聚苯胺-蛭石插层复合材料 1468-6996_9_2_025010 唐群委
聚苯胺席巴纳米复合材料的原位还原法制备及表征

其细化分散负载在不同的载体上来解决 这一问题 , 取 得 了很好 的效 果 。 目前 , 常 用 的 载 体 包 括 碳 纳 米
管、 活性 炭 、 多孔 S i O 、 石 墨烯 等 。 聚 苯胺 ( P o l y a n i l i n e , P A N I ) 作 为 一 种 常 见 的 导
1 . 2 聚苯胺 的 合成及 后处 理
拥 有 良好 前 景 的 电催 化 剂 载 体 材 料 。 近 年 的 研 究还表明 , 钯 纳 米 粒 子 与 聚 苯 胺 之 间存 在 一 定 的 相 互作 用 ¨ J , 使 得 聚苯胺 在 催 化 剂 载 体 方 面 拥 有 了
广 泛 的应 用 ” J 。
察 了掺 杂态及脱掺杂 态聚 苯胺 对原位还原反应 的影 响 , 通过 红外光谱 、 紫外光谱 、 扫描 电镜和 x射线衍射对所得复合材料 的结
构进行 了表征 , 并 用循 环伏安法测定 了复 合材料 对 甲酸的催 化性 能。结果表 明所制备 的复合 材料对 甲酸 具有一 定的催 化性
能。 且 脱掺 杂 态聚 苯胺 复合 材 料 的性 能要 优 于掺 杂 态聚 苯 胺 复合 材 料 的性 能 。 关 键 词 :原 位 还 原 法 ; 聚 苯 胺 ;钯 纳 米 粒 子 ; 复合 材 料 中 图 分 类 号 :T B 3 3 2 ; T B 3 3 3 文 献 标 识 码 :A 文 章 编 号 :1 0 0 3— 0 9 9 9 ( 2 0 1 3 ) 0 6— 0 0 5 3—0 5
液, 0 . 2 %, D u p o n t 公 司; 氢 氧化钠 ( A . R . ) 、 浓 氨 水 ( A . R . ) 、 氯化 钾 ( A . R . ) 、 丙酮( A . R . ) , 北 京 化 工
原位溶液聚合聚苯胺与聚苯乙烯溶液共混复合材料的制备及表征

原位溶液聚合聚苯胺与聚苯乙烯溶液共混复合材料的制备及表征祝宏;李胜松;邝生鲁【摘要】以十二烷基萘磺酸(DNSA)为掺杂剂,在醇(或酮)-水介质中采用原位溶液聚合法制备了聚苯胺,以溶液共混法制备了聚苯胺/聚苯乙(PANI/PS)烯复合材料,采用红外光谱、热失重、元素分析和扫描电镜等手段对产物进行了表征.结果表明,掺杂的聚苯胺电导率最高为0.65 S/cm,优于常用的十二烷基苯磺酸(DBSA),具有一定的实用价值和理论意义.该复合材料的表面电阻率最低为101 Ω/□数量级,并在一定范围内可调,可用于电磁屏蔽,适合于聚合物表面使用.【期刊名称】《高等学校化学学报》【年(卷),期】2010(031)012【总页数】8页(P2474-2481)【关键词】聚苯胺;复合物;原位溶液聚合;掺杂【作者】祝宏;李胜松;邝生鲁【作者单位】武汉工程大学湖北省新型反应器与绿色化学工艺重点实验室,武汉,430073;武汉工程大学湖北省新型反应器与绿色化学工艺重点实验室,武汉,430073;武汉工程大学湖北省新型反应器与绿色化学工艺重点实验室,武汉,430073【正文语种】中文【中图分类】O6311984年,MacDiamid等[1]首先发现聚苯胺(PANI)经质子酸掺杂后能够导电.由于PANI具有密度轻、导电性好、化学稳定性好以及物理化学性能独特等特点,被广泛应用于许多领域.且已成为最热门的有机导电材料之一,目前它的电导率最高已达到102S/cm数量级[2].但因为PANI分子链的刚性和分子链间作用力很强,导致PANI难溶、难熔和难以成膜,研究人员为了改善其溶解性及可加工性,采取了掺杂、结构改性和制备复合材料等方法,但至今可产业化的品种还不多[3,4],仅有导电PANI防腐涂料、抗静电涂料和电磁屏蔽涂料等3个品种.在金属防腐方面,世界各大国家都已开始采用PANI,但在抗静电和电磁屏蔽方面还未大规模使用[5],法国将PANI应用于军事,制成了PANI复合材料隐身潜艇.目前,商业化的PANI电导率一般为10-1~100S/cm[3].鉴于聚苯胺应用前景广阔和产业化刚刚起步,其继续开发的潜力还很大[6~13].本文以十二烷基萘磺酸(DNSA)为掺杂剂,过硫酸铵(APS)作氧化剂,在醇(或酮)-水溶剂中,以原位溶液聚合法一步合成掺杂态PANI,采用溶液共混法制备了聚苯胺/聚苯乙烯(PANI/PS)导电复合材料,通过红外光谱、热失重、元素分析和扫描电镜等对产物进行了表征.1.1 试剂与仪器实验用水为自来水;苯胺(分析纯,上海展云化工有限公司);过硫酸铵(分析纯,上海实验试剂有限公司);无水乙醇(分析纯,上海振兴化工一厂);1-甲基-2-吡咯烷酮(NMP)、N,N-二甲基甲酰胺(DMF)和二甲亚砜(DMSO)均为分析纯试剂.用NYL-500型压片机(无锡建筑材料仪器机械厂)在20 MPa压力下,将PANI 粉末压成直径为16 mm、厚度为2~3 mm的圆片,在圆片的两个侧面涂上导电银胶,使用TH2828型精密数字电桥(常州市同惠电子有限公司),在室温下测试电导率.使用409 PC/PG热分析仪(NETZSCH STA公司)测试热重(TG)曲线:采用空气气氛,流量40 mL/min,温度范围40~650℃,升温速率10℃/min.用Vario ELⅢ型元素分析仪(德国Elementar公司)测试C,N,S和H等元素的含量.用Nesus型FTIR光谱仪(美国Thermo Nicolet公司),室温下以KBr压片法制样,在400~4000 cm-1范围内测试红外光谱.1.2 导电聚苯胺的制备1.2.1 DNSA的合成将192 g(1.5 mol)精萘和400 mL环己烷装入1000 mL四口玻璃反应瓶中,搅拌升温至萘完全溶解后,加入10 g(0.075 mol)AlCl3,在搅拌下缓缓滴加187 g(0.75 mol)溴代十二烷,继续搅拌回流反应2 h.反应完毕后,加入10%的盐酸冰水溶液,分除水层后,将有机层用水洗至中性,加入无水CaC12干燥过夜,于80~81℃常压蒸馏除去环己烷,在85℃/0.5 kPa左右减压蒸除萘,于160~170℃/0.5 kPa精馏收集十二烷基萘98.8 g(0.33 mol).将所得十二烷基萘转入三口玻璃反应瓶中,搅拌并控制体系温度在0℃左右,缓缓滴加20%发烟硫酸93.5 g(1 mol)后,于室温继续搅拌反应30 min,再升温至50℃反应1.5 h.反应完毕后,加入少量水,搅拌10 min,用250 mL乙醚提取产物,得到棕色粘稠液体99.5 g,总产率为17.2%.用Agilent 1100型高效液相色谱仪[Diamond C18色谱柱(250 mm×4.6 mm,5 μm),流动相为V(乙腈)∶V(乙醇)=30∶70,检测波长220 nm,流速1.0 mL/min]对所得产物进行色谱分析,得到其中DNSA的含量为95.1%,杂质含量为4.9%.1.2.2 原位溶液聚合法一步合成掺杂态PANI 称取9.3 g(0.1 mol)苯胺和35.6 g (0.1 mol)DNSA,放入500 mL三口玻璃反应瓶中,倒入330 mL乙醇-水(2/1,体积比),磁力搅拌至混合液澄清,控制体系温度在0℃左右,缓慢滴加含28.5 g(0.125 mol)(NH4)2S2O8的50 mL水溶液,保温反应24 h,用G3玻璃砂芯漏斗抽滤不溶物,用0.01 mol/L相应的有机磺酸水溶液洗涤至滤液无色,置于60℃烘箱中干燥24 h,研磨得到墨绿色掺杂态导电PANI粉末.1.3 PANI/PS复合材料的制备1.3.1 PANI-DNSA溶液的制备称取5.0 g PANI-DNSA粉末,加入到1000 mL 单口瓶中,加入500 mL氯仿-间甲酚(9∶1,体积比),于50℃搅拌回流1 h.冷却至室温,用G3玻璃砂芯漏斗过滤,收集滤液.此溶液为墨绿色,浓度为0.009 g/mL.1.3.2 聚苯乙烯(PS)溶液的制备称取10.0 g PS加入至500 mL氯仿中,常温下搅拌使其完全溶解,溶液浓度为0.020 g/mL.1.3.3 PANI/PS复合材料的制备按照m(PANI-DNSA)/[m(PANI-DNSA)+m(PS)]的质量比为0~100%配制系列混合溶液各50 mL,将每一种混合溶液于40℃下进行旋转蒸发,浓缩至约25 mL,浓缩后溶液变稠,无沉淀产生,类似于胶体,有利于涂刷,将以上系列溶液涂刷于3 cm×1 cm大小的聚氯乙烯薄片上,于室温下水平放置,自然挥发,待氯仿挥发完毕后,再涂一次.如此重复3~5次,最后于鼓风干燥箱中于60℃下放置48 h,使间甲酚挥发,得到系列PANI/PS导电复合材料.1.4 性能测试1.4.1 表面电阻率测试将制备好的PANI/PS导电复合材料样品的两端涂上导电银胶,每端的银胶宽为0.5 cm,则样品实际测量的尺寸为2 cm×1 cm,如图1所示,图1中白色部分为导电银胶,黑色部分为复合材料,复合材料实际呈透明墨绿色.测试时将DT930F型数字万用表(山创仪器仪表有限公司)设定为0~200 MΩ档,其两极分别与两端导电银胶部分任一点紧密接触,即可测得样品的表面电阻,导电银胶部分的电阻忽略不计,表面电阻率按照下式计算:式中,ρ为表面电阻率(Ω或Ω/□);R为表面电阻(Ω);W为试样宽度(cm);L为试样长度(cm).1.4.2 材料表面形态分析将样品切割成尺寸为5 mm×5 mm的小块,在样品表面镀上一层金膜,使用JSM-5510LV型扫描电子显微镜(日本电子株式会社)观察样品表面形态.1.4.3 溶解性的测定称取一定量的PANI样品分别加入到100 mL甲醇、丙酮、甲苯、二甲苯、三氯甲烷、1,2-二氯乙烷、NMP、DMF、DMSO、间甲酚、三氯甲烷-间甲酚(9∶1,体积比)和浓硫酸中,于50℃下充分搅拌回流1 h,冷却至室温后,用50 mL G3漏斗抽滤,将漏斗置于鼓风干燥箱内使溶剂挥发,称量过滤前后漏斗质量差即为100 mL溶剂中溶解的PANI.1.5 产率的计算产率的计算公式为式中,m(PANI)为产物PANI的质量(g);m(ANI)为原料苯胺的质量(g);m(SA)为有机磺酸的加入量(g).2.1 单体浓度对聚苯胺电导率和产率的影响实验选定在n(APS)/n(ANI)=1,n(DNSA)/n(ANI)=1,乙醇-水(体积比2/1)为溶剂,反应温度0~5℃及反应时间24 h条件下进行.改变单体浓度,考察单体浓度对PANI电导率和产率的影响,结果见图2.由图2(A)可见,单体浓度为0.3 mol/L时电导率最大(为0.44 S/cm),之后,随着单体浓度的增加,PANI电导率下降,但在单体浓度为0.1~0.5 mol/L范围内PANI电导率变化很小;由图2(B)可知,PANI的产率在单体浓度为0.3~0.9 mol/L时变化不大,单体浓度为0.5 mol/L时产率最高,单体浓度为0.1 mol/L时产率最低.聚合反应速率与单体浓度有关.苯胺单体浓度较大时,则聚合反应速率也较大,容易发生暴聚,生成的PANI结构缺陷多,分子链不够规整,分子量小,多为无定形结构,因此电导率小.单体浓度也是影响聚合物产率的因素之一.单体浓度越小,则活性中心(阳离子自由基)的浓度越小,与单体分子碰撞的机率越小,因此有一部分单体未参加反应或是生成小分子量聚合物而被洗去,导致产率较低.2.2 有机磺酸用量对聚苯胺电导率和产率的影响固定苯胺单体(ANI)浓度为0.3 mol/L,其它条件不变,改变DNSA用量,考察了PANI电导率和产率随DNSA/ANI摩尔比的变化情况,结果如图3.在适当的酸度条件下,有机磺酸可为苯胺聚合提供质子,并保证聚合体系有足够的酸度作用,使反应按1,4-偶联方式发生.酸度过低时,聚合按1,4-偶联和1,2-偶联两种方式进行;酸度过高时,发生1,4-偶联和芳环上的取代反应.酸度过低或过高均会使产物中含有支链PANI和其它副产物,使产物电导率和产率均有所下降. 有机磺酸还作为掺杂剂,当掺杂率为50%时电导率最高.有机磺酸浓度过低(掺杂率<50%)时,PANI链结构中亚胺氮原子未被完全质子化,影响大共轭π键的连续性,电导率降低;有机磺酸浓度过高时,残留在产物中的过量有机磺酸会阻断PANI链间形成的导电通道,使产物的电导率降低;同时因为能与PANI掺杂结合的有机磺酸量是有限的,有机磺酸用量越大,流失的越多,产率就会越低.由图3(A)可见,随着DNSA用量的增加,PANI的电导率先增加后减少,在n (DNSA)/n(ANI)=1时电导率最大(0.44 S/cm),而当n(DNSA)/n (ANI)=1.5时,电导率为0.38 S/cm,下降幅度较小.由图3(B)可见,随着DNSA用量的增加,PANI的产率先增加后减少,在n (DNSA)/n(ANI)=0.75时PANI的产率最大.由图3还可见,电导率和产率达到最高值时DNSA用量并不相同.2.3 氧化剂用量对聚苯胺电导率和产率的影响固定n(DNSA)/n(ANI)=1,ANI浓度为0.3 mol/L,其它条件不变,考察氧化剂过硫酸铵(APS)的用量对聚苯胺电导率和产率的影响,结果见图4.当n(APS)/n(ANI)=1.25时,电导率达到最大值(0.6 S/cm),继续增加APS用量,电导率则下降,因为过量的氧化剂会对主链进一步氧化,破坏主链的共轭结构.当n(APS)/n(ANI)<1.25时,氧化剂用量不足,PANI多以还原单元存在,随着氧化剂用量的增加,有利于共轭结构的形成,致使电导率增加.由PANI聚合反应方程式可知,n(APS)/n(ANI)理论值应为1.25,理论与实验正好吻合.聚合反应方程式如下:PANI的产率也随着氧化剂用量的增加而先增后减,当n(APS)/n(ANI)=1.5时产率最高.当氧化剂与苯胺的摩尔比较低时,苯胺聚合反应体系的活性中心相对较少,随着氧化剂用量的增加,活性中心越来越多,每个活性中心又各自生成高分子量PANI,故生成的PANI量增加;而当氧化剂用量过多时,体系的活性中心相对较多,不但不利于生成高分子量的PANI,还易生成副产物而被洗去,因此产率下降.2.4 不同溶剂对聚苯胺电导率和产率的影响固定n(DNSA)/n(ANI)=1,n(APS)/n(ANI)=1.25,ANI浓度为0.3 mol/L,其它条件不变,使用不同的溶剂进行实验,结果见表1.APS是水溶性的氧化剂,因此溶剂中必须要有水才能进行反应,实验所用溶剂醇(或酮)与水的体积比均为2∶1,如果醇(或酮)比例太少,在搅拌的作用下会形成乳液,造成后处理过程中过滤困难.质子酸在不同溶剂中表现出不同的酸性,过硫酸铵在不同溶剂中表现出不同的氧化性,因此影响聚合产物的结构,导致电导率和产率有所不同. 当以甲醇-水为溶剂时,合成的PANI电导率最小、产率最高;以异丙醇-水为溶剂时,合成的PANI电导率最大、产率也较高,为最佳溶剂.实验结果表明,除甲醇-水外,使用其它4种溶剂合成的PANI在电导率和产率方面均相差不大,均为优良溶剂.当以乙醇-水为溶剂时所得PANI的电导率与以异丙醇-水为溶剂时相比稍低,但产率要高,考虑到乙醇价廉易得,而且对环境污染最小,本文选用乙醇-水为溶剂.该方法制备的掺杂PANI的导电率最高为0.65 S/cm,与乳液法相当,既简化了工艺,降低了成本,又减少了环境污染.2.5 PANI-DNSA元素分析PANI-DNSA和本征态PANI元素分析结果见表2.根据PANI-DNSA元素分析结果计算,掺杂率n(S)/n(N)=0.49,表明掺杂剂DNSA还有一小部分为游离态,因此实际掺杂率低于0.49,理论上最佳掺杂率为0.50,不过实测值与理论值已十分接近,说明该方法制备的PANI掺杂良好.从本征态PANI元素分析结果可以看出,本征态PANI中含有少量S元素,说明DNSA不易脱掺杂,大分子磺酸与PANI分子链有较强的作用力.2.6 PANI-DNSA的溶解性能使用大分子质子磺酸掺杂能改善PANI的溶解性,PANI-DNSA的溶解性能见表3. PANI-DNSA在所选多数溶剂中有一定溶解度.PANI-DNSA在浓硫酸中溶解度达到5.3 g/100 mL,是因为浓硫酸破坏了PANI-DNSA的长分子链,以分子链碎片的形式溶解在浓硫酸中.PANI-DNSA在间甲酚中溶解度为1.0 g/100 mL,在氯仿中加入间甲酚也能较大地提高PANI-DNSA的溶解性,当氯仿与间甲酚的体积比为9∶1时,能使PANI-DNSA溶解度由0.4 g/100 mL提高到0.9 g/100 mL,由于间甲酚熔点高、不易挥发且毒性大,应尽量减少使用量,因此选择氯仿-间甲酚混合溶剂.2.7 PANI-DNSA的热性能分析对于导电聚合物,其热稳定性是一个很重要的指标,掺杂剂对导电PANI的热稳定性有较大影响,本文对本征态PANI和掺杂态PANI-DNSA的热稳定性进行了比较.由图5的TG曲线可见,本征态PANI的起始分解温度约为350℃,在650℃时分解完全.PANI-DNSA的起始分解温度约为230℃,在230℃之前也有少量的失重,是由水分的蒸发和小分子量PANI分解所致;随着温度的升高,少量游离DNSA先发生分解(TG曲线出现一小台阶),随后PANI-DNSA缓慢分解,整个聚合物在650℃时分解完全.结果表明,掺杂对PANI热稳定性影响很大,起始分解温度相差达120℃,但PANI-DNSA热稳定性还是很高的.2.8 PANI-DNSA的电导率稳定性考察将PANI-DNSA样品自然放置于室内,按不同时间点测试其电导率,电导率变化情况见表4.由表4可知,PANI-DNSA的电导率在180 d的考察期内基本稳定,说明它在空气中抗氧化性良好,后期测试的电导率均高于第一天,是因为PANI-DNSA粉末有吸湿性,水分会使电导率略微增大.2.9 PANI-DNSA的红外光谱分析由图6(A)可见,本征态PANI的红外光谱中1587 cm-1处的吸收峰为苯醌环(—N Q N—)的骨架伸缩振动特征吸收峰;1498 cm-1处的吸收峰为苯环(—B—)的骨架伸缩振动特征吸收峰;1319 cm-1处的吸收峰为苯二胺(—N—B—N—)结构中的C—N伸缩振动吸收峰;1166 cm-1处的吸收峰为苯醌环的C—H 面外弯曲振动特征吸收峰;835 cm-1处的吸收峰为苯环中的C—H面外弯曲振动特征吸收峰.而掺杂态PANI-DNSA相应的红外光谱中吸收峰[图6(B)]的位置(位移)分别为1575 cm-1(12 cm-1),1484 cm-1(14 cm-1),1307 cm-1(12 cm-1),1141 cm-1(25 cm-1)和809 cm-1(26 cm-1).另外,PANI-DNSA在3460 cm-1处有一较为明显的吸收峰,应为—N—B—N—和—N+ Q N+—结构中N—H的伸缩振动;而本征态PANI在此处为一宽的弱吸收峰,特征不明显.以上结果表明,DNSA对PANI起到了良好的掺杂作用,而不是简单的混合.掺杂使聚合物分子链中的电子云密度下降,降低了原子间的力常数;同时掺杂作用使分子链中的电子云及电荷的离域化作用增加,形成共振结构,产生共轭效应.两种作用的结果使得基团的振动频率下降,因此,用DNSA掺杂PANI后红外特征吸收峰均向低频方向移动.2.10 PANI-DNSA的用量对复合材料表面电阻率的影响在室温下测试了系列PANI/PS复合材料的电阻率,测试结果见图7,将质量分数定义为PANIDNSA占PANI-DNSA和PS总质量的百分比.复合型导电高分子的导电机理主要有导电通道理论和隧道效应学说[14]两个理论.导电通道机理是指当导电填料含量达到“渗滤阈值”时,导电微粒相互接触形成无限网链,从而形成导电通道,载流子可以在体系内自由移动,从而使复合材料导电,在导电填料浓度较高时起主要作用;隧道效应是指当导电粒子间存在一定的间距时(一般认为<10 nm),电子在热振动作用下产生迁移运动,从而形成导电网络,使复合高分子导电[4],一般在导电填料浓度较低时起作用.由图7可知,本方法可根据实际需要制得不同电导率的复合材料,随着导电PANI 在复合材料中含量的增加,表面电阻率随之减小,8%(PANI质量分数)为表面电阻率的突变点,也就是渗滤阈值,当导电PANI在复合材料中质量分数达到8%时,复合材料突然变得导电,表面电阻率为105Ω/□数量级,PANI微粒相互接触形成了导电通道,当导电PANI在复合材料中质量分数超过8%时,随着导电PANI量的增加,表面电阻率逐渐变小,但变化渐趋平缓.特别是当导电PANI含量≥30%时,表面电阻率变化很小,说明导电PANI含量为30%时,微粒已充分接触,形成了足够的导电网络,过多的PANI对复合材料的表面导电性影响不明显. 当导电PANI在复合材料中的质量分数≥20%时,表面电阻率在101~102Ω/□范围内可调,可用作电磁屏蔽材料.当导电PANI的质量分数为100%时,所制得材料实际上是纯粹的PANI-DNSA材料,因PANI-DNSA材料的力学性能稍差,无实际用途,此处仅作数据对比.2.11 不同溶剂对复合材料表面电阻率的影响使用不同的溶剂溶解PANI-DNSA,聚苯乙烯的溶剂不变,按PANI-DNSA在复合材料中的质量分数为30%制备复合材料,溶剂对复合材料表面电阻率的影响见表5.由表5可见,使用不同的溶剂溶解PANI-DNSA,复合材料表面电阻率相差2个数量级,由于间甲酚具有“二次掺杂”的作用,使PANI-DNSA卷曲的分子链展开,有利于提高导电性,从而降低表面电阻率;碱性溶剂使PANI-DNSA导电性降低,因此NMP作溶剂表面电阻率最高;以氯仿-间甲酚(体积比9∶1)为溶剂,不仅使PANI-DNSA溶解度增大,而且制备的复合材料导电性最好,因此,该溶剂为最佳溶剂.2.12 PANI/PS导电复合材料表面电阻率的稳定性将3号复合材料样品自然放置于室内,考察其在120 d内表面电阻率的变化情况,结果见表6.由表6可见,复合材料在120 d内表面电阻率基本稳定且基本不受环境湿度的影响,该复合材料在空气中抗氧化性较好.2.13 PANI/PS导电复合材料的表面形态PANI/PS复合材料的表面SEM观察照片如图8所示.图中已分不出单纯的PANI-DNSA和PS,PANI-DNSA在PS中高度分散,表明PANI-DNSA和PS具有较好的相容性,片状结构的尺寸约为几个微米,“片”与“片”之间虽然存在缝隙或小孔,但每片之间还是相互接触,形成了较好的导电网络,因此具有较好的导电性. 本文实验结果表明,以电导率和产率为评价指标,用DNSA作掺杂剂,制备导电聚苯胺的优化条件是:单体浓度为0.3 mol/L,质子酸/单体摩尔比为1,过硫酸铵/单体摩尔比为1.25;实验证明,醇(酮)-水为优良的溶剂体系,该溶剂体系简化了后处理操作,并降低了成本,反应完成后只需过滤、洗涤即可得产品,综合考虑原料和环境的因素,推荐乙醇-水(体积比2∶1)作为反应溶剂系统.所制备的PANI-DNSA电导率最高为0.65 S/cm,并且在6个月的考察期内基本稳定,PANI-DNSA的溶解性和热稳定性良好,红外光谱和元素分析表明DNSA 对PANI起到了良好的掺杂作用.采用溶液共混法,即以氯仿溶解PS,将PANI-DNSA也制成溶液,两种溶液混合后浓缩,在基体材料表面涂刷3~5次,制成PANI/PS导电复合涂层材料.混合溶液浓缩后变稠,不仅有利于涂刷,也减少了溶剂的挥发量,有利于保护环境和降低成本,该方法可操作性强,简化了制备工艺,有较强的实用价值.实验证明,PANI/PS复合材料渗滤阈值为8%,最佳溶剂为氯仿-间甲酚(体积比9∶1),复合材料表面导电性在4个月考察期内保持稳定,SEM图片显示PANI-DNSA和PS具有较好的相容性,形成了较好的导电网络.当PANI-DNSA在复合材料中质量分数≥20%时,表面电阻率在101~102Ω/□范围内可调,可用于电磁屏蔽[15].该复合材料适合于聚合物表面使用.【相关文献】[1] MacDiarmid A.G.,Chiang J.C..Polymer Preprints[J],1984,25:248[2] LI Yuan-Xun(李元勋),LIU Ying-Li(刘颖力),ZHANG Huai-Wu(张怀武),LING Wei-Wei(凌味未),XIE Yun-Song(谢云松),XIAO John-Qiang.Chem.J.Chinese Universities(高等学校化学学报)[J],2008,29(3):640—644[3] CHAO Dan-Ming(晁单明),CHEN Jing-Yu(陈靖禹),LU Xiao-Feng(卢晓峰),CHEN Liang(陈梁),ZHANG Wan-Jin(张万金).Chem.J.Chinese Universities(高等学校化学学报)[J],2005,26(11):2176—2178[4] HUANG Mei-Rong(黄美荣),LI Xin-Gui(李新贵).Petrochemical Technology(石油化工)[J],2003,32(9):820—825[5] Yao P.,Xu J.,Wang Y.,Zhu C..Journal of Materials Science:Materials in Electronics [J],2009,20(9):891—898[6] Zhang J.,Kong L.B.,Wang B.,Luo Y.C.,Kang L..Synthetic Metals[J],2009,159(3/4):260—266[7] Hsieh T.H.,Ho K.S.,Bi X.T.,Han Y.K.,Chen Z.L.,Hsu C.H.,Chang Y.C..European Polymer Journal[J],2009,45(3):613—620[8] Xu J.,Yao P.,Li X.,He F..Materials Science & Engineering,B:Advanced Functional Solid-State Materials[J],2008,151(3):210—219[9] Ji S.Z.,Li Y.,Yang M.J..Sensors and Actuators,B:Chemical[J],2008,B133(2):644—649[10] Grigoras M.,Chitanu G.C.,Popescu I.,Pelin I.M.,Rimbu G.A..Macromolecular Symposia[J],2008,263:30—37[11] Kong L.B.,Zhang J.,An J.J.,Luo Y.C.,Kang L..Journal of Materials Science[J],2008,43(10):3664—3669[12] Qi Y.N.,Xu F.,Ma H.J.,Sun L.X.,Zhang J.,Jiang T..Journal of Thermal Analysis and Calorimetry[J],2008,91(1):219—223[13] Zhao D.L.,Zeng X.W.,Xia Q.S.,Tang J.T..Key Engineering Materials[J],2007,334:1189—1192[14] WANG Yang-Yong(王杨勇),QIANG Jun-Feng(强军锋),JING Xin-Li(井新利),YAO Sheng(姚胜).New Chemical Materials(化工新型材料)[J],2003,31(3):1—3 [15] ZHANG Peng-Tao(张澎涛),SUN Li-Ping(孙丽萍).Forestry Machinery&Woodworking Equipment(林业机械与木工设备)[J],2006,34(6):24—26。
聚苯胺纳米复合材料用于癌症诊疗的研究进展
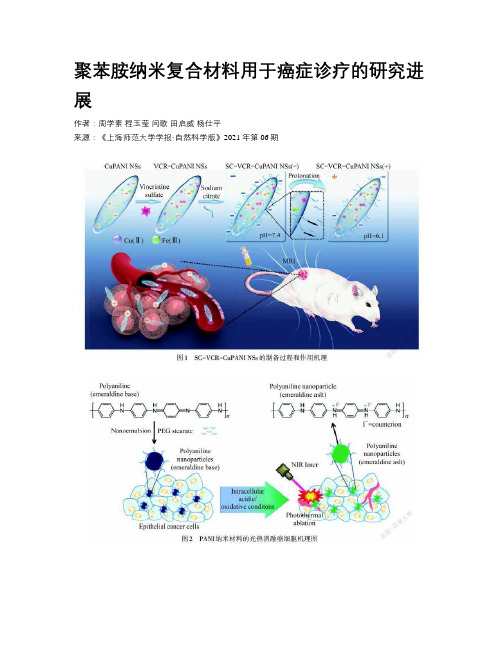
聚苯胺纳米复合材料用于癌症诊疗的研究进展作者:周学素程玉莹闫歌田启威杨仕平来源:《上海师范大学学报·自然科学版》2021年第06期摘要:共轭聚合物以其独特的结构和性能得到了广泛的关注.聚苯胺(PANI)纳米复合材料制备工艺简单、成本低廉、毒性低、易于功能化,从而在癌症治疗方面取得了巨大的进展.通过不同功能的化合物修饰制备的PANI纳米复合材料极大地拓宽了癌症治疗领域.基于PANI 纳米复合材料,文章总结了其在癌症诊疗领域的光热治疗、协同治疗、多模态成像引导治疗和智能响应治疗的研究进展,并分析了其发展趋势.关键词:聚苯胺(PANI)纳米复合材料; 光热治疗; 协同治疗; 多模态成像; 智能响应中图分类号: O 611.3 文献标志码: A 文章编号: 1000-5137(2021)06-0721-07Abstract: Conjugated polymers have attracted much attention due to their unique structure and properties. Polyaniline(PANI) nanocomposites have the advantages of simple preparation process, low cost, low toxicity, easy functionalization, etc., and have made great progress in tumor treatment. PANI nanocomposites prepared by modifying different functional compounds have greatly broadened the field of tumor treatment. Based on PANI nanocomposites, this article reviews their research progress in photothermal therapy, collaborative therapy, multimodal imaging guided therapy, and intelligent response therapy in the field of cancer diagnosis and treatment, and analyzes its development trends.Key words: polyaniline(PANI) nanocomposite; photothermal therapy; synergy therapy; multimodal imaging; intelligent response0 引言癌症作为世界上高死亡率的疾病之一,一直以来都是人类面临的最严重的健康问题之一.在2018年,全世界因癌症死亡的人数就达到960万.目前在癌症临床治疗中应用比较多的是传统方法,包括手术、化学疗法和放射疗法.虽在杀死癌细胞方面具有明显效果,但传统疗法也会杀死正常细胞及组织,给病人带来副作用大、缺乏特异性等问题.光热疗法是一种通过外部刺激从而杀死癌细胞,侵入性小的微创疗法[1].由于近红外光具有很好的组织穿透能力,光能量可以充分传递到作用部位,光热疗法采用能够吸收近红外光的纳米材料,将光能转化为热能,从而在体内实现局部高温,杀死癌细胞,并且不损害其他正常组织[2].共轭聚合物是一类特殊的聚合物,存在π电子共轭主链和高度离域化的结构,具有很高的光热转换效率,可以作为近红外光诱导的光热转换纳米材料.目前,共轭聚合物已广泛用于癌症治疗的研究领域[3],主要分为两大类:一类是有机共轭聚合物纳米粒子,例如聚吡咯(Pyy)、聚苯胺(PANI)、聚多巴胺[4]等;第二类是基于π共轭和离域电子的供体-受体(DA)体系设计的供体-受体共轭聚合物纳米粒子.共轭聚合物纳米粒子具有很高的光热转换效率,远远超过其他的纳米粒子,且具有优异的光稳定性和良好的生物降解性.聚苯胺掺杂后其离域轨道电子易发生迁移,导带与价带之间的能隙减小,导致紫外可见吸收峰发生红移.当受到近红外光(NIR)照射时,PANI价带中的电子将受激发,跃迁至导带,具有显著的光电转换效应,实现光热转换,使得PANI可作为诊疗一体化试剂进行癌症治疗[5].PANI具有制备工艺简单、成本低廉、毒性低,以及可调节的结构和表面形态,有增强肿瘤治疗的效果.本文主要概述了PANI纳米复合材料的制备和改性,以及其在肿瘤诊断领域的研究进展.1 PANI纳米复合材料的制备及改性1.1 制备PANI纳米粒子合成的常用方法有化学氧化聚合法[6-7].化学氧化法是在酸性条件下使用氧化剂将苯胺单体氧化聚合成PANI,广泛应用的氧化剂有过硫酸铵、过氧化氢、氯化铁、高锰酸钾[8]等.通过改变质子酸的种类、氧化剂、原料浓度、反应时间、反应环境等因素,获得不同结构和形貌的PANI纳米粒子,从而实现不同的功能.例如,LIU等[7]采用氧化铁作为氧化剂,并改变氧化剂与苯胺之间的浓度比,制备得到纳米梭状的PANI. MONDAL等[9]将掺杂剂改为芳族羧酸,并改变有机酸中的-COOH基团数目,获得不同长径比的管状PANI纤维,其吸附效果良好,可用于油水分离.1.2 表面改性对纳米粒子表面改性,可以减少材料在体内的聚集,降低细胞毒性,阻止免疫系统对材料的清除,延長材料在血液的循环周期等,从而实现功能化治疗癌症.由于纳米材料进入体内后,在其表面会形成蛋白冠,粒子破坏蛋白冠后被体内的免疫系统识别,并当作有害物质从体内清除,降低了治疗效果[10].表面改性可以掩盖纳米粒子或阻止蛋白冠的形成,延长PANI纳米粒子在血液中的循环时间,并到达作用部位,提高治疗效果[11].目前对PANI纳米材料的表面改性,主要通过物理吸附或采用共价键将配体结合在纳米颗粒表面等方式以实现其功能化.较常用于纳米颗粒改性的化合物是聚乙二醇.通过聚乙二醇改性可改善材料的亲水性,改变材料药代动力学效果,同时具有实现其他功能的优势[12].为了改善PANI在水中的分散性,WANG等[13]设计了一种核-壳结构的复合材料聚苯胺-聚吡咯烷酮(PANi@PVP),PVP作为空间稳定剂对PANI进行改性,在水中有优异的溶解性和良好的细胞相容性.LIU等[7]提出了一种分级靶向策略,设计了pH敏感的柠檬酸钠修饰的铜掺杂PANI 纳米梭(SC-VCR-CuPANI NSs),如图1所示,该材料在血液中呈负电,显示出增强的隐身效果,血液循环周期延长,且在肿瘤微酸环境中实现质子化,提高细胞内在化效果.通过这种分级靶向的策略,材料在血液中的循环半衰期从4.35 h增加到7.33 h,磁共振成像分辨率和信号强度得到显著提高.为了提高肿瘤的治疗效果,基于透明质酸(HA)可以特异性结合受体(CD44)靶向肿瘤细胞[14], JIANG等[15]设计了一种水溶性透明质酸-杂交PANI纳米材料,靶向光热治疗肿瘤.通过细胞实验,材料可以针对性地杀死癌细胞HeLa和HCT-116,而对正常细胞HFF没有影响.将HA修饰的二氧化硅荧光纳米颗粒连接到PANI,在靶向肿瘤的同时,二氧化硅提供的荧光成像可以引导光热治疗.2 PANI纳米复合材料在肿瘤诊疗上的应用2.1 光热治疗PANI经掺杂后在近红外区(700~900 nm)有很强的吸收,PANI具有成为光热剂的可能[16].如图2所示,YANG等[5]首次报道了PANI在酸性条件下进行掺杂,具有有机光热剂的潜力.用808 nm,2.45 W·cm-2的NIR激光照射5 min,掺杂态PANI温度升高了54.8 ℃,采用相同条件,本征态PANI温度升高了15 ℃左右,表明了掺杂后的PANI光热效果明显.为评估PANI对癌细胞在体外和体内的光热消融能力,将本征态的PANI用上皮癌细胞A431处理后发现,PANI颜色从紫色变成绿色,并在808 nm NIR激光下照射,台盼蓝染色实验发现大量细胞被破坏.同样在体内实验中,经瘤内注射材料,用激光照射治疗后,肿瘤组织切片显示有严重的细胞损伤.这说明PANI可以在癌细胞中掺杂,并诱导产生光热效应,从而杀死癌细胞.2.2 协同治疗光热疗法是微创治疗技术,通过外部激光刺激富集到肿瘤部位的材料导致局部升温,致使细胞结构发生破坏,但研究表明:受制于纳米材料在肿瘤部位的富集程度及机体代谢的影响,光热治疗的效果并不佳,同时材料也会给机体带来一定的毒性.对于深部肿瘤,激光强度会由于深度而依赖性改变,这些因素迫使光热疗法同其他的疗法相结合,从而增强肿瘤治疗的效果,降低治疗对机体正常组织的损伤[17-18].将光热疗法和光动力疗法相结合,两种疗法均采用外部激光照射激发.光动力疗法是利用光敏剂和组织细胞中的氧气(O2)的共同存在,经激光照射产生活性氧(ROS),ROS会氧化生物大分子,从而破坏细胞结构,促使细胞死亡[19-20].由于肿瘤中低氧的限制,会降低光动力疗法的治疗效果,将光动力疗法和光热疗法相结合,可以提高整体治疗效果,同时光热疗法会促进血液的流动,提高肿瘤中的氧气浓度,得到1+1>2的效果.TAN等[21]合成了一种PANI包覆吲哚菁绿载银纳米复合材料(ICG-Ag@PANI),银/聚苯胺核壳纳米粒子(Ag@PANI)通过π-π堆积和疏水相互作用的方式负载光敏剂吲哚菁绿(ICG),实现单光触发的光热疗法和光动力疗法协同治疗.将光热剂与化疗药物结合,可控制药物在肿瘤部位的释放[22],降低药物对正常细胞的损伤,协同化疗和光热疗法能很好地提高癌症治疗能力.结合药物的方法有两种:一是将光热剂同可载药的载体结合;二是将光热剂自身设计为药物载体.SILVA等[23]设计了装载5-FU的二甲基咪唑结合PANI纳米粒子(PANI@ZIF-8),在NIR和pH=5.2的缓冲溶液中,5-FU的累计释放量达到80%,PANI吸收NIR会促使温度的升高,增强了5-FU的释放,具有很好的化学光热效应.如图3所示,XIA等[24]采用多孔硅包覆阿霉素(DOX),并将PANI共价接枝到其表面,通过pH和NIR响应控制药物释放,多孔硅可降解为無毒氢氧化硅(Si(OH)4)排出体外,解决了光热剂不可生物降解所带来的长期毒性问题,化学与光热结合疗法展现了巨大的潜力.2.3 多模式成像引导治疗通过成像的方式来引导治疗可以有效地提高肿瘤治疗效率.掺杂态PANI在近红外区具有独特的光吸收特性,是一种优异的光声成像剂[25].光声成像是探测激光照射产生的光声信号后,产生组织分布图像的成像方式,具有高信噪比和高分辨率的优点[26].但是单一的成像依旧存在缺陷,尤其对深部肿瘤,光声成像对激光的强度存在依赖,激光过强会损伤正常的组织细胞.多模式成像方式引导治疗来提高治疗效果是目前研究的趋势.如图4所示,WANG等[27]制备一种PANI包覆二硫化钼(MoS2@PANI-PEG)量子点复合材料,MoS2量子点可产生强荧光,可用于体内成像的探针,同时MoS2是放射增敏剂,结合PANI的光热疗法和光声成像能力,协同增强癌症的治疗效果.2.4 智能响应治疗基于PANI易被质子酸掺杂和肿瘤微环境具有微酸性的特点,设计智能响应探针用于癌症治疗.由于肿瘤微酸性环境(pH为5.0~7.4)低于PANI掺杂要求的pH值(pH<3.0),限制了PANI在癌症治疗中光热治疗和光声成像的应用.JU等[28]合成了金/聚苯胺核壳纳米材料(Au@PANI),基于Au到PANI的电荷转移以及PANI掺杂过程诱导的电子传递效率的提高,Au@PANI在pH=6.5时即可实现掺杂,显示出优异的光热效应.但金离子在体内具有长期毒性,对人体会造成很大的危害.为提高智能治疗剂的治疗性能,如图5所示,LI等[29]采用牛血清白蛋白(BSA)包覆PANI,设计了BSA-PANI纳米粒子,可以实现肿瘤内源性触发的诊断和治疗,光热转换效率达到37%,且纳米粒子具有很好的生物相容性,降低体内毒性.目前对PANI纳米材料的表面改性,主要通过物理吸附或采用共价键将配体结合在纳米颗粒表面等方式以实现其功能化.较常用于纳米颗粒改性的化合物是聚乙二醇.通过聚乙二醇改性可改善材料的亲水性,改变材料药代动力学效果,同时具有实现其他功能的优势[12].为了改善PANI在水中的分散性,WANG等[13]设计了一种核-壳结构的复合材料聚苯胺-聚吡咯烷酮(PANi@PVP),PVP作为空间稳定剂对PANI进行改性,在水中有优异的溶解性和良好的细胞相容性.LIU等[7]提出了一种分级靶向策略,设计了pH敏感的檸檬酸钠修饰的铜掺杂PANI 纳米梭(SC-VCR-CuPANI NSs),如图1所示,该材料在血液中呈负电,显示出增强的隐身效果,血液循环周期延长,且在肿瘤微酸环境中实现质子化,提高细胞内在化效果.通过这种分级靶向的策略,材料在血液中的循环半衰期从4.35 h增加到7.33 h,磁共振成像分辨率和信号强度得到显著提高.为了提高肿瘤的治疗效果,基于透明质酸(HA)可以特异性结合受体(CD44)靶向肿瘤细胞[14], JIANG等[15]设计了一种水溶性透明质酸-杂交PANI纳米材料,靶向光热治疗肿瘤.通过细胞实验,材料可以针对性地杀死癌细胞HeLa和HCT-116,而对正常细胞HFF没有影响.将HA修饰的二氧化硅荧光纳米颗粒连接到PANI,在靶向肿瘤的同时,二氧化硅提供的荧光成像可以引导光热治疗.2 PANI纳米复合材料在肿瘤诊疗上的应用2.1 光热治疗PANI经掺杂后在近红外区(700~900 nm)有很强的吸收,PANI具有成为光热剂的可能[16].如图2所示,YANG等[5]首次报道了PANI在酸性条件下进行掺杂,具有有机光热剂的潜力.用808 nm,2.45 W·cm-2的NIR激光照射5 min,掺杂态PANI温度升高了54.8 ℃,采用相同条件,本征态PANI温度升高了15 ℃左右,表明了掺杂后的PANI光热效果明显.为评估PANI对癌细胞在体外和体内的光热消融能力,将本征态的PANI用上皮癌细胞A431处理后发现,PANI颜色从紫色变成绿色,并在808 nm NIR激光下照射,台盼蓝染色实验发现大量细胞被破坏.同样在体内实验中,经瘤内注射材料,用激光照射治疗后,肿瘤组织切片显示有严重的细胞损伤.这说明PANI可以在癌细胞中掺杂,并诱导产生光热效应,从而杀死癌细胞.2.2 协同治疗光热疗法是微创治疗技术,通过外部激光刺激富集到肿瘤部位的材料导致局部升温,致使细胞结构发生破坏,但研究表明:受制于纳米材料在肿瘤部位的富集程度及机体代谢的影响,光热治疗的效果并不佳,同时材料也会给机体带来一定的毒性.对于深部肿瘤,激光强度会由于深度而依赖性改变,这些因素迫使光热疗法同其他的疗法相结合,从而增强肿瘤治疗的效果,降低治疗对机体正常组织的损伤[17-18].将光热疗法和光动力疗法相结合,两种疗法均采用外部激光照射激发.光动力疗法是利用光敏剂和组织细胞中的氧气(O2)的共同存在,经激光照射产生活性氧(ROS),ROS会氧化生物大分子,从而破坏细胞结构,促使细胞死亡[19-20].由于肿瘤中低氧的限制,会降低光动力疗法的治疗效果,将光动力疗法和光热疗法相结合,可以提高整体治疗效果,同时光热疗法会促进血液的流动,提高肿瘤中的氧气浓度,得到1+1>2的效果.TAN等[21]合成了一种PANI包覆吲哚菁绿载银纳米复合材料(ICG-Ag@PANI),银/聚苯胺核壳纳米粒子(Ag@PANI)通过π-π堆积和疏水相互作用的方式负载光敏剂吲哚菁绿(ICG),实现单光触发的光热疗法和光动力疗法协同治疗.将光热剂与化疗药物结合,可控制药物在肿瘤部位的释放[22],降低药物对正常细胞的损伤,协同化疗和光热疗法能很好地提高癌症治疗能力.结合药物的方法有两种:一是将光热剂同可载药的载体结合;二是将光热剂自身设计为药物载体.SILVA等[23]设计了装载5-FU的二甲基咪唑结合PANI纳米粒子(PANI@ZIF-8),在NIR和pH=5.2的缓冲溶液中,5-FU的累计释放量达到80%,PANI吸收NIR会促使温度的升高,增强了5-FU的释放,具有很好的化学光热效应.如图3所示,XIA等[24]采用多孔硅包覆阿霉素(DOX),并将PANI共价接枝到其表面,通过pH和NIR响应控制药物释放,多孔硅可降解为无毒氢氧化硅(Si(OH)4)排出体外,解决了光热剂不可生物降解所带来的长期毒性问题,化学与光热结合疗法展现了巨大的潜力.2.3 多模式成像引导治疗通过成像的方式来引导治疗可以有效地提高肿瘤治疗效率.掺杂态PANI在近红外区具有独特的光吸收特性,是一种优异的光声成像剂[25].光声成像是探测激光照射产生的光声信号后,产生组织分布图像的成像方式,具有高信噪比和高分辨率的优点[26].但是单一的成像依旧存在缺陷,尤其对深部肿瘤,光声成像对激光的强度存在依赖,激光过强会损伤正常的组织细胞.多模式成像方式引导治疗来提高治疗效果是目前研究的趋势.如图4所示,WANG等[27]制备一种PANI包覆二硫化钼(MoS2@PANI-PEG)量子点复合材料,MoS2量子点可产生强荧光,可用于体内成像的探针,同时MoS2是放射增敏剂,结合PANI的光热疗法和光声成像能力,协同增强癌症的治疗效果.2.4 智能响应治疗基于PANI易被质子酸掺杂和肿瘤微环境具有微酸性的特点,设计智能响应探针用于癌症治疗.由于肿瘤微酸性环境(pH为5.0~7.4)低于PANI掺杂要求的pH值(pH<3.0),限制了PANI在癌症治疗中光热治疗和光声成像的应用.JU等[28]合成了金/聚苯胺核壳纳米材料(Au@PANI),基于Au到PANI的电荷转移以及PANI掺杂过程诱导的电子传递效率的提高,Au@PANI在pH=6.5时即可实现掺杂,显示出优异的光热效应.但金离子在体内具有长期毒性,对人体会造成很大的危害.为提高智能治疗剂的治疗性能,如图5所示,LI等[29]采用牛血清白蛋白(BSA)包覆PANI,设计了BSA-PANI纳米粒子,可以实现肿瘤内源性触发的诊断和治疗,光热转换效率达到37%,且纳米粒子具有很好的生物相容性,降低体内毒性.目前对PANI纳米材料的表面改性,主要通过物理吸附或采用共价键将配体结合在纳米颗粒表面等方式以实现其功能化.较常用于纳米颗粒改性的化合物是聚乙二醇.通过聚乙二醇改性可改善材料的亲水性,改变材料药代动力学效果,同时具有实现其他功能的优势[12].为了改善PANI在水中的分散性,WANG等[13]设计了一种核-壳结构的复合材料聚苯胺-聚吡咯烷酮(PANi@PVP),PVP作为空间稳定剂对PANI进行改性,在水中有优异的溶解性和良好的细胞相容性.LIU等[7]提出了一种分级靶向策略,设计了pH敏感的柠檬酸钠修饰的铜掺杂PANI 纳米梭(SC-VCR-CuPANI NSs),如图1所示,该材料在血液中呈负电,显示出增强的隐身效果,血液循环周期延长,且在肿瘤微酸环境中实现质子化,提高细胞内在化效果.通过这种分级靶向的策略,材料在血液中的循环半衰期从4.35 h增加到7.33 h,磁共振成像分辨率和信号强度得到显著提高.为了提高肿瘤的治疗效果,基于透明质酸(HA)可以特异性结合受体(CD44)靶向肿瘤细胞[14], JIANG等[15]设计了一种水溶性透明质酸-杂交PANI纳米材料,靶向光热治疗肿瘤.通过细胞实验,材料可以针对性地杀死癌细胞HeLa和HCT-116,而对正常细胞HFF没有影响.将HA修饰的二氧化硅荧光纳米颗粒连接到PANI,在靶向肿瘤的同时,二氧化硅提供的荧光成像可以引导光热治疗.2 PANI纳米复合材料在肿瘤诊疗上的应用2.1 光热治疗PANI经掺杂后在近红外区(700~900 nm)有很强的吸收,PANI具有成为光热剂的可能[16].如图2所示,YANG等[5]首次报道了PANI在酸性条件下进行掺杂,具有有机光热剂的潜力.用808 nm,2.45 W·cm-2的NIR激光照射5 min,掺杂态PANI温度升高了54.8 ℃,采用相同条件,本征态PANI温度升高了15 ℃左右,表明了掺杂后的PANI光热效果明显.为评估PANI对癌细胞在体外和体内的光热消融能力,将本征态的PANI用上皮癌细胞A431处理后发现,PANI颜色从紫色变成绿色,并在808 nm NIR激光下照射,台盼蓝染色实验发现大量细胞被破坏.同样在体内实验中,经瘤内注射材料,用激光照射治疗后,肿瘤组织切片显示有严重的细胞损伤.这说明PANI可以在癌细胞中掺杂,并诱导产生光热效应,从而杀死癌细胞.2.2 协同治疗光热疗法是微创治疗技术,通过外部激光刺激富集到肿瘤部位的材料导致局部升温,致使细胞结构发生破坏,但研究表明:受制于纳米材料在肿瘤部位的富集程度及机体代谢的影响,光热治疗的效果并不佳,同时材料也会给机体带来一定的毒性.对于深部肿瘤,激光强度会由于深度而依赖性改变,这些因素迫使光热疗法同其他的疗法相结合,从而增强肿瘤治疗的效果,降低治疗对机体正常组织的损伤[17-18].将光热疗法和光动力疗法相结合,两种疗法均采用外部激光照射激发.光动力疗法是利用光敏剂和组织细胞中的氧气(O2)的共同存在,经激光照射产生活性氧(ROS),ROS会氧化生物大分子,从而破坏细胞结构,促使细胞死亡[19-20].由于肿瘤中低氧的限制,会降低光动力疗法的治疗效果,将光动力疗法和光热疗法相结合,可以提高整体治疗效果,同时光热疗法会促进血液的流动,提高肿瘤中的氧气浓度,得到1+1>2的效果.TAN等[21]合成了一种PANI包覆吲哚菁绿载银纳米复合材料(ICG-Ag@PANI),银/聚苯胺核壳纳米粒子(Ag@PANI)通过π-π堆积和疏水相互作用的方式负载光敏剂吲哚菁绿(ICG),实现单光触发的光热疗法和光动力疗法协同治疗.将光热剂与化疗药物结合,可控制药物在肿瘤部位的释放[22],降低药物对正常细胞的损伤,协同化疗和光热疗法能很好地提高癌症治疗能力.结合药物的方法有两种:一是将光热剂同可载药的载体结合;二是将光热剂自身设计为药物载体.SILVA等[23]设计了装载5-FU的二甲基咪唑结合PANI纳米粒子(PANI@ZIF-8),在NIR和pH=5.2的緩冲溶液中,5-FU的累计释放量达到80%,PANI吸收NIR会促使温度的升高,增强了5-FU的释放,具有很好的化学光热效应.如图3所示,XIA等[24]采用多孔硅包覆阿霉素(DOX),并将PANI共价接枝到其表面,通过pH和NIR响应控制药物释放,多孔硅可降解为无毒氢氧化硅(Si(OH)4)排出体外,解决了光热剂不可生物降解所带来的长期毒性问题,化学与光热结合疗法展现了巨大的潜力.2.3 多模式成像引导治疗通过成像的方式来引导治疗可以有效地提高肿瘤治疗效率.掺杂态PANI在近红外区具有独特的光吸收特性,是一种优异的光声成像剂[25].光声成像是探测激光照射产生的光声信号后,产生组织分布图像的成像方式,具有高信噪比和高分辨率的优点[26].但是单一的成像依旧存在缺陷,尤其对深部肿瘤,光声成像对激光的强度存在依赖,激光过强会损伤正常的组织细胞.多模式成像方式引导治疗来提高治疗效果是目前研究的趋势.如图4所示,WANG等[27]制备一种PANI包覆二硫化钼(MoS2@PANI-PEG)量子点复合材料,MoS2量子点可产生强荧光,可用于体内成像的探针,同时MoS2是放射增敏剂,结合PANI的光热疗法和光声成像能力,协同增强癌症的治疗效果.2.4 智能响应治疗基于PANI易被质子酸掺杂和肿瘤微环境具有微酸性的特点,设计智能响应探针用于癌症治疗.由于肿瘤微酸性环境(pH为5.0~7.4)低于PANI掺杂要求的pH值(pH<3.0),限制了PANI在癌症治疗中光热治疗和光声成像的应用.JU等[28]合成了金/聚苯胺核壳纳米材料(Au@PANI),基于Au到PANI的电荷转移以及PANI掺杂过程诱导的电子传递效率的提高,Au@PANI在pH=6.5时即可实现掺杂,显示出优异的光热效应.但金离子在体内具有长期毒性,对人体会造成很大的危害.为提高智能治疗剂的治疗性能,如图5所示,LI等[29]采用牛血清白蛋白(BSA)包覆PANI,设计了BSA-PANI纳米粒子,可以实现肿瘤内源性触发的诊断和治疗,光热转换效率达到37%,且纳米粒子具有很好的生物相容性,降低体内毒性.目前对PANI纳米材料的表面改性,主要通过物理吸附或采用共价键将配体结合在纳米颗粒表面等方式以实现其功能化.较常用于纳米颗粒改性的化合物是聚乙二醇.通过聚乙二醇改性可改善材料的亲水性,改变材料药代动力学效果,同时具有实现其他功能的优势[12].为了改善PANI在水中的分散性,WANG等[13]设计了一种核-壳结构的复合材料聚苯胺-聚吡咯烷酮(PANi@PVP),PVP作为空间稳定剂对PANI进行改性,在水中有优异的溶解性和良好的细胞相容性.LIU等[7]提出了一种分级靶向策略,设计了pH敏感的柠檬酸钠修饰的铜掺杂PANI 纳米梭(SC-VCR-CuPANI NSs),如图1所示,该材料在血液中呈负电,显示出增强的隐身效果,血液循环周期延长,且在肿瘤微酸环境中实现质子化,提高细胞内在化效果.通过这种分级靶向的策略,材料在血液中的循环半衰期从4.35 h增加到7.33 h,磁共振成像分辨率和信号强度得到显著提高.为了提高肿瘤的治疗效果,基于透明质酸(HA)可以特异性结合受体(CD44)靶向肿瘤细胞[14], JIANG等[15]设计了一种水溶性透明质酸-杂交PANI纳米材料,靶向光热治疗肿瘤.通过细胞实验,材料可以针对性地杀死癌细胞HeLa和HCT-116,而對正常细胞HFF没有影响.将HA修饰的二氧化硅荧光纳米颗粒连接到PANI,在靶向肿瘤的同时,二氧化硅提供的荧光成像可以引导光热治疗.2 PANI纳米复合材料在肿瘤诊疗上的应用2.1 光热治疗PANI经掺杂后在近红外区(700~900 nm)有很强的吸收,PANI具有成为光热剂的可能[16].如图2所示,YANG等[5]首次报道了PANI在酸性条件下进行掺杂,具有有机光热剂的潜力.用808 nm,2.45 W·cm-2的NIR激光照射5 min,掺杂态PANI温度升高了54.8 ℃,采用相同条件,本征态PANI温度升高了15 ℃左右,表明了掺杂后的PANI光热效果明显.为评估PANI对癌细胞在体外和体内的光热消融能力,将本征态的PANI用上皮癌细胞A431处理后发现,PANI颜色从紫色变成绿色,并在808 nm NIR激光下照射,台盼蓝染色实验发现大量细胞被破坏.同样在体内实验中,经瘤内注射材料,用激光照射治疗后,肿瘤组织切片显示有严重的细胞损伤.这说明PANI可以在癌细胞中掺杂,并诱导产生光热效应,从而杀死癌细胞.。
导电高分子材料聚苯胺

苯胺简介及结构聚苯胺是一种具有金属光泽的粉末,因分子内具有大的线型共轭π电子体系,其自由电子可随意迁移和传递,而成为最具代表性的有机半导体材料。
与其他导电聚合物相比,聚苯胺具有结构多样化、耐氧化和耐热性好等特点,同时还具有特殊的掺杂机制。
MacDiarmid 重新开发聚苯胺后,在固体13C-NMR及IR研究的基础上提出聚苯胺是一种头尾连接的线性聚合物,由苯环-醌环交替结构所组成,但这种结构和后来出现的大量实验数据相矛盾。
1987年,MacDiarmid进一步提出了后来被广泛接受的苯式-醌式结构单元共存的模型,两种结构单元通过氧化还原反应相互转化。
即本征态聚苯胺由还原单元:和氧化单元:构成,其结构为:其中y值用于表征聚苯胺的氧化还原程度,不同的y值对应于不同的结构、组分和颜色及电导率,完全还原型(y=1)和完全氧化型(y=0)都为绝缘体。
在0<y<1的任一状态都能通过质子酸掺杂,从绝缘体变为导体,仅当y=0.5时,其电导率为最大。
聚苯胺的导电原理物质的导电过程是载流子(电子、离子等带电粒子) 在电场作用下定向移动的过程。
通常认为, 高分子聚合物导电必须具备两个条件:一是要能产生足够数量的载流子, 二是大分子链内和链间要能够形成导电通道。
纯的聚苯胺是绝缘体, 要使它变为导体需要掺杂, 就是掺入少量其他元素或化合物。
0<y<1的聚苯胺, 掺杂后能变为导体, y为0.5的中间氧化态聚苯胺(苯式-醌式交替结构) 掺杂后的导电性最好。
而y为1的完全还原态聚苯胺(全苯式结构) 和y为0的完全氧化态聚苯胺(全醌式结构) 即使掺杂也不能变为导体。
一种掺杂聚苯胺的结构式如图所示, x代表掺杂程度, A-是掺杂剂质子酸中的阴离子, y仍代表还原程度。
向聚苯胺中掺入质子酸是一种有效的掺杂方式, 但是使用普通有机酸及无机弱酸获得的掺杂产物电导率不高, 必须用酸性较强的质子酸(如H2SO4、H3PO4、HBr和HCl) 作掺杂剂才可得到电导率较高的掺杂态聚苯胺, 盐酸是最常用的无机掺杂酸。
对甲苯磺酸掺杂聚苯胺材料的导电性能及其机理研究

对甲苯磺酸掺杂聚苯胺材料的导电性能及其机理研究
吴唯;骆巧玲;阮明珠
【期刊名称】《中国塑料》
【年(卷),期】2014(0)4
【摘要】通过再掺杂法制备了对甲苯磺酸(TSA)掺杂的导电聚苯胺(PANI),探究掺杂时间及对甲苯磺酸水溶液的浓度对PANI的电导率和结构的影响,通过四探针法和傅里叶变换红外光谱仪(FTIR)分析表征了掺杂态PANI的电导率和结构,并探究掺杂时间对掺杂态PANI导电性能影响的机理,还利用热重分析仪(TG)探究TSA对PANI热稳定性的影响.结果表明,掺杂时间为12 h、TSA水溶液浓度为0.5 mol/L 所制得的掺杂态PANI具有最好的电导率.
【总页数】5页(P23-27)
【作者】吴唯;骆巧玲;阮明珠
【作者单位】华东理工大学材料科学与工程学院,中德先进材料联合研究中心,上海200237;华东理工大学材料科学与工程学院,中德先进材料联合研究中心,上海200237;华东理工大学材料科学与工程学院,中德先进材料联合研究中心,上海200237
【正文语种】中文
【中图分类】TQ324.8
【相关文献】
1.磺基水杨酸掺杂导电聚苯胺纳米材料的制备与性能研究 [J], 陈德贤;郑玉婴;张通;李宝铭
2.氨基磺酸掺杂导电聚苯胺/OMMT纳米复合材料的制备与性能研究 [J], 冯辉霞;邵亮;王毅;张国宏;赵阳;邱建辉
3.溴掺杂聚苯胺的导电性能及机理研究 [J], 吴玲娟;叶葏;韩菲菲;梁旦;高建生;盛玮;徐学诚
4.基于灰色理论盐酸掺杂聚苯胺/凹凸棒石材料导电性能的研究 [J], 冯辉霞;罗梓轩;鲁华涛;陈姣;刘生丽;张建强
5.不同酸掺杂聚苯胺电极材料的制备及其导电性能研究 [J], 杨铁金;江姗姗
因版权原因,仅展示原文概要,查看原文内容请购买。
导电高分子聚苯胺及其应用

3、传感器领域
3、传感器领域
聚苯胺作为一种敏感材料,在传感器领域有着广泛的应用。通过化学或电化 学掺杂,聚苯胺的导电性能发生变化,利用这种特性可以制造出各种传感器。例 如,基于聚苯胺的酸碱传感器可以用来检测溶液的酸碱度,而聚苯胺基的压力传 感器则可以用于监测压力变化。
Байду номын сангаас
三、研究方法
1、化学反应机理
导电高分子聚苯胺的合成
3、聚合反应:将苯胺单体、氧化剂和催化剂混合在一起,在适当的温度和压 力条件下进行聚合反应。
导电高分子聚苯胺的合成
4、后处理:通过后处理步骤,如脱色、干燥等,得到纯净的导电高分子聚苯 胺。
4、后处理:通过后处理步骤, 如脱色、干燥等,得到纯净的导 电高分子聚苯胺。
4、后处理:通过后处理步骤,如脱色、干燥等,得到纯净的导电高 分子聚苯胺。
导电高分子聚苯胺及其应用
01 引言
目录
02 一、研究现状
03 二、应用领域
04 三、研究方法
05 参考内容
引言
引言
导电高分子材料在当代科技领域具有广泛的应用前景,其中聚苯胺作为一种 新型的高分子导电材料备受。聚苯胺具有优异的导电性能、良好的化学稳定性和 易于制备等优点,成为一种极具潜力的导电高分子材料。本次演示将详细介绍聚 苯胺的研究现状、应用领域及研究方法,并展望其未来发展方向。
4、后处理:通过后处理步骤,如脱色、干燥等,得到纯净的导电高 分子聚苯胺。
3、功能性应用研究:除了传统的电子、航天、建筑等领域,探索聚苯胺在新 能源、生物医学等领域的应用,如作为电池材料、生物传感器等。
4、后处理:通过后处理步骤,如脱色、干燥等,得到纯净的导电高 分子聚苯胺。
4、理论计算模拟:通过理论计算和模拟,深入了解聚苯胺的分子结构和性能 之间的关系,为材料的设计和优化提供指导。
银/聚苯胺复合材料的制备及抗菌性能研究

复合 材料 。Li等 利 用 紫 外 照 射 ,在 正 己醇 、环 己 烷微 乳 液 体 系 中 制 备 了粒 径 不 到 100 nm 的核 壳 式 Ag/PANI复 合材 料 。Dix等¨钊通 过 在 N 气 氛 下 对 苯 胺及 硝酸 银溶 液超 声处 理 ,得到 了对 络氨 酸氧 化有 极 高 电催化 活 性 的 Ag/PANI复 合 材 料 。Detsri等 ¨ 用 分层 沉积 的方 法 制 备 的 Ag/PANI复 合 薄 膜 ,在 氨 气 存在 下能 迅速从 橘 红色转 变 黄色 ,是 理想 的氨 气传感 器材 料 。
氧化 剂使 苯胺 聚合 ,制备聚苯胺完全包裹银的复合材料 。通过扫描 电镜 、透射 电镜 、x射 线衍射分析 仪 、傅里 叶变 换
红外 光谱 仪 ,x射线光 电子能谱分析仪对复合材料进行 测试与分 析 ,结果 显示银 纳米粒 子粒径 在 5—10 nm左 右 ,分
散性 良好且被 聚苯胺 完全包裹。银纳米粒子与聚苯胺有着较 强的相互 作用。抗菌性 能测试结果 显示 银/聚苯胺 复合 材
show that the diameter range of silver nanoparticles iS about 5 to 10 nm. The silver nanoparticles are covered by polyaniline and disperse wel1. The silver nanoparticles have strong interaction with polyaniline. The antibacterial perform ance test results show that the antibacterial ratio of the silver/polyaniline composites iS 99.7% .
27129445_聚苯胺复合材料的制备及掺杂酸的影响研究

第48卷 第7期·6·作者简介:明皓(1988-),男,博士研究生,讲师,主要从事环境功能材料、电化学工业水处理技术方面工作研究。
基金项目:辽宁省教育厅科学研究经费项目(202013 62101);沈阳科技学院科学研究项目(ZD-2021-06);沈阳科技学院校级创新团队。
收稿日期:2022-04-27聚苯胺(PANI )因其诸多特性,在生活和生产的各个领域均得到广泛应用,如二次电池和电极材料、导电材料和防静电材料、防腐材料、防污材料、发光二极管、光学器件及非线性光学器件等[1~2]。
作为防腐涂料,无论从实验室结果还是从实际检测结果来看,聚苯胺都是较为理想的,尤其是其特有的抗点蚀、抗划伤能力更是单纯有机涂层不可比的[3]。
因此,开发聚苯胺防腐蚀涂料已成为导电高分子材料的应用和涂料研究开发领域的一个新的热点[4]。
我国从1985年起开展了聚苯胺的研究,在聚苯胺导电性研究方面取得了重大突破。
我国从事聚苯胺研究开发的单位有中国科学院化学研究所、中国科学院长春应用化学研究所、华南理工大学、南京大学、华中理工大学等。
现在已从对聚苯胺的链结构、掺杂反应、导电机理等基础理论的研究,发展到改进其溶解性和加工性的合成方法的研究上,并取得了突破性的进展[5]。
科研工作者们针对聚苯胺的优异特性进行了广泛深入的探讨,并逐渐深入到了聚苯胺的应用领域。
对聚苯胺的链的结构有过许多的争论MacDarmid 在1987年提出了目前人们普遍接受的结构式,即结构中不但含有苯-醌交替的氧化形式,而且含有苯-苯连续的还原形式。
通过质子酸掺杂可以改变结构的氧化状态,而且又陆续发现了其他种类的掺杂方式[6]。
聚苯胺复合材料的制备及掺杂酸的影响研究明皓1,2,沈佳慧1,范文玉3(1.沈阳科技学院,辽宁 沈阳 110167;2.沈阳工业大学,辽宁 沈阳 110870;3.沈阳化工大学,辽宁 沈阳 110142)摘要:聚苯胺(PANI )因其诸多特性,在生活和生产的各个领域均得到广泛应用,如二次电池和电极材料、导电材料和防静电材料、防腐材料、防污材料、发光二极管、光学器件及非线性光学器件等。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Home Search Collections Journals About Contact us My IOPscienceSynthesis, characterization and properties of polyaniline/expanded vermiculite intercalated nanocompositeThis content has been downloaded from IOPscience. Please scroll down to see the full text.2008 Sci. Technol. Adv. Mater. 9 025010(/1468-6996/9/2/025010)View the table of contents for this issue, or go to the journal homepage for moreDownload details:IP Address: 218.196.194.131This content was downloaded on 27/11/2013 at 03:11Please note that terms and conditions apply.IOP P UBLISHING S CIENCE AND T ECHNOLOGY OF A DV ANCED M ATERIALS Sci.Technol.Adv.Mater.9(2008)025010(6pp)doi:10.1088/1468-6996/9/2/025010Synthesis,characterization and properties of polyaniline/expanded vermiculite intercalated nanocompositeJianming Lin,Qunwei Tang,Jihuai Wu and Hui SunThe Key Laboratory of Functional Materials for Fujian Higher Education,Institute of Material PhysicalChemistry,Huaqiao University,Quanzhou362021,People’s Republic of ChinaE-mail:jhwu@Received9December2007Accepted for publication8April2008Published10July2008Online at /STAM/9/025010AbstractThe synthesis characterization and conductivities of polyaniline/expanded vermiculiteintercalated nanocomposite are presented in this paper.The conductive emeraldine salt formof polyaniline is inserted into the interlayer of expanded vermiculite to produce thenanocomposite with high conductivity.The structures and properties are characterized bytransmission electron microscopy x-ray diffraction spectroscopy fourier transform infraredspectroscopy thermogravimetry analysis and by the measurements of conductivity andstability.The results show that an intercalated nanocomposite with high conductivity andstability is obtained.The synthesis conditions are optimized to obtain the highest conductivitywhich is6.80S cm−1.Keywords:polyaniline,expanded vermiculite,intercalated nanocomposite,conductivity,stability(Somefigures in this article are in colour only in the electronic version)1.IntroductionDuring the last decade,considerable attention has been paid to the synthesis and evaluation of clay/polymer nanocomposites[1–3]via the intercalation polymerization of special monomers such as aniline,pyrrole,thiophene or N-vinylcarbazole.Among these synthetic materials, polyaniline(PANI)nanocomposites have attracted special attention,because by the intercalation polymerization,it is possible to obtain structure with a more ordered chain and better properties than those of bulk ones[4].The most common inorganic host used to prepare PANI nanocomposites is clay[5–7],owing to clay’s swelling capacity and exchange cations.In this paper,PANI is chosen as a conducting component and expanded vermiculite(EVMT)as the host on the basis of the following.(i)EVMT is a natural mineral and has a layered structure;the stacking of the layers of about.1nm thickness by weak dipolar forces leads to interlayers or galleries between the layers. The galleries normally occupied by hydrated cations that balance the charge deficiency are generated by the isomorphous substitution in the tetrahedral sheets.The aniline monomer can be introduced into the galleries by ion exchange,and consequently,it becomes barely separable from the galleries.(ii)EVMT is an inactive inorganic host without a redox character,so the situ polymerization can be controlled.(iii)It is a well-ordered host in two dimensions after the intercalation of the aniline monomer; the extrinsic initiator,potassium persulfate,can enter and initiate the polymerization in the interlayers[8].To improve the exfoliation effect of EVMT,an ultrasonic technique is introduced,and a polyaniline/expanded vermiculite intercalated nanocomposite is prepared by aqueous solution polymerization.The structures of the nanocomposite are determined by transmission electron microscopy(TEM), x-ray diffraction analysis(XRD),and Fourier transform infrared spectroscopy(FTIR).2.Experimental method2.1.MaterialsThe aniline monomer purchased from ShanghaiChemical Reagent Co.,China was distilled twice underreduced pressure prior to use.Expanded vermiculite{EVMT,[(Mg,Ca)0.7(Mg,Fe,Al)6[(Al,Si8)](OH4.8H2O)]}, consisting of38.41%SiO2,23.42%Fe2O3,14.57%Al2O3,11.15%MgO,and0.89%CaO was purchased from HebeiMineral Co.,China and prepared from natural vermiculiteheated at500◦C.Potassium persulfate(KPS),a radicalinitiator for the synthetic reaction of PANI,was purifiedby recrystallization from66wt.%ethanol/water solution.Sodium dodecyl benzene sulfate(SDBS)as dopant andemulsifier were used as received.Both materials werepurchased from Shanghai Chemical Reagent Co.,China.2.2.Preparation of PANI/EVMT nanocompositesEVMT was pretreated with HCl according to the methoddescribed in[9].Fifty grams of EVMT was added to1000mlof HCl solution(2mol l−1)and the resulting slurry was stirredcontinuously for20h.Then,the acid-treated EVMT wasfiltered and rinsed for5min with distilled water until the pHof thefiltrate reached approximately7.0.The obtained EVMTwas dried at80◦C overnight and stored in a desiccator.The ultrasonic technique was introduced to expand theinterlayer of EVMT and intercalate PANI into the interlayer ofEVMT in the same manner as that for graphite oxide[10].Thesteps are as follows:5g of expanded EVMT was mixed with300ml of distilled water.The suspension was ultrasonicallyvibrated under a power of59kHz for10h,then centrifugedand dried at70◦C in a vacuum oven.The resultant expandedEVMT sheets were kept in a desiccator prior to test and furtheruse.PANI/EVMT nanocomposites were prepared as follows:a mixed solution of predetermined amounts of EVMT,SDBS,aniline monomer(2ml),hydrochloric acid solution(100ml,1.5mol l−1)and distilled water(250ml)was madein a three–mouthflask by stirring with a rotation speedof450rpm in an80◦C water bath for90min.Then,theflask was cooled to room temperature.KPS acid solutionprepared by dissolving KPS in80ml of distilled waterand50ml of hydrochloric acid solution(1.5mol l−1)wasplaced in theflask within15min under450rpm stirring atambient temperature.The product was washed5times withhydrochloric acid solution(1.5mol l−1)and distilled water,and then dried in an80◦C oven for24h.Thus,a PANI/EVMTnanocomposite was obtained.2.3.Conductivity measurementFor the conductivity measurement,all the test samples wereprepared in pellet form(diameter:13mm,thickness:1mm)at pressure of14MPa using a Carver model C Press.Thefour-probe method was used to measure the conductivity ofthe PANI/EVMT nanocomposites.2.4.CharacterizationsFor TEM observations,a small amount of powder sample was dispersed in high-purity methanol solution by ultrasonic vibration for20min to ensure the complete separation of the particles.A drop of the suspension was pipetted onto a copper grid coated with holey carbonfilm or gold,and the solvent was allowed to evaporate.The TEM morphologies of the samples were observed with a JEM-2010at an acceleration voltage of 100kV.The powder XRD analysis of the samples was carried out using a D8ADV ANCE x-ray diffractometer of Germany BRUKER Co.,CuKαof0.1540nm wavelength,running at 40kV and30mA,scanning from2◦to40◦at5◦min−1. The sample surface was observed using a scanning electron microscope(S-3500N,HITACHI).The FTIR spectroscopy of the samples was carried out with a Nicolet Impact410FTIR spectrophotometer using KBr pellets.The thermogravimetric (TG)curves of the samples were measured using an SDT2960 simultaneous DSC-TGA device(USA TA Instrument).3.Results and discussion3.1.TEM observationThe TEM images of untreated EVMT and PANI/EVMT nanocomposite are shown infigure1.The layered structure of the EVMT is perceptible infigure1(a).The typical diffraction spots in its selected-area diffraction pattern are shown in the top right corner offigure1(a).From thefigure,it can be seen that the untreated EVMT as received is a single crystal with some impurities that result from the VMT mineral(the mineral was purchased from Hebei Mineral Co.,China and not purified before use).The particle size is large before the acid treatment,while in the PANI/EVMT nanocomposite, the size of the EVMT particles decreases to approximately 100nm and the layered structures of the EVMT are also exfoliated.This is consistent with result of Liu et al[9].3.2.XRD patternsFigure2shows the XRD patterns of the untreated EVMT,ultrasonically treated EVMT,and PANI/EVMT nanocomposites.The as-received EVMT(curve a infigure2) has diffraction peaks at2θ=8.78◦(d001)and27.22◦(d003), which belong to phlogopite in the EVMT and represent an interlayer spacing of EVMT(d=1.01nm).The exfoliation of the EVMT can be determined from the XRD spectra.For the sample acid-treated longer than20h and subsequently sonicated(curve b infigure2),the peak corresponding to a layer gap of1.01nm moves to2θ=7.53◦with an interlayer distance of1.17nm.Moreover,the intensity also markedly decreases,which indicates that the EVMT is delaminated and the platelet of the EVMT is less than ten cells or layers of single crystals[9].For the PANI/EVMT nanocomposite, the diffraction peaks at2θ=8.78◦and27.22◦shift to 2θ=6.33◦,25.93◦(curve c infigure2)and7.52◦,26.49◦(curve d infigure2),respectively.The shift of the diffraction peaks to low angles and the diffraction intensity decreaseFigure1.TEM image of untreated EVMT(a)and PANI/EVMT nanocomposite(b).compared with curve b reveal that the interlayer is furtherexpanded(the interlayer distance is d=1.40nm)during thesitu polymerization of aniline monomers.The new diffractionpeak at approximately3.37◦is attributed to PANI.The sharppeak indicates a well-ordered crystallization of PANI in theinterlayers of EVMT[8].3.3.FTIR spectraInfigure3,the absorption peak at3457cm−1is attributedto the H–O–H stretching,3715cm−1is for O–H in Mg3OHstretching,1641cm−1is for H–O–H in absorbed waterbending,and990and450cm−1are for the Si–O–Si stretchingof EVMT.The absorption peak at1557cm−1is attributed tothe quinoid ring stretching,1480cm−1is for the benzene ringstretching,1300and1243cm−1are for the N–H stretchingin the connecting benzene ring-quinoid ring and benzenering-benzene ring,respectively,and1124and814cm−1are asymmetric peak stretchings of SO3−.There are two absorption peaks at1020and664cm−1,which is consistentwith the results given in a previous report[11].Owing tothe doping of SDBS into the PANI,a-CH2-absorption at10203040 Intensity(arb.units)2θ (°)(a)(b)(c)8.78o7.53ο27.22ο25.86ο6.33ο3.37οFigure2.XRD patterns of untreated EVMT(a),ultrasonically treated EVMT(b),PANI/EVMT(c).Transmittance(arb.units)Wavenumber (cm–1)Figure3.FTIR spectra of EVMT and PANI/EVMT nanocomposite.Preparation conditions:molar ratio of SDBS to aniline of0.5(3.83g of SDBS),KPS to aniline of0.8and mass ratio of EVMT to aniline of3.0.2992cm−1is observed.In the PANI/EVMT nanocomposite, adsorption bands at wave numbers similar to those of PANI are observed.This suggests that PANI was in the form of emeraldine salt during the situ polymerization of aniline monomers.The formation of emeraldine salt in PANI/EVMT nanocomposites is expected to lead to high conductivity.3.4.TG curvesThe TG curves of pure PANI and PANI/EVMT were determined and shown infigure4.It can be seen that pure PANI almost burned up at600◦C,while the PANI/EVMT composite still maintained72wt.%of the original weight at this temperature,which is approximately equal to the weight of EVMT in the composite.The TG experimental results imply that25wt.%of PANI is intercalated into the interlayer of EVMT or deposited on the EVMT surface to form aW e i g h t l o s s (%)Temperature (o C)Figure 4.TG curves of pure PANI andPANI /EVMTnanocomposite.Preparation conditions:molar ratio of SDBS to aniline of 0.5,KPS to aniline of 0.8and mass ratio of EVMT to aniline of 3.0.C o n d u c t i v i t y (S c m –1)KPS/aniline (mol mol –1)Figure 5.Conductivity of PANI /EVMT nanocomposites as a function of KPS under preparation conditions of molar ratio of SDBS to aniline of 0.5(3.83g of SDBS),mass ratio of EVMT to aniline of 5.0,reaction temperature of 15◦C and reaction time of 8h.PANI /EVMT composite,which correlates with the feed ratio for this sample.3.5.Effect of initiator on conductivity of PANI /EVMT nanocompositesThe polymerization of PANI /EVMT nanocomposites dispersed in an aqueous medium proceeds with a typical color change from blue to dark green,indicating the formation of PANI emeraldine salt.As shown in figure 5,the conductivity of the PANI /EVMT nanocomposite increases with increasing KPS dosage in the range of 0.4–0.8;beyond a molar ratio for 0.8,the conductivities of the nanocomposites decrease gradually.The conductivity of the nanocomposite depends on the PANI chain,and the formation of the PANI chain is initiated by KPS;thus,the amount of initiator KPS affects theconductivity of the composite.Clearly,a lower amount of KPS does not produce sufficient crosslink points to construct a PANI chain and a conducting channel,which results in the decline of the conductivity of the nanocomposites.On the other hand,KPS is not only an initiator,but also an oxidizer;excessive KPS causes a side reaction for oxidizing PANI,which results in the disruption of the PANI chain to some extent.A conducting channel cannot run through the material effectively;therefore,the conductivity of the nanocomposite decreases.According to figure 5,the mass ratio of KPS to aniline monomer of 0.8is the better for a high conductivity.3.6.Effect of SDBS on conductivity of PANI /EVMT nanocompositeThe conductivity of the PANI /EVMT nanocomposite also depends on the amount of the SDBS dopant [12–15].Figure 6shows the dependence of conductivity of the PANI /EVMT nanocomposite on the amount of SDBS under the same preparation conditions.It can be seen that with the increase in the SDBS amount,the conductivity of PANI /EVMT increases and then decreases.The classical protonation (doping)concept assumes that the acid reacts with the imine nitrogens in the emeraldine PANI base;as a result,PANI salt is produced.The two electrons from the electron pairs located at the imine nitrogen are injected into an adjacent quininoid ring,and the latter is converted to a benzenoid ring.The remaining unpaired electron present in the imine nitrogen and cation radicals act as carriers in the electric conduction.The increase in the amount of SDBS dopant causes an increase in the degree of protonation and leads to an increase in the conductivity when the molar ratio of SDBS to aniline is lower than 1.5.However,much SDBS may protect against H +doping of the chains of PANI,which leads to the decrease in the doping degree of PANI when the molar ratio of SDBS to aniline is higher than 1.5.Moreover,the electrostatic repulsion forces of the dopant make it unstable for conducting chains and restrict electron conduction.Under our conditions,the molar ratio of SDBS to aniline of 1.5is the better for high conductivity.3.7.Effect of EVMT on conductivity of PANI /EVMT nanocompositeThe effect of EVMT content on the electrical conductivity of the PANI /EVMT nanocomposites is shown in figure 7.When the mass ratio of aniline monomer to EVMT changes from 0.14to 0.25,the conductivity of the nanocomposite markedly increases from 0.33to 6.10S cm −1.When the mass ratio of aniline monomer to EVMT increases to 1,the conductivity reaches 6.80S cm −1.The nanocomposites prepared by aqueous solution polymerization show a typical percolation phenomenon in terms of electrical conductivity as a function of PANI content.It has been reported that the percolation phenomenon occurs in polymer-matrix-conducting composites [16–20],and theC o n d u c t i v i t y (S c m –1)SDBS/aniline (mol mol –1)Figure 6.Conductivity of PANI /EVMT nanocomposites as a function of SDBS under preparation conditions of mass ratio of EVMT to aniline of 3.0,molar ratio of KPS to aniline of 1.0,reaction temperature of 15◦C,and reaction time of 8h.C o n d u c t i v i t y (S c m –1)Aniline/EVMT (g g –1)Figure 7.Conductivity of PANI /EVMT nanocomposites as a function of EVMT content under preparation conditions of molar ratio of SDBS to aniline of 0.5(3.83g of SDBS),KPS to aniline of 0.8,reaction temperature of 15◦C and reaction time of 8h.percolation theory is introduced in equation (1):(1−φ)(σ1/t1−σ1/tm )σ1/t 1+A σ1/t m +φ(σ1/t h −σ1/tm )σ1/t h +σ1/t m=0,A =1−φcφc .(1)Here,σm ,σ1and σh are the conductivities of the medium and the low-and high-conductivity components,respectively.ϕis the volume fraction of the high-conductivity component (PANI in this case)and ϕc is the critical concentration (i.e.percolation threshold).t is an exponent related to both the percolation threshold and the shapes of the grains making up the medium.From the variation of the electrical conductivity of the composite with PANI content,the percolation threshold seems to occur at 0.14(mass ratio of aniline monomer to EVMT).If the mass ratio is higher than 0.14,the electrical conductivity of the PANI /EVMT nanocomposites increases markedly.C o n d u c t i v i t y (S c m –1)Reaction time (h)Figure 8.Conductivity of PANI /EVMT nanocomposites as a function of reaction temperature and time under preparation conditions of molar ratio of SDBS to aniline of 0.5(3.83g of SDBS),KPS to aniline of 0.8,and mass ratio of EVMT to aniline of 3.0.3.8.Effect of reaction temperature and time on conductivity of nanocompositeThe reaction temperature and time also affect the structure and conductivity of the composite.From figure 8,it is clear that the conductivity of the composite increases and then decreases with reaction time for two temperature curves,and the higher the temperature,the shorter is the reaction time for reaching the highest conductivity.Higher reaction temperature resulting in a shorter reaction time is in accordance with the general rule of chemical reactions shown in the equation (2):n (aniline )←KPSin situ polymerization →polyaniline +Q .(2)However,KPS is a stronger oxidant for initiating aniline monomer to form PANI.A longer reaction time leads to a side reaction for oxidizing PANI,which results in the breakage of the PANI conducting chain to some extent and a decrease in the conductivity of the composite.Because of an exothermic polymerization reaction,the higher temperature would produce PANI with more short chains and decrease the conductivity.To obtain a high conductivity composite,the reaction temperature should be reduced,as has been implemented in PANI systems by other groups [21,22].3.9.Stability of nanocompositesIn our work,the conducting PANI was prepared by directly doping PANI with SDBS.Because of the hydrophilic nature of the doped PANI,it is soluble or dispersible in water.Moreover,the hydrophilicity increases with increasing length of the hydrophilic chain in the dopant,and this also leads to increased solubility in water [23].To investigate the environmental stability of the PANI /EVMT conducting nanocomposites,the samplesC o n d u c t i v i t y (S c m –1)Display time (days)Figure 9.Effects of display time on conductivity of PANI /EVMT nanocomposites at 25◦C under preparation conditions of molar ratio of SDBS to aniline of 0.5(3.83g of SDBS),KPS to aniline of 0.8,and mass ratio of EVMT to aniline of 3.0.prepared under different conditions (reacted at 15◦C for 8h (sample a)and at 45◦C for 6h (sample b))were displayed at room temperature and 35%humidity for days,and their conductivities were then determined.From figure 9,it can be seen that the composites have good stability,and the stability depends on the reaction temperature.The conductivities of samples (a)and (b)after 50days of display were maintained at 90.6and 82.4%,respectively.The nanocomoposite synthesized at low temperature shows a better stability.4.ConclusionsA PANI /EVMT intercalated conducting nanocomposite was prepared by aqueous solution polymerization with aniline monomer and exfoliated EVMT powder in the presence of SDBS as dopant and KPS as initiator.When the preparation conditions were optimized at a molar ratio of SDBS to aniline of 0.5,KPS to aniline of 0.8,mass ratio of EVMT to aniline of 3.0,and polymerization at 15◦C for 8h,the PANI /EVMT nanocomposite possessed a conductivity of 6.667S cm −1.The nanocomposite exhibited a typical percolation phenomena and the percolation threshold was approximately 0.14interms of the mass ratio of aniline monomer to EVMT.Moreover,the PANI /EVMT conducting nanocomposites have good environmental stability.AcknowledgmentsWe are grateful for the joint support of the National Natural Science Foundation of China (Nos 50572030and 50372022)and the Key Scientific Technology Program of Fujian,China (Nos 2005HZ01-4and 2007HZ0001-3).Reference[1]LeBaron P C,Wang Z and Pinnavaia T J 1999Appl.Clay Sci.1511[2]Akane O and Arimitsu U 1995Mater.Sci.Eng.C 3109[3]Carrado K A and Xu L 1999Micropor.Mesopor.Mater.2787[4]Do Nascimento G M,Constantino V R L,Landers R andTemperini M L A 2006Polymer 476131[5]Chang K C,Jang G W,Peng C W,Lin C Y ,Shieh J C,Yeh J M,Yang J C and Li W T 2007Electrochim.Acta 525191[6]Chang K C,Lai M C,Peng C W,Chen Y T,Yeh J M,Lin C Land Yang J C 2006Electrochim.Acta 515645[7]Jia W,Segal E,Kornemandel D,Lamhot Y ,Narkis M andSiegmann A 2002Synth.Met.128115[8]Wu Q,Xue Z,Qi Z and Wang F 2000Polymer 412029[9]Liu D F,Du X S and Meng Y Z 2006Mater.Lett.601847[10]Chen G H,Weng W G,Wu D J and Wu C L 2004Carbon42753[11]Huang J and Wang M X 1999J.Polym.Sci.A 37151[12]Sudha J D and Sasikala T S 2007Polymer 48338[13]Singla M L,Awasthi S and Srivastava A 2007SensorsActuator A 136604[14]Lekha P C,Subramanian E and Padiyan D P 2007SensorsActuator B 122274[15]Wu X M,Wang X H,Li J and Wang F S 2007Synth.Met.157176[16]Shekhar S,Prasad V and Subramanyam S V 2006Carbon44334[17]Li J R,Xu J R,Zhang M Q and Rong M Z 2003Carbon412353[18]Emmerich F G,Desousa J C and Torriani I L 1987Carbon25417[19]Malmonge L F and Mattoso L H C 1995Polymer 36245[20]Ghosh P and Chakrabarti A 2000Eur.Polym.J.361043[21]Yan H and Toshima N 1995Synth.Met.69151[22]Cao Y ,Andreatta A,Heeger A J and Smith P 1989Polymer302305[23]Geng Y H,Sun Z C and Li J 1999Polymer 405723。
