体细胞胚胎发生副本共40页文档
Chapter4体细胞胚胎发生

植物细胞的群体培养比较困难,受到溶氧 量、营养供给、对剪切力的适应性差等方 面的限制,目前还没有比较成熟的技术体 系。
2、意义:P81 、意义:P81 ① ② ③ ④ ⑤ ⑥
3、局限性: ①贮藏困难:易萌发,易脱水; ②成本较高:生产规模小、技术不成熟; ③体cell无性系变异:优良性状丢失; ③体cell无性系变异:优良性状丢失; 体cell无性系变异:在不施加任何选择压的情 cell无性系变异:在不施加任何选择压的情 况下,由离体的体细胞产生再生植株所表 现的变异。
(2)、生产应用: ①快繁 ②脱毒 单cell培养 cell培养 ③细胞培养 cell群培养 cell群培养 ④人工种子 次生产物 人工改良
三.长期培养物形态发生潜力的 丧失
有些愈伤组织或悬浮培养物起初具有器官 发生或胚胎发生的潜力, 发生或胚胎发生的潜力 , 但经过反复继代 保存之后喧种形态发生能力常常逐渐下降, 保存之后喧种形态发生能力常常逐渐下降 , 有些甚至完全损失。 为了解释这种现象, 有些甚至完全损失 。 为了解释这种现象 , 现在有三种假说。 现在有三种假说。
Chapter 5 体细胞胚胎发生
概念 形成及结构 发生途径 影响因子 人工种子
体细胞胚胎的概念 胚胎:自然状态下,高等植物的胚胎是受精 胚胎:自然状态下,高等植物的胚胎是受精 作用后由雌雄配子结合而成的合子发育而 来的,称为合子胚。 来的,称为合子胚。 1、合子胚(生命周期) 合子胚(生命周期) 2、非合子胚 i、无融合生殖胚 ii、体细胞胚(组培周期) ii、体细胞胚(组培周期)
胚状体与合子胚的比较
合子胚 质量 来源 胚柄 形态 胚状体 萌发率高,质量 萌发率低,质量 好 差 受精卵 体细胞 or 离体生殖细胞 有,明显 即使有也不明显 固定,体积相对 复杂,常有两个 较小 以上的子叶体积 相对较大 低 高
体细胞胚发生

植物物种和品种基因型影响 愈伤组织的分化
• 程林梅等(2001)的研究表明,利用不同 基因型大豆品种的下胚轴作外植体进行组培时, 培养基对愈伤组织的影响不明显;细胞分裂素 (KT)和NAA的配比浓度对其愈伤组织的分 化影响显著;不同基因型大豆植株再生作用差 异明显;外植体取材时间和取材部位对再生植 株的形成也具有较大的影响,再生能力最强的 取材部位时从子叶节到下胚轴6mm区段,当下 胚轴长到2~3cm时取材最佳。
2 氮源 除生长素外,培养基中氮源的形态也会影响离 体条件下的胚胎发生。只有当培养基中含有一定量 的还原态氮时,才能出现胚胎发生过程。 在以KNO3为唯一氮源的培养基上建立起来的 愈伤组织,去掉生长素后不能形成胚。然而,若在 55mmol/LKNO3的培养基中加入少量的 (5mmol/L)NH4Cl形态的氮,胚胎发生过程就会出 现。
不同浓度的激素对烟草愈伤组织的影响
愈伤分化
器官分化的植物激素控制理论
• 大量的实验结果表明:
大多数植物组织或器官的再生作用符合器 官分化的植物激素控制理论。
即:生长素与细胞分裂素的比例小时则产 生苗,比例大时则生根,而两种激素的比例适中 时,则产生无结构的愈伤组织。
器官发生途径
外植体 脱分化 愈伤组织 再分化
• 1.4.1.2位置效应
1.4.2 器官分化信息传递
1.4.2.1 胞外信号分子
• 胞外通讯信号分子又称第一信使(first messenger)。在植物细胞外的通讯信号分子,, • 如植物激素主要通过维管束系统在细胞与器官间 进行调节。它在植株的某些发育阶段,于特, • 部位发生作用。植物激素信号由位于膜或其它部 位的受体所接收,进一步直接或间接地影响: • 因表达。当植物激素以直接的形式影响基因表达 时,就会产生在转录、翻译等水平上的不同, • 应,从而控制酶的合成,影响代谢变化,最终产 生某些生理反应。
第十二章 植物体细胞胚胎发生

(A) 伸长期合子;(B) 合子经过第一次分裂, 产生1 个顶细胞(绿色)和一个 基细胞(粉红色);(C) 四分体胚: 顶细胞经历2 次纵向分裂,产生含有4 个 细胞的胚体和2 个细胞的胚柄;(D) 16 细胞球形胚: 8 细胞胚的所有细胞经 过1 次平周分裂, 产生包含8 个细胞的表皮原和8 个细胞的内部组织;(E) 早期心形胚;(F) 心形胚: 子叶和胚根原基已经出现;(G) 成熟胚胎。 1: 顶细胞; 2 : 基细胞; 3: 胚根原细胞, 形成未来的ROC 和根冠中央区; RM: 根尖生长点; SM: 茎尖生长点 图6.2 双子叶植物拟南芥的胚胎发育过程
品种Jack Jack(光照强度 Jack 50-60 µE m2 s-1)
品种 Fayette Fayette(光照强度50-60 µE m2 s-1)
品种Jack Jack(光照强度5-10 Jack µE m2 s-1)
品种Fayette Fayette(光照强度 Fayette 5-10 µE m2 s-1)
于非胚性愈伤组织,而且mRNA种类丰富 mRNA种类丰富 mRNA种类丰富、不同发育时期mRNA种类
不同,因而转译形成探针。
**Sengupta等早在20世纪80年代初就发现,胡萝卜体细胞胚发生 中,RNA合成始于胚性细胞培养的2-4小时 RNA合成始于胚性细胞培养的 RNA合成始于胚性细胞培养的2 小时,两天后细胞分裂较快,
总RNA和蛋白质含量增加 ,而且在 胚性细胞中主要合成poly(A)+ mRNA,并认为它的出现是体细胞胚分化的重要遗传信息。
(六)、DNA代谢动态
DNA代谢动态与体细胞胚的形态学极性密切有关,
它的合成为细胞分裂奠定了物质基础,为细胞分化提 供了条件。
体细胞胚胎发生和人工种子

(二)、局限性
1、一些具有重要性状的植物目前发生体细胞胚的能力较弱, 再生系统尚不健全,难以形成有活力的胚并同步化发育。
2、制作的人工种子成本远高于自然种子,无竞争力。
3、人工种子的贮藏、运输以及机械化播种等问题尚未解决 。
第34页,此课件共39页哦
第35页,此课件共39页哦
第29页,此课件共3凝胶中直接加入大量元素、碳水化合物及防病用抗生 素。
• 微型包裹法 将碳水化合物和大量元素包裹在微型胶囊内,然 后再把微型胶囊和种胚一起包裹在海藻酸钠中。
第30页,此课件共39页哦
2、植物组织培养的应用:
现在已有胡萝卜、芹菜、柑橘、咖啡、棉花、玉米、水 稻、橡胶等几十种植物的人工种子试种成功,但由于成本 较高,中国尚未应用于生产。
第10页,此课件共39页哦
二、愈伤组织器官的发生
• 不定芽方式
• 胚状体形成的方式
第11页,此课件共39页哦
• 不定芽方式(adventitious bud type)是指
愈伤组织培养物,通过形成不定芽再生成 植株。这是愈伤组织中常见的器官发生方 式。
第12页,此课件共39页哦
• 愈伤组织通过不定芽方式再分化形成再生植株的方式主 要有四种:
第23页,此课件共39页哦
人工种皮需要满足的条件:
• 1、具有一定的透气性,不能影响胚状体的呼吸;
• 2、具有一定的硬度,经得起种子储存和运输中的挤 压和机械播种中的磨损;
• 3、易于降解;
• 4、含有其他有利于胚状体存活、生长的成分。
第24页,此课件共39页哦
B. 体细胞胚包裹的材料:
• 凝胶 • 可以使人工种皮具有柔软性,不仅适于胚
体细胞胚胎发生

心形胚
心形胚 原胚、球形胚、心形胚 球形胚、心形胚 原胚 原胚 原胚 原胚 球形胚、心形胚、鱼雷胚 球形胚、心形胚 胚性细胞、原胚 球形胚 原胚、球形胚、心形胚、鱼 雷胚 胚性细胞、球形胚、心形胚 胚性细胞、球形胚、心形胚 、鱼雷胚、小植株 球形胚、心形胚
第七章 植物体细胞胚胎发生
本章主要内容
体细胞胚胎的概念、特点和意义 影响体细胞胚发生的因素 体细胞胚胎发生的生物学 人工种子
一、体细胞胚胎的概念、特点和意义
概念
离体培养下没有经过受精过程,但经过胚 胎发生和胚胎发育过程所形成的类胚结构,称 为胚状体(embryoid)或体细胞胚(somatic embryo)。
饥饿;有丝分裂阻止
2、人工种子包埋
包埋材料: 海藻酸钠、明胶、果胶酸钠、琼脂、树胶
包埋方法: 液胶包埋法;干燥包埋法;水凝胶法
人工胚乳研制
木薯淀粉与海藻酸钠混合胚乳是目前比较理想 的材料,还可以在胚乳中加入激素(GA3等)、 缓释物(活性炭等)、固化剂(CaCl2等)、抗生 素、其他混合物等制作全能性人工胚乳。
人工种皮研制
壳聚糖、El-vax-4260(10%环己烷)等
三、人工种子转换实验
转换指人工种子在一定条件下,萌发、生长、 形成完整植株的过程。可以分为无菌条件下的转 换和土壤条件下的转换。
2、培养基的影响
碳源
碳水化合物的种类和浓度可以影响体细胞胚的生长 发育,蔗糖是体胚发生最有效的还原碳源。但在一些 试验中,某些糖的培养效果优于蔗糖。还可添加半乳 糖、可溶性淀粉等促进体胚的生长发育。
糖类还可作渗透调节剂对体胚发育或成熟起作用。
第七章器官发生与体细胞胚胎发生ppt课件
器官发生
• 概念:离体培养的组织、细胞在诱导 条件下经分裂和增殖再分化形成不定 根和不定芽等器官的过程。
• 发生方式:
– 直接发生:(具有初生分生能力的)外 植体直接分化成器官的过程。如落地生 根.
2023/12/31
– 间接发生:外植体(已分化的成熟组织 )经脱分化形成愈伤组织再分化形成器 官的过程。
2023/12/31
2023/12/31
组织的分化与器官建成
• 维管组织的分化
• 早期研究发现(Wetmore,1955),丁香的芽可 以诱导邻近的组织分化出维管束,后证明这是芽中 IAA的作用。将一块含有14C-IAA和蔗糖的琼胶楔 形物插入到愈伤组织切口中,14C-IAA表明蔗糖和 IAA通过琼胶扩散到愈伤组织,从而在愈伤组织中 造成这两种物质的梯度,在楔形物一颇有同感形成 维管束的瘤状物,瘤状物含有一形成层带,一面形 成韧皮部,一面形成木质部,与正常的维管束有类 似的排列。增加IAA的浓度,导致木质部形成,增 加蔗糖浓度则导致韧皮部形成。生长素水平恒定时 ,2%蔗糖则全部分化出木质部,4%蔗糖几乎全部 分化出韧皮部,3%蔗糖则可以分化出二者。所以, 生长素和蔗糖浓度决定愈伤组织中维管束的类型与
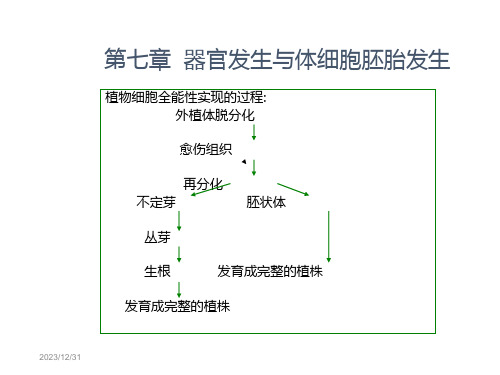
第七章 器官发生与体细胞胚胎发生
植物细胞全能性实现的过程: 外植体脱分化
愈伤组织
再分化
不定芽
胚状体
丛芽
生根
发育成完整的植株
发育成完整的植株
2023/12/31
• 脱分化:成熟细胞转变成分生状态并 形成愈伤组织的过程.
• 再分化:由分生状态的愈伤组织或外 植体分化出各种器官的过程.
2023/12/31
2023/12/31
影响体细胞胚胎发生的因素
第2节植物体细胞胚胎发生

表 在MS固体培养基上不同浓度2,4-D对木豆幼苗叶片 外植体诱导和原胚细胞形成的影响
2,4-D(μM) 0.00 2.26 4.52 6.78 Callus induction(%) 0.0 3.2d 7.4c 38.6a Proembryonic cell*(%) 0.0 3.4d 10.2c 37.4a
**总之,体细胞胚胎的发生是一个非常复杂的生理过程,仍 有许多问题尚需进一步研究。
大多数体细胞胚起源于单细胞,通过体细胞胚胎 形成的再生植株,其遗传特性相对稳定.而器官 发生途径的再生植株来源复杂,形成的个体常为 嵌合植株;
从体细胞胚形成的过程可知,体细胞胚的发育过 程与合子胚基本相同.但在形态结构和生理特性 与合子胚有一定的区别.
a 胚性叶片 愈伤组织;b 球形细胞;c 伸长细胞;d 两个细胞时 期; e 四个细胞时 期; f 原胚; g 球形胚;h 心形胚;i 早期鱼雷胚; j 成熟鱼雷 胚; k 不同时期的 体细胞胚;l 体细胞胚的 萌发; m 再生小植株。
图 木豆幼苗叶片愈伤组织悬浮培养体细胞胚发育过程
2 体细胞胚的多细胞起源
四、体细胞胚的起源 **体细胞胚胎发生途径有直接途径和间接途径, 但无论哪一种发生途径,绝大多数研究报道认 为体细胞胚胎起源于单细胞,从多种植物中都 观察到单个胚性细胞,有不均等分裂的二细胞
原胚和均等分裂的二细胞原胚、多细胞原胚、
球形胚直到成熟胚。也有研究认为起源于多细
胞。
1 体细胞胚的单细胞起源
**有的学者观察到体细胞胚来源于多细胞组成的胚性细胞复合
体,王亚馥等人也观察到这种胚性细胞团,但用同位素脉冲标
记实验即可证明这些细胞团也是由一个单细胞连续分裂而形成,
经进一步分化和分裂形成不同发育时期的体细胞胚,因而这些 体细胞胚实质也可能是起源于单细胞。当然也不排除有些植物
第5章 体细胞胚胎发生 - 副本

(图4-6),在制造单胚人工种子的时候, 主要采用诸如藻酸钠一类的水凝胶作为体细 胞胚的包被物质。
当体细胞胚与藻酸钠混合以后,再滴人到硝
酸钙或氯化钙溶液(100 mmol/L)中,30 min内表面即可完全络合,形成一层持久的 种皮(图4-7 ,图4-8 )。
8.5 代谢产物的合成
重复体细胞胚胎发生体系在合成代谢产物如
药物和油脂中有潜在的应用前景。如玻璃苣 种子含有高水平的γ-亚麻酸,用作前列腺素 前体或治疗遗传性过敏症。玻璃苣体细胞胚 也产生该代谢产物,而且通过重复体细胞胚 胎发生,能连续形成γ-亚麻酸,否则这种代 谢产物会局限在合子胚生长的季节。
欲使胡萝卜培养细胞形成体细胞胚,必须有
一个最低数量的内源NH4+存在(每千克鲜重 的组织约5 mmol)。如果内源NH4+达不到这 个临界值,细胞就不能进行胚胎分化。而要 使细胞内NH4+达到这个水平,不但需要有很 少量的外源NH4+存在(2.5 mmol/L),还需 要供应相对浓度很高的NO3-(60 mmol/L)。
Kohlenbach关于胚的分类: (1)合子胚:由受精卵或合子形成的胚。
(2)非合子胚:由合子之外的细胞形成的胚。
体细胞胚—由孢子体细胞(除合子外)离体
培养形成的胚。 非体细胞胚—即指芽、短枝、愈伤组织、花 粉胚、块茎、球茎等繁殖体。 孤雌生殖胚—由未受精卵形成的胚。 孤雄生殖胚—由雄配子体(小孢子花粉粒) 形成的胚。
卜细胞胚胎发生的频率 。
某些挥发性和非挥发性物质:抑制愈伤组织
中的体细胞胚胎发生。
4. 体细胞胚胎发生的解剖学和细胞学
在胡萝卜悬浮培养中,对愈伤组织形成和生
体细胞胚胎发生

SOMATIC EMBRYOGENESIS AND PLANT REGENERATION FROM CALLUS CULTURES OF SEVERAL SPECIES IN THE GENUS TRICYRTISMASARU NAKANO 1*,KEIKO MIZUNASHI 1,SHIGEFUMI TANAKA 1,TOSHINARI GODO 2,MASASHI NAKATA 2,ANDHIROYUKI SAITO 31Faculty of Agriculture,Niigata University,2-8050Ikarashi,Niigata 950-2181,Japan2Botanic Gardens of Toyama,42Kamikutsuwada,Fuchu-machi,Nei-gun,Toyama 939-2713,Japan3Plant Functions Laboratory,RIKEN (The Institute of Physical and Chemical Research),2-1Hirosawa,Wako-shi,Saitama 351-0198,Japan(Received 22May 2003;accepted 16September 2003;editor C.L.Armstrong)SummaryThe liliaceous perennial plants,Tricyrtis spp.,are cultivated as ornamental plants in Japan.Natural populations of several Japanese Tricyrtis spp.are severely threatened by indiscriminate collection and habitat destruction.In this study,a plant regeneration system based on somatic embryogenesis has been developed for efficient clonal propagation of T.hirta ,T.hirta var.albescens ,T.formosana ,T.formosana cv.Fujimusume,T.flava ssp.ohsumiensis ,and T.macrantha ssp.macranthopsis .Flower tepal explants of these genotypes were cultured on media containing 2,4-dichlorophenoxyacetic acid (2,4-D)or 4-amino-3,5,6-trichloropicolinic acid (picloram,PIC)alone or in combination with N -(1,2,3-thiadiazol-5-yl)-N 0-phenylurea (thidiazuron,TDZ).Calluses induced on media containing 2,4-D produced somatic embryos following their transfer to a plant growth regulator-free medium,indicating that these calluses were embryogenic.A combination of 4.5m M 2,4-D and 0.45m M TDZ was most effective for inducing embryogenic calluses from tepal explants.Among various explant sources,filaments were most suitable for inducing embryogenic calluses on a medium containing 4.5m M 2,4-D and 0.45m M TDZ.Embryogenic calluses were only obtained from filament for T.macrantha ssp.macranthopsis .Embryogenic calluses could be maintained by subculturing monthly onto the same medium,and a 1.5–3.5-fold increase in fresh weight was obtained after 1mo.of subculture.Depending on the plant genotype,50–500somatic embryos per 0.5g fresh weight of embryogenic callus was obtained 1mo.after transfer to a plant growth regulator-free medium.Most of the embryos developed into plantlets,and they were successfully acclimatized to greenhouse conditions.Regenerated plants showed no alteration in the ploidy level as indicated by chromosome observation and flow cytometric analysis.Key words:embryogenic callus;endangered plant;liliaceous ornamental;micropropagation;Tricyrtis .IntroductionThe liliaceous perennial plants,Tricyrtis spp.,are native to East Asia.Some of these species have recently become popular in Japan as ornamental plants for pot and garden use because of their beautiful foliage,attractive flowers,and the ability to grow in the shade.On the other hand,natural populations of several Tricyrtis spp.,such as T.formosana ,T.macrantha ssp.macranthopsis ,and T.flava ssp.ohsumiensis ,are severely threatened in Japan by indiscriminate collection for commercial purposes and habitat destruction.These three species are listed as vulnerable (VU),VU,and near threatened (NT),respectively,on the Red Data List of Japan (Environment Agency of Japan,2000).Generally,Tricyrtis spp.are propagated vegetatively by cutting or division of lateral shoots,but the efficiency is relatively low (Nagamura,1989).Tissue culture offers increased rates of vegetative propagation as well as maintenance of pathogen-free plants (Saito and Nakano,2002).In addition,tissue culture is an efficient method for ex situ conservation of plant diversity becausemany endangered plant species can be quickly propagated and preserved from a minimum of plant material with low impact on wild populations (Fay,1992,1994).Tissue culture techniques could be applied to clonal propagation of selected strains and for ex situ conservation of Tricyrtis spp.,however,no studies on tissue culture have yet been reported for these species.In the present study,we examined the development of an efficient plant regeneration system based on somatic embryogenesis in several Tricyrtis spp.,T.hirta ,T.hirta var.albescens ,T.formosana ,T.flava ssp.ohsumiensis ,and T.macrantha ssp.macranthopsis .Materials and MethodsPlant material and callus induction .Potted plants of T.hirta (Thunb.)Hook.,T.hirta var.albescens Makino,T.formosana Bak.,T.formosana cv.Fujimusume,T.flava ssp.ohsumiensis (Masam.)Kitam.,and T.macrantha ssp.macranthopsis (Masam.)Kitam.were cultivated in the greenhouse without heating.Fully expanding leaves (35–50mm in length)were harvested 1–2mo.before anthesis,and flower buds (12–20mm in length)were harvested 3–5d before anthesis.They were washed in running water for 1min,surface-sterilized with a sodium hypochlorite solution containing 1%active chlorine for 20min,and rinsed three times with sterile,distilled water.Leaves were cut transversely into 5–7mm segments.Tepals,*Author to whom correspondence should be addressed:Email mnakano@agr.niigata-u.ac.jpIn Vitro Cell.Dev.Biol.—Plant 40:274–278,May–June 2004DOI:10.1079/IVP2003506q 2004Society for In Vitro Biology 1054-5476/04$18.00+0.00274filaments,anthers,styles,and ovaries were isolated fromflower buds and transversely cut into halves.Tepal explants were cultured on half-strength MS medium(Murashige and Skoog,1962)lacking plant growth regulators(PGRs),or containing0.45 or 4.5m M2,4-dichlorophenoxyacetic acid(2,4-D)or0.41or 4.1m M 4-amino-3,5,6-trichloropicolinic acid(picloram,PIC)alone or in combination with0.45m M N-(1,2,3-thiadiazol-5-yl)-N0-phenylurea(thidia-zuron,TDZ)to examine the effect of PGRs on callus formation(Table1). Various explants,leaf,tepal,filament,anther,style,and ovary explants were cultured on half-strength MS media containing4.5m M2,4-D and0.45m M TDZ.All media used were supplemented with30g l21sucrose and2g l21 gellan gum and adjusted to pH5.8before autoclaving at1218C and105kPa for15min.Plastic Petri dishes(9cm in diameter)werefilled with30ml of the medium,and20explants were cultured per dish.Cultures were incubated at258C in the dark.Data on callus formation were recorded2mo. after culture initiation.Primary calluses induced on a medium containing 4.5m M2,4-D and0.45m M TDZ were then separated from the explants and transferred to plastic Petri dishes(9cm in diameter)each containing30ml of fresh medium of the same composition.They were subcultured monthly onto the same fresh medium at258C in the dark.To determine fresh mass, calluses were transferred onto pre-weighed aluminum foil and immediately weighed again.Plant regeneration.After1mo.of subculture,calluses were transferred to plastic Petri dishes(9cm in diameter)each containing 30ml of half-strength MS medium lacking PGRs.Cultures were maintained at258C under continuous illumination withfluorescent lighting(50m mol m22s21).Data on the production of somatic embryos were recorded1mo.after transfer.Somatic embryos were transferred to test tubes(2.5£13cm)each containing10ml of fresh medium of the same composition and capped with aluminum foil.Cultures were incubated under the same conditions.Regenerated plantlets with a well-established root system were washed carefully with tap water to remove gellan gum and transferred to pots containing moist vermiculite.They were acclimatized in a transparent plastic cabinet covered with a polyethylene sheet at258C under a16/8-h (light/dark)photoperiod withfluorescent lighting(50m mol m22s21).Small holes were punched in the polyethylene sheet after1wk,and then the sheet was gradually removed in a stepwise fashion.The plantlets were next transplanted to planters containing soil and cultivated in the greenhouse as the mother plants.Flow cytometric analysis.Ploidy level of regenerated plants was determined by measuring relative DNA content of isolated nuclei using a flow cytometer(Partec PA,cytometer(Partec,PA,GmbH,Mu¨nster, Germany)as previously described(Saito et al.,2003).Chromosome observation.Root tips(ca.5mm)were incubated in2m M 8-hydroxyquinoline at208C for5h and thenfixed in ethanol:acetic acid (3:1,v/v)at48C for over24h.After hydrolyzing with1N HCl:45%acetic acid(1:2,v/v)at608C for30–50s,root tips were stained with2%aceto-orcein for20min and squashed under a cover glass.At leastfive root tips per plant were observed under a light microscope(Leica DMLB,Leica,Wetzlar, Germany).Results and DiscussionEstablishment of embryogenic callus cultures.In preliminary experiments,we tried to induce direct morphogenesis from various organs of Tricyrtis spp.on media containing various concentrations and combinations of2,4-D,PIC,and TDZ,but direct regeneration of adventitious shoots and somatic embryos was not obtained on any media tested.Therefore,we next examined the development of embryogenic callus cultures,since somatic embryogenesis offers several advantages for micropropagation(Ammirato and Styer, 1985;Durzan and Durzan,1991).Effects of2,4-D,PIC,and TDZ on callus formation was initially examined using tepal explants(Table1).Three to6wk after culture initiation,calluses were initiated mainly from the cut end of explants.Among six genotypes,T.hirta most frequently produced calluses,and callus formation of this genotype was observed on all media tested except for PGR-free medium.For T.hirta,T.hirta var. albescens,and T.formosana,two visibly distinguishable types of callus were produced:one was a white to yellowish,friable callus occasionally containing a few globular to oval structures(Fig.1A), and the other was yellow compact callus.The former developed somatic embryos following transfer to PGR-free medium,indicating that these calluses were embryogenic.Similar appearances of embryogenic calluses,i.e.,friable and white to yellowish in color, have been reported for other liliaceous species,such as Asparagus officinalis(Kunitake and Mii,1998),Muscari armeniacumTABLE1EFFECT OF PGRs ON THE PERCENTAGE OF TEPAL EXPLANTS FORMING CALLUSES IN SEVERAL TRICYRTIS SPP.PGRs(m M)T.hirta T.hirta var.albescens T.formosanaT.formosanacv.FujimusumeT.flava ssp.ohsumiensisT.macranthassp.macranthopsis2,4-D PIC TDZ0000000000.450023.8EC000000.4500.4532.5EC 1.3EC 2.3EC026.8EC04.50047.1EC000004.500.4561.4EC7.7EC9.5EC 3.3EC33.3EC000.41012.30000000.410.4536.5 1.200000 4.1023.6000000 4.10.4531.808.3000Significance Callus formation Embryogenic callus formationPGR(P)***Genotype(G)****P£G***Data were recorded2mo.after culture initiation.Values represent the mean of three independent experiments,each of which consisted of20explants. EC indicates that induced calluses were embryogenic because they produced somatic embryos following transfer to PGR-free medium.*and**indicate significant at P¼0.05and P¼0.01,respectively(F test).SOMATIC EMBRYOGENESIS AND REGENERATION OF TRICYRTIS275(Suzuki and Nakano,2001),and Agapanthus praecox ssp.orientalis (Suzuki et al.,2002).Because calluses with such appearance were all embryogenic in the three Tricyrtis genotypes,it might be possible to visibly select embryogenic calluses in Tricyrtis spp.The yellow compact calluses did not produce any differentiated structures on PGR-free medium,and regenerated a few adventitious shoots after transfer to media containing 4.5–45m M TDZ (data not shown).Regardless of the plant genotype,all the calluses produced on 2,4-D-containing media were white to yellowish,friable,and embryogenic,whereas those produced on PIC-containing media were all yellow,compact,and non-embryogenic.In liliaceous species,two types of synthetic auxins,2,4-D (Krikorian and Kann,1981;Buiteveld et al.,1994;Kim and Soh,1996;Kunitake and Mii,1998)and PIC (Beyl and Sharma,1983;Phillips and Luteyn,1983;Eady et al.,1998;Nakano et al.,2000;Suzuki et al.,2002),have mainly been used for inducing embryogenic parative studies of these two auxins have been reported for Gasteria verrucosa and Haworthia fasciata (Beyl and Sharma,1983)and Allium cepa (Phillips and Luteyn,1983;Eady et al.,1998),all in which PIC was comparable or superior to 2,4-D for efficient induction of embryogenic calluses.In this study,embryogenic calluses of three Tricyrtis genotypes were only produced on 2,4-D-containing media.In the Liliaceae ,the type of auxin appropriate for inducing somatic embryogenesis may be different according to the species.For all genotypes except for T.macrantha ssp.macranthopsis ,embryogenic calluses were most frequently obtained on a medium containing 4.5m M 2,4-D and 0.45m M TDZ.No calluses were obtained from tepal explants of T.macrantha ssp.macranthopsis on any media tested.The explant source was then examined for callus formation (Table 2)using a medium containing 4.5m M 2,4-D and 0.45m M TDZ,on which embryogenic calluses were most frequently induced from tepal explants.Regardless of the plant genotype and explant source,all the induced calluses were white to yellowish,friable,and developed somatic embryos following transfer to PGR-free medium,indicating that they were embryogenic.T.hirta showed efficient formation of embryogenic calluses compared with the other genotypes,and for this genotype embryogenic calluses were obtained from all types of explants tested.Among different explant sources,filaments showed the best response for embryogenic callus formation in all six genotypes.For T.macrantha ssp.macranthopsis ,embryogenic calluses were obtained only from filamentexplants.F IG .1.Somatic embryogenesis and plant regeneration from callus cultures of Tricyrtis formosana .A ,Callus formation from a filament explant 2mo.after culture initiation on a medium containing 4.5m M 2,4-D and 0.45m M TDZ (bar ¼5mm).B ,Embryogenic callus cultures subcultured on a medium containing 4.5m M 2,4-D and 0.45m M TDZ (bar ¼1cm).C ,Development of somatic embryos 3wk after transfer of embryogenic calluses onto PGR-free medium (bar ¼5mm).D ,Germination of somatic embryos on PGR-free medium (bar ¼1cm).E ,Somatic embryo-derived plantlets on PGR-free medium (bar ¼1cm).F ,Somatic embryo-derived plants established in the greenhouse (bar ¼5cm).G ,Chromosomes in a root tip cell of a somatic embryo-derived plant (2n ¼2x ¼26)(bar ¼10m m).276NAKANO ET AL.Efficient induction of embryogenic calluses fromfilaments has already been reported for Brassica campestris(Choi et al.,1998)and Vitis vinifera(Iocco et al.,2001).Apart from high potential to form embryogenic calluses,filaments are appropriate as a starting material for tissue culture of Tricyrtis spp.,especially of threatened ones,because explants can be prepared after horticultural evaluation of the mother plants with minimal damage. Embryogenic calluses produced on a medium containing4.5m M 2,4-D and0.45m M TDZ were subcultured monthly onto fresh medium of the same composition(Fig.1B).After stabilization of the growth rate(after three or four subcultures),one callus line for each genotype was selected for vigorous growth and high ability to produce somatic embryos.Tepal-derived calluses were selected for T.hirta,T.hirta var.albescens,and T.flava ssp.ohsumiensis, whereasfilament-derived calluses were selected for the other genotypes.Six months after the initiation of callus culture,the growth rate of embryogenic calluses of selected lines was determined on a medium containing4.5m M2,4-D and0.45m M TDZ(Table3).According to the plant genotype, 1.5–3.5-fold increases in fresh weight were obtained after1mo.of subculture. T.macrantha ssp.macranthopsis calluses were less friable and showed poor growth as compared with the other genotype.Plant regeneration from embryogenic callus cultures.About 2wk after transfer onto PGR-free medium,embryogenic calluses of all six genotypes started to develop somatic embryos.Somatic embryos of Tricyrtis spp.were oval to club-shaped and cream to yellow in color,and easily detached from the original callus (Fig.1C),as in the cases of other liliaceous species,Muscari armeniacum(Suzuki and Nakano,2001)and Agapanthus praecox ssp.orientalis(Suzuki et al.,2002).Embryogenic calluses of selected lines,which had been subcultured for6mo.,were analyzed for their ability to form somatic embryos(Table3).Depending on the plant genotype,50to over500somatic embryos per0.5g fresh weight of embryogenic callus were obtained1mo.after transfer to PGR-free medium.Embryogenic calluses of T.hirta and its variety produced many somatic embryos,whereas T.macrantha ssp. macranthopsis produced a few embryos.For a wide range of plant species,a decline in the regeneration potential of callus cultures with increasing age of cultures has been demonstrated(Vasil and Vasil,1985).Further studies are needed to examine the regeneration potential of callus cultures established in this study after long-term subculture.Somatic embryos simultaneously developed a shoot and root (Fig.1D),and plantlets were established from them(Fig.1E). Conversion of somatic embryos into plantlets was promoted by subculturing the embryos onto fresh PGR-free medium.For all six genotypes,almost all of the somatic embryos developed into plantlets. Irrespective of the plant genotype,somatic embryo-derived plantlets were readily acclimatized and successfully transplanted to green-house conditions(Fig.1F).At least20somatic embryo-derived plants for each genotype have so far been established in the greenhouse,and some plants of T.hirtaflowered3–4mo.after transplantation.No morphological alterations have yet been observed in the regenerated plants.Detailed morphological characterization of the regenerated plants is needed for all Tricyrtis genotypes tested.TABLE2EFFECT OF EXPLANT SOURCE ON THE PERCENTAGE OF EXPLANTS FORMING CALLUSES IN SEVERAL TRICYRTIS SPP.Explant source T.hirta T.hirta var.albescens T.formosanaT.formosanacv.FujimusumeT.flava ssp.ohsumiensisT.macrantha ssp.macranthopsisLeaf15.3EC 1.4EC0000Tepal61.4EC7.7EC9.5EC 3.3EC33.3EC0 Filament90.3EC16.3EC36.1EC40.3EC36.3EC33.3EC Anther 1.4EC00000Style 1.7EC0 3.4EC 3.4EC 5.6EC0Ovary 2.9EC012.5EC25.0EC16.7EC0Significance Embryogenic callus formationPGR(P)*Genotype(G)*P£G*Explants were cultured on a medium containing4.5m M2,4-D and0.45m M TDZ.Data were recorded2mo.after culture initiation.Values represent the mean of three independent experiments each of which consisted of20explants.EC indicates that induced calluses were embryogenic because they produced somatic embryos following transfer to PGR-free medium.*indicates significant at P¼0.05(F test).TABLE3GROWTH AND SOMATIC EMBRYO PRODUCTION OF EMBRYOGENICCALLUSES IN SEVERAL TRICYRTIS SPP.Genotype %Increasein FWNo.of somaticembryosT.hirta291^32533^29T.hirta var.albescens264^33342^56T.formosana354^25185^24T.formosana cv.Fujimusume286^48203^36T.flava ssp.ohsumiensis249^45136^51T.macrantha ssp.macranthopsis154^1253^16Data on the%increase in fresh weight(FW)and the number of somaticembryos were recorded1mo.after transfer of0.5g FW of embryogeniccallus onto a medium containing4.5m M2,4-D and0.45m M TDZ,and PGR-free medium,respectively.Tepal-derived calluses were used for T.hirta,T.hirta var.albescens,and T.flava ssp.ohsumiensis,andfilament-derivedcalluses were used for the other genotypes.Values represent the mean^SE offive independent experiments. SOMATIC EMBRYOGENESIS AND REGENERATION OF TRICYRTIS277In order to detect somaclonal variation in the ploidy level,flow cytometric analysis was performed on10regenerated plants for each Tricyrtis genotype.For all genotypes,no variability in the relative DNA content was observed between the mother plants and all the10regenerated plants(data not shown).Also no difference in the chromosome number was detected between the mother plants and the regenerated plants.All the regenerants analyzed had the expected diploid number of chromosomes(2n¼2x¼26;Fig.1G). In conclusion,we have succeeded for thefirst time in the establishment of a plant regeneration system based on somatic embryogenesis in Tricyrtis spp.The auxin2,4-D andfilament explants were found to be appropriate for efficient induction of embryogenic calluses.Although some genotypic differences were observed in the efficiency of embryogenic callus formation from explants,the growth rate of embryogenic calluses,and the efficiency of somatic embryo production from the calluses,the system may be applicable for both clonal propagation of selected strains and ex situ conservation of plant diversity in a wide range of Tricyrtis spp.Embryogenic callus cultures established in this study may be useful as a target material for Agrobacterium-mediated transformation as for other liliaceous species,such as Agapanthus praecox ssp.orientalis(Suzuki et al., 2001)and Muscari armeniacum(Suzuki and Nakano,2002).ReferencesAmmirato,A.V.;Styer,D.J.Strategies for large scale manipulation of somatic embryos in suspension culture.In:Zaitlin,M.;Day,P.;Hollaender,A.,eds.Biotechnology in plant science.New York: Academic Press;1985:161–178.Beyl,C.A.;Sharma,G.C.Picloram induced somatic embryogenesis in Gasteria and Haworthia.Plant Cell an Cult.2:123–132;1983.Buiteveld,J.;Fransz,P. F.;Creemers-Molenaar,J.Induction and characterization of embryogenic callus types for the initiation of suspension cultures of leek(Allium ampeloprasum L.).Plant Sci.100:195–202;1994.Choi,P.S.;Min,S.R.;Ahn,M.Y.;Soh,W.Y.;Liu,J.R.Somatic embryogenesis and plant regeneration in immature zygotic embryo, ovule,and antherfilament cultures of Chinese cabbage.Sci.Hort.72:151–155;1998.Durzan,D.J.;Durzan,P.E.Future technologies:model reference control for the scale up of embryogenesis and polyembryogenesis in cell suspension culture.In:Debey,H.;Zimmermann,R.H.,eds.Micropropagation technology and application.Kluwer Academic Publishers:Boston;1991:389–424.Eady, C. C.;Butler,R. C.;Suo,Y.Somatic embryogenesis and plant regeneration from immature embryo cultures of onion(Allium cepa L.).Plant Cell Rep.18:111–116;1998.Environment Agency of Japan.Threatened wildlife of Japan—Red Data book,2nd edn,vol.8,Vascular plants.Japan:Japan WildlifeResearch Center;2000.Fay,M. F.Conservation of rare and endangered plants using in vitro methods.In Vitro Cell.Dev.Biol.Plant28:1–4;1992.Fay,M. F.In what situation is in vitro culture appropriate to plant conservation?Biodiv.Conserv.3:176–183;1994.Iocco,P.;Franks,T.;Thomas,M.R.Genetic transformation of major wine grape cultivars of Vitis vinifera L..Trans.Res.10:105–112;2001. Kim,J.W.;Soh,W.Y.Plant regeneration through somatic embryogenesis from suspension cultures of Alliumfistulosum.Plant Sci.114:215–220;1996.Krikorian,A.D.;Kann,R.P.Plantlet production from morphologically competent cell suspension cultures of daylily.Ann.Bot.47:679–686;1981.Kunitake,H.;Mii,M.Somatic embryogenesis and its application for breeding and micropropagation in asparagus(Asparagus officinalisL.).Plant Biotechnol.15:51–61;1998.Murashige,T.;Skoog, F.A revised medium for rapid growth and bioassays with tobacco tissue cultures.Physiol.Plant.15:473–497;1962.Nagamura,Y.Tricyrtis Wall.,nom.cons.In:Tsukamoto,Y.,ed.The grand dictionary of horticulture,vol. 4.Tokyo:Shogakukan;1989:431–433[in Japanese].Nakano,M.;Sakakibara,T.;Suzuki,S.;Saito,H.Decrease in the regeneration potential of long-term cell suspension cultures of Liliumformosanum Wallace and its restoration by the auxin transportinhibitor,2,3,5-triiodobenzoic acid.Plant Sci.158:129–137;2000. Phillips,G.C.;Luteyn,K.J.Effects of picloram and other auxins on onion tissue culture.J.Am.Soc.Hort.Sci.108:948–953;1983. Saito,H.;Mizunashi,K.;Tanaka,S.;Adachi,Y.;Nakano,M.Ploidy estimation in Hemerocallis species byflow cytometry.Sci.Hort.97:185–192;2003.Saito,H.;Nakano,M.Plant regeneration from suspension cultures of Hosta sieboldiana.Plant Cell an Cult.71:23–28;2002. Suzuki,S.;Nakano,anogenesis and somatic embryogenesis from callus cultures in Muscari armeniacum Leichtl.ex.Bak.In VitroCell.Dev.Biol.Plant37:382–387;2001.Suzuki,S.;Nakano,M.Agrobacterium-mediated production of transgenic plants of Muscari armeniacum Leichtl.ex Bak.Plant Cell Rep.20:835–841;2002.Suzuki,S.;Oota,M.;Nakano,M.Embryogenic callus induction from leaf explants of the Liliaceous ornamental plant,Agapanthus praecox ssp.orientalis(Leighton)Leighton—histological study and response toselective agents.Sci.Hort.95:123–132;2002.Suzuki,S.;Supaibulwatana,K.;Mii,M.;Nakano,M.Production of transgenic plants of the Liliaceous ornamental plant Agapanthuspraecox ssp.orientalis(Leighton)Leighton via Agrobacterium-mediated transformation of embryogenic calli.Plant Sci.161:89–97;2001.Vasil,V.;Vasil,I.K.Isolation and maintenance of embryogenic cell suspension cultures of graminae.In:Vasil,I.K.,ed.Cell culture andsomatic cell genetics of plants.Orlando,FL:Academic Press;1985:152–157.278NAKANO ET AL.。
21人体胚胎发生

7.受精意义
受精是生殖过程中的一个关键环节,受精卵是精子和卵子 相互融合的产物,是新个体的开端。受精使父系和母系的 遗传物质融合,形成了新的染色体组合和基因组合,促进 了个体的遗传多样性。受精决定了新个体的遗传性别。如 果性染色体为X的精子与卵子受精,新个体的遗传性别就 会是女性(46,XX);如果性染色体为Y的精子与卵子受 精,新个体的遗传性别就会是男性(46,XY)。受精激 活了卵细胞的代谢过程,启动了受精卵的卵裂。没有受精 的卵细胞不能完成第二次减数分裂,排卵24小时后即退变 死亡。但受精后得卵30小时就会完成第一次卵裂,40小时 左右达4个卵裂球,3天左右便形成由12~16个卵裂球构成 的桑葚胚。
8.羊膜囊的形成
受精后第8天,随着上胚层细胞的增生,在细胞间出现了 一个小的腔隙并逐渐扩大,于是上胚层被分隔成了两层细 胞:贴近细胞滋养层内面的一层细胞为成羊膜细胞 (amnioblast),后形成羊膜(amniotic membrane);与下 胚层相贴的一层细胞仍为上胚层。这两层细胞的边缘相延 续,环绕中央的羊膜腔(amniotic cavity),共同构成了羊 膜囊(amnion),羊膜腔中充满了羊水(amniotic fluid) (图21-8)。
植入的完成
受精后第9天,胚泡已深入子宫内膜,表面上皮的植入口 由纤维蛋白凝栓(fibrin coagulation plug)封堵(图21-9)。 与此同时,合体滋养层增厚并形成若干陷窝,称滋养层陷 窝(trophoblastic lacunae)(图21-9)。
受精后第12天左右,胚泡已完全进入子宫内膜,内膜表面 的植入口已被表面上皮完全覆盖,从子宫腔内可以看到一 个轻微突起。此时,合体滋养层内的陷窝增多,并相互沟 通成网。子宫内膜中的小血管被合体滋养层侵蚀而破裂, 血液流入陷窝网(图21-10)。
体细胞胚胎发生与人工种子共39页共41页

体细胞胚胎发生与人工种子 共39页
41、实际上,我们想要的不是针对犯 罪的法 律,而 是针对 疯狂的 法律。 ——马 克·吐温 42、法律的力量应当跟随着公民,就 像影子 跟随着 身体一 样。— —贝卡 利亚 43、法律和制度必须跟上人类思想进 步。— —杰弗 逊 44、人类受制于法律,法律受制于情 理。— —托·富 勒
53、 伟 大 的 事 业,需 要决心 ,能力 ,组织 和责任 感。 ——易 卜 生 54、 唯 书 籍 不 朽。——乔 特
55、 为 中 华 之 崛起而 读书。 ——周 恩来
器官发生与体细胞胚胎发生PPT参考幻灯片共51页

46、我们若已接受最坏的,就再没有什么损失。——卡耐基 47、书到用时方恨少、事非经过不知难。——陆游 48、书籍把我们引入最美好的社会,使我们认识各个时代的伟大智者。——史美尔斯 49、熟读唐诗三百首,不会作诗也会吟。——孙洙 50、谁和我一样用功,谁就会和我一样成功。—参考幻灯片
6、纪律是自由的第一条件。——黑格 尔 7、纪律是集体的面貌,集体的声音, 集体的 动作, 集体的 表情, 集体的 信念。 ——马 卡连柯
8、我们现在必须完全保持党的纪律, 否则一 切都会 陷入污 泥中。 ——马 克思 9、学校没有纪律便如磨坊没有水。— —夸美 纽斯
