Biotin-Nick Translation Mix说明书
核酸探针技术

核酸探针技术化学及生物学意义上的探针(probe),是指与特定的靶分子发生特异性相互作用,并可被特殊的方法探知的分子。
抗体-抗原、生物素-抗生物素蛋白、生长因子-受体的相互作用都可以看作是探针与靶分子的相互作用。
核酸探针技术原理是碱基配对。
互补的两条核酸单链通过退火形成双链,这一过程称为核酸杂交。
核酸探针是指带有标记物的已知序列的核酸片段,它能和与其互补的核酸序列杂交,形成双链,所以可用于待测核酸样品中特定基因序列的检测。
每一种病原体都具有独特的核酸片段,通过分离和标记这些片段就可制备出探针,用于疾病的诊断等研究。
(一) 核酸探针的种类1.按来源及性质划分可将核酸探针分为基因组DNA探针、cDNA探针、RNA 探针和人工合成的寡核苷酸探针等几类。
作为诊断试剂,较常使用的是基因组DNA探针和cDNA探针。
其中,前者应用最为广泛,它的制备可通过酶切或聚合酶链反应(PCR)从基因组中获得特异的DNA后将其克隆到质粒或噬菌体载体中,随着质粒的复制或噬菌体的增殖而获得大量高纯度的DNA探针。
将RNA进行反转录,所获得的产物即为cDNA。
cDNA探针适用于RNA病毒的检测。
cDNA探针序列也可克隆到质粒或噬菌体中,以便大量制备。
将信息RNA(mRNA)标记也可作为核酸分子杂交的探针。
但由于来源极不方便,且RNA极易被环境中大量存在的核酸酶所降解,操作不便,因此应用较少。
用人工合成的寡聚核苷酸片段做为核酸杂交探针应用十分广泛,可根据需要随心所欲合成相应的序列,可合成仅有几十个bp的探针序列,对于检测点突变和小段碱基的缺失或插入尤为适用2.按标记物划分有放射性标记探针和非放射性标记探针两大类。
放射性标记探针用放射性同位素做为标记物。
放射性同位素是最早使用,也是目前应用最广泛的探针标记物。
常用的同位素有32P、3H、35S。
其中,以32P应用最普遍。
放射性标记的优点是灵敏度高,可以检测到Pg级;缺点是易造成放射性污染,同位素半衰期短、不稳定、成本高等。
TUNEL细胞凋亡检测试剂盒显色法-碧云天
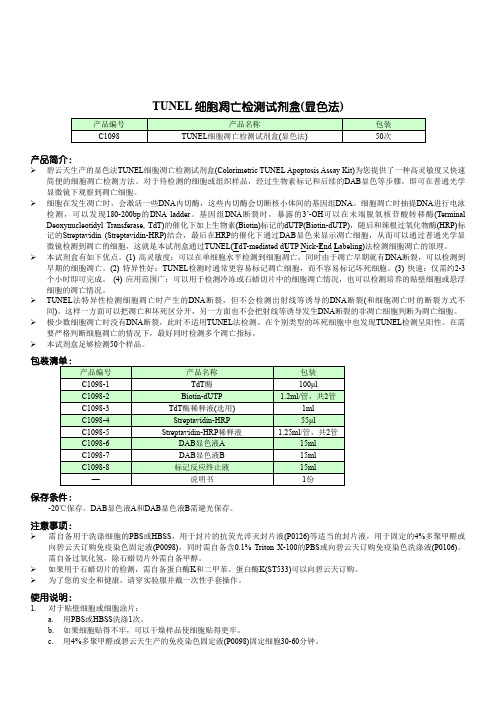
TUNEL细胞凋亡检测试剂盒(显色法)产品简介:碧云天生产的显色法TUNEL细胞凋亡检测试剂盒(Colorimetric TUNEL Apoptosis Assay Kit)为您提供了一种高灵敏度又快速简便的细胞凋亡检测方法。
对于待检测的细胞或组织样品,经过生物素标记和后续的DAB显色等步骤,即可在普通光学显微镜下观察到凋亡细胞。
细胞在发生凋亡时,会激活一些DNA内切酶,这些内切酶会切断核小体间的基因组DNA。
细胞凋亡时抽提DNA进行电泳检测,可以发现180-200bp的DNA ladder。
基因组DNA断裂时,暴露的3’-OH可以在末端脱氧核苷酸转移酶(Terminal Deoxynucleotidyl Transferase, TdT)的催化下加上生物素(Biotin)标记的dUTP(Biotin-dUTP),随后和辣根过氧化物酶(HRP)标记的Streptavidin (Streptavidin-HRP)结合,最后在HRP的催化下通过DAB显色来显示凋亡细胞,从而可以通过普通光学显微镜检测到凋亡的细胞,这就是本试剂盒通过TUNEL(T dT-mediated d U TP N ick-E nd L abeling)法检测细胞凋亡的原理。
本试剂盒有如下优点。
(1) 高灵敏度:可以在单细胞水平检测到细胞凋亡,同时由于凋亡早期就有DNA断裂,可以检测到早期的细胞凋亡。
(2) 特异性好:TUNEL检测时通常更容易标记凋亡细胞,而不容易标记坏死细胞。
(3) 快速:仅需约2-3个小时即可完成。
(4) 应用范围广:可以用于检测冷冻或石蜡切片中的细胞凋亡情况,也可以检测培养的贴壁细胞或悬浮细胞的凋亡情况。
TUNEL法特异性检测细胞凋亡时产生的DNA断裂,但不会检测出射线等诱导的DNA断裂(和细胞凋亡时的断裂方式不同)。
这样一方面可以把凋亡和坏死区分开,另一方面也不会把射线等诱导发生DNA断裂的非凋亡细胞判断为凋亡细胞。
TUNEL-roche使用流程

In situ cell death detection kit-POD法一、原理:TUNEL(TdT-mediated dUTP nick end labeling)细胞凋亡检测试剂盒是用来检测组织细胞在凋亡早期过程中细胞核DNA的断裂情况。
其原理是脱氧核糖核苷酸末端转移酶(TdT Enzyme)把荧光素(fluorescein)标记的dUTP(三磷酸脱氧尿甘)连接到凋亡细胞中断裂DNA的3’-OH末端,并与连接辣根过氧化酶(HRP,horse-radish peroxidase)的荧光素抗体特异性结合,HRP又与HRP底物二氨基联苯胺(DAB)反应产生很强的颜色反应(呈深棕色),特异准确地定位正在凋亡的细胞,因而在光学显微镜下即可观察凋亡细胞;由于正常的或正在增殖的细胞几乎没有DNA断裂,因而没有3‘-OH形成,很少能够被染色。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)在单细胞水平上的凋亡原位检测。
还可应用于抗肿瘤药的药效评价,以及通过双色法确定细胞死亡类型和分化阶段。
二、器材与试剂器材:光学显微镜及其成像系统、小型染色缸、湿盒(塑料饭盒与纱布)、塑料盖玻片或封口膜、吸管、各种规格的加样器及枪头等;试剂:试剂盒含TdT 10×、荧光素标记的dUTP 1×、标记荧光素抗体的HRP;自备试剂:PBS、双蒸水、二甲苯、梯度乙醇(100、95、90、80、70%)、DAB工作液(临用前配制,5 μl 20×DAB+1μL 30%H2O2+94 μl PBS)、Proteinase K工作液(10-20 μg/ml in 10 mM Tris/HCl, pH 7.4-8)或细胞通透液(0.1% Triton X-100 in 0.1% sodium citrate,临用前配制)、苏木素或甲基绿、DNase 1(3000 U/ml– 3 U/ml in 50 mM Tris-HCl,pH 7.5, 10 mM MgCl2,1 mg/ml BSA)等。
原位末端转移酶标记技术

原位末端转移酶标记技术在现代生物学研究领域中,原位标记技术已是一项非常重要的技术。
一些特殊的实验需要通过特定的手段对样品进行标记,使原有的未标记结构物变得可以被观测和检测到,进而更准确地掌握样品的内部组成和结构情况。
其中,“原位末端转移酶标记技术”也就是一项重要的技术之一。
“原位末端转移酶标记技术”(TdT-mediated dUTP-biotinnick end labeling,TUNEL)是指用末端转移酶(TdT)与生物素掺杂的dUTP核苷酸(或其他标记的核苷酸),标记组织或细胞DNA片段断裂处。
在染色体研究、细胞凋亡等研究中,TUNEL技术广泛应用。
下面,将对“原位末端转移酶标记技术”的具体实施步骤进行阐述:第一步,准备样品。
TUNEL技术主要用于富含核酸的样品,如细胞核和细胞。
首先,需将待检测的组织或细胞取出,用生理盐水洗涤,去掉表面的皮脂、油脂等杂质。
通过某些特殊的手段可以对样品进行固定。
第二步,制作反应混合物。
取10 μL 1×反应缓冲液、8 μL dUTP-biotin标记混合物、1 μL TdT酶(10 U/μL),20 μL混合均匀即可。
第三步,反应处理。
取适量DNA样品,加入反应混合物,进行反应。
反应温度可根据研究要求在37℃-40℃之间进行,通常反应时间为1-2h。
第四步,准备样品切片。
处理的不同样品需要进行不同的制片处理。
对于细胞,可以通过离心和固定等方式。
对于组织,需要进行组织的包埋操作等。
第五步,进行特殊染色。
通过特殊染色操作,对样品进行标记。
标记后可通过荧光显微镜或其他仪器进行显示和观察。
总之,“原位末端转移酶标记技术”在生物学研究中应用广泛,其标记出的样品可以直观地提供样品DNA断裂情况,是一项十分重要的生物学技术。
上述操作流程可以作为“原位末端转移酶标记技术”的简单技术手段,具体实施过程中还需根据研究对象等具体情况进行不同的调整和研究。
Nick Translation Kit(1)

2.
2.1
How to use this product
Before you begin
3
dCTP
DNA • The DNA must be in low-salt solution. 2.2 Procedure for standard (radioactive) labeling
4
dGTP
5
dTTP
Fragment size of the nick translated DNA and also the proportion of “snapback” DNA should be taken in account. Too much of DNase I will result in the DNA fragments being excessively short and correspondingly poor hybridization reactions. The proportion of snapback DNA is also increased in this case, reducing the amount of DNA available for hybridization (6). 3.2 Product Characteristics Labeling Efficiency The degree of radioactive labeling is determined by taking an aliquot of the reaction and comparing incorporated radioactivity to total radioactivity in that aliquot. The kinetics of the reaction may be followed by removing aliquots at various times during the reaction, precipitating the DNA with trichloroacetic acid, and determining the amount of radioactivity in the precipitate. Radioactive dNTP for labeling • [␣-32P]dCTP is usually used due to its greater stability in comparison to other labeled deoxyribonucleoside triphosphates. • [␣-32P] deoxyribonucleoside triphosphates with a specific activity of 3000 Ci/mmol give better incorporation and higher levels of labeling than those with a specific activity of 400 Ci/mmol. Specific Activity • The level of specific labeling and the incorporation rate are dependent on the ratio of substrate DNA to labeled deoxyribonucleoside triphosphate, e.g. the kinetics and labeling levels obtained are identical in assays containing 0.1 g DNA and 20 Ci dXTP or 0.5 g DNA and 100 Ci dXTP. • The standard assay described here will label different substrate DNAs (e.g. pBR322, DNA, DNA fragments) to a specificity of 3ϫ 108 dpm/g, corresponding to 65% incorporation in 35 min (see figure 1). Fig. 1: Labeling kinetics of 0.1 g DNA with 20 Ci[␣32P]dCTP, 3000 Ci/mmol.
TUNEL细胞凋亡检测试剂盒(显色法)说明书

TUNEL 细胞凋亡检测试剂盒(显色法)产品编号 产品名称包装 C1098TUNEL 细胞凋亡检测试剂盒(显色法)50次产品简介:碧云天生产的显色法TUNEL 细胞凋亡检测试剂盒(Colorimetric TUNEL Apoptosis Assay Kit)为您提供了一种高灵敏度又快速简便的细胞凋亡检测方法。
对于待检测的细胞或组织样品,经过生物素标记和后续的DAB 显色等步骤,即可在普通光学显微镜下观察到凋亡细胞。
细胞在发生凋亡时,会激活一些DNA 内切酶,这些内切酶会切断核小体间的基因组DNA 。
细胞凋亡时抽提DNA 进行电泳检测,可以发现180-200bp 的DNA ladder 。
基因组DNA 断裂时,暴露的3'-OH 可以在末端脱氧核苷酸转移酶(Terminal Deoxynucleotidyl Transferase, TdT)的催化下加上生物素(Biotin)标记的dUTP(Biotin-dUTP),随后和辣根过氧化物酶(HRP)标记的Streptavidin (Streptavidin-HRP)结合,最后在HRP 的催化下通过DAB 显色来显示凋亡细胞,从而可以通过普通光学显微镜检测到凋亡的细胞,这就是TUNEL(TdT-mediated dUTP Nick-End Labeling)法检测细胞凋亡的原理。
本试剂盒有如下优点。
(1) 高灵敏度:背景染色极低,阳性染色强,可以在单细胞水平检测到细胞凋亡,同时由于凋亡早期就有DNA 断裂,可以检测到早期的细胞凋亡。
(2) 特异性好:TUNEL 检测时通常更容易标记凋亡细胞,而不容易标记坏死细胞。
(3) 快速:仅需约2-3个小时即可完成。
(4) 应用范围广:可以用于检测冷冻或石蜡切片中的细胞凋亡情况,也可以检测培养的贴壁细胞或悬浮细胞的凋亡情况。
(5) 实测效果好:参考图1。
图1. 本试剂盒的检测效果图。
A. HeLa 细胞未处理或用DNase I 室温孵育10分钟后的检测效果图。
第6章 核酸分子杂交(林小聪)

DNA 聚 合 酶 ⅠKlenow 片 段 : 保 留 5’→3’
DNA 聚 合 酶 活 性 , 弱 3’→5’外切酶活性, 无5’→3’外切酶活性。
通常,产物平均长度为400-600个核苷酸。
2020年10月14日9时57分
18
(三) DNA探针的末端标记
1. DNA的3′末端标记
5′ 3′ 5′
限制性内切酶消化
酶切DNA样品的电泳分离
凝胶中DNA带被 转移到NC膜上
标记探 针杂交
Southern印迹
凝胶中DNA分离带
凝胶中DNA的 NaOH变性
探针杂交带曝光显影
思考题2 North-South 杂交原理和操作流程(地高辛标记)
⑤底物与抗体-HRP 反应, 显色或发光
③杂交:Dig 标记的探针与 靶DNA结合
The method used to produce nonradioactive DNA molecules
carried a specific chemical marker could be detected with an
appropriate antibody or avidin.
2020年10月14日9时57分
3′
53′′ 5′
3′
2020年10月14日9时57分
3′ 5′ 限制性内切酶
3′ 5′
Klenow 聚合酶
完整双链DNA
5 ′末端突出 的DNA
Bio-dNTP
其他3 种dNTPs 35 ′′
变性
3 ′末端标记的 DNA
3 ′ 末端标记的单
5′
链DNA探针
19
Dig-dUTP
用一种标记核苷酸代替原料核苷酸中对应的非 标记核苷酸,使标记核苷酸掺入到合成新链中。
Imino Biotin Resin产品说明书

IminoBiotin Resin Cat. No. L00272 Technical Manual No. TM0246 Version 07112010 Index1.Product Description2.Operation3.Purification Procedure4.Related Products1.Product DescriptionGenscript IminoBiotin Resin is an affinity chromatography medium designed for easy, single-step purification of avidin, streptavidin or their conjugates. The iminobiotin ligand is covalently coupled to 4% highly cross-linked agarose. The total binding capacity of IminoBiotin Resin is greater than 12 mg streptavidin/ml settled resin. Table 1 lists the characteristics of IminoBiotin Resin.Iminobiotin is the cyclic guanido analog of biotin that has a lower affinity constant for binding avidin, streptavidin. The Iminobiotin analog can be used in situations requiring mild dissociation of the avidin-biotin complex. Normally, disrupting an avidin-biotin interaction requires 6-8 M guanidine•HCl, pH 1.5, an environment that is often too harsh for proteins to maintain native structure or activity. Iminobiotin binds at pH values above 9.5 and elution is performed at pH 4.0.Table 1. Characteristics of IminoBiotin ResinResin Volume 5 ml settled resin (10 ml 50% slurry)Ligand IminoBiotinTotal binding capacity > 12 mg streptavidin/ml settled resin.Matrix spherical agarose, 4% cross-linkedAverage particle size 90 μm (45-165 μm)Storage solution 1X PBS containing 20% ethanolStorage conditions 2-8 °CShelf life 18 months when stored unopened2.OperationBuffer PreparationWater and chemicals used for buffer preparation should be of high purity. It is recommended filtering the buffers by passing them through a 0.45 μm filter before use.Binding/Wash Buffer: 50 mM sodium borate, 0.3 M NaCl, pH 11.0Elution Buffer: 50 mM sodium acetate, pH 4.03. Purification ProcedureThis procedure is optimized for a column of 0.5 ml bed volume. The volumes of the reagents can be scaled up or down according to the size of the column.Sample PreparationThe sample should be adjusted to contain 0.3 M NaCl with pH between 9.5 and 11.Packing of Columnpletely resuspend the resin and transfer 1 ml slurry to a new column, in which 1 ml Binding/Wash Buffer wasadded in advance.2.Allow the resin to settle down and the buffer to drain from the column.3.Add 5 ml Binding/Wash Buffer onto the column to equilibrate the resin with a flow speed of about 2ml/min.Column Purification1.Apply the sample onto the column with the flow speed of 1 ml/min.2.Wash the column with 10 - 20 bed volumes of Binding/Wash Buffer with a flow speed of about 1ml/min.3.Elute the bound target molecules by 10 - 20 bed volumes of the Elution Buffer with a flow speed of about 0.5 -1ml/min.4.Collect the elution fraction and immediately neutralize it with 1M NaOH to avoid loss of activity resulting fromextended exposure to acidic pH.4.Related ProductsCat. No. Product NameL00210 Protein A ResinL00400 Ultra Protein A ResinL00209 Protein G ResinL00239 Protein L ResinL00405 Chicken IgY Precipitating ResinL00223 High Affinity Ni-Charged ResinL00206 Glutathione ResinL00353 Streptavidin ResinL00207 GST Fusion Protein Purification KitL00208 Protein Expression and Purification kitL00403 High-Affinity Iodoacetyl ResinL00404 High-Affinity Antibody Purification KitFor Research Use OnlyGenScript USA Inc.860 Centennial Ave.,Piscataway, NJ 08854Tel: 732-885-9188, 732-885-9688Fax: 732-210-0262, 732-885-5878Email:*********************Web: 。
原位杂交技术(单独一章)

第十三章原位杂交技术原位杂交(in situ hybridization,ISH)是分子生物学、组织化学和细胞学相结合的产物。
该技术是根据核酸碱基互补配对原则,将放射性或非放射性标记的外源核酸(探针)与染色体经过变性的单链DNA互补配对,结合成专一的核酸杂交分子。
经过一定的检测手段,可利用显微镜直接观察到所研究的目的序列在细胞或染色体上的位置和分布。
为宏观的细胞学与微观的分子生物学研究架起了一座桥梁,形成了一门新的交叉学科――分子细胞遗传学(molecular cytogenetics)。
原位杂交技术是由Gall 和Pardue利用同位素标记的rDNA探针与非洲爪蟾细胞核杂交,于60年代末首次获得成功。
第一节原位杂交基本程序原位杂交的基本程序包括5个基本步骤:第一步是制作用来进行原位杂交的染色体制片,方法与普通染色体制片完全相同。
材料可以是体细胞(如根尖细胞),也可以是花粉母细胞。
以3:1甲醇/乙醇:冰醋酸固定材料,而后做直接压片法或酶解火焰干燥法制片,选择具有理想的细胞分裂时期的片子以备后用。
第二步是对染色体DNA进行变性(denaturation)处理。
先把盖玻片揭开,用RNA酶处理制片,消化掉染色体上的内源RNA,而后用碱、酸或甲酰胺或高温处理,使DNA变性,成为单链状态。
第三步进行杂交。
用以进行杂交的DNA或RNA分子探针通常利用切刻平移法或随机引物标记法标记上放射性的3H、125I或非放射性的生物素(biotin)、地高辛(digoxingenin)。
目前常用的是,把非放射性探针、甲酰胺、硫酸葡聚糖、鲑鱼精DNA(ssDNA)、磷酸缓冲液(SSC)配成杂交液,置于制片上,盖上盖玻片,在37℃右的温箱中保温过夜,使探针同与其碱基序列具有互补性的单链DNA分子进行充分杂交,形成双链。
而后打开盖片,冲洗掉多余未杂交的探针。
第四步信号检出和对染色体进行染色。
对于使用放射性标记探针的,先将制片浸入一定浓度的感光乳剂溶液,并缓慢取出,使制片表面涂上一层均匀的感光乳剂,而后把制片放入暗箱,进行2-3天的感光,接着进行显影和定影。
TUNEL-roche使用流程

In situ cell death detection kit-POD法一、原理:TUNEL(TdT-mediated dUTP nick end labeling)细胞凋亡检测试剂盒是用来检测组织细胞在凋亡早期过程中细胞核DNA的断裂情况。
其原理是脱氧核糖核苷酸末端转移酶(TdT Enzyme)把荧光素(fluorescein)标记的dUTP(三磷酸脱氧尿甘)连接到凋亡细胞中断裂DNA的3’-OH末端,并与连接辣根过氧化酶(HRP,horse-radish peroxidase)的荧光素抗体特异性结合,HRP又与HRP底物二氨基联苯胺(DAB)反应产生很强的颜色反应(呈深棕色),特异准确地定位正在凋亡的细胞,因而在光学显微镜下即可观察凋亡细胞;由于正常的或正在增殖的细胞几乎没有DNA断裂,因而没有3‘-OH形成,很少能够被染色。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)在单细胞水平上的凋亡原位检测。
还可应用于抗肿瘤药的药效评价,以及通过双色法确定细胞死亡类型和分化阶段。
二、器材与试剂器材:光学显微镜及其成像系统、小型染色缸、湿盒(塑料饭盒与纱布)、塑料盖玻片或封口膜、吸管、各种规格的加样器及枪头等;试剂:试剂盒含TdT 10×、荧光素标记的dUTP 1×、标记荧光素抗体的HRP;自备试剂:PBS、双蒸水、二甲苯、梯度乙醇(100、95、90、80、70%)、DAB工作液(临用前配制,5 μl 20×DAB+1μL 30%H2O2+94 μl PBS)、Proteinase K工作液(10-20 μg/ml in 10 mM Tris/HCl, pH 7.4-8)或细胞通透液(0.1% Triton X-100 in 0.1% sodium citrate,临用前配制)、苏木素或甲基绿、DNase 1(3000 U/ml– 3 U/ml in 50 mM Tris-HCl,pH 7.5, 10 mM MgCl2,1 mg/ml BSA)等。
Santa Cruz Biotechnology产品说明书

In complete Ad j u v ant (10x10ml): s c -24019CH EMICAL IDENTIFICATIO NSynonyms: Celex, Celloidin, Cellulose Tetranitrate, Kodak LR 115, Paralodion, Pyralin, Proxylin, and Xyloidin.Description: Complete Freund’s Adjuvant (CFA) is a water-in-oil emulsion t hat contains Mycobacterium tuberculosis cell wall components. A djuvant activity results from sustained release of immunogen from the oily deposit and stimulation of a local immune response. CFA is used for t he initial inject ions. T o minimize side-effects, Incomplete Freund’s Adjuvant, lacking Mycobacterium tuberculosis cell wall components, is used for subsequent boosts.References:Tubercle 58,4: 221-224 (1977).CO MPOSITION/INFO RMATIO N O N INGRED IENTS CAS #: noneStorage: Store container in a cool, well-ventilated area.HAZARD S IDENTIFICATIONPhysical State and Appearance: Liquid. (liquid until emulsified with antigen when it takes on a milky color.)Medical Conditions Aggravated by O verexposure: Repeated or prolonged exposure is not known to aggravate medical condition.FIRST-AID MEASURESIngestion: Do not induce vomiting unless directed to do so by medical personnel. N ever give anythin g by mou th to an unconscious person. If large quantities of t his material are swallowed, call a physician immediately. Loosen tight clothing such as a collar, tie, belt or waistband.Eye Contact: Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15minutes. Get medical attention.Skin Contact: In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing before reuse. Thoroughly clean shoes before reuse. Get medical attention.Inhalation: If inhaled, remove to fresh air. If not breathing, giv e artificial respiration. If breathing is difficult, give oxygen. Get medical attention.ACCID ENT AL RELEASE MEASURESWear protective equipment. Absorb on sand or vermiculite and place in closed containers for disposal. Ventilate area and wash spill site after material pickup is complete.FIRE EXTINGUISHING MEASURESExtinguishing media: carbon dioxide, dry chemical powder or appropriate foam.Special firefighting procedures: wear self-contained breathing apparatus and protective clothing to prevent contact with skin and eyes.H ANDLING AND STO RAGEHandling: Keep container tightly closed. Keep container in a cool,well-ventilated area.Storage: Avoid breathing vapors or spray mists.EXPOSURE CO NTROLS/PERSONAL PROTECTIO N Wear appropriate NIOSH /MSH A -approved respirator, chemical-resistan t gloves, safet y goggles, ot her protect ive clothin g.Mechanical exhaust required.PHYSICAL AND CHEMICAL PROPERTIES Appearance and odor: amber oil STABILITY AND REACTIVITY Stability: stable.Protect from light.H azardous combustion or decomposition products: nature of decomposition products not known; hazardous polymerization will not occur.TO XICOLOGICAL IN FORMATIONAcute effects: may be harmful by inhalation, ingestion, or skin absorption. the toxicological properties have not been thoroughly investigated.ECO LO GICAL INFORMATION Data not yet available.DISPOSAL CONSIDERATIONSDissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. O bserve all federal, state and local environmental regulations.TRANSPORT INFORMATIONContact Santa Cruz Biotechnology for transportation information.Material Safety Data SheetREGULATORY INFORMATIONU.S. Federal RegulationsTSCA: No products were found.Clean Water Act (CWA) 307: No products were found.Clean Water Act (CWA) 311: No products were found.Clean air act (CAA) 112 accidental release prevention: N o products were found.Clean air act (CAA) 112 regulated flammable substances: N o products were found.Clean air act (CAA) 112 regulated toxic substances: No products were found.SARA 302/304/311/312 extremely hazardous substances: N o products were found.SARA 302/304 emergency planning and notification: No products were found.SARA 302/304/311/312 hazardous chemicals: No products were found.SARA 311/312 MSDS distribution - chemical inventory - hazard identification: No products were found.SARA313 toxic chemical notification and release reporting: No products were found.OTHER INFORMATIONThe above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. Santa Cruz Biotechnology shall n ot be held liable for any damage resulting from handling or from contact with the product.。
核酸分子杂交(DNADNA or DNARNA)

优点:简单、快速、可同时检测多个样品
液相杂交
指待测核酸和探针都存在于杂交液中,碱基互补 的单链核酸分子在液体中配对形成杂交分子的过程。
(一)RNA酶保护分析法
1、RNA酶保护分析法原理
RNaseA和RNaseT1专一水解杂交体系中的单 链RNA,不水解探针RNA与待测RNA互补形成 的双链RNA,使杂交分子得到保护,称RNA酶 保护分析法。
核酸分子杂交的基本原理
DNA变性
• 方法
热变性:90~100℃ 酸碱变性:常采用碱变性 化学试剂变性:尿素、甲酰胺、甲醛等
• 特点:粘度增加、密度增加、增色效应、溶 解温度(melting temperature,Tm)
DNA复性或杂交(hybridization)
• DNA/DNA,DNA/RNA,RNA/RNA等
2、凝胶过滤柱层析法:利用凝胶的分子筛作用, 将大分子DNA和小分子dNTP、磷酸根离子及寡 核苷酸(<80bp)等物质分离,常用凝胶基质是 Sephadex G-50
3、微柱离心法:其原理与上述凝胶过滤柱层析法 相同,不同的是上述采用洗脱的方式纯化探针, 而此法则是利用离心的方式来纯化探针
Biotin
Avidin
(2)探针标记物 理想标记物应具备的特性:
• 高度灵敏性 • 不影响碱基配对的特异性 • 不影响探针分子的主要理化性质 • 对酶促反应活性无影响或影响不大 • 检测方法具有高度灵敏性和高度特异性
BCIP/NBT
紫蓝色沉淀
碱性磷酸酶
抗Dig抗体 Dig
(3)探针标记方法
影响杂交的因素
核酸分子的浓度和长度
• 浓度大,复性快;分子量大,复性慢
亨廷顿舞蹈症概述

亨廷顿舞蹈症概述摘要亨廷顿舞蹈症(HD)是由携带了更多扩增的多聚谷氨酰胺的突变亨廷顿蛋白所造成的。
在神经细胞的水平,野生型亨廷顿蛋白功能的丧失与突变亨廷顿蛋白毒性功能的获得被认为是亨廷顿病的病因。
进一步而言,兴奋性毒性,多巴胺毒性,代谢损伤,线粒体功能紊乱,细胞凋亡以及自体吞噬涉及于亨廷顿病的渐进性病理发展过程。
尽管提出了多种治疗策略,目前对于这种破坏性的神经退行性病变还没有有效的治疗。
随着对亨廷顿病的病理机制的进一步认识,以及相关技术的进一步发展,我们可能会找到一种有效的治疗方案。
1.简介亨廷顿舞蹈症(HD)是一种渐进性的常染色体显性的神经退行性病变,是由在亨廷顿蛋白基因的CAG三核苷酸序列发生扩增所致(>35个重复序列)。
这就使亨廷顿蛋白N末端带有一个扩增的多聚谷氨酰胺束(形成突变亨廷顿蛋白)。
亨廷顿病最显著的特征是不能控制的舞蹈样运动,痴呆,精神异常,以及早期死亡,这通常发生在中年时期。
这些症状与在纹状体的中等棘状神经元(神经细胞)更倾向于发生退行性病变密切相关,当然这种病变在晚期也会累及其它脑区。
2.神经退行性病变的机制野生型亨廷顿蛋白(htt)被认为具有许多细胞内的功能,比如蛋白运输,囊泡转运,与细胞骨架的锚定等。
当它发生了突变(加上了一个扩增的多聚谷氨酰胺束),亨廷顿蛋白将赋予一个新的对细胞具有毒性的功能(毒性功能的获得),同时原有的功能丧失(野生型功能的丧失)。
相关的胞内信号通路的失调包括蛋白酶的活化[1],蛋白质错折叠与蛋白质降解途径的抑制[2],转录失调,轴突运输的干扰[3],以及突触功能紊乱[4]。
由突变亨廷顿蛋白介导的细胞内功能紊乱逐渐造成在不同脑区的神经元发生神经退行性病变与死亡,这包括纹状体。
已经发现多种机制涉及于亨廷顿病的病理过程,诸如兴奋性毒性,多巴胺毒性,代谢损伤,线粒体功能紊乱,细胞凋亡以及自体吞噬。
2.1 皮质纹状体功能紊乱与兴奋性毒性纹状体接收来自整个大脑皮质的兴奋性谷氨酸能信号输入,因此在亨廷顿病中所表现的纹状体神经元选择性的易损性可能归因于它们接收了大量的谷氨酸能信号输入,以及(或者)是由于这些细胞所表达的谷氨酸受体类型的特殊性。
TUNEL法检测细胞凋亡实验原理和方法

TUNEL 法检测细胞凋亡实验原理和方法细胞凋亡中染色体 DNA 的断裂是个渐进的分阶段的过程,染色体 DNA 首先在内源性的核酸水解酶的作用下降解为 50-300kb 的大片段。
然后大约 30 ﹪的染色体 DNA 在 Ca ²+和 Mg²+依赖的核酸内切酶作用下, 在核小体单位之间被随机切断, 形成 180~200bp 核小体 DNA 多聚体。
DNA 双链断裂或只要一条链上出现缺口而产生的一系列 DNA 的3’ -OH 末端可在脱氧核糖核苷酸末端转移酶(TdT 的作用下,将脱氧核糖核苷酸和荧光素、过氧化物酶、碱性磷酸化酶或生物素形成的衍生物标记到 DNA 的3’ -末端,从而可进行凋亡细胞的检测, 这类方法一般称为脱氧核糖核苷酸末端转移酶介导的缺口末端标记法(TUNEL 。
由于正常的或正在增殖的细胞几乎没有 DNA 的断裂,因而没有3’ -OH 形成,很少能够被染色。
低分子量的DNA 分离后,也可使用 DNA 聚合酶进行缺口翻译(nick translation ,使低分子量的DNA 标记或染色,然后分析凋亡细胞。
TUNEL 或缺口翻译法实际上是分子生物学与形态学相结合的研究方法, 对完整的单个凋亡细胞核或凋亡小体进行原位染色,能准确的反应细胞凋亡最典型的生物化学和形态特征, 可用于石蜡包埋组织切片、冰冻组织切片、培养的细胞和从组织中分离的细胞的细胞凋亡测定, 并可检测出极少量的凋亡细胞, 灵敏度远比一般的组织化学和生物化学测定法要高,因而在细胞凋亡的研究中已被广泛采用。
一、过氧化物酶标记测定法原理:脱氧核糖核苷酸衍生物地高辛 [(digoxigenin -11-dUTP]在 TdT 酶的作用下, 可以掺入到凋亡细胞双链或单链 DNA 的 3-OH 末端, 与 dATP 形成异多聚体, 并可与连接了报告酶(过氧化物酶或碱性磷酸酶的抗地高辛抗体结合。
在适合底物存在下,过氧化物酶可产生很强的颜色反应, 特异准确的定位出正在凋亡的细胞, 因而可在普通光学显微镜下进行观察。
TUNEL 法测凋亡操作步骤

TUNEL 法测凋亡操作步骤一、TUNEL检测原理TUNEL 细胞凋亡检测试剂盒是采用非同位素方法来检测细胞在凋亡过程中DNA 的断裂情况,可在原位快速准确地检测单个凋亡细胞。
本试剂盒适用于组织样本(石蜡包埋、冰冻和超薄切片)和细胞样本(细胞涂片)的凋亡原位检测。
其原理是细胞凋亡发生时,内源性核酸酶激活,使DNA的双链断裂或一条链出现缺口,产生一系列3’-OH末端,在脱氧核糖核苷酸末端转移酶(TdT)作用下,组织细胞原位DNA切口可被标记上生物素-dUTP,即TUNEL(Terminal deoxynucleotidyl TransferaseBiotin-dUTP Nick End Labeling),可与连接了的辣根过氧化酶的链霉亲和素(Streptavidin-HRP)特异结合,在辣根过氧化酶底物二氨基联苯胺(DAB)的存在下,显示为棕色(过氧化物酶活性),特异准确地定位正在凋亡的细胞,在普通显微镜下即可观察和计数凋亡细胞;由于正的或正在增殖的细胞几乎没有DNA 的断裂,因而没有3'-OH 形成,很少能够被染色。
二、试剂盒组份组份10 assays25 assays 储存条件A:Equilibration Buffer 1.0 mL 2.5 mL -20 ℃B:Biotinylated Nucleotide Mix 10 μL 25 μL -20 ℃C:TdT Enzyme 10 μL 25 μL -20 ℃D:500×Proteinase K 10 μL 25 μL -20 ℃20×SSC 15 mL 38mL 4 ℃E:Streptavidin-HRP 10 μL 25 μL 4 ℃F:20×DAB Substrate Buffer 50 μL 125 μL 4 ℃G:20×DAB Chromogen 50 μL 125 μL 4 ℃H:20×Hydrogen Peroxide 50 μL 125 μL4 ℃三、操作流程1.操作流程概览2.细胞样本制备准备:● PBS:8.0g NaCl, 0.2 g KCl, 1.44g Na2HPO4,0.24g KH2PO4,溶于800ml去离子水中,HCl调pH至7.4,加水定容至1L。
核酸标记各种标记物及标记方法

1.核素标记物放射性核素是目前应用最多的一类探针标记物。
放射性核素的灵敏度极高,可以检测到10-14~10-18克的物质,在最适条件下可以测出样品中少于1000个分子的核酸含量。
常用标记核酸探针的核素有32P、3H、35S,在Southern印迹杂交中以“P最常用。
还应根据标记方法的不同选择相应标记方位的核素,一般情况下,大多数标记方法需要的是a—32P,而5,末端标记必须是r-32P。
2.非放射性标记物多年来,科学家们致力于寻找一些安全、可靠、灵敏度高的物质代替放射性核素用于核酸分子杂交,并取得了一定的进展,部分非放射性标记物已在国内外逐步推广使用。
其优点是无放射性污染,可以较长时间存放,从而更便于临床诊断等方面的应用。
但此类非放射性标记物的缺点是灵敏度及特异性都不太高。
根据其检测方法,非核素标记物可分为以下4类。
(1)半抗原:生物素(biotin)和地高辛(DIG)都是半抗原,可以利用这些半抗原的抗体进行免疫学检测,根据显色反应检测杂交信号。
这两种物质是目前使用较普遍的非核素标记物。
(2)配体:生物素不仅是半抗原,故可用生物素与亲和素反应进行杂交信号的检测。
(3)荧光素:如FITC、罗丹明类等,可以发出紫荧光进行观察,主要适用于细胞原位杂交。
(4)化学发光探针:一些标记物可与某种物质反应产生化学发光现象,通过化学发光可以像核素一样直接使X线胶片上的乳胶颗粒感光。
化学发光探针可能是今后非核素标记物研究的主要方向。
三、标记方法体内标记法:将核素标记的化合物作为合成代谢的底物,在细胞合成代谢时使核素掺入到新合成的核酸分子中,如3H-胸苷可掺入到DNA中,3H-尿苷可掺入到RNA中体外标记法:化学标记法:标记物分子上的活性基因与探针分子上的某些基因反应,标记物直接结合到探针分子上酶促标记法:标记物预先标记核苷酸,然后利用酶促法将标记的核苷酸掺入到探针上酶促标记法(一)切口平移法(nick translation)1、利用DNaseI在DNA双链上造成单链切口2、利用大肠杆菌DNA聚合酶I的5′→3′核酸外切酶活性在切口处将旧链从5′末端逐步切除3、在DNA聚合酶I的5′→3′聚合酶催化下,以互补的DNA单链为模板依次将dNTP 连接到切口的3′末端-OH上,合成新的DNA链,同时将标记的核苷酸掺入到新的DNA 链中(二) 随机引物法其原理是随机引物能与各种单链DNA模板结合,作为合成新链的引物,在DNA聚合酶的催化下,按5′→3′方向合成一新的DNA链,其核苷酸序列与模板DNA完全互补。
急性白血病M4EO型患者ETV6基因重排及其意义知识讲解

急性白血病M4E O型患者E T V6基因重排及其意义【摘要】目的探讨在急性髓细胞白血病(aml)m4eo型患者中检测到etv6基因重排的临床意义。
方法对3例aml m4eo患者骨髓标本进行细胞培养,制备染色体,分析核型,split signal fish技术分析12p13染色体易位,rt pcr检测etv6/arg融合基因产物。
结果核型分析显示3例患者均有inv(16),split signal fish检出12p13染色体易位1例,rt pcr证实患者etv6/arg融合基因产物阳性。
这是国际上第2例报道etv6/arg融合基因在aml m4eo中表达。
结论伴有etv6/arg融合基因的患者对化疗不敏感,预后较etv6/arg融合基因阴性者差。
split signal fish是检测etv6基因重排准确可靠的手段。
【关键词】白血病;etv6;原位杂交;聚合酶链反应1 资料与方法1.1 病例资料1.2 骨髓细胞培养及染色体制备方法取患者骨髓标本,在无刺激条件下培养16~24?h后收获细胞,常规制备骨髓细胞染色体,进行r显带处理,核型根据《人类细胞遗传学国际命名体制(iscn 1995)》识别和描述。
1.3 split signal fish分析对3例aml m4eo患者染色体标本进行荧光原位杂交检测其etv6重排情况。
荧光原位杂交试剂盒、human cot i dna、链霉亲和素hrp工作液、dapi均购自美国invitrogen公司,缺口平移法标记探针dig nick translation mix试剂盒、biotin nick translation mix试剂盒、抗dig ap、hnpp/fast red tr均为roche公司产品,质粒dna提取试剂盒为qiagen公司产品,去离子甲酰胺为amresco公司产品,严格按操作说明书进行操作。
1.3.1 探针选取位于etv6基因两侧的基因序列(bac)作为探针。
brentuximab vedotin 英文版说明书

HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ADCETRIS safely and effectively. See full prescribing information for ADCETRIS.ADCETRIS TM (brentuximab vedotin) for InjectionFor intravenous infusionInitial U.S. approval: 2011WARNING: PROGRESSIVE MULTIFOCALLEUKOENCEPHALOPATHY (PML)See Full Prescribing Information for complete boxed warning. JC virus infection resulting in PML and death can occur in patients receiving ADCETRIS (5.5, 6.2).---------------------------------RECENT MAJOR CHANGES--------------------------Boxed Warning 01/2012 Dosage and Administration (2.1) 01/2012 Contraindications (4) 01/2012 Warnings and Precautions, PML (5.5) 01/2012 ----------------------------------INDICATIONS AND USAGE---------------------------ADCETRIS is a CD30-directed antibody-drug conjugate indicated for:• The treatment of patients with Hodgkin lymphoma after failure of autologous stem cell transplant (ASCT) or after failure of at least two prior multi-agent chemotherapy regimens in patients who are notASCT candidates (1.1).• The treatment of patients with systemic anaplastic large cell lymphoma after failure of at least one prior multi-agent chemotherapy regimen(1.2).These indications are based on response rate. There are no data available demonstrating improvement in patient reported outcomes or survival with ADCETRIS.-----------------------------DOSAGE AND ADMINISTRATION ----------------------• The recommended dose is 1.8 mg/kg administered only as an intravenous infusion over 30 minutes every 3 weeks (2).• Continue treatment until a maximum of 16 cycles, disease progression or unacceptable toxicity.---------------------------DOSAGE FORMS AND STRENGTHS --------------------50 mg single-use vial (3).-------------------------------------CONTRAINDICATIONS------------------------------Concomitant use of ADCETRIS and bleomycin is contraindicated due to pulmonary toxicity (4). -------------------------WARNINGS AND PRECAUTIONS ---------------------• Peripheral neuropathy: Treating physicians should monitor patients for neuropathy and institute dose modifications accordingly (5.1). • Infusion reactions: If an infusion reaction occurs, the infusion should be interrupted and appropriate medical managementinstituted. If anaphylaxis occurs, the infusion should bediscontinued immediately and appropriate medical managementinstituted (5.2).• Neutropenia: Monitor complete blood counts prior to each dose of ADCETRIS. If Grade 3 or 4 neutropenia develops, manage by dose delays, reductions or discontinuation (5.3).• Tumor Lysis Syndrome: Patients with rapidly proliferating tumor and high tumor burden are at risk of tumor lysis syndrome andthese patients should be monitored closely and appropriatemeasures taken (5.4).• Progressive Multifocal Leukoencephalopathy (PML): Monitor neurologic function; hold ADCETRIS if PML is suspected anddiscontinue ADCETRIS if PML is confirmed. (5.5).• Stevens-Johnson syndrome: If Stevens-Johnson syndrome occurs, discontinue ADCETRIS and administer appropriate medical therapy(5.6).• Use in pregnancy: Fetal harm can occur. Pregnant women should be advised of the potential hazard to the fetus (5.7).--------------------------------ADVERSE REACTIONS ----------------------------The most common adverse reactions (≥20%) are neutropenia, peripheral sensory neuropathy, fatigue, nausea, anemia, upper respiratory tract infection, diarrhea, pyrexia, rash, thrombocytopenia, cough, and vomiting (6.1).To report SUSPECTED ADVERSE REACTIONS, contact Seattle Genetics, Inc. at 1-855-473-2436 or FDA at 1-800-FDA-1088 or /medwatch.---------------------------------DRUG INTERACTIONS-----------------------------Patients who are receiving strong CYP3A4 inhibitors concomitantly with ADCETRIS should be closely monitored for adverse reactions (7.1).-------------------------USE IN SPECIFIC POPULATIONS ---------------------None (8).See 17 for PATIENT COUNSELING INFORMATION.Revised January 2012FULL PRESCRIBING INFORMATION: CONTENTS WARNING: PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY (PML)1 INDICATIONS AND USAGE1.1 Hodgkin Lymphoma1.2 Systemic Anaplastic Large Cell Lymphoma2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information2.2 Dose Modification2.3 Instructions for Preparation and Administration3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS5.1 Peripheral Neuropathy5.2 Infusion Reactions5.3 Neutropenia5.4 Tumor Lysis Syndrome5.5 Progressive Multifocal Leukoencephalopathy5.6 Stevens-Johnson Syndrome5.7 Use in Pregnancy6 ADVERSE REACTIONS6.1 Clinical Trial Experience6.2 Post Marketing Experience6.3 Immunogenicity7 DRUG INTERACTIONS7.2 Effect of ADCETRIS on Other Drugs8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.3 Nursing Mothers8.4 Pediatric Use8.5 Geriatric Use8.6 Renal Impairment8.7 Hepatic Impairment10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.2 Pharmacodynamics12.3 Pharmacokinetics13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility14 CLINICAL STUDIES14.1 Hodgkin Lymphoma14.2 Systemic Anaplastic Large Cell Lymphoma15 REFERENCES16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied16.2 Storage16.3 Special Handling17 PATIENT COUNSELING INFORMATIONFULL PRESCRIBING INFORMATIONWARNING: PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY (PML)JC virus infection resulting in PML and death can occur in patients receiving ADCETRIS [see Warnings and Precautions (5.5), Adverse Reactions (6.1)].1 INDICATIONS AND USAGEThese indications are based on response rate. There are no data available demonstrating improvement in patient reported outcomes or survival with ADCETRIS.Lymphoma1.1 HodgkinADCETRIS (brentuximab vedotin) is indicated for treatment of patients with Hodgkin lymphoma (HL) after failure of autologous stem cell transplant (ASCT) or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates.1.2 Systemic Anaplastic Large Cell LymphomaADCETRIS is indicated for treatment of patients with systemic anaplastic large cell lymphoma (sALCL) after failure of at least one prior multi-agent chemotherapy regimen.2 DOSAGE AND ADMINISTRATION2.1 General Dosing InformationThe recommended dose is 1.8 mg/kg administered only as an intravenous infusion over30 minutes every 3 weeks. The dose for patients weighing greater than 100 kg should be calculated based on a weight of 100 kg.Do not administer as an intravenous push or bolus.Continue treatment until a maximum of 16 cycles, disease progression or unacceptable toxicity.Modification2.2 DosePeripheral Neuropathy: Peripheral neuropathy should be managed using a combination of dose delay and reduction to 1.2 mg/kg. For new or worsening Grade 2 or 3 neuropathy, dosing should be held until neuropathy improves to Grade 1 or baseline and then restarted at1.2 mg/kg. For Grade 4 peripheral neuropathy, ADCETRIS should be discontinued. Neutropenia: Neutropenia should be managed by dose delays and reductions. The dose of ADCETRIS should be held for Grade 3 or 4 neutropenia until resolution to baseline or Grade 2 or lower. Growth factor support should be considered for subsequent cycles in patients who experience Grade 3 or 4 neutropenia. In patients with recurrent Grade 4 neutropenia despite the use of growth factors, discontinuation or dose reduction of ADCETRIS to 1.2 mg/kg may be considered.2.3 Instructions for Preparation and AdministrationProcedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published [see References (15)].Use appropriate aseptic technique for reconstitution and preparation of dosing solutions.ReconstitutionCalculate the dose (mg) and number of vials of ADCETRIS required. The dose for patients weighing greater than 100 kg should be calculated based on a weight of 100 kg. Reconstitute each 50 mg vial of ADCETRIS with 10.5 mL of Sterile Water for Injection, USP, to yield a single-use solution containing 5 mg/mL brentuximab vedotin. Direct the stream toward wall of vial and not directly at the cake or powder. Gently swirl the vial to aid dissolution. DO NOT SHAKE. Inspect the reconstituted solution for particulates and discoloration. The reconstituted solution should be clear to slightly opalescent, colorless, and free of visible particulates. Following reconstitution, dilute immediately into an infusion bag, or store the solution at 2-8˚C (36-46˚F) and use within 24 hours of reconstitution. DO NOT FREEZE. Discard any unused portion left in the vial.DilutionCalculate the required volume of 5 mg/mL reconstituted ADCETRIS solution needed and withdraw this amount from the vials. The dose for patients weighing greater than 100 kg should be calculated based on a weight of 100 kg. Immediately add the reconstituted solution to an infusion bag containing a minimum volume of 100 mL to achieve a final concentration of 0.4mg/mL to 1.8 mg/mL brentuximab vedotin. ADCETRIS can be diluted into 0.9% Sodium Chloride Injection, 5% Dextrose Injection or Lactated Ringer's Injection. Gently invert the bag to mix the solution. ADCETRIS contains no bacteriostatic preservatives. Following dilution, infuse the ADCETRIS solution immediately, or store the solution at 2-8˚C (36-46˚F) and use within 24 hours of reconstitution. DO NOT FREEZE.Do not mix ADCETRIS with, or administer as an infusion with, other medicinal products.3 DOSAGE FORMS AND STRENGTHSADCETRIS (brentuximab vedotin) for Injection single-use vial containing 50 mg of brentuximab vedotin as a sterile, white to off-white lyophilized, preservative-free cake or powder.4 CONTRAINDICATIONSPulmonary toxicity: Concomitant use of ADCETRIS and bleomycin is contraindicated due to pulmonary toxicity. In a clinical trial that studied ADCETRIS with bleomycin as part of a combination regimen, the rate of non-infectious pulmonary toxicity was higher than the historical incidence reported with ABVD (adriamycin, bleomycin, vinblastine, dacarbazine). Patients typically reported cough and dyspnea. Interstitial infiltration and/or inflammation were observed on radiographs and computed tomographic imaging of the chest. Most patients responded to corticosteroids.5 WARNINGS AND PRECAUTIONSNeuropathy5.1 PeripheralADCETRIS treatment causes a peripheral neuropathy that is predominantly sensory. Cases of peripheral motor neuropathy have also been reported. ADCETRIS-induced peripheral neuropathy is cumulative. In the HL and sALCL clinical trials, 54% of patients experienced any grade of neuropathy. Of these patients, 49% had complete resolution, 31% had partial improvement, and 20% had no improvement. Of the patients who reported neuropathy, 51% had residual neuropathy at the time of their last evaluation. Monitor patients for symptoms of neuropathy, such as hypoesthesia, hyperesthesia, paresthesia, discomfort, a burning sensation, neuropathic pain or weakness. Patients experiencing new or worsening peripheral neuropathy may require a delay, change in dose, or discontinuation of ADCETRIS [see Dose Modification (2.2)].Reactions5.2 InfusionInfusion-related reactions, including anaphylaxis, have occurred with ADCETRIS. Monitor patients during infusion. If anaphylaxis occurs, immediately and permanently discontinue administration of ADCETRIS and administer appropriate medical therapy. If an infusion-related reaction occurs, the infusion should be interrupted and appropriate medical management instituted. Patients who have experienced a prior infusion-related reaction should be premedicated for subsequent infusions. Premedication may include acetaminophen, an antihistamine and a corticosteroid.5.3 NeutropeniaComplete blood counts should be monitored prior to each dose of ADCETRIS and more frequent monitoring should be considered for patients with Grade 3 or 4 neutropenia. Prolonged (≥1 week) severe neutropenia can occur with ADCETRIS. If Grade 3 or 4 neutropenia develops, manage by dose delays, reductions, or discontinuations [see Dose Modification (2.2)].5.4 Tumor Lysis SyndromeTumor lysis syndrome may occur. Patients with rapidly proliferating tumor and high tumor burden may be at increased risk of tumor lysis syndrome. Monitor closely and take appropriate measures.5.5 Progressive Multifocal LeukoencephalopathyJC virus infection resulting in PML and death has been reported in ADCETRIS-treated patients. In addition to ADCETRIS therapy, other possible contributory factors include prior therapies and underlying disease that may cause immunosuppression.Consider the diagnosis of PML in any patient presenting with new-onset signs and symptoms of central nervous system abnormalities. Evaluation of PML includes, but is not limited to, consultation with a neurologist, brain MRI, and lumbar puncture or brain biopsy. Hold ADCETRIS dosing for any suspected case of PML and discontinue ADCETRIS dosing if a diagnosis of PML is confirmed.5.6 Stevens-JohnsonSyndromeStevens-Johnson syndrome has been reported with ADCETRIS. If Stevens-Johnson syndrome occurs, discontinue ADCETRIS and administer appropriate medical therapy.5.7 Use in PregnancyThere are no adequate and well-controlled studies of ADCETRIS in pregnant women. However, based on its mechanism of action and findings in animals, ADCETRIS can cause fetal harm when administered to a pregnant woman. Brentuximab vedotin caused embryo-fetal toxicities, including significantly decreased embryo viability and fetal malformations, in animals at maternal exposures that were similar to human exposures at the recommended doses for patients with HL and sALCL. If this drug is used during pregnancy, or if the patient becomes pregnant while receiving the drug, the patient should be apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)].REACTIONS6 ADVERSE6.1 Clinical Trial ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.ADCETRIS was studied as monotherapy in 160 patients in two phase 2 trials. Across both trials, the most common adverse reactions (≥20%), regardless of causality, were neutropenia, peripheral sensory neuropathy, fatigue, nausea, anemia, upper respiratory tract infection, diarrhea, pyrexia, rash, thrombocytopenia, cough and vomiting. The most common adverse reactions occurring in at least 10% of patients in either trial, regardless of causality, using the NCI Common Toxicity Criteria Version 3.0, are shown in Table 1.Experience in Hodgkin LymphomaADCETRIS was studied in 102 patients with HL in a single arm clinical trial in which the recommended starting dose and schedule was 1.8 mg/kg intravenously every 3 weeks. Median duration of treatment was 27 weeks (range, 3 to 56 weeks) [see Clinical Studies (14)].The most common adverse reactions (≥20%), regardless of causality, were neutropenia, peripheral sensory neuropathy, fatigue, upper respiratory tract infection, nausea, diarrhea, anemia, pyrexia, thrombocytopenia, rash, abdominal pain, cough, and vomiting.Experience in Systemic Anaplastic Large Cell LymphomaADCETRIS was studied in 58 patients with sALCL in a single arm clinical trial in which the recommended starting dose and schedule was 1.8 mg/kg intravenously every 3 weeks. Median duration of treatment was 24 weeks (range, 3 to 56 weeks) [see Clinical Studies (14)].The most common adverse reactions (≥20%), regardless of causality, were neutropenia, anemia, peripheral sensory neuropathy, fatigue, nausea, pyrexia, rash, diarrhea, and pain.Combined ExperienceTable 1: Most Commonly Reported (≥10%) Adverse ReactionsHL Total N = 102% of patients sALCLTotal N = 58 % of patientsAdverse ReactionAnyGradeGrade3Grade4AnyGradeGrade3Grade4Blood and lymphatic systemdisordersNeutropenia* 54 15 6 55 12 9 Anemia* 33 8 2 52 2 -Thrombocytopenia* 28 7 2 16 5 5 LymphadenopathyNervous system disorders11 --10 --Peripheral sensoryneuropathy52 8 -53 10 -Peripheral motorneuropathy16 4 -7 3 -Headache 19 --16 2 -DizzinessGeneral disorders andadministration site conditions11 --16 --Fatigue 49 3 -41 2 2 Pyrexia 29 2 -38 2 -Chills 13 --12 --Pain 7 --28 - 5Edema peripheralInfections and infestations4 --16 --Upper respiratory tractinfectionGastrointestinal disorders47 --12 --Nausea 42 --38 2 -Diarrhea 36 1 -29 3 -Abdominal pain 25 2 1 9 2 -Vomiting 22 --17 3 -ConstipationSkin and subcutaneoustissue disorders16 --19 2 -Rash 27 --31 --Pruritus 17 --19 --Alopecia 13 --14 --Night sweats 12 --9 --Dry skin 4 --10 --HL Total N = 102% of patients sALCLTotal N = 58 % of patientsAdverse ReactionAnyGradeGrade3Grade4AnyGradeGrade3Grade4Respiratory, thoracic andmediastinal disordersCough 25 --17 --Dyspnea 13 1 -19 2 -Oropharyngeal painMusculoskeletal andconnective tissue disorders11 --9 --Arthralgia 19 --9 --Myalgia 17 --16 2 -Back pain 14 --10 2 -Pain in extremity 10 --10 2 2Muscle spasmsPsychiatric disorders9 --10 2 -Insomnia 14 --16 --AnxietyMetabolism and nutritiondisorders11 2 -7 --Decreased appetiteInvestigations11 --16 2 -Weight decreased 6 --12 3 -*Derived from laboratory values and adverse reaction dataInfusion reactionsTwo cases of anaphylaxis were reported in phase 1 trials. There were no Grade 3 or 4 infusion-related reactions reported in the phase 2 trials, however, Grade 1 or 2 infusion-related reactions were reported for 19 patients (12%). The most common adverse reactions (≥2%) associated with infusion-related reactions were chills (4%), nausea (3%), dyspnea (3%), pruritus (3%), pyrexia (2%), and cough (2%).Serious adverse reactionsIn the phase 2 trials, serious adverse reactions, regardless of causality, were reported in 31% of patients receiving ADCETRIS. The most common serious adverse reactions experienced by patients with HL include peripheral motor neuropathy (4%), abdominal pain (3%), pulmonary embolism (2%), pneumonitis (2%), pneumothorax (2%), pyelonephritis (2%), and pyrexia (2%). The most common serious adverse reactions experienced by patients with sALCL were septic shock (3%), supraventricular arrhythmia (3%), pain in extremity (3%), and urinary tract infection(3%). Other important serious adverse reactions reported include PML, Stevens-Johnson syndrome and tumor lysis syndrome.Dose modificationsAdverse reactions that led to dose delays in more than 5% of patients were neutropenia (14%) and peripheral sensory neuropathy (11%) [see Dose Modification (2.2)].DiscontinuationsAdverse reactions led to treatment discontinuation in 21% of patients. Adverse reactions that led to treatment discontinuation in 2 or more patients with HL or sALCL were peripheral sensory neuropathy (8%) and peripheral motor neuropathy (3%).6.2 Post Marketing ExperienceThe following adverse reactions have been identified during post-approval use of ADCETRIS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.Cases of PML have been reported [see Boxed Warning, Warnings and Precautions (5.5)].6.3 ImmunogenicityPatients with HL and sALCL in the phase 2 trials [see Clinical Studies (14)] were tested for antibodies to brentuximab vedotin every 3 weeks using a sensitive electrochemiluminescent immunoassay. Approximately 7% of patients in these trials developed persistently positive antibodies (positive test at more than 2 timepoints) and 30% developed transiently positive antibodies (positive in 1 or 2 post-baseline timepoints). The anti-brentuximab antibodies were directed against the antibody component of brentuximab vedotin in all patients with transiently or persistently positive antibodies. Two of the patients (1%) with persistently positive antibodies experienced adverse reactions consistent with infusion reactions that led to discontinuation of treatment. Overall, a higher incidence of infusion related reactions was observed in patients who developed persistently positive antibodies.A total of 58 patient samples that were either transiently or persistently positive for anti-brentuximab vedotin antibodies were tested for the presence of neutralizing antibodies. Sixty-two percent of these patients had at least one sample that was positive for the presence of neutralizing antibodies. The effect of anti-brentuximab vedotin antibodies on safety and efficacy is not known.Immunogenicity assay results are highly dependent on several factors including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of incidence of antibodies to ADCETRIS with the incidence of antibodies to other products may be misleading.7 DRUGINTERACTIONSIn vitro data indicate that monomethyl auristatin E (MMAE) is a substrate and an inhibitor of CYP3A4/5.7.1 Effect of Other Drugs on ADCETRISCYP3A4 Inhibitors/Inducers: MMAE is primarily metabolized by CYP3A [see Clinical Pharmacology (12.3)]. Co-administration of ADCETRIS with ketoconazole, a potent CYP3A4 inhibitor, increased exposure to MMAE by approximately 34%. Patients who are receiving strong CYP3A4 inhibitors concomitantly with ADCETRIS should be closely monitored for adverse reactions. Co-administration of ADCETRIS with rifampin, a potent CYP3A4 inducer, reduced exposure to MMAE by approximately 46%.7.2 Effect of ADCETRIS on Other DrugsCo-administration of ADCETRIS did not affect exposure to midazolam, a CYP3A4 substrate. MMAE does not inhibit other CYP enzymes at relevant clinical concentrations [see Clinical Pharmacology (12.3)]. ADCETRIS is not expected to alter the exposure to drugs that are metabolized by CYP3A4 enzymes.8 USE IN SPECIFIC POPULATIONS8.1 PregnancyPregnancy Category D [see Warnings and Precautions (5.7)].There are no adequate and well-controlled studies with ADCETRIS in pregnant women. However, based on its mechanism of action and findings in animals, ADCETRIS can cause fetal harm when administered to a pregnant woman. Brentuximab vedotin caused embryo-fetal toxicities in animals at maternal exposures that were similar to human exposures at the recommended doses for patients with HL and sALCL. If this drug is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus.In an embryo-fetal developmental study, pregnant rats received 2 intravenous doses of 0.3, 1, 3, or 10 mg/kg brentuximab vedotin during the period of organogenesis (once each on Pregnancy Days 6 and 13). Drug-induced embryo-fetal toxicities were seen mainly in animals treated with 3 and 10 mg/kg of the drug and included increased early resorption (≥99%), post-implantation loss (≥99%), decreased numbers of live fetuses, and external malformations (i.e., umbilical hernias and malrotated hindlimbs). Systemic exposure in animals at the brentuximab vedotin dose of 3 mg/kg is approximately the same exposure in patients with HL or sALCL who received the recommended dose of 1.8 mg/kg every three weeks.Mothers8.3 NursingIt is not known whether brentuximab vedotin is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ADCETRIS a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.8.4 PediatricUseThe safety and effectiveness of ADCETRIS have not been established in the pediatric population. Clinical trials of ADCETRIS included only 9 pediatric patients and this number is not sufficient to determine whether they respond differently than adult patients.Use8.5 GeriatricClinical trials of ADCETRIS did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Safety and efficacy have not been established.Impairment8.6 RenalThe kidney is a route of excretion for MMAE. The influence of renal impairment on the pharmacokinetics of MMAE has not been determined.Impairment8.7 HepaticThe liver is a route of clearance for MMAE. The influence of hepatic impairment on the pharmacokinetics of MMAE has not been determined.10 OVERDOSAGEThere is no known antidote for overdosage of ADCETRIS. In case of overdosage, the patient should be closely monitored for adverse reactions, particularly neutropenia, and supportive treatment should be administered.11 DESCRIPTIONADCETRIS (brentuximab vedotin) is a CD30-directed antibody-drug conjugate (ADC) consisting of three components: 1) the chimeric IgG1 antibody cAC10, specific for human CD30, 2) the microtubule disrupting agent MMAE, and 3) a protease-cleavable linker that covalently attaches MMAE to cAC10.Brentuximab vedotin has an approximate molecular weight of 153 kDa. Approximately4 molecules of MMAE are attached to each antibody molecule. Brentuximab vedotin is produced by chemical conjugation of the antibody and small molecule components. The antibody is produced by mammalian (Chinese hamster ovary) cells, and the small molecule components are produced by chemical synthesis.ADCETRIS (brentuximab vedotin) for Injection is supplied as a sterile, white to off-white, preservative-free lyophilized cake or powder in single-use vials. Following reconstitution with10.5 mL Sterile Water for Injection, USP, a solution containing 5 mg/mL brentuximab vedotin is produced. The reconstituted product contains 70 mg/mL trehalose dihydrate, 5.6 mg/mL sodium citrate dihydrate, 0.21 mg/mL citric acid monohydrate, and 0.20 mg/mL polysorbate 80 and water for injection. The pH is approximately 6.6.12 CLINICAL PHARMACOLOGY12.1 Mechanism of ActionBrentuximab vedotin is an ADC. The antibody is a chimeric IgG1 directed against CD30. The small molecule, MMAE, is a microtubule disrupting agent. MMAE is covalently attached to the antibody via a linker. Nonclinical data suggest that the anticancer activity of ADCETRIS is due to the binding of the ADC to CD30-expressing cells, followed by internalization of theADC-CD30 complex, and the release of MMAE via proteolytic cleavage. Binding of MMAE to tubulin disrupts the microtubule network within the cell, subsequently inducing cell cycle arrest and apoptotic death of the cells.12.2 PharmacodynamicsQT/QTc Prolongation PotentialThe effect of brentuximab vedotin (1.8 mg/kg) on the QTc interval was evaluated in an open-label, single-arm study in 46 evaluable patients with CD30-expressing hematologic malignancies. Administration of brentuximab vedotin did not prolong the mean QTc interval>10 ms from baseline. Small increases in the mean QTc interval (<10 ms) cannot be excluded because this study did not include a placebo arm and a positive control arm.12.3 PharmacokineticsThe pharmacokinetics of brentuximab vedotin were evaluated in phase 1 trials and in a population pharmacokinetic analysis of data from 314 patients. The pharmacokinetics of three analytes were determined: the ADC, MMAE, and total antibody. Total antibody had the greatest exposure and had a similar PK profile as the ADC. Hence, data on the PK of the ADC and MMAE have been summarized.AbsorptionMaximum concentrations of ADC were typically observed close to the end of infusion. A multiexponential decline in ADC serum concentrations was observed with a terminal half-life of approximately 4 to 6 days. Exposures were approximately dose proportional from 1.2 to2.7 mg/kg. Steady-state of the ADC was achieved within 21 days with every 3-week dosing of ADCETRIS, consistent with the terminal half-life estimate. Minimal to no accumulation of ADC was observed with multiple doses at the every 3-week schedule.The time to maximum concentration for MMAE ranged from approximately 1 to 3 days. Similar to the ADC, steady-state of MMAE was achieved within 21 days with every 3 week dosing of ADCETRIS. MMAE exposures decreased with continued administration of ADCETRIS with approximately 50% to 80% of the exposure of the first dose being observed at subsequent doses.。
基因定位常用的方法

DMD基因克隆的方法
研究者们通过对几个女性患者的细胞遗传学研究发现每个病例受累的常染色体不同但在X染色体上的断裂点总是p21。 同时一个无任何遗传缺陷家族史的男孩被发现患有DMD等4种X连锁隐性遗传病,在这个独一无二个体的引发下,经过仔细的遗传学研究,最终发现了Xp21.1的微小缺失。
DMD女性患者的核型
*
2.原位杂交和荧光原位杂交
原位杂交(in situ hybridization):是最直接的基因定位方法之一,是分子生物学技术在基因定位中的应用,胰岛素基因用此方法定位于11p15。 原理:碱基的互补配对,同源的DNA-DNA双链或DNA-RNA双链在一定条件下能结合成双链。用放射性或非放射性物质标记的DNA、RNA或与mRNA互补的cDNA作探针,可检测细胞基因组中的同源部分。
多重PCR筛查抗肌萎缩蛋白基因缺失
FOR WATCHING
X染色体与常染色体易位时X染色体失活的结果
两个研究小组分别采用两种不同的方法克隆了DMD基因: 一组是通过X常染色体易位,克隆了该基因的一部分。 另一研究组使用有Xp21.1微小缺失的男孩的DNA,利用消减技术,获得了在正常X染色体存在而在这个男孩DNA中缺乏的DNA克隆片段。
*
一名患有DMD等4种X连锁隐性遗传病的男孩存在Xp21.2的微小臂内缺失
定位克隆鉴定的第一批基因利用了特定基因座的染色体畸变,Duchenne肌营养不良(DMD)基因的克隆是一个重要的例子。
*
假肥大型肌营养不良症(DMD/BMD)
由于抗肌萎缩蛋白(dystrophin)遗传性缺陷所致的进行性肌萎缩的致死性神经肌肉性遗传病,发病率为1/3500,XR遗传。
主要症状:通常3---5岁发病,开始走路不稳,鸭型步态,上楼梯困难,Gower征阳性.进行性肌萎缩伴有腓肠肌假性肥大,10多岁下肢瘫,一般在20多岁之前死于心衰或呼吸衰竭.
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Biotin-Nick Translation Mix
for in situ probes For generation of highly sensitive probes for in situ hybridization labeled with biotin-16-dUTP Premixed solution for 40 labeling reactions
Cat. No. 11 745 824 910 160 l
y Version 09
Content version: August 2007 Store at Ϫ15 to Ϫ25° C
Application Probes prepared with the Biotin-Nick Translation Mix for in situ probes are especially qualified for in situ hybridization applications but can also be used for filter hybridization techniques. Note: For highly sensitive filter hybridization probes we recommend to use Biotin-High Prime*. For non-radioactive labeling of in situ probes with other haptens Roche Applied Science offers the DIG-Nick Translation Mix*.
Stability material 2.1 Standard labeling reaction
Additional • heating block equipment and • 0.5 M EDTA (pH 8.0) reagents required Procedure In the following table please find a protocol for the standard labeling reaction. Step 1 2 3 Action Add 1 µg template DNA to sterile, double distilled water and end up with a final volume of 16 l. Add 4 l Biotin-Nick Translation Mix, mix and centrifuge briefly. Incubate for 90 min at +15°C. Stop the reaction by adding 1 l 0.5 M EDTA (pH 8.0) and heating to +65°C for 10 min.
1. Product overview
Contents Label Sufficient for 40 labeling reactions Content
Biotin-Nick Transla- • 160 l tion Mix for in situ • 5× conc. probes, • stabilized reaction buffer in 50% glycerol (v/v) and 5 × conc. DNA Polymerase I, DNase I, 0.25 mM dATP, 0.25 mM dCTP, 0.25 mM dGTP, 0.17 mM dTTP and 0.08 mM Biotin-16-dUTP. Labeling principle The nick translation method (1) is based on the ability of DNase I to introduce randomly distributed nicks into DNA at low enzyme concentrations in the presence of MgCl2. E.coli DNA Polymerase I synthesizes DNA complementary to the intact strand in a 5' 3' direction using the 3'-OH termini of the nick as a primer (2). The 5' 3' exonucleolytic activity of DNA polymerase I simultaneously removes nucleotides in the direction of synthesis (3). The polymerase activity sequentially replaces the removed nucleotides with isotope-labeled or hapten-labeled deoxyribonucleoside triphosphates (1). At low temperature (15°C), the unlabeled DNA in the reaction is thus replaced by newly synthesized labeled DNA. in situ hybridization In in situ hybridization experiments, the fragment length distribution influences severely the efficiency of hybridization (4). The use of probes showing fragment lengths above the optimal range of 200-500 nucleotides usually results in enhanced spotty background signals due to unspecific sticking of the probe to the glass surface. It also can result in reduced accessibility to the target nucleic acid (like metaphase chromosomes or cellular and tissue targets). On the other hand use of very short probes will result in poor hybridization efficiency and sensitivity. This is due to fast rehybridization kinetics of short fragments yielding a high proportion of "snapback" probe DNA that reduces the amount of probe being available for hybridization to the target (5). Thus the level of DNase I is of high relevance in probe labeling for in situ applications. The use of the premixed nick solution reduces pipetting steps and increases the reproducibility of the labeling reaction. • supercoiled and linearized plasmid DNA • supercoiled and linearized cosmid DNA • purified PCR products Note: Denaturation of the template before nick translation is not required.
Length of labeled The labeled fragments obtained in the standard labeling reaction show a length distribution maximum fragments in the range of 200 to 500 nucleotides. Molar ratio The molar ratio of biotin-16-dUTP to dTTP is adjusted to ensure that every 20th-25th nucleotide in the newly synthesized DNA is modified with biotin This density of haptens in the DNA yields the highest sensitivity in the immunological detection reaction. The unopened vial is stable at Ϫ15 to Ϫ25°C until the control date printed on the label. Note: Repeated freezing and thawing should be avoided. To avoid contamination we recommend to aliquot the Biotin-Nick translation Mix solution and to store in 2-3 portions.
Sample material
4
0511.11746 812001➈
2.2 Determination of fragment length of the labeled probe
