2011版FDA最新工艺验证指南
FDA工艺验证指南(中文)

工艺验证:通则和实践本指南代表FDA对这一话题的当前观点,它不会创造或授予任何人任何权利,并不会对FDA或公众起约束作用,你可以使用另外的能够满足法律法规的替代方法,如果你要讨论替代方法,联系FDA负责该指南实施的人员。
如果你不认识特定的FDA 工作人员,请拨打本指南标题页的电话号码。
Ⅰ序言本指南概括了FDA认为的制造人用药和兽用药和生物制品的工艺验证通则和方法,包括APIs和药品。
在本指南中被称为药物或产品。
所有生产企业都可将这些原理和方法用于制造过程的工艺验证。
本指南结合产品生命周期观点和FDA现行的指南,包括FDA/ICH的工业指南,Q8(R2)制药开发,Q9风险管理,和QQ10制药质量体系。
尽管本指南没有重复上述指南中的观点和原理,但FDA鼓励在产品生命周期的所有阶段使用现在的药物开发理念,质量风险管理,和质量体系管理。
1、本指南由药品评估和研究中心(CDER)的生产和产品质量部起草,在FDA下属的药品评价和研究中心的药学办公室(PS),生物制品评估和研究中心(CBER),法规事务办公室(ORA),兽药中心(CVM)的协助下完成。
2、确认你有的指南是最新版的,在CDER指南浏览页查找(网址省略):生命周期观点和产品和工艺开发过程,商业化生产确认,如何在日常商业化生产过程确保工艺处于受控制的状态相关联,本指南支持用合理的技术和方法对工艺进行改进和创新。
本指南适用于以下类别的药品●人用药●兽用药●生物和生物技术产品●制剂和API(API’s或药用物质)●组合药物(药物和医疗器械)中的药物成分本指南不适用于以下类型的产品●含A型药物的产品和含药饲料●医疗器械●膳食补充剂●公共卫生法令361章节用于移植手术的人体组织本指南没有指定哪些信息为注册资料的一部分。
利害关系人可参考适当的指南或联系有关中心决定那些信息应注册时提交。
本指南也没有特别的讨论自动化过程控制系统的验证(比如,计算机硬件和软件界面),通常它们整合在新的制造设备中,本指南存在相关性,不论如何,在加工过程验证中应包括自动化设备的验证。
2011-FDA行业指南_工艺验证(中英文对照):一般原则与规范

Guidance for Industry行业指南Process Validation: GeneralPrinciples and Practices工艺验证:一般原则与规范U.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)Center for Biologics Evaluation and Research (CBER)Center for Veterinary Medicine (CVM)January 2011Current Good Manufacturing Practices (CGMP)Revision 1美国卫生与人类服务部食品药品管理局药物评价和研究中心(CDER)生物制品评价和研究中心(CBER)兽药中心(CVM)2011年1月现行药品质量生产管理规范(CGMP)修订版 1Guidance for Industry行业指南Process Validation: GeneralPrinciples and Practices工艺验证:一般原则与规范Additional copies are available from:Office of CommunicationsDivision of Drug Information, WO51, Room 220110903 New Hampshire Ave.Silver Spring, MD 20993Phone: 301-796-3400; Fax: 301-847-8714druginfo@/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htmand/orOffice of Communication, Outreach and Development, HFM-40Center for Biologics Evaluation and ResearchFood and Drug Administration1401 Rockville Pike, Rockville, MD 20852-1448(Tel) 800-835-4709 or 301-827-1800/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htmand/orCommunications Staff, HFV-12Center for Veterinary MedicineFood and Drug Administration7519 Standish Place,Rockville, MD 20855(Tel) 240-276-9300/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/default.htm另外的副本可从以下部门得到:马里兰州银泉市新罕布什尔大道10193号2201室药品信息处,对外信息办公室,邮政编码:20993电话:301-796-3400; 传真:301-847-8714druginfo@/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm和/或马里兰州洛克维尔市洛克维尔大道1401号 HFM-40 FDA生物制品评价和研究中心对外信息、外联与发展办公室邮政编码:20852-1448电话:800-835-4709 或 301-827-1800/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm和/或马里兰州洛克维尔市Standish Place 7519号食品药品管理局兽药中心HFV-12通讯处,邮政编码:20885电话:240-276-9300/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/default.htmU.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)Center for Biologics Evaluation and Research (CBER)Center for Veterinary Medicine (CVM)January 2011Current Good Manufacturing Practices (CGMP)Revision 1美国卫生与人类服务部食品药品管理局药物评估和研究中心(CDER)生物制品评估和研究中心(CBER)兽药中心(CVM)2011年1月现行药品质量生产管理规范(CGMP)修订版 1Table of Contents目录I. INTRODUCTION (1)一. 简介 (1)II. BACKGROUND (3)二. 背景 (3)A. Process Validation and Drug Quality (4)A. 工艺验证与药品质量 (4)B. Approach to Process Validation (5)B. 工艺验证方法 (5)III. STATUTORY AND REGULATORY REQUIREMENTS FOR PROCESS VALIDATION (7)三. 对工艺验证的法规和监管要求 (7)IV. RECOMMENDATIONS (9)四. 建议 (9)A. General Considerations for Process Validation (9)A. 对工艺验证的总体考虑 (9)B. Stage 1 - Process Design (10)B. 第一阶段 - 工艺设计 (10)1. Building and Capturing Process Knowledge and Understanding (11)1. 建立和捕获工艺知识与理解 (11)2. Establishing a Strategy for Process Control (12)2. 建立工艺控制策略 (12)C. Stage 2 - Process Qualification (14)C. 第二阶段 - 工艺确认 (14)1. Design of a Facility and Qualification of Utilities and Equipment (14)1. 厂房设施设计以及公用设施与设备确认 (14)2. Process Performance Qualification (16)2. 工艺性能确认 (16)3. PPQ Protocol (17)3. 工艺性能确认方案 (17)4. PPQ Protocol Execution and Report (19)4. 工艺性能确认执行与报告 (19)D. Stage 3 - Continued Process Verification (20)D. 第三阶段 - 持续工艺验证 (20)V. CONCURRENT RELEASE OF PPQ BATCHES (22)五. 工艺性能确认批次的同时放行 (22)VI. DOCUMENTATION (24)六. 文件记录 (24)VII. ANALYTICAL METHODOLOGY (24)七. 分析方法 (24)GLOSSARY (26)术语表 (26)REFERENCES (28)参考资料 (28)Guidance for Industry1行业指南1Process Validation: General Principles and Practices工艺验证:一般原则与实施This guidance represents the Food and Drug Administration’s (FDA’s) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance.本指南体现了食品药品管理局(FDA)关于这一主题的最新见解。
欧洲药监局--工艺验证指南更新意向书

Concept Paper on the Revision of the Guideline on Process Validation工艺验证指南更新意向书欧洲药品管理局,2010年2月25日发布1. IntroductionThis concept paper addresses the need to update the guideline on Process Validation1. This guideline was originally adopted in February 2001. With the development of new ICH guidelines Q8, Q9 and Q10, this guideline is being reviewed in order to implement the concepts highlighted in the ICH guidelines.该指南旨在适应ICH不断提高的要求,改动后将Q8,Q9和Q10的一些理念也融合了进去。
2. Problem statementThe current guideline does not reflect the recent regulatory developments on Process Analytical Technology (PAT), Quality by Design (QbD) and Real-Time Release Testing (RTRT).当前的指南没有考虑目前生产过程控制技术、质量源于设计和实时放行测试方面的发展。
3. Discussion (on the problem statement) 讨论(基于上述问题)即将出台的这份指南是基于ICHQ8、Q9和Q10这些先进指南的综合考虑而出台的。
根据这些新指南的出台,对于工艺控制以及传统验证批的概念提出了全新的理解(根据这些指南的定义,可能颠覆对传统的工艺验证批次以及工艺过程控制的理解)。
工艺验证指南简介201102

工艺验证指南简介201102FDA《工艺验证:一般原则与惯例》简介编者按:美国食品药品管理局(FDA)于1月24日在其官方网站上发布了名为《工艺验证:一般原则与惯例》工业指南的最终版本(这个指南的草案版曾于20XX年11月在FDA官方网站上发布)。
这个指南一经发布,就在美国以及许多国家的药政与企业间产生了强烈的反响,世界制药行业正积极讨论该指南的内涵,并为相应工作的开展做准备。
本报第一时间得到了业内专家对该文件概要的翻译及简析,现刊载于此,以读者。
《工艺验证:一般原则与惯例》工业指南(以下简称《指南》)在两年多的征求意见过程中,一些国际相关组织,如国际制药工程协会(ISPE)、美国注射剂协会(PDA)及一些大型制药公司等,都对《指南》的草案发表了相关的意见,足见世界制药行业对此文件的关注程度。
在1987年5月11日,FDA发布了一个通告,宣布了《工艺验证的一般原则》指南(1987年版)的问世。
日前发布的《指南》作为1987年版本的第一修订版,表达了FDA关于制药工艺验证的现行思考,并与1987年指南首先引入的基本原则相一致。
《指南》同样提供了能反映出FDA倡议的“21世纪制药cGMP-基于风险的办法”中对某些目标的建议,特别是提供了关于在药品生产中使用先进技术,以及实施现代风险管理和质量管理体系的工具和概念。
纵观《指南》,可以清楚地发现,其理念就是这些年来国际上一直推荐的,基于科学、基于风险的质量体系化过程。
但这个文件并没有在工艺验证方面规定证明工艺有效性所需要的验证次数,因此,相关的具体操作还要参考其他的指南性文件。
指南概要《指南》的正文部分有七章。
在正文部分以外还有“术语”与“参考文件”两个章节。
总体上来说,《指南》与草案版指南在基本理念、基本要素方面区别不大,但更严谨。
从文件中,我们可以看到其整合了ICH-Q8、Q9与Q10的基本理念。
笔者现简单介绍如下:第一章:简介简介中指出,工艺验证与产品生命周期相联系。
FDA工艺验证指南的特点分析_康建磊

现代药物与临床 Drugs & Clinic 第31卷第7期 2016年7月·1110·• 药事管理 • FDA工艺验证指南的特点分析康建磊1,李亮2*1. 国家食品药品监督管理总局药品审评中心,北京 1000382. 中国医学科学院北京协和医学院放射医学研究所天津市分子核医学重点实验室,天津 300192摘 要:有效的工艺验证对保证药品质量至关重要,2011年1月24日,FDA发布了工艺验证指南《Process Validation: General Principles and Practices》。
该指南概况了工艺验证的一般原则和方法,将工艺验证与产品生命周期概念以及现有的FDA/ICH 指南进行了整合,如Q8(R2)药品研发、Q9质量风险管理和Q10药品质量体系。
从技术审评的角度,对FDA新版工艺验证指南的主要特点进行讨论分析。
关键词:FDA;工艺验证;特点;产品生命周期中图分类号:R951 文献标志码:A 文章编号:1674 - 5515(2016)07 - 1110 - 03DOI: 10.7501/j.issn.1674-5515.2016.07.043Discussion on characteristics of FDA guidance for process validationKANG Jian-lei1, LI Yi-liang21. Center for Drug Evaluation, China Food and Drug Administration, Beijing 100038, China2. Tianjin Key Laboratory of Radiation Medicine and Molecular Nuclear Medicine, Institute of Radiation Medicine, Peking UnionMedical College & Chinese Academy of Medical Sciences, Tianjin 300192, ChinaAbstract: Effective process validation contributes significantly to assuring drug quality. On January 24, 2011, the process validation guidance “Process Validation: General Principles and Practices” was issued by FDA. This guidance overviews general principles and methods of process validation, aligns process validation activities with a product lifecycle concept and with existing FDA/ICH guidance, including Q8(R2) Pharmaceutical Development, Q9 Quality Risk Management, and Q10 Pharmaceutical Quality System. In this article, the main characteristics of FDA new guidance for process validation are discussed from the view of technical review.Key words: FDA; process validation; characteristics; product lifecycle美国食品药品监督管理局(FDA)于1987年首次发布了药品工艺验证的指南,伴随着现代科学技术、药品质量及风险管理理念的不断提升,FDA 对工艺验证指南逐步进行修订和完善,2008年11月更新了工艺验证指南的草案,并对工艺验证的内涵、总体原则等进行了大幅度的改进和更新。
美国FDA生产过程(工艺)验证总则指南

美国FDA生产过程(工艺)验证总则指南1 9 8 7年I.目的 3II.范围 3III.序言 3Ⅳ. 总概念 4V.现行药品生产质量管理规范(CGMP)法规 6Ⅵ.医疗器械的生产质量管理规范法规7Ⅶ.验证预备阶段所需考虑的事情7Ⅷ.生产过程验证的内容8Ⅸ.产品检验的可接受性11I.目的本指南概述了人用和兽用药品和医疗器械的生产过程(工艺)验证的总则,其验证的基本原理是得到fdA认可的。
II.范围本指南是根据21CFR10-90颁布的,适用于药品和医疗器械的生产。
本指南阐述了一般适用范围的原则和方法,这些原则和方法在法律上未做规定要求,但是得到了fdA认可。
本指南可以作为依据,并保证可以得到FDA的批准,但也可以按照其他方法进行验证。
在使用不同方法进行验证时,可事前与(但也可以不与)fdA讨论所要进行的验证工作,以避免在以后被FDA认为不合格而浪费了财力和精力。
总而言之,本指南列述的有关药品和医疗器械的生产过程验证原则和方法,是得到FdA认可的。
但不是在所有情况下都必须使用本指南所列述的原则和方法以符合法律。
本指南是要经常进行修订的。
对此有兴趣的人士可对本文件及随后的任一版本提出意见。
书面意见应向FDA的Dockets Maragement Branch(HFA—305)上报。
地址为:Room 462,5600FishersLane,Rockville,Maryland20847。
在星期—至星期五,上午9:00到下午4:00可在该办公处查阅所收到的意见,III.序言生产过程验证是药品生产管理规范法规21CFR210•211和医疗器械生产管理规范法规21CFR820的规定要求,所以适用于药品和医疗器械的生产。
有些生产厂商曾向FDA要求提供具体的指导:关寸FDA要求生产商做些什么工作,以保证生产过程验证符合规定的要求。
本指南讨沦了生产过程验证的原理和概念,FDA认为这些原理和概念是符合验证方案要求的。
GMP 工艺验证

生产工艺在使用规定的原辅料和设备条件下 应 当
能够始终生产出符合预定用途和注册要求的产品。
三、新版GMP对工艺验证的要求
当
影 响
产 品 质 量 的 主 要 因 素
原辅料 与药品直接接触的包装材料
生产设备 生产环境(或厂房)
生产工艺 检验方法
发生变更时
应当进行 确认或验证
必要时
还应当经药品监 督管理部门批准
书面化的证据,证明工艺在所建立的参数 范围内能有效和重复生产出符合预期标准
和质量属性的医药产品。
确认的定义
证明厂房、设施、设备能正确运行并可
达到预期结果的一系列活动。
验证的定义
证明任何操作规程(或方法)、生产工艺 或系统能够达到预期结果的一系列活动。
三、新版GMP对工艺验证的要求
企业
确认或验证的范围和程度
五、FDA 2011版工艺验证基本内容
1)厂房设计及公用系统及设备的确认 2)工艺性能确认(PPQ) 3)PPQ方案 4)PPQ方案实施与报告
五、FDA 2011版工艺验证基本内容
• 第三阶段-持续工艺核查 • ������ 目标:持续保证工艺能保持在商业化生产中的可控性
(即验证状态)。 • -恪守GMP要求。 • -一个或多个系统用来探测与所设计的工艺非计划性偏离。 • -收集、评估关于工艺的信息和数据,查出漂移和漂移的程
五、FDA 2011版工艺验证基本内容
• -由统计专家建立用于衡量与评价工艺稳定和工艺能力的数 据收集方案。
• -收集生产数据以便评估工艺的稳定性与生产能力。 • -质量管理部门应当审核这些资料。 • -做法得当应能发现问题。 • -及时发现漂移,警告生产单位对工艺进行完善。 • -建立报警与行动限度。 • -对工艺变异进行定期的评估,取样和/或监测也应做相应的
工艺验证新版GMP附录

工艺验证流程:工艺验证的生命周期
1.工艺设计 设计,研发和放大 知识和技术转移 关键质量属性确定 关键工艺参数确定
11
工艺验证流程:工艺验证的生命周期
2.工艺确认 调试和确认厂房、设备、公用设施,人 员培训…… 工艺确认方案并成功执行 产品质量一致性与工艺能力的分析和评 估
12
工艺验证流程:工艺验证的生命周期
3.持续工艺确认 产品质量与工艺能力的监控与趋势分析 产品质量一致性与工艺能力的持续分析 和评估 再验证和后续确认 工艺验证应在足够的设计,研发和放 大后,才能成功进行
13
产品和工艺的控制策略关键
• 控制策略Control Strategy(CS)
基于当前对产品和工艺的了解,为确保工艺性能和产品质量而计 划进行的一系列控制。这些控制可包括与原料药以及药物制剂的 材料和组分相关的参数和属性,设施和设备运行条件,过程控制 ,成品质量标准,以及相关的监测和控制方法与频率
9
什么时候工艺验证?
• 首次验证,当引入新的产品 • 变更后的验证: 当现有的产品,现有工艺从一个生产线、生产车间 或工厂转移到其它的生产线,生产车间或工厂时-技术 转移 当产品批量发生变更时 当关键工艺变更,关键工艺参数变更,关键设备等 等变更…… 是否需要工艺验证: 应建立在对产品和工艺进行基于风险,基于科学的系 统和综合评估之上! 10 应建立在法规要求上(CDE变更技术指南)!
GMP对工艺验证的要求
第一百四十二条当影响产品质量的主要因素,如原辅 料、与药品直接接触的包装材料、生产设备、生产环境 (或厂房)、生产工艺、检验方法等发生变更时,应当 进行确认或验证。必要时,还应当经药品监督管理部门 批准。 第一百四十四条确认和验证不是一次性的行为。首次 确认或验证后,应当根据产品质量回顾分析情况进行再 确认或再验证。关键的生产工艺和操作规程应当定期进 行再验证,确保其能够达到预期结果。
最新美FDA药品工艺验证指南草案摘要

美FDA新的药品工艺验证指南草案摘要2008年11月中旬,美FDA推出了新的工艺验证指南草案(以下简称指南草案)。
随即,这项草案成为业界关注的焦点。
指南草案共分七章,勾勒出的一般原则与方法,将是FDA进行工艺验证的恰当要素。
这些工艺被用于生产人用药物、兽药、生物和生物技术产品、制剂产品与活性药物成分(API或药用物质)、复合产品(药物和医疗设备)中的药物成分类别的药品。
此次的指南草案与上一个版本[《工艺验证的一般原则指南(1987年指南)》]不同的是,没有针对具体的工艺验证进行规范,而是将ICH (国际人用药品注册和医药技术协调会议)-Q8(药物研发)、Q9(质量风险管理)与Q10(制药质量体系)的理念整合到了新的指南中。
其中最大的变化是,将整个验证过程分成了工艺设计、工艺确认、持续工艺核实3个阶段,制药企业需要在目前验证管理的基础上,将前期研发、中试阶段的数据进行分析整理,作为验证的一部分或作为对第三阶段验证的支持。
同时,指南草案将工艺验证活动与产品生命周期概念以及现有的FDA指南进行了调整,将产品与工艺开发、商业生产工艺的确认以及保持工艺在日常商业生产中处于受控状态连接在一起。
指南草案还明确,工艺验证对于药品生产来说是一个法定的、强制性的要求。
指南草案传达了FDA关于工艺验证的现行思考,特别是提供了关于在药品生产中使用先进技术,以及实施现代风险管理和质量管理体系的工具和概念。
当正式公布后,该指南将取代1987年的指南。
所有的药品生产企业都可以将这些原则与方法用于生产工艺的验证。
本版刊登的是FDA新的工艺验证指南草案的主要内容,虽然该指南还仅是草案,但对于不断开拓国际市场的我国药企来说,其理念很值得重视,利于未雨绸缪。
工艺验证的法规要求药品(制剂与其成分)的工艺验证是《食品、药品与化妆品法案》之501(a)(2)(B)节规定的一个法定的、强制性的要求,其规定如下:“某一药品……如果在其生产、加工、包装或持有中所使用的方法、设施或管理控制不符合cGMP要求,或未按照cGMP要求来操作或管理,从而不能保证药品符合本法规所要求的安全性、鉴别、含量并符合其声称的质量与纯度特性,该药品将被视为伪劣药品”。
最新附录工艺验证检查条款解读

最新附录工艺验证检查条款解读作者:阿郎,转载请与作者联系。
什么是工艺验证?指为证明工艺在设定参数范围内能有效稳定地运行并生产出符合预定质量标准和质量特性药品的验证活动。
它包括有书面记录、预先设定工艺、验证方案、验证标准、工艺一致性和产品质量一致性等内容。
对此,2011年FDA行业指南“工艺验证:一般原则与规范”中定义工艺验证为“收集和评估从工艺设计、研发到商业化生产阶段的数据,并用这些数据科学的证明该工艺能够有能力持续稳定地生产出优质产品”。
两个定义的区别是,中国“工艺验证”还是基于传统的工艺验证活动,但本版附录已经和国外最新要求接轨了,但基于国情也做了某些方面的妥协;而欧美的“工艺验证”,更多基于产品的生命周期考虑,国外通行把工艺验证分为“工艺设计、工艺确认(传统的工艺验证)、持续工艺确认”三个阶段。
工艺验证的目的,不仅为监管机构所要求,更重要的是产品质量自身的需要,是为能够持续、可靠、稳健地生产出质量受控、有竞争力、高盈利的产品。
内容提纲如下:一、条款解读:一般要求(8条)二、工艺验证:生命周期三、工艺验证:QbD四、问题讨论一、条款解读:一般要求(8条)1、第19条:工艺验证应当证明一个生产工艺按照规定的工艺参数能够持续生产出符合预定用途和注册要求的产品。
工艺验证应当包括首次验证、影响产品质量的重大变更后的验证、必要的再验证以及在产品生命周期中的持续工艺确认,以确保工艺始终处于验证状态。
解读:本条款提出了工艺验证的总体要求,既要符合预定用途(满足目标产品质量概况QTPP)也要符合法定批准的注册要求。
另外,工艺验证包括的类型有首次验证、变更验证、再验证、持续工艺确认,取消了原来的回顾性验证。
1)回顾性验证做为一种事后的验证手段,和现代质量管理概念中的“必须建立一个持续和不断发展的程序、收集和分析与产品质量有关的产品和工艺数据”的持续改进的概念是冲突的,因此,新的欧盟验证指南,希望企业建立的是一个能够持续改进的验证系统。
FDA工艺验证指南深度解析

FDA工艺验证指南深度解析FDA (美国食品药品监管局) 工艺验证指南是为了确保在食品和药品制造过程中运行的设备和工艺能够满足质量标准和法规要求而制定的。
这份指南提供了详细的步骤和要求,以帮助制造商进行工艺验证,并确保产品的可靠性和一致性。
工艺验证是一个系统性的过程,旨在验证制造过程和设备能否实现其预期的目标和要求。
它涉及到收集和分析数据,以确保每一个生产步骤都能按预期进行,保证产品质量和安全性。
首先,工艺验证需要明确定义验证目标和范围。
这意味着制造商需要确保他们准确地了解他们希望验证的产品和过程,并明确标识验证所需的关键参数和指标。
其次,制造商需要制定验证计划,明确工艺验证所需的样本数量和测试方法。
这包括确定所需的数据收集点和统计学方法,以便得出可靠的结论。
然后,制造商需要收集和分析数据。
他们应该记录每个生产步骤的关键参数,并使用实验数据和统计学分析方法进行数据分析。
这将确保验证结果符合预期,并能够证明产品和过程的一致性。
最后,制造商需要编制验证报告,总结验证过程和结果。
这个报告应该包括验证计划、数据分析和结论,并指出是否满足验证目标。
验证报告是证明制造过程和设备符合质量标准和法规要求的重要文件。
总之,FDA工艺验证指南为制造商提供了详细的步骤和要求,以确保食品和药品制造过程中的设备和工艺能够满足质量标准和法规要求。
遵循这些指南将确保产品的可靠性和一致性,保护公众的健康和安全。
对于制造商来说,进行工艺验证是确保产品质量和符合法规要求的重要步骤。
工艺验证是美国食品药品监管局(FDA)在食品和药品制造过程中的一项重要要求。
通过工艺验证,制造商能够验证生产过程的可靠性和一致性,并确保所生产的食品和药品符合质量标准和法规要求。
在工艺验证的过程中,制造商需要进行一系列的步骤和操作,以确定关键参数,收集数据,进行数据分析,并编制验证报告。
首先,制造商需要明确定义工艺验证的目标和范围。
在这个阶段,制造商需要详细了解他们要验证的产品和生产过程,并明确验证所需的关键参数和指标。
FDA 最新工艺验证指南深度解析

FDA 最新工艺验证指南深度解析(一)验证工作是实施 GMP 规范的基础,而工艺验证又是验证工作中的关键性环节。
1987 年,FDA 发布了关于工艺验证的指南文件;FDA 关于工艺验证的要求和理解深深地影响着世界各国药政当局和制药行业。
2008 年 11 月,FDA 再次发布最新的工艺验证指南(草案),对工艺验证的概念和文件要求进行了大幅度地修改和更新。
毫无疑问,这个指南文件将对 GMP 实施产生巨大影响,尤其对于以国际市场为主的外向型制药企业,影响也必将是深远的。
笔者通过查阅 FDA 网站资料,并结合其他著名制药企业和行业协会对于这个指南地评价意见,对这个指南文件进行系统的分析和解读。
一、工艺验证指南文件结构FDA 工艺验证验证指南全名为《工艺验证:一般原则和规范》。
指南全文分为 7 部分,依次是:简介、背景、工艺验证的法规和法规要求、建议、性能确认批次的同步放行、文件和分析方法学。
二、工艺验证指南文件解读为了方便读者阅读,本部分顺序按照 FDA 工艺验证指南正文顺序进行依次解读和评价,并且采用了表格形式进行比较。
第一部分:简介工艺验证指南原文翻译I.简介本指南概括了一般的原则与方法,这些原则与方法是FDA 认为进行工艺验证的恰当要素,这些工艺被用于生产人用药、动物用药以及生物制品,包括活性药物成分(API 或药用物质),在本指南中以上统称为药品或产品。
本指南整合了一般的原则和方法,所有的生产企业都可以将这些原则和方法应用于生产工艺的验证。
评价意见应该在划线部分后面增加生物技术产品,应用范围和本指南后面描述保持一致。
工艺验证指南原文翻译本指南将工艺验证活动与产品生命周期概念以及现有FDA 相关指南(这里指得是Q8、Q9和Q10指南)进行了协调。
生命周期概念将产品与工艺开发、商业生产工艺的确认以及维护工艺在日常商业生产中处于受控状态连结在一起。
本指南可促进现代生产的原则、工艺改进、创新并且形成完善的科学。
FDA最新工艺验证指南(2011.1版)(中文版)

Guidance for Industry 行业指南Process Validation: General Principles and Practices工艺验证:一般原则与规范U.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)Center for Biologics Evaluation and Research (CBER)Center for Veterinary Medicine (CVM)January 2011Current Good Manufacturing Practices (CGMP)Revision 1美国卫生与人类服务部食品药品管理局药物评价和研究中心(CDER)生物制品评价和研究中心(CBER)兽药中心(CVM)2011年1月现行药品质量生产管理规范(CGMP)修订版1包含不具约束力的建议中文译稿:北京大学药物信息与工程研究中心************** Guidance for Industry 行业指南Process Validation: General Principles and Practices工艺验证:一般原则与规范Additional copies are available from:Office of CommunicationsDivision of Drug Information, WO51, Room 220110903 New Hampshire Ave.Silver Spring, MD 20993Phone: 301-796-3400; Fax: 301-847-8714****************.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htmand/orOffice of Communication, Outreach and Development, HFM-40Center for Biologics Evaluation and ResearchFood and Drug Administration1401 Rockville Pike, Rockville, MD 20852-1448(Tel) 800-835-4709 or 301-827-1800/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm and/orCommunications Staff, HFV-12Center for Veterinary MedicineFood and Drug Administration7519 Standish Place,Rockville, MD 20855(Tel) 240-276-9300/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/default.htm包含不具约束力的建议中文译稿:北京大学药物信息与工程研究中心**************另外的副本可从以下部门得到:马里兰州银泉市新罕布什尔大道10193号2201室药品信息处,对外信息办公室,邮政编码:20993电话:301-796-3400; 传真:301-847-8714****************.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm和/或马里兰州洛克维尔市洛克维尔大道1401号HFM-40 FDA生物制品评价和研究中心对外信息、外联与发展办公室邮政编码:20852-1448电话:800-835-4709 或301-827-1800/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm和/或马里兰州洛克维尔市Standish Place 7519号食品药品管理局兽药中心HFV-12通讯处,邮政编码:20885电话:240-276-9300/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/default.htmU.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)Center for Biologics Evaluation and Research (CBER)Center for Veterinary Medicine (CVM)January 2011Current Good Manufacturing Practices (CGMP)Revision 1美国卫生与人类服务部食品药品管理局药物评估和研究中心(CDER)生物制品评估和研究中心(CBER)兽药中心(CVM)2011年1月现行药品质量生产管理规范(CGMP)修订版 1包含不具约束力的建议中文译稿:北京大学药物信息与工程研究中心**************Table of Contents目录I. INTRODUCTION (1)一. 简介 (1)II. BACKGROUND (3)二. 背景 (3)A. Process Validation and Drug Quality (4)A. 工艺验证与药品质量 (4)B. Approach to Process Validation (5)B. 工艺验证方法 (5)III. STATUTORY AND REGULATORY REQUIREMENTS FOR PROCESS VALIDATION (7)三. 对工艺验证的法规和监管要求 (7)IV. RECOMMENDATIONS (9)四. 建议 (9)A. General Considerations for Process Validation (9)A. 对工艺验证的总体考虑 (9)B. Stage 1 - Process Design (10)B. 第一阶段- 工艺设计 (10)1. Building and Capturing Process Knowledge and Understanding (11)1. 建立和捕获工艺知识与理解 (11)2. Establishing a Strategy for Process Control (12)2. 建立工艺控制策略 (12)C. Stage 2 - Process Qualification (14)C. 第二阶段- 工艺确认 (14)1. Design of a Facility and Qualification of Utilities and Equipment (14)1. 厂房设施设计以及公用设施与设备确认 (14)2. Process Performance Qualification (16)2. 工艺性能确认 (16)3. PPQ Protocol (17)3. 工艺性能确认方案 (17)4. PPQ Protocol Execution and Report (19)4. 工艺性能确认执行与报告 (19)D. Stage 3 - Continued Process Verification (20)D. 第三阶段- 持续工艺验证 (20)V. CONCURRENT RELEASE OF PPQ BATCHES (22)五. 工艺性能确认批次的同时放行 (22)VI. DOCUMENTATION (24)六. 文件记录 (24)VII. ANALYTICAL METHODOLOGY (24)七. 分析方法 (24)GLOSSARY (26)术语表 (26)REFERENCES (28)参考资料 (28)包含不具约束力的建议中文译稿:北京大学药物信息与工程研究中心**************1Guidance for Industry1行业指南1Process Validation: General Principles and Practices工艺验证:一般原则与实施This guidance represents the Food and Drug Administration’s (FDA’s) current thin king on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance.本指南体现了食品药品管理局(FDA)关于这一主题的最新见解。
FDA工艺验证指南(2023年.1版)(中文版)

FDA最新工艺验证指南(2011.1版)(中文版)1000字食品药品监管局(FDA)发布了2011年1月的最新工艺验证指南(中文版),该指南包含了关于工艺验证的详细信息和建议。
工艺验证是一个验证过程,旨在确保产品的性能符合特定的规格要求。
以下是指南中重要的一些提要:工艺验证的定义工艺验证是一种验证过程,它通过控制和监测生产过程的关键要素(如温度、时间和物料)来证明产品性能的一致性,并确定任何很少出现的过程偏差的控制方法和监测程序。
此外,工艺验证有助于识别和消除潜在的质量问题,并确保产品的质量和安全性。
工艺验证的类型指南中提到了三种类型的工艺验证:安装资格验证、功能性验证和进程验证。
1.安装资格验证安装资格验证是确保关键设备和系统的安装、操作和性能符合要求的过程。
2.功能性验证功能性验证是证明产品在链接到现实的生产环境中,能够满足性能要求的过程。
3.进程验证进程验证是确保每次生产批次的过程参数符合规格要求的过程。
工艺验证的步骤指南中提到了四个主要的工艺验证步骤。
1.设计验证方案首先,必须设计一个包括验证示例、过程和程序检查和监测方法的验证方案。
2.执行验证执行验证时必须记录和监测所有的步骤和数据,特别是关键参数。
3.收集和分析数据通过收集和分析数据以确认生产过程的稳定性和一致性。
4.确定验证完成和维护验证状态一旦数据收集和分析完成,并验证的结果可以证明产品的一致性,就可以确定验证工作已经完成。
验证后,工厂必须确保维护验证状态,并定期进行监测。
总之,食品药品监管局(FDA)最新工艺验证指南(2011.1版)(中文版)提供了在生产过程中进行工艺验证的详细说明,这有助于确保生产的产品质量达到极高的水平,同时也有利于确保产品的安全性。
FDA 2011工艺验证指南培训
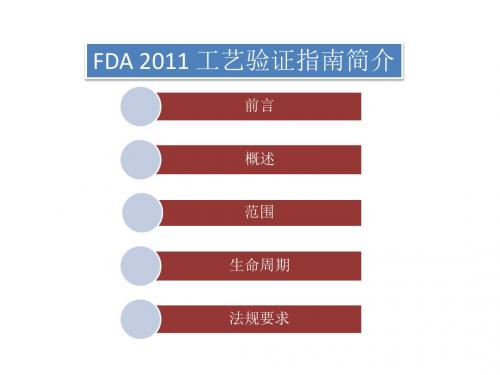
前言 概述 范围 生命周期 法规要求
前言
• 验证工作是实施 GMP 规范的基础,而工艺验证又是验证工作中的关键 性环节。1987年FDA发布了关于工艺验证的指南文件,FDA关于工艺验 证的要求和理解深深地影响着世界各国药政当局和制药行业。2008年 11月,FDA再次发布最新的工艺验证指南(草案),对工艺验证的概 念和文件要求进行了大幅度修改和更新。经过2年多的收集制药行业 的意见和激烈争论,2011年1月24日,FDA发布了工艺验证指南的正式版 指 南 文 件 《Process Validation: General Principles and Practices》。修订后的指南文件更加明确和具体 , 毫无疑问 , 这个指 南文件将对GMP实施产生巨大影响,尤其对于以国际市场为主的外向型 制药企业,影响必将是深远的。新指南对原来的观念有了颠覆性的转 变,尤其是将验证概念延伸到了研发阶段,这对中国企业是个很有难 度的课题 。最大的变化是将整个验证过程分成3个阶段,制药企业需 要在目前验证管理的基础上,将前期在研发和中试阶段的数据进行分 析整理,作为验证的第一部分。与上一版本不同的是,没有针对具体 的工艺验证进行规范,而是将ICH-Q8,Q9和Q10的理念整合到了新的指 南中。不再从技术指南文件上提出具体措施,而是鼓励制药企业在原 则范围内进行技术革新。
概述
• 此版指南将产品生命周期概念和工艺验证活动结合 起来,将工艺验证分为工艺设计、工艺确认、持续 的工艺核实三个阶段。 • 工艺验证是指从工艺设计阶段到商业生产的整个过 程中,对数据进行收集和评价,建立能够使工艺始 终如一的传递到优质产品中的科学证据。 • 对已经上市的产品则直接执行持续工艺核实这一阶 段的工作。制作商应该保持持续的信息收集和对工 艺的定期评价,以发现常见的工艺变异情况,进而 增加对工艺和变异的理解,评价和控制工艺参数, 并建立科学的参数评价方法,在商品生产这一阶段 内做到对工艺的逐步改进(如缩小参数范围等)。 在此阶段如发现有重大变异或工艺有较大改动,而 现有数据不足以进行分析时,可以回到工艺设计或 工艺确认阶段。
最新附录工艺验证检查条款解读

最新附录工艺验证检查条款解读原创2015-09-08阿郎蒲公英本文为蒲公英巍信智会第7期主题分享作者:阿郎,转载请与作者联系。
什么是工艺验证?指为证明工艺在设定参数范围内能有效稳定地运行并生产出符合预定质量标准和质量特性药品的验证活动。
它包括有书面记录、预先设定工艺、验证方案、验证标准、工艺一致性和产品质量一致性等内容。
对此,2011年FDA行业指南“工艺验证:一般原则与规范”中定义工艺验证为“收集和评估从工艺设计、研发到商业化生产阶段的数据,并用这些数据科学的证明该工艺能够有能力持续稳定地生产出优质产品”。
两个定义的区别是,中国“工艺验证”还是基于传统的工艺验证活动,但本版附录已经和国外最新要求接轨了,但基于国情也做了某些方面的妥协;而欧美的“工艺验证”,更多基于产品的生命周期考虑,国外通行把工艺验证分为“工艺设计、工艺确认(传统的工艺验证)、持续工艺确认”三个阶段。
工艺验证的目的,不仅为监管机构所要求,更重要的是产品质量自身的需要,是为能够持续、可靠、稳健地生产出质量受控、有竞争力、高盈利的产品。
内容提纲如下:一、条款解读:一般要求(8条)二、工艺验证:生命周期三、工艺验证:QbD四、问题讨论一、条款解读:一般要求(8条)1、第19条:工艺验证应当证明一个生产工艺按照规定的工艺参数能够持续生产出符合预定用途和注册要求的产品。
工艺验证应当包括首次验证、影响产品质量的重大变更后的验证、必要的再验证以及在产品生命周期中的持续工艺确认,以确保工艺始终处于验证状态。
解读:本条款提出了工艺验证的总体要求,既要符合预定用途(满足目标产品质量概况QTPP)也要符合法定批准的注册要求。
另外,工艺验证包括的类型有首次验证、变更验证、再验证、持续工艺确认,取消了原来的回顾性验证。
1)回顾性验证做为一种事后的验证手段,和现代质量管理概念中的“必须建立一个持续和不断发展的程序、收集和分析与产品质量有关的产品和工艺数据”的持续改进的概念是冲突的,因此,新的欧盟验证指南,希望企业建立的是一个能够持续改进的验证系统。
药品确认与验证

确认与验证的关系
风险评估与确认/验证
• 第一百三十八条 企业应当确定需要进 行的确认或验证工作,以证明有关操作的 关键要素能够得到有效控制。确认或验证 的范围和程度应当经过风险评估来确定。
确认或验证风险评估的范围
• 对复杂的系统、工艺或设备应该进行风险评估, 不是每个IQ/OQ/PQ都需要做风险评估。 • 对于多年生产的产品,且工艺没有变更,产品验 证前,通过工艺回顾分析很容易找到存在的风险 和风险源,采取措施后即能消除风险或使风险降 低到可接受的程度,在这种情况下,产品验证前 的风险评估可采用只列出存在风险,采用的措施, 措施实施的效果以及结论。
• 一、前确认或前验证 • 4、附录1:无菌药品 第十八条 因吹灌封技术的 特殊性,应当特别注意设备的设计和确认、在线 清洁和在线灭菌的验证及结果的重现性…. • 5、附录1:无菌药品 第四十条 关键设备,如灭 菌柜、空气净化系统和工艺用水系统等,应当经 过确认,并进行计划性维护,经批准方 可使用。 • 6、附录1:无菌药品 第六十二条 可采用湿热、 干热、离子辐射、环氧乙烷或过滤除菌的方式进 行灭菌。每一种灭菌方式都有其特定的适用范围, 灭菌工艺必须与注册批准的要求相一致,且应当 经过验证。
• 1、FDA2011 版工艺验证指南的启示
• 2、2010 版GMP 对验证和确认的要求 • 3、验证和确认的新思路 • 4、验证和确认的检查
验证与确认的应用范围关系
• 验证和确认本质上是相同的概念 • 确认通常应用于厂房、设施、设备和检验 仪器 • 验证则应用于操作规程(或方法)、生产 工艺或系统 • 通常情况下,确认是验证的前提,验证是 确认的延伸。
工艺验证的生命周期
• 工艺验证生命周期的概念:将产品与工艺 开发、商业生产工艺确认、日常商业生产 中处于受控状态连接在一起。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
FDA工艺验证指南新旧版透彻比较解读【整理者提醒】1-左侧文本为2011年1月最新修订版本,右侧文本为2008年11月草案版本。
2-蓝色文本为修订后文本或者新增加文本。
3-下划线文本是比旧版本增加的部分内容。
4-删除线文本表示该部分存在于旧版本中,在新版本中删除。
5-注释前面加【注释】2字注明。
6-Zhulikou431关于FDA2008年11月草案彻底解读版本可以在丁香园论坛搜索到,欢迎下载阅读、讨论。
7-不得用于商业用途,转载请注明丁香园信息。
8-增加了新旧版本的中文译文。
9-欢迎各位朋友提出宝贵建议,联系邮箱zhulikou431@.Guidance for IndustryProcess Validation: General Principles and PracticesFinal Version January 2011 Draft 2008I. INTRODUCTIONI. INTRODUCTION简介This guidance outlines the general principlesand approaches that FDA considers appropriate elements of process validation for the manufacture of human and animal drug and biological products, including active pharmaceutical ingredients (APIs or drug substances), collectively referred to in thisguidance as drugs or products. This guidanceincorporates principles and approaches thatall manufacturers can use to validate manufacturing processes.本指南概括了一般的原则与方法,这些原则与方法是FDA 认为进行工艺验证的恰当要素,这些工艺被用于生产人用药、动物用药以及生物制品,包括活性药物成分(API 或药用物质),在本指南中以上统称为药品或产品。
本指南整合了一般的原则和方法,所有的生产企业都可以将这些原则和方法应用于生产工艺的验证。
This guidance outlines the general principles and approaches that FDA considers to be appropriate elements of process validation for the manufacture of human and animal drug and biological products, including active pharmaceutical ingredients (API or drug substance),collectively referred to in this guidance as drugs or products. This guidance incorporates principles and approaches that all manufacturers can use in validating amanufacturing process. 本指南概括了一般的原则与方法,这些原则与方法是FDA 认为进行工艺验证的恰当要素,这些工艺被用于生产人用药、动物用药以及生物制品,包括活性药物成分(API 或药用物质),在本指南中以上统称为药品或产品。
本指南整合了一般的原则和方法,所有的生产企业都可以将这些原则和方法应用于生产工艺的验证。
This guidance aligns process validation activities with a product lifecycle concept and with existing FDA guidance, including the FDA/International Conference on Harmonisation (ICH) guidances for industry, Q8(R2) Pharmaceutical Development, Q9 Quality Risk Management, and Q10 Pharmaceutical Quality System.2 Although this guidance does not repeat the concepts and principles explained in those guidances, FDA encourages the use of modern pharmaceutical development concepts, quality risk man- agement, and quality systems at all stages of the manufacturing process lifecycle. 本指南将工艺验证活动与产品生命周期概念以及现有FDA 相关指南进行了协调;这些存在的FDA指南包括FDA/ICH指南,Q8(R2)药品研发指南、Q9质量风险管理指南和Q10制药质量体系指南。
尽管本指南没有重复上述指南解释的概念和This guidance aligns process validation activities with the product lifecycle concept and with existing FDA guidance.2 2 See the FDA/International Conference on Harmonisation (ICH) guidances for industry: Q8 Pharmaceutical Development, Q9 Quality Risk Management, and when finalized, Q10 Pharmaceutical Quality System (a notice of availability for the May 2007 ICH draft guidance, Q10 Pharmaceutical Quality System, published in the Federal Register on July 13, 2007 (72 FR 38604)). We update guidance documents periodically. To make sure you have the most recent version of a guidance, check the CDER guidance page at /cder/guidance/index.htm, the CBER guidance page at原则,FDA鼓励在药品制造工艺生命周期的各个阶段使用现代药品研发概念、质量风险管理和质量体系概念。
注释2To make sure you have the most recent version of a guidance, check the CDER guidance page at /Drugs/GuidanceComplianceR egulatoryInformation/Guidances/default.htm, the CBER guidance page at/BiologicsBloodVaccines/Guida nceComplianceRegulatoryInformation/Guidances/default.htm, or the CVM guidance page at/AnimalVeterinary/GuidanceCo mplianceEnforcement/GuidanceforIndustry/default.htm/cber/guidelines.htm, or the CVM guidance page at /cvm/Guidance/published.htm . 本指南将工艺验证活动与产品生命周期概念以及现有FDA 相关指南(这里指得是Q8、Q9和Q10指南)进行了协调。
The lifecycle concept links product and process development, qualification of the commercial manufacturing process,3 andmaintenance of the process in a state of controlduring routine commercial production.This guidance supports process improvementand innovation through sound science.生命周期概念将产品与工艺开发、商业生产工艺的确认以及维护工艺在日常商业生产中处于受控状态连结在一起。
本指南通过足够科学知识来支持工艺优化和革新。
注释 3 In this guidance, the term commercialmanufacturingprocess refers to the manufacturingprocess resulting in commercial product (i.e., drugthat is marketed, distributed, and sold or intended to be sold). For the purposes of this guidance, the term commercial manufacturing process does not include clinical trial or treatment IND material. The lifecycle concept links product andprocess development, qualification of thecommercial manufacturing process, andmaintenance of the process in a state of controlduring routine commercial production.This guidance promotes modern manufacturingprinciples, process improvement, innovation,and sound science.生命周期概念将产品与工艺开发、商业生产工艺的确认以及维护工艺在日常商业生产中处于受控状态连结在一起。
