美国环保局 EPA 试验 方法 3520c
二氯硫化碳检测标准

二氯硫化碳检测标准二氯硫化碳(CS2)是一种有机化合物,其检测通常涉及空气、水或土壤中的含量。
对于不同的环境介质,可能存在不同的检测标准和方法。
以下是一些常见的检测标准和方法:1.空气中的检测:•美国职业安全与健康管理局(OSHA):OSHA规定了空气中二氯硫化碳的允许接触限值(Permissible ExposureLimit,PEL),以确保工作场所空气中的CS2浓度在安全范围内。
PEL通常以时间加权平均(Time-WeightedAverage,TWA)和短期曝露限值(Short-Term ExposureLimit,STEL)表示。
•美国国家职业安全卫生研究所(NIOSH):NIOSH也提供了关于二氯硫化碳的安全指南和检测方法。
2.水中的检测:•美国环境保护署(EPA):EPA规定了水中二氯硫化碳的最大容许浓度,以确保饮用水和环境水体中的CS2浓度处于安全水平。
•国际标准化组织(ISO):ISO可能也提供了一些关于水质标准和检测方法的国际标准。
3.土壤中的检测:•美国环境保护署(EPA):EPA可能规定了土壤中二氯硫化碳的安全限值和检测方法,以确保土壤中的CS2浓度对人类和环境没有危害。
4.其他国家和地区的标准:•根据不同国家和地区的法规和标准,对于二氯硫化碳的检测和限值可能存在差异。
因此,在具体的应用中,应该参考相应国家或地区的法规和标准。
需要注意的是,CS2的检测方法通常使用气相色谱法(Gas Chromatography,GC)等分析技术。
实际的检测程序和标准可能根据不同的监管机构和研究机构而有所不同。
在进行检测之前,建议咨询专业的环境监测和分析服务提供商。
EN及EPA等系列标准 中文名称

BS EN1122:2001:湿法消解测定塑料中的镉BS EN 1122:2001:Plastics-determination of cadmium-Wet decomposition method方法3005A:FLAA,ICP方法分析酸式消解水中的总溶解金属METHOD 3005A:ACID DIGESTION OF WATERS FOR TOTAL RECVERABLE OR DISSOLVED METALS FOR ANALYSIS BY FLAA OR ICP SPECTROSCOPY方法3010A: FLAA,ICP方法分析酸式消解水样和蒸馏样中的总金属METHOD 3010A:ACID DIGESTION OF AQUEOUS SAMPLES AND EXTRACTS FOR TOTAL METALS FOR ANALYSIS BY FLAA OR ICP SPECTROSCOPY方法3015:微波酸式消解水样和蒸馏液METHOD 3015:MICROWAVE ASSISTED ACID DIGESTION OF AQUEOUS SAMPLES AND EXTRACTS 方法3020A:G FAA方法分析酸式消解水样和蒸馏样中的总金属METHOD 3010A:ACID DIGESTION OF AQUEOUS SAMPLES AND EXTRACTS FOR TOTAL METALS FOR ANALYSIS BY GFAA SPECTROSCOPY方法3031:AAS,ICP分析酸式消解石油中的金属元素METHOD 3031:ACID DIGESTION OF OILS FOR METALS ANALYSIS BY ATOMIC ABSORPTION OR ICP SPECTROMETRY方法3040A:石油,油脂,石蜡的消解程序METHOD 3040A:DISSOLUTION PROCEDURE FOR OILS, GREASES, OR WAXES方法3050B:沉淀物,淤泥,土壤的酸式消解METHOD 3050B:ACID DIGESTION OF SEDIMENTS, SLUDGES, AND SOILS方法3051:沉淀物,淤泥,土壤石油的微波酸式消解METHOD 3051:MICROWAVE ASSISTED ACID DIGESTION OF SEDIMENTS SLUDGES, SOILS, AND OILS方法3052:硅酸盐和有机质的微波酸式消解METHOD 3052:MICROWAVE ASSISTED ACID DIGESTION OF SILICEOUS AND ORGANICALLY BASED MATRICES方法3060:六价铬的碱式消解METHOD 3060A:ALKALINE DIGESTION FOR HEXAVALENT CHROMIUM方法3500B:有机萃取和样品制备METHOD 3500B:ORGANIC EXTRACTION AND SAMPLE PREPARATION方法3510C:分液漏斗的液—液萃取METHOD 3510C :SEPARATORY FUNNEL LIQUID-LIQUID EXTRACTION方法3520C:连续液-液萃取METHOD3520C:CONTINUOUS LIQUID-LIQUID EXTRACTION方法3535:固相萃取METHOD 3535:SOLID-PHASE EXTRACTION (SPE)方法3540C:索氏萃取SOXHLET EXTRACTION方法3541:AUTOMATED SOXHLET EXTRACTION方法3542:用方法0010收集半挥发性分析物的萃取物METHOD 3542:EXTRACTION OF SEMIVOLATILE ANALYTES COLLECTED USING METHOD 0010(MLDIFIED METHOD 5 SAMPLING TRAIN)方法3545:密闭流动萃取METHOD 3545:PRESSURIZED FLUID EXTRACTION (PFE)方法3546:微波萃取METHOD 3546:MICROWAVE EXTRACTION方法3550B:超声波萃取METHOD 3550B:ULTRASONIC EXTRACTION方法3560:超临界流动萃取石油中的中回收物METHOD 3560:SUPERCRITICAL FLUID EXTRACTION OF TOTAL RECOVERABLE PETROLEUM HYDROCARBONS方法3561:多环芳烃的超临界流动萃取METHOD 3561:SUPERCRITICAL FLUID EXTRACTION OF POLYNUCLEAR AROMATIC HYDROCARBONS方法3580:废物稀释METHOD 3580A:WASTE DILUTION方法3585:挥发性有机物的废物稀释METHOD 3585:WASTE DILUTION FOR VOLATILE ORGANICS方法3600C:清除METHOD 3600C:CLEANUP方法3610B:矾土的清除METHOD 3610B:ALUMINA CLEANUP方法3611B:柱状矾土的清除和石油废弃物的分离METHOD 3611B:ALUMINA COLUMN CLEANUP AND SEPARATION OF PETROLEUM WASTES方法3520B:硅酸镁的清除METHOD 3520B:FLORISIL CLEANUP方法3630C:硅胶的清除METHOD 3630C:SILICA GEL CLEANUP方法3640A:渗入硅胶的清除METHOD 3640A:GEL-PERMEATION CLEANUP方法3650B:酸碱的分离清除METHOD 3650B:ACID-BASE PARTITION CLEANUP方法3660B:硫磺的清除METHOD3660B:SULFUR CLEANUP方法3665A:硫酸,高锰酸的清除METHOD 3665A:SULFURIC ACID/PERMANGANATE CLEANUP方法3810:顶部空间METHOD 3810:HEADSPACE方法3820:16烷萃取和净化有机物的屏蔽METHOD 3820:HEXADECANE EXTRACTION AND SCREENING OF PURGEABLE ORGANICS方法7000A:原子吸收方法METHOD 7000A:ATOMIC ABSORPTION METHODS方法7130:镉(原子吸收,直接吸收)METHOD7130:CADMIUM (ATOMIC ABSORPTION, DIRECT ASPIRATION)方法7130A:镉(原子吸收,炉子技术)METHOD7131A:CADMIUM (ATOMIC ABSORPTION, FURNACE TECHNIQUE)方法7190:铬(原子吸收,直接吸收)METHOD7190:CHROMIUM (ATOMIC ABSORPTION, DIRECT ASPIRATION)方法7191:铬(原子吸收,炉子技术)METHOD7191:CHROMIUM (ATOMIC ABSORPTION, FURNACE TECHNIQUE)方法7196A:六价铬(比色)METHOD 7196A:CHROMIUM, HEXAVALENT (COLORIMETRIC)方法7420:铅(原子吸收,直接吸收)方法7420:LEAD (ATOMIC ABSORPTION, DIRECT ASPIRATION)方法7421:铅(原子吸收,炉子技术)METHOD 7421:LEAD (ATOMIC ABSORPTION, DIRECT ASPIRATION)方法7470A:废水中的汞(冷原子蒸汽技术)METHOD 7470A:MERCURY IN LIQUID WASTE (MANUAL COLD-VAPOR TECHNIQUE)方法7471A:固体和固体废弃物中的汞(冷原子蒸汽技术)METHOD 7471A:MERCURY IN SOLID OR SEMISOLID WASTE (MANUAL COLD-VAPOR TECHNIQUE) 方法7473 :热分解原子吸收光谱法测定固体和液体样品中的汞METHOD 7473:MERCURY IN SOLIDS AND SOLUTIONS BY THERMAL DECOMPODITION AMALGAMATION, AND ATOMIC ABSORPTION SPECTROPHOTOMETRY方法8000B:限定色谱分离METHOD 8000B:DETERMINATIVE CHROMATOGRAPHIC SEPARATIONS方法8081A:气相色谱分析有机氯沙虫剂METHOD 8081A:ORGANOCHLORINE PESTICIDES BY GAS CHROMATOGRAPHY方法8081B:气相色谱分析有机氯沙虫剂METHOD 8081B:ORGANOCHLORINE PESTICIDES BY GAS CHROMATOGRAPHY方法8082:气相色谱分析多氯联苯METHOD 8082:POLYCHLORINATED BIPHENYLS (PCBs) BY GAS CHROMATOGRAPHY方法8082A:气相色谱分析多氯联苯METHOD 8082A:POLYCHLORINA BIPHENYLS (PCBs) BY GAS CHROMATOGRAPHY方法8260B:气相色谱/质谱分析挥发性有机化合物METHOD 8260B:VOLATILE ORGANIC COMPOUNDS BY GAS CHROMATOGRAPHY/MASS SPECTROMETRY (GC/MS)。
epa standard method 533

epa standard method 533
EPA(美国环境保护局)Standard Method 533是关于气体监测的方法,具体名为《Method 533:Determination of Volatile Organic Compounds (VOCs) in Air by Adsorption on Activated Carbon and Gas Chromatography》。
这个方法主要用于测定空气中的挥发性有机化合物(VOCs)。
在这个方法中,样品通过吸附在活性炭上收集,然后使用气相色谱法进行分析。
方法涵盖了吸附剂的选择、采样设备、样品处理和分析等步骤。
活性炭吸附剂具有较高的吸附能力,可以有效地捕获空气中的VOCs。
气相色谱法用于分离和定量吸附剂上的VOCs,从而得出空气中VOCs的浓度。
EPA Standard Method 533为监测空气中的挥发性有机化合物提供了可靠的方法,有助于评估空气质量并制定相应的环境保护措施。
该方法在我国环保领域也有广泛应用,以保障空气质量和人民健康。
EPA-Method-3501[1]
![EPA-Method-3501[1]](https://img.taocdn.com/s3/m/3da4fd8ecc22bcd126ff0c4a.png)
DETERMINATION OF AMMONIA NITROGEN BY SEMI-AUTOMATEDCOLORIMETRYEdited by James W. O'DellInorganic Chemistry BranchChemistry Research DivisionRevision 2.0August 1993ENVIRONMENTAL MONITORING SYSTEMS LABORATORY OFFICE OF RESEARCH AND DEVELOPMENTU.S. ENVIRONMENTAL PROTECTION AGENCYCINCINNATI, OHIO 45268350.1-1DETERMINATION OF AMMONIA NITROGEN BY SEMI-AUTOMATEDCOLORIMETRY1.0SCOPE AND APPLICATION1.1This method covers the determination of ammonia in drinking, ground,surface, and saline waters, domestic and industrial wastes.1.2The applicable range is 0.01-2.0 mg/L NH as N. Higher concentrations can be3determined by sample dilution. Approximately 60 samples per hour can beanalyzed.1.3This method is described for macro glassware; however, micro distillationequipment may also be used.2.0SUMMARY OF METHOD2.1The sample is buffered at a pH of 9.5 with a borate buffer in order to decreasehydrolysis of cyanates and organic nitrogen compounds, and is distilled into asolution of boric acid. Alkaline phenol and hypochlorite react with ammoniato form indophenol blue that is proportional to the ammonia concentration.The blue color formed is intensified with sodium nitroprusside and measuredcolorimetrically.2.3Reduced volume versions of this method that use the same reagents and molarratios are acceptable provided they meet the quality control and performancerequirements stated in the method.2.4Limited performance-based method modifications may be acceptable providedthey are fully documented and meet or exceed requirements expressed inSection 9.0, Quality Control.3.0DEFINITIONS3.1Calibration Blank (CB) -- A volume of reagent water fortified with the samematrix as the calibration standards, but without the analytes, internalstandards, or surrogate analytes.3.2Calibration Standard (CAL) -- A solution prepared from the primary dilutionstandard solution or stock standard solutions and the internal standards andsurrogate analytes. The CAL solutions are used to calibrate the instrumentresponse with respect to analyte concentration.350.1-23.3Instrument Performance Check Solution (IPC) -- A solution of one or moremethod analytes, surrogates, internal standards, or other test substances usedto evaluate the performance of the instrument system with respect to a definedset of criteria.3.4Laboratory Fortified Blank (LFB) -- An aliquot of reagent water or other blankmatrices to which known quantities of the method analytes are added in thelaboratory. The LFB is analyzed exactly like a sample, and its purpose is todetermine whether the methodology is in control, and whether the laboratoryis capable of making accurate and precise measurements.3.5Laboratory Fortified Sample Matrix (LFM) -- An aliquot of an environmentalsample to which known quantities of the method analytes are added in thelaboratory. The LFM is analyzed exactly like a sample, and its purpose is todetermine whether the sample matrix contributes bias to the analytical results.The background concentrations of the analytes in the sample matrix must bedetermined in a separate aliquot and the measured values in the LFMcorrected for background concentrations.3.6Laboratory Reagent Blank (LRB) -- An aliquot of reagent water or other blankmatrices that are treated exactly as a sample including exposure to allglassware, equipment, solvents, reagents, internal standards, and surrogatesthat are used with other samples. The LRB is used to determine if methodanalytes or other interferences are present in the laboratory environment, thereagents, or the apparatus.3.7Linear Calibration Range (LCR) -- The concentration range over which theinstrument response is linear.3.8Material Safety Data Sheet (MSDS) -- Written information provided byvendors concerning a chemical's toxicity, health hazards, physical properties,fire, and reactivity data including storage, spill, and handling precautions.3.9Method Detection Limit (MDL) -- The minimum concentration of an analytethat can be identified, measured and reported with 99% confidence that theanalyte concentration is greater than zero.3.10Quality Control Sample (QCS) -- A solution of method analytes of knownconcentrations that is used to fortify an aliquot of LRB or sample matrix. TheQCS is obtained from a source external to the laboratory and different fromthe source of calibration standards. It is used to check laboratory performancewith externally prepared test materials.3.11Stock Standard Solution (SSS) -- A concentrated solution containing one ormore method analytes prepared in the laboratory using assayed referencematerials or purchased from a reputable commercial source.4.0INTERFERENCES350.1-34.1Cyanate, which may be encountered in certain industrial effluents, willhydrolyze to some extent even at the pH of 9.5 at which distillation is carriedout.4.2Residual chorine must be removed by pretreatment of the sample with sodiumthiosulfate or other reagents before distillation.4.3Method interferences may be caused by contaminants in the reagent water,reagents, glassware, and other sample processing apparatus that bias analyteresponse.5.0SAFETY5.1The toxicity or carcinogenicity of each reagent used in this method have notbeen fully established. Each chemical should be regarded as a potential healthhazard and exposure should be as low as reasonably achievable. Cautions areincluded for known extremely hazardous materials or procedures.5.2Each laboratory is responsible for maintaining a current awareness file ofOSHA regulations regarding the safe handling of the chemicals specified inthis method. A reference file of Material Safety Data Sheets (MSDS) should bemade available to all personnel involved in the chemical analysis. Thepreparation of a formal safety plan is also advisable.5.3The following chemicals have the potential to be highly toxic or hazardous,consult MSDS.5.3.1Sulfuric acid (Section 7.6)5.3.2Phenol (Section 7.7)5.3.3Sodium nitroprusside (Section 7.10)6.0EQUIPMENT AND SUPPLIES6.1Balance - Analytical, capable of accurately weighing to the nearest 0.0001 g.6.2Glassware - Class A volumetric flasks and pipets as required.6.3An all-glass distilling apparatus with an 800-1000 mL flask.6.4Automated continuous flow analysis equipment designed to deliver and reactsample and reagents in the required order and ratios.6.4.1Sampling device (sampler)6.4.2Multichannel pump350.1-46.4.3Reaction unit or manifold6.4.4Colorimetric detector6.4.5Data recording device7.0REAGENTS AND STANDARDS7.1Reagent water - Ammonia free: Such water is best prepared by passagethrough an ion exchange column containing a strongly acidic cation exchangeresin mixed with a strongly basic anion exchange resin. Regeneration of thecolumn should be carried out according to the manufacturer's instructions.Note: All solutions must be made with ammonia-free water.7.2Boric acid solution (20 g/L): Dissolve 20 g H BO (CASRN 10043-35-3) in33reagent water and dilute to 1 L.7.3Borate buffer: Add 88 mL of 0.1 N NaOH (CASRN 1310-73-2) solution to 500mL of 0.025 M sodium tetraborate solution (5.0 g anhydrous Na B O [CASRN2471330-43-4] or 9.5 g Na B O10H O [CASRN 1303-96-4] per L) and dilute to 1 L2472with reagent water.7.4Sodium hydroxide, 1 N: Dissolve 40 g NaOH in reagent water and dilute to 1L.7.5Dechlorinating reagents: A number of dechlorinating reagents may be used toremove residual chlorine prior to distillation. These include:7.5.1Sodium thiosulfate: Dissolve 3.5 g Na S O5H O (CASRN 10102-17-7)2232in reagent water and dilute to 1 L. One mL of this solution willremove 1 mg/L of residual chlorine in 500 mL of sample.7.5.2Sodium sulfite: Dissolve 0.9 g Na2SO (CASRN 7757-83-7) in reagent3water and dilute to 1 L. One mL removes 1 mg/L Cl per 500 mL ofsample.7.6Sulfuric acid 5 N: Air scrubber solution. Carefully add 139 mL of conc.sulfuric acid (CASRN 7664-93-9) to approximately 500 mL of reagent water.Cool to room temperature and dilute to 1 L with reagent water.7.7Sodium phenolate: Using a 1-L Erlenmeyer flask, dissolve 83 g phenol(CASRN 108-95-2) in 500 mL of distilled water. In small increments,cautiously add with agitation, 32 g of NaOH. Periodically cool flask underwater faucet. When cool, dilute to 1 L with reagent water.7.8Sodium hypochlorite solution: Dilute 250 mL of a bleach solution containing5.25% NaOCl (CASRN 7681-52-9) (such as "Clorox") to 500 mL with reagent350.1-5water. Available chlorine level should approximate 2-3%. Since "Clorox" is aproprietary product, its formulation is subject to change. The analyst mustremain alert to detecting any variation in this product significant to its use inthis procedure. Due to the instability of this product, storage over an extendedperiod should be avoided.7.9Disodium ethylenediamine-tetraacetate (EDTA) (5%): Dissolve 50 g of EDTA(disodium salt) (CASRN 6381-92-6) and approximately six pellets of NaOH in 1L of reagent water.7.10Sodium nitroprusside (0.05%): Dissolve 0.5 g of sodium nitroprusside (CASRN14402-89-2) in 1 L of reagent water.7.11Stock solution: Dissolve 3.819 g of anhydrous ammonium chloride, NH Cl4 (CASRN 12125-02-9), dried at 105°C, in reagent water, and dilute to 1 L.1.0 mL = 1.0 mg NH-N.37.12Standard Solution A: Dilute 10.0 mL of stock solution (Section 7.11) to 1 Lwith reagent water. 1.0 mL = 0.01 mg NH-N.37.13Standard Solution B: Dilute 10.0 mL of standard solution A (Section 7.12) to100.0 mL with reagent water. 1.0 mL = 0.001 mg NH-N.38.0SAMPLE COLLECTION, PRESERVATION AND STORAGE8.1Samples should be collected in plastic or glass bottles. All bottles must bethoroughly cleaned and rinsed with reagent water. Volume collected should besufficient to insure a representative sample, allow for replicate analysis (ifrequired), and minimize waste disposal.8.2Samples must be preserved with H SO to a pH <2 and cooled to 4°C at the24time of collection.8.3Samples should be analyzed as soon as possible after collection. If storage isrequired, preserved samples are maintained at 4°C and may be held for up to28 days.9.0QUALITY CONTROL9.1Each laboratory using this method is required to operate a formal qualitycontrol (QC) program. The minimum requirements of this program consist ofan initial demonstration of laboratory capability, and the periodic analysis oflaboratory reagent blanks, fortified blanks and other laboratory solutions as acontinuing check on performance. The laboratory is required to maintainperformance records that define the quality of the data that are generated.9.2INITIAL DEMONSTRATION OF PERFORMANCE350.1-6350.1-79.2.1The initial demonstration of performance is used to characterizeinstrument performance (determination of LCRs and analysis of QCS)and laboratory performance (determination of MDLs) prior toperforming analyses by this method.9.2.2Linear Calibration Range (LCR) -- The LCR must be determinedinitially and verified every six months or whenever a significant changein instrument response is observed or expected. The initialdemonstration of linearity must use sufficient standards to insure thatthe resulting curve is linear. The verification of linearity must use aminimum of a blank and three standards. If any verification dataexceeds the initial values by ± 10%, linearity must be reestablished. Ifany portion of the range is shown to be nonlinear, sufficient standardsmust be used to clearly define the nonlinear portion.9.2.3Quality Control Sample (QCS) -- When beginning the use of thismethod, on a quarterly basis or as required to meet data-quality needs,verify the calibration standards and acceptable instrument performancewith the preparation and analyses of a QCS. If the determinedconcentrations are not within ±10% of the stated values, performance ofthe determinative step of the method is unacceptable. The source ofthe problem must be identified and corrected before either proceedingwith the initial determination of MDLs or continuing with on-goinganalyses.9.2.4Method Detection Limit (MDL) -- MDLs must be established for allanalytes, using reagent water (blank) fortified at a concentration of twoto three times the estimated instrument detection limit. To determine9MDL values, take seven replicate aliquots of the fortified reagent waterand process through the entire analytical method. Perform allcalculations defined in the method and report the concentration valuesin the appropriate units. Calculate the MDL as follows:where,t = Student's t value for a 99% confidence level and astandard deviation estimate with n-1 degrees offreedom [t = 3.14 for seven replicates]S = standard deviation of the replicate analyses MDLs should be determined every six months, when a new operatorbegins work or whenever there is a significant change in thebackground or instrument response.9.3ASSESSING LABORATORY PERFORMANCE9.3.1Laboratory Reagent Blank (LRB) -- The laboratory must analyze at leastone LRB with each batch of samples. Data produced are used to assess contamination from the laboratory environment. Values that exceed the MDL indicate laboratory or reagent contamination should be suspectedand corrective actions must be taken before continuing the analysis.9.3.2Laboratory Fortified Blank (LFB) -- The laboratory must analyze at leastone LFB with each batch of samples. Calculate accuracy as percentrecovery (Section 9.4.2). If the recovery of any analyte falls outside therequired control limits of 90-110%, that analyte is judged out of control, and the source of the problem should be identified and resolved beforecontinuing analyses.9.3.3The laboratory must use LFB analyses data to assess laboratoryperformance against the required control limits of 90-110%. Whensufficient internal performance data become available (usually aminimum of 20-30 analyses), optional control limits can be developedfrom the percent mean recovery (x) and the standard deviation (S) ofthe mean recovery. These data can be used to establish the upper andlower control limits as follows:UPPER CONTROL LIMIT = x + 3SLOWER CONTROL LIMIT = x - 3SThe optional control limits must be equal to or better than the requiredcontrol limits of 90-110%. After each five to 10 new recoverymeasurements, new control limits can be calculated using only the most recent 20-30 data points. Also, the standard deviation (S) data shouldbe used to established an on-going precision statement for the level ofconcentrations included in the LFB. These data must be kept on fileand be available for review.9.3.4Instrument Performance Check Solution (IPC) -- For all determinationsthe laboratory must analyze the IPC (a mid-range check standard) anda calibration blank immediately following daily calibration, after every10th sample (or more frequently, if required) and at the end of thesample run. Analysis of the IPC solution and calibration blankimmediately following calibration must verify that the instrument iswithin ±10% of calibration. Subsequent analyses of the IPC solutionmust verify the calibration is still within ±10%. If the calibration cannot be verified within the specified limits, reanalyze the IPC solution. If the second analysis of the IPC solution confirms calibration to be outsidethe limits, sample analysis must be discontinued, the cause determinedand/or in the case of drift, the instrument recalibrated. All samplesfollowing the last acceptable IPC solution must be reanalyzed. Theanalysis data of the calibration blank and IPC solution must be kept onfile with the sample analyses data.350.1-8350.1-99.4ASSESSING ANALYTE RECOVERY AND DATA QUALITY9.4.1Laboratory Fortified Sample Matrix (LFM) -- The laboratory must add aknown amount of analyte to a minimum of 10% of the routine samples.In each case the LFM aliquot must be a duplicate of the aliquot usedfor sample analysis. The analyte concentration must be high enough tobe detected above the original sample and should not be less than fourtimes the MDL. The added analyte concentration should be the sameas that used in the laboratory fortified blank.9.4.2Calculate the percent recovery for each analyte, corrected forconcentrations measured in the unfortified sample, and compare thesevalues to the designated LFM recovery range 90-110%. Percentrecovery may be calculate using the following equation:where,R =percent recoveryC =fortified sample concentrationsC =sample background concentrations =concentration equivalent of analyte added tosample9.4.3If the recovery of any analyte falls outside the designated LFM recoveryrange and the laboratory performance for that analyte is shown to be incontrol (Section 9.3), the recovery problem encountered with the LFM isjudged to be either matrix or solution related, not system related.9.4.4Where reference materials are available, they should be analyzed toprovide additional performance data. The analysis of referencesamples is a valuable tool for demonstrating the ability to perform themethod acceptably.10.0CALIBRATION AND STANDARDIZATION10.1Prepare a series of at least three standards, covering the desired range, and ablank by diluting suitable volumes of standard solutions (Sections 7.12 and7.13) to 100 mL with reagent water.10.2Process standards and blanks as described in Section 11.0, Procedure.10.3Set up manifold as shown in Figure 1.10.4Prepare flow system as described in Section 11.0, Procedure.10.5Place appropriate standards in the sampler in order of decreasingconcentration and perform analysis.10.6Prepare standard curve by plotting instrument response against concentrationvalues. A calibration curve may be fitted to the calibration solutionsconcentration/response data using computer or calculator based regressioncurve fitting techniques. Acceptance or control limits should be establishedusing the difference between the measured value of the calibration solutionand the "true value" concentration.10.7After the calibration has been established, it must be verified by the analysis ofa suitable QCS. If measurements exceed ±10% of the established QCS value,the analysis should be terminated and the instrument recalibrated. The newcalibration must be verified before continuing analysis. Periodic reanalysis ofthe QCS is recommended as a continuing calibration check.11.0PROCEDURE11.1Preparation of equipment: Add 500 mL of reagent water to an 800 mLKjeldahl flask. The addition of boiling chips that have been previously treatedwith dilute NaOH will prevent bumping. Steam out the distillation apparatusuntil the distillate shows no trace of ammonia.11.2Sample preparation: Remove the residual chorine in the sample by addingdechlorinating agent (Section 7.5) equivalent to the chlorine residual. To 400mL of sample add 1 N NaOH (Section 7.4), until the pH is 9.5, check the pHduring addition with a pH meter or by use of a short range pH paper.11.3Distillation: Transfer the sample, the pH of which has been adjusted to 9.5, toan 800 mL Kjeldahl flask and add 25 mL of the borate buffer (Section 7.3).Distill 300 mL at the rate of 6-10 mL/min. into 50 mL of 2% boric acid (Section7.2) contained in a 500 mL Erlenmeyer flask.Note: The condenser tip or an extension of the condenser tip must extendbelow the level of the boric acid solution.11.4Since the intensity of the color used to quantify the concentration is pHdependent, the acid concentration of the wash water and the standardammonia solutions should approximate that of the samples.11.5Allow analysis system to warm up as required. Feed wash water throughsample line.11.6Arrange ammonia standards in sampler in order of decreasing concentration ofnitrogen. Complete loading of sampler tray with unknown samples.11.7Switch sample line from reagent water to sampler and begin analysis.350.1-1012.0DATA ANALYSIS AND CALCULATIONS12.1Prepare a calibration curve by plotting instrument response against standardconcentration. Compute sample concentration by comparing sample responsewith the standard curve. Multiply answer by appropriate dilution factor.12.2Report only those values that fall between the lowest and the highestcalibration standards. Samples exceeding the highest standard should bediluted and reanalyzed.12.3Report results in mg NH-N/L.313.0METHOD PERFORMANCE13.1In a single laboratory (EMSL-Cincinnati), using surface water samples atconcentrations of 1.41, 0.77, 0.59, and 0.43 mg NH-N/L, the standard3deviation was ±0.005.13.2In a single laboratory (EMSL-Cincinnati), using surface water samples atconcentrations of 0.16 and 1.44 mg NH-N/L, recoveries were 107% and 99%,3respectively.13.3The interlaboratory precision and accuracy data in Table 1 were developedusing a reagent water matrix. Values are in mg NH-N/L.314.0POLLUTION PREVENTION14.1Pollution prevention encompasses any technique that reduces or eliminates thequantity or toxicity of waste at the point of generation. Numerousopportunities for pollution prevention exist in laboratory operation. The EPAhas established a preferred hierarchy of environmental management techniquesthat places pollution prevention as the management option of first choice.Whenever feasible, laboratory personnel should use pollution preventiontechniques to address their waste generation. When wastes cannot be feasiblyreduced at the source, the Agency recommends recycling as the next bestoption.14.2The quantity of chemicals purchased should be based on expected usageduring its shelf life and disposal cost of unused material. Actual reagentpreparation volumes should reflect anticipated usage and reagent stability.14.3For information about pollution prevention that may be applicable tolaboratories and research institutions, consult "Less is Better: LaboratoryChemical Management for Waste Reduction", available from the AmericanChemical Society's Department of Government Regulations and Science Policy,1155 16th Street N.W., Washington, D.C. 20036, (202)872-4477.15.0WASTE MANAGEMENT350.1-1115.1The U.S. Environmental Protection Agency requires that laboratory wastemanagement practices be conducted consistent with all applicable rules andregulations. Excess reagents, samples and method process wastes should becharacterized and disposed of in an acceptable manner. The Agency urgeslaboratories to protect the air, water and land by minimizing and controllingall releases from hoods, and bench operations, complying with the letter and spirit of any waste discharge permit and regulations, and by complying with all solid and hazardous waste regulations, particularly the hazardous wasteidentification rules and land disposal restrictions. For further information onwaste management consult the "Waste Management Manual for LaboratoryPersonnel", available from the American Chemical Society at the address listed in Section 14.3.350.1-1216.0REFERENCES1.Hiller, A., and Van Slyke, D., "Determination of Ammonia in Blood", J. Biol.Chem. 102, p. 499 (1933).2.O'Connor, B., Dobbs, R., Villiers, B., and Dean. R., "Laboratory Distillation ofMunicipal Waste Effluents", JWPCF 39, R 25 (1967).3.Fiore, J., and O'Brien, J.E., "Ammonia Determination by Automatic Analysis",Wastes Engineering 33, p. 352 (1962).4. A Wetting Agent Recommended and Supplied by the Technicon Corporationfor Use in AutoAnalyzers.5.ASTM "Manual on Industrial Water and Industrial Waste Water", 2nd Ed.,1966 printing, p. 418.6.Booth, R.L., and Lobring. L.B., "Evaluation of the AutoAnalyzer II: A ProgressReport" in Advances in Automated Analysis: 1972 Technicon InternationalCongress, Vol. 8, p. 7-10, Mediad Incorporated, Tarrytown, N.Y., (1973).7.Standards Methods for the Examination of Water and Wastewater, 18thEdition, p. 4-77, Methods 4500 NH3 B and H (1992).8.Annual Book of ASTM Standards, Part 31, "Water", Standard D1426-79(C).9.Code of Federal Regulations 40, Ch. 1, Pt. 136, Appendix B.350.1-1317.0TABLES, DIAGRAMS, FLOWCHARTS, AND VALIDATION DATATABLE 1. INTERLABORATORY PRECISION AND ACCURACY DATA Number of True StandardValues Value Mean Residual Deviation ResidualReported(T)(X)for X(S)for S 1340.2700.2670-0.00110.03420.00151570.6920.69720.00590.0476-0.0070136 1.20 1.20080.00010.0698-0.0112195 1.60 1.60950.00760.10230.0006142 3.00 3.01280.00690.1677-0.0067159 3.50 3.4991-0.00830.21680.0165156 3.60 3.5955-0.01220.1821-0.0234200 4.20 4.22710.01770.28550.04881968.768.7257-0.05680.4606-0.012715611.011.07470.04570.5401-0.049514213.012.9883-0.04650.69610.002719918.017.9727-0.0765 1.16350.2106 REGRESSIONS: X = 1.003T - 0.003, S = 0.052T + 0.019350.1-14。
epa3500方法

epa3500方法
EPA 3500方法是美国环境保护局(EPA)所制定的一种分析化
学方法,用于土壤、废水、废物和其它环境样品中有机化合物的测定。
该方法通常用于环境监测和污染物检测。
EPA 3500方法主要涉
及样品的制备、提取和分析,以便准确测定有机化合物的含量。
具
体来说,该方法通常包括以下步骤:
1. 样品的准备,样品需要根据方法要求进行适当的处理和准备,以确保分析的准确性和可重复性。
2. 提取过程,样品中的有机化合物需要通过适当的提取方法提
取出来,以便后续的分析。
3. 分析方法,EPA 3500方法通常使用气相色谱-质谱联用
(GC-MS)或液相色谱-质谱联用(LC-MS)等高灵敏度的分析技术,
以确保对有机化合物的准确测定。
4. 质量控制,在整个分析过程中,需要进行质量控制以确保分
析结果的可靠性。
总的来说,EPA 3500方法是一种标准化的分析化学方法,用于环境样品中有机化合物的测定。
通过严格遵循该方法,可以获得准确可靠的分析结果,从而评估环境中有机污染物的水平,为环境保护和管理提供重要的数据支持。
美国国家环保局EPA方法要点和推荐仪器

美国国家环保局EPA方法要点和推荐仪器EPA方法218.6离子色谱测定在饮用水、地下水和工业废水中的水溶性铬(1994年修订版3.3)应用范围测定饮用水、地下水和工业废水中的水溶性六价铬(如CrO2-4),这种方法的检测下限为0.4μg/L。
样品中如果含有大量的阴离子物质如硫酸或氯离子可能会引起色谱柱过载。
样品如果含有大量有机物或硫离子可能会引起可溶性的六价铬快速还原为三价铬。
样品贮存在4℃,在24小时内分析。
方法采用离子色谱法分析。
方法要点:水样经0.45μm滤膜过滤后,用浓缓冲溶液调节pH为9-9.5。
样品的测量体积为50-250μL进样到离子色谱。
保护柱去除样品中的有机物,六价铬以CrO2-4形式,在高容量的阴离子交换分离柱上分离,六价铬用双苯基苄巴脲柱后衍生,然后在530nm波长下检测有色络合物。
建议采用的仪器条件保护柱:Dionex IonPac NG1或与之相同的色谱柱分离柱:Dionex IonPac AS7或与之相同的色谱柱阴离子抑制器装置:Dionex Anion MicroMembrane Suppressor,其它抑制器必须有足够低的检测限和足够的基线稳定性。
色谱条件:色谱柱:保护柱-Dionex IonPac NG1, 分离柱-Dionex IonPac AS7淋洗液:250mM (NH4)2SO4, 100mM NH4OH, 流速=1.5 mL/min柱后试剂:2mM双苯基苄巴脲,10% v/v甲醇,1N 硫酸,流速=0.5 mL/min 检测器:可见光530nm保留时间:3.8 分钟离子色谱测定无机阴离子(1993年八月,修订版2.2)应用范围1.可测定的阴离子包括A部分:溴离子,氯离子,氟离子,硝酸根,亚硝酸根,磷酸根,硫酸B部分:溴酸根,亚氯酸根,氯酸根2.基体包括:饮用水,地表水,民用水和工业废水,地下水,试剂用水,固体浸出液方法要点1.小量样品,一般2-3mL注入离子色谱,阴离子采用一个系统含有保护柱,分离柱,抑制器和电导检测器进行分离和检测。
美国EPA通用土壤筛选值

75-68-3
1,1-二氟-1-氯乙烷
5.8E+04
ns
2.4E+05
nms
5.2E+01
1.0E+05
n
126-99-8
2-氯-1,3-丁二烯
8.4E+00
n
3.6E+01
n
7.5E-03
1.4E+01
n
3165-93-3
1.5E+02
n
1.5E+03
n
4.7E+01
9.1E+01
n
7664-41-7
氨
7790-98-9
高氯酸铵
5.5E+01
n
7.2E+02
n
2.6E+01
n
7773-06Leabharlann 0氨基磺酸铵1.6E+04
n
2.0E+05
nm
7.3E+03
n
62-53-3
苯胺
8.5E+01
c**
3.0E+02
c*
4.0E-03
c
542-88-1
二氯甲基醚
7.7E-05
c
3.9E-04
c
1.5E-08
6.2E-05
c
80-05-7
双酚A
3.1E+03
n
3.1E+04
n
1.4E+02
1.8E+03
n
7440-42-8
硼及硼酸盐
1.6E+04
n
2.0E+05
美国EPA 关于空气自动监测系统性能指标的规定和测试方法
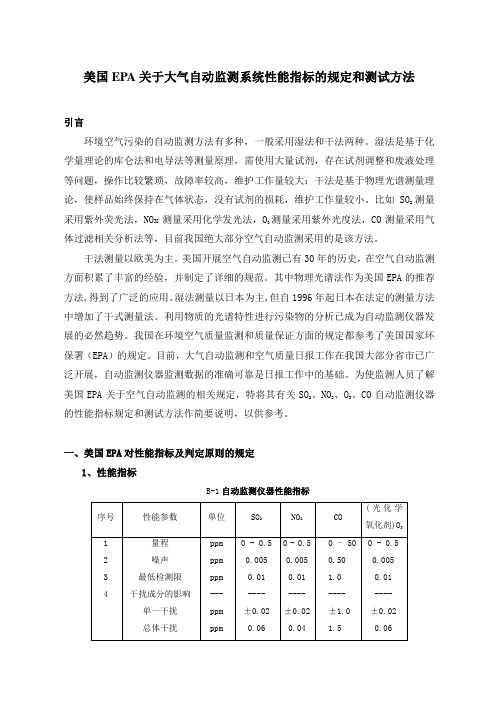
美国EPA关于大气自动监测系统性能指标的规定和测试方法引言环境空气污染的自动监测方法有多种,一般采用湿法和干法两种。
湿法是基于化学量理论的库仑法和电导法等测量原理,需使用大量试剂,存在试剂调整和废液处理等问题,操作比较繁琐,故障率较高,维护工作量较大;干法是基于物理光谱测量理论,使样品始终保持在气体状态,没有试剂的损耗,维护工作量较小。
比如SO2测量采用紫外荧光法,NOx测量采用化学发光法,O3测量采用紫外光度法,CO测量采用气体过滤相关分析法等,目前我国绝大部分空气自动监测采用的是该方法。
干法测量以欧美为主。
美国开展空气自动监测已有30年的历史,在空气自动监测方面积累了丰富的经验,并制定了详细的规范。
其中物理光谱法作为美国EPA的推荐方法,得到了广泛的应用。
湿法测量以日本为主,但自1996年起日本在法定的测量方法中增加了干式测量法。
利用物质的光谱特性进行污染物的分析已成为自动监测仪器发展的必然趋势。
我国在环境空气质量监测和质量保证方面的规定都参考了美国国家环保署(EPA)的规定。
目前,大气自动监测和空气质量日报工作在我国大部分省市已广泛开展,自动监测仪器监测数据的准确可靠是日报工作中的基础。
为使监测人员了解美国EPA关于空气自动监测的相关规定,特将其有关SO2、NO2、O3、CO自动监测仪器的性能指标规定和测试方法作简要说明,以供参考。
一、美国EPA对性能指标及判定原则的规定1、性能指标B-1自动监测仪器性能指标M/0.02447,M是该气体的摩尔质量。
2、判定原则对于每个性能指标(量程除外),测试程序从开始起要重复7次,得到7组测试结果。
每组结果要和表B-1中的规定指标相比较,高于或超出规定指标的值是一个超标值。
每个参数的7个结果说明如下:(1)0次超标:被测的参数合格;(2)3次或更多次超标:该参数不合格;(3)1次或2次超标:再重复测试该参数 8次,得到共15个测试结果。
将此15个测试结果说明如下:a:1次或2次超标:通过测试;b:3次以上:该参数不合格。
美国EPA最新参考方法标准

特别规定的样品采集过滤器。
手动参考方法: 配备 RAAS-10 PM10 进气口或
RFPS-0699-131 40 联邦法规(CFR)第 50 部分,
附录 L, 图 L-2 到 L-19 中特定的
联邦公告:卷 64, 有通气孔的进口,作为 PM10
第 33481 页 , 参考方法配置,流量为 16.67 升
图 L-2 参考方法
第 33481 页 , 配置,流量为 16.67 升/分钟,24
BGI 公司 BGI 公司 DKK-TOA 公司 Ecotech 公司
PQ100 型空气采样器
PQ200 型空气采样器
FPM-222/222C,FPM223 /223C 及 DUB-222(S)型 PM10 监测器 3000 型 PM10 大容量空 气采样器
或
12/01/87 及卷 53, GMW-IP-10-8000 中的任一型号
第 1062 页 , 大容量采样器,这些采样器含有
01/15/88
以下部件:带有丙烯腈-丁二烯-
苯乙烯塑胶过滤器托架和电机/
鼓风机外壳或不锈钢过滤器托
架和酚醛塑料电机/鼓风机外壳
的阳极氧化处理铝制大容量外
壳;0.6 大功率电机/鼓风机; 压
06/23/99
小时连续采样周期操作。符合
RAAS105-300 操作说明书,遵
循 40 CFR 第 50 部分,附录 J
或附录 M 中有关要求和特别规
定的样品采集过滤器。
手动参考方法: 配备 BGI16.7 进气口装置或附
RFPS-0699-132 录 L,40 联邦法规(CFR)50,
图 L-2 到 L-19 中特定的有通气
7.0 说明书,适当的还带有特制
EPA方法索引范文

EPA方法索引范文EPA方法索引是指美国环境保护署(Environmental Protection Agency)使用的一种方法或指南的集合。
这些方法和指南被广泛应用于环境监测、控制和评估等领域,以确保环境和公共健康的保护。
以下是一些常见的EPA方法索引。
1.环境监测方法-EPA方法200.7:用于痕量金属分析的集中器分析方法。
-EPA方法353.2:用于水中氨氮的连续流动分析方法。
-EPA方法8010:挥发性有机化合物(VOCs)在土壤、固体废物和水样中的分析方法。
2.大气排放测量方法-EPA方法1:测量排放源气流量的方法。
-EPA方法3:使用热式测速计测量气流速度的方法。
-EPA方法25A:测量总有机气态污染物(TO-9A)的方法。
3.水质监测方法-EPA方法200.8:通过电感耦合等离子体发射光谱法(ICP-OES)测量地下水和饮用水中痕量金属的方法。
-EPA方法160.2:测量水和废水中总悬浮颗粒物(TSS)的方法。
-EPA方法300.0:用于汞浓度分析的氢化物发生-冷蒸汽原子吸收光谱法的方法。
4.土壤和固体废物分析方法-EPA方法3050B:提取土壤和固体废物中的金属的方法。
-EPA方法3540C:挥发性有机物在土壤、底泥和固体样品中的提取方法。
-EPA方法8260B:环境样品中挥发性有机物(VOCs)的气相色谱/质谱(GC/MS)分析法。
5.生物监测方法-EPA方法1605:用于大肠杆菌和菌落总数的微生物分析方法。
-EPA方法821-R-02-012:基于鱼类激素和特征蛋白的鱼类暴露评估方法。
-EPA方法821-R-02-013:用于粪臭强度测量的无尘试纸法。
需要注意的是,这只是EPA方法索引中的一小部分,并且每个方法都有详细的操作规程和分析步骤。
研究人员和环境监测单位可以根据需要,选择合适的方法来进行各种环境样品的分析和监测工作,以确保结果的准确性和可比性。
EPA方法索引

EPA方法索引EPA(Environmental Protection Agency,环境保护局)是美国联邦政府机构,负责制定环境保护政策和监督执行,旨在保护人类健康和自然环境。
EPA通过开发和更新一系列的方法和准则来评估和监测环境中的各种污染物。
以下是EPA方法的索引,其中包含了一些常用的方法。
1.水质分析方法:-EPA方法6010:使用电感耦合等离子体质谱仪对水样中的重金属进行测定。
-EPA方法160.2:测定饮用水中总溶解性氟化物的浓度。
-EPA方法200.7:使用火焰原子吸收光谱法测定水样中的金属。
-EPA方法365.2:测定地下水中40种有机化合物的浓度。
2.大气质量监测方法:-EPA方法305:测定大气中颗粒物(PM10)的质量浓度。
-EPA方法1664:对水和底泥中的油脂进行提取和测定。
-EPA方法321.8:通过气浓度梯度法测定大气中的苯系化合物。
-EPA方法327:使用红外光谱法测定大气中的多环芳烃。
3.土壤和底泥分析方法:-EPA方法3540:对土壤和底泥中的有机物进行提取。
-EPA方法8000:使用气相色谱质谱法分析土壤和底泥中的挥发性有机化合物。
-EPA方法3051:测定土壤样品中重金属的浓度。
-EPA方法8240:使用气相色谱质谱法分析土壤和底泥中的半挥发性有机化合物。
4.垃圾和固体废物分析方法:-EPA方法8015:使用气相色谱质谱法分析固体废物中的多环芳烃。
-EPA方法8082:使用气相色谱质谱法分析土壤、底泥和固体废物中的戴奥辛和类似化合物。
-EPA方法8260:使用气相色谱质谱法分析固体废物中的挥发性有机化合物。
-EPA方法8280:使用气相色谱质谱法分析固体废物中的多氯联苯。
5.生物监测方法:-EPA方法1600:测定饮用水和海水中的大肠杆菌和肠球菌数量。
-EPA方法1613:使用液相色谱质谱法测定鱼类组织中的多氯联苯和多溴联苯醚。
-EPA方法2050:测定水和生物体中蓝绿藻的数量和类群组成。
EPA方法索引

EPA方法索引根据您的要求,以下是EPA(美国环保局)使用的一些常见的方法索引。
这些方法涵盖了环境监测、风险评估、废物管理、空气质量评估和水质评估等各个领域。
请注意,这只是一个简要的索引,详细的方法描述和操作程序可以在EPA的官方网站上找到。
1.环境监测方法:-EPA方法1:样品获取和保留方法-EPA方法2:采样口和尾气采集系统评估方法-EPA方法3:抽样方法-EPA方法4:大气沉降物的抽样和分析方法-EPA方法5:大气礁石沉积物中颗粒物的采样和分析方法-EPA方法6:大气颗粒物的测定方法-EPA方法7:废气流中氮氧化物的测定方法-EPA方法8:高温、高湿废气流中苯/甲苯浓度的测定方法2.风险评估方法:-EPA方法9:风险评估基础指南-EPA方法10:风险评估的质量保证3.废物管理方法:-EPA方法11:可回收物品处理-EPA方法12:生物治理/垃圾填埋申请-EPA方法13:废物水处理系统操作4.空气质量评估方法:-EPA方法14:大气污染源排放计算-EPA方法15:大气质量模型基础指南-EPA方法16:大气氨浓度的测定方法-EPA方法17:大气细颗粒物的测定方法-EPA方法18:大气湿沉降物的收集和分析方法5.水质评估方法:-EPA方法19:水质评估基础指南-EPA方法20:废水处理工艺-EPA方法21:水样处理和分析方法-EPA方法22:饮用水质量监测这些方法索引只是EPA使用的一小部分方法。
EPA还有其他方法用于地下水监测、土壤污染评估、生物毒性评估和生态风险评估等。
为了确保准确性和合规性,使用这些方法时应仔细阅读相关的方法说明和操作程序,以确保正确的实施和数据采集。
美国环保局 EPA 试验 方法 3500b
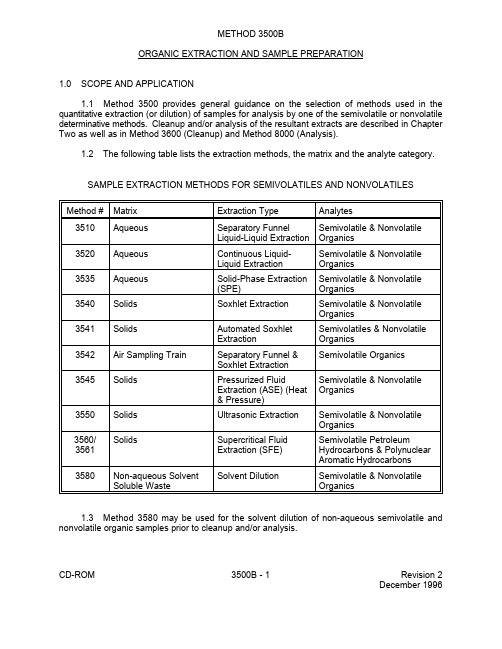
METHOD 3500BORGANIC EXTRACTION AND SAMPLE PREPARATION1.0SCOPE AND APPLICATION1.1Method 3500 provides general guidance on the selection of methods used in the quantitative extraction (or dilution) of samples for analysis by one of the semivolatile or nonvolatile determinative methods. Cleanup and/or analysis of the resultant extracts are described in Chapter Two as well as in Method 3600 (Cleanup) and Method 8000 (Analysis).1.2The following table lists the extraction methods, the matrix and the analyte category.SAMPLE EXTRACTION METHODS FOR SEMIVOLATILES AND NONVOLATILESMethod #Matrix Extraction Type Analytes3510Aqueous Separatory Funnel Semivolatile & NonvolatileLiquid-Liquid Extraction Organics3520Aqueous Continuous Liquid-Semivolatile & NonvolatileLiquid Extraction Organics3535Aqueous Solid-Phase Extraction Semivolatile & Nonvolatile(SPE)Organics3540Solids Soxhlet Extraction Semivolatile & NonvolatileOrganics3541Solids Automated Soxhlet Semivolatiles & NonvolatileExtraction Organics3542Air Sampling Train Separatory Funnel &Semivolatile OrganicsSoxhlet Extraction3545Solids Pressurized Fluid Semivolatile & NonvolatileExtraction (ASE) (Heat Organics& Pressure)3550Solids Ultrasonic Extraction Semivolatile & NonvolatileOrganics3560/Solids Supercritical Fluid Semivolatile Petroleum3561Extraction (SFE)Hydrocarbons & PolynuclearAromatic Hydrocarbons 3580Non-aqueous Solvent Solvent Dilution Semivolatile & Nonvolatile Soluble Waste Organics1.3Method 3580 may be used for the solvent dilution of non-aqueous semivolatile and nonvolatile organic samples prior to cleanup and/or analysis.CD-ROM3500B - 1Revision 2December 19961.4Methods 3545, 3560, and 3561 are techniques that utilize pressurized solvent extraction to reduce the amount of solvent needed to extract target analytes and reduce the extraction time when compared to more traditional techniques such as Soxhlet extraction.1.5Prior to employing this method, analysts are advised to consult the disclaimer statement at the front of the manual and the information in Chapter Two for guidance on the allowed flexibility in the choice of apparatus, reagents, and supplies. In addition, unless specified in a regulation, the use of SW-846 methods is not mandatory in response to Federal testing requirements. The information contained in this procedure is provided by EPA as guidance to be used by the analyst and the regulated community in making judgments necessary to meet the data quality objectives or needs for the intended use of the data.2.0SUMMARY OF METHOD2.1 A sample of a known volume or weight is extracted with solvent or diluted with solvent. Method choices for aqueous samples include liquid-liquid extraction by separatory funnel or by continuous extractor and solid-phase extraction (SPE). Method choices for soil/sediment and solid waste samples include standard solvent extraction methods utilizing either Soxhlet, automated Soxhlet, or ultrasonic extraction. Solids may also be extracted using pressurized extraction techniques such as supercritical fluid extraction or heated pressurized fluid extraction.2.2The resultant extract is dried and concentrated in a Kuderna-Danish (K-D) apparatus. Other concentration devices or techniques may be used in place of the Kuderna-Danish concentrator if the quality control requirements of the determinative methods are met (Method 8000, Sec. 8.0).NOTE:Solvent recovery apparatus is recommended for use in methods that require the use of Kuderna-Danish evaporative concentrators. EPA recommends theincorporation of this type of reclamation system as a method to implement anemissions reduction program.2.3See Sec. 7.0 for additional guidance to assist in selection of the appropriate method.3.0INTERFERENCES3.1Solvents, reagents, glassware, and other sample processing hardware may yield artifacts and/or interferences to sample analysis. All these materials must be demonstrated to be free from interferences under the conditions of the analysis by analyzing method blanks. Specific selection of reagents and purification of solvents by distillation in all-glass systems may be necessary. Refer to each method for specific guidance on quality control procedures and to Chapter Four for guidance on the cleaning of glassware.3.2Interferences coextracted from the samples will vary considerably from source to source. If analysis of an extracted sample is prevented due to interferences, further cleanup of the sample extract may be necessary. Refer to Method 3600 for guidance on cleanup procedures.3.3Phthalate esters contaminate many types of products commonly found in the laboratory. Plastics, in particular, must be avoided because phthalates are commonly used as plasticizers and are easily extracted from plastic materials. Serious phthalate contamination may result at any time if consistent quality control is not practiced.CD-ROM3500B - 2Revision 2December 19963.4Soap residue (e.g. sodium dodecyl sulfate), which results in a basic pH on glassware surfaces, may cause degradation of certain analytes. Specifically, Aldrin, Heptachlor, and most organophosphorus pesticides will degrade in this situation. This problem is especially pronounced with glassware that may be difficult to rinse (e.g., 500-mL K-D flask). These items should be hand-rinsed very carefully to avoid this problem.4.0APPARATUS AND MATERIALS4.1Refer to the specific method of interest for a description of the apparatus and materials needed.4.2Solvent recovery apparatus is recommended for use in methods that require the use of Kuderna-Danish evaporative concentrators. Incorporation of this apparatus may be required by State or local municipality regulations that govern air emissions of volatile organics. EPA recommends the incorporation of this type of reclamation system as a method to implement an emissions reduction program. Solvent recovery is a means to conform with waste minimization and pollution prevention initiatives.5.0REAGENTS5.1Refer to the specific method of interest for a description of the solvents needed.5.2Organic-free reagent water. All references to water in this method refer to organic-free reagent water as defined in Chapter One.5.3Stock standards for spiking solutions - Stock solutions may be prepared from pure standard materials or purchased as certified solutions. The stock solutions used for the calibration standards are acceptable (dilutions must be made in a water miscible solvent) except for the quality control check sample stock concentrate which must be prepared independently to serve as a check on the accuracy of the calibration solution.5.3.1Prepare stock standard solutions by accurately weighing about 0.0100 g of purecompound. Dissolve the compound in a water miscible solvent (i.e., methanol, acetone, 2-propanol, etc.) and dilute to volume in a 10-mL volumetric flask. If compound purity is 96 percent or greater, the weight can be used without correction to calculate the concentration of the stock standard solution. Commercially-prepared stock standard solutions can be used at any concentration if they are certified by the manufacturer or by an independent source.5.3.2Stock standard solutions should be stored in polytetrafluoroethylene(PTFE)-sealed containers at 4E C or below. The solutions should be checked frequently for stability. Refer to the determinative method for holding times of the stock solutions.5.4Surrogate standards - A surrogate (i.e., a compound that is chemically similar to the analyte group but is not expected to occur in an environmental sample) should be added to each sample, blank, laboratory control sample (LCS), and matrix spike sample just prior to extraction or processing. The recovery of the surrogate standard is used to monitor for unusual matrix effects, gross sample processing errors, etc. Surrogate recovery is evaluated for acceptance by determining whether the measured concentration falls within the acceptance limits.CD-ROM3500B - 3Revision 2December 19965.4.1Recommended surrogates for certain analyte groups are listed in Table 1. Formethods where no recommended surrogates are listed, the lab is free to select compounds that fall within the definition provided above. Even compounds that are on the method target analyte list may be used as a surrogate as long as historical data are available to ensure their absence at a given site. Normally one or more standards are added for each analyte group.5.4.2Prepare a surrogate spiking concentrate by mixing stock standards preparedabove and diluting with a water miscible solvent. Commercially prepared spiking solutions are acceptable. The concentration for semivolatile/nonvolatile organic and pesticide analyses should be such that a 1-mL aliquot into 1000 mL of a sample provides a concentration of 10 times the quantitation limit or near the mid-point of the calibration curve. Where volumes of less than 1000 mL are extracted, adjust the volume of surrogate standard proportionately. For matrices other than water, 1 mL of surrogate standard is still the normal spiking volume.However, if gel permeation chromatography will be used for sample cleanup, 2 mL should be added to the sample. See Table 1 for recommended surrogates. The spiking volumes are normally listed in each extraction method. Where concentrations are not listed in a method,a concentration of 10 times the quantitation limit is recommended. If the surrogate quantitationlimit is unknown, the average quantitation limit of method target analytes may be utilized to estimate a surrogate quantitation limit. As necessary or appropriate to meet project objectives, the surrogates listed in Table 1 may be modified by the laboratory. The concentration of the surrogate in the sample (or sample extract) should either be near the middle of the calibration range or approximately ten times the quantitation limit.5.5Matrix spike standards - The following are recommended matrix spike standard mixtures for a few analyte groups. Prepare a matrix spike concentrate by mixing stock standards prepared above and diluting with a water miscible solvent. Commercially-prepared spiking solutions are acceptable. The matrix spike standards should be independent of the calibration standard. A few methods provide guidance on concentrations and the selection of compounds for matrix spikes (see Table 2).5.5.1Base/neutral and acid matrix spiking solution - Prepare a spiking solution inmethanol that contains each of the following base/neutral compounds at 100 mg/L and the acid compounds at 200 mg/L for water and sediment/soil samples. The concentration of these compounds should be five times higher for waste samples.Base/neutrals Acids1,2,4-Trichlorobenzene PentachlorophenolAcenaphthene Phenol2,4-Dinitrotoluene2-ChlorophenolPyrene4-Chloro-3-methylphenolN-Nitroso-di-n-propylamine4-Nitrophenol1,4-Dichlorobenzene5.5.2Organochlorine pesticide matrix spiking solution - Prepare a spiking solution inacetone or methanol that contains the following pesticides in the concentrations listed for water and sediment/soil. The concentration should be five times higher for waste samples.CD-ROM3500B - 4Revision 2December 1996Pesticide Concentration (mg/L)Lindane0.2Heptachlor0.2Aldrin0.2Dieldrin0.5Endrin0.54,4'-DDT0.55.5.3For methods with no guidance, select five or more analytes (select all analytesfor methods with five or less) from each analyte group for use in a spiking solution. Where matrix spike concentrations in the sample are not listed it should be at or below the regulatory concentration or action level, or 1 to 5 times higher than the background concentration, whichever, concentration would be larger.5.5.4Sec. 8.3.3 provides guidance on determining the concentration of the matrix spikecompounds in the sample. As necessary or appropriate to meet project objectives, the matrix spiking compounds listed in Secs. 5.5.1, 5.5.2, and/or the concentrations listed in the spiking solutions may be modified by the laboratory. When the concentration of an analyte is not being checked against a regulatory limit or action level (see Sec. 8.3.3.3) the concentration of the matrix spike compound in the sample (or sample extract) should be near the middle of the calibration range or approximately ten times the quantitation limit.5.6Laboratory control spike standard - Use the matrix spike standard prepared in Sec. 5.5 as the spike standard for the laboratory control sample (LCS). The LCS should be spiked at the same concentration as the matrix spike.6.0SAMPLE COLLECTION, PRESERVATION, AND HANDLINGSee Chapters Two and Four for guidance on sample collection.7.0PROCEDURE7.1Water, soil/sediment, sludge, and waste samples requiring analysis for semivolatile and nonvolatile organic compounds (within this broad category are special subsets of analytes, i.e., the different groups of pesticides, explosives, PCBs etc.), must undergo solvent extraction prior to analysis. This manual contains method choices that are dependent on the matrix, the physical properties of the analytes, the sophistication and cost of equipment available to a given laboratory, and the turn-around time required for sample preparation.7.1.1The laboratory should be responsible for ensuring that the method chosen forsample extraction will provide acceptable extraction efficiency for the target analytes in a given matrix. There are several approaches that may be employed to ensure the appropriateness of the extraction method.7.1.1.1Prior to employing any extraction procedure on samples submitted forregulatory compliance monitoring purposes, the laboratory should complete the initialdemonstration of proficiency described in Sec. 8.2. This demonstration applies to allSW-846 extraction methods, including those for which specific performance data areprovided in a determinative method.CD-ROM3500B - 5Revision 2December 19967.1.1.2In addition, when a new or different extraction technique is to be appliedto samples, the laboratory should also demonstrate that their application of the techniqueprovides acceptable performance in the matrix of interest for the analytes of interest.One approach to demonstrating extraction method performance is to make a directcomparison between the chosen method and either Method 3520 (continuous liquid-liquidextraction of aqueous samples) or Method 3540 (Soxhlet extraction of solid samples),as these methods have the broadest applicability to environmental matrices.When direct comparisons are performed, they should be conducted using either standardreference materials derived from real-world matrices or samples from a given site thatcan be reasonably expected to contain the analytes of interest. Because of concerns withthe incorporation of spiking materials into samples, the use of samples spiked by thelaboratory is generally a less useful comparison relative to either real-world contaminatedsamples or standard reference materials, and thus should generally only be employedwhen neither of these latter materials are available. Analyze at least four portions of awell homogenized sample by the extraction method of interest and either Method 3520or Method 3540, depending on the matrix.7.1.1.3When direct comparisons between methods are conducted, thelaboratory may use statistical tests such as an F-test to determine if the results arecomparable between the methods. The laboratory may employ the method of interestprovided that the demonstrated performance can be shown to be either as good or betterthan that of the "reference" method, or adequate for project needs, that is, meeting therequirements of the QA Project Plan for a specific project.7.1.1.4Whatever approaches are taken to ensure the adequacy of theextraction procedure for the matrix of interest, it is the responsibility of the laboratory todocument the results and maintain records of such demonstrations.7.1.2Each method has QC requirements that normally include the addition ofsurrogates to each analytical sample and QC sample as well as the inclusion of a matrix spike/matrix spike duplicate (or matrix spike and duplicate sample), a laboratory control sample, and a method blank in each sample extraction batch. As defined in Chapter One, a "batch" consists of up to 20 environmental samples processed as a unit. In the case of samples that must undergo extraction prior to analysis, each group of 20 samples extracted together by the same method constitutes an extraction batch.The decision of whether to prepare and analyze a matrix spike/matrix spike duplicate pair ora matrix spike and a duplicate sample should be based on knowledge of the samples in theextraction batch. If the samples are expected to contain the analytes of interest, then the analysis of a duplicate sample may yield data on the precision of the analytical process and the analysis of the matrix spike will yield data on the accuracy of the process. In contrast, when the samples are not known or expected to contain the analytes of interest, then the batch should include a matrix spike/matrix spike duplicate pair to ensure that both accuracy and precision data will be generated within the extraction batch.7.2Method 3510 - Applicable to the extraction and concentration of water-insoluble and slightly water-soluble organics from aqueous samples. A measured volume of sample is solvent extracted using a separatory funnel. The extract is dried, concentrated and, if necessary, exchanged into a solvent compatible with further analysis. Separatory funnel extraction utilizes relatively inexpensive glassware and is fairly rapid (three, 2-minute extractions followed by filtration) but is labor intensive, uses fairly large volumes of solvent and is subject to emulsion problems. Method CD-ROM3500B - 6Revision 2December 19963520 should be used if an emulsion forms between the solvent-sample phases, which cannot be broken by mechanical techniques.7.3Method 3520 - Applicable to the extraction and concentration of water-insoluble and slightly water-soluble organics from aqueous samples. A measured volume of sample is extracted with an organic solvent in a continuous liquid-liquid extractor. The solvent must have a density greater than that of the sample. The extract is dried, concentrated and, if necessary, exchanged into a solvent compatible with further analysis. Continuous extractors are excellent for samples with particulates (of up to 1% solids) that cause emulsions, provide more efficient extraction of analytes that are more difficult to extract and once loaded, require no hands-on manipulation. However, they require more expensive glassware, use fairly large volumes of solvent and extraction time is rather lengthy (6 to 24 hours).7.4Method 3535 - Applicable to the extraction and concentration of water-insoluble and slightly water-soluble organics from aqueous samples. A measured volume of water is pumped through an appropriate medium (e.g., disk or cartridge) containing a solid phase that effects the extraction of organics from water. A small volume of extraction solvent is passed through the medium to elute the compounds of interest. The eluant is dried, concentrated and, if necessary, exchanged into a solvent compatible with further analysis. Appropriate solid-phase extraction media allow extraction of water containing particulates, are relatively fast and use small volumes of solvent. However, they do require some specialized pieces of equipment.7.5Method 3540 - This method is applicable to the extraction of nonvolatile and semivolatile organic compounds from solids such as soils, relatively dry sludges, and solid wastes. A solid sample is mixed with anhydrous sodium sulfate, placed into an extraction thimble or between two plugs of glass wool, and extracted using an appropriate solvent in a Soxhlet extractor. The extract is concentrated and, if necessary, exchanged into a solvent compatible with further analysis. Soxhlet extraction uses relatively inexpensive glassware, once loaded requires no hands-on manipulation, provides efficient extraction, but is rather lengthy (16 to 24 hours) and uses fairly large volumes of solvent. It is considered a rugged extraction method because there are very few variables that can adversely affect extraction efficiency.7.6Method 3541 - This method utilizes a modified Soxhlet extractor and is applicable to the extraction of semivolatile/nonvolatile organic compounds from solids such as soils, relatively dry sludges, and solid wastes. A solid sample is mixed with anhydrous sodium sulfate, placed into an extraction thimble or between two plugs of glass wool, and extracted using an appropriate solvent in an automated Soxhlet extractor. This device allows the extraction thimble to be lowered into the boiling liquid for the first hour and then extracted in the normal thimble position for one additional hour. The automated Soxhlet allows equivalent extraction efficiency in 2 hours, combines the concentration step within the same device but requires a rather expensive device.7.7Method 3542 - This method is applicable to the extraction of semivolatile organic compounds from the Method 0010 air sampling train. The solid trapping material (i.e., glass or quartz fiber filter and porous polymeric adsorbent resin) are extracted using Soxhlet extraction and the condensate and impinger fluid are extracted using separatory funnel extraction.7.8Method 3545 - This method is applicable to the extraction of nonvolatile/semivolatile organic compounds from solids such as soils, relatively dry sludges, and solid wastes. A solid sample is mixed with anhydrous sodium sulfate, placed into an extraction cell and extracted under pressure with small volumes of solvent. The extract is concentrated and, if necessary, exchanged into a solvent compatible with further analysis. The method is rapid and efficient, in that it uses small volumes of solvent, but does require the use of an expensive extraction device.CD-ROM3500B - 7Revision 2December 1996CD-ROM 3500B - 8Revision 2December 19967.9Method 3550 - This method is applicable to the extraction of nonvolatile and semivolatile organic compounds from solids such as soils, sludges, and wastes using the technique of ultrasonic extraction. Two procedures are detailed depending upon the expected concentration of organics in the sample; a low concentration and a high concentration method. In both, a known weight of sample is mixed with anhydrous sodium sulfate and solvent extracted using ultrasonic extraction.The extract is dried, concentrated and, if necessary, exchanged into a solvent compatible with further analysis. Ultrasonic extraction is fairly rapid (three, 3-minute extractions followed by filtration) but uses relatively large volumes of solvent, requires a somewhat expensive device and requires following the details of the method very closely to achieve acceptable extraction efficiency (proper tuning of the ultrasonic device is very critical). This technique is much less efficient than the other extraction techniques described in this section. This is most evident with very non-polar organic compounds (e.g., PCBs, etc.) that are normally strongly adsorbed to the soil matrix. EPA has not validated Method 3550 for the extraction of organophosphorus compounds from solid matrices. In addition, there are concerns that the ultrasonic energy may lead to breakdown of some organophosphorus compounds (see Reference 1). As a result, this extraction technique should not be used for organophosphorous compounds without extensive validation on real-world samples.Such studies should assess the precision, accuracy, ruggedness, and sensitivity of the technique relative to the appropriate regulatory limits or project-specific concentrations of interest.7.10Methods 3560 and 3561 - These methods are applicable to the extraction of total recoverable petroleum hydrocarbons and PAHs from solids such as soils, sludges, and wastes using the technique of supercritical fluid extraction (SFE). SFE normally uses CO (which may contain very 2small volumes of solvent modifiers). Therefore, there is no solvent waste for disposal, may be automated, provides relatively rapid extraction, but, is currently limited to total recoverable petroleum hydrocarbons and PAHs. It also requires a rather expensive device and sample size is more limited.Research on SFE is currently focusing on optimizing supercritical fluid conditions to allow efficient extraction of a broader range of RCRA analytes in a broad range of environmental matrices.7.11Method 3580 - This method describes the technique of solvent dilution of non-aqueous waste samples. It is designed for wastes that may contain organic chemicals at a level greater than 20,000 mg/kg and that are soluble in the dilution solvent. When using this method, the analyst must use caution in the addition of surrogate compounds, so as not to dilute out the surrogate response when diluting the sample.7.12Sample analysis - Following preparation of a sample by one of the methods described above, the sample is ready for further analysis. Samples prepared for semivolatile/nonvolatile analysis may, if necessary, undergo cleanup (See Method 3600) prior to application of a specific determinative method.8.0QUALITY CONTROL8.1Refer to Chapter One for specific guidance on quality control procedures. Each laboratory using SW-846 methods should maintain a formal quality assurance program. Each extraction batch of 20 or less samples should contain: a method blank; either a matrix spike/matrix spike duplicate or a matrix spike and duplicate samples; and a laboratory control sample, unless the determinative method provides other guidance.8.2Initial Demonstration of Proficiency - Each laboratory must demonstrate initial proficiency with each sample preparation and determinative method combination it utilizes, by generating data of acceptable accuracy and precision for target analytes in a clean reference matrix. This will include a combination of the sample extraction method (usually a 3500 series method for extractableorganics) and the determinative method (an 8000 series method). The laboratory should also repeat the following operations whenever new staff are trained or significant changes in instrumentation are made.8.2.1The reference samples are prepared from a spiking solution containing eachanalyte of interest. The reference sample concentrate (spiking solution) may be prepared from pure standard materials, or purchased as certified solutions. If prepared by the laboratory, the reference sample concentrate should be made using stock standards prepared independently from those used for calibration.8.2.2The procedure for preparation of the reference sample concentrate is dependentupon the method being evaluated. Guidance for reference sample concentrations for certain methods are listed below. In other cases, the determinative methods contain guidance on preparing the reference sample concentrate and the reference sample. If no guidance is provided, prepare a reference sample concentrate in methanol (or other water miscible solvent). Spike the reference sample at the concentration on which the method performance data are based. The spiking volume added to water should not exceed 1 mL/L so that the spiking solvent will not decrease extraction efficiency. If the method lacks performance data, prepare a reference standard concentrate at such a concentration that the spike will providea concentration in the clean matrix that is 10 - 50 times the MDL for each analyte in that matrix.The concentration of target analytes in the reference sample may be adjusted to more accurately reflect the concentrations that will be analyzed by the laboratory. If the concentration of an analyte is being evaluated relative to a regulatory limit or action level, see Sec. 8.3.1 for information on selecting an appropriate spiking level.8.2.3To evaluate the performance of the total analytical process, the referencesamples must be handled in exactly the same manner as actual samples. Therefore, 1 mL (unless the method specifies a different volume) of the reference sample concentrate is spiked into each of four (minimum number of replicates) 1-L aliquots of organic-free reagent water (now called the reference sample), extracted as per the method. For matrices other than water or for determinative methods that specify a different volume of water, add 1.0 mL of the reference sample concentrate to at least four replicates of the volume or weight of sample specified in the method. Use a clean matrix for spiking purposes (one that does not have any target or interference compounds) e.g., organic-free reagent water for the water matrix or sand or soil (free of organic interferences) for the solid matrix.8.2.4Preparation of reference samplesThe following sections provide guidance on the QC reference sample concentrates for many SW-846 determinative methods. The concentration of the target analytes in the QC reference sample for the methods listed below may need to be adjusted to more accurately reflect the concentrations of interest in different samples or projects. If the concentration of an analyte is being evaluated relative to a regulatory limit or action level, see Sec. 8.3.3 for information on selecting an appropriate spiking level. In addition, the analyst may vary the concentration of the spiking solution and the volume of solution spiked into the sample.However, because of concerns about the effects of the spiking solution solvent on the sample, the total volume spiked into a sample should generally be held to no more than 1 mL.8.2.4.1Method 8041 - Phenols: The QC reference sample concentrate shouldcontain each analyte at 100 mg/L in 2-propanol.CD-ROM3500B - 9Revision 2December 1996。
usepa5030c(2003)

主题:USEPA5030C(2003)分析方法及其应用1. 简介USEPA5030C(2003)是美国环保局(USEPA)发布的一项环境分析方法,该方法用于土壤、沉积物和固体样品中重金属和其他有机化合物的分析。
该方法对环境保护和监测具有重要意义,也在环境科学研究和工程实践中得到广泛应用。
2. 方法原理USEPA5030C(2003)方法主要是通过溶剂提取和分析仪器测定来获取样品中重金属和有机化合物的含量。
该方法主要包括以下步骤:(1)样品的制备:将样品研磨、干燥并粉碎,以获得均匀的样品。
(2)溶剂提取:使用适当的溶剂将目标化合物从样品中提取出来。
(3)仪器分析:使用分析仪器(如气相色谱-质谱联用仪器、液相色谱-质谱联用仪器等)测定样品中目标化合物的含量。
3. 应用领域USEPA5030C(2003)方法可广泛应用于环境监测、土壤污染评价、固体废物处理等领域。
常见的应用包括:(1)环境监测:对大气沉降物、水体底泥等环境样品中的污染物进行监测和分析。
(2)土壤污染评价:评估土壤中重金属和有机污染物的程度,为土壤修复和保护提供依据。
(3)固体废物处理:对固体废物中的有害物质进行分析,指导固体废物的安全处置和处理。
4. 方法优势USEPA5030C(2003)方法具有以下优势:(1)全面性:能够有效检测多种重金属和有机化合物。
(2)灵敏度高:能够对样品中微量的污染物进行准确测定。
(3)操作简便:样品制备和分析步骤相对简单,操作方便。
5. 方法发展与应用现状随着环境监测和污染防治的需求不断增长,USEPA5030C(2003)方法得到了不断的改进和应用扩展。
在国际上,许多国家和地区也采用了该方法进行环境分析。
在我国,该方法也得到了广泛应用,并已成为环境保护部门和科研机构的标准分析方法之一。
6. 结语USEPA5030C(2003)方法作为一种重要的环境分析方法,对于环境保护和监测具有重要的意义。
随着环境污染问题日益突出,该方法的应用将在未来得到进一步的拓展和深化,为保护地球环境作出更大的贡献。
EPA3540C方法验证

微波萃取-氣相色譜/質譜聯用儀檢測塑膠材料中的PBBs或PBDEs1 采用方法:US EPA3540C-20002 試劑和儀器設備2.1 試劑2.1.1丙酮(AR),正己烷(AR) ,甲醇(AR)。
2.1.2 PBB&PBDE標準溶液2.1.3 萃取液(1:1丙酮:正己烷)2.2 儀器設備2.2.1分析天平(精確至0.0001g)2.2.2 過濾針筒(濾膜0.45um)2.2.3 索氏萃取系統2.2.4 氣相色譜/質譜聯用儀(GC-MS)3前處理:3.1用剪刀將樣品盡可能地剪碎至約1mm*1mm*1mm大小,稱取1.000g樣品進行萃取,並平行做8次。
3.2 將樣品依據《3540C作業規范》進行萃取操作。
4方法曲線配制1.0mg/l, 3mg/l, 5.0mg/l的10-PBDE標准溶液建立曲線,方程為:10-PBDE : y =61412.8x, 相關係數γ為0.9959。
5方法回收率稱取1.0000g PBT塑膠樣品(SY7100501)加入1ml25mg/l的標准溶液按3要求前處理後檢測,其結果如下:回收率=(加標測量值-樣品測量)/加標量6.0方法檢出限(mg/kg)因報出結果為總含量PBB(多溴聯苯)和PBDE(多溴聯苯醚),所以檢測限之核算取該類物質的最大標准偏差放大3倍再乘以種類數作為方法檢出限,即PBB檢出限為0.018×3×3= 0.17mg/kg , PBDE檢出限為0.007×3×4=0.08mg/kg。
為確保結果更加安全,本中心檢測限暫定為5mg/kg,以該濃度除以數量較多的聯苯醚種類(4種)進行驗證(5mg/kg÷4=1.25mg/kg),取1mg/kg濃度進行驗證,其結果如下:標准偏差:0.11;平均值為:1.033;回收率:103.3%;相對標准偏差10.6%標准偏差:0.079;平均值:0.993;回收率:99.3%;相對標准偏差7.9%標准偏差0.091;平均值0.965;回收率96.5%:相對標准偏差9.43%標准偏差0.13;平均值0.985;回收率98.5%:相對標准偏差13.2%標准偏差0.155;平均值0.989;回收率98.9%:相對標准偏差15.7%標准偏差0.096;平均值1.018;回收率101.8%:相對標准偏差9.4%標准偏差0.127;平均值0.957;回收率95.7%:相對標准偏差13.3%7.結論由以上統計分析結果得知,本中心采用US EPA3540C方法前處理,GC/MS檢測塑膠材料中的多溴聯苯和多溴聯苯醚,回收率符合標准70~130%的要求;檢出限驗證RSD<20%(符合US EPA8000中8.3.1的要求),方法可行。
美国环保局 EPA 试验 方法 EPA 3550c

METHOD 3550CULTRASONIC EXTRACTIONSW-846 is not intended to be an analytical training manual. Therefore, method procedures are written based on the assumption that they will be performed by analysts who are formally trained in at least the basic principles of chemical analysis and in the use of the subject technology.In addition, SW-846 methods, with the exception of required method use for the analysis of method-defined parameters, are intended to be guidance methods which contain general information on how to perform an analytical procedure or technique which a laboratory can use as a basic starting point for generating its own detailed Standard Operating Procedure (SOP), either for its own general use or for a specific project application. The performance data included in this method are for guidance purposes only, and are not intended to be and must not be used as absolute QC acceptance criteria for purposes of laboratory accreditation.1.0SCOPE AND APPLICATION1.1This method describes a procedure for extracting nonvolatile and semivolatile organic compounds from solids such as soils, sludges, and wastes. The ultrasonic process ensures intimate contact of the sample matrix with the extraction solvent.1.2This method is divided into two procedures, based on the expected concentration of organic compounds. The low concentration procedure (Sec. 11.3) is for individual organic components expected at less than or equal to 20 mg/kg and uses the larger sample size and three serial extractions (lower concentrations are more difficult to extract). The medium/high concentration procedure (Sec. 11.4) is for individual organic components expected at greater than 20 mg/kg and uses the smaller sample and a single extraction.1.3It is highly recommended that the extracts be subject to some form of cleanup(e.g., using a method from the 3600 series) prior to analysis.1.4Because of the limited contact time between the solvent and the sample, ultrasonic extraction may not be as rigorous as other extraction methods for soils/solids. Therefore, it is critical that the method (including the manufacturer's instructions) be followed explicitly, in order to achieve the maximum extraction efficiency. See Sec. 11.0 for a discussion of the critical aspects of the extraction procedure. Consult the manufacturer's instructions regarding specific operational settings.1.5This method describes at least three extraction solvent systems that may be employed for different groups of analytes (see Sec. 7.4). Other solvent systems may be employed, provided that adequate performance can be demonstrated for the analytes of interest. The choice of extraction solvent will depend on the analytes of interest and no single solvent is universally applicable to all analyte groups. As a result of concerns about the efficiency of ultrasonic extraction, particularly at concentrations near or below about 10 µg/kg, it is imperative that the analyst demonstrate the performance of the specific solvent system and operating conditions for the analytes of interest and the concentrations of interest. This demonstration applies to any solvent system that is employed, including those specifically listed in this method. At a minimum, such a demonstration will encompass the initial demonstration of proficiency described in Method 3500, using a clean reference matrix. Method 8000 describesprocedures that may be used to develop performance criteria for such demonstrations as well as for matrix spike and laboratory control sample results.1.6EPA notes that there are limited published data on the efficiency of ultrasonic extraction with regard to organophosphorus pesticides at low part-per-billion (ppb) concentrations and below. As a result, use of this method for these compounds in particular should be supported by performance data such as those discussed above and in Method 3500.1.7Prior to employing this method, analysts are advised to consult the base method for each type of procedure that may be employed in the overall analysis (e.g., Methods 3500, 3600, 5000, and 8000) for additional information on quality control procedures, development of QC acceptance criteria, calculations, and general guidance. Analysts also should consult the disclaimer statement at the front of the manual and the information in Chapter Two for guidance on the intended flexibility in the choice of methods, apparatus, materials, reagents, and supplies, and on the responsibilities of the analyst for demonstrating that the techniques employed are appropriate for the analytes of interest, in the matrix of interest, and at the levels of concern.In addition, analysts and data users are advised that, except where explicitly specified in a regulation, the use of SW-846 methods is not mandatory in response to Federal testing requirements. The information contained in this method is provided by EPA as guidance to be used by the analyst and the regulated community in making judgments necessary to generate results that meet the data quality objectives for the intended application.1.8Use of this method is restricted to use by, or under the supervision of, appropriately experienced and trained analysts. Each analyst must demonstrate the ability to generate acceptable results with this method. As noted above, such demonstrations are specific to the analytes of interest and the solvent system used, as well as to the procedures for low and medium/high concentration samples.2.0SUMMARY OF METHOD2.1Low concentration procedure -- The sample is mixed with anhydrous sodium sulfate to form a free-flowing powder. The mixture is extracted with solvent three times, using ultrasonic extraction. The extract is separated from the sample by vacuum filtration or centrifugation. The extract is ready for final concentration, cleanup, and/or analysis.2.2Medium/high concentration procedure -- The sample is mixed with anhydrous sodium sulfate to form a free-flowing powder. This is extracted with solvent once, using ultrasonic extraction. A portion of the extract is collected for cleanup and/or analysis.3.0DEFINITIONSRefer to Chapter One and the manufacturer's instructions for definitions that may be relevant to this method.4.0INTERFERENCES4.1Solvents, reagents, glassware, and other sample processing hardware may yield artifacts and/or interferences to sample analysis. All of these materials must be demonstrated to be free from interferences under the conditions of the analysis by analyzing method blanks.Specific selection of reagents and purification of solvents by distillation in all-glass systems may be necessary. Refer to each method to be used for specific guidance on quality control procedures and to Chapter Four for general guidance on the cleaning of glassware.4.2Interferences are usually specific to the analytes of interest. Therefore, refer to Method 3500 and the appropriate determinative methods for specific guidance on extraction interferences.5.0SAFETYThis method does not address all safety issues associated with its use. The laboratory is responsible for maintaining a safe work environment and a current awareness file of OSHA regulations regarding the safe handling of the chemicals listed in this method. A reference file of material safety data sheets (MSDSs) should be available to all personnel involved in these analyses.6.0EQUIPMENT AND SUPPLIESThe mention of trade names or commercial products in this manual is for illustrative purposes only, and does not constitute an EPA endorsement or exclusive recommendation for use. The products and instrument settings cited in SW-846 methods represent those products and settings used during method development or subsequently evaluated by the Agency. Glassware, reagents, supplies, equipment, and settings other than those listed in this manual may be employed provided that method performance appropriate for the intended application has been demonstrated and documented.This section does not list common laboratory glassware (e.g., beakers and flasks).6.1Apparatus for grinding dry waste samples.6.2Ultrasonic preparation -- A horn-type device equipped with a titanium tip, or a device that will give appropriate performance, must be used.6.2.1Ultrasonic disrupter -- The disrupter must have a minimum power wattageof 300 watts, with pulsing capability. A device designed to reduce the cavitation sound is recommended. Follow the manufacturers instructions for preparing the disrupter forextraction of samples with low and medium/high concentrations.6.2.2Use a 3/4-inch horn for the low concentration method procedure and a1/8-inch tapered microtip attached to a 1/2-inch horn for the medium/high concentration method procedure.6.3Sonabox -- Recommended with the above disrupters for decreasing cavitation sound (Heat Systems - Ultrasonics, Inc., Model 432B or equivalent).6.4Apparatus for determining percent dry weight6.4.1Drying oven -- Capable of maintaining 105 E C.6.4.2Desiccator.6.4.3Crucibles -- Porcelain or disposable aluminum.6.5Pasteur pipets -- 1-mL, glass, disposable.6.7Vacuum or pressure filtration apparatus6.7.1Buchner funnel6.7.2Filter paper -- Whatman No. 41 or equivalent.6.8Kuderna-Danish (K-D) apparatus6.8.1Concentrator tube -- 10-mL, graduated (Kontes K-570050-1025 orequivalent). A ground-glass stopper is used to prevent evaporation of extracts.6.8.2Evaporation flask -- 500-mL (Kontes K-570001-500 or equivalent). Attachthe flask to the concentrator tube with springs, clamps, or equivalent.6.8.3Snyder column -- Three-ball macro (Kontes K-503000-0121 orequivalent).6.8.4Snyder column -- Two-ball micro (Kontes K-569001-0219 or equivalent).6.8.5Springs -- 1/2-inch (Kontes K-662750 or equivalent).6.9Solvent vapor recovery system (Kontes K-545000-1006 or K-547300-0000, Ace Glass 6614-30, or equivalent).NOTE:This glassware is recommended for the purpose of solvent recovery during the concentration procedures requiring the use of Kuderna-Danish evaporativeconcentrators. Incorporation of this apparatus may be required by Federal, State orlocal municipality regulations that govern air emissions of volatile organics. EPArecommends the incorporation of this type of reclamation system as a method toimplement an emissions reduction program. Solvent recovery is a means to conformwith waste minimization and pollution prevention initiatives.6.10Boiling chips -- Solvent-extracted, approximately 10/40 mesh (silicon carbide or equivalent).6.11Water bath -- Heated, with a concentric ring cover, capable of temperature control to ± 5 E C. The bath should be used in a hood.6.12Balance -- Top-loading, capable of accurately weighing to the nearest 0.01 g.6.13Vials -- 2-mL, for GC autosampler, equipped with polytetrafluoroethylene (PTFE)-lined screw caps or crimp tops.6.14Glass scintillation vials -- 20-mL, equipped with PTFE-lined screw caps.6.15Spatula -- Stainless steel or PTFE.6.16Drying column -- 20-mm ID borosilicate glass chromatographic column with glass wool at the bottom.NOTE:Columns with fritted glass discs are difficult to decontaminate after they have been used to dry highly-contaminated extracts. Columns without frits may be purchased.Use a small pad of glass wool to retain the adsorbent. Prewash the glass wool padwith 50 mL of acetone followed by 50 mL of the elution solvent prior to packing thecolumn with adsorbent.6.17Nitrogen evaporation apparatus (optional) -- N-Evap, 12- or 24-position(Organomation Model 112, or equivalent).7.0REAGENTS AND STANDARDS7.1Reagent-grade chemicals must be used in all tests. Unless otherwise indicated, it is intended that all reagents conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit its use without lessening the accuracy of the determination. Reagents should be stored in glass to prevent the leaching of contaminants from plastic containers.7.2Organic-free reagent water. All references to water in this method refer to organic-free reagent water, as defined in Chapter One.7.3Sodium sulfate (granular, anhydrous), Na 2SO 4. Purify by heating at 400 E C for 4hrs in a shallow tray, or by precleaning the sodium sulfate with methylene chloride. If the sodium sulfate is precleaned with methylene chloride, a method blank should be analyzed,demonstrating that there is no interference from the sodium sulfate.7.4Extraction solventsSamples should be extracted using a solvent system that gives optimum, reproducible recovery of the analytes of interest from the sample matrix, at the concentrations of interest. The choice of extraction solvent will depend on the analytes of interest and no single solvent is universally applicable to all analyte groups. Whatever solvent system is employed, including those specifically listed in this method, the analyst must demonstrate adequate performance for the analytes of interest, at the levels of interest. At a minimum, such a demonstration will encompass the initial demonstration of proficiency described in Method 3500, using a cleanreference matrix. Method 8000 describes procedures that may be used to develop performance criteria for such demonstrations as well as for matrix spike and laboratory control sample results.Many of the solvent systems described below include the combination of a water-miscible solvent, such as acetone, and a water-immiscible solvent, such as methylene chloride orhexane. The purpose of the water-miscible solvent is to facilitate the extraction of wet solids by allowing the mixed solvent to penetrate the layer of water of the surface of the solid particles. The water-immiscible solvent extracts organic compounds with similar polarities. Thus, a non-polar solvent such as hexane is often used for non-polar analytes such as PCBs, while a polar solvent like methylene chloride may be used for polar analytes. The polarity of acetone may also help extract polar analytes in mixed solvent systems.Table 1 provides example recovery data for selected semivolatile organic compounds extracted from an NIST SRM using various extraction solvent systems. The following sections provide guidance on the choice of solvents for various classes of analytes.All solvents should be pesticide quality or equivalent. Solvents may be degassed prior to use.7.4.1Semivolatile organics may be extracted with acetone/hexane (1:1, v/vCH 3COCH 3/C 6H 14), or acetone/methylene chloride (1:1,v/v CH 3COCH 3/CH 2Cl 2).7.4.2Organochlorine pesticides may be extracted with acetone/hexane (1:1,v/v CH 3COCH 3/C 6H 14), or acetone/methylene chloride (1:1,v/v CH 3COCH 3/CH 2Cl 2).7.4.3PCBs may be extracted with acetone/hexane (1:1, v/v CH 3COCH 3/C 6H 14),acetone/methylene chloride (1:1, v/v CH 3COCH 3/CH 2Cl 2) or hexane (C 6H 14).7.4.4Other solvent systems may be employed, provided that the analyst candemonstrate adequate performance for the analytes of interest, at the concentrations of interest, in the sample matrix (see Method 3500).7.5Exchange solvents -- With the use of some determinative methods, the extraction solvent will need to be exchanged to a solvent compatible with the instrumentation used in that determinative method. Refer to the determinative method to be used for selection of theappropriate exchange solvent. All solvents must be pesticide quality or equivalent. Examples of exchange solvents are given below.7.5.1Hexane, C 6H 147.5.22-Propanol, (CH 3)2CHOH 7.5.3Cyclohexane, C 6H 127.5.4Acetonitrile, CH 3CN 7.5.5Methanol, CH 3OH 8.0SAMPLE COLLECTION, PRESERVATION, AND STORAGE8.1See the introductory material to Chapter Four, "Organic Analytes," Method 3500,and the specific determinative methods to be employed.8.2Solid samples to be extracted by this procedure should be collected and stored like any other solid samples containing semivolatile organics.9.0QUALITY CONTROL9.1Refer to Chapter One for additional guidance on quality assurance (QA) andquality control (QC) protocols. When inconsistencies exist between QC guidelines, method-specific QC criteria take precedence over both technique-specific criteria and those criteria given in Chapter One, and technique-specific QC criteria take precedence over the criteria in Chapter One. Any effort involving the collection of analytical data should include development of a structured and systematic planning document, such as a Quality Assurance Project Plan (QAPP) or a Sampling and Analysis Plan (SAP), which translates project objectives andspecifications into directions for those that will implement the project and assess the results. Each laboratory should maintain a formal quality assurance program. The laboratory should also maintain records to document the quality of the data generated. All data sheets and quality control data should be maintained for reference or inspection.9.2Initial demonstration of proficiencyEach laboratory must demonstrate initial proficiency with each sample preparation and determinative method combination it utilizes by generating data of acceptable accuracy and precision for target analytes in a clean matrix. The laboratory must also repeat the demonstration of proficiency whenever new staff members are trained or significant changes in instrumentation are made. See Method 8000 for information on how to accomplish a demonstration of proficiency.9.3Initially, before processing any samples, the analyst should demonstrate that all parts of the equipment in contact with the sample and reagents are interference-free. This is accomplished through the analysis of a method blank. As a continuing check, each time samples are extracted, cleaned up, and analyzed, and when there is a change in reagents, a method blank should be extracted and analyzed for the compounds of interest as a safeguard against chronic laboratory contamination.9.4Any method blanks, matrix spike samples, or replicate samples should be subjected to the same analytical procedures (Sec. 11.0) as those used on actual samples.9.5Standard quality assurance practices should be used with this method as included in appropriate systematic planning documents and laboratory SOPs. All instrument operating conditions should be recorded.9.6Also refer to Method 3500 for extraction and sample preparation quality control procedures and the determinative methods to be used for determinative QC procedures.9.7When listed in the appropriate determinative method, surrogate standards should be added to all samples prior to extraction. See Methods 3500 and 8000, and the appropriate determinative methods for more information.9.8As noted earlier, use of any extraction technique, including ultrasonic extraction, should be supported by data that demonstrate the performance of the specific solvent system and operating conditions for the analytes of interest, at the levels of interest, in the sample matrix.10.0CALIBRATION AND STANDARDIZATIONThere are no calibration or standardization steps directly associated with this sample extraction procedure.11.0PROCEDUREAs noted in Sec. 1.4, ultrasonic extraction may not be as rigorous a method as other extraction methods for soils/solids. Therefore, it is critical that this method be followed explicitly (including the manufacturer's instructions) to achieve the maximum extraction efficiency. At a minimum, for successful use of this technique:•The extraction device must have a minimum of 300 watts of power and be equipped with appropriate size disrupter horns (see Sec. 6.2).•The horn must be properly maintained, including tuning according to the manufacturer's instructions prior to use, and inspection of the horn tip for excessive wear.•The sample must be properly prepared by thoroughly mixing it with sodium sulfate, so that it forms a free-flowing powder prior to the addition of the solvent.•The extraction horns used for the low concentration and high concentration protocols (Secs. 11.3 and 11.4, respectively) are not interchangeable. Results indicate that the use of the 3/4-inch horn is inappropriate for the high concentration procedure, particularly for extraction of very non-polar organic compounds such as PCBs, which are stronglyadsorbed to the soil matrix.•For low concentration samples, three extractions are performed with the appropriate solvent, the extraction is performed in the designated pulse mode, and the horn tip ispositioned just below the surface of the solvent, yet above the sample. The sameapproach is used for high concentration samples, except that only one extraction may be needed.•Very active mixing of the sample and the solvent must occur when the ultrasonic pulse is activated. The analyst must observe such mixing at some point during the extractionprocess.11.1Sample handling11.1.1Sediment/soil samples -- Decant and discard any water layer on asediment sample. Discard any foreign objects such as sticks, leaves, and rocks. Mix the sample thoroughly, especially composited samples.11.1.2Waste samples -- Samples consisting of multiple phases must beprepared before extraction by the phase separation procedure described in Chapter Two.This extraction procedure is for solids only.11.1.3Dry waste samples amenable to grinding -- Grind or otherwise subdividethe waste so that it either passes through a 1-mm sieve or can be extruded through a 1-mm hole. Introduce sufficient sample into the grinding apparatus to yield at least 10 gafter grinding.CAUTION:Drying and grinding should be performed in a hood, to avoid contamination of the laboratory.11.1.4Gummy, fibrous, or oily materials not amenable to grinding -- Cut, shred,or otherwise reduce in size these materials to allow mixing and maximum exposure of the sample surfaces for the extraction.11.2Determination of percent dry weight -- When sample results are to be calculated ona dry weight basis, a separate portion of sample should be weighed out at the same time as the portion used for analytical determination.CAUTION:The drying oven should be contained in a hood or vented. Significant laboratory contamination may result from a heavily contaminated hazardous waste sample.Immediately after weighing the sample aliquot to be extracted, weigh an additional 5- to10-g aliquot of the sample into a tared crucible. Dry this aliquot overnight at 105 E C. Allow to cool in a desiccator before weighing.Calculate the percent dry weight as follows:%dry weight'g of dry sampleg of sample×100This oven-dried aliquot is not used for the extraction and should be appropriately disposed of once the dry weight is determined.11.3Low concentration extraction procedureThis procedure applies to solid samples that are expected to contain less than or equal to 20 mg/kg of organic analytes.NOTE:Add the surrogates and matrix spiking compounds to the sample aliquot prior to mixing the sample with the sodium sulfate drying agent. Spiking the sample first increasesthe contact time of the spiked compounds and the actual sample matrix. It should also lead to better mixing of the spiking solution with the sample when the sodium sulfateand sample are mixed to the point of free-flowing.11.3.1The following steps should be performed rapidly to avoid loss of the morevolatile extractables.11.3.1.1Weigh approximately 30 g of sample into a 400-mL beaker.Record the weight to the nearest 0.1 g.11.3.1.2For the sample in each batch selected for spiking, add 1.0 mLof the matrix spiking solution. Consult Method 3500 for guidance on theappropriate choice of matrix spiking compounds and concentrations. Also see thenote in Sec. 11.3.11.3.1.3Add 1.0 mL of the surrogate standard solution to all samples,spiked samples, QC samples, and blanks. Consult Method 3500 for guidance onthe appropriate choice of surrogate compounds and concentrations. Also see thenote in Sec. 11.3.11.3.1.4If gel permeation cleanup (see Method 3640) is to beemployed, the analyst should either add twice the volume of the surrogate spikingsolution (and matrix spiking solution, where applicable), or concentrate the finalextract to half the normal volume, to compensate for the half of the extract that islost due to loading of the GPC column. Also see the note in Sec. 11.3.11.3.1.5Nonporous or wet samples (gummy or clay type) that do nothave a free-flowing sandy texture must be mixed with 60 g of anhydrous sodiumsulfate, using a spatula. If needed, more sodium sulfate may be added. Afteraddition of sodium sulfate, the sample should be free flowing. Also see the note inSec. 11.3.11.3.1.6Immediately add 100 mL of the extraction solvent or solventmixture (see Sec. 7.4 and Table 2 for information on the choice of solvents).11.3.2Place the bottom surface of the tip of the 3/4-inch disrupter horn about1/2-inch below the surface of the solvent, but above the sediment layer.NOTE:Be sure that the horn is properly tuned according to the manufacturer'sinstructions.11.3.3Extract the sample ultrasonically for 3 min, with output control knob set at10 (full power) or at the manufacturer’s recommended power setting, the mode switch onPulse (pulsing energy rather than continuous energy), and the percent-duty cycle knob set at 50% (energy on 50% of time and off 50% of time). Do not use the microtip probe.11.3.4Decant the extract and filter it through Whatman No. 41 filter paper (orequivalent) in a Buchner funnel that is attached to a clean 500-mL filtration flask.Alternatively, decant the extract into a centrifuge bottle and centrifuge at low speed toremove particles.11.3.5Repeat the extraction two more times with two additional 100-mL portionsof clean solvent. Decant off the solvent after each ultrasonic extraction. After the finalultrasonic extraction, pour the entire sample into the Buchner funnel, rinse the beaker with extraction solvent, and add the rinse to the funnel. Apply a vacuum to the filtration flask, and collect the solvent extract. Continue filtration until all visible solvent is removed from the funnel, but do not attempt to completely dry the sample, as the continued application of a vacuum may result in the loss of some analytes. Alternatively, if centrifugation is used in Sec. 11.3.4, transfer the entire sample to the centrifuge bottle. Centrifuge at low speed, and then decant the solvent from the bottle.11.3.6If necessary, concentrate the extract prior to analysis following theprocedure in Sec. 11.5. Otherwise, proceed to Sec. 11.7.11.4Medium/high concentration extraction procedureThis procedure applies to solid samples that are expected to contain more than 20 mg/kg of organic analytes.11.4.1Transfer approximately 2 g of sample to a 20-mL vial. Wipe the mouth ofthe vial with a tissue to remove any sample material. Cap the vial before proceeding with the next sample to avoid any cross-contamination. Record the weight to the nearest 0.1 g.11.4.2For the sample in each batch selected for spiking, add 1.0 mL of thematrix spiking solution. Consult Method 3500 for guidance on the appropriate choice of matrix spiking compounds and concentrations. Also see the note in Sec. 11.3.11.4.3Add 1.0 mL of surrogate spiking solution to all samples, spiked samples,QC samples, and blanks. Consult Method 3500 for guidance on the appropriate choice of matrix spiking compounds and concentrations. Also see the note in Sec. 11.3.11.4.4If gel permeation cleanup (see Method 3640) is to be employed, theanalyst should either add twice the volume of the surrogate spiking solution (and matrix spiking solution, where applicable), or concentrate the final extract to half the normalvolume, to compensate for the half of the extract that is lost due to loading of the GPCcolumn.11.4.5Nonporous or wet samples (gummy or clay type) that do not have a free-flowing sandy texture must be mixed with 2 g of anhydrous sodium sulfate, using aspatula. If needed, more sodium sulfate may be added. After addition of sodium sulfate, the sample should be free flowing (see the note in Sec. 11.3).。
国外农药残留检测技术一瞥

国外农药残留检测技术一瞥作者:佚名来源:转载发布时间:2008-7-26 16:08:57减小字体增大字体轻轻一点,立刻拥有一本安全工具书!收藏本篇文章,方便以后查看随着国外不断发布更加严格的农药残留最大允许限量,以及日本肯定列表制度的出台,我国农产品、食品进出口贸易正面临严重的农残困扰。
欧盟、美国、日本、加拿大等发达国家非常重视构建食品安全保障体系,健全相关的法规和标准,完善人员、装备力量,并形成了一套科学有效的模式。
本文对国外先进的农药残留管理体系和检测技术进展进行论述。
农药分类农药用于防止、破坏、引诱、排拒、控制昆虫和有毒有害病菌,或控制动物的外寄生虫,其种类繁多。
迄今为止,在世界各国注册的农药大约1500种,其中常用的就有300多种。
根据用途、来源、化学结构等不同有多种分类方式,常用的按用途不同可分为4种:(1)杀虫剂,主要有有机氯类、有机磷类、拟除虫菊酯类、氨基甲酸酯类、杀蚕毒素类等;(2)杀菌剂,主要有有机汞类、苯并咪唑类、有机氯类等;(3)除草剂,主要有麦田除草剂、玉米除草剂、豆除草剂、棉田除草剂等;(4)熏蒸剂,主要有磷化氢、溴甲烷、二硫化碳等。
样品预处理技术农产品和食品样品组分比较复杂,农药残留含量极低,一般在PPm和PPb,而且还存在农药的同系物、异构体、降解产物、代谢产物和轭合物影响,要想除去与目标物同时存在的杂质,减少色谱干扰峰,避免检测器和色谱柱污染,样品预处理十分重要,大约占工作量的 70%左右。
国际上相继出现了一系列公认的标准分析方法,主要有美国分析化学协会(AOAC)方法;美国环保署(EPA)方法;美国食品和药品监督管理局(FDA)方法;美国加州食品与农业部分析化学中(CDFA)方法;食品法典委员会(CAC)方法;联合国农粮组织和世界卫生组织(FAOC/WHO)方法;欧盟委员会方法;加拿大和日本等国家注册和颁布的标准方法。
现在常用的食品中农残预处理方法有:(1)固相萃取法(SPE),主要通过吸附填料和吸脱液互相作用,实现组分分离净化,例如有机氯和有机磷农药预处理常用的Florisil柱。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
METHOD 3520CCONTINUOUS LIQUID-LIQUID EXTRACTION1.0SCOPE AND APPLICATION1.1This method describes a procedure for isolating organic compounds from aqueous samples. The method also describes concentration techniques suitable for preparing the extract for the appropriate determinative steps described in Sec. 4.3 of Chapter Four.1.2This method is applicable to the isolation and concentration of water-insoluble and slightly soluble organics in preparation for a variety of chromatographic procedures.1.3Method 3520 is designed for extraction solvents with greater density than the sample. Continuous extraction devices are available for extraction solvents that are less dense than the sample. The analyst must demonstrate the effectiveness of any such automatic extraction device before employing it in sample extraction.1.4This method is restricted to use by or under the supervision of trained analysts. Each analyst must demonstrate the ability to generate acceptable results with this method.2.0SUMMARY OF METHOD2.1 A measured volume of sample, usually 1 liter, is placed into a continuous liquid-liquid extractor, adjusted, if necessary, to a specific pH (see Table 1), and extracted with organic solvent for 18 - 24 hours.2.2The extract is dried, concentrated (if necessary), and, as necessary, exchanged into a solvent compatible with the cleanup or determinative method being employed (see Table 1 for appropriate exchange solvents).3.0INTERFERENCES3.1Refer to Method 3500.3.2The decomposition of some analytes has been demonstrated under basic extraction conditions required to separate analytes. Organochlorine pesticides may dechlorinate, phthalate esters may exchange, and phenols may react to form tannates. These reactions increase with increasing pH, and are decreased by the shorter reaction times available in Method 3510. Method 3510 is preferred over Method 3520 for the analysis of these classes of compounds. However, the recovery of phenols may be optimized by using Method 3520 and performing the initial extraction at the acid pH.4.0APPARATUS AND MATERIALS4.1Continuous liquid-liquid extractor - Equipped with polytetrafluoroethylene (PTFE) or glass connecting joints and stopcocks requiring no lubrication (Kontes 584200-0000, 584500-0000, 583250-0000, or equivalent).CD-ROM3520C - 1Revision 3December 19964.2Drying column - 20 mm ID Pyrex® chromatographic column with Pyrex® glass wool at bottom and a PTFE stopcock.NOTE:Fritted glass discs are difficult to decontaminate after highly contaminated extracts have been passed through. Columns without frits may be purchased.Use a small pad of Pyrex® glass wool to retain the adsorbent. Prewash theglass wool pad with 50 mL of acetone followed by 50 mL of elution solvent priorto packing the column with adsorbent.4.3Kuderna-Danish (K-D) apparatus4.3.1Concentrator tube - 10-mL graduated (Kontes K-570050-1025 or equivalent).A ground glass stopper is used to prevent evaporation of extracts.4.3.2Evaporation flask - 500-mL (Kontes K-570001-500 or equivalent). Attach toconcentrator tube with springs, clamps, or equivalent.4.3.3Snyder column - Three-ball macro (Kontes K-503000-0121 or equivalent).4.3.4Snyder column - Two-ball micro (Kontes K-569001-0219 or equivalent).4.3.5Springs - 1/2 inch (Kontes K-662750 or equivalent).NOTE:The following glassware is recommended for the purpose of solvent recovery during the concentration procedures requiring the use of Kuderna-Danishevaporative concentrators. Incorporation of this apparatus may be requiredby State or local municipality regulations that govern air emissions of volatileorganics. EPA recommends the incorporation of this type of reclamationsystem as a method to implement an emissions reduction program. Solventrecovery is a means to conform with waste minimization and pollutionprevention initiatives.4.4Solvent vapor recovery system (Kontes K-545000-1006 or K-547300-0000, Ace Glass 6614-30, or equivalent).4.5Boiling chips - Solvent-extracted, approximately 10/40 mesh (silicon carbide or equivalent).4.6Water bath - Heated, with concentric ring cover, capable of temperature control (± 5E C). The bath should be used in a hood.4.7Vials - 2-mL, glass with PTFE-lined screw-caps or crimp tops.4.8pH indicator paper - pH range including the desired extraction pH.4.9Heating mantle - Rheostat controlled.4.10Syringe - 5-mL.CD-ROM3520C - 2Revision 3December 1996CD-ROM 3520C - 3Revision 3December 19965.0REAGENTS5.1Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it isintended that all reagents shall conform to the specifications of the Committee on AnalyticalReagents of the American Chemical Society, where such specifications are available. Other gradesmay be used, provided it is first ascertained that the reagent is of sufficiently high purity to permit itsuse without lessening the accuracy of the determination. Reagents should be stored in glass toprevent the leaching of contaminants from plastic containers.5.2Organic-free reagent water - All references to water in this method refer to organic-freereagent water, as defined in Chapter One.5.3Sodium hydroxide solution (10N), NaOH. Dissolve 40 g NaOH in organic-free reagentwater and dilute to 100 mL. Other concentrations of hydroxide solutions may be used to adjustsample pH, provided that the volume added does not appreciably change (e.g., <1%) the totalsample volume.5.4Sodium sulfate (granular, anhydrous), Na SO . Purify by heating at 400E C for 4 hours24in a shallow tray, or by precleaning the sodium sulfate with methylene chloride. If the sodium sulfateis precleaned with methylene chloride, a method blank must be analyzed, demonstrating that thereis no interference from the sodium sulfate.5.5Sulfuric acid solution (1:1 v/v), H SO . Slowly add 50 mL of H SO (sp. gr. 1.84) to 5024 24mL of organic-free reagent water. Other concentrations of acid solutions may be used to adjustsample pH, provided that the volume added does not appreciably change (e.g., <1%) the totalsample volume.5.6Extraction/exchange solvents - All solvents must be pesticide quality or equivalent.5.6.1Methylene chloride, CH Cl , boiling point 39E C. 225.6.2Hexane, C H , boiling point 68.7E C.6145.6.32-Propanol, CH CH(OH)CH , boiling point 82.3E C. 335.6.4Cyclohexane, C H , boiling point 80.7E C. 6125.6.5Acetonitrile, CH CN, boiling point 81.6E C.36.0SAMPLE COLLECTION, PRESERVATION, AND HANDLINGSee the introductory material to this chapter, Organic Analytes, Sec. 4.1.7.0PROCEDURE7.1Using a 1-liter graduated cylinder, measure 1 liter (nominal) of sample. Alternatively, ifthe entire contents of sample bottle are to be extracted, mark the level of sample on the outside ofthe bottle. If high concentrations are anticipated, a smaller sample volume may be taken and dilutedto 1-L with organic-free reagent water. It is recommended that if high analyte concentrations areanticipated, samples should be collected in smaller sample bottles and the whole sample used.7.2Pipet 1.0 mL of the surrogate spiking solution into each sample in the graduated cylinder (or sample bottle) and mix well. (See Method 3500 and the determinative method to be used for details on the surrogate standard solution and matrix spiking solution).7.2.1For the sample in each batch (see Chapter One) selected for use as a matrixspike sample, add 1.0 mL of the matrix spiking standard.7.2.2If Method 3640, Gel-Permeation Cleanup, is to be employed, add twice thevolume of the surrogate spiking solution and the matrix spiking standard, since half of the extract is not recovered from the GPC apparatus. (Alternatively, use 1.0 mL of the spiking solutions and concentrate the final extract to half the normal volume, e.g., 0.5 mL instead of1.0 mL).7.3Check the pH of the sample with wide-range pH paper and adjust the pH, if necessary, to the pH indicated in Table 1, using 1:1 (v/v) sulfuric acid or 10 N sodium hydroxide. Lower concentrations of acid or base solution may be employed, provided that they do not result in a significant change (<1%) in the volume of sample extracted (see Secs. 5.3 and 5.5).7.4Add 300 - 500 mL of methylene chloride to the distilling flask of the extractor. Add several boiling chips to the flask.7.5Quantitatively transfer the sample from the graduated cylinder (or sample bottle) to the extractor. Use a small volume of methylene chloride to rinse the cylinder (or bottle) and transfer this rinse solvent to the extractor. Add organic-free reagent water to the extractor, if needed, to ensure proper operation and extract for 18-24 hours. If the sample was transferred directly from the sample bottle, refill the bottle to the mark made in Sec. 7.1 with water and then measure the volume of sample that was in the bottle.7.6Allow the extractor to cool, then detach the boiling flask. If extraction at a secondary pH is not required (see Table 1), the extract is dried and concentrated using one of the techniques described in Secs. 7.10 - 7.11.7.7If a pH adjustment and second extraction is required (see Table 1), carefully, while stirring, adjust the pH of the aqueous phase to the second pH indicated in Table 1. If the extracts are to be analyzed separately (see Sec. 7.8), attach a clean distilling flask containing 500 mL of methylene chloride to the continuous extractor. Extract for 18-24 hours, allow to cool, and detach the distilling flask. If the extracts are not to be analyzed separately, then the distilling flask and solvent need not be changed and may be used for the second pH extraction.7.8If performing GC/MS analysis (Method 8270), the acid/neutral and base extracts may be combined prior to concentration. However, in some situations, separate concentration and analysis of the acid/neutral and base extracts may be preferable (e.g., if for regulatory purposes the presence or absence of specific acid/neutral and base compounds at low concentrations must be determined, separate extract analyses may be warranted).7.9Perform concentration (if necessary) using the Kuderna-Danish technique (Secs. 7.10.1 through 7.10.6).7.10K-D technique7.10.1Assemble a Kuderna-Danish (K-D) concentrator (Sec. 4.3) by attaching a 10-mLconcentrator tube to a 500-mL evaporation flask.CD-ROM3520C - 4Revision 3December 19967.10.2Attach the solvent vapor recovery glassware (condenser and collection device)(Sec. 4.4) to the Snyder column of the K-D apparatus following manufacturer's instructions.7.10.3Dry the extract by passing it through a drying column containing about 10 cm ofanhydrous sodium sulfate. Collect the dried extract in a K-D concentrator. Rinse the Erlenmeyer flask, which contained the solvent extract, with 20 - 30 mL of methylene chloride and add it to the column to complete the quantitative transfer.7.10.4Add one or two clean boiling chips to the flask and attach a three-ball Snydercolumn. Prewet the Snyder column by adding about 1 mL of methylene chloride to the top of the column. Place the K-D apparatus on a hot water bath (15 - 20E C above the boiling point of the solvent) so that the concentrator tube is partially immersed in the hot water and the entire lower rounded surface of the flask is bathed with hot vapor. Adjust the vertical position of the apparatus and the water temperature, as required, to complete the concentration in 10 -20 minutes. At the proper rate of distillation the balls of the column will actively chatter, butthe chambers will not flood. When the apparent volume of liquid reaches 1 mL, remove the K-D apparatus from the water bath and allow it to drain and cool for at least 10 minutes.7.10.5If a solvent exchange is required (as indicated in Table 1), momentarily removethe Snyder column, add 50 mL of the exchange solvent, a new boiling chip, and reattach the Snyder column. Alternatively, pour the exchange solvent into the top of the Snyder column while the concentrator remains on the water bath in Sec. 7.10.4. Concentrate the extract, as described in Sec. 7.10.4, raising the temperature of the water bath, if necessary, to maintain proper distillation.7.10.6Remove the Snyder column and rinse the flask and its lower joints into theconcentrator tube with 1 - 2 mL of methylene chloride or exchange solvent. If sulfur crystals are a problem, proceed to Method 3660 for cleanup. The extract may be further concentrated by using the techniques outlined in Sec. 7.11 or adjusted to 10.0 mL with the solvent last used.7.11If further concentration is indicated in Table 1, either the micro-Snyder column technique (7.11.1) or nitrogen blowdown technique (7.11.2) is used to adjust the extract to the final volume required.7.11.1Micro-Snyder column techniqueAdd another one or two clean boiling chips to the concentrator tube and attacha two-ball micro-Snyder column. Prewet the column by adding 0.5 mL of methylenechloride or exchange solvent to the top of the column. Place the K-D apparatus in a hotwater bath so that the concentrator tube is partially immersed in the hot water. Adjustthe vertical position of the apparatus and the water temperature, as required, to completethe concentration in 5 - 10 minutes. At the proper rate of distillation the balls of thecolumn will actively chatter, but the chambers will not flood. When the apparent volumeof liquid reaches 0.5 mL, remove the K-D apparatus from the water bath and allow it todrain and cool for at least 10 minutes. Remove the Snyder column, rinse the flask andits lower joints into the concentrator tube with 0.2 mL of methylene chloride or theexchange solvent, and adjust the final volume as indicated in Table 1, with solvent. CD-ROM3520C - 5Revision 3December 19967.11.2Nitrogen blowdown technique7.11.2.1Place the concentrator tube in a warm bath (35E C) and evaporate thesolvent to the final volume indicated in Table 1, using a gentle stream of clean, drynitrogen (filtered through a column of activated carbon).CAUTION:New plastic tubing must not be used between the carbon trap andthe sample, since it may introduce contaminants.7.11.2.2The internal wall of the tube must be rinsed several times withmethylene chloride or appropriate solvent during the operation. During evaporation, thetube must be positioned to avoid water condensation (i.e., the solvent level should bebelow the level of the water bath). Under normal procedures, the extract must not beallowed to become dry.CAUTION:When the volume of solvent is reduced below 1 mL, semivolatileanalytes may be lost.7.12The extract may now be analyzed for the target analytes using the appropriate determinative technique(s) (see Sec. 4.3 of this chapter). If analysis of the extract will not be performed immediately, stopper the concentrator tube and store refrigerated. If the extract will be stored longer than 2 days it should be transferred to a vial with a PTFE-lined screw-cap or crimp top, and labeled appropriately.8.0QUALITY CONTROL8.1Any reagent blanks, matrix spikes, or replicate samples should be subjected to exactly the same analytical procedures as those used on actual samples.8.2Refer to Chapter One for specific quality control procedures and Method 3500 for extraction and sample-preparation procedures.9.0METHOD PERFORMANCERefer to the determinative methods for performance data.10.0REFERENCESNone.CD-ROM3520C - 6Revision 3December 1996CD-ROM 3520C - 8Revision 3December 1996METHOD 3520CCONTINUOUS LIQUID-LIQUID EXTRACTION。
