第8章药理药效研究 动物模型
药理学研究中的动物模型

药理学研究中的动物模型药理学是研究药物对生物系统的作用、药物相互作用及其在人体内的代谢和排泄等方面的科学。
而动物模型则是药理学研究的重要手段之一,是在药理学研究中广泛应用的实验方法。
本文将探讨动物模型在药理学研究中的运用以及存在的问题。
一、动物模型在药理学研究中的运用1.药效学研究药物剂量-反应关系是药一个药物治疗效果的关键参数。
通过对动物模型进行药效学研究,可以帮助研究者确定药物的剂量和给药方法,及其对特定疾病产生的治疗作用。
2.药代动力学研究药代动力学研究涉及药物在生物体内的吸收、分布、代谢及排泄等过程。
通过使用动物模型,研究者可以更好地理解人体内药物相互作用的机制,以及药物在体内的代谢及排泄吸收规律。
3.毒理学研究另外,动物模型也广泛应用于毒理学研究方面。
通过使用动物模型,研究者可以评估药物的毒性,并为药物的临床使用提供指导。
二、动物模型存在的问题1.模型转化性能的问题研究者对动物模型的应用需要深刻认识到动物模型本身具有的局限性。
动物模型无法完全反映人体内部各种细微变化的复杂性,因此,能否将动物的结果转化为人体的治疗方案存疑。
2.道德问题另外,动物模型研究也存在一定的道德问题。
因为动物在实验中往往会受到一定程度的折磨,所以必须确保实验的道德可接受,避免动物受到过度转化。
3.统计学意义上的问题最后,动物模型的应用还可能存在统计学意义上的问题,研究者必须严格控制实验中的各种环境因素,以确保研究结果的可靠性。
三、结语总体来说,动物模型在药理学研究中扮演着重要的角色。
尽管存在一些问题,但研究者仍需要认真对待这种研究方法,尽力避免它的局限性,改善其缺陷,并为临床应用提供可靠的依据。
同时,必须通过科学的伦理道德评估来确保研究过程的公正公平。
只有慎重对待,才能更好地补充人类已知的药理学知识,为发现从动物实验中发现的新药物奠定基础。
4 A phase I II study of external 药理药效研究 动物模型

A Phase I/II Study of External Beam Radiation, Brachytherapy,and Concurrent Chemotherapy for Patients with Localized Carcinoma of the Esophagus (Radiation Therapy Oncology Group Study9207)Final ReportLaurie E.Gaspar,M.D.1Kathryn Winter,M.S.2Walter I.Kocha,M.D.3Lawrence R.Coia,M.D.4Arnold Herskovic,M.D.5Mary Graham,M.D.61University of Colorado Health Sciences Center, Denver,Colorado.2Radiation Therapy Oncology Group Headquarters, Philadelphia,Pennsylvania.3University of Western Ontario,London,Ontario, Canada.4Community Medical Center,Toms River,New Jersey.5Department of Radiation,Northwest Community Hospital,Arlington Heights,Illinois.61000West10th Street,Rolla,Missouri. Presented at a meeting of the American RadiumSociety,Monte Carlo,Monaco,May1998. Address for reprints:Laurie E.Gaspar,M.D.,Ra-diation Oncology,University of Colorado Health Sciences Center,4200East Ninth Avenue,Box A031,Denver,CO80262.Received March29,1999;revision received Sep-tember14,1999;accepted September14,1999.BACKGROUND.A multiinstitutional,prospective study of the Radiation Therapy Oncology Group(RTOG)was designed to determine the feasibility and toxicity of chemotherapy,external beam radiation,and esophageal brachytherapy(EB)in a potentially curable group of patients with adenocarcinoma or squamous cell carcinoma of the esophagus.A preliminary analysis indicated a17%1-year actu-arial risk of treatment-relatedfistulas.Afinal analysis of this study was considered important to determine the median survival time,local control,and late toxicity associated with this treatment regimen.METHODS.Planned treatment was50grays(Gy)of external beam radiation(25 fractions given over5weeks)followed2weeks later by EB(either high-dose-rate5 Gy during Weeks8,9,and10,for a total of15Gy,or low-dose-rate20Gy during Week8).Chemotherapy was given during Weeks1,5,8,and11,with cisplatin75 mg/m2and5-fluorouracil1000mg/m2/24hours in a96-hour infusion. RESULTS.Of the49eligible patients,45(92%)had squamous histology and4(6%) had adenocarcinoma.Forty-seven patients(96%)completed external beam radi-ation plus at least2courses of chemotherapy,whereas34patients(69%)were able to complete external beam radiation,EB,and at least2courses of chemotherapy. The estimated survival rate at12months was49%,with an estimated median survival of11months.Life-threatening toxicity or treatment-related death oc-curred in12(24%)and5(10%)cases,respectively.Treatment-related esophageal fistulas occurred in6cases(12%overall,14%of patients starting EB)at0.5–6.2 months from thefirst day of brachytherapy,leading to death in3cases. CONCLUSIONS.In this study,severe toxicity,including treatment-relatedfistulas, occurred within7months of brachytherapy.Based on the12%incidence of fistulas,the authors continue to urge caution in employing EB,particularly when used in conjunction with chemotherapy.Cancer2000;88:988–95.©2000American Cancer Society.KEYWORDS:esophageal carcinoma,brachytherapy,chemoradiation.R adiation therapy with concurrent chemotherapy is considered the standard nonsurgical treatment for cancer of the esophagus.A prospective randomized trial(Radiation Therapy Oncology Group [RTOG]8501,Southwest Oncology Group[SWOG]8598,North Cen-tral Cooperative Tumor Group[NCCTG]88-40-51)demonstrated a statistically significant survival advantage for radiation and concur-988©2000American Cancer Societyrent chemotherapy compared with radiation therapy alone.1Following chemoradiation,3-year survival rates were30%,compared with0%with radiation alone.Nevertheless,locoregional tumor control re-mained a major problem,with25%of patients having persistence and20%recurrence of locoregional dis-ease following combined modality treatment.In view of the suboptimal locoregional tumor con-trol,intensification of the radiation dose was thought to be reasonable.Esophageal brachytherapy(EB)was proposed as a“boost”to the primary tumor,allowing relative sparing of the surrounding normal tissues. Several retrospective studies had suggested that EB combined with external beam radiation offered im-proved local control and relief from dysphagia com-pared with historical controls treated with external beam radiation only.2–4Few data were available re-garding the outcome of chemoradiation followed by an intracavitary EB boost.The purpose of RTOG9207was to assess the fea-sibility,toxicity,and efficacy of external beam radia-tion and EB with concurrent chemotherapy.A prelim-inary report concluded that the study was feasible; 70%of patients were able to complete external beam radiation,EB,and at least2courses of chemotherapy.5 The estimated survival rate at12months was48%,with an estimated11-month median survival rate. However,the development of6fistulas in the35pa-tients starting EB was of concern.A follow-up report was deemed important to determine whether there were other unexpected late toxicities.METHODSPatient SelectionAll patients had histologically confirmed squamous cell or adenocarcinoma of the thoracic esophagus measuring10cm or less in length.The esophageal primary tumor had to be clinically limited to the esophagus,with or without the presence of involved regional lymph nodes,i.e.,T1–2,NX–1,M0according to the1983International Union Against Cancer (UICC)clinical staging system(Table1).To be en-rolled,a patient had to have a Karnofsky performance status(KPS)of60or greater,a white blood cell count ofՆ4.0ϫ109cells per liter and plateletsՆ150ϫ109 cells per liter,total serum bilirubinՅ1.5mg%,and serum creatinineՅ1.5mg%and/or creatinine clear-anceՆ65cc/minute.There had to have been no his-tory of prior malignancy other than nonmelanoma cancers of the skin or in situ cervical carcinoma, within5years of entrance to the study.The study protocol was approved by the National Institutes of Health and the Investigational Review Boards at all the participating institutions.All patients gave informed written consent.Ineligibility criteria included cervical esophageal tumors(upper tumor borderϽ18cm from the incisor teeth),extension of tumor to within1cm of gastro-esophageal junction,radiologic or clinical evidence of lymph node metastases to supraclavicular or more distant lymph node groups,and invasion of the tra-cheobronchial tree proven by bronchoscopy. Pretreatment EvaluationsThe following were required within4weeks preced-ing registration:complete medical history and physi-cal examination;complete blood count;biochemical screening profile including alkaline phosphatase,total bilirubin,and creatinine;chest radiography;a barium contrast esophagogram including the proximal stom-ach;abdominal and thoracic computed tomography (CT)scan;and esophageal endoscopy with biopsy. Bronchoscopy was required for all patients with pri-mary tumors situated less than29cm from the inci-sors.Bone scan was required only if the serum alkaline phosphatase was above normal or the patient had complaints of new bone pain.Swallowing was graded according to the scale in Table2.TABLE11983TNM Staging SystemT0No evidence of primary tumorT1InvolvesՅ5cm lengthNo obstructionNot circumferentialConfined to esophageal wallT2InvolvesϾ5cm lengthObstructionCircumferentialConfined to esophageal wallT3Extra esophageal spreadNX Regional lymph nodes cannot be assessed N0No regional lymph node metastasisN1Regional lymph node metastasisM0No distant metastasisM1Distant metastasisTABLE2Grading of Swallowing StatusGrade1No dysphagia—able to eat any solidsGrade2Mild dysphagia—semisolids and liquidsGrade3Moderate dysphagia—able to take liquids only Grade4Complete obstruction—unable to take even liquidsBrachytherapy and Concurrent Chemotherapy for Carcinoma of the Esophagus/Gaspar et al.989Radiation TherapyExternal beamMegavoltage radiation therapy units were used,with a minimum source-to-axis distance of80cm.The radi-ation therapyfield extended at least5cm above and below the tumor,with at least a2cm lateral margin. The radiation therapyfield included the supraclavic-ular fossae if the tumor originated above the level of the carina.Multifield techniques were used to limit the maximum dose to the spinal cord toՅ45grays(Gy).Radiation treatments were delivered5days a week at2Gy per fraction.The initial anterior-posterior par-allel-opposedfields received30Gy.The off-cordfields received20Gy.The total dose was50Gy in25frac-tions over5weeks.All doses were calculated without correction for inhomogeneity of tissue.The dose gra-dient within the volume of tissue treated was not to vary more than10%.BrachytherapyThe EB could be either low-dose-rate(LDR)or high-dose-rate(HDR)iridium-192,although institutions were required to declare themselves HDR or LDR for the entire duration of the study.The ranges of accept-able dose rates for HDR and LDR were0.2–5.0Gy/ minute and0.5–1.0Gy/hour,respectively.The HDR brachytherapy treatment consisted ini-tially of15Gy in3fractions of5Gy during Weeks8,9, and10.The HDR dose was specified at a1cm depth from the middwell position.Equal dwell times were to be used.Center-to-center separations of the dwell po-sitions of the stepping source was to be no more than 1cm.HDR fractions were6–8days apart,given con-currently with the5-fluorouracil(5-FU)in Week8, 24–48hours after starting the5-FU infusion of the third cycle of chemotherapy.Following the observa-tion of severalfistulas in the HDR group,the HDR brachytherapy dose was reduced in December1994to 10Gy in2fractions of5Gy,1week apart in Weeks8 and9.The LDR group received20Gy during Week8, given concurrently with the5-FU infusion of the third cycle of chemotherapy.The LDR dose was specified1 cm from the midsource position.In December1994 the LDR alternative was discontinued,having accrued only19patients in2.5years.The HDR and LDR brachytherapy were delivered with a10–12French applicator(external diameter, 4–6mm),inserted transnasally or transorally.Only single channel applicators were allowed.The active treatment length,i.e.,the distance from the proximal and distal HDR dwell positions or LDR source posi-tions,was the pretreatment esophageal tumor length plus a1cm proximal and distal margin.Given that the protocol only allowed primary tumors up to10cm in length,the maximum active treatment length was12 cm.The esophageal tumor length was determined from the CT scans,barium swallow,and endoscopy findings,taking the longest of the three available de-terminations.Prior to enrolling patients,each radia-tion facility had to submit a“dummy”case,which was reviewed by the Physics Committee of the RTOG to assure correct applicator type and size,radioactive source,and dosimetry.Following the identification of an unexpectedly high rate of esophagealfistulas,the radiation therapy study chairs(L.E.G.and A.H.)reviewed the pretreat-ment CT scans,brachytherapy simulationfilms,and dosimetry data to determine potential contributing factors.Chemotherapy5-FU1000mg per square meter of body-surface area per day was administered as a continuous intravenous infusion for thefirst4days of Weeks1,5,8,and11. Cisplatin75mg per square meter was given at a rate of 1mg per minute on thefirst day of each course.The dose of5-FU was reduced by25%if severe stomatitis or diarrhea developed.The dose of cisplatin was not reduced for transient changes in renal function but was reduced by25%or50%if the granulocyte count fell below3.0ϫ109cells per liter or1.0ϫ109cells per liter,respectively,or a platelet count below75ϫ109 cells per liter or50ϫ109cells per liter.If the renal function did not return to normal by the time of the next scheduled dose of cisplatin,the cisplatin was discontinued.Follow-UpPatients were seen3months posttherapy and then at minimum follow-up intervals of6months.Barium swallow;CT scan of the chest and abdomen;chest X-ray;complete blood count;serum biochemistry;his-tory;and physical examination including assessment of KPS,weight,and swallowing status were to be done at each visit.Endoscopy was required1–3months following completion of therapy.Posttreatment biop-sies were required only if suspicious areas were iden-tified at endoscopy.Patients with no evidence of tumor upon barium swallow,CT,and endoscopy were considered re-sponders to therapy.Those with histologically con-firmed tumor posttreatment were considered to have experienced failure of therapy.There was no defini-990CANCER March1,2000/Volume88/Number5tion of partial or complete response for this clinical trial.Data Collection and Statistical AnalysisThe study endpoints were1)patient tolerance of the proposed treatment in terms of life-threatening acute esophagitis and latefistula,2)the percentage of pa-tients receiving at least75%of the treatment,3)local control and patterns of failure,and4)survival.In RTOG8501,a study of50Gy external beam radiation and concurrent chemotherapy in which the entry criteria were similar to RTOG92-07,life-threat-ening esophageal toxicity occurred in5%of patients. The life-threatening toxicity of RTOG92-07was not expected to be above10%.The1-year local control rate was expected to be approximately56%,similar to the local control found in RTOG8501with chemora-diation.A sample of50patients for each dose rate(LDR and HDR)was planned.With this sample size,the lower bound of the95%1-sided confidence interval would be1)65%for an hypothesized feasibility of75% of patients receiving the designated protocol treat-ment;2)44%for an hypothesized local control at1 year of56%;and3)39%for an hypothesized1-year survival of51%.Hypothesized rates for feasibility,lo-cal control,and survival were derived from the RTOG 8501data base.The target accrual for each dose rate was55patients,allowing for an additional10%of the required sample to guard against ineligible cases or cases that could not be evaluated.Interim reports with statistical analyses,including toxicities,were prepared every6months.It was pre-determined that if the accrual for a particular dose rate was less than12patients per year,further patient accrual to that dose rate was to be terminated. RESULTSDemographic DataBetween June1992and February1995,56patients were entered on the HDR option and19patients onthe LDR option.Because accrual was much slower than expected for the LDR arm,new patient entries to the LDR arm were discontinued as of December1994. Fifty-six patients were entered to the HDR alternative, although7patients were later deemed ineligible for the following reasons:primary tumor extension to the gastroesophageal-esophageal junction(3patients), suspected tumor involvement of the celiac lymph nodes(2patients),primary tumor involving the cervi-cal esophagus(1patient),and KPSϽ60(1patient). The demographic data and tumor characteristics of the49eligible patients are given in plianceForty-seven patients(96%)completed the planned external beam radiation therapy concurrent with2 courses of chemotherapy.Forty-one patients(84%) received1or more fraction(s)of brachytherapy;34 (69%)had all fractions,i.e.,15Gy in3fractions before the protocol change(28patients)and10Gy in2frac-tions afterward(6patients).Seven patients had only one of the planned fractions of brachytherapy.Eight patients never started the brachytherapy;2due to refusal,2due to disease progression,1because of deterioration in medical condition,1due to death, TABLE3Patient and Tumor Characteristics of the49Evaluable Patients on RTOG9207Characteristic No.(%)of patientsAge(yrs)Mean63.6Range40.1–75.7GenderMale32(65%)Female17(35%)RaceWhite32(65%)Black15(31%)Hispanic2(4%)KPS70–8022(45%)90–10027(55%) HistologySquamous45(92%)Adenocarcinoma4(8%)%Weight lossMean 6.5%Range0–22%Ն10%16(32%)Ͻ10%30(62%)Missing data3(6%)Current weight(kg)Mean67Range40–159Primary length(cm)Mean 6.0Range 1.5–10Ͻ5cm15(31%)Ն5cm34(69%)Clinical T classificationT112(25%)T237(75%)Clinical N classificationN038(78%)N18(16%)NX3(1%)Clinical M classificationM049(100%)RTOG:Radiation Therapy Oncology Group;KPS:Karnofsky performance status.Brachytherapy and Concurrent Chemotherapy for Carcinoma of the Esophagus/Gaspar et al.991and 2for reasons unknown.Thirty-nine patients (80%)started the third course of chemotherapy;28(57%)started the fourth course.Reasons for not start-ing or completing the third or fourth cycle of chemo-therapy included patient refusal (7patients),poor general condition (1patient),disease progression (2patients),treatment toxicity (3patients),death (2pa-tients),or unidentified reason (5patients).Of the ini-tial 49eligible patients,24(49%)finished the external beam radiation therapy,chemotherapy,and brachy-therapy as per protocol.Survival and Sites of FailureThe median follow-up for 13patients alive at the time of analysis was 29months.With a median follow-up of 10months for the 49eligible patients,36deaths have been reported.The 1-year survival rate was estimated to be 49%,with a 95%confidence interval of 34–63%.The estimated survival rates at 2and 3years were 31%and 29%,respectively.Figure 1is the survival curve with log-transformed 95%confidence intervals and censoring marks for the 49eligible patients.Information regarding tumor response and first site of failure was available in all 49eligible patients (Tables 4and 5).Persistent disease was found in 26%of patients;complete response was seen in 74%.Of the 74%with initial complete response,locoregional fail-ure occurred at the time of first relapse in 37%:29%with local failure alone,and 8%with combined local and distant failure.The final local persistence or re-currence rate was 63%:13patients (26%)with persis-tence,and 18patients (37%)with initial complete response followed by local recurrence.Therefore,local control was ultimately 37%.Tumor response and siteof first failure in the chemoradiation patients on RTOG 85-01is also presented in Tables 4and plete response and locoregional control rates appear com-parable between the two studies.Treatment ToxicitySevere toxicity (RTOG Grade 3)was documented in 29(59%)of the patients,mostly in the form of hemato-logic or gastrointestinal side effects.Life-threatening (RTOG Grade 4)or fatal toxicity was seen in 12(24%)and 5(10%)of patients,respectively.Toxicities are summarized in Table 6.Life-threatening esophageal strictures occurred in three patients.Esophageal fistulas,thought to be treatment-related rather than tumor-related,occurred in six patients,leading to death in three.All patients with fistula had endoscopy and attempted biopsy at the time of fistula or had autopsy at death.The fistulas were considered treatment-related only if biopsy or autopsy found no local tumor persistence or recur-rence.The fistula was aortoesophageal in one patient,in the esophageal-bronchotracheal tree in three pa-tients,and esophageal-mediastinal in two.A compar-ison of the pretreatment studies and brachytherapy simulation films with the postfistula studies suggested that the fistulas occurred in the region of the brachy-therapy.Fistulas occurred in patients with primary tumors in both the middle and lower esophagus (Ta-ble 7).However,of the six fistualas,only two were not in the region of the carina,and only one was intheFIGURE 1.A Kaplan–Meier survival curve for patients in Radiation TherapyOncology Group Study 9207is shown.TABLE 4Tumor Response in RTOG 9207and RTOG 8501RTOG 9207(n ؍49)RTOG 8501(n ؍130)Persistent disease 13(26%)34(26%)No information 0(0%)7(5%)Complete response36(74%)89(68%)RTOG:Radiation Therapy Oncology Group.TABLE 5First Site of Failure in RTOG 9207and RTOG 8501Patients with Initial Complete ResponseRTOG 9207(n ؍36)RTOG 8501(n ؍89)Locoregional 14(29%)23(18%)Distant4(8%)14(11%)Local/distant simultaneous 4(8%)7(5%)Failure,not specified 0(0%)5(4%)No failure 14(29%)40(31%)RTOG:Radiation Therapy Oncology Group.992CANCER March 1,2000/Volume 88/Number 5distal esophagus.The cumulative yearly incidence es-timate for fistula was 17.5%per year,compared with the crude rate of 14.6%(6of 41)for those patients who had at least 1fraction of brachytherapy,or 12%(6of 49)for all study patients (Figure 2).The interval from first brachytherapy to diagnosis of esophageal fistula ranged from 0.5to 6.2months,with a median of 3.9months.Five of the 6patients developing fistulas re-ceived a brachytherapy dose of 15Gy.The other pa-tient received just 1fraction of 5Gy and developed a fistula within 0.5months.None of the 10patients treated after the reduction of brachytherapy dose from 15Gy to 10Gy have developed fistulas.No correlation was found between the develop-ment of fistula and the location of the primary tumor,brachytherapy active length,or applicator diameter (4mm vs.6mm).DISCUSSIONEB has long been advocated as a radiation therapy technique enabling irradiation of locoregional disease to a high dose with relative sparing of the surrounding normal tissues,potentially increasing tumor control while minimizing acute and chronic toxicity.6–9Com-pared with external beam radiation with dose escala-tion,other possible advantages include increased speed of relief of dysphagia,shortening of treatment time,and patient convenience.10Several retrospective studies 2,4,11–14and two pro-spective studies 15,16comparing external beam radia-tion therapy with or without a brachytherapy boost claim improved survival,local control,and swallowing ability,favoring patients treated with high-,interme-diate-,or low-dose rate brachytherapy.Concurrent chemotherapy was not an established therapy during the years that many of these studies were conducted.Chemoradiation is now considered standard therapy for patients with carcinoma of the thoracic esopha-gus.1Previous studies claiming that brachytherapy has benefits are now of questionable relevance if chemotherapy was not included in the treatment reg-imen.Prior to this report,there were few published re-sults of external beam radiation,brachytherapy boost,and concurrent chemotherapy for meaningful num-bers of patients.17,18Only one of the two published reports was in full manuscript form.18Several reports of brachytherapy boost following concurrent chemoradiation have appeared since the publication of the preliminary results of RTOG 9207.19–21Table 8summarizes these experiences.Di-TABLE 6Life-Threatening and Fatal Toxicities in RTOG 9207(n ؍49)Life-threateningFatal All (%)Upper aerodigestive tract excluding fistulas a 303(6%)Fistula336(12%)Gastrointestinal tract b 202(4%)Hematologic c 819(18%)Infection 235(10%)Skin d 011(2%)Renal 011(2%)Cardiac011(2%)aFor the lungs and esophagus,life-threatening effects included ulceration,necrosis,perforation,and formation of a stricture.bLife-threatening gastrointestinal side effects were nausea and vomiting for more than 6days,requir-ing hospitalization.cLife-threatening hematologic effects included a leukocyte count below 1.0ϫ109cells per liter,a platelet count below 25ϫ109cells per liter,and a hemoglobin concentration below 50g per liter.dFatal side effects of skin involved moist desquamation.TABLE 7Patient and Tumor Characteristics in Six Patients Who Developed FistulasCase no.Tumor length Distance a Stage Appl diam (cm)Brachydose (Gy)1 5.5cm 25cm T2,N00.61529.0cm 25cm T2,N00.6153 3.0cm 26cm T2,N00.6154 4.0cm 19cm T1,N00.6155 4.0cm 36cm T2,N00.61563.0cm25cmT1,N00.65Appl diam:applicator diameter;Brachydose:total brachytherapy dose received 1cm from the middwellposition.aDistance between tumor and incisors.FIGURE 2.The cumulative incidence of esophageal fistulas in RadiationTherapy Oncology Group Study 9207is shown.Brachytherapy and Concurrent Chemotherapy for Carcinoma of the Esophagus/Gaspar et al.993rect comparison among these clinical series is ham-pered by the differences in staging,response end-points,and duration of follow-up.For example,the 37%local control observed in RTOG9207is much lower than the73–80%reported in the other series. This difference in local control may be explained by the requirement for esophagoscopy1–3months fol-lowing completion of therapy in RTOG9207.Differ-ences in treatment regimens,i.e.,differences in the outer diameter of the EB applicator,or sequencing of brachytherapy,may also account for the observed dif-ferences in esophageal toxicity.For example,to deliver 10Gy to a10cm active length,the dose at the appli-cator surface for a0.6cm catheter and a1.0cm cath-eter would be36.6Gy and21.4Gy,respectively.RTOG9207documented treatment-related esopha-gealfistulas in12%of the patients;by comparison,none were reported in several other brachytherapy series.17–21 Compared with the experience of Montravadi18and Cal-ais,19RTOG9207called for1)more courses of chemo-therapy,2)chemotherapy concurrent with brachyther-apy,3)a smaller outer diameter of the EB applicator,and 4)a higher brachytherapy dose(initially15Gy in3frac-tions).The lack offistula formation in the10patients treated on RTOG9207following a reduction of HDR dose(10Gy in2fractions)suggests that the brachyther-apy dose was a contributing factor.Tables4and5,in which response rate and sites of first failure are tabulated for RTOG9207and RTOG 8501,suggest little benefit for brachytherapy boost over external beam radiation plus chemotherapy alone.A direct comparison of tumor response and local control between RTOG8501and RTOG9207is difficult because response was assessed without en-doscopy in RTOG8501.Despite the high risk offistula identified in the preliminary report of RTOG9207,there is a wide vari-ation in the reported indications,treatment regimens, and dosimetry for brachytherapy in the treatment of carcinoma of the esophagus.20–22Therefore,the American Brachytherapy Society(ABS)developed guidelines to encourage consistent,safe treatment policies for the use of brachytherapy in the definitive treatment of patients with this disease.23The ABS guidelines recommend a maximum HDR brachyther-apy dose of10Gy in2weekly fractions of5Gy each, utilizing an EB applicator with an external diameter of 6–10mm.The ABS recommended that brachytherapy not be given concurrently with chemotherapy.This recommendation was based on the reported lack of fistulas in patients treated with chemoradiation fol-lowed by a brachytherapy boost.18,19Compared with these other clinical series,RTOG9207also utilized more cycles of chemotherapy.TABLE8Clinical Results of External Beam Radiation,Brachytherapy Boost,and Concurrent ChemotherapyStaar et al.17Montravadi et al.18RTOG9207No.of patients324050Pathology NS Adenoca—29Adenoca—4Squamous—11Squamous—46Extent of disease Stage II–III Stage II–III Chemotherapy Mito C10mg/m2Days2,29Mito C10mg/m2i.v.DDP75mg/m2Day15-FU1000mg/m2/dayϫ4days Days1,29Wk1,5,8,115-FU1000mg/m2/dayϫ4days5-FU1000mg/m2/dayϫ4daysWk1,5,8,11XRT(Gy/wks)55.8Gy/6wks40–55Gy/4–6wks50Gy/5wksHDR a dose/fraction7Gy5Gy5GyNo.of fractions222–3Interfraction interval2wks2wks1wkApplicator diameter NS1cm4–6mmComplicationStricture NS23%4%Fistula NS0%12%Local control NS85%Complete response74%Complete response78%Local control37%Local controlSurvival1yr48%,2yrs24%3yrs40%1yrs49%Median15mos3yrs29%RTOG:Radiation Therapy Oncology Group;XRT:radiation therapy;HDR:high-dose-rate;NS:not specified;Adenoca:adenocarcinoma;Mito C:mitomycin C;DDP:cisplatin;5-FU:5-fluorouracil.a All doses specified at1cm from middwell position.994CANCER March1,2000/Volume88/Number5Thisfinal analysis of RTOG9207,with a longer duration of follow-up,has not revealed late toxicity occurring beyond7months after treatment.Neverthe-less,the high incidence of esophagealfistulas is alarm-ing.In the absence of clear benefits of brachytherapy boost in terms of tumor response,local control,or patient survival rates,there are no plans to take this treatment regimen to the Phase III setting.Pending the results of other prospective studies,extreme cau-tion is urged in the use of external beam radiation, brachytherapy boost,and concurrent chemotherapy as utilized in this study.REFERENCES1.Al-Sarraf M,Martz K,Herskovic A,Leichman L,Brincle JS,Vaitkevicius VK,et al.Progress report of combined chemo-radiotherapy(CT-RT)versus radiotherapy(RT)alone in pa-tients with esophageal cancer:an intergroup study.J Clin Oncol1997;15:277–84.2.Hyden EC,Langholz B,Tilden T,Lam K,Luxton G,AstrahanM,et al.External beam and intraluminal radiotherapy in the treatment of carcinoma of the esophagus.Thorac Cardiovas Surg1988;96:237–41.3.Flores AD,Nelems B,Evans KG,Hay JH,Stoller JL,JacksonSM.Impact of new radiotherapy modalities on the surgical management of cancer of the esophagus and cardia.Int J Radiat Oncol Biol Phys1989;17:937–44.4.Hishikawa Y,Kurisu K,Taniguchi M,Kamikonya N,Miura T.High-dose-rate intraluminal brachytherapy for esophageal cancer:10years experience in Hyogo College of Medicine.Radiother Oncol1991;21:107–14.5.Gaspar LE,Qian C,Kocha WI,Coia LR,Herskovic A,GrahamM.A phase I/II study of external beam radiation,brachy-therapy and concurrent chemotherapy in localized cancer of the esophagus(RTOG92-07):preliminary toxicity report.Int J Radiat Oncol Biol Phys1997;37:593–9.6.Barcat J,Guisez J.Essais de traitement de quelques casd’epithelioma de l’oesophage par les applications locales directes de radium.Bull Mem Soc Med Hop Par1909;26: 712–7.7.Bottrill DO,Plane JH,Newaishy GA.A proposed afterloadingtechnique for irradiation of the oesophagus.Br J Radiol 1979;52:573–4.8.Dickson RJ.Radiation therapy in carcinoma of the esopha-gus:a review.Am J Med Sci1971;241:662–77.9.Guisez J.Malignant tumors of the esophagus.J LaryngolOtol1925;40:213–32.10.Nori D,Allison R.Prospects for improved treatment resultswith radiation dose escalation in esophageal cancer.En-docuriether Hyperthermia Oncol1993;9:63–7.11.Hareyama M,Nishio M,Kagami Y,Narimatsu N,Saito A,Sakurai T.Intracavitary brachytherapy combined with ex-ternal beam irradiation for squamous cell carcinoma of thethoracic esophagus.Int J Radiat Oncol Biol Phys1992;24: 235–40.12.Hishikawa Y,Kurisu K,Taniguchi M,Kamikonya N,Miura T.High-dose-rate intraluminal brachytherapy(HDRIBT)for esophageal cancer.Int J Radiat Oncol Biol Phys1991;21: 1133–5.13.Sur RK,Kochhar R,Negi PS,Gupta BD.High-dose-rateintraluminal brachytherapy in palliation of esophageal car-cinoma.Endocuriether Hyperthermia Oncol1994;10:25–9.14.Okawa T,Nishio M,Kita OM,Hirokawa Y,Dokiya T,YamadaS,et al.JASTRO Study Group.Prospective study of external radiotherapy with and without intracavitary brachtherapy for esophageal cancer in Japan.Int J Radiat Oncol Biol Phys 1998;42:199.15.Yin W.Brachytherapy of carcinoma of the esophagus inChina,1970–1974and1982–1984.Proceedings Remote Af-terloading.State of the Art1989:52–6.16.Kharadi MY,Qadir A,Khan FA,Khuroo parative eval-uation of therapeutic approaches in Stage III and IV squamous cell carcinoma of the thoracic esophagus with conventional radiotherapy and endoscopic treatment in combination and endoscopic treatment alone:a randomized prospective trial.Int J Radiat Oncol Biol Phys1997;39:309–20.17.Staar S,Mueller RP,Achterrath W.Intensified treatment forinoperable esophagus cancer:simultaneous radiochemo-therapy combined with HDR intraluminal brachytherapy—results of a phase II trial[abstract].Proc ASCO1993;12:223.18.Montravadi RVP,Gates JO,Bajpai D,Crawford JN,TrenknerJD,Trenkner bined chemotherapy and external radiation therapy plus intraluminal boost with high dose rate brachytherapy for carcinoma of the esophagus.En-docuriether Hyperthermia Oncol1995;11:223–33.19.Calais G,Dorval E,Louisot P,Bourlier P,Klein V,Chapet S,et al.Radiotherapy with high dose rate brachytherapy boost and concomitant chemotherapy for Stages IIB and III esophageal carcinoma:results of a pilot study.Int J Radiat Oncol Biol Phys1997;38:769–75.20.Dosoretz DE,Rubenstein JH,Katin MJ,Blitzer PH,GartonGR,Penuel JW,et al.External beam radiation therapy and high dose rate intraluminal brachytherapy with and without chemotherapy for esophageal cancer.J Brachyther Int1997;13:207–17.21.Murakami M,Kuroda Y,Okamoto Y,Kono K,Yoden E,Kusumi F,et al.Neoadjuvant concurrent chemoradiother-apy followed by definitive high-dose radiotherapy or surgery for operable thoracic esophageal carcinoma.Int J Radiat Oncol Biol Phys1998;40:1049–59.22.Micaily B,Miyamoto CT,Freire JE,Brady LW.Intracavitarybrachytherapy for carcinoma of the esophagus.Semin Surg Oncol1997;13:185–9.23.Gaspar LE,Nag S,Herskovic A,Mantravadi R,Speiser B,andthe Clinical Research Committee of the American Brachy-therapy Society.American Brachytherapy Society(ABS) consensus guidelines for brachytherapy of esophageal can-cer.Int J Radiat Oncol Biol Phys1997;38:127–32.Brachytherapy and Concurrent Chemotherapy for Carcinoma of the Esophagus/Gaspar et al.995。
药理实验选择动物模型的原则

药理实验选择动物模型的原则药理实验选择动物模型的原则药理学是一门研究药物在生物体内作用、代谢和副作用的学科,制药企业和学术研究者们需要经常进行药理学实验来评估药物的疗效和副作用。
在进行药理实验时,选择适合的动物模型非常重要,它直接关系到实验结果的准确性和真实性。
下面,我们将按照不同类型,分享药理实验选择动物模型的原则。
1. 哺乳动物模型哺乳动物包括鼠类、大鼠、兔子、狗和猴子等,它们具有和人类相似的器官和生理功能,适合于研究和评价药物的疗效和安全性。
常见药理实验的哺乳动物模型选择原则如下:(1)选择和人类代谢和药物反应相似的物种,有利于药物的有效性和毒副作用的测定。
(2)考虑动物的体型和代谢速率,这有助于确定药物的剂量和给药方式。
(3)确定动物的性别和年龄,避免物种差异以及性别和年龄的影响。
(4)考虑动物的基因型和遗传背景,有助于模拟人类的遗传多态性和药物反应的异质性。
2. 禽类模型禽类模型可用于研究一些病原体引起的感染疾病,如病毒和细菌感染,常用的有家禽和鸽子。
常见药理实验的禽类模型选择原则如下:(1)选择敏感或易感染的禽类物种,有助于观察不同病原体的感染症状和病理变化。
(2)考虑禽类的年龄和性别,这会影响禽类抵御病原体的能力。
(3)确定禽类的疫苗接种史和健康状态,以避免健康状况不良的禽类干扰实验结果。
3. 爬行动物模型爬行动物包括蜥蜴和蛇等,它们对温度变化和环境因素的适应能力很强,有助于研究某些药物对环境适应和耐受性的影响。
常见的药理实验的爬行动物模型选择原则如下:(1)选择能够适应实验环境的爬行动物,它们对温度和湿度的要求必须与实验要求相匹配。
(2)确定爬行动物的年龄、性别和体型,这会影响药物剂量的选择和实验的结果。
(3)考虑爬行动物的代谢速率和药物吸收,确定最佳的给药途径和适当的剂量。
总之,选择适合的动物模型是药理实验的关键之一,它直接决定了药效和安全性的评价。
在选择动物模型时,要考虑到物种差异、性别和年龄、药物代谢和吸收、剂量和给药方式等因素,确保实验结果的可靠性和准确性。
药学研究中动物模型的选择与应用

药学研究中动物模型的选择与应用在药学研究中,动物模型是评估药物安全性和有效性的重要工具。
它们帮助科研人员理解疾病机制、评估治疗效果以及预测临床治疗的结果。
合理地选择和应用动物模型对于药物研发的成功至关重要。
本文将探讨动物模型在药学研究中的重要性,以及如何科学合理地选择和应用这些模型。
动物模型的基本概念动物模型是指在实验条件下模拟人类疾病或药物反应的动物。
这些模型通过不同的手段被诱发或基因改造,以便于研究特定的人类疾病过程和评估药物干预的效果。
常见的动物模型包括小鼠、大鼠、兔子、猪以及非人类灵长类动物等。
动物模型的分类自然发病模型:这类模型是指那些自然发生特定疾病的动物。
例如,某些腺体肿瘤在特定品系的小鼠中自然发生,适合用于研究肿瘤相关药物。
诱导性模型:通过化学、物理或生物方法诱导动物产生某种特定疾病。
例如,通过注射化合物来引起糖尿病或心脏病等疾病,可以用于新型药物的筛选和验证。
基因工程模型:借助基因编辑技术,如CRISPR/Cas9,创建具有特定遗传缺陷的动物。
这些模型常用于癌症、自身免疫性疾病等复杂疾病的研究。
选择动物模型的原则在选择适合的动物模型时,有几个关键因素需要考虑:1. 生物相似性选用的动物种类需在生理、生化及病理特征上与人类有高度相似性。
例如,非人灵长类动物因为与人类更接近,会被用于复杂神经系统疾病的研究。
2. 模型的可重复性一个良好的动物模型应具备可重复性,即其他研究者能够通过相同的方法和条件再现先前的发现。
因此,选择已有广泛验证和接受的标准化模型尤为重要。
3. 操作简便性实验人员对操作简单、管理便捷的动物更为青睐,以确保实验环境一致并减少操作误差。
同时也要考虑到伦理问题,保障实验动物的福利。
4. 成本效益不同动物模型所需资源成本各异,选择时需考虑预算,比如小鼠通常相对比较经济,而大型动物如猪则涉及更高的照料及管理费用。
动物模型在药学研究中的应用动物模型在药学研究中扮演多重角色,它们不仅用于基础科学研究,也广泛用于药物开发、毒性检测以及临床试验设计等领域。
中药在动物模型中的药效学研究

中药在动物模型中的药效学研究中药在动物模型中的药效学研究引言:中药作为中国传统医学的重要组成部分,具有悠久的历史和丰富的临床应用经验。
随着现代科学技术的发展,越来越多的研究开始关注中药在动物模型中的药效学研究。
通过动物模型的研究,可以更好地理解中药的药理学特性、药效机制以及临床应用的科学依据。
本文将介绍中药在动物模型中的药效学研究的重要性、方法和应用。
一、中药在动物模型中的药效学研究的重要性中药在动物模型中的药效学研究对于中药的临床应用具有重要意义。
首先,通过动物模型的研究,可以评估中药的药效和安全性。
动物模型可以模拟人体疾病的发生和发展过程,通过给予中药治疗,观察其对疾病的疗效以及对动物的毒副作用。
这对于评估中药的疗效和安全性具有重要意义,为临床应用提供科学依据。
其次,动物模型可以帮助揭示中药的药效机制。
通过对动物模型中中药的作用机制的研究,可以更好地理解中药的药理学特性,为中药的开发和应用提供理论基础。
此外,动物模型还可以用于筛选中药的活性成分和优化中药的配方,提高中药的疗效和安全性。
二、中药在动物模型中的药效学研究的方法中药在动物模型中的药效学研究主要包括药效评价、药代动力学研究和药物安全性评价。
药效评价是中药在动物模型中的主要研究内容之一,通过给予动物一定剂量的中药,观察其对疾病的疗效以及对动物的影响。
常用的药效评价方法包括行为学观察、生理指标检测和组织病理学分析等。
药代动力学研究是研究中药在动物体内的吸收、分布、代谢和排泄过程,通过测定中药在动物体内的浓度-时间曲线,了解其在体内的代谢和排泄动力学特性。
药物安全性评价是评估中药对动物的毒副作用的研究,通过观察动物在给予中药后的生理指标变化、组织病理学变化以及行为学变化等,评估中药的安全性。
三、中药在动物模型中的药效学研究的应用中药在动物模型中的药效学研究广泛应用于临床医学和药物研发领域。
在临床医学中,中药在动物模型中的药效学研究可以为中药的临床应用提供科学依据。
药理学研究中的动物模型选择

药理学研究中的动物模型选择概述:动物模型在药理学研究中发挥着重要的作用。
通过合适的动物模型可以更好地理解药物的机制、效果以及安全性。
然而,在选择适当的动物模型时需要考虑多个因素,包括相似性、成本和可行性等。
本文将探讨药理学研究中动物模型选择的原则和常见的应用。
一、相似性1.1 物种相似性:选择与人类生理相似度较高的动物作为研究对象,如大鼠和小鼠。
因为这些动物在生命活动、器官结构和代谢途径等方面与人类有较好的相似性,能够提供更可靠的数据。
1.2 疾病模型:根据所研究的药物治疗目标来选择与之相关的疾病模型。
例如,在心血管领域,可以使用高胆固醇饮食诱导小鼠产生高血压或者缺血再灌注损伤等模型来评估药物对这些情况下心脏功能改善效果。
二、成本和可行性2.1 成本:动物模型的建立和维护需要耗费大量资金,因此在选择时需要考虑经济实用性。
多个相关研究中常用的小鼠是较经济实惠的选择。
2.2 可行性:动物模型的选取还要根据实验室条件、技术设备和人员配备等方面进行考虑。
对于一些高度特化的模型,如果缺乏相关资源,则不宜选择。
三、常见应用3.1 药效学评价:通过动物模型可以评估药物在生物体内的药效学参数,包括药代动力学和药效学等。
这些数据对于了解药物吸收、分布、代谢和排泄过程非常重要。
3.2 治疗策略验证:在新药开发阶段,动物模型常被用来验证治疗策略的有效性。
例如,在癌症治疗研究中,使用小鼠移植瘤模型可以评估抗肿瘤药物的疗效。
3.3 安全评价:动物模型能够帮助检测潜在毒副作用,并评估药物在各种毒理学指标上的影响。
这对于药物安全性评价以及制定适当的用药指南至关重要。
四、局限性和新技术4.1 物种差异:尽管动物模型在探索药理学的研究中有很大帮助,但人类与动物之间仍然存在一定的生理、代谢差异。
因此,不能直接将动物实验结果推广到人体。
4.2 替代方法:随着科技的发展,出现了许多替代动物模型的技术,如体外细胞培养和计算机模拟等。
这些新技术可以减少对动物实验的需求,并提供更快速、精确、经济高效的研究手段。
药理药效研究 动物模型subsequent erlotinib

Lung Cancer 73 (2011) 211–216Contents lists available at ScienceDirectLungCancerj o u r n a l h o m e p a g e :w w w.e l s e v i e r.c o m /l o c a t e /l u n g c anDuration of prior gefitinib treatment predicts survival potential in patients with lung adenocarcinoma receiving subsequent erlotinibKazuhiro Asami a ,∗,Masaaki Kawahara b ,Shinji Atagi a ,Tomoya Kawaguchi a ,Kyoichi Okishio aa Kinki-chuo Chest Medical Center,1180Nagasone-cho,Kita-ku,Sakai City,Osaka 591-8555,Japan bOtemae Hospital,1-5-34Otemae,Chuo-ku,Osaka,Japana r t i c l ei n f oArticle history:Received 9August 2010Received in revised form 19October 2010Accepted 18December 2010Keywords:Gefitinib ErlotinibEGFR mutationResistance to EGFR-TKIsTime to progression of gefitiniba b s t r a c tPurpose:We investigated survival potential in patients receiving erlotinib after failure of gefitinib,focus-ing on response and time to progression (TTP)with gefitinib.Methods:We retrospectively reviewed lung adenocarcinoma patients who received erlotinib after expe-riencing progression with gefitinib.Our primary objective was to evaluate the prognostic significance of erlotinib therapy.Results:A total 42lung adenocarcinoma patients were included in this study.Overall disease control rate was 59.5%(partial response [PR],2.4%;stable disease [SD],57.1%).Median overall survival was 7.1months,and median progression-free survival was 3.4months.The number of patients who achieved PR and non-PR (SD+progressive disease [PD])with gefitinib were 22(52%)and 20(48%),respectively.Patients with PR for gefitinib showed significantly longer survival times than those with non-PR (9.2vs.4.7months;p =0.014).In particular,among PR patients,those with TTP <12months on gefitinib showed significantly longer survival times than those with TTP ≥12months (10.3vs.6.4months;p =0.04).Conclusions:Erlotinib may exert survival benefit for lung adenocarcinoma patients with less than 12months of TTP of prior gefitinib who achieved PR for gefitinib.© 2011 Elsevier Ireland Ltd. All rights reserved.1.IntroductionGefitinib and erlotinib are oral epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs).Gefitinib has been reported to be effective in limited populations such as never smokers,Asians,and patients with adenocarcinoma,and is particularly effective in patients with EGFR mutations [1–3].Erlotinib,which has a sim-ilar quinazoline frame to gefitinib,is the first EGFR-TKI shown to provide survival benefit in patients with non-small cell lung cancer (NSCLC)[4]:the BR.21trial revealed significantly longer sur-vival times among patients who received erlotinib compared with a placebo group [4].In addition,these two EGFR-TKIs have been found to occasionally induce a particularly significant response in EGFR-mutant patients.However,despite this documented efficacy,most cancer clones acquire resistance to these particular com-pounds over time [5].Previous studies have demonstrated that amplified MET onco-gene and secondary EGFR T790M mutations are most commonly responsible for resistance to gefitinib and erlotinib [6,7].Indeed,several previous studies showed that secondary EGFR T790M muta-tion and MET amplification occurred in nearly half and 20%of lung∗Corresponding author.Tel.:+81722523021;fax:+81722511372.E-mail address:kazu.taizo@ (K.Asami).cancer specimens that had become resistant to EGFR-TKIs,respec-tively [8–11].In addition,the majority of patients who showed secondary resistance had EGFR mutations such as exon 19dele-tion mutations or L858R point mutation,which have been found to be sensitive to EGFR-TKIs [12,13].Several reports have demonstrated clinical benefits when administering erlotinib to NSCLC patients following failure of gefi-tinib [14–18];in contrast,one previous report has suggested that no erlotinib-derived clinical benefit can be expected in patients who failed gefitinib [19].However,reports thus far have all had small sample sizes,and clear findings regarding efficacy of erlotinib in patients who failed gefitinib have yet to be obtained.Consequently,whether or not erlotinib is useful in these patients remains contro-versial.We hypothesize that tumor clones may require exposure to gefi-tinib treatment with a positive response for a specific duration to acquire secondary common resistance to EGFR-TKIs.Even if a patient experiences tumor progression on gefitinib therapy,sub-sequent erlotinib therapy may nevertheless still be able to inhibit progression,provided the tumor clones did not acquire secondary resistance.As such,in positive-responder patients with confirmed progression within a specific duration of gefitinib treatment,some tumor clones may remain sensitive to erlotinib,and therefore these patients may still experience survival benefit with erlotinib treat-ment.0169-5002/$–see front matter © 2011 Elsevier Ireland Ltd. All rights reserved.doi:10.1016/j.lungcan.2010.12.014212K.Asami et al./Lung Cancer73 (2011) 211–216Here,we conducted a retrospective study primarily aimed at assessing overall survival(OS)of patients who received erlotinib therapy after failure with gefitinib.We also attempted to charac-terize the clinical features of patients who benefited from erlotinib treatment.2.Patients and methodsWe retrospectively reviewed records for patients with histopathologically diagnosed lung adenocarcinoma who received erlotinib after experiencing progression on gefitinib at Kinki-chuo Chest Medical Center between December2008and October2009. Responses were evaluated based on patient records and radio-graphic studies,such as chest roentgenograms and computed tomographic(CT)and magnetic resonance imaging(MRI)scans. We examined EGFR mutation status using the PCR-invader method with paraffin sections of biopsy specimens from patients.Time to progression(TTP)with gefitinib was defined as the period from initiation of gefitinib therapy to the date when dis-ease progression was confirmed.Overall survival was defined as the period from initiation of erlotinib therapy to the date of death or last follow-up.Disease control rate(DCR)was defined as com-plete response(CR)plus partial response(PR)plus stable disease (SD).Evaluation of response to gefitinib and erlotinib therapy by CT scan was performed according to the response evaluation crite-ria in solid tumors(RECIST).Stable disease plus progressive disease (PD)with prior gefitinib treatment was defined as“non-PR.”Categorical outcomes,including DCRs,were compared using the 2test,and survival distribution was estimating using the Kaplan–Meier method.Overall survival and progression-free sur-vival(PFS)were compared with regard to demographic factors such as gender,performance status,EGFR mutation status,response to gefitinib,TTP with gefitinib,and toxicity grade of skin rash,which may be associated with survival,using the log-rank test.Values were considered statistically significant for p<0.05.A multivariate Cox-proportional-hazards model was used to determine the clini-cal variables which influenced OS.Statistical analyses were carried out using SPSS software ver.11.0for Windows(IBM,Chicago,IL, USA).3.Results3.1.Patient characteristicsForty-two patients with lung adenocarcinoma were reviewed in the present study.All patients became refractory to gefitinib dur-ing the course of treatment and were subsequently switched to erlotinib therapy.Patient characteristics are described in detail in Table1.Thirty patients(71%)had received1or2regimens before Table1Patient characteristics.Number(%)Median age,years(range)65(31–85) SexMale13(31)Female29(69)Smoking historyNever28(67)Former/current14(33)ECOG score0–124(57)2–418(43)Cancer stageIIIB8(19)IV34(81) Number of previous treatments with erlotinib1–230a(71)3≤12(29) EGFR mutationExon19deletion mutation14(33)L858R14(33)Exon18point mutation1(2)Wild13(32)TTP with gefitinib treatment,months(range)8.1(0.9–40.7) <1229(69)≤1213(31)Response to gefitinibCR0(0)PR22(53)SD17(40)PD3(7)EGFR,epidermal growth factor receptor;TTP,time to progression;CR,complete response;PR,partial response;SD,stable disease;PD,progressive disease;ECOG, Eastern Cooperative Oncology Group.a Two patients received gefitinib asfirst-line treatment.initiation of erlotinib and2(7%)had received gefitinib asfirst-line treatment.EGFR mutations were detected in29(69%)patients:14(33%) had exon19deletions,14(33%)had L858R mutations,and1(2%) had an exon18point mutation.The median TTP with gefitinib treatment was8.1months.Thirteen(31%)patients had TTPs of12 months or more,while29(69%)had TTPs of less than12months. Twenty-two(53%)patients receiving gefitinib achieved PR,and17 (40%)achieved SD.None achieved CR while receiving gefitinib ther-apy.The response rate(RR)and DCR for gefitinib were53%(22of 42patients)and93%(39of42patients),respectively.Of the22 patients who achieved PR with gefitinib,19(86%)were found to have EGFR mutations.Of the20patients who had SD or PD(non-PR)Table2Response to erlotinib according to the response to prior gefitinib and EGFR mutation status.EGFR mutation Response to gefitinibPR(n=22)Non-PR a(n=20)SD(n=17)PD(n=3)Positive,n(%)Negative b,n(%)Positive,n(%)Negative,n(%)Positive,n(%)Negative,n(%) Response to erlotinib PR(N=1)1(4.5)0(0)0(0)0(0)0(0)0(0) SD(N=24)14(64)1(4.5)3(18)5(29)0(0)1(33)PD(N=17)4(18)2(9)6(35)3(18)1(33)1(33)EGFR,epidermal growth factor receptor;PR,partial response;SD,stable disease;PD,progressive disease.Overall disease control rate(PR+SD)was73%(EGFR mutation-positive:15/22[68%],EGFR mutation-negative:1/22[5%])among patients who achieved PR with gefitinib and45%(EGFR mutation-positive:3/20[15%],EGFR mutation-negative:6/20[30%])among patients with non-PR(SD+PD for gefitinib)with gefitinib treatment.Overall disease control rate was62%(PR for gefitinib:15/29[52%],non-PR for gefitinib:3/29[10%])in EGFR mutation-positive patients and54%(PR for gefitinib:1/13[8%],non-PR for gefitinib:6/13[46%])in EGFR mutation-negative patients.a Defined as SD plus PD with prior gefitinib therapy.b EGFR wild-type.K.Asami et al./Lung Cancer73 (2011) 211–216213 Table3Response to erlotinib stratified by TTP with prior gefitinib treatment.(A)TTP with gefitinib<12monthsResponse to gefitinib TTP with gefitinib(months)<12(n=29)PR(n=11)n(%)Non-PR(n=18)SD(n=15)n(%)PD(n=3)n(%) Response to erlotinib PR(n=1)1b(9)0(0)0(0)SD(n=17)8a,b(73)8a(44)1(6)PD(n=11)2(18)7(39)2(11)B.TTP with gefitinib≥12monthsResponse to gefitinib TTP with gefitinib(months)≥12(n=13)PR(n=11)n(%)Non-PR(n=2)SD(N=2)n(%)PD(N=0)n(%) Response to erlotinib PR(n=0)0(0)0(0)0(0)SD(n=7)7(64)0(0)0(0)PD(n=6)4(36)2(100)0(0)EGFR,epidermal growth factor receptor;PR,partial response;SD,stable disease;PD,progressive disease;TTP,time to progression.Overall disease control rate was62%(PR for gefitinib:9/29[31%],non-PR for gefitinib:9/29[31%])in patients with TTP of gefitinib<12months.Overall disease control rate was54%(PR for gefitinib:7/13[54%], non-PR for gefitinib:0/13[0%])in patients with TTP of gefitinib≥12months.a Ten patients showed improvement of target lesions,but not to PR standards.Seven and three patients achieved PR and SD,respectively,with gefitinib treatment.b A second biopsy from progression lesions was performed in three patients(one had PR and2had SD with erlotinib)who achieved PR with gefitinib.Exon19deletion mutations which were the same pattern as detected infirst biopsy specimen for primary diagnosis of NSCLC were identified,whereas EGFR T790M mutation,which endowed secondary common resistance to EGFR-TKIs,was not identified in those biopsy specimens.with gefitinib,EGFR mutations were detected in10(50%).Among patients with EGFR mutations,only one showed PD with gefitinib therapy,and RR and DCR in this group were66%(19of29)and97% (28of29),respectively.3.2.ResponseOn erlotinib therapy,1of42patients achieved PR,and24had SD.No patients achieved CR with erlotinib.Overall RR and DCR for erlotinib were2.4%(one of42)and59.5%(25of42),respectively.Response to erlotinib categorized by response to prior gefi-tinib duration and EGFR mutation status is described in Table2. Among patients who achieved PR with gefitinib,one achieved PR and15patients achieved SD with erlotinib therapy.Patients who achieved PR with gefitinib showed higher DCRs with erlotinib than patients who had non-PR with gefitinib(16[73%]of22vs.9[45%] of20),albeit without statistical significance(p=0.07).In addi-tion,EGFR mutation status was not found to be associated with response to erlotinib;in terms of DCR,no significant difference was noted between EGFR-mutant patients(18/29)and EGFR non-mutant patients group(7/13)(62%vs.54%,p=0.616).Time to progression with prior administration of gefitinib was not found to be associated with achieving a response with subse-quent erlotinib.Details regarding response to erlotinib categorized by TTP with gefitinib are shown in Table3.DCR among patients experiencing progression after less than12months of gefitinib therapy was18/29(62%).In contrast,DCR among patients with TTPs of12months or more was7/13(54%).No statistical significant difference in DCR was noted between these two groups accord-ing to TTP with prior administration of gefitinib(p=0.62).Of the 24patients who achieved SD with erlotinib therapy,10showed improvement in target lesions which had been exacerbated during gefitinib treatment;all10were EGFR-mutant patients(4L858R, 5exon19deletion mutations,and1exon18point mutation),and TTPs with gefitinib were all less than12months.Of the two patients who received gefitinib asfirst-line treatment,one had an EGFR L858R mutation and showed responses to gefitinib and subsequent erlotinib of PR and SD,respectively.While this particular patient showed a relatively long TTP(39.5months)with gefitinib,disease progression was confirmed4months after initiation of erlotinib therapy,and OS was58.6months.The other patient who received gefitinib asfirst-line treatment had EGFR-wild type,and responses to both gefitinib and subsequent erlotinib treatment were PD.TTP and OS in this patient were3and7.4months,respectively.A second biopsy of the progressed lesions was performed in three patients after gefitinib therapy failed.While exon19deletion mutations of the same pattern as noted in thefirst biopsy specimen for primary diagnosis were also detected on this second biopsy, we noted no EGFR T790M mutations.Of note,however,was the fact that imagingfindings for lesions after erlotinib therapy were improved on the second biopsy(Table3).3.3.SurvivalMedian OS and median progression-free survival(PFS)were 7.1months(95%confidence interval[CI]:4.4–9.8months)and3.4 months(95%CI:1.1–5.7months),respectively(Fig.1).Multivariate analysis of prognostic factors was performed using a Cox propor-tional hazards model to determine which clinical variables were most strongly associated with OS(Table4).Response to gefitinibTable4Multivariate analysis of prognostic variables for OS by use of a Cox proportional-hazards model.Multivariate analysisp a Hazard ratio95%CISex0.51 1.350.55–3.31 ECOG score0.190.580.25–1.31 EGFR mutation0.78 1.130.48–2.70 Response to gefitinib0.0050.230.80–0.64 TTP of gefitinib0.050.340.12–1.01 Grade of skin rash0.290.640.27–1.47EGFR,epidermal growth factor receptor;TTP,time to progression;CI,confidence interval;ECOG,Eastern Cooperative Oncology Group;PR,partial response.Response to gefitinib was the only independent prognostic factor.TTP with gefitinib showed borderline significance.Variables were compared as paired categories:sex(female vs.male),ECOG score(0–1vs.2–4),response to gefitinib(PR vs.non-PR),TTP of gefitinib(<12months vs.≥12months),grade of skin rash(3vs.1–2).a p<0.05was considered significant.214K.Asami et al./Lung Cancer73 (2011) 211–216Fig.1.Kaplan–Meier plot of survival time with erlotinib.(A)Overall survival rates and(B)progression-free survival rates of42patients.MST:median survival time; mPFS:median progression-free survival.was found to be the only independent prognostic factor(hazard ratio=0.23;95%CI:0.08–0.64,p=0.005),and time to progression with gefitinib showed borderline significance(hazard ratio=0.34; 95%CI:0.12–1.01,p=0.05).Kaplan–Meier curves of survival time according to response to prior gefitinib therapy are shown in Fig.2.Patients who achieved PR while receiving gefitinib therapy showed significantly longer OS(p=0.014).However,no significant difference was noted in PFS between patients with PR for gefitinib and those with non-PR(4.7 months[95%CI:2.9–6.5months]vs.1.8months[95%CI:1.4–2.2 months];p=0.122).Time to progression with gefitinib showed a borderline significant impact on survival with erlotinib therapy. However,among patients who achieved PR with gefitinib,TTP with gefitinib therapy was strongly correlated with survival time. Kaplan–Meier curves of survival time for patients who achieved PR with gefitinib stratified according to TTP are shown in Fig.3. Patients with TTPs of less than12months with gefitinib ther-apy were found to have significantly longer OS(10.3months[95% CI:7.0–13.6months]vs.6.4months[95%CI:2.6–10.2months]; p=0.04)and longer PFS(6.4months[95%CI:3.6–9.2months]vs.3.4 months[95%CI:1.2–5.6months];p=0.19)than patients with TTPs of12months or more.However,no statistically significant differ-ence was noted between the two groups in terms of PFS(p=0.19).Fig.2.Kaplan–Meier plot of survival time with erlotinib.(A)Overall survival rates and(B)progression-free survival rates stratified by response to prior gefitinib.Non-PR is defined as SD plus PD with gefitinib therapy.In addition,we found that skin rash was not predictive of sur-vival with erlotinib therapy.All patients in the present study were affected by rash of some grade while receiving erlotinib.The degree of skin rash toxicity due to erlotinib exceeded the grade noted dur-ing gefitinib treatment in32patients.Seven patients required dose reduction of erlotinib due to grade3skin ing a Cox propor-tional hazard model,we determined that skin rash grade had no impact on survival(hazard ratio=0.64[95%CI:0.27–1.47];p=0.29).4.DiscussionHere,we investigated survival potential in patients receiv-ing erlotinib after failure of gefitinib,focusing on response and TTP with gefitinib.Ourfindings suggest that administration of erlotinib subsequent to gefitinib may exert survival benefit in for-mer gefitinib-positive responders.Further,among those former responders,most with TTP<12months may not yet have sec-ondary resistance to EGFR-TKIs.Ourfindings suggest little chance for patients to achieve a high response with erlotinib therapy after experiencing progression with gefitinib therapy.This observation may be due to these two EGFR TKIs sharing the same mechanism of EGFR blockade or to cross resistance[5].Our retrospective study showed that response achieved with prior administration of gefitinib was the only prognostic factor for subsequent erlotinib therapy after experiencing progression on gefitinib therapy.In particular,among patients who achieved PR with gefitinib,patients with TTPs of less than12months with gefitinib therapy were found to have significantly longer OS thanK.Asami et al./Lung Cancer73 (2011) 211–216215Fig.3.Kaplan–Meier plot of survival time for patients who achieve PR with gefitinib.(A)Overall survival rates and(B)progression-free survival rates stratified by TTP with gefitinib.patients with TTPs of12months or more.In addition,most of these patients showed some degree of improvement in imagefind-ings after subsequent erlotinib therapy.We noted no EGFR T790M mutations in any of three patients who underwent a second biopsy of their progressed lesions after failure with gefitinib therapy.We therefore supposed that most patients with TTP<12months may have not yet acquired the EGFR T790M mutation.However,we only investigated the presence of a secondary EGFR T790M mutation in three patients in the present study.Validation of this hypothesis will require collection of more molecular information from patients who are no longer responsive to gefitinib in the future.Shepherd et al.demonstrated that TTP was2.6months in NSCLC patients who had previously been treated with docetaxel therapy [20].We observed that PFS was3.4months in patients with TTP≥12 months who achieved PR in our study,a duration which appears improved over that demonstrated by Shepherd et al.Given these findings,we posited that,regardless of duration of gefitinib ther-apy,subsequent erlotinib may be able to prolong PFS compared to chemotherapy with cytotoxic agent provided the patients demon-strated a positive response with gefitinib.However,given that our results were obtained in a retrospective study with an extremely small sample population,a prospective study is warranted to clar-ify whether or not erlotinib administered subsequent to gefitinib can elicit greater survival benefit in gefitinib-positive responders than chemotherapy with cytotoxic agents.We noted here that treatment with erlotinib following gefitinib resulted in more toxic grades of skin rash in patients,findings which suggest that erlotinib may have greater biological activity than gefitinib.Several other investigators have also suggested based on their ownfindings that erlotinib may have higher biological activity than gefitinib.Costa et al.showed that differing efficacy between gefitinib and erlotinib was due to differences in commonly admin-istered dosages between the two drugs[21].Gefitinib(250mg per day)is typically administered at one third of its maximum-tolerated dose,whereas erlotinib(150mg per day)is administered at its maximum tolerated dose.In vitro data showed that the mean concentration of gefitinib was0.24g/ml at the300-mg daily dose and1.1g/ml at1000mg/day.In contrast,median concentration of erlotinib at150mg/day was1.26g/ml.These previousfindings suggest that erlotinib(150mg/day)has a higher biological dose of EGFR inhibition than gefitinib(250mg/day).Recent studies have demonstrated that the increased biological activity of EGFR-TKIs is associated with control of tumor clones. Yoshimasu et al.reported observing a dose-response relationship between inhibition rates and gefitinib concentration[22].Clarke et al.reported that high-dose erlotinib was effective in controlling leptomeningeal metastases progression while receiving standard erlotinib therapy in EGFR-mutant patients[23].These authors demonstrated that a weekly1200-mg dose of erlotinib controlled leptomeningeal metastases in a patient who was no longer respon-sive to a standard daily dose of erlotinib(150mg).Ourfindings here suggest that a treatment duration of12 months of gefitinib therapy may be the borderline period for tumor clones to attain resistance to EGFR-TKIs.However,speculation as to whether or not previously EGFR-TKI-sensitive clones gradually grow resistant to EGFR-TKIs has not been resolved.Further studies are necessary to validate ourfindings.In conclusion,gefitinib responders may achieve survival ben-efits from erlotinib therapy after experiencing progression with gefitinib.Among patients who have been receiving gefitinib ther-apy for less than12months,tumor clones may not yet have acquired a secondary mutation.However,further studies are needed to clarify precisely how tumor clones attain such secondary resistance to EGFR-TKIs.Conflicts of interest statementNone declared.AcknowledgementsWe are grateful to the staff of Kinki-Chuo Chest Medical Cen-ter for their helpful comments.We are especially indebted to Dr. Masahiko Ando of Kyoto University for support on statistical anal-yses.References[1]Moscatello DK,Holgado-Madruga M,et al.Frequent expression of a mutantepidermal growth factor receptor in multiple human tumors.Cancer Res 1995;55(23):5536–9.[2]Janne PA,Engelman JA,et al.Epidermal growth factor receptor mutations innon-small-cell lung cancer:implications for treatment and tumor biology.J Clin Oncol2005;23(14):3227–34.[3]Baselga J,Arteaga CL.Critical update and emerging trends in epidermal growthfactor receptor targeting in cancer.J Clin Oncol2005;23(11):2445–59.[4]Pao W,Miller V,Zakowski M,et al.EGF receptor gene mutations are commonin lung cancers from“never smokers”and are associated with sensitivity of tumors to gefitinib and erlotinib.Proc Natl Acad Sci2004;101:13306–11. [5]Mitsudomi T,Kosaka T,Endoh H,et al.Mutations of the epidermal growth factorreceptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence.J Clin Oncol 2005;23(11):2513–20.[6]Mok TS,Wu Y-L,et al.Gefitinib or carboplatin–paclitaxel in pulmonary adeno-carcinoma.N Engl J Med2009;361(September(10)):947–57.[7]Shepherd FA,Rodrigues Pereira J,et al.Erlotinib in previously treated non-small-cell lung cancer.N Engl J Med2005;353(2):123–32.216K.Asami et al./Lung Cancer73 (2011) 211–216[8]Johnson JR,Cohen M,et al.Approval summary for erlotinib for treatmentof patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen.Clin Cancer Res 2005;11(18):6414–21.[9]Allan S,et al.Efficacy of erlotinib in patients with advanced non-small celllung cancer(NSCLC)relative to clinical characteristics:subset analysis from the TRUST study.In:Poster presented at ASCO.2008.[10]Baselga J,Rischin D,et al.Phase I safety,pharmacokinetic,and pharmaco-dynamic trial of ZD1839,a selective oral epidermal growth factor receptor tyrosine kinase inhibitor,in patients withfive selected solid tumor types.J Clin Oncol2002;20(November(21)):4292–302.[11]Hidalgo M,Siu LL,Nemunaitis J,Rizzo J,et al.Phase I and pharmacologic studyof OSI-774,an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies.J Clin Oncol2001;19(July(13)):3267–79.[12]Riely GJ,Pao W,et al.Clinical course of patients with non-small cell lung cancerand epidermal growth factor receptor exon19and exon21mutations treated with gefitinib or erlotinib.Clin Cancer Res2006;12(3(Pt1)):839–44.[13]Choong NW,Dietrich S,Seiwert TY,Tretiakova MS.Gefitinib response oferlotinib-refractory lung cancer involving meninges-role of EGFR mutation.Nat Clin Pract Oncol2006;3(January(1)):50–7[quiz1p following57].[14]Mitsudomi T,Kosaka T,Endoh H,Yoshida K.Mutational analysis of theEGFR gene in lung cancer with acquired resistance to gefitinib.J Clin Oncol 2006;24(18S):7074.[15]Pao W,Balak MN,Riely GJ,Li AR.Molecular analysis of NSCLC patients withacquired resistance to gefitinib or erlotinib.J Clin Oncol2006;24(18S):7078.[16]Bean J,Brennan C,Shih JY,Riely G,Viale A.MET amplification occurs with orwithout T790M mutations in EGFR mutant lung tumors with acquired resis-tance to gefitinib or erlotinib.Proc Natl Acad Sci USA2007;104(December(52)):20932–27.[17]Engelman JA,Zejnullahu K,Mitsudomi T.MET amplification leads to gefitinibresistance in lung cancer by activating ERBB3signaling.Science2007;316(May (5827)):1039–43.[18]Engelman JA,Zejnullahu K,Mitsudomi T,et al.MET amplification leads togefitinib resistance in lung cancer by activating ERBB3signaling.Science 2007;316(May(5827)):1039–43[Epub2007April26].[19]Balak MN,Gong Y,Riely GJ,et al.Novel D761Y and common secondary T790Mmutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors.Clin Cancer Res2006;12(Nov(21)):6494–501.[20]Shepherd FA,Dancey J,Ramlau R,et al.Prospective randomized trial ofdocetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy.J Clin Oncol 2000;18(10):2095–103.[21]Costa DB,Schumer ST,Tenen DG,et al.Differenctial responses to erlotinib inepidermal growth factor receptor(EGFR)-mutated lung cancers with acquired resistance to gefitinib carrying the L747S or T790M secondary mutations.J Clin Oncol2008;26(7):1182–4.[22]Yoshimasu T,Ohta F,Oura S,et al.Histoculture drug response assay for gefi-tinib in non-small-cell lung cancer.Gen Thorac Cardiovasc Surg2009;57(March(3)):138–43[Epub2009March12].[23]Clarke JL,Pao W,Wu N,et al.High dose weekly erlotinib achieves thera-peutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer.J Neurooncol 2010;February.。
药理学研究中的动物模型优化

药理学研究中的动物模型优化药理学研究中的动物模型是衡量药物疗效和安全性的一种重要工具。
然而,传统的动物模型存在一些缺陷,包括代谢差异、物种间变异和伦理限制等问题。
因此,如何优化动物模型,提高其预测药物效果的准确性和可靠性,成为了药理学研究的一个重要课题。
一、借鉴人类疾病特征设计动物模型传统的动物模型往往采用小鼠或大鼠,但其代谢和疾病模式与人类存在差异。
因此,优化动物模型的首要任务是借鉴人类疾病特征,设计与之相符合的动物模型。
例如,在研究癌症新药时,可以使用转基因小鼠模型,将人类癌细胞移植到小鼠体内,以更真实地模拟人类肿瘤环境。
二、利用基因编辑技术构建精准遗传动物模型近年来,基因编辑技术的迅猛发展为动物模型的优化提供了新的途径。
通过利用CRISPR/Cas9等基因编辑技术,可以精确改变动物基因组,构建与人类疾病具有高度相似性的动物模型。
例如,可以通过编辑小鼠基因,使其表达人类特定基因突变,以模拟人类遗传疾病。
三、采用体外细胞模型辅助动物模型研究除了传统的动物模型,体外细胞模型在药理学研究中发挥着越来越重要的作用。
通过使用基因编辑技术构建人类来源的细胞系,可以模拟人类疾病的特征,进行高通量筛选和药物效果评估。
优化动物模型时,可以借助体外细胞模型的结果指导动物实验的设计,提高动物模型的选择性和预测能力。
四、结合计算机模拟优化动物模型计算机模拟在药理学研究中也扮演着重要角色。
通过建立数学模型,模拟药物在动物体内的药代动力学和药效学过程,可以更好地理解药物在体内的作用机制。
此外,计算机模拟还可以帮助设计动物实验的方案,减少动物数量,节约研究成本并提高实验效率。
五、权衡优化动物模型的可行性和代表性在优化动物模型时,需要权衡其可行性和代表性。
确保动物模型的可行性,即能够满足实验目的,操作方便且可重复,保证实验结果的准确性。
同时,要保证动物模型的代表性,即模型能够准确反映人类疾病的特征,提高药物研究的可靠性。
综上所述,优化动物模型在药理学研究中具有重要的意义。
60 Management of adenocarcinoma 药理药效研究 动物模型

0 Phase I/II
ቤተ መጻሕፍቲ ባይዱ
Trials
MANAGEMENT OF ADENOCARCINOMA OF THE ESOPHAGUS WITH CHEMORADIATION ALONE OR CHEMORADIATION FOLLOWED BY ESOPHAGECTOMY: RESULTS OF SEQUENTIAL NONRANDOMIZED PHASE II STUDIES
Fox ChaseCancerCenter, *Departmentof RadiationOncology, +Department of SurgicalOncology, *Department of Medical Oncology, Philadelphia,PA
Purpose: The incidence of adenocarcinoma of the esophagus is increasing, but the optimal treatment for thisase is unknown. We evaluated the efficacy of chemoradiation and chemoradiation followed by esophagectomy as treatment for adenocarcinoma of the esophagus in sequential prospective nonrandomized nhase II studies. Methods and Materials: Between May 1981 and June 1992, all previously untreated patients (N = 35) with potentially resectable adenocarcinoma of the esophagus (clinical Stage I or II) were treated with curative intent in sequential prospective Phase II studies. From May 1981 to August 1987,ll patients (median age 66) were treated with concurrent chemotherapy [mitomycin C, and 5-fluorouracil(5FU)] and radiotherapy to a median dose of 60 Gy (CRT group). From September 1987 to June 1992,24 patients (median age 65) were treated with the same regimen of chemoradiation followed by planned esophagectomy (CRT + PE group). Of these, 12 patients (median age 62) actually underwent esophagectomy (CRT + E subgroup). Results: The median overall survival was 19 months for the CRT group and 15 months for the CRT + PE group. For the CRT + E subgroup, the median overall survival was 33 months. The 3-year actuarial overall survival for the CRT and the CRT + PE groups were 36 and 28% @ = 0.949). The subset of patients treated with chemoradiation followed by esophagectomy had a 3-year actuarial overall survival of 33% (p = 0.274). The 3-year actuarial freedom from local failure rates were similar: 62% in the CRT group vs. 58% in the CRT + PE group. Of the 12 patients who underwent esophagectomy (CRT + E group), 9 (75%) were free of local failure. Four of 12 (33%) patients had no pathologic evidence of malignancy in their surgical specimen. Six of 11 patients (55%) in the CRT group were free of local failure at the time of analysis. Two of five patients in this group who had local recurrence at 2 and 10 months underwent surgical salvage with subsequent survivals of 20 and 100 months, respectively. Treatment-related mortality was 0 out of 11 in the CRT group and 2 out of 24 in the CRT + PE group. Dysphagia relief was similar in the CRT group vs. the CRT + E subgroup; however, a greater percentage of patients treated with chemoradiation alone had normal long-term swallowing function when compared to those patients also undergoing esophagectomy (100% vs. 73%). Conclusion: High-dose chemoradiation alone appears to provide similar survival and relief of dysphagia compared with high-dose chemoradiation followed by esophagectomy for patients with potentially resectable esophageal adenocarcinoma. Local failure may be higher in patients undergoing chemoradiation compared to chemoradiation followed by esophagectomy, but surgical salvage is possible, thus providing similar overall local control. However, because of the small number of patients in each group, these treatment modalities need to be further evaluated in a prospective randomized Phase III study. Adenocarcinoma of the esophagus, Chemoradiation, Chemoradiation and esophagectomy.
table药理药效研究 动物模型
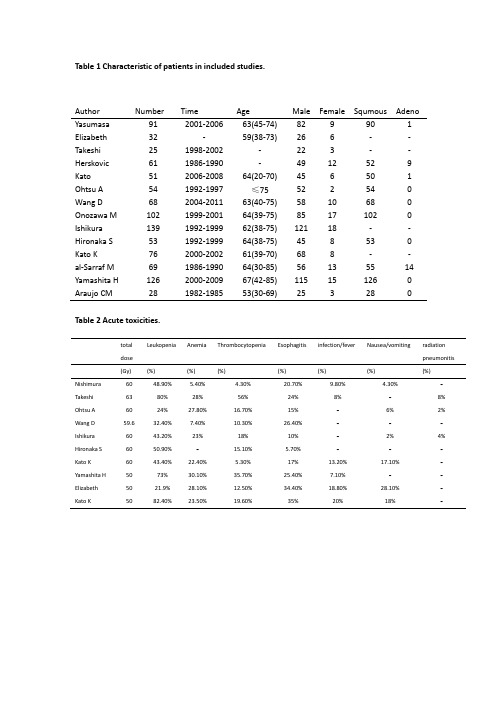
Table 1 Characteristic of patients in included studies.Table 2 Acute toxicities.Author Number Time Age Male Female Squmous Adeno Yasumasa 91 2001-200663(45-74) 82 9 90 1 Elizabeth 32 -59(38-73)26 6 - - Takeshi 25 1998-2002 - 22 3 - - Herskovic 61 1986-1990 - 49 12 52 9 Kato 51 2006-2008 64(20-70) 45 6 50 1 Ohtsu A 54 1992-1997 ≤75 52 2 54 0 Wang D 68 2004-2011 63(40-75) 58 10 68 0 Onozawa M 102 1999-2001 64(39-75) 85 17 102 0 Ishikura 139 1992-1999 62(38-75) 121 18 - - Hironaka S 53 1992-1999 64(38-75) 45 8 53 0 Kato K 76 2000-2002 61(39-70) 68 8 - - al-Sarraf M 69 1986-1990 64(30-85) 56 13 55 14 Yamashita H 126 2000-2009 67(42-85) 115 15 126 0 Araujo CM281982-198553(30-69)25328total doseLeukopeniaAnemiaThrombocytopeniaEsophagitisinfection/feverNausea/vomitingradiation pneumonitis(Gy) (%) (%) (%)(%) (%)(%)(%)Nishimura 60 48.90% 5.40% 4.30% 20.70% 9.80% 4.30%-Takeshi 63 80% 28% 56% 24% 8%-8% Ohtsu A 60 24% 27.80% 16.70% 15% - 6%2%Wang D 59.6 32.40% 7.40% 10.30% 26.40% - --Ishikura 60 43.20% 23%18% 10% - 2%4%Hironaka S 60 50.90% -15.10% 5.70% --- Kato K 60 43.40% 22.40% 5.30% 17% 13.20% 17.10%- Yamashita H 50 73% 30.10% 35.70% 25.40% 7.10% -- Elizabeth 50 21.9% 28.10% 12.50% 34.40% 18.80% 28.10% - Kato K5082.40%23.50%19.60%35%20%18%-Table 3 Late toxicities of grade 3 or greater (esophagus: dysphagia, stenosis,fistula;heart:pericarditis,pericardialeffusion,cardiactamponade;)Author Number Esophagus Heart Lung Pleural Gastrointestinal (%) (%) (%) (%) hemorrhage (%)Nishimura 91 2.2 6.6 1.1 4.4 -Kato 51 3.9 - 5.9 - 2Wang D 68 4.4 - 1.5 - -Hironaka 53 - 7.5 5.7 9.4 -Kato K 76 13.2 15.8 3.9 - -Yamashita 126 - 1 1.6 - -Table 4. Treatment related death.Author Number Total death P neumonitis Esophagus Upper-gastrointestinal Pericarditis Pleural effusion Infarction Renal failure Neutropenic Others rate fistula hemorrhage sepsis(%) (%) (%) (%) (%) (%) (%) (%) (%) (%)Elizabeth 32 12.5 --0.031 - - 3.1 -- 6.3 Takeshi 25 8 8 --- -----Herskovic 61 1.6 ---- -- 1.6 --Ohtsu A 54 7.4 1.9 3.7 -- --- 1.9 -Wang D 68 7.4 - 2.9 4.4 - -----Kato K 76 5.3 2.6 -- 1.3 1.3 ----al-Sarraf 69 1.4 - 1.4 -------Yamashita H 126 3.2 2.4 0.8 -------others 464 0 ---------in total 975 2.6 0.8 0.6 0.4 0.1 0.1 0.1 0.1 0.1 0.2Table 5 failure pattern of ENI. LRFR1:the incidence of localregional failure without distantfailure ; LRFR2: the incidence of localregional failure both with and without distant failure.Author Number LRFR1 LRFR2 (%) (%)Takeshi 25 - 56 Herskovic 61 39.3 44.3 Kato 51 27.5 35.3 Ohtsu 54 35.2 57.4 Wang 68 26.5 29.4 Onozawa 102 - 52 Ishikura 139 54.7 56.1 Hironaka 53 - 35.9 Kato 76 34.2 - al-Sarraf 69 47.8 58 Yamashita 126 - 47 Araujo 28 - 61 In total 827 45.6 52.3Table 6 Survival outcome; SD=standard deviationOverall survivalstudies withavailable datamean±SD(%)median (range)(%)1-year 7 62.5±15.4 59 (41-88)2-year 8 41.5±8.1 41 (31-56)3-year 11 40.7±11.5 43 (26-64)5-year 8 29.2±9.9 27.5 (14-46) Table 7 Survival outcome according to total dose delivered; SD=standard deviationOverall Survival GyStudies withavailable dataMean±SD(%)Median(range)(%)1-year 50 3 66.3±19.1 59(52-88)60 3 58.5±17.3 59(41-75.5)2-year 50 3 41±13.2 36(31-56)60 3 46±0.0 46(46-46)3-year 50 3 45.7±17.2 43(30-64)60 7 40.6±8.6 43(23-49)5-year 50 1 26±0.0 26(26-26)60 5 33.9±8.7 35(22.7-46)。
CTR-S-14-00173药理药效研究 动物模型

Elsevier Editorial System(tm) for Cancer Treatment ReviewsManuscript DraftManuscript Number:Title: The Value of Elective Lymph Node Irradiation in Definitive Concurrent Chemoradiotherapy for Esophageal Cancer.Article Type: Review ArticleSection/Category: Anti-tumour TreatmentKeywords: Esophageal cancer; chemoradiotherapy; elective lymph node irradiation. Corresponding Author: Dr.Med. Shixiu Wu, M.D.Corresponding Author's Institution: Hangzhou cancer hospitalFirst Author: X.D. LiangOrder of Authors: X.D. Liang; R.F. Xie; T. Song; Y.B. Gao; Shixiu Wu, M.D.Abstract: Background: Esophageal cancer remains one of the most lethal carcinomas and concurrent chemoradiotherapy has been accepted as the standard non-surgical treatment. However, no consistent conclusions have been reached whether elective lymph node irradiation (ENI) should be delivered. Therefore, we performed a systematic review of the literature on the feasibility and value of ENI during definitive concurrent chemoradiotherpay for esophageal cancer.Methods: A search based on PubMed electronic databases was carried out to select studies including definitely concurrent chemoradiotherapy with ENI for esophageal cancer. All of the studies were evaluated carefully regarding with acute and late toxicities, treatment-related death, patterns of failure and overall survival.Results: Fourteen studies were identified with a total of 975 patients included. Concurrent chemoradiotherapy with ENI was feasible with acceptable acute and late toxicities. The localregional control rate seems to be higher with ENI, comparing with studies which omitted ENI. However, no obvious overall survival benefit with ENI was indicated in this review.Conclusion: the localregional control rate seems to be higher with ENI in concurrent chemoradiotherapy for esophageal cancer and no obvious better OS results were indicated in this review. Therefore, the value of ENI remains controversial and further prospective phase III trials in this setting are highly warranted.Suggested Reviewers:Conflict of Interest StatementCompeting interestsThe authors declare that they have no competing interests.Cover LetterJun 1, 2014Dear Editor:We would like to submit the manuscript entitled ‘The value of elective lymph nodeirradiation in definitive concurrent chemoradiotherapy for esophageal cancer.’ whichwe sincerely wish to be considered for publication in Cancer Treatment Reviews.Esophageal cancer remains one of the most lethal carcinomas and concurrentchemoradiotherapy has been accepted as the standard non-surgical treatment.However, no consistent conclusions have been reached whether elective lymph nodeirradiation (ENI) should be delivered. Therefore, we performed a systematic review ofthe literature on the feasibility and value of ENI during definitive concurrentchemoradiotherpay for esophageal cancer.We confirm that this paper has not been published elsewhere, nor is it currently underconsideration by any other journal. We hereby acknowledge that all mentionedauthors have contributed substantially to conception and design of the study, draftingor revising the paper as well as giving final approval for submission. We confirm thatwe had full access to all the data in the study and we take final responsibility for thedecision to submit the manuscript for publicationYours sincerely,S.X. Wu*Highlights (for review)Highlights.Definitive CCRT with ENI is feasible with acceptable acute and late toxicities foresophageal cancer. The local-regional control rate seems to be higher with ENI andimproved control of local and regional tumors could lengthen OS, Multicenter phaseIII clinical trials are urgently needed and our ongoing prospective randomized clinicaltrial (NCT00686114) is awaited.*ManuscriptClick here to view linked ReferencesArticle type: Review;Title: The Value of Elective Lymph Node Irradiation in DefinitiveConcurrent Chemoradiotherapy for Esophageal Cancer.Authors: X.D. Liang1, R.F. Xie1, T. Song1, Y.B. Gao1, S.X. Wu1*Author’s affiliation:1. Department of Radiation Oncology, Hangzhou Cancer Hospital,Hangzhou 310000, Zhejiang, P. R. China.*Full address for correspondence:Department of Radiation Oncology, Hangzhou Cancer Hospital, No.34, Yanguan Lane, Shangcheng District, Hangzhou 310000, P. R. China,TEL: +86-571-86826086; FAX: +86-571-86062281; E-mail:wushixiu@The authors indicated no potential conflicts of interest.AbstractBackground: Esophageal cancer remains one of the most lethal carcinomas and concurrent chemoradiotherapy has been accepted as the standard non-surgical treatment. However, no consistent conclusions have been reached whether elective lymph node irradiation (ENI) should be delivered. Therefore, we performed a systematic review of the literature on the feasibility and value of ENI during definitive concurrent chemoradiotherpay for esophageal cancer.Methods: A search based on PubMed electronic databases was carried out to select studies including definitely concurrent chemoradiotherapy with ENI for esophageal cancer. All of the studies were evaluated carefully regarding with acute and late toxicities, treatment-related death, patterns of failure and overall survival. Results: Fourteen studies were identified with a total of 975 patients included. Concurrent chemoradiotherapy with ENI was feasible with acceptable acute and late toxicities. The localregional control rate seems to be higher with ENI, comparing with studies which omitted ENI. However, no obvious overall survival benefit with ENI was indicated in this review.Conclusion: the localregional control rate seems to be higher with ENI in concurrent chemoradiotherapy for esophageal cancer and no obvious better OS results were indicated in this review. Therefore, the value of ENI remains controversial and further prospective phase III trials in this setting are highly warranted.Key Words: Esophageal cancer; chemoradiotherapy; elective lymph node irradiation.IntroductionEsophageal carcinoma is the eighth most common cancer and sixth cause of cancer death with approximately 480 000 new cases and 400 000 deaths annually worldwide 1-3. Esophageal cancer remains one of the most lethal carcinomas and the prognosis is dismal with surgery or radiotherapy (RT) alone. Surgery is the standard treatment for patients with resectable esophageal cancer currently. Concurrent chemoradiotherapy (CCRT) has been considered as the standard non-surgical treatment for esophageal cancer based on the results of Radiation Therapy Oncology Group (RTOG) 85-01 trial 4and 94-05 trial 5. However, there were disagreements between two trials such as elective lymph node irradiation (ENI) was used in RTOG 85-01 but was omitted in RTOG 94-05. Subsequently, the incidence of local-regional failure rate (44.3%) was apparently decreased in RTOG 85–01 than the standard arm in RTOG 94-05 (55%), which suggested that ENI could improve local-regional control rate. Better local-regional control rate with ENI was also reported in some studies 4, 6-10but in other studies 11-16, and thus no consistent conclusions could be reached. To date, only one small sample sized prospective clinical trial 17 has been focused on the value of ENI in CCRT for cervical and upper-thoracic esophageal cancer and the conclusions were inconclusive. Therefore, the role of ENI in CCRT for esophageal cancer remains controversial.Esophageal carcinomas are prone to spread axially to regional lymphatics and high incidence of occult regional lymph node metastasis was revealed by surgery 18-21. A total of 20 patients (26%) were found to have node metastasis in the dissected neck lymph nodes while the primary lesions were located at upper/middle/lower esophagus (7/42/28 patients respectively) 18. Better overall survival (OS) with prophylactic three-field lymph node dissection had been reported in esophageal cancer 18, 19, 22. In theory, CCRT with ENI may improve local control and thus improve OS in line with the benefit of three-field lymph node dissection in curative surgery.The purpose of this study was to review the value of ENI in CCRT for esophageal cancer regarding with toxicities,local-regional control rate and OS. Only articleswith ENI in definitely CCRT were reviewed.Methods and materialsThis review was designed to investigate the feasibility and value of ENI in definitively CCRT for patients with esophageal cancer. The literature search was conducted with assistance from a research librarian in the database of PubMed, to identify all clinical trials since 1980 including definitely CCRT with ENI in esophageal cancer. The following terms were explored and used for search: (esophageal OR esophagus OR oesophageal) AND (cancer OR carcinoma OR neoplasm) AND chemotherapy AND radiotherapy. Studies were excluded as following: neoadjuvant or adjuvant chemoradiotherapy combined with surgery; combined with target therapy; not published in English; not published in full text; phase I study; with other site of cancers. The abstracts of articles were reviewed by the two investigators. Irrelevant citations were removed according to the criteria mentioned above, thus creating a preliminary set of potentially relevant publications. Secondly, the full text articles were distributed to the two reviewers along with an evaluation form customized for reviewing the treatment outcomes for ENI in CCRT. Two reviewers independently evaluated a number of allocated articles and extracted information regarding study design, study population, methods, outcome measures, results and conclusions for each article. The evaluation results were compared and re-evaluated until consensus was reached between two reviewers or decided by the corresponding author. When complete information was not available, attempts were made to contact the corresponding authors of the studies for additional information. Fourteen studies with a total of 975 patients were included and the characteristic was shown in table 1.Results1.Clinical target volume (CTV) for ENI.There are some slight differences in literature regarding the CTV of ENI. At large, the CTV of ENI included the whole thoracic esophagus and periesophageal lymph nodes. For the boost field, margins were usually at least 2-5 cm in thecraniocaudal direction for the primary tumor, and 1-2 cm in the lateral direction for adjacent mediastinum.For cervical esophageal cancer, supraclavicular region were included in the CTV of ENI. However, lower mediastinal lymph nodal region was excluded by some authors 23.For upper thoracic esophageal carcinoma, the CTV of ENI included superior mediastinal lymph nodes alone by some authors7. Supraclavicular fossa nodes were included in the CTV for ENI in most studies 4, 6, 9, 10, 12, 13, 24.The CTV of ENI included mediastinal and perigastric lymph nodes for carcinoma of the middle thoracic esophagus7. Supraclavicular fossa was also included in the CTV of ENI in many studies 4, 9, 10, 12, 24.The supraclavicular nodes were not included while lesions were located in the lower third of the esophagus 4, 6, 16, 24. Perigastric nodes were included in ENI for lower thoracic esophageal carcinoma 7, 10, 23 and celiac lymph nodal region were also included in many studies 6, 7, 10, 13, 23.2.Feasibility of CCRT with ENI2.1 acute toxicities.Although acute toxicities of grade 3 or higher were frequently reported (table 2), CCRT applied with ENI was feasible. Leukopenia and esophagitis were the most common toxicities noted in literature. The incidence of leukopenia (grade 3 or higher) varied from 24% to 82.3%, and that of esophagitis varied from 6% to 35%. Other common toxicities included nausea, vomitting, anemia, infection, stomatitis, thrombocytopenia and cardiac ischemia.2.2 late toxicities.Only 6 papers detailed late toxicities (table 3). Cardiac related toxicities were the most common late toxicities and the incidence varied from 0 to 16%, most of patients suffered from pericardial effusion. The incidence of grade 3 or greater esophagus-related toxicities (dysphagia, stenosis, fistula) varied from 0 to 13%. The incidence of pneumonitis varied from 0 to 5.9%. Pleural effusion occurred in 0 to 5% patients. One case of gastrointestinal hemorrhage was reported.2.3 treatment-related death.A total of 25 deaths were reported to be associated with treatment and the incidence of treatment-related death varied from 0 to 12.5% (table 4) with pooled estimates of 2.6% 4, 6, 8, 10-12, 15, 24. On the whole, eight patients died from pneumonitis, 6 died from esophagus related (mainly fistula), 4 from upper-gastrointestinal hemorrhage. Each one patient died from pericarditis, pleural effusion, myocardial infarction, renal failure and neutropenic sepsis respectively. In addition, two patients died at home without defined cause 4 and 10 weeks after treatment 24.plete response rateComplete remission (CR) rate with ENI in this review varied from 33% to 75%. It seems that CR rates were comparable between higher dose (around 60Gy) and lower dose (around 50Gy) with ENI.4.Patterns of failure with ENIIn order to evaluate the patterns of failure, localregional failure was defined as the first site of failure (local persistence plus localregiongal recurrence) in this review. Distant failure was defined as failure in any site beyond the primary tumor and regional lymph nodes accordingly. Some authors defined localregional failure as localregional failure without distant failure, while other authors defined localregional failure as localregional failure both with and without distant failure. We defined localregional failure rate 1 (LRFR1) as the incidence of localregional failure without distant failure and localregional failure rate 2 (LRFR2) as the incidence of localregional failure both with and without distant failure. Localregional failure pattern in all of the articles included in this review was shown in table 5. For all of the studies included in this review (patients in stage I-IV), LRFR1 ranged from 26.5% to 54.68% and LRFR2 ranged from 29.4% to 61%. We analyzed these articles as a whole group, the pooled estimates of LRFR1 and LRFR2 were 45.6% and 52.3% respectively. A total of 6 studies were identified which including patients in stage I-III only (mainly stage II-III) 4, 6, 7, 9, 15, 16, the LRFR1 and LRFR2 were 27% to 47.8% and 35% to 61% respectively and the failure pattern was shown in table 5. LRFR1 and LRFR2 were 37.7% and 46.2% for a pooled analysis of all of the 6 studies.5.OS with ENIThe mean and median OS were 62.5% and 59% at 1 year; 41.5% and 41% at 2 year; 40.7% and 43% at 3 year; 29.2% and 27.5% at 5 year respectively (table 6). It was comparable between higher dose group (around 60 Gy) and lower dose group (around 50 Gy) with ENI regarding OS (table 7).6.Dose of ENI and boostIn RTOG 8501 and another report 16, 24, the dose of ENI was 30Gy in 15 fractions, and additional 20 Gy was delivered to the boost field. Kodaira et al 11delivered 36 Gy for ENI and the total dose to the boost field was 63Gy. The highest dose delivered to the CTV of ENI was 50.4 Gy and no boost was delivered in that article 10. The dose of ENI varied from 40 to 41.4Gy and the total dose varied from 50.4 to 60 Gy in the other studies 6-9, 12-14, 23.7.Chemotherapy regimenNo difference was documented between protracted infusion chemotherapy and standard short-term infusion chemotherapy for esophageal cancer 23. Two cycles of chemotherapy were concurrently administered in all of the studies and additional two cycles of chemotherapy were delivered in some studies 7, 12, 24. 5-Fu combined with cisplatin was used both in CCRT and consolidated chemotherapy by most authors. Some authors replaced cisplatin with nedaplatin 10, 11.DiscussionThere is a lack of data consistently support or against the delivering of ENI in patients with esophageal cancer treated with CCRT, and the value of ENI in CCRT remains uncertain. Different UICC stage system and toxicities evaluation criterion were used, different races and stages were included in this review, it is difficult to compare the results because of heterogeneity of these studies. However, based on these results included in this review, the localregional control rate seems to be higher with ENI compared with that in RTOG 94-05 which omitted ENI. Nevertheless, we suggest that there is not a large OS difference between the treatment approaches with or without ENI.In this review, LRFR1 and LRFR2 were comparable with those in the combined modality arm of RTOG 8501. However, LRFR1 and LRFR2 with stage I-III (37.7% and 46.2%) were much lower than those in RTOG 94-05 (53.2% and 55%) while ENI was omitted in that study, which indicates that ENI could improve localregional control. However, in the RTOG 95–04 trial, patients with T1-4N0-1 were included (7 patients with T4), the prognosis could be worse than that in the RTOG 8501, in which patients with T1-3N0-1 were included. In addition, the regional failure rate (7% and 9%) in RTOG 94-05 was consistent with other report 25. Only one patient of elective nodal failure was noted in patients with ENI by Onozawa et al., therefore ENI is effective for localregional controlling 13.Studies of CCRT without ENI 26-33 have yielded an OS of 28–56% at 2 years, and 20–35% at 3-5 years, including the trial RTOG 95-04, in which 2 year OS of 31% and 40% were reported. So the results of OS are comparable to that in this review and it seems that a better localregional control could not translate into an obvious OS benefit. Due to the difference of study design, the relatively small number of enrolled patients, it was difficult to compare the OS results between studies with ENI and without ENI. On the other hand, the main failure after CCRT was local failure and distant metastases. Therefore, it is difficult to translate a better regional control with ENI into an obvious OS benefit although an OS benefit may exist. Dissection of regional lymph nodes seems to improve the OS of patients for esophageal cancer 19. In addition, the 5-year OS in this review was comparable to that (15–40%) reported in patients with resectable esophageal cancer who underwent surgery 34, though many patients with unresectable disease were included in this review.The OS results were comparable between higher dose group (around 60 Gy) and lower dose group (around 50 Gy) with ENI in this review. However, the median 5 year OS was 35% in the higher dose group was much higher comparing with historical data and further research is needed. We suggest that total radiation dose of 50 Gy was reasonable in CCRT and 50-64.8 Gy was also acceptable.Herskovic et al 4 noted the LRFR2 of 44% with 30 Gy of ENI and Yamashita etal 10 noted the LRFR2 of 47% with 50-50.4 Gy of ENI, which indicates that 30 Gy of ENI may be comparable with 50-50.4Gy of ENI and that 30 Gy could be enough dose for ENI. The incidence of grade 3 or greater acute toxicities was high in the study by Yamashita et al 10, which indicates more severe acute toxicities with higher radiation dose (50-50.4 Gy) for ENI. However, the late toxicities and death rate in that study were comparable to those studies with lower dose of ENI. The 5-year OS was also satisfactory with 30 Gy dose of ENI in RTOG 8501 10. Therefore, 30 Gy of radiation dose could be adequate for ENI and 30 to 50 Gy of ENI was acceptable.Studies in this review showed that CCRT with ENI was feasible with acceptable acute and late toxicities. Acute and late toxicities were comparable between high dose group (total dose of 60Gy) and low dose group (total dose of 50Gy) with ENI. However, most studies with high total dose had a 1-2 week treatment break. In RTOG 9504, more treatment-related deaths (11 patients, 10%) occurred in the high-dose arm without ENI. However, 7 of these 11 treatment-related deaths occurred in patients who had received 50.4 Gy or less. Therefore, it seems that more deaths were not resulted from higher dose of irradiation.On contrary, Zhao et al 25argued that ENI was unnecessary. However, regional nodal recurrence (out-of-field) was 8% in that study consistent with that in RTOG 95-04, which could be prevented with ENI. ENI may be necessary in definitive CCRT for esophageal cancer in order to reach a higher localregional control.We did not include the only one published prospective randomized trial 17 in this review. The causes are that, firstly, the localregional failure rate of 13.6% with ENI and 17.6% without ENI was much lower than those included in this review; secondly, the 1 year and 2 year OS was much higher than that in this review, however, the 4 year OS fall to nearly zero abruptly; thirdly, patients with cervical and upper-thoracic esophageal cancer were included only, superior mediastinal lymphatic regions could receive 60-70% of the prescribed dose in the group without ENI 17; so we did not consider the patients characteristic in that study were consistent with those in this review.ConclusionsIn conclusion, definitive CCRT with ENI is feasible with acceptable acute and late toxicities for esophageal cancer. The localregional control rate seems to be higher with ENI and improved control of local and regional tumors could lengthen OS, although no obvious better OS results were indicated in this review. It seems appropriate to enlarge the radiation field to cover the lymph node areas at high risk and lower the radiation dose to 30 Gy for ENI in CCRT for esophageal cancer. The incidence and severity of toxicities will be improved with modern radiation technique and better supportive care. Consequently, the treatment related death rate may be reduced further. To date, there is no large phase III study has been published evaluating the role of ENI in definitive CCRT for esophageal cancer and its role has not been defined. Therefore, multicenter phase III clinical trials are urgently needed and our ongoing prospective randomized clinical trial (NCT00686114) is awaited. DisclosureThe authors have declared no conflicts of interest.Reference1. Parkin DM, Bray F. Evaluation of data quality in the cancer registry: principles and methods Part II. Completeness. Eur J Cancer. 2009;45:756-64.2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA: a cancer journal for clinicians. 2009;59:225-49.3. Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol. 2012;13:790-801.4. Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593-8.5. Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167-74.6. Kato K, Muro K, Minashi K, Ohtsu A, Ishikura S, Boku N, et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys. 2011;81:684-90.7. Kato K, Nakajima TE, Ito Y, Katada C, Ishiyama H, Tokunaga SY, et al. Phase II study of concurrent chemoradiotherapy at the dose of 50.4 Gy with elective nodal irradiation for Stage II-III esophageal carcinoma. Jpn J Clin Oncol. 2013;43:608-15.8. Wang D, Yang J, Zhu J, Li B, Zhai L, Sun M, et al. Elective lymph node irradiation late course accelerated hyper-fractionated radiotherapy plus concurrent cisplatin-based chemotherapy for esophageal squamous cell carcinoma: a phase II study. Radiation oncology. 2013;8:108.9. Hironaka S, Ohtsu A, Boku N, Muto M, Nagashima F, Saito H, et al. Nonrandomized comparison between definitive chemoradiotherapy and radical surgery in patients with T(2-3)N(any) M(0) squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 2003;57:425-33.10. Yamashita H, Okuma K, Wakui R, Kobayashi-Shibata S, Ohtomo K, Nakagawa K. Details ofrecurrence sites after elective nodal irradiation (ENI) using 3D-conformal radiotherapy (3D-CRT) combined with chemotherapy for thoracic esophageal squamous cell carcinoma--a retrospective analysis. Radiother Oncol. 2011;98:255-60.11. Kodaira T, Fuwa N, Kamata M, Furutani K, Tachibana H, Yamazaki T. Single-institute phase I/II trial of alternating chemoradiotherapy with 5-FU and nedaplatin for esophageal carcinoma. Anticancer research. 2006;26:471-8.12. Ohtsu A, Boku N, Muro K, Chin K, Muto M, Yoshida S, et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915-21. 13. Onozawa M, Nihei K, Ishikura S, Minashi K, Yano T, Muto M, et al. Elective nodal irradiation (ENI) in definitive chemoradiotherapy (CRT) for squamous cell carcinoma of the thoracic esophagus. Radiother Oncol. 2009;92:266-9.14. Ishikura S, Nihei K, Ohtsu A, Boku N, Hironaka S, Mera K, et al. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:2697-702.15. al-Sarraf M, Martz K, Herskovic A, Leichman L, Brindle JS, Vaitkevicius VK, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15:277-84.16. Araujo CM, Souhami L, Gil RA, Carvalho R, Garcia JA, Froimtchuk MJ, et al. A randomized trial comparing radiation therapy versus concomitant radiation therapy and chemotherapy in carcinoma of the thoracic esophagus. Cancer. 1991;67:2258-61.17. Ma JB, Song YP, Yu JM, Zhou W, Cheng EC, Zhang XQ, et al. Feasibility of involved-field conformal radiotherapy for cervical and upper-thoracic esophageal cancer. Onkologie. 2011;34:599-604.18. Kato H, Watanabe H, Tachimori Y, Iizuka T. Evaluation of neck lymph node dissection for thoracic esophageal carcinoma. The Annals of thoracic surgery. 1991;51:931-5.19. Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Annals of surgery. 1994;220:364-72; discussion 72-3.20. Huang W, Li B, Gong H, Yu J, Sun H, Zhou T, et al. Pattern of lymph node metastases and its implication in radiotherapeutic clinical target volume in patients with thoracic esophageal squamous cell carcinoma: A report of 1077 cases. Radiother Oncol. 2010;95:229-33.21. Chen J, Liu S, Pan J, Zheng X, Zhu K, Zhu J, et al. The pattern and prevalence of lymphatic spread in thoracic oesophageal squamous cell carcinoma. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2009;36:480-6.22. Ye T, Sun Y, Zhang Y, Zhang Y, Chen H. Three-field or two-field resection for thoracic esophageal cancer: a meta-analysis. The Annals of thoracic surgery. 2013;96:1933-41.23. Nishimura Y, Mitsumori M, Hiraoka M, Koike R, Nakamatsu K, Kawamura M, et al. A randomized phase II study of cisplatin/5-FU concurrent chemoradiotherapy for esophageal cancer: Short-term infusion versus protracted infusion chemotherapy (KROSG0101/JROSG021). Radiother Oncol. 2009;92:260-5.24. Poplin EA, Jacobson J, Herskovic A, Panella TJ, Valdivieso M, Hutchins LF, et al. Evaluation of multimodality treatment of locoregional esophageal carcinoma by Southwest Oncology Group 9060. Cancer. 1996;78:1851-6.25. Zhao KL, Ma JB, Liu G, Wu KL, Shi XH, Jiang GL. Three-dimensional conformal radiation therapy for esophageal squamous cell carcinoma: is elective nodal irradiation necessary? Int J Radiat Oncol Biol Phys. 2010;76:446-51.。
P20943648药理药效研究 动物模型

Invasive breast carcinomas are characterized by their strong heterogeneity, reflecting tumor histology and response to
epithelial–mesenchymal transition semantics
Epithelial–mesenchymal transition (EMT) consists of a rapid and often reversible change of cell phenotype. Epithelial cells loosen cell–cell adhesion structures including adherens junctions and desmosomes, modulate their polarity and rearrange their cytoskeleton: intermediate filaments typically switch from cytokeratins to vimentin. Cells become isolated, motile and resistant to apoptosis. The EMT was initially defined as the cellular remodeling occurring during heart morphogenesis, but the concept was extended to analogous transformation occurring during the formation of mesoderm and neural crest [1, 2]. In the past 20 years, EMT has been studied in detail in vitro allowing the characterization of numerous pathways, typically involving socalled ‘EMT master genes’, genes of a family of transcriptional factors including Snail, Twist, Zeb and E47 [3]. Based on the similarities between transducing pathways, the EMT concept has been extended to the physiological process of partial EMT taking place during wound healing and mammary tubulogenesis [4]. The link between EMT and stemness has been recently explored in several publications [5, 6]. Even though this link is somehow confusing when considering cell population renewal and dynamics, it emphasizes the plasticity of cells undergoing an EMT. In vivo, the EMT process generates poorly differentiated and potentially pluripotent cells rather than true fibroblasts, a differentiated cell type expressing tissue and organ specificity. This
33 Definitive Chemoradiotherapy 药理药效研究 动物模型

Copyright � The Korean Academy of Medical Sciences
Definitive Chemoradiotherapy with Capecitabine and Cisplatin in Patients with Esophageal Cancer: A Pilot Study
121
patients were 20-75 yr of age with a performance status of 0-2 on the Eastern Cooperative Oncology Group (ECOG) scale. Plus, adequate hematological (WBC count ≥4×109/L, platelet count ≥100×109/L, hemoglobin ≥9 g/dL), renal (serum creatinine ≤1.5 mg/dL and creatinine clearance ≥ 50 mL/min), and hepatic (total bilirubin ≤2.0 mg/dL and serum transaminase level ≤3 times the upper limit of the normal range) levels were also required. Patients were ineligible if they had previously received chemotherapy or radiation therapy, or had other severe medical illnesses, distant metastasis, another active malignancy in the last 5 yr, except treated nonmelanoma skin cancer or cervical dysplasia, or a history of anaphylaxis to drugs. The institutional review board of the authors’ institution approved the protocol, and written informed consent was obtained from all patients before enrollment.
实验动物学第八章实验动物的选择和应用

动物性别对药物敏感性的比较
动 物 敏感性比较 雄 >雌 雄 >雌 药 物
小鼠 大鼠
乙醇、氨基比林、氨基喋啶、氯仿、麦角 固醇、烟碱 肾上腺素、麦角、野白合碱、哇巴因、
雄 <雌
兔 猫 犬 雄 <雌 雄 <雌 雄 >雌
2018/4/5
8
二、 根据解剖和生理学特点选择实验动物
1. 选用解剖特点符合实验目的和要求的动物
兔的胸腔结构 兔的减压神经 犬和兔的甲状旁腺 大鼠、仓鼠无胆囊
2.选用生理特点符合实验目的和要求的动物 犬的红绿色盲 兔对致热原十分敏感 犬和猫对呕吐反应敏感 豚鼠易于致敏 致敏比较:豚鼠> 兔> 犬> 小鼠> 猫> 青蛙 豚鼠对维生素C缺乏敏感 家兔对射线十分敏感
2018/4/5
17
注意事项
根据年龄判断体重或根据体重判断年龄要注意品 种品系的不同
同批动物的年龄、体重尽可能一致,相差不应超 过10%。
在选择动物时,必须了解所选动物的寿命、怀孕 期、哺乳期等一些指标,这些指标对保证研究工 作的顺利进行及试验结果的分析是非常有用的。
2018/4/5
18
六、选用的动物性别应符合实验要求
巴比妥类、印防已毒素、乙基硫氨酸、固 醇类激素
苯芘比 二硝基苯酚 地高辛
2018/4/5
19
下列动物实验可选用单性别 1.与性别有关的解剖、生理、病理实验; 2.与性别无关的解剖、生理、病理实验; 3.已知药物作用与性别无关,如糖尿病、高血压;
4.性器官对实验的影响,如热板法筛选镇痛药物时 不用雄性小鼠。
1药理药效研究 动物模型

(一)生物大分子主要包括核酸、蛋白质、多糖等,其主要特征是由小分子的构件分子组成,具有复杂的空间结构,而且结构与生物活性密切相关。
生物小分子包括游离核苷酸、辅基辅酶、寡糖、寡肽、寡核苷酸、类脂、维生素、信号分子以及许多生物大分子的代谢产物等等。
乳糖操纵子包括:启动子;操纵基因;结构基因(Z, Y, A)(二)基因按其功能可分为:结构基因、调节基因、rRNA基因与tRNA基因。
常见的调节基因有microRNAs,siRNAs,piRNAs ……真核生物的RNA聚合酶(3种):RNA聚合酶I,II,III。
种类I II III分布核仁核基质核基质产物rRNA mRNA, microRNA 5S rRNA, tRNA, siRNA任一链上的可读框(ORF)方向总是由5,→3,。
原核生物转座因子主要有二类:插入序列(IS);复合型转座子(Tn)根据转座的的机制和结果,可将转座分为:复制型转座、保守型转座。
质粒DNA可以有共价闭合环状DNA、缺口的环状DNA、线性DNA三种结构状态。
琼脂糖凝胶电泳时涌动速度的快慢:超螺旋<线性<环状DNA(与分子量有关)按质粒复制的调控及其拷贝数可分两类:严紧控制型质粒、松弛控制型质粒。
影响质粒稳定性的因素:①宿主细胞分裂时质粒能否均衡地分配到子代细胞。
②质粒分子自身结构的稳定性。
真核生物基因组可分为细胞核基因组和细胞器基因组。
真核生物基因组存在大量的重复序列:单拷贝序列、中度重复序列(Alu家族)、高度重复序列。
DNA多态性主要包括单核苷酸多态性(SNP)和串联重复序列多态性。
真核基因组的另一特点是存在多基因家族与假基因真核生物有两类细胞器能携带遗传物质:线粒体和叶绿体。
高等动物线粒体基因组具有独特的特点:①母系遗传;②线粒体DNA损伤后不易修复;(三) ③遗传密码与通用遗传密码存在差别。
蛋白质组学研究需要两条互补的实验工作流程:基于凝胶的工作流程和基于液相色谱的工作流程。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
网络可靠性主要指系统的容错能力,即当网络系统突然发生 故障时,系统能够继续工作及迅速恢复的能力。
网络系统可靠性的相关概念 (1) 软件容错 (2) 硬件容错 (3) 容错存储 (4) 数据备份 (5) 容错电源
服务器
心跳线
服务器
双机容错技术
(1)共享磁盘阵列柜方式 (2)镜像磁盘方式
服务器
硬盘
硬盘
服务器
8.1.5 安全接入技术
2.局域网的安全接入 PPPoE
特点: 第一,PPPoE很容易检查到用户下线,可通过一个PPP会话的建
立和释放对用户进行基于时长或流量的统计,计费方式灵活方便。 第二,PPPoE可以提供动态IP地址分配方式,用户无需任何配置,
网管维护简单,无需添加设备就可解决IP地址短缺问题,同时根据分 配的IP地址,可以很好地定位用户在本网内的活动。
信息加密技术
数据加密过程就是通过加密系统把原始的数字信息(明文),按照加 密算法变换成与明文完全不同的数字信息(密文)的过程。
数字签名技术
对文件进行加密只解决了传送信息的保密问题,而防止他人对传输的 文件进行破坏,以及如何确定发信人的身份还需要采取其它的手段, 这一手段就是数字签名。
8.1.3 网络攻击与网络病毒
UDP IP
OSI数据链路层 物理层
SNMP有5种消息类型: Get Request Get Response Get Next Request Set Request Trap
Hale Waihona Puke ● 5.SNMP的弱点没有伸缩型,在大型网络中,轮询会产生巨大的网络管理通信 量,因而导致通信拥挤的情况发生。它将收集数据的负担加在 网络管理控制台上,在管理几个网段时也许能轻松的收集网络 信息,当它们监控许多网段时,就非常困难了。
WWW、E-mail)。
相隔离。
● 防火墙产品 1 防火墙分类 2 防火墙特性比较
● 入侵监测与入侵保护 1 入侵监测与入侵保护的概念 2 入侵监测、入侵保护与防火墙的区别
8.1.5 安全接入技术
1.远程访问的安全接入
远程访问系统
远程访问系统模型
PC
PSTN
Moderm
IP
PPP 拨号访问服务器
网络层防火墙
应用层防火墙
价格
便宜,可设置在内部网络与 比网络层防火墙要高得多,需要购
Internet相连接的路由器上, 置专门的软件及高性能的硬件系统,
无需另购
否则会产生严重的通信瓶颈
安装、 配置与
管理
功能
简单,过滤规则易于维护
功能单一。按照规则表对数 据包进行过滤;只能提供基 本的统计记录
复杂。管理员不仅需要清楚地了解 TC P/IP,还需要为存储、监视和报 告功能作出最合理的设置
● Sircam病毒 ● Netsky病毒 ● 冲击波病毒 ● 冲击波杀手病毒 ●冲击波杀手变种病毒 ● 震荡波病毒
8.1.4 网络安全设施
● 防火墙的基本概念
什么是防火墙 防火墙是一种访问控制技术,用于加强两个或多个网 络间的边界防卫能力。
内部网络
防火墙
外部网络 (Internet)
设置防火墙的原因
受控存取保护级,实施比C1级更精细的自主访问控制 标记安全保护级,从本级开始,不仅具有自主访问控制,而且具 有强制访问控制 结构化保护级
安全域级 验证设计级
网络安全基础 1 网络安全的内涵 2 可能受到威胁的网络资源 3 网络安全问题日益突出的主要原因 4 攻击网络安全的主要途径
网络安全控制措施 1 物理安全 2 访问控制 3 传输安全
应用层防火墙 应用层防火墙也称为代理服务器,它能够代替网络用 户完成特定的TCP/IP功能,控制对应用程序的访问。
应用层防火墙
get
请求 内部 外部 x.html
Web Web
Internet
返回
Web 浏览器
服务 代理
服务 代理
return x.html
网络层防火墙和应用层防火墙比较 (1)
项目 分类
● 4.简单网络管理协议的工作原理
SNMP代理和管理站通过标准消息通信,这些消息中的每一个都 是一个单个的包。因此,SNMP使用UDP作为传输协议。
SNMP的OSI参考模型
Layer7 Layer6 Layer5 Layer4 Layer3 Layer2 Layer1
SNMP OSI表示层 OSI会话层
企业级
RMON
8.2.4 计算机网络管理的实施 1 网络管理员 2 实施网络管理 3 布线系统的日常维护 4 关键设备的管理 5 IP地址的管理
8.2.5 计算机网络管理的发展趋势 ● 网络管理层次化 ● 网络管理集成化 ● 网络管理Web化
8.3 网络热点技术
8.3.1 目录服务
什么是目录
目录就是一个数据库,它包含了网络以及在网络上运行 的应用程序所需的信息。每一个网络,即使是简单的网 络,也会有某种形式的目录,而且通常不只一个。
网络层防火墙和应用层防火墙比较 (2)
项目 分类
网络层防火墙
应用层防火墙
安全性
使用路由器过滤数据包可以 有效地阻止罪犯进入内部网 络,但罪犯有可能绕过过滤 器。整个内部网络容易受到 来自Internet的入侵。
可以使内部网络与外部网络完全隔 离。可以监视外部主机的连接企图, 并使用启发式分析,确定是否有罪 犯要闯入网络。防火墙系统中还存 有可疑活动的详细报告,管理人员 可以利用该报告调查有潜在危险的 Internet主机,并采取预防措施。
第8章药理药效研究 动物模型
8.1 计算机网络安全
计算机网络安全是指通过采取各种技术的和管理的 措施,确保网络数据的可用性、完整性和保密性,其目 的是确保经过网络传输和交换的数据不会发生增加、修 改、丢失和泄漏等 。
8.1.1 网络系统安全概述
计算机安全基础
1. 什么是计算机安全 国际标准化组织ISO对计算机安全作了如下定义:计算机 安全是指为保护数据处理系统而采取的技术的和管理的安 全措施,保护计算机硬件、软件和数据不会因偶然或故意 的原因而遭到破坏、更改和泄密。
8.2.3 远程监控(RMON) RMON分为嵌入式和分布式两种:
嵌入式RMON 当RMON嵌入到网络设备(如集线器)之中时,它的作用效率
更高、经济上更划算,可以一次监控所有连通的局域网网段。
分布式RMON RMON I及RMON Ⅱ在7层模型中的层次
标准 RMON I I
标准 RMON I
应用层 表示层 会话层 运输层 网络层 数据链路层 物理层
8.1.2 信息安全技术
PKI(Public Key Infrastructure,意为“公钥基础设 PKI技术就是利用公钥理论和技术施建”立)的提供信息安全服务的基础设施, 是CA认证、数字证书、数字签名以及相关安全应用组件模块的集合。
CA(Certificate Authority)认证技术
在电子商务中,必须从技术上保证在交易过程中能够实现:身份认证、 安全传输、不可否认性、数据一致性。CA证书认证技术采用了加密 传输和数字签名技术,能够实现上述要求。
为什么使用目录 (1) 一次登录 (2) (2) 设备识别和 定位
(3) (3) 与位置无关 (4) (4) 简化管理 (5) (5) 可靠性
LDAP简介
LDAP(轻型目录访问 协议)是促使目录流行 的关键技术,是目录服 务器的Internet标准协议。
活动目录与Novell目录服务、Windows NT 4域的区别
网络管理应用软件 网络管理工具 网络管理平台 网络管理协议 网络平台
8.2.2 简单网络管理协议SNMP ● 1.SNMP管理模型
● 3.SNMP协议
Trap GetResponse Set GetNext Get
Trap GetResponse Set GetNext Get
管理应用进程
SNMP管理者 UDP IP
功能多,应用灵活。能够提供许多 报告、统计和监控的手段,以控制 网络的运行;提供有益的内部网络 功能,如存储频繁访问页,设置拒 绝与缺乏商业价值的站点建立连接
编址 方式
要求所有的内部主机都要有
正确分配的地址。这些地址 在Internet上都是可见的,可 以被Internet上的系统访问
允许内部网络使用任何寻址模式, 因为能够与Internet实现真正通信的 唯一系统就是应用层防火墙。这就 使管理员可以不受任何约束地对其 内部网络进行灵活的编址
2. 计算机安全的主要内容
计算机硬件的安全性;软件安全性;数据安全性;计算机 运行安全性。
3. 破坏计算机安全的途径
4. 保护计算机安全的措施
5. 计算机安全等级
安全等级 D1级 C1级 C2级 B1级
B2级 B3级 A1级
主要内容 安全保护欠缺级,无安全保护级,计算机安全的最低一级 自主安全保护级
认证服务器
设备端提供的服务 客户端PAE
认证服务器
授控端口
端口非授权 非授控端口
LAN/WAN
802.1x协议仅仅关注端口的打开与关闭,对于合法用户(根据帐号和 密码)接入时,该端口打开,而对于非法用户接入或没有用户接入时, 则该端口处于关闭状态。认证的结果在于端口状态的改变。
8.1.6 网络系统可靠性
第三,用户通过免费的PPPoE客户端软件(如EnterNet),输入 用户名和密码就可以上网,跟传统的拨号上网差不多,最大程度地延 续了用户的习惯,从运营商的角度来看,PPPoE对其现存的网络结构 进行变更也很小。
8.1.5 安全接入技术 2.局域网的安全接入 802.1X
客户端
客户端PAE
设备端
网络黑客与入侵者 黑客一词是指程序员,而不是那些非法破坏系统安
