奥美拉唑-欧洲8.0药典
奥美拉唑 (Omeprazole) 抗酸药物

奥美拉唑 (Omeprazole) 抗酸药物奥美拉唑 (Omeprazole) 抗酸药物奥美拉唑(Omeprazole)是一种广泛应用的抗酸药物,被用于治疗胃酸相关的疾病。
它属于质子泵抑制剂(Proton Pump Inhibitors,简称PPIs)的一种,通过抑制胃酸的分泌来缓解相关症状。
本文将详述奥美拉唑的作用机制、临床应用、副作用与注意事项。
一、作用机制奥美拉唑通过抑制胃壁中的质子泵酶(质子转运ATP酶)活性,从而减少胃酸的分泌。
该药物在酸性环境中快速转化为活性形式,并与质子泵酶结合,使其失去催化作用,从而抑制酸的产生。
奥美拉唑的作用可持续数天,因此它一般作为长期治疗消化性溃疡和胃食管反流病的首选药物。
二、临床应用1. 消化性溃疡奥美拉唑常用于治疗胃溃疡和十二指肠溃疡。
它能有效减少胃酸分泌,促进溃疡愈合。
通常建议患者在饭前服用奥美拉唑,以提高药物的吸收效果。
2. 胃食管反流病胃食管反流病是指胃酸逆流至食管和喉部,引起不适症状,如胸骨后灼热感、咳嗽等。
奥美拉唑通过抑制胃酸分泌,减少胃酸对食管的刺激,缓解相关症状。
3. Zollinger-Ellison综合征Zollinger-Ellison综合征是一种罕见的疾病,患者体内存在胃酸分泌过多的肿瘤,导致消化性溃疡和其他症状。
奥美拉唑可用于控制胃酸分泌,帮助控制疾病的进展。
三、副作用与注意事项1. 副作用奥美拉唑一般耐受性良好,但长期使用时可能会出现一些副作用。
常见的副作用包括头痛、腹痛、恶心、腹泻等。
偶尔会出现肝功能异常、皮疹和关节炎等不良反应。
2. 注意事项(1)奥美拉唑不适用于对本药物过敏的患者。
(2)在使用奥美拉唑前,应告知医生有无其他慢性病、药物过敏等情况。
(3)长期使用奥美拉唑时,应定期进行胃镜检查,以评估疾病状况和确认疗效。
(4)奥美拉唑可与其他药物发生相互作用,例如抗凝药物、抗癫痫药物等,服用时应咨询医生。
结语奥美拉唑是一种常用的抗酸药物,通过抑制胃酸的分泌来治疗消化性溃疡和胃食管反流病等疾病。
质量标准奥美拉唑

AS-NAI 质量标准
检查项目方法标准规定
性状目测
本品应为白色或类白色的冻干块状物或
粉末
鉴别一般鉴别试
验
本品显钠盐的鉴别反应(中国药典2010
年版二部附录Ⅲ)
HPLC
在对映体纯度测定项下记录的高效液相
色谱图中,供试品溶液主峰的保留时间
应与对照品溶液主峰的保留时间一致。
HPLC
在含量测定项下记录的高效液相色谱图
中,供试品溶液主峰的保留时间应与对
照品溶液主峰的保留时间一致。
溶液的颜色与澄清度溶液颜色检
查法
澄清度检查
法
取本品1瓶,加水1ml使溶解,溶液
应澄清无色,如显浑浊,与1号浊度标准
液(中国药典2010版二部附录IX B)比
较,不得更浓;如显色,与黄色1号标准
比色液(中国药典2010版二部附录IX A)
比较,不得更深。
碱度碱度检查法
取本品1瓶,加水1ml溶解后,依
法测定(中国药典2010版二部附录VI
H),pH值应为10.0~11.5
水分水分测定法
取本品适量,以无水甲醇为溶剂,照
水分测定法(中国药典2010年版二部附
录Ⅷ M第一法)测定,含水分不得过
0.5%。
有关物质HPLC 按面积归一化法计算,杂质C(碸)的量不得过0.2%、杂质A(咪唑), 杂质B (氮氧化物)和杂质D(硫醚)的量均不得过0.1%,其他单个杂质的量不得过0.1%,杂质总量不得过0.5%。
对映体纯度HPLC 纯度不低于99.5%
含量HPLC 含埃索美拉唑钠(C17H18N3NaO3S)应为标示量的90%~110%。
奥美拉唑钠检验标准操作规程

奥美拉唑钠是一种常见的质子泵抑制剂,用于治疗胃酸过多相关的疾病。
在药典或实验室标准操作规程中,对奥美拉唑钠的检验通常包括纯度检查、含量测定、有关物质检查等项目。
以下是一个简化的奥美拉唑钠检验标准操作规程示例:
1. 试剂与仪器准备
奥美拉唑钠原料药或注射液。
盐酸、氢氧化钠等溶剂。
高效液相色谱仪(HPLC)。
分析天平、移液器等实验室常规仪器。
2. 纯度检查
通过高效液相色谱法(HPLC)测定奥美拉唑钠的纯度。
制备适宜的样品溶液,注入HPLC仪,设置合适的色谱条件(如柱温、流动相组成、流速、检测波长等)。
记录色谱图,计算奥美拉唑钠的峰面积,与标准品比较,确保样品纯度符合药典规定。
3. 含量测定
采用紫外分光光度法或HPLC法测定奥美拉唑钠的含量。
制备标准系列溶液,分别测定吸光度或峰面积,绘制标准曲线。
取适量样品溶液,按照与标准系列相同的方法测定吸光度或峰面积。
根据标准曲线计算样品中奥美拉唑钠的含量。
4. 有关物质检查
通过HPLC检查奥美拉唑钠中的有关物质,如降解产物、杂质等。
设置合适的色谱条件,使奥美拉唑钠主峰与其他峰分离良好。
记录色谱图,确保有关物质的含量在规定的限值之内。
5. 结果判断
根据药典或实验室标准,综合纯度、含量和有关物质检查的结果,判断样品是否符合规定。
6. 记录与报告
记录所有实验数据,包括仪器参数、实验条件、结果等。
编写检验报告,将结果提交给相关部门或人员。
奥美拉唑注射液的变色原因分析及对策

奥美拉唑注射液的变色原因分析及对策陈金玉【摘要】Omeprazole is a pair of active enantiomers of racemic mixtures,with the chemical structure of sulfonyl benzimidazole. Omeprazole chemical structure is easy to change, especially under the acidic condition, its stability is affected by the solvent type,dosage,pH value,light,metal ions,temperature and other factors , and clinical compatibility also affects the clinical application of omeprazole injection. Incompatibility results in polymerization and discoloration, influencing the normal clinical application. Here is to make a review on the causes and prevention measures of the discoloration.%奥美拉唑(洛赛克)是一对活性旋光对映体的消旋混合物,化学结构为磺酰基苯并咪唑类.奥美拉唑的化学结构易发生变化,特别是在酸性条件下,其稳定性受溶剂的种类、用量、pH值、光线、金属离子、温度等多种因素的影响,同时临床配伍也会影响奥美拉唑注射液的临床应用.配伍不当会出现聚合和变色现象,影响其临床的正常应用.现就其变色的原因与防范措施予以综述.【期刊名称】《医学综述》【年(卷),期】2012(018)024【总页数】3页(P4206-4208)【关键词】奥美拉唑注射;变色现象;原因;防范措施【作者】陈金玉【作者单位】解放军第一八〇医院药学科,福建,泉州,362000【正文语种】中文【中图分类】R917奥美拉唑注射液是苯咪唑的衍生物,为临床常用的质子泵抑制剂。
注射用奥美拉唑钠制剂分析及使用中对变色的防范措施

此推荐使用 0 . 9 %氯化钠注射液作溶媒 _ 4 _ 。
3 . 2 金 属离子 的存在 :制剂 中微量金属 离子主要来 自原辅 料、 溶剂 、 容器 以及操作过程 中使 用的工具等 。 微 量金属离子 对 自动氧化反 应有显 著催 化作用 。为 了避 免金属离 子的影
响, 除应选择纯度较高 的原辅料 , 尽量不使用金属器具外 , 常
更为稳定 。 陈玫芬等 [ 4 ] 研究表明 , 虽然静脉滴注用奥美拉唑说
明书显 示用 1 0 0 ml 5 %葡萄 糖注射 液或 1 0 0 ml 0 . 9 %氯化钠 注射液均可做稀释溶媒 , 但研究 资料表明注射用奥美拉唑钠 在0 . 9 %氯化钠注射液 中更稳定 , 保存 时间相对较长一些 , 因
在药液 中加入 金属离子络合剂 , 如依地 酸盐 、 枸橼酸 、 酒石酸 等。 金属络合剂可与溶液 中的金属离子生成稳定 的水 溶性络
和混 浊 , 但未记录详细信息 。
1 . 2 方法 : 收集我院正在使用的和可得的注射用奥美拉唑钠 药品说 明书 , 将说 明书 中与制 剂设计 、 配 置方法及存 放时 间 等内容录入表格进行 比较分析 。 确定影 响变 色现 象产生 的相 关影 响因素。
进行溶解 。注射用奥美拉 唑冻干瓶内充有氮气保护 , 瓶内压
力较高 , 导致无法将溶媒一次性全部注入小瓶 中。此 时如果 将药液 回吸至针管 内 , 则针管 内的酸性环境会导致药 液质量 发生改变 , 变成黄色 、 粉红色直至黑色 , 此 时溶液必须弃 去[ 6 ] 。
因此 . 在 进 行 配 制 时 可 将 注 射 器从 安 瓿 中抽 取 l 0 ml 溶媒 , 将
颜色 随 p H降低可依 次表现为淡蓝紫 色 、 蓝紫色 、 淡 紫红色 、 紫红色 、 紫色 、 淡黄 、 深棕色 ; p H值 在 7 . 0 ~ 8 . 0时 , 颜色 可变化 如淡紫 色 , 但 降解不 明显 ; 而溶液 p H值 在 8 . 0 ~ 1 0 . 0时 , 奥 美 拉唑在 4 h内是稳 定的 。奥美拉唑注射液 的稳定性随 p H值
奥美拉唑钠药品说明书

核准日期:2006年10月16日修改日期:2007年02与09日奥西康?注射用奥美拉唑钠说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名:注射用奥美拉唑钠商品名:奥西康英文名:omeprazole sodium for injection汉语拼音:zhusheyong aomeilazuona本品主要成份为:奥美拉唑钠。
其化学名称为:5—甲氧基-2-{[(4—甲氧基—3,5—二甲基—2—吡啶基)—甲基]—亚磺酰基}—1h—苯并咪唑钠一水合物。
其化学结构式为:分子式:c17h18n3nao3s·h2o分子量:385.41【性状】本品为白色疏松块状物或粉末,专用溶剂为无色的透明液体。
【适应症】适用于十二指肠溃疡、胃溃疡。
急性胃粘膜病变。
复合性溃疡等急性上消化出血。
【规格】 42.6mg(相当于奥美拉唑40mg)【用法用量】静脉滴注。
一次一支,每日1~2次。
临用前将10ml专用溶剂注入冻干粉小瓶内,禁止用其它溶剂溶解。
溶解后及时加入0.9%氯化钠注射液100ml或5%葡萄糖注射液100ml中稀释后供静脉滴注,经稀释后的奥美拉唑钠溶液静注时间不得少于20分钟。
本品溶解和稀释后必须在4小时内用完。
【不良反应】奥美拉唑的耐受性良好,不良反应多为轻度和可逆。
下列不良反应为临床试验或常规使用中所报告,但在许多病例中与奥美拉唑治疗本身的因果关系尚未确定。
下述不良反应中:“常见”是指发生率≥1/100;“不常见”是指发生率≥1/1000,但<1/100;“罕见”是指发生率<1/1000。
曾有文献报道,个例重症患者接受高剂量奥美拉唑静脉注射后出现不可逆性视觉损伤。
【禁忌】对本品过敏者禁用。
【注意事项】(1)本品抑制胃酸分泌的作用强,时间长,故应用本品时不宜同时再服用其它抗酸剂或抑酸剂。
为防止抑酸过度,在一般消化性溃疡等疾病,不建议大剂量长期应用(zollinger-ellison综合征患者除外)。
奥美拉唑

3.代谢/内分泌系统:长期应用奥美拉唑可导致维生素B12缺乏。
4.致癌性:动物实验表明奥美拉唑可引起胃底部和胃体部主要内分泌细胞-肠嗜铬细胞增生,长期用药还可 发生胃部类癌。
5.其他:可有皮疹、男性乳腺发育、溶血性贫血等。
1.对奥美拉唑过敏者。 2.严重肾功能不全者。 3.婴幼儿。 4.孕妇、哺乳妇女禁用。
奥美拉唑
目录
01 化合物简介
03 药物说明
02 药典标准 04 专家点评
奥美拉唑,主要用于十二指肠溃疡和卓-艾综合征,也可用于胃溃疡和反流性食管炎;静脉注射可用于消化性 溃疡急性出血的治疗。与阿莫西林和克林霉素或与甲硝唑与克拉霉素合用,以杀灭幽门螺杆菌。
化合物简介
物化性质
基本信息
安全信息ቤተ መጻሕፍቲ ባይዱ
中文名称:奥美拉唑 中文别名:埃索美拉唑镁杂质F;奥克;奥咪拉唑;奥西康;福尔丁奥美拉唑;洛赛克;欧麦亚砜;沃必唑; 渥咪哌唑;涯米哌唑;亚砜咪唑;安胃哌唑;甲氧磺唑 英文名称:(R)-omeprazole 英文别名:LOSEC;MEPRAL;GASTROGARD;OMEPRAZOLE;Loec;Moprial;Omeprazolum CAS号:-89-8 分子式:C17H19N3O3S 结构式: 分子量:345. 精确质量:345. PSA:96.
【检查】二氯甲烷溶液的澄清度与颜色取本品0.5g,加二氯甲垸25ml溶解,溶液应澄清无色;如显色,立即 照紫外-可见分光光度法(附录IV A),在440nm的波长处测定吸光度,不得过0.10。有关物质避光操作。
药物说明
01
分类
02
性状
03
剂型
注射用奥美拉唑标准——2008年修订(暂未审批)

国家食品药品监督管理局国家药品标准注射用奥美拉唑钠Zhusheyong AomeilazuonaOmeprazole Sodium for Injection本品为奥美拉唑钠的无菌冻干品。
含奥美拉唑钠以奥美拉唑(C17H19N3O3S)计应为标示量的93.0%~107.0%。
【性状】本品为白色或类白色疏松块状物或粉末。
【鉴别】(1)在含量测定项下记录的色谱图中,供试品溶液主峰的保留时间应与对照品溶液主峰保留时间一致。
(2)取本品,加0.1mol/L的氢氧化钠溶液制成每1ml中约含奥美拉唑20μg的溶液,照紫外-可见分光光度法(中国药典2005年版二部附录Ⅳ A)测定,在305nm与276nm的波长处有最大吸收,其吸收度比值应为1.6~1.8。
(3)本品的水溶液显钠盐的鉴别反应(中国药典2005年版二部附录Ⅲ)。
【检查】溶液的澄清度与颜色取本品5瓶,加注射用水适量使溶解并制成每1ml中含奥美拉唑4mg的溶液,溶液应澄清,如显浑浊,与1号浊度标准液(中国药典2005年版二部附录Ⅸ B)比较,不得更浓;取溶液,照紫外-可见分光光度法(中国药典2005年版二部附录Ⅳ A),在440nm的波长处测定,吸光度不得过0.1。
碱度取溶液澄清度与颜色项下的溶液,依法测定(中国药典2005年版二部附录ⅥH),pH值应为10.1~11.1。
有关物质避光操作。
取奥美拉唑磺酰化物(5-甲氧基-2{[4-甲氧基-3,5-二甲基-2-吡啶基]-甲基]-磺酰基}-1H-苯并咪唑)对照品约6mg,精密称定,置100ml量瓶中,加乙腈5ml使溶解,加溶剂稀释至刻度,摇匀,精密量取适量,加溶剂制成每1ml中约含0.6μg的溶液,作为杂质对照品溶液。
另精密量取含量测定项下的供试品溶液1ml,置100ml量瓶中,加溶剂稀释至刻度,作为对照溶液。
照含量测定项下的色谱条件,量取对照溶液20μl注入液相色谱仪,调节检测灵敏度,使主成分色谱峰的峰高为满量程的10%~20%。
最新-奥美拉唑注射液说明书 精品

奥美拉唑注射液说明书临床用药应本着安全、有效、经济的原则,为此,请临床医师在使用药品前,认真阅读并严格按照药品说明书及《中华人民共和国药典——临床用药须知》的要求使用药品要严格按照药品说明书中所列适应症用药,各种研究成果不具有法律效力,不能等同于药品说明书严格按照说明书中用法和用量开药,不得超剂量用药,不得违反给药途径;如指明肌肉注射或不许静脉给药则不能用于静脉熟悉所开药品的配伍禁忌,询问用药史,避免不合理的药物合用;对药品说明书中所列的注意事项,要多加关注;严格掌握说明书中所列的禁忌症,不得违反规定,对于已经明确列入禁忌症的疾病,绝对不允许使用该药熟悉说明书中所列药品不良反应,对于患者主述的不良反应,应能够鉴别诊断现对部分药品的使用注意事项重申如下1、维生素1注射液注射时偶见过敏反应,个别可发生过敏性休克,故除急需补充的情况外,很少采用注射,且应注射前,用其10倍稀释液01作皮试,以防止过敏反应本品不宜静脉注射给药本品在碱性溶液中易分解,遇碱性药物如碳酸氢钠、枸橼酸钠配伍易引起变质同时本品应注意遮光、密闭保存2、根据《中华人民共和国药典》2019年版《临床用药须知》,甲硝唑注射液静脉滴注用于厌氧菌感染,一次最大剂量不应超过1本药不应与含铝的针头和套管接触,滴注速度宜慢,每次滴注时间应超过1小时,并避免与其他药物一起滴注3、乳酸环丙沙星氯化钠注射液,为了调节等渗,注射液中加有氯化钠如果在使用中与其他药物接瓶使用,可能出现药液浑浊或产生沉淀为此请使用时避免与其他药物接瓶使用本药与同是喹诺酮类的其他药物共用属不合理用药;与头孢菌素类药物抗菌谱相似,建议避免合用本药对光敏感,应注意避光保存4、青霉素及其口服制剂的使用注意事项《中华人民共和国药典》2019年版《临床用药须知》中明确规定,使用青霉素类抗菌药物前需做青霉素皮肤试验,阳性反应者禁用用药前需详细询问病史,包括用药史,是否有青霉素类、头孢菌素类或其他内酰胺类抗菌药物过敏史,或过敏性疾病史,有无易为病人忽略的反应症状,如胸闷、瘙痒、面部发麻、发热等,以及有无个人或家属有变态反应性疾病等有青霉素过敏史者一般不宜进行皮试,应该用其他药物皮试液浓度为500,皮内注射01对可疑阳性者,应在另一前臂用生理盐水做对照实验5、根据《中华人民共和国药典》2019年版《临床用药须知》,对青霉素类、青霉素衍生物或青霉胺过敏者也可能对头孢菌素过敏,头孢菌素本身也可引起过敏,为了保证头孢菌素在临床使用的安全性,建议头孢菌素类药物使用前做皮试对有青霉素过敏性休克或即刻反应者,不宜再选用头孢菌素类药物皮试液浓度为300μ,皮下注射016、根据《中华人民共和国药典》2019年版《临床用药须知》,阿奇霉素用于治疗小于6个月小儿中耳炎、社区获得性肺炎及小于2岁小儿咽炎或扁桃体炎的疗效与安全性尚未确立,应慎用维路德、维宏干混悬剂、抒罗康注射用阿奇霉素新锋达齐、齐宏说明书中明确指出由于16周岁以下儿童或青少年使用阿奇霉素注射剂的安全性和有效性还没有建立,所以目前不推荐使用7、痰热清注射液在说明书中明确规定,不得与酸性成分的注射剂混合使用提示中药注射液应避免与其他药物配伍使用,以免出现沉淀等药物相互作用8、注射用奥美拉唑钠必须使用药品自带的专用溶媒。
奥美拉唑说明书

奥美拉唑说明书很多人买完药品之后都没有看过,自然就不知道本品的食用禁忌和功效了,又怎能发挥本品的作用呢。
下面就一起来了解下的主要内容吧。
请仔细阅读说明书并在医师指导下使用1、通用名称:奥美拉唑肠溶胶囊2、批准文号:国药准字H202134443、汉语拼音:AoMeiMaMuoJiaoNang4、英文名称:OmeprazoleEnteric-coatedCapsules5、成份:本品主要成份为奥美拉唑。
6、剂型:胶囊剂7、形状:本品内容物为白色或类白色肠溶小丸或颗粒。
8、功能主治:适用于胃溃疡,十二指肠溃疡,应激性溃疡,反流性食管炎和卓-艾综合症胃泌素瘤。
9、规格/中西药品:20mg*14粒10、用法用量:口服,不可咀嚼。
详情见说明书11、不良反应:本品耐受性良好,常见不良反应是腹泻、头痛、恶心、腹痛、胃肠胀气及便秘,偶见血清氨基转移酶ALT,AST增高、皮疹、眩晕、嗜睡、失眠等,上述不良反应通常较轻,可自动消失。
长期治疗,在有些病例中可发生胃粘膜细胞增生和萎缩性胃炎。
12、禁忌:对本品过敏者、严重肾功能不全者禁用。
13、注意事项:治疗胃溃疡时,应首先排除溃疡型胃癌的可能,因用本品治疗可减轻其症状,从而延误治疗。
肝肾功能不全者慎用。
本品为肠溶胶囊,服用时请注意不要嚼碎,以防止药物颗粒过早在胃内释放而影响疗效。
14、药物相互作用:本品可延缓经肝脏代谢药物在体内的消除,如安定、苯妥英钠、华法令、硝苯啶等,当本品和上述药物一起使用时,应减少后者用量。
15、药理毒理:质子泵抑制剂。
本品为脂溶性弱碱性药物,易浓集于酸性环境中,因此口服后可特异地分布于胃粘膜壁细胞的分泌小管中,并在此高酸环境下转化为亚磺酰胺的活性形式,然后通过二硫键与壁细胞分泌膜中的H+,K+-ATP酶又称质子泵的巯基呈不可逆的结合,生成亚磺酰胺与质子泵的复合物,从而抑制该酶活性,阻断胃酸分泌的最后步骤,因此本品对各种原因引起的胃酸分泌具有强而持久的抑制作用。
奥美拉唑

奥美拉唑【药物名称】中文通用名称:奥美拉唑英文通用名称:Omeprazole其它名称:奥克、奥咪拉唑、奥西康、福尔丁奥美拉唑、金洛克、洛凯、洛赛克、欧麦亚砜、沃必唑、渥米哌唑、涯米哌唑、亚砜咪唑、Antra、Aoxikang、Audazol、H-168/68、Logastric、Losec、Mepral、Miol、Mopral、Moprial、Norpramin、Omapren、Omepral、Omeprazen、Omeprazolum、Parizac、Prilosec、Zoltum 【临床应用】1.用于胃、十二指肠溃疡等消化性溃疡。
2.用于反流性食管炎、胃泌素瘤。
3.本药注射剂可用于:(1)消化道出血,如消化性溃疡出血、吻合口溃疡出血等。
(2)应激状态时并发或由非甾体类抗炎药引起的急性胃粘膜损伤。
(3)全身麻醉或大手术后以及昏迷患者,以防止胃酸反流及吸入性肺炎。
【药理】1.药效学本药为具有脂溶性的质子泵抑制药,呈弱碱性,易浓集于酸性环境中,能特异性地作用于胃壁细胞顶端膜构成的分泌性微管和胞质内的管状泡上(即胃壁细胞质子泵所在部位),并转化为亚磺酰胺的活性形式,然后通过二硫键与质子泵的巯基呈不可逆结合,生成亚磺酰胺与质子泵(H+-K+·ATP酶)的复合物,从而抑制该酶活性,使壁细胞内的H+不能转运到胃腔中,阻断了胃酸分泌的最后步骤,使胃液中的胃酸量大为减少。
实验证明,本药对基础胃酸分泌和由组胺、五肽胃泌素及刺激迷走神经引起的胃酸分泌具有强而持久的抑制作用,对H2受体阻断药不能抑制的由二丁基环腺苷酸引起的胃酸分泌亦有明显的抑制作用。
由于本药对质子泵的抑制作用具有不可逆性,故本药的抑酸作用时间长,只有待新的质子泵形成后,才能恢复其泌酸作用。
健康志愿者单次口服本药,其抗酸作用可维持24小时;多次口服(1周)可使基础胃酸和五肽胃泌素刺激引起的胃酸分泌抑制70%-80%。
随着胃酸分泌量的明显下降,胃内pH值迅速升高,一般停药后3-4天胃酸分泌可恢复到原有水平。
注射用奥美拉唑钠 申报书

第一组:文洁、谢婷、杨丽云、林文华、李玲仪、梁炜婷、冯锦荣、康涛注射用奥美拉唑钠注射用奥美拉唑钠说明书样稿国家食品药品监督管理局二○○六年十月二十八日注射用奥美拉唑钠说明书请仔细阅读说明书并在医师指导下使用。
【药品名称】通用名称:注射用奥美拉唑钠英文名称:Omeprazole Sodium for Injecfior汉语拼音:Zhusheyong Aomeilazuona【成份】本品主要成份为:奥美拉唑钠。
分子式:C17H18N3NaO3S·H2O分子量:385.41【辅料】辅料为甘露醇和依地酸二钠。
【性状】本品为白色或类白色疏松块状物或粉末。
【适应症】作为当口服疗法不适用时下列病症的替代疗法:十二指肠溃疡、胃溃疡、反流性食管炎及Zollinger-Ellison综合征。
【规格】40mg(以C17H19N3O3S计)【用法用量】静脉滴注:临用前将瓶中的内容物溶于l00毫升0.9%氯化钠注射液或100毫升5%葡萄糖注射液中,本品溶解后静脉滴注时间应在20一30分钟或更长。
禁止用其他其溶剂或其他药物溶解和稀释。
当口服疗法不适用于十二指肠溃疡、胃溃疡和反流性食营炎的患者时,推荐静脉滴注本品的剂量为40mg,每日一次。
Zollinger-Ellison综合征患者推荐静脉滴注奥美拉唑60mg作为起始剂量,每日一次。
Z ollinger-Ellison综合征患者每日剂量可能要求更高,剂量应个体化。
当每日剂量超过60mg 时分两次给药。
【不良反应】奥美拉唑的耐受性良好,不良反应多为轻度和可逆。
下列不良反应为临床试验或常规使用中所报告,但在许多病例中与奥美拉唑治疗本身的因果关系尚未确定。
下述不良反应中:“常见”是指发生率≥l/100“不常见”是指发生率≥l/1000,但<1/100“罕见”是指发生率<1/1000常见中枢和外周神经系统:头痛消化系统:腹泻、便秘、腹痛、恶心/呕吐和气胀不常见中枢和外周神经系统:头晕、感觉异样、嗜睡、失眠和眩晕肝脏:肝酶升高皮肤:皮疹和(或)瘙痒、荨麻疹其他:不适罕见中枢和外周神经系统:可逆性精神错乱、激动、攻击性行为、抑郁和幻觉,多见于重症患者内分泌系统:男子乳房女性化消化系统:口干、口炎和胃肠道念珠菌感染血液系统:白细胞减少、血小板减少、粒细胞缺乏症和各类血细胞减少肝脏:脑病(见于先前有严重肝病患者),肝炎或黄疸性肝炎、肝脏衰竭肌肉与骨骼:关节痛、肌力减弱和肌痛皮肤:光敏性、多形牲红斑、Stevens—Johnson综合征、毒性表皮坏死(TEN)、脱发其他:过敏反应,例如血管睦水肿、发热、支气管痉挛、间质性肾炎和过敏性休克。
奥美拉唑简介

2.1奥美拉唑的结构特点
苯并咪唑环
3
吡啶基
O
2
1
O
5
N
2
O
1
亚磺酰基
亚 砜 基 的 S具 有 光 学 活 性
骨架结构
2.2 奥美拉唑的构效及药代
奥美拉唑是一种单烷氧基吡啶化合物,与H+/K+-ATP酶 (质子泵)有2个结合部位,可选择性、非竞争性地抑制壁细 胞膜中的H+/K+-ATP酶。 在肝脏经由细胞色素P450代谢,代谢物为砜、sulfide、羟 基接合的奥美拉唑,它们对胃酸分泌没有影响。
• PPIs即质子泵抑制剂,可抑制H+/K+-ATP酶的活性,抑制 各种因素引起的胃酸分泌. • 经过长期的研究发现,抑制H+/K+-ATP酶的药物必须具有 3个结构部分:吡啶环、SO基和苯并咪唑环。 • 目前几种上市的PPIs多为苯并咪唑类衍生物,它通过对吡 啶环或苯并咪唑环进行不同的修饰而增强其抑制胃酸的功 能。
H F CO F N N Na+
-
O S
N O OCH3
二氟甲氧基
由美国百克顿公司(Gooden Byk)开发, 1994年上市
3.3 雷贝拉唑 Rabeprazole
O N N H S O OCH3 N
甲氧丙氧基
又名波利特,由日本卫材公司开发, 1997年上市
3.4 埃索美拉唑 Esomeprazole
奥美拉唑的生产工艺原理
一、概述
奥美拉唑的命名及简介 奥美拉唑结构、构效及药代
奥美拉唑衍生物的介绍
1.1奥美拉唑的命名
O O
5 1 5 4 2 3
N O
N
奥美拉唑溶出度
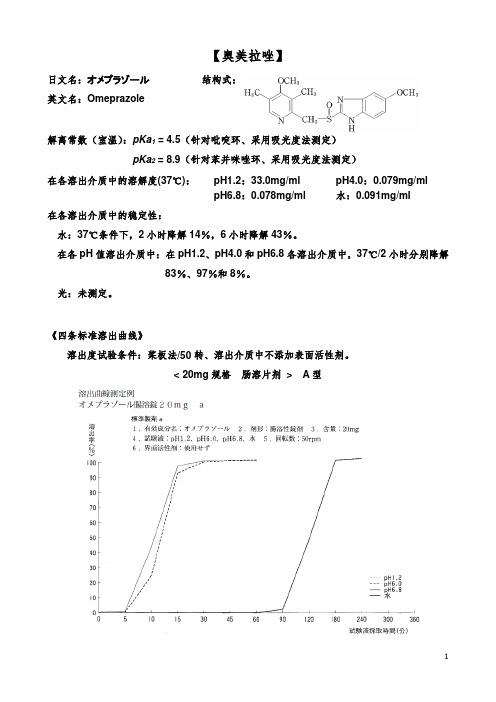
【奥美拉唑】日文名:オメプラゾール英文名:Omeprazole结构式:解离常数(室温):pKa1 = 4.5(针对吡啶环、采用吸光度法测定)pKa2 = 8.9(针对苯并咪唑环、采用吸光度法测定)在各溶出介质中的溶解度(37℃):pH1.2:33.0mg/ml pH4.0:0.079mg/mlpH6.8:0.078mg/ml 水:0.091mg/ml在各溶出介质中的稳定性:水:37℃条件下,2小时降解14%,6小时降解43%。
在各pH值溶出介质中:在pH1.2、pH4.0和pH6.8各溶出介质中,37℃/2小时分别降解83%、97%和8%。
光:未测定。
《四条标准溶出曲线》溶出度试验条件:桨板法/50转、溶出介质中不添加表面活性剂。
< 20mg规格肠溶片剂> A型< 20mg规格肠溶片剂> B型《质量标准》【在pH1.2介质中】取本品,照溶出度测定法(桨板法),以盐酸溶液(氯化钠2.0g溶于盐酸7.0ml中,加水稀释至1000ml,即得)900ml为溶剂,转速为每分钟50转,依法操作,经120分钟时,取溶液适量滤过,弃去至少10ml初滤液,精密量取续滤液适量,加溶出介质稀释制成每1ml中含22μg的溶液,作为供试品溶液。
另精密称取奥美拉唑对照品0.022g,置50ml量瓶中,加乙醇溶解并稀释至刻度,摇匀,精密量取5ml,置100ml 量瓶中,加溶出介质稀释至刻度,摇匀,作为对照品溶液。
取上述两种溶液照紫外-可见分光光度法,分别在323nm波长处测定吸光度,计算每片溶出量,不得过标示量的5%,应符合规定。
【在pH6.8介质中】取本品,照溶出度测定法(桨板法),以磷酸盐缓冲液(pH6.8) 900ml 为溶剂,转速为每分钟50转,依法操作,经15分钟时,取溶液适量滤过,弃去至少10ml 初滤液,精密量取续滤液适量,加溶出介质稀释制成每1ml中含22μg的溶液,作为供试品溶液。
另精密称取奥美拉唑对照品0.022g,置50ml量瓶中,加乙醇溶解并稀释至刻度,摇匀,精密量取5ml,置100ml量瓶中,加溶出介质稀释至刻度,摇匀,作为对照品溶液。
奥美拉唑杂质分析

奥美拉唑杂质Omeprazole Imp. G (EP) Omeprazole Imp. H(EP)奥美拉唑(omeprazole ),化学名为5-甲氧基-2 - [ [ ( 4-甲氧基-3, 5-二甲基-2-吡啶基)甲 基]亚硫酰基]-1-苯并咪唑,是英国 AstraZeneca 公司研发的质子泵抑制剂类抗溃疡药,临床 主要用于治疗消化道溃疡和反食性胃炎。
欧洲药典(European Pharmacopeia ,缩写为EP )共列出其杂质 9个。
奥美拉唑及其九 个杂质分别为: HS O N N O S O HNONNNO OOSON NNNNOClS O ONSONHN NHHNNOmeprazole Imp. A (EP) Omeprazole Imp. C (EP) Omeprazole Imp. E(EP) Omeprazole Imp. B (EP)Omeprazole Imp. D (EP)Omeprazole Imp. F (EP)O/SO -OO由于合成方法和步骤的不同,所产生的杂质也有所不同。
根据本实验所采用的方法及所使用的原料情况,现分析其可能产生杂质如下:Omeprazole Imp. A (EP):化学名5-Methoxy-1H-be nzimidazole-2-thiol 合成奥美拉唑中间体奥美拉唑硫醚的一个原料。
该方法的具体步骤为:颈圆底烧瓶1只,安装搅拌轮,100°C温度计和加液管置于恒温水槽中。
(颜国和,王飞武.奥美拉唑合成路线图解[J].中国医药工业杂志,佃91,2 2( 6 ):2 83-28)本反应与其使用方法不同,反应历程也不相同,最终结果未检测到该杂质,所以不对该杂质进行研究。
3.Omeprazole Imp. C (EP) : 化学名5-Methoxy-2-[[(4-methoxy-3,5-dimethylpyridi n-2-yl)methyl]-sulpha nyl]-1H-be nzimidazole,即奥美拉唑硫醚,又名Ufiprazole。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
EUROPEAN PHARMACOPOEIA 8.0Omeprazole04/2013:0942OMEPRAZOLEOmeprazolum C 17H 19N 3O 3S M r 345.4[73590-58-6]DEFINITION 5-Methoxy-2-[(RS )-[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl]-1H -benzimidazole.Content :99.0per cent to 101.0per cent (dried substance).CHARACTERSAppearance :white or almost white powder.Solubility :very slightly soluble in water,soluble in methylene chloride,sparingly soluble in ethanol (96per cent)and in methanol.It dissolves in dilute solutions of alkali hydroxides.It shows polymorphism (5.9).IDENTIFICATIONInfrared absorption spectrophotometry (2.2.24).Comparison :omeprazole CRS .If the spectra obtained in the solid state show differences,dissolve the substance to be examined and the referencesubstance separately in methanol R ,evaporate to dryness and record new spectra using the residues.TESTSSolution S .Dissolve 0.50g in methylene chloride R and dilute to 25mL with the same solvent.Appearance of solution .Solution S is clear (2.2.1).Impurities F and G :maximum 350ppm for the sum of the contents.The absorbance (2.2.25)of solution S determined at 440nm is not greater than 0.10.Related substances .Liquid chromatography (2.2.29).Prepare the solutions immediately before use .Test solution .Dissolve 3mg of the substance to be examined in the mobile phase and dilute to 25.0mL with the mobile phase.Reference solution (a).Dissolve 1mg of omeprazole CRS and 1mg of omeprazole impurity D CRS in the mobile phase and dilute to 10.0mL with the mobile phase.Reference solution (b).Dilute 1.0mL of the test solution to 100.0mL with the mobile phase.Dilute 1.0mL of this solution to 10.0mL with the mobile phase.Reference solution (c).Dissolve 3mg of omeprazole for peak identification CRS (containing impurity E)in the mobile phase and dilute to 20.0mL with the mobile phase.Column :–size :l =0.125m,Ø=4.6mm;–stationary phase :octylsilyl silica gel for chromatography R (5μm).Mobile phase :mix 27volumes of acetonitrile R and 73volumes of a 1.4g/L solution of disodium hydrogen phosphate R previously adjusted to pH 7.6with phosphoric acid R .Flow rate :1mL/min.Detection :spectrophotometer at 280nm.Injection :40μL.Run time :5times the retention time of omeprazole.Identification of impurities :use the chromatogram obtained with reference solution (a)to identify the peak due to impurity D;use the chromatogram supplied with omeprazole for peak identification CRS and the chromatogram obtainedwith reference solution (c)to identify the peak due toimpurity E.Relative retention with reference to omeprazole(retention time =about 9min):impurity E =about 0.6;impurity D =about 0.8.System suitability :reference solution (a):–resolution :minimum 3.0between the peaks due toimpurity D and omeprazole;if necessary,adjust the pH of the aqueous part of the mobile phase or the concentration of acetonitrile R ;an increase in the pH will improve theresolution.Limits :–impurities D,E :for each impurity,not more than 1.5times the area of the principal peak in the chromatogramobtained with reference solution (b)(0.15per cent);–unspecified impurities :for each impurity,not more than thearea of the principal peak in the chromatogram obtained with reference solution (b)(0.10per cent);–total :not more than 5times the area of the principal peak in the chromatogram obtained with reference solution (b)(0.5per cent);–disregard limit :0.5times the area of the principal peak in the chromatogram obtained with reference solution (b)(0.05per cent).Loss on drying (2.2.32):maximum 0.2per cent,determined on 1.000g by drying under high vacuum at 60°C for 4h.Sulfated ash (2.4.14):maximum 0.1per cent,determined on1.0g.ASSAYDissolve 0.250g in a mixture of 10mL of water R and 40mL of ethanol (96per cent)R .Titrate with 0.1M sodium hydroxide ,determining the end-point potentiometrically (2.2.20).1mL of 0.1M sodium hydroxide is equivalent to 34.54mg of C 17H 19N 3O 3S.STORAGEIn an airtight container,protected from light,at a temperature of 2°C to 8°C.IMPURITIESSpecified impurities:D,E,F,G .Other detectable impurities (the following substances would,if present at a sufficient level,be detected by one or other of the tests in the monograph.They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034).It is therefore not necessary to identify these impurities for demonstration of compliance.See also 5.10.Control of impurities in substances for pharmaceutical use ):A,B,C,H,I.A.5-methoxy-1H-benzimidazole-2-thiol,B.2-[(RS )-[(3,5-dimethylpyridin-2-yl)methyl]sulfinyl]-5-methoxy-1H -benzimidazole,General Notices (1)apply to all monographs and other texts2911Omeprazole magnesium EUROPEAN PHARMACOPOEIA8.0C.5-methoxy-2-[[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfanyl]-1H-benzimidazole(ufiprazole),D.5-methoxy-2-[[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfonyl]-1H-benzimidazole(omeprazolesulfone),E.4-methoxy-2-[[(RS)-(5-methoxy-1H-benzimidazol-2-yl)sulfinyl]methyl]-3,5-dimethylpyridine1-oxide,F.8-methoxy-1,3-dimethyl-12-thioxopyrido[1′,2′:3,4]-imidazo[1,2-a]benzimidazol-2(12H)-one,G.9-methoxy-1,3-dimethyl-12-thioxopyrido[1′,2′:3,4]-imidazo[1,2-a]benzimidazol-2(12H)-one,H.2-[(RS)-[(4-chloro-3,5-dimethylpyridin-2-yl)methyl]sulfinyl]-5-methoxy-1H-benzimidazole,I.4-methoxy-2-[[(5-methoxy-1H-benzimidazol-2-yl)sulfonyl]methyl]-3,5-dimethylpyridine1-oxide.01/2009:2374corrected6.7OMEPRAZOLE MAGNESIUMOmeprazolummagnesicumC34H36MgN6O6S2Mr713[95382-33-5]DEFINITIONMagnesium bis[5-methoxy-2-[(RS)-[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl]-1H-benzimidazol-1-ide].It contains a variable quantity of water.Content:97.5per cent to102.0per cent(anhydrous substance).CHARACTERSAppearance:white or almost white,hygroscopic powder.Solubility:very slightly soluble in water,sparingly soluble inmethanol,practically insoluble in heptane.IDENTIFICATIONCarry out either tests A,B,C or tests A,B,D.A.Optical rotation(2.2.7):−0.10°to+0.10°.Dissolve0.250g in methanol R and dilute to25.0mL withthe same solvent.B.Infrared absorption spectrophotometry(2.2.24).Comparison:omeprazole magnesium CRS.C.Atomic absorption spectrometry(2.2.23)as described inthe test for magnesium.The test solution shows the absorption maximum at285.2nm.D.Ignite about0.5g of the substance to be examinedaccording to the procedure for the sulfated ash test(2.4.14).Dissolve the residue in10mL of water R.2mL of thissolution gives the reaction of magnesium(2.3.1).TESTSAbsorbance(2.2.25):maximum0.10at440nm.Dissolve0.500g in methanol R and dilute to25.0mL with thesame solvent.Filter the solution through a membranefilter(nominal pore size0.45μm).Related substances.Liquid chromatography(2.2.29):use thenormalisation procedure.Prepare the solutions immediatelybefore use.Test solution.Dissolve3.5mg of the substance to be examinedin the mobile phase and dilute to25.0mL with the mobilephase.Reference solution(a).Dissolve1mg of omeprazole CRS and1mg of omeprazole impurity D CRS in the mobile phase anddilute to10.0mL with the mobile phase.Reference solution(b).Dissolve3mg of omeprazole for peakidentification CRS(containing impurity E)in the mobile phaseand dilute to20.0mL with the mobile phase.Reference solution(c).Dilute1.0mL of the test solution to100.0mL with the mobile phase.Dilute1.0mL of this solutionto10.0mL with the mobile phase.Column:–size:l=0.125m,Ø=4.6mm;–stationary phase:octylsilyl silica gel for chromatography R(5μm).2912See the information section on general monographs(cover pages)。
