Rational targeting of Notch signaling in cancerTargeting Notch in the treatment of cancer
干细胞研究必看的经典综述

⼲细胞研究必看的经典综述⼲细胞是⼀个已经⽕了好⼏年的热点,上次我们梳理过()。
我们可以参考中专项实施⽅案部署8个⽅⾯的研究任务:1. 多能⼲细胞建⽴与⼲性维持;2. 组织⼲细胞获得、功能和调控;3. ⼲细胞定向分化及细胞转分化;4. ⼲细胞移植后体内功能建⽴与调控;5. 基于⼲细胞的组织和器官功能再造;6. ⼲细胞资源库;7. 利⽤动物模型的⼲细胞临床前评估;8. ⼲细胞临床研究。
在以⼲细胞为主题的研究中,肿瘤⼲细胞和间充质⼲细胞是两个⼤的⽅向,常见于肿瘤的复发转移耐药研究和组织损伤修复研究,⽐如肿瘤⼲细胞ISL1介导的肝癌⼲细胞与⾮癌⼲细胞转换在转移中的作⽤和分⼦机制(重点)全反式维甲酸(ATRA)调控肝癌⼲细胞抑制肝癌合并门静脉癌栓化疗耐药机制、敏感标志物筛选及其临床应⽤(重点)⾎管微环境与淋巴瘤⼲细胞的相互作⽤(优青)⽩⾎病⼲细胞⼲性维持与⾃我更新的分⼦机制(优青)间充质⼲细胞间质⼲细胞治疗慢性移植物抗宿主病的免疫作⽤机制(重点)下丘脑-垂体-睾丸轴对间质⼲细胞的调节以及其异常导致性功能低下的机制研究(重点)IL-10基因修饰⾻髓间充质⼲细胞对⾓膜移植排斥的作⽤和机制研究(⾯上)今天我们就来分别梳理这两个⽅向的经典⾼分综述,下期我们来介绍这两个⽅向各⾃的研究套路。
肿瘤⼲细胞⽅向:1. The cancer stem cell niche: how essential is the niche in regulating stemness of tumorcells? (⼲细胞与微环境,2015)2. DNA Damage in Stem Cells.(⼲细胞与DNA损伤,2017)3. RNA editing-dependent epitranome diversity in cancer stem cells.(RNA甲基化、编辑与⼲细胞,2017)4. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update.(信号通路与⼲细胞,2015)5. Cancer stem cell metabolism: a potential target for cancer therapy(⼲细胞代谢与治疗,2016).6. The cancer stem-cell signaling network and resistance to therapy(⼲细胞通路和治疗,2016).7. Cancer stem cell metabolism(⼲细胞代谢,2016)8. Nanomedicine-mediated cancer stem cell therapy(纳⽶药物与⼲细胞治疗,2016).9. Combination of chemotherapy and cancer stem cell targeting agents: Preclinical andclinical studies(⼲细胞治疗,2017).间充质⼲细胞(MSC)⽅向:1. The Therapeutic Promise of Mesenchymal Stem Cells for Liver Restoration(肝脏,2015).2. Mesenchymal stromal cells and liver fibrosis: a complicated relationship.(肝纤维化,2016)3. Mesenchymal stromal cells in renal transplantation: opportunities and challenges(肾移植,2016).4. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, andEngineered Heart Tissue(⼼脏,2016).5. Use of mesenchymal stem cells for therapy of cardiac disease(⼼脏病,2015).6. Mesenchymal Stem Cells in Fibrotic Disease(纤维病变,2017).7. Interactions between mesenchymal stem cells and the immune system.(免疫系统,2017)8. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets.(肿瘤相关间充质⼲/基质细胞,2017)。
肿瘤Notch信号通路的研究进展

肿瘤Notch信号通路的研究进展王晓清;袁国强;潘亚文【摘要】Notch gene encodes a class of highly conserved cell surface receptors that determine the fate of cells in embryonic development and mature tissue, which is an important pathway of communication between adjacent cells, and then regulates of cell development, proliferation and apoptosis. The occurrence and progression of multiple tumors are associated with abnormal Notch signaling pathways. For different tumors, it is important significance for anti-tumor therapy to effectively regulate the Notch participants and target molecules.%Notch基因编码一类高度保守的细胞表面受体,决定胚胎发育和成熟组织中的细胞命运,是相邻细胞之间通讯进而调控细胞发育、增殖和凋亡的重要通路.多种肿瘤的发生与进展和Notch信号通路异常有关.针对不同肿瘤,有效调控靶向Notch的参与者与靶分子,对抗肿瘤治疗具有重要研究意义.【期刊名称】《基础医学与临床》【年(卷),期】2018(038)007【总页数】4页(P1025-1028)【关键词】Notch信号通路;肿瘤;异常【作者】王晓清;袁国强;潘亚文【作者单位】兰州大学第二医院神经病学研究所, 甘肃兰州730030;兰州大学第二医院神经病学研究所, 甘肃兰州730030;兰州大学第二医院神经病学研究所, 甘肃兰州730030;兰州大学第二医院神经外科临床医学中心, 甘肃兰州730030【正文语种】中文Notch基因最早在1917年黑腹果蝇中发现,因其功能部分缺失造成果蝇翅膀边缘缺刻(notch)而命名。
Notch信号通路与肝纤维化发生发展的关系

综述Notch 信号通路与肝纤维化发生发展的关系张旭,刘平,慕永平(上海中医药大学附属曙光医院,肝病研究所,上海201203)摘要:Notch 信号通路主要由Notch 受体和配体、转录因子以及DNA 结合蛋白共同组成,其决定机体细胞的增殖、分化和凋亡。
近年研究表明,Notch 信号通路在肝纤维化发生发展过程中起重要作用,阻断或激活该信号通路可以影响肝纤维化的进展。
对Notch 信号通路的构成、活化机制及其与肝纤维化关系的研究进展进行了综述。
关键词:肝硬化;受体,Notch ;信号传导;综述中图分类号:R575.2文献标志码:A文章编号:1001-5256(2018)01-0181-03Relationship between the Notch signaling pathway and the development and progression of liver fibrosisZHANG Xu ,LIU Ping ,MU Yongping.(Institute of Liver Diseases ,Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine ,Shanghai 201203,China )Abstract :The Notch signaling pathway mainly includes Notch receptors and ligands ,transcription factors ,and DNA binding proteins.Itdecides the way of cell proliferation ,differentiation ,and apoptosis.Recent studies have shown that the Notch signaling pathway plays an im-portant role in the development and progression of liver fibrosis ,and blocking or activating the Notch signaling pathway can influence the pro-gression of liver fibrosis.This article reviews the research advances in the composition of the Notch signaling pathway ,the mechanism by which it is activated ,and its association with liver fibrosis.Key words :liver cirrhosis ;receptors ,Notch ;signal transduction ;reviewdoi :10.3969/j.issn.1001-5256.2018.01.039收稿日期:2017-08-07;修回日期:2017-09-27。
Notch Signaling Pathway
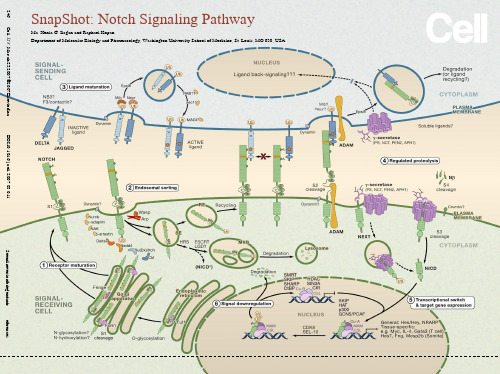
SnapShot: Notch Signaling Pathway Ma. Xenia G. Ilagan and Raphael Kopan Department of Molecular Biology and Pharmacology, Washington University School of Medicine, St. Louis, MO 630, USASeeonlineversionforlegendandreferences.246Cell128,March23,2007©2007ElsevierInc.DOI10.1016/j.cell.2007.03.011SnapShot:Notch Signaling PathwayMa. Xenia G. Ilagan and Raphael KopanDepartment of Molecular Biology and Pharmacology, Washington University School of Medicine, St. Louis, MO 63110, USAThe Notch signaling pathway is a short-range communication transducer that is involved in regulating many cellular processes (proliferation, stem cell and stem cell niche maintenance, cell fate specification, differentiation, and cell death) during development and renewal of adult tissues. Notch signaling is mediated by proteolysis and does not appear to involve any secondary messengers. However, depending on cellular context, the amplitude and timing of Notch activity can be regulated by posttranslational modifications to ligands and receptors and their trafficking.(1) Receptor MaturationNotch receptors are large single pass type I transmembrane proteins. Upon translation, the Notch protein is fucosylated by the chaperone O-fut, a modification essential for the production of a functional receptor. In cells expressing Fringe, the fucose is extended by the glycosyltransferase activity of Fringe, altering the ability of specific ligands to activate Notch (see below). The mature receptor undergoes proteolytic cleavage by protein convertases (PC5; Furin) at site 1 (S1) and then is targeted to the cell surface as a heterodimer held together by noncovalent interactions.(2) Endosomal SortingSeveral mechanisms control the steady-state levels of the Notch receptor at the cell surface and therefore regulate its availability for binding of ligand. For example, Numb, in cooperation with the AP2 component α-adaptin and AP2- or Numb-associated kinase (NAK), can promote Notch endocytosis and degradation. Several E3 ubiquitin ligases—Deltex, Nedd4, Su(Dx)/Itch, Cbl—target Notch, shifting receptor trafficking toward degradation or recycling. Other proteins prevent inappropriate receptor activation in the absence of ligand binding. Mutations in certain ESCRT complex proteins lead to accumulation of Notch in endosomal vesicles, which surprisingly permits ectopic activation of Notch via γ-secretase-dependent proteolysis (NICD*). Another protein, Lethal Giant Discs (LGD), is also required to maintain Notch in the OFF state. Therefore, ESCRT and LGD complexes are normally involved in Notch downregulation, indicating that endosomal sorting is a key way to restrict activation of Notch to the cell surface and that defects in endosomal sorting may contribute to pathogenesis. SE, sorting endosome; RE, recycling endosome; MVB, multivesicular body.(3) Ligand MaturationNotch ligands are also type I transmembrane proteins characterized by an N-terminal DSL domain. The two major classes of ligands are Delta and Jagged (Serrate in Drosophila), the latter containing a cysteine-rich domain. In addition to Delta and Jagged, the neural adhesion molecule F3/contactin, the related NB-3 protein, the EGF repeat protein DNER, and a diffusible protein in C. elegans have been identified as potential Notch ligands. Endocytic trafficking of the DSL ligands is crucial for enhancing their signaling activity: Ligands are ubiquitinated by the E3 ubiquitin ligases, Neur and Mib, triggering Epsin-mediated endocytosis; an undefined modification produces an active ligand that recycles to the cell surface in a Rab11-dependent process. Current models explaining the nature of ligand modification include ligand clustering, posttranslational modifications, and/or recycling into specific membrane domains.(4) Regulated ProteolysisThe Notch receptor is activated by binding to a ligand presented by a neighboring cell. Productive receptor-ligand interactions depend on the glycosylation state, for example, a Fringe-modified receptor may favor binding of Delta. Ligand endocytosis is thought to generate sufficient force to produce partial or complete domain dissociation, thereby exposing Notch to cleavage at site S2 by ADAM metalloproteases (perhaps following heterodimer dissociation at S1). The Notch extracellular domain is transendocytosed into the signal-sending cell, whereas the membrane-anchored NEXT (N otch ex tracellular t runcation) fragment is recognized by the inactive aminopeptidase domain of nicastrin (NCT), which transfers NEXT to the active site of γ-secretase, an enzymatic complex composed of presenilin (PS), NCT, PEN2, and APH1. γ-secretase then cleaves the Notch transmembrane domain sequentially starting near the cytosolic surface (sites S3 and S4) to release the Notch intracellular domain (NICD) and Nβ peptides, respectively. γ-secretase cleavage can occur at the cellsurface or in endosomal compartments, perhaps following monoubiquitination. The apical polarity protein Crumbs appears to play a role in restricting γ-secretase activity thereby limiting the extent of Notch activation. Like many type I proteins, Notch ligands are also subject to extracellular cleavage by ADAM proteases followed by transmembrane domain cleavage by γ-secretase. Ligand processing may be important to reduce its ability to antagonize Notch signaling in cis and for its downregulation and membrane clearance. Alternatively, it could generate biologically active fragments, e.g., soluble ligands (that may act as antagonists of Notch signaling) and/or ligand intracellular domain fragments.(5) Transcriptional Switch and Target Gene ExpressionIn the absence of NICD, the DNA-binding protein CSL associates with ubiquitous corepressor (Co-R) proteins and histone deacetylases (HDACs) to repress transcription of target genes. When NICD enters the nucleus, its binding to CSL may trigger an allosteric change that facilitates displacement of transcriptional repressors. The NICD/CSL interface is then recognized by Mastermind (MAM), and this tri-protein complex recruits coactivators (Co-A)—such as histone acetylases (HATs), chromatinremodeling factors, and a mediator complex— to assemble an active transcription complex on target promoters.(6) Signal DownregulationDuring transcriptional activation, NICD is phosphorylated on its PEST domain by kinases such as CDK8 and targeted for proteasomal degradation by E3 ubiquitin ligases such as Sel10/Fbw7. This terminates the Notch signal and resets the cell for the next round of signaling.ReferencesBray, S.J. (2006). Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678–689. Ehebauer, M., Hayward, P., and Martinez-Arias, A. (2006). Notch signaling pathway. Sci. STKE 2006, cm7. Haines, N., and Irvine, K.D. (2003). Glycosylation regulates Notch signalling. Nat. Rev. Mol. Cell Biol. 4,786–797.Kadesch, T. (2004). Notch signaling: the demise of elegant simplicity. Curr. Opin. Genet. Dev. 14, 506–512. Kovall, R.A. (2007). Structures of CSL, Notch and Mastermind proteins: piecing together an active transcription complex. Curr. Opin. Struct. Biol. 17, 117–127.Le Borgne, R. (2006). Regulation of Notch signalling by endocytosis and endosomal sorting. Curr. Opin. Cell Biol. 18, 213–222.Lubman, O.Y., Korolev, S.V., and Kopan, R. (2004). Anchoring notch genetics and biochemistry; structural analysis of the ankyrin domain sheds light on existing data.Mol. Cell 13, 619–626.Mumm, J.S., and Kopan, R. (2000). Notch signaling: from the outside in. Dev. Biol. 228, 151–165. Schweisguth, F. (2004). Regulation of notch signaling activity. Curr. Biol. 14, R129–R138.Wilkin, M.B., and Baron, M. (2005). Endocytic regulation of Notch activation and down-regulation (review). Mol. Membr. Biol. 22, 279–289.AcknowledgmentsM.X.G.I., R.K., and Washington University may receive income based on a license of Notch-related technology by the University to Merck. Merck did not support this work.1246.e1 Cell 128, March 23, 2007 .2007 Elsevier Inc. DOI 10.1016/j.cell.2007.03.011。
Notch Signaling in the Immune System

Notch Signaling in the Immune SystemFreddy Radtke,1,*Nicolas Fasnacht,1and H.Robson MacDonald21Ecole Polytechnique Fe´de´rale de Lausanne(EPFL),Swiss Institute for Experimental Cancer Research(ISREC),Station19,1015Lausanne,Switzerland2Ludwig Institute for Cancer Research,Lausanne Branch,University of Lausanne,1066Epalinges,Switzerland*Correspondence:freddy.radtke@epfl.chDOI10.1016/j.immuni.2010.01.004The Notch signaling pathway regulates many aspects of embryonic development,as well as differentiation processes and tissue homeostasis in multiple adult organ systems.Disregulation of Notch signaling is asso-ciated with several human disorders,including cancer.In the last decade,it became evident that Notch signaling plays important roles within the hematopoietic and immune systems.Notch plays an essential role in the development of embryonic hematopoietic stem cells and influences multiple lineage decisions of developing lymphoid and myeloid cells.Moreover,recent evidence suggests that Notch is an important modulator of T cell-mediated immune responses.In this review,we discuss Notch signaling in hematopoi-esis,lymphocyte development,and function as well as in T cell acute lymphoblastic leukemia.Overview of Notch SignalingThe Notch signaling cascade is highly conserved and found in organisms as diverse as worms and humans.In1917,the genet-icist Thomas Hunt Morgan and his colleagues described fruit flies with notches at the margins of their wing blades(Morgan, 1917).It turned out that this notched wing phenotype is the result of a partial loss of function of the Drosophila Notch gene,which was cloned in the mid eighties(Kidd et al.,1986;Wharton et al., 1985).Drosophila Notch encodes an unusual type I transmem-brane receptor that is activated by two different membrane-bound ligands called Delta and Serrate.Mammals posses four Notch receptors(Notch1–4)that are bound byfive ligands (Delta-like1,3,and4and Jagged1and2)(Figure1;Bray, 2006).The molecular and biochemical details of Notch signaling have recently been covered by excellent reviews(Gordon et al., 2008;Kopan and Ilagan,2009).In brief,newly synthesized Notch receptors are proteolytically cleaved in the Golgi(at site S1) during their transport to the cell surface by a furin-like protease. This cleavage generates a heterodimeric receptor consisting of an extracellular subunit(N EC)that is noncovalently linked to a second subunit containing the extracellular heterodimerization domain and the transmembrane domain followed by the cyto-plasmic region of the Notch receptor(NÔ).The extracellular part of the receptors contains29–36epidermal growth factor-like repeats involved in ligand binding,followed by three cysteine-rich LIN12repeats that prevent ligand-independent activation and a hydrophobic stretch of amino acids mediating heterodimerization between N EC and NÔ.The cytoplasmic tail of the receptor harbors multiple conserved elements including nuclear localization signals,as well as protein-protein interaction and transactivation domains.Notch signaling is initiated by ligand-receptor interaction between neighboring cells,leading to two successive proteolytic cleavages of the receptor.Thefirst is mediated by metallopro-teases of the ADAM family,which cleave the receptors12–13 amino acids external to the transmembrane domain(at site S2).The shedded extracellular domain is endocytosed by the ligand-expressing cell,a process that is dependent on monoubi-quitinylation of the cytoplasmic tail of the ligands by E3-ubiquitin ligases of the mind bomb and neuralized family.Ligand binding to N EC presumably induces a conformational change within the Notch receptors to expose the S2cleavage site for proteolysis. After shedding of the extracellular domain,a second cleavage within the transmembrane domain(at site S3)is mediated by the g-secretase activity of a multiprotein complex.This liberates the intracellular domain of Notch receptors(NICD),which subse-quently traffics to the nucleus and heterodimerizes with the DNA binding transcription factor CSL in order to form a short-lived nuclear transcription complex.The transcription factor CSL is also known as CBF-1in humans,Suppressor of hairless in Drosophila,Lag in Caenorhabditis elegans,and RBP-J in the mouse.Once bound to CSL,NICD recruits other coactivators including mastermind proteins(MAML1-3),which in turn recruit the MED8-mediator transcription activation complex in order to induce transcriptional expression of downstream target genes (Figure2).Members of the Hairy enhancer of split(Hes)or Hairy related(Hey or Hrt)genes have been identified as Notch target genes in many tissues,while other targets are more tissue restricted.Recent studies via genome-wide expression and chromatin immunoprecipitation(ChIP)arrays point to the exis-tence of a large number of genes that can be directly regulated by Notch(Palomero et al.,2006;Weng et al.,2006).The chal-lenge will now be to distinguish the drivers from the passengers among the large number of target genes.Moreover,there is emerging data suggesting that Notch can crosstalk to or coop-erate with other signaling pathways(including NF-k B,hypoxia, or TGF-b)and thereby broaden the spectrum of target genes that are influenced by Notch signaling(Poellinger and Lendahl, 2008;Samon et al.,2008).Notch signaling is regulated at multiple levels.For example, cell type-specific and spatial expression of ligands and Notch receptors can restrict signaling to a certain cell population or context.The ability of Jagged ligands to trigger Notch receptor-mediated signaling is dependent on the glycosylation status of the extracellular domain of Notch.Fringe proteins are glycosyl transferases that add N-Acetylglucosamine to O-fucose residues present within certain epidermal growth factor-like repeats of the receptors(Haines and Irvine,2003).Notch14Immunity32,January29,2010ª2010Elsevier Inc.receptors carrying these additional sugar moieties preferentially signal via Delta ligands,while Jagged-mediated Notch signaling is inhibited.Another level of regulation is to ensure that a Notch signal is short lived.Notch receptors carry a PEST domain at the very C terminus that is responsible for rapid turnover of the acti-vated NICD via E3-ubiquitin ligase (including Fbw7)-mediated proteosomal degradation (Figure 2;O’Neil et al.,2007;Thomp-son et al.,2007).Notch in Hematopoietic Stem Cell Development and HomeostasisThe blood system originates from different sites during embry-onic development and is generally closely associated with vas-culogenesis.The most primitive hematopoietic cells are found within the extraembryonic yolk sac before hematopoiesis shifts to intraembryonic sites including the para-aortic splanchno-pleiura and aorta-gonad mesonephros (P-sP and AGM).Later hematopoiesis occurs in the fetal liver before it is finally estab-lished in the bone marrow (Godin and Cumano,2002).The first hematopoietic stem cells capable of long-term repopulation of all blood lineages upon transplantation are found within the AGM region.These cells are generated from a bipotent heman-gioblast by budding off from the dorsal aorta of midgestation embryos (de Bruijn et al.,2002).Germline mutant embryos defi-cient for Notch1or RBP-J have been shown not to generate in-traembryonic HSCs,whereas yolk sac hematopoiesis of these mutant mice was unperturbed (Kumano et al.,2003;Robert-Moreno et al.,2005).These studies led to the suggestion that Notch signaling is important for definitive but not primitive hema-topoiesis.However,Notch signaling is also important for arterial cell fate specification in developing blood vessels.Hence,these mutant embryos displayed severe vasculogenic defects charac-terized by the loss of arterial cell fate (Krebs et al.,2004).There-fore,it was not clear whether the inability to generate intraem-bryonic HSC is a cell-autonomous defect of hemangioblasts or simply a secondary effect resulting from the absence of arteries.This uncertainty was recently resolved by studies analyzing germline mutant mice for the Jagged ligand family.Jagged1but not Jagged2null embryos failed to generate hematopoietic cells in the AGM,without losing the arterial cell fate (Robert-Moreno et al.,2008).Moreover,the same study linked Jagged1-mediated Notch signaling to GATA2and Runx1expression,two important transcription factors for hema-topoiesis.These observations were important because they were the first studies showing that Notch signaling is directly associated with the generation of hematopoietic cells indepen-dently of its role in arterial development.Thus,Notch signaling is indeed essential for definitive hematopoiesis in the developing embryo.Whether Notch signaling plays a similar role during the gener-ation or maintenance of HSC in the adult bone marrow compart-ment was debated for several years.Jagged1was suggested to be part of the HSC stem cell niche,because osteoblast-specific expression of the parathyroid hormone-related protein receptor (PTHRP)resulted in increased numbers of Jagged1-expressing osteoblasts,which correlated with increased numbers of HSCs.This result led to the suggestion that Jagged1-mediated Notch signaling might regulate HSC homeostasis (Calvi et al.,2003).Moreover,multiple gain-of-function studies support a role for Notch in HSC maintenance.Overexpression of N1-ICD or its downstream target gene Hes1in bone marrow pro-genitors resulted in increased HSC numbers and/or enhanced self-renewal (Kunisato et al.,2003;Stier et al.,2002).Coculture experiments of murine hematopoietic progenitor cells with im-mobilized Notch ligands promoted early T cell differentiation and generation of multilog increases in the number of hemato-poietic progenitor cells with short-term lymphoid and myeloid re-populating activity (Varnum-Finney et al.,2003).The dose of Notch signaling determines the in vitro process of hematopoietic progenitor cell expansion versus B and/or T cell differentiation.Coculture of hematopoietic progenitor cells in the presence of high densities of Notch ligands increases the propensity todriveFigure 1.Notch Ligands and ReceptorsTo date,five conventional Notch ligands are known:Jagged1(J1),Jagged2(J2),Delta-like1(Dll1),Delta-like3(Dll3),and Delta-like4(Dll4).A common structural feature of all ligands is an amino-terminal domain called DSL (Delta,Serrate,and Lag-2)involved in receptor binding followed by EGF-like repeats.A cysteine-rich domain (CR)is located downstream of the EGF-like repeats of J1and J2close to the plasma membrane (PM).Vertebrates have four Notch receptors (Notch1–Notch4;N1–N4).The extracellular domain of the receptors contains EGF-like repeats (36in N1and N2,34in N3,and 29in N4)followed by three cysteine-rich LIN domains that prevent ligand-independent activation and the heterodimerization domain (HD).The cytoplasmic domain contains a RAM domain followed by six ankyrin repeats (ANK)that bind to the CSL tran-scription factor,two nuclear localization signals (NLS),a transactivation domain (TAD;present in N1and N2),and a PEST sequence involved in regu-lating protein stability.Immunity 32,January 29,2010ª2010Elsevier Inc.15differentiation toward the T cell lineage (Dallas et al.,2005).In particular,the finding that human umbilical cord blood cells (UCB)could also be expanded ex vivo when cocultured with Delta-like1-IgG fusion proteins and that these cells showed a marked increase (approximately 15-fold)in repopulating cell frequency in xenotransplantation assays (Delaney et al.,2005)may be exploited for clinical purposes (Bernstein et al.,2008).Although it is very encouraging that Notch ligands are currently used to expand murine and human hematopoietic progenitors,there is limited evidence that Notch can be used to expand long-term HSCs.Thus,the question remains whether this is a physiological role of Notch signaling.This has been addressed by analyzing several conditional gene-targeted mice for different components of the Notch pathway.Mice lacking Notch1or Jagged1or both did not reveal any defects in HSC maintenance or in the capacity to repopulate the hematopoietic compartment after transplantation (Mancini et al.,2005;Radtke et al.,1999).These results do not exclude the possibility that other Notch receptors or ligands might functionally compensate for the loss of Notch1and/or Jagged1.Two complementary approaches were recently used to block canonical Notch signaling.The first used a dominant-negative form of the Mastermind-like protein,which inhibits the formation of a functional Notch transactivation complex in HSCs and bone marrow (BM)progenitors,whereas the second inactivated the Rbp-j gene within HSCs.These experimental approaches block Notch signaling independently of Notch receptor or ligand usage.Notch signaling-deprived progenitors did not reveal any HSC defects;they showed normal long-term reconstitution even in secondary competitive trans-plantation assays (Maillard et al.,2008).Taken together,these experiments show that canonical Notch signaling is dispensable for HSC homeostasis in the bone marrow.Moreover,the identi-fication of the proto-oncogene LRF (Leukemia/lymphoma Related Factor,encoded by the Zbtb7a gene and also known as Pokemon )as a negative regulator of Notch signaling in BM progenitors indicates that Notch signaling must be repressedor under very stringent control in HSCs in order to prevent ectopic T cell differentiation in the BM (Maeda et al.,2007).How LRF represses Notch signaling in HSC or progenitor cells is currently unknown.Notch in T Cell DevelopmentThe essential role of Notch signaling during thymic T cell lineage commitment and maturation is the best-studied function of Notch in hematopoiesis.Via the blood stream,BM progenitors constantly seed the thymus,where they adopt a T cell fate and further differentiate into mature ab and gd T cells before emigrating to the periphery.Multiple genetic loss-and gain-of-function studies highlight the importance of Notch1for T cell lineage commitment.Inducible inactivation of Notch1or Rbp-j results in a block in T cell development accompanied by the accu-mulation of ectopic B cells in the thymus (Han et al.,2002;Radtke et al.,1999).These results were initially interpreted to mean that canonical Notch1signaling instructs a bipotent early thymic progenitor to adopt a T cell as opposed to a B cell fate because no other myeloid or lymphoid lineages were affected.Neverthe-less,recent loss of Notch1function combined with lineage tracing experiments reveal that the inhibitory functions of Notch1are broader.Notch1inhibits multiple cell fate potentials of thymus-seeding cells including myeloid and B cells,as well as conventional and plasmacytoid dendritic cell potential (both in a cell-intrinsic and -extrinsic manner)and thereby ensures effi-cient T cell lineage commitment (Bell and Bhandoola,2008;Feyerabend et al.,2009;Wada et al.,2008).Similarly,interference with Notch signaling by transgenic expression of Notch modula-tors (including Fringe,Deltex1,or Nrarp)or dominant-negative forms of the transcriptional coactivator MAML-1also blocks T cell development concomitant with B lymphopoiesis in the thymus (Izon et al.,2002;Koch et al.,2001;Maillard et al.,2004;Yun and Bevan,2003).Reciprocal gain-of-function studies involving overexpressing N1-ICD in BM progenitors result in ectopic T cell development at the expense of B cell developmentGolgiFurin clevage at S1ADAM clevage at S2γ-secretase clevage at S3NotchNotchGlycosylation by FringeHDNICDNotch ligandCorepressorCoactivators (e.g. MAML)Polyubiquitination and proteosomal degradationCSLFigure 2.Notch SignalingNotch proteins are synthesized as single precursor proteins,which are cleaved in the Golgi by a Furin-like convertase at site S1.Cleavage at S1generates two subunits held together non-covalently by the N-and C-terminal subunits of the heterodimerization domains (HD).EGF-like repeats are glycosylated by Fringe proteins in the Golgi before receptors are transported to the cell surface.Notch signaling is initiated by ligand receptor interaction,which induces a second cleavage at site S2(close to the transmembrane domain)mediated by ADAM-type metallopro-teases followed by a third cleavage at S3within the transmembrane domain mediated by the g -secretase activity of a multiprotein complex containing presenilins.This last proteolytic cleavage liberates the cytoplasmic domain of Notch receptors (NICD),which translocate to the nucleus and bind to the transcription factor CSL (CBF1,Suppressor of hairless,and Lag-1),con-verting it from a transcriptional repressor into a transcriptional activator by recruiting coactiva-tors including mastermind-like proteins (MAML).NICD is polyubiquitinated by E3ubiquitin ligases (including Fbw7),which marks NICD for proteoso-mal degradation.16Immunity 32,January 29,2010ª2010Elsevier Inc.in the BM(Pui et al.,1999).Taken together,these results demon-strate that Notch1is the key receptor expressed on thymus-seeding cells responsible for T cell lineage commitment.The question of the ligand(s)required for this process was recently addressed.Historically,Dll1and somewhat later Dll4 have been favored as potential Notch1ligands for T cell fate specification based on their capacity to support complete devel-opment of mature T cells from BM precursors in vitro(Hozumi et al.,2004;Jaleco et al.,2001;Schmitt and Zu´n˜iga-Pflu¨cker, 2002).Nevertheless,inactivation of Dll4but not Dll1in thymic epithelial cells(TECs)resulted in a complete block in T cell devel-opment accompanied by ectopic B cell development within the thymus,which phenocopies mice with loss of Notch1function in BM progenitors(Hozumi et al.,2008;Koch et al.,2008).These results demonstrate an essential interaction between Dll4-ex-pressing TECs and thymus-seeding Notch1-expressing hema-topoietic progenitors for T lineage commitment.Previous studies of the thymic epithelium of gene-targeted mice in which T cell development is arrested at early developmental stages showed that the thymocyte progenitors also influence TEC maturation and function.Thus,lymphostromal interactions between devel-oping thymocytes and TECs are bidirectional,a concept known as‘‘thymus crosstalk’’(van Ewijk et al.,1994).In this context, a recent report showed that maturation of thymocytes to the CD4+CD8+stage induced downregulation of Dll4on cortical TECs suggesting a negative-feedback loop between developing thymocytes and cortical TECs(Fiorini et al.,2008).This coincides with the maturation and the ability of medullary TECs to mediate positive and negative selection,a trait that is acquired in a thymo-cyte-dependent manner(Alves et al.,2009).How and whether downregulation of Dll4on cortical TECs is essential to allow posi-tive and/or negative selection remains an open question.Once the T cell lineage has been specified,developing thymo-cytes must choose between the ab and gd T cell lineage.gd T cell development is mostly driven by the successful rearrangement of T cell receptor g(TCR-g)and TCR-d genes and appears to be Notch independent(Ciofani et al.,2006;Wolfer et al.,2002). Interestingly,the helix-loop-helix protein Id3can induce promo-tion of the gd T cell fate as well as rendering gd T cell maturation independent of Notch signaling(Lauritsen et al.,2009).In con-trast,ab T cell development requires continuous Notch signaling up to the DN3stage,where cells have to pass a critical check-point known as b-selection(Wolfer et al.,2002).Although it is well established that signaling via the pre-TCR (consisting of productively rearranged TCR-b chain associated with CD3components and an invariant pT a chain)is essential for b-selection and further thymocyte development,in vitro experiments suggest that successful transition through this checkpoint requires cooperative signaling of both Notch and pre-TCR(Ciofani et al.,2006).This leads to the question of how this functional cooperativity is established at the molecular level and whether Notch and pre-TCR signaling influence each other. Loss-of-function experiments for both Notch signaling and components of the pre-TCR highlight the essential role of each individual signaling pathway during thymocyte development. For example,the consequences of a loss of Notch signaling in vivo(via a dominant-negative MAML-1)in immature thymo-cytes prior to the b-selection checkpoint cannot be overcome by TCR-b or TCR-ab transgenes,suggesting that the require-ment for early Notch signaling is absolute and independent of the pre-TCR(Maillard et al.,2006).Similarly,RAG2-deficient thymocytes(which lack a pre-TCR because of the inability to re-arrange a functional TCR-b chain)cannot progress to the DP stage even if they receive a Notch signal(Allman et al.,2001). Nevertheless,successful transition through b-selection requires the cooperative action of both Notch and the pre-TCR.As thymo-cytes pass through b-selection,Notch assures survival by regu-lating glucose metabolism(Ciofani and Zu´n˜iga-Pflu¨cker,2005). Moreover,transcriptional reporter assays combined with ChIP experiments suggest a direct crosstalk between Notch and pre-TCR because Notch1and/or Notch3(which is a Notch1 target gene)can directly activate the transcription of the pT a gene(Bellavia et al.,2007;Reizis and Leder,2002).Additional, indirect regulation of pT a gene expression by Notch involves Notch3and Ikaros.Ikaros functions as a transcriptional repressor and recognizes the same DNA binding sites as Rbp-j.Thus, Ikaros and Rbp-j can potentially compete for the same DNA binding site,a process that has been shown to be important during T cell leukemogenesis(Beverly and Capobianco,2003; Dumortier et al.,2006).Notch3activation results in the expres-sion of HuD,a RNA binding protein that can trigger the generation of non-DNA binding Ikaros isoforms through alternative splicing. These isoforms competitively block the activity of full-length Ikaros and thereby facilitate the upregulation of pT a directly through Notch-Rbp-j-mediated transcriptional complexes(Bel-lavia et al.,2007).Thus,Notch1signaling directly and indirectly participates in the generation of the pre-TCR.This leads to the question of how Notch1itself is regulated during thymocyte development.Notch1(in an autoregulatory loop)together with the transcription factor E2A directly contrib-utes to the progressive increase of Notch1expression at the earliest stages of thymocyte development,prior to the b-selec-tion stage.Thymocytes that successfully pass b-selection imme-diately downregulate the expression of Notch1(Taghon et al., 2006;Yashiro-Ohtani et al.,2009).This process is driven via the pre-TCR-mediated induction of the HLH transcription factor Id3.Id3is an inhibitor of E-proteins and as such inhibits E2A-dependent activation of Notch1transcription,leading to a decrease in Notch1mRNA.Taken together,Notch1-mediated signaling is necessary for assembling a functional pre-TCR and as soon as thymocytes pass b-selection,the pre-TCR ensures the transcriptional repression of Notch1,a mechanism that is presumably essential to avoid the oncogenic properties of Notch signaling and its targets(see below)(Weng et al.,2006).Interest-ingly,the abrupt downregulation of Notch1transcription after b-selection is not reflected at the protein level.Surface expression of Notch1receptor remains at equally high amounts from DN3 stage until the ISP stage and decreases only subsequently in DP thymocytes(Fiorini et al.,2009).These results suggest that the decrease in Notch1transcription and Notch1target gene expression after b-selection may occur independently of the regulation of Notch1surface expression.Thus,it is conceivable that downregulation of Notch1target genes is not simply the result of absence of Notch signaling resulting from lack of Notch1surface expression,but may also implicate additional repressive mechanisms.Interestingly,Ikaros is implicated in the negative regulation of the Notch target gene Hes1in thymo-cytes that successfully passed b-selection.DN4thymocytes Immunity32,January29,2010ª2010Elsevier Inc.17thereby lose their capacity to transcribe Hes1in response to Notch signaling.This event correlates with epigenetic silencing of the Hes1locus,suggesting that Ikaros might help to shut down Notch target genes once thymocytes passed b-selection (Kleinmann et al.,2008).Notch in Marginal Zone B Cell DevelopmentA second well-characterized role for Notch signaling in the lymphoid system involves the specification of marginal zone (MZ)versus follicularB cell fate in the spleen.Mature splenic B cells are comprised of two principal subsets,follicular B cells and MZ B cells(Pillai and Cariappa,2009).Follicular B cells,which are the most abundant subset,are recirculating cells that home to B cell follicles and participate in T cell-dependent immune responses to protein antigens.In contrast,MZB cells are not re-circulating and localize in the outer region of the splenic white pulp between the marginal sinus and the red pulp.MZB cells provide an important line of defense against blood-borne patho-gens by mounting T cell-independent antibody responses.In addition,MZB cells express high amounts of CD1d,which allows them to capture lipid antigens from the circulation and present them to CD1d-restricted V a14invariant natural killer T cells.In some respects,MZB cells can thus be considered to be an ‘‘innate-like’’population because they exhibit a constitutively activated phenotype similar to NK cells,NKT cells,and gd T cells. Both MZB cells and follicular B cells in the spleen are derived from B lineage progenitors in the BM.During development, immature B cells that productively rearrange heavy-and light-chain immunoglobulin genes express a B cell receptor(BCR) at the cell surface.Similar to T cells,immature B cells that express strongly self-reactive BCR undergo clonal deletion or receptor editing.Further B cell maturation proceeds through transient transitional stages(T1and T2),ultimately leading to the differentiation of mature follicular or MZB cells in the spleen. The specification of splenic follicular versus MZB cell fate from immature T2B cells is determined by several factors and has been reviewed in detail recently(Pillai and Cariappa,2009).In this section,we will concentrate on Notch signaling,which has a critical and nonredundant role in specifying MZB cell fate.It is now widely accepted that MZB cell fate specification in the spleen depends upon nonredundant interaction between Notch2and Dll1.Thus mice conditionally deficient in either Notch2or Dll1have greatly reduced numbers of MZB cells(Ho-zumi et al.,2004;Saito et al.,2003).Further evidence supporting a requirement for Notch signaling in MZB cell development comes from the analysis of mice deficient in other components of the Notch signaling pathway such as Rbp-j(Tanigaki et al., 2002)or MAML1(Oyama et al.,2007;Wu et al.,2007),which also fail to generate MZB cells.In reciprocal experiments,dele-tion of MINT(a negative regulator of Notch signaling)led to an increase in splenic MZB cells(Kuroda et al.,2003).Collectively these loss-of-function experiments provide compelling evidence that the strength of signaling via Notch2:Dll1interactions controls the rate of development of MZB cells.Although it has been known for some time that Dll1is the rele-vant ligand of Notch2in MZB cell development,the identity of Dll1-expressing cells in the spleen remained elusive.Nonhema-topoietic cells(Hozumi et al.,2004),and in particular endothelial cells located in the red pulp and MZ of the spleen(Tan et al.,2009),selectively express Dll1.It seems likely that endocytosis of Dll1by these ligand-expressing endothelial cells may be required for efficient signaling via Notch2on MZB cells or their precursors,because deletion of Mindbomb1(Mib1),an E3 ligase known to regulate Dll1endocytosis,phenocopies condi-tional Notch2and Dll1mutant mice(Song et al.,2008). Another modulator of Notch signaling that plays an important role in MZB cell development is the Fringe family of glycosyl-transferases.As discussed earlier,Fringe can enhance interac-tions of Notch receptors and Dll ligands by adding N-acetyl glucosamine to O-linked fucose residues on Notch.Two members of the Fringe family(Lunatic fringe and Manic fringe) function cooperatively to strengthen the presumably weak inter-action between Notch2on MZB cells(or their precursors)and Dll1-expressing endothelial cells in splenic niches,thereby promoting development of the MZB cell lineage(Tan et al., 2009).According to this scenario,MZB cell homeostasis depends upon Fringe-regulated competition between Notch2-expressing precursor cells for access to Dll1ligands.Notch in Other Hematopoietic LineagesAlthough much attention has been focused on the controversial role of Notch signaling in HSC homeostasis and differentiation, Notch may also play a role in other cell fate decisions within the hematopoietic system.Thus megakaryocyte development is enhanced in vitro when BM precursors are cocultured with Dll1-expressing OP9stromal cells(Mercher et al.,2008).In recip-rocal experiments,inhibition of canonical Notch signaling in vivo by a dominant-negative MAML1decreased megakaryocyte numbers,but(somewhat surprisingly)did not affect platelet counts,even after challenge with5-Fluorouracil(5-FU)(Mercher et al.,2008).Further studies will be needed to identify the phys-iologically relevant Notch receptors and ligands implicated in the development of the megakaryocyte lineage in vivo.Dendritic cells(DCs)appear to be another hematopoietic lineage that is influenced by Notch signaling during develop-ment.Several in vitro systems have demonstrated that Dll1-mediated Notch signaling promotes the development of either plasmacytoid or conventional DCs at the expense of other line-ages such as T cells or macrophages(Ohishi et al.,2001;Olivier et al.,2006).Moreover,differentiation of ES cells or BM progen-itors into DCs in vitro was shown to depend upon Notch signaling via downstream activation of the Wnt pathway(Zhou et al., 2009).More compellingly,loss of function of canonical Notch signaling via Rbp-j inactivation specifically in the DC lineage led to a selective impairment of the splenic CD8ÀDC subset (Caton et al.,2007).Intriguingly,CD8ÀDCs in the spleen were found adjacent to unidentified Dll1-expressing cells in the mar-ginal zone,raising the possibility that CD8ÀDCs may compete with MZB cells for Notch signals during development(see previous section).In a genetic fate mapping model,inactivation of Notch1in early intrathymic precursors revealed their potential to develop into DCs in the thymus(Feyerabend et al.,2009). Taken together,these experiments indicate that at least some aspects of DC development may depend upon Notch signaling. Notch in Peripheral T Cell Differentiation and Function Once T cells leave the thymus,they migrate to the periphery, where they orchestrate immunity against different pathogens.18Immunity32,January29,2010ª2010Elsevier Inc.。
EnMT

2008 Nov;135(21):3611-22. Epub 2008 Oct 2.LinksZhang J, Lin Y, Zhang Y, Lan Y, Lin C, Moon AM, Schwartz RJ, Martin JF, Wang F.Center for Cancer and Stem Cell Biology, Institute of Biosciences and Technology, Texas A&M Health Science Center, 2121 W. Holcombe Boulevard, Houston, TX 77030, USA.The cardiac outflow tract (OFT) is a developmentally complex structure derived from multiple lineages and is often defective in human congenital anomalies. Although emerging evidence shows that fibroblast growth factor (FGF) is essential for OFT development, the downstream pathways mediating FGF signaling in cardiac progenitors remain poorly understood. Here, we report that FRS2alpha (FRS2), an adaptor protein that links FGF receptor kinases to multiple signaling pathways, mediates crucial aspects of FGF-dependent OFT development in mouse. Ablation of Frs2alpha in mesodermal OFT progenitor cells that originate in the second heart field (SHF) affects their expansion into the OFT myocardium, resulting in OFT misalignment and hypoplasia. Moreover, Frs2alpha mutants have defective endothelial-to-mesenchymal transition and neural crest cell recruitment into the OFT cushions, resulting in OFT septation defects. These results provide new insight into the signaling molecules downstream of FGF receptor tyrosine kinases in cardiac progenitors.PMID: 18832393 [PubMed - in process]2008 Jul 28;182(2):315-25.LinksNiessen K, Fu Y, Chang L, Hoodless PA, McFadden D, Karsan A. Department of Medical Biophysics, British Columbia Cancer Agency, Vancouver V5Z 1L3, Canada.Snail family proteins are key regulators of epithelial-mesenchymal transition, but their role in endothelial-to-mesenchymal transition (EMT) is less well studied. We show that Slug, a Snail family member, is expressedby a subset of endothelial cells as well as mesenchymal cells of the atrioventricular canal and outflow tract during cardiac cushion morphogenesis. Slug deficiency results in impaired cellularization of the cardiac cushion at embryonic day (E)-9.5 but is compensated by increased Snail expression at E10.5, which restores cardiac cushion EMT. We further demonstrate that Slug, but not Snail, is directly up-regulated by Notch in endothelial cells and that Slug expression is required for Notch-mediated repression of the vascular endothelial cadherin promoter and for promoting migration of transformed endothelial cells. In contrast, transforming growth factor beta (TGF-beta) induces Snail but not Slug. Interestingly, activation of Notch in the context of TGF-beta stimulation results in synergisticup-regulation of Snail in endothelial cells. Collectively, our data suggest that combined expression of Slug and Snail is required for EMT in cardiac cushion morphogenesis.PMID: 18663143 [PubMed - indexed for MEDLINE]Potenta S, Zeisberg E, Kalluri R.[1] 1Division of Matrix Biology, Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, USA [2] 2Department of Cell Biology, Harvard Medical School, Boston, MA, USA.Recent evidence has demonstrated that endothelial-to-mesenchymal transition (EndMT) may have a significant role in a number of diseases. Although EndMT has been previously studied as a critical process in heart development, it is now clear that EndMT can also occur postnatally in various pathologic settings, including cancer and cardiac fibrosis. During EndMT, resident endothelial cells delaminate from an organised cell layer and acquire a mesenchymal phenotype characterised by loss of cell-cell junctions, loss of endothelial markers, gain of mesenchymal markers, and acquisition of invasive and migratory properties.Endothelial-to-mesenchymal transition -derived cells are believed to function as fibroblasts in damaged tissue, and may therefore have an important role in tissue remodelling and fibrosis. In tumours, EndMT is an important source of cancer-associated fibroblasts (CAFs), which are known to facilitate tumour progression in several ways. These new findings suggest that targeting EndMT may be a novel therapeutic strategy, which is broadly applicable not only to cancer but also to various other disease states.British Journal of Cancer advance online publication, 16 September 2008;doi:10.1038/sj.bjc.6604662 .PMID: 18797460 [PubMed - as supplied by publisher]2008 Jul;24(4):462-8.LinksRieder F, Fiocchi C.Department of Internal Medicine I, University of Regensburg, Regensburg, Germany.PURPOSE OF REVIEW: Intestinal fibrosis is a potentially serious complication of inflammatory bowel disease and its pathophysiology is still unclear. This review will discuss recent developments relating to sources of fibroblasts in intestinal inflammation, mediators that modulate fibroblast activation and function, as well as new clinical, laboratory, endoscopic and radiological studies aimed at improving diagnosis and management of intestinal fibrosis in inflammatory bowel disease. RECENT FINDINGS: The fibroblast remains the central cell responsible for intestinal fibrosis in inflammatory bowel disease and transforming growth factor-beta1 is still the most potent pro-fibrogenic cytokine. Novel mediators, however, are being identified that modulate fibroblast function, such as interleukin-13, interleukin-21, galectin-3, osteopontin, Wnt and toll-like receptor ligands, and anti-tumor necrosis factor-alpha agents. New fibroblast sources are being identified, such as fibrocytes, and new mechanisms of fibroblast generation, like epithelial- and endothelial-to-mesenchymal transition. Animal models of intestinal fibrosis are still few, but new ways to induce gut fibrosis are being explored. Serological markers indicating a clinically complicated course that includes intestinal fibrosis are promising and are being tested in adult and pediatric populations, particularly in Crohn's disease. Video capsule endoscopy, the Given Patency capsule, double balloon enteroscopy, and computed tomographic enteroscopy are some of the new modalities being developed to assess the risk and improve the diagnosis of intestinal fibrosis. Novel therapeutic approaches include endoscopic balloon dilatation with conventional and double balloon enteroscopy, and local injection of glucocorticoids and tumor necrosis factor-alpha blockers, showing partial but encouraging success. SUMMARY: More studies are needed to improve knowledge of the pathophysiology of intestinal fibrosis if better preventive, diagnostic and therapeutic measures are to be expected in the near future.PMID: 18622160 [PubMed - indexed for MEDLINE]Nat Med. 2007 Aug;13(8):952-61. Epub 2007 Jul 29.LinksZeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R.Division of Matrix Biology, Department of Medicine, Beth Israel Deaconess Medical Center & Harvard Medical School, Boston, Massachusetts 02215, USA.Cardiac fibrosis, associated with a decreased extent of microvasculature and with disruption of normal myocardial structures, results from excessive deposition of extracellular matrix, which is mediated by the recruitment of fibroblasts. The source of these fibroblasts is unclear and specificanti-fibrotic therapies are not currently available. Here we show that cardiac fibrosis is associated with the emergence of fibroblasts originating from endothelial cells, suggesting an endothelial-mesenchymal transition (EndMT) similar to events that occur during formation of the atrioventricular cushion in the embryonic heart. Transforming growth factor-beta1 (TGF-beta1) induced endothelial cells to undergo EndMT, whereas bone morphogenic protein 7 (BMP-7) preserved the endothelial phenotype. The systemic administration of recombinant human BMP-7 (rhBMP-7) significantly inhibited EndMT and the progression of cardiac fibrosis in mouse models of pressure overload and chronic allograft rejection. Our findings show that EndMT contributes to the progression of cardiac fibrosis and that rhBMP-7 can be used to inhibit EndMT and to intervene in the progression of chronic heart disease associated with fibrosis.PMID: 17660828 [PubMed - indexed for MEDLINE]2006 Jul;74(6):277-92.LinksArciniegas E, Neves YC, Carrillo LM.Servicio Autónomo Instituto de Biomedicina, Facultad de Medicina,Universidad Central de Venezuela, Apartado de correos 4043, Carmelitas,Caracas 1010, Venezuela. earciniegasbeta@Endothelial-to-mesenchymal transition (EndoMT) is a process throughwhich certain subsets of endothelial cells lose endothelial characteristicsand transform into mesenchymal or smooth muscle-like cells. Emergingevidence suggests that this process plays an important role during vasculardevelopment and in many vascular pathologies. As inepithelial-mesenchymal transition, EndoMT seems to progress through aseries of important steps whose interdependence and order are not clear, andthat some of them are regulated by soluble growth factors. Insulin-likegrowth factor II (IGFII), apart from being considered important in cancer,angiogenesis, and atherosclerotic lesions, is also considered as essential toembryonic development. Here, we report that addition of IGFII promotedthe EndoMT process in the presence of very low amounts of chicken serumto arrested primary embryonic aortic chicken endothelial cells attached tofibronectin (FN), gelatin, or native type I collagen. This was demonstratedby cell spreading, loss of cell-cell contacts, detachment, migration, andtransformation. These cellular events also occurred when IGFII was addedto medium containing vitronectin (VN). Additionally, we demonstrated thatthese proteins were present in the spontaneous intimal thickenings that areobserved at day 11-13 of chicken embryo development. We also show thatalterations in the distribution of VE-cadherin and beta-catenin occur afterIGFII and serum or VN stimulation, and propose that the via VN IGFIIeffects may be facilitated by interaction of the mannose-6-phosphate/IGFIIreceptor (M6P/IGFIIR) with the urokinase-type plasminogen activatorreceptor (uPAR) and its ligand (uPA). Collectively, these findings providethe first evidence for a potential role of the IGFII-VN complex during theEndoMT process. From our observations and previous studies, we postulatea working hypothesis supporting a fundamental role for these moleculesduring EndoMT.PMID: 16831197 [PubMed - indexed for MEDLINE]Rac regulates integrin-mediated endothelial cell adhesion and migration on laminin-8Hironobu Fujiwara a, b, Jianguo Gu a and Kiyotoshi Sekiguchi a, b, ,a Institute for Protein Research, Osaka University, Suita, Osaka 565-0871, Japanb Sekiguchi Biomatrix Signaling Project, ERATO, Japanese Science and Technology Corporation, Aichi Medical University, Nagakute-cho, Aichi480-1195, JapanReceived 6 February 2003;revised 1 July 2003.Available online 3 October 2003.AbstractBlood vessel formation requires endothelial cell interactions with the extracellular matrix through cell surface receptors, and signaling events that control endothelial cell adhesion, migration, and lumen formation. Laminin-8 (α4β1γ1) is present in all basement membranes of blood vessels in fetal and adult tissues, but despite its importance in vessel formation, its role in endothelial cell adhesion and migration remains undefined. We examined adhesion and migration of HMEC-1 human microvascular endothelial cells on laminin-8 with an emphasis on the integrin-mediated signaling events, as compared with those on laminin-10/11 and fibronectin. We found that laminin-8 was less potent in HMEC-1 cell adhesion than laminin-1, laminin-10/11, and fibronectin, and mediated cell adhesion through α6β1 integrin. Despite its weak cell-adhesive activity, laminin-8 was as potent as laminin-10/11 in promoting cell migration. Cells adhering to laminin-8 displayed streaks of thin actin filaments and formed lamellipodia at the leading edge of the cells, as observed with cells adhering to laminin-10/11, while cells on fibronectinshowed thick actin stress fibers and large focal adhesions. Pull-down assays of GTP-loaded Rho, Rac, and Cdc42 demonstrated that Rac, but not Rho or Cdc42, was preferentially activated on laminin-8 and laminin-10/11, when compared with fibronectin. Furthermore, a dominant-negative mutant of Rac suppressed cell spreading, lamellipodial formation, and migration on laminin-8, but not on fibronectin. These results, taken together, indicate that Rac is activated during endothelial cell adhesion to laminin-8, and is pivotal for α6β1 integrin-mediated cell spreading and migration on laminin-8.Author Keywords: Basement membrane; Laminin; Endothelial cell; Integrin; RacAbbreviations: FBS, fetal bovine serum; HUVECs, human umbilical vein endothelial cells; mAb, monoclonal antibody; PBS, phosphate-buffered saline; GST-RBD, a fusion protein of glutathione S-transferase to the Rho-binding domain of rhotekin; GST-CRIB, a fusion protein of glutathione S-transferase to the Cdc42/Rac-interactive-binding domain of PAK1; BSA, bovine serum albuminArticle Outline• Introduction• Materials and methods• Cell culture• Reagents and antibodies• Cell-adhesive proteins• Purification of laminin-8• SDS-PAGE and immunoblotting• Expression vectors• Cell spreading assay• Cell migration assay and microinjection• Immunofluorescence staining• Detection of GTP-loaded Rho, Rac, and Cdc42• Results• HMEC-1 cell adhesion to laminin-8• Laminin-8 stimulates HMEC-1 migration through α6β1 inTransdifferentiation of pulmonary arteriolar endothelial cells into smooth muscle-like cells regulated by myocardin involved in hypoxia-induced pulmonary vascular remodelling.P Zhu, L Huang, X Ge, F Yan, R Wu, and Q AoInt J Exp Pathol, December 1, 2006; 87(6): 463-74.AbstractF ull text via InfotrieveAlert me when citedF ind more like thisDepartment of Pathology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan City, China.Myocardin gene has been identified as a master regulator of smooth muscle cell differentiation. Smooth muscle cells play a critical role in the pathogenesis of hypoxia-induced pulmonary hypertension (PH) and pulmonary vascular remodelling (PVR). The purpose of this study was to investigate the change of myocardin gene expression in the pulmonary vessels of hypoxia-induced PH affected by Sildenafil treatment and the involvement of endothelial cells transdifferentiation into smooth muscle cells in the process of hypoxia-induced PH and PVR. Myocardin and relative markers were investigated in animal models and cultured endothelial cells. Mean pulmonary artery pressure (mPAP) was measured. Immunohistochemistry and immunofluorescence were used to show the expression of smooth muscle alpha-actin (SMA), in situ hybridization (ISH) and reverse transcription polymerase chain reaction (RT-PCR) were performed respectively to detect the myocardin and SMA expression at mRNA levels. Small interfering RNA (siRNA) induced suppression of myocardin in cultured cells. We confirmed that hypoxia induced the PH and PVR in rats. Sildenafil could attenuate thehypoxia-induced PH. We found that myocardin mRNA expression is upregulated significantly in the hypoxic pulmonary vessels and cultured cells but downregulated in PH with Sildenafil treatment. The porcine pulmonary artery endothelial cells (PAECs) transdifferentiate into smooth muscle-like cells in hypoxic culture while the transdifferentiation did not occur when SiRNA of myocardin was applied. Our results suggest that myocardin gene, as a marker of smooth muscle cell differentiation, was expressed in the pulmonary vessels in hypoxia-induced PH rats, which could be downregulated by Sildenafil treatment, as well as in hypoxic cultured endothelial cells. Hypoxia induced the transdifferentiation of endothelial cells of vessels into smooth muscle-like cells which was regulated by myocardin.Erratum in Int J Exp Pathol. 2007 Apr;88(2):127-8Publication Types。
Notch信号通路与血管发育

Notch信号通路与血管发育【关键词】血管形成; Notch信号; 血管发生血管发育是复杂的血管网络形成的过程,在个体发育、组织再生、肿瘤发生发展中发挥重要作用,因此具有重要的研究价值。
以往研究已经证明,血管发育与细胞因子、组织缺氧、基因调控等多种因素有关。
现就Notch信号通路在血管发育中的作用的研究进展作一综述。
1 Notch信号通路Notch信号通路是进化中高度保守的信号转导通路,其调控细胞增殖、分化和凋亡的功能涉及几乎所有组织和器官[1]。
哺乳动物中有4个notch基因,编码4种Notch受体(Notch1, 2, 3, 4)。
Notch前体蛋白经内质网O�惭以逄腔�转移酶(POFUT1)作用后,在高尔基体中被Furin蛋白酶裂解成两部分,二者通过非共价键相连,形成细胞表面的异二聚体受体。
胞外结构域(NECD)含29~36个EGF样重复序列(EGF��like repeats)和3个富含半胱氨酸的Notch/LIN��12重复序列(Notch/LIN��12 repeats),其中, EGF样重复序列是配体结合所必需的,而Notch/LIN��12重复序列与抑制配体非依赖的Notch信号活化有关。
胞内结构域(NICD)主要由核定位信号序列(NLS), 6个串联的富含天冬酰胺的锚蛋白重复序列(tandem ankyrin repeats)和羧基端的PEST序列组成,其中锚蛋白重复序列介导胞内结构域与下游信号分子结合, PEST序列有助于加速蛋白水解酶对NICD的降解。
目前在哺乳动物发现5种Notch配体,分别为Delta��like1、 3、 4(Dll1、 3、 4)和Jagged1、 2(与果蝇Serrate/Lag��2蛋白同源),亦可被共同称为DSL(Delta/Serrate/Lag��2)。
该配体的胞外部分由氨基端的DSL结构域和下游数目可变的EGF样重复序列构成, DSL结构域主要介导与受体的结合,该结构域的泛素化是Notch配体活化的关键步骤,这一过程需要E3泛素连接酶Mindbomb(Mib)的催化。
激活Notch信号通路对人滋养细胞氧化应激损伤的影响及机制

激活Notch信号通路对人滋养细胞氧化应激损伤的影响及机制任艳芳;姜永杰;张秀玲;姜姗;王玉红【摘要】目的观察激活Notch信号通路对滋养细胞氧化应激损伤的影响, 并探讨其作用机制.方法将人早孕期绒毛外滋养细胞HTR8/SVneo分为正常对照组、缺氧/复氧(H/R) 组和rh NF-κB+H/R组.正常对照组常规培养;H/R组进行H/R处理, 先缺氧培养8 h, 再常规培养16 h, 2个循环, 共48 h;rh NF-κB+H/R组先加入1 gsu/m L的Notch信号通路激活剂rh NF-κB孵育2 h后, 再进行H/R处理.采用流式细胞仪检测细胞凋亡率和活性氧簇(ROS) 水平, Western blotting法检测各组细胞中Notch1、发状分裂相关增强子1(Hes1) 、可溶性血管内皮生长因子受体1(s Flt-1) 、血管内皮生长因子(VEGF) 蛋白表达.结果 H/R组细胞凋亡率、ROS水平及s Flt-1、VEGF蛋白表达高于正常对照组, Notch1、Hes1蛋白表达低于正常对照组(P均<0.01);rh NF-κB+H/R组细胞凋亡率、ROS水平及s Flt-1、VEGF蛋白表达低于H/R组, Notch1、Hes1蛋白表达高于H/R组(P均<0.01) .结论激活Notch信号通路可降低氧化应激导致的滋养细胞凋亡, 其机制可能与降低细胞ROS水平、s Flt-1、VEGF的表达而发挥细胞保护作用有关.%Objective To investigate the role of Notch signaling pathway in oxidative stress of human trophoblast cells of preeclampsia and its mechanism. Methods HTR8/SVneo cells were divided into the normal control group,hypoxia/reoxygenation (H/R) treatment group, and recombinant human neurotrophic factor (rh NF) -κB + H/R treatment group.The normal control group was cultured routinely; the H/R group was cultured under hypoxia for 8 h, followed by routine culture for 16 h, two cycles for 48 h; the rh NF-κB + H/R group was first incubated with 1 gsu/m L the NOTCH signal pathway activator rh NF-κB for 2 h, and then treated with H/R. Flow cytometry was used to detect the apoptotic rate and reactive oxygen species (ROS) level. Western blotting was used to detect the expression of Notch1, hes1, soluble vascular endothelial growth factor receptor (s Flt) -1, and vascular endothelial growth factor (VEGF) in each group. Results The apoptotic rate, ROS level, s Flt-1, and VEGF protein expression in the H/R group were higher than those in the normal control group, while the protein expression of Notch1 and Hes1 was lower than that in the normal control group (all P <0.01); the apoptotic rate, ROS level, s Flt-1, and VEGF protein expression in the rh NF-κB + H/R group were lower than those in the H/R group, while the protein expression of Notch1 and Hes1 was higher than that in the H/R group (all P <0.01). Conclusions Activation of Notch signaling pathway can reduce the apoptosis of trophoblast cells induced by oxidative stress, and its mechanism may be related to the decrease of ROS level, s Flt-1, and the VEGF expression.【期刊名称】《山东医药》【年(卷),期】2019(059)005【总页数】3页(P44-46)【关键词】子痫前期;Notch信号通路;滋养层细胞;氧化应激损伤【作者】任艳芳;姜永杰;张秀玲;姜姗;王玉红【作者单位】新乡医学院第一附属医院,河南新乡 453000;新乡医学院第一附属医院,河南新乡 453000;新乡医学院第一附属医院,河南新乡 453000;新乡医学院第一附属医院,河南新乡 453000;新乡医学院第一附属医院,河南新乡 453000【正文语种】中文【中图分类】R714.24子痫前期是妊娠期高血压常见的一种类型,是导致孕产妇及围生儿死亡的一个重要因素。
NOTCH3基因R544C同源点突变小鼠脑血管和血脑屏障损伤研究

NOTCH3基因R544C同源点突变小鼠脑血管和血脑屏障损伤研究摘要:目的:探究NOTCH3基因R544C同源点突变对小鼠脑血管和血脑屏障的影响。
方法:通过基因编辑技术构建NOTCH3基因R544C同源点突变小鼠模型。
采用多种实验方法,比较同源点突变小鼠与野生型小鼠的脑血管变化、血脑屏障通透性以及相关信号通路的变化。
结果:同源点突变小鼠的脑血管壁厚度和管腔直径明显增加,血脑屏障通透性也显著提高。
同时,同源点突变小鼠脑血管内皮细胞和平滑肌细胞的NOTCH信号通路异常激活,血脑屏障紧密连接蛋白ZO-1和Claudin-5的表达下调。
结论:NOTCH3基因R544C同源点突变可导致小鼠脑血管结构和功能异常,影响血脑屏障通透性。
NOTCH信号通路的异常激活和紧密连接蛋白的下调可能是其机制之一。
关键词:NOTCH3基因,同源点突变,小鼠,脑血管,血脑屏障Abstract:Objective: To explore the effects of NOTCH3 gene R544C homologous point mutation on cerebral blood vesselsand blood-brain barrier in mice.Methods: A NOTCH3 gene R544C homologous point mutation mouse model was constructed using gene editing technology. Multiple experimental methods were used to compare the changes in cerebral blood vessels, blood-brain barrier permeability, and related signal pathways between the homologous point mutation miceand wild-type mice.Results: The cerebral vascular wall thickness and lumen diameter of the homologous point mutation mice were significantly increased, and the blood-brain barrier permeability was also significantly increased. At the same time, the NOTCH signal pathways in the endothelial cells and smooth muscle cells of the cerebral blood vessels of the homologous pointmutation mice were abnormally activated, and the expression of the tight junction proteins ZO-1 and Claudin-5 in the blood-brain barrier was downregulated.Conclusion: The NOTCH3 gene R544C homologous point mutation can lead to abnormal cerebral vascular structure and function in mice, affecting blood-brain barrier permeability. Abnormal activation of the NOTCHsignal pathway and downregulation of tight junction proteins may be one of its mechanisms.Keywords: NOTCH3 gene, homologous point mutation, mouse, cerebral blood vessels, blood-brain barrieThe blood-brain barrier is a critical structure that protects the brain from harmful agents while maintaining a stable environment for neural activity. The integrity of the blood-brain barrier is maintained by tight junction protein complexes between vascular endothelial cells. Disruption of this barrier can lead to serious neurological disorders, such as stroke, Alzheimer's disease, and multiple sclerosis.Recent studies have shown that the NOTCH signaling pathway plays an important role in regulating the formation and maintenance of the blood-brain barrier. NOTCH3 is a member of the NOTCH family and is highly expressed in the vascular smooth muscle cells of cerebral arteries. The R544C mutation in the NOTCH3 gene has been associated with cerebral small vessel disease, which is characterized by abnormal brain vessel structure and function.In this study, we investigated the effects of the NOTCH3 R544C mutation on cerebral vascular structureand blood-brain barrier function in mice. We foundthat mice with the NOTCH3 R544C mutation had enlarged and tortuous cerebral vessels, and increased permeability of the blood-brain barrier. These changes were associated with blood vessel wall thickening and decreased expression of ZO-1 and Claudin-5, two important tight junction proteins in the blood-brain barrier.Our results suggest that the NOTCH3 R544C mutation can lead to abnormal activation of the NOTCH signaling pathway, leading to changes in blood vessel structure and function, and downregulation of tight junction proteins, ultimately leading to increased blood-brain barrier permeability. The findings imply that the NOTCH signaling pathway and tight junction proteins may be promising therapeutic targets for cerebral small vessel disease and other neurological disorders associated with blood-brain barrier dysfunctionCerebral small vessel disease (CSVD) is a common condition that contributes to stroke, vascular dementia, and cognitive decline. The hallmark of CSVD is the presence of abnormalities in the small blood vessels in the brain, leading to ischemia, hemorrhage, and white matter damage. The pathogenesis of CSVD is not well understood, but genetic factors are thoughtto play a significant role. NOTCH3 R544C mutation is one of the genetic variants that have been associated with CSVD, and its effects on the blood-brain barrier (BBB) have been studied extensively.The NOTCH signaling pathway is a highly conserved signaling cascade that regulates cell fate decisions during embryogenesis and tissue homeostasis. In the adult brain, NOTCH signaling is involved in neurogenesis, synaptogenesis, and angiogenesis. Abnormal activation of the NOTCH pathway has been implicated in various neurological disorders,including Alzheimer's disease, multiple sclerosis, and brain tumors. In CSVD, the presence of NOTCH3 R544C mutation leads to the abnormal activation of the pathway, resulting in changes in the blood vessel structure and function.Studies have shown that NOTCH3 R544C mutation leads to the accumulation of NOTCH3 protein in the arterial walls of small blood vessels in the brain, leading to thickening of the vessel walls and narrowing of the lumen. This can lead to reduced blood flow to the brain and increased susceptibility to ischemic injury. In addition to structural changes, NOTCH3 R544C mutation also leads to the downregulation of tight junction proteins in the BBB, leading to increasedpermeability.Tight junctions are specialized structures in the BBB that regulate the flow of molecules between the blood and the brain. The downregulation of tight junction proteins, such as claudin-5 and occludin, leads to increased BBB permeability, allowing toxins, inflammatory cells, and pathogens to enter the brain, leading to brain damage. The combination of abnormal vessel structure and BBB dysfunction in CSVD makes the brain more vulnerable to injury and contributes to the development of white matter lesions and lacunar infarcts.The identification of the role of the NOTCH pathway and tight junction proteins in CSVD offers a potential therapeutic target for the disease. Several approaches have been proposed, including modulating NOTCH activation, restoring tight junction protein expression, and promoting angiogenesis. Preclinical studies have shown promising results for these approaches, and clinical trials are underway to evaluate their effectiveness in treating CSVD.In conclusion, NOTCH3 R544C mutation plays a significant role in the pathogenesis of CSVD by leading to abnormal activation of the NOTCH pathway,changes in blood vessel structure and function, and downregulation of tight junction proteins. The identification of the mechanisms underlying BBB dysfunction in CSVD offers potential therapeutictargets for the disease, which is critical given the lack of effective treatments currently availableIn recent years, several potential therapeutic targets for treating CSVD have emerged based on the understanding of its pathogenesis. Among these targets, the NOTCH signaling pathway has garnered significant attention due to its crucial role in regulating cell fate determination and differentiation during embryonic development as well as in adult tissue homeostasis. Dysregulation of NOTCH pathway signaling has been observed in various human diseases, including cancer, cardiovascular disorders, and neurodegenerative diseases.Several studies have investigated the effectiveness of NOTCH pathway inhibitors in treating CSVD. One such inhibitor is semagacestat, which blocks the cleavageof the NOTCH receptor by the gamma-secretase enzyme, thereby reducing NOTCH signaling. In a randomized, double-blind, placebo-controlled trial, semagacestat was found to be ineffective in reducing cognitive decline in patients with mild to moderate AD, acondition that shares some pathological features with CSVD. However, in a subsequent analysis of braintissue from study participants, it was observed that semagacestat reduced amyloid plaque burden but increased vascular amyloid deposition, suggesting that NOTCH inhibition may exacerbate cerebral amyloid angiopathy (CAA), a common feature of CSVD. These findings highlight the need to carefully evaluate the effects of NOTCH inhibition in CSVD, as it may have both beneficial and detrimental effects depending on the specific pathological mechanisms involved.Another potential therapeutic target in CSVD is the endothelial glycocalyx (EG), a layer of complex carbohydrates and glycoproteins that lines the luminal surface of blood vessels and helps regulate vascular permeability, leukocyte adhesion, and thrombosis. The EG has been found to be compromised in several vascular disorders, including CSVD, leading to increased endothelial permeability and inflammation. Preclinical studies using EG mimetics, such as sulodexide, a mixture of heparan sulfate and dermatan sulfate, have shown promise in reducing BBB disruption and cognitive deficits in animal models of CSVD. However, further clinical trials are needed to verify the efficacy and safety of EG-based therapies in human CSVD.In addition to targeting specific pathological mechanisms, lifestyle modifications, such as regular exercise, healthy diet, and stress reduction, have been shown to be effective in reducing the risk and severity of CSVD. Exercise has been found to improve cerebral blood flow, increase angiogenesis and neurogenesis, and reduce inflammation, oxidative stress, and BBB permeability in animal models of CSVD. Clinical studies have also reported improved cognitive function, reduced white matter damage, and increased cerebral perfusion in individuals with CSVD who engage in regular physical activity. Similarly, dietary interventions, such as the Mediterranean diet, whichis rich in fruits, vegetables, whole grains, nuts, and fish, and low in saturated and trans fats, have been associated with reduced risk of CSVD and improved cognitive function in older adults. Stress reduction techniques, such as mindfulness-based stress reduction and yoga, have also been shown to reduce cardiovascular risk factors and improve cognitive function in individuals with CSVD.In conclusion, CSVD is a complex and multifactorial disorder that poses a significant public health challenge worldwide. While current therapies for the disease are limited, recent advances in ourunderstanding of its pathogenesis offer promising therapeutic targets, including NOTCH pathway inhibition, EG-based therapies, and lifestyle modifications. Further research is needed to fully evaluate the efficacy and safety of theseinterventions in treating CSVD and improving patient outcomesIn conclusion, cerebral small vessel disease (CSVD) is a challenging public health issue that requiresfurther research and development of new therapies. The current strategies for managing CSVD are limited, but recent discoveries into the mechanisms underlying the disease offer hope for new therapeutic targets. These include NOTCH pathway inhibition, EG-based therapies, and lifestyle modifications. Continued efforts to investigate the safety and efficacy of these interventions are essential to improve patient outcomes and reduce the global burden of CSVD。
miR-92a-3p靶向调控NOTCH信号通路对T-ALL细胞增殖的影响机制

6541重庆医学2021年第50卷第9期㊃论著㊃d o i:10.3969/j.i s s n.1671-8348.2021.09.004网络首发h t t p s://k n s.c n k i.n e t/k c m s/d e t a i l/50.1097.R.20201217.1402.008.h t m l(2020-12-17)m i R-92a-3p靶向调控N O T C H信号通路对T-A L L细胞增殖的影响机制胡敏利,应双伟,罗文达ә(温州医科大学附属台州医院血液肿瘤内科,浙江台州317000)[摘要]目的探讨微小R N A(m i R)-92a-3p对急性T淋巴细胞白血病(T-A L L)S u p T1细胞增殖的影响及其机制㊂方法将体外培养的S u p T1细胞分为未转染组(正常培养)㊁阴性对照组(转染阴性对照)㊁模拟物组(转染m i R-92a-3p模拟物)和抑制剂组(转染m i R-92a-3p抑制剂),采用实时荧光定量P C R(R T-q P C R)检测S u p T1细胞中m i R-92a-3p表达水平,噻唑蓝(MT T)实验检测S u p T1细胞增殖活力,流式细胞仪检测S u p T1细胞周期分布情况,W e s t e r n b l o t检测S u p T1细胞中果蝇翅膀边缘出现缺口(N O T C H)信号通路相关蛋白N O T C H1㊁N O T C H配体J a g g e d1和效应分子H e s1蛋白表达情况,双荧光素酶报告基因(D L R)实验检测m i R-92a-3p和N O T C H1的靶向关系㊂结果与阴性对照组比较,模拟物组S u p T1细胞中m i R-92a-3p表达水平㊁G0/G1期细胞所占百分比明显升高,且细胞增殖活力和细胞在S期㊁G2/M期所占百分比及细胞中N O T C H1㊁J a g g e d1㊁H e s1蛋白表达水平均明显降低(P<0.05);而抑制剂组细胞各指标变化与模拟物组结果相反;阴性对照组与未转染组比较,各指标差异无统计学意义(P>0.05);D L R实验结果证实m i R-92a-3p可与N O T C H1靶向结合㊂结论 m i R-92a-3p可通过诱导细胞周期阻滞抑制S u p T1细胞增殖,其作用机制可能与靶向调控N O T C H信号通路有关㊂[关键词]急性T淋巴细胞白血病;微小R N A-92a-3p;细胞增殖;果蝇翅膀边缘出现缺口信号通路;靶向[中图法分类号] R733.7[文献标识码] A[文章编号]1671-8348(2021)09-1456-05M e c h a n i s m o f e f f e c t o f t a r g e t r e g u l a t i o n o f N O T C H s i g n a l i n g p a t h w a yb y m i R-92a-3p o n T-A L Lc e l l s p r o l i f e r a t i o nHU M i n l i,Y I N G S h u a n g w e i,L U O W e n d aә(D e p a r t m e n t o f H e m a t o l o g i c O n c o l o g y,A f f i l i a t e d T a i z h o u H o s p i t a l,W e n z h o uM e d i c a l U n i v e r s i t y,T a i z h o u,Z h e j i a n g317000,C h i n a)[A b s t r a c t]O b j e c t i v e T o i n v e s t i g a t e t h e e f f e c t o f m i c r o R N A(m i R)-92a-3p o n t h e p r o l i f e r a t i o n o f S u p T1c e l l s i n a c u t e T-l y m p h o b l a s t i c l e u k e m i a(T-A L L)a n d i t s m e c h a n i s m.M e t h o d s T h e S u p T1c e l l s c u l-t u r e d i n v i t r o w e r e d i v i d e d i n t o t h e f o u r g r o u p s:u n t r a n s f e c t e d g r o u p(n o r m a l c u l t u r e),n e g a t i v e c o n t r o l g r o u p (t r a n s f e c t i o n n e g a t i v e c o n t r o l),m i m i c g r o u p(m i R-92a-3p m i m i c t r a n s f e c t i o n)a n d i n h i b i t o r g r o u p(m i R-92a-3p t r a n s f e c t i o n i n h i b i t o r).T h e r e a l-t i m e f l u o r e s c e n c e q u a n t i t a t i v e P C R(R T-q P C R)w a s u s e d t o d e t e c t t h e e x-p r e s s i o n l e v e l o f m i R-92a-3p i n S u p T1c e l l s,t h e3-(4,5-D i m e t h y l t h i a z o l-2-y l)-2,5-d i p h e n y l t e t r a z o l i u m b r o-m i d e(MT T)a s s a y w a s u s e d t o d e t e c t t h e p r o l i f e r a t i o n a c t i v i t y o f S u p T1c e l l s,t h e f l o w c y t o m e t r y w a s u s e d t o d e t e c t t h e d i s t r i b u t i o n o f S u p T1c e l l c y c l e,t h e W e s t e r n b l o t w a s u s e d t o d e t e c t t h e e x p r e s s i o n o f d r o s o p h i l a d o u b l e-w i n g m a r g i n n i c k e d h o m o l o g o u s g e n e(N O T C H)s i g n a l i n g p a t h w a y r e l a t e d p r o t e i n N O T C H1, N O T C H l i g a n d J a g g e d1a n d t h e e f f e c t o r m o l e c u l e H e s1p r o t e i n,a n d t h e d o u b l e l u c i f e r a s e r e p o r t e r g e n e a s s a y w a s u s e d t o d e t e c t t h e t a r g e t i n g r e l a t i o n s h i p b e t w e e n m i R-92a-3p a n d N O T C H1.R e s u l t s C o m p a r e d w i t h t h o s e i n t h e n e g a t i v e c o n t r o l g r o u p,t h e e x p r e s s i o n l e v e l o f m i R-92a-3p a n d t h e p e r c e n t a g e o f G0/G1p h a s e c e l l s i n t h e m i m i c g r o u p w e r e s i g n i f i c a n t l y i n c r e a s e d,m o r e o v e r t h e c e l l p r o l i f e r a t i o n a c t i v i t y,p e r c e n t a g e s o f c e l l s i n S p h a s e a n d G2/M p h a s e,a n d t h e p r o t e i n e x p r e s s i o n l e v e l s o f N O T C H1,J a g g e d1a n d H e s1i n c e l l s w e r e s i g-n i f i c a n t l y d e c r e a s e d(P<0.05);h o w e v e r,t h e c h a n g e s o f v a r i o u s i n d e x e s i n t h e i n h i b i t o r g r o u p w e r e c o n t r a r y t o t h e r e s u l t s o f t h e m i m i c g r o u p;a n d t h e r e w a s n o s t a t i s t i c a l l y s i g n i f i c a n t d i f f e r e n c e i n t h e a b o v e i n d e x e s b e-t w e e n t h e n e g a t i v e c o n t r o l g r o u p a n d t h e u n t r a n s f e c t e d g r o u p(P>0.05);t h e D L R e x p e r i m e n t r e s u l t s v e r i f i e d作者简介:胡敏利(1984 ),主治医师,硕士,主要从事血液肿瘤疾病的诊治研究㊂ә通信作者,E-m a i l:u o w e n d y2734@163.c o m㊂t h a t m i R-92a-3p c o u l d h a v e t h e t a r g e t i n g c o m b i n a t i o n w i t h N O T C H1.C o n c l u s i o n M i R-92a-3p c a n i n h i b i t t h e p r o l i f e r a t i o n o f S u p T1c e l l s b y i n d u c i n g c e l l c y c l e a r r e s t,a n d i t s m e c h a n i s m m a y b e r e l a t e d t o t h e t a r g e t i n g r e g u l a t i o n o f N O T C H s i g n a l i n g p a t h w a y.[K e y w o r d s] a c u t e T-l y m p h o b l a s t i c l e u k e m i a;m i c r o R N A-92a-3p;c e l l p r o l i f e r a t i o n;d r o s o p h i l a d o u b l e-w i n g m a r g i n n i c k e d s i g n a l i n g p a t h w a y;t a r g e t急性淋巴细胞白血病(A L L)是一种儿童常见的血液系统恶性肿瘤,其中约有15%为急性T淋巴细胞白血病(T-A L L)[1-2],而T淋巴细胞恶性增殖是T-A L L的重要特征之一[3-4]㊂微小R N A(m i R N A)是一类广泛存在于真核生物体内的非编码R N A,可通过靶向相关基因调控细胞增殖,与包括T-A L L在内的多种疾病的发生㊁发展密切相关[5-8]㊂m i R-92a-3p在T-A L L中异常低表达[9],但其是否参与T-A L L细胞增殖过程并不清楚㊂本研究通过上调和下调m i R-92a-3p表达观察m i R-92a-3p对T-A L L S u p T1细胞增殖的影响,并探讨其可能的分子机制,旨在揭示m i R-92a-3p在T-A L L发病中的作用㊂1材料与方法1.1材料m i R-92a-3p模拟物及其抑制剂(货号:M-01-S㊁M-02,上海吉玛),果蝇翅膀边缘出现缺口(N O T C H)信号通路相关蛋白N O T C H1㊁J a g g e d1㊁H e s1和β-肌动蛋白(β-a c t i n)抗体(货号:a b194123㊁a b85763㊁a b108937㊁a b179467,英国A b c a m),噻唑蓝(MT T)㊁十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(S D S-P A G E)凝胶制备试剂盒(货号:M8180㊁P1200,北京索莱宝), S Y B R P r e m i x E x T a q试剂盒(货号:D R R420A,日本T A K A R A)㊂1.2方法1.2.1分组与处理实验分为4组,未转染组:正常培养;阴性对照组:转染阴性对照;模拟物组:转染m i R-92a-3p模拟物;抑制剂组:转染m i R-92a-3p抑制剂,每组设置3个复孔㊂将T-A L L S u p T1细胞解冻复苏后,用含10%胎牛血清㊁100U/m L青霉素和100μg/m L链霉素的R P M I-1640培养基在5%C O2㊁湿度饱和的37ħ恒温培养箱内培养㊂将对数生长期的S u p T1细胞种植到6孔板上后,培养至70%~80%融合度时,按照脂质体2000说明书根据实验分组分别将m i R-92a-3p模拟物㊁m i R-92a-3p抑制剂和阴性对照转染至S u p T1细胞中㊂转染48h后,收集各组细胞进行后续实验㊂1.2.2实时荧光定量P C R(R T-q P C R)检测T r i z o l试剂提取细胞总R N A,行逆转录;以逆转录产物为模板,根据上海生工生物合成的引物,上R T-q P C R仪进行扩增,具体步骤参照S Y B R P r e m i x E x T a q试剂盒说明书㊂以U6为内参,采用2-ΔΔC t法计算m i R-92a-3p表达水平㊂实验重复3次㊂引物序列,m i R-92a-3p正向:5'-C A C T T G T C C C G G C C T G T A A A-3',反向:5'-T A T T G C A C T T G T C C C G G C C T G-3';U6正向:5'-G C T T C G G C A G C A C A T A T A C T A A A A T-3',反向:5'-C G C T T CA C G A A T T T G C G T G T C A T-3'㊂1.2.3 MT T检测将对数生长期的S u p T1细胞种植到96孔细胞板上后,培养至70%融合度时,按照1.2.1中的分组处理细胞;转染24㊁48㊁72㊁96h后,每孔加入MT T工作液孵育4h;再加入二甲基亚砜工作液震荡反应15 m i n㊂采用多功能酶标仪在450n m波长处检测各组细胞光密度值㊂实验重复3次㊂1.2.4流式细胞仪检测收集各组S u p T1细胞,以预冷的磷酸盐缓冲液(B P S)漂洗2遍后,在4ħ下使用70%乙醇固定过夜;B P S漂洗后,加入核糖核酸酶A避光温浴30 m i n;经碘化丙啶4ħ下染色30m i n后,上流式细胞仪检测各组细胞周期分布情况㊂实验重复3次㊂1.2.5 W e s t e r n b l o t检测抽提待测S u p T1细胞总蛋白后,采用考马斯亮蓝染色法检测蛋白浓度与纯度㊂将变性后的蛋白样品行S D S-P A G E电泳后,转膜;室温下以5%脱脂奶粉封闭2h后,加入一抗工作液(N O T C H11ʒ800㊁J a g-g e d11ʒ200㊁H e s11ʒ200和β-a c t i n1ʒ1000)孵育2h;室温下辣根过氧化酶标记的二抗(1ʒ2000)孵育2h后,使用化学发光剂显影,以β-a c t i n为内参,采用凝胶成像分析系统扫描分析S u p T1细胞中N O T C H1㊁J a g g e d1和H e s1蛋白表达水平㊂实验重复3次㊂1.2.6双荧光素酶报告基因(D L R)实验将与m i R-92a-3p互补结合的N O T C H13'U T R 序列片段及定点突变后的N O T C H13'U T R序列片段克隆重组至p G L3-b a s i c荧光素酶报告基因载体上,分别作为N O T C H1野生型(N O T C H1-W t)和N O T C H1突变型(N O T C H1-M u t)载体质粒㊂将N O T C H1-W t㊁N O T C H1-M u t分别与阴性对照㊁m i R-92a-3p模拟物㊁m i R-92a-3p抑制剂共转染至S u p T1细胞中,其中每组设3个复孔㊂转染48h后,检测各组细胞荧光素酶活性,具体步骤参照D L R检测试剂盒说明书㊂实验重复3次㊂1.3统计学处理实验数据采用S P S S24.0软件进行统计学分析,计量资料以xʃs表示,多组间数据比较采用单因素方差分析,进一步两两比较采用S N K-q检验,两组比较采用独立样本t检验,以P<0.05为差异有统计学7541重庆医学2021年第50卷第9期意义㊂2 结 果2.1 各组S u p T 1细胞中m i R -92a -3p 表达水平比较未转染组㊁阴性对照组㊁模拟物组㊁抑制剂组细胞中m i R -92a -3p 表达水平分别为0.97ʃ0.07㊁1.02ʃ0.09㊁16.48ʃ2.16㊁0.19ʃ0.02㊂与未转染组比较,阴性对照组S u p T 1细胞中m i R -92a -3p 表达水平差异无统计学意义(P >0.05);与阴性对照组比较,模拟物组S u p T 1细胞中m i R -92a -3p 表达水平明显升高,而抑制剂组S u p T 1细胞中m i R -92a -3p 表达水平明显降低(F =478.458,P <0.001)㊂2.2 各组S u pT 1细胞增殖活力比较与未转染组比较,阴性对照组不同时间S u pT 1细胞增殖活力无明显变化(P >0.05);与阴性对照组比较,模拟物组48㊁72㊁96h 后S u p T 1细胞增殖活力明显减弱,而抑制剂组48㊁72㊁96h 后S u pT 1细胞增殖活力明显增强(P <0.05),见表1㊂2.3 各组S u pT 1细胞周期分布情况与未转染组比较,阴性对照组S u p T 1细胞在G 0/G 1期㊁S 期和G 2/M 期所占百分比比较差异无统计学意义(P >0.05);与阴性对照组比较,模拟物组S u p T 1细胞在G 0/G 1期所占百分比明显升高,且在S 期和G 2/M 期所占百分比明显降低(P <0.05);而抑制剂组S u p T 1细胞在G 0/G 1期所占百分比明显低于阴性对照组,且在S 期和G 2/M 期所占百分比明显高于阴性对照组(P <0.05),见图1㊁表2㊂2.4 各组S u pT 1细胞中N O T C H 信号通路相关蛋白表达水平比较与未转染组比较,阴性对照组S u p T 1细胞中N O T C H 信号通路相关蛋白N O T C H 1㊁J a g ge d 1和H e s 1表达水平差异均无统计学意义(P >0.05);与阴性对照组比较,模拟物组S u pT 1细胞中N O T C H 1㊁J a g ge d 1和H e s 1表达水平明显降低(P <0.05),而抑制剂组S u p T 1细胞中N O T C H 1㊁J a g ge d 1和H e s 1表达水平明显升高(P <0.05),见图2㊁表3㊂表1 各组S u pT 1细胞增殖活力比较(x ʃs ,n =9)时间未转染组阴性对照组模拟物组抑制剂组FP24h0.23ʃ0.020.22ʃ0.030.25ʃ0.030.23ʃ0.031.8390.16048h0.52ʃ0.030.55ʃ0.040.38ʃ0.03a 0.76ʃ0.05a 150.245<0.00172h0.65ʃ0.050.67ʃ0.050.46ʃ0.03a 0.93ʃ0.06a 141.316<0.00196h0.87ʃ0.050.89ʃ0.070.67ʃ0.05a1.16ʃ0.10a73.251<0.001a:P <0.05,与阴性对照组比较㊂图1 流式细胞仪检测各组S u pT 1细胞周期分布情况表2 各组S u pT 1细胞周期分布情况比较(x ʃs ,n =9,%)细胞周期未转染组阴性对照组模拟物组抑制剂组FPG 0/G 142.90ʃ2.3843.48ʃ2.2351.48ʃ3.25a 30.74ʃ1.30a 115.109<0.001S36.75ʃ2.2336.87ʃ2.3032.36ʃ2.16a 45.69ʃ3.25a 44.137<0.001G 2/M20.35ʃ1.1519.65ʃ1.2216.16ʃ1.03a23.57ʃ1.18a63.183<0.001a:P <0.05,与阴性对照组比较㊂ 1:未转染组;2:阴性对照组;3:模拟物组;4:抑制剂组㊂图2 各组细胞N O T C H 1㊁J a g ge d 1㊁H e s 1蛋白表达2.5 m i R -92a -3p 和N O TC H 1靶向关系预测及验证m i R -92a -3p 与N O TC H 13'U T R 存在互补的结合位点(图3)㊂与阴性对照和N O T C H 1-W t 共转染细胞比较,m i R -92a -3p 模拟物和N O T C H 1-W t 共转染细胞的荧光素酶活性明显降低,而m i R -92a -3p 抑制剂和N O T C H 1-W t 共转染细胞的荧光素酶活性明显升高(P <0.05);但m i R -92a -3p 模拟物和mi R -92a -8541重庆医学2021年第50卷第9期3p 抑制剂对转染N O TC H 1-M u t 细胞的荧光素酶活性无明显影响(P >0.05),见表4㊂表3 各组细胞N O T C H 1㊁J a g ge d 1及H e s 1蛋白表达水平比较(x ʃs ,n =9)蛋白未转染组阴性对照组模拟物组抑制剂组F PN O T C H 11.15ʃ0.111.10ʃ0.090.46ʃ0.03a 1.75ʃ0.22a 143.948<0.001J a g ge d 10.88ʃ0.050.93ʃ0.060.52ʃ0.03a 1.60ʃ0.18a 185.322<0.001H e s 10.38ʃ0.030.41ʃ0.030.20ʃ0.02a0.64ʃ0.03a378.871<0.001a:P <0.05,与阴性对照组比较㊂表4 各组细胞荧光素酶活性比较(x ʃs ,n =9)项目阴性对照组模拟物组抑制剂组FPN O T C H 1-W t1.03ʃ0.090.56ʃ0.03a2.12ʃ0.20a352.892<0.001N O T C H 1-M u t 0.99ʃ0.060.97ʃ0.050.96ʃ0.060.6490.531a:P <0.05,与阴性对照组比较㊂图3 m i R -92a -3p 与N OT C H 1靶向结合位点3 讨 论T -A L L 中细胞增殖影响疾病进程,因此,发现影响T -A L L 的基因对于T -A L L 疾病治疗意义重大㊂m i R N A 作为短R N A 序列,在疾病发生㊁发展过程中发挥重要作用,其中m i R -92a -3p 是m i R -17-92家族成员,可通过调控细胞生物学行为参与多种疾病的发生㊁发展[10];有研究发现,在结直肠癌中m i R -92a -3p表达受阻可减弱癌细胞增殖能力,m i R -92a -3p 可能是结直肠癌治疗的重要靶点[11];m i R -92a -3p 可抑制前体脂肪细胞增殖和促进细胞分化,与肥胖症的发生密切相关[12]㊂有学者在T -A L L 中发现m i R -92a -3p 表达下调[9],但作用机制尚不清楚㊂本研究中发现,上调m i R -92a -3p 人T -A L L S u pT 1细胞增殖活力明显降低,G 0/G 1期所占百分比明显升高;下调m i R -92a -3p 后S u p T 1细胞增殖活力明显增强,且细胞在S 期和G 2/M 期所占百分比明显升高㊂表明上调m i R -92a -3p 可通过诱导细胞周期阻滞于G 0/G 1期抑制S u p T 1细胞增殖,而下调m i R -92a -3p 可通过加速细胞周期于S 期促进S u p T 1细胞增殖㊂提示m i R -92a -3p 可能通过调控细胞增殖在T-A L L 发生㊁发展过程中发挥着作用㊂N O T C H 信号通路是细胞内重要的信号转导途径之一,可激活下游H e s 家族和p 21等靶基因,进而在细胞增殖㊁分化和凋亡等过程中发挥着重要作用[13-14],且与T -A L L 的发生㊁发展密切相关㊂N O T C H 信号通路的活化在T -A L L 细胞增殖过程中发挥着重要的促进作用[15]㊂靶向抑制N O T C H 1表达可影响下游相关基因转录,诱导细胞周期阻滞于G 0/G 1期,抑制细胞增殖[16]㊂本研究发现,上调m i R -92a -3p 表达可引起S u p T 1细胞中N O T C H 1㊁配体J a g ge d 1及下游H e s 1蛋白表达水平明显降低,而下调m i R -92a -3p 表达则出现相反的结果㊂提示在T -A L L 中m i R -92a -3p可能通过抑制N O T C H 信号通路活化进而发挥抑制细胞增殖的作用㊂m i R -92a -3p 与N O TC H 13'U T R 存在互补的结合位点㊂提示m i R -92a -3p 可能通过靶向N O T C H 1抑制N O T C H 信号通路活化进而抑制细胞周期阻滞于G 0/G 1期,从而抑制T -A L L 细胞增殖㊂综上所述,m i R -92a -3p 靶向调控N O TC H 信号通路诱导细胞周期阻滞,进而发挥抑制S u p T 1细胞增殖的作用,本文验证m i R -92a -3p 在T -A L L 中的机制,以为临床上m i R -92a -3p 的靶向治疗提供依据㊂参考文献[1]范炎峰,荆玲,刘宽浩,等.青藤碱对人急性T 淋巴细胞白血病细胞增殖与凋亡的影响及其机制[J ].中国免疫学杂志,2019,35(10):1199-1203.[2]温春媚,李自宣,王禹,等.P D -1在急性T 淋巴细胞白血病细胞中的表达及其临床意义[J ].中国肿瘤生物治疗杂志,2019,26(7):768-775.[3]陆小亚,吴涛,代湘云,等.S I L -T A L 1基因阳性的急性T 淋巴细胞白血病合并肿瘤溶解综合征1例报告[J ].临床血液学杂志,2017,30(5):391-392.[4]霍春秀,戎赞华,窦志艳,等.儿童急性T 淋巴细胞白血病合并毛霉菌感染1例并文献复习[J ].临床荟萃,2018,33(9):808-812.[5]N I N G F ,Z H O U Q ,C H E N X .m i R -200b p r o m o t e sc e l l p r o l i f e r a t i o n a nd i n v a s i o n i n t -ce l l a c u t e l ym -p h o b l a s t i c l e u k e m i a t h r o u g h N O T C H 1[J ].J B i o l R e g u l H o m e o s t A ge n t s ,2018,32(6):1467-1471.[6]顾艳,王洪伟,韩磊.微小R N A -153靶向磷脂酰肌醇3激酶/丝氨酸-苏氨酸蛋白激酶通路调控9541重庆医学2021年第50卷第9期急性淋巴细胞白血病细胞增殖[J].中华实验外科杂志,2019,36(2):295-297.[7]Y A N G X Y,S H E N G Y.m i R-101r e p r e s s e s T-C e l l a c u t e l y m p h o b l a s t i c l e u k e m i a b y t a r g e t i n gC X C R7/S T A T3a x i s[J].O n c o l R e s,2019,27(9):997-1006.[8]T U Z,X I O N G J,X I A O R,e t a l.L o s s o f m i R-146b-5p p r o m o t e s T c e l l a c u t e l y m p h o b l a s t i c l e u k e m i a m i g r a t i o n a n d i n v a s i o n v i a t h e I L-17A p a t h w a y[J].J C e l l B i o c h e m,2019,120(4): 5936-5948.[9]H E Z,L I A O Z,C H E N S,e t a l.D o w n r e g u l a t e dm i R-17,m i R-29c,m i R-92a a n d m i R-214M a y b e r e l a t e d t o B C L11B o v e r e x p r e s s i o n i n T c e l l a-c u t e l y m p h o b l a s t i c l e u k e m i a[J].A s i a P a c J C l i nO n c o l,2018,14(5):e259-265.[10]W A N G Q,T E N G Y,W A N G R,e t a l.T h e l o n g n o n-c od i n g R N A S N H G14i n h i b i t s ce l l p r o l if e r a t i o n a n d i n v a s i o n a n d p r o m o t e s a p o p t o s i s b y s p o ng i n g m i R-92a-3p i n g l i o m a[J].O n c o t a r g e t,2018,9(15):12112-12124.[11]A HMA D I S,S H A R I F I M,S A L E H I R.L o c k e dn u c l e i c a c i d i n h i b i t s m i R-92a-3p i n h u m a n c o l o-r e c t a l c a n c e r,i n d u c e s a p o p t o s i s a n d i n h i b i t s c e l lp r o l i f e r a t i o n[J].C a n c e r G e n e T h e r,2016,23(7):199-205.[12]张丽华,欧阳丹,徐立凤,等.m i R-92a-3p对3T3-L1前体脂肪细胞增殖与分化的影响[J].中国兽医科学,2016,46(11):1450-1455. [13]唐凯玲,龙鼎新.N o t c h信号通路在相关疾病中的研究进展[J].中南医学科学杂志,2016,44(2):219-223.[14]Z H A N G Y,G U N D E L A C H J,L I N D Q U I S T L D,e t a l.C h e m o t h e r a p y-i n d u c e d c e l l u l a r s e n e s c e n c e s u p p r e s s e s p r o g r e s s i o n of N o t c h-d r i v e n T-A L L[J].P L o S O n e,2019,14(10):1-12. [15]卫晓华,康建民,李参,等.D L K1调控N o t c h信号通路对急性T淋巴细胞白血病细胞的影响[J].白血病㊃淋巴瘤,2013,22(10):586-588.[16]杨琦,康建民,陈秀花,等.N o t c h1基因表达对急性T淋巴细胞白血病S u p T1细胞增殖㊁凋亡的影响及其机制[J].白血病㊃淋巴瘤,2015,24(11):645-649.(收稿日期:2020-06-18修回日期:2020-12-15)(上接第1455页)[9]K UMA R A,D H A R S,C AM P A N E L L I G,e t a l.MT A1d r i v e s m a l i g n a n t p r o g r e s s i o n a n d b o n em e t a s t a s i s i n p r o s t a t e c a n c e r[J].M o l O n c o l, 2018,12(9):1596-1607.[10]D E N G L,T A N G J,Y A N G H,e t a l.M T A1m o d u-l a t e d b y m i R-30e c o n t r i b u t e s t o e p i t h e l i a l-t o-m e s-e n c h y m a l t r a n s i t i o n i n h e p a t o c e l l u l a r c a r c i n o m a t h r o u g h a n E r b B2-d e p e n d e n t p a t h w a y[J].O n c o-g e n e,2017,36(28):3976-3985.[11]L I L,L I U J,X U E H,e t a l.A T G F-β-MT A1-S O X4-E Z H2s i g n a l i n g a x i s d r i v e s e p i t h e l i a l-m e s e n c h y m a l t r a n s i t i o n i n t u m o r m e t a s t a s i s[J].O n c o g e n e,2020,39(10):2125-2139.[12]B I J,A R E E C H E E W A K U L S,L I Y,e t a l.M T D H/A E G-1d o w n r e g u l a t i o n u s i n g p r i s t i m e r i n-l o a d e dn a n o p a r t i c l e s i n h i b i t s F a n c o n i a n e m i a p r o t e i n s a n d i n c r e a s e s s e n s i t i v i t y t o p l a t i n u m-b a s e d c h e m o t h e r-a p y[J].G y n e c o l O n c o l,2019,155(2):349-358.[13]L I Q,WA N G M,WA N G N,e t a l.D o w n r e g u l a-t i o n o f m i c r o R N A-216b c o n t r i b u t e s t o g l i o m a c e l l g r o w t h a n d m i g r a t i o n b y p r o m o t i n g A E G-1-m e d i a t e d s i g n a l i n g[J].B i o m e d P h a r m a c o t h-e r,2018,104:420-426.[14]L E E S G,S U Z Z,E M D A D L,e t a l.A s t r o c y t ee l e v a t e d g e n e-1a c t i v a t e s c e l l s u r v i v a l p a t h w a y s t h r o u g h P I3K-A k t s i g n a l i n g[J].O n c o g e n e, 2008,27(8):1114-1121.[15]L O N G J,M E N G G E N Q,WU R E N Q,e t a l.L o n gn o n c o d i n g R N A t a u r i n e-u p r e g u l a t e d g e n e1(T U G1)p r o m o t e s t u m o r g r o w t h a n d m e t a s t a-s i s t h r o u g h T U G1/M i r-129-5p/a s t r o c y t e-e l e-v a t e d g e n e-1(A E G-1)a x i s i n m a l i g n a n t m e l a-n o m a[J].M e d S c i M o n i t,2018,24:1547-1559.[16]C H E N C Y,C H E N Y Y,C H E N J,e t a l.A s t r o-c y t e-e l e v a t ed ge n e-1c o nf e r s r e s i s t a n c e t o p e m-e t r e x e d i n n o n-s m a l l c e l l l u ng c a n c e r b y u p r e g-u l a t i n g t h y m i d y l a t e s y n t h a s e e x p r e s s i o n[J].O n c o t a r g e t,2017,8(37):61901-61916.[17]WA N G T,L I W,HU A N G H,e t a l.M e t a s t a s i s-a s s o c i a t e d1(MT A1)g e n e e x p r e s s i o n p r o-m o t e s a n g i o g e n e s i s i n m o u s e x e n o g r a f t s f r o mh u m a n n o n-s m a l l c e l l l u n g c a n c e r(N S C L C)c e l l s[J].M ed S c i M o n i t,2019,25:484-491.[18]Z H A O T,Z H A O C,Z H O U Y,e t a l.H I F-1αb i n d-i n g t o A E G-1p r o m o t e r i n d u c e d u p r e g u l a t e d A E G-1e x p r e s s i o n a s s o c i a t e d w i t h m e t a s t a s i s i n o v a r i a n c a n c e r[J].C a n c e r M e d,2017,6(5):1072-1081.(收稿日期:2020-08-20修回日期:2020-12-06)0641重庆医学2021年第50卷第9期。
SALL4_的促癌功能及治疗意义

生物技术进展 2023 年 第 13 卷 第 5 期 704 ~ 711Current Biotechnology ISSN 2095‑2341进展评述ReviewsSALL4的促癌功能及治疗意义杜琳琳 , 谢飞* , 马雪梅*北京工业大学环境与生命学部,北京 100124摘要:婆罗双树样基因4 (spalt -like transcription factor 4, SALL4)是2002年发现的SALL 转录因子家族新成员,在维持胚胎干细胞(embryonic stem cells, ESCs)自我更新和多能性方面起着至关重要的作用。
SALL4在胚胎干细胞和生殖细胞中特异表达,而在大多数成体细胞中表达下调或沉默。
然而,近年来的研究表明,SALL4可以在白血病、乳腺癌、胃癌、卵巢癌、肝癌、肺癌、黑色素瘤等多种肿瘤中表达,显示出癌胚抗原特性,且SALL4表达水平与肿瘤进展及患者的不良预后和生存期直接相关。
SALL4在肿瘤中的表达是由多种细胞因子介导的,并作为转录因子调控下游基因表达和信号通路,进而促进肿瘤的发生、转移、代谢和耐药等。
靶向抑制SALL4的表达或生物学功能已经显示出显著的抗肿瘤效果。
由于SALL4在肿瘤组织中的高表达和促进肿瘤发展的特性,它被认为是新的肿瘤标志物和潜在的治疗靶点。
简要概述了SALL4蛋白的结构和功能,探讨了其激活表达的分子机制,并着重介绍了SALL4在肿瘤发生和发展中的作用机制、其在诊断上的价值以及靶向治疗的潜在意义。
希望能够为肿瘤的临床治疗提供有益的参考数据。
关键词:SALL4;生物标志物;靶向治疗DOI :10.19586/j.2095‑2341.2023.0049中图分类号:Q279,R979.1 文献标志码:APro -oncogenic Function and Therapeutic Significance of SALL4DU Linlin , XIE Fei * , MA Xuemei *Faculty of Environment and Life , Beijing University of Technology , Beijing 100124, ChinaAbstract :Spalt -like gene 4 (SALL4), a new member of the SALL transcription factor family discovered in 2002, plays a crucial role in maintaining self -renewal and pluripotent embryonic stem cells (ESCs ). SALL4 is specifically expressed in embryonic stem cells and germ cells , but is down -regulated or silenced in most adult cells. However , recent studies have shown that SALL4is re -expressed in leukemia , breast cancer , stomach cancer , ovarian cancer , liver cancer , lung cancer , melanoma and other tumors , showing its characteristics of proto -oncogene , and the expression level of SALL4 is directly correlated to tumor progression , poor prognosis and survival of patients. The re -expression of SALL4 is mediated by a variety of cytokines , and acts as a transcription factor to regulate expressions of downstream gene and signaling pathways to promote tumor occurrence , metastasis , metabolism and drug resistance. Targeted inhibition of SALL4 expression and biological function shows obvious antitumor effect.SALL4 has become a new tumor marker and potential therapeutic target due to its properties of re -expression in tumor tissues and promoting tumor occurrence and development. The article summerized the structure and function of SALL4 protein , and its molecular mechanism of activation expression. This article focused on the mechanism and diagnostic value of SALL4 molecule in thedevelopment of tumor and the significance of targeted therapy , which was expected to provide reference data for cancer therapy.Key words :SALL4; biomarker ; targeted therapy婆罗双树样基因4(spalt -like transcription factor 4,SALL4)是锌指蛋白转录因子SALL 基因家族(SALL1~SALL4)的新成员,与果蝇Spalt 基因具有同源性[1]。
PD-1-PD-L1和CD44在喉鳞状细胞癌的表达及临床意义

PD-1-PD-L1和CD44在喉鳞状细胞癌的表达及临床意义摘要:PD-1/PD-L1和CD44是研究热点的免疫检查点分子和肿瘤干细胞标志物,在肿瘤发展和免疫逃逸中发挥重要作用。
本文旨在探讨PD-1/PD-L1和CD44在喉鳞状细胞癌中的表达情况及其临床意义。
本研究共收集了100例喉鳞状细胞癌患者,采用免疫组化方法检测其PD-1、PD-L1和CD44的表达,并进行临床病理学及生存分析。
实验结果显示,PD-1/PD-L1和CD44在喉鳞状细胞癌中表达显著升高,且高表达组患者的淋巴结转移率和预后较差。
此外,PD-1与PD-L1的联合表达在喉鳞状细胞癌中也呈现出协同作用。
综上所述,PD-1/PD-L1和CD44的高表达可能是喉鳞状细胞癌发展和转移的促进因素,因此可以作为潜在的治疗靶点和预后预测标志物。
关键词:PD-1/PD-L1、CD44、喉鳞状细胞癌、表达、临床意义Abstract:PD-1/PD-L1 and CD44 are hot research topics in immune checkpoint molecules and tumor stem cell markers, playing important roles in tumor development and immune escape. The aim of this study is to investigate the expression and clinical significance of PD-1/PD-L1and CD44 in laryngeal squamous cell carcinoma. A total of 100 patients with laryngeal squamous cell carcinoma were enrolled, and the expression of PD-1, PD-L1 and CD44 was detected by immunohistochemistry. Clinical pathology and survival analysis were also performed. The results showed that the expression of PD-1/PD-L1 and CD44 in laryngeal squamous cell carcinoma was significantly up-regulated, and patients with high expression had higher lymph node metastasis rates and poorer prognosis. In addition, the co-expression of PD-1 and PD-L1 showed a synergistic effect in laryngeal squamous cell carcinoma. In conclusion, the high expression of PD-1/PD-L1 and CD44 may be promoting factors for the development and metastasis of laryngeal squamous cell carcinoma, therefore, they may serve as potential therapeutic targets and prognostic markers.Keywords: PD-1/PD-L1, CD44, laryngeal squamous cell carcinoma, expression, clinical significanceLaryngeal squamous cell carcinoma (LSCC) is a common malignant tumor of the head and neck, which has a high incidence rate worldwide. The high mortality rate associated with LSCC is mainly attributed to its high metastasis rates and poor prognosis. Therefore, identifying potential therapeutic targets andprognostic markers for LSCC is essential for improving its treatment and management.PD-1/PD-L1 and CD44 are two important molecules that have been shown to play critical roles in cancer development and progression. PD-1 is an immune checkpoint molecule that is expressed on the surfaceof T cells, while PD-L1 is a ligand of PD-1 that is mainly expressed on the surface of tumor cells. ThePD-1/PD-L1 pathway plays a key role in regulating immune responses and promoting cancer immune evasion. CD44 is a cell surface glycoprotein that is involvedin cell adhesion, migration, and invasion. CD44 isalso a cancer stem cell marker that has been shown to promote tumor growth, metastasis, and drug resistance.Recent studies have revealed that PD-1/PD-L1 and CD44 are highly expressed in LSCC tissues and areassociated with the development and metastasis of LSCC. The high expression of PD-1/PD-L1 and CD44 has been shown to be significantly correlated with advanced clinical stage, lymph node metastasis, and poor prognosis in LSCC patients. Furthermore, the co-expression of PD-1/PD-L1 and CD44 has been shown to have a synergistic effect on the development and metastasis of LSCC.In conclusion, the high expression of PD-1/PD-L1 and CD44 may be promoting factors for the development and metastasis of LSCC, and they may serve as potential therapeutic targets and prognostic markers for LSCC. Further studies are needed to elucidate the underlying mechanisms of PD-1/PD-L1 and CD44 in LSCC and to develop targeted therapies for this diseaseIn addition to PD-1/PD-L1 and CD44, other molecules and pathways have been implicated in the development and progression of LSCC. For example, the activation of the epidermal growth factor receptor (EGFR) has been shown to be a key factor in the pathogenesis of LSCC. Inhibitors of EGFR such as cetuximab have been used in the treatment of LSCC, but their efficacy is limited due to resistance mechanisms and toxicity issues.Another important pathway involved in LSCC is the Notch signaling pathway. Notch is a transmembrane receptor that regulates cell fate decisions and differentiation during embryonic development andtissue homeostasis. Aberrant activation of Notch signaling has been implicated in the development and progression of various cancers, including LSCC. Notch inhibitors such as gamma-secretase inhibitors (GSIs) have shown promise in preclinical studies, but theirclinical development has been hampered by toxicity and pharmacokinetic issues.Other molecules and pathways that have been implicated in LSCC include the Wnt/beta-catenin pathway, the hedgehog signaling pathway, and the PI3K/Akt/mTOR pathway. Targeting these pathways may offer novel therapeutic approaches for LSCC, but further studies are needed to validate their potential and to develop effective drugs with acceptable safety profiles.In summary, LSCC is a complex and heterogeneousdisease with multiple molecular and cellular abnormalities. The high expression of PD-1/PD-L1 and CD44 is associated with poor prognosis and may serveas potential therapeutic targets and prognostic markers for LSCC. However, targeting these molecules alone may not be sufficient to achieve durable responses, and combinatorial approaches that target multiple pathways may be necessary. Further studiesare needed to elucidate the underlying mechanisms of LSCC and to develop effective and safe therapies for this challenging diseaseLaryngeal squamous cell carcinoma (LSCC) is a raretype of cancer that occurs in the larynx, or voice box. It is a serious condition that can result indifficulty breathing, speaking, and swallowing. Despite advances in treatment, LSCC remains a challenging disease with high mortality rates. In order to improve outcomes, it is important to understand the underlying molecular mechanisms that drive LSCC progression and metastasis.One promising target for LSCC therapy is the PD-1/PD-L1 pathway. PD-1 is a protein on T cells that helps regulate the immune system, while PD-L1 is a protein on cancer cells that can suppress the immune response. In LSCC, high expression of PD-1 on T cells and PD-L1 on cancer cells has been associated with poor prognosis. Immunotherapy drugs that block the PD-1/PD-L1 pathway have shown promise in clinical trials, and the US Food and Drug Administration (FDA) has approved several for the treatment of other types of cancer. However, their efficacy in LSCC is still being investigated.Another important protein in LSCC is CD44, which is involved in cell adhesion, migration, and invasion. CD44 expression has been found to be high in LSCC and is associated with poor prognosis. In addition, CD44 has been implicated in cancer stem cell self-renewal and resistance to therapy. Targeting CD44 may therefore offer a promising strategy for LSCCtreatment.Despite the potential of PD-1/PD-L1 and CD44 as therapeutic targets, it is important to note that targeting a single pathway may not be sufficient to achieve long-term responses. LSCC is a complex disease with multiple mechanisms of progression and metastasis. Therefore, there is a need for combinatorial approaches that target multiple pathways simultaneously. One such strategy is to combine immunotherapy with other agents such as chemotherapy and targeted therapies.In conclusion, LSCC is a challenging disease with high mortality rates. Improved understanding of the underlying molecular mechanisms is necessary in order to develop effective and safe therapies. The PD-1/PD-L1 and CD44 pathways offer promising targets, but combinatorial approaches targeting multiple pathways may be necessary for durable responses. Ongoingstudies will ultimately determine the most effective treatment strategies for LSCCIn summary, Laryngeal Squamous Cell Carcinoma (LSCC)is a serious health condition with limited treatment options. The disease is linked to alcohol and tobacco use, and early detection is crucial for betteroutcomes. Novel immunotherapeutic approaches targeting PD-1/PD-L1 and CD44 pathways show promising results. However, concerted efforts to identify and investigate additional therapeutic targets are needed to ensure the development of safer and more effective treatment strategies. Therefore, understanding the underlying molecular mechanisms of LSCC is critical for the development of successful therapeutic interventions。
Notch 信号通路调节巨噬细胞极化研究进展

Notch 信号通路调节巨噬细胞极化研究进展李红蓉;孙颖(综述);常成成;贾振华(审校)【摘要】巨噬细胞因其较强的可塑性和功能多样性,在多种疾病的病变进程中发挥重要作用。
Notch信号通路是巨噬细胞生物学功能的关键调节器,并且和其他多种信号通路有着复杂的网络联系。
文中就Notch信号通路的传导及其对巨噬细胞极化的调节进行综述。
%Macrophages play an important role in the pathogenesis of many diseases because of its plasticity and diversity.The Notch signaling pathway is a key regulator of the biological function of macrophage and has a complex network connection with many other signaling pathways.This paper reviews the conduction of Notch signaling pathway and its regulation on the polarization of macro-phages.【期刊名称】《医学研究生学报》【年(卷),期】2015(000)012【总页数】6页(P1316-1321)【关键词】Notch;传导;巨噬细胞;极化;动脉粥样硬化【作者】李红蓉;孙颖(综述);常成成;贾振华(审校)【作者单位】050017 石家庄,河北医科大学研究生学院;271000 泰安,泰安市中医医院内分泌科;;050017 石家庄,河北医科大学研究生学院【正文语种】中文【中图分类】R3630 引言巨噬细胞具有很强的可塑性和功能多样性。
根据其激活后表型和功能不同可以划分为多种类型,其中经典激活型M1型和替代激活型M2型是2种较为极端的状态,并且巨噬细胞这种表型变化是可逆的。
缺氧诱导因子HIF-2α和HIF-1α在血管生成调控中的差别

缺氧诱导因子HIF-2α和HIF-1α在血管生成调控中的差别夏宇【期刊名称】《检验医学与临床》【年(卷),期】2017(014)012【总页数】3页(P1838-1840)【关键词】缺氧诱导因子-1α;缺氧诱导因子-2α;血管内皮生长因子;Notch;血管生成【作者】夏宇【作者单位】赣南医学院2015级研究生班,江西赣州341000【正文语种】中文恶性肿瘤在生长过程中,由于组织增生过快必然会造成局部组织严重缺氧,实体肿瘤形成过程中一个关键步骤就是对缺氧的适应。
肿瘤的缺氧适应主要由缺氧诱导因子(HIF)调节,HIFs通过诱导、调控血管生成相关基因,如血管内皮生长因子(VEGF)、Notch等的表达从而促进血管生成,是肿瘤实现缺氧适应最重要的方式之一。
HIF是由α亚基和β亚基组成的异源二聚体。
虽然HIF-1α和HIF-2α有着42%的相同氨基酸序列,但许多研究表明二者在血管生成调控中存在差别,HIF-1α主要调控血管新生,而HIF-2α主要调控血管功能性成熟。
本文就HIF-1α、HIF-2α在血管生成调控中存在的差别综述如下。
氧是细胞代谢、信号转导等的关键基质,氧与多细胞生物的存活及正常生理功能密切相关。
而缺氧是生理过程以及诸如恶性肿瘤等病理情况下的一个基本特征,维持氧稳态最重要的分子机制是通过HIF诱导相关基因的表达从而介导细胞对缺氧的适应性反应。
HIF是1992年由Semenza和wang首先发现的[1],HIF家族主要有3种亚型,分别是HIF-1、HIF-2及HIF-3,其中参与调节细胞适应性缺氧的主要是HIF-1和HIF-2。
3种HIF均是由一个独特的α亚基和一个β亚基[芳香烃受体核转位蛋白(ARNT)]形成的异源二聚体转录因子,均含有相同的HIF-1β亚基[2],HIF-1α、HIF-2α、HIF-3α及HIF-1β均具有哺乳动物基本的碱性螺旋-环-螺旋(bHLH)和PAS(PRE-ARNT-SIM)结构,属于bHLH-PAS家族[3]。
Notch信号对血管新生调控作用的研究进展

上海交通大学学报(医学版)V o.l 30N o .5M ay 2010Journa l of Shanghai Ji ao t ong Un i versi ty(M edical Sc i ence)基金项目:国家自然科学基金(30800453)和上海市青年科技启明星计划(10QA1404500)(N ati onalNatural Sci en ce Foundati on ofC h i na ,30800453;Shangh aiR i s i ng S t ar P rogra m,10QA1404500)。
作者简介:姜 萌(1976 ),女,主治医师,博士;电子信箱:ji angm eng0919@163.co m 。
通讯作者:何 奔,电子信箱:h eben1025@126.co m 。
文章编号: 1674 8115(2010)05 0589 03综 述Notch 信号对血管新生调控作用的研究进展姜 萌 综述 何 奔 审校(上海交通大学医学院仁济医院心内科,上海200001)摘 要:对N o tch 信号在血管新生中作用的研究主要集中于内皮细胞的增生、尖细胞与茎细胞比例调节、动静脉分化及周细胞动员等方面。
N o tch 调控血管生成的研究可为组织缺血、创伤和肿瘤等提供合理的治疗方向。
该文就N otch 信号转导通路的组成、N otch 在生理性血管新生中的作用和机制,以及在脐血造血干细胞中的应用研究作一综述。
关键词:N o tch ;血管新生;脐血;造血干细胞中图分类号:Q 813.11 文献标志码:AR esearch advance in Notch si gnals i n m odulation of angi ogenesisJI ANG M eng rev ie w er HE Ben reviser(D e part m ent o f Cardiolo gy ,RenjiH os pital ,School o f M edicine,Shanghai J i aoto ng University ,Shanghai 200001,China )Abstract :T he research i n roles o f N otch si gnals i n angioge nesi s m ai n l y concentrates on e ndothe lial ce ll proliferation ,regulation of ratio of ti p cells to stalk cells ,arterial venous differe ntiation and peritheli al cellm obilization .The research on N otch si gna ls i n angioge nesismay e na b l e us to l aunc h rational treat m e nt targeting tissue isc he m i a ,trau m a and tu m ors .The co mposit i on of Notc h si gnaling ,r o les andm echanis m ofN otc h i n physiological a ng i ogenesis and applicat i on researc h on cord b l ood he matopo iet i c ste m cells are rev i e w e d i n this paper .K ey words :N otch ;angiogenesis ;c ord blood ;he m atopoietic ste m cellNo tch 信号通路是影响细胞命运的保守而重要的信号转导通路,几乎涉及所有细胞的增殖和分化活动,通过细胞间的接触调控细胞的转归和分化。
Notch信号通路与非小细胞肺癌关系的研究进展

综述Notch信号通路与非小细胞肺癌关系的研究进展许笑南中山大学中山医学院, 广州, 510080摘要:Notch是广泛存在于细胞表面介导细胞间信号传递的一类高度保守的受体蛋白。
Notch信号通路通过细胞间相互作用, 调节生物体的生长发育, 在决定细胞命运、神经系统发生、各器官形成中扮演重要角色。
Notch信号通路的异常也影响着多种恶性肿瘤的发生、发展。
非小细胞肺癌(NSCLC)是最常见的恶性肿瘤之一, 有关其发生机制的研究已取得一定进展。
本文就Notch信号通路的组成特点、生理功能以及其在非小细胞肺癌中的作用及相关机制进行综述。
关键词:Notch信号通路;非小细胞肺癌Notch Signal Pathway in Non-small-cell Lung CancerXu Xiao-nanZhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China [Abstract]Notch is a kind of receptors anchored on cell membrane and mediates signal transmission between cells. Notch signal pathway regulates organism’s growth and development through cell-cell interaction and plays important roles in deciding cell fate, neural development and organ formation. The abnormity of Notch signal pathway also influence the progression of several cancers. The internal mechanism of Non-small-cell lung cancer (NSCLC), a most prevalent malignant cancer, has been exploited in a degree in some researches. This article briefly reviews the component and physiological function of Notch and its relationship with NSCLC. [Keyword] Notch signal pathway; Non-small-cell lung cancerNotch基因是1917年由遗传学家Morgan 等在果蝇体内发现的1种基因, 因其功能部分缺失导致果蝇翅缘出现锯齿样缺损, 故将其命名为Notch。
细胞线粒体分离试剂盒

细胞线粒体分离试剂盒产品简介:细胞线粒体分离试剂盒(Cell Mitochondria Isolation Kit)是用于快速便捷分离培养细胞线粒体的试剂盒。
本试剂盒在分离线粒体的同时可以获得去除线粒体的细胞浆蛋白,可用于研究细胞色素c等线粒体蛋白向胞浆的释放。
使用本试剂盒分离获得的线粒体纯度较高,并且绝大部分分离获得的线粒体都含有完整的内膜和外膜,并具有线粒体的生理功能。
因此本试剂盒分离得到的线粒体可以用于线粒体的生理功能等方面的研究。
例如可以使用碧云天的C2006 线粒体膜电位检测试剂盒(JC-1)测定分离得到的线粒体的膜电位。
本试剂盒分离得到的线粒体也可以被试剂盒中的线粒体裂解液或其它适当裂解液裂解后用于SDS-PAGE、Western、双向电泳等蛋白分析。
本试剂盒提供了线粒体制备过程中匀浆程度的重要判断指标,即台盼蓝染色,使分离得到的线粒体的质量更加有保证。
台盼蓝染色液为选用试剂,在实验条件成熟后可以不必使用。
本试剂盒提供了蛋白酶抑制剂PMSF,使匀浆时蛋白酶的活性被适当抑制,这样在获得线粒体的同时还可以获得有时有用的去除了大量线粒体的蛋白,在进行蛋白或酶活分析时可以作为对照。
如果每个样品的细胞数量为2000-5000万,本试剂盒可以处理50-100个样品。
保存条件:-20℃保存,一年有效。
其中台盼蓝染色液也可以4℃保存,PMSF(晶体)和PMSF(溶剂)在配制成100mM PMSF溶液前可以室温保存。
注意事项:试剂盒中的试剂对于不同的实验目的不必全部使用。
如果不是用于制备线粒体蛋白样品,线粒体分离试剂和线粒体裂解液中不必加入PMSF。
如果用于制备线粒体蛋白样品,线粒体分离试剂和线粒体裂解液中需添加PMSF。
PMSF一定要在线粒体分离试剂或线粒体裂解液加入到样品中前2-3分钟内加入,以免PMSF在水溶液中很快失效。
分离线粒体的所有步骤均需在冰上或4℃进行,所用溶液需冰浴或4℃预冷。
通常在分离线粒体时前后两次离心速度选取600g和11,000g,如果希望纯度更高,但对线粒体的得率要求不高,前后两次离心速度可以采用1000g和3500g。
动物水平的绿原酸通过Notch1信号通路调控非小细胞肺癌凋亡及机制研究

动物水平的绿原酸通过Notch1信号通路调控非小细胞肺癌凋亡及机制研究李维;刘旭;张国倩;张琳琳【摘要】Background and objective It has been proven that chlorogenic acids can produce anticancer effects by regulating cell cycle, inducing apoptosis, inhibiting cell growth, Notch signaling pathways are closely related to many human tumors. The aim of this study is to study the mechanism of chlorogenic acid on apoptosis of non-small lung cancer through Notch1 pathway in animal level, and hope to provide theory basis on clinical treatment and research aimed at targeting Notch1 signaling in non-small cell carcinoma (NSCLC).Methods MTT assay was used to evaluate the A549 cell proliferation under the treatment of chlorogenic acid. The effect of chlorogenic acid on apop-totic and cell cycle were detected by flow cytometry. The animal model of A549 cell transplanted in nude was estab-lished, tumer size and weight were detected. The mRNA level of Notch1 signal pathway related facter were detected by RT-PCR; the expression of Notch1 signal pathway related facter in tumor tissue was detected by western blot.Results Chlorogenic acid inhibited the A549 cell proliferation. incresed cell apoptotic and cell percentagein G2/M (P<0.05), and in a dose-dependent manner. In animal model, tumer size and weight were lower than control group, the difference was statistically significant (P<0.05). The relative expression of mRNA of Notch1, VEGF, Delta4, HES1 and HEY1 were decreaced (P<0.05) in tumor tissue which treated withchlorogenic. The expression of Notch1 were decreaced, PTEN, p-PTEN, p-AKT were increced significantly in tumor tissue which treated with chlorogenic (P<0.05).Con-clusion Chlorogenic acid can regulate theapoptosis of non-small lung cancer through Notch pathway in animal level,which may be associated with the down-regulating the expression of VEGF and Delta4. Notch pathway may cross talk with PI3K/AKT pathway through PTEN in NSCLC.%背景与目的绿原酸类物质可通过调节细胞周期、诱导凋亡、抑制细胞生长等途径产生抗癌作用,Notch信号通路与人类许多肿瘤都存在密切的关系,本研究旨在探讨绿原酸通过Notch1信号通路控制非小细胞肺癌细胞凋亡的作用机制,为临床应用以及Notch1靶向药物的研究提供依据.方法 MTT法检测不同浓度的绿原酸对非小细胞肺癌细胞系A549细胞形态和细胞增殖的影响;流式细胞仪检测绿原酸对A549细胞的凋亡和细胞周期的影响;建立A549细胞的裸鼠荷瘤模型;测量肿瘤大小和重量;实时荧光定量PCR法检测Notch信号通路相关因子的mRNA表达水平;免疫印迹法检测Notch信号通路相关因子的蛋白表达水平.结果绿原酸抑制A549细胞增殖,诱导A549细胞凋亡,增加细胞G2期/M期细胞百分比增加(P<0.05),并且呈现剂量依赖趋势.在A549细胞的裸鼠荷瘤模型中,实验组肿瘤大小和体积明显小于对照组,差异具有统计学意义(P<0.01).试验组Notch1、VEGF、Delta4、HES1、HEY1 mRNA表达量较对照组明显减少(P<0.05).实验组Notch1蛋白明显减少,PTEN、p-PTEN、p-AKT明显增加(P<0.05).结论在动物水平,绿原酸可能通过Notch1信号通路调控非小细胞肺癌的凋亡,可能是通过减少VEGF的表达,下调Delta 4水平,从而抑制Notch1信号通路的活化.Notch1信号通路可能通过PTEN与PI3K/AKT通路存在交叉调控作用.【期刊名称】《中国肺癌杂志》【年(卷),期】2017(020)008【总页数】7页(P555-561)【关键词】肺肿瘤;肺癌细胞A549;绿原酸;Notch1信号通路;PI3K/AKT通路【作者】李维;刘旭;张国倩;张琳琳【作者单位】300193 天津,天津中医药大学第一附属医院检验科;300193 天津,天津中医药大学第一附属医院检验科;300193 天津,天津中医药大学第一附属医院检验科;300052 天津,天津医科大学总医院肿瘤科【正文语种】中文肺癌是严重危害人类健康的恶性肿瘤之一,无论其发病率还是死亡率均居恶性肿瘤之首,已经成为严重危害人类生命健康的常见疾病.非小细胞肺癌(non-small cell lung cancer, NSCLC)是肺癌的一种,其患者数量为肺癌患者总数的80%以上[1].目前NSCLC的治疗措施主要有手术、放疗、化疗及生物治疗等,早期手术可以治愈NSCLC,但大部分NSCLC患者确诊时已属晚期,目前尚无治愈的根本疗法.研究[2]表明,血管内皮生长因子(vascular endothelial growth factor, VEGF)及Notch信号通路,对肿瘤的形成和发展具有重要作用.Notch信号通路高度保守,由Notch受体、Notch配体、CSL DNA结合蛋白(CBF-1, suppressor of hairless, Lag)、其他的效应物和Notch的调节分子等组成.当相邻细胞的Notch受体、配体相互接触时,γ-分泌酶催化裂解形成Notch胞内段,之后Notch胞内段进入细胞核内结合并激活转录因子RBP-Jk,调控转录因子超级组成员Hes和Hey分子的转录,从而调节细胞的分化、发育、增殖、凋亡等过程.Notch通路还与其他信号通路存在交叉调控,从而扩大其生物调节作用[3].目前已有不少研究Notch通路与肺癌的关系[4,5],可是尚未形成一个明确的结论,而Notch通路生物学作用下的具体的机制更是十分缺乏,仍然有待进一步研究.近几年来,中药活性成分的抑癌作用得到了广泛关注,在诱导细胞凋亡方面的作用具有重要意义.绿原酸(chlorogenic acid, CGA)由奎尼酸(quinic acid, QA)与反式肉桂酸(trans cinnamic acidst-CA)缩合而成的酯类化合物家族,是多种中草药中的一种活性成分,具有抗炎作用、抗病毒作用、降血脂和血糖作用、抗氧化作用、增强机体免疫力等诸多生物学活性.已有研究[6]证明,绿原酸具有抑制肺癌细胞增值和转移,诱导肺癌细胞凋亡的作用.本研究建立了NSCLC细胞裸鼠模型,通过检测Notch 信号通路及相互作用信号通路的关键蛋白的表达,进一步阐明绿原酸通过Notch1信号通路调控NSCLC凋亡的作用机制.1 材料和方法1.1 药品和试剂绿原酸(250 mg, >98%)购自Sigma-Aldrich公司(溶解于1640培养基,0.22 μm滤膜过滤),胎牛血清购自上海沪峰化工有限公司,RPMI-1640培养液购自上海博麦德生物技术有限公司,胰蛋白酶购自GIBCO公司,Annexin V-FITC 细胞凋亡检测试剂盒购自碧云天生物技术研究所,TRizol购自北京百奥森泰生物科技有限公司,总蛋白提取试剂盒购自Sigma-Aldrich公司,BCA蛋白浓度测定试剂盒购自上海美吉生物医药科技有限公司,反转录试剂盒、SYBR Green Mix、DNA Marker购自北京全式金生物科技有限公司,PTEN抗体(兔抗)、p-PTEN抗体(p-Ser370,兔抗)、p-Akt(p-Ser473,兔抗)抗体购自Santa Cruz公司,Notch1抗体(兔抗)、GAPDH抗体(兔抗)、HRP-二抗(羊抗兔)等相关抗体购自Exapha Biologicals公司,其他试剂购自北京鼎国昌盛生物试剂公司.引物由上海英骏生物技术公司合成.1.2 主要设备多功能酶标仪(Wallac公司);TS-100倒置相差显微镜购自日本Nikon公司;Real-time PCR仪购自BIO-RAD公司,二氧化碳培养箱(RCO3000TVBA)购自美国 REVCO公司.1.3 实验动物实验用裸鼠BALB/c-nu由北京华阜康生物科技有限公司提供许可证号:[SCXK(京)2014-0004],均为4周龄的健康SPF级雄性小鼠,体重为(18.77±1.02)g,裸鼠在无特定病原体条件下的层流架内饲养,无菌操作下定期更换笼具、垫料、饮用水和标准饲料.1.4 细胞培养 NSCLC细胞株A549购自中国医学科学院中国协和医科大学细胞库,细胞培养液为含10%胎牛血清RPMI-1640,其中含终浓度为100 μg/mL青霉素和链霉素,2 mmol/L的L-谷氨酰胺;将细胞置于37 oC、5% CO2孵箱中培养;2 d传代一次,取对数生长期细胞用于实验.1.5 MTT法检测细胞增殖抑制率将对数生长期A549细胞接种于96孔板中,每孔接种1X105个细胞;培养24 h之后,在培养基加入梯度浓度的绿原酸(终浓度为:0、10 μg/mL、50 μg/mL、100 μg/mL),每个浓度设6个平行孔,绿原酸终浓度为0 μg/mL的为对照组;分别在药物处理A549细胞24 h、48 h、72 h后,每孔加入20 μL的5 mg/mL的MTT试剂,置于细胞培养箱中孵育4 h后吸出,加入150 μL 的DMSO试剂,充分震荡后,用多功能酶标仪在490 nm波长下测定吸光度.计算细胞增殖抑制率.抑制率(%)=(1-药物处理组OD值/对照组OD值)X100%.1.6 流式细胞仪检测细胞凋亡检测和细胞周期分析将对数生长期A549细胞接种于6孔板,调节细胞密度,2 mL/孔,每孔细胞数目大约为4X105个.24 h后加入系列浓度梯度的绿原酸培养基,使绿原酸的终浓度为:0、10 μg/mL、50 μg/mL、100μg/mL;常规培养72 h后收集细胞,用195 μL的Bind Buffier重悬细胞,加入5 μL Annexin V-FITC混匀,室温避光孵育10 min.离心去上清后,用190 μL的Bind Buffier重悬细胞,再加入10 μL碘化丙啶(100 mg/L),并与避光放置,随即进行流式细胞仪检测细胞凋亡情况,并用软件进行数据分析.另一组细胞采用梯度绿原酸处理细胞72 h后收集细胞,PBS清洗后用预冷的95%乙醇处理,离心去上清后,碘化丙啶(100 mg/L)(含RNase 1 g/L)0.5 mL,室温避光孵育30 min,随即进行流式细胞仪进行细胞周期分析.1.7 建立裸鼠成瘤模型大量培养A549肺癌细胞,将生长状态良好的细胞消化后计数,将2X106的肿瘤细胞种植于裸鼠皮下,共接种30只裸鼠,饲养观察裸鼠成瘤情况.在接种2周-3周后,肿瘤大小约1 cm3时,选择肿瘤大小接近的20只小鼠,根据随机分层法分组,分为2组.实验组每天在肿瘤区域注射绿原酸溶液(100 μg/mL);对照组每天注射相同体积的生理盐水;每次注射体积为100 μL.连续观察并测量肿瘤的生长情况:每周测肿瘤长径(L),短径(W).肿瘤大小按公式V=1/2LW2计算.给药4周后处死裸鼠取瘤称重,检查肝脏肺部肿瘤的转移情况.每个肿瘤取两部分,一部分用于提取瘤组织蛋白,另一部分提取组织RNA.1.8 实时荧光定量PCR(Real-time PCR)检测mRNA相对含量取100 mg左右肿瘤组织,加入1 mL TRIzol,用研钵研磨至无明显肉眼可见固体.按照说明书操作步骤,提取RNA.然后按照反转录试剂盒步骤,将提取的RNA进行反转录,得到的cDNA 用SYBR Green染料结合法,进行Real-time PCR.目的基因的相对表达量经过内参标化后分析(表1).1.9 免疫印迹(Western blot)检测蛋白表达取少量肿瘤组织,按照总蛋白提取试剂盒步骤,提取组织总蛋白,用BCA法进行蛋白浓度测定(具体步骤见商品说明书).将蛋白液与蛋白上样缓冲液混合后,100 oC变性10 min,然后进行SDS-聚丙烯酰胺凝胶电泳.电泳后,取下凝胶,标记方向后进行转膜,封闭,孵育一抗(兔源Notch1、p-PTEN、PTEN、p-Akt抗体按照1:800浓度稀释比稀释,GAPDH抗体按照1:2,000浓度比稀释),孵育二抗(羊抗兔二抗按照1:5,000浓度稀释比稀释),最后经过充分洗膜后,进行曝光,分析结果.1.10 统计学分析采用SPSS 18.0 统计学软件进行数据分析,数据均以均数±标准差(Mean±SD)表示,组间比较采用Student's t检验,P<0.05表示差异具有统计学意义.2 结果2.1 绿原酸对NSCLC A549细胞增殖的影响绿原酸处理A549细胞48 h后,对照组细胞生长状态较好;而给药组细胞随着绿原酸剂量的升高,细胞脱落悬浮,破碎增多,部分细胞呈凋亡状态.在绿原酸处理A549细胞24 h、48 h、72 h后,分别采用MTT法检测细胞增殖的影响.结果显示,与对照组比较,绿原酸对A549细胞的抑制率明显升高(P<0.05),并且呈现剂量依赖趋势.在100 μg/mL绿原酸作用在72 h时,抑制率达最高(61.63%)(表2).2.2 绿原酸对NSCLC A549细胞凋亡和细胞周期的影响流式细胞仪检测绿原酸作用于A549细胞72 h后,随着绿原酸浓度增加,G2期/M期细胞百分比逐渐增加,且均高于对照组,差异显著(P<0.05或P<0.01);随着给药浓度增加,细胞的凋亡率逐渐增加,均高于对照组,差异显著(P<0.05或P<0.01)(图1,表3).2.3 绿原酸对A549细胞裸鼠荷瘤模型肿瘤增殖的影响通过A549细胞裸鼠荷瘤模型发现,绿原酸能够抑制裸鼠肿瘤的生长增殖,实验组裸鼠肿瘤大小明显小于对照组,具有统计学差异(P<0.05),见表4、图2、图3.将裸鼠处死后,发现试验组裸鼠无转移瘤出现,而在对照组肺中发现有6例微小转移瘤.实验组肿瘤重量为(0.48±0.12)g,对照组为(0.83±0.22)g,两组比较差异具有统计学意义(P<0.01).2.4 绿原酸对Notch信号通路相关分子mRNA表达量的影响实验组Notch1的配体Delta4、VEGF的mRNA相对表达量明显小于与对照组(P<0.05).实验组Notch1信号通路下游效应分子HES1、HEY1的mRNA相对表达量明显降低(P<0.05).见图4.2.5 绿原酸对PTEN-PI3K/AKT通路相关因子蛋白表达的影响检测Notch1及PTEN-PI3K/AKT通路相关因子的蛋白表达水平,结果显示,与对照组比较,试验组Notch1蛋白表达明显降低,且p-PTEN、PTEN蛋白表达量升高,总的PTEN蛋白表达量增多;试验组p-Akt的表达量减少.见图5.表 1 相关基因PCR引物Tab 1 Related gene PCR primersGene name 5'-3'3'-5'GAPDH GAGTCAACGGATTTGGTCGT TTGATTTTGGAGGGATCTCG Delta 4 GCAGAACTTACATCACCTCA GCATTGCTGCCTCTAGTTAT VEGF TCGGGCCTCCGAAACCATGA CCTGGTGAGAGATCTGGTTC Notch1 ACCTCTTTGGGCTGGTATTG AACGGACAGCTTTGGATTTC HES1 TGAAGAAAGATAGCTCGCGG GGTACTTCCCCAGCACACTT HEY1 TGGATCACCTGAAAATGCTG CGAAATCCCAAACTCCGATA表 2 绿原酸对A549细胞增殖的抑制作用(Mean±SD)Tab 2 Inhibitory effect of chlorogenic acid on proliferation of A549 cells (Mean±SD)The experimental group compared with the control group, *P<0.05,**P<0.01.Group CGA(μg/mL)24 h 48 h 72 h OD490 Inhibitory ra te OD490 Inhibitory rate OD490 Inhibitory rate NC 0 0.75±0.02 0.81±0.03 0.86±0.03 Experimental group 10 0.70±0.05* 6.67% 0.71±0.05** 12.35% 0.68±0.07** 20.93%50 0.59±0.04** 21.33% 0.61±0.07** 24.69% 0.52±0.05** 39.53%100 0.44±0.03** 41.33% 0.45±0.08** 44.44% 0.33±0.09** 61.63%表 3 绿原酸对A549细胞周期分布时相的影响(Mean±SD)Tab 3 Effect of chlorogenic acid on the cell cycle profile of A549 cells (Mean±SD)The experimental group compared with the control group, *P<0.05,**P<0.01.Group CGA (μg/mL)Cell cycle G0/G1 S G2/M NC 0 66.7±0.69 25.9±0.60 7.2±0.74 Experimental group 10 57.1±0.72 28.9±0.6114.6±0.65*50 47.9±0.61 31.0±0.32 21.0±0.36**100 41.1±0.62 28.0±0.63 30.7±0.57**表 4 不同时间点裸鼠肿瘤大小的比较(Mean±SD, cm3)Tab 4 Comparison of tumor size in nude mice at different time points (Mea n±SD, cm3)The experimental group compared with the control group, *P<0.05,**P<0.01.Time NC Experimental group P 1st week 1.671±0.1241.222±0.048* <0.05 2nd week 1.960±0.171 1.401±0.078* <0.05 3rd week2.196±0.132 1.541±0.098** <0.01 4th week 2.472±0.118 1.706±0.112**<0.013 讨论绿原酸类物质是植物体中重要的次生代谢产物,广泛存在于高等双子叶植物和蕨类植物中,杜仲、金银花、咖啡等植物中绿原酸类物质含量较高.绿原酸类物质可通过调节细胞周期、诱导凋亡、抑制细胞生长等途径产生抗癌作用,对肺癌、乳腺癌、肝癌具有显著的抑制作用,被认为是癌症的有效化学防护剂[6-8].但绿原酸对肺癌的抑制作用机制的研究并不多见.Notch信号通路与人类许多肿瘤都存在密切的关系.Notch信号的产生是通过相邻细胞的Notch配体与受体相互作用,Notch蛋白经过三次剪切,由胞内段释放入胞质,激活HES、HEY、HERP等碱性-螺旋-环-螺旋(bHLH)转录抑制因子家族的靶基因,发挥生物学作用.Notch信号通路不仅在组织器官的正常发育中起作用,还与一些肿瘤的发生、发展密切相关[9-13].有研究表明Notch信号通路在肺癌的发生发展起着重要作用[14],但是具体的作用机制一直没有达成共识.本文通过研究Notch受体、配体以及相关因子在非小细胞肺癌细胞裸鼠荷瘤模型中的表达,探讨Notch1信号通路与肺癌发生发展的关系,阐明绿原酸抑制非小细胞肺癌细胞凋亡的原理,同时为以Notch1信号通路为靶点的治疗提供分子机制方面的理论支持.本研究发现,在细胞及动物水平上,绿原酸能有效抑制非小细胞肺癌细胞的生长增殖,促进细胞的凋亡.同时本研究发现实验组肺癌组织的Notch1 mRNA的平均表达水平显著下降,同时下调下游的HES1和HEY1 mRNA的表达,提示绿原酸可以通过Notch1信号通路转录水平调控非小细胞肺癌凋亡的.有研究[15]发现,抑制VEGF 表达可以达到抑制人非小细胞肺癌A549细胞裸鼠移植瘤的血管生成的作用.VEGF 可诱导Notch1配体Delta4表达增高,从而可启动Notch信号传导通路.研究发现Notch信号通路配体Delta4以及VEGF的表达水平,发现实验组减少Delta4以及VEGF的mRNA水平的表达.因此,推测绿原酸可能是通过减少VEGF的表达,下调Delta4水平,从而抑制Notch1信号通路的活化.图 1 绿原酸对A549细胞凋亡率的影响.实验组与对照组比较,*P<0.05,**P<0.01.Fig 1 Effect of chlorogenic acid on apoptosis rate of A549 cells. The experimental group compared with the control group,*P<0.05, **P<0.01.图 2 绿原酸对肿瘤生长增殖的影响Fig 2 Effect of chlorogenic acid on tumor growth and proliferation图 3 两组裸鼠肿瘤比较Fig 3 Comparison of nude mice tumers of two groups图 4 Real-time PCR检测Delta4、VEGF、Notch1、HES1、HEY1的mRNA表达水平.实验组与对照组比较,**P<0.01,*P<0.05.Fig 4 mRNA expression of Delta4, VEGF, Notch1, HES1,HEY1 detected by Real-time PCR. The experimental group compared with the control group, *P<0.05,**P<0.01. 图 5 Western blot检测Notch1、PTEN、p-PTEN、p-Akt蛋白表达水平Fig 5 Expression of Notch1, PTEN, p-PTEN, p-Akt protein detected by Western blotNotch信号通路不仅能够直接调控大量的基因表达,而且还可以与其他信号通路(包括TGF-β、NF-κB、Hif-1α等)相互作用而扩大其调控范围[16-18].近年来,Notch通路与PI3K-AKT等通路之间的互相作用受到广泛的关注抑癌基因PTEN在PI3K-AKT信号通路中发挥着至关重要的作用,PTEN的失活会导致PI3K-AKT通路活化,PTEN能够使磷脂酰肌醇(3,4,5)-三磷酸(PIP3)脱磷酸,使PIP3磷酸化呈低水平,抑制AKT的活化,从而下调PI3K/AKT通路相关因子表达.在PI3K-AKT通路中,PTEN有时候以磷酸化形式(p-PTEN)出现,继而启动下游级联反应,导致一系列生物学行为的发生.AKT磷酸化激活下游信号因子活化.本研究检测Notch1信号通路与PTEN-PI3K/AKT通路的相互作用是否存在交叉调控,结果发现,试验组的 p-PTEN、PTEN蛋白表达量都出现了一定程度的升高,总的PTEN蛋白表达量增多;与对照组比较,试验组p-Akt的表达量减少.提示Notch1通路可能通过对抑癌基因PTEN的调控来影响肺癌细胞的生物学功能,通过PTEN与PI3K/AKT通路存在交叉调控作用.Notch信号通路非常复杂,且调控机制目前仍未清楚阐明.Notch信号通路在肿瘤的发生发展中非常重要,但是目前该通路的研究以及Notch信号通路与其他信号通路的交叉调控方面的研究相对较少,希望能后续进一步详细阐明Notch信号通路的生物学作用机制,填补这一块研究的空白,为肺癌的靶向治疗提供更有力的理论基础. 参考文献【相关文献】1 Pisters KM, Chevalier TL. Adjuvant chemotherapy in completely resectednon-small-cell lung cancer. J Clin, 2005, 23(14): 3270-3278.2 Balint K, Xiao M, Pinnix CC, et al. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest, 2005, 115(11): 3166-3176.3 Mungamuri SK, Yang X, Thor AD, et al. Survival signaling by Notch1:mammalian target of Rapamycin (mTOR)-dependent inhibition of p53.Cancer Res, 2006, 66(9): 4715-4724.4 Zheng YF, Zheng ZH. Notch and Ras/MAPK signaling pathways in embryonic development and tumor occurrence. Yi Xue Fen Zi Sheng Wu Xue Za Zhi, 2006, 3(6): 460-463.[郑莺凤, 郑志竤. Notch与Ras/MAPK信号通路在胚胎发育及肿瘤发生中的串话. 医学分子生物学杂志, 2006,3(6): 460-463.]5 Sriuranpong V, Borges MW, Ravi RK, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res, 2001, 61(7):3200-3205.6 Tian W, Dou YW, Wang HT, et al. Study on apoptosis of lung cancer cells induced by chlorogenic acid and its mechanism. Jie Fang Jun Yu Fang Yi Xue Za Zhi, 2016, 34(6): 854-857.[田伟, 豆亚伟, 王宏涛, 等. 绿原酸诱导肺癌细胞凋亡及其机制研究. 解放军预防医学杂志, 2016,34(6):854-857.]7 Ye XL, Liu Y, Qiu G, et al. Inhibitory effiect of chlorogenic acid on mouse EMT-6 breast cancer. Zhong Yao Yao Li Yu Lin Chuang, 2012(1): 51-52.[叶晓林, 刘艳, 邱果, 等. 绿原酸对小鼠EMT-6乳腺癌抑制作用研究.中药药理与临床, 2012(1): 51-52.]8 Lai LL, Xiao Y, Peng XF, et al. Effiects of chlorogenic acid extract from eucommia ulmoides oliv. leaves combined with crocin on cholesterol metabolism in hepatocellular carcinoma cells. Shandong Yi Yao, 2017(8): 17-20.[赖玲林, 肖苑, 彭小芳, 等. 杜仲叶绿原酸提取物联合西红花苷对肝癌细胞胆固醇代谢的影响. 山东医药, 2017(8): 17-20.]9 Artavanistsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science, 1999, 284(5415): 770-776.10 Deftos ML, Bevan MJ. Notch signaling in T cell development. Curr Opin Immunol, 2000, 12(2): 166-172.11 Maillard I, Pear WS. Notch and cancer: Best to avoid the ups and downs.Cancer Cell, 2003, 3(3): 203-205.12 Balint K, Xiao M, Pinnix CC, et al. Activation of Notch l signaling is required for beta-caten in-mediated human primar melanom a progression. J Clin Invest, 2005, 115(11): 3166-3176.13 Weng AP, Aster JC. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science, 2004, 306(5694): 269-271.14 Collins BJ, Kleeberger W, Ball DW. Notch in lung development and lung cancer. Semin Cancer Biol, 2004, 14(5): 357-364..15 Jiang P, Cen HF, Mao Y, et al. Study on Notch signaling pathway and expression of VEGF protein in non-small cell lung cancer. Zhongguo Lin Chuang Yao Li Za Zhi, 2011,27(4): 265-267.[蒋鹏, 岑浩锋, 毛勇, 等. 非小细胞肺癌中Notch信号传导通路及VEGF蛋白表达的研究. 中国临床药理学杂志, 2011, 27(4): 265-267.]16 Palomero T, Wei KL, Odom DT, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci U S A, 2006, 103(48): 18261-18266.17 Poellinger L, Lendahl U. Modulating Notch signaling by pathway-intrinsic and pathway-extrinsic mechanisms. Curr Opin Genet Dev, 2008, 18(5):449-454.18 Samon JB, Champhekar A, Minter LM, et al. Notch1 and TGF beta1 cooperatively regulate Foxp3 expression and the maintenance of peripheral regulatory T cells. Blood, 2008, 112(5): 1813-1821.。
葫芦素B对人MCF-7细胞生物学行为的影响

济宁医学院学报2021年2月第44卷第1期 JJiningMedUniv,February2021,Vol 44,No.1DOI:10.3969/j.issn.1000 9760.2021.01.003·基础医学·葫芦素B对人MCF 7细胞生物学行为的影响黄擎擎1 王丹凤1 马 毅1 田力平2△(1江苏省原子医学研究所附属江原医院,无锡214063;2苏州大学附属第一医院,苏州215006) 摘 要 目的 探讨葫芦素B对激素受体阳性的人MCF 7乳腺癌细胞生物学行为的影响及其机制。
方法 培养人MCF 7细胞,随机分为对照组和20μM、50μM、100μM葫芦素B组。
SRB法观测葫芦素B对人MCF 7细胞增殖活性的影响,Hoechst33258凋亡实验观察葫芦素B对人MCF 7细胞凋亡的影响,WesternBlotting检测自噬相关蛋白。
结果 与对照组相比,葫芦素B各浓度组能显著抑制人MCF 7细胞增殖,增强其细胞凋亡,差异具有统计学意义(均P<0.05);葫芦素B各浓度组LC3 Ⅱ表达水平显著升高,LC3 Ⅰ、P62表达水平显著下降(均P<0.05)。
结论 葫芦素B可诱导人MCF 7细胞自噬和凋亡,抑制细胞增殖。
关键词 人MCF 7细胞;葫芦素B;细胞增殖;自噬中图分类号:Q786 文献标识码:A 文章编号:1000 9760(2021)02 010 04TheeffectofcucurbitacinBonthebiologicalbehaviorofMCF 7cellsHUANGQingqing1,WANGDanfeng1,MAYi1,TIANLiping2△(1JiangYuanHospitalAffiliatedtoJiangsuInstituteofNuclearMedcine,Wuxi214063,China;2TheFirstHospitalAffiliatedtoSoochowUniversity,Suzhou215006,China)Abstract:Objective ToinvestigatetheeffectofcucurbitacinBonthebiologicalbehaviorofhormonere ceptorpositivehumanMCF 7breastcancercellsanditsmechanism.Methods HumanMCF 7cellswerecul turedandrandomlydividedintocontrolgroupandcucurbitacinBgroups(20μM,50μM,100μM).TheeffectofcucurbitacinBontheproliferationofMCF 7cellswasobservedbySRBmethod,theeffectofcucurbitacinBontheapoptosisofMCF 7cellswasobservedbyHoechst33258apoptosisassay,andautophagyrelatedproteinsweredetectedbyWesternblotting.Results Comparedwiththecontrolgroup,cucurbitacinBcouldsignificantlyinhibittheproliferationrateofhumanMCF 7cellsandenhancetheirapoptosis(allP<0.05);theexpressionlevelsofLC3 ⅡincucurbitacinBgroupsweresignificantlyincreased,whiletheexpressionlevelsofLC3 IandP62weresignificantlydecreased(allP<0.05).Conclusion CucurbitacinBcaninhibitthepro liferationofMCF 7cellsandinduceautophagyandapoptosis.Keywords:MCF 7cells;CucurbitacinB;Cellproliferation;Autophagy 葫芦素B(CucurbitacinB)是从葫芦科植物中提取的四环三萜类化合物,是葫芦素家族中含量最丰富的成员,具有广泛的药理活性,如抗炎、保肝、提高机体免疫力等作用[1 2]。
经眼动脉介入化学疗法治疗视网膜母细胞瘤的研究进展

经眼动脉介入化学疗法治疗视网膜母细胞瘤的研究进展崔雪皓;季迅达;赵培泉【摘要】经眼动脉介入化学药物治疗(intra-arterial chemotherapy,IAC)是通过介入的方法从眼动脉向患眼注射化学治疗(化疗)药物,对视网膜母细胞瘤(retinoblastoma,RB)进行局部化疗的一种治疗方法.目前IAC治疗RB的常用药物有美法仑、拓扑替康和卡铂.经眼动脉注射化疗药物,可在局部形成高浓度药物作用,有效杀伤肿瘤细胞,同时可显著降低静脉化疗全身并发症的发生率.应用IAC治疗RB国际分期中B、C、D期及部分E期患者,可有效提高保眼率.IAC的主要并发症包括眼睑、结膜水肿,眼睑下垂,眼球运动障碍,玻璃体出血,视网膜、脉络膜缺血和一过性骨髓抑制等.目前,IAC技术已在国际上广泛开展,本文主要对其临床应用研究进行综述.%Intra-arterial chemotherapy (IAC) refers to the injection of chemotherapeutics into patients' eyes through arteria ophthalmica in order to local chemotherapy for retinoblastoma (RB).The common drugs in the treatment of RB include melphalan,topotecan and carboplatin.IAC can form the high-concentration drug effects on local areas.It can effectively kill tumors and significantly reduce the occurrence rate of complications after intravenous chemotherapy.Application of IAC can cure patients with RB in B,C,D and E stage in international criteria,which can effectively improve eye protection plications of IAC include eyelids edema,chemosis,blepharoptosis,ocular motility disorders,vitreous hemorrhage,ischemia of choroid or retina,transient myelosuppression,etc.It should be further conduction of long-term and large-sample cases studies on how to improve curative effects and reduce serious eye complications.【期刊名称】《眼科新进展》【年(卷),期】2018(038)004【总页数】4页(P385-388)【关键词】视网膜母细胞瘤;治疗;化学疗法;介入【作者】崔雪皓;季迅达;赵培泉【作者单位】200091 上海市,上海交通大学医学院附属新华医院眼科;200091 上海市,上海交通大学医学院附属新华医院眼科;200091 上海市,上海交通大学医学院附属新华医院眼科【正文语种】中文【中图分类】R739.72视网膜母细胞瘤(retinoblastoma,RB)是儿童期最常见的眼内原发恶性肿瘤,发病率为1/20 000~1/15 000[1-5]。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
REVIEWRational targeting of Notch signaling in cancerP Rizzo 1,C Osipo 1,K Foreman 1,T Golde 2,B Osborne 3and L Miele 11Breast Cancer Program,Cardinal Bernardin Cancer Center,Loyola University Chicago,Chicago,IL,USA;2Department ofNeurology,Mayo Clinic at Jacksonville,Jacksonville,FL,USA and 3Department of Veterinary and Animal Sciences,University of Massachusetts at Amherst,Amherst,MA,USAAccumulating preclinical and clinical evidence supports a pro-oncogenic function for Notch signaling in several solid tumors,particularly but not exclusively in breast cancer.Notch inhibitory agents,such as c -secretase inhibitors,are being investigated as candidate cancer therapeutic agents.Interest in therapeutic modulation of the Notch pathway has been increased by recent reports,indicating that its role is important in controlling the fate of putative ‘breast cancer stem cells’.However,as is the case for most targeted therapies,successful targeting of Notch signaling in cancer will require a considerable refinement of our understanding of the regulation of this pathway and its effects in both normal and cancer cells.Notch signaling has bidirectional ‘cross talk’interaction with multiple other pathways that include candidate therapeutic targets.Understanding these interactions will greatly increase our ability to design rational combination regimens.To determine which patients are most likely to benefit from treatment with Notch inhibitors,it will be necessary to develop molecular tests to accurately measure pathway activity in specific tumors.Finally,mechanism-based toxicities will have to be addressed by a careful choice of therapeutic agents,combinations and regimens.This article summarizes the current state of the field,and briefly describes opportunities and challenges for Notch-targeted therapies in oncology.Oncogene (2008)27,5124–5131;doi:10.1038/onc.2008.226Keywords:Notch;cancer;targeted therapyIntroduction:why target the Notch pathway?For those of us involved in the pharmacological treatment of human cancers,whether experimental or standard of care,the most frustrating feature of neoplastic diseases is the inherent ability of cancer cells to respond to therapy by adapting and/or selecting resistant subclones.In clinical practice,this results in drug-resistant recurrence of disease,often in a meta-static form even after apparent eradication.Much of themorbidity and mortality from cancer is the result of such recurrences.For decades,we have been searching for a ‘magic bullet’that could hit an elusive target only cancer cells depend upon.Yet,to date most of pharmacological cancer treatments still rely on the relatively blunt instruments of traditional chemotherapy.Targeted agents are slowly but steadily being added to our therapeutic arsenal.These are directed largely at growth factor receptors or their associated kinase activities,as well as angiogenic growth factors.Monoclonal anti-bodies (mAbs)such as trastuzumab,pertuzumab,rituximab,bevacizumab and others,as well as tyrosine kinase inhibitors,such as imatinib,gefitinib,lapatinib and others have gained clinical acceptance as part of combination regimens.Some of these agents have proven to be remarkably effective.Yet,even with these agents resistance is observed.Our increasing understanding of cancer biology is beginning to explain the reasons for these therapeutic failures (Hanahan and Weinberg,2000).First,decades of signal transduction research have revealed that the receptors,enzymes and transcription factors that regulate cell fate are virtually all connected into an amazingly complex network of cross-regulatory interac-tions.The Internet rather than an old-fashioned machine may be an apt metaphor for the workings of cell signaling.Even if one or several ‘routers’are taken out of commission,Internet traffic can be rerouted around them.To completely stop Internet traffic,a very large number of routers or cables need to be disabled.Second,we have learned that the cell-fate control system is not only interconnected but also highly redundant,such that if a gene or protein is disabled,another can perform a similar function.Connectivity and redun-dancy mean that if the system is stressed (for example,by a targeted drug that blocks a growth factor receptor),the system can reset itself to a new status that works around the block or replaces the disabled receptor with a similar one.All this makes evolutionary sense.Eukaryotic cells are very complex and delicate objects with tens of thousands of moving parts.To allow survival in an often forbidding environment,evolution has created a robust system of cell-fate regulation that is highly resistant to the loss of one or even a few components.Unfortunately,this adaptability is hijacked by cancer cells,making the search for ‘magic bullets’a very frustrating task.Even though cancers can becomeCorrespondence:Professor L Miele,Breast Cancer Program,Cardinal Bernardin Cancer Center,Loyola University,2160South First Avenue,Room 236,Maywood,Chicago,IL 60163,USA.E-mail:lmiele@Oncogene (2008)27,5124–5131&2008Macmillan Publishers Limited All rights reserved 0950-9232/08$30.00/onc‘addicted’to specific pathways,they are remarkably adept at weaning themselves from their addictions if the need arises,andfind alternatives to pathways hit by therapeutics.This is,in part,because the genomic instability resulting from loss of DNA repair check-points,in transformed cells makes these cells capable of selecting drug-resistant mutants by utilizing the built-in connectivity and redundancy in signaling.Thus,the reason why‘magic bullets’have been so hard tofind is that there are very few,if any,individual,irreplaceable targets that are uniquely associated with cancer cells. Is there any hope offinding an Achilles’heel in cancer?Perhaps.Not all pathways are created equal. Some of the most ancient cell-fate control pathways which evolved with the initial appearance of multi-cellular organisms are conserved in all living organisms. These pathways function as‘Internet service providers’in the signaling network.That is,they are multi-functional and control key nodes in the traffic of signaling information that regulates differentiation, proliferation and survival.The Wnt,Hedgehog and Notch pathways belong to this‘aristocracy’of signaling systems.Not surprisingly,they are important in devel-opmental biology and come into play whenever critical cell-fate decisions are made.Learning how to safely and effectively manipulate these pathways in cancer cells may bring us the next quantum leap in cancer therapy. The Notch pathway,the subject of this article,is a short-range communication system in which contact between a cell expressing a membrane-associated ligand and a cell expressing a transmembrane receptor sends the receptor-expressing cell(and quite possibly both cells)a cell-fate regulatory signal.This signal takes the form of a cascade of transcriptional regulatory events that affects the expression of hundreds if not thousands of genes,and has profound phenotypic consequences that are context dependent.The basic features of the pathway and the possible biological roles of Notch signaling in human malignancies have been discussed in several recent reviews(Allenspach et al.,2002;Miele, 2006;Miele et al.,2006;Berman and Look,2007;Koch and Radtke,2007;Roy et al.,2007;Shih and Wang, 2007).In most cases,its deregulation has oncogenic effects,with the notable exception of epidermal kerati-nocytes where Notch-1functions as a tumor suppressor. In several malignancies going from T-cell acute lympho-blastic leukemia(Roy et al.,2007)to breast cancer (Reedijk et al.,2005;Dickson et al.,2007)to melanoma (Pinnix and Herlyn,2007)to lung adenocarcinoma (Chen et al.,2007)and others,the inappropriate activation of Notch signaling results in signals that stimulate proliferation,restrict differentiation and pre-vent apoptosis in cancer cells.As a result,Notch signaling inhibitors are being actively investigated for the treatment of a variety of malignancies.An additional reason for focusing on Notch and other ancient developmental pathways is that in recent years, a distinct cellular hierarchy has been identified in hematopoietic and some solid tumors.Many cancers appear to contain a small population of pluripotent ‘stem cells’or‘tumor-initiating cells’that give rise to the bulk population of cancer cells through a process of aberrant differentiation that recapitulates that of normal tissues(Song and Miele,2007).These‘cancerstem cells’are characterized by properties of normalstem cells,such as indefinite self-replication through asymmetric cell division,very slow proliferation ratesand resistance to toxic agents due in part to high-level expression of ABC transporters.Whether cancer stemcells are derived from the malignant transformation of normal tissue stem cells or from the‘dedifferentiation’of normal non-stem cells is a matter of considerable debate.What seems likely is that these cells are uniquely capable of resisting anticancer agents,surviving for along time in a nearly quiescent status and produce recurrences and metastases.Thus,a complete eradica-tion of these cells will be necessary to attain a cure.Thiswill require targeting of pathways that participate in the survival,replication and differentiation decisions in undifferentiated,pluripotent cells,such as the Wnt, Hedgehog and Notch pathways(Song and Miele,2007). Indeed,there is significant evidence that Notch is relevant to the survival of breast cancer‘stem cells’(Farnie et al.,2005,2007;Farnie and Clarke,2007; Sansone et al.,2007).Finally,the interaction between cancer cells and the surrounding stroma is receiving increasing attention as akey factor in tumor progression.‘Tumor stroma’includes endothelial cells,necessary for tumor angiogen-esis,fibroblasts that can produce growth factors and cytokines,as well as many subtypes of immunocytes,from T cells to dendritic cells to NK cells that can affect tumor progression either favorably or unfavorably. There is significant evidence that bidirectional inter-cellular communication involving Notch signals takesplace between tumor cells and stromal cells in some malignancies(Jundt et al.,2002,2004;Houde et al., 2004;Zeng et al.,2005),suggesting that targeting the Notch–ligand interaction in endothelial cells can have therapeutic applications(Yan and Plowman,2007). Moreover,Notch signaling has multifaceted functionsin the immune system that need to be taken into accountwhen planning therapeutic interventions(Dallman et al., 2005;McKenzie et al.,2005;Minato and Yasutomo, 2005;Minter et al.,2005;Tu et al.,2005).This article discusses current efforts to develop Notch-targeted cancer therapeutics by both small molecules and biologics,including pros and cons of different targeting strategies as well as identifying challenges to overcome. Targeting Notch in cancerFor targeting purposes,some features of the Notch pathway have unique relevance.First,the fact that the signaling cascade triggered by Notch–ligand interactionsdoes not include an enzymatic amplification step(for example,a nucleotide cyclase or a kinase)means that‘signal intensity’can be modulated very precisely by cellular regulatory mechanisms.As a result,the down-stream effects of Notch activation are exquisitelydose Targeting Notch in the treatment of cancerP Rizzo et al5125Oncogenedependent (Miele et al .,2006).This means that complete shutdown of the pathway may not always be necessary to achieve a therapeutic effect.A second key feature is that the intracellular half-life of the active form of Notch is generally very short,in the order of minutes,though it may be longer in transformed cells (Weijzen et al .,2002).The Notch signal is essentially a short pulse of gene regulation (Miele et al .,2006).This implies that sustained inhibition may not be always necessary and that intermittent inhibition may be successful.A third important feature to keep in mind is that the effects of Notch are remarkably context dependent.This means that Notch signals can be used for different purposes in different cell types,and for each cell type the effects of Notch manipulations need to be investigated without preconceived assumptions.Systemic inhibition of Notch signaling is likely to have a multitude of effects in different cell types.Thus,for therapeutic purposes we shall have to determine whether there is a level (or timing)of Notch inhibition that is sufficient to attain efficacy in disease control without causing intolerable adverse effects.An alternate strategy may be to identify more context-specific targets within the Notch pathway,selectively effective drug combinations (based on cell-specific cross talk)or designing more selective delivery strategies for Notch inhibitors.Inhibition of Notch signaling can be achieved theoretically at many different levels.Agents targeted to some of these levels have been described,whereas others remain hypothetical possibilities (Figure 1).It is possible to interfere with Notch–ligand interactions by using receptor decoys (Nickoloff et al .,2002),blocking ligand ubiquitination/trans-endocytosis (Pitsouli and Delidakis,2005)or Notch receptor fucosylation (Okajima and Irvine,2002).It is possible to interfere with receptor activation by blocking ligand-induced conformational changes in Notch receptors (Gordon et al .,2007),receptor cleavage by ADAM proteases (Brou et al .,2000)or g -secretase (Kopan and Ilagan,2004;Miele et al .,2006),as well as Notch mono-ubiquitination (Gupta-Rossi et al .,2004).Finally,it is conceivable that Notch signaling could be inhibited by disrupting protein–protein interactions involved in Notch-dependent nuclear events (Nam et al .,2006,2007),including assembly of co-activators with the Notch transcriptional complex (NTC)and formation of higher order DNA-bound complexes.As of this writing,g -secretase inhibitors (GSIs)are in early clinical trials in various institutions,and mAbs that ‘lock’Notch receptors in an inactive conformation by binding to the ‘negative regulatory region’(NRR)are in preclinical development (Li et al .,2008).Moreover,mAbs that target Notch ligand DLL4(Ridgway et al .,2006)have been shown to inhibit Notch signaling in endothelial cells and cause disorganized angiogenesis.These mAbs are currently being developed as antineoplastic agents (Noguera-Troise et al .,2007;Thurston et al .,2007;Yan and Plowman,2007).Each of these approaches has potential advantages and disadvantages.GSIs are active in several experi-mental models and have the advantage of relative easeof administration,oral bioavailability and low cost.In general,small molecules can be dosed more precisely than biologics because of their relatively short biological half-life and simpler dose–response relationships,and this may be important in a field where the therapeutic window may be small.An additional potential advan-tage is the fact that a single agent can block the activation of all four Notch homologues.In our experience and that of others,some solid tumors such as breast cancers and melanomas co-express several Notch homologues,and it is conceivable that redun-dancy between them could blunt the effects of more selective inhibitors and lead to resistance.On the other hand,there is some evidence that different Notch homologues may have opposite effects.For instance,Notch-2appears to counteract the pro-oncogenic effects of Notch-1and Notch-4in breast cancer cells (O’NeillADAM γ-secretaseUQ ligaseUQActivation EndocytosisUQLigand-N EC Trans-endocytosisNeuralized, MindbombMAML1SKIPCBF-1Notch target genesNTC, monomericor dimeric**ADAM inh.Conformation-stabilizing mAbReceptor decoyGSIs, GSMsCross-talk, non-canonical pathways?p300Ligand-blocking mAbFucosylation inh.***miRNAs siRNAsNotch expression controlNumbα-adaptinEndocytosisKurzDeltex Arrestin-mediated DegradationFigure 1Diagram of putative therapeutic targets in the Notch pathway.(Red)Flat-tipped arrows indicate inhibitors.(Green)Sharp-pointed arrows indicate stimulation of Notch degradation by the Numb pathway (e.g.,by inducing Numb expression)or the b -arrestin/Kurz pathway (e.g.,by inducing expression of b -arrestin or Kurz).Asterisks indicate targets for which specific agents have been developed to date.Question marks indicate potential targets,yet unverified.Theoretically,inhibition of Notch signaling could be achieved by targeting ligand ubiqutination (UQ),ligand-receptor interaction,N EC /N IC dissociation,a disintegrin and metalloprotease (ADAM)-mediated cleavage,receptor ubiquitination/endocytosis,g -secretase cleavage,assembly of the co-activator complex with Notch andC-promoter binding factor 1(CBF-1)and heterodimer-ization of DNA-bound Notch transcriptional complexes (NTCs).In addition,expression of receptors and ligands as well as expression of selected downstream Notch targets could be conceivably targeted (e.g.,by modulating pathways that regulate Notch or ligand expression,or by using RNA-targeted therapies to modulate translation of Notch targets.Targeting Notch in the treatment of cancerP Rizzo et al5126Oncogeneet al.,2007),and its expression correlates with better differentiated tumors(Parr et al.,2004)unlike that of Notch-1and Jagged-1,which correlate with poor prognosis(Reedijk et al.,2005;Dickson et al.,2007). Similar differences have been noted in embryonal brain tumors(Fan et al.,2004),where conversely it is Notch-2 that has an oncogenic role.Another obvious potential disadvantage of GSIs is off-target effects.Most GSIs in development are competitive inhibitors that mimic the structure of short hydrophobic peptides.Given the fact that g-secretase has numerous targets other than Notch receptors and a rather promiscuous cleavage specificity (Kopan and Ilagan,2004),it is to be expected that GSIs will not specifically inhibit the cleavage of Notch receptors.It should be noted that off-target effects are the rule rather than the exception for most approved small molecule drugs.The mere fact that an agent can have off-target effects should not discourage its therapeutic development,unless it can be demonstrated that a more selective agent offers safety or efficacy advantages.The recent discovery that some nonsteroidal antiinflammatory drugs and structurally related com-pounds can allosterically modify the substrate specificity of g-secretase and decrease or increase the production of A b42amyloid peptide(K ukar and Golde,2008)raises the prospect that a class of g-secretase modifiers(GSM) can be developed that are capable of selectively modulate the cleavage of Notch receptors as opposed to other substrates,though specificity for individual Notch homologues is unlikely.Is there a role for biologics(mAbs or recombinant decoys)in Notch modulation?If specificity for an individual receptor or ligand is desirable,biologics are more likely to deliver such specificity than small molecules.Particularly mAbs,if epitopes are carefully selected,can achieve exquisite specificity and very high affinities.One situation in which a specific biologic may be preferable is if the target has a relatively restricted expression pattern compared to other Notch pathway members and/or can be replaced through redundancy in nontarget tissues.This may limit mechanism-based toxicities.For example,the expression pattern of Notch-4appears to be considerably more restricted than that of Notch-1,suggesting that agents targeting Notch-4may have less systemic toxicity than agents targeting Notch-1.The mAbs to DLL4(Noguera-Troise et al.,2007;Thurston et al.,2007;Yan and Plowman, 2007)represent a promising approach in this direction. These mAbs exploit the role of DLL4–Notch interac-tions in endothelial cell activation.In xenograft models, they appear to target tumor angiogenesis by causing disorganized blood vessel development but do not have the same side effects caused by systemic Notch inhibitors.An additional potential advantage of bio-logics and mAbs in particular is the ability to conjugate them with radionuclides or toxins to selectively target cells that overexpress their targets.In the case of Notch pathway components,this approach would require a target that is at least relatively specific to cancer cells or significantly overexpressed by them(for example, Jagged-2in multiple myeloma(Houde et al.,2004).The potential disadvantages of biologics in this setting include their generally complex dose–response curves invivo and their long biological half-lives.If intermittent inhibition of Notch signaling is desirable to minimize adverse effects,using an mAb that will remain in circulation for days or weeks may prove challenging in terms of regimen design.Of course,the biologicalhalf-lives of mAbs can be modulated recombinant engineering or generation of F(ab)2s,F(ab)s or even single chain Fvs.Indirect mechanisms of modulating Notch signaling without directly engaging pathway members with drugsor biologics can be envisioned.Modulating the expres-sion of Notch receptors,ligands or downstream mediators is conceivable.This approach will require a detailed understanding of the transcriptional,transla-tional and post-translational regulation of Notch pathway component expression in specific cellular contexts.At the moment,our knowledge in this area isstill limited,with few exceptions.Expression of Jagged-1was reported to be increased by nuclear factor(NF)-k B (Bash et al.,1999).Transcription of Notch-4in endothelial cells is induced by AP-1(Wu et al.,2005)and the glucocorticoid receptor(Wu and Bresnick, 2007),which uncharacteristically act synergistically atthe Notch-4promoter.Notch-1has been reported to upregulate its own transcription(Deftos et al.,1998)and that of Notch-4(Weijzen et al.,2002).The Ras (Weijzen et al.,2002),AKT(Liu et al.,2003)and mitogen-activated protein kinases(Zeng et al.,2005) pathways have been reported to stimulate Notch activityin some experimental models,suggesting that inhibitorsof these pathways may affect Notch signaling in some situations.Some isoforms of transcriptional regulator Ikaros inhibit Notch-dependent transcription in normaland neoplastic T cells(Beverly and Capobianco,2003; Bellavia et al.,2007;Kathrein et al.,2008).Corepressor SHARP(Oswald et al.,2002,2005)associates with the Notch transcriptional complex and recruits CtIP/CtBPto block Notch-dependent transcription.If expression of Ikaros or SHARP could be modulated in specific cell types,this would result in selective Notch inhibition.In Drosophila,various microRNAs(miRNAs)regulate Notch signaling through the30-untranslated sequencesof numerous Notch target genes(Kwon et al.,2005;Lai et al.,2005),and conversely miRNAs have been suggested to mediate some Notch effects in Caenorhab-ditis elegans(Yoo and Greenwald,2005),raising the possibility that RNA-based therapeutics(siRNAs or miRNAs)could be used to modulate Notch signaling by targeting specific subsets of Notch targets.At the post-transcriptional level,Notch-1protein levels and activityare regulated by several ubiquitin ligases that mediate either the degradation of Notch by polyubiquitinationor conversely,its activation by monoubiqutination (Miele et al.,2006).One of these ligases(SEL10/Fbw7/Ago/hCDC4),responsible for the polyubiquitinationand degradation of nuclear Notch-1is mutated in someT-cell acute lymphoblastic leukemia(T-ALL)cases, resulting in prolonged Notch-1signals(Malyukovaet al.,2007;O’neil et al.,2007;Thompson et al.,2007). Targeting Notch in the treatment of cancerP Rizzo et al5127OncogeneDesensitization of Notch-1signaling by nonvisual b -arrestin and Kurz (Mukherjee et al .,2005)or endocytosis and degradation of Notch-1mediated by Numb (Santolini et al .,2000;Pece et al .,2004)are also potential avenues to regulate Notch signaling if expres-sion of Kurz or Numb can be modulated in cell-specific fashions.Are there situations in which stimulation of Notch signaling can be envisioned as a therapeutic strategy in cancer?The strongest evidence for a tumor-suppressive role of Notch-1is in epidermal keratinocytes (Nicolas et al .,2003;Koch and Radtke,2007),because kerati-nocyte-targeted Notch-1knockout in mice increases chemical carcinogenesis in the skin.However,it is difficult to envision chronic treatment of basal cell carcinomas with Notch ligands,given that these lesions can be generally cured by excision.In the context of tumor immunology,it is conceivable that Notch activation in T cells or other immunocytes may promote tumor rejection induced by a therapeutic tumor vaccine.This may be achieved,for example,by expressing a Notch ligand in antigen-presenting cells such as dendritic cells.We still do not have a clear under-standing of the multiple functions of Notch signaling in the peripheral immune system,where it could modulate Th1-type (Minter et al .,2005)or Th-2-type (Tu et al .,2005)T-cell responses.It is conceivable that slightly different strategies for stimulation or slightly different signal strengths could produce either effect.Systemic delivery of a Notch stimulator may pose pharmacolo-gical challenges,because physiologically Notch ligands are transmembrane proteins.The mechanism of Notch activation requires trans-endocytosis of the Notch extracellular subunit N EC into the ligand-expressing cell,which in turn requires monoubiquitination of ligand intracellular tails (Miele et al .,2006).This is because the dissociation of N EC from the transmembrane subunit N TM requires significant mechanical strength to disrupt hydrophobic interactions in the NRR at the interaction site between the two subunits (Gordon et al .,2007).Thus,cell-associated or at least solid phase-bound ligands (for example,ligand-coated beads)are expected to be more effective than soluble ligands.Having said that,oligopeptides mimicking the conserved DSL region of Notch ligands can activate Notch receptors,albeit at high concentrations (Nickoloff et al .,2002).Fc fusion constructs that may associate with Fc receptors may be another strategy,particularly if the objective is immuno-modulation.ToxicityAlthough there is wide agreement that targeting cell-fate modulatory pathways is one of the most attractive new avenues in experimental cancer therapy,it would be naive to expect the modulation of such ancient,pervasively important pathways to have no adverse effects.Thus,key components of studying the possibility of targeting Notch in cancer must be the identificationof clinically relevant toxicities and the development of strategies to prevent or ameliorate them.Early clinical experience with GSIs indicates that the main adverse event in patients is dose-limiting secretory diarrhea caused by goblet cell metaplasia of the small intestine,which was first observed in preclinical models (Wong et al .,2004).The absence of myelotoxicity is welcome news in the setting of cancer chemotherapy.In mice,other adverse effects of systemic GSI treatment include reversible thymic suppression (Wong et al .,2004)and,in our hands,reversible hair depigmentation.Hair loss in dose-escalation experiments is an indication that a toxic dose has been reached and is associated with diarrhea and weight loss.Skin tumors have not been observed in humans and we have not observed them in mice treated for several weeks,though patches of mild,reversible hyperkeratosis are observed in nude mice.It is possible that life-long and/or complete shutdown of Notch signaling is required for more severe skin proliferative phenotypes.In both preclinical models and clinical studies,intermittent rather than continuous oral administration of GSIs greatly ameliorates the intestinal toxicity,presumably because it allows at least some intestinal stem cells to correctly differentiate as enterocytes.Parenteral administration of GSIs in mouse xenograft models in our hands was associated with less severe side effects,and doses that caused significant antineoplastic effects did not cause diarrhea or weight loss (Nickoloff et al .,2005),(Rizzo et al .,submitted for publication).In mice,GSIs have immunosuppressive effects that may be undesirable under some circum-stances.However,these effects may find clinical applica-tions of their own in autoimmune disorders such as multiple sclerosis (Minter et al .,2005),and could potentially be indirectly useful in oncology in the treatment of graft-versus-host disease after bone marrow transplantation (Minter et al .,2005).GSIs are not significantly myelotoxic,making such a potential appli-cation at least theoretically feasible.Whether prolonged Notch inhibition by agents that pass the blood–brain barriers is associated with neurological toxicity is unknown at this time,but possible based on mouse data (Wang et al .,2004).At this time,it would appear that relatively short-term (days to weeks)systemic suppres-sion of Notch signaling is consistent with a reasonable safety profile,especially in the context of cancer therapy.Perhaps the best way to use systemic Notch inhibitors in cancer may turn out to be as multiple cycles separated by ‘drug holidays’.Chronic,long-term suppression of Notch signaling is likely to require more selective agents,such as DLL4antibodies in the context of antiangiogenic therapy,or more targeted delivery strategies of Notch inhibitors (for example,encapsulated within tumor-targeted nanocarriers).Looking to the future:rational combinations including Notch inhibitors and individualized medicineLong-term therapeutic success in cancer is rarely achieved with monotherapy,and eventargetingTargeting Notch in the treatment of cancerP Rizzo et al5128Oncogene。
