢化合物及其衍生物organic chemistry 有机化学structure of molecule 分子结构chemical bond 化学键covalent bond 共价键hybrid orbital 杂化轨道bond length 键长bond angle 键角bond energy键能polarity 极性dissociation energy 离解能constitution构造contiguration构型conformation构象stereochemistry立体化学tetrahedral正四面体cis-顺trans-反isomerism同分异构现象isomer异构体stereoisomer立体异构constitutional isomer构造异构structural formula 结构式octet八隅体perspective 透视式eclipsed conformation重叠式构象staggered conformation交叉式构象newman projection纽曼投影式functional group 官能团chain compoud 链状化合物carbocyclic compound碳环化合物heterocyclic compound杂环化合物dipole-dipole interactions 偶极-偶极相互作用van der Waals forces 范德华力hydrogen bonding 氢键dipole moment偶极矩electronegativity 电负性physical property物理性质melting point熔点boiling point 沸点reaction mechanism反应机理homolysis均裂free redical自由基heterolysis异裂ionic type离子型electrophilic reagent亲电试剂electrophilic reaction亲电反应nucleophilic reagent亲核试剂nucleophilic reaction 亲核反应1. The Ideal-Gas Equation 理想气体状态方程2. Partial Pressures 分压3. Real Gases: Deviation from Ideal Behavior 真实气体:对理想气体行为的偏离4. The van der Waals Equation 范德华方程5. System and Surroundings 系统与环境6. State and State Functions 状态与状态函数7. Process 过程8. Phase 相9. The First Law of Thermodynamics 热力学第一定律10. Heat and Work 热与功11. Endothermic and Exothermic Processes 吸热与发热过程12. Enthalpies of Reactions 反应热13. Hess’s Law 盖斯定律14. Enthalpies of Formation 生成焓15. Reaction Rates 反应速率16. Reaction Order 反应级数17. Rate Constants 速率常数18. Activation Energy 活化能19. The Arrhenius Equation 阿累尼乌斯方程20. Reaction Mechanisms 反应机理21. Homogeneous Catalysis 均相催化剂22. Heterogeneous Catalysis 非均相催化剂23. Enzymes 酶24. The Equilibrium Constant 平衡常数25. the Direction of Reaction 反应方向26. Le Chatelier’s Principle 列•沙特列原理27. Effects of V olume, Pressure, Temperature Changes and Catalystsi. 体积,压力,温度变化以及催化剂的影响28. Spontaneous Processes 自发过程29. Entropy (Standard Entropy) 熵(标准熵)30. The Second Law of Thermodynamics 热力学第二定律31. Entropy Changes 熵变32. Standard Free-Energy Changes 标准自由能变33. Acid-Bases 酸碱34. The Dissociation of Water 水离解35. The Proton in Water 水合质子36. The pH Scales pH值37. Bronsted-Lowry Acids and Bases Bronsted-Lowry 酸和碱38. Proton-Transfer Reactions 质子转移反应39. Conjugate Acid-Base Pairs 共轭酸碱对40. Relative Strength of Acids and Bases 酸碱的相对强度41. Lewis Acids and Bases 路易斯酸碱42. Hydrolysis of Metal Ions 金属离子的水解43. Buffer Solutions 缓冲溶液44. The Common-Ion Effects 同离子效应45. Buffer Capacity 缓冲容量46. Formation of Complex Ions 配离子的形成47. Solubility 溶解度48. The Solubility-Product Constant Ksp 溶度积常数49. Precipitation and separation of Ions 离子的沉淀与分离50. Selective Precipitation of Ions 离子的选择沉淀51. Oxidation-Reduction Reactions 氧化还原反应52. Oxidation Number 氧化数53. Balancing Oxidation-Reduction Equations 氧化还原反应方程的配平54. Half-Reaction 半反应55. Galvani Cell 原电池56. V oltaic Cell 伏特电池57. Cell EMF 电池电动势58. Standard Electrode Potentials 标准电极电势59. Oxidizing and Reducing Agents 氧化剂和还原剂60. The Nernst Equation 能斯特方程61. Electrolysis 电解62. The Wave Behavior of Electrons 电子的波动性63. Bohr’s Model of The Hydrogen Atom 氢原子的波尔模型64. Line Spectra 线光谱65. Quantum Numbers 量子数66. Electron Spin 电子自旋67. Atomic Orbital 原子轨道68. The s (p, d, f) Orbital s(p,d,f)轨道69. Many-Electron Atoms 多电子原子70. Energies of Orbital 轨道能量71. The Pauli Exclusion Principle 泡林不相容原理72. Electron Configurations 电子构型73. The Periodic Table 周期表74. Row 行75. Group 族76. Isotopes, Atomic Numbers, and Mass Numbers 同位素,原子数,质量数77. Periodic Properties of the Elements 元素的周期律78. Radius of Atoms 原子半径79. Ionization Energy 电离能80. Electronegativity 电负性81. Effective Nuclear Charge 有效核电荷82. Electron Affinities 亲电性83. Metals 金属84. Nonmetals 非金属85. Valence Bond Theory 价键理论86. Covalence Bond 共价键87. Orbital Overlap 轨道重叠88. Multiple Bonds 重键89. Hybrid Orbital 杂化轨道90. The VSEPR Model 价层电子对互斥理论91. Molecular Geometries 分子空间构型92. Molecular Orbital 分子轨道93. Diatomic Molecules 双原子分子94. Bond Length 键长95. Bond Order 键级96. Bond Angles 键角97. Bond Enthalpies 键能98. Bond Polarity 键矩99. Dipole Moments 偶极矩100. Polarity Molecules 极性分子101. Polyatomic Molecules 多原子分子102. Crystal Structure 晶体结构103. Non-Crystal 非晶体104. Close Packing of Spheres 球密堆积105. Metallic Solids 金属晶体106. Metallic Bond 金属键107. Alloys 合金108. Ionic Solids 离子晶体109. Ion-Dipole Forces 离子偶极力110. Molecular Forces 分子间力111. Intermolecular Forces 分子间作用力112. Hydrogen Bonding 氢键113. Covalent-Network Solids 原子晶体114. Compounds 化合物115. The Nomenclature, Composition and Structure of Complexes 配合物的命名,组成和结构116. Charges, Coordination Numbers, and Geometries 电荷数、配位数、及几何构型117. Chelates 螯合物118. Isomerism 异构现象119. Structural Isomerism 结构异构120. Stereoisomerism 立体异构121. Magnetism 磁性122. Electron Configurations in Octahedral Complexes 八面体构型配合物的电子分布123. Tetrahedral and Square-planar Complexes 四面体和平面四边形配合物124. General Characteristics 共性125. s-Block Elements s区元素126. Alkali Metals 碱金属127. Alkaline Earth Metals 碱土金属128. Hydrides 氢化物129. Oxides 氧化物130. Peroxides and Superoxides 过氧化物和超氧化物131. Hydroxides 氢氧化物132. Salts 盐133. p-Block Elements p区元素134. Boron Group (Boron, Aluminium, Gallium, Indium, Thallium) 硼族(硼,铝,镓,铟,铊)135. Borane 硼烷136. Carbon Group (Carbon, Silicon, Germanium, Tin, Lead) 碳族(碳,硅,锗,锡,铅)137. Graphite, Carbon Monoxide, Carbon Dioxide 石墨,一氧化碳,二氧化碳138. Carbonic Acid, Carbonates and Carbides 碳酸,碳酸盐,碳化物139. Occurrence and Preparation of Silicon 硅的存在和制备140. Silicic Acid,Silicates 硅酸,硅酸盐141. Nitrogen Group (Phosphorus, Arsenic, Antimony, and Bismuth) 氮族(磷,砷,锑,铋)142. Ammonia, Nitric Acid, Phosphoric Acid 氨,硝酸,磷酸143. Phosphorates, phosphorus Halides 磷酸盐,卤化磷144. Oxygen Group (Oxygen, Sulfur, Selenium, and Tellurium) 氧族元素(氧,硫,硒,碲)145. Ozone, Hydrogen Peroxide 臭氧,过氧化氢146. Sulfides 硫化物147. Halogens (Fluorine, Chlorine, Bromine, Iodine) 卤素(氟,氯,溴,碘)148. Halides, Chloride 卤化物,氯化物149. The Noble Gases 稀有气体150. Noble-Gas Compounds 稀有气体化合物151. d-Block elements d区元素152. Transition Metals 过渡金属153. Potassium Dichromate 重铬酸钾154. Potassium Permanganate 高锰酸钾155. Iron Copper Zinc Mercury 铁,铜,锌,汞156. f-Block Elements f区元素157. Lanthanides 镧系元素158. Radioactivity 放射性159. Nuclear Chemistry 核化学160. Nuclear Fission 核裂变161. Nuclear Fusion 核聚变162. analytical chemistry 分析化学163. qualitative analysis 定性分析164. quantitative analysis 定量分析165. chemical analysis 化学分析166. instrumental analysis 仪器分析167. titrimetry 滴定分析168. gravimetric analysis 重量分析法169. regent 试剂170. chromatographic analysis 色谱分析171. product 产物172. electrochemical analysis 电化学分析173. on-line analysis 在线分析。
化学化工专业英语1

Chapter I Nomenclature of inorganic compounds
c.阴离子: 单原子阴离子,词根 + ide ,同时有“某化物”的意思, 如 chloride 氯化物。 其实很多元素的并不能形成真正游离态的阴离子,不过基 于下文二元化合物命名的需要,所以仍然将“词根+ide” 形式列入,表示“某化物”的含义,如O2-对应的氧化物 MgO(magnesium oxide)。
Chapter I Nomenclature of inorganic compounds
七 hepta-;八 octa-;九 nona- ;十 decab.命名时在相应多原子的元素名称前加上数字前缀即可: CS2 carbon disulfide; SnCl4 tin tetrachloride。 也可以明化合价:tin(IV) chloride; CO carbon oxide(carbon monoxide) ; CO2 carbon dioxide; CrO3 chromium trioxide; As2S2雄黄diarsenic disulfide; As2S3雌黄diarsenic trisulfide; As2O5砒霜diarsenic pentaoxide 。
Chapter I Nomenclature of inorganic compounds
5.不同氧化度的二元化合物 过氧化物 peroxide O22-: H2O2 hydrogen peroxide;CaO2 calcium peroxide;Na2O2 sodium peroxide 超氧化物 superoxide O2-:KO2 potassium superoxide 三、含氧酸和含氧酸盐 1.含氧酸有高酸per+正酸”、正酸“词根+ic”、亚酸“词 根+ous”、次酸“hypo+亚酸”、过酸“peroxo+正酸”、 代酸“thio+对应酸”等形态,最后加“acid”:
生物化学 专业英语单词

单词表第一章Prokaryote 原核生物Eukaryote 真核生物fractionation 分级、分馏biomolecule 生物分子organism 生物体、有机体membrane 膜nucleus 细胞核cocci 球菌bacilli 杆菌spirilla 螺旋菌Eubacteria 真细菌Archaebacteria 原细菌Gram-positive 革兰氏阳性菌Gram negative bacteria 革兰氏阴性菌Cyanobacteria 蓝细菌Plasma 细胞浆Mesosome 间体Nuleoid 拟核Sytosol 细胞质、原生质Bilayer 双分子层(膜)Protein 蛋白质Lipid 脂类Carbohydrate 糖类、碳水化合物osmotic pressure 渗透压Peptidoglycan 肽聚糖Subcellular 亚细胞的Ganelle 细胞器Genetic 遗传的Chromosome 染色体ribosomal ribonucleic acid rRNA Endoplasmic reticulum 内质网Phospholipid 磷脂Detoxification 解毒Golgi apparatus 高尔基体Refresh 更新Mitochondria 线粒体oxidative phosphorylation 氧化磷酸化fatty acid 脂肪酸degradation 降解Chloroplasts 叶绿体thylakoid vesicles 类囊体photosynthesis 光合作用Lysosomes 溶酶体Macromolecule 大分子Enzyme 酶Cytoskeleton 细胞支架Metabolic 新陈代谢的Centrifugation 离心Isolate 分离Equilibrium 平衡Density 密度Friction 摩擦力Velocity 速率Supernatant 上清夜Pellet 沉淀第二章Amino acid 氨基酸Enantiomers 对映体Tetrahedral 正四面体的Hydrophobic 疏水的、憎水的Aliphatic 脂肪族的Aromatic 芳香族的Polar 极性的Charged 带电荷的Glycine Gly,甘氨酸alanine Ala,丙氨酸valine Val,缬氨酸leucine Leu,亮氨酸isoleucine Ile,异亮氨酸methionine Met,甲硫氨酸proline Pro,脯氨酸cystine Cys,半胱氨酸Phenylalanine Phe,苯丙氨酸Tyrosine Tyr,酪氨酸Tryptophan Trp,色氨酸Asparagines Asn, 天冬酰胺Glutamine Gln,谷氨酰胺Serine Ser,丝氨酸Threonine Thr,苏氨酸Varginine Arg, 精氨酸Lysine Lys,赖氨酸Histidine His,组氨酸aspartic acid Asp,天冬氨酸glutamic acid Glu,谷氨酸base 碱carboxyl 羧基isoelectric point 等电点positive 正的、阳性的negative 负的、阴性的buffering 缓冲physiological 生理的Primary structure 一级结构Secondary structure 二级结构Tertiary structure 三级结构Quaternary structure 四级结构peptide bond 肽键sequence 顺序、序列covalent Bond 共价键polypeptide 多肽terminal 末端carbonyl 羰基resonance structures 共振结构rigid 刚性的rotate 旋转trans configuration 顺式构象disulfide bonds 二硫键α-helix α-落选hydrogen bond 氢键β-pleated sheet β-折叠片parallel 平行的antiparallel 反平行的random coil 无规卷曲unique 唯一的spatial 空间的arrangement 排列、安排linear sequence 线性序列residue 残基Hydrophobic interaction疏水相互作用Interior 内部的Electrostatic force 静电力salt bridge 盐桥、盐键van der Waals force 范德华力subunit 亚基allosteric effect 变构效应Noncovalent interactions 非共价相互作用protein stability 蛋白质的稳定dimensional 空间的、维的proton 质子donor 供体、赠与者lone pair of electrons 孤对电子collinear 在同一直线上Hydrophobic force 疏水力Nonpolar 非极性Minimize 最小化protein folding 蛋白质折叠Accessory protein 辅助蛋白质molecular chaperones 分子伴侣Myoglobin 肌红蛋白Hemoglobin 血红蛋白prosthetic group 辅基essential 必需的heme 血红素crevice 缝隙protoporphyrin 原卟啉porphyrin 卟啉ferrous 含铁的proximal 最接近的cooperative 协同的noncooperative 非协同的dissociation curve 解离曲线sigmoidal S形曲线hyperbolic 双曲线affinity 亲和性blood capillaries 血管Bohr effect 波尔效应2,3-biphosphoglycerate 2,3-二磷酸甘油酸Mechanism 机制Relaxed state 松弛状态tense state 紧张状态hemoglobinopathies 血红蛋白分子病Sickle-cell anemia 镰刀形细胞贫血症Erythrocyte 红血球sticky patch 粘性小区therapeutic 治疗的Collagen 胶原蛋白Skin 皮肤Bone 骨骼Tendon 腱Cartilage 软骨blood vessel 血管mammal 哺乳动物fibrous 纤维状的tripeptide 三肽的triple-helical 三股螺旋的cross-linke 交联Allysine 醛基赖氨酸Antibodie 抗体immune system 免疫系统pathogen 病原体trigger 引发、触发response 响应、应答antigen 抗原antigenic determine 抗原决定簇epitope 抗原决定簇Immunolocalization 免疫定位Antibody 抗体Enzyme-linked immunosorbent assayELISA酶联免疫吸附测定purification 提纯、纯化Homogenization 匀浆solubilization 溶解Ammonium sulfate 硫酸铵Precipitation 沉淀Dialysis 透析Chromatographic techniques 层析技术gel filtration 凝胶过滤affinity chromatography 亲和层析Electrophoretic techniques 电泳技术isoelectric focusing 等电聚焦SDS polyacrylamide gel eletrophoresisSDS聚丙烯酰胺凝胶电泳semi-permeable 半透性ligand 配基inert 惰性的matrix 基质elute 洗出、流出lectin 外源凝集素glycoprotein 糖蛋白molecular sieve 分子筛polyampholytes 聚两性电解质gradient 梯度migrate 迁移、移动chymotrypsin 胰凝乳蛋白酶sequencing 测序2-mercaptoethanol 2-巯基乙醇ninhydrin 茚三酮fluorescamine 荧光胺fluorodinitrobenzene 二硝基氟苯dansyl chloride 丹磺酰氯phenyl isothiocyanate PITC苯异硫氰酸酯fragment 片断、碎片encoding 编码decipher 解读、破译anchor 锚定第三章biocatalyst 生物催化剂active site 活性中心substrate 底物The induced –fit model 诱导契合学说Stereospecificity 立体异构专一性Specificity 专一性Trypsin 胰蛋白酶Elastase 弹性蛋白酶Oxidoreductase 氧化还原酶Transferase 转移酶Hydrolase 水解酶Lyase 裂合酶Isomerase 异构酶Ligase 连接酶Ribozyme 核酶Abzyme 抗体酶catalytic antibody 抗体酶analog 类似物assay 化验、测定optimal 最佳的Coenzyme 辅酶Cofactor 辅因子apoenzyme 脱辅酶holoenzyme 全酶acetylcholinesterase 乙酰胆碱酯酶Nicotinamide 烟酰胺Adenine 腺嘌呤Dinucleotide 二核苷酸Phosphate 磷酸Oxidation 氧化reduction 还原Flavin 黄素Mononucleotide 单核苷酸Acyl 酰基thiamine pyrophosphate 焦磷酸硫胺素decarboxylase 脱羧酶Pyridoxal 吡哆醛Pyridoxamine 吡哆胺Pyridoxine 吡哆醇Ubiquinone 泛醌Isoenzymes 同功酶Kinetic 动力学lactate dehydrogenase 乳酸脱氢酶proportional 成比例的saturate 使饱和thermal 热的denaturation 变性optimum 最适宜的diversity 多样性Michaelis-Menten equation 米氏方程double-reciprocal plot 双倒数作图法inhibition 抑制Inhibitor 抑制剂Metabolite 代谢物Irreversible 不可逆的Reversible 可逆的Competitive 竞争性的Noncompetitive 非竞争性的Probe 探测Clinically 临床上Regulation 调节committed step 关键步骤activator 激活剂Adjust 调节Feedback 反馈Sequential 连续的Branched 分支的Conformational 构象的homotropic effect 同促效应heterotropic effect 异促效应Phosphofructokinase 磷酸果糖激酶Citrate 柠檬酸盐Fructose 2,6 bisphosphate 2,6-二磷酸果糖phosphorylation 磷酸化dephosphorylation 去磷酸化hydroxyl 羟基hormone 激素Glycogen phosphorylase 糖原磷酸化酶Phosphorylate 使磷酸化glycogen synthase 糖原合酶unphosphorylate 使去磷酸化proteolytic 蛋白质水解的proenzymes 酶原zymogen 酶原hydrolysis 水解pancreatic 胰腺的pancreas 胰腺small intestine 小肠blood clotting 血液凝固amplification 扩大cascade 级联第四章boundary 边界compartments 小室Mechanical 机械的signaling 发信号insoluble 不可溶的glycerophospholipids 甘油磷脂类sphingolipids 鞘脂类sterols 固醇类glycerol 甘油sphingosine 鞘氨醇sphingomyelins 鞘磷脂cholesterol 胆固醇steroid 类固醇Amphipathic 两性的Hydrophilic 亲水的Bulky 体积大的self-assemble 自组装的fluidity 流动性rotational 转动的lateral 侧向的Fluid mosaic model 流体镶嵌模型Integral 整体的、内在的Flip 翻跟头integral membrane proteins 内在膜蛋白peripheral membrane proteins外周膜蛋白asymmetry 不对称asymmetrically 不对称地membrane-spaning protein 跨膜蛋白Multiple 多重的Lipid-anchored proteins 脂锚定蛋白Heterokaryon 异核体Fusion 融合Reconstitution 重建Reincorporated 重新合并Extracellular 细胞外的Intercellular 细胞内的Passive transport 被动运输active transport 主动运输concentration 浓度diffusion 扩散saturable 可饱和的facilitated 协助的、推动的symport 同向运送antiport 逆向运送epithelial cells 上皮细胞exocytosis 分泌作用endocytosis 内吞作用phagocytosis 吞噬作用pinocytosis 胞饮作用Receptor mediated endocytosis fusion受体介导的内吞作用debris 碎片transduction 转导Lipophilic 亲脂性的Receptors 受体second messengers 第二信使第五章Nucleic acid 核酸Replication 复制Nucleotide 核苷酸Pyrimidine 嘧啶Guanine 鸟嘌呤Thymine 胸腺嘧啶Cytosine 胞嘧啶Nucleoside 核苷Deoxyribonucleoside 脱氧核糖核苷ribonucleoside 核糖核苷deoxyribonucleotide 脱氧核糖核苷酸genes 基因complementarily 互补地nucleosome 核小体loop 突环rosette 玫瑰花结semi-conservative 半保留的polymerase 聚合酶template 模板primer 引物fork 叉Bidirectional 双向的Okazaki fragments 冈崎片段semi-discontinuous 半不连续的strand 链、一股hybridization 杂交melting temperature 熔融温度renaturation 复性labeled 标记的fluorescent 荧光的tag 标记、标签annealing 退火amplify 增强、扩大The central dogma 中心法则Transcription 转录initiation 起始Elongation 延伸termination 终止promoters 启动子palindrome 回文结构processing 加工splicing 拼接reverse transcription 逆转录第六章genetic code 遗传密码intermediate 中间的、媒介codons 密码子unambiguous 明确的correspond 相应、符合degenerate 简并的mutation 变异incorporation 合并nonoverlapping 不相重叠的reading frames 阅读框aminoacyl-tRNA 氨酰-tRNA peptidyl-tRNA 肽酰-tRNA stem 茎、干、臂anticodon 反密码子translocation 移位第七章metabolism 代谢Saccharides 糖类monosaccharides 单糖aldehyde group 醛基ketone group 酮基Stereoisomers 立体异构体Oligosaccharides 寡糖Glycosidic bond 糖苷键Polysaccharides 多糖Starch 淀粉Cellulose 纤维素Dextran 葡聚糖Amylose 直链淀粉amylopectin 支链淀粉Glycolysis 糖酵解Cytoplasm 细胞质Glucose 葡萄糖Galactose 半乳糖Mannose 甘露糖Sucrose 蔗糖Trehalose 海藻糖Lactose 乳糖Hexokinase 己糖激酶Fructose 果糖Phosphoglucoisomerase 磷酸葡萄糖变位酶Bisphosphate 二磷酸glyceraldehydes 甘油醛dihydroxyacetone 二羟丙酮aldolase 醛缩酶triose 丙糖1,3-bisphosphoglycerate 1,3 二磷酸甘油酸dehydrogenase 脱氢酶3-phosphoglycerate 3-磷酸甘油酸kinase 激酶mutase 变位酶phosphoenolpyruvate 磷酸烯醇式丙酮酸enolase 烯醇化酶pyruvate 丙酮酸Gluconeogenesis 糖异生Noncarbhydrate 非糖的Liver 肝脏skeletal muscle 骨骼肌phosphorylase 磷酸化酶Phosphorolysis 磷酸化pyrophosphorylase 焦磷酸化酶glucosyl 葡萄糖基nonreducing end 非还原端Epinephrine 肾上腺素glucagon 胰高血糖素Insulin 胰岛素第八章fatty acid 脂肪酸hydrocarbon 烃、碳氢化合物carboxylic acid 羧酸Unsaturated 不饱和的Triacylglycerol 三酰甘油Acetyl 乙酰基Thioester 硫酯Carnitine 肉(毒)碱Hydration 水合作用Thiolysis 硫解Consume 消耗ketone bodies 酮体acetoacetate 乙酰乙酸D-3-hydroxybutyrate D-3-羟基丁酸Acetone 丙酮diabetes 糖尿病toxic 有毒的lethal 致命的multifunctional 多功能的malonyl 丙二酰基carboxylation 羧化condensation 缩合acetoacetyl 乙酰乙酰基hydroxybutyryl 羟丁酰基crotonyl 丁烯酰基butyryl 丁酰基hydrolyzation 水解作用palmitoyl 软脂酰基palmitate 软脂酸lipoproteins 脂蛋白globular 球状的micelle 胶束、微囊第九章Respiration 呼吸作用citric acid cycle 柠檬酸循环、三羧酸循环concomitant 伴随的isocitrate 异柠檬酸酸盐α-ketoglutarate α-酮戊二酸succinate 琥珀酸盐succinyl 琥珀酰基fumarate 延胡索酸盐malate 苹果酸盐oxaloacetate 草酰乙酸盐cytochrome 细胞色素oxidase 氧化酶reductase 还原酶Rotatory 旋转的engine 发动机第十章Nitrogen 氮Diet 常吃的食物Erythrose 赤藓糖Ribose 核糖Transamination 转氨基作用Deamination 脱氨基作用Transdeamination 联合脱氨基作用Ammonia 氨Excrete 排泄Aquatic 水生uric acid 尿酸terrestrial 陆生的reptile 爬行动物urea 尿素vertebrates 脊椎动物ornithine 鸟氨酸arginine 精氨酸citrullin 瓜氨酸permanently 不变地。
有机化学专业英语词汇

有机化学专业英语词汇(精)acetal 醛缩醇-ene 烯acetal- 乙酰epi-表acid 酸epoxy-环氧-al 醛-ester 酯alcohol 醇-ether 醚-aldehyde 醛ethoxy-乙氧基ethyl乙基alkali-碱allyl烯丙基[propenyl(丙烯基)]alkoxy-烷氧基fluoro-氟代-amide酰胺form 仿amino-氨基的-glycol 二醇-amidine 月米-amine 胺-ane 烷hemi-半anhydride 酐hendeca-十^一anilino-本胺基hepta-七aquo-含水的heptadeca-十七-ase 酶hexa-六-ate含氧酸的盐、酯hexadeca-十六-atriyne 三炔-hydrin 醇azo-偶氮hydro-氢或水hydroxyl 羟基benzene 苯hypo-低级的,次bi-在盐类前表示酸式盐hyper-高级的,高bis-双-borane 硼烷-ic酸的,高价金属bromo-澳-ide无氧酸的盐,酰替胺,酐butyl 」基-il偶酰-imine亚胺-carbinol 甲醇iodine 碘carbonyl 羰基iodo-碘代-carboxylic acid 羧酸iso-异,等,同centi- 10-2-ite亚酸盐chloro-氯代cis-顺式keto-酮ketone 酮condensed缩合的、冷凝的cyclo-环-lactone 内酯deca-十deci 10-1mega- 106-dine 啶meta-间,偏dodeca-十二methoxy-甲氧基methyl甲基micro- 10-6milli- 10-3mono- ( mon-) 一,单nano- 10-9 nitro-硝基nitroso-亚硝基nona-九nonadeca-十九octa-八octadeca-十八-oic酸的-ol醇-one 酮ortho-邻,正,原-ous亚酸的,低价金属oxa-氧杂-oxide氧化合物-oxime月亏oxo- 酮oxy-氧化-oyl 酰para-对位,仲penta-五pentadeca-十五per-高,过petro-石油phenol苯酚phenyl苯基pico- 10-12 poly-聚,多quadri-四quinque-五semi-半septi-七sesqui 一个半sulfa-磺胺sym-对称syn-顺式,同,共ter-三tetra-四tetradeca-十四tetrakis-四个thio-硫代trans-反式,超,跨thio-硫代tri-三trideca-十三tris-三个undeca-十一uni-单,一unsym-不对称的,偏位-yl基-ylene撑(二价基,价在不同原子上) -yne 炔organic compounds 有机化合物compounds of carbon 碳化合物hydrocarbons and their derivatives 碳氢化合物及其衍生物organic chemistry 有机化学structure of molecule 分子结构chemical bond 化学键covalent bond 共价键hybrid orbital 杂化轨道bond length 键长bond angle 键角bond energy 键能polarity 极性dissociation energy 离解能constitution 构造contiguration 构型conformation 构象stereochemistry 立体化学tetrahedral正四面体cis-顺trans-反isomerism同分异构现象isomer异构体stereoisomer立体异构constitutional isomer 构造异构structural formula 结构式octet八隅体perspective 透视式eclipsed conformation 重叠式构象staggered conformation 交叉式构象newman projection 纽曼投影式functional group 官能团chain compoud链状化合物carbocyclic compound 碳环化合物heterocyclic compound 杂环化合物dipole-dipole interactions 偶极-偶极相互作用van der Waals forces 范德华力hydrogen bonding 氢键dipole moment 偶极矩electronegativity 电负性physical property 物理性质melting point 熔点boiling point 沸点reaction mechanism 反应机理homolysis 均裂free redical 自由基heterolysis 异裂ionic type 离子型electrophilic reagent 亲电试剂electrophilic reaction 亲电反应nucleophilic reagent 亲核试剂nucleophilic reaction 亲核反应英文名汉文名Angular methyl group 角甲基Alkylidene group 亚烷基Allyl group 烯丙基Allylic烯丙型[的]Phenyl group 苯基Aryl group 芳基Benzyl group 苄基Benzylic 苄型[的]Activating group 活化基团Chromophore 生色团Auxochrome 助色团Magnetically anisotropic group 磁各向异性基团Smally ring 小环Common ring 普通环Medium rimg 中环Large ring 大环Bridged-ring system 桥环体系Spiro compound螺环化合物Helical molecule螺旋型分子Octahedral compound八面体化合物Conjugation 共轭Conjugated-system 共轭体系Acyl cation酰[基]正离子Benzylic cation 苄[基]正离子Arenirm ion 芳[基]正离子Ketyl radical羰自由基Radical ion自由基离子Radical cation自由基正离子Radical anion自由基负离子Isomerism异构[现象] Aci form 酸式Fluxional structure 循变结构Stereochemistry 立体化学Optical activity 光学活性Dextro isomer 右旋异构体Laevo isomer左旋异构体Tetrahedral configuration 四面体构型Stereoisomerism 立体异构[现象]Asymmetric atom不对称原子Asymmetric carbon 不对称碳Pseudoasymmetric carbon 假不对称碳Phantom atom 虚拟原子Homotopic 等位[的] Heterotopic 异位[的] Enantiotopic对映异位[的]Diastereotopic非对映异位[的]Configuration 构型Absolute configuration 绝对构型Chirality 手性Chiral手性[的]Chiral center手性中心Chiral molecule 手性分子Achiral非手性[的]Fischer projection费歇尔投影式Neoman projection 纽曼投影式D-L system of nomenclature D-L 命名体系R-S syytem of nomenclature R-S 命名体系Cahn-Ingold-Prelon sequence 顺序规则Symmetry factor 对称因素Plane of symmetry 对称面Mirror symmetry 镜面对称Enantiomer对映[异构]体Diastereomer非对映[异构]体Epimer差向异构体Anomer端基[差向]异构体Erythro configuration 赤型构型Erythro isomer赤型异构体Threo configuration 苏型构型Threo isomer苏型异构体Trigonal carbon 三角型碳Cis-trans isomerism 顺反异构 E isomer E异构体Z isomer Z异构体Endo isomer内型异构体Exo isomer外型异构体Prochirality 前手性Pro-R group 前R 基团Pro-S proup 前S 基团Re face Re 面Si face Si 面Racemic mixture外消旋混合物Racemic compound夕卜消旋化合物Racemic solid solution 外消旋固体溶液Meso compound内消旋化合物Quasi recemate准外消旋体Conformation 构象Conformational 构象分析Torsion angle 扭转角Rotamer旋转异构体Anti conformation 反式构象Bisecting conformation 等分构象Anti periplanar conformation 反叠构象Synperiplanar conformation 顺叠构象Synclinal conformation 反错构象Synclinal conformation 顺错构象Eclipsed conformation 重叠构象Gauche conformation, skew con-formation邻位交叉构象Staggered conformation 对位交叉构象Steric effect空间效应Steric hindrance 位阻Atropismer阻转异构体Puckered ring 折叠环Conformational inversion 构象反转Chair conformation 椅型构象Boat conformation 船型构象Twist conformation 扭型构象Skew boat conformation 扭船型构象Half-chair conformation 半椅型构象Pseudorotation 假旋转Envelope conformation 信封[型]构象Axial bond 直[立]键Equatorial bond 平[伏]键Cisoid conformation 顺向构象Transoid conformation 反向构象Retention of configuration 构型保持Regioselectivity 区域选择性Regiospecificity 区域专一性Stereocelectivity 立体选择性Stereospecificty 立体专一性Conformer构象异构体Conformational effect 构象效应Cram,s rube克拉姆规则Prelog,rule普雷洛格规则Stereochemical orientation 立体[化学]取向Conformational transmission 构象传递Homolog同系物Ipso position 本位Ortho position 邻位Meta position 间位Para position 对位Amphi position 远位Peri position 近位Trigonal hybridization 三角杂化Molecular orbiral method 分子轨道法Valence bond method 价键法Delocalezed bond 离域键Cross conjugation 交叉共轭Vinylog插烯物Mesomeric effect 中介效应Resonance 共振Resonance effect 共振效应Hyperconjugation 超共轭Isovalent hyperconjugation 等价超共轭No-bond resonance 无键共振Aromaticity 芳香性Aromatic sexter 芳香六隅 Huckel’ru咻克尔规则Paramagnetic ring current 顺磁环电流Diamagnetic ring cruuent 抗磁环电流Homoaromaticity 同芳香性Antiaromaticity 反芳香性Alternant hydrocarbon 交替烃Non-alternant hydrocarbon 非交替烷Pericyclic reaction 周环反应Electrocyclic rearrangement 电环[化]重排Conrotatory 顺旋Disroatatory 对旋Cycloaddition 环力口成Symmetry forbidden-reaction 对称禁阻反应Synfacial reaction 同面反应Antarafacial reaction 异面反应Mobius system默比乌斯体系Leois structure路易斯结构Coordinate-covalent bond 配位共价键Banana bond 香蕉键Pauling electronegativity scale 鲍林电负性标度Polarizability 可极化性Inductive effect 诱导效应Field effect 场效应Electrical effect 电场效应tautomerism互变异构Tautomerization互变异构化Keto-enol tautomerism 酮-烯醇互变异构Phenol-keto tautomerism 酚-酮互变异构Imine-enamine atutomerism 亚胺-烯胺互变异构Ring-chain tautomerism 环-链互变异构Valence tautomerism 价互变异构Ambident 两可[的]Solvent effect 溶剂效应Acid-base catalyxed reaction 酸性溶剂Basic solvent碱性溶剂Dielectric constant 介电常数Solvated electron 溶剂化电子Acid-base catalyzed reaction 酸碱催化反应Conjugate base 共轭酸Conjugate base 共轭碱Therm odynamic acidity 热力学酸度Kinetic acidity动力学酸度Electron donof-acceptor complex,EDAcomplex 电子给[体]受体络合物Host主体Guest客体Primary isotope effect 一级同位素效应Secondary isotope effect 二级同位数效应Inverse isotope effect 逆同位素效应Kinetic control动力学控制Thermodynamic control 热力学控制Substrate 底物Intermediate 中间体Reactive intermediate 活泼中间体Microscopic reversibility 微观可逆性Hammond postulate 哈蒙德假说Linear free energy 线性自由能Non-bonded interaction 非键相互作用Torsional effect 扭转效应Pitzer strain皮策张力Restricted rotation 阻碍旋转Eclipsing effect 重叠效应Eclipsing strain 重叠张力Small-angle strain 小角张力Large angle strain 大角张力Transannular interaction 跨环相互作用Transannular strain 跨环张力I strain内张力F strain前张力B strain后张力Anomeric effect端基异构效应Walden inversion瓦尔登反转Racemization外消旋化Isoinversion 等反转Isoracemization 等消旋Homochiral纯手性[的] Mechanism 机理Unimolecular nucleophilic 单分子亲核取代Bimolecular nucleophilic sub-stitution 双分子亲核取代Bimolecular nucleophilic substi-tution(with allylic rearrange-ment) 双分子亲核取代(含烯丙型重排) Internal nucleophilic substiru-tion 分子内亲核取代Aromatic nucleophilic substitu-tion 芳香亲核取代Unimolecular electrophilic sub-stitution 单分子亲电取代Bimolecular electrophilic substi-tution 双分子亲电取代Nucleophile-assisted unimolecu-larelectrophilic substitution 亲核体协助单分子亲电取代Unimolecular elimination 单分子消除Bimolecular elimination 双分子消除Unimolecular elimination through theconjugate base单分子共轭碱消除Bimolecular elimination through theconjugate base双分子共轭碱消除Bimolecular elimination with for-mation ofa carbonyl group双分子羰基形成消除Unimolecular acid-catalyzed acyl-oxygencleavage单分子酸催化酰氧断裂Bimolecular base-catalyzed acyl-oxygencleavage双分子碱催化酰氧断裂Unimolecular acid-catalyzed alkyl-oxygencleavage 单分子酸催化烷氧断裂Bimllecular base-catalyzed al- kyl-oxygencleavage双分子碱催化烷氧断裂n-allyl complex mechanism 兀烯丙型络合机理Borderline mechanism 边理机理Homolysis 均裂Heterolysis 异裂Heterolytic michanism 异裂机理Counrer[gegen]ion 反荷离子Ion pair离子对Carbocation碳正离子Nonclassical carbocation 非经典碳正离子Carbanion碳负离子Masked carbanion掩蔽碳负离子Carbenoid卡宾体Carbene 卡宾Nitrene 氮宾Carbine 碳炔Electrophilic addition 亲电加成Electrophile 亲电体Diaxial addition双直键加成Markovnikov,s rube马尔科夫尼科规则Anti-Markovnikov addition 反马氏力口成Michael addition迈克尔加成Substitution 取代Electrophilic substitution 亲电取代Addition-elimination mechanism 加成消除机理Electrophilic aromatic substitu-tion 亲电芳香取代Electron transfer 电子转移Electron-donating group 给电子基团Electron-Withdrawing group 吸电子基团Deactivating group 钝化基团Orinentation 取向Ortho-para directing group 邻对位定位基Meta directing group 间位定位基Ortho effect邻位效应Partial rate factor 分速度系数Nucleophilic reaction 亲核反应Internal return 内返Nucleophilicity 亲核体Nucleophilicity 亲核性a-effect a-效应Backside attack 背面进攻Inversion 反转Umbrella effect 伞效应Push-pull effect 推拉效应Leaving group 离去基团Electrofuge 离电体Nucleofuge 离核体Phase-transfer catalysis 相转移催化Neighboring group participation 邻基基参与Neighboring proupassistance,anchimeric assistance 邻助作用Neighboring group effect 邻基效应Apofacial reaction 反面反应Briddgehead displacement 桥头取代Aryl action芳正离子Benzyne 苯炔Zaitsev rule札依采夫规则Anti-Zaitsev orientation 反札依采夫定向Hofmann,s rule霍夫曼规则Bredt rule布雷特规则Initiation 引发Anionic cleavage负离子裂解Partial bond fixation键[的]部分固定化02. 3有机化学反应Alkylation 烷基化C- alkylation C-烷基化O- alkylation O-烷基化N-alkylation N-烷基化Silylation硅烷[基]化Exhaustive methylation 彻底甲基化Seco alkylation断裂烷基化Demethylation 脱甲基化Ethylation 乙基化Arylation芳基化Acylation 酰化Formylation 甲酰化Carbalkoxylation 烷氧羰基化Carboamidation 氨羰基化Carboxylation 较基化Amination 氨基化Bisamination 双氨基化Cine substitution 移位取代Transamination 氨基交换Hydroxylation羟基化acyloxyation 酰氧基化Decarboxylative nitration 脱竣卤化Allylic halogenation 烯丙型卤化Dehalogenation 脱卤Nitration 硝化Decarboxylative nitration 脱竣硝化Nitrosation 亚硝化Sulfonation 磺化Chlorosulfonation 氯磺酰化Desulfonation 脱磺酸基Sulfenylation 亚磺酰化Sulfonylation 磺酰化Chlorosulfenation 氯亚磺酰化Chlorocarbonylation 氯羰基化Diazotization 重氮化Diazo transfer重氮基转移Coupling reaction 偶联反应Diazonium coupling 重氮偶联Cross-coupling reaction 交叉偶联反应1,4-addition 1, 4-加成Conjugate addition 共轭力口成Dimerization 二聚Trimefization 三聚Additive dimerization 加成二聚sulfurization 硫化Selenylation 硒化Hydroboration 硼氢化Oxyamination 羟氨基化Insertion 插入carbonylation 较基化Hydroformylation加氢甲酰基化Hydroacylation 加氢酰化Oxo process羰基合成Decarbonylation 脱羰Hydrocarboxylation 氢较基化Homologization 同系化Cyanoethylation 氰乙基化Decyanoethylation 脱氧乙基Ring clsure 环合Diene synthesis 双烯合成Dienophile亲双烯体Endo addition内型加成Exo addition夕卜型力口成Diels-Alder reaction第尔斯-尔德反应Retro Diels-Alder reaction 逆第尔斯- 阿尔德反应Ene synthesis单烯合成Anionic cycloaddition 负离子环力口成Dipolar addition 偶极加成-elimination -消除-elimination -消除-elimination -消除-elimination -消除Dehydrohalogenation 脱卤化氢Deamination 脱氨基Pyrolytic elimination 热解消除Elimination-addition 消除-力口成Decarboxylation 脱羧Decarboxamidation脱酰胺Decyanation 脱氧基Alkylolysis,alkyl cleavage 烷基裂解Acylolysis,acyl cleavage 酰基裂解Flash pyrolysis 闪热裂Fragmentation 碎裂Chiletropic reaction 螯键反应Chelation 螯环化Esterification 酯化Transesterification 酯交换Saponification 皂化Alcoholysis 醇解Ethanolysis 乙醇解Cyanomethylation 氟甲基化Aminomethylation 氨甲基化Hydroxymethylation 羟甲基化Hydroxyalkylation 羟烷基化Cholromethylation 氯甲基化Haloalkylation 卤烷基化Transacetalation 缩醛交换Enolization 烯醇化Haloform reaction 卤仿反应Condensation 缩合Aldol condensation 羟醛缩合Cross aldol condensation 交叉羟醛缩合Retrograde aldol condensation 逆羟醛缩合Acyloin condensation 偶姻缩合Cyclization 环化Annulation,annelation 增环反应Spiroannulation 螺增环Autoxidation 自氧化Allylic hydroperoxylation 烯丙型氢过氧化Epoxidation 环氧化Oxonolysis 臭氧解Electrochemical oxidation 电化学氧化Oxidative decarboxylation 氧化脱竣Aromatization 芳构化Catalytic hydrogenation 催化氢化Heterogeneous hydrogenation 多相氢化Homogeneous hydrogenation 均相氢化Catalytic dehydrogenation 催化脱氢Transfer hydrogenation 转移氢化Hydrogenolysis 氢解Dissolving metal reduction 溶解金属还原Single electron transfer 单电子转移Bimolecular reduction 双分子还原Electrochemical reduction 电化学还原Reductive alkylation 还原烷基化Reductive acylation 还原酰化Reductive dimerization 还原二聚Deoxygenation 脱氧Desulfurization 脱硫Deselenization 脱石西Mitallation 金属化Lithiation 锂化Hydrometallation 氢金属化Mercuration 汞化Oxymercuration 羟汞化Aminomercuration 氨汞化Abstraction 夺取[反应] Internal abstraction 内夺取[反应] Rearrangement 重排Prototropic rearrangement 质了转移重排Double bond migration 双键移位Allylic migration 烯丙型重排Allylic migration 烯丙型迁移Ring contraction 环缩小[反应] Ring expansion,ring enlargement 扩环[反应]-ketol rearrangement -酮醇重排Pinacol rearrangement 频哪醇重排Retropinacol rearrangement 逆频哪醇重排Semipinacol rearrangement 半频哪醇重排Benzilic rearrangement 二苯乙醇酸重排Acyl rearrangement 酰基重排Migratory aptitude 迁移倾向Transannular insertion 跨环插入Transannular rearrangement 跨环重排Migration 迁移Prototropy质子转移Cationotropic rearrangement 正离子转移重排Anionotropy负离子转移Anionotropic rearrangement 负离子转移重排Sigmatropic rearrangement -迁移重排Homosigmatropic rearrangement 同迁移重排Electrophilic rearrangement 亲电重排Photosensitization 光敏化Forbidden transition 禁阻跃迁photooxidation 光氧化Photoisomerization 光异构化Photochemical rearrangement 光化学重排2. 4有机化合物类名Aliphatic compound脂肪族化合物Hpdrocarbon碳氢化合物Alkane 烷Wax蜡Paraffin wax 石蜡Alkene 烯Alkyen 炔Acetylide炔化物Active hydrogen compounds 活泼氢化合物Carbon acid 碳氢酸Super acid 超酸Diene双烯Triene三烯Allene 丙二烯Ccumulene累积多烯Enyne烯炔Diyne 二炔Alkyl halide 卤代烷Alcohol 醇Homoallylic alcohol 高烯丙醇Ether 醚Epoxide 环氧化物Cellosolve 溶纤剂Crown ether 冠醚Netro compound硝基化合物Amine 胺Quaternaryammonium com-pound 季铵化合物Amine oxide 氧化胺Diazoalkane 重氮烷Mercaptan 硫醇Sulfonic acid 磺酸Sulfoxide 亚飒Sulfone 飒Aldehyde 醛Detone 酮Aldehyde hydrate 醛水合物Ketone hydrate 酮水合物Hemiacetal 半缩醛Acetal缩醛Ketal 缩酮Dithiane 二口塞烷Aminal 缩醛胺imine 亚胺Aldimine 醛亚胺Oxime月亏Aldimine 醛肟Oxime 亚硝基化合物aldoxime 硝酮Hydrazone 腙Azine 嗪Semicarbazone 缩氯基脲Cyanohydrin 羟月青Pinacol频哪醇Enol烯醇Enol ether烯醇醚Enol ester烯醇酯Enamine 烯胺Ynamine 炔胺Mannich base曼尼希碱Carboxylic acid 较酸Ester 酯orthoester 原酸酯Acyl halide 酰卤Acyl fluoride 酰氟Acyl chloride 酰氯Acyl rtomide 酰澳Acyl iodide 酰碘Carbobenzoxy chloride 苄氧甲酰氯Acyl tosylate酰基对甲苯磺酸酐Ketene乙烯酮Peracid 过酸Perester过酸酯Acyl peroxide酰基过氧化物Nitrile 月青Nitrile oxide氧化月青Isonitrile 异月青Amide酰胺Imide二酰亚胺N-bromo compound N-澳化物Hydrazide 酰肼Acyl azide酰叠氮Amidine 月米Keto ester酮酸酯Acyl cyanide 酰月青Carbon suboxide 二氧化三碳Glycidic acid环氧丙酸Carbammic acid 氨基甲酸Carbamate氨基甲酸酯Urea 脲Cyanamide氨月青Carbodiimide 碳二亚胺Allophanate脲基甲酸酯Thioester硫代酸酯Thiol acid硫羰酸Lactone 内酯Lactol内半缩醛Macrolide大环内酯Amino acid氨基酸Zwitterions两性离子Inner salt 内盐Betaine甜菜碱Lactam内酰胺Hydantion乙内酰脲Peptide 肽Glycol 二醇Aldol 羟醛Acyloin 偶姻Carbohydrate碳水化合物Aldose醛糖Ketose酮糖Furanose呋喃糖Pyranose毗喃糖Glycoside 糖昔Glucoside葡[萄]糖昔Aglycon 苷元Saccharide 糖类Oligosaccharide 寡糖Polysaccharide 多糖Alditol 糖醇Osazone 月杀Alicyclic compound 脂环化合物Cycloalkene 环烷Spirane 环烯Cage compound 螺烷Propellane笼型化合物Rotazane螺桨烷Catenane 轮烷Rused ring 索烃。
应用化学专业英语 -化合物命名

樊海梅
LOGO
有机化合物的命名
链 烃
饱和烃:烷烃 不饱和烃:烯烃,炔烃 脂环烃 芳香烃
烃
有 机 物
环 烃
卤代烃
烃 的 衍 生 物
醇
含 氧 衍 生 物
酚 醛 酮 羧酸
酯等
烷烃(alkanes) 直链烷烃:英文名称除了含1到4个碳原子以外,其余均用希腊
90 alkane:nonacontane
100 alkane:hectane
含支链烷烃和烷基 烷基:只需要把烷烃的后缀ane换成-yl加在相应烷烃的字 首后 如:CH3- methyl CH3-CH2- ethyl CH3-(CH2)9-CH2 undecyl
还有一些烷基也可以在相应的烃名前加iso-(异)、sec-仲、tert-叔、
和拉丁文的数词加上相应的词尾来命名(烷烃:ane;烯烃: ene;炔 烃:yne),10个碳原子以上的则在数词前加前缀un、do、 tri、 tetra、 penta等。 例如:甲烷:methane 乙烷:ethane 丙烷:propane 丁烷:butane 庚烷:heptane 癸烷:decane 具体来说:11~19:数字前缀-decane 十一烷:undecane 十二烷:dodecane 十三烷:tridecane 十四烷:tetradecane 戊烷:pentane 己烷:hexane 辛烷:octane 壬烷:nonane
丁二烯 butadiene
丁三烯 butatriene
同时还有双键和三键的烷类成为烯炔,命名时烯在前,炔在后, 双键的编号写在前面,三键的定位号写在表示炔烃词尾前
CH3-CH=CH-C ≡ CH
化学专业英语复习
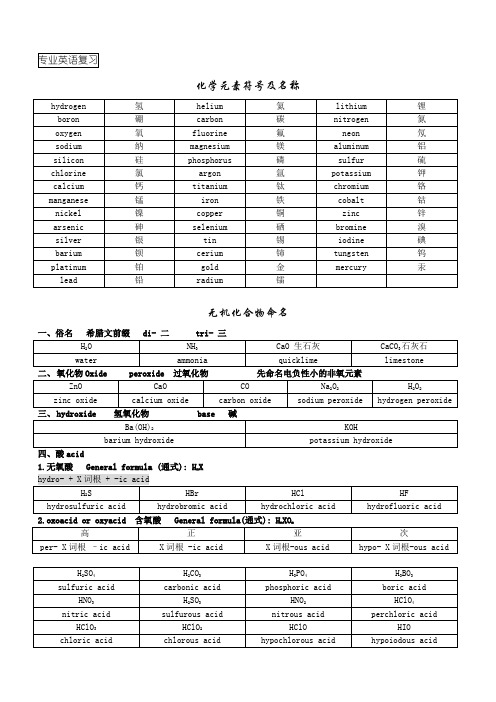
化学元素符号及名称无机化合物命名二、氧化物Oxide peroxide 过氧化物先命名电负性小的非氧元素1.无氧酸 General formula (通式): H n Xn m五、盐salt1.binary salt 二元盐 General formula(通式) : M n X m2.oxysalt 含氧酸盐 General formula(通式): M n XO manion 阴离子, 负离子 cation 阳离子, 正离子 poly 多, 聚 polymer n.聚合物polymerize v.聚合 polymerization n. 聚合(作用) heteropoly acid 杂多酸cyanide 氰化物 thio- 硫代…,硫的,含硫的 borate n.硼酸盐 silane 硅烷 (Si n H2n+2) brane n.硼烷ortho- [希腊词头] :正、原(无机酸用)邻(位)(有机化合物命名)meta-[希腊词头] :偏(无机酸用)间(位)(有机化合物命名)ortho-acid 原酸;meta-acid 偏酸有机化合物命名2.烯烃-ene alkene4.烷基-yl alkyl用数字表示取代基或重键的位置用 di-(二)、tri-(三)、tetra-(四) 等词缀表示重键或相同(简单)基团的数目的位置从与 O 原子连接的 C 原子开始排序, 用希腊字母α、β、γ、δ等表示取代基(Br)nitro-硝基14.hydro- 含氢, 水16.iso- 异 n-正 isomer 异构体 isomerism 异构现象17.iso- 同,等论文摘要重点“5 Steps” for abstract writing1.Underling key words and sentences2.Listing essential points of the paper3.Boiling down each section to a sentence or two4.Drafting the abstract (Borrow some words or phrases from the others)5.Checking the final draft文献阅读的五个问题“5A Strategy”Q1: What is the general knowledge of your topic in the academic field?Q2: What research topic is the paper to focus on?Q3:What method or materials do you use to support your main point of view? Q4: What conclusion will you draw?Q5: What is the main contribution of the paper?。
化学专业英语译文

化学专业英语(修订版)译文目录:1、THE ELEMENTS AND THE PERIODIC TABLE(元素和周期表)........................................6、THE CLASSIFICATION OF INORGANIC COMPOUNDS(无机化合物的分类).................................................................................................................................................................................7、The Nomenclature of Inorganic Compounds(无机化合物的命名) ....................................................9、The coordination complex(配位化合物) ...........................................................................................10、Alkanes(烷烃) ..............................................................................................................................................11、Unsaturated Compounds不饱和化合物...............................................................................................12、The Nomenclature of Cyclic Hydrocarbons(环烃的命名) ...........................................................13、Subsitutive Nomencalture(取代基命名法)......................................................................................14、The Compounds Containing Oxygen(含氧化合物).......................................................................15、Preparation of A Carboxylic Acid by the Grignard Method (格式法制备羧酸) .....................................................................................................................................................18、Synthesis of alcohols and design of organic synthesis (醇的合成及有机合成的设计)........................................................................................................................................22、Polymers(聚合物).................................................................................................................................1、THE ELEMENTS AND THE PERIODIC TABLE(元素和周期表)在原子核中质子的数目被称为原子序数,或质子数,Z。
有机化学专业英语

1, oxygen ['ɔksidʒən] n. 氧气,氧2, alkenes n. 烯属烃3, methyl ['mi:θail, 'miθil] n. 甲基;木精4, alkanes ['ælkeinz] n. 烷类,链烷烃;烷属烃(alkane的复数)5, turpentine ['tə:pəntain] n. 松节油;松脂 vt. 涂松节油 vi. 提取松节油6, benzene ['benzi:n]n. [化]苯7, chlorine ['klɔ:ri:n]n. 氯(17号化学元素)8, nickle alloy nickle alloy: 而非不含金之镍合金 | 而非不含金的镍合金9, brominate ['brəumineit]vt. 用溴处理,溴化10, haloalkane n. [有化] 卤代烷11, decolourise [di:'kʌləraiz]vt. 使…脱色;将…漂白 vi. 漂白(等于decolorize)12, copper ['kɔpə]n. 铜;铜币;[俚]警察 adj. 铜的 vt. 镀铜于13, hydrolysis [hai'drɔlisis]n. [化]水解作用14, derived adj. 导出的;衍生的,派生的 v. 得到;由…而来;推断(derive的过去分词)15, detergent [di'tə:dʒənt]n. 清洁剂;去垢剂16, sheet [ʃi:t]n. 薄片,纸张;床单;薄板 vt. 盖上被单;覆盖;使成大片 vi. 成片流动;大片落下adj. 片状的17, glycol ['ɡlaikɔl]n. 乙二醇;甘醇;二羟基醇18, bromide ['brəumaid]n. 溴化物;庸俗的人;陈词滥调19, styrene ['stairi:n, 'sti-]n. [有化] 苯乙烯,苏合香烯20, insulation [,insju'leiʃən, 'insə-]n. 绝缘;隔离,孤立21, antiseptics [,ænti'septiks]n. 防腐剂(antiseptic的复数)22, hydration [hai'dreiʃən]n. [化学] 水合作用23, halogenation [,hælədʒi'neiʃən]n. 卤化,加卤作用24, adhesive [əd'hi:siv]n. 粘合剂;胶黏剂 adj. 带粘性的;粘着的25, clay [klei]n. 泥土;粘土;肉体;似黏土的东西 vt. 用黏土处理26, sugarcane ['ʃugə,kein]n. [作物] 甘蔗;糖蔗27, fermentation [,fə:men'teiʃən]n. 发酵28, molasses [mə'læsiz]n. 糖蜜,糖浆29, intermediate [,intə'mi:djət, -dieit]vi. 起媒介作用 adj. 中间的,中级的 n. 中间物;媒介30, volatile ['vɔlətail]adj. 爆炸性的;不稳定的;挥发性的;反覆无常的 n. 挥发物;有翅的动物31, carbonisation n. 碳化,碳化作用32, liquefaction [,likwi'fækʃən]n. 液化;熔解33, synthesis ['sinθisis]n. 综合,合成;综合体34, optimum ['ɔptiməm]adj. 最适宜的 n. 最佳效果;最适宜条件35, usage ['ju:zidʒ]n. 用法;使用;惯例36, empirical [em'pirikəl]adj. 经验主义的,完全根据经验的37, endothermic [,endəu'θə:mik,-məl]adj. [热] 吸热的;温血的38, radical ['rædikəl]adj. 根本的;激进的;彻底的 n. 激进分子;原子团;根数;基础39, prefix [,pri:'fiks, 'pri:fiks]n. 前缀vt. 加前缀;将某事物加在前面40, initiator [i'niʃieitə]n. 发起人,创始者;教导者;启动程序;引爆器41, dimer ['daimə]n. [有化] 二聚物;二量体42, activation [,ækti'veiʃən]n. 激活;活化作用43, propagation [,prɔpə'ɡeiʃən]n. 传播;繁殖;增殖44, rigid ['ridʒid]adj. 严格的;僵硬的,死板的;坚硬的;精确的45, amorphous [ə'mɔ:fəs]adj. 无定形的;无组织的;非晶形的46, viscous ['viskəs]adj. 粘性的;黏的47, bracket ['brækit]n. 支架;括号;墙上凸出的托架 vt. 括在一起;把…归入同一类;排除48, caustic ['kɔ:stik]adj. [化]腐蚀性的;苛性的;刻薄的;[物]焦散的n. [医]腐蚀剂;[化]苛性钠;[物]焦散曲线49, moderate ['mɔdərət, 'mɔdəreit]adj. 适度的,中等的;有节制的;稳健的,温和的vi. 变缓和,变弱 vt. 节制;减轻50, brittle ['britl]adj. 易碎的,脆弱的;易生气的51, stiffiness n. 硬度52, gutter ['ɡʌtə]n. 排水沟;槽;贫民区 vi. 流;形成沟vt. 开沟于…;弄熄 adj. 贫贱的;粗俗的;耸人听闻的53, upholstery [ʌp'həulstəri]n. 家具装饰用品业;垫衬物54, curtain ['kə:tən]n. 窗帘;幕 vt. 遮蔽;装上门帘55, ultraviolet [,ʌltrə'vaiələt]adj. 紫外的;紫外线的 n. 紫外线辐射,紫外光56, decomposition [,di:kɔmpə'ziʃən]n. 分解,腐烂;变质57, cassettes n. 盒式录音带(cassette的复数)58, cassette [kæ'set]n. 盒式磁带;暗盒;珠宝箱;片匣59, disposable [dis'pəuzəbl]adj. 可任意处理的;可自由使用的;用完即可丢弃的60, autoclave ['ɔ:təkleiv]n. 高压灭菌器;高压锅 vt. 用高压锅烹饪 vi. 用高压锅烹饪。
化学及化工专业英语词汇(O)

化学及化工专业英语词汇(O)化学及化工专业英语词汇(O)化学及化工专业英语词汇(O)obermayer's reagent奥伯迈耶试剂object glass物镜objective color实在彩色objective function目标函数objective lens物镜obscure glass不透茫璃obscure radiation暗辐射observable可观察量observation观测observation error观测误差obsidian黑曜岩occluded gas吸留气体occlusion吸留occupational disease职业病ocean floor spreading theory海底扩张说ocher舣octadecane十八烷octadecylene十八烯octafluorocyclobutane过氟化环丁烷octahedron八面体octance value辛烷值octane辛烷octane number辛烷值octanoic acid辛酸octanol辛醇octant rule八区律octose辛糖octyl acetate乙酸辛酯octyl alcohol辛醇octyl mercaptan正辛硫醇octylene辛烯octylene oxide氧化辛烯octylic acid辛酸octyne辛炔ocular目镜ocular dichroscope接眼二色镜ocular examination目视检查法ocular micrometer目镜测微计odd even nucleus奇偶核odor气味odorant着嗅剂odorimeter气味计odorimetry气味测定法odoriphore生臭团oenometer酒度计ohmic loss电阻损失oil油oil absorbency吸油性能oil absorption吸油量oil absorptiveness吸油性能oil and fat油脂oil bath油浴oil cake豆饼oil cleaner油净化器oil coke石油焦炭oil color油溶性染料oil diffusion pump油扩散泵oil emulsion油品乳化液oil extended rubber油增塑橡胶oil field油田oil filled capacitor充油电容器oil film油膜oil firing油燃烧oil foot油渣oil gas油气oil gas tar油气焦油oil gasification油的气化oil hardening steel淬焦钢oil hydrometer油比重计oil immersion test油浸试验oil insulator油类绝缘体oil lubrication油润滑oil meal油粉oil modified resin油改性尸oil of vitriol硫酸oil paint油涂料oil printing油印法oil purifier油净化器oil putty油灰oil reactive ester resin油反应性酯尸oil reclaiming process废油再生法oil removing脱油oil resistance耐油性oil resistant rubber耐油橡胶oil separation油分离oil separator油分离器oil shale油页岩oil soluble dyes油溶染料oil soluble resin油溶尸oil stain油着色剂oil sugars油糖剂oil thief取油样器oil vapor velocity油汽速度oil varnish清油漆oiliness油性oiliness improver油性添加剂oiling涂油oily matter油状物ointment软膏old fustic黄颜木oleate油酸盐olefin烯olefin complex烯烃复体olefin polymer oil烯烃聚合油oleic acid油酸oleic acid nitrile油腈oleic alcohol油醇olein油精oleinic acid油酸oleo resinous varnish油基尸清漆oleomargarine人造奶油oleonitrile油腈oleorefractometer油折射计oleoresin含油尸oleum发烟硫酸oleyl alcohol油醇oligomer低聚物oligomeric protein低聚蛋白质oligonucleotide低核苷酸oligopeptide低聚肽oligosaccharide低聚糖olive infused oil橄榄泡制油olive oil橄榄油oliver filter连续式转鼓过滤机olivine橄榄石on line operation联机操作on off control双位置控制one bath dyeing单浴染色one component system单组分系one dimensional chromatography单向色谱one side printing单面印花法one way vision glass单向观察玻璃onethrough operation单程操作opacifier遮光剂opacity不透萌opal蛋白石opal glass乳色玻璃opalizer遮光剂open chain开链open flash point tester开方引火点试验器open hearth furnace平炉open pot开口坩埚open steam直接蒸汽open steam vulcanization直接蒸汽硫化open system开系operating method操捉法operating system控制系统operation工作操作operator算符opianic acid鸦片酸opiate鸦片制剂opium鸦片oppanol欧巴诺尔opposing reaction对抗反应opposite pole异性极optic axial angle光轴角optic axis光轴optical active polymer光学活性聚合物optical activity旋光性optical anomaly光学反常optical bleaching agent荧光增白剂optical center光中心optical density光密度optical depth光深度optical fiber光学纤维optical glass光学玻璃optical isomer旋光异构体optical isomerism光学异构性optical microscope光学显微镜optical path difference光程差optical property光学性能optical pyrometer光学高温计optical rotation旋光度optical rotatory power旋光强度optical sensitizer光学增感剂optical transfer function光学传递函数optically active substance旋光物optically functional materials光功能性材料optimal control最佳控制optimum cure最适硫化optimum temperature最适温度optimum value最佳值optimum vulcanization最适硫化optoacoustic detection method光声检测法oral contraceptive口服避孕药orange flower oil橙花油orange oil橙油orange peel oil橙皮油orange pigment橙色颜料orbital electron轨道电子orbital elements轨道要素orbital function轨道函数orbital quantum number轨道量子数orbital symmetry轨道对称orbital valence轨道原子价orcin蓄黑酚orcinol蓄黑酚order次order of perturbation微扰阶数order of phase transition相变的级order of reaction反应级数ordinary light普通光ordinary rays寻常光线ordinary sheathed explosive常规安全炸药ordinary state常态ordination number原子序ore矿石ore assaying矿石分析ore burner烧矿炉ore deposit矿床ore dressing选矿ore flotation promoter矿石浮选促进剂organic accelerator有机促进剂organic acid有机酸organic analysis有机分析organic base有机碱organic catalyst有机催化剂organic chemistry有机化学organic colloid有机胶体organic coloring matter有机色素organic compound有机化合物organic electrochemistry有机电化学organic fertilizer有机肥料organic glass有机玻璃organic group有机基organic molecular compound有机分子化合物organic peroxide有机过氧化物organic pigment有机颜料organic plastics有机塑料organic precipitant有机沉淀剂organic radical有机基organic reagent有机试药organic semiconductor有机半导体organic solvent有机溶剂organic substance有机物质organic superconductor有机超导体organism有机体organism of fermentation发酵微生物organized ferment活体酶organoalkoxysilane有机烷氧基硅烷organoaluminium compound有机铝化合物organoaluminium polymer有机铝聚合物organoboron compound有机硼化合物organogel有机凝胶organoleptic test感官试验organomagnesium compound有机镁化合物organomercurous fungicide有机汞杀菌剂organomercury compound有机汞化合物organometallic compound有机金属化合物organophosphor有机磷organosilicate有机硅酸盐organosilicon compound有机硅化合物organosol有机溶胶organotin compound有机锡化合物orientation取向orientation force定向力orientation polarization定向极化orifice遮光板orifice meter孔板量计origin of elements元素的起源original coal原煤original color原色original mold原型orlon腈纶ornamental glass装饰用玻璃ornithine鸟氨酸ornithuric acid鸟尿酸orotic acid乳清酸orpiment雄黄orsat apparatus奥萨特气体分析器orsellinic acid苷色酸orthanilic acid邻氨基苯磺酸orthite褐帘石ortho effect邻位效应ortho helium正氦ortho hydrogen正氢ortho para orientation邻对位定向ortho position邻位orthobaric density正压密度orthochem原生化学沉积orthochromatic plate定色板orthoclase正长石orthoform凹栓因orthoformic acid原甲酸orthogonal coordinates直角坐标orthogonal function正交函数orthogonal matrix正交矩阵orthogonal transformation正交变换orthonormal system标准正交系orthophosphate磷酸盐orthophosphoric acid磷酸orthophosphorous acid亚磷酸orthorhombic system正交系orthosilicate正硅酸盐osazone脎oscillation振荡oscillatory reaction振荡反应oscillogram示波图oscillograph示波器oscillographic polarography示波极谱法oscillometric titration高频滴定oscillometry示波测量术oscilloscope示波器oscillotitrator示波滴定仪osmate锇酸盐osmic acid锇酸osmiridium铱锇矿osmium锇osmium oxide氧化锇osmium sulfide硫化锇osmochemistry渗析化学osmolality克分子渗透压重量浓度osmolarity克分子渗透压浓度osmole渗透压摩尔osmometer渗压计osmophore渗泳群osmophore group发香团osmoscope渗透计osmosis渗透osmotic coefficient渗透系数osmotic pressure渗透压力osmotropism向渗性ossein骨素osteolith土磷灰石ostwald ripening奥斯特瓦尔德成熟ostwald's dilution law奥斯特瓦尔德稀释定律ostwald's viscometer奥斯特瓦尔德粘度计otto of rose蔷薇油ouabain哇巴因outer diameter外直径outer flame外焰outflow瘤outflux瘤outgas放气outlet出口outlet pressure排出压力output输出功率outside indicator外指示剂ovalbumin卵白蛋白oven炉over cure过度硫化over exposure过度照射overall coefficient of heat transfor总传热系数overall plate efficiency总塔板效率overall reaction rate总反应速度overall stability constant总稳定常数overbleaching漂白过度overcharge过度充电overcooling过冷overdevelopment过度显影overflow外溢overflow mold溢粒overflow pipe下导管overhead塔顶馏出物overheated vapour过热蒸汽overheating过热overlap integral重叠积分overoxidation过氧化overpotential超电势overproduction过度生产oversaturation过饱和overvoltage超电势ovoflavin核黄素oxadiazon恶草灵oxalacetic acid草乙酸oxalate草酸盐oxalic acid草酸oxalic nitrile氰oxaluric acid草酰酸oxalyl chloride乙二酰氯oxalylurea乙二酰脲oxamic acid草氨酸oxamide草酰胺oxanilide草酰替苯胺oxanthrone蒽酚酮oxazine dye恶嗪染料oxazole恶唑oxazoline恶唑啉oxidability可氧化性oxidant氧化剂oxidase氧化酶oxidation氧化oxidation bleaching氧化漂白oxidation capacity氧化能力oxidation catalyst氧化催化剂oxidation color氧化染料oxidation inhibitor抗氧化剂oxidation number氧化值oxidation of coal碳氧化oxidation polymerization氧化聚合oxidation potential氧化电势oxidation reduction氧化还原oxidation reduction cell氧化还原电池oxidation reduction electrode氧化还原电极oxidation reduction indicator氧化还原指示剂oxidation reduction potential氧化还原电位oxidation reduction reaction氧化还原反应oxidation reduction system氧化还原系统oxidation reduction titration氧化还原滴定oxidation resistance耐氧化性oxidation rinsing氧化洗涤oxidation stage氧化阶段oxidation style氧化法oxidation test氧化试验oxidation wave氧化波oxidation zone氧化区oxidative condensation氧化缩合oxidative degradation氧化降解oxidative ferment氧化酶oxidative phosphorylation氧化磷酸化oxide氧化物oxide cathode氧化物阴极oxide salt氧化物盐oxidimetry氧化还原滴定法oxidized form氧化型oxidized starch氧化淀粉oxidizing氧化oxidizing agent氧化剂oxidizing enzyme氧化酶oxidizing flame氧化焰oxidizing reagent氧化剂oxidizing roasting氧化焙烧oxidoreductase氧化还原酶oxime肟oximetry测氧法oxindol羟吲哚oxine喔星oxirane氧杂环丙烷oxo compound氧基化合物oxo synthesis羰基合成法oxonium base氧碱oxonium compound氧化合物oxonium ion水合氢离子oxonium salt氧盐oxozone双氧气oxy acid含氧酸oxyacetylation氧氯净化oxyacetylene flame氧乙炔焰oxyazo color氧化叠氨色素oxybenzone氧苯酮oxybromide溴氧化物oxycalorimeter氧量热计oxycarboxin氧化萎锈灵oxycellulose氧化纤维素oxychloride cement氯氧化水泥oxycompound氧基化合物oxygen氧oxygen bomb氧气瓶oxygen bomb test氧气瓶试验oxygen bonding properties氧结合性能oxygen carrier载氧体oxygen convertible alkyd resin氧化型醇酸尸oxygen convertible phthalic resin氧化型苯二甲酸尸oxygen enriched air富氧空气oxygen flask method氧瓶法oxygen hydrogen cell氧氢电池oxygen inhalator氧吸入器oxygen number氧价oxygen permeable membrane富氧膜oxygen point氧点oxygen pole氧极oxygenase氧合酶oxyhemoglobin氧合血红蛋白oxyhydrogen blowpipe氢氧气吹管oxyhydrogen flame氢氧火焰oxyhydrogen light氢氧爆气光oxyhydrogen welding氢氧焊接oxyliquit液氧炸药oxymeter氧气计oxysalt含氧盐oxytetracycline氧四环素oxytocin氧毒素ozalid熏晒图ozalid paper氨熏晒图纸ozocerite天然地蜡ozokerite木炭ozonation臭氧化ozone臭氧ozone bleaching臭氧漂白ozone generator臭氧发生器ozonide臭氧化物ozonizer臭氧发生器ozonolysis臭氧分解ozonometer臭氧计ozonometry臭氧测定术ozonoscope臭氧测量仪化学及化工专业英语词汇(O) 相关内容:。
有机化学专业英语中英文对照

有机化学专业英语中英文对照第一章无机化学英文命名1.1元素的英文命名S-block ElementIAH Hydrogen Li Lithium Na Sodium K PotassiumRb Rubidium Cs Cesium Fr FranciumIIABe Beryllium Mg Magnesium Ca Calcium Sr Strontium Ba Barium Ra RadiumP-block ElementIIIA IV A V AB BoronC Carbon N NitrogenAl Aluminium Si Silicon P PhosphorusGa Gallium Ge Germanium As ArsenicIn Indium Sn Tin Sb AntimonyTl Thallium Pb Lead Bi BismuthVIA VIIA0He Helium O Oxygen F Fluorine Ne NeonS Sulfur Cl Chlorine Ar ArgonSe Selenium Br Bromine Kr KryptonTe Tellurium I Iodine Xe XenonPo Polonium At Astatine Rn RadonCommon Transition ElememtFe:iron Mn:manganese Cu:copper Zn:zincHg:mercury Ag:silver Au:gold1.2离子、氧化物、酸、碱、盐及其他化合物的英文命名Na+Sodium Al3+AluminumK+Potassium Ca2+CalciumFe2+Iron(II)or FerrousFe3+Iron(III)or FerricCr2+Chromium(II)Cr3+Chromium(III)Mn4+Manganese(IV)Mn2+Manganese(II)FeO:iron(II)oxide或ferrous oxideFe2O3:iron(III)oxide或ferric oxideCu2O:copper(I)oxide或cuprous oxideCuO:copper(II)oxide或cupric oxideCl-Chloride O=OxideBr-Bromide OH-HydroxideI-Iodide CN-CyanideS=Sulfide H-Hydride HF hydrogen fluoride HCl hydrogen chloride HBr hydrogen bromide HI hydrogen iodideH2S hydrogen sulfide H2Se hydrogen selenideH2Te hydrogen telluridePH3:phosphine或phosphane AsH3:arsine或arsaneSbH3:stibine或stibane BiH3:bismuthaneCH4:methane SiH4:silaneB2H6:diboraneHCl:hydrochloric acidH2S:hydrosulfuric acidAl(OH)3Aluminum hydroxideNaOH Sodium hydroxideCa(OH)2Calcium hydroxideBa(OH)2Barium hydroxideCo(OH)2Cobalt(II)hydroxideHgSO4Mercury(II)sulfateHg2SO4Mercury(I)sulfateKNO3Potassium nitrateNa2CO3Sodium carbonateNaClO Sodium hypochloriteFeSO4iron(II)sulfateKMnO4potassium permanganateNaHSO4Sodium hydrogen sulfateNa2HPO4Disodium hydrogen phosphateNaH2PO4Sodium dihydrogen phosphateCa(HSO4)2Calcium bisulfateNaHCO3Sodium hydrogencarbonate或Sodium bicarbonateAlCl3·6H2O:aluminum chloride6-water或aluminum chloride hexahydrateAlK(SO4)2·12H2O:aluminium potassium sulfate12-waterH2CO3Carbonic acidH2SO4Sulfuric acidH3PO4Phosphoric acidHNO3Nitric acidHClO4Perchloric acidHCl Hydrochloric acidH2SO3Sulfurous acidH3PO3Phosphorous acidHNO2Nitrous acidHClO Hypochlorous acidHClO2Chlorous acidNaming coordination complexLigandsCN-Cyano NO2-NitroF-Fluoro NO3-NitratoCl-Chloro CO3=CarbonatoBr-Bromo CH3COO-AcetatoO=Oxo H-HydridoOH-Hydroxo-O2CCO2-Oxalato1.3配位化学中常见配体的名称1.4常用无机化学专业英语词汇相对原子质量relative atomic mass消去反应elimination reaction硝化反应nitratlon reaction硝酸钡barium nitrate硝酸银silver nitrate溴的四氯化碳溶液solution of bromine in carbon tetrachloride溴化钠sodium bromide溴水bromine water溴水bromine water盐类的水解hydrolysis of salts盐析salting-out焰色反应flame test氧化剂oxidizing agent氧化铝aluminium oxide氧化铁iron(III)oxide 乙醇ethanol乙醛ethana1乙炔ethyne乙酸ethanoic acid乙酸乙酯ethyl acetate乙烯ethene银镜反应silver mirror reaction硬脂酸stearic acid油脂oils and fats有机化合物organic compound元素周期表periodic table of elements 元素周期律periodic law of elements 原电池primary battery原子序数atomic number皂化反应saponification粘合剂adhesive蔗糖sucrose指示剂Indicator酯ester酯化反应esterification周期period族group(主族:main group)Bunsen burner本生灯product化学反应产物flask烧瓶apparatus设备PH indicator PH值指示剂,氢离子(浓度的)负指数指示剂matrass卵形瓶litmus石蕊litmus paper石蕊试纸graduate,graduated flask量筒,量杯reagent试剂test tube试管burette滴定管retort曲颈甑still蒸馏釜cupel烤钵crucible pot,melting pot坩埚pipette吸液管filter滤管stirring rod搅拌棒element元素body物体compound化合物atom原子gram atom克原子atomic weight原子量atomic number原子数atomic mass原子质量molecule分子electrolyte电解质ion离子anion阴离子cation阳离子electron电子isotope同位素isomer同分异物现象polymer聚合物symbol复合radical基structural formula分子式valence,valency价monovalent单价bivalent二价halogen成盐元素bond原子的聚合mixture混合combination合成作用compound合成物alloy合金organic chemistry有机化学inorganic chemistry无机化学derivative衍生物series系列acid酸hydrochloric acid盐酸sulphuric acid硫酸nitric acid硝酸aqua fortis王水fatty acid脂肪酸organic acid有机酸hydrosulphuric acid 氢硫酸hydrogen sulfide氢化硫alkali碱,强碱ammonia氨base碱hydrate水合物hydroxide氢氧化物,羟化物hydracid氢酸hydrocarbon碳氢化合物,羟anhydride酐alkaloid生物碱aldehyde醛oxide氧化物phosphate磷酸盐acetate醋酸盐methane甲烷,沼气butane丁烷salt盐potassium carbonate碳酸钾soda苏打sodium carbonate碳酸钠caustic potash苛性钾caustic soda苛性钠ester酯gel凝胶体analysis分解fractionation分馏endothermic reaction吸热反应exothermic reaction放热反应precipitation沉淀to precipitate沉淀to distil,to distill蒸馏distillation蒸馏to calcine煅烧to oxidize氧化alkalinization碱化to oxygenate,to oxidize脱氧,氧化to neutralize中和to hydrogenate氢化to hydrate水合,水化to dehydrate脱水fermentation发酵solution溶解combustion燃烧fusion,melting熔解alkalinity碱性isomerism,isomery同分异物现象hydrolysis水解electrolysis电解electrode电极anode阳极,正极cathode阴极,负极catalyst催化剂catalysis催化作用oxidization,oxidation氧化reducer还原剂dissolution分解synthesis合成reversible可逆的摩尔mole摩尔质量molar mass品红magenta或fuchsine葡萄糖glucose气体摩尔体积molar volume of gas 铅蓄电池lead storage battery强电解质strong electrolyte氢氟酸hydrogen chloride氢氧化铝aluminium hydroxide取代反应substitution reaction醛aldehyde炔烃alkyne燃料电池fuel cell弱电解质weak electrolyte石油Petroleum水解反应hydrolysis reaction四氯化碳carbon tetrachloride塑料plastic塑料的降解plastic degradation塑料的老化plastic ageing酸碱中和滴定acid-base neutralization titration酸雨acid rain羧酸carboxylic acid碳酸钠sodium carbonate碳酸氢铵ammonium bicarbonate碳酸氢钠sodium bicarbonate糖类carbohydrate烃hydrocarbon烃的衍生物derivative of hydrocarbon烃基hydrocarbonyl同分异构体isomer同素异形体allotrope同位素isotope同系物homo1og涂料coating烷烃alkane物质的量amount of substance物质的量浓度amount-of-substance concentration of B烯烃alkene洗涤剂detergent纤维素cellulose相对分子质量relative molecular mass 极性键polar bond加成反应addition reaction加聚反应addition polymerization甲烷methane碱金属alkali metal碱石灰soda lime结构式structural formula聚合反应po1ymerization可逆反应reversible reaction空气污染指数air pollution index勒夏特列原理Le Chatelier's principle离子反应ionic reaction离子方程式ionic equation离子键ionic bond锂电池lithium cell两性氢氧化物amphoteric hydroxide两性氧化物amphoteric oxide裂化cracking裂解pyrolysis硫氰化钾potassium thiocyanate硫酸钠sodium sulphide氯化铵ammonium chloride氯化钡barium chloride氯化钾potassium chloride氯化铝aluminium chloride氯化镁magnesium chloride氯化氢hydrogen chloride氯化铁iron(III)chloride氯水chlorine water麦芽糖maltose煤coal酶enzyme二氧化氮nitrogen dioxide二氧化硅silicon dioxide二氧化硫sulphur dioxide二氧化锰manganese dioxide芳香烃arene放热反应exothermic reaction非极性分子non-polar molecule非极性键non-polar bond肥皂soap分馏fractional distillation酚phenol复合材料composite干电池dry cell干馏dry distillation甘油glycerol高分子化合物polymer共价键covalent bond官能团functional group光化学烟雾photochemical fog过氧化氢hydrogen peroxide合成材料synthetic material合成纤维synthetic fiber合成橡胶synthetic rubber核电荷数nuclear charge number核素nuclide化学电源chemical power source化学反应速率chemical reaction rate氨ammonia氨基酸amino acid铵盐ammonium salt饱和链烃saturated aliphatic hydrocarbon苯benzene变性denaturation不饱和烃unsaturated hydrocarbon超导材料superconductive material臭氧ozone醇alcohol次氯酸钾potassium hypochlorite醋酸钠sodium acetate蛋白质protein氮族元素nitrogen group element碘化钾potassium iodide碘化钠sodium iodide电化学腐蚀electrochemical corrosion电解质electrolyte电离平衡ionization equilibrium电子云electron cloud淀粉starch淀粉碘化钾试纸starch potassium iodide paper化学键chemical bond化学平衡chemical equilibrium 还原剂reducing agent磺化反应sulfonation reaction霍尔槽Hull Cell极性分子polar molecule 2.5常用无机元素英文Actinium(Ac)锕Aluminium(Al)铝Americium(Am)镅Antimony(Sb)锑Argon(Ar)氩Arsenic(As)砷Astatine(At)砹Barium(Ba)钡Berkelium(Bk)锫Beryllium(Be)铍Bismuth(Bi)铋Boron(B)硼Bromine(Br)溴Cadmium(Cd)镉Caesium(Cs)铯Calcium(Ca)钙Californium(Cf)锎Carbon(C)碳Cerium(Ce)铈Chlorine(Cl)氯Chromium(Cr)铬Cobalt(Co)钴Copper(Cu)铜Curium(Cm)锔Dysprosium(Dy)镝Einsteinium(Es)锿Erbium(Er)铒Europium(Eu)铕Fermium(Fm)镄Fluorine(F)氟Francium(Fr)钫Gadolinium(Gd)钆Gallium(Ga)镓Germanium(Ge)锗Gold(Au)金Hafnium(Hf)铪Helium(He)氦Holmium(Ho)钬Hydrogen(H)氢Indium(In)铟Iodine(I)碘Iridium(Ir)铱Iron(Fe)铁Krypton(Kr)氪Lanthanum(La)镧Lawrencium(Lr)铹Lead(Pb)铅Lithium(Li)锂Lutetium(Lu)镥Magnesium(Mg)镁Manganese(Mn)锰Mendelevium(Md)钔Mercury(Hg)汞Molybdenum(Mo)钼Neodymium(Nd)钕Neon(Ne)氖Neptunium(Np)镎Nickel(Ni)镍Niobium(Nb)铌Nitrogen(N)氮Nobelium(No)锘Osmium(Os)锇Oxygen(O)氧Palladium(Pd)钯Phosphorus(P)磷Platinum(Pt)铂Plutonium(Pu)钚Polonium(Po)钋Potassium(K)钾Praseodymium(Pr)镨Promethium(Pm)钷Protactinium(Pa)镤Radium(Ra)镭Radon(Rn)氡Rhenium(Re)铼Rhodium(Rh)铑Rubidium(Rb)铷Ruthenium(Ru)钌Samarium(Sm)钐Scandium(Sc)钪Selenium(Se)硒Silicon(Si)硅Silver(Ag)银Sodium(Na)钠Strontium(Sr)锶Sulphur(S)锍Tantalum(Ta)钽Technetium(Tc)锝Tellurium(Te)碲Terbium(Tb)铽Thallium(Tl)铊Thorium(Th)钍Tin(Sn)锡Thulium(Tm)铥Titanium(Ti)钛Tungsten(W)钨Uranium(U)铀Vanadium(V)钒Xenon(Xe)氙Ytterbium(Yb)镱Yttrium(Y)钇Zinc(Zn)锌Zirconium(Zr)锆第二章有机化学英文命名2.1有机化学系统命名中主要官能团的优先顺序1.鎓盐(onium and similar cations)2.酸(acids):按COOH、(C)OOH次序,然后是它们的S和Se的衍生物,再次为磺酸、亚磺酸等。
【有机化学专业英语词汇】
v1.0可编辑可修改有机化学专业英语词汇(精)acetal 醛缩醇acetal- 乙酰acid 酸-al 醛alcohol 醇-aldehyde 醛alkali- 碱allyl 烯丙基[prope nyl(丙烯基)]alkoxy- 烷氧基-amide 酰胺amino- 氨基的-amidine 脒-amine 胺-ane 烷an hydride 酐an ili no- 苯胺基bromo-溴butyl 丁基-carb inol 甲醇carb onyl 羰基-carboxylic acid 羧酸centi- 10 -2chloro-氯代cis- 顺式con de nsed缩合的、冷凝的cyclo-环deca-十deci 10 -1-di ne 啶dodeca-十二aquo-含水的-ase 酶-ate含氧酸的盐、酯-atri yne 三炔azo-偶氮benzene 苯bi-在盐类前表示酸式盐bis-双-borane 硼烷-ene 烯epi-表epoxy- 环氧-ester 酯-ether 醚ethoxy- 乙氧基ethyl乙基fluoro-氟代form 仿v1.0可编辑可修改-lact one 内酯-ide无氧酸的盐,酰替胺,酐-il 偶酰octa-八-imi ne 亚胺iodi ne 碘iodo-碘代iso- 异,等,同-ite 亚酸盐keto-酮ketone 酮octadeca-十八-oic酸的-ol醇-one 酮ortho- 邻,正,原-ous亚酸的,低价金属oxa-氧杂-oxide氧化合物-oxime 肟hemi-半hen deca- 十一hepta-七heptadeca-十七hexa-六hexadeca-十六-hydri n 醇hydro-氢或水hydroxyl 羟基hypo-低级的,次hyper-高级的,高mega- 106 meta-间,偏methoxy-甲氧基methyl 甲基micro- 10 -6milli- 10 -3mono- ( mon-) 一,单-ic酸的,高价金属nano- 10 一n itro- 硝基n itroso- 亚硝基nona-九nonadeca-十九-glycol 二醇v1.0可编辑可修改covale nt bond 共价键hybrid orbital 杂化轨道bond len gth 键长oxo-酮 tetrakis- 四个oxy-氧化thio- 硫代-oyl 酰 trans- 反式,超,跨para- 对位,仲 tri-三 penta-五 trideca- 十三 pentadeca-十五tris-三个per- 高,过petro- 石油 undeca- 十^一- phenol 苯酚 uni-单,一phenyl 苯基un sym-不对称的,偏位-12pico- 10poly-聚,多 -yl 基quadri- 四 -yne 炔quinque-五organic compounds 有机化合物semi-半 compo unds of carb on 碳化合物 septi- 七 hydrocarbons and their derivatives sesqui 一个半 碳氢化合物及其衍生物 sulfa- 磺胺 orga nic chemistry有机化学sym- 对称structure of molecule 分子结构syn- 顺式,同,共chemical bond 化学键 ter- tetra-tetradeca- 十四bond angle 键角thio- 硫代-ylene 撑(二价基,价在不同原子上)v1.0可编辑可修改bond energy 键能hydroge n bonding 氢键polarity 极性dipole moment 偶极矩dissociati on en ergy 离解能electr on egativity 电负性con stituti on 构造physical property 物理性质con tigurati on 构型melt ing point 熔点conformation 构象boili ng point 沸点stereochemistry 立体化学react ion mecha nism 反应机理tetrahedral 正四面体homolysis 均裂cis-顺free redical 自由基trans-反heterolysis 异裂isomerism同分异构现象ion ic type 离子型isomer异构体electrophilic reage nt 亲电试剂stereoisomer 立体异构electrophilic react ion 亲电反应con stituti onal isomer 构造异构nu cleophilic reage nt 亲核试剂structural formula 结构式nu cleophilic react ion 亲核反应octet八隅体英文名汉文名perspective 透视式An gular methyl group 角甲基eclipsed con formati on 重叠式构象Alkylide ne group 亚烷基staggered con formati on 交叉式构象Allyl group 烯丙基n ewma n project ion 纽曼投影式Allylic 烯丙型[的]functional group 官能团Phe nyl group 苯基chain compoud链状化合物Aryl group 芳基carbocyclic compo und碳环化合物Ben zyl group 苄基彳heterocyclic compo und 杂环化合物Benzylic 苄型[的]dipole-dipole in teract偶极- Activat ing group 活化基团ions偶极相互作用Chromophore 生色团van der Waals forces 范德华力Auxochrome 助色团v1.0可编辑可修改Magn etically ani sotropic group 磁各向异性基团Smally ring 小环Com mon ring 普通环Medium rimg 中环Large ring 大环Bridged-ri ng system 桥环体系Spiro compound螺环化合物Helical molecule 螺旋型分子Octahedral compound八面体化合物Con jugatio n 共轭Con jugated-system 共轭体系Acyl cation 酰[基]正离子Benzylic cation 苄[基]正离子Arenirm ion 芳[基]正离子Ketyl radical 羰自由基Radical ion 自由基离子Radical catio n 自由基正离子Radical a nio n 自由基负离子Isomerism 异构[现象]Aci form 酸式Fluxi onal structure 循变结构Stereochemistry 立体化学Optical activity 光学活性Dextro isomer 右旋异构体Laevo isomer 左旋异构体Tetrahedral con figuratio n 四面体构型Stereoisomerism 立体异构[现象]Asymmetric atom 不对称原子Asymmetric carb on 不对称碳Pseudoasymmetric carb on 假不对称碳Phantom atom虚拟原子Homotopic 等位[的]Heterotopic 异位[的]Enantiotopic 对映异位[的]Diastereotopic 非对映异位[的]Con figurati on 构型Absolute con figurati on 绝对构型Chirality 手性Chiral手性[的]Chiral ce nter 手性中心Chiral molecule 手性分子Achiral非手性[的]Fischer projectio n 费歇尔投影式Neoma n projectio n 纽曼投影式D-L system of nomen clature D-L 命名体系v1.0可编辑可修改R-S syytem of nomen clature R-S 命名体系Cahn-Ingold-Prelon sequence 顺序规则v1.0可编辑可修改Symmetry factor 对称因素Pla ne of symmetry 对称面Mirror symmetry 镜面对称Enantiomer对映[异构]体Diastereomer 非对映[异构]体Epimer差向异构体Anomer端基[差向]异构体Erythro con figurati on 赤型构型Erythro isomer 赤型异构体Threo con figurati on 苏型构型Threo isomer 苏型异构体Trigonal carb on 三角型碳Cis-tra ns isomerism 顺反异构E isomer E 异构体Z isomer Z 异构体Endo isomer内型异构体Exo isomer外型异构体Prochirality 前手性Pro-R group 前R基团Pro-S proup 前S 基团Re face Re 面Si face Si 面Racemic mixture 外消旋混合物Racemic compo und外消旋化合物Racemic solid solutio n 外消旋固体溶液Meso compou nd内消旋化合物Quasi recemate 准外消旋体Con formati on 构象Con formati onal 构象分析Torsi on an gle 扭转角Rotamer旋转异构体Anti con formati on 反式构象Bisect ing con formatio n 等分构象Anti peripla nar con formati on 反叠构象Syn peripla nar con formati on 顺叠构象Syn cli nal con formatio n 反错构象Syn cli nal con formatio n 顺错构象Eclipsed con formati on 重叠构象Gauche con formati on, skew con-formatio n 邻位交叉构象Staggered con formati on 对位交叉构象Steric effect 空间效应Steric hindrance 位阻Atropismer 阻转异构体v1.0可编辑可修改Puckered ring 折叠环Con formati onal inv ersi on 构象反转Chair con formati on 椅型构象Boat con formati on 船型构象Twist con formati on 扭型构象Skew boat co nformatio n 扭船型构象v1.0可编辑可修改Half-chair con formatio n 半椅型构象Pseudorotati on 假旋转Envelope conformation 信圭寸[型]构象Axial bond 直[立]键Equatorial bond 平[伏]键Cisoid con formati on 顺向构象Tran soid con formati on 反向构象Retention of configuration 构型保持Regioselectivity 区域选择性Regiospecificity 区域专-性Stereocelectivity 立体选择性Stereospecificty 立体专性Con former构象异构体Con formati onal effect 构象效应Cram s rube克拉姆规则Prelog ' rule普雷洛格规则Stereochemical orie ntati on 立体[化学]取向Con formati onal tran smissi on传递Homolog同系物构象Ipso positi on 本位Ortho positi on 邻位Meta positi on 间位Para positi on 对位Amphi positi on 远位Peri positi on 近位Trigo nal hybridizati on 三角杂化Molecular orbiral method 分子轨道法Vale nee bond method 价键法Delocalezed bond 离域键Cross con jugation交叉共轭Viny log插烯物Mesomeric effect 中介效应Resonance 共振Resonance effect 共振效应Hyperconjugati on 超共轭Isovale nt hyperc on jugati on 等价超共轭No-bond resonance 无键共振Aromaticity 芳香性Aromatic sexter 方香八隅Huckel' rule休克尔规则Paramag netic ring curre nt 顺磁环电流Diamag netic ring cruue nt 抗磁环电流Homoaromaticity 同芳香性An tiaromaticity 反芳香性Alter nant hydrocarb on 交替烃v1.0可编辑可修改Non-alter nant hydrocarb on 非交替烷Pericyclic reaction 周环反应Electrocyclic rearra ngeme nt 电环[化]重排Con rotatory 顺旋Disroatatory 对旋Cycloadditio n 环加成Symmetry forbidde n-reactio n 对称禁阻反应Syn facial reacti on 同面反应An tarafacial reacti on 异面反应Mobius system默比乌斯体系Leois structure 路易斯结构Coord in ate-covale nt bond 配位共价键Banana bond 香蕉键Pauli ng electro negativity scale鲍林电负性标度Polarizability 可极化性In ductive effect 诱导效应Field effect 场效应Electrical effect 电场效应tautomerism 互变异构Tautomerizati on 互变异构化Keto-e nol tautomerism 酮-烯醇互变异构Phe no l-keto tautomerism 酚-酮互变异构Imin e-e namine atutomerism 亚胺-烯胺互变异构Rin g-cha in tautomerism 环-链互变异构Vale nee tautomerism 价互变异构Ambident 两可[的]Solve nt effect 溶剂效应Acid-base catalyxed react ion 酸性溶剂Basic solve nt 碱性溶剂Dielectric con sta nt 介电常数Solvated electro n 溶剂化电子Acid-base catalyzed reaction 酸碱催化反应Con jugate base 共轭酸Con jugate base 共轭碱Therm odyn amic acidity 热力学酸度Kin etic acidity 动力学酸度Electr on dono f-acceptorcomplex,EDAcomplex 电子给[体]受体络合物Host主体Guest客体Primary isotope effect 一级同位素效应Secon dary isotope effect 二级同位数效应Inv erse isotope effect 逆同位素效应Kin etic control 动力学控制Thermod yn amic con trol 热力学控制9Substrate 底物In termediate 中间体Reactive in termediate 活泼中间体Microscopic reversibility 微观可逆性Hammond postulate 哈蒙德假说Lin ear free energy 线性自由能Non-bon ded in teracti on 非键相互作用Torsional effect 扭转效应Pitzer strain 皮策张力Restricted rotati on 阻碍旋转Eclipsi ng effect 重叠效应Eclipsi ng strain 重叠张力Small-a ngle strain 小角张力Large an gle stra in 大角张力Transannu lar in teracti on 跨环相互作用Transannu lar strain跨环张力I strain 内张力F strain 前张力B strain 后张力Ano meric effect 端基异构效应Walde n inv ersi on 瓦尔登反转Racemizati on 夕卜消旋化Isoinv ersi on 等反转Isoracemizati on 等消旋Homochiral纯手性[的]Mecha nism 机理Un imolecular nu cleophilic 单分子亲核取代Bimolecular nu cleophilic sub-stitutio n双分子亲核取代Bimolecular nu cleophilicsubsti-tuti on( with allylic rearra nge-me nt) 双分子亲核取代(含烯丙型重排)Internal nu cleophilic substiru-tio n 分子内亲核取代Aromatic nu cleophilic substitu-tio n 芳香亲核取代Un imolecular electrophilic sub-stitution单分子亲电取代Bimolecular electrophilic substi-tutio n双分子亲电取代Nucleophile-assisted unim olecu-lar electrophilicv1.0可编辑可修改substituti on 亲核体协助单分子亲电取代Unim olecular elim in ati on 单分子消除Bimolecular elim in ati on 双分子消除Unim olecular elim in ati on through the con jugate base 单分子共轭碱消除Bimolecular elim in atio n through the con jugate base 双分子共轭碱消除Bimolecular elim in atio n withfor-matio n of a carb onyl group 双分子羰基形成消除Unim olecular acid-catalyzed acyl-oxyge n cleavage 单分子酸催化酰氧断裂Bimolecular base-catalyzed acyl-oxyge n cleavage 双分子碱催化酰氧断裂Unim olecular acid-catalyzed alkyl-oxyge n cleavage 单分子酸催化烷氧断裂Bimllecular base-catalyzed alkyl-oxyge n cleavage 双分子碱催化烷氧断裂n - allyl complex mechanism n 烯丙型络合机理Borderl ine mecha nism 边理机理Homolysis 均裂Heterolysis 异裂Heterolytic micha nism 异裂机理Coun rer[gege n]i on 反荷离子Ion pair 离子对Carbocati on 碳正离子Non classical carbocatio n 非经典碳正离子Carbanion碳负离子Masked carbanion掩蔽碳负离子Carbenoid卡宾体Carbe ne 卡宾Nitre ne 氮宾Carbine 碳炔Electrophilic addition 亲电加成Electrophile 亲电体Diaxial addition 双直键加成Markovnikov ' s rube马尔科夫尼科规则An ti-Marko vn ikov additi on 反马氏v1.0可编辑可修改加成Michael addition 迈克尔加成Substituti on 取代v1.0可编辑可修改Electrophilic substituti on 亲电取代Additi on-elim in ati on mecha nism 力卩成消除机理Electrophilic aromaticsubstitu-ti on 亲电芳香取代Electro n tran sfer 电子转移Electro n-do nati ng group 给电子基团Electro n-Withdrawi ng group 吸电子基团Deactivati ng group 卡屯化基团Orinen tati on 取向Ortho-para direct ing group 令B对位定位基Meta direct ing group 间位定位基Ortho effect 邻位效应Partial rate factor 分速度系数Nucleophilic reacti on 亲核反应In ternal return 内返Nucleophilicity 亲核体Nucleophilicity 亲核性a - effect a -效应Backside attack 背面进攻Inv ersi on 反转Umbrella effect 伞效应Push-pull effect 推拉效应Leav ing group 离去基团Electrofuge 离电体Nucleofuge 离核体Phase-tra nsfer catalysis 相转移催化Neighbori ng group participati on邻基基参与Neighbori ng proupassista nce,a nchimeric assista nee邻助作用Neighbori ng group effect 令B基效应Apofacial reacti on 反面反应Briddgehead displaceme nt 桥头取代Aryl action 芳正离子Benzyne 苯炔Zaitsev rule 札依采夫规则An ti-Zaitsev orie ntati on 反札依采夫定向Hofmann s rule 霍夫曼规贝UBredt rule 布雷特规则In itiatio n 引发Anionic cleavage 负离子裂解Partial bo nd fixation 键[的]部分固定化v1.0可编辑可修改02. 3有机化学反应Alkylation 烷基化C- alkylatio n C-烷基化v1.0可编辑可修改O- alkylatio n O- 烷基化N-alkylation N-烷基化Silylation 硅烷[基]化Exhaustive methylatio n 彻底甲基化Seco alkylatio n 断裂烷基化Demethylatio n 脱甲基化Ethylation 乙基化Arylation 芳基化Acylation 酰化Formylation 甲酰化Carbalkoxylatio n 烷氧羰基化Carboamidati on 氨羰基化Carboxylation 羧基化Amination 氨基化Bisam in ati on 双氨基化Cine substitutio n 移位取代Tran sami natio n 氨基交换Hydroxylati on 羟基化acyloxyati on 酰氧基化Decarboxylative n itrati on 脱羧卤化Allylic haloge nation 烯丙型卤化Dehaloge nati on 脱卤Nitration 硝化Decarboxylative n itrati on 脱羧硝化Nitrosatio n 亚硝化Sulfo natio n 磺化Chlorosulfo natio n 氯磺酰化Desulfo nati on 脱磺酸基Sulfe nylation 亚磺酰化Sulfo nylation 磺酰化Chlorosulfe natio n 氯亚磺酰化Chlorocarb on ylatio n 氯羰基化Diazotizati on 重氮化Diazo tran sfer 重氮基转移Coupli ng reaction 偶联反应Diaz on ium coupli ng 重氮偶联Cross-coupli ng reaction 交叉偶联反应1,4-addition 1 ,4-加成Conjugate addition 共轭加成Dimerizatio n 二聚Trimefizati on 三聚Additive dimerizati on 力卩成二聚sulfurizati on 硫化Sele ny lati on 硒化Hydroborati on 硼氢化Oxyam in ation羟氨基化In serti on 插入v1.0可编辑可修改carb ony lati on 羧基化Hydroformylatio n 加氢甲酰基化Hydroacylati on 加氢酰化Oxo process 羰基合成v1.0可编辑可修改Decarb ony lati on 脱羰Hydrocarboxylati on 氢羧基化Homologizati on 同系化Cyan oethylation 氰乙基化Decya no ethylati on 脱氰乙基Ri ng clsure 环合Die ne syn thesis 双烯合成Die nophile 亲双烯体Endo addition 内型加成Exo addition 外型加成Diels-Alder reactio n 第尔斯-尔德反应Retro Diels-Alder reaction 逆第尔斯-阿尔德反应Ene syn thesis 单烯合成Anionic cycloadditi on 负离子环力卩成Dipolar addition 偶极加成-elim in ati on - 消除-elim in ati on - 消除-elim in ati on - 消除-elim in ati on - 消除Dehydrohaloge nati on 脱卤化氢Deamination 脱氨基Pyrolytic elim in ati on 热解消除Elimi natio n-addition 消除-加成Decarboxylati on 脱羧Decarboxamidati on 脱酰胺Decyanation 脱氰基Alkylolysis,alkyl cleavage 烷基裂解Acylolysis,acyl cleavage 酰基裂解Flash pyrolysis 闪热裂Fragme ntati on 碎裂Chiletropic react ion 螯键反应Chelation 螯环化Esterificati on 酉旨化Tran sesterificatio n 酯交换Sapon ificati on 皂化Alcoholysis 醇解Etha nolysis 乙醇解Cyano methylati on 氰甲基化Ami no methylatio n 氨甲基化Hydroxymethylatio n 羟甲基化Hydroxyalkylatio n 羟烷基化Cholromethylatio n 氯甲基化Haloalkylatio n 卤烷基化Tran sacetalati on 缩醛交换En olization 烯醇化Haloform reaction 卤仿反应Condensation 缩合Aldol c onden satio n 羟醛缩合Cross aldol conden sati on 交叉羟醛缩合Retrograde aldol conden sati on 逆羟醛缩合Acyl oin conden sati on 偶姻缩合Cyclizati on 环化Annu lati on,ann elati on 增环反应Spiroa nnu latio n 螺增环Autoxidati on 自氧化Allylic hydroperoxylati on 烯丙型氢过氧化Epoxidati on 环氧化Oxono lysis 臭氧解Electrochemical oxidati on 电化学氧化Oxidative decarboxylati on 氧化脱羧Aromatizati on 芳构化Catalytic hydroge nati on 催化氢化Heteroge neous hydroge nati on 多相氢化Homogeneous hydrogenation 均相氢化Catalytic dehydroge natio n 催化脱氢Transfer hydroge nati on 转移氢化Hydroge no lysis 氢解Dissolvi ng metal reduct ion 溶解金属还原Sin gle electro n transfer 单电子转移Bimolecular reducti on 双分子还原Electrochemical reduction 电化学还原Reductive alkylatio n 还原烷基化Reductive acylati on 还原酰化Reductive dimerizatio n 还原二聚Deoxygenation 脱氧Desulfurizati on 脱硫Desele ni zati on 脱硒Mitallati on 金属化Lithiation 锂化Hydrometallati on 氢金属化Mercurati on 汞化Oxymercurati on 羟汞化Ami nomercuration 氨汞化Abstractio n 夺取[反应]In ternal abstract ion 内夺取[反应]Rearrangement 重排Prototropic rearra ngeme nt 质了转移重排Double bond migrati on 双键移位Allylic migrati on 烯丙型重排Allylic migrati on 烯丙型迁移Ring contraction 环缩小[反应]Ring expa nsion,ring enl argeme nt Electrophilic rearra ngeme nt 亲电扩环[反应]重排-ketol rearra ngeme nt - 酮醇重排Photose nsitizatio n 光敏化Pin acol rearra ngeme nt 频哪醇重排Forbidde n tran siti on 禁阻跃迁Retrop in acol rearra ngeme nt 逆频哪photooxidati on 光氧化醇重排Photoisomerizati on 光异构化Semip in acol rearra ngeme nt 半频哪E Photochemical rearra ngeme光化nt醇重排学重排Ben zilic rearra ngeme nt 二苯乙醇酸2. 4有机化合物类名重排Aliphatic compou nd 脂肪族化合物Acyl rearran geme nt 酰基重排Hpdrocarbon碳氢化合物Migratory aptitude 迁移倾向Alkane 烷Transannu lar in serti on 跨环插入Wax蜡Transannu lar rearra ngeme nt 跨环重Paraffi n wax 石蜡排Alkene 烯Migrati on 迁移Alkyen 炔Prototropy 质子转移Acetylide 炔化物Cati ono tropic rearra ngeme nt 正离Active hydroge n compo unds 活泼氢子转移重排化合物An io notropy 负离子转移Carbon acid 碳氢酸Aniono tropic rearra ngeme nt 负离子Super acid 超酸转移重排Diene双烯Sigmatropic rearra ngeme nt - 迁移重Triene 三烯排Alle ne 丙二烯Homosigmatropic rearra ngeme nt 同Ccumule ne累积多烯迁移重排Enyne烯炔Diyne 二炔v1.0可编辑可修改Alkyl halide 卤代烷Alcohol 醇Homoallylic alcohol 高烯丙醇Ether 醚Epoxide环氧化物Cellosolve 溶纤剂Crown ether 冠醚Netro compound硝基化合物Ami ne 胺Quater naryam monium com-po und 季铵化合物Amine oxide 氧化胺Diazoalkane 重氮烷Mercaptan 硫醇Sulfo nic acid 磺酸Sulfoxide 亚砜Sulfone 砜Aldehyde 醛Deto ne 酮Aldehyde hydrate 醛水合物Keto ne hydrate 酮水合物Hemiacetal 半缩醛Acetal缩醛Ketal缩酮Dithia ne 二噻烷Aminal缩醛胺imine 亚胺Aldimi ne醛亚胺Oxime 肟Aldimi ne 醛肟Oxime亚硝基化合物aldoxime 硝酮Hydraz one 腙Azine 嗪Semicarbazone 缩氯基脲Cyanohydrin 羟腈Pinacol频哪醇Enol烯醇Enol ether 烯醇醚Enol ester 烯醇酯Enamine 烯胺Yn ami ne 炔胺Mannich base 曼尼希碱Carboxylic acid 羧酸Ester 酯orthoester 原酸酯Acyl halide 酰卤Acyl fluoride 酰氟Acyl chloride 酰氯Acyl rtomide 酰溴Acyl iodide 酰碘v1.0可编辑可修改Carbobe nzoxy chloride 苄氧甲酰氯Acyl tosylate 酰基对甲苯磺酸酐Ketene乙烯酮v1.0可编辑可修改Peracid 过酸Perester过酸酯Acyl peroxide 酰基过氧化物Nitrile 腈Nitrile oxide 氧化腈Ison itrile 异腈Amide酰胺Imide二酰亚胺N-bromo compo und N-溴化物Hydrazide 酰肼Acyl azide 酰叠氮Amidine 脒Keto ester 酮酸酯Acyl cyanide 酰腈Carbon suboxide 二氧化三碳Glycidic acid 环氧丙酸Carbammic acid 氨基甲酸Carbamate氨基甲酸酯Urea 脲Cyan amide 氨腈Carbodiimide 碳二亚胺Allopha nate 脲基甲酸酯Thioester 硫代酸酯Thiol acid 硫羰酸Lactone 内酯Lactol内半缩醛Macrolide 大环内酯Ami no acid 氨基酸Zwitterio ns 两性离子Inner salt 内盐Betaine甜菜碱Lactam内酰胺Hydantion乙内酰脲Peptide 肽Glycol 二醇Aldol羟醛Acyloin 偶姻Carbohydrate碳水化合物Aldose醛糖Ketose酮糖Furanose呋喃糖Pyranose吡喃糖Glycoside 糖苷Glucoside 葡[萄]糖苷Aglycon 苷元Saccharide 糖类Oligosaccharide 寡糖Polysaccharide 多糖Alditol 糖醇Osazone 脎Alicyclic compo und 脂环化合物Cycloalke ne 环烷Spirane 环烯Cage compo und 螺烷Propella ne 笼型化合物Rotaza ne螺桨烷Cate nane 轮烷Rused ring 索烃。
(完整版)有机化学专业英语词汇(精)

有机化学专业英语词汇(精)acetal 醛缩醇acetal- 乙酰acid 酸-al 醛alcohol 醇-aldehyde 醛alkali- 碱allyl 烯丙基 [propenyl(丙烯基)] alkoxy- 烷氧基-amide 酰胺amino- 氨基的-amidine 脒-amine 胺-ane 烷anhydride 酐anilino- 苯胺基aquo- 含水的-ase 酶-ate 含氧酸的盐、酯-atriyne 三炔azo- 偶氮benzene 苯bi- 在盐类前表示酸式盐bis- 双-borane 硼烷bromo- 溴butyl 丁基-carbinol 甲醇carbonyl 羰基-carboxylic acid 羧酸centi- 10-2chloro- 氯代cis- 顺式condensed 缩合的、冷凝的cyclo- 环deca- 十deci 10-1-dine 啶dodeca- 十二-ene 烯epi- 表epoxy- 环氧-ester 酯-ether 醚ethoxy- 乙氧基ethyl 乙基fluoro- 氟代form 仿-glycol 二醇hemi- 半hendeca- 十一hepta- 七heptadeca- 十七hexa- 六hexadeca- 十六-hydrin 醇hydro- 氢或水hydroxyl 羟基hypo- 低级的,次hyper- 高级的,高-ic 酸的,高价金属-ide 无氧酸的盐,酰替胺,酐-il 偶酰-imine 亚胺iodine 碘iodo- 碘代iso- 异,等,同-ite 亚酸盐keto- 酮ketone 酮-lactone 内酯mega- 106meta- 间,偏methoxy- 甲氧基methyl 甲基micro- 10-6milli- 10-3mono- ( mon-) 一,单nano- 10-9nitro- 硝基nitroso- 亚硝基nona- 九nonadeca- 十九octa- 八octadeca- 十八-oic 酸的-ol 醇-one 酮ortho- 邻,正,原-ous 亚酸的,低价金属oxa- 氧杂-oxide 氧化合物-oxime 肟oxo- 酮oxy- 氧化-oyl 酰para- 对位,仲penta- 五pentadeca- 十五per- 高,过petro- 石油phenol 苯酚phenyl 苯基pico- 10-12poly- 聚,多quadri- 四quinque- 五semi- 半septi- 七sesqui 一个半sulfa- 磺胺sym- 对称syn- 顺式,同,共ter- 三tetra- 四tetradeca- 十四tetrakis- 四个thio- 硫代trans- 反式,超,跨thio- 硫代tri- 三trideca- 十三tris- 三个undeca- 十一uni- 单,一unsym- 不对称的,偏位-yl 基-ylene 撑(二价基,价在不同原子上) -yne 炔organic compounds 有机化合物compounds of carbon 碳化合物hydrocarbons and their derivatives 碳氢化合物及其衍生物organic chemistry 有机化学structure of molecule 分子结构chemical bond 化学键covalent bond 共价键hybrid orbital 杂化轨道bond length 键长bond angle 键角bond energy键能polarity 极性dissociation energy 离解能constitution构造contiguration构型conformation构象stereochemistry立体化学tetrahedral正四面体cis-顺trans-反isomerism同分异构现象isomer异构体stereoisomer立体异构constitutional isomer构造异构structural formula 结构式octet八隅体perspective 透视式eclipsed conformation重叠式构象staggered conformation交叉式构象newman projection纽曼投影式functional group 官能团chain compoud 链状化合物carbocyclic compound碳环化合物heterocyclic compound杂环化合物dipole-dipole interactions 偶极-偶极相互作用van der Waals forces 范德华力hydrogen bonding 氢键dipole moment偶极矩electronegativity 电负性physical property物理性质melting point熔点boiling point 沸点reaction mechanism反应机理homolysis均裂free redical自由基heterolysis异裂ionic type离子型electrophilic reagent亲电试剂electrophilic reaction亲电反应nucleophilic reagent亲核试剂nucleophilic reaction 亲核反应英文名汉文名Angular methyl group 角甲基Alkylidene group 亚烷基Allyl group 烯丙基Allylic 烯丙型[的]Phenyl group 苯基Aryl group 芳基Benzyl group 苄基Benzylic 苄型[的]Activating group 活化基团Chromophore 生色团Auxochrome 助色团Magnetically anisotropic group 磁各向异性基团Smally ring 小环Common ring 普通环Medium rimg 中环Large ring 大环Bridged-ring system 桥环体系Spiro compound 螺环化合物Helical molecule 螺旋型分子Octahedral compound 八面体化合物Conjugation 共轭Conjugated-system 共轭体系Acyl cation 酰[基]正离子Benzylic cation 苄[基]正离子Arenirm ion 芳[基]正离子Ketyl radical 羰自由基Radical ion 自由基离子Radical cation 自由基正离子Radical anion 自由基负离子Isomerism 异构[现象]Aci form 酸式Fluxional structure 循变结构Stereochemistry 立体化学Optical activity 光学活性Dextro isomer 右旋异构体Laevo isomer 左旋异构体Tetrahedral configuration 四面体构型Stereoisomerism 立体异构[现象] Asymmetric atom 不对称原子Asymmetric carbon 不对称碳Pseudoasymmetric carbon 假不对称碳Phantom atom 虚拟原子Homotopic 等位[的]Heterotopic 异位[的] Enantiotopic 对映异位[的] Diastereotopic 非对映异位[的] Configuration 构型Absolute configuration 绝对构型Chirality 手性Chiral 手性[的]Chiral center 手性中心Chiral molecule 手性分子Achiral 非手性[的]Fischer projection 费歇尔投影式Neoman projection 纽曼投影式D-L system of nomenclature D-L命名体系R-S syytem of nomenclature R-S命名体系Cahn-Ingold-Prelon sequence 顺序规则Symmetry factor 对称因素Plane of symmetry 对称面Mirror symmetry 镜面对称Enantiomer 对映[异构]体Diastereomer 非对映[异构]体Epimer 差向异构体Anomer 端基[差向]异构体Erythro configuration 赤型构型Erythro isomer 赤型异构体Threo configuration 苏型构型Threo isomer 苏型异构体Trigonal carbon 三角型碳Cis-trans isomerism 顺反异构E isomer E异构体Z isomer Z异构体Endo isomer 内型异构体Exo isomer 外型异构体Prochirality 前手性Pro-R group 前R基团Pro-S proup 前S基团Re face Re面Si face Si面Racemic mixture 外消旋混合物Racemic compound 外消旋化合物Racemic solid solution 外消旋固体溶液Meso compound 内消旋化合物Quasi recemate 准外消旋体Conformation 构象Conformational 构象分析Torsion angle 扭转角Rotamer 旋转异构体Anti conformation 反式构象Bisecting conformation 等分构象Anti periplanar conformation 反叠构象Synperiplanar conformation 顺叠构象Synclinal conformation 反错构象Synclinal conformation 顺错构象Eclipsed conformation 重叠构象Gauche conformation, skewcon-formation 邻位交叉构象Staggered conformation 对位交叉构象Steric effect 空间效应Steric hindrance 位阻Atropismer 阻转异构体Puckered ring 折叠环Conformational inversion 构象反转Chair conformation 椅型构象Boat conformation 船型构象Twist conformation 扭型构象Skew boat conformation 扭船型构象Half-chair conformation 半椅型构象Pseudorotation 假旋转Envelope conformation 信封[型]构象Axial bond 直[立]键Equatorial bond 平[伏]键Cisoid conformation 顺向构象Transoid conformation 反向构象Retention of configuration 构型保持Regioselectivity 区域选择性Regiospecificity 区域专一性Stereocelectivity 立体选择性Stereospecificty 立体专一性Conformer 构象异构体Conformational effect 构象效应Cram’s rube 克拉姆规则Prelog’rule 普雷洛格规则Stereochemical orientation 立体[化学]取向Conformational transmission 构象传递Homolog 同系物Ipso position 本位Ortho position 邻位Meta position 间位Para position 对位Amphi position 远位Peri position 近位Trigonal hybridization 三角杂化Molecular orbiral method 分子轨道法Valence bond method 价键法Delocalezed bond 离域键Cross conjugation 交叉共轭Vinylog 插烯物Mesomeric effect 中介效应Resonance 共振Resonance effect 共振效应Hyperconjugation 超共轭Isovalent hyperconjugation 等价超共轭No-bond resonance 无键共振Aromaticity 芳香性Aromatic sexter 芳香六隅Huckel’rule 休克尔规则Paramagnetic ring current 顺磁环电流Diamagnetic ring cruuent 抗磁环电流Homoaromaticity 同芳香性Antiaromaticity 反芳香性Alternant hydrocarbon 交替烃Non-alternant hydrocarbon 非交替烷Pericyclic reaction 周环反应Electrocyclic rearrangement 电环[化]重排Conrotatory 顺旋Disroatatory 对旋Cycloaddition 环加成Symmetry forbidden-reaction 对称禁阻反应Synfacial reaction 同面反应Antarafacial reaction 异面反应Mobius system 默比乌斯体系Leois structure 路易斯结构Coordinate-covalent bond 配位共价键Banana bond 香蕉键Pauling electronegativity scale 鲍林电负性标度Polarizability 可极化性Inductive effect 诱导效应Field effect 场效应Electrical effect 电场效应tautomerism 互变异构Tautomerization 互变异构化Keto-enol tautomerism 酮-烯醇互变异构Phenol-keto tautomerism 酚-酮互变异构Imine-enamine atutomerism 亚胺-烯胺互变异构Ring-chain tautomerism 环-链互变异构Valence tautomerism 价互变异构Ambident 两可[的]Solvent effect 溶剂效应Acid-base catalyxed reaction 酸性溶剂Basic solvent 碱性溶剂Dielectric constant 介电常数Solvated electron 溶剂化电子Acid-base catalyzed reaction 酸碱催化反应Conjugate base 共轭酸Conjugate base 共轭碱Therm odynamic acidity 热力学酸度Kinetic acidity 动力学酸度Electron donof-acceptor complex,EDAcomplex 电子给[体]受体络合物Host 主体Guest 客体Primary isotope effect 一级同位素效应Secondary isotope effect 二级同位数效应Inverse isotope effect 逆同位素效应Kinetic control 动力学控制Thermodynamic control 热力学控制Substrate 底物Intermediate 中间体Reactive intermediate 活泼中间体Microscopic reversibility 微观可逆性Hammond postulate 哈蒙德假说Linear free energy 线性自由能Non-bonded interaction 非键相互作用Torsional effect 扭转效应Pitzer strain 皮策张力Restricted rotation 阻碍旋转Eclipsing effect 重叠效应Eclipsing strain 重叠张力Small-angle strain 小角张力Large angle strain 大角张力Transannular interaction 跨环相互作用Transannular strain 跨环张力I strain 内张力F strain 前张力B strain 后张力Anomeric effect 端基异构效应Walden inversion 瓦尔登反转Racemization 外消旋化Isoinversion 等反转Isoracemization 等消旋Homochiral 纯手性[的] Mechanism 机理Unimolecular nucleophilic 单分子亲核取代Bimolecular nucleophilicsub-stitution 双分子亲核取代Bimolecular nucleophilicsubsti-tution(with allylic rearrange-ment) 双分子亲核取代(含烯丙型重排)Internal nucleophilicsubstiru-tion 分子内亲核取代Aromatic nucleophilicsubstitu-tion 芳香亲核取代Unimolecular electrophilicsub-stitution 单分子亲电取代Bimolecular electrophilicsubsti-tution 双分子亲电取代Nucleophile-assistedunimolecu-lar electrophilic substitution 亲核体协助单分子亲电取代Unimolecular elimination 单分子消除Bimolecular elimination 双分子消除Unimolecular elimination through the conjugate base 单分子共轭碱消除Bimolecular elimination through the conjugate base 双分子共轭碱消除Bimolecular elimination withfor-mation of a carbonyl group 双分子羰基形成消除Unimolecular acid-catalyzedacyl-oxygen cleavage 单分子酸催化酰氧断裂Bimolecular base-catalyzedacyl-oxygen cleavage 双分子碱催化酰氧断裂Unimolecular acid-catalyzedalkyl-oxygen cleavage 单分子酸催化烷氧断裂Bimllecular base-catalyzed al- kyl-oxygen cleavage 双分子碱催化烷氧断裂π-allyl complex mechanism π烯丙型络合机理Borderline mechanism 边理机理Homolysis 均裂Heterolysis 异裂Heterolytic michanism 异裂机理Counrer[gegen]ion 反荷离子Ion pair 离子对Carbocation 碳正离子Nonclassical carbocation 非经典碳正离子Carbanion 碳负离子Masked carbanion 掩蔽碳负离子Carbenoid 卡宾体Carbene 卡宾Nitrene 氮宾Carbine 碳炔Electrophilic addition 亲电加成Electrophile 亲电体Diaxial addition 双直键加成Markovnikov’s rube 马尔科夫尼科规则Anti-Markovnikov addition 反马氏加成Michael addition 迈克尔加成Substitution 取代Electrophilic substitution 亲电取代Addition-elimination mechanism 加成消除机理Electrophilic aromaticsubstitu-tion 亲电芳香取代Electron transfer 电子转移Electron-donating group 给电子基团Electron-Withdrawing group 吸电子基团Deactivating group 钝化基团Orinentation 取向Ortho-para directing group 邻对位定位基Meta directing group 间位定位基Ortho effect 邻位效应Partial rate factor 分速度系数Nucleophilic reaction 亲核反应Internal return 内返Nucleophilicity 亲核体Nucleophilicity 亲核性α-effect α-效应Backside attack 背面进攻Inversion 反转Umbrella effect 伞效应Push-pull effect 推拉效应Leaving group 离去基团Electrofuge 离电体Nucleofuge 离核体Phase-transfer catalysis 相转移催化Neighboring group participation 邻基基参与Neighboring proupassistance,anchimeric assistance 邻助作用Neighboring group effect 邻基效应Apofacial reaction 反面反应Briddgehead displacement 桥头取代Aryl action 芳正离子Benzyne 苯炔Zaitsev rule 札依采夫规则Anti-Zaitsev orientation 反札依采夫定向Hofmann’s rule 霍夫曼规则Bredt rule 布雷特规则Initiation 引发Anionic cleavage 负离子裂解Partial bond fixation 键[的]部分固定化02.3有机化学反应Alkylation 烷基化C- alkylation C-烷基化O- alkylation O-烷基化N-alkylation N-烷基化Silylation 硅烷[基]化Exhaustive methylation 彻底甲基化Seco alkylation 断裂烷基化Demethylation 脱甲基化Ethylation 乙基化Arylation 芳基化Acylation 酰化Formylation 甲酰化Carbalkoxylation 烷氧羰基化Carboamidation 氨羰基化Carboxylation 羧基化Amination 氨基化Bisamination 双氨基化Cine substitution 移位取代Transamination 氨基交换Hydroxylation 羟基化acyloxyation 酰氧基化Decarboxylative nitration 脱羧卤化Allylic halogenation 烯丙型卤化Dehalogenation 脱卤Nitration 硝化Decarboxylative nitration 脱羧硝化Nitrosation 亚硝化Sulfonation 磺化Chlorosulfonation 氯磺酰化Desulfonation 脱磺酸基Sulfenylation 亚磺酰化Sulfonylation 磺酰化Chlorosulfenation 氯亚磺酰化Chlorocarbonylation 氯羰基化Diazotization 重氮化Diazo transfer 重氮基转移Coupling reaction 偶联反应Diazonium coupling 重氮偶联Cross-coupling reaction 交叉偶联反应1,4-addition 1,4-加成Conjugate addition 共轭加成Dimerization 二聚Trimefization 三聚Additive dimerization 加成二聚sulfurization 硫化Selenylation 硒化Hydroboration 硼氢化Oxyamination 羟氨基化Insertion 插入carbonylation 羧基化Hydroformylation 加氢甲酰基化Hydroacylation 加氢酰化Oxo process 羰基合成Decarbonylation 脱羰Hydrocarboxylation 氢羧基化Homologization 同系化Cyanoethylation 氰乙基化Decyanoethylation 脱氰乙基Ring clsure 环合Diene synthesis 双烯合成Dienophile 亲双烯体Endo addition 内型加成Exo addition 外型加成Diels-Alder reaction 第尔斯-尔德反应Retro Diels-Alder reaction 逆第尔斯-阿尔德反应Ene synthesis 单烯合成Anionic cycloaddition 负离子环加成Dipolar addition 偶极加成- elimination -消除- elimination -消除- elimination -消除-elimination -消除Dehydrohalogenation 脱卤化氢Deamination 脱氨基Pyrolytic elimination 热解消除Elimination-addition 消除-加成Decarboxylation 脱羧Decarboxamidation 脱酰胺Decyanation 脱氰基Alkylolysis,alkyl cleavage 烷基裂解Acylolysis,acyl cleavage 酰基裂解Flash pyrolysis 闪热裂Fragmentation 碎裂Chiletropic reaction 螯键反应Chelation 螯环化Esterification 酯化Transesterification 酯交换Saponification 皂化Alcoholysis 醇解Ethanolysis 乙醇解Cyanomethylation 氰甲基化Aminomethylation 氨甲基化Hydroxymethylation 羟甲基化Hydroxyalkylation 羟烷基化Cholromethylation 氯甲基化Haloalkylation 卤烷基化Transacetalation 缩醛交换Enolization 烯醇化Haloform reaction 卤仿反应Condensation 缩合Aldol condensation 羟醛缩合Cross aldol condensation 交叉羟醛缩合Retrograde aldol condensation 逆羟醛缩合Acyloin condensation 偶姻缩合Cyclization 环化Annulation,annelation 增环反应Spiroannulation 螺增环Autoxidation 自氧化Allylic hydroperoxylation 烯丙型氢过氧化Epoxidation 环氧化Oxonolysis 臭氧解Electrochemical oxidation 电化学氧化Oxidative decarboxylation 氧化脱羧Aromatization 芳构化Catalytic hydrogenation 催化氢化Heterogeneous hydrogenation 多相氢化Homogeneous hydrogenation 均相氢化Catalytic dehydrogenation 催化脱氢Transfer hydrogenation 转移氢化Hydrogenolysis 氢解Dissolving metal reduction 溶解金属还原Single electron transfer 单电子转移Bimolecular reduction 双分子还原Electrochemical reduction 电化学还原Reductive alkylation 还原烷基化Reductive acylation 还原酰化Reductive dimerization 还原二聚Deoxygenation 脱氧Desulfurization 脱硫Deselenization 脱硒Mitallation 金属化Lithiation 锂化Hydrometallation 氢金属化Mercuration 汞化Oxymercuration 羟汞化Aminomercuration 氨汞化Abstraction 夺取[反应]Internal abstraction 内夺取[反应] Rearrangement 重排Prototropic rearrangement 质了转移重排Double bond migration 双键移位Allylic migration 烯丙型重排Allylic migration 烯丙型迁移Ring contraction 环缩小[反应] Ring expansion,ring enlargement 扩环[反应]-ketol rearrangement -酮醇重排Pinacol rearrangement 频哪醇重排Retropinacol rearrangement 逆频哪醇重排Semipinacol rearrangement 半频哪醇重排Benzilic rearrangement 二苯乙醇酸重排Acyl rearrangement 酰基重排Migratory aptitude 迁移倾向Transannular insertion 跨环插入Transannular rearrangement 跨环重排Migration 迁移Prototropy 质子转移Cationotropic rearrangement 正离子转移重排Anionotropy 负离子转移Anionotropic rearrangement 负离子转移重排Sigmatropic rearrangement -迁移重排Homosigmatropic rearrangement 同迁移重排Electrophilic rearrangement 亲电重排Photosensitization 光敏化Forbidden transition 禁阻跃迁photooxidation 光氧化Photoisomerization 光异构化Photochemical rearrangement 光化学重排2.4 有机化合物类名Aliphatic compound 脂肪族化合物Hpdrocarbon 碳氢化合物Alkane 烷Wax 蜡Paraffin wax 石蜡Alkene 烯Alkyen 炔Acetylide 炔化物Active hydrogen compounds 活泼氢化合物Carbon acid 碳氢酸Super acid 超酸Diene 双烯Triene 三烯Allene 丙二烯Ccumulene 累积多烯Enyne 烯炔Diyne 二炔Alkyl halide 卤代烷Alcohol 醇Homoallylic alcohol 高烯丙醇Ether 醚Epoxide 环氧化物Cellosolve 溶纤剂Crown ether 冠醚Netro compound 硝基化合物Amine 胺Quaternaryammonium com-pound 季铵化合物Amine oxide 氧化胺Diazoalkane 重氮烷Mercaptan 硫醇Sulfonic acid 磺酸Sulfoxide 亚砜Sulfone 砜Aldehyde 醛Detone 酮Aldehyde hydrate 醛水合物Ketone hydrate 酮水合物Hemiacetal 半缩醛Acetal 缩醛Ketal 缩酮Dithiane 二噻烷Aminal 缩醛胺imine 亚胺Aldimine 醛亚胺Oxime 肟Aldimine 醛肟Oxime 亚硝基化合物aldoxime 硝酮Hydrazone 腙Azine 嗪Semicarbazone 缩氯基脲Cyanohydrin 羟腈Pinacol 频哪醇Enol 烯醇Enol ether 烯醇醚Enol ester 烯醇酯Enamine 烯胺Ynamine 炔胺Mannich base 曼尼希碱Carboxylic acid 羧酸Ester 酯orthoester 原酸酯Acyl halide 酰卤Acyl fluoride 酰氟Acyl chloride 酰氯Acyl rtomide 酰溴Acyl iodide 酰碘Carbobenzoxy chloride 苄氧甲酰氯Acyl tosylate 酰基对甲苯磺酸酐Ketene 乙烯酮Peracid 过酸Perester 过酸酯Acyl peroxide 酰基过氧化物Nitrile 腈Nitrile oxide 氧化腈Isonitrile 异腈Amide 酰胺Imide 二酰亚胺N-bromo compound N-溴化物Hydrazide 酰肼Acyl azide 酰叠氮Amidine 脒Keto ester 酮酸酯Acyl cyanide 酰腈Carbon suboxide 二氧化三碳Glycidic acid 环氧丙酸Carbammic acid 氨基甲酸Carbamate 氨基甲酸酯Urea 脲Cyanamide 氨腈Carbodiimide 碳二亚胺Allophanate 脲基甲酸酯Thioester 硫代酸酯Thiol acid 硫羰酸Lactone 内酯Lactol 内半缩醛Macrolide 大环内酯Amino acid 氨基酸Zwitterions 两性离子Inner salt 内盐Betaine 甜菜碱Lactam 内酰胺Hydantion 乙内酰脲Peptide 肽Glycol 二醇Aldol 羟醛Acyloin 偶姻Carbohydrate 碳水化合物Aldose 醛糖Ketose 酮糖Furanose 呋喃糖Pyranose 吡喃糖Glycoside 糖苷Glucoside 葡[萄]糖苷Aglycon 苷元Saccharide 糖类Oligosaccharide 寡糖Polysaccharide 多糖Alditol 糖醇Osazone 脎Alicyclic compound 脂环化合物Cycloalkene 环烷Spirane 环烯Cage compound 螺烷Propellane 笼型化合物Rotazane 螺桨烷Catenane 轮烷Rused ring 索烃11。
化学专业英语有机化合物中英文命名二

Trivial name: glycol(乙二醇), glycerol (丙三醇)…
OH OH CH2 CH2
C C C OH OH
OH HO-C-C-C-OH
glycerol ethylene glycol propylene glycol 1,2-ethanediol 1,2-propanediol 1,2,3-propanetriol
H2 H3C C H2 SH
CH3 SH
1-propanethiol
0-mercaptotoluene
mercapto 含巯基的,含硫氢基的
3、硫醇-Thiols -SH
systematic names: C-C-SH ethanethiol C-C-C-C=C SCH 3 C C-C-C-C-SH 2-methylbutanethiol SH
有机化合物的命名
Nomenclature of Organic Compounds
有机化合物的命名
非官能团化合物 Nonfunctional Compounds
1烃类------(烷烃《 Alkane》烯烃《 Alkene》炔烃 《Alkyne》)
2单环化合物(Monocyclic compounds)
OH C-C-C
OH
isopropyl alcohol
C C-C-C OH
OH
tert-butyl alcohol
2-cyclopenten-1-ol
phenol
2、醇- Alcohols
◆
Alcohols containing more than one OH group Systematic name: suffix: -diol, -triol…
有机化学专业英语词汇

有机化学专业英语词汇(精) acetal 醛缩醇acetal- 乙酰acid 酸-al 醛alcohol 醇-aldehyde 醛alkali- 碱allyl 烯丙基[propenyl(丙烯基)] alkoxy- 烷氧基-amide 酰胺amino- 氨基的-amidine 脒-amine 胺-ane 烷anhydride 酐anilino- 苯胺基aquo- 含水的-ase 酶-ate 含氧酸的盐、酯-atriyne 三炔azo- 偶氮benzene 苯bi- 在盐类前表示酸式盐bis- 双-borane 硼烷bromo- 溴butyl 丁基-carbinol 甲醇carbonyl 羰基-carboxylic acid 羧酸-2 centi- 10 -2 chloro- 氯代cis- 顺式condensed 缩合的、冷凝的cyclo- 环deca- 十deci 10 -1 -dine 啶dodeca- 十二-ene 烯epi- 表epoxy- 环氧-ester 酯-ether 醚ethoxy- 乙氧基ethyl 乙基fluoro- 氟代form 仿-glycol 二醇hemi- 半hendeca- 十一hepta- 七heptadeca- 十七hexa- 六hexadeca- 十六-hydrin 醇hydro- 氢或水hydroxyl 羟基hypo- 低级的,次hyper- 高级的,高-ic 酸的,高价金属-ide 无氧酸的盐,酰替胺,酐-il 偶酰-imine 亚胺iodine 碘iodo- 碘代iso- 异,等,同-ite 亚酸盐keto- 酮ketone 酮-lactone 内酯mega- 106 meta- 间,偏methoxy- 甲氧基methyl 甲基-6micro- 10milli- 10 mono- ( mon-) 一,单nano- 10 nitro- 硝基nitroso- 亚硝基nona- 九nonadeca- 十九octa- 八octadeca- 十八-oic 酸的-ol 醇-one 酮ortho- 邻,正,原-ous 亚酸的,低价金属oxa- 氧杂-oxide 氧化合物-oxime 肟oxo- 酮oxy- 氧化-oyl 酰para- 对位,仲penta- 五pentadeca- 十五per- 高,过petro- 石油phenol/phenoxide 苯酚phenyl 苯基-12pico- 10-12 poly- 聚,多quadri- 四quinque- 五semi- 半septi- 七sesqui 一个半sulfa- 磺胺sym- 对称syn- 顺式,同,共ter- 三tetra- 四tetradeca- 十四tetrakis- 四个thio- 硫代trans- 反式,超,跨thio- 硫代tri- 三trideca- 十三tris- 三个undeca- 十一uni- 单,一unsym- 不对称的,偏位-yl 基-ylene 撑( 二价基,价在不同原子上) -yne 炔organic compounds 有机化合物compounds of carbon 碳化合物hydrocarbons and their derivatives 碳氢化合物及其衍生物Derivative 衍生物organic chemistry 有机化学structure of molecule 分子结构Chemical bond 化学键covalent bond 共价键hybrid orbital 杂化轨道bond length 键长bond angle 键角bond energy 键能polarity 极性dissociation energy 离解能constitution 构造contiguration 构型conformation 构象stereochemistry 立体化学tetrahedral 正四面体cis- 顺trans- 反isomerism 同分异构现象isomer 异构体stereoisomer 立体异构constitutional isomer 构造异构structural formula 结构式octet 八隅体perspective 透视式eclipsed conformation 重叠式构象staggered conformation 交叉式构象newman projection 纽曼投影式functional group 官能团chain compound 链状化合物carbocyclic compound 碳环化合物heterocyclic compound 杂环化合物dipole-dipole interactions 偶极- 偶极相互作用van der Waals forces 范德华力hydrogen bonding 氢键dipole moment 偶极矩electronegativity 电负性physical property 物理性质melting point 熔点boiling point 沸点reaction mechanism 反应机理homolysis 均裂free redical 自由基heterolysis 异裂ionic type 离子型electrophilic reagent 亲电试剂electrophilic reaction 亲电反应nucleophilic reagent 亲核试剂nucleophilic reaction 亲核反应英文名汉文名Angular methyl group 角甲基Alkylidene group 亚烷基Allyl group 烯丙基Allylic 烯丙型[的] Phenyl group 苯基Aryl group 芳基Benzyl group 苄基Benzylic 苄型[的]Activating group 活化基团Chromophore 生色团Auxochrome 助色团Magnetically anisotropic group 磁各向异性基团Smally ring 小环Common ring 普通环Medium rimg 中环Large ring 大环Bridged-ring system 桥环体系Spiro compound 螺环化合物Helical molecule 螺旋型分子Octahedral compound 八面体化合物Conjugation 共轭Conjugated system 共轭体系Acyl cation 酰[基]正离子Benzylic cation 苄[基]正离子aromatic ion 芳[基]正离子Ketyl radical 羰自由基Radical ion 自由基离子Radical cation 自由基正离子Radical anion 自由基负离子Isomerism 异构[现象] Acid form 酸式Fluxional structure 循变结构Stereochemistry 立体化学Optical activity 光学活性Dextro isomer 右旋异构体Laevo isomer 左旋异构体Tetrahedral configuration 四面体构型Stereoisomerism 立体异构[现象]Asymmetric atom 不对称原子Asymmetric carbon 不对称碳Pseudo asymmetric carbon 假不对称碳Phantom atom 虚拟原子Homotopic 等位[的] Heterotopic 异位[的]Enantiotopic 对映异位[的]Diastereotopic 非对映异位[的]Configuration 构型Absolute configuration 绝对构型Chirality 手性Chiral 手性[的] Chiral center 手性中心Chiral molecule 手性分子Achiral 非手性[的] Fischer projection 费歇尔投影式Newman projection 纽曼投影式D-L system of nomenclature D-L 命名体系R-S system of nomenclature R-S 命名体系Cahn-Ingold-Prelon sequence 顺序规则Symmetry factor 对称因素Plane of symmetry 对称面Mirror symmetry 镜面对称Enantiomer 对映[异构]体Diastereomer 非对映[异构]体Epimer 差向异构体Anomer 端基[差向]异构体Erythro configuration 赤型构型Erythro isomer 赤型异构体Threo configuration 苏型构型Threo isomer 苏型异构体Trigonal carbon 三角型碳Cis-trans isomerism 顺反异构E isomer E 异构体Z isomer Z 异构体Endo isomer 内型异构体Exo isomer 外型异构体Prochirality 前手性Pro-R group 前R 基团Pro-S proup 前S 基团Re face Re 面Si face Si 面Racemic mixture 外消旋混合物Racemic compound 外消旋化合物Racemic solid solution 外消旋固体溶液Meso compound 内消旋化合物Quasi recemate 准外消旋体Conformation 构象Conformational 构象的Torsion angle 扭转角Rotamer 旋转异构体Anti conformation 反式构象Bisecting conformation 等分构象Antiperiplanar conformation 反叠构象Synperiplanar conformation 顺叠构象anticlinal conformation 反错构象Synclinal conformation 顺错构象Eclipsed conformation 重叠构象Gauche conformation/skew conformation 邻位交叉构象Staggered conformation 对位交叉构象Steric effect 空间效应Steric hindrance 位阻Atropisomer 阻转异构体Puckered ring 折叠环Conformational inversion 构象反转Chair conformation 椅型构象Boat conformation 船型构象Twist conformation 扭型构象Skew boat conformation 扭船型构象Half-chair conformation 半椅型构象Pseudorotation 假旋转Envelope conformation 信封[型]构象Axial bond 直[立]键Equatorial bond 平[伏]键Cisoid conformation 顺向构象Transoid conformation 反向构象Retention of configuration 构型保持Regioselectivity 区域选择性Regiospecificity 区域专一性Stereoselectivity 立体选择性Stereospecificty 立体专一性Conformer 构象异构体Conformational effect 构象效应Cram's rube 克拉姆规则Prelog 'rule 普雷洛格规则Stereochemical orientation 立体[化学]取向Conformational transmission 构象传递Homolog 同系物Ipso position 本位Ortho position 邻位Meta position 间位Para position 对位Amphi position 远位Peri position 近位Trigonal hybridization 三角杂化Molecular orbital method 分子轨道法Valence bond method 价键法Delocalezed bond 离域键Cross conjugation 交叉共轭Vinylog 插烯物Mesomeric effect 中介效应Resonance 共振Resonance effect 共振效应Hyperconjugation 超共轭Isovalent hyperconjugation 等价超共轭No-bond resonance 无键共振Aromaticity 芳香性Aromatic sexter 芳香六隅Huckel 'rule 休克尔规则Paramagnetic ring current 顺磁环电流Diamagnetic ring current 抗磁环电流Homoaromaticity 同芳香性Antiaromaticity 反芳香性Alternant hydrocarbon 交替烃Non-alternant hydrocarbon 非交替烷Pericyclic reaction 周环反应Electrocyclic rearrangement 电环[ 化] 重排Conrotatory 顺旋Disroatatory 对旋Cycloaddition 环加成Symmetry forbidden-reaction 对称禁阻反应Synfacial reaction 同面反应Antarafacial reaction 异面反应Mobius system 默比乌斯体系Lewis structure 路易斯结构Coordinate-covalent bond 配位共价键Banana bond 香蕉键Pauling electronegativity scale 鲍林电负性标度Polarizability 可极化性Inductive effect诱导效应Field effect 场效应Electrical effect 电场效应tautomerism 互变异构Tautomerization 互变异构化Keto-enol tautomerism 酮- 烯醇互变异构Phenol-keto tautomerism 酚- 酮互变异构Imine-enamine atutomerism 亚胺-烯胺互变异构Ring-chain tautomerism 环- 链互变异构Valence tautomerism 价互变异构Ambident 两可[ 的]Solvent effect 溶剂效应Acidic solvent 酸性溶剂Basic solvent 碱性溶剂Dielectric constant 介电常数Solvated electron 溶剂化电子Acid-base catalyzed reaction 酸碱催化反应Conjugate acid 共轭酸Conjugate base 共轭碱Thermodynamic acidity 热力学酸度Kinetic acidity 动力学酸度Electron donor-acceptor complex,EDAcomplex 电子给[ 体] 受体络合物Host 主体Guest 客体Primary isotope effect 一级同位素效应Secondary isotope effect 二级同位数效应Inverse isotope effect 逆同位素效应Kinetic control 动力学控制Thermodynamic control 热力学控制Substrate 底物Intermediate 中间体Reactive intermediate 活泼中间体Microscopic reversibility 微观可逆性Hammond postulate 哈蒙德假说Linear free energy 线性自由能Non-bonded interaction 非键相互作用Torsional effect 扭转效应Pitzer strain 皮策张力Restricted rotation 阻碍旋转Eclipsing effect 重叠效应Eclipsing strain 重叠张力Small-angle strain 小角张力Large angle strain 大角张力Transannular interaction 跨环相互作用Transannular strain 跨环张力I strain 内张力F strain 前张力B strain 后张力Anomeric effect 端基异构效应Walden inversion 瓦尔登反转Racemization 外消旋化Isoinversion 等反转Isoracemization 等消旋Homochiral 纯手性[ 的] Mechanism 机理Unimolecular nucleophilic substitution 单分子亲核取代Bimolecular nucleophilic substitution 双分子亲核取代Bimolecular nucleophilic substitution(with allylic rearrangement) 双分子亲核取代(含烯丙型重排) Internal nucleophilic substirution 分子内亲核取代Aromatic nucleophilic substitution 芳香亲核取代Unimolecular electrophilic substitution 单分子亲电取代Bimolecular electrophilic substitution 双分子亲电取代Nucleophile-assisted unimolecular electrophilic substitution 亲核体协助单分子亲电取代Unimolecular elimination 单分子消除Bimolecular elimination 双分子消除Unimolecular elimination through the conjugate base 单分子共轭碱消除Bimolecular elimination through the conjugate base 双分子共轭碱消除Bimolecular elimination with formation of a carbonyl group 双分子羰基形成消除Unimolecular acid-catalyzed acyl-oxygen cleavage 单分子酸催化酰氧断裂Bimolecular base-catalyzed acyl-oxygen cleavage 双分子碱催化酰氧断裂Unimolecular acid-catalyzed alkyl-oxygen cleavage 单分子酸催化烷氧断裂Bimolecular base-catalyzed alkyl-oxygen cleavage 双分子碱催化烷氧断裂n -allyl complex mechanism n 烯丙型络合机理Borderline mechanism 边理机理Homolysis 均裂Heterolysis 异裂Heterolytic michanism 异裂机理Counrer[gegen]ion 反荷离子Ion pair 离子对Carbocation 碳正离子Nonclassical carbocation 非经典碳正离子Carbanion 碳负离子Masked carbanion 掩蔽碳负离子Carbenoid 卡宾体Carbene 卡宾, 碳烯Nitrene 氮宾Carbine 碳炔Electrophilic addition 亲电加成Electrophile 亲电体Diaxial addition 双直键加成Markovnikov 's rube 马尔科夫尼科规则Anti-Markovnikov addition 反马氏加成Michael addition 迈克尔加成Substitution 取代Electrophilic substitution 亲电取代Addition-elimination mechanism 加成消除机理Electrophilic aromatic substitution 亲电芳香取代Electron transfer 电子转移Electron-donating group 给电子基团Electron-Withdrawing group 吸电子基团Deactivating group 钝化基团Orinentation 取向Ortho-para directing group 邻对位定位基Meta directing group 间位定位基Ortho effect 邻位效应Partial rate factor 分速度系数Nucleophilic reaction 亲核反应Internal return 内返Nucleophilicity 亲核体Nucleophilicity 亲核性a -effect a -效应Backside attack 背面进攻Inversion 反转Umbrella effect 伞效应Push-pull effect 推拉效应Leaving group 离去基团Electrofuge 离电体Nucleofuge 离核体Phase-transfer catalysis 相转移催化Neighboring group participation 邻基基参与Neighboring proupassistance/anchimeric assistance 邻助作用Neighboring group effect 邻基效应Apofacial reaction 反面反应Briddgehead displacement 桥头取代Aryl action 芳正离子Benzyne 苯炔Zaitsev rule 札依采夫规则Anti-Zaitsev orientation 反札依采夫定向Hofmann's rule 霍夫曼规则Bredt rule 布雷特规则Initiation 引发Anionic cleavage 负离子裂解Partial bond fixation 键[的]部分固定化2.3 有机化学反应Alkylation 烷基化C- alkylation C- 烷基化O- alkylation O- 烷基化N-alkylation N- 烷基化Silylation 硅烷[基]化Exhaustive methylation 彻底甲基化Seco alkylation 断裂烷基化Demethylation 脱甲基化Ethylation 乙基化Arylation 芳基化Acylation 酰化Formylation 甲酰化Carbalkoxylation 烷氧羰基化Carboamidation 氨羰基化Carboxylation 羧基化Amination 氨基化Bisamination 双氨基化Cine substitution 移位取代Transamination 氨基交换Hydroxylation 羟基化acyloxyation 酰氧基化Decarboxylative halogenation 脱羧卤化Allylic halogenation 烯丙型卤化Dehalogenation 脱卤Nitration 硝化Decarboxylative nitration 脱羧硝化Nitrosation 亚硝化Sulfonation 磺化Chlorosulfonation 氯磺酰化Desulfonation 脱磺酸基Sulfenylation 亚磺酰化Sulfonylation 磺酰化Chlorosulfenation 氯亚磺酰化Chlorocarbonylation 氯羰基化Diazotization 重氮化Diazo transfer 重氮基转移Coupling reaction 偶联反应Diazonium coupling 重氮偶联Cross-coupling reaction 交叉偶联反应1,4-addition 1 ,4- 加成Conjugate addition 共轭加成Dimerization 二聚Trimerization 三聚Additive dimerization加成二聚sulfurization 硫化Selenylation 硒化Hydroboration 硼氢化Oxyamination 羟氨基化Insertion 插入carbonylation 羧基化Hydroformylation 加氢甲酰基化Hydroacylation 加氢酰化Oxo process 羰基合成Decarbonylation 脱羰Hydrocarboxylation 氢羧基化Homologization 同系化Cyanoethylation 氰乙基化Decyanoethylation 脱氰乙基Ring clsure 环合Diene synthesis 双烯合成Dienophile 亲双烯体Endo addition 内型加成Exo addition 外型加成Diels-Alder reaction 第尔斯- 尔德反应Retro Diels-Alder reaction 逆第尔斯-阿尔德反应Ene synthesis 单烯合成Anionic cycloaddition 负离子环加成Dipolar addition 偶极加成Dehydrohalogenation 脱卤化氢Deamination 脱氨基Pyrolytic elimination 热解消除Elimination-addition 消除-加成Decarboxylation 脱羧Decarboxamidation 脱酰胺Decyanation 脱氰基Alkylolysis/alkyl cleavage 烷基裂解Acylolysis,acyl cleavage 酰基裂解Flash pyrolysis 闪热裂Fragmentation 碎裂Chelatropic reaction 螯键反应Chelation 螯环化Esterification 酯化Transesterification 酯交换Saponification 皂化Alcoholysis 醇解Ethanolysis 乙醇解Cyanomethylation 氰甲基化Aminomethylation 氨甲基化Hydroxymethylation 羟甲基化Hydroxyalkylation 羟烷基化Cholromethylation 氯甲基化Haloalkylation 卤烷基化Transacetalation 缩醛交换Enolization 烯醇化Haloform reaction 卤仿反应Condensation 缩合Aldol condensation 羟醛缩合Cross aldol condensation 交叉羟醛缩合Retrograde aldol condensation 逆羟醛缩合Acyloin condensation 偶姻缩合Cyclization 环化Annulation,annelation 增环反应Spiroannulation 螺增环Autoxidation 自氧化Allylic hydroperoxidation 烯丙型氢过氧化Epoxidation 环氧化Oxonolysis 臭氧解Electrochemical oxidation 电化学氧化Oxidative decarboxylation 氧化脱羧Aromatization 芳构化Catalytic hydrogenation 催化氢化Heterogeneous hydrogenation 多相氢化Homogeneous hydrogenation 均相氢化Catalytic dehydrogenation 催化脱氢Transfer hydrogenation 转移氢化Hydrogenolysis 氢解Dissolving metal reduction 溶解金属还原Single electron transfer 单电子转移Bimolecular reduction 双分子还原Electrochemical reduction 电化学还原Reductive alkylation 还原烷基化Reductive acylation 还原酰化Reductive dimerization 还原二聚Deoxygenation 脱氧Desulfurization 脱硫Deselenization 脱硒Mitallation 金属化Lithiation 锂化Hydrometallation 氢金属化Mercuration 汞化Oxymercuration 羟汞化Aminomercuration 氨汞化Abstraction 夺取[ 反应]Internal abstraction 内夺取[ 反应]Rearrangement 重排Prototropic rearrangement 质了转移重排Double bond migration 双键移位Allylic rearrangement 烯丙型重排Allylic migration 烯丙型迁移Ring contraction 环缩小[ 反应] Ring expansion,ring enlargement 扩环[ 反应] ketol rearrangement 酮醇重排Pinacol rearrangement 频哪醇重排Retropinacol rearrangement 逆频哪醇重排Semipinacol rearrangement 半频哪醇重排Benzilic rearrangement 二苯乙醇酸重排Acyl rearrangement 酰基重排Migratory aptitude 迁移倾向Transannular insertion 跨环插入Transannular rearrangement 跨环重排Migration 迁移Prototropy 质子转移Cationotropic rearrangement 正离子转移重排Anionotropy 负离子转移Anionotropic rearrangement 负离子转移重排Sigmatropic rearrangement 单键迁移重排Homosigmatropic rearrangement 同迁移重排Electrophilic rearrangement 亲电重排Photosensitization 光敏化Forbidden transition 禁阻跃迁photooxidation 光氧化Photoisomerization 光异构化Photochemical rearrangement 光化学重排2.4 有机化合物类名Aliphatic compound 脂肪族化合物Hydrocarbon 碳氢化合物Alkane 烷Wax 蜡Paraffin wax 石蜡Alkene 烯Alkyen 炔Acetylide 炔化物Active hydroge n compo unds 活泼氢化合物Carbon acid 碳氢酸Super acid 超酸Diene双烯Triene 三烯Alle ne 丙二烯Ccumule ne累积多烯Enyne烯炔Diyne 二炔Alkyl halide 卤代烷Alcohol 醇Homoallylic alcohol 高烯丙醇Ether 醚Epoxide环氧化物Cellosolve 溶纤剂Crown ether 冠醚Netro compound硝基化合物Ami ne 胺Quater naryam monium compo und 季铵化合物Amine oxide 氧化胺Diazoalkane 重氮烷Mercaptan 硫醇Sulfo nic acid 磺酸Sulfoxide 亚砜Sulfone 砜Aldehyde 醛Deto ne 酮Aldehyde hydrate 醛水合物Keto ne hydrate 酮水合物Hemiacetal半缩醛Acetal缩醛Ketal缩酮Dithia ne 二噻烷Aminal缩醛胺imine 亚胺Aldimine醛亚胺Oxime 肟Aldimine 醛肟n itroso compo und 亚硝基化合物aldoxime 硝酮Hydrazone 腙Azine吖嗪,连氮Semicarbazone 缩氯基脲Cyanohydrin 羟腈Pinacol频哪醇Enol烯醇Enol ether 烯醇醚Enol ester 烯醇酯Enamine 烯胺Yn ami ne 炔胺Mannich base 曼尼希碱Carboxylic acid 羧酸Ester 酯orthoester 原酸酯Acyl halide 酰卤Acyl fluoride 酰氟Acyl chloride 酰氯Acyl bromide 酰溴Acyl iod ine 酰碘Carbobe nzoxy chloride 苄氧甲酰氯Acyl tosylate 酰基对甲苯磺酸酐Ketene乙烯酮Peracid 过酸Perester过酸酯Acyl peroxide 酰基过氧化物Nitrile 腈Nitrile oxide 氧化腈Ison itrile 异腈Amide酰胺Imide二酰亚胺N-bromo compou nd N-溴化物Hydrazide 酰肼Acyl azide 酰叠氮Amidine 脒Keto ester 酮酸酯Acyl cyanide 酰腈Carbon suboxide 二氧化三碳Glycidic acid 环氧丙酸Carbammic acid 氨基甲酸Carbamate 氨基甲酸酯Urea 脲Cyan amide 氨腈Carbodiimide 碳二亚胺Allopha nate 脲基甲酸酯Thioester 硫代酸酯Thiol acid 硫羰酸Lactone 内酯Lactol 内半缩醛Macrolide 大环内酯Amino acid 氨基酸Zwitterions 两性离子Inner salt 内盐Betaine 甜菜碱Lactam 内酰胺Hydantion 乙内酰脲Peptide 肽Glycol 二醇Aldol 羟醛Acyloin 偶姻Carbohydrate 碳水化合物Aldose 醛糖Ketose 酮糖Furanose 呋喃糖Pyranose 吡喃糖Glycoside 糖苷Glucoside 葡[萄]糖苷Aglycon 苷元Saccharide 糖类Oligosaccharide 寡糖Polysaccharide 多糖Alditol 糖醇Osazone 脎Alicyclic compound 脂环化合物Cycloalkene 环烷Spirane 环烯Cage compound 螺烷Propellane 笼型化合物Rotazane 螺桨烷Catenane 轮烷Rused ring 索烃。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
化学专业英语之含氧有机化合物THE COMPOUNDS CONTAINING OXYGENAlcohols and phenols. In substitutive and conjunctive nomenclature of alcohols the hydroxyl group (OH)as principal group is indicated by a suffix"—ol", with elision of terminal "e"(if present)from the name of the parent compound, for example .methanol, 2-propanol, triphenylmethanol, etc.The following are examples of trivial names which are retained: allyl alcohol, tert-butyl alcohol, benzyl alcohol, ethylene glycol, glycerol, etc.Hydroxy derivatives of benzene and other aromatic carbocyclic systems are named by adding the suffix "-ol ", "-diol ", etc. , to the name of the hydrocarbon, with elision of terminal "e"(if present) before "ol", e.g. , 1,2,4-benzenetriol. The following are examples of trivial names of aromatic hydroxy compounds which are retained, for example, phenol, cresol. naphthol, pyrocatechol, resorcinol, hydroquinone ,etc. Radicals RO- are named by adding "oxy" as a suffix to the name of the radical R, e. g., pentyloxy, allyloxy, benzyloxy, etc. Only the following contractions for oxygen-containing radical names are recommended as exceptions to this rule: methoxy, ethoxy, propoxy, butoxy, phenoxy, isopropoxy.Except when forming part of a ring system, bivalent radicals of the form -O-X-O- are named by adding "dioxy" to the name of the bivalent radical -X-,e. g. , ethylenedioxy, trimethylenedioxy.Salts. Anions derived from alcohols or phenols are named by changing the final"-ol" of the name of the alcohol or phenol to "-olate ". This applies to substitutive, radicofunctional, and trivial names, e.g. , potassium methanolate, sodium phenolate.Ethers. Compounds R1—O—R2 have the generic name "ethers" and may be named by either the substitutive or the radicofunctional method. Substitutive names of unsymmetrical ethers are formed by using names of radicals R'O- as prefixes to the names of the hydrocarbons corresponding to the second radical R2. The senior component is selected as the parent compound. Radicofunctional names of ethers are formed by citing the names of the radicals R1 and R2followed by the word "ether”, for example, 1-isopropoxypropane, ethyl methyl ether, diethyl ether, ethyl vinyl ether.Aldehydes. The term "aldehyde" is applied to compounds which contain the group — C(=O)H attached to carbon. Aldehydes are named by means of the suffixes "-al", "-aldehyde",or "-carbaldehyde", or by the prefix "formyl-" [[representing the —C( = O)H group when present as the terminal group of a carbon chain},or, in connexion with trivial names, by the prefix "oxo-"(representing = O). The name of an unbranched acyclic mono-ordi-aldehyde is formed by adding the suffix"-al"(for a monoaldehyde) , with elision of a terminal "e" (if present), or "-dial" (for a dialdehyde ) to the name of the hydrocarbon containing the same number of carbon atoms, e.g. , ethanal, hexanal.Trivial Names. When the corresponding monobasic acid has a trivial name, the name of the aldehyde may be formed from the trivial name of the acid by changing the ending~-ic acid" or "-oic acid " to "-aldehyde ", for example,' formaldehyde, acetaldehyde, propionaldehyde, acrylaldehyde(or acrolein), benzaldehyde, cinnamaldehyde, furfural.Ketones. The generic name "ketone" is given to compounds containing an oxygen atom doubly bound to a single carbon atom with the carbonyl group =C=O joined to two carbon atoms1. Ketones are named by means of the suffix "-one", the prefix "oxo-", the functional class name "ketone", or, inspecial cases, the suffix "-quinone". In substitutive nomenclature the name of an acyclic ketone is formed by adding thesuffix"-one"or"-dione",etc. to the name of the hydrocarbon corresponding to the principal chain, with elision of terminal "e" (if present) before "one", e. g. , 2-butanone, 2, 4-pentanedione.Radicofunctional names of ketones, R^OR2, are formed, by citing the names of the radicals R l and R2followed by the word "ketone", for example, ethyl methyl ketone, diethyl ketone.at the end of the Carboxylic acids. Carboxyl groups COOH replacing CH3main chain of an acyclic hydrocarbon are denoted by adding "-oic acid" or "-dioic acid" to the name of this hydrocarbon, with elision of terminal "e"(if present) before "oic". Alternatively, the name may be formed by substitutive use of the suffix "-carboxylic acid". In the aliphatic series the numbering of the chain dose not then include the carbon atom of the carboxyl group, e.g., heptanoic acid, heptanedioic acid, cyclohexanecarboxylic acid.The name of a univalent or bivalent acyl radical formed by removal of hydroxyl from all the carboxyl groups is derived from the name of the corresponding acid by changing the ending -oic to –oyl.Systematic and trivial names for some acids and their acyl radicals are given in Table 14Neutral salts of carboxylicamic, imidic, carboximidic, hydroxamic, carbohydroxamic, etc. , acids are named by citing the cation (s) and then the anion or, when, the carboxyl groups of the acid are not all named as affixes, by use of a periphrase, such as "(metal) salt of (the acid)"2,e.g. , sodium heptanoate, potassium acetate.Neutral esters of carboxylic acids, etc., are named in the same way astheir neutral salts except that (a) the name of the alkyl or aryl, etc., radical replaces the name of the cation and (b) a periphrase such as "(alkyl or aryl) ester" replaces " (metai)salt", e.g. , ethyl acetate, diethyl malonate, ethyl chloroformate.Symmetrical anhydrides of monocarboxylic acids, when unsubstituted, are named by replacing the word "acid" by "anhydride", e.g., acetic anhydride, phthalic anhydride,Acyl halides, that is, compounds in which the hydroxyl group of a carboxyl group is replaced by halogen, are named by placing the name of the corresponding halide after that of the acyl radical, e.g., acetyl chloride, benzoyl iodide.。
