Change Control Process 变更控制流程
变更控制管理流程

变更控制管理流程背景在业务和项目管理中,变更是一种常见的现象。
为了确保变更能够被有效地管理和控制,变更控制管理流程被引入。
本文将介绍一个标准的变更控制管理流程,以帮助组织和团队在实施变更时更加高效和有序。
目标- 确保所有变更都被审查、评估和批准,以避免未经授权或未经评估的变更对业务或项目带来风险和问题。
- 确定变更的影响范围和风险,在变更实施前进行充分的准备工作,以降低不必要的风险和影响。
- 提供透明和可追踪的变更控制过程,便于审计和追踪变更历史。
- 促进有效的沟通和协作,在变更实施过程中各方能够及时获得相关信息和决策。
流程步骤1. 变更请求- 发起人提交变更请求,并提供详细的变更描述和理由。
- 变更请求应包括所涉及的业务或项目范围、影响和风险评估。
2. 变更评估- 变更评估小组(Change Assessment Group)对变更请求进行评估。
- 评估包括变更的商业影响、技术可行性和资源需求。
- 评估结果将用于确定变更是否应被批准、拒绝或需要进一步的审查。
3. 变更批准- 变更委员会(Change Control Board)对变更请求进行审议和决策。
- 变更委员会由相关利益相关方组成,包括业务代表、项目经理、技术专家等。
- 变更委员会根据评估结果和变更的优先级等因素,决定是否批准变更。
- 批准的变更将进入下一步骤,否则将结束变更流程。
4. 变更计划- 变更计划包括变更的详细实施计划、资源分配和沟通计划等。
- 变更计划应充分考虑风险管理和变更影响管理。
5. 变更实施- 根据变更计划进行变更实施。
- 变更实施过程中需确保变更的正确性和可追溯性。
- 变更实施后,进行验证和确认,确保变更的预期效果和质量。
6. 变更关闭- 在变更实施后进行变更关闭。
- 特定文件和记录将被归档和存档,便于审计和追踪。
总结变更控制管理流程提供了一种系统化、透明和可控的方式来管理和处理业务和项目中的变更。
通过严格遵循这个流程,组织和团队可以更有效地处理变更,并减少变更可能带来的风险和问题。
工程变更流程(中英文)
实施变更 Implement the changing
跟踪变更 实施情 况 Follow up the result of changing implementation
生产部/品管 部/采购及 相关 部门
Production Dept. /QA Dept.
/Purchasing Dept. and related Dept.
8.0 9.0 /
确定变更 实施计 划 Define the changing implement
plan
变更小组/技 术部/ 开发 部 Changing Team
/Technical Dept. /Engineering Dept.
变更验证 Changing validation
变更小组/品 管部/ 生产 部 Changing team /QA Dept.
工程变更控制程序 Engineering Change Control Procedure
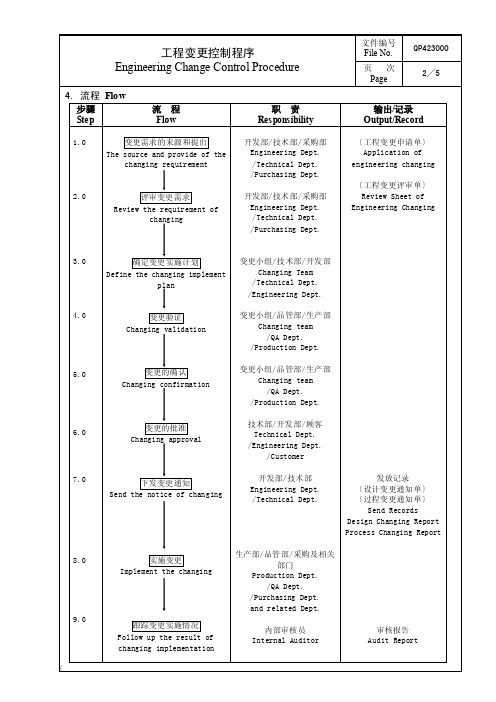
4. 流程 Flow 步骤 Ste p
流程 Flow
1.0
变更需求 的来源 和提出
The source and provide of the
changing requirement
2.0
评审变更 需求
Review the requirement of
/Engineering Dept. /Customer
下发变更 通知 Send the notice of changing
开发部/ 技术 部 Engineering Dept. /Technical Dept.
发放记录 〔设计变 更通知 单〕 〔过程变 更通知 单〕
Send Records Design Changing Report Process Changing Report
药品变更控制的流程

药品变更控制的流程英文回答:Process for Drug Change Control.Introduction.Drug change control is a critical process in the pharmaceutical industry that ensures the safety andefficacy of drugs throughout their lifecycle. It involves managing any changes made to a drug's formulation, manufacturing process, or labeling. A robust drug change control process helps prevent medication errors, adverse drug events, and regulatory non-compliance.Key Steps in the Drug Change Control Process.1. Initiation: A change request is initiated when a need for a change is identified. The request should include a detailed description of the proposed change, itsrationale, and potential impact.2. Assessment: A review team assesses the proposed change to determine its potential risks and benefits. The team may include representatives from quality assurance, regulatory affairs, and clinical development.3. Approval: If the change is deemed necessary and safe, it is approved by the appropriate authorities. This may include management, regulatory agencies, and ethics committees.4. Implementation: The change is implemented accordingto a predefined plan. This may involve updating manufacturing processes, label specifications, or clinical protocols.5. Monitoring: The implemented change is closely monitored to assess its impact on the drug's safety and efficacy. Regular reviews and risk assessments are conducted.6. Documentation: All aspects of the drug changecontrol process are documented meticulously. This includes the change request, assessment report, approval records, and monitoring data.Regulatory Requirements for Drug Change Control.Regulatory agencies such as the FDA and EMA have established guidelines for drug change control. These guidelines ensure that changes to drugs are made in a safe and controlled manner. Key regulatory requirements include:Prior approval: Certain types of changes, such as those affecting the drug's safety or efficacy, requireprior approval from regulatory authorities.Documentation: Comprehensive documentation is required at each stage of the change control process.Risk assessment: A thorough risk assessment must be conducted to identify and mitigate potential risks associated with the change.Monitoring: Post-implementation monitoring isessential to assess the impact of the change on the drug's safety and efficacy.Best Practices for Effective Drug Change Control.Establish a Clear Change Control Policy: Define the scope, responsibilities, and procedures for drug change control within the organization.Appoint a Change Control Committee: Form a multidisciplinary team responsible for reviewing and approving change requests.Use a Risk-Based Approach: Prioritize change requests based on their potential risks and benefits.Communicate Effectively: Ensure timely and effective communication among all stakeholders involved in the change control process.Continuously Improve: Regularly review and update the drug change control process to enhance its effectiveness.中文回答:药物变更控制流程。
软件配置管理

软件配置管理1、简介软件配置管理,贯穿于整个软件生命周期,它为软件研发提供了一套管理办法和活动原则。
软件配置管理无论是对于软件企业管理人员还是研发人员都有着重要的意义。
软件配置管理可以提炼为三个方面的内容:●VersionControl-版本控制●ChangeControl-变更控制●ProcessSupport-过程支持关键活动包括:配置项、工作空间管理、版本控制、变更控制、状态报告、配置审计等。
2、软件配置管理技术软件配置管理是一组活动,是设计用来标识变更的工作产品、建立它们之间的关系、定义管理这些工作产品不同版本、控制变更以及审计和报告所发生的变更。
每一个涉及到软件工程过程的人员均在某种程度上和SCM相关联。
一般情况下需要专门的SCM小组或专门的技术人员来管理和支持。
下面通过依次介绍配置管理过程中的主要活动来描述配置管理过程。
2.1识别配置项在项目开发过程中,程序、数据和文档都可以作为配置管理的对象,下面以图的形式来列举可能的配置项,如图2-1所示,由图可以看出配置项之间是组合关系或者相互关系。
图2-1可能的配置项2.2基于配置项版本控制版本控制是将规程和工具相结合来管理在软件工程过程中所创建的配置对象的不同版本,通过“属性元组”等其它技术来控制完整版本中的“变体”,采用不同的工具不同的技术,版本控制的机制会有一些不同。
2.3变更控制变更在软件开发过程中是不可避免的,但过于频繁的变更也会对项目的开发产生负面的影响,如影响项目的进度、浪费人力物力,因此需要对变更进行控制。
变更控制可以依照如下的步骤来进行:(1)提交变更请求;(2)审核变更请求;(3)分配和确定任务;(4)提取变更项;(5)执行变更;(6)审核变更;(7)更新配置管理库。
整个变更控制的产物主要是变更请求单、变更报告单、工程变更单或变更确认单等。
2.4配置审计配置审计一般包括两种,一种是正式的技术评审,另一种是软件配置审计。
在正式的技术评审中,将关注已经被修改的配置项的正确性,配置项的评估配置项,以确定它与其他一致性、遗漏及潜在的副作用。
变更控制流程英语作文

变更控制流程英语作文Title: Implementing Change Control Processes。
In today's dynamic business environment, change is inevitable. Whether it's due to technological advancements, market demands, or internal reorganizations, organizations must adapt to stay competitive. Implementing effective change control processes is crucial to manage these transitions smoothly and minimize disruptions. In this essay, we will explore the importance of change control processes and outline steps to implement them successfully.Firstly, let's discuss why change control processes are essential. These processes provide a structured approach to manage changes within an organization. They help in assessing the impact of proposed changes, identifying potential risks, and ensuring proper communication among stakeholders. By establishing clear procedures for requesting, reviewing, approving, and implementing changes, organizations can maintain stability while fosteringinnovation.The implementation of change control processes involves several key steps:1. Assessment of Current Processes: Before implementing any changes, it's essential to evaluate the existing processes. This includes identifying strengths, weaknesses, and areas for improvement. Gathering feedback from stakeholders across various departments can provide valuable insights into the effectiveness of current procedures.2. Defining Change Categories: Not all changes are equal. It's essential to categorize changes based on their impact and urgency. For example, minor changes may only require approval from a department head, while major changes may need endorsement from the executive team. By classifying changes into different categories, organizations can streamline the approval process and allocate resources more efficiently.3. Establishing Change Review Board: A change review board (CRB) plays a crucial role in overseeing the change control process. This board typically consists of representatives from different departments or functional areas. Its primary responsibility is to review change requests, assess their impact, and make informed decisions about their implementation. Having a diverse CRB ensures that changes are evaluated from various perspectives, minimizing the risk of oversight.4. Documentation and Communication: Clear documentation is essential throughout the change control process. This includes documenting change requests, approvals, implementation plans, and post-implementation reviews. Additionally, effective communication is key to ensuring that all stakeholders are informed about upcoming changes and their potential impact on operations. Utilizing communication channels such as emails, meetings, and intranet portals can help disseminate information efficiently.5. Training and Education: Implementing change controlprocesses requires buy-in from employees at all levels of the organization. Providing training and education on the new procedures is essential to ensure widespread adoption. This training should not only cover the technical aspects of the process but also emphasize the rationale behind it and its benefits to the organization as a whole.6. Continuous Monitoring and Improvement: Change control processes should not be static. Continuous monitoring and evaluation are necessary to identify bottlenecks, address issues, and incorporate lessons learned from past changes. Regular reviews of the change control process enable organizations to adapt to evolving needs and optimize their change management capabilities.In conclusion, implementing effective change control processes is essential for organizations to navigate through periods of transition successfully. By following the steps outlined above, organizations can establish structured procedures for managing changes, minimize disruptions, and foster a culture of adaptability and innovation. While the process may require initialinvestment in terms of time and resources, the long-term benefits far outweigh the costs. Embracing change control processes is not just about managing change; it's about empowering organizations to thrive in an ever-changing world.。
变更控制Change control WHO

13
PRINCIPLES OF CHANGE CONTROL
• Change control is not department-specific, but encompasses the whole company.
The change control • monitors all types of changes, which can influence the process or product quality and • states the measures necessary for implementing the change or • decides that a change should not be implemented.
8
LIFE CYCLE
CHANGES AND APR
OOS
Deviations Audits Complaints
Changes in materials
Improvement (equipment, systems, procedures, etc.)
Root Cause Analysis CAPA proposal Risk assessupferman, Industry Pharmacist
Alain Kupferman
Pharmacist (ULB, Brussels, Belgium) Industry Pharmacist (Qualified Person)
比利时
Alain Kupferman
11
The task of change control
PRINCIPLES OF CHANGE CONTROL
• In the WHO-GMP guideline glossary there is the definition of the term “change control”:
Change control变更控制

Pharmaceutical Services Corporation 美国医药服务有限公司Change Control变更控制Topics题目•What is change?什么是变更?•What is change control?什么是变更控制?•Why do we need change control?为什么需要变更控制?•What should be covered by change control?变更控制应包括什么?•What are the requirements of the change control process?变更控制过程的要求是什么?Definition定义•What is change?什么是变更?•To alter, to become different改变,变得不同•Changes can be either planned or unplanned 变更可能是有计划的,也可能是没有计划的What is Change Control?什么是变更控制?• A formal system for evaluating all changes that may affect the quality, safety and efficacy of the product一个正式的系统,用于评价所有可能影响产品质量、安全和效力的变更。
Why?为什么?•It is a regulatory requirement法规要求•It is a way in which the correct personnel can be made aware of impending changes能够使合适的人员知道即将发生变更的一种途径•It is a way of identifying the work needed to support a change识别为支持变更所需要进行的工作的一种途径•It prevents constant trivial “evolutionary”change 防止不断的没有价值的“进化”变更•Raw materials, specifications, analytical methods, facilities, support systems, equipment (including computer hardware), processing steps, labeling and packaging materials, and computer software原料、标准、分析方法、厂房、支持系统、设备(包括计算机硬件)、工艺步骤、标签、包装材料和计算机软件•Must include:必须包括:–Identification of changes subject to change control 确认需要经过变更控制进行的变更–Documentation of proposed changes提议变更的文件–Evaluation by Quality质量部的评估–Work required to support change支持变更所需要进行的工作–Record of changes变更记录–Audits of Change Control system变更控制系统的审计EU GMP•The basic requirements of GMP are that:GMP的基本要求是–(ii) critical steps of manufacturing processes and significant changes to the process are validated要对生产工艺的关键步骤和工艺的重要变更进行验证•Regular periodic or rolling quality reviews of all licensed medicinal products should be conducted and should include:对所有注册药品要进行定期或周而复始的质量审核,其包括:–A review of all changes carried out to the processes or analytical methods对所有工艺或分析方法进行的变更要进行审核FDA Oral Solid Dosage guidanceFDA口服固体制剂指南•As with other dosage forms, it is important for the firm to carefully control how changes are made in the production of topical products. Firms should be able to support changes which represent departures from approved and validated manufacturing processes.同其他制剂一样,企业认真地控制外用药产品生产的变更是十分重要的。
TS-2018 06 工程变更控制程序

1.0PURPOSE目的This procedure defined the processes of ECN and the responsibilities of related departments to assure ECN effective execution and control and avoid the risks of product quality generated by ECN.本程序定义了工程变更的流程及工程变更过程中相关部门的职责,确保工程变更过程有效地实施和控制,避免工程变更中产生产品质量风险。
2.0SCOPE范围This procedure is applicable for the ECN control of silicon, ingot, casting, wafer, cell and module of Changzhou Trina Solar Energy Co., Ltd.本程序适用于常州天合光能从硅料到单晶、多晶、硅片、电池和组件ECN的控制。
3.0DEFINITION 定义3.1ME: Manufacturing Engineering 制造工程3.2TECN: Temporary Engineering Change Notice 临时工程变更通知单3.3ECN: Engineering Change Notice工程变更通知单3.4ECCB: Engineering Change Control Board 工程变更管控平台Before engineering change formally initiated, Manufacturing engineering, Quality, Process, Production, TD and other relevant departments should participate in and assess engineering change proposal preliminarily.由制造工程、质量、工艺、生产、技术等有关部门参与的,在工程变更正式发起前对工程变更提案进行初步评估。
中译文 变更控制Change control WHO

为大家提供优质的药品
变更控制定义和审批 示例变更
示例 等级
按比例放大批次大小
清洗材料-产品接触 服装-无菌 电脑系统 承包实验室 承包制造商 对等的设备 不同的设备 工作时间 工艺规程-变更 配制方法
2
2 1 2 2或3 3 1 2 1 3 2或3
包装(不同材料的主要成分)
3
为大家提供优质的药品
变更控制定义和审批 示例变更
• •
修正 •审核 •提供文档进行佐证
•
示例
制造商变更:原始 •更换装置(其为同 材料的其它合成路线 种设计的一部分) (其它杂质) •地面清洁剂的变更 •将工艺转移至另一 •工作服洗衣室的变 个工作场址; 更(无菌的或抗菌 •产品成分的变更 的区域) •工艺参数的变更
工作时间的变 更
•
在行政区域安 装空调
• 鉴于变更控制被视作为药品质量保证系统必不可少的一部 分,那么对其质量保证承担部分责任则应当是符合逻辑的 。(质量保证代表和质量保证主任)
10
为大家提供优质的药品
变更控制的原则 • 变更控制的范围并非仅局限于某个专门的部门, 而是覆盖整个公司的。
变更控制 • 监控可能会影响工艺或产品质量的所有变更类型 ;以及 • 称述执行变更所必需的措施;或 • 决定不应当执行某个变更。
为大家提供优质的药品
变更控制项目
•
公司为何种以及多少变更种类进行了分级不是决定性的 ,但是公司是如何保证这些需要控制的变更则是被公认 为具有决定性的并且应当按照定义的程序进行实施。
23
为制造商和药品生产质量管理规范检查员 精选的药品生产质量管理规范主题 内罗比,2011年5月9日-12日
为大家提供优质的药品
项目管理中常用英语

项目管理中常用英语转载学习工程项目管理常用英语工艺包 process package工艺设计 process design工艺发表 process release工艺预发表 initial process release工作范围;项目任务范围 scope of work ; project scope工作包 work package工作项 work item任务单项 line item分解结构 breakdown structure工作分解结构 work breakdown structure组织分解机构 organizational breakdown structure项目大项工作分解结构 project summary work breakdown structure承包商标准工作分解结构;工程公司标准工作分解结构 contractor's standard work breakdown structure责任分工矩阵 responsibility assignment matrix风险 risk风险分析 risk analysis风险备忘录 risk memorandum公司本部 home office公司本部服务 home office service公用工程 utility计划 plan项目计划 project plan项目设计计划 projectengineering plan项目采购计划 projectprocurement plan项目施工计划 projectconstruction plan项目开车计划 projectstart-up plan专利 patent专利权 patent right产权技术 proprietary technology专利技术 licensed technology专有技术;技术诀窍 know-how许可证 license专利商;许可方 licensor受许可放;受让方 licensee技术转让费;提成费 royalty许可证费 license fee专有技术费 know-how fee专业 discipline工艺 process design ; process engineering 系统 systems engineering设备 equipment engineering布置 plant layout engineering管道设计 piping design管道机械 piping mechanical engineering仪表 instruments engineering电气 electrical engineering建筑 architectural engineering土建 civil engineering开车 start-up试车 commissioning投料试车 start-up ; test run ; initial operations性能考核,生产考核 performance test run ; performance guarantee tests用户验收 client acceptance支付条件;付款条件 terms of payment ; conditions of payment ; terms and conditions of payment预付款 advance payment ; down payment按实物进度付款 progress payment按日工程进度付款 schedule payment保留金;扣留款 retention money最终付款 final payment代码;编码 code ; number组码 group code标准分类记帐码;记帐码 standard classification of account numbersSCAN;account codes ; code of accounts可编码 variable code ; optional variable code通用型活动码 generic activity typenumbersGAT不可预见费 contingency发表 issue ; release汇票 bill of exchange ; draft议付汇票 bill for negotiation业主 owner用户;客户 client设计;工程设计 design ; engineering设计阶段 engineering phase工艺设计阶段 process design phase基础工程设计阶段 basic engineering phase分析设计阶段 analytical engineering phase平面设计阶段 planning engineering phase详细工程设计阶段 detailed engineering phase ; final engineering phase ; production engineering phase会议 meeting开工会议 kick-off meeting报价开工会议 proposal kick-off meeting用户开工会议 client kick-off meeting项目开工会议 project kick-off meeting设计开工会议 project kick-off meeting施工动员会议 construction mobilization meeting审核会 review meeting合同;承包 contract合同生效日期 effective date of the contract合同终止 termination of contract合同失效 frustration of contract总价合同 lump-sum contractL-S固定单价合同 fixed unit price contract偿付合同;成本加抽筋合同 cost reimbursable contract ; cost-plus fee contract C-P成本加固定酬金合同 cost plus fixed fee contract CPFF成本加浮动酬金合同 cost plus fluctuating fee contract ; cost plus sliding scale fee contract目标成本加奖罚合同 target cost plus fee contract , with bonus or penalty conditions限定最高价偿付合同;限定最高成本加抽筋合同 reimbursable guaranteed maximum price contract RGMP ; guarantee maximum cost plus fee contract承包商 contractor分包商 subcontractor合营企业 joint venture JV交货 delivery交货日期 delivery date ; date of delivery交货周期 lead time交货到现场 delivery to job-site交货单 delivery note提货单 delivery order提单 bill of lading交货条件 delivery terms离岸价 free on board FOB铁路交货价;敞车上交货价 free on rail FOR ; free on truck FOT成本加运费价 cost and freight CFR or C&F到岸价 cost insurance and freight ; cost insurance freight CIF船边交货价 free alongside ship FAS货交承运人价 free carrier FCA工厂交货价 ex works EXW估算;费用估算 estimate ' cost estimate估算方法类别 types of estimate详细估算发 detailed estimate ; defined estimate设备详细估算发;确切估算发 defined equipment estimate ; definitive estimate设备估算 equipment estimate分析估算 analysis estimate报价估算 proposal estimate控制估算 control estimate初期控制估算 interim control estimate ; initial control estimate ICE批准的控制估算 initial approved cost IAC核定估算 check estimate首次核定估算 first check estimate FCE二次核定估算 production check estimate PCE人工时估算 man-hour estimate证书;证明书 certificate产地证明书 certificate of origin机械竣工证书 mechanical completion certificate用户验收证书;合同项目验收证书 client acceptance certificate of plant材料 material设备 equipment散装材料 bulk materials ; commodities材料分类 material class材料统计 material take-off MTO材料表;材料清单 bill of materials BOM材料管理 material management材料控制 material control进度;进度表;进度计划 schedule进展;进度;实物进度 progress ; physical progress编制进度计划 scheduling ; time scheduling项目初期工作进度计划 starter schedule ; early work schedule项目主进度计划 project master schedule ; master project schedule 详细进度计划;详细进度表 detailed schedule网络图;网络进度计划 network diagram里程碑网络图 milestone network详细网络图 detailed network关键线路法 critical path method CPM关键工序;关键活动 critical activity工序;活动 activity里程碑 milestone进度控制 schedule control ; progress control进度曲线;S曲线 progress curve ; "S" curve资源负荷曲线 resource loading curve ; Bell curve进度提前 ahead of schedule进度拖延 schedule delay违约 default违约通知 default notice违约罚款条款 penalty clause违约罚金 liquidated damages运费 freight Frt. ; carriage运费付讫 freight paid ; carriage free ; carriage paids运费待收;货到收运费 freight collect ; freight to be collected ; freight payable at destination运费预付 freight prepaid ; advanced freight货到付运费;运费未付 freight forward ; carriage forward运费担保函 freight indemnity运费单 freight note运输商;承运商 forwarding agent采购 procurement采买 purchasing催交 expediting检验 inspection运输 traffic ; transport报关单 bill of entry报告 report费用和进展月报告 monthly cost and progress report项目进展月报 job monthly progress report设计进展月报 engineering monthly progress report项目费用汇总报告 project cost summary report项目实施费用状态报告 project operation cost status report进度报告 schedule report控制索引 control index执行效果报告 performance report劳动生产率报告 productivity report异常报告 exception report材料异常报告 material exception report MER ; equipment & materials exception report采购状态报告 procurement status report材料状态报告 material status report请购单和订单状态报告 purchase order and requisition status report供货厂商图纸状态报告 vendor drawing status report ; vendor data scheduling status report检验报告 inspection report项目完工报告 close-out report报价;投标 bid ; quotation ; proposal ; tender ; offer讯价文件评审委员会 inquiry review committee IRC报价策略会议 bid strategy meeting报价经理 proposal manager投标书;标书;建议书 proposal ; quotation ; bid ;tender ;offer标书评审;报价评审 bid evaluation标书评选表;报价评选表 bid tabulation form ; tabulation of bids谈判 negotiation厂商协调会议;协调会议 vendor coordination meeting VCM ; coordination meeting订货合约;定约;成交 commitment订货电传;订货通知 telex order ; notification of commitment订单即定单;采买订单;订货合同 purchase order签订合同;合同签约 contract award询价;招标 inquiry ; invitation to bid资格预审 pre-qualification合格投标商表;合格供货商表 qualified bidders list ; qualified vendors list询价书;招标文件 inquirypackage ; request for quotationpackage RFQ; request for proposal RFP ; invitation to bid ITB请购文件;请购单 requisition package ; requisition documents采购规格书;采购说明书 purchasing specification PS投标者须知;报价须知 instructions to bidders ITB变更 change用户变更;合同变更 client change ;contract change待定的用户变更 pending client change认可的用户变更 approved client change用户变更通知单;合同变更通知单 client change notice CCB ; contract change order CCO项目变更;内部变更 project change ; internal change项目变更通知单;内部变更通知单 project change notice PCN ;internal change order ICO变更申请单;偏差通知单 change request ; deviation notice DN质量 quality质量方针 quality policy质量管理 quality management质量策划 quality planning质量控制 quality control QC质量保证 quality assurance QA质量体系 quality system质量改进 quality improvement质量手册 quality manual质量计划 quality plan图纸 drawing工艺流程图 process flow diagram PFD工艺控制图 process control diagram PCD管道仪表流程图 piping and instrument diagram PID P&ID装置布置图 plot plan管道平面设计图 piping planning drawing管道平面布置图 piping layout drawingsort out herez O G {/e a+M 管段图;管道空视图 isometric drawing |.X$h }7v C ^ s7s"批准用于详细工程设计"图纸 drawing issued "Approved for Design" AFDY$Q L u d"批准用于施工"图纸 drawings issued "Approved for construction" AFC供货厂商先期确认图纸 advanced certified final drawings ACF ; advanced certified vendors' drawings ; preliminary vendor drawings PD供货厂商最终确认图纸 certified final drawings CF ; certified vendor drawingsCD图表 diagram ; chart直方图 histogram横道图 bar ; bar-chart ; Gantt chart进度趋势展示图 schedule trend display chart费用趋势展示图 cost trend display chart项目;工程项目 project项目实施费用状态报告 project execution项目实施阶段 project phase项目初始阶段 initial phase of project execution施工阶段 construction phase开车阶段 start-up phase项目建设周期 project duration's项目管理 project management项目控制 project control项目组 project team专责项目组 task force项目经理 project manager项目设计经理 project engineering manager项目采购经理 project procurement manager项目施工经理 project construction manager项目工艺经理 project process manager项目控制经理 project controls manager项目进度计划工程师 project scheduling engineer ; project scheduler项目估算师 project estimator项目费用控制工程师 project cost control engineer ; project cost engineer项目材料控制工程师 project material control engineer项目财务经理 project financial manager项目质量经理 project quality manager项目开车经理 project start-up manager项目安全工程师 project safety engineer项目秘书 project secretary项目控制手段 project controlling ; tools for project control控制基准;执行效果测量基准;实物进度基准 control baseline ; performance measurement baseline ; progress baseline偏差;差异 deviation ; variance执行效果;效绩;性能 performance跟踪 tracking ; follow up监控 monitoring趋势分析;趋势预测 trending ; trend projection偏差预测值 projected deviations预测 projection ; forecasting纠正措施 corrective action ; corrective measures项目综合控制 integrated project control赢得值原理 earned value concept EVC计划工作量的预算费用 budgeted cost for work scheduled BCWS已完工作量的预算费用 budgeted cost for work performed BCWP已完工作量的实耗费用 actual cost for work performed ACWP费用差异 cost variance CV进度差异 schedule variance SV费用指数 cost index CI进度指数 schedule index SI费用执行效果指数 cost performance index CPI进度执行效果指数 schedule performance index SPI竣工差异 at completion variance ACV竣工预算费用 budgeted cost at completion BAC竣工预测费用 estimated cost at completion EAC费用;成本 cost建设总费用 total installed cost TIC材料费用;直接材料费用 material cost ; direct material cost设备费用;设备购买费用 equipment cost ; purchased cost of equipment散装材料费用;散装材料购买费用 bulk materials cost ; purchased cost of bulk materials ; commodities cost直接材料相关费用运费和保险费等 cost of material related freight ; insurance , etc.施工费用 construction cost施工人工费用;施工劳力费用 labor cost ; erection labor cost ; construction force cost设备安装人工费用 labor cost associated with equipment散装材料施工人工费用 labor cost associated with bulk materials人工时估算定额;施工人工时估算定额;标准工时定额 standard man-hours ; standard labor man-hours ; standard construction man-hours ; standardhours劳动生产率;劳动生产率系数 labor productivity ; productivity factor ; productivity ratio修正的人工事估算值 adjusted man-hours人工时单价 man-hour rate施工监督费用;施工监督和管理费用cost of construction supervision ; cost of construction management and supervision ; field administration and direct supervision cost施工间接费用 cost of construction indirect分包合同费用;现场施工分包合同费用 subcontract cost ; field subcontract cost公司本部服务费用 home office cost ; cost of home office servers公司管理费 overhead ; home office overheads非工资费用 non-payroll ; home-office expenses试车费用 commissioning activities cost投料试车费用 start-up cost其他费用 other costs利润;预期利润 profit ; expected profit服务酬金 service gains内部费用转换 internal transfer认可的预计费用 anticipated approved cost费用控制;成本控制 cost control预算值;项目控制预算 budget ; project control budget预算结余值;费用结余值 under-run ; cost under-run预算超支值;费用超支值 over-run ; cost over-run施工 construction施工工种 construction craft试车准备 pre-commissioning机械竣工 mechanical completion MC界区 battery limit BL工艺界区 process battery limit ; inside battery limit ISBL界外设施区;辅助设施界区 offsite battery limit工艺装置 process section ; process unit界外设施;辅助设施;通用设施 offsite section ; offsite unit ; offsite facilities ; general facilities保证;担保 guarantee ; warranty性能保证 performance guarantees绝对保证 absolute guarantees违约罚款保证 penaltiable guarantees工作质量保证 workmanship guarantees机械保证;机械担保 mechanical guarantees ; mechanical warranties保函;担保书 bond投标保函 bid bond履约保函;履约担保书;为履约出具的银行保证书 performance bond ; performance bank guarantee预付款保函;为预付款出具的银行保证书 advance payment bond ; bank guarantee for advance payment保证书;保险公司出具的保函 surety bond银行保证书;信用保证书 bank guarantee ; letter of guarantee安慰信 letter of comfort保密协议 secrecy agreement保险 insurance ; assurance保险单 insurance policy保险证书 insurance certificate保险费 insurance premium保税货物 bonded goods保税仓库 bonded warehouse信用证 letter of credit L/C不可撤销信用证 irrevocable L/C可撤销信用证 revocable L/C即期信用证 sight credit ; sight L/C L 远期信用证 term credit ; term L/C保兑信用证 confirmed L/C可转让信用证 transferable L/C备用信用证 stand-by L/C信用证提款 drawing on L/C信贷 CREDIT卖方信贷 SUPPLIER'S CREDIT S/C买方信贷 buyer's credit B/C信贷额度 line of credit项目融资;工程项目筹资 project finance 贷款 loan即期贷款 demand loan索赔 claim ; claim indemnity培训 training税;税金 taxes所得税 income taxes关税 duties合法避税 tax avoidance程序 procedure项目协调程序 project coordination procedure项目实施程序 project execution procedure项目设计程序 project engineering procedure项目采购程序 project procurement procedure项目检验程序 project inspection procedure项目控制程序 project control procedure变更控制程序 change control procedure预算变更程序 budget change procedure项目试车程序;项目开车程序 project commissioning procedure ; project start-up procedure ; project test run procedure化学清洗程序 chemical cleaning procedure性能考核程序;生产考核程序 performance test run procedure ; performance guarantee test procedure装置验收程序 plant acceptance procedure数据 data条件数据;设计条件 supporting data返回的条件数据;返回的设计条件 resultant feedback of data 意向书 letter of intent。
SOP 变更管理程序 (联邦)

批准人/日期Approved
Prepared By/Date
By/Date
By/Date
文件编号 File No. SOP-Q00011.05
执行日期 Effective Date
化学纯度和微生物质量证书的变更
Changes of certificates for chemical purity and microbiological quality.
文件名称File
变更管理程序
Name
Change Control Procedure
起草人/日期
审核人/日期Checked
批准人/日期Approved
Prepared By/Date
By/Date
By/Date
文件编号 File No. SOP-Q00011.05
执行日期 Effective Date
销售部门负责向其他客户提供变更的有关信息。
Sales Department should report the relevant information to other customers.
控制文件 Controlled Document
第 1 页 共 15 Page 1 of 15页
文件名称File
Purchase department should apply and manage the changes for materials.
质量管理部负责向国内药政机构如SFDA 传递相关信息;
QA department should report the relevant information to domestic authorities (such as SFDA).
CCCCRCC认证产品变更控制程序中英文版

CCCCRCC认证产品变更控制程序中英文版第一篇:CCC CRCC 认证产品变更控制程序中英文版认证产品变更控制程序Authentication Product Change Control Procedure 1 目的Purpose 为了满足CCC、CRCC产品认证质量保证能力和相关的法律、法规要求,确保申请认证的产品与认证标准的符合性和与型式试验样机的一致性,特制定本程序。
In order to meet CCC, CRCC product certificate quality assurance capability and related law, regulation requirements, ensure the conformity and consistency of the authentication product with certificate standard and type test sample, specially formulated the procedure.2 适用范围 Application Scope 本程序适用于动车组和高速车辆、重型铁路车辆、地铁轻轨的风挡、公交客车折棚和航空登机桥前端折棚等强制性认证产品和铁路客车重要零部件(A类)生产条件的审核认证,自愿性认证和“四防”(防燃、防裂、防脱、防松)要求的产品可以参考执行。
The procedure is applied to the audit certificate of production condition for CRH train, High Speed train, heavy railway, light railway metro gangway, bus bellows and Air bridge front bellow etc.mandatory authentication product and important parts(A class)of railway passenger car.Voluntary certific ate and product with “four prevents”(burning resistance, cracking resistance, fall-off resistance, loosen-preventive”)can reference to it.规范性引用文件 Normative Documents 下列文件中的条款通过本标准的引用而成为本标准的条款。
变更控制Change control

1、目的Purpose贯彻实施正式的书面的变更控制程序是为了有计划地评价所有变更可能对中间体、最终产品质量和SHE带来的影响。
A formal, written change control procedure will be implemented and followed that is designed to evaluated all changes that may affect the SHE, the quality of intermediates and final product.2、范围Scope2.1本规程适用于司。
This procedure is applicable to Xinhua-Chemferm.2.2该程序适用于公司各部门对已确立的所有程序所进行的变更。
This procedure is applicable to all departments’ established document for changing.2.3该规程适用于对产品质量或SHE有影响的工序或系统的变更, 包括, 但不限于:This procedure applies to the changes that involve effects to the products quality or the SHE aspects in the following process and /or systems, including, but not limited:2.3.1原料和供应商, 标准和有效期, 工艺过程, 分析方法, 标签和包材, 设备, 设施, 辅助系统, 计算机的硬件和软件Raw materials and suppliers, Specifications and valid date, Process steps, Analytical methods, Labeling and packaging materials, Equipment, Facilities,内部使用Internal use 正式文件OfficialSupport systems,Computer hardware and software.3、职责Responsibility3.1 总经理负责批准本程序。
项目变更控制流程图

项目变更控制流程图在项目管理中,变更控制是指对项目计划、范围、时间、成本、质量等方面的任何变化进行跟踪、评价和记录,并对其进行审批、控制和管理的过程。
项目变更可出于各种原因,如需求变更、范围调整、技术进展等,因此建立一个有效的项目变更控制流程图是十分必要的。
一、变更请求的提出项目中的任何人员,包括项目经理、项目团队成员、业务代表等,都有权利提出变更请求。
变更请求可以以书面或口头的形式提出,并应包括详细的描述、原因、预期的结果等信息。
二、变更请求的评估在收到变更请求后,项目团队首先将对该请求进行评估。
评估过程包括对变更请求进行可行性分析、风险评估、影响分析等。
团队需要综合考虑项目目标、进度安排、资源利用以及风险管理等因素,以确保变更是否对项目产生积极的影响,并确定变更的优先级。
三、变更请求的审批在评估变更请求后,项目经理将会召集相关方进行讨论并做出决策。
审批过程通常由项目变更控制委员会(Change Control Board,CCB)来完成。
CCB由项目经理、项目发起人、项目团队成员等组成,他们将根据项目目标、资源约束、变更影响等因素,对变更请求进行审批或拒绝。
四、变更的执行与监控一旦变更请求被批准,项目团队将按照变更要求进行相应的执行。
在执行过程中,团队需要及时记录变更的执行情况、耗时、资源使用情况等,并进行定期的监控和反馈。
五、变更的验收与评估在变更请求执行完毕后,项目团队将对变更的结果进行验收和评估。
验收过程包括确认变更是否达到预期的效果以及是否满足项目的需要。
评估结果将作为项目后续决策的依据,并为类似变更请求的处理提供经验教训。
六、变更的文档管理除了对变更过程进行跟踪和记录外,还需要对相关的文档进行有效的管理。
这包括对变更请求、审批决策、执行情况等进行归档和存档,以备将来参考和审计。
通过以上的流程图,可以清晰地了解项目变更控制的流程和各个环节的关系。
在项目管理中,准确地把握变更的控制和管理对于项目的成功至关重要。
变更控制Change-control

1、目的 Purpose贯彻实施正式的书面的变更控制程序是为了有计划地评价所有变更可能对中间体、最终产品质量和SHE带来的影响。
A formal, written change control procedure will be implemented and followed that is designed to evaluated all changes that may affect the SHE, the quality of intermediates and final product.2、范围 Scope2.1本规程适用于司。
This procedure is applicable to Xinhua-Chemferm.2.2该程序适用于公司各部门对已确立的所有程序所进行的变更。
This procedure is applicable to all departments’established document for changing. 2.3该规程适用于对产品质量或SHE有影响的工序或系统的变更, 包括, 但不限于:This procedure applies to the changes that involve effects to the products quality or the SHE aspects in the following process and /or systems, including, but not limited:2.3.1原料和供应商, 标准和有效期, 工艺过程, 分析方法, 标签和包材, 设备, 设施, 辅助系统, 计算机的硬件和软件Raw materials and suppliers, Specifications and valid date, Process steps, Analytical methods, Labeling and packaging materials, Equipment, Facilities,Support systems,Computer hardware and software.3、职责 Responsibility3.1 总经理负责批准本程序。
浅谈药品生产的变更控制流程

浅谈药品生产的变更控制流程张文云;齐景瑞;李恒宁;王霞【摘要】新版《药品生产质量管理规范》要求制药企业必须建立完善的质量管理体系,在质量管理中变更管理是保证药品质量的重要组成部分,强调在实施 GMP 时要求企业建立完善的变更控制流程,以保证产品质量。
从发起、分析、规划和审批、执行、实施等方面介绍了变更流程。
指出制药企业的任何变更都应遵守国家药监局新版 GMP变更控制的要求,确保制药标准的权威性,防止随意变换,便于质量追溯及产品质量跟踪,为质量信息系统提供基础信息。
%According to the new edition GMP,pharmaceutical enterprises should establish a complete quality management system,in which a change control system is one of the major components. During the GMP implementation process, enterprises are also required to set up a complete control flow for changes to ensure product quality. This paper describes the changing process from the aspects of initiation,plan & approval,execution & following up. It points out that any quality-related change in the companies shall conform to the requirements on change control in the new edition GMP issued by the State Food and Drug Administration(SFDA)to maintain the authority of the standard and guard against any freely changing. This will help in the quality tracing and following-up of relative products and provide basic information to the quality infor-mation system.【期刊名称】《天津科技》【年(卷),期】2015(000)006【总页数】3页(P5-6,9)【关键词】《药品生产质量管理规范》;GMP;变更控制管理【作者】张文云;齐景瑞;李恒宁;王霞【作者单位】天津百特医疗用品有限公司天津 300402;天津百特医疗用品有限公司天津 300402;天津百特医疗用品有限公司天津 300402;天津百特医疗用品有限公司天津 300402【正文语种】中文【中图分类】R954变更控制是药品生产质量管理系统中的重要组成部分,2011年3月1日正式施行的《药品生产质量管理规范》,即新版GMP对变更控制做出了明确的法规规定。
范围变更控制的6个流程

范围变更控制的6个流程Change control processes are crucial for ensuring that any changes made to a system or product are done in a controlled and documented manner. 范围变更控制流程对于确保系统或产品的任何变更都以受控且有文档记录的方式进行至关重要。
These processes help to minimize risks, ensure compliance with regulations, and maintain the overall stability and quality of the system or product. 这些流程有助于最小化风险,确保遵守法规,并保持系统或产品的整体稳定性和质量。
There are typically six main steps involved in change control processes, each of which plays a critical role in managing and implementing changes effectively. 通常,范围变更控制流程涉及六个主要步骤,每个步骤在有效管理和实施变更方面都起着关键作用。
The first step in the change control process is the identification of the need for change. 范围变更控制流程中的第一步是识别变更的需求。
This involves identifying any potential issues or areas for improvement that may require changes to be made to the system or product. 这涉及识别可能需要对系统或产品进行变更的任何潜在问题或改进领域。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Change Control ProcessPurposeThe purpose of this document is to provide the project manager, sponsors, steering committee members and all other stakeholders with the standard process for managing changes on the [project name] project.Related DocumentsThe scope of the [project name] has been defined in the approved Project Charter dated [date]. The work breakdown of the project and the timeline are detailed in the approved project plan dated [date].Purpose and ObjectivesThe purpose of this change management procedure is to manage change requests so that approved changes will be controlled, ensuring the project remains on schedule, within budget and provides the agreed deliverables.The primary objectives of change management are to:∙manage each change request from initiation through to closure;∙process change requests based upon direction from the appropriate authority;∙communicate the impact of changes to appropriate personnel; and∙allow small changes to be managed with a minimum of overhead.ScopeThe Change Management Process is the mechanism used to initiate, record, assess, approve and resolve project changes. Project changes are needed when it is deemed necessary to change the scope, time or cost of one or more previously approved project deliverables. Most changes will affect the budget and/or schedule of the project. PolicyThe use of the formal change management procedure will be required when any changes are discovered or requested which impact previously reviewed, approved and published project deliverables.The documentation and tracking of all change requests will be managed using the defined procedure and facilitated by the use of the change management log.A multi-tiered approach will be used to approve change requests:∙The Project Manager will make decisions to analyze and decisions to proceed with changes if the changes do not impact scope, budget or schedule or result in an increase in risk for the project.∙Changes which do impact scope, budget or schedule will be forwarded to the Steering Committee for review. The Steering Committee will advise the Project Sponsor.∙Where the [functional owner] has the resources to absorb the impact of the change, the Project Sponsor will make the final decision, based upon the information provided by the Project Manager and the input of the Steering Committee. The Project Sponsor, the [advisor], and [advisor] will discuss requests that may result in a significant change in scope, schedule, and budget, i.e. the impact of the change cannot be covered by [functional owner] resources. This group will advise the Steering Committee.∙The Steering Committee will make the final decision based upon the information providedDecision Matrixs c op e ch a n g ei n c r e a s e b u d g e tn o t i nc r e a s e b ud g eti n c r e a s e r i s kn o t i nc r e a s e r i s ki n c r e a s e s c h e d u l en o t i nc r e a s e s c h ed u le Decision by Project ManagerN N Y N Y N Y Vet with Steering CommitteeY Y N Y N Y N Decision by Project SponsorY Y Y Y Y Y Y Vet with [advisor names]Y Y n/a Y n/a Y n/a Decision by Steering CommitteeYYYYYYYT h e i m p a c t o f t h e c h a n g e m a y b e a b s o r b e d b y [f u n c t i o n a l o w n e r ]T h e i m p a c t o f t h e c h a n g e c a n n o t b e a b s o r b e d b y [f u n c t i o n a l o w n e r ]The following is a general guideline for the change management process. Most changes will require a subset of the steps listed.ProceduresEach request will be tracked from the time of presentation through: 1. Identify (identify and document the required change)2. Validate (verify the change is valid and requires management)3. Analyze (analyze and record schedule, cost and effort impact of change)4. Control (decide whether to execute the change)5. Action (execute decision, including revision to project plans if necessary)6. Close (verify that action is complete and close change request)Identify Change Request ActionResponsibility1. Identify and record the issue (in [location]). Project Manager or Team LeadValidate Change RequestAction Responsibility2. Identify member of the management team as the issue owner.3. Validate change request with project team members as appropriate.4. Assess and evaluate change for necessity to project.5. Update change request with target date for completion of analysis.Project ManagerAnalyze ImpactAction Responsibility6. Triage w/ consultation of the Project Sponsor7. Meet contract requirements for responding to Change Requests.8. Assign resources to review the impact of the change request.9. Direct activity to assess the scope, cost and schedule impact of thechange.10. Update change request with impact analysis and estimates in terms ofscope, cost, schedule and effort impacts.11. Update change request with target date for decision.Project ManagerControl Change RequestAction Responsibility12. Meet Sigma contract requirements for responding to Change Requests.13. Determine required approvals and assign priority to the change request.14. If changes impact scope, budget or schedule place request on agenda fornext Steering Committee meeting.15. If changes do not impact scope, budget or schedule decide whether toproceed with the change.Project Manager16. Review and discuss analysis of change request.17. Develop recommendation for the Project Sponsor.Steering Committee18. Decide whether to proceed with the change.19. If impact of change cannot be absorbed by [functional owner], schedulediscussion with [advisor names].Project Sponsor20. Review and discuss analysis of change request.21. If change request should be escalated to Steering Committee, placerequest on agenda for next meeting (or email if request is urgent).22. Develop recommendation for the Steering Committee.Project Sponsor, [advisor names]23. Review and discuss analysis of change request.24. Decide whether to proceed with the change.Steering Committee25. Generate approval signature sheets for each outstanding change request.26. Update status of change request with control decision.Project SponsorAction Change RequestAction Responsibility27. Negotiate contract changes.28. Execute contract changes. Project Manager, Project Sponsor, Technology Acquisition Manager29. Incorporate change request into appropriate plans and work plan.30. Update work plan baseline for agreed changes.Project ManagerClose Change RequestAction Responsibility31. Close change request..32. Communicate work plan change to project team.33. Monitor and report progress against project plan.34. Confirm all updates have been recorded and file all Change RequestDocuments.Project Manager。
