Angiotensin II Induces Epithelial-to-Mesenchymal Transition through activation of EGFR
传统肺减容术和经支气管镜肺减容术的比较

传统肺减容术和经支气管镜肺减容术的比较邹雷 刘翱作者单位:650032 解放军昆明总医院呼吸内科 慢性阻塞性肺疾病(COP D )是严重危害人类健康的多发性疾病之一,也是继心血管疾病、癌症、脑血管疾病之后第4位引起死亡的重要疾病[1]。
WHO 近期公布资料表明,2000年有274万人死于C OP D 。
由于大气污染和吸烟等危害因素,其发病率和死亡率在未来10年内将继续升高。
1990年COP D 造成的医疗经济负担为第12位,至2020年将上升为第5位[2]。
由于C OP D 严重危害人类健康,已引起了世界各国政府及卫生机构的重视并加大了防治的研究力度。
COP D 的治疗主要以对症治疗为主,对晚期患者的治疗目前主要有两种方法,第一种为传统肺减容术(lung volu me reducti on surgery ,LVRS ),国内开展该手术近十年,国外二十余年。
但随时间的推移人们也越来越认识到LVRS 的不足,很多术式都被淘汰,从而在LVRS 的发展道路上也变得越来越谨小慎微了[3]。
另一种为近几年在国外开始出现的经支气管镜肺减容术(B r onchoscop ic lung volu me reducti on,BLVR ),它的出现似乎解决了LVRS 所面临的尴尬境地。
下面就目前国内外这两种最主要的治疗方法进行一个综述和对比。
肺减容术发展简介肺减容术(LVRS ):是指通过外科手术切除严重充气的肺组织,使其它相对正常的肺组织得以膨胀,从而改善肺功能、改善通气功能的手术。
早在1957年B rantigan 就提出了切除部分气肿肺组织,可使胸腔压力下降,肺功能获得明显改善,用于治疗严重肺气肿,这种手术称为肺减容术。
但由于术后严重并发症和手术近期的高死亡率(16%)使得人们望而却步,从而未获重视。
直到1995年,Cooper 等[4]在多年从事肺移植的基础上,经胸骨正中切开入路行双侧肺减容术,取得类似于肺移植术的疗效。
血管紧张素 II 通过细胞外信号调节激酶1/2通路调控过氧化氢酶的表达及促进成纤维细胞表型转化

血管紧张素 II 通过细胞外信号调节激酶1/2通路调控过氧化氢酶的表达及促进成纤维细胞表型转化沈凯;陈芬;林卓明;陈士良;袁国裕;刘晓光【摘要】目的:探讨细胞外信号调节激酶1/2( ERK1/2)在高血压大鼠模型动脉外膜血管重塑中的作用。
方法:利用血管紧张素II ( Ang II)微泵灌注制备高血压大鼠模型,随机分为未处理组、生理盐水灌注组和Ang II灌注组。
分别检测各组大鼠尾动脉收缩压及血管形态学改变;Western blotting技术检测外膜成纤维细胞过氧化氢酶( CAT)蛋白在未处理组、单纯Ang II、ERK1/2抑制剂PD98059和Ang II+PD98059培养下的表达。
结果:大鼠颈动脉HE染色和收缩压结果显示,与未处理组及生理盐水灌注组相比,Ang II组大鼠颈动脉中膜厚度和收缩压明显增加(P<0.01),动脉形态结构有明显改变,并且有显著的病理性血管重塑发生。
Western blotting 检测结果显示, PD98059作用下CAT比单纯Ang II明显增高(P<0.05),表明ERK1/2信号通路能够恢复Ang II诱导的CAT表达下调。
结论:Ang II可能通过ERK1/2信号通路下调血管外膜CAT的表达,进而促进血管细胞表型转化,导致血管病理性重塑发生。
%AIM:To explore the effect of extracellular signal-regulated kinase 1/2 ( ERK1/2) on the vascular adventitial remodeling in a hypertension ratmodel .METHODS:The rats were randomly divided into control group , mini-pump infusion of saline group and mini-pump infusion of angiotensin II ( Ang II) group as the hypertension model .The sys-tolic pressure and vascular morphology of the rats were examined .Adventitial fibroblasts were treated with Ang II , PD98059 ( ERK1/2 inhibitor) and Ang II+PD98059.The catalase ( CAT) expression in the cells was detected byWestern blotting . RESULTS:Compared with control group and mini-pump infusion of saline group , the systolic pressure and carotid media thickness (stained by HE) in mini-pump infusion of Ang II group were significantly increased (P<0.01).Meanwhile, ar-tery morphology in mini-pump infusion of Ang II group had obviously changed with a significant occurrence of pathological vascular remodeling .The result of Western blotting showed that the expression of CAT in the adventitial fibroblasts treated with Ang II+PD98059 was much higher than that in the cells treated with Ang II alone (P<0.05), indicating that down-regulation of CAT induced by Ang II was restored by ERK 1/2 signaling pathway .CONCLUSION:Ang II down-regulates CAT through ERK1/2 pathway and promotes cell phenotype transformation , which lead to pathological vascular remodeling .【期刊名称】《中国病理生理杂志》【年(卷),期】2014(000)004【总页数】4页(P711-714)【关键词】血管紧张素II;ERK1/2通路;过氧化氢酶;表型转化【作者】沈凯;陈芬;林卓明;陈士良;袁国裕;刘晓光【作者单位】温州医科大学附属舟山医院心血管内科,浙江舟山316004;温州医科大学附属舟山医院细胞分子生物学实验室,浙江舟山316004;温州医科大学附属舟山医院细胞分子生物学实验室,浙江舟山316004;温州医科大学附属舟山医院心血管内科,浙江舟山316004;温州医科大学附属舟山医院心血管内科,浙江舟山316004;温州医科大学附属舟山医院细胞分子生物学实验室,浙江舟山316004【正文语种】中文【中图分类】R329.21血管重塑是指血管内径和血管壁厚度的适应性变化,是高血压病显著病理特征和并发症产生的主要原因[1]。
肾素-血管紧张素-醛固酮系统与妊娠高血压病的关系

肾素-血管紧张素-醛固酮系统与妊娠高血压病的关系杨昱;钟群英;陈瑞坚【摘要】目的探讨肾素-血管紧张素-醛固酮系统(RAAS)与妊娠高血压疾病(HDCP)的关系.方法选取2014年2月~ 2015年8月在我院住院分娩的孕产妇92例,其中合并HDCP 38例,设为妊高症组,正常孕产妇54例,设为对照组.比较两组孕产妇产前、产后血浆活性肾素(PRA)水平、血管紧张素Ⅱ(AngⅡ)及醛固酮(ALD)水平的差异.结果与对照组比较,妊高症组孕产妇产前血浆PRA、AngⅡ水平均明显降低(P <0.05),ALD水平明显升高(P<0.05);与产前比较,妊高症组孕产妇产后血浆PRA、AngⅡ均明显升高,ALD水平明显降低(P<0.05),而对照组无统计学差异(P>0.05);与对照组比较,妊高症组孕产妇产后AngⅡ水平明显升高(P<0.05),而PRA、ALD 水平比较差异无显著性(P>0.05);妊高症组孕产妇胎盘组织中AT1受体表达明显强于对照组(P<0.05),组间肾素表达差异无统计学意义(P>0.05).结论 HDCP的发病与胎盘组织局部肾素-血管紧张素系统相关,其机制可能与胎盘组织中AT1受体表现显著增多,通过多种途径影响胎盘血流灌注,使胎盘发生缺血缺氧有关.【期刊名称】《中国医药科学》【年(卷),期】2016(006)008【总页数】4页(P20-22,131)【关键词】妊高症;肾素;血管紧张素Ⅱ;醛固酮【作者】杨昱;钟群英;陈瑞坚【作者单位】广东省清远市妇幼保健院产科,广东清远511500;广东省清远市妇幼保健院产科,广东清远511500;广东省清远市妇幼保健院产科,广东清远511500【正文语种】中文【中图分类】R544.1妊娠期高血压疾病(hypertensive disorder complicating pregnancy,HDCP)是妊娠期特有的疾病,其在我国的发病率高达9.4%。
HDCP主要表现为,高血压,伴或不伴有蛋白尿,严重者可伴全身脏器损害,是导致孕产妇死亡的主要原因之一[1]。
细胞高糖模型的建立

Angiotensin II mediates the high-glucose-induced endothelial-to-mesenchymal transition in human aortic endothelial cellsRining Tang,Qing Li,Linli Lv,Houyong Dai,Min Zheng,Kunling Ma,Bicheng Liu *BackgroundVascular complications,such as cardiomyopathy and nephropathy,are the leading cause of morbidity and mortality in patients with diabetes.Because the initial injury by hyperglycemia occurs in the blood vessels,endothelial cells are considered to be the first target,and,furthermore,endothelial damage plays an impor-tant role in the development and progression of diabetic vascular complications [1-3].Four main molecular mechanisms have been implicated in glucose-mediated vascular disease:the glucose-induced activation ofprotein kinase C isoforms,an increased formation of glucose-derived advanced glycation end-products (AGEs),an increased glucose flux through the aldose reductase pathway,and an increased production of reac-tive oxygen species [4];however,the mechanisms of endothelial injury by high glucose (HG)are not fully understood.Recent studies have indicated that the endothelial-to-mesenchymal transition (EndMT)could contribute to the progression of diabetic nephropathy,diabetic renal fibro-sis,and cardiac fibrosis [5-7],and that the rennin-angio-tensin system (RAS)may be involved.Irbesartan is an angiotensin II (Ang II)receptor type 1blocker (ARB)and has been shown to reduce vascular endothelial damage,improve hyperglycemia-induced endothelial dysfunction,*Correspondence:liubc64@Institute of Nephrology,Zhong Da Hospital,Southeast University,Nanjing 210009,China Tang et al .Cardiovascular Diabetology 2010,9:31/content/9/1/31C ARDIO V ASCULAR DIABETOLOGY©2010Tang et al;licensee BioMed Central Ltd.This is an Open Access article distributed under the terms of the Creative Commons Attribution License (/licenses/by/2.0),which permits unrestricted use,distribution,and reproduction in any medium,provided the original work is properly cited.and inhibit endothelial transdifferentiation into myofibro-blasts in valve leaflets[8-11].The aim of this study was to explore the influence of HG on the EndMT and its rele-vance in the activation of the RAS in HAECs.Materials and methodsCell cultureHAECs were purchased from Sciencell(No.6100)and grown in a Sciencell endothelial basal medium(ECM, No.1001).This ECM consists of500ml of basal med-ium,25ml of fetal bovine serum(No.0025),5ml of endothelial cell growth supplement(No.1052),and 5ml of a penicillin/streptomycin solution(No.0503). Cells were cultured at37°C in a humidified atmosphere with5%CO2.The medium was changed every other day until the culture was approximately50%confluent. When the culture reached50%confluence,the medium was changed every day until the culture was approxi-mately80%confluent.HAECs were performed between the2-4passages.The culture medium was changed to a serum-free solution for24h,and the HAECs were trea-ted with normal glucose(NG;5.5mM),HG(15mM or 30mM D-glucose)[12],or5.5mM NG+24.5mM man-nitol for48h.These cells were exposed to HG(media that contained5.5,15,or30mM D-glucose)for0,6,12, 24,48,and72h.Some of the cells that were exposed to HG(30mM)were also incubated with irbesartan(1μM, Sanofi-aventis,France)[13]for48h.Ang II measurementAng II was measured in the supernatant by radioimmu-noassay,as previously described[14].A commercial radioimmunoassay kit(Beifang,China)was used for the Ang II measurement.On the basis of the time course of Ang II synthesis,HAECs were exposed to HG(30mM) for48h.RT-PCR analysisTotal RNA was prepared from the HAECs using TRIzol (Key GEN).Total RNA was prepared using TRIzol(Key GEN)from HAEC.PCR reactions were performed using specific primer pairs:a FSP1sense primer:5′TTGGGGAAAAG GACAGATGAAG3′,anti-sense pri-mer:5′TGAAGGAGCCAGGGTGGAAAAA3′),a-SMA sense prime:5′ATAACATCAAGCCCAAATCTGC3′, anti-sense primer:5′TTCCTTTTTTCTTTCCCAACA 3’)and a GADPH sense primer:5′AAGGTCG GAGT-CAACGGATTT3′,antisense primer:5′AGATGAT-GACCCTTTTGGCTC3′).Western blot analysisEqual amounts of cell lysate proteins(30μg)were sepa-rated on4-20%SDS-polyacrylamide gels and transferred onto nitrocellulose membranes(Pall,USA).The membranes were incubated overnight with poly-clonal rabbit anti-rat FSP1and the polyclonal rabbit anti-rat a-SMA(Abcam,England),followed by a horse-radish peroxidase-labeled goat anti-rabbit IgG(Key GEN,China).The signals were detected using an ECL advance system(GE Healthcare,UK). Immunofluorescent StainingFor a double immunofluorescence procedure,we incu-bated the HAECs with two primary antibodies at4°C overnight.The primary antibodies were monoclonal mouse anti-CD31(Santa Cruz Biotechnology,Europe) and polyclonal rabbit anti-FSP1(Abcam,England).We incubated cells in1%BSA for1h at room temperature in the dark with a mixture of two secondary antibodies and two different fluorochromes:Rhod red-conjugated goat anti-rabbit and FITC green-conjugated goat anti-mouse.As a negative control,the primary antibody was replaced with non-immune IgG,and no staining could be observed.FSP1+cells were observed to have oval and elongated shapes in the HG group.The pictures were captured by the LSM5image browser(Zeiss)and ana-lyzed using a laser scanning confocal microscope(LSM 510META,Zeiss).Morphological analysisUltra-thin cells were counter-stained with uranyl acetate and lead citrate and were examined with a transmission electron microscope(HITACHI H600,TEM).The LSM5image browser(Zeiss)was used to capture images of morphological changes in the HAECs using CD31 immunofluorescence staining,as previously mentioned. Statistical analysisData were expressed as mean±standard deviation(SD) and analyzed by one-way analysis of the variance (ANOVA)using SPSS,version13.0.Data were consid-ered significant if P<0.05.ResultsHG exposure dose response and time course on HAEC angiotensin II productionTo demonstrate that enhanced Ang II production depended on the concentration and duration of HG exposure,we incubated HAECs in a medium that con-tained5.5,15,or30mM glucose for48h.Mannitol was added to the control cell incubation medium to equalize the osmolarity.Ang II was observed to increase in a dose-dependent manner in response to HG exposure (Fig.1A).The concentration of Ang II in HG-exposed cells(30mM)increased as early as12h and continued to increase until48h after exposure(Fig.1B).As can be observed in Fig.1C,irbesartan partially inhibited Ang II production in the culture medium.The Effect of irbesartan on the mRNA expression of FSP1 and a-SMAAs shown in Fig.2,FSP1and a-SMA mRNA expres-sions in HAECs exposure to HG were markedly up-regulated in comparison to NG group,which were inhibited by treatment with irbesartan(P<0.05).The effect of irbesartan on the protein expression of FSP1 and a-SMAAccording to Fig.3A-B,after exposing a confluent mono-layer of cells with HG at different concentrations and periods of time,it can be observed that after48h of exposure to increased HG concentrations,the FSP1pro-tein was progressively up-regulated(NG5.5mM:0.08±0.01,HG15mM:0.57±0.04,HG30mM:1.25±0.06; #P<0.05vs.NG),reaching a peak at30mM HG with a15.62-fold increase in comparison to that with NG expo-sure(Fig.3A).In response to30mM HG,the introduc-tion of HG time-dependently induced the synthesis of FSP1protein(0h:0.04±0.001,12h:0.652±0.04,24h: 0.98±0.04,and48h:1.22±0.02;P<0.05vs.the con-trol;Fig.3B).Furthermore,FSP1and a-SMA protein expression in HAECs exposure to HG were markedly up-regulated in comparison to NG group,which were inhib-ited by treatment with irbesartan(P<0.05).Confocal microscopic analysisWe performed labeling experiments using antibodies to CD31(endothelial cell marker;green)and fibroblast markers FSP1(red,also termed S100A4).Confocal microscopy revealed the co-localization of both FSP1 and CD31(Fig.4B).An analysis of FSP1/CD31double labeling revealed that some cells acquired FSP1staining and lost CD31staining,which suggests that the EndMT occurred.The administration of irbesartan markedly reduced the number of such double-staining cells(Fig. 4C,P<0.05).In the control cells,FSP1expression was confined to sparsely scattered fibroblasts(Fig.4A).Morphological analysisNormal endothelial monolayers displayed a typical cob-blestone morphology.We observed thatHAECsexposure to HG for48h exhibited profound changes with cells becoming elongated,spindle-shaped and lost cobblestone morphologies according to fluorescence microscopic analysis(Fig.5B).Interestingly,treatment with irbesartan was observed to significantly prevent these morphological changes(Fig.5C).Electron microscopy analysis of the control demon-strated that the endothelial cells therein exhibited normal structures(Fig.6A,×6000).In contrast,the HG group that was treated with HG(30mM)for48h exhibited endothelial protrusion,a significantly rough-ened endoplasmic reticulum,and microfilamentation (arrows,Fig.6B,×6000).These changes were attenu-ated by treatment with irbesartan(Fig.6C,×6000). DiscussionMicroangiopathy is the most common complication in diabetes,wherein endothelial cell injury is an early fea-ture of microvascular lesions.Studies have shown that endothelial injury accelerates atherosclerosis and subse-quently causes cardiovascular events[15].Emerging evi-dence has shown that hyperglycemia may have a direct role in endothelial cell injury[16-18],which is charac-terized by cell apoptosis.More recently,it has been shown that the endothelium may develop the EndMT, which has been found to be involved in cardiac fibrosis and tubulointerstitial fibrosis in animal models [6,7,19,20];however,the potential mechanisms therein are still largely assumptive.In this study,we found that when HAECs were exposed to HG,they developed a series of phenotypical changes,such as a spindle-shaped morphology,an increasingly roughened endoplasmic reticulum,and microfilamentation.Moreover,these cells expressed FSP1and a-SMA,which suggests the occur-rence of the EndMT.Although the EndMT was first investigated as a criti-cal process in heart development[21],it is now clear that the EndMT can also postnatally occur in various pathological settings,including cardiac fibrosis,renal fibrosis,and diabetic nephropathy.Recent studies have shown that the EndMT also contributes to the develop-ment of diabetic renal interstitial fibrosis,diabetic nephropathy,and cardiac fibrosis[5,7,19],which indi-cate a relationship between the EndMT and fibrosis. Cardiac and renal fibroses are also the most common diabetic vascular complications[22-24].Chronic hyper-glycemia is a major initiator of diabetic vascular compli-cations.Indeed,HG via various mechanisms,such as an increased production of oxidative stress,AGEs,and the activation of the RAS and protein kinase C[4,25],pro-motes cardiac and renal fibroses.Therefore,whether HG directly induces the EndMT in HAECs is an inter-esting question that has not been previously addressed. In this study,our findings demonstrate that double-stained HAECs exposure to high glucose exhibited the co-localization of CD31and FSP1,and some cells acquired spindle-shaped morphologies and a loss of CD31staining.Furthermore,the expressions of FSP1 and a-SMA were significantly increased in the HG group,which strongly indicates an HG-induced EndMT and could be an important mechanism in diabetic vas-cularcomplications.How did HG induce EndMT?In our study,we observed that irbesartan as an ARB significantly inhib-ited the EndMT.Furthermore,other studies have demonstrated the antiproteinuric effects and the preser-vation of endothelial function that derive from ARB, which translate into cardiovascular and renoprotective benefits that extend beyond the lowering of blood pres-sure[26,27].In vitro and in vivo studies have found that irbesartan could ameliorate endothelial function in hypertension and diabetes,which are two frequent dis-eases where endothelium homeostasis alterations are typically present[27].In addition,irbesartan therapy has been demonstrated to improve metabolic risk factors in clinical settings[28,29];however,the exact mechanisms of the cardiovascular and renoprotective benefits that derive from irbesartan therapy are not fully understood. In this study,we found that HG directly stimulated angiotensin II synthesis in HAECs,and irbesartan mark-edly protected endothelial cells from HG-induced injury. Because the EndMT may be an early event in the patho-genesis of fibrosis[19,30],our findings suggest that early treatment with ARB might be an important strategy in the prevention of microvascular disease that is compli-cated by diabetes.Figure4Irbesartan inhibited the high glucose-induced EndMT in HAECs according to laser-scanning confocal microscopy. Representative immunofluorescence staining of CD31(green)and FSP1(red)were observed.A merging of both images reveals populations of cells acquired FSP1expression and lost CD31expression(arrows,B).The administration of irbesartan reduced the number of co-localization of CD31and FSP1(C,P<0.05).A:normal glucose as controls;B:Treated with HG(30mM)for48h.Treated with HG(30mM)+irbesartan(1μM)for48h.Experiments were repeated three times.NG:normal glucose.HG:high glucose.HI+Irb:high glucose+Irbesartan.Figure5Immunofluorescence staining of HAECs with CD31in various groups.The Incubation of HAECs with high glucose(30mM)for 48h resulted in a fibroblast-like phenotype(B).Treatment with irbesartan could significantly prevent the morphological changes(C).NG:normal glucose.HG:high glucose.HI+Irb:high glucose+Irbesartan.ConclusionsThese findings suggest a novel and early mechanism concerning HG-induced endothelial damage via an angiotensin II-mediated EndMT,which provides new insight into the early application of ARB in the protec-tion of blood vessels and the prevention of organ failure in diabetes.AbbreviationsEndMT:endothelial-to-mesenchymal transition;HAECs:human aortic endothelial cells;HG:high glucose;Irb:Irbesartan;FSP1:fibroblast-specific protein1;AGEs:advanced glycation end-products;RAS:rennin-angiotensin system;Ang II:angiotensin II;ARB:angiotensin II receptor type1blocker. AcknowledgementsThis work was supported in part by grants from the National Natural Science Foundation of the P.R.China,Grant Number:30870953,and the Jiangsu Natural key protect project of the P.R.China;Grant Number:2007709. Authors’contributionsTR performed the experiments,analyzed data,interpreted results,and wrote the manuscript.LQ participated in the HAEC culture and analysis.LL and DH carried out the RT-PCR and Western blotting.ZM helped to carry out the immunofluorescent staining.MK coordinated the study and was involved in the data interpretation.LB participated in the study design and coordinationFigure6Cellular ultrastructure following HG treatment.Transmission following HG(30mM)exposure(magnification×6,000).It can be seen endoplasmic reticulum(A).After exposure to HG,microfilamentation and These changes were attenuated by treatment with irbesartan(C).1barand helped review the manuscript.All authors read and approved the final manuscript.Competing interestsThe authors declare that they have no competing interests.Received:17June 2010Accepted:27July 2010Published:27July 2010References1.Okon EB,Szado T,Laher I,McManus B,van Breemen C:Augmentedcontractile response of vascular smooth muscle in a diabetic mouse model.J Vasc Res 2003,40:520-530.gaud GJ,Masih-Khan E,Kai S,van Breemen C,Dube GP:Influence oftype II diabetes on arterial tone and endothelial function in murine mesenteric resistance arteries.J Vasc Res 2001,38:578-589.3.Yu Y,Ohmori K,Kondo I,Yao L,Noma T,Tsuji T,Mizushige K,Kohno M:Correlation of functional and structural alterations of the coronary arterioles during development of type II diabetes mellitus in rats.Cardiovasc Res 2002,56:303-311.4.Nishikawa T,Kukidome D,Sonoda K,Fujisawa K,Matsuhisa T,Motoshima H,Matsumura T,Araki E:Impact of mitochondrial ROS production on diabetic vascular complications.Diabetes Res Clin Pract 2007,77:S41-45.5.Kizu A,Medici D,Kalluri R:Endothelial-mesenchymal transition as a novelmechanism for generating myofibroblasts during diabetic nephropathy.Am J Pathol 2009,175:1371-1373.6.Zeisberg EM,Potenta SE,Sugimoto H,Zeisberg M,Kalluri R:Fibroblasts inkidney fibrosis emerge via endothelial-to-mesenchymal transition.J Am Soc Nephrol 2008,19:2282-2287.7.Zeisberg EM,Tarnavski O,Zeisberg M,Dorfman AL,McMullen JR,Gustafsson E,Chandraker A,Yuan X,Pu WT,Roberts AB,Neilson EG,Sayegh MH,Izumo S,Kalluri R:Endothelial-to-mesenchymal transition contributes to cardiac fibrosis.Nat Med 2007,13:952-961.8.Rizzoni D,Rosei EA:Small artery remodeling in diabetes mellitus.NutrMetab Cardiovasc Dis 2009,19:587-592.9.Croom KF,Plosker GL:Irbesartan a review of its use in hypertension anddiabetic nephropathy.Drugs 2008,68:1543-1569.10.Willemsen JM,Westerink JW,Dallinga-Thie GM,van Zonneveld AJ,Gaillard CA,Rabelink TJ,de Koning EJ:Angiotensin II type 1receptor blockade improves hyperglycemia-induced endothelial dysfunction and reduces proinflammatory cytokine release from leukocytes.J Cardiovasc Pharmacol 2007,49:6-12.11.Arishiro K,Hoshiga M,Negoro N,Jin D,Takai S,Miyazaki M,Ishihara T,Hanafusa T:Angiotensin receptor-1blocker inhibits atherosclerotic changes and endothelial disruption of the aortic valve inhypercholesterolemic rabbits.J Am Coll Cardiol 2007,49:1482-1489.12.Mohan S,Hamuro M,Koyoma K,Sorescu GP,Jo H,Natarajan M:Highglucose induced NF-kappaB DNA-binding activity in HAEC is maintained under low shear stress but inhibited under high shear stress:role of nitric oxide.Atherosclerosis 2003,171:225-234.13.Batenburg WW,Garrelds IM,Bernasconi CC,Juillerat-Jeanneret L,vanKats JP,Saxena PR,Danser AH:Angiotensin II type 2receptor-mediated vasodilation in human coronary microarteries.Circulation 2004,109:2296-2301.14.Liu BC,Gao J,Li Q,Xu LM:Albumin caused the increasing production ofangiotensin II due to the dysregulation of ACE/ACE2expression in HK2cells.Clin Chim Acta 2009,403:23-30.15.Schafer K,Kaiser K,Konstantinides S:Rosuvastatin exerts favourable effectson thrombosis and neointimal growth in a mouse model of endothelial injury.Thromb Haemost 2005,93:145-152.16.Han J,Mandal AK,Hiebert LM:Endothelial cell injury by high glucose andheparanase is prevented by insulin,heparin and basic fibroblast growth factor.Cardiovasc Diabetol 2005,4:12.17.Mandal AK,Ping T,Caldwell SJ:Electron microscopic analysis of glucose-induced endothelial damage in primary culture:possible mechanism and prevention.Histol Histopathol 2006,21:941-950.18.Oyama T,Miyasita Y,Watanabe H,Shirai K:The role of polyol pathway inhigh glucose-induced endothelial cell damages.Diabetes Res Clin Pract 2006,73:227-234.19.Li J,Qu X,Bertram JF:Endothelial-myofibroblast transition contributes tothe early development of diabetic renal interstitial fibrosis instreptozotocin-induced diabetic mice.Am J Pathol 2009,175:1380-1388.20.Widyantoro B,Emoto N,Nakayama K,Anggrahini DW,Adiarto S,Iwasa N,Yagi K,Miyagawa K,Rikitake Y,Suzuki T,Kisanuki YY,Yanagisawa M,Hirata KI:Endothelial cell-derived endothelin-1promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition.Circulation 2010,121:2407-2418.21.Eisenberg LM,Markwald RR:Molecular regulation of atrioventricularvalvuloseptal morphogenesis.Circ Res 1995,77:1-6.22.Ban CR,Twigg SM:Fibrosis in diabetes complications:pathogenicmechanisms and circulating and urinary markers.Vasc Health Risk Manag 2008,4:575-596.23.Ares-Carrasco S,Picatoste B,Benito-Martín A,et al :Myocardial fibrosis andapoptosis,but not inflammation,are present in long-term experimental diabetes.Am J Physiol Heart Circ Physiol 2009,297:H2109-2119.24.Aneja A,Tang WH,Bansilal S,Garcia MJ,Farkouh ME:Diabeticcardiomyopathy:insights into pathogenesis,diagnostic challenges,and therapeutic options.Am J Med 2008,121:748-757.25.Yamagishi S,Imaizumi T:Diabetic vascular complications:pathophysiology,biochemical basis and potential therapeutic strategy.Curr Pharm Des 2005,11:2279-2299.26.Burnier M,Zanchi A:Blockade of the renin-angiotensin-aldosteronesystem:a key therapeutic strategy to reduce renal and cardiovascular events in patients with diabetes.J Hypertens 2006,24:11-25.27.Negro R:Endothelial effects of antihypertensive treatment:focus onirbesartan.Vasc Health Risk Manag 2008,4:89-101.28.Parhofer KG,Munzel F,Krekler M:Effect of the angiotensin receptorblocker irbesartan on metabolic parameters in clinical practice:the DO-IT prospective observational study.Cardiovasc Diabetol 2007,6:36.29.Kintscher U,Bramlage P,Paar WD,Thoenes M,Unger T:Irbesartan for thetreatment of hypertension in patients with the metabolic syndrome:a sub analysis of the Treat to Target post authorization survey.Prospective observational,two armed study in 14,200patients.Cardiovasc Diabetol 2007,6:12.30.Schaefer C,Biermann T,Schroeder M,Fuhrhop I,Niemeier A,Ruther W,Algenstaedt P,Hansen-Algenstaedt N:Early microvascular complications of prediabetes in mice with impaired glucose tolerance and dyslipidemia.Acta Diabetol 2009.。
ACE2_基因缺失对止血带休克后主动脉收缩反应性的影响(英文)

Effect of ACE2 deletion on vasoconstriction reactivity of aortic segmentsin mice with tourniquet shock *FANG Fang 1, WANG Lijun 1,2, YANG Ling 3, ZHANG Wenli 3,ZHANG Xiaofu 1, YANG Xiuhong 1,3△(1Hebei Key Laboratory for Chronic Diseases , School of Basic Medical Sciences , North China University of Science and Tech⁃nology , Tangshan 063210, China ; 2Department of Pathology , Dongzhimen Hospital Beijing University of Chinese Medi⁃cine , Beijing 100007, China ; 3School of Public Health , North China University of Science and Technology , Tangshan063210, China. E -mail : yangxiuhong@ )[ABSTRACT ] AIM : To observe the effect of angiotensin -converting enzyme 2 (ACE2) deletion on vasoconstric‑tion reactivity of aortic segments in ACE2 knockout (KO ) mice with tourniquet shock (TS ). METHODS : The 8-month -old male mice with C57BL/6 background were divided into wild -type (WT ) control group , WT -TS group , KO group and KO -TS group , with 10 mice in each group , of which five were used for determination of vascular reactivity , and the other five for the other assays. The hindlimbs of the mice in WT -TS group and KO -TS group were ligated with tourniquet for 2 h and loosened for 4 h. The mice in WT group and KO group were subjected to the same treatment except for tourniquet liga‑tion. The vasoconstriction reactivity of the aorta was measured on tensiometer. The morphological damage of the aorta was evaluated by vascular histopathology. Western blot was used to detect the expression of AT1, MAS , ACE and ACE2 pro‑teins in aorta. The serum levels of angiotensin (Ang ) II and Ang -(1-7) were determined by enzyme -linked immunosorbent assay. RESULTS : Compared with WT group , the mice in WT -TS group had lower vascular reactivity to norepinephrine(NE ) and obvious vascular lesions. The expression of ACE protein increased significantly (P <0.01), while the expres‑sion of ACE2 decreased (P <0.05). The expression of AT1 protein in aorta decreased significantly , the expression of MAS protein increased significantly , and the AT1/MAS ratio decreased (P <0.01). Serum Ang II level increased , serum Ang -(1-7) level decreased , and Ang II/Ang -(1-7) ratio increased (P <0.05). Compared with WT group , vascular reactivity in KO group increased at low concentration of NE (<10−7 mol/L ), and decreased at high concentration (>10−7 mol/L ) without vascular lesion. The expression levels of aortic AT1, MAS and ACE were all elevated (P <0.05). The serum level of Ang II increased (P <0.05), but the level of Ang -(1-7) had no obvious change. Compared with KO and WT -TS groups , the aortic reactivity in KO -TS group subtracted apparently (P <0.05), representing its curve shifting to the right obviously.The morphological damage aggravated slightly , and the expression of AT1 and ACE increased slightly in KO -TS group com‑pared with WT -TS group (P <0.05). However , the expression of MAS increased significantly in vascular tissue (P <0.01). The serum levels of Ang II and Ang -(1-7) further increased and decreased , respectively , and the Ang II/Ang -(1-7) ratio increased (P <0.01). CONCLUSION : Deficiency of ACE2 induces severe aortic hyporeactivity to NE duringTS , which may be related to the increased imbalance of renin -angiotensin system in ACE2 gene knockout mice.[KEY WORDS ] angiotensin -converting enzyme 2; tourniquet shock ; vascular hyporeactivity ; gene knockout[CLC number ] R363.2; R543.1 [Document code ] Adoi : 10.3969/j.issn.1000-4718.2023.05.005Vascular hyporeactivity is usually secondary to the late stage of severe shock. It refers to vasoconstrictionor relaxation disorder , and blood vessels are insensitiveor even non -responsive to the application of vasoactive drugs , resulting in poor elevation of blood pressure , tis‑sue insufficiency , and further ischemia and hypoxia of[文章编号] 1000-4718(2023)05-0802-09[Received date ] 2022-11-11 [Accepted date ] 2023-04-03 * [Foundation item ] Supported by the National Natural Science Foundation of China (No. 81970359; No. 81372029), the NaturalScience Foundation of Hebei Province (No. H2022209081) and the Foundation of Key R&D Program of Hebei Province (No. 20277718D )△Corresponding author Tel : 0315-*******; E -mail : yangxiuhong@··802cells. The renin-angiotensin system (RAS) is an impor‑tant humoral regulation system that exists in both circu‑lation and local organs,including the two opposite axes:angiotensin-converting enzyme (ACE)/angioten‑sin (Ang) II/Ang II type 1 (AT1) receptor and ACE2/ Ang-(1-7)/MAS receptor,which together regulate the functions of many organs in vivo[1]. Targeting the forma‑tion and action of Ang II by ACE inhibitor (ACEI) and AT1 receptor inhibitor has been the key therapeutic tar‑get for last three decades. In recent years, people have paid more and more attention to ACE2 with organ-pro‑tective properties, known to degrade Ang II to Ang-(1-7)[2].In previous animal experiments,we found that RAS showed a noticeable imbalance after limb ischemia-reperfusion, demonstrating over-activation of ACE/Ang II, suppression of ACE2/Ang-(1-7), and induction of acute injury of distant organs such as kidneys and lungs[3-4]. The experimental study of isolated blood ves‑sels in mice showed that vascular reactivity decreased after tourniquet shock (TS),and ACE2 agonists im‑proved the contractile reactivity of aortas[5-6]. So, we sus‑pected that vascular hyporeactivity in severe shock may be related to the concurrent RAS imbalance. In the present study, we observed the changes of vascular reactivity and expression of RAS components in the aorta of ACE2 gene knockout mice with TS,so as to explore the effect of ACE2 gene deletion on vascular reactivity during shock. MATERIALS AND METHODS1 Animal experiment schemeThe animal experiment was approved by the Animal Ethics Committee of North China University of Science and Technology [Certificate No:SYXK (Beijing)2016-0039] and the procedures were carried out in ac‑cordance with Guidance on the Care and Use of Labora‑tory Animals and Management Regulations on Medical Waste Disposal established by the university.The C57BL/6 background ACE2 knockout mice were sup‑plied by the Laboratory Animal Institute of Chinese Academy of Medical Sciences, and bred in the specific pathogen-free (SPF) animal facility of the Laboratory Ani‑mal Center of North China University of Science and Tech‑nology, with the room temperature maintained at (23±2) ℃, standard rodent chows and free drinking water. Forty 8-month-old male mice weighing about 30 g were divided into wild-type (WT)group,WT-TS group,ACE2gene knockout group (KO group)and KO-TS group, with 10 mice in each group. The mice in WT-TS group and KO-TS group were anesthetized by in‑traperitoneal injection of pentobarbital sodium,ligated with hemorrhoid alligator (M-M-0084,Jintan Jiangsu Medical Instrument Co.,Ltd.)and rubber bands (5 mm wide and 1 cm in diameter) at the root of both hind limbs to cause ischemia for 2 h,and then the rubber bands were cut to restore blood for 4 h perfusion[5, 8]. The mice in WT group and KO group were subjected to the same treatment except for tourniquet ligation, taken as sham operation control.At the end of 4 h reperfu‑sion,mice were euthanized and serum samples were collected for analysis of Ang II/Ang-(1-7) content. The abdominal aortas of 5 mice were separated for determi‑nation of vascular reactivity.The aortas of the other 5 mice were used for other assays.2 Determination of vascular reactivityThe prepared aortic rings were placed into the bath containing K-H solution with sustained input of a mix‑ture comprised of 95% oxygen and 5% carbon dioxide at a constant temperature of 37 ℃. Two wires with a diameter of about 40 µm and length about 3 cm were put through the vascular rings to fix them to the pressure sensor un‑der the corresponding nuts,and the two wires were maintained parallel straight under a particular tension. The solution of 100 µL KCl at 3 mol/L was added to the bath and 60 mmol/L KCl solution was used to stimulate the vascular rings to contract. The magnitude of contraction was recorded,and then the artery rings were eluted for 3 times by K-H solution at 5 min inter‑vals. The above steps were repeated for totally 3 times. If the contraction magnitude was greater than 1 mN and the difference between two values was less than 10%, it indicated that the vasoactivity remained well. Besides, 10−6 mol/L norepinephrine (NE) solution was used to stimulate the vascular rings. When the va‑soconstriction reached equilibration after 20 min,10−6 mol/L acetylcholine was added for vasodilation. If vas‑cular relaxation magnitude was greater than 80%,it suggested that the endothelial integrity was kept well. Aortic rings were stimulated by different concen‑trations (10−9 to 10−4.5 mol/L) of NE, and contraction of vascular rings was recorded using the PowerLab system.··8033 Pathological examination of vascular tissueThe aortas of mice were treated with routine histologi‑cal procedures including fixation,dehydrating,embed‑ding, slicing and HE staining. Two experienced patholo‑gists observed the vascular tissue structure under a light microscope,and scored the vascular injury according to the literature[7] from 20 visual fields randomly select‑ed from each group of sections under high power lens. Scoring criteria are as follows: 0 point, internal elastic membrane integrity;1 point,internal elastic mem‑brane rupture; 2 points, middle elastic membrane rup‑ture; 3 points, external elastic membrane rupture.The aortic tissue was treated following transmis‑sion electron microscope (TEM;H-7650,HITACHI)specimen preparation procedures, including fixation, de‑hydrating,infiltration,embedding,aggregation,slicing and staining with lead citrate and uranyl acetate,and the vascular ultrastructural changes were observed un‑der TEM.4 Assay of vascular ACE/ACE2 and AT1/MAS ex⁃pression by Western blottingFrozen vascular tissue (50 mg) was ground, and ho‑mogenized on ice using the electric disperser after addi‑tion of 300 µL radio immunoprecipitation assay (RIPA)lysis buffer. It was then centrifuged at 12 000 r/min for 15 min at 4 ℃.The concentration of supernatant pro‑tein was assayed using BCA method. The sample of 50 µg protein was mixed with loading buffer,boiled in a water bath at 100 ℃ for 5 min to denature the protein. It then underwent electrophoresis for about 30 min in stacking gel and for about 2 h in separation gel.The protein was transferred to a nitrocellulose (NC)mem‑brane.The NC membrane was placed into 5% bovine serum albumin,blocked for 1 h,followed by TBST rinse for 3 times. Primary antibodies were then incubated at 4 ℃ overnight, the concentrations of which were 1∶1 000 for GAPDH (Beyotime), 1∶500 for ACE (Epito‑mics)and ACE2 (Santa Cruz),and 1∶200 for AT1 (Santa Cruz)and MAS (Alomone Labs),followed by TBST rinse for 3 times. All the secondary antibody (Be‑yotime) incubation was conducted at room temperature for 30 min and the concentration was all 1∶1 000. En‑hanced chemiluminescence (ECL)agent was adopted to detect proteins. ImageJ processing software was then applied for strip assay and analysis.5 Determination of serum Ang II/Ang-(1-7) content by enzyme-linked immunosorbent assay (ELISA)Serum Ang II and Ang-(1-7)levels were deter‑mined by ELISA according to the instructions of the kit (USCN Life)[3-4]. The absorbance of the reaction system was measured under a wavelength of 450 nm to calculate the concentration of the target protein in the sample. 6 Statistical analysisStatistical analysis of the data was conducted using SPSS 26.0 software.Continuous variables were de‑scribed as mean±standard deviation (SD). Comparison between groups was performed by two-tailed t-test or one-way ANOVA.P<0.05 indicated the statistical sig‑nificance of the difference.RESULTS1 Results of vascular reactivityThe vessels in WT group did not show obvious con‑traction under the stimulation by NE at the concentra‑tions of 10−9, 10−8.5, 10−8 and 10−7.5 mol/L. The percentage of vascular contraction in WT group gradually increased under the stimulation by the elevated NE with concen‑trations above10−7.5mol/L (P<0.05).In contrast,the vasoconstriction rate in WT-TS group gradually in‑creased with the increase in NE concentration between 10−7.5 mol/L and 10−6.5 mol/L (P<0.05), but significant‑ly decreased with the increase in NE concentration above 10−6.5 mol/L (P<0.05). See Figure 1A.The vasoconstriction percentage in KO group in‑creased with the increase in NE concentration less than 10−7 mol/L (P<0.05). Compared with WT group, vaso‑constriction response curve in KO group shifted to the left. The vascular contractile reactivity in KO group sig‑nificantly increased between 10−8mol/L and 10−7mol/L NE (P<0.05).When the concentration of NE was higher than 10−7mol/L,the constriction percentage gradually declined with the increase in NE concentra‑tion (P<0.05). See Figure 1B.Compared with KO and WT-TS groups,the con‑traction curve of KO-TS group moved significantly to the right (Figure 1C and 1D).The contraction ampli‑tude was significantly lower than that in WT-TS group when the concentration of NE was between 10−9 mol/L to 10−6 mol/L (P<0.05), and the constriction became ob‑vious when the stimulation concentration was above··80410−6.5mol/L (P <0.05). The above results indicate that ACE2 knockout further reduced the vascular reactivityof TS mice.2 Morphological changes of vascular injuryUnder the light microscope , the vessel wall of themice in WT group and KO group was smooth , the endo‑thelial cells were orderly arranged , and elastic mem‑brane of each layer showed even thickness with goodcontinuity. The images in WT -TS group and KO -TS group showed vascular injury , including rough vascular intima , incomplete endothelial cells , ruptured elasticfibers , loosened arrangement of elastic fibers with non -uniform thickness and even a mass protruding into the lumen. The score of vascular injury was increased afterreperfusion (P <0.01). See Figure 2A.Under TEM , the endothelial cells of the mice in WT group and KO group were tightly arrayed , and rough endoplasmic reticulum and mitochondria were clearly visible in the cytoplasm , and internal elasticmembrane , smooth muscle cells and elastic fibers wereregularly arranged. The images in WT -TS group and KO -TS group showed serious injury , mainly including the disappearance of intercellular tight junctions ,stained intracellular cytoplasm , fuzzy organelles , swol‑len mitochondria with internal vacuoles or broken cris‑ta , reduction of rough endoplasmic reticulum , non -uni‑form internal elastic membranes , hyperplasia of some fiber tissues with disordered arrangement , and prolifer‑ation of smooth muscle cells. The injury status of the two groups was similar (Figure 2B ).3 Results of vascular ACE and ACE2 protein ex⁃pressionCompared with WT group , the expression of ACE was greatly enhanced (P <0.01), the expression ofACE2 was significantly reduced (P <0.05)in the aortaFigure 1. Changes of mouse vascular reactivity in different groups. A : the vascular reactivity in WT -TS group was lower thanthat in WT group ; B : the vascular reactivity in KO group was greater than that in WT group at NE concentrations between 10−8 mol/L and 10−7 mol/L ; C : the vascular reactivity in KO -TS group was lower than that in WT -TS group ; D : the vascularreactivity in KO -TS group lower than that in KO group. Mean±SD. n =5. *P <0.05 vs WT group ; #P <0.05 vs WT -TSgroup ; △P <0.05 vs KO group.··805of WT-TS group.The ACE expression was also in‑creased (P<0.05)in KO group compared with WT group. The expression of vascular ACE protein was fur‑ther elevated in KO-TS group compared with KO group (P<0.05). However, the difference of ACE expression between KO-TS group and WT-TS group was not statis‑tically significant.ACE2 expression was not detected in KO group and KO-TS group (Figure 3).4 Results of AT1 and MAS protein expression Compared with WT group,AT1 receptor protein expression reduced (P<0.01) while MAS receptor pro‑tein expression rose (P<0.01) in WT-TS group, caus‑ing the decline of AT1/MAS ratio (P<0.01).Com‑pared with WT group,AT1 and MAS receptorprotein Figure 2. Changes of mouse vascular injury in different groups. A: HE staining of mouse aortic tissues (scale bar=20 µm) and scoring of vascular injury; B: ultrastructural changes of endothelial cells and endo-elastic membrane of blood vessels un‑der transmission electron microscope (scale bar=2 µm). Mean±SD.n=5.**P<0.01 vs WT group;##P<0.01 vsKO group.Figure 3. Changes of ACE and ACE2 protein expression in vascular tissue of mice in different groups. Western blot was used to detect the expression of ACE and ACE2 in vascular tissues. Mean±SD.n=5.*P<0.05,**P<0.01 vs WT group;#P<0.05 vs WT-TS group.··806expression in KO group significantly increased (P< 0.05),resulting in increased AT1/MAS ratio (P< 0.01).Compared with KO group,AT1 receptor pro‑tein expression in KO-TS group decreased (P<0.01)but was more than that in WT-TS group, while MAS re‑ceptor expression increased (P<0.01)exceeding that in WT-TS group. The end result of AT1/MAS ratio de‑creased close to that in WT-TS group (Figure 4).5 Changes of serum Ang II and Ang-(1-7) levels Compared with WT group,the serum content of Ang II in WT-TS group increased greatly (P<0.05),and the level of Ang-(1-7) decreased measurably (P< 0.01).Ang II/Ang-(1-7)ratio turned out to increase apparently (P<0.05). The Ang II content of serum in KO group was higher than that in WT group (P< 0.05),but Ang-(1-7)content was similar to that in WT group.Finally,Ang II/Ang-(1-7)ratio in KO group increased without statistical significance com‑pared with WT pared with KO group and WT-TS group,the serum content of Ang II in KO-TS group increased (P<0.01),and Ang-(1-7)content was decreased (P<0.01). The Ang II/Ang-(1-7) ratio in KO-TS group was higher than that in KO and WT-TS groups (P<0.01),leading to more serious imbalance between the two peptides. See Figure 5. DISCUSSIONThe RAS is activated after TS,and the expres‑sions of Ang II and Ang-(1-7) in the circulatory system become substantially unbalanced,which can alone or collaboratively with NE,induce vascular hyporeactivi‑ty.There are more Ang II and less Ang-(1-7)in the circulation following TS,which helps maintain blood pressure and ensure the blood supply to vital organs. As in previous animal experiments[5], it was found that the expression of AT1 receptor in blood vessels de‑creased,and the expression of MAS receptor in‑creased,and the vascular reactivity to NE decreased. Studies have shown that vascular reactivity is temporari‑ly elevated during the compensatory phase of shock.In Figure 4. Changes of AT1 and MAS receptor protein expression in mouse vascular tissues in different groups.Western blot was used to detect the expression of AT1 and MAS receptors in vascular tissues. Mean±SD.n=5.*P<0.05,**P<0.01 vs WT group;#P<0.05 vs WT-TS group;△△P<0.01 vs KO group.··807the late stage of shock , the body's compensatory mecha‑nism is insufficient to alleviate shock caused by allkinds of damage , decreasing vascular reactivity , and refractory hypotension. At this time , both systolic and diastolic functions of the blood vessel are disordered. Blood vessels are not sensitive or even no reaction to vascular active drugs , which results in poor improve‑ment and ineffective tissue perfusion further leading to cell hypoxia and injury [9-10].ACE2 is a key enzyme that catalyzes Ang II to pro‑duce Ang -(1-7). In this study , however , the serum levels of Ang II and Ang -(1-7) in KO group did not change significantly compared with WT group , which may be related to bypass activation. Berenyiova et al [11] found that treatment with MLN -4760, an ACE2 inhibi‑tor , did not affect the expression levels of Ang I , Ang II , Ang -(1-7), Ang -(1-5) and Ang IV in serum of es‑sential hypertensive rats (SHR ). Inhibition of ACE2 or gene deletion did not affect the production of Ang -(1-7). Ang -(1-7) formation bypass may exist in ACE2 gene knockout mice. Prolyl carboxypeptidase (PCP )and prolyl oligopeptidase (PEP ) can catalyze the trans‑formation of Ang II into Ang -(1-7). Ang I was also di‑rectly catalyzed by thimet oligopeptidase (THOP ), PCP and PEP to produce Ang -(1-7)[12]. Compensatoryactivation of THOP , PCP and PEP pathways in ACE2 gene knockout mice was hypothesized to maintain nor‑mal levels of Ang -(1-7). In addition , it has been re‑ported that ACE2 plasmid DNA transfection can inhibit AT1 receptor expression and downstream activating transcription factor 3 signaling pathway in rat smooth muscle cells in a concentration - and time -dependentmanner [13]. The expression of ACE protein and AT1 and MAS receptors increased in the aorta of SHR treated with MLN -4760. In the present study , it was found that the expression of ACE protein , AT1 receptor and MASreceptor in ACE2 gene knockout mice increased , whichis consistent with the results reported before [13].In the present study , the vascular reactivity ofACE2 gene knockout mice was increased at low concen‑trations of NE , and decreased at high concentrations ofNE. We suppose that it may be related to its high ex‑pression of AT1 and MAS receptors. However , the ex‑pression of AT1 decreased , and expression of MAS fur‑ther increased after suffering TS. The vascular reactivi‑ty of KO -TS mice decreased greatly , and its vascular in‑jury significantly aggravated , simultaneously showingmore severe imbalance of Ang II/Ang -(1-7) ratio. Through the analysis of literatures , it is found that sup‑pression of the binding between Ang -(1-7) and MAS re‑ceptor can reduce the production of nitric oxide (NO ) in rat hemorrhagic shock. It is also reported that the production of reactive oxygen species (ROS ) reducesthe activity of NO [14-15], and antioxidative treatment at‑tenuates lipopolysaccharide -induced vascular hyporeac‑tivity in the rat aorta [16]. NO is an important endoge‑nous vasodilator , and the higher the content of NO , the weaker the vasoconstriction reactivity [17]. Other scholars found that inhibition of ACE2 can inhibit NO [18] and H 2S pathway. When the aortic ring is co -incubated with NaHS , the increasing level of H 2S can inhibit the vaso‑tonic effect [19-20]. Inhibition of H 2S pathway in ACE2 gene knockout mice may enhance the vasoconstriction. We previously demonstrated that with the gradualin‑Figure 5. Changes of serum Ang II and Ang -(1-7) levels in mice from different groups. A : serum Ang II content in KO groupwas higher than that in WT group , with further elevation after reperfusion ; B : no obvious difference of Ang -(1-7) content in KO group and WT group , with a decrease in KO -TS group ; C : marked increase in Ang II/Ang -(1-7) ratio in KO -TSgroup compared with KO group. Mean±SD. n =5. *P <0.05, **P <0.01 vs WT group ; ##P <0.01 vs KO group.··808crease in serum Ang II/Ang-(1-7) ratio, the level of oxi‑dative stress in the limbs of mice with ischemia-reperfu‑sion injury increased significantly[6, 21]. In the present ex‑periment, we hypothesized that the increase in vasocon‑strict reactivity in ACE2 KO group and the decrease in vasoconstrict reactivity in KO-TS group may be due to changes of production of NO,H2S and ROS,which needs to be further investigated.In short,ACE2 gene knockout mice showed the ob‑vious vasoconstriction hyporeactivity and serious injury with RAS imbalance after TS, which suggested that cor‑rection of the RAS imbalance targeting ACE2 may at‑tenuate vascular hyporeactivity and injury by influencing the expression of downstream molecules.[REFERENCES][1]Correa BHM, Becari L, Fontes MAP, et al. Involvement of the renin-angiotensin system in stress:state of the artand research perspectives[J].Curr Neuropharmacol,2022, 20(6):1212-1228.[2]Marquez A,Wysocki J,Pandit J,et al.An update on ACE2 amplification and its therapeutic potential[J]. ActaPhysiol (Oxf), 2021, 231(1):e13513.[3]Yang XH, Wang YH, Wang JJ, et al. Role of angioten‑sin-converting enzyme (ACE and ACE2)imbalance ontourniquet-induced remote kidney injury in a mousehindlimb ischemia-reperfusion model[J].Peptides,2012, 36(1):60-70.[4]Chen LN, Yang XH, Nissen DH, et al. Dysregulated re‑nin-angiotensin system contributes to acute lung injurycaused by hind-limb ischemia-reperfusion in mice[J].Shock, 2013, 40(5):420-429.[5]王丽君,王建辉,王玉娟,等.止血带休克后主动脉血管收缩反应性及RAS成分的变化[J].中国病理生理杂志, 2016, 32(3):405-410.Wang LJ,Wang JH,Wang YJ,et al.Changes of aorticcontractile reactivity and RAS components after tourniquetshock[J]. Chin J Pathophysiol, 2016, 32(3):405-410.[6]吴婕,王丽君,方芳,等.血管紧张素转换酶2激动剂对止血带休克小鼠主动脉收缩反应性的影响[J].慢性病学杂志, 2022,23(06):874-878.Wu J, Wang LJ, Fang F, et al. Effect of ACE2 agonist onaortic contractile reactivity in mice with tourniquet shock[J]. Chronic Pathematol J, 2022, 23(6):874-878.[7]Schwartz RS,Huber KC,Murphy JG,et al.Restenosis and the proportional neointimal response to coronary ar‑tery injury: results in a porcine model[J]. J Am Coll Car‑diol, 1992, 19(2):267-274.[8]Crawford RS, Hashmi FF, Jones JE, et al. A novel mod‑el of acute murine hindlimb ischemia[J].Am J PhysiolHeart Circ Physiol, 2007, 292(2):H830-H837.[9]Zhao H, Kuang L, He J, et al. Role of tumor necrosis fac‑tor-α in vascular hyporeactivity following endotoxic shockand its mechanism[J]. J Trauma Acute Care Surg, 2019,87(6):1346-1353.[10]Duan C, Yang G, Li T, et al. Advances in vascular hypo‑reactivity after shock:the mechanisms and managements[J]. Shock, 2015, 44(6): 524-534.[11]Berenyiova A, Bernatova I, Zemancikova A, et al. Vas‑cular effects of low-dose ACE2 inhibitor MLN-4760-bene‑fit or detriment in essential hypertension?[J].Biomedi‑cines, 2021, 10(1):38-61.[12]Kuriakose J, Montezano AC, Touyz RM. ACE2/Ang-(1-7)/Mas1 axis and the vascular system:vasoprotection toCOVID-19-associated vascular disease[J].Clin Sci(Lond), 2021, 135(2):387-407.[13]龚晶婧,李荣通,卢卓强,等. ACE2小干扰RNA对平滑肌细胞AT1受体蛋白表达及其下游信号通路的影响[J].中国药理学通报, 2020, 36(3):354-359.Gong JJ, Li RT, Lu ZQ, et al. Expression of AT1 recep‑tor protein and its downstream signaling pathways in vas‑cular smooth muscle cells after ACE2 siRNA interference[J]. Chin Pharmacol Bull, 2020, 36(3):354-359.[14]Jin M, Cao B, Lin C, et al. Tianma gouteng decoction ex‑erts pregnancy-protective effects against preeclampsia reg‑ulation of oxidative stress and NO signaling[J].FrontPharmacol, 2022, 13:849074.[15]Zhang C, Wang J, Ma X, et al. ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injurythrough the miR-18a/Nox2/ROS pathway[J].J Cell MolMed, 2018, 22(3):1873-1882.[16]Bai YY, Yan D, Zhou HY, et al. Betulinic acid attenu‑ates lipopolysaccharide-induced vascular hyporeactivity inthe rat aorta by modulating Nrf2 antioxidative function[J]. Inflammopharmacology, 2020, 28(1):165-174.[17]Pan Y,Sun S,Wang X,et al.Improvement of vascular function by knockdown of salusin-β in hypertensive ratsvia nitric oxide and reactive oxygen species signaling path‑way[J]. Front Physiol, 2021, 12:622954.[18]Da Silva MC, Dos Santos VM, Da Silva MVB, et al. In‑volvement of shedding induced by ADAM17 on the nitricoxide pathway in hypertension[J].Front Mol Biosci,2022, 9:1032177.[19]Sun C,Yu W,Lv B,et al.Role of hydrogen sulfide in sulfur dioxide production and vascular regulation[J].PLoS One, 2022, 17(3):e0264891.[20]Zhang D, Wang X, Tian X, et al. The increased endoge‑nous sulfur dioxide acts as a compensatory mechanism for··809。
血管紧张素Ⅱ通过抑制人心房成纤维细胞BKCa通道诱导心房纤维化

基金项目:泸州市人民政府西南医科大学科技战略合作项目(2021LZXNYD Z07,2021LZXNYD J26)通信作者:于风旭,E mail:yuluchuan@163.com·论著·血管紧张素Ⅱ通过抑制人心房成纤维细胞BKCa通道诱导心房纤维化贾春森 李磊 李劲平 谭宏伟 周伟 聂永梅 于风旭(西南医科大学附属医院心脏大血管外科,四川泸州646000)【摘要】目的 探讨在血管紧张素Ⅱ(AngⅡ)诱导心房纤维化的过程中,大电导钙激活钾通道(BKCa通道)的作用机制。
方法 通过组织块贴壁法获取原代人心房成纤维细胞,使用免疫荧光染色进行鉴定。
用浓度为500nmol/L的AngⅡ处理人心房成纤维细胞24h,实时荧光定量PCR与蛋白质印迹法用于检测处理前后纤维化标志基因α 平滑肌肌动蛋白(α SMA)、胶原蛋白Ⅰ(collagenⅠ)和胶原蛋白Ⅲ(collagenⅢ),以及BKCa通道的α与β亚基的mRNA和蛋白表达水平,全细胞膜片钳技术检测AngⅡ处理前后的BKCa通道的电流变化。
结果 (1)人心房成纤维细胞经AngⅡ处理后,α SMA、collagenⅠ和collagenⅢ的mRNA和蛋白表达水平升高;(2)经过AngⅡ处理后,BKCa通道α及β亚基mRNA和蛋白表达水平降低;(3)人心房成纤维细胞存在功能正常的BKCa通道,具有电压依赖性;(4)人心房成纤维细胞BKCa通道的宏观电流幅度在经AngⅡ处理后降低;(5)在人心房成纤维细胞上过表达BKCa通道α亚基后,纤维化标志物α SMA、collagenⅠ和collagenⅢ的表达受到了明显抑制。
结论 AngⅡ可能通过抑制人成纤维细胞BKCa通道的表达和功能来诱导人心房成纤维细胞的纤维化,并最终导致心房纤维化。
【关键词】心房纤维化;血管紧张素Ⅱ;人心房成纤维细胞;大电导钙激活钾通道【DOI】10 16806/j.cnki.issn.1004 3934 2023 11 020AngiotensinⅡInducesAtrialFibrosisbyInhibitingBKCaChannelinHumanAtrialFibroblastJIAChunsen,LILei,LIJinping,TANHongwei,ZHOUWei,NIEYongmei,YUFengxu(DepartmentofCardiovascularSurgery,TheAffiliatedHospitalofSouthwestMedicalUniversity,Luzhou646000,Sichuan,China)【Abstract】Objective Toinvestigatethemechanismoflargeconductancecalcium activatedpotassiumchannel(BKCa)inangiotensinⅡ(AngⅡ) inducedatrialfibrosis.Methods Primaryhumanatrialfibroblastswereobtainedbytissueblockattachmentmethodandidentifiedbyimmunofluorescencestaining.HumanatrialfibroblastsweretreatedwithAngⅡ(500nmol/L)for24h.Real timefluorescentquantitativePCRandWesternblotwereusedtodetectthemRNAandproteinexpressionlevelsoffibrosismarkergenesα SMA,collagenⅠandcollagenⅢ,aswellasαandβsubunitsofBKCachannelsbeforeandaftertreatment.AndwholecellpatchclamptechniquewasusedtodetectthecurrentchangesofBKCachannelsbeforeandafterAngⅡtreatment.Results (1)AfterAngⅡtreatmentofhumanatrialfibroblasts,themRNAandproteinexpressionlevelsofα SMA,collagenⅠandcollagenⅢincreased;(2)AfterAngⅡtreatment,themRNAandproteinexpressionofBKCachannelαandβsubunitsdecreased;(3)HumanatrialfibroblastsexistnormalBKCachannel,whicharevoltagedependent;(4)MacrocurrentamplitudeofBKCachannelinhumanatrialfibroblastsdecreasedafterAngⅡtreatment;(5)AfteroverexpressionofBKCachannelαsubunitonhumanatrialfibroblasts,themRNAandproteinexpressionlevelsoffibrosismarkerα SMA,collagenⅠandcollagenⅢdecreasedsignificantly.Conclusion AngⅡmayinducefibrosisinhumanatrialfibroblastsbyinhibitingtheexpressionandfunctionofBKCachannel,andfinallyinduceatrialfibrosis.【Keywords】Atrialfibrosis;AngiotensinⅡ;Humanatrialfibroblast;Largeconductancecalcium activatedpotassiumchannel 心房颤动(房颤)是临床上最为常见的心律失常,有较高的发病率与死亡率,其发病机制复杂,其中以心房纤维化为代表的结构重构是重要始动因素[1]。
p38MAPK在糖尿病肾脏疾病中的作用研究进展

p38MAPK在糖尿病肾脏疾病中的作用研究进展沈霞蔚;邓德明;孙爱萍【摘要】介绍了p38MAPK家族成员、信号通知及P-p38(磷酸化的p38)MAPK 在肾脏组织的分布;p38MAPK通路是细胞内一条重要的信号转导通路,其发挥作用的方式复杂多样.它可通过调节转化生长因子、葡萄糖转运体蛋白等多种途径影响新生血管形成及纤维组织增殖过程来影响着糖尿病肾脏疾病的进程.【期刊名称】《长江大学学报(自然版)理工卷》【年(卷),期】2011(008)001【总页数】4页(P206-209)【关键词】糖尿病肾脏疾病;p38MAPK;细胞因子;葡萄糖转运体蛋白;炎症反应【作者】沈霞蔚;邓德明;孙爱萍【作者单位】长江大学医学院,湖北,荆州,434023;长江大学医学院,湖北,荆州,434023;长江大学医学院,湖北,荆州,434023【正文语种】中文【中图分类】R587.1糖尿病肾脏疾病( diabetic kidney disease,DKD)是糖尿病最常见的微血管并发症之一,在欧美目前是导致终末期肾脏疾病的首要原因。
近30年研究显示,我国糖尿病患病率呈逐渐上升趋势,目前患病率高达10%,由此发展而来的DKD发生比例也将显著提高。
在糖尿病状态下,由于糖代谢紊乱、肾脏血流动力学改变和许多细胞因子的作用,肾脏细胞内信号转导通路发生改变。
其中p38MAPK信号转导通路的激活,在一定程度上导致和加速了糖尿病肾脏疾病的发生发展。
现将p38MAPK(p38 mitogen-activated protein kinase)家族成员及其信号通路与糖尿病肾脏疾病关系方面的文献综述如下。
Han 等[1]首先从小鼠肝脏cDNA中分离出编码p38MAPK的基因,并克隆出p38MAPK分子,发现其为由360个氨基酸构成的38KD蛋白,故被称为p38MAPK。
现已克隆出6种p38MAPK异构体亚型,分别为:p38α1MAPK,p38α2MAPKp38β1MAPK,p38β2MAPK,p38γMAPK和p38δMAPK,其中α、β亚型分布于各种组织细胞中,γ亚型分布在骨骼机中,δ亚型主要分布于腺体中。
α2-巨球蛋白通过调控血管内皮细胞改善小鼠激素性股骨头坏死

糖皮质激素(GC )广泛应用于严重感染、血液病和自身免疫性疾病,发挥其抗炎、代谢调节和免疫抑制的作用[1]。
然而,超生理剂量GC 的应用可导致库欣综合征、骨质疏松和心血管反应等一系列的副作用。
激素性股骨头坏死(SANFH )是过量使用糖皮质激素的严重后α2-macroglobulin alleviates glucocorticoid-induced avascular necrosis of the femoral head in mice by promoting proliferation,migration and angiogenesis of vascular endothelial cellsZHU Qi,LU Yunxiang,PENG You,HE Jiale,WEI Zeyu,LI Zhiyong,CHEN YuxianDepartment of Joint Surgery,Third Affiliated Hospital,Sun Yat-Sen University,Guangzhou 510630,China摘要:目的探讨α2-巨球蛋白(A2M )是否对激素性股骨头坏死(SANFH )具有保护作用。
方法体外实验:用梯度浓度(10-8~10-5mol/L )地塞米松(DEX )处理人脐静脉内皮细胞(HUVECs )建立糖皮质激素(GC )诱导内皮细胞损伤体外模型,设置对照组、DEX 组、DEX+A2M (0.05mg/mL )和DEX+A2M (0.1mg/mL )4组,采用CCK-8法检测细胞活性,Transwell 实验和划痕愈合实验检测HUVECs 迁移,血管形成实验检测HUVECs 血管形成能力,Western blot 检测HUVECs 中CD31和VEGF-A 蛋白表达水平。
体内实验:将24只BALB/c 小鼠分为对照组、模型组(GC )和干预组(GC+A2M ),Micro-CT 检测骨小梁情况,HE 染色观察组织学特征,免疫组化染色检测CD31的表达。
肾素-血管紧张素系统在骨生物学活性中的研究进展

对来源于新生大鼠颅盖骨的成骨细胞研究发 现,AngII下调成骨细胞骨钙素mRNA水平,并抑制 碱性磷酸酶的活性,von Kossa染色结果表明,Angll (1×10’7 M)显著减少矿化结节数及矿化总面积, 降低细胞内及间质层的钙含量,说明Ang II抑制成 骨细胞的分化以及矿化能力旧1。然而,Ang lI对成
1 RAS系统各组分及其体内分布
在循环系统中,主要的RAS组分包括肾素 (renin)、血管紧张素原(AGT)、血管紧张素I(AngI)
作者单位:200093 上海.上海理工大学系统生物医学工程重 点实验室
通讯作者:张岩,Email:medicineyan@yahoo.com.cn
万方数据
和血管紧张素Ⅱ(An91I)。AGT不断地由肝脏释放 人血,肾素由肾小球入球动脉的球旁细胞分泌,分解 AGT生成10肽物质AngI。位于血管和肺部的内皮 细胞上的血管紧张素转化酶(ACE)分解AngI的C 末端两个氨基酸,生成含8肽的AngII。Angll进而 通过与其I型受体(ATl)和Ⅱ型受体(AT2)结合发 挥其体内生物活性…。除了以上经典的RAS途径 外,各组织中都能局部生成AGT和renin,或者从血 循环中摄取。大部分的组织细胞中都表达ACE和 肾素前体受体(PRR),PRR与肾素前体(PR)结合, 能够诱导AngII的局部生成¨。,而Ang II是RAS中 主要的活性物质。总的来看,胞外AngⅡ的合成主 要是ACE依赖性和PR依赖性。
主垦量亟堕塑盘查垫!!笙!旦蔓!!鲞筮!塑竺!垫』堕!!望!翌!:!苎璺!!翌垫!!:!堂!!:盟!:! —P——u——b———li—shed——on——l—ine www.wanfaagdate,com.cn doi:10.3969/j.issn.1006-7108.2010.01.018
慢性肾脏病与肾脏纤维化研究进展

[8】Fofine
M,Torregrossa R,Ced M.et a1.TGF—betal induce epithelail
transition
mesenchymal
but
not
myofibmblast transdifferentiation of
A
A.Molecular basis of renal
fibrosis[J J.Pediatr Nephrol, II,
2000,15(3-4):290-301.
[4]Seceia
T M。Maniem C,Belloni
A
s,et a1.Role of angiotensin
in
endothelin一1 and L-type calcium channel
于Smad信号通路在RIF中的可能机制。我们进一步探讨防治
RIF的新策略。
3.了越来越多的
研究证实.它不但能加速急性肾衰中肾小管上皮细胞的再生. 而且能减缓慢性肾衰的进程。在肾脏。HGF主要表达于肾间质 细胞、肾小球内皮细胞和巨噬细胞,它以内分泌、自分泌和旁分 泌的形式。特异性作用于靶细胞一TEC。发挥其对肾脏的保护作 用.Docherty等【12]报道HGF可以阻止培养的人肾小管上皮细 胞凋亡。在不同的细胞中,HGF通过不同的方式阻断TGF.Bl 的信号转导。 调控ECM代谢的降解酶系统主要包括:(1)基质金属蛋 白酶(MMPs)是舍有锌指结构的一个酶家族,对多种细胞外蛋 白具有溶解作用;(2)TIMPs,其特异性抑制物是MMPs,MMPs 与1rIMPs相互作用,是影响ECM降解或堆积的重要因素。 体内外实验证实HGF是一种重要的抗纤维化的因子。 HGF延缓IuF的可能机制如下:(1)HGF可中和TGF.B,的促纤 维化作用,如HGF抑制TEC及内皮细胞凋亡,促使TEC分支 形成,保护和重建肾小管结构、功能的完整性.刺激或诱导蛋白 酶及一些基质金属蛋白酶降解ECM:(2)HGF可抑制TEC表达 d一平滑肌肌动蛋白(d.SMA)。阻止TEC向肌成纤维细胞转化。 并减弱由TGF.B,诱导的E一钙依赖蛋白的表达抑制,且呈剂量 依赖性,还可减少FN在细胞外沉积:(3)HGF可促进基质金属 蛋白酶一9(MMp-9)表达.同时抑制rnMP.1和TIMP一2表达,从
电针调控Nrf2HO-1通路对缺血缺氧性脑损

电针调控Nrf2/HO-1通路对缺血缺氧性脑损伤大鼠小胶质细胞活化的影响张赟,李珂,补王珍摘要:目的探究电针调控核因子E2相关因子2(Nrf2)/血红素加氧酶1(HO-1)通路对缺血缺氧性脑损伤(HIBD)大鼠小胶质细胞活化的影响。
方法随机将40只SD大鼠均分为假手术组、模型组、电针组、电针+全反式维甲酸组。
除假手术组外其余组均通过颈总动脉结扎及缺氧建立HIBD大鼠模型,假手术组大鼠仅暴露左侧颈总动脉及迷走神经,不进行结扎及缺氧处理。
造模结束后,电针组于百会穴进行电针刺激;电针+全反式维甲酸组大鼠经电针刺激后腹腔注射全反式维甲酸(7mg/kg)。
通过Longa评分进行大鼠神经行为学评分;旷场及水迷宫实验检测大鼠自主活动能力和认知功能;TUNEL法检测大鼠神经细胞凋亡情况;免疫组化法检测大鼠脑组织中小胶质细胞标志蛋白离子钙结合衔接分子1(Iba1)表达;试剂盒检测大鼠脑组织中白细胞介素10(IL-10)、IL-1β、活性氧(ROS)及丙二醛(MDA)水平;Western blot检测脑组织CD68、诱导型一氧化氮合酶(iNOS)和精氨酸酶(Arginase)及Nrf2/HO-1通路相关蛋白表达。
结果与假手术组比较,模型组大鼠神经行为学评分、逃避潜伏期、大脑皮质神经细胞凋亡率、Iba1、IL-1β、MDA水平、ROS荧光强度、CD68、iNOS表达增加,运动路程、站立次数、穿越平台次数、IL-10、Arginase、Nrf2及HO-1水平降低(P<0.05);电针可降低HIBD大鼠神经行为学评分、逃避潜伏期、神经细胞凋亡率、Iba1、IL-1β、MDA 水平、ROS荧光强度、CD68、iNOS表达,增加运动路程、站立次数、穿越平台次数、IL-10、Arginase、Nrf2及HO-1水平(P<0.05);电针+全反式维甲酸组可逆转电针对上述指标的影响。
结论电针可能通过激活Nrf2/HO-1通路抑制HIBD大鼠小胶质细胞过度活化,并促进其由M1型向M2型转变。
以血管紧张素Ⅱ为作用靶点的新药研究进展

以血管紧张素Ⅱ为作用靶点的新药研究进展血管紧张素Ⅱ(Angiotensin II,简称AngⅡ)是一种重要的肽类激素,在肾脏、血管、心脏和大脑等多个组织和器官中起着调节血压、体液平衡和电解质代谢的作用。
然而,AngⅡ在一些病理状态下的过度激活和异常表达会导致心血管疾病的发生和发展。
因此,针对AngⅡ作为作用靶点的新药研究成为一个备受关注的领域。
近年来,关于以AngⅡ为作用靶点的新药研究取得了一些进展。
以下是一些研究的重要方面:1. 血管紧张素转化酶(Angiotensin-converting enzyme,简称ACE)抑制剂:ACE是将AngⅠ转化为AngⅡ的关键酶,ACE抑制剂可以有效地抑制AngⅡ的合成。
已经有多种ACE抑制剂被广泛应用于临床,如依那普利(Enalapril)、雷米普利(Ramipril)等。
这类药物通过降低血管阻力和促进尿液排除来降低血压,并且对心脏和肾脏具有保护作用。
2. 血管紧张素受体拮抗剂(Angiotensin receptor blockers,简称ARBs):ARBs是结合AngⅡ受体,阻断其活性的药物。
目前已有多种ARBs被应用于临床,如缬沙坦(Valsartan)和洛沙坦(Losartan)等。
ARBs可以通过抑制AngⅡ对血管平滑肌的收缩和促进尿液排泄来降低血压,并且对心脏和肾脏有保护作用。
3. 血管紧张素Ⅱ受体偶联酶(Angiotensin II type 1 receptor-associated protein,简称ATRAP):ATRAP是一种新发现的蛋白质,它可以结合AngⅡ受体,并抑制AngⅡ的信号传导。
研究发现,ATRAP的表达水平在高血压患者中显著降低。
进一步的实验研究表明,通过增加ATRAP的表达或使用与ATRAP相关的药物,可以有效地抑制AngⅡ的功能,并且具有降低血压和减轻心血管疾病的作用。
4. 血管紧张素Ⅱ型2受体(Angiotensin II type 2 receptor,简称AT2R)激动剂:AT2R是AngⅡ受体的一种亚型,与AT1R相比,AT2R的功能较为复杂且具有更为广泛的组织分布。
肠道菌群参与糖尿病肾病的发病机制研究进展

- 184 -collagen-induced arthritis through suppression of vascular endothelial growth factor and its receptors[J].International Immunopharmacology,2016,34:71-77.[27] Pope R M,Shahrara S.Possible roles of IL-12-family cytokinesin rheumatoid arthritis[J].Nature Reviews Rheumatology,2012,9(4):252-256.[28]张传峰,苑海涛.病毒性心肌炎患者血清IL17、IL35水平变化及意义[J].山东医药,2014,58(31):28-30.[29] Kempe S,Heinz P,Kokai E,et al.Epstein-Barr Virus-Induced Gene-3 Is Expressed in Human Atheroma Plaques[J].American Journal of Pathology,2009,175(1):440-447.[30]修晶辉.白细胞介素35(IL-35)转基因小鼠的建立及过表达IL-35改善小鼠哮喘症状的机制研究[D].北京:北京协和医学院,2014.[31] Hu Y,Dong C,Yue Y,et al.In vivo delivery of interleukin-35relieves coxsackievirus-B3-induced viral myocarditis byinhibiting Th17 cells[J].Archives of Virology,2014,159(9):2411-2419.[32] Olson B M,Jankowska-Gan E,Becker J T,et al.Humanprostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockade[J].Journal of Immunology,2012,189(12):5590.[33] Huang C,Li N,Li Z,et al.Tumour-derived Interleukin 35promotes pancreatic ductal adenocarcinoma cell extravasation and metastasis by inducing ICAM1 expression[J].Nature Communications,2017,8:14035.[34] Jia Wang,Qianshan Tao,Huiping Wang,et al.Elevated IL-35 in bone marrow of the patients with acute myeloid leukemia[J].Human Immunology: Official Journal of the American Society for Histocompatibility and Immunogenetics,2015,76(9):681-686.(收稿日期:2020-08-10) (本文编辑:张爽)*基金项目:山西省卫生健康委科研课题(2018127)①长治医学院 山西 长治 046000②长治医学院附属和平医院通信作者:吴飞飞肠道菌群参与糖尿病肾病的发病机制研究进展*郭鑫① 吴飞飞②【摘要】 2型糖尿病(type 2 diabetes mellitus,T2DM)是常见疾病,随着患病率不断上升,其慢性并发症也给患者的生活、家庭以及社会带来了沉重的负担。
Actin-Tracker Green(微丝绿色荧光探针) 产品说明书
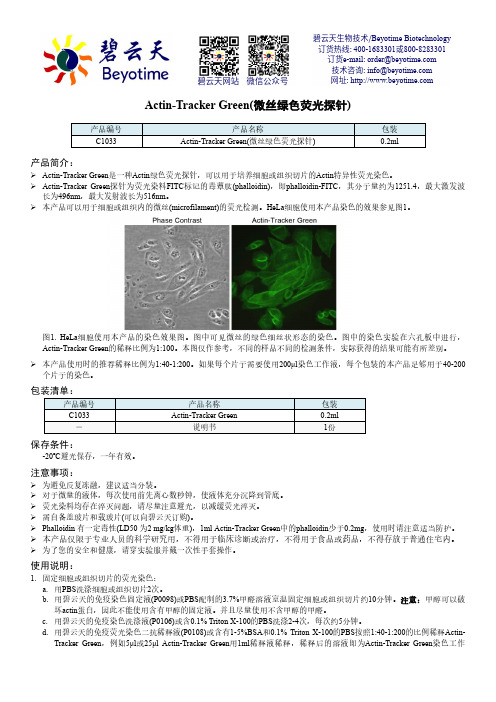
Actin-Tracker Green(微丝绿色荧光探针) 产品编号产品名称包装C1033 Actin-Tracker Green(微丝绿色荧光探针) 0.2ml产品简介: Actin-Tracker Green 是一种Actin 绿色荧光探针,可以用于培养细胞或组织切片的Actin 特异性荧光染色。
Actin-Tracker Green 探针为荧光染料FITC 标记的毒蕈肽(phalloidin),即phalloidin-FITC ,其分子量约为1251.4,最大激发波长为496nm ,最大发射波长为516nm 。
本产品可以用于细胞或组织内的微丝(microfilament)的荧光检测。
HeLa 细胞使用本产品染色的效果参见图1。
图1. HeLa 细胞使用本产品的染色效果图。
图中可见微丝的绿色细丝状形态的染色。
图中的染色实验在六孔板中进行,Actin-Tracker Green 的稀释比例为1:100。
本图仅作参考,不同的样品不同的检测条件,实际获得的结果可能有所差别。
本产品使用时的推荐稀释比例为1:40-1:200。
如果每个片子需要使用200μl 染色工作液,每个包装的本产品足够用于40-200个片子的染色。
包装清单:产品编号产品名称 包装 C1033Actin-Tracker Green 0.2ml - 说明书 1份保存条件:-20ºC 避光保存,一年有效。
注意事项:为避免反复冻融,建议适当分装。
对于微量的液体,每次使用前先离心数秒钟,使液体充分沉降到管底。
荧光染料均存在淬灭问题,请尽量注意避光,以减缓荧光淬灭。
需自备盖玻片和载玻片(可以向碧云天订购)。
Phalloidin 有一定毒性(LD50 为2 mg/kg 体重),1ml Actin-Tracker Green 中的phalloidin 少于0.2mg ,使用时请注意适当防护。
本产品仅限于专业人员的科学研究用,不得用于临床诊断或治疗,不得用于食品或药品,不得存放于普通住宅内。
浅析AngⅡ对腹膜间皮细胞ROS和NADPH氧化酶亚基p47phox

浅析AngⅡ对腹膜间皮细胞ROS和NADPH氧化酶亚基p47phox背景和研究意义腹膜间皮细胞(peritoneal mesothelial cells,PMC)是腹腔内最具代表性的细胞类型,也是腹膜水平隔离的关键细胞。
目前已知,PMC具有多种功能如发挥局部免疫作用、维持腹腔内环境平衡以及参与肿瘤转移等。
腹膜的损伤或者炎症反应引起的病理过程都会导致PMC受到损伤。
因此,寻找PMC的损伤机制并探讨PMC的保护措施对于防治相关疾病具有重要意义。
Angiotensin Ⅱ(AngⅡ)是肾素-血管紧张素系统的重要产物之一。
多年来的研究表明,AngⅡ在炎症反应和纤维化过程中发挥着重要的生理和病理作用。
最近的研究还表明,AngⅡ参与PMC损伤的机制是通过ROS的产生和开启NADPH氧化酶(NOX)信号通路来实现的。
p47phox是原始的NOX亚基之一,它的表达水平可以反映NOX信号通路的活性。
因此,探讨AngⅡ对PMC产生ROS和NOX亚基p47phox的影响,对于深入了解AngⅡ在PMC损伤中的作用机制具有重要价值。
方法本研究选取来自Sprague-Dawley雄性大鼠的PMC,培养于DMEM/F12培养基中,添加10%脐血血清,孵育于37℃、5%CO2中。
将PMC分为三组:对照组、AngⅡ组和AngⅡ+射频组(射频是制备核酸和蛋白质电泳的一种技术)。
对照组PMC没有任何处理,AngⅡ组PMC培养液中加入10^-7mol/L AngⅡ,AngⅡ+射频组PMC培养液中加入10^-7mol/L AngⅡ和260 kHz 15 W的射频放射治疗30min,每组PMC培养72小时后采用Western blot、DCFH-DA荧光染色和imminofluorescence染色等方法分别检测NOX亚基p47phox、ROS含量和活性。
结果在本研究中,我们发现AngⅡ组PMC的ROS含量和活性显著升高,同时NOX亚基p47phox的表达水平也有明显增加。
肾素抑制剂阿利吉仑对阿霉素肾病小鼠肾损伤及足细胞nephrin表达的影响

肾素抑制剂阿利吉仑对阿霉素肾病小鼠肾损伤及足细胞nephrin表达的影响伍巧源;廖蕴华;杨桢华;郭谊;周颖川;陈一强【摘要】目的观察肾素抑制剂阿利吉仑对阿霉素肾病小鼠肾损伤的保护作用及对足细胞nephrin表达的影响.方法通过尾静脉注射阿霉素建立小鼠阿霉素肾病模型后给予阿利吉仑,另设正常对照组及阿霉素肾病对照组;比较不同组别尿蛋白及肾功能的差异;肾组织石蜡切片行PAS、HE和Masson染色,观察各组肾小球硬化程度和肾小管损害程度.RT-PCR及Western blot检测各组nephrin的表达.结果阿霉素肾病小鼠表现为蛋白尿、低蛋白血症并伴有进行性肾功能下降,病理表现为肾小球硬化及小管间质损害;阿利吉仑可使蛋白尿状况显著改善,血清白蛋白上升,肾功能恶化得以延缓(P<0.05),肾小球硬化和小管间质损害程度明显改善(P<0.05).阿霉素肾病小鼠nephrinmRNA及蛋白表达水平均显著低于正常对照组,给予阿利吉仑干预后nephrin mRNA及蛋白表达水平有显著上升(P<0.05).结论阿利吉仑对小鼠阿霉素肾病有良好的保护作用,该作用至少部分与其上调肾小球足细胞nephrin 的表达有关.【期刊名称】《西安交通大学学报(医学版)》【年(卷),期】2014(035)005【总页数】5页(P665-668,713)【关键词】肾素抑制剂;阿利吉仑;阿霉素肾病;足细胞;nephrin【作者】伍巧源;廖蕴华;杨桢华;郭谊;周颖川;陈一强【作者单位】广西医科大学第一附属医院肾内科,广西南宁530021;广西医科大学研究生院,广西南宁530021;广西医科大学第一附属医院肾内科,广西南宁530021;广西医科大学第一附属医院肾内科,广西南宁530021;广西医科大学第一附属医院检验科,广西南宁530021;广西医科大学第一附属医院病理科,广西南宁530021;广西医科大学第一附属医院呼吸科,广西南宁530021【正文语种】中文【中图分类】R692肾素-血管紧张素系统(renin-angiotensin system,RAS)过度激活是肾脏疾病发生发展的重要机制之一,抑制RAS系统是治疗肾脏病的有效途径。
血管生成素-2和内皮抑素在子宫内膜异位症发生发展中的作用

血管生成素-2和内皮抑素在子宫内膜异位症发生发展中的作用范俊;董文韬;孙宝治;栾少红;马慧敏;李晓林【期刊名称】《现代妇产科进展》【年(卷),期】2008(17)11【摘要】目的:探讨血管生成素-2(angiopoietin-2,Ang-2)和内皮抑素(endostatin,ENS)在EMs发生、发展中的作用。
方法:选择25例OEMs病人作为研究组,28例同期非内膜疾病妇女作为对照组。
均留取子宫内膜,使用免疫组化染色方法检测Ang-2和ENS在研究组异位、在位子宫内膜及对照组正常子宫内膜中的表达。
结果:Ang-2和ENS蛋白主要定位于子宫内膜腺上皮细胞的胞浆中。
Ang-2在OEMs组在位子宫内膜组织中的阳性表达率为80.00%,显著高于异位内膜(52.00%)和对照组(21.48%)(P<0.05);异位内膜组阳性表达率为52.00%,显著高于对照组(21.48%)(P<0.05);OEMs组在位内膜和对照组子宫内膜中Ang-2在分泌期(90%、30.75%)和增生期(73.33%、13.33%)的表达均无显著性差异(P>0.05);ENS在OEMs异位内膜组织中的表达为84.00%,明显高于在位内膜(56.00%)和对照组(25.00%)(P<0.05);在位内膜(56.00%)明显高于对照组(25.00%)(P<0.05)。
OEMs组在位内膜中ENS分泌期的表达为50.00%,与增生期(60.00%)比较无统计学差异(P>0.05);对照组中子宫内膜ENS在分泌期的表达为23.08%,与增生期(26.67%)比较无统计学差异(P>0.05)。
结论:Ang-2和ENS在子宫内膜异位症发生发展中起到重要作用。
【总页数】4页(P835-838)【关键词】子宫内膜异位症;血管生成素-2;内皮抑素;血管生成【作者】范俊;董文韬;孙宝治;栾少红;马慧敏;李晓林【作者单位】青岛市市立医院,青岛266071;莱西市人民医院【正文语种】中文【中图分类】R737.31【相关文献】1.血管生成素-2和内皮抑素在子宫内膜异位症中的高表达 [J], 范俊;董文韬;孙宝治;李晓林;刘波;王纯2.血管内皮生长因子、血管内皮抑素与子宫内膜异位症 [J], 张俊辉;熊正爱3.血管内皮生长因子和促血管生成素及其受体Tie2在肝细胞癌发生发展中的作用[J], 张中林;刘志苏;孙权4.血管生成素-2及其受体在子宫内膜异位症发生发展中的作用 [J], 胡利霞;涂雪松;陈玉环;黄彩彩5.血管内皮生长因子、血管生成素-2及巨噬细胞移动抑制因子在子宫内膜异位症患者血清中的表达水平及在疾病发生发展中的意义 [J], 陈薇;陆月梅;王琛琛;丁其培;章杨韦因版权原因,仅展示原文概要,查看原文内容请购买。
血管紧张素Ⅱ对乳腺癌细胞迁移的影响

血管紧张素Ⅱ对乳腺癌细胞迁移的影响曹令森;沈玮;曹祥荣【摘要】乳腺癌是女性发病率高且恶性程度高的肿瘤,肿瘤侵袭转移是导致患者死亡的重要原因,研究与癌细胞转移相关的因子或指标具有重要意义.本实验采用血管紧张素Ⅱ(AngⅡ)处理乳腺癌细胞MDA-MB-231,可促进肿瘤细胞迁移,增强到1.51倍.Q-PCR检测结果表明,AngⅡ能够明显刺激MDA-MB-231细胞中uPA(2.97倍)和uPAR的表达.因此,AngⅡ刺激乳腺癌细胞uPA和uPAR的表达,可能是AngⅡ促进乳腺癌细胞迁移的原因之一.【期刊名称】《南京师大学报(自然科学版)》【年(卷),期】2018(041)001【总页数】5页(P83-87)【关键词】血管紧张素Ⅱ;uPA;乳腺癌细胞【作者】曹令森;沈玮;曹祥荣【作者单位】南京师范大学生命科学学院,江苏省分子医学生物技术重点实验室,江苏南京210023;南京师范大学生命科学学院,江苏省分子医学生物技术重点实验室,江苏南京210023;南京师范大学生命科学学院,江苏省分子医学生物技术重点实验室,江苏南京210023【正文语种】中文【中图分类】Q28血管紧张素Ⅱ(Angiotensin Ⅱ,AngⅡ)是肾素-血管紧张素系统中主要的效应肽,通过受体(Angiotensin Ⅱ receptor,AT)来发挥生物学作用. 已有报道证明,AngⅡ能够刺激实体瘤的生长,包括胃癌[1]、乳腺癌[2]、卵巢癌[3]等. 过表达血管紧张素受体1(AT 1)或用AT1拮抗剂处理,都证明AngⅡ-AT1系统的增强可以诱导上皮细胞间质化转变(epithelial-mesenchymal transition,EMT),促进肿瘤生长和血管生成[4]. 用AT1拮抗剂处理,可抑制食道腺癌细胞的增殖和肿瘤的生长[5]. AT1拮抗剂还可抑制乳腺癌细胞增殖和血管形成[6]. miR-410过表达可抑制AT1表达,或干扰AT1表达水平,都可抑制胰腺癌细胞生长和侵袭转移[7].uPA(尿激酶型纤溶酶原激活物,urokinase plasminogen activator)系统包括uPA、uPA受体(uPAR)、纤溶酶原激活物抑制子(plasminogen activator inhibitor,PAI),参与多种生理或病理活动过程,如细胞分化、血管生成、细胞迁移、细胞外基质降解和组织重建等过程. 分析2780例原发性乳腺癌中uPA系统成员(uPA,uPAR,PAI-1和PAI-2)的表达量,发现它们与患者的无复发存活率相关[8]. 目前研究结果已表明,uPA在肿瘤侵袭转移过程中扮演更重要的角色[9]. 神经营养蛋白受体相关黑色素瘤抗原同源物(neurotrophin receptor-interacting MAGE homolog,NRAGE)具有明显的促凋亡作用[10],抑制胰腺癌和恶性黑色素瘤细胞的浸润和转移,是肿瘤生长和转移相关基因[11].本实验采用血管紧张素Ⅱ(AngⅡ)处理乳腺癌细胞MDA-MB-231,检测血管紧张素Ⅱ(AngⅡ)对肿瘤细胞迁移以及uPA-uPAR和NRAGE等肿瘤细胞迁移有关基因表达的影响,探讨血管紧张素Ⅱ(AngⅡ)促进乳腺癌细胞迁移的机制.1 材料与方法1.1 主要药品与试剂人乳腺癌细胞系MDA-MB-231为本实验室保存;RPMI 1640和DMEM购自Gibco BRL公司;PrimeScript RT reagent Kit 试剂盒、总RNA 抽提试剂Trizol 购自Invitrogen 公司,SYBR Premix EX Taq(Perfect Real Time)试剂为TAKARA 公司产品. 所用引物由上海生工合成.1.2 细胞及培养乳腺癌细胞MDA-MB-231的培养条件分别为含10%胎牛血清的RPMI 1640、DMEM高糖培养液,于37 ℃、5%的CO2培养箱培养.1.3 细胞划痕愈合实验收集对数生长期乳腺癌细胞MDA-MB-231,计数. 在6孔板中,铺细胞数量约4×105. 37 ℃,5% CO2培养24 h. 吸去培养液,用移液枪枪尖在培养基表面划一条痕迹(长约2 cm),用PBS洗3次,加入无血清的培养基及含0.1 μm及1 μm的AngⅡ继续培养. 分别在0 h、24 h时拍照,测量划痕愈合的距离,取4个视野尺寸进行数据统计.1.4 细胞总RNA的提取和cDNA的合成收集MDA-MB-231 细胞,按Trizol法说明提取细胞总RNA,加入RNase-free的水10 μL~20 μL溶解沉淀. 测定260 nm和280 nm光吸收值的比值,A260/280比值在1.8~2.0之间. 取总RNA各500 ng,按PrimeScript RT reagent Kit试剂盒说明,合成cDNA,所得cDNA在-20 ℃保存备用.1.5 Q-PCR检测乳腺癌细胞中mRNA的表达选取对数生长期乳腺癌细胞系MDA-MB-231. 无血清培养细胞饥饿12 h后,换含AngⅡ无血清培养基(终浓度分别为0.1 μm和1 μm)培养. 处理24 h后,提取其总RNA,合成cDNA. 以actin基因为内参,Q-PCR 检测uPA-uPAR及NRAGE mRNA的表达量.Q-PCR所用引物:uPA上:5′-GCGACCCTGGTGCTATGT-3′;uPA下:5′-CCCTTGCGTGTTGGAGTT-3′.uPAR上:5′-GTGGGAAGAAGGAGAAGAGC-3′;uPAR下:5′-GATGAGCCACAGGAAATGC-3′.PAI-2上:5′-GGAGCATCTCGTCCACCA-3′;PAI-2下:5′-ATCGCATCAGGATAACTACC-3′.NRAGE上:5′-GCTCGGTCTCCTCTTGGT-3′;NRAGE下:5′-GTTGCTGTTGGGCACTCG-3′.Actin上:5′-TGACGTGGACATCCGCAAAG-3′;Actin下:5′-CTGGAAGGTGGACAGCGAGG-3′.具体步骤如下:(1)细胞总RNA抽提取6孔板贴壁培养细胞,移去培养基,用冰冷PBS洗两次后加入TRIZOL试剂1 mL,室温下放置5 min,使其充分裂解后转移至DEPC处理的EP管中,每管加氯仿200 μL,剧烈振荡15 S,室温放置5 min,于4 ℃,12 000 g离心15 min. 小心吸取上层透明层于DEPC处理的EP管中,加等体积异丙醇(约500 μL),混匀后室温下放置10 min以沉淀RNA. 然后于4 ℃,12 000 g离心10 min,弃上清液,加入冰预冷的75%乙醇洗涤RNA沉淀,室温下干燥沉淀10 min至沉淀半透明,加入RNase-free的水10 μL~20 μL溶解沉淀并保存于-80 ℃. (所用器具和试剂用DEPC处理)(2)RNA浓度测定取2 μL上述提取的RNA,用分光光度计测定RNA浓度,并读取在260 nm和280 nm波长的光吸收值的比值,A260/280比值应在1.8~2.0之间.(3)cDNA合成依次加入如下试剂,形成10 μL的反转录反应体系5×PrimeScriptTM Buffer(for Real Time) 2 μLPri meScriptTM RT Enzyme MixI 0.5 μLOligo dT Primer(50 μmol/L) 0.5 μLRandom 6 mers(100 μmol/L) 0.5 μLTotal RNA 500 ngRNase-free的水X μL补足10 μL将样品于37 ℃加热15 min,然后于85 ℃加热5 s. 合成的cDNA可于-20 ℃保存备用.(4)Q-PCR将上述逆转录cDNA稀释10倍用于Q-PCRcDNA 1 μLSRBR Premix Ex TaqTM Ⅱ 7.5 μLPCR Forward Primer(10 μmol/L) 1 μLPCR Reverse Primer(10 μmol/L) 1 μLRNase-free的水 4.5 μL总体积15 μL反应体系15 μL,反应条件:95 ℃变性1 min,95 ℃ 30 s、60 ℃ 1 min,40 个循环.2 结果2.1 Q-PCR检测乳腺癌细胞中uPA和NRAGE的mRNA表达水平选取对数生长期乳腺癌细胞系MDA-MB-231及MCF-7,提取其总RNA,Q-PCR检测uPA系统各成员及NRAGE mRNA的表达. 从结果(图1)中可以看出,在乳腺癌细胞中uPA、uPAR,NRAGE转录水平有差异,而PAI-2基因在两个细胞系中的表达量均很低.图1 Q-PCR检测乳腺癌细胞中uPA-uPA和NRAGE的mRNA表达(actin为内参)Fig.1 The expression of the uPA-uPAR and NRAGE in MDA-MB-231 and MCF-7 cells by Q-PCR measurement2.2 血管紧张素Ⅱ可促进乳腺癌细胞的迁移将乳腺癌细胞株MDA-MB-231细胞培养在六孔板上,待细胞长满后,用移液枪在中央区域画一条直线,用PBS将细胞清洗3次,除去这条直线区域内的细胞,用含0.1 μmol/L或1 μm的AngⅡ无血清培养基继续培养,测量细胞向无细胞的划痕区域迁移的距离,来判断细胞的迁移能力. 结果(图2)表明,AngⅡ能够明显地刺激MDA-MB-231细胞的迁移,当浓度为0.1 μmol/L时促进细胞迁移的作用更为明显(1.51倍),1 μm浓度处理时,迁移能力为1.31 倍.图2 细胞划痕愈合实验检测AngⅡ对MDA-MB-231细胞迁移的影响Fig.2 The effect of the angiotensin Ⅱ on the migration of MDA-MB-231 cells by wound healing assay图3 Q-PCR检测AngⅡ对MDA-MB-231细胞中uPA系统及NRAGE基因mRNA的影响(actin为内参)Fig.3 The expression of the uPA-uPAR and NRAGE in MDA-MB-231 cells treated with angiotensin Ⅱ by Q-PCR measurement2.3 AngⅡ增强乳腺癌细胞中uPA-uPAR和NRAGE表达水平选取对数生长期乳腺癌细胞系MDA-MB-231,细胞经0.1 μm及1 μm浓度的AngⅡ处理后,提取细胞总RNA,以actin转录水平为内参,Q-PCR检测uPA-uPAR 及NRAGE mRNA的表达量变化(图3). 实验结果显示,当AngⅡ的浓度为0.1 μm 和1 μm处理时,乳腺癌细胞系MDA-MB-231中uPA的转录水平分别增加到2.97倍和2.38倍;同时刺激uPAR mRNA有一定量的升高. 用0.1 μm和1 μm AngⅡ处理时,MDA-MB-231细胞中NRAGE的RNA表达量分别提高到3.55倍和2.73倍.3 讨论乳腺癌是女性发病率高且恶性程度高的肿瘤,有研究结果表明乳腺癌细胞MCF-7和MDA-MB-231细胞表面发现uPA的增多会增加纤溶酶原的活化,引起细胞蛋白水解活力的提高[12]. 在载脂蛋白E缺陷的小鼠中,AngⅡ能增加uPA的表达率并诱导腹主动脉瘤[13]. 血管紧张素Ⅱ可促进乳腺癌细胞金属蛋白酶MMP2、MMP9的表达水平,增强细胞表面蛋白水解能力,继而促进乳腺癌细胞迁移和局部侵袭作用[14]. 血管紧张素Ⅱ可降低整联蛋白integrin α3和β1,继而增强乳腺癌细胞黏附和侵袭能力[15]. 因此认为肿瘤患者血浆中血管紧张素Ⅱ可以作为癌症恶性的指标[16].本研究结果已显示AngⅡ可以促进乳腺癌MDA-MB-231细胞的迁移. 当用不同浓度的AngⅡ刺激细胞时,在乳腺癌细胞系MDA-MB-231中AngⅡ能够明显地刺激uPA的转录,uPAR RNA量也有一定的增长,增强细胞的迁移能力. 有研究结果显示,NRAGE在细胞生命活动中的作用是多元化的,但其功能的发挥与其相互作用的配体蛋白有关,NRAGE具有促进凋亡和抑制恶性黑色素瘤转移的能力[11]. 本实验中AngⅡ促进乳腺癌MDA-MB-231细胞的迁移情况下,也能够促进NRAGE的表达. 在肝细胞癌中也观察到,NRAGE高水平表达时,促进肝细胞癌的恶性表型[17]. AngⅡ刺激乳腺癌细胞uPA和uPAR的表达,可能是AngⅡ促进乳腺癌细胞迁移,继而促进肿瘤血管生成和肿瘤生长转移的原因之一.致谢:感谢南京师范大学生命科学学院温传俊老师对本实验的指导和支持![参考文献][1] KINOSHITA J,FUSHIDA S,HARADA S,et al. Local angiotensin Ⅱ-generation in human gastric cancer:correlation with tumor progression through the activation of ERK1/2,NF-kappaB and surviving[J]. Int J Oncol,2009,34(6):1 573-1 582.[2] PUDDEFOOT J R,UDEOZO U K I,BARKER S,et al. The role of angiotensin Ⅱ in the regulation of breast cancer cell adhesion and invasion[J]. Endocrine-related cancer,2006,13:895-903.[3] SUGANUMA T,INO K,SHIBATA K,et al. Functional expression of the angiotensin Ⅱ type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumorinvasion,angiogenesis,and peritoneal dissemination[J]. Clin Cancer Res,2005,11(7):2 686-2 694.[4] OH E,KIM J Y,CHO Y,et al. Over-expression of angiotensin Ⅱ type 1 receptor in breast cancer cells induces epithelial-mesenchymal transition and promotes tumor growth and angiogenesis[J]. Biochim Biophys Acta,2016,1863(6 Pt A):1 071-1 081.[5] FUJIHARA S,MORISHITA A,OGAWA K,et al. The angiotensin Ⅱ type 1 receptor antagonist telmisartan inhibits cell proliferation and tumor growth of esophageal adenocarcinoma via the AMPKa/mTOR pathway in vitro and in vivo[J]. Oncotarget,2017,8(5):8 536-8 549.[6] CHEN X,MENG Q,ZHAO Y,et al. Angiotensin Ⅱ type 1 receptor antagonists inhibit cell proliferation and angiogenesis in breast cancer[J]. Cancer Lett,2013,328(2):318-324.[7] GUO R,GU J,ZHANG Z,et al. MicroRNA-410 functions as a tumor suppressor by targeting angiotensin Ⅱ type 1 receptor in pancreatic cancer[J]. IUBMB Life,2015,67(1):42-53.[8] FOEKENS J A,PETERS H A,LOOK M P,et al. The urokinase system of plasminogen activation and prognosis in 2780 breast cancer patients[J].Cancer Res,2000,60(3):636-643.[9] 王学谦,林洪生. uPA/uPAR与恶性肿瘤关系的研究进展[J]. 医学研究杂志,2016,45(9):16-17.[10] SALEHI A H,XANTHOUDAKIS S,BARKER P A. NRAGE,a p75 neurotrophin receptor interacting protein,induces caspase activation and cell death through a JNK dependent mitochondrial pathway[J]. J Biol Chem,2002,277(50):48 043-48 050.[11] CHU,C S,CHU C S,XUE B,et al. NRAGE suppresses metastasis of melanoma and pancreatic cancer in vitro and in vivo[J]. CancerLett,2007,250(2):268-275.[12] STILLFRIED G E,SAUNDERS D N,RANSON M. Plasminogen binding and activation at the breast cancer cell surface:the integral role of urokinase activity[J]. Breast Cancer Res,2007,9(1):R14.[13] WANG Y X,MARTIN-MCNULTY B,FREAY A D,et al. Angiotensin Ⅱ increases urokinase-type plasminogen activator expression and induces aneurysm in the abdominal aorta of apolipoprotein E-deficient mice[J]. Am J Pathol,2001,159(4):1 455-1 464.[14] RODRIGUES-FERREIRA S R,ABDELKARIM M,DILLENBURG-PILLA P,et al. Angiotensin Ⅱ facilitates breast cancer ce ll migration and metastasis[J]. PLoS ONE,2012,7(4):e35667.[15] PUDDEFOOT J R,UDEOZO U K I,BARKER S,et al. The role of angiotensin Ⅱ in the regulation of breast cancer cell adhesion and invasion[J]. Endocrine-related cancer,2006,13:895-903.[16] PENAFUERTE C A,GAGNON B,SIROIS J,et al. Identification ofneutrophil-derived proteases and angiotensin Ⅱ as biomarkers of cancer cachexia[J]. Br J Cancer,2016,114(6):680-687.[17] SHIMIZU D,KANDA M,SUGIMOTO H,et al. NRAGE promotes the malignant phenotype of hepatocellular carcinoma[J]. OncologyLett,2016,11:1 847-1 854.。
尿激酶治疗大鼠急性深静脉血栓的实验研究

尿激酶治疗大鼠急性深静脉血栓的实验研究马建华; 汪坤; 李鑫【期刊名称】《《中国现代医药杂志》》【年(卷),期】2019(021)008【总页数】4页(P20-23)【关键词】尿激酶; 急性深静脉血栓【作者】马建华; 汪坤; 李鑫【作者单位】271600 山东省肥城市人民医院血管外科; 271600 山东省肥城市人民医院神经内科【正文语种】中文深静脉血栓形成(deep venous thrombosis,DVT)是临床上常见、多发的静脉疾病,发病率达100/10万[1],DVT并发症包括肺栓塞、血栓后综合征、静脉性坏疽,对患者健康及生活质量有很大影响。
因此,如何采用更为有效的治疗方法促进血栓再通成为当前研究的热点。
尿激酶亦称为尿激酶型纤溶酶原激活物,能催化裂解纤溶酶原成纤溶酶,降解纤维蛋白凝块和纤维蛋白原、凝血因子Ⅴ和凝血因子Ⅷ等,从而发挥溶栓作用[2]。
由于其价廉、高效、出血并发症少等优点,是主要的溶栓药物,溶栓效果已得到肯定,但其作用机制还有待进一步研究。
本研究通过建立大鼠下腔静脉血栓模型,观察尿激酶对血管内皮细胞、新生毛细血管、VEGF 表达及细胞凋亡的影响,并探讨其可能的机制。
1 材料与方法1.1 一般资料1.1.1 实验动物 SPF级SD大鼠20只,雄性,体质量250~280g,购自北京维通利华实验动物技术有限公司,均遵循动物实验管理办法,通过动物实验伦理验证。
1.1.2 主要试剂及仪器兔抗血管性血友病因子(vWF)、兔抗血管内皮生长因子(VEGF),TUNEL(瑞士Roche公司);石蜡切片机(德国莱卡公司),共聚焦显微镜(德国莱卡公司)。
1.2 实验方法1.2.1 血栓模型的制备 36g/L水合氯醛(1ml/100g)麻醉大鼠,仰卧固定于手术台上,严格无菌操作。
游离左肾静脉与下腔静脉汇合处和髂总静脉分叉处之间的下腔静脉段。
分别在左肾静脉汇合下腔静脉处下方、髂总静脉分叉处上方用显微血管夹暂时阻断下腔静脉2h,经25G细针穿刺无血液流出,确认血栓形成(血管由红色变为暗红色)后取下血管夹关腹[3]。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Angiotensin II Induces Epithelial-to-Mesenchymal Transition in Renal Epithelial Cells through Reactive Oxygen Species/Src/Caveolin-Mediated Activation of an Epidermal Growth FactorReceptor–Extracellular Signal-Regulated Kinase Signaling PathwayJianchun Chen,a Jian-Kang Chen,a and Raymond C.Harris a,b,cDepartments of Medicine a and Molecular Physiology and Biophysics,b Vanderbilt University School of Medicine,and Department of Veterans Affairs,c Nashville, Tennessee,USAChronic activation of the renin-angiotensin system plays a deleterious role in progressive kidney damage,and the renal proximal tubule is known to play an important role in tubulointerstitialfibrosis;however,the underlying molecular mechanism is un-clear.Here we report that in the proximal tubule-like LLCPKcl4cells expressing angiotensin II(Ang II)type1receptor,Ang II induced changes in cell morphology and expression of epithelial-to-mesenchymal transition(EMT)markers,which were inhib-ited by the miotogen-activated protein(MAP)kinase/extracellular signal-regulated kinase(ERK)-activating kinase(MEK)inhib-itor PD98059or the Src kinase inhibitor PP2.Ang II-stimulated phosphorylation of caveolin-1(Cav)at Y14and epidermal growth factor receptor(EGFR)at Y845and induced association of these phosphoproteins in caveolin-enriched lipid rafts, thereby leading to prolonged EGFR-ERK signaling that was inhibited by Nox4small interfering RNA(siRNA)and Src siRNA. Two different antioxidants not only inhibited phosphorylation of Src at Y416but also blocked the EGFR-ERK signaling.More-over,erlotinib(the EGFR tyrosine kinase inhibitor),EGFR siRNA,and Cav siRNA all inhibited both prolonged EGFR-ERK sig-naling and phenotypic changes induced by Ang II.Thus,this report provides thefirst evidence that reactive oxygen species (ROS)/Src-dependent activation of persistent Cav-EGFR-ERK signaling mediates renal tubular cell dedifferentiation and identi-fies a novel molecular mechanism that may be involved in progressive renal injury caused by chronic exposure to Ang II.C hronic kidney disease(CKD)is considered to be an irrevers-ible process that eventually leads to end-stage renal disease (ESRD).In addition to glomerular injury,it is now generally ac-cepted that progressive injury to the tubulointerstitial compart-ment is an essential factor in progressive kidney injury.In this regard,the dedifferentiation of epithelial cells,with decreased ex-pression of epithelial markers and the appearance of a mesenchy-mal phenotype,is thought to play an essential role in mediating the increased deposition of extracellular matrix(ECM)produced by myofibroblasts(24)and possibly providing a source for a mi-nority of the interstitial myofibroblasts through frank epithelial-to-mesenchymal transition(EMT)(18).Among the potential me-diators inducing this renal epithelial cell dedifferentiation,the renin-angiotensin system is widely acknowledged to play a central role.The cellular actions of angiotensin II are mediated by two subtypes of seven-transmembrane G protein-coupled receptors (GPCR),AT1and AT2(37).Renal cells express primarily the AT1 receptor,which mediates most of the known physiological and pathological effects of Ang II.However,the signaling events downstream of the AT1activation that mediates renal epithelial cell dedifferentiation are still under investigation.The epidermal growth factor receptor(EGFR)is a member of the ErbB family of receptor tyrosine kinases;this family includes EGFR(ErbB1/HER1),ErbB2/Neu/HER2,ErbB3/ HER3,and ErbB4/HER4(35).EGFR is widely expressed in the mammalian kidney,including the glomeruli,proximal tubules, and cortical and medullary collecting ducts(3,15,16).There is increasing evidence that EGFR transactivation serves as an im-portant signaling response to numerous hormones,growth factors,and cytokines.This transactivation can occur as a re-sponse to metalloproteinase-dependent cleavage and release of soluble EGFR ligands from membrane-associated precursors. In addition,non-ligand-mediated transactivation of EGFR may occur in response to cellular stress(17).EGFR has also been implicated in the pathogenesis of progressive renalfibro-sis induced by angiotensin II(Ang II)(21),but the detailed molecular mechanisms underlying renal injury following chronic Ang II treatment remain to be clarified.The current study demonstrates that renal proximal tubule epithelial cells undergo EMT in response to chronic Ang II treatment through AT1receptor-mediated production of reactive oxygen species (ROS)and activation of Src kinase,thereby leading to phos-phorylation and association of EGFR and caveolin-1(Cav)and resulting in prolonged ERK activation.MATERIALS AND METHODSReagents and antibodies.Antibodies against EGFR,extracellular signal-regulated kinase(ERK),Shc,GRB2,Cav,N-cadherin,phospho-EGFR (Y1173,Y845),phospho-Src(Y416),and phospho-ERK were from Cell Signaling Technology(Beverly,MA).Antibody against-actin and all secondary antibodies were from Santa Cruz Biotechnology(Santa Cruz, CA).Antibodies to phospho-Cav(Y14)and E-cadherin were from BDReceived10October2011Returned for modification11November2011Accepted22December2011Published ahead of print3January2012Address correspondence to Raymond C.Harris,ray.harris@.Copyright©2012,American Society for Microbiology.All Rights Reserved.doi:10.1128/MCB.06410-110270-7306/12/$12.00Molecular and Cellular Biology p.981–981Bioscience(Franklin Lakes,NJ).Antibodies against Nox2and Nox4were from Novus Biological(Littleton,CO).Alexa594-conjugated donkey anti-rabbit antibody and Alexa488-conjugated donkey anti-mouse antibody were from Invitrogen Corporation(Carlsbad,CA).OptiPrep was purchased from Accurate Chemical&Scientific Corp.(Westbury, NY).Erlotinib was purchased from LC Laboratories(Woburn,MA).Ang II,4-hydroxy-2,2,6,6-tetramethylpiperidine1-oxyl(tempol),phalloidin-fluorescein isothiocyanate(phalloidin-FITC),4=,6-diamidino-2-phenyl-indole(DAPI),Percoll,and all other reagents were purchased from Sigma-Aldrich(St.Louis,MO).Cell culture.Clone4of LLC-PK1cells(LLCPKcl4),expressing the characteristics of renal proximal tubular epithelial cells,was subcloned from cells of the parental porcine renal proximal tubule LLC-PK1cell line and stably transfected with AT1receptor(AT1R/Cl4)or empty vector (Vector/Cl4)and maintained in culture medium as we described previ-ously(5).Cells were made quiescent in serum-free culture medium for24 h followed by treatment with different reagents for different times as in-dicated in Results.For induction of EMT in cultured cells,AT1R/Cl4cells were cultured in Dulbecco’s modified Eagle’s medium/F-12(DMEM/F-12)containing0.5%serum and left untreated or treated with100nM Ang II;this induction medium was prepared and applied to the cells daily for up to4to7days.Preparation of caveolin-enriched membranes.Membranes were pu-rified using a detergent-free method as described previously(38).Briefly, AT1/Cl4cells were scraped into cold buffer A(0.25M sucrose,1mM EDTA,20mM Tricine;pH7.8)and pelleted by centrifugation at1,400ϫg for5min in an IEC Centra-4B centrifuge,followed by resuspension in0.5ml of buffer A and homogenization using a1-ml Wheaton Dounce tissue grinder.The suspensions were centrifuged at1,000ϫg for10min. The supernatant fraction was transferred into a clean1.5-ml centrifuge tube and resuspended in0.5ml of cold buffer A,followed by homogeni-zation and recentrifugation.The two supernatants were combined and brought to a2-ml volume with cold buffer A,followed by layering the2ml of supernatant onto23ml of30%Percoll in buffer A and centrifuging at 84,000ϫg for30min.The plasma membrane fractions were collected and adjusted to2.0ml with cold buffer A.The isolated plasma membrane fractions were sonicated on ice followed by mixing with0.16ml of buffer A and1.84ml of buffer C,50%OptiPrep(Accurate Chemical&Scientific Corp.,Westbury,NY)in buffer B(0.25M sucrose,6mM EDTA,120mM Tricine;pH7.8).The mixture was loaded into a centrifuge tube followed by pouring a linear OptiPrep gradient(20%to10%;prepared by diluting buffer C with buffer A)onto the sample and then centrifuging at52,000ϫg for90min to obtain OptiPrep1.The top5ml(containing fractions1to7)of the OptiPrep1solution was collected,placed in a fresh centrifuge tube,and mixed with4ml of buffer C.The sample was overlaid with2ml of5%OptiPrep and centrifuged at52,000ϫg for another90min at4°C to obtain OptiPrep2.A distinct opaque band,fraction3,was present about 4to5mm above the interface.Fraction3had previously been demon-strated to be a highly purified preparation of caveolin-enriched mem-branes(38).In our subsequent experiments,we utilized fraction3in AT1/Cl4cells treated with Ang II,EGF,or vehicle alone to study protein-protein associations by immunoprecipitation(IP)and immunoblotting (IB)analysis.Transfection of Src,EGFR,Nox4,and Cav siRNA.Duplexes(21-bp) of EGFR small interfering RNA(siRNA),Cav siRNA,or Silencer Negative Control1siRNA(catalogue no.AM4611;Applied Biosystems/Ambion, Austin,TX)or Src or NOX4siRNA(Thermo Fisher Scientific,Lafayette,CO)were transfected into subconfluent AT1R/Cl4cells by the Lipo-fectamine method(Invitrogen Corporation,Carlsbad,CA)as we de-scribed previously(4).Three different duplex sequences were examined for knocking down EGFR(for EGFR siRNA1,5=-CGCUGGAGGAGAAG AAAGUTT-3=;for EGFR siRNA2,5=-GAAGGAGACGGAAUUCAAAT T-3=;and for EGFR siRNA3,5=-CACCGUGGAGAAGAUCCCUTT-3=) or caveolin-1(for caveolin-1siRNA1,5=-GGAGAUAGACUUGGUCAAC TT-3=;for caveolin-1siRNA2,5=-AACCAGAAGGGACACACAGTT-3=;and for caveolin-1siRNA3,5=-CCAGAAGGGACACAVAUUUTT-3=) (see Fig.4C and9A).A pool of the EGFR siRNA1and EGFR siRNA3 duplex sequences or a pool of caveolin-1siRNA1and caveolin-1siRNA2 duplex sequences was used for later experiments.For inhibition of Nox4 expression,either Thermo Scientific Dharmacon ON-TARGET plus SMARTpool L-010194-00-0005or siGenome smartPool M-010194-00-0005siRNAs were used.For Src expression inhibition,either Thermo Scientific Dharmacon ON-TARGET plus SMARTpoolL-003175-00-0005 FIG1Chronic Ang II treatment induced AT1R/Cl4cells to undergo EMT by a Src-and MEK-dependent signaling pathway.AT1R/Cl4cells were cultured in6-well plates and made quiescent by serum deprivation for24h and then maintained in DMEM/F12medium containing0.5%serum and Ang II(10Ϫ7 M)with or without the Src kinase inhibitor PP2(5M)or the MEK inhibitor PD98059(5M)for4days;during this period,such an EMT-inducing me-dium with or without inhibitors was prepared and changed daily for the cells.(A)Photographs were taken atϫ100magnification.(B)Cell lysates were sub-jected to immunoblotting with an antibody against E-cadherin or FSP1,fol-lowed by stripping and reprobing with an antibody to-actin,respectively. (All of the data shown in these studies are representative of at least three separate experiments with similar results).Chen et al. Molecular and Cellular Biologyor siGenome smartPool M-003175-03-0005siRNAs (Thermo Fisher Sci-entific,Lafayette,CO)were used.For signaling studies,48h after trans-fection,cells were made quiescent in serum-free medium for another 24h followed by the indicated treatments.For monitoring the progress of EMT,after 48h of transfection,medium was changed with 0.5%serum and 100nM Ang II daily for another 3to 7days.Immunoprecipitation and immunoblotting.These procedures were performed as we previously described (5,6).Briefly,cells were made qui-escent in serum-free medium for 24h,treated with the indicated reagents,and lysed with lysis buffer (0.5%Nonidet P-40,50mm NaCl,10mmTris-HCl [pH 7.4],2mm EDTA,2mm EGTA,0.5%sodium deoxycholate,0.1%sodium dodecyl sulfate [SDS],100m Na 3VO 4,100mm NaF,30mm sodium pyrophosphate,1mm phenylmethylsulfonyl fluoride,10g/ml aprotinin,10g/ml leupeptin).After centrifugation of the cell lysates at 10,000ϫg for 15min at 4°C,equal amounts of protein were subjected to immunoprecipitation with the indicated antibodies as de-scribed previously (7).Immunoprecipitation samples were resuspended and boiled in sample buffer before separation using 7%to 15%SDS-PAGE and immunoblotted onto Immobilon-P transfer membranes (Mil-lipore,Bedford,MA).After blocking with 3%bovine serum albumin in 150mm NaCl–50mm Tris-HCl (pH 7.4)(TBS)for 1h at room temper-ature,blots were probed with the indicated primary antibodies.The blots were washed 3times at room temperature with 0.05%Tween 20–TBS,incubated with the appropriate secondary antibody conjugated with horseradish peroxidase,and detected with enhanced chemiluminescence (Amersham Biosciences,Little Chalfont,Buckinghamshire,United Kingdom).Measurement of intracellular ROS generation.AT1R/Cl4cells were cultured in a 24-well plate and made quiescent in serum-free culture me-dium for 24h,followed by washing once with HEPES buffered salt solu-tion (HBSS;pH ϭ7.4)(0.5ml/well)containing 25mM HEPES,120mMNaCl,5.4mM KCl,1.8mM CaCl 2,25mM NaHCO 3,and 5.5mM glucose.The cells then were then left untreated or treated with apocynin for 30min before addition of 10Ϫ7M Ang II and 2=,7=-dichlorodihydrofluorescin diacetate (DCFH-DA)(100uM)for 2h,and the fluorescence intensity was measured by using a fluorescence multiwell plate reader with excita-tion and emission wavelengths of 485nm and 530nm.Immunofluorescence staining.Cells cultured in a 16-well Lab-Tek chamber slide system (Nalge Nunc International,Rochester,NY)were fixed with 4%paraformaldehyde–phosphate-buffered saline (PBS),per-meabilized with 0.1%Triton X-100–PBS,and washed three times with PBS.After incubation with phalloidin-FITC (50g/ml)at room temper-ature for 40min,the cells were washed three times with PBS and covered with coverslips.In additional experiments,cells were incubated with rab-bit anti-phospho-EGFR (Y845;1:50)and mouse anti-phospho-Cav(Y14;FIG 2Ang II treatment induced persistent EGFR phosphorylation at tyrosine 845(Y845)but not tyrosine 1173(Y1173)in AT1R/Cl4cells by an Src-dependent mechanism.(A)AT1R/Cl4cells were rendered quiescent and ex-posed to Ang II (10Ϫ7M),EGF (30nM),or vehicle alone for 10min,30min,3h,or 6h.(B)Quiescent AT1R/Cl4cells were pretreated with the Src kinase inhibitor PP2(5M)or with vehicle alone for 30min prior to exposure to Ang II (10Ϫ7M),EGF (30nM),or vehicle alone for 10min.Cell lysates were subjected to immunoblotting with antibodies against Y845-or Y1173-phosphorylated EGFR,followed by stripping and reprobing of the blots with an antibody recognizing total EGFR,asindicated.FIG 3Ang II treatment induced Src-mediated prolonged ERK activation in AT1R/Cl4cells.(A)Quiescent AT1R/Cl4cells were treated as described for Fig.2A.(B)Quiescent AT1R/Cl4cells were pretreated with PP2as indicated in Fig.2B and treated with Ang II (10Ϫ7M)or vehicle alone for 10min or 3h.Cell lysates were subjected to immunoblotting with indicated antibodies.At 48h after transfection with Src siRNA or scrambled control siRNA,quiescent AT1R/Cl4cells were exposed to Ang II (10Ϫ7M)or vehicle alone for 10min or 3h.(C)Cell lysates were subjected to immunoblotting with indicated antibodies.Persistent EGFR-ERK Signaling and EMTMarch 2012Volume 32Number 9831:50)antibodies for 1h at room temperature following fixation and per-meability experiments and then incubated with Alexa 594-conjugated donkey anti-rabbit antibody or Alexa 488-conjugated donkey anti-mouse antibody for 1h.Nuclei were counterstained with 4=,6-diamidino-2-phenylindole (DAPI).Images were captured using a Nikon TE300fluo-rescence microscope and a Spot-Cam digital camera (Diagnostic Instru-ments).RESULTSChronic Ang II treatment induced AT1R/Cl4cells to undergo EMT by a Src-and MEK-dependent signaling pathway.In vivo ,the proximal tubule expresses high levels of AT1receptors (25);we therefore utilized stable transfectants of LLCPKcl4,the renal proximal tubular epithelial cell line,that expressed functionalAngFIG 4Chronic Ang II treatment induced EMT in AT1R/Cl4cells through an EGFR-ERK activation-dependent but HB-EGF-independent pathway.AT1R/Cl4cells were pretreated with erlotinib at 100nM for 30min,followed by exposure to indicated stimuli for 10min or 3h.(A)Cell lysates were subjected to immunoblotting with indicated antibodies.(B)Cells were treated as described for Fig.1A with or without erlotinib (100nM)for 4days,and photographs were taken at ϫ400magnification following FITC-conjugated phalloidin staining.(C and D)At 48h after transfection with different EGFR siRNAs or scrambled control siRNA,quiescent AT1R/Cl4cells were exposed to indicated stimuli for 10min or 3h and cell lysates were subjected to immunoblotting analysis with the indicated antibodies.(E and F)At 48h after siRNA transfection as indicated,AT1R/Cl4cells were subjected to induction of EMT with Ang II as indicated in Fig.1and cell lysates were immunoblotted with indicated antibodies (E)or cell photographs were taken at ϫ400magnification following FITC-conjugated phalloidin staining (F).(G)AT1R/Cl4cells were treated as indicated for either 10min or 3h followed by immunoblotting analysis of cell lysates with indicated antibodies.Chen et al.Molecular and Cellular BiologyII type1receptors(AT1R/Cl4cells)(5).We have previously re-ported that AT1R/Cl4cells responded to acute Ang II treatment by increasing EGFR phosphorylation due in part to release of heparin-binding EGF-like growth factor(HB-EGF)within10min (5).Dysregulated or sustained activation of the renin-angiotensin system is known to play a detrimental role in progressive kidney damage.Accordingly,the present study examined chronic effects of Ang II in renal proximal tubular epithelial cells.When we ex-tended the exposure of AT1R/Cl4cells to Ang II for4days,we observed striking morphological changes from an epithelial to a fibroblast-like morphology(Fig.1A,top panels).The Ang II-induced morphological changes were largely prevented by either the Src kinase inhibitor,PP2(Fig.1A,middle panels),or the ERK-activating kinase(MEK)inhibitor,PD98059(Fig.1A,bottom panels).Immunoblotting analysis revealed decreased expression of the adherent junction protein,E-cadherin,and increased ex-pression of thefibroblast specific protein,FSP-1,in the Ang II-treated AT1R/Cl4cells;these Ang II-induced alterations in gene expression were largely prevented by the presence of either the Src kinase inhibitor,PP2,or the MEK inhibitor,PD98059(Fig.1B). These data suggest that chronic Ang II exposure causes AT1R/Cl4 cells to undergo EMT through a Src-and MEK-dependent mech-anism.Ang II treatment induced persistent EGFR phosphorylation at tyrosine845(Y845)but not tyrosine1173(Y1173)in AT1R/ Cl4cells by a Src-dependent mechanism.In our previous study, using immunoprecipitation(IP)with anti-phosphotyrosine anti-bodies(anti-PY)and immunoblotting(IB)with an EGFR anti-body(anti-EGFR),we found that Ang II induced EGFR tyrosine phosphorylation in AT1R/Cl4cells within5min;however,we did not determine the specific tyrosine residues of EGFR that were phosphorylated in response to Ang II treatment(5).In the present study,using phosphotyrosine residue-specific antibodies,we found that although Ang II induced EGFR phosphorylationat FIG5Antioxidants blocked Ang II-induced Src activation and prolonged EGFR-ERK signaling in AT1R/Cl4cells.(A and B)Nox4siRNA-or scrambled control siRNA-transfected AT1R/Cl4cells were treated as indicated,and the cell lysates were subjected to immunoblotting with indicated antibodies(A)or cell photographs were taken atϫ400magnification after FITC-conjugated phalloidin staining(B).(C)ROS production was measured by thefluorescence intensity of2=,7=-dichlorodihydrofluorescin(DCFH)in Ang II-treated AT1R/Cl4cells with or without a NADPH oxidase inhibitor,apocynin pretreatment.(D and E) AT1R/Cl4cell lysates were immunoblotted with indicated antibodies after the cells were exposed to indicated stimuli following pretreatment with or without an antioxidant,N-acetylcysteine(NAC;5mM)(D)or tempol(3mM)(E).Persistent EGFR-ERK Signaling and EMT 985both tyrosine845(Y845)and tyrosine1173(Y1173)in AT1R/Cl4 cells within10min,Ang II-stimulated EGFR Y1173phosphoryla-tion peaked within10min,decreased within0.5h,and returned to basal level within3h whereas Ang II-stimulated EGFR Y845phos-phorylation remained elevated even after6h(Fig.2A).In con-trast,administration of EGF did not induce EGFR phosphoryla-tion at Y845but did stimulate EGFR phosphorylation at the autophosphorylation site,Y1173,with a rapid peak and a return to basal levels within3h(Fig.2A).EGFR can be directly phosphorylated at Y845by Src kinase activity(2,22,26).We therefore pretreated AT1R/Cl4cells with the Src kinase inhibitor,PP2,before exposing the cells to Ang II. As indicated in Fig.2B,PP2pretreatment markedly inhibited Ang II-induced EGFR Y845phosphorylation but did not inhibit EGFR Y1173phosphorylation induced by either Ang II or EGF.These data suggest that EGFR Y845phosphorylation is mediated by Src kinase activity but that EGFR Y1173phosphorylation is mediated by autophosphorylation through the intrinsic tyrosine kinase ac-tivity of EGFR in response to Ang II,which induces rapid HB-EGF shedding to act as a ligand for EGFR(5).Src kinase mediates prolonged ERK1/2activation in AT1R/ Cl4cells in response to Ang II.Previous studies have suggested that activation of the MEK-ERK pathway is a mediator of EMT(14,41) and that this pathway is a classic downstream effecter of EGFR acti-vation(17,27).Our observation that the MEK inhibitor,PD98059, inhibited the morphological changes and EMT marker expression alterations suggested the involvement of the MEK-ERK pathway in the EMT process in AT1R/Cl4cells in response to chronic Ang II treatment(Fig.1).We examined the phosphorylation of ERK1/2at different times after administration of Ang II to AT1R/Cl4cells and found that Ang II treatment induced persistent phosphorylation of ERK1/2.In contrast,administration of EGF led to transient ERK1/2 activation,which was diminished within0.5h and returned to basallevels by3h(Fig.3A).pp60c-src(Src)is the prototype of a family of nine cytosolic nonreceptor tyrosine kinases that function as cotransducers of transmembrane signals emanating from a variety of growth fac-tor/hormone receptors(11),including AT1receptors in vascular smooth muscle(34)and in kidney cells(40).We found that pre-treatment of the cells with PP2partially inhibited the early phase (10min of treatment)of ERK1/2activation but completely blocked the late phase(3h of treatment)of ERK1/2activation induced by Ang II(Fig.3B).Knocking down the Src gene expres-sion by its specific siRNA sequences markedly blunted the Ang II treatment-induced persistent EGFR Y845and ERK1/2phosphor-ylation(Fig.3C),suggesting that Ang II treatment induced persis-tent EGFR-ERK1/2activation by a Src kinase-dependent mecha-nism.Chronic Ang II treatment induced EMT in AT1R/Cl4cells through an EGFR-ERK activation-dependent but HB-EGF-independent pathway.To determine whether EGFR transactiva-tion is essential in the prolonged ERK1/2activation and epithelial cell dedifferentiation in response to chronic Ang II treatment,we pretreated the cells with erlotinib,the specific EGFR tyrosine ki-nase inhibitor,and found that pretreatment of the cells with erlo-tinib not only inhibited prolonged phospho-EGFR and phospho-ERK signaling but also eliminated the morphological changes (Fig.4A and B).Furthermore,we knocked down EGFR expression with different EGFR sequence-specific siRNAs(Fig.4C and D) and found that downregulation of EGFR expression inhibited the prolonged ERK1/2activation(Fig.4D)and reversed the altera-tions in E-cadherin and FSP-1expression in response to Ang II (Fig.4E)and prevented the morphological changes(Fig.4F). These data confirmed that EGFR is located upstream of the ERK1/2activation in response to Ang II treatment in AT1R/Cl4 cells.Because our previous studies had indicated that the immediate activation of ERK1/2in response to Ang II is partially mediated by EGFR transactivation due to release of soluble HB-EGF(5),we further determined whether prolonged ERK1/2activation by Ang II was also mediated by release of soluble HB-EGF.We therefore preincubated AT1R/Cl4cells with CRM197,a nontoxic and cata-lytically inactive(Glu-52)mutant of diphtheria toxin that binds to the extracellular HB-EGF domain and inhibits the mitogenic ac-tivity of HB-EGF.As shown Fig.4G,CRM197almost completely inhibited the transient ERK1/2activation induced by exogenously administered HB-EGF but only partially inhibited the early phase (10min of treatment)of ERK1/2activation induced by Ang II and had no effect on the late phase(3h after treatment)of Ang II-mediated ERK1/2activation.These results suggest that the Ang II-induced early phase of ERK1/2activation is partially mediated by HB-EGF but that the late phase of ERK1/2activation is inde-pendent of HB-EGF.Antioxidants blocked Ang II-induced Src activation and pro-longed EGFR-ERK signaling.NADPH oxidase-dependent reac-tive oxygen species(ROS)are important mediators of Ang II sig-naling and known to activate Src(12,13,33).Althoughmultiple FIG6The early phase of ERK activation in response to Ang II treatment was mediated through both ROS production and HB-EGF release in AT1R/Cl4 cells.Quiescent AT1R/Cl4cells were pretreated with or without CRM197(10g/ml)and with or without NAC(5mM)(A)or tempol(3mM)(B),followed by treatment with Ang II(10Ϫ7M),HB-EGF(15ng/ml),or vehicle alone for10 min.Cell lysates were immunoblotted with anti-phospho-ERK1/2,followed by stripping and reprobing with an antibody to total ERK1/2.Chen et al. Molecular and Cellular Biologyisoforms are potentially present in the kidney,the constitutively active isoform Nox4is predominantly expressed in epithelial cells (8).In AT1R/Cl4cells,we utilized Nox4sequence-specific siRNAs to knock down Nox4gene expression without affecting Nox2ex-pression (Fig.5A)and found that downregulation of Nox4ex-pression markedly inhibited prolonged EGFR-ERK activation (Fig.5A)and reversed fibroblast cell morphological changes in response to Ang II treatment (Fig.5B).In addition,Ang II in-creases in ROS production were inhibited by apocynin,the NAD(P)H oxidase inhibitor (Fig.5C).Phosphorylation of Src at tyrosine 416(Y614)is a well-established readout of Src kinase activity (20),and immunoblotting with an antibody that recog-nizes Y416-phosphorylated Src indicated that Ang II activated Src in AT1R/Cl4cells within 10min and remained activated at 3h after addition of the stimuli (Fig.5D and E).Furthermore,pre-treatment of the cells with an antioxidant (n -acetylcysteine [NAC]or tempol)blocked both Src Y416phosphorylation and EGFR Y845phosphorylation (Fig.5D and E),consistent with ROS acti-vation of Src,which then phosphorylates EGFR at Y845(2,22,26).HB-EGF did transiently activate ERK1/2phosphorylation but did not induce Src phosphorylation of EGFR Y845phosphorylation.Although HB-EGF-induced ERK1/2activation was not altered in the presence of the antioxidant,both NAC and tempolpartiallyFIG 7Ang II-induced phosphorylation of both EGFR at Y845and caveolin-1at Y14was mediated by AT1receptors in an Src-dependent manner.(A)Phos-phorylation of caveolin-1(Cav)at Y14was induced by Ang II in a concentration-dependent manner in AT1R/Cl4cells but not in empty vector-transfected LLCPKcl4cells (Vector/Cl4).(B)A selective AT1R antagonist,losartan (10Ϫ6M),but not the selective AT2R antagonist,PD123319(10Ϫ6M),inhibited Ang II-induced phosphorylation of EGFR at Y845and Cav at Y14.(C)An Src kinase inhibitor,PP2(5M),inhibited phosphorylation of Cav atY14.FIG 8Ang II treatment-induced phosphorylation of Cav and EGFR and their association in lipid rafts.(A)Lipid raft fractions were prepared as described in Materials and Methods followed by immunoblotting analysis with an antibody to Cav or EGFR along with plasma membrane (10g)and cell lysates (10g)as controls.(B)AT1R/Cl4cells were exposed to different stimuli for 10min,and then the lipid rafts were isolated and subjected to immunoprecipitation (IP)with an anti-EGFR antibody and immunoblotting (IB)with indicated antibodies.(C)AT1R/Cl4cell lysates were prepared after treatment with the indicated stimuli and were subjected to immunoprecipitation (IP)with anti-phosphotyrosine antibod-ies (anti-PY),followed by immunoblotting (IB)with an antibody against EGFR (top panel).Another aliquot of cell lysates was directly immunoblotted with indi-cated antibodies.(D)Quiescent AT1R/Cl4cells cultured in a 16-well Lab-Tek chamber slide system were exposed to Ang II (10Ϫ7M)or vehicle alone for 3h.Y845-phosphorylated EGFR (red)and Y14-phosphorylated Cav (green)were vi-sualized and photographed by fluorescence microscopy at ϫ400magnification.Nuclei were visualized with DAPI (blue).Persistent EGFR-ERK Signaling and EMTMarch 2012Volume 32Number 5 987。
