美国高中化学酸碱反应 Acid-Base Reactions
高中化学英文词汇

高中化学英文词汇High School Chemistry English VocabularyChemistry is a fascinating subject that explores the composition, properties, and behavior of matter. Whether you are a high school student diving into the world of chemistry for the first time or a seasoned science enthusiast, it is essential to have a good understanding of the English vocabulary commonly used in this field. In this article, we will introduce some key terms and concepts in high school chemistry, along with their English equivalents.1. Elements and AtomsIn chemistry, an element is a pure substance that cannot be broken down into simpler substances by chemical means. Each element is made up of atoms, which are the smallest units of matter that retain the properties of the element. Some common elements include hydrogen, oxygen, carbon, and nitrogen.2. Compounds and MoleculesCompounds are substances composed of two or more elements chemically combined in fixed proportions. The smallest unit of a compound is called a molecule, which consists of two or more atoms bonded together. Water (H2O) and carbon dioxide (CO2) are examples of compounds.3. Chemical ReactionsChemical reactions involve the breaking and forming of chemical bonds between atoms. During a chemical reaction, reactants are transformed intoproducts through the rearrangement of atoms. Some common types of chemical reactions include synthesis, decomposition, combustion, and single displacement.4. Acids and BasesAcids are substances that donate protons (H+) in a chemical reaction, while bases are substances that accept protons. The strength of an acid or base is determined by its pH level, with acids having a pH below 7 and bases having a pH above 7. Examples of acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4), while common bases include sodium hydroxide (NaOH) and ammonia (NH3).5. Chemical EquationsChemical equations are symbolic representations of chemical reactions, with reactants on the left side and products on the right side. Coefficients are used to balance chemical equations and ensure the conservation of mass. For example, the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water can be represented as 2H2 + O2 → 2H2O.6. States of MatterMatter can exist in three states: solid, liquid, and gas. Solids have a fixed shape and volume, liquids have a fixed volume but can change shape, and gases have neither a fixed shape nor volume. Changes in temperature and pressure can cause substances to change states, such as melting, freezing, boiling, and condensation.7. The Periodic TableThe periodic table is a tabular arrangement of the elements based on their atomic number and chemical properties. Elements are organized into groups and periods, with similar properties grouped together. The periodic table provides valuable information about the properties of elements, such as their atomic mass, symbol, and electron configuration.8. StoichiometryStoichiometry is the branch of chemistry that deals with the calculation of quantities of reactants and products in a chemical reaction. It involves balancing chemical equations, determining the limiting reactant, and calculating the theoretical yield of a reaction. Stoichiometry is essential for understanding the composition of compounds and predicting the outcome of reactions.In conclusion, mastering the English vocabulary of high school chemistry is crucial for success in the subject. By familiarizing yourself with key terms and concepts, you can enhance your understanding of chemical principles and communicate effectively with peers and educators. Remember to review and practice these terms regularly to solidify your knowledge and confidence in the exciting world of chemistry.。
美国高中化学酸碱反应 Acid-Base Reactions

• What types of acid-base reactions do these titration graphs show?
Titrations practice
Titrations practice
• Graphs shows titration of 0.5 M NaOH with 50ml of an unknown acid. After titration NaBr salt crystals were isolated from the solution. a) What is the acid used? Is it strong or e produced from an acid-base reaction
Regulating pH
• Living things interact with acids and bases all the time; their pH must be regulated • Buffer
• How do we know the Standard solution is neutral? • pH Indicators酸碱指示剂
• End point • Equivalence point • Neutral point
– Volume of acid/base used gives us molarity – MAVA = MBVB
Titrations practice
• If 15.0 mL of 0.50 M NaOH is used to neutralize 25.0 mL of HC2H3O2, what is the molarity of the acid solution? NaOH + HC2H3O2 H2O + NaC2H3O2; 1:1 ratio • MAVA = MBVB • MA= MBVB/VA = (0.50 M)(15.0 ml)/25.0 ml = 0.30 M • If 25.0 mL of a standard 0.05 M HCl solution is required to neutralize 20.0 mL of a solution of Sr(OH)2, what is the concentration of the base? 2 HCl + Sr(OH)2 SrCl2 + 2H2O; 2:1 ratio • MAVA = 2 MBVB • MB = MAVA/2VB = (0.05 M)(25.0 ml)/(2)(20.0 ml) = 0.03 M
SAT化学词汇

附录三名词总结第二章正电荷(positive charge)原子核(atomic nucleus)负电荷(negative charge)质子(proton)中子(neutron)道尔顿模型(Dalton Model)汤姆逊模型(Thomason Model)库伦(coulomb)卢瑟福模型(Rutherford Model)波尔模型(N.Bohr Model)原子轨道学说(Atomic Orbital Hypothesis) 基态(ground state)激发态(excited state)电离(ionization)普朗克常数(Planck’s constant)频率(frequency)波长(wavelength)物质波假设(The L.de Broglie Hypothesis) 电子衍射实验(electron diffraction experiment)海森堡测不准原则(Heisenberg Uncertainty Principle)位置(position)动量(momentum)量子数(quantum number)电子层(shells)亚层(subshells)轨道(orbit)主量子数(Principal Quantum Number)角量子数(Azimuthal Quantum Number)磁量子数(Magnetic Quantum Number)自旋量子数(Spin Quantum Number)电子排布(Electron Configuration)保利不相容原理(Pauli’s Exclusion Principle)能量最低原理(Lowest Energy Principle, Aufbau Principle) 价电子(valence electron)洪特规则(Hund's Rules)能级交错原理(energy overlay)元素周期表(the Periodic Table)原子序数(Atomic Number)同位素(Isotope)质量数(Mass Number)原子质量(Atomic Masses)周期(period)族(group)主族(main group)碱金属(alkali metal)碱土金属(alkaline earth metal)卤素(halogen)惰性气体(noble gas)硼族元素(boron group element)碳族元素(carbon group element)氮族元素(nitrogen group element)氧族元素(oxygen group element)。
酸碱理论
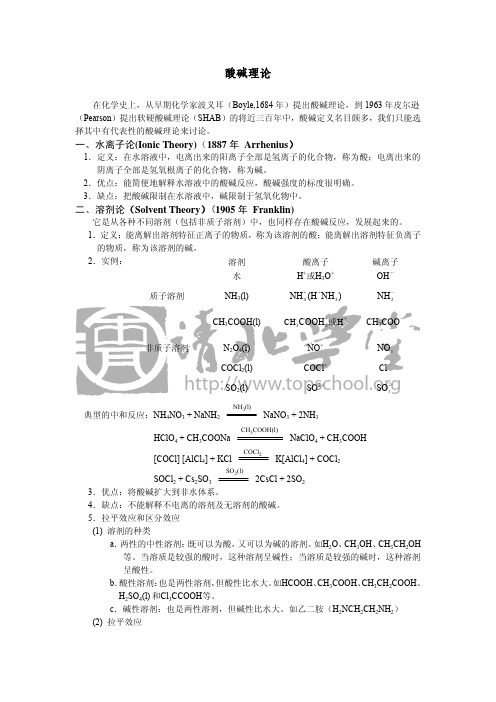
_
4.用质子理论来解释一些反应:
(1) 气相中的酸碱反应: HCl(g)
A1
(2) 离解反应(dissociation reactions):
H+
a. auto ionization : H2O
+ H+
H2O
Kw
H3O
+
+
OH
_
b. acid ionization : HAc
− 、 HCO 3 ? What is the conjugate acid of each of the following bases: CN − 、 H 2S 、 PH + 4 − − 、 H 2 O 、 HCO 3 ? SO 2 4
-
H+ + NH3(g) B2
+
Lewis base
-
acid−base adduct H2O
(1) adduct reaction:H + : OH
Cu 2 + + 4NH + 4 2+ (3) substitution reaction of base: Cu(NH 3 ) 4 + 2OH Cu(OH) 2 + 4NH 3 (4) both substitution reaction:Ba(OH)2 + H2SO4 BaSO4↓+2H2O 4.优点: (1) 它包括了水离子论、溶剂论、质子论三种理论; (2) 它扩大了酸的范围。 5.缺点:无统一的酸碱强度的标度。 由于电子论包括了所有的酸碱理论, 所以该理论又称为广义酸碱理论。 酸碱电子论的提 出,可以把所有的化学反应分为三大类:
酸碱滴定法acid-basetitration

① 强酸(碱)溶液
C mol/L HCl
[H+]=[OH-]+[Cl-]=
Kw [H
]
C HCl
CHCl > 10-6 mol/l → [H+] = CHCl
② 弱酸(碱)溶液
a. 一元弱酸(碱)
HA Ka H+ + A-
[H+][A-] Ka = [HA]
[H+]=[]+[A-]=
Kw [H
1. 指示剂+惰性染料(背景)
甲基橙色
PH≥4.4 黄 PH=4.1 橙 PH≤3.1 红
甲基橙+靛蓝色
绿 浅灰 紫
2. 二种以上指示剂混合, 使变色点更符合计量点
溴甲酚绿-甲基红: PH<3.8: 黄 + 红→ 酒红(橙) PH=5.1:接近无色 PH>6.2:蓝 + 黄→ 绿
酸碱溶液酸碱度的计算
TE% [OH- ]-[HA] -[H ] 100% Csp
由于强碱滴定弱酸化学计量点溶液呈碱性, 故[H+]可略去,又因为[HA]/CSP=δHA
上式可简化为:
TE%
[OH (
Csp
]
HA
)
100%
Csp=C0·V0/Vep, C0, V0为被测物质的原始浓度 和体积, Vep为计量点时溶液的体积。
酚酞:有机弱酸Pka=9.1
羟式
醌式
甲基橙:有机弱碱
偶氮式 醌式
现以HIn代表弱酸指示剂,其离解平衡表示如下:
HIn H+ + In酸式色 碱式色
以InOH代表弱碱指示剂,其离解平衡表示如下:
生物化学考研精解名词解释答案(上)版

生化考研精解名词解释答案(上)温馨提示:部分解释不是采自教材,如有疑问,请参考课本!第一章糖类(p6)6.构型(configuration):在立体化学中,因分子中存在不对称中心而产生的异构体中的原子或取代基团的空间排列关系。
有D型和L型两种。
构型的改变要有共价键的断裂和重新组成,从而导致光学活性的变化。
7.构象(conformation):分子中由于共价单键的旋转所表现出的原子或基团的不同空间排列。
指一组结构而不是指单个可分离的立体化学形式。
构象的改变不涉及共价键的断裂和重新组成,也无光学活性的变化。
12.差向异构体(epimer):在立体化学中,含有多个手性碳原子的立体异构体中,只有一个手性碳原子的构型不同,其余的构型都相同的非对映体叫差向异构体。
14.异头碳(anomeric carbon):单糖由直链变成环状结构时,羰基碳原子成为新的手性中心,导致C1差向异构化,产生两个非对映异构体。
在环状结构中,半缩醛碳原子称为异头碳原子。
15.半缩醛(hemiacetal):醛基和一个醇基缩合形成的产物。
通过该反应,使单糖形成环状结构。
16.变旋(mutarotation):当一种旋光异构体如糖,溶于水中转变成几种不同旋光异构体的平衡混合物时,随着时间而发生的旋光变化。
18.糖苷键(glycosidic bond):一个单糖或糖链还原端半缩醛上的羟基与另一个分子(如醇、糖、嘌呤或嘧啶)的羟基、胺基或巯基之间缩合形成的缩醛键或缩酮键。
常见的糖苷键有O-糖苷键和N-糖苷键。
19.还原糖(reducing sugar):能够还原斐林(H.von Fehling)试剂或托伦斯(B.Tollens)试剂的糖称为还原糖,所有的单糖(除二羟丙酮),不论醛糖、酮糖都是还原糖。
大部分双糖也是还原糖,蔗糖例外。
22.淀粉(starch):由D-葡萄糖单体组成的同聚物。
包括直链淀粉和支链淀粉两种类型,为植物中糖类的主要贮存形式。
Lewis酸碱理论

Lewis酸碱理论理论发展布朗斯特酸碱理论概念的核心系于分子或离子间的质子转移,显然无法对不涉及质子转移,但却具有酸碱特征的反应做解释.这一不足在布朗斯特概念提出的同年由美国化学家路易斯提出的酸碱电子理论(the electronic theory of acid and alkali),也称广义酸碱理论、路易斯(lewis)酸碱理论,是1923年美国物理化学家吉尔伯特·牛顿·路易斯(Lewis G N)提出的一种酸碱理论,它认为:凡是可以接受外来电子对的分子、基团或离子为酸;凡可以提供电子对的分子、离子或原子团为碱。
这种理论包含的酸碱范围很广,但是,它对确定酸碱的相对强弱来说,没有统一的标度,对酸碱的反应方向难以判断。
后来,皮尔逊提出的软硬酸碱理论弥补了这种理论的缺陷。
电子酸碱该理论认为:凡是能够接受外来电子对的分子、离子或原子团称为路易斯酸(Lewis acid),即电子对接受体,简称受体;凡是能够给出电子对的分子、离子或原子团称为路易斯碱(Lewis base),即电子对给予体,简称给体。
或者说:路易斯酸(Lewis acid)是指能作为电子对接受体(Electron pair acceptor)的原子,分子,离子或原子团;路易斯碱(Lewis base)则指能作为电子对给予体(Electron pair donor)的原子,分子,离子或原子团;酸碱反应是电子对接受体与电子对给予体之间形成配位共价键的反应.路易斯酸的分类1、配位化合物中的金属阳离子,例如[Fe(H2O)6]3+和[Cu(NH3)4]2+中的Fe3+离子和Cu2+离子.2、有些分子和离子的中心原子尽管满足了8电子结构,仍可扩大其配位层以接纳更多的电子对.如 SiF4 是个路易斯酸,可结合2个F–的电子对形成[SiF6]2–.3、另一些分子和离子的中心原子也满足8电子结构,但可通过价层电子重排接纳更多的电子对.再如CO2能接受OH–离子中O 原子上的孤对电子4、某些闭合壳层分子可通过其反键分子轨道容纳外来电子对.碘的丙酮溶液呈现特有的棕色,是因为I2分子反键轨道接纳丙酮中氧原子的孤对电子形成配合物(CH3)2COI2.再如四氰基乙烯(TCNE)的π*轨道能接受一对孤对电子。
分析化学英文课件04 酸碱平衡Acid-Base Equilibria

Autoprotolysis
As the elemental unit of positive charge, the proton possesses a charge density which makes its independent existence in a solution extremely unlikely. Thus, in order to transform HB into B-, a proton acceptor must be present. Often as in the dissociation of acetic acid in water, this base may be the solvent(H2O) itself.
HAc = H+ + Ac- , H2O + H+ = H3O+
Combination of this two equations, HAc + H2O = H3O+ + Ac-
The protonated water molecule or hydrated proton H3O+, may be called a “hydronium ion”, but it is usually designated simply by “hydrogen ion” and often written as “H+”.
According to Arrhenius theory, acids dissociate into
hydrogen ions and anions, and bases dissociate into
hydroxide ions and cations.
常见化学反应英文单词

acetate 醋酸盐acid 酸Actinium(Ac) 锕aldehyde 醛alkali 碱,强碱alkalinity 碱性alkalinization 碱化alkaloid 生物碱alloy 合金Aluminium(Al) 铝Americium(Am) 镅ammonia 氨analysis 分解anhydride 酐anion 阴离子anode 阳极,正极Antimony(Sb) 锑apparatus 设备aqua fortis 王水Argon(Ar) 氩Arsenic(As) 砷asphalt 沥青Astatine(At) 砹atom 原子atomic mass 原子质量atomic number 原子数atomic weight 原子量Barium(Ba) 钡base 碱benzene 苯Berkelium(Bk) 锫Beryllium(Be) 铍Bismuth(Bi) 铋bivalent 二价body 物体bond 原子的聚合Boron(B) 硼Bromine(Br) 溴Bunsen burner 本生灯burette 滴定管butane 丁烷Cadmium(Cd) 镉Caesium(Cs) 铯Calcium(Ca) 钙Californium(Cf) 锎Carbon(C) 碳catalysis 催化作用catalyst 催化剂cathode 阴极,负极cation 阳离子caustic potash 苛性钾caustic soda 苛性钠Cerium(Ce) 铈chemical fiber 化学纤维Chlorine(Cl) 氯Chromium(Cr) 铬Cobalt(Co) 钴combination 合成作用combustion 燃烧compound 合成物compound 化合物Copper(Cu) 铜cracking 裂化crucible pot, melting pot 坩埚crude oil, crude 原油cupel 烤钵Curium(Cm) 锔derivative 衍生物dissolution 分解distillation column 分裂蒸馏塔distillation 蒸馏Dysprosium(Dy) 镝Einsteinium(Es) 锿electrode 电极electrolysis 电解electrolyte 电解质electron 电子element 元素endothermic reaction 吸热反应Erbium(Er) 铒ester 酯Europium(Eu) 铕exothermic reaction 放热反应fatty acid 脂肪酸fermentation 发酵Fermium(Fm) 镄filter 滤管flask 烧瓶Fluorine(F) 氟fractional distillation 分馏fractionating tower 分馏塔fractionation 分馏Francium(Fr) 钫fuel 燃料fusion, melting 熔解Gadolinium(Gd) 钆Gallium(Ga) 镓gas oil 柴油gel 凝胶体Germanium(Ge) 锗Gold(Au) 金graduate, graduated flask 量筒,量杯gram atom 克原子Hafnium(Hf) 铪halogen 成盐元素Helium(He) 氦high-grade petrol, high-octane petrol 高级汽油,高辛烷值汽油Holmium(Ho) 钬hydracid 氢酸hydrate 水合物hydrocarbon 碳氢化合物,羟hydrocarbon 烃,碳氢化合物hydrochloric acid 盐酸hydrogen sulfide 氢化硫Hydrogen(H) 氢hydrolysis 水解hydrosulphuric acid 氢硫酸hydroxide 氢氧化物,羟化物Indium(In) 铟inorganic chemistry 无机化学Iodine(I) 碘ion 离子Iridium(Ir) 铱Iron(Fe) 铁isomer 同分异物现象isomerism, isomery 同分异物现象isotope 同位素kerosene, karaffin oil 煤油Krypton(Kr) 氪Lanthanum(La) 镧Lawrencium(Lr) 铹Lead(Pb) 铅Lithium(Li) 锂litmus paper 石蕊试纸litmus 石蕊LNG, liquefied natural gas 液化天然气LPG, liquefied petroleum gas 液化石油气lubricating oil 润滑油Lutetium(Lu) 镥Magnesium(Mg) 镁Manganese(Mn) 锰matrass 卵形瓶Mendelevium(Md) 钔Mercury(Hg) 汞metal 金属metalloid 非金属methane 甲烷,沼气mixture 混合molecule 分子Molybdenum(Mo) 钼monovalent 单价natural gas 天然气Neodymium(Nd) 钕Neon(Ne) 氖Neptunium(Np) 镎Nickel(Ni) 镍Niobium(Nb) 铌nitric acid 硝酸Nitrogen(N) 氮Nobelium(No) 锘Nuclear Fusion 核聚变octane number 辛烷数,辛烷值olefin 烯烃organic acid 有机酸organic chemistry 有机化学Osmium(Os) 锇oxide 氧化物oxidization, oxidation 氧化Oxygen(O) 氧Palladium(Pd) 钯paraffin 石蜡petrol 汽油(美作:gasoline)PH indicator PH值指示剂,氢离子(浓度的)负指数指示剂phosphate 磷酸盐Phosphorus(P) 磷pipette 吸液管plastic 塑料Platinum(Pt) 铂Plutonium(Pu) 钚Polonium(Po) 钋polymer 聚合物polymerizing, polymerization 聚合potassium carbonate 碳酸钾Potassium(K) 钾Praseodymium(Pr) 镨precipitation 沉淀product 产物electrochemical analysis 电化学分析on-line analysis 在线分析macro analysis 常量分析characteristic 表征micro analysis 微量分析deformation analysis 形态分析semimicro analysis 半微量分析systematical error 系统误差routine analysis 常规分析random error 偶然误差arbitration analysis 仲裁分析gross error 过失误差normal distribution 正态分布accuracy 准确度deviation 偏差precision 精密度relative standard deviation 相对标准偏差(RSD)coefficient variation 变异系数(CV)confidence level 置信水平confidence interval 置信区间significant test 显著性检验significant figure 有效数字standard solution 标准溶液titration 滴定stoichiometric point 化学计量点end point 滴定终点titration error 滴定误差primary standard 基准物质amount of substance 物质的量standardization 标定chemical reaction 化学反应concentration 浓度chemical equilibrium 化学平衡titer 滴定度general equation for a chemical reaction 化学反应的通式proton theory of acid-base 酸碱质子理论acid-base titration 酸碱滴定法dissociation constant 解离常数conjugate acid-base pair 共轭酸碱对acetic acid 乙酸hydronium ion 水合氢离子electrolyte 电解质ion-product constant of water 水的离子积ionization 电离proton condition 质子平衡zero level 零水准buffer solution 缓冲溶液methyl orange 甲基橙acid-base indicator 酸碱指示剂phenolphthalein 酚酞coordination compound 配位化合物center ion 中心离子cumulative stability constant 累积稳定常数alpha coefficient 酸效应系数overall stability constant 总稳定常数ligand 配位体ethylenediamine tetraacetic acid 乙二胺四乙酸side reaction coefficient 副反应系数coordination atom 配位原子coordination number 配位数lone pair electron 孤对电子chelate compound 螯合物metal indicator 金属指示剂chelating agent 螯合剂masking 掩蔽demasking 解蔽electron 电子catalysis 催化oxidation 氧化catalyst 催化剂reduction 还原catalytic reaction 催化反应reaction rate 反应速率electrode potential 电极电势activation energy 反应的活化能 redox couple 氧化还原电对potassium permanganate 高锰酸钾 iodimetry 碘量法potassium dichromate 重铬酸钾 cerimetry 铈量法redox indicator 氧化还原指示oxygen consuming 耗氧量(OC)chemical oxygen demanded 化学需氧量(COD) dissolved oxygen 溶解氧(DO)precipitation 沉淀反应argentimetry 银量法heterogeneous equilibrium of ions 多相离子平衡 aging 陈化postprecipitation 继沉淀coprecipitation 共沉淀ignition 灼烧fitration 过滤decantation 倾泻法 chemical factor 化学因数spectrophotometry 分光光度法 colorimetry 比色分析transmittance 透光率 absorptivity 吸光率calibration curve 校正曲线 standard curve 标准曲线monochromator 单色器 source 光源wavelength dispersion 色散absorption cell 吸收池detector 检测系统 bathochromic shift 红移Molar absorptivity 摩尔吸光系数 hypochromic shift 紫移acetylene 乙炔ethylene 乙烯acetylating agent 乙酰化剂acetic acid 乙酸adiethyl ether 乙醚ethyl alcohol 乙醇acetaldehtde 乙醛β-dicarbontl compound β–二羰基化合物bimolecular elimination 双分子消除反应bimolecular nucleophilic substitution 双分子亲核取代反应open chain compound 开链族化合物molecular orbital theory 分子轨道理论chiral molecule 手性分子tautomerism 互变异构现象reaction mechanism 反应历程chemical shift 化学位移Walden inversio 瓦尔登反转nEnantiomorph 对映体addition rea ction 加成反应dextro- 右旋levo- 左旋stereochemistry 立体化学stereo isomer 立体异构体Lucas reagent 卢卡斯试剂covalent bond 共价键conjugated diene 共轭二烯烃conjugated double bond 共轭双键conjugated system 共轭体系conjugated effect 共轭效应isomer 同分异构体isomerism 同分异构现象organic chemistry 有机化学hybridization 杂化hybrid orbital 杂化轨道heterocyclic compound 杂环化合物peroxide effect 过氧化物效应tvalence bond theory 价键理论sequence rule 次序规则electron-attracting grou p 吸电子基Huckel rule 休克尔规则Hinsberg test 兴斯堡试验infrared spectrum 红外光谱Michael reacton 麦克尔反应halogenated hydrocarbon 卤代烃haloform reaction 卤仿反应systematic nomenclatur 系统命名法eNewman projection 纽曼投影式aromatic compound 芳香族化合物aromatic character 芳香性rClaisen condensation reaction克莱森酯缩合反应Claisen rearrangement 克莱森重排Diels-Alder reation 狄尔斯-阿尔得反应Clemmensen reduction 克莱门森还原Cannizzaro reaction 坎尼扎罗反应positional isomers 位置异构体unimolecular elimination reaction 单分子消除反应unimolecular nucleophilic substitution 单分子亲核取代反应benzene 苯functional grou 官能团pconfiguration 构型conformation 构象confomational isome 构象异构体electrophilic addition 亲电加成electrophilic reagent 亲电试剂nucleophilic addition 亲核加成nucleophilic reagent 亲核试剂nucleophilic substitution reaction亲核取代反应active intermediate 活性中间体Saytzeff rule 查依采夫规则cis-trans isomerism 顺反异构inductive effect 诱导效应tFehling’s reagent 费林试剂phase transfer catalysis 相转移催化作用aliphatic compound 脂肪族化合物elimination reaction 消除反应Grignard reagent 格利雅试剂 nuclear magnetic resonance 核磁共振alkene 烯烃 allyl cation 烯丙基正离子leaving group 离去基团 optical activity 旋光性boat confomation 船型构象 silver mirror reaction 银镜反应Fischer projection 菲舍尔投影式 Kekule structure 凯库勒结构式Friedel-Crafts reaction 傅列德尔-克拉夫茨反应 Ketone 酮carboxylic acid 羧酸 carboxylic acid derivative 羧酸衍生物hydroboration 硼氢化反应 bond oength 键长bond energy 键能 bond angle 键角carbohydrate 碳水化合物 carbocation 碳正离子carbanion 碳负离子 alcohol 醇Gofmann rule 霍夫曼规则 Aldehyde 醛Ether 醚Polymer 聚合物product 化学反应产物Promethium(Pm) 钷Protactinium(Pa) 镤purification 净化qualitative analysis 定性分析quantitative analysis 定量分析chemical analysis 化学分析instrumental analysis 仪器分析titrimetry 滴定分析gravimetric analysis 重量分析法regent 试剂chromatographic analysis 色谱分析radical 基Radium(Ra) 镭Radon(Rn) 氡reagent 试剂reducer 还原剂refinery 炼油厂refining 炼油reforming 重整retort 曲颈甑reversible 可逆的Rhenium(Re) 铼Rhodium(Rh) 铑Rubidium(Rb) 铷Ruthenium(Ru) 钌salt 盐Samarium(Sm) 钐Scandium(Sc) 钪Selenium(Se) 硒separation 分离series 系列Silicon(Si) 硅Silver(Ag) 银soda 苏打sodium carbonate 碳酸钠Sodium(Na) 钠solution 溶解solvent 溶剂still 蒸馏釜stirring rod 搅拌棒Strontium(Sr) 锶structural formula 分子式Sulphur(S) 锍sulphuric acid 硫酸symbol 复合synthesis 合成synthetic rubber 合成橡胶Tantalum(Ta) 钽Technetium(Tc) 锝Tellurium(Te) 碲Terbium(Tb) 铽test tube 试管Thallium(Tl) 铊Thorium(Th) 钍Thulium(Tm) 铥Tin(Sn) 锡Titanium(Ti) 钛to calcine 煅烧to dehydrate 脱水to distil, to distill 蒸馏to hydrate 水合,水化to hydrogenate 氢化to neutralize 中和to oxidize 氧化to oxygenate, to oxidize 脱氧,氧化to precipitate 沉淀Tungsten(W) 钨Uranium(U) 铀valence, valency 价Vanadium(V) 钒vaseline 凡士林Xenon(Xe) 氙Ytterbium(Yb) 镱Yttrium(Y) 钇Zinc(Zn) 锌Zirconium(Zr) 锆理想气体状态方程Partial Pressures 分压Real Gases: Deviation from Ideal Behavior 真实气体:对理想气体行为的偏离The van der Waals Equation 范德华方程System and Surroundings 系统与环境State and State Functions 状态与状态函数Process 过程Phase 相The First Law of Thermodynamics 热力学第一定律Heat and Work 热与功Endothermic and Exothermic Processes 吸热与发热过程Enthalpies of Reactions 反应热Hess’s Law 盖斯定律Enthalpies of Formation 生成焓Reaction Rates 反应速率Reaction Order 反应级数Rate Constants 速率常数Activation Energy 活化能The Arrhenius Equation 阿累尼乌斯方程Reaction Mechanisms 反应机理Homogeneous Catalysis 均相催化剂Heterogeneous Catalysis 非均相催化剂Enzymes 酶The Equilibrium Constant 平衡常数the Direction of Reaction 反应方向Le Chatelier’s Principle 列·沙特列原理Effects of Volume, Pressure, Temperature Changes and Catalysts 体积,压力,温度变化以及催化剂的影响Spontaneous Processes 自发过程Entropy (Standard Entropy) 熵(标准熵)The Second Law of Thermodynamics 热力学第二定律Entropy Changes 熵变Standard Free-Energy Changes 标准自由能变Acid-Bases 酸碱The Dissociation of Water 水离解The Proton in Water 水合质子The pH Scales pH值Bronsted-Lowry Acids and Bases Bronsted-Lowry 酸和碱Proton-Transfer Reactions 质子转移反应Conjugate Acid-Base Pairs 共轭酸碱对Relative Strength of Acids and Bases 酸碱的相对强度Lewis Acids and Bases 路易斯酸碱Hydrolysis of Metal Ions 金属离子的水解Buffer Solutions 缓冲溶液The Common-Ion Effects 同离子效应Buffer Capacity 缓冲容量Formation of Complex Ions 配离子的形成Solubility 溶解度The Solubility-Product Constant Ksp 溶度积常数Precipitation and separation of Ions 离子的沉淀与分离Selective Precipitation of Ions 离子的选择沉淀Oxidation-Reduction Reactions 氧化还原反应Oxidation Number 氧化数Balancing Oxidation-Reduction Equations 氧化还原反应方程的配平Half-Reaction 半反应Galvani Cell 原电池Voltaic Cell 伏特电池Cell EMF 电池电动势Standard Electrode Potentials 标准电极电势Oxidizing and Reducing Agents 氧化剂和还原剂The Nernst Equation 能斯特方程Electrolysis 电解The Wave Behavior of Electrons 电子的波动性Bohr’s Model of The Hydrogen Atom 氢原子的波尔模型Line Spectra 线光谱Quantum Numbers 量子数Electron Spin 电子自旋Atomic Orbital 原子轨道The s (p, d, f) Orbital s(p,d,f)轨道Many-Electron Atoms 多电子原子Energies of Orbital 轨道能量The Pauli Exclusion Principle 泡林不相容原理Electron Configurations 电子构型The Periodic Table 周期表Row 行Group 族Isotopes, Atomic Numbers, and Mass Numbers 同位素,原子数,质量数Periodic Properties of the Elements 元素的周期律Radius of Atoms 原子半径Ionization Energy 电离能Electronegativity 电负性Effective Nuclear Charge 有效核电荷Electron Affinities 亲电性Metals 金属Nonmetals 非金属Valence Bond Theory 价键理论Covalence Bond 共价键Orbital Overlap 轨道重叠Multiple Bonds 重键Hybrid Orbital 杂化轨道The VSEPR Model 价层电子对互斥理论Molecular Geometries 分子空间构型Molecular Orbital 分子轨道Diatomic Molecules 双原子分子Bond Length 键长Bond Order 键级Bond Angles 键角Bond Enthalpies 键能Bond Polarity 键矩Dipole Moments 偶极矩Polarity Molecules 极性分子Polyatomic Molecules 多原子分子Crystal Structure 晶体结构Non-Crystal 非晶体Close Packing of Spheres 球密堆积Metallic Solids 金属晶体Metallic Bond 金属键Alloys 合金Ionic Solids 离子晶体Ion-Dipole Forces 离子偶极力Molecular Forces 分子间力Intermolecular Forces 分子间作用力Hydrogen Bonding 氢键Covalent-Network Solids 原子晶体Compounds 化合物The Nomenclature, Composition and Structure of Complexes 配合物的命名,组成和结构Charges, Coordination Numbers, and Geometries 电荷数、配位数、及几何构型Chelates 螯合物Isomerism 异构现象Structural Isomerism 结构异构Stereoisomerism 立体异构Magnetism 磁性Electron Configurations in Octahedral Complexes 八面体构型配合物的电子分布Tetrahedral and Square-planar Complexes 四面体和平面四边形配合物General Characteristics 共性s-Block Elements s区元素Alkali Metals 碱金属Alkaline Earth Metals 碱土金属Hydrides 氢化物Oxides 氧化物Peroxides and Superoxides 过氧化物和超氧化物Hydroxides 氢氧化物Salts 盐p-Block Elements p区元素Boron Group (Boron, Aluminium, Gallium, Indium, Thallium) 硼族(硼,铝,镓,铟,铊)Borane 硼烷Carbon Group (Carbon, Silicon, Germanium, Tin, Lead) 碳族(碳,硅,锗,锡,铅)Graphite, Carbon Monoxide, Carbon Dioxide 石墨,一氧化碳,二氧化碳Carbonic Acid, Carbonates and Carbides 碳酸,碳酸盐,碳化物Occurrence and Preparation of Silicon 硅的存在和制备Silicic Acid,Silicates 硅酸,硅酸盐Nitrogen Group (Phosphorus, Arsenic, Antimony, and Bismuth) 氮族(磷,砷,锑,铋)Ammonia, Nitric Acid, Phosphoric Acid 氨,硝酸,磷酸Phosphorates, phosphorus Halides 磷酸盐,卤化磷Oxygen Group (Oxygen, Sulfur, Selenium, and Tellurium) 氧族元素(氧,硫,硒,碲)Ozone, Hydrogen Peroxide 臭氧,过氧化氢Sulfides 硫化物Halogens (Fluorine, Chlorine, Bromine, Iodine) 卤素(氟,氯,溴,碘)Halides, Chloride 卤化物,氯化物The Noble Gases 稀有气体Noble-Gas Compounds 稀有气体化合物d-Block elements d区元素Transition Metals 过渡金属Potassium Dichromate 重铬酸钾Potassium Permanganate 高锰酸钾Iron Copper Zinc Mercury 铁,铜,锌,汞f-Block Elements f区元素Lanthanides 镧系元素Radioactivity 放射性Nuclear Chemistry 核化学Nuclear Fission 核裂变analytical chemistry 分析化学。
化学 倍半反应 英文

化学倍半反应英文Chemical reactions can be classified into various types, including redox reactions, acid-base reactions, and precipitation reactions. One particular type of redox reaction is known as a half-reaction or a half-equation.Half-reactions are essential in understanding and balancing redox reactions. In this article, we will explore the concept of half-reactions, their significance in chemistry, and how they can be balanced.A half-reaction represents either the reduction or oxidation process that occurs during a redox reaction. In a redox reaction, one species loses electrons (oxidation)while another species gains electrons (reduction). By separating the redox reaction into two half-reactions, it becomes easier to balance the overall equation. Each half-reaction focuses on either the reduction or oxidation process, allowing for a step-by-step approach to balancing the reaction.Balancing a half-reaction involves ensuring that the number of atoms and charges on both sides of the equation are equal. To balance the atoms, coefficients are added to the reactants and products. Additionally, electrons are added to balance the charges. The number of electrons transferred in the half-reaction is determined by the change in oxidation states of the elements involved. For example, if an element goes from an oxidation state of +2 to 0, it has gained two electrons.Half-reactions are particularly useful in electrochemistry, as they allow for the calculation of cell potentials. The cell potential, also known as the electromotive force (EMF), is a measure of the driving force behind a redox reaction. By knowing the half-reactions and their standard reduction potentials, it is possible to determine the overall cell potential. This information is crucial in understanding the feasibility and directionality of a redox reaction.In addition to their importance in balancing redox reactions and calculating cell potentials, half-reactionshave practical applications in various fields. For instance, they are used in the electroplating industry to deposit a layer of metal onto a substrate. By controlling the reduction half-reaction, it is possible to selectively deposit a desired metal onto a surface, providingprotection or enhancing its appearance.Furthermore, half-reactions are essential in understanding the corrosion process. Corrosion is an electrochemical process that involves the oxidation of a metal. By identifying the half-reaction responsible for the oxidation process, it becomes possible to implement measures to prevent or mitigate corrosion. This knowledgeis crucial in industries such as construction, automotive, and aerospace, where corrosion can lead to structural integrity issues.In conclusion, half-reactions play a vital role in understanding and balancing redox reactions. They allow for a step-by-step approach to balancing equations by focusing on either the reduction or oxidation process. Half-reactions are also crucial in electrochemistry, as theyenable the calculation of cell potentials. Additionally, they have practical applications in fields such as electroplating and corrosion prevention. By mastering the concept of half-reactions, chemists can gain a deeper understanding of redox reactions and their significance in various chemical processes.。
酸碱 酸碱反应

水、液氨的自偶电离是质子转移反应
H+
H 2O + H 2O 酸I 碱II
H+
H3 酸II
O+
+ OH 碱I
–
NH3 + NH3 酸I 碱II
NH4+ + NH2– 酸II 碱I
③酸碱中和反应是质子转移反应
H+
H3O+ (aq )
H+
+ OH− (aq = H2O(l) ) =
+
H2O(l)
H3O+ (aq )
H+
H+ + F-(aq) H3O+(aq) H3O+ + F– 酸II 碱I NH4+ + OH– 酸II 碱I
H+ + H2O(l) HF + H2O 酸I 碱II
H+
H2O + NH3 碱II 酸I
高氯酸在冰醋酸中的解离平衡是质子转移反应
H+
HClO4 + HAc 酸I 碱II
H2Ac+ + ClO4– 酸II 碱I
H+
+ N 3(aq H )
+ == NH4 (aq)
+ H2O(l)
H Ac(aq )
+ OH−(aq = H2O(l) ) =
+
Ac− (aq)
非水溶液中的酸碱反应,例如 非水溶液中的酸碱反应,例如NH4Cl的生成 的生成
H+
HCl 酸I
+
NH 3 碱Ⅱ
+ NH 4
+
chapter 4 acid base reaction

In a dilute solution of a weak acid, the great majority of HA molecules are undissociated: [H3O+] << [HA]init or [HA]eq [H3O+][A-] Qc = [HA][H2O] at equilibrium, Qc = Kc << 1
第四章 酸、碱及酸碱反应
Chapter 4 Acid, Base and Acid-Base Reaction
Contents
4.1 酸碱理论概述
The introduction of acid-base theories
4.2 弱酸、弱碱水溶液的质子转移平衡
The transfer equilibrium of the proton in weak acid and base aqueous solution
Graphical representation of the behavior of acids of different strengths in aqueous solution
A Strong Acid
A Weak Acid
The Extent of Dissociation for Strong and Weak Acids
Brønsted-Lowry Acid-Base Definition
An acid is a proton donor, any species that donates an H+ An acid must contain H in its formula; HNO3 and H2PO4examples, all Arrhenius acids are Brønsted-Lowry acids
生物化学考研精解名词解释答案(上)免费版

生化考研精解名词解释答案(上)温馨提示:部分解释不是采自教材,如有疑问,请参考课本!第一章糖类(p6)6.构型(configurati on):在立体化学中,因分子中存在不对称中心而产生的异构体中的原子或取代基团的空间排列关系。
有D型和L型两种。
构型的改变要有共价键的断裂和重新组成,从而导致光学活性的变化。
7.构象(conformation):分子中由于共价单键的旋转所表现出的原子或基团的不同空间排列。
指一组结构而不是指单个可分离的立体化学形式。
构象的改变不涉及共价键的断裂和重新组成,也无光学活性的变化。
12.差向异构体(epimer):在立体化学中,含有多个手性碳原子的立体异构体中,只有一个手性碳原子的构型不同,其余的构型都相同的非对映体叫差向异构体。
14.异头碳(anomeric carbon):单糖由直链变成环状结构时,羰基碳原子成为新的手性中心,导致C1差向异构化,产生两个非对映异构体。
在环状结构中,半缩醛碳原子称为异头碳原子。
15.半缩醛(hemiacetal):醛基和一个醇基缩合形成的产物。
通过该反应,使单糖形成环状结构。
16.变旋(mutarotation):当一种旋光异构体如糖,溶于水中转变成几种不同旋光异构体的平衡混合物时,随着时间而发生的旋光变化。
18.糖苷键(glycosidic bond):一个单糖或糖链还原端半缩醛上的羟基与另一个分子(如醇、糖、嘌呤或嘧啶)的羟基、胺基或巯基之间缩合形成的缩醛键或缩酮键。
常见的糖苷键有O-糖苷键和N-糖苷键。
19.还原糖(reducing sugar):能够还原斐林(H.von Fehling)试剂或托伦斯(B.Tollens)试剂的糖称为还原糖,所有的单糖(除二羟丙酮),不论醛糖、酮糖都是还原糖。
大部分双糖也是还原糖,蔗糖例外。
22.淀粉(starch):由D-葡萄糖单体组成的同聚物。
包括直链淀粉和支链淀粉两种类型,为植物中糖类的主要贮存形式。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Weak Acid + Strong Base
• HC2H3O2(aq) + NaOH(aq) • Weak acid wont completely dissociate
– Wont breakdown into ions on reaction side HC2H3O2(aq) + Na+(aq)+OH-(aq)Na+(aq)+C2H3O2-(aq)+ H2O(l)
H+(aq)+Cl-(aq)+Al(OH)3(s)Al+3(aq)+Cl-(aq)+H2O(l) H+(aq)+Al(OH)3(s)Al+3(aq)+H2O(l) • End solution is slightly acidic • What about NH3?
– Considered a weak base but has no OH– Does not produce water H+(aq)+Cl-(aq)+NH3(aq)NH4++Cl-(aq) H+(aq))+NH3(aq)NH4+ – HCl(aq)+NH3(aq)NH4Cl(aq)
Strong Acid + Strong Base
• HCl + NaOH NaCl + HOH • Double replacement reaction • Both compounds completely dissociate
HCl Cl- + H+ NaOH Na+ + OH-
• Complete equation (aq) can be written: H++Cl-+Na++OH-Cl-+Na++H2O • Spectator Ions
Strong Acid + Strong Base
• What is the net ionic equation: HCl(aq) + NaOH(aq) 1) H+(aq) +Cl-(aq) +Na+(aq) +OH-(aq) Cl-(aq) +Na+(aq) +H2O(aq) 2) H+(aq) +OH-(aq) H2O(l) • All strong acid and strong base reactions have this as a base net ionic equation KOH(aq)+HNO3(aq)KNO3(aq)+H2O(l) K++OH-+H++NO3- K++NO3-+H2O(l) OH-+H++ H2O(l)
– H2CO3 increases making blood more acidic
• What kind of blood pH results in yawning?
– Acidic blood; body needs to release large amount of CO2 by taking in large amount of O2
• HCl(aq) + Al(OH)3(s) • Weak bases wont completely dissociate
Strong Acid + Weak Base
– Cannot write them as ions on reaction side of net ionic equation
Bronsted-Lowery Acids and Bases
H2O(l)+NH3(aq)NH4+(aq)+OH-(aq)
ACID BASE Conjugate acid Conjugate base
• • • •
Acid= Any compound that releases H+ Base= Any compound that takes H+ Water can act as an acid or a base Conjugate Acid/Base
Acid-Base Reactions Ch. 15
• Neutralization reactions中和反应
– pH is changed
Acid-Base Reactions
• Produce a salt and H2O
• 2 types of Acids
– Strong and Weak
HC2H3O2(aq) +OH-(aq)C2H3O2-(aq)+ H2O(l) • End solution is slightly basic Weak Acid + Weak Base ???Not clear??? • Both the acid and base are so unreactive there is little change • Not common reaction type in nature
Add a strong base: HC2H3O2(aq)+NaOH(aq) C2H3O2-(aq)+H2O(l) HC2H3O2(aq)+OH-(aq) C2H3O2-(aq)+H2O(l)
• Weak acid/base + salt of that acid/base NaC2H3O2 and HC2H3O2
– Solution that adjusts to the addition of acids and bases to slowly change the pH Add a strong acid: - and H+ ions – Free OH NaC2H3O2(aq)+HCl(aq)
HC2H3O2(aq)+NaCl(l) C2H3O2-(aq)+H+(aq)HC2H3O2(aq)
HBr: NaOH + HBr NaBr + H2O; strong
b) what is the concentration of the acid used?
MA= MBVB/VA = (0.5M)(35ml)/50ml = 0.35 M
• What types of acid-base reactions do these titration graphs show?
Titrations practice
Titrations practice
• Graphs shows titration of 0.5 M NaOH with 50ml of an unknown acid. After titration NaBr salt crystals were isolated from the solution. a) What is the acid used? Is it strong or weak?
NaOH + HC2H3O2H2O + NaC2H3O2
Buffers in the Blood
• Blood must keep a pH of 7.4 to allow the best exchange of CO2 and O2 • Blood buffer is HCO3-/H2CO3 • Add Base: H2CO3+OH-HCO3-+H2O • Add Acid: HCO3-+H+H2CO3 • What happens when you take in too much CO2?
– Ions that do not take part in the reaction
Net Ionic Equations净离子方程式
1) Write Complete Ionic Equation
All soluble compounds are shown as free ions
2) Remove Spectator Ions Ions not directly evolved in the rxn. 3) Balance the remaining rxn.
• How do we know the Standard solution is neutral? • pH Indicators酸碱指示剂
• End point • Equivalence point • Neutral point
– Volume of acid/base used gives us molarity – MAVA = MBVB
H2O
• Test to determine the molarity of an acid or a base • Process:
Titrations滴定
– Find the Standard Solution标准溶液 – Standard solution of an acid/base is slowly added to an acid/base of unknown molarity – When the unknown acid/base is neutral, the [H+]=[OH-]
– Salts are ionic compounds
• 2 types of Bases
– Strong and Weak
• 4 possible combinations of Acids and Bases
– – – – Strong A + Strong B Strong A + Weak B Weak B + Strong B Weak B + Weak A
