欧洲药典的更新
各国药典最新版及出版周期

中国药典(ChP):最新版2015版自家药典,每五年更新一次,很了解,不用多说。
官方网站:/美国药典(USP):最新版USP39-NF34美国药典-国家处方集第39版于2015年12月份出版,2016年5月1日生效,是由美国政府所属的美国药典委员会编辑出版的关于药典标准的公开出版物。
它包含关于药物、剂型、原料药、辅料、医疗器械和食物补充剂的标准。
美国药典-国家处方集每年出版一次。
2016版《美国药典》包含4卷及2个增补版。
官方网站:/英国药典(BP):最新版BP2016英国药典是英国药品委员会(British Pharmacopoeia Commission)的正式出版物,是英国制药标准的重要来源。
英国药典不仅为读者提供了药用和成药配方标准以及公式配药标准,而且也向读者展示了许多明确分类并可参照的欧洲药典专著。
英国药典出版周期不定,最新的版本为2016版,英国药典2016版共6卷。
官方网站:https:///欧洲药典(EP):最新版EP8.8欧洲药典(EP):每3年1版,中间有8次增补版,如:8.1~8.8。
欧洲药典8为欧洲药典最新版本(2013年7月出版,2014年1月生效),欧洲药典第8版包括两个基本卷,于2013年7月出版发行,以后在每次欧洲药典委员会全会做出决定后,通过非累积增补本更新,每年出3个增补本。
第8版累计共有8个非累积增补本(8.1~8.8)。
各增补版的出版日期及执行的日期。
官方网站:http://online.edqm.eu/EN/entry.htm日本药典(JP):最新版JP16日本药局方:(The Japanese Pharmacopoeia)由日本药局方编集委员会编篡,由厚生省颁布执行。
分两部出版,第一部收载原料药及其基础制剂,第二部主要收载生药,家庭药制剂和制剂原料,日本药典最新版是第十六改正版,每五年出版一次。
JP14,2001年3月31号公布,4月1号使用;JP15,2006年3月31号公布,4月1号使用;JP16,2011年4月1号使用。
欧洲药典标准 -回复

欧洲药典标准-回复欧洲药典标准是一套用于药品质量控制的指导原则和方法。
它通过确立药品的质量标准,保证了药品的安全性和有效性。
本文将逐步解答欧洲药典标准的详细内容。
欧洲药典标准(European Pharmacopoeia,EP)是由欧洲药典委员会(European Pharmacopoeia Commission)制定和管理的,作为欧洲药理学领域权威的国际药典。
它于1964年第一次出版,至今已经是第10版。
欧洲药典标准不仅适应欧洲国家的需求,还被众多非欧洲国家所采用。
欧洲药典标准包括药品的物质标准、质量标准和生产标准等。
首先,药品的物质标准规定了药品中所含的特定成分以及其含量范围。
这些物质标准是根据科学研究和临床实践得出的,旨在确保药品的质量和效力。
其次,药品的质量标准规定了药品在生产过程中需要满足的各项质量要求。
这些质量标准包括药品的外观、溶解度、含量一致性、微生物污染、重金属含量等。
药品必须符合这些质量标准才能进行生产和销售。
最后,欧洲药典标准还规定了药品的生产标准。
这包括药品的生产工艺、质量控制措施、灭菌方法等。
通过严格遵守这些生产标准,可以确保药品的制备过程符合质量要求,有助于预防药品污染和缺陷。
欧洲药典标准的制定过程非常严谨和透明。
欧洲药典委员会负责制定、修订和更新药典标准,他们的工作是基于科学实证和广泛的专家讨论。
药典标准的修订需要通过多个阶段的公众咨询,以确保制定过程的透明度和合理性。
欧洲药典标准不仅适用于药品的生产和销售,还应用于药品检验和注册审批过程中。
很多国家和地区在药品审批过程中要求参考欧洲药典标准进行药品质量评估。
这不仅有助于确保药品的质量和安全,还方便了国际贸易和合作。
与其他国际药典相比,欧洲药典标准具有一定的特点和优势。
首先,欧洲药典标准对药品的质量要求非常严格,保证了药品的安全性和稳定性。
其次,欧洲药典标准的制定是基于科学研究和实践经验,因此更加可靠和实用。
此外,欧洲药典标准的更新速度也相对较快,使其能够适应新的科学进展和药品研发。
欧洲药典7.5版

EUROPEAN PHARMACOPOEIA 7.5
INDEX
To aid users the index includes a reference to the supplement in which the latest version of a text can be found. For example : Amikacin sulfate...............................................7.5-4579 means the monograph Amikacin sulfate can be found on page 4579 of Supplement 7.5. Note that where no reference to a supplement is made, the text can be found in the principal volume.
EUROPEAN PHARMACOPOEIA 7.5
Index
Numerics 1. General notices ................................................................... 7.5-4453 2.1.1. Droppers................................................................................... 15 2.1.2. Comparative table of porosity of sintered
欧洲药典——精选推荐

欧洲药典◇欧洲药典8为欧洲药典最新版本;◇ 2013年7⽉出版;◇ 2014年1⽉⽣效。
欧洲药典第8版包括两个基本卷,于2013年7⽉出版发⾏,以后在每次欧洲药典委员会全会做出决定后,通过⾮累积增补本更新,每年出3个增补本。
第8版累计共有8个⾮累积增补本(8.1~8.8)。
各增补版的出版⽇期及执⾏的⽇期。
最初的两卷包括第7版完整的内容,以及欧洲药典委员会在2012年12⽉全会上通过或修订的内容,共收载了2224个个论,345个含插图或⾊谱图的总论,以及2500种试剂的说明。
变化的内容(插⼊或删除的内容)在页边标注出⾃2014年1⽉起,在欧洲药典成员国,包括欧盟国家,将执⾏第8版并取代第7版。
第7版⾄12⽉31⽇都是有效的。
欧洲药典有英⽂版与法⽂版,英语与法语是欧洲委员会的官⽅语⾔。
欧洲药典有印刷版、USB闪存版和在线版。
其西班⽛⽂版正在翻译之中,将来包括在在线版中,不再另收取费⽤。
欧洲药典:⼀部药品与药⽤物质的标准欧洲药典是欧洲药品质量控制的标准。
已有多项法律⽂件使欧洲药典成为法定标准:2009年经36个欧洲国家和欧盟批准的编撰欧洲药典协议;关于⼈⽤或兽⽤药品的欧盟指令2001/82/EC、2001/83/EC(修正案)和2003/63/EC,维持了欧洲药典对在欧洲上市药品的强制执⾏性。
这些标准规定了药品、⽣产⽤原材料与合成⽤中间体成份的定性、定量和所⽤的检验项⽬。
所有药品、药⽤物质⽣产企业在欧洲销售或使⽤其产品时,都必须遵循欧洲药典标准。
欧洲药典的内容具有法律约束⼒,由⾏政管理或司法部门强制要求符合欧洲药典。
成员国的国家当局必须采⽤欧洲药典,必要时可替代相同物质国家标准中的个论。
欧洲药典的内容包括活性物质、辅料、化学、动物、⼈或植物来源的药⽤物质或制品、顺势疗法制剂和顺势疗法原料、抗⽣素,以及制剂和容器等。
欧洲药典还适⽤于⽣物制品、⾎液和⾎浆制品、疫苗和放射药品。
欧洲药典8 EP8相关内容Index 8.0 EP8.0索引Index 8.3 EP8.3索引Index 8.5 EP8.5索引Index 8.6 EP8.6索引欧洲药典8.6内容变更NEW TEXTSThe texts below appear for the first time in the European Pharmacopoeia. They will be implemented on 1 January 2016 at the latest.MONOGRAPHSRadiopharmaceutical preparations and starting materials for radiopharmaceutical preparations Copper tetramibi tetrafluoroborate for radiopharmaceuticalpreparations (2547)Herbal drugs and herbal drug preparationsAnemarrhena asphodeloides rhizome (2661)Hamamelis bark (2532)Indigo plant leaf (2727)Homoeopathic preparationsBelladonna for homoeopathic preparations (2489)Petroleum rectificatum for homoeopathic preparations (2683)Staphysagria for homoeopathic preparations (2289)MonographsExemestane (2766)Nicorandil (2332)Pirfenidone (2856)Sodium selenite (2740)Solifenacin succinate (2779)Somatropin solution for injection (2370)REVISED TEXTSThe texts below have been technically revised since their last publication. They will be implemented on 1 January 2016.GENERAL CHAPTERS2.2.4. Approximate pH of solutions2.2.19. Amperometric titration2.2.20. Potentiometric titration2.2.34. Thermal analysis2.2.36. Potentiometric determination of ionic concentration using ion-selective electrodes 2.4.29. Composition of fatty acids in oils rich in omega-3 acids2.5.5. Peroxide value2.5.32. Water: micro determination2.9.3. Dissolution test for solid dosage forms2.9.40. Uniformity of dosage units4. Reagents (new, revised, corrected)5.2.4. Cell cultures for the production of veterinary vaccines5.8. Pharmacopoeial harmonisation5.22. Names of herbal drugs used in traditional Chinese medicineMONOGRAPHSVaccines for veterinary use Brucellosis vaccine (live) (Brucella melitensis Rev. 1 strain) for veterinary use (0793)Radiopharmaceutical preparations and starting materialsfor radiopharmaceutical preparationsPentetate sodium calcium for radiopharmaceuticalpreparations (2353)Technetium (99mTc) medronate injection (0641)Herbal drugs and herbal drug preparationsBenzoin, Siam (2158)Bilberry fruit, dried (1588)Bilberry fruit, fresh (1602)Centella (1498)Fresh bilberry fruit dry extract, refined and standardised (2394)Ginseng (1523)Java tea (1229)Homoeopathic preparationsMethods of preparation of homoeopathic stocks and potentisation (2371)MonographsAluminium phosphate, hydrated (1598)Amidotrizoic acid dihydrate (0873)Amiloride hydrochloride dihydrate (0651)Amlodipine besilate (1491)Anticoagulant and preservative solutions for human blood (0209)Aprotinin (0580)Aprotinin concentrated solution (0579)Bromhexine hydrochloride (0706)Buserelin (1077)Carbomers (1299)Carnauba wax (0597)Chymotrypsin (0476)Crospovidone (0892)Demeclocycline hydrochloride (0176)Dihydralazine sulfate, hydrated (1310)Diphenhydramine hydrochloride (0023)Dithranol (1007)Doxapram hydrochloride (1201)Filgrastim concentrated solution (2206)Fluticasone propionate (1750)Fructose (0188)Fulvestrant (2443)Galactose (1215)Glimepiride (2223)Glucose, anhydrous (0177)Glucose monohydrate (0178)Hexylresorcinol (1437)Human coagulation factor IX (rDNA) concentrated solution (2522) Hypromellose (0348)Iopanoic acid (0700)Ioxaglic acid (2009)Isoleucine (0770)Lactose, anhydrous (1061)Lactose monohydrate (0187)Leucine (0771)Lysine hydrochloride (0930)Methionine (1027)Methylcellulose (0345)Methylprednisolone acetate (0933)Methylprednisolone hydrogen succinate (1131) Methylthioninium chloride (1132)Naftidrofuryl hydrogen oxalate (1594)Nicotinamide (0047)Orphenadrine citrate (1759)Orphenadrine hydrochloride (1760)Oxeladin hydrogen citrate (1761)Oxolinic acid (1353)Pancreas powder (0350)Phenazone (0421)Phentolamine mesilate (1138)Polysorbate 80 (0428)Potassium hydroxide (0840)Povidone, iodinated (1142)Propylene glycol dicaprylocaprate (2122)Quinidine sulfate (0017)Quinine hydrochloride (0018)Quinine sulfate (0019)Risedronate sodium 2.5-hydrate (2572)Rivastigmine hydrogen tartrate (2630)Sodium amidotrizoate (1150)Sodium hydroxide (0677)Sodium nitroprusside (0565)Sodium selenite pentahydrate (1677)Spirapril hydrochloride monohydrate (1766)Sucrose (0204)Sugar spheres (1570)Sulfacetamide sodium (0107)Theophylline-ethylenediamine hydrate (0301)Thiamine hydrochloride (0303)Thiamine nitrate (0531)Thiamphenicol (0109)Tribenoside (1740)Trypsin (0694)CORRECTED TEXTSThe texts below have been corrected and are republished in their entirety. These corrections are to be taken into account from the publication date of Supplement 8.6 (1 July 2015), unless otherwise indicated.GENERAL CHAPTERS2.4.22. Composition of fatty acids by gas chromatography2.5.1. Acid value2.7.14. Assay of hepatitis A vaccine2.8.13. Pesticide residues5.7. Table of physical characteristics of radionuclides mentioned in the European PharmacopoieaMONOGRAPHSRadiopharmaceutical preparations and starting materials for radiopharmaceutical preparations Gallium (68Ga) edotreotide injection (2482)MonographsCimetidine (0756)Cimetidine hydrochloride (1500)Flucytosine (0766)Goserelin (1636)Human antithrombin III concentrate (0878)(1)Insulin, bovine (1637)Insulin, human (0838)Insulin, porcine (1638)Insulin preparations, injectable (0854)Isomalt (1531)Miconazole nitrate (0513)Nitric acid (1549)Oxaliplatin (2017)Polyoxypropylene stearyl ether (2602)HARMONISED TEXTSThe texts below have undergone pharmacopoeial harmonisation (see chapter 5.8. Pharmacopoeial harmonisation).GENERAL CHAPTERS2.2.34. Thermal analysis2.9.3. Dissolution test for solid dosage forms2.9.40. Uniformity of dosage unitsMONOGRAPHSMonographsCrospovidone (0892)Glucose, anhydrous (0177)Glucose monohydrate (0178)Hypromellose (0348)Methylcellulose (0345)Polysorbate 80 (0428)TEXTS WHOSE TITLE HAS CHANGEDThe titles of the following texts have been changed in Supplement 8.6.GENERAL CHAPTERS2.2.4. Approximate pH of solutions (previously Relationship between reaction of solution, approximate pH and colour of certain indicators)MONOGRAPHSMonographsAmiloride hydrochloride dihydrate (0651) (previously Amiloride hydrochloride)DELETED TEXTSThe following texts are deleted as of 1 January 2016.MONOGRAPHSImmunosera for veterinary useClostridium novyi alpha antitoxin for veterinary use (0339)Clostridium perfringens beta antitoxin for veterinary use (0340)Clostridium perfringens epsilon antitoxin for veterinary use (0341)The following text is deleted as of 1 April 2015.MONOGRAPHSMonographsLiquorice ethanolic liquid extract, standardised (1536)下载PDF格式订购欧洲药典版本货期⽣效⽇期价格欧洲药典8[2卷]及增补1、2 [印刷/英⽂]现货2014年1⽉¥4950.00欧洲药典8 增补3、增补4、增补5 [印刷/英⽂]现货2014年4⽉¥4950.00欧洲药典8.3、8.4、8.5 [U盘/英⽂/含8.0-8.2内容]现货2014年4⽉¥4950.00欧洲药典8.6、8.7、8.8 [U盘/英⽂/含8.0-8.5内容]现货2016年1⽉¥4950.00。
欧洲药典标准 -回复

欧洲药典标准-回复欧洲药典标准——提高医药品质的指南导论:欧洲药典标准是制药和医学领域的一项重要参考,为确保制药工业生产过程的质量和安全性提供了明确指导。
本文将逐步介绍欧洲药典标准,包括其背景、作用、更新过程以及对制药行业和患者的影响。
第一部分:背景1. 欧洲药典的起源欧洲药典由欧洲药典委员会(EDQM)负责管理,成立于1964年。
其初衷是通过制定和推广共享卓越的药物质量控制方法,促进药品的质量、安全性和疗效。
2. 欧洲药典的目标欧洲药典的目标是确保欧洲范围内生产和分发的药品质量符合高标准,以保障患者使用的药品的有效性、安全性和一致性。
第二部分:作用1. 药物质量控制欧洲药典标准提供了一套严格的化学、物理和生物学测试方法,用于检验制药品质量。
药品生产商可以依据这些标准确保其产品的质量和一致性。
2. 环境和设备要求欧洲药典标准还规定了制药工业所需的环境和设备要求,以确保药品在生产、包装和储存过程中不受到污染。
3. 药品注册要求欧洲药典标准是制药行业在欧洲市场上注册药品所必需遵守的要求之一。
符合这些标准的药品可以获得欧洲药品注册的批准。
第三部分:更新过程1. 欧洲药典更新委员会欧洲药典标准每年都会进行更新和修订,以跟上科学和技术的发展。
这项工作由欧洲药典更新委员会负责,该委员会由来自各个国家的专家组成。
2. 更新内容和程序更新的内容包括新增的药品、检验方法的修订和新的法规要求。
更新程序包括对提案的评估、公开咨询和最终修订版本的制定。
第四部分:对制药行业和患者的影响1. 制药企业欧洲药典标准的遵循是制药企业获得药品注册批准的前提。
遵守这些标准有助于企业确保产品质量和合规性,提升企业的竞争力。
2. 患者利益欧洲药典标准的严格要求确保了药品的品质和一致性,提高了患者对药品疗效和安全性的信任。
患者能够获得符合标准的高质量药品,从而更有效地治疗和管理疾病。
结论:欧洲药典标准是欧洲范围内制药和医学领域的重要参考文献。
各国药典更新周期和特点比较

各国药典更新周期和特点⽐较药典是⼀个国家记载药品标准和规格的法典,⼀般由国家药典委员会编纂、国家药品监督管理机构批准并颁布实施。
⽽国际性药典则由公认的国际组织或者有关国家协商编订。
各个国家或者地区药典更新周期不同,同时也有不同的特点,本⽂对其进⾏⼀个总结和对⽐。
中国药典(CP):⼤家都⽐较熟悉。
/cms/home/发⾏历史和最新版本《中华⼈民共和国药典》(下称《中国药典》) 是中国药典委员会编制完成。
当前更新周期为每五年出版更新⼀次,⽬前最新版本为2015年版中国药典,也是新中国成⽴以来第⼗版药典。
中国药典的特点2015年版《中国药典》分四部,收载品种共计5608个,⼀部中药收载品种总数2598个,其中新增品种440个,修订品种517个,不收载品种7个;⼆部化学药收载品种总数2603个,其中新增品种492个,修订品种415个,不收载品种28个;三部⽣物制品收载品种总数137个,其中新增品种13个,修订品种105个;新增⽣物制品通则1个、⽣物制品总论3个;不收载品种6个;四部收载通则(附录)总数317个,其中整合和修订⼀部、⼆部、三部制剂通则38个,检测⽅法附录278个,新增检测⽅法18个、指导原则15个。
收载辅料品种总数270个,其中新增137个,修订97个,不收载2个(表1) 。
美国药典/国家处⽅集(USP/NF)U.S. Pharmacopeia / National Formulary:Pharmacopeia/发⾏历史和最新版本由美国政府所属的美国药典委员会(The United States Pharmacopeial Convention)编辑出版。
USP于1820年出第⼀版,1950年以后每5年出⼀次修订版,⼀直到2002年的USP25。
从2002年开始,以后每⼀年出版,到2017年11⽉已出⾄第41版。
NF于1883年出第⼀版,1980年15版起并⼊USP,但仍分两部分,前⾯为USP,后⾯为NF,于是出版了第⼀部USP20-NF15合订本。
欧洲药典第5版增修订的概貌

欧洲药典第5版增修订的概貌
朱济广
【期刊名称】《中国药品标准》
【年(卷),期】2005(6)1
【摘要】首先,欧洲药典第5版在编排上在较大改变,开始分一、二两册.原排在第4版各论之后的人用疫苗、兽用疫苗、人用免疫血清、兽用免疫血清、人用缝线、兽用缝线和顺势疗法制品的各论,统一移至第一册.第一册排在版首顺序仍为"前言"、"绪论"(introduction)、"欧洲药典委"(包括主席、委员、专家及秘书处高级技术人
员名单等)、"第5版内容"、"通则篇"(包括凡例、分析方法、包装容器原材料与包
装容器、通用文本).通用文本包括各论通则与制剂通则等.第二册全部为化学药和天然药各论.
【总页数】2页(P60-61)
【作者】朱济广
【作者单位】国家药典委员会,北京,100061
【正文语种】中文
【中图分类】R921
【相关文献】
1.教育部修订高考考纲:增古文化常识、数学文化 [J], ;
2.《中华人民共和国药典(2015年版)》(四部)通则中有关生物测定部分的增、修订情况介绍 [J], 唐黎明;陈桂良
3.威远药业受邀参与伊维菌素欧洲药典修订 [J],
4.第一讲《中图法》(第3版)修订的指导思想、修订原则与修订概貌 [J], 卜书庆;张涵
5.威远参与伊维菌素欧洲药典修订 [J],
因版权原因,仅展示原文概要,查看原文内容请购买。
欧洲药典10.0 胆固醇

欧洲药典10.0中胆固醇标准的重要更新及其影响一、引言欧洲药典10.0近日发布了关于胆固醇标准的重要更新,这一变化不仅对医药界,而且对整个社会都具有深远的影响。
胆固醇,一种在人体内自然存在的脂质物质,既是生物膜的重要组成部分,又是合成多种激素和维生素D的前体。
然而,胆固醇水平的异常也与一系列心血管疾病有关。
因此,对胆固醇的准确检测和标准化管理至关重要。
本文将详细讨论欧洲药典10.0中胆固醇的新标准,以及这些变化可能带来的影响。
二、欧洲药典10.0中的胆固醇新标准欧洲药典10.0对胆固醇的标准进行了重要的更新。
首先,对于胆固醇的纯度要求更加严格,以确保药品的质量和安全性。
其次,新标准对胆固醇的检测方法进行了优化,提高了检测的准确性和可靠性。
这些新标准的设立,无疑将为欧洲甚至全球的医药界带来新的挑战和机遇。
三、新标准对医药界的影响1. 药品研发:新标准的实施将推动药品研发行业对胆固醇相关药物进行更深入的研究和开发。
制药公司需要重新评估和调整他们的研发策略,以满足新的纯度和检测标准。
2. 临床试验:新的胆固醇标准也将对临床试验产生影响。
在新标准的指导下,临床试验需要更准确地评估药物对胆固醇水平的影响,这将有助于更精确地预测药物的疗效和安全性。
3. 药品生产:对于药品生产行业来说,新标准的实施意味着他们需要升级生产设备和技术,以满足新的纯度要求。
这将增加生产成本,但也为那些能够成功适应新标准的制药公司带来了市场机会。
四、新标准对社会的影响1. 提高公众健康意识:欧洲药典10.0对胆固醇标准的更新,将有助于提高公众对胆固醇及其相关疾病的认识和重视程度。
这将进一步推动公众采取更健康的生活方式,以降低患心血管疾病的风险。
2. 促进医药创新:新标准的实施将激励医药界进行创新,开发出更有效的药物和治疗方法,以满足新标准的要求。
这将推动医药行业的进步,为公众提供更优质的医疗服务。
3. 增强国际竞争力:欧洲药典10.0的新标准将使欧洲的医药产品和服务在国际市场上更具竞争力。
中国、美国、欧洲药典比较

姓名:徐涛学号:14211020462 专业:中药生物技术学《中国药典》、《美国药典》、《欧洲药典》比较1、各国药典概况1.1 历史沿革《中国药典》英文名称Pharmacopoeia of The People’s Republic of China;简称Ch .P。
1950年4月,成立了第一届中国药典编纂委员会,药典委员会分设名词、化学药、制剂、植物药、生物制品、动物药、药理、剂量8个小组,第一版《中国药典》于1953年由卫生部编印发行。
1957年出版《中国药典》1953年增补本。
1953年药典共收载药品531中,其中化学药215种,植物药与油脂类65种,动物药13种,抗生素2种,生物制品25种,各类制剂211种。
1965年1月26日卫生部颁布《中国药典》1963年版(第二版)发行通知和实施办法。
本版药典收载药品1310种,分一、二部,各有凡例和有关的目录,一部收载中医常用的中药材446种和中药成方制剂197;二部收载化学药品667种。
此外,一部记载药品的“功能主治”,二部增加了药品的“作用与用途”。
1979年10月4日卫生部颁布《中国药典》1977年版(第三版),自1980年1月1日起执行。
本版药典共收载药品1925种,其中一部收载中草药材(包括少数民族药材)、中草药提取物、植物油脂以及单味药材制剂等882种,成方制剂(包括少数民族药成方)270种,共1152种;二部收载化学药品、生物制品等773种。
1985年9月出版《中国药典》1985年版(第四版),1986年4月1日起执行。
本版收载药品1489种,其中一部收载中药材、植物油脂及单味制剂506种,成方制剂207种,共713种,二部收载化学药品、生物制品等776种。
1990年12月3日卫生部颁布《中国药典》1990年版(第五版),自1991年7月1日起执行。
1990年版的第一、第二增补本先后于1992、1993年出版,英文版于1993年7月出版。
欧洲药典适用性证书的变更更新的管理程序 中英对照 2013.07.13

Procedures for management of revisions/renewals of certificates of suitability to the European Pharmacopoeia monographs Certification of suitability to Monographs of the European Pharmacopoeia欧洲药典适用性证书PROCEDURES FOR MANAGEMENT OF REVISIONS/RENEWALS OF CERTIFICATES OF SUITABILITY TO THE EUROPEAN PHARMACOPOEIAMONOGRAPHS欧洲药典适用性证书的变更/更新的管理程序Introduction:介绍This document should be read in conjunction with the EDQM “Guideline on Requirements on Revision/Renewal of Certificates of Suitability to the European Pharmacopoeia monographs”(PA/PH/CEP (04) 2, as amended), which describes the conditions to be fulfilled as well as the documentation to be submitted for each request for revision.此文件应该与EDQM的“欧洲药典适用性证书修订与更新规定指南” (PA/PH/CEP (04) 2)联合起来阅读,后者描述了每个变更所要求满足的条件,以及要提供的文件资料。
The procedures for the management of revisions of certificates of suitability (CEPs) are described below and have been revised according to the revised European Regulation for Variations to Marketing Authorisation Applications.对于CEP证书变更管理的程序,在下面进行了描述,并且按照新修订的欧洲市场授权申请的有关法规进行了修订。
各国药典比较

ChP、USP、Ph.Eur.中药/天然药物质量标准比较及评述—以芦荟为例by14211第一部分中美欧药典简介1.中国药典《中华人民共和国药典》,简称《中国药典》。
是由国家药典委员会(原名卫生部药典委员会成立于1950年),根据《中华人民共和国药品管理法》的规定,负责组织编纂《中华人民共和国药典》及制定、修订国家药品标准,是法定的国家药品标准。
由国家食品药品监督管理部门批准颁布实施。
《中华人民共和国药典》(简称《中国药典》)2010年版,分一部、二部和三部,收载品种总计4567种,其中新增1386种。
药典一部收载药材和饮片、植物油脂和提取物、成方制剂和单味制剂等,品种共计2165种,其中新增1019种(包括439个饮片标准)、修订634种;药典二部收载化学药品、抗生素、生化药品、放射性药品以及药用辅料等,品种共计2271种,其中新增330种、修订1500种;药典三部收载生物制品,品种共计131种,其中新增37种、修订94种。
2010版药典收载的附录亦有变化,其中药典一部新增14个、修订47个;药典二部新增15个、修订69个;药典三部新增18个、修订39个。
一、二、三部共同采用的附录分别在各部中予以收载,并尽可能做到统一协调、求同存异。
中国药典包括凡例、正文及附录,是药品研制、生产、经营、使用和监督管理等均应遵循的法定依据。
所有国家药品标准应当符合中国药典凡例及附录的相关要求。
作为我国保证药品质量的法典,本版药典在保持科学性、先进性、规范性和权威性的基础上,着力解决制约药品质量与安全的突出问题,着力提高药品标准质量控制水平,充分借鉴了国际先进技术和经验,客观反映了中国当前医药工业、临床用药及检验技术的水平,必将在提高药品质量过程中起到积极而重要的作用,并将进一步扩大和提升我国药典在国际上的积极影响。
2.美国药典《美国药典/国家处方集》U.S. Pharmacopeia / National Formulary(简称USP/NF)。
欧洲药典在线查询全文
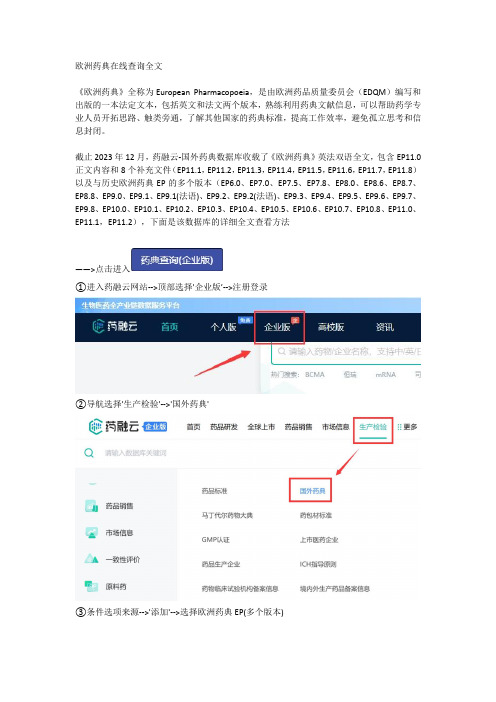
欧洲药典在线查询全文《欧洲药典》全称为European Pharmacopoeia,是由欧洲药品质量委员会(EDQM)编写和出版的一本法定文本,包括英文和法文两个版本,熟练利用药典文献信息,可以帮助药学专业人员开拓思路、触类旁通,了解其他国家的药典标准,提高工作效率,避免孤立思考和信息封闭。
截止2023年12月,药融云-国外药典数据库收载了《欧洲药典》英法双语全文,包含EP11.0正文内容和8个补充文件(EP11.1,EP11.2,EP11.3,EP11.4,EP11.5,EP11.6,EP11.7,EP11.8)以及与历史欧洲药典EP的多个版本(EP6.0、EP7.0、EP7.5、EP7.8、EP8.0、EP8.6、EP8.7、EP8.8、EP9.0、EP9.1、EP9.1(法语)、EP9.2、EP9.2(法语)、EP9.3、EP9.4、EP9.5、EP9.6、EP9.7、EP9.8、EP10.0、EP10.1、EP10.2、EP10.3、EP10.4、EP10.5、EP10.6、EP10.7、EP10.8、EP11.0、EP11.1,EP11.2),下面是该数据库的详细全文查看方法——>点击进入①进入药融云网站-->顶部选择'企业版’-->注册登录②导航选择'生产检验'-->'国外药典'③条件选项来源-->'添加'-->选择欧洲药典EP(多个版本)-----最后说下关于欧洲药典新版本EP11---《欧洲药典》EP11.0于2023年1月1日开始实施,是欧洲药品行业的权威参考书,EP11.0版本对之前版本的内容进行了全面更新和重新组织,新增和修改了600多个单体和试剂。
因此欧洲药典2024版将涵盖超过4000个全新的认证试剂,并提供7000多个化学物质的简要概述。
EP11.0版本的欧洲药典引入了一些重要的改进。
2013.9.1欧洲药典CEP证书修订更新规定指南PAPHCEP_(04)_2__4R[1]-翻译
![2013.9.1欧洲药典CEP证书修订更新规定指南PAPHCEP_(04)_2__4R[1]-翻译](https://img.taocdn.com/s3/m/da0f62d5d15abe23482f4d99.png)
Guideline on Requirements for Revision/Renewal of Certificates of Suitability to the European Pharmacopoeia MonographsEDQM CEP证书变更/更新指南要求目录1.INTRODUCTION: (1)1.介绍 (1)2.CLASSIFICATION OF CHANGES: (1)2.变更分类 (1)3.DOCUMENTATION TO BE PROVIDED: (2)3.需提供的文件 (2)4.LIST OF CHANGES: (3)4.变更清单 (3)4.I.ADMINISTRATIVE CHANGES (4)4.I.行政变更 (4)4.II.QUALITY CHANGES (7)4.II.质量变更 (7)4.II.1Manufacture (8)4.II.1生产 (8)4.II.2Control of the final substance (16)4.II.2产品的控制 (17)4.II.3Container closure system (20)4.II.3容器密封系统 (20)4.II.4Stability (22)4.II.4稳定性 (23)4.II.5Design Space and Post-Approval Change Management Protocols (24)4.II.5设计空间与批准后变更管理方案 (24)4.III.TSE CHANGES (26)4.III.TSE变更 (27)5.RENEWAL (30)5.更新 (32)6.TRANSFER OF HOLDERSHIP (33)6.CEP证书持有人转让 (33)Date of implementation: 1st September 2013实行日期:2013-9-11.INTRODUCTION:1.介绍The holder of a certificate of suitability (CEP) shall inform the EDQM of any change to information in the CEP application by sending an appropriate request for revision demonstrating that the conditions laid down in the present guideline are met. In addition, this guideline describes the requirements for the renewal of CEPs and for a transfer of holdership.欧洲药典适用性证书持有人必须向EDQM 报告所有与申报文件有关的变更,申报时应寄送合理的变更请求,证明变更符合现行指南的规定。
欧洲药典文档

欧洲药典1. 简介欧洲药典(European Pharmacopoeia,简称EP)是欧洲药典委员会(European Pharmacopoeia Commission)发布的一项药典标准,用于指导欧洲成员国家在医学及药学方面的药品标准、质量控制和药品监管工作。
欧洲药典于1964年首次发布,目前已经成为欧洲卫生和药品监管的法定标准。
2. 发布周期欧洲药典每年都会进行一次更新,以确保其与最新的科学和技术进展保持一致。
这意味着欧洲药典每年都会发布一次新版,其中包括新增的药品标准、更新的药品质量要求等内容。
3. 标准内容欧洲药典包含了广泛的药品标准和质量要求,涵盖了药物的各个方面,包括但不限于药品的质量、纯度、药理学活性、适应症以及适用于特定制剂的生产工艺等。
通过这些标准,欧洲药典旨在确保在欧洲市场上销售的药品具有一致的质量和效能。
3.1 药品质量标准欧洲药典确立了一系列的药品质量标准,包括药品的标识、纯度、含量、微生物限度和残留溶剂等方面的要求。
这些标准旨在确保药品在制造、贮存和使用过程中的质量和纯度不受到干扰,以保证药物的安全性和有效性。
3.2 药品生产工艺标准欧洲药典还规定了一系列的药品生产工艺标准,包括药品的制备方法、工艺步骤、原料选择和加工等。
这些标准的制定旨在确保药品的制造过程符合一定的质量要求,以保证药品的一致性和稳定性。
3.3 药理学标准除了药品质量和生产工艺标准外,欧洲药典还包含了一些药理学活性的标准。
这些标准通常是通过对药物在体内的作用机制和效果进行研究得出的,旨在为开发新的药物和评估已有药物的疗效提供指导。
4. 应用范围欧洲药典的应用范围广泛,包括但不限于药品制造商、药品监管机构、医药研究机构等。
对于药品制造商来说,欧洲药典的标准是必须遵守和符合的,以获得在欧洲市场上销售的资格。
对于药品监管机构来说,欧洲药典的标准是评估和监督药品质量与安全性的重要依据。
对于医药研究机构来说,欧洲药典的标准是开展临床研究和药物开发的参考依据。
欧洲药典2034药用物质

欧洲药典2034药用物质欧洲药典(European Pharmacopoeia,简称Ph. Eur.)是欧洲共同体委员会(Commission of the European Communities)出版的欧洲药品标准集。
它是欧洲药品质量评估、药品生产、进口、销售和使用等方面的主要参考文件。
关于欧洲药典2034中的药用物质,以下是一些更详细的信息:1.定义与分类:药用物质(Substances for Pharmaceutical Use)通常指的是用于药品制造过程中的活性成分(Active Substances)和辅料(Excipients)。
活性成分是指药品中对疾病产生治疗效果的部分。
辅料则是除了活性成分以外的其他成分,如填充剂、崩解剂、稳定剂等,它们对于药品的成型、稳定性和患者接受度都至关重要。
2.质量标准与纯度要求:欧洲药典对药用物质设定了详细的质量标准,包括纯度、粒度、杂质含量、溶解性、稳定性等。
这些标准确保了药用物质在制造过程中的一致性,从而确保最终药品的质量和安全性。
药用物质必须符合欧洲药典中规定的限度,否则将被视为不合格。
3.来源与制造:药用物质可以来自多种来源,包括天然提取(如植物、动物或矿物来源)、化学合成或生物技术制造。
无论来源如何,药用物质的制造过程都必须符合药品生产质量管理规范(GMP)的要求,以确保产品的质量和安全性。
4.监管与合规:在欧洲,药用物质的生产、进口、销售和使用都受到欧洲药品管理局(European Medicines Agency,EMA)的严格监管。
EMA会定期评估药用物质的安全性、有效性和质量,并根据需要更新欧洲药典中的标准。
制药公司必须遵守欧洲药典和相关法规,确保药用物质的质量符合标准,否则将面临法律责任和市场禁入等后果。
5.药品评估与批准:在欧洲,新药申请(Marketing Authorization Applications,MAAs)必须包含药用物质的详细信息和质量控制数据,以证明其符合欧洲药典和相关法规的要求。
欧洲药典标准

欧洲药典标准欧洲药典标准是欧洲药学界广泛接受并使用的药物质量标准,它对于保障药物质量的稳定性和一致性起着至关重要的作用。
随着全球化的不断发展,欧洲药典标准也逐渐成为世界范围内广泛应用的参考依据。
本文将深入探讨欧洲药典标准的重要性、制定过程以及对于全球制药行业和患者安全的意义。
一、欧洲药典标准的重要性1.1 保障患者用药安全作为医疗保健行业不可或缺的一环,药物质量对于患者用药安全至关重要。
欧洲药典标准通过确立严格而细致的规范,确保了每一批次生产出来的制剂在质量上具备稳定性和可靠性。
这使得患者能够放心使用这些制剂,降低了因为不合格或次品造成病情恶化或其他不良后果发生的风险。
1.2 促进国际贸易与合作欧洲药典标准作为一种国际公认的药物质量标准,为全球制药行业提供了一个统一的参考框架。
这使得不同国家和地区的制药企业可以基于相同的标准进行生产,降低了贸易壁垒和技术壁垒。
同时,欧洲药典标准也为国际间的合作提供了基础,使得不同国家和地区之间可以进行相互认可和交流,共同提高制药行业的整体水平。
1.3 保护公众利益欧洲药典标准不仅仅关注于保护患者利益,同时也关注于保护整个公众利益。
通过确保制剂质量的稳定性和可靠性,欧洲药典标准有效地防止了次品或假冒伪劣产品流入市场。
这不仅有助于维护消费者权益,还有助于维持市场秩序和公平竞争环境。
二、欧洲药典标准的制定过程2.1 专家委员会欧洲药典委员会是负责制定、修订和发布欧洲药典标准的机构。
该委员会由来自欧洲各国的专家组成,代表了药学、医学和相关领域的权威。
这些专家通过研究、讨论和评估,确保欧洲药典标准的科学性和可靠性。
2.2 药物评估制定欧洲药典标准的过程中,委员会会对各种类型的药物进行评估。
这些评估包括对原料药、制剂、辅料等方面进行全面研究,以确保其质量符合要求。
同时,委员会还会考虑到不同类型患者(如儿童、老年人等)对于药物质量要求的特殊性。
2.3 草案讨论和修订在制定欧洲药典标准过程中,专家委员会将根据评估结果起草相应的标准文本。
欧洲药典10.0 2.8.13 20813 农药残留

01/2019:208132.8.13. 农药残留定义:本药典规定:农药是在草药生产、加工、储藏、运输或销售的过程中用来阻止、杀灭或控制任何害虫、不良植物或动物品种的物质或物质混合物。
若不控制这些害虫、不良植物或动物品种,就会干扰草药生产、加工、储藏、运输或销售顺利进行。
农药品种包括用作生长调节剂、脱叶剂或干燥剂和任何在农作物收割前后农作物所适用的物质,以便防止商品在储藏和运输期间变质。
农药残留会出现在草药原料和草药制剂中,残留量需加以控制。
限度: 除非本各论另有明确指示,供试草药至少应符合表2.8.13.-1中所指明的限度。
未列入本表的农药适用限度和它们因任何原因而受到人们怀疑的含量,则符合按欧盟(EC )396/2005号指导(包括它们的附录和以后更新文件)设立的限度。
既没有列入表2.8.13.-1,也没有列入欧盟指导文件的农药限度,则按下列公式计算:100⨯⨯HD MDD M ADIADI =联合国粮农组织-世界卫生组织(FAO-WHO)发表的日容许摄取量(mg/kg 人体总重)。
M =人体公斤重量(60kg ) MDD HD =药物的日公斤剂量。
草药制剂中农残的限度按下式计算: 若DER ≤10:DER MRD HD ⨯若DER >10:100⨯⨯HP MDD M ADIMRL HD =表2.8.13.-1或欧盟(EU)测试或用上式计算的草药中最大农残限度; DER =药品/提取率,如,用于生产草药制剂的草药原料的量和所得的草药制剂量的比值。
MDD HP=药品制剂的日使用剂量,以kg计。
如果了解该批的完整处理记录(使用的农药的性质和数量,培养期间和收获后的每个处理日期)且根据GACP可以精确地检查,则主管当局可能免除整个或部分的测试。
表2.8.13.-1物质限度(mg/kg)Acephate乙酰甲胺磷0.1 Alachlor甲草胺0.05 Aldrin and dieldrin(sum of)艾氏剂和狄氏剂(总计) 0.05 Azinphos-ethyl谷硫磷0.1 Azinphos-methyl谷速松 1 Bromophos-ethyl溴硫磷0.05 Bromophos-methyl甲基溴磷松0.05 Brompropylate溴螨酯 30.05 Chlordane(sum of cis-,trans-and oxychlordane)氯丹(以其顺式、反式和氧化氯丹的总和计)Chlrofenvinphos毒虫畏0.5 Chlorpyriphos-ethyl氯蜱硫磷0.2 Chlorpyriphos-methyl甲基陶斯松0.1 Chlorthal-dimethyl氯酞酸甲酯0.01 Cyfluthrin氟氯氰菊酯(总计)0.1λ-Cyhalothrin λ-格林奈 1 Cypermethrin and isomers(sum of)氯氰菊酯及其同分异构体(总和) 11DDT(sum of o,p’-DDE,p,p’-DDE,o,p’-DDT,p,p’-DDT,o,p’-TDE,p,p’TDE)DDT(以o,p’-DDE,p,p’-DDE,o,p’-DDT,p,p’-DDT,o,p’-TDE,p,p’TDE的总和计)Deltamethrin溴氰菊酯0.5 Diazinon二嗪农0.5 Dichlofluanid抑菌灵0.1 Dichlorvos敌敌畏 1Dicofol三氯杀螨醇0.5 Dimethoate and omethoate(sum of)乐果和氧(化)乐果(总计)0.1 Dithiocarbamates(expressed ad CS2)二硫代氨基甲酸盐类(以CS2形式) 23 Endosulfan(sum of isomers and endosulfan sulphate)硫丹(以其同分异构体及其硫酸盐的总和计)Endrin异狄氏剂0.05 Ethion乙硫磷 2 Etrimphos乙嘧硫磷0.05 Fenchlorophos(sum of fenchlorophos and fenchlorophos-oxon) (以皮蝇磷及0.1 其氧化物的总和计)Fennitrothion杀螟松0.5 Fenpropathrin甲氰菊酯0.03 Fensulfothion(sum of0.05 fensulfothion,fensulfothion-oxon,fensulfothion-oxonsulfon and fensulfothion-sulfon) 丰索磷(以丰索磷、丰索磷-oxon、丰索磷-oxonsulfon及丰索磷-sulfon的总和计)0.05 Fenthion(sum of fenthion , fenthion-oxon, fenthion-oxon-sulfon,fenthion-oxon-sulfoxid, fenthion-sulfon and fenthion-sulfoxid) 倍硫磷(以倍硫磷,倍硫磷-oxon,倍硫磷-oxon-sulfon,倍硫磷-oxon-sulfoxid,倍硫磷-sulfoxid总和计)Fenvalerate氰戊菊酯 1.5 Flucytrinate氟氰戊菊酯0.05 τ-Fluvalinate τ-氟胺氰菊酯0.05 Fonophos地虫磷0.05 Heptachlor(sun of heptachlor,cis-heptachlroepoxide and0.05 trans-heptachlorepoxide) 七氯(以七氯,顺式和反式七氯总和计) Hexachlorbenzene六氯苯0.1 Hexachlorocyclohexane(sum of isomers α-,β-,δ-andε)六氯环己烷0.3 Lindan(γhexachlorocyclohexane) 林丹(γ-六六六)0.6 Malathion and malaoxon(sum of ) 褪黑激素和马拉氧磷(总计) 1 Mecarbam灭蚜磷0.05Methacriphos虫酰肼0.05 Methamidophos甲胺磷0.05 Methidathion杀扑磷0.2 Methoxychlor甲氧滴滴涕0.05 Mirex灭蚁灵0.01 Monocrotophos久效磷0.10.5 Parathion-ethyl and Paraoxon-ethyl(sum of ) 乙基对硫磷和乙基对氧磷(总计)0.2 Parathion-methyl and Paraoxon-methyl(sum of ) 甲基对硫磷和甲基对氧磷(总计)Pendimethalin二甲戊乐灵0.5 Pentachloranisol五氯苯甲醚0.01 Permethrin and isomers(sum of) 扑灭司林及其异构体(总计) 1 Phosalone伏杀硫磷0.1 Phosmet亚胺硫磷0.05 Piperomyl butoxide胡椒基丁醚 3 Pirimiphos-ethyl乙基嘧啶磷0.054 Pirimiphos-methyl(sum of pirimiphos-methyl and N-desethyl-pirimiphos-methyl) 甲基嘧啶磷(甲基嘧啶磷和N-去乙基-甲基嘧啶磷的总计)Procymidone腐霉利0.1 Profenophos丙溴磷0.1 Prothiophos丙硫磷0.053 Pyrethrum(sum of cinerinⅠ,cinerinⅡ,jasmolinⅠ, jasmolinⅡ,pyrethrinⅠand pyrethrin Ⅱ) 除虫菊(除虫菊酯I、除虫菊酯II、茉莉菊酯I、茉莉菊酯II、除虫菊素I、除虫菊素II的总计)Quinalphos喹噁啉0.051 Quintozene(sum of quintozene,pentachloraniline and methylpenthachlorphenyl sulfide) 五氯硝基苯(五氯硝基苯、五氯苯胺、甲基-五氯苯硫酸盐的总计)S-421 0.02 Tecnazene四氯硝基苯0.05 Tetradifon四氯二苯砜0.3 Vinclozonlin乙烯菌核利0.4草药的抽样:根据总章2.8.20草药:抽样及样品的制备进行抽样。
欧洲药典CEP证书修订更新规定指南PAPHCEP (04) 2, 4R[1]
![欧洲药典CEP证书修订更新规定指南PAPHCEP (04) 2, 4R[1]](https://img.taocdn.com/s3/m/b9be8bbbfd0a79563c1e7278.png)
AN6 成品或中间体批量变化:变小 条件: - 生产方法所有变动只与批量变小有关,如:使用不同大小的设备 - 至少有符合已批准质量标准的两个成品批号检验结果(新批量) - 产品不是生物或无菌产品 - 变动不影响生产工艺的重现性 - 该变动原因不是生产过程的异常或稳定性实验出现问题引起的 - 原批量不是经过 通知 批准的
NB: 若新包装比原包装更严格,三个月的稳定性数据不是必须的
如果复测期(按新包装)后不符合标准或可能不符合标准,须尽快完成这些研究 并立即将数据提交给EDQM T - 产品不是无菌、液体或生物产品t 文件要求: - 若有的话,新旧内包装标准对比 -新包装的一些适当的数据,包括证明材料符合相关药典要求或欧盟对与食品接 触的塑料材料或物体的法规 - 证书持有者申明:要求的稳定性实验已经按照ICH条件(指明批号)进行,执 行时可提供符合要求的最少稳定性数据且数据没有问题。确定将完成实验,并且 若在复测期(新标准)结束后出现不合格时会立即将数据上报给相关部门
通知((IN/AN) 通知分为立即通知和每年报告的通知 1. 立即通知 IN
IN1 证书持有人或生产厂原名原址变更 条件: - 证书持有人/生产法人地位不变(公司出让或被兼并除外)。 文件要求: - 官方出具(如:商会)的有关新名称和新地址的正式文件
- 更新所有声明(见申请表附录)
IN2 生产场所名称或地址改变 条件:生产场所具体位置应保持不变。 文件要求: - 官方出具(如:商会)的有关新名称和新地址的正式文件 - 声明更新:按申报文件组织生产、GMP 声明或愿意接受检查的声明
欧洲药典的第11版及增补内容

《探讨欧洲药典第11版及增补内容》近年来,随着医药领域的不断发展,欧洲药典第11版及增补内容备受关注。
本文将从多个角度全面评估欧洲药典第11版及增补内容,以及其对医药行业的影响。
一、欧洲药典第11版及增补内容概述1.1 欧洲药典的历史和发展在探讨欧洲药典第11版及增补内容之前,我们有必要回顾一下欧洲药典的历史和发展。
欧洲药典始于1964年,至今已经经历了数次重要的版本更新与修订。
在第11版中,除了延续之前的药典标准,还增加了大量新的内容和标准。
1.2 新增标准和内容欧洲药典第11版及增补内容的一个重要特点就是新增了大量标准和内容。
这些新增标准和内容可能涉及到新的药物成分、药物制剂、检测方法等多个方面,对医药行业的发展具有重要意义。
二、欧洲药典第11版及增补内容对医药行业的影响2.1 质量标准的提升欧洲药典第11版及增补内容的发布将会对医药行业的质量标准产生一定的影响。
新增的标准和内容将有助于提升药品的质量控制和生产标准,从而保障患者的用药安全。
2.2 市场需求和产业发展随着欧洲药典第11版及增补内容的实施,市场对符合新标准的药品需求将逐渐增加。
这必将对医药行业的产业发展产生重大影响,推动行业向更加规范、科学和可持续的方向迈进。
三、个人观点和理解3.1 对欧洲药典第11版及增补内容的期待作为一名医学工作者,我对欧洲药典第11版及增补内容寄予厚望。
我期待这一次的更新能够更好地兼顾传统和创新,为医药行业的发展注入更多活力与动力。
3.2 对医药行业的影响我相信欧洲药典第11版及增补内容的发布将会对医药行业产生深远的影响。
这种影响不仅来自于其新增的标准和内容,更来自于对医药行业的规范化和标准化推动。
结语文章通过对欧洲药典第11版及增补内容的概述、对医药行业的影响和个人观点和理解的深入探讨,希望能够使读者更全面、深刻和灵活地理解并关注这一重要议题。
欧洲药典第11版及增补内容的发布是医药领域的重要事件,相信它将对医药产业产生积极推动和引领作用,推动医药行业迈向更加健康、可持续的发展道路。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
110
European Regulatory Network
©2012 EDQM, Council of Europe, All rights reserved
欧洲立法网络
©2012 EDQM, Council of Europe, All rights reserved
111
European Pharmacopoeia (Ph. Eur.)
©2012 EDQM, Council of Europe, All rights reserved 13
欧洲药典委员会
• 每个成员国或观察员一名代表 • 36个成员国以及一名欧盟代表(卫生与消费者总局和EMA代表); 24个观察国和WHO。. • 卫生部、卫生部门、药典、大学或企业代表,由国家主管当局根 据其专业水平任命。 • 每年三次会议:内容经过不记名投票决定采纳。 • 现在20个永久性专家组&52个特设工作组:每年超过250天专家会 议;由药典委员会决定专家组成员 • 一个秘书处:EDQM/Ph. Eur. Department (EPD)
©2012 EDQM, Council of Europe, All rights reserved
©2012 EDQM, Council of Europe, All rights reserved
工作通则
• 基于由欧洲药典委员会(药典管理主体)指定的不同 专家组和工作组成员之间的科学合作,药典专论或其 它文本的起草和批准经过了一个高效、透明和顺利运 作的过程。
©2012 EDQM, Council of Europe, All rights reserved
©2012 EDQM, Council of Europe, All rights reserved
欧洲药品质量管理局(EDQM)
• 欧 洲 指 导 委 员 会 , 基 于 发 展 欧 洲 药 典 的 共 识 (PA, 1964) • 使命:为基本人权做贡献:良好 药品和卫生保健质量
©2012 EDQM, Council of Europe, All rights reserved
©2012 EDQM, Council of Europe, All rights reserved 18
115
General Working Principles
• Elaboration and approval of monographs and other texts proceeds through an efficient and transparent, smooth-running process, based on scientific cooperation between the members of the various Groups of Experts and Working Parties assigned by the European Pharmacopoeia Commission, the governing body of the Pharmacopoeia.
©2012 EDQM, Council of Europe, All rights reserved
工作通则(2)
• 专家们贡献出时间、专业和经验来建立公众可获得的 最高水平的质量标准,这些质量标准随着科技发展进 行持续改进。 • 来自于法规部门、官方药品控制实验室、学院和企业 等的专家的合作,代表了科学合作的最高峰,以得到 高质量的技术专论和章节。
2
107
Agenda
• • • • • • • • The EDQM and the Ph. Eur. General working principles Control of impurities Heavy metals P4 procedure: a success story Ph. Eur. and “Quality by Design” Ph. Eur. strategy in the biological field Ph. Eur. and adulterants
©2012 EDQM, Council of Europe, All rights reserved 3
日程
• • • • • • • • EDQM及欧洲药典 工作通则 杂质控制 重金属 P4规程:一个成功的故事 欧洲药典和“质量源于设计” 生物领域内的欧洲药典策略 欧洲药典和掺杂物
©2012 EDQM, Council of Europe, All rights reserved 4
©2012 EDQM, Council of Europe, All rights reserved
11
欧洲药典
• 保护公共健康:一种普遍的义务标准 • 欧洲药典是欧洲的正式官方药典–以各成员国的国家药典作为补 充,该国家药典内容仅对该国有用 • 在36个成员国(欧洲委员会)和欧盟内同一天强制生效(欧洲 药典委员会决策). • 对欧盟成员国内所有药品标准具有法定约束力,如原辅料,制 剂,剂型,容器必须符合欧洲药典现存标准的规定。
– protection of human rights – pluralist democracy & the rule of law
©2012 EDQM, Council of Europe, All rights reserved
5
欧洲理事会
– 建于1949年 – 欧洲共同和民主原则的发 展 – 47个成员国 – 总部位于斯特拉斯堡 – 核心价值:
欧洲药典:超过2187个专论
©2012 EDQM, Council of Europe, All rights reserved
114
GENERAL WORKING PRINCIPLES
©2012 EDQM, Council of Europe, All rights reserved
17
工作通则
116
General Working Principles (2)
• Experts give of their time, expertise and experience to produce the highest-level quality standards available to the public, standards that are continually revised in line with scientific developments. • This co-operation between experts from regulatory authorities, OMCLs, academia, and industry represents the pinnacle of scientific co-operation to produce a high standard of technical monographs and chapters.
Update on the European Pharmacopoeia
Dr. Susanne Keitel Workshop on GMP/Pharmacopoeia/API Beijing, 11-12 July 2012
1
欧洲药典的更新
Susanne Keitel博士 GMP/药典/API研讨会 北京, 11-12 July 2012
• Protecting public health - one common compulsory standard • The Ph. Eur. is the official pharmacopoeia in Europe – complemented by national pharmacopoeias for texts of interest to only one Member State • Mandatory at the same date in 36 Member States (CoE) and the EU (decision of Ph. Eur. Commission). • Legally binding quality standards for ALL medicinal products in its member states, i.e. raw material, preparations, dosage forms, containers must comply with the Ph. Eur. requirements when they exist.
– 人权保护 – 多元化民主&法制
©2012 EDQM, Council of Europe, All rights reserved
6
109
European Directorate for the பைடு நூலகம்uality of Medicines & HealthCare (EDQM)
• A Council of Europe Directorate, based on the Convention on the Elaboration of a European Pharmacopoeia (PA, 1964) • Mission: to contribute to a basic human right: access to good quality medicines and healthcare
©2012 EDQM, Council of Europe, All rights reserved
12
112
Ph. Eur. Commission
• One delegation per member state or observer • 36 member states plus a delegation from the EU (representatives from DG Health & Consumers and the EMA); 24 Observer countries and World Health Organization (WHO). • Delegates come from health ministries, health authorities, pharmacopoeias, universities, or industry and are appointed by the national authorities on the basis of their expertise. • Three sessions a year; texts are adopted by unanimous vote. • Currently 20 permanent Groups of Experts & 52 ad hoc Working Parties - > 250 meeting days/ year for experts; Composition of groups of experts decided by Ph. Eur. Commission • One Secretariat: EDQM/Ph. Eur. Department (EPD)
