跨膜蛋白抽提试剂盒说明书_Merck 英文版
MERCK D-Tube -- TMTMTM 透析 电洗脱管 产品说明书

产品使用说明书D -Tube TM透析透析//电洗脱管电洗脱管::Mini/Midi/Maxi/Mega/D Mini/Midi/Maxi/Mega/D--Tube96TM*建议同时参考本说明书英文原文(说明书编号TB422)。
产品名称产品名称包装包装 货号货号 D-Tube™ Dialyzer Mini, 截留分子量 6–8kDa D-Tube Dialyzer Mini,截留分子量 12–14kDa 10个 10个 71504-3 71505-3 D-Tube Dialyzer Midi, 截留分子量 3.5kDa D-Tube Dialyzer Midi, 截留分子量6–8kDa 10个 10个 71506-3 71507-3 D-Tube Dialyzer Maxi, 截留分子量 3.5kDa D-Tube Dialyzer Maxi, 截留分子量6–8kDa D-Tube Dialyzer Maxi, 截留分子量12-14kDa 10个 10个 10个 71508-3 71509-3 71510-3 D-Tube96™ Dialyzer, 截留分子量6-8kDa D-Tube96 Dialyzer, 截留分子量12-14kDa1 1 71712-3 71713-3 D-Tube Electroelution Accessory Kit 1 71511-3截留分子量截留分子量体积体积 试剂盒包装试剂盒包装目录号目录号 D-Tube™ Dialyzer Mega D-Tube Dialyzer Mega D-Tube Dialyzer Mega D-Tube Dialyzer Mega 3.5kDa(蓝色) 3.5kDa(蓝色) 6-8kDa(粉色) 6-8kDa(粉色) 3-10ml 3-10ml 3-10ml 3-10ml 10个 50个 10个 50个 71739-3 71739-4 71740-3 71740-4 D-Tube Dialyzer Mega D-Tube Dialyzer Mega D-Tube Dialyzer Mega D-Tube Dialyzer Mega 3.5kDa(蓝色) 3.5kDa(蓝色) 6-8kDa(粉色) 6-8kDa(粉色) 10-15ml 10-15ml 10-15ml 10-15ml 10个 50个 10个 50个 71742-3 71742-4 71743-3 71743-4 D-Tube Dialyzer Mega D-Tube Dialyzer Mega D-Tube Dialyzer Mega D-Tube Dialyzer Mega 3.5kDa(蓝色) 3.5kDa(蓝色) 6-8kDa(粉色) 6-8kDa(粉色) 15-20m 15-20ml 15-20ml 15-20ml10个 50个 10个 50个 71745-3 71745-4 71746-3 71746-4 Floating Racks,Mega - -10个71748-3试剂盒概述试剂盒概述D-Tube™ 透析管为生物样品制备提供了极为方便、通用的操作系统。
细胞跨膜蛋白提取方法

1
4
4
图片说明:用细胞跨膜蛋白提取试剂盒提取细胞样本的SDA-PAGE的电泳考染照片和Western照片。
图1泳道中1、4分别为Marker、膜蛋白样本。图2为以上样品的EGFR的WB结果照片。
试剂盒以外自备试剂和仪器
● 移液器、吸头
● 离心机及离心管
● 涡旋振荡器
● 冰箱,冰盒
使用方法: A. 细胞跨膜蛋白提取 1. 取 5-10×106 个以上细胞①,在 4℃,500g 条件下离心 2-3 分钟,小心吸取培养 基,尽可能吸干,收集细胞。 2. 用冷 PBS 洗涤细胞两次,每次洗涤后尽可能吸干上清。 3. 细胞样品中加入 200μl 冷的试剂 A,2ul 蛋白酶抑制剂混合物,2ul 磷酸酶抑制 剂混合物,高速涡旋振荡 15 秒,置冰上 10-15 分钟。 4. 再次高速涡旋振荡 5 秒,然后在 4℃,13000×g 条件下离心 5 分钟。
贝博细胞跨膜蛋白提取试剂盒是一种基于化学而非去污剂方法的高产跨膜蛋白提取 试剂盒。本试剂盒提供全套试剂,适用于从细胞和动物组织温和、高效地抽提膜/跨膜蛋白 和膜蛋白复合物。用本试剂盒抽提和富集到的蛋白包括从分子量很小到很大的蛋白,单次跨 膜至 7 次以上的蛋白,甚至一些大的蛋白复合物。提取过程简单方便,可在 1 小时内完成。 提取的膜蛋白不仅纯度高,保持天然活性,而且绝少交叉污染。提取的蛋白可用于 Western Blotting、转录活性分析、Gel shift 凝胶阻滞实验、免疫共沉淀、酶活性测定等蛋白研究。
B. 组织跨膜蛋白提取 1. 取适量组织样本剪碎,加冷 PBS,用组织匀浆器匀浆至无明显肉眼可见固体(或 用液氮研磨),冰上静置 5 分钟,小心将匀浆吸入另一预冷的干净离心管。 2. 在 4℃,500g 条件下离心 2-3 分钟,弃上清。 3. 按照细胞蛋白的提取方法的第 3 步骤往下操作即可。
GENMED可溶性细胞膜蛋白(粗提)制备试剂盒 产品说明书(中文版)

GENMED SCIENTIFICS INC. U.S.A GMS30039.1 v.A GENMED可溶性细胞膜蛋白(粗提)制备试剂盒产品说明书(中文版)主要用途GENMED可溶性细胞膜蛋白(粗提)制备试剂是一种旨在使用物理或化学方法快速充分裂解细胞,并在蛋白酶抑制混合剂的帮助下,通过超速离心,分离和收集所有可溶性细胞膜蛋白质(完整蛋白和跨膜蛋白以及膜相关蛋白等)的权威而经典的技术方法。
该技术由大师级科学家精心研制、成功实验证明的。
其适用于各种细胞(动物、人体)等样品。
可直接用于特定蛋白后续纯化、酶谱分析、二维蛋白电泳、西方杂交、免疫沉淀、功能鉴定和质谱分析以及蛋白组学研究等。
产品即到即用,性能稳定,操作便捷,收集效果显著。
技术背景通过物理匀浆和化学裂解以及蛋白酶抑制混合剂的共同作用,既防止裂解后细胞内各种活性蛋白成分遭到活化的内源性蛋白酶的破坏,充分保留细胞内蛋白质的免疫和生物学活性,又确保膜蛋白(完整蛋白integral protein和跨膜蛋白transmembrane protein)的高产萃取。
产品内容GENMED清理液(Reagent A)毫升GENMED裂解液(Reagent B)毫升GENMED活性液(Reagent C)微升GENMED强化液(Reagent D)毫升GENMED保存液(Reagent E)毫升产品说明书1份保存方式保存GENMED活性液(Reagent C)在-20℃冰箱里, 其余的保存在4℃冰箱里, 有效保证12月用户自备PBS缓冲溶液(GMS12033):用于清理细胞胰蛋白酶乙二胺四乙酸混合液(GMS12024):用于细胞脱离完全细胞培养液(GMS12052):用于细胞处理所需的培养基50毫升锥形离心管:用于细胞收集后离心15毫升锥形离心管:用于样品操作的容器1.5毫升离心管:用于样品保存4℃台式离心机:用于细胞和蛋白收集以及去除杂质组织匀浆器:用于细胞裂解实验步骤实验开始前,将试剂盒里的GENMED活性液(Reagent C)从℃的冰箱里取出,置入冰槽里冻融,然后移出毫升GENMED裂解液(Reagent B)到毫升离心管,加入微升GENMED活性液(Reagent C),充分混匀,置于冰槽里,标记为裂解工作液。
蛋白组分抽提试剂盒说明书
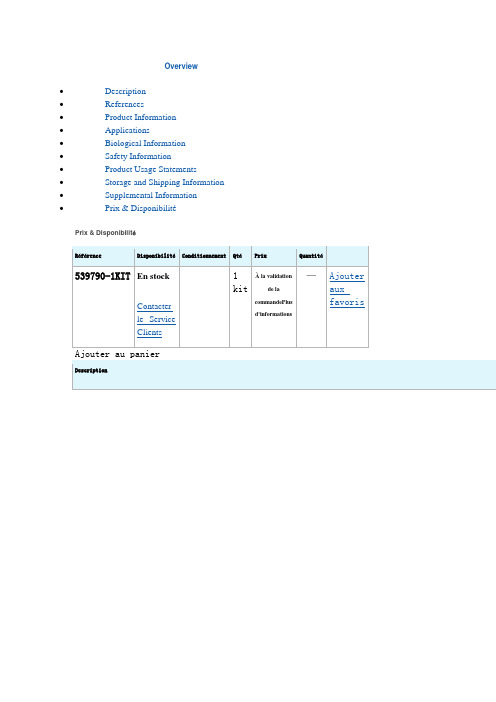
Overview∙Description∙References∙Product Information∙Applications∙Biological Information∙Safety Information∙Product Usage Statements∙Storage and Shipping Information∙Supplemental Information∙Prix & DisponibilitéPrix & DisponibilitéRéférence DisponibilitéConditionnement QtéPrix Quantité539790-1KIT En stockContacterle ServiceClients 1kitÀ la validationde lacommandePlusd'informations—AjouterauxfavorisAjouter au panier DescriptionOverviewFast and reproducible extraction of subcellular proteomes from mammalian cellsProteoExtract® Subcellular Proteome Extraction Kit (S-PEK) is designed for fast andreproducible extraction of subcellular proteomes from adherent and suspension-grownmammalian cells. The S-PEK takes advantage of the different solubilities of certain subcecompartments in the four selected reagents. In the case of adherent cells, the procedure isperformed directly in the tissue culture dish without the need for cell removal. Cells or theparts of the cells remain attached to the plate during sequential extraction of subcellularcompartments, until the appropriate extraction reagent is used. Thus, the early destructionthe cellular structure by enzymatic or mechanical detachment of cells from the tissue cultuplate and any mixing of different subcellular compartments is prevented. Forsuspension-grown cells, extraction starts with gentle sedimentation and washing of the celThe stepwise extraction delivers four distinct protein fractions from one sample:∙Cytosolic fraction (F1)∙Membrane/organelle protein fraction (F2)∙Nucleic protein fraction (F3)∙Cytoskeletal fraction (F4)Proteins are obtained in the native state making the S-PEK suitable for many downstreamapplications such as 1D and 2D gel electrophoresis, immunoblotting, enzyme activity assaand protein microarrays.Sample size: 3-5x106or 25-50 mg tissue.CatalogueNumber539790BrandFamilyCalbiochem®Synonyms S-PEK KitFeatures and benefits ∙Stepwise extraction resulting in four distinct subcellular proteomes from one sample ∙Highly reproducible∙No ultracentrifugation steps∙Fast—needs just 2 hours with 45 minutes hands-on time∙Produces proteins suitable for functional studiesApplicationDataA:Adherent SAOS cells were extracted according to the Detailed ProtocolSubcellular Extraction of Proteins From Adherent Cells as outlined above. Imi-iv show the morphology of the cells before and after each extraction s(200-fold enlarged). The SAOS cells remained attached throughout the extracprocedure. B:An aliquot of each fraction from A were subjected to SDS-analysis (F1-F4 = fractions 1-4). The data demonstrate clear differences inprotein banding patterns among the 4 fractions. C:Aliquots of each fracfrom A were separated by SDS-PAGE and transferred to PVD membrane for blotwith the indicated antibodies. For c-Fos, the fractions were subjected timmunoprecipitation, prior to Western blotting, to enrich for any c-Fos prein each fraction. The data demonstrate that each marker protein is specificenriched within the appropriate fraction.A431 cel ls were stimulated with 0.2 µg/ml TNF-αfor the indicated times. At end of each induction period the cells were extracted as outlined in the Deta Protocol for Subcellular Extraction of Proteins from Adherent Cells. The prot from an aliquot of each fraction were separated by SDS-PAGE and transferre PVD membrane for Western blot analysis using an antibody specific for NF-The data indicate that there is measurable translocation of NF-κ B from cytoplasm to the nucleas as early as 5 min after TNF-α stimulation.*Tested on rat liver and bovine liver tissue.。
英文特生物技术(北京)有限公司-植物质膜蛋白提取试剂盒说明书

Minute TM 植物质膜蛋白提取试剂盒目录号:SM-005-P描述:植物膜蛋白占植物细胞总蛋白的很小一部分,但是在植物生理学中起着非常重要的作用。
传统的植物膜组分分离纯化方法是蔗糖密度梯度离心法和双液相法。
这些方法虽然比较有效,但是需要超高速离心和大量的起始原材料,操作过十分繁琐和费时。
为了克服植物膜组分提取中的缺点,我们特别开发了此款植物膜组分提取试剂盒。
植物组织首先通过缓冲液A中致敏,匀浆,然后通过一个特殊的离心管柱,在此过程中匀浆的组织通过柱子特有的Z字形通路后细胞膜被切割成大小相等的碎片,后续无需超高速离心,通过差速离心法和密度梯度离心法将天然的质膜组分从未破裂的细胞,细胞核,细胞浆和细胞器的混合物中分离出来。
在每次实验中仅需使用相同量的起始材料,离心力和离心时间,即可高度富集膜组分,并保证一致性良好。
整个操作过程大约1小时可以完成。
应用:试剂盒用于快速从植物组织中分离天然膜组分,可应用于SDS-PAGE,immunoblottings,ELISA,IP,膜蛋白质结构分析,2-D,酶活性测定及其他应用。
试剂盒组份(50次):1. 25ml Buffer A2. 10ml Buffer B3. 50个离心管柱4. 50个收集管5. 2根塑料研磨棒6. 组织分离粉储存:Buffer A和Buffer B 需在-20℃储存所需附加材料1XPBS涡旋震荡仪台式离心机重要产品信息1.仔细阅读整个操作说明。
将缓冲液A和缓冲液B完全解冻后摇匀,放置于冰上。
将离心管柱和接收管套管放置于冰上预冷。
2.离心机请调整成RCF/Xg模式,按照离心力设置离心机,所有离心步骤都需要在4℃室温下或者低温离心机中进行。
3.研究蛋白磷酸化,磷酸酶抑制剂应在使用前加入缓冲液A中。
蛋白酶抑制剂可以选择添加或不添加,如添加在使用前加入缓冲液A中(请按照蛋白酶或磷酸酶抑制剂母液比例,例如母液是100x,添加时按照1:100添加,1ml缓冲液A添加10ul抑制剂)。
人红细胞膜蛋白提纯分离试剂盒 说明书

人红细胞膜蛋白提纯分离试剂盒说明书好,先从“人红细胞膜蛋白提纯分离试剂盒”的说明书开始,咱们这篇内容得做得自然些,像是聊聊天一样。
首先,咱们要明白这试剂盒是干啥的。
其实很简单,说白了就是帮助咱们从血液中把那些红细胞膜上的蛋白提取出来,干啥用呢?很多实验都需要这些蛋白,比如做一些生物学研究、探究蛋白质和细胞相互作用的机制之类的。
能不能做出来,就得看你使用这款试剂盒的技巧了。
记得第一次使用这个试剂盒的时候,我特别紧张。
因为就像我去做菜一样,明明看过无数次做法,实际操作的时候总觉得心里没底。
你看,说明书上写得都挺清楚的:“将血液样本与试剂混合,轻轻摇晃均匀,离心……”一看这些步骤,心想:“这不就是做饭嘛,似乎也没什么特别的。
”结果,刚开始操作的时候,我的手有点不听使唤,试剂倒不小心洒了出来,弄得台面上都是,还得重新整理一下。
后来才发现,原来这种东西真的得细心,毕竟血液中的每一个小细胞,都是关键。
接着进入重点——提取红细胞膜蛋白。
关键步骤是离心,离心是整个过程的核心,成功与否就在这一步。
每次我都站在离心机旁边,眼睁睁看着试管里的液体被快速旋转,心里都想着:“能不能分得更清楚点?”有时候试剂盒给的离心条件如果设置得不太对,结果就很容易分不清各层,试管里的东西都混在一起了,那就得重新来过,浪费时间又心累。
不过,做几次下来,你会发现其实这些都不是事儿。
就像我现在再做,操作得已经越来越顺手了,试剂盒里的配方和步骤其实挺合理的,你只要按照它的要求走,基本上问题不大。
其实就是需要点耐心,再加上一点点细心。
因为不小心错过了哪个环节,提取出来的蛋白质量会差很多,实验的结果就不准确了,这就是“细节决定成败”的意思了。
说实话,每次看到离心后沉淀物清晰地分层,蛋白提取得一干二净的时候,心里那种成就感真是没法形容,简直是“哇塞,居然做出来了!”你会觉得所有的麻烦和小失误都不算什么,反倒成了回忆的一部分。
好了,讲了这些步骤,也别忘了这款试剂盒的优点。
GST resin产品使用说明书

这相当于50ml培养液采用2.5ml抽提试剂。如果是小规模培养,则采用约1/5培养体积的抽提试剂重悬 沉淀(例如,1.5ml培养液采用300µl抽提试剂)。如果抽提试剂过量也没有什么副作用。 选做:加蛋白酶抑制剂。BugBuster Master Mix与蛋白酶抑制剂兼容。如果目的蛋白后续要用凝血酶 (货号69671),Xa因子(货号69036)或重组肠激酶(货号69066)处理,就应该避免使用丝氨酸蛋白 酶抑制剂。尽管纯化过程可能去除活性抑制剂,建议在酶切前最好做透析或凝胶过滤。 3. 室温下将重悬的细胞液在摇板或低速搅拌器上孵育10-20分钟。 注意:孵育后获得的抽提物不是粘稠的。 4. 4℃下16,000g离心20分钟以去除不溶的细胞碎片。如果需要,沉淀可以留作“包涵体纯化”(见以 下说明)的材料。
可溶蛋白制备 采用此操作抽提到的蛋白包括来自细胞周质和细胞质的可溶蛋白。如果欲仅收集周质部分蛋白,可以参 考Novagen的TB055“PET系统操作手册”中提到的渗压休克方法或其它合适的方法。渗透休克方法中 得到的沉淀也可以用于以下操作以获得细胞质中的可溶蛋白。 1. 用经预先称重的离心管10,000g离心10分钟从液体培养体系收集细胞。如果是小规模培养(如1.5ml或
GST•Bind™ 缓冲液试剂盒
GST•Bind缓冲液试剂盒包括了从GST•Bind树脂和GST•Mag™磁性琼脂糖树脂纯化目的蛋白所需的结 合,冲洗和洗脱所需的缓冲液。试剂盒所含成分足够用于10个2.5ml柱的纯化。具体包括: • 2×100ml 10×GST Bind/Wash Buffer (43mM Na2HPO4,14.7mM KH2PO4,1.37M NaCl,27mM KCl,pH7.3) • 40ml 10×Glutathione Reconstitution Buffer (500mM Tris-HCl,pH8.0) • 1g Glutathione,Reduced,Free Acid
Thermo-Scientific-NE-PER核蛋白-胞浆蛋白抽提试剂盒

Thermo-Scientific-NE-PER核蛋白-胞浆蛋白抽提试剂盒描述Thermo Scientific NE-PER核蛋白-胞浆蛋白抽提试剂盒提供了高效的细胞裂解和抽提方法,可在两个小时之内完成胞浆蛋白与核蛋白的分离操作。
NE-PER核蛋白-胞浆蛋白抽提试剂盒的特性:• 快速—在两个小时之内获得胞核与胞浆内的可溶性蛋白组分• 可靠—NE-PER试剂盒已经被950篇以上的发表文献引用• 通用–适用于培养细胞或组织(仅适用于新鲜样本)的核蛋白抽提• 可扩展—两种规格试剂盒,满足从细胞和组织中抽提样本的应用需求• 方便—操作简单,无需超速梯度离心• 兼容性好—获得样本适合于多种下游应用,包括免疫印迹、凝胶迁移分析、蛋白分析、报告基因分析以及酶活性分析NE-PER试剂盒为用户提供了高效的核蛋白抽提方法,用户只需拥有台式微量离心机、离心管和移液器,进行简单的分步式细胞裂解及核蛋白与胞浆蛋白离心分离即可。
NE-PER试剂盒能够高效地将胞浆蛋白与核蛋白溶解和分离为不同组分,交叉污染以及基因组DNA/mRNA 的干扰均降到了最低。
一旦经过脱盐或稀释,分离后的蛋白即可用于免疫分析和蛋白相互作用实验,如迁移率分析(EMSA)、免疫共沉淀(Co-IP)和拉下实验。
分离胞核和制备核蛋白抽提物的方法很多,但大多数制备核蛋白抽提物的制备方法都很耗时,并需要机械匀浆、冻融循环、反复离心或透析,而这些都可能影响核蛋白的完整性。
NE-PER 核蛋白-胞浆蛋白抽提试剂盒使用了基于试剂的分离方案,能够分步裂解细胞并从胞浆中分离出完整的细胞核,然后再从基因组DNA与mRNA中抽提核蛋白。
这一温和的过程可在两小时之内完成,且使用培养细胞时仅需标准的桌台式离心机即可。
此外,用户还可以从细胞培养物或组织样本中分离得到具有活性的核蛋白与胞浆蛋白。
本试剂盒能够从两百万个细胞中提出200至500µg胞浆蛋白和100至200µg的核蛋白(浓度为1mg/mL)。
总蛋白提取试剂盒说明书

总蛋白提取试剂盒说明书总蛋白提取试剂盒(Total Protein Extraction Kit)P1250:蛋白抽提试剂盒-I (实体组织和细胞蛋白抽提, 100 extractions)描述:从组织细胞中提取总蛋白是Western Blot的关键步骤。
实体软组织如脑脊髓富含磷脂,神经血管含大量结缔组织,而脂肪则有大量油脂,常规的方法难以有效从这些组织提取蛋白。
本试剂盒为组织或培养细胞总蛋白提取提供完整的解决方案。
用裂解-结合缓冲液匀浆裂解实体组织,或直接用裂解-结合缓冲液重悬培养细胞,然后加入抽提试剂去除非蛋白成分,离心、干燥后即可得到总蛋白。
蛋白沉淀溶解后用常规方法进行蛋白定量。
提取过程可在30-60分钟内完成,可在1.5ml离心管微量提取也可大规模制备,极为简便高效。
通常一次提取10-100mg 组织所获得的总蛋白可进行几十到上百次分析如蛋白质电泳、Western Blot、免疫共沉淀。
组成 1. 裂解-结合缓冲液100 ml 2. 抽提试剂100 ml每毫升裂解缓冲液和抽提试剂可提取10-100mg组织或1 ? 107细胞。
试剂盒的裂解缓冲液是过量的。
适用各种实体软组织(> 1 mg),如脑、脊髓、神经结或纤维、脂肪、肝脏、消化道组织、肾脏、心脏、肌肉、血管、结缔组织等,以及各种原代细胞和永生化细胞系。
保存:室温或4oC保存2年固体组织蛋白提取1.每10-100mg固体组织剪碎后加入0.5-1 ml裂解液,放入玻璃匀浆器内上下手动匀浆15次;或用高速机械匀浆器12,000 rpm 破碎组织。
注意:初始组织的量应在10-100mg范围。
肝肾组织蛋白含量高,提取时应加较少量的组织,否则形成的蛋白膜厚、致密且难溶。
少量小的未破碎组织不影响后续抽提。
2.仅取0.5ml组织匀浆液转移到1.5ml离心管,弃去剩余的组织匀浆液。
每0.5ml组织匀浆液加入2倍体积的(1ml)抽提试剂充分混匀。
室温或4oC静置10分钟,偶尔晃动。
哺乳动物细胞全蛋白提取试剂盒说明书

INSTRUCTIONSM-PER ®Mammalian Protein Extraction ReagentNumber Description78503 M-PER Mammalian Protein Extraction Reagent , 25mL, sufficient reagent to extract protein from ~2.5g of cells78501 M-PER Mammalian Protein Extraction Reagent , 250mL, sufficient reagent to extract protein from ~25g of cells78505M-PER Mammalian Protein Extraction Reagent , 1L, sufficient reagent to extract protein from ~100g of cellsStorage: Upon receipt store product at room temperature.IntroductionThe Thermo Scientific M-PER Mammalian Protein Extraction Reagent extracts cytoplasmic and nuclear protein from cultured mammalian cells using a proprietary detergent in 25mM bicine buffer (pH 7.6). The simple composition of this reagent is compatible with many different applications, such as reporter assays (e.g., luciferase, β-galactosidase,chloramphenicol acetyltransferase), protein assays (e.g., PKA, PKC, tyrosine kinase), immunoassays (e.g., Western blot, ELISA, RIA) and protein purification. M-PER Reagent enables rapid, mild and efficient lysis. The reagent is dialyzable and the cell lysate is compatible with protein assays such as the Thermo Scientific Coomassie Plus (Bradford) Assay and the Pierce BCA Protein Assay.Important Product Information•Adherent Cells vs. Cell Pellets: M-PER Reagent effectively lyses both plated cells and cells pelleted from suspension cultures or scraped cells. For direct, in-plate lysis of adherent cells, protein extraction efficiency using M-PER Reagent is similar to freeze/thaw methods. For lysis of pelleted cells, either from cell suspension or scraped adherent cells, protein extraction efficiency is typically 25% higher than that achieved with freeze-thaw (three cycles) and 20% higher than sonication (2 minutes with 50% pulse) methods.•Cell Lines: M-PER Reagent has been tested on cell lines representing several different cell types. Complete lysis of adherent cells is observed with, but is not limited to, the following cell lines: COS-7, NIH3T3, Hepa 1-6, 293, CHO, MDA, MB 231 and FM2 cells. For protein extraction from tissues, greater efficiency may be achieved using Thermo Scientific T-PER Tissue Protein Extraction Reagent (Product No. 78510).•Additives: Protease inhibitors, such as Thermo Scientific Halt Protease Inhibitor Cocktail, EDTA-Free (Product No. 87785) may be added to the reagent. For immunoassays, such as ELISA or RIA, extracts prepared in M-PER Reagent alone generate satisfactory results; however, adding 150mM NaCl to the cell lysate often improves results.•Volume for Cell Lysis: Volumes indicated in Table 1 are optimal for maximum cell lysis without scraping cells. If more concentrated extracts are preferred, use a smaller volume; however, scraping the cells is necessary for maximal recovery. If cell volume is unknown, it may be estimated. For example, 2 × 106 of HeLa cells equals ~10μL of a packed cell volume, which is equivalent to 20 mg of cells and requires 200 μl of M-PER Reagent.• Compatibility with Protein Assays: M-PER Reagent is compatible with Coomassie Plus (Bradford) Assay (Protein No. 23236) and the Pierce ® BCA Protein Assay Kit (Product No 23225).Procedure for Lysis of Monolayer-cultured Mammalian CellsNote: M-PER Reagent does not contain protease inhibitors. If desired, add Halt™ Protease Inhibitor Cocktail, EDTA-Free (Product No. 87785) to the reagent.1.Carefully remove (decant) culture medium from adherent cells.Note: If the culture medium contained phenol red or other reagents that could interfere with subsequent protein analysis,wash cells once in wash buffer (e.g., PBS).2.Add the appropriate amount of M-PER Reagent to the plate or to each plate well (see Table 1). Shake gently for5 minutes.Table 1. Suggested volume of Thermo Scientific M-PER Reagent to use for different sizes ofstandard culture plates.Plate Size/Surface Area M-PER Reagent Volume100mm* 500-1,000μL60mm 250-500µL 6-well plate 200-400µL per well24-well plate 100-200µL per well96-well plate 50-100µL per well*Cells grown in 100mm plates typically contain 10 cells (50mg) and yield ~3mg total protein depending oncell type.3.Collect the lysate and transfer to a microcentrifuge tube. Centrifuge samples at ~14,000 ×g for 5-10 minutes to pellet thecell debris.4.Transfer the supernatant to a new tube for analysis.Procedure for Lysis of Suspension-cultured Mammalian Cells1.Pellet the suspension of cells by centrifugation at 2,500 ×g for 10 minutes. Discard the supernatant.2.Optional Wash: If the culture medium contained phenol red or other reagents that could interfere with subsequent proteinanalysis, wash the cells once by resuspending the cell pellet in wash buffer (e.g., PBS). Pellet cells by centrifugation at2,500 ×g for 10 minutes.3.Add M-PER Reagent to the cell pellet. Use at least 1mL of M-PER Reagent for each 100 mg (~100μL) of wet cell pellet.If a large amount of cells is used, first add 1/10 the final recommended volume of M-PER Reagent to the cell pellet.Pipette the mixture up and down to resuspend pellet. Add the rest of the M-PER Reagent to the cell suspension.Note: Total protein yield for 100mg of wet cell pellet is approximately 6 mg depending on cell type.4.Shake mixture gently for 10 minutes. Remove cell debris by centrifugation at ~14,000 ×g for 15 minutes.5.Transfer the supernatant to a new tube for analysis.TroubleshootingProblem PossibleCause SolutionProtein expression was low Optimize the transfection procedureInsufficient amount of M-PER Reagent was used Add more M-PER ReagentLow protein yieldM-PER Reagent was unable to penetrate the cell membrane Increase incubation time and shake more vigorously during incubationUnable to retrieve membrane protein M-PER Reagent extracts only nuclear andcytoplasmic proteinsUse Thermo Scientific Mem-PER MembraneProtein Extraction Reagent (Product No.89826)Related Thermo Scientific Products87785 Halt Protease Inhibitor Cocktail, EDTA-Free (100X), 1mL87786 Halt Protease Inhibitor Cocktail, contains sufficient reagents to treat 100mL of sample78248 B-PER® Bacterial Protein Extraction Reagent, 500mL78990 Y-PER® Yeast Protein Extraction Reagent, 500mLProtein Extraction Reagent Kit89826 Mem-PERMembrane23236 Coomassie Plus (Bradford) Assay23227 Pierce BCA Protein Assay Kit78833 NE-PER® Nuclear and Cytoplasmic Extraction Kit26148 Pierce Direct IP Kit34080 SuperSignal® West Pico Chemiluminescent Substrate, 500mL, Western blot substrate for HRP 34076 SuperSignal West Dura Extended Duration Substrate, 200mL, Western blot substrate for HRP Product ReferencesCampa, M.J., et al. (2003). Protein expression profiling identifies macrophage migration inhibitory factor and cyclophilin A as potential molecular targets in non-small cell lung cancer. Cancer Res63:1652-6.Deng, W., et al. (2003). LPA protects intestinal epithelial cells from apoptosis by inhibiting the mitochondrial pathway. Amer J Physiol-Gastrointest L 284:821-9.Phiel, C.J., et al. (2001). Differential binding of an SRF/NK-2/MEF2 transcription factor complex in normal versus neoplastic smooth muscle tissues. Biol Chem.276(37):34637-50.Waite, K.A. and Eng, C. (2003). BMP2 exposure results in decreased PTEN protein degradation and increased PTEN levels. Hum Mol Genet12(6):679-84. B-PER® Technology is protected by U.S. Patent # 6,174,704.SuperSignal® Technology is protected by U.S. Patent #6,432,662.This product (“Product”) is warranted to operate or perform substantially in conformance with published Product specifications in effect at the time of sale, as set forth in the Product documentation, specifications and/or accompanying package inserts (“Documentation”) and to be free from defects in material and workmanship. Unless otherwise expressly authorized in writing, Products are supplied for research use only. No claim of suitability for use in applications regulated by FDA is made. The warranty provided herein is valid only when used by properly trained individuals. Unless otherwise stated in the Documentation, this warranty is limited to one year from date of shipment when the Product is subjected to normal, proper and intended usage. This warranty does not extend to anyone other than the original purchaser of the Product (“Buyer”).No other warranties, express or implied, are granted, including without limitation, implied warranties of merchantability, fitness for any particular purpose, or non infringement. Buyer’s exclusive remedy for non-conforming Products during the warranty period is limited to replacement of or refund for the non-conforming Product(s).There is no obligation to replace Products as the result of (i) accident, disaster or event of force majeure, (ii) misuse, fault or negligence of or by Buyer, (iii) use of the Products in a manner for which they were not designed, or (iv) improper storage and handling of the Products.Current versions of product instructions are available at /pierce. For a faxed copy, call 800-874-3723 or contact your local distributor.© 2010 Thermo Fisher Scientific Inc. All rights reserved. Unless otherwise indicated, all trademarks are property of Thermo Fisher Scientific Inc. and its subsidiaries. Printed in the USA.。
Invent Biotechnologies, Inc. MinuteTM 核膜蛋白提取试剂盒说明书

Minute TM核膜蛋白提取试剂盒目录号:NE-013描述:核膜蛋白是一种非常复杂的膜蛋白系统,由于核膜与细胞核细胞质相连所以非常难以分离和纯化。
传统方法提取和纯化需要非常大的样本量,并且过程乏味冗长。
Minute TM核膜蛋白提取试剂盒是第一个可以快速分离天然核膜蛋白及相连蛋白的商业试剂盒,无需使用梯度离心,超速离心。
由于使用专利离心管柱技术,使得操作简单,快速,易用,并有效富集核膜蛋白。
不像传统方法那样需要大量样品,起始样品量仅需10-20million个细胞,缓冲液不含表面活性剂和EDTA,操作时间<45分钟,并可获得10-50ug蛋白/样品。
应用:试剂盒提取的核膜蛋白可应用于SDS-PAGE,immunoblottings,ELISA,IP,蛋白质运输分析,酶活性测定及其他应用。
试剂盒提供了一个快速富集天然核膜蛋白的方法。
试剂盒组份:1. 25 ml裂解液A2. 15 ml 裂解液B3. 50个离心管柱4. 50个收集管储存:-20℃储存所需附加材料1XPBS涡旋震荡仪台式离心机重要产品信息1.仔细阅读整个操作说明。
将缓冲液A和缓冲液B完全解冻后摇匀,放置于冰上。
将离心管柱和接收管套管放置于冰上预冷。
2.所有离心步骤都需要在4℃室温下或者低温离心机中进行。
3.研究蛋白磷酸化,磷酸酶抑制剂应在使用前加入缓冲液A中。
蛋白酶抑制剂可以选择添加或不添加。
4.推荐使用BCA试剂盒用于蛋白浓度测定。
核膜蛋白分离步骤1.将离心管柱放入接收管中,放置冰上预冷2.低速离心(500-600Xg,5分钟)收集10-20million细胞或组织中分离出的细胞。
从动物组织中获取单细胞推荐使用Minute TM 单细胞分离试剂盒(Cat# SC-012)。
3.用1ml预冷的PBS清洗细胞一次,去除上清,加入0.5ml Buffer A中重悬细胞。
冰上孵育10分钟。
涡旋大力震荡10-20秒。
迅速将细胞悬液转入离心管柱中。
细胞膜蛋白与细胞浆蛋白抽提试剂盒 说明书

¾ 约90分钟即可完成培养细胞或组织的细胞膜蛋白与细胞浆蛋白的分离和抽提。抽提得到的蛋白可以用于SDS-PAGE, Western、酶活性测定等后续实验。
—
产品名称 膜蛋白抽试剂A 膜蛋白抽提试剂B
说明书
包装 100ml 30ml
1份
保存条件:
-20℃保存,一年有效。
注意事项: ¾ 需 自 备 PMSF 。 PMSF 一 定 要 在 抽 提 试 剂 加 入 到 样 品 中前2-3分钟内加入,以免PMSF在水溶液中很快失效。
PMSF(ST506)可以向碧云天订购。 ¾ 使 用 本 试 剂 盒 抽 提 到 的 细 胞 膜 蛋 白 与 细 胞 浆 蛋 白 均 可 直 接 用 碧 云 天 生 产 的 BCA 法 蛋 白 浓 度 测 定 试 剂 盒
2. 准备细胞或组织样品: a. 对于细胞 (1) 收集细胞 对于贴壁细胞:培养约2000-5000万细胞,用PBS洗一遍,用细胞刮子刮下细胞或用含有EDTA但不含胰酶的细胞消化 液处理细胞使细胞不再贴壁很紧,并用移液器吹打下细胞。离心收集细胞,吸除上清,留下细胞沉淀备用。尽量避 免用胰酶消化细胞,以免胰酶降解需抽提的目的膜蛋白。 对于悬浮细胞:培养约2000-5000万细胞,直接离心收集细胞,吸除上清,留下细胞沉淀备用。 (2) 洗涤细胞:用适量冰浴预冷的PBS轻轻重悬细胞沉淀,取少量细胞用于计数,剩余细胞4℃,600g离心5分钟沉淀 细胞。弃上清,随后4℃,600g离心1分钟,以沉淀离心管管壁上的残留液体并进一步沉淀细胞,尽最大努力吸尽残 留液体。 (3) 细胞预处理:把1毫升临用前添加了PMSF的膜蛋白抽提试剂A加入至2000-5000万细胞中,轻轻并充分悬浮细胞, 冰浴放置10-15分钟。 b. 对于组织: 取约100毫克组织,用剪刀尽量小心剪切成细小的组织碎片。加入1毫升临用前添加了PMSF的膜蛋白抽提试剂A,轻 轻悬浮组织碎片,冰浴放置10-15分钟。注:如果组织样品比较少,也可以使用更少的组织量,例如30-50mg,后续 试剂的用量及操作步骤不变;组织用量较少时,最后获得的膜蛋白也较少。
PH0710 膜蛋白和胞浆蛋白提取试剂盒操作手册
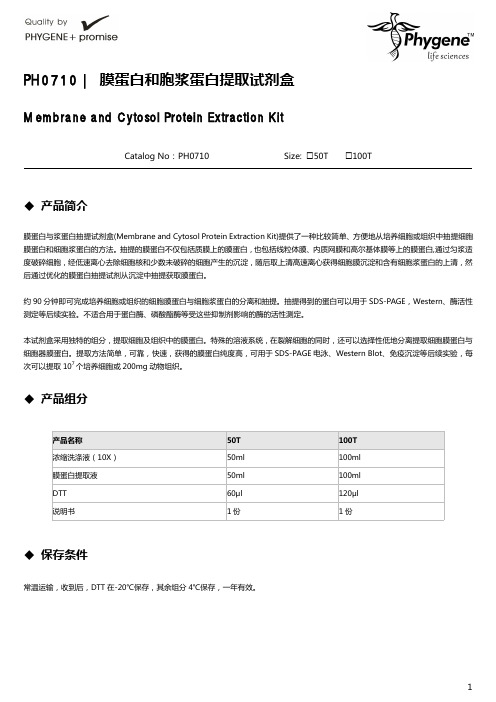
PH0710|膜蛋白和胞浆蛋白提取试剂盒Membrane and Cytosol Protein Extraction KitCatalog No:PH0710Size:□50T □100T◆产品简介膜蛋白与浆蛋白抽提试剂盒(Membrane and Cytosol Protein Extraction Kit)提供了一种比较简单、方便地从培养细胞或组织中抽提细胞膜蛋白和细胞浆蛋白的方法。
抽提的膜蛋白不仅包括质膜上的膜蛋白,也包括线粒体膜、内质网膜和高尔基体膜等上的膜蛋白,通过匀浆适度破碎细胞,经低速离心去除细胞核和少数未破碎的细胞产生的沉淀,随后取上清高速离心获得细胞膜沉淀和含有细胞浆蛋白的上清,然后通过优化的膜蛋白抽提试剂从沉淀中抽提获取膜蛋白。
约90分钟即可完成培养细胞或组织的细胞膜蛋白与细胞浆蛋白的分离和抽提。
抽提得到的蛋白可以用于SDS-PAGE,Western、酶活性测定等后续实验。
不适合用于蛋白酶、磷酸酯酶等受这些抑制剂影响的酶的活性测定。
本试剂盒采用独特的组分,提取细胞及组织中的膜蛋白。
特殊的溶液系统,在裂解细胞的同时,还可以选择性低地分离提取细胞膜蛋白与细胞器膜蛋白。
提取方法简单,可靠,快速,获得的膜蛋白纯度高,可用于SDS-PAGE 电泳、Western Blot、免疫沉淀等后续实验,每次可以提取107个培养细胞或200mg 动物组织。
◆产品组分◆保存条件常温运输,收到后,DTT 在-20℃保存,其余组分4℃保存,一年有效。
产品名称50T 100T 浓缩洗涤液(10X)50ml 100ml 膜蛋白提取液50ml 100ml DTT60μl 120μl 说明书1份1份使用说明1.准备细胞或组织样品:A.细胞贴壁细胞:培养约2000-5000万细胞,用PBS洗一遍,用细胞刮子刮下细胞或用含有EDTA但不含胰酶的细胞消化液处理细胞使细胞不再贴壁很紧,并用移液器吹打下细胞。
离心收集细胞,吸除上清,留下细胞沉淀备用。
蛋白重折叠试剂盒说明书

随盒附送的简明操作流程使得分离的包涵体能够在温和的变性条件下重溶。蛋白重溶后,将可
溶部分用含有还原试剂的中性缓冲液透析促进二硫键正确折叠,多余的还原剂可以再次用透析
除去并将中性缓冲液替换成所需蛋白缓冲液。经验证,这个操作流程适用于从包涵体中重溶得
到的CBD•TagTM融合蛋白及其他重组蛋白的重折叠。
蛋白重折叠中的透析操作
试剂盒中包含有50×透析缓冲液和1M DTT溶液用于透析溶解包涵体。 1. 准备透析用的所需体积的缓冲液。通常来讲,要用大于50倍体积的透析缓冲液透析2次以
上。比如,如果样品体积是15ml,则每次透析至少要用750ml,那么总共需要准备至少1.5L 透析缓冲液。制备此体积的缓冲液时,将30ml 50×透析缓冲液稀释在1.5L去离子水中,加 入150μL 1M DTT(使其终浓度为0.1mM)。 注意:如果目标蛋白是带CBDclos融合标签的,推荐使用添加了100mM NaCl(用5M储液稀 释)的透析缓冲液。因为在一些情况下,CBDclos标签对低盐缓冲液敏感。 2. 4℃透析至少3小时,替换缓冲液并继续透析3小时或更长时间。 3. 按照步骤1中操作准备另外的缓冲液,但是不要加DTT。 4. 用不含DTT的透析缓冲液继续透析两次(每次3小时)。 5. 如果透析后样品中有可见的不溶物,4℃ 10,00×g离心10分钟去除沉淀。需要对目标蛋白 进行功能检测从而确定是否需要另外用硫醇试剂处理蛋白氧化并异构二硫键。
液。缓冲液体积需要 25 倍于可溶蛋白样品体积。冷却至 4℃。 2. 4℃过夜透析重折叠蛋白。 3. 取出一些样品检测蛋白活性。 4. 如果需要,室温或 37℃继续透析提高二硫键重形成速率。 注意:也可以直接将高浓度的氧化和还原型谷胱甘肽储液直接加入目标蛋白溶液中,孵育(间 隔不同时间取出样品检测蛋白活性)后透析去除多余的氧化还原试剂。
完整版膜蛋白提取

1别离组织膜蛋白的方法1、取组织,参加10ml Buffer A 于冰上充分匀浆.2、J6-HC离心机800rpm , 4 c离心10min后,所得上清液转入超速离心管.3、100000g , 4c离心1hr.弃去上清,沉淀用适量的Buffer B重悬,冰上孵育2hr后分装至EP 管, Eppendorf 台式离心机10000rpm , 4 c离心30min.4、收集所得上清液即为膜组份.Buffer A : 0.32M 蔗糖,5mM Tris-HCl (PH 7.5), 120mM KCl , 1mM EDTA,1mM EDTA,0.2mM PMSF, 1ug/ml Leupeptin, 1ug/ml Pepstatin A, 1ug/ml Aprotinin .冰上预冷.Buffer B : 20mM HEPES (PH 7.5 ) , 10% 甘油,2% Triton X-100, 1mM EDTA, 1mM EDTA,0.2mM PMSF, 1ug/ml Leupeptin, 1ug/ml Pepstatin A, 1ug/ml Aprotinin .冰上预冷.2取约0.1g肝组织,参加2ml粉碎缓冲液,冰浴中超声粉碎,每次20秒,间隔30秒,共3 次,4° c 105xg条件下离心2小时,上清液为胞浆蛋白,沉淀局部参加1ml胞膜蛋白提取液,超声粉碎,4.c 105xg条件下离心2小时,上清液为胞膜蛋白.样品蛋白含量测定采用酚试剂法.3我们实验室提取膜蛋白的方法如下:1.将细胞种于T75或T175的培养瓶中培养数天,细胞铺满瓶底后,吸去培养液.将PBS/EDTA 溶液(NaCl:0.1M,NaH2PO4:0.01M,EDTA:0.04%) 参加量以覆盖细胞为止,置于培养箱或在超净台内消化3〜5分钟,如仍未脱落瓶底,用吸管吹打,使细胞完全脱落.2.收集细胞悬液于10毫升或50毫升的离心管中,1000rpm离心10分钟,去上清液.3. 按2.5毫升(T75 ) /每瓶或5毫升(T175/每瓶参加冰冷的匀浆液(HEPES:50mM,PH7.4,MgCl2:3mM,EGTA:1mM )于离心管中,将溶液和沉淀转移到匀浆器中,匀浆.4.将匀浆液转入离心管中, 参加适量的Tris - HCl (50mM, PH7.4)4oC, 18000rpm (40000g) 10分钟.在沉淀中参加同前量的匀浆液,再匀浆,匀浆后参加适量的tris-HCl buffer. 4度18000rpm(40000g)离心,共离心三次.5.在最后一次离心彳#到沉淀中按250微升每瓶(T75)或500微升每瓶(T175)参加tris-HClbuffer,匀浆,取50微升匀浆液测量蛋白浓度.6.按实验要求,将匀浆分装于1毫升的Eppendoff管中,其于-80oC冰箱中待用.主要基于膜蛋白分子量较大,在40000g时易沉降来别离.做膜蛋白的免疫荧光, 可以用荧光抗体染色, 然后用荧光显微镜或共聚焦显微镜观察,具体的protocol可以找到,就不多说了.需要注意的是,要弄清楚你所使用的一抗结合部位是在膜蛋白的胞外结构域还是胞内结构域,如果是胞外,可以直接染色;如果在胞内,那么需要用无水甲醇在-20度处理10min,使细胞膜通透,然后再用抗体孵育,这样抗体才能进入细胞内与蛋白结合. 一般对膜蛋白的提取都是采用梯度离心或者高速离心的方法别离,可是我们现有的离心机都无法到达所需要的转速,所以我们试着采用分布溶解分布沉淀的方法.1)预冷的pbs洗去组织血液,除去结缔组织、脂肪.2) 4 C剪碎,加bufferA玻璃匀浆器匀浆至无大块状物,10 C超声波10min ,重悬,再破碎5—10min.3)12000rpm 离心10min,沉淀用bufferB 重悬,20 C超声波10min.4)12000rpm 离心10min,沉淀用bufferC 抽提.5)12000rpm离心10min,所得上清含有9%的膜蛋白.6)12000rpm离心10min,沉淀加bufferD沸水煮5min , 12000rpm离心10min,上清含有1%的膜蛋白. bufferA: 40mM Tris basebufferB: 8M urea ,100mM DTT, 40mM Tris basebufferC: 6-7M urea, 0.2mmol/L PMSF,40mM Tris basebufferD: 1% SDS, 50mM DTT,25%Glycerol,in 0.4M Tris-HCl pH8.8我们做的是动物组织的膜蛋白,提出来电泳条带还可以,只是我们没有专一膜蛋白抗体来检测提出来的是否是膜蛋白.但是这个方法是可行的5三、蛋白提取操作步骤I实体组织蛋白的提取1、组织样本(200〜300mg)尽量去除脂肪组织和结缔组织等非目的组织,于冰上剪碎;2、组织样本中参加1mL Lysis Buffer (注:使用前,每mL Lysis Buffer参加1科L蛋白酶抑制剂和1科L 1M DTT ),置玻璃均浆器冰上均质30〜50次(或超声破碎细胞, 每次30 S ,3〜4次,每次间隔1 min),置于冰上冷却.均质或超声破碎细胞后应镜检,细胞破碎率不小于90%;3、将均浆液转移至冷的离心管中,于4C, 3000 rpm离心10 min,弃沉淀;4、取上清转移至新冷离心管中,于4 C , 14 000 rpm离心30 min ,所得上清转至新管中, 即为胞浆蛋白,分装冷冻保存;5、取沉淀,参加1mL冷的抽提Buffer,涡旋振荡混匀后,4c放置10〜15min;【注:因抽提Buffer在室温时会分层,请务必于4c混匀后参加】6、4 ℃ , 3000rpm离心5min ,取上清转移至新管(注意勿将沉淀带入上清),进行下步提取;7、置于37 c水浴10min;8、室温,13 000rpm离心5min ,样品分成上层和下层〔含膜蛋白〕;【注:建议使用进口透明性较好的微量离心管,可见下层为含膜蛋白的有机相. 上下两层因均为透明,只在交界处有一折光线,需仔细观察才可见到.或室温静置30分钟〜1小时亦可见分层.以下亦同.】9、取下层,参加500d L冰冷灭菌水,4c放置5min; 10、置于37 c水浴10min; 11、室温,13 000rpm离心5min ,样品分成上层和下层〔含膜蛋白〕;12、取下层,参加500d L冰冷灭菌水,4c放置5min;13、置于37 c水浴10min;14、室温,13 000rpm离心5min ,样品分成上层和下层〔含膜蛋白〕;15、最终得到的下层即为膜蛋白提取物,BCA法测定蛋白含量〔因残留有机相可能影响测定结果,此含量为相对参考值〕,分装冷冻保存.6用RIPA强配方,把脱氧胆酸钠浓度调整至1.0%,同时参加NP-40和Triton X-100 ,浓度均为1%.我刚做了一个7次跨膜蛋白,LHR的WB.7提取总蛋白的方法不能用于膜蛋白的提取, 提供一个常用的方法:先别离膜,在提取膜蛋白. 这类方法在园子里有不少介绍. 我们实验室曾经采用蔗糖梯度超速离心别离膜,据说效果还可以.您可以试一下.呵呵——8细胞破碎后,先低速离心,除去未破碎的细胞和大的细胞碎片.保存上清,超速离心,可获得细胞膜.然后用detergent溶液萃取,就可以得到膜蛋白溶液了.9我有新的问题了...哪位仁兄有好的提取细胞膜蛋白的裂解液配方...我的膜蛋白总提不出来.我的细胞是HEPG2;目的蛋白是LDL受体;我的配方是这样的:4%CHAPS , 8 mol/L urea, 0.1 mmol/L leupeptin, and 1.5 mmol/L phenylmethylsulfonyl fluoride但是总提不出来....急! ! ! ! !呵呵,CHAPS提取膜蛋白水平较差,尝试参加1%ASB-14 ,或者参加1 % Triton X-100,与CHAPS联合抽提.另外最好参加2M thiourea.祝好运1.6% Triton X-100, 5 mol/L urea, 0.1 mmol/L leupeptin, and 1.5 mmol/L phenylmethylsulfonyl fluoride ;2M thiourea; thiourea很贵吗我现在没有,又要急着用,能不能不用thiourea??thiourea不是很贵,merck250g才300多rmb.电泳时可以考虑用ASB-14 , sb3-10.没有的话,提取最好加点NP-40, 2M thiourea还是要的,要是有TBP就更好了.NP lysis buffer.NP 配方,stock solution 20ml (50mM Tris-HCL (PH8.0), 5mMEDTA, 0.05%NaN3, 0.14M NaCL, 100mM NaF), 1% Nonidet P40, 0.2TIU/ml aprotinin, 1.5uM pepstatin A, 20mM Iodoacetamide.NP配好后分装,-80度保存.用之前,参加NaV和PMSF.混匀后立即置于冰上.10如果你研究的蛋白表达比拟丰富没有必要提取膜蛋白,直接用RIPA裂解液裂解后离心取可溶性蛋白变性做WB就可以了;如果你蛋白的含量低或抗体特异性很差的话,你应该用试剂盒或超速离心提取膜蛋白,可以起到富集和纯化的作用,使你更容易得到好的结果!11RAPI的裂解液只能说是强的非变性裂解液,而算不上是强的变性裂解液.提取膜蛋白一般都在变性条件下才可以.所以,你应该改用其他更适合的强的变性裂解液,如含SB3-10、DM、ASB-14等膜助溶剂,或者SDS-Bolied loading bufffer,2-D 裂解液7M thiourea+2M urea 也可以12组织膜蛋白提取:所在操作冰上进行(1)取组织,用PBS冲洗干净组织上的血液.参加10ml Buffer A ,用剪刀尽可能剪碎组织,于冰上充分匀浆.每次30秒,重复3-5次.(2)离心机1000rpm , 4 C离心10min后,所得上清液转入超速离心管.(去掉大块组织及结缔组织)(3)100000g, 4c离心1hr,弃去上清.(此为胞浆蛋白,假设只提取胞浆蛋白,可用10000rpm, 4 度,30分钟离心,取上清即可).沉淀用适量的Buffer B重悬,4度过夜后,分装至EP管, Eppendorf 台式离心机10000rpm, 4 C 离心30min.(4)收集所得上清液即为膜组份.Buffer A :0.32M 蔗糖,5mM Tris-HCl (PH 7.5), 120mM KCl , 1mM EDTA, 1mM EGTA,0.2mM PMSF, 1ug/ml Leupeptin, 1ug/ml Pepstatin A, 1ug/ml Aprotinin .冰上预冷.Buffer B 20mM HEPES (PH 7.5), 10% 甘油,2% Triton X-100, 1mM EDTA, 1mM EGTA, 0.2mM , PMSF, 1ug/ml Leupeptin, 1ug/ml Pepstatin A, 1ug/ml Aprotinin .冰上预冷.所得蛋白分装EP管(每管约50ul),取一管测蛋白浓度,其它放入-70度深冻冰箱保存.如果要定量的话,最好能立即根据浓度,参加上样缓冲,煮沸, 4度保存.反复冻融蛋白会降解,浓度不准.13我用来抽提大鼠脑组织细胞膜蛋白方法:取两只大鼠的整个脑组织,参加10ml Buffer A 于冰上充分匀浆.J6-HC离心机800rpm , 4 C离心10min后,所得上清液转入超速离心管.100000g, 4c离心1hr.弃去上清,沉淀用适量的Buffer B重悬,冰上孵育2hr后分装至EP管,Eppendorf台式离心机10000rpm, 4 C 离心30min.所得上清液即为膜组份.Buffer A : 0.32M 蔗糖,5mM Tris-HCl 〔PH 7.5〕,120mM KCl , 1mM EDTA,1mM EDTA, 0.2mMPMSF, 1ug/ml Leupeptin, 1ug/ml Pepstatin A, 1ug/ml Aprotinin .冰上预冷.Buffer B : 20mM HEPES 〔PH 7.5〕, 10%甘油,2% Triton X-100, 1mM EDTA, 1mM EDTA, 0.2mM PMSF, 1ug/ml Leupeptin, 1ug/ml Pepstatin A, 1ug/ml Aprotinin .冰上预冷.CHAPS〔一种离子去污剂〕是提取膜蛋白的常用试剂一般用10mMol的浓度即可,你可以试试加到你的Buffer中,看效果如何.不过此药品很贵!lysis buffer的选择依赖于你所研究蛋白质的位置和性质:1〕细胞浆蛋白〔可溶性〕:Tris Buffer2〕细胞浆蛋白〔结合到细胞骨架〕:Tris-Triton buffer3〕膜结合蛋白:Np-40或RIPA4〕核蛋白:RIPA5〕线粒体蛋白:特殊的抽提裂解液6〕全细胞蛋白:Np-40 or RIPAWestern blotAdipose tissue membrane protein was extracted from approx. 20mg of adipose tissue. PAGE was performed according to Laemmli [ 8]. A portion of 2 g of adipose t issue membrane protein was applied per lane. In addition, 10 q of mononuclear cell membrane protein 〔corresponding to 1.7 106 mononuclear cells〕 was applied and used as a positive control for verification of blotting, incubation and detection. Tank blotting to nitrocellulose was performed according to standard protocols, with transfer for 3h at 70V and 15 C.°The blots were incubated with 1 闵/ml rabbit anti-〔human b2-adrenoceptor〕 IgG 〔Santa Cruz Biotechnology Inc.〕, and were then re-incubated with alkaline phosphatase-labelled goat anti-rabbit antibody. Detection was performed using DDAO [9H-(1,3-dichloro-9,9-dimethylacridin-2-one-7-yl)] phosphate (Pro-Q Western Blot Stain Kit; Molecular Probes Inc.) on a Fujifilm FLA-3000 instrument.Quantification of b2-adrenoceptor protein was performed by determining the fluorescence intensity of b2-adrenoceptor-antibody-immunoreactive bands. The concentration of b2-adrenoceptor protein is expressed as the intensity of the immunoreactive bands of the tissue samples in relation to the intensity of the immunoreactive bands of the mononuclear cell membrane protein internal standard. Like others before us trying to isolate the b2-adrenoceptor [9], we observed several immunoreactive bands on our Western blots ( Figure 1). On all blots we found a single major band at 57kDa and several smaller immunoreactive bands (38-52kDa). Theb2-adrenoceptor is known to have a molecular mass of 64kDa and to be very fragile, so the immunoreactive bands on our blots must represent proteolytic degradation products and/or secondarily modified b2-adrenoceptor. When trying toquantify the receptor it is therefore uncertain whether to include all visible bands [total band area (T)] or to include only the single major band (S). We have done both. Figure 1 shows the area included in determining the intensity of the single major band and total band area intensity.All blots were loaded with the same amount of membrane protein per well, but since we wished to determine the level of protein expression in relation to individual cells, the concentration is expressed as intensity of b2-adrenoceptor protein per ng of DNA. The coefficient of variation based on 13 double determinations was 28% for total band area determinations and 29% for single major band determinations14凯基膜蛋白提取试剂盒凯基膜蛋白和胞浆蛋白提取试剂盒一、描述:本试剂盒提供独特的组份提取细胞及组织中的膜蛋白.其原理是裂解细胞后,先离心别离出质膜粗提物,再利用特殊的抽提Buffer,选择性地别离提取膜蛋白,抽提Buffer含一种特殊的去污剂,在4c时所有的蛋白质均可都溶于抽提Buffer,但在37c时,抽提Buffer分为水相和去污相;此时亲水性蛋白溶于水相,疏水的膜蛋白溶于去污剂相中,根楣该性质别离出膜蛋白.产物不仅含细胞膜蛋白,也含胞器质膜蛋白.提取方法简单,可靠, 快速.获得的膜蛋白纯度高,可用于PAGE电泳、Western Blot、免疫共沉淀等后续研究. 二、试剂盒组份三、蛋白提取操作步骤I实体组织蛋白的提取1、组织样本〔200〜300mg〕尽量去除脂肪组织和结缔组织等非目的组织,于冰上剪碎;2、组织样本中参加1mL Lysis Buffer 〔注:使用前,每mL Lysis Buffer参加1科L蛋白酶抑制剂和1 ^L 1M DTT 〕,置玻璃均浆器冰上均质30〜50次〔或超声破碎细胞,每次30 S ,3〜4次,每次间隔1 min〕,置于冰上冷却.均质或超声破碎细胞后应镜检,细胞破碎率不小于90%;3、将均浆液转移至冷的离心管中,于4C, 3000 rpm离心10 min,弃沉淀;4、取上清转移至新冷离心管中,于 4 C , 14 000 rpm离心30 min ,所得上清转至新管中, 即为胞浆蛋白,分装冷冻保存;5、取沉淀,参加1mL冷的抽提Buffer,涡旋振荡混匀后,4c放置10〜15min;【注:因抽提Buffer在室温时会分层,请务必于4c混匀后参加】6、4 ℃ , 3000rpm离心5min ,取上清转移至新管〔注意勿将沉淀带入上清〕,进行下步提取;7、置于37 c水浴10min;8、室温,13 000rpm离心5min ,样品分成上层和下层〔含膜蛋白〕;【注:建议使用进口透明性较好的微量离心管,可见下层为含膜蛋白的有机相. 上下两层因均为透明,只在交界处有一折光线,需仔细观察才可见到.或室温静置30分钟〜1小时亦可见分层.以下亦同.】9、取下层,参加500d L冰冷灭菌水,4c放置5min;10、置于37 c水浴10min;11、室温,13 000rpm离心5min ,样品分成上层和下层〔含膜蛋白〕;12、取下层,参加500d L冰冷灭菌水,4c放置5min;13、置于37 c水浴10min;14、室温,13 000rpm离心5min ,样品分成上层和下层〔含膜蛋白〕;15、最终得到的下层即为膜蛋白提取物,BCA法测定蛋白含量〔因残留有机相可能影响测定结果,此含量为相对参考值〕,分装冷冻保存.四、SDS PAGE电泳操作步骤1、进彳T PAGE电泳前,取该提取物,每100 dL膜蛋白提取物,参加约300 dL的溶解Buffer 和约100 dL三氯乙酸〔TCA〕试剂, 混匀后置冰上20〜30 min后,13 000rpm ,离心15 min ,尽可能除去上清;2、沉淀参加1mL丙酮,室温静置10min后,13 000rpm离心15 min;3、弃上清,沉淀真空旋干或置冰上枯燥约10 min 〔敞开离心管盖〕,参加适当体积的LoadingBuffer 〔使用前每100 ^L Loading Buffer参加2〜5dL疏基乙醇〕溶解,彻底分散〔枪头反复吹吸或剧烈涡旋〕;【注:参加Loading Buffer后如有局部难溶物,可取上清继续上样;如参加Loading Buffer 后澳酚蓝转呈黄色,此为少量TCA残留所致,不影响电泳结果.请参照Marker标准.】4、上样进行SDS PAGE电泳.五、考前须知:1、所有的试剂及器具均需预冷后使用.细胞或组织量需到达要求.2、抽提Buffer 4 C时,为混悬状态,请于此温度下混匀后吸取参加粗提物中.3、室温25c〜37c时,抽提Buffer需静置30分钟以上可看到分上下两层,其中约9/10 至4/5为上层水相,下层只占1/10至1/5为有机相.4、因产品采用不透明的包装瓶,因此无法观察到上述分层现象,可将该BUFFER倒入玻璃烧杯或试管中静置30分钟〜1小时可见分层.5、TCA试剂具强腐蚀性,操作时请带适宜的手套并注意防护.六、储存蛋白酶抑制剂-200C, Loading Buffer室温保存,其余40C,保存一年。
麦克瑞His标签蛋白纯化试剂盒(包涵体蛋白) His-Tagged Protein Purifica
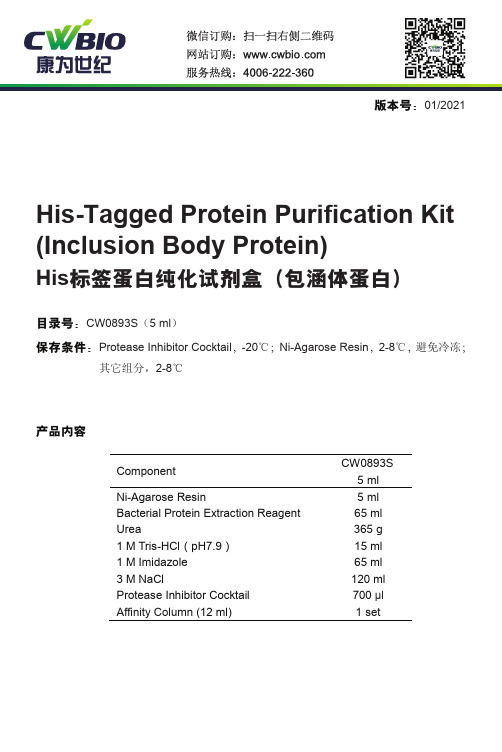
版本号:01/2021ComponentCW0893S 5 ml Ni-Agarose Resin 5 ml Bacterial Protein Extraction Reagent65 ml Urea365 g 1 M Tris-HCl (pH7.9)15 ml 1 M Imidazole65 ml 3 M NaCl120 ml Protease Inhibitor CocktailAffinity Column (12 ml) 700 μl 1 setHis 标签蛋白纯化试剂盒(包涵体蛋白)His-Tagged Protein Purification Kit (Inclusion Body Protein)目录号:CW0893S (5 ml )保存条件:Protease Inhibitor Cocktail ,-20℃;Ni-Agarose Resin ,2-8℃,避免冷冻;其它组分,2-8℃产品内容产品简介 该产品为镍柱纯化系统,对6×His-tag蛋白具有显著特异吸附能力,能够高效一步纯化带有6个组氨酸亲和标签的蛋白。
该系统具有4个Ni2+螯合位点,较只有3个螯合位点的Ni-IDA结合Ni2+更为牢固,有效防止纯化过程中Ni2+脱落且增强对His标签蛋白的结合能力,提高纯化效率。
较高的基团密度,大大提高了蛋白载量。
该系统在天然或变性条件下,对来源于各种表达系统(如杆状病毒,哺乳细胞,酵母以及细菌)中的His标签蛋白,均有很好的纯化效果。
本产品已螯合镍离子,可直接用于包涵体蛋白的纯化,使用方便,快捷。
支持物: CL-6B琼脂糖凝胶载量: 20-30 mg His标签蛋白/ml填料粒径: 50-160 μm注意事项1.在纯化之前采用电泳检测蛋白的可溶性,本试剂盒只适合于包涵体蛋白的纯化,如需纯化可溶性蛋白,请选择我公司的可溶性蛋白纯化试剂盒,货号为CW0894。
2.缓冲液中不建议使用 β-巯基乙醇、DTT和EDTA。
BCA法蛋白测定试剂盒(BCA)英文说明书

BCA Protein Assay Reagent(bicinchoninic acid)The Thermo Scientific Pierce BCA Protein Assay Kit remains one of the most popular protein quantization methods worldwide.Used in more labs than any other detergent-compatible protein assay, Pierce BCA Reagents provide accurate determination of protein concentration with most sample types encountered in protein research. The Pierce BCA Assay can be used to assess yields in whole cell lysates and affinity-column fractions, as well as to monitor protein contamination in industrial applications. Compared to most dye-binding methods, the BCA Assay is affected much less by protein compositional differences, providing greater protein-to-protein uniformity.Highlights:∙Colorimetric– estimate visually or measure with a standard spectrophotometer or plate reader (562nm)∙Excellent uniformity– exhibits less protein-to-protein variation than dye-binding methods∙Compatible– unaffected by typical concentrations of most ionic and nonionic detergents∙Moderately fast– much easier and four times faster than the classical Lowry method∙High linearity– linear working range for BSA equals 20 to 2000µg/mL∙Sensitive– detect down to 5µg/mL with the enhanced protocolBCA Protein Assay Applications:∙Studying protein:protein interactions∙Measuring column fractions after affinity chromatography∙Estimating percent recovery of membrane proteins from cell extractsHigh-throughput screening of fusion proteinsStandard curves. Typical standard curves for bovine serum albumin(BSA) and bovine gamma globulin (BGG) in the BCA Protein Assay.Kits include ampules of Albumin Standard.How the BCA Protein Assay Detects Protein:The BCA Protein Assay combines the well-known reduction of Cu2+ to Cu1+ by protein in an alkaline medium with the highly sensitive and selective colorimetric detection of the cuprous cation (Cu1+) by bicinchoninic acid. The first step is the chelation of copper with protein in an alkaline environment to form a light blue complex. In this reaction, known as the biuret reaction, peptides containing three or more amino acid residues form a colored chelate complex with cupric ions in an alkaline environment containing sodium potassium tartrate.In the second step of the color development reaction, bicinchoninic acid (BCA) reacts with the reduced (cuprous) cation that was formed in step one. The intense purple-colored reaction product results from the chelation of two molecules of BCA with one cuprous ion. The BCA/copper complex is water-soluble and exhibits a strong linear absorbance at 562 nm with increasing protein concentrations. The BCA reagent is approximately 100 times more sensitive (lower limit of detection) than the pale blue color of the first reaction.The reaction that leads to BCA color formation is strongly influenced by four amino acid residues (cysteine or cystine, tyrosine, and tryptophan) in the amino acid sequence of the protein. However, unlike the Coomassie dye-binding methods, the universal peptide backbone also contributes to color formation, helping to minimize variability caused by protein compositional differences.For more information, see the article "Chemistry of Protein Assays" in the Protein Methods Library.References:o Smith, P.K., et al. (1985). Anal. Biochem.150, 76-85.o Sorensen, K. (1992). BioTechniques12(2), 235-236.o Ju, T., et. al. (2002). J. Biol. Chem.277, 178-186.o Shibuya, T., et. al. (1989). Tokyo Ika Daigaku Zasshi47(4), 677-682.o Hinson, D.L. and Webber, R.J. (1988). BioTechniques6(1), 14, 16, 19.o Akins, R.E. and Tuan, R.S. (1992). BioTechniques12(4), 496-7, 499.o Tylianakis, P.E., et. al.(1994). Anal. Biochem.219(2), 335-340.o Gates, R.E. (1991). Anal. Biochem.196(2), 290-295.o Stich, T.M. (1990). Anal. Biochem.191, 343-346.o Tuszynski, G.P. and Murphy, A. (1990). Anal. Biochem.184(1), 189-191.。
小鼠跨膜蛋白98TMEM98ELISA检测试剂盒

本试剂盒只能用于科学研究,不得用于医学诊断版号:18-09小鼠跨膜蛋白98(TMEM98)ELISA检测试剂盒货号:M-KMLJM220871m检测原理试剂盒采用双抗体一步夹心法酶联免疫吸附试验(ELISA)。
往预先包被跨膜蛋白98(TMEM98)抗体的包被微孔中,依次加入标本、标准品、HRP标记的检测抗体,经过温育并彻底洗涤。
用底物TMB 显色,TMB在过氧化物酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的跨膜蛋白98(TMEM98)呈正相关。
用酶标仪在450nm波长下测定吸光度(OD值),计算样品浓度。
样品收集、处理及保存方法1.血清:使用不含热原和内毒素的试管,操作过程中避免任何细胞刺激,收集血液后,3000转离心10分钟将血清和红细胞迅速小心地分离。
2.血浆:EDTA、柠檬酸盐或肝素抗凝。
3000转离心30分钟取上清。
3.细胞上清液:3000转离心10分钟去除颗粒和聚合物。
4.组织匀浆:将组织加入适量生理盐水捣碎。
3000转离心10分钟取上清。
5.保存:如果样本收集后不及时检测,请按一次用量分装,冻存于-20℃,避免反复冻融,在室温下解冻并确保样品均匀地充分解冻。
自备物品1.酶标仪(450nm)2.高精度加样器及枪头:0.5-10uL、2-20uL、20-200uL、200-1000uL3.37℃恒温箱操作注意事项1.试剂盒保存在2-8℃,使用前室温平衡20分钟。
从冰箱取出的浓缩洗涤液会有结晶,这属于正常现象,水浴加热使结晶完全溶解后再使用。
2.实验中不用的板条应立即放回自封袋中,密封(低温干燥)保存。
3.浓度为0的标准品可视为阴性对照或者空白;按照说明书操作时样本已经稀释5倍,最终结果乘以5才是样本最终浓度。
4.严格按照说明书中标明的时间、加液量及顺序进行温育操作。
5.所有液体组分使用前充分摇匀。
6.若中英文说明书有误,请以中文说明书为准。
试剂盒组成名称96孔配置48孔配置备注微孔酶标板12孔×8条12孔×4条无标准品(8ng/ml)0.5mL0.5mL按说明书进行稀释标准品稀释液6mL3mL按说明书进行稀释样本稀释液6mL3mL按说明书进行稀释检测抗体-HRP6mL3mL无20×洗涤缓冲液20mL20mL按说明书进行稀释底物A6mL3mL无底物B6mL3mL无终止液6mL3mL无封板膜2张2张无说明书1份1份无自封袋1个1个无注:标准品用标准品稀释液依次稀释为:8、4、2、1、0.5、0.25、0ng/ml.试剂的准备20×洗涤缓冲液的稀释:蒸馏水按1:20稀释,即1份的20×洗涤缓冲液加19份的蒸馏水。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
NovagenUSA and Canada Europe All Other CountriesTel (800) 526-7319 novatech@FranceFreephone0800 126 461GermanyFreecall0800 100 3496IrelandToll Free1800 409 445United KingdomFreephone0800 622 935All otherEuropean Countries+44 115 943 0840Contact Your Local Distributornovatech@ProteoExtract® Transmembrane Protein Extraction Kit Table of ContentsAbout the Kits (2)Description 2Components 2Storage 2Equipment and materials required but not supplied 3ProteoExtract®Transmembrane Protein Extraction Protocol (3)Extraction of membrane proteins from adherent cultured cells 3Extraction of membrane proteins from suspension cells or frozen cell pellets 4Extraction of membrane proteins from tissue 6Frequently Asked Questions (8)Appendix (8)Example extractions 8Examples of total protein yields using TM-PEK 9© 2009 EMD Chemicals Inc., an affiliate of Merck KGaA, Darmstadt, Germany. All rights reserved. The Novagen® logo and Novagen® name are registered trademarks of EMD Chemicals Inc. in the United States and in certain other jurisdictions. ProteoExtract® is a registered trademark of Merck KGaA, Darmstadt, Germany. TRITON® is a registered trademark of Dow Chemical Company.About the KitsProteoExtract® Transmembrane Protein Extraction Kit 1 kit 71772-3DescriptionThe ProteoExtract® Transmembrane Protein Extraction Kit (TM-PEK) uses a novel, detergent-freechemistry for extraction of peripherally-associated and integral membrane proteins, such asG-Protein Coupled Receptors (GPCRs), from mammalian cells and tissues. The membrane protein fractionis directly compatible with enzyme assays, native and denaturing gel electrophoresis, Western blotting,immunoprecipitation, and (following in-gel tryptic digestion) mass spectrometry.The TM-PEK method comprises a two-step protocol for the enrichment of transmembrane (TM) proteins. Inthe first step, cells or homogenized tissues are permeablized using Extraction Buffer 1 and their soluble(cytoplasmic) fraction separated from the insoluble (membrane) fraction by centrifugation. In the secondstep, membrane proteins are extracted from the lipid bilayer using one of two novel extraction buffers.These buffers, Extraction Buffer 2A and Extraction Buffer 2B, are prepared by diluting TM-PEK Reagent Aor TM-PEK Reagent B into Extraction Buffer 2. The optimal extraction reagent must be determinedempirically, as it will depend on characteristics of the protein(s) of interest and intended downstreamapplications. Extraction Buffer 2A is a very mild extraction agent, allowing for recovery of fragile proteincomplexes. Extraction Buffer 2B, by comparison, is a highly efficient extraction agent and facilitatesrecovery of difficult-to-extract transmembrane proteins, including those with multiple transmembranesegments.If using the TM-PEK kit for the first time, prepare duplicate samples. Extract the first set of replicates withTM-PEK Reagent A, diluted 2-fold with Extraction Buffer 2. Extract the second set of replicates with TM-PEK Reagent B, diluted 2-fold with Extraction Buffer 2. Extraction conditions can be optimized further byvarying the dilution range of each reagent into Extraction Buffer, from undiluted to a 10-fold dilution.Unlike alternative membrane protein extraction methods, the TM-PEK kit does not require sonication,rigorous vortexing, time-consuming ultracentrifugation, or incubation at elevated temperatures. The absenceof such harsh treatments minimizes potential damage or changes to the target protein.Each TM-PEK kit provides sufficient reagents to process a total of 40 samples (20 samples using ExtractionBuffer 2A at the recommended dilution, and 20 samples using Extraction Buffer 2B at the recommendeddilution). Each sample may be comprised of 1–5 x 107 cultured cells or 25–50 mg of tissueComponents•••••40 ml Extraction Buffer 150 ml Extraction Buffer 22.0 ml TM-PEK Reagent A2.0 ml TM-PEK Reagent B0.4 ml Protease Inhibitor Cocktail Set IIIStorageStore all components at 4°C for up to 6 months. For prolonged storage, dispense the components in working aliquots and store at –20°C. Before performing extractions, thaw all kit components at room temperature and mix completely. Avoid repeated freezing and thawing.Note that the protease inhibitors are provided in DMSO and must be kept at room temperature during the extraction procedure to prevent freezing.Equipment and materials required but not supplied•••••Rocking platform or elliptical mixer (required at room temperature and 4°C; the device can be transferred from 4°C to room temperature during the course of the experiment)Homogenizer (e.g., Dounce or Potter-Elvehjem) or mortar and pestle (for tissue samples)Refrigerated centrifuge with rotor accommodating a 15 ml and/or a 50 ml tubeRefrigerated centrifuge with rotor accommodating a 2 ml tube and generating 16,000 × gPhosphate Buffered Saline (PBS) (137 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl, 1.8 mM KH2PO4,pH 7.4)ProteoExtract®Transmembrane Protein Extraction ProtocolTable 1. Buffer volumes required for membrane protein extraction from cultured cells or tissue.Source Material Cultured cells* Fresh or frozen tissueAmount 1.0–5.0 x 10725–50 mg 100–200 mg 200–1000 mgExtraction Buffer 1 (ml) 1.0 1.0 1.0 2.0Extraction Buffer 2A or 2B (ml) 0.2 0.20.51.0Protease Inhibitor (µl) 5 5 20 40*Adherent, suspension, or frozen pelletExtraction of membrane proteins from adherent cultured cellsConsiderations Before You BeginThe following protocol is optimized for extraction of membrane proteins from adherent cells grown in oneT-75 culture flask. Cells should be of high viability (>90%) and 70–90% confluent(1.0–2.0 x 107 cells). Different cell types yield considerably different amounts of protein in the membrane protein fraction (see Table 2 on p 9 in Appendix). If low yield is anticipated, the total number of cells can be increased to 5.0 x 107 without increasing reagent volumes (Table 1). For membrane protein extraction from larger cell numbers, we recommend performing replicate extractions from aliquots of 1.0–5.0 x 107 cells. Alternatively, buffer volumes may be scaled up appropriately.The kit is supplied with two transmembrane solubilization agents, TM-PEK Reagents A and B(see Description on p 2). Depending on the unique characteristics of the target membrane protein, Extraction Buffer 2A or Extraction Buffer 2B may prove to be more efficient. As a starting point, we recommend testing both membrane extraction buffers. If performing trial extractions using both reagents, carry out Steps 2–9 in parallel on each of two sets of sample replicates.Extraction Buffer 2A is a mild extraction reagent which can facilitate recovery of protein complexes. Thus, protein yields obtained using Extraction Buffer 2A may be lower than those obtained using Extraction Buffer 2B (see Table 2 on p 9). Extracts can be concentrated with a Vivaspin 2 or Vivaspin 500 Ultrafiltration Centrifugal Device.Large volumes of TM-PEK 2B reagent can interfere with protein migration in SDS-PAGE. If high resolution is desired, Extraction Buffer 2B samples for SDS-PAGE may be prepared in 2X SDS sample buffer. Alternatively, employ a buffer exchange step.For most transmembrane proteins, a 2-fold dilution of TM-PEK Reagent A or B in Extraction Buffer 2 results in efficient extraction. For some transmembrane proteins, extraction efficiency may be improved by using TM-PEK Reagent A or B at a different concentration, from undiluted to a 10-fold dilution in Extraction Buffer 2.Protocol1.Prepare Extraction Buffer(s) 2A and/or 2B by diluting the appropriate TM-PEK Reagent 2-fold withExtraction Buffer 2. For membrane protein extraction from 1.0–5.0 x 107 cells, 0.2 ml of ExtractionBuffer 2A or 0.2 ml of Extraction Buffer 2B is required.- To prepare 0.2 ml of Extraction Buffer 2A, mix 0.1 ml Extraction Buffer 2 and 0.1 mlTM-PEK Reagent A.- To prepare 0.2 ml of Extraction Buffer 2B, mix 0.1 ml Extraction Buffer 2 and 0.1 mlTM-PEK Reagent B.Notes: We recommend testing both Extraction Buffer 2A and Extraction Buffer 2B to determine which is optimal for your protein of interest.Samples prepared with Extraction Buffer 2A may require concentration, depending ondownstream application.High levels of Reagent B can interfere with protein migration on SDS-PAGE. Buffer exchange ordilution in sample buffer may be required.A 2-fold dilution of TM-PEK Reagent A orB in Extraction Buffer 2 results in an efficient extractionof most proteins, but reagent dilutions may be optimized (from undiluted to a 10-fold dilution).2.Discard medium from the culture flask.3.Wash cells two times with PBS at 4°C.4.Add 3 ml PBS to the culture vessel. Using a cell scraper or a rubber policeman, free cells from theculture vessel. Transfer cells to a 15 ml conical tube.5.Centrifuge cells at 1000 × g for 5 min at 4°C. (Alternatively, collect cells by centrifuging at 500 × g for10 min at 4°C.)6.Resuspend cells in 1 ml Extraction Buffer 1 + 5 µl Protease Inhibitor Cocktail Set III.7.Incubate 10 min at 4°C with gentle agitation to avoid formation of cell clumps.8.Centrifuge at 1000 × g for 5 min at 4°C.9.Carefully remove supernatant. Store on ice. This is referred to as ‘cytosolic (soluble)’ protein fraction.10.Resuspend pellet in 0.2 ml Extraction Buffer 2A + 5 µl of Protease Inhibitor Cocktail Set IIIor 0.2 ml Extraction Buffer 2B + 5 µl of Protease Inhibitor Cocktail Set III.11.Incubate for 45 min at room temperature with gentle agitation.Note: The length and temperature of the incubation at Step 11 can be varied to improve extraction efficiency or to preserve target protein activity. Increasing the incubation time (up to 120 min) canincrease the protein recovery, but may result in decreased target protein activity. Conversely,incubation at low temperature (4°C) may better preserve activity, but may lower the extractionefficiency.12.Centrifuge at 16,000 × g for 15 min at 4°C.13.Transfer the supernatant, which is enriched in integral membrane proteins, to a fresh tube.Note: Store cytosolic and membrane fractions on ice if they will be analyzed on the same day. For long-term storage, dispense into aliquots and store at –20°C.14.Determine total protein concentration of the cytosolic and membrane protein fractions with the BCAassay.Note: For some cell types, total protein concentration of the membrane protein fraction (Step 13 above) will be >1.0 mg/ml. A two- to four-fold dilution in sterile, deionized water may be required to bringthe concentration of these samples within the linear region of the BCA standard curve.Extraction of membrane proteins from suspension cells or frozen cell pelletsConsiderations Before You BeginThe following protocol is optimized for extraction of membrane proteins from 1.0–2.0 x 107 cells cultured insuspension. Cells should be of high viability (>90%). Different cell types yield considerably differentamounts of protein in the membrane protein fraction (see Table 2 on p 9 in Appendix). If low yield isanticipated, increase the total number of cells to 5.0 x 107 without increasing reagent volumes (see Table 1on p 3). For extracting membrane proteins from larger cell numbers, perform replicate extractions fromaliquots of 1.0–5.0 x 107 cells. Alternatively, scale up buffer volumes.The TM-PEK kit is also compatible with frozen cell pellets (1.0–5.0 x 107 cells per extraction). Cells shouldbe washed a minimum of two times with an appropriate buffer (e.g., PBS) prior to freezing in liquidnitrogen. If using frozen cell pellets, begin at Step 7 below.The kit is supplied with two transmembrane solubilization agents, TM-PEK Reagents A and B(see Description on p 2). Depending on the unique characteristics of the target membrane protein, ExtractionBuffer 2A or Extraction Buffer 2B may prove to be more efficient. As a starting point, we recommendtesting both membrane extraction buffers. If performing trial extractions using both reagents, carry out Steps2–9 in parallel on each of two sets of sample replicates.Extraction Buffer 2A is a mild extraction reagent which can facilitate recovery of protein complexes. Thus,protein yields obtained using Extraction Buffer 2A may be lower than those obtained using Extraction Buffer2B (see Table 2 on p 9). Extracts can be concentrated with a Vivaspin 2 or Vivaspin 500 UltrafiltrationCentrifugal Device.Large volumes of TM-PEK 2B reagent can interfere with protein migration in SDS-PAGE. If high resolutionis desired, Extraction Buffer 2B samples for SDS-PAGE may be prepared in 2X SDS sample buffer.Alternatively, employ a buffer exchange step.For most transmembrane proteins, a 2-fold dilution of TM-PEK Reagent A or B in Extraction Buffer 2results in efficient extraction. For some transmembrane proteins, extraction efficiency may be improved byusing TM-PEK Reagent A or B at a different concentration, from undiluted to a 10-fold dilution inExtraction Buffer 2.Protocol1.Prepare Extraction Buffer(s) 2A and/or 2B by diluting the appropriate TM-PEK Reagent 2-fold withExtraction Buffer 2. For membrane protein extraction from 1.0–5.0 x 107 cells, 0.2 ml of ExtractionBuffer 2A or 0.2 ml of Extraction Buffer 2B is required.- To prepare 0.2 ml of Extraction Buffer 2A, mix 0.1 ml Extraction Buffer 2 and 0.1 mlTM-PEK Reagent A.- To prepare 0.2 ml of Extraction Buffer 2B, mix 0.1 ml Extraction Buffer 2 and 0.1 mlTM-PEK Reagent B.Notes: We recommend testing both Extraction Buffer 2A and Extraction Buffer 2B to determine which is optimal for your protein of interest.Samples prepared with Extraction Buffer 2A may require concentration, depending ondownstream application.High levels of Reagent B can interfere with protein migration on SDS-PAGE. Buffer exchange ordilution in sample buffer may be required.A 2-fold dilution of TM-PEK Reagent A orB in Extraction Buffer 2 results in an efficient extractionof most proteins, but reagent dilutions may be optimized (from undiluted to a 10-fold dilution).1.Transfer 1.0–5.0 x 107 cells to a centrifuge tube.2.Centrifuge cells at 1000 × g for 5 min at 4°C. (Alternatively, collect cells by centrifuging at500 × g for 10 min at 4°C.)3.Discard supernatant and gently resuspend cells in 5 ml PBS (4°C).4.Centrifuge cells at 1000 × g for 5 min at 4°C.5.Repeat Steps 3 and 4 two times, for a total of three washes.6.Centrifuge cells at 1000 × g for 5 min at 4°C.Note: At this point, cells may be frozen in liquid nitrogen and stored at –70°C.7.Resuspend cells in 1 ml Extraction Buffer 1 + 5 µl Protease Inhibitor Cocktail Set III.8.Incubate 10 min at 4°C with gentle agitation to avoid formation of cell clumps.9.Centrifuge at 1000 × g for 5 min at 4°C.10.Carefully remove supernatant. Store on ice. This is referred to as the ‘cytosolic (soluble)’ proteinfraction.11.Resuspend pellet in 0.2 ml Extraction Buffer 2A + 5 µl Protease Inhibitor Cocktail Set IIIor 0.2 ml Extraction Buffer 2B + 5 µl Protease Inhibitor Cocktail Set III.12.Incubate for 45 min at room temperature with gentle agitation.Note: The length and temperature of the incubation at Step 12 can be varied to improve extraction efficiency or to preserve target protein activity. Increasing the incubation time (up to 120 min) canincrease the protein recovery, but may result in decreased target protein activity. Conversely,incubation at low temperature (4°C) may better preserve activity, but may lower the extractionefficiency.13.Centrifuge at 16,000 × g for 15 min at 4°C.14.Transfer supernatant, which is enriched in integral membrane proteins, to a fresh tube.Note: Store cytosolic and membrane fractions on ice if they will be analyzed on the same day. For long-term storage, dispense into aliquots and store at –20°C.15.Determine the total protein concentration of the cytosolic and membrane protein fractions with the BCAassay.Note: For some cell types, total protein concentration of the membrane protein fraction (Step 15 above) will be >1.0 mg/ml. A two- to four-fold dilution in sterile, deionized water may be required to bringthe concentration of these samples within the linear region of the BCA standard curve.Extraction of membrane proteins from tissueConsiderations Before You BeginThe following protocol is optimized for extracting membrane proteins from 25–50 mg of fresh or frozentissue. If tissue is not limiting, start with 100–1000 mg of tissue to offset sample loss during homogenization.When using > 50 mg of tissue, increase volumes of the extraction buffers (see Table 1 on p 3 for buffervolume guidelines). As an example, ~2 mg total protein can be extracted from 35 mg bovine liver (see Table3 on p 9 in the Appendix for representative yields from other tissue sources and types). Yields from varioustissue types can vary considerably, however. Certain transmembrane proteins are expressed at very lowlevels in various tissues, and thus may be below the lower limit of detection by immunological methods. Inthis circumstance, analysis by mass spectrometry may prove beneficial.Protocol1.Prepare membrane Extraction Buffer(s) 2A and/or 2B by diluting the appropriate TM-PEK Reagent 2-fold with Extraction Buffer 2. For membrane protein extraction from 25–50 mg of tissue, 0.2 ml ofExtraction Buffer 2A or 0.2 ml of Extraction Buffer 2B is required.- To prepare 0.2 ml of Extraction Buffer 2A, mix 0.1 ml Extraction Buffer 2 and 0.1 mlTM-PEK Reagent A.- To prepare 0.2 ml of Extraction Buffer 2B, mix 0.1 ml Extraction Buffer 2 and 0.1 mlTM-PEK Reagent B.Note: We recommend testing both Extraction Buffer 2A and Extraction Buffer 2B to determine which is optimal for your protein of interest.Samples prepared with Extraction Buffer 2A may require concentration, depending ondownstream application.High levels of Reagent B can interfere with protein migration on SDS-PAGE. Buffer exchange ordilution in sample buffer may be required.A 2-fold dilution of TM-PEK Reagent A orB in Extraction Buffer 2 results in an efficient extractionof most proteins, but reagent dilutions may be optimized (from undiluted to a 10-fold dilution).2.Ensure that all buffers are thawed and well mixed. Keep Extraction Buffers 1, 2A, and/or 2B on iceduring the extraction procedure. Keep the Protease Inhibitor Cocktail Set III at room temperature toprevent DMSO from freezing.3.Following dissection of the tissue of interest, quickly remove unwanted materials(e.g., connective tissue, fat, blood vessels, etc). To slow proteolysis, keep tissue at 4°C while refiningthe dissection.4.Quickly slice the tissue into ~2 mm3 pieces. Add tissue slices to a tube containing 2 ml ice-cold PBS.5.Gently flick tube to dislodge blood cells and other loosely attached material.6.Collect tissue pieces by centrifuging at 100 × g for 2 min at 4°C. Remove and discard the supernatant.7.Repeat Steps 5 and 6 for a total of two washes. After the second wash, ensure that all PBS has beenremoved completely.Notes: At this point, the tissue can be frozen on liquid nitrogen and stored at –70°C.During tissue extraction, it is important to work quickly, but carefully. Keep the sample cool (<4°C)and store buffers on ice throughout the extraction procedure.8.Transfer the tissue (fresh or frozen) to a pre-cooled homogenizer. We recommend ideally, a glassPotter-Elvehjem or Dounce homogenizer.9.Add 5 µl Protease Inhibitor Cocktail Set III to the wall of the homogenizer.10.Add 2 ml ice-cold Extraction Buffer 1 to the homogenizer.11.Carefully homogenize until tissue is completely homogenized and intact pieces are no longer visible.Use as few strokes as possible (e.g., ~10 strokes for 50 mg mouse liver). The number of strokes willdepend on the type of tissue used. If desired, homogenization efficiency can be monitored by phasecontrast microscopy. An efficient homogenization should generate small cell clumps rather thatfragmentation of individual cells.Note: Some tissues (e.g., heart, muscle, brain) may be difficult to completely dissociate by mechanical homogenization. As an alternative, the ProteoExtract®Tissue Dissociation Buffer Kit(Cat. No. 539720) including collagenase may be used. Other tissue-specific protocols may becompatible with the TM-PEK kit.12.Incubate 10 min at 4°C with gentle agitation.13.Centrifuge at 1000 × g for 5 min at 4°C.14.Carefully remove supernatant. Store on ice. This is referred to as the ‘cytosolic (soluble)’ proteinfraction.15.Add 5 ml ice-cold PBS. Gently resuspend pellet. Collect membranes by centrifuging at 1000 × g for 5min at 4°C. Carefully remove supernatant.Note: When using > 200 mg tissue, perform an extra wash at this point to remove additional cytosolic proteins. Repeat Step 15 for a total of two washes.pletely and carefully resuspend pellet in 0.2 ml Extraction Buffer 2A + 5 µl of Protease InhibitorCocktail Set IIIor 0.2 ml Extraction Buffer 2B + 5 µl Protease Inhibitor Cocktail Set III.17.Incubate 15 min at 4°C with gentle agitation to avoid formation of cell clumps.18.Centrifuge at 16,000 × g for 15 min at 4°C.19.Transfer supernatant, which is enriched in integral membrane proteins, to a fresh tube.Note: Store cytosolic and membrane fractions on ice if they will be analyzed on the same day.For long-term storage, dispense into aliquots and store at –20°C.20.Determine the total protein concentration of the cytosolic and membrane fractions with the BCA assay. Note: For some tissue types, total protein concentration of the membrane protein fraction (Step 19 above) will be >1.0 mg/ml. A two- to ten-fold dilution in sterile, deionized water may be required tobring the concentration of these samples within the linear region of the BCA standard curve.Frequently Asked QuestionsQuestion AnswerHow do I determine the proteinconcentration of the membrane proteinextract? The components in the extraction buffers are directly compatible with common protein assays. We recommend using the BCA Protein Assay Kit (Cat. No. 71285-3). Note that the Extraction Buffer 2B fraction may require a 2-4-fold dilution to bring the total protein concentration within the linearrange of the BCA assay.How do I prepare the TM-PEK fraction for one-dimensional SDS-PAGE? The TM-PEK fractions can be analyzed directly by one-dimensional SDS-PAGE. For samplesextracted with Reagent A, add SDS-PAGE sample buffer to a 1X final concentration and loaddirectly on to the gel. Samples extracted with Reagent B should be prepared using a 2Xconcentration of SDS-PAGE sample buffer.How can I concentrate the TM-PEK extracted proteins? It is possible to reduce the volume of Extraction Buffer 2A or 2B used, but this may decrease thetotal protein yield. We recommend using the ProteExtract ® Protein Precipitation Kit (Cat. No.539180) to concentrate samples for use in downstream applications not requiring native protein.For applications that do require native protein, we recommend using an ultrafiltration device suchas Vivaspin 2 or Vivaspin 500.How should the membrane fractions be treated prior to mass spectrometry analysis?The membrane extracts should be applied to a one-dimensional or two-dimensional gel, spots orbands cut from the gel, and then digested with trypsin.Appendix Example extractionsIn the examples below, the SDS extract (total cell lysate) serves as a positive control. The Extraction Buffer 2A recovers EGFR, which has a single transmembrane span, with efficiency comparable to TRITON ® X-100. However, Extraction Buffer 2A recovers Frizzled-4 and CELSR-3, both of which contain seven transmembrane domains, with far greater efficiency than does TRITON X-100.A: Figure 1. Ex ction of transmembrane prote EGFRB: Frizzled-4 C: CELSR-3tra ins from MDA-MB-468 breast adenocarcinoma cultured ells. Transmembrane proteins were extracted from MDA-MB 468 cells using the TM-PEK kit. In the first two identical pools of 1 x 107 cells were treated with Extraction Buffer 1, which recovers proteins from the cytosolic fraction. The insoluble material was then treated with TM-PEK Extraction Buffer 2A or 0.5% TRITON ® X-100. Lane 1 shows the expected size of the target proteins and is not a quantitative control. Lanes 2–4 contain the extracted protein from 1 x 106 cells. Fractions were separated using a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. Membranes were blocked and incubated with primary antibody to EGFR (panel A), Frizzled-4 (panel B) or CELSR-3 (panel C). Blots were developed using an HRP-conjugated secondary antibody and a chemiluminescent substrate. Lane 1, 0.5% SDS (total cell lysate); Lane 2, cytoplasmic fraction; Lane 3, membrane fraction (TRITON X-100); Lane 4, membrane fraction (Extraction Buffer 2A). Arrows indicate size of the full-length version of each protein.c step,Examples of total protein yields using TM-PEKThe values presented in Tables 2 and 3 below are intended to serve as a guide to estimate the required amount of starting material. For each cultured cell type, proteins were extracted according to the TM-PEK protocol from cell monolayers grown to 80% confluency in a T-75 flask. All tissues were disrupted mechanically using a Dounce homogenizer. Table 2. Protein yields for cytosolic and membrane fractions recovered from cultured cells using the TM-PEK kit.Total Protein (mg/107 cells)*Cell TypeBuffer 1(cytosolic) Buffer 2A (membrane) Buffer 2B (membrane) ExtractionExtraction Extraction MDA-MB-468 (breast adenocarcinoma)0.62 0.15 0.78 MCF 7 (breast adenocarcinoma)0.68 0.26 1.27 A-431 (epidermoid carcinoma)0.55 0.12 0.75 CHO-K1 (Chinese hamster ovary)0.52 0.22 0.94 NCI-H292 (mucoepidermoid carcinoma)0.06 0.04 0.47 HEP-G2 (hepatocellular carcinoma)0.97 76 0.13 0.Mia PaCa-2 (pancreatic carcinoma)0.23 0.05 0.52 HCT 116 (colon carcinoma) 0.60 0.10 0.76 *Protein concentrations were determined using the BCA .Table 3. olic and membrane fractions recovered from mouse tissue Total Protein g/mg tissue)*assayProtein yields for cytos TM-PEK kit.s using the (μTissue TypeExtractionB(cytos Extraction Bu A (me ne) Extraction Bu B (memb uffer 1olic)ffer 2mbra ffer 2rane)Liver §55.6 1.7 11.4 Heart28.2 2.9 17.3 Brain18.4 0.9 5.8 Spleen 31.6 8.2 8.8 §Skeletal Muscle13.6 2.1 6.3 Kidney 33.6.9 5.4 16*Protein concentrations were determined using the B §Average of two tr CA assay.ials.。
