活性自由基聚合,TEMPO
tempo的质谱

Tempo的质谱:探索分子精神的奥秘
Tempo是一种含有自由基的小分子化合物,该化合物因其强大的自由基化学活性而被广泛用于有机化学和生物化学中。
尽管它的应用非常广泛,但我们对其分子结构和局部化学性质的认识仍然不完整。
为了更好地理解Tempo分子的特性,科学家们用质谱技术研究了Tempo分子的质谱,并发现了令人惊叹的结果。
Tempo分子的质谱图显示出其分子式为C9H18NO,并且有一个特征峰出现在m/z = 172处。
这说明Tempo分子的分子量为172,而其结构与N-tert-丁基尿素的相似,有一个氧化乙酰氨基团。
进一步的研究表明,Tempo分子具有一个较为稳定的自由基。
在分析Tempo分子的质谱图时,我们能够清楚地发现其电子自旋共振信号,这表明自由基存在于分子中。
另外,还可发现一些碎片离子,表明Tempo分子很容易分解。
在质谱分析中,可以通过不同的碎片质量-to-charge比例来推断化合物的部分结构。
在Tempo分子的质谱图中,可以清楚地看到其主要的分解模式为α C-H断裂和α C-N断裂。
这意味着Tempo分子的α-C-H基团和α-C-N基团更倾向于分解,这对于了解其基本结构和化学性质是至关重要的。
通过对Tempo分子的质谱分析,科学家能够更好地了解其分子结构和性质。
这些研究有助于深入探索分子精神的奥秘,为生物医学、有机化学和材料科学的发展提供有力支撑。
活性自由基聚合

Wang, J. S.; Matyjaszewski, K. Macromolecules 1995, 28, 7901-7910
11
Iniferter试剂 氮氧自由基 引发剂,催化剂/配体 链转移剂(RAFT试剂)
引发-转移-终止剂法聚合
(Initiator-Transfer agent-Terminator, Iniferter )
G. K. Hamer, Macromolecules 26, 2987 (1993).
10
Iniferter试剂 氮氧自由基
引发-转移-终止剂法聚合
(Initiator-Transfer agent-Terminator, Iniferter )
稳定自由基聚合
(Stable Free Radical Polymerization, SFRP)
Vol. 38, 2121–2136 (2000)
4
Dr. Takayuki Otsu is a Professor Emeritus, Osaka City University. He was born in Osaka in 1929 and received his B.Sc. degree from the Osaka Institute of Science and Technology in 1951. He then was appointed as an instructor at Osaka City University and started his research work on radical polymerization under the late Professor Minoru Imoto.
This being the case, I had an interest in new initiators and their mechanisms, and I focused my attention on the unique reaction behavior of organic sulfur compounds, which have been used as a thiyl radical source, an accelerator, a modifier, a terminator for vinyl or diene polymerization, and an accelerator for vulcanization in the rubber industry.
活性自由基聚合

活性自由基聚合活性自由基聚合是一种在化学合成中非常有效和重要的方法。
它包括一系列彼此之间相互作用的活性自由基和共价化合物,从而形成新的高分子化合物。
活性自由基聚合的这种特性使其在生物合成中得到越来越多的应用。
此外,活性自由基聚合还可以用于制备有用材料,如塑料,橡胶,和聚合物复合材料。
活性自由基聚合的基本过程可以分为几个步骤,即催化剂的应用,反应物的配对,活性自由基的形成,活性自由基的反应以及合成产物的分离和纯化。
在催化剂应用方面,通常需要采用表面活性剂和金属离子来促进反应,从而改善活性自由基聚合的效率。
在反应物配对方面,它们通常以不同的物种形式存在,如卤素和烃类,碳酸根和烃类,或氧化物和烃类,聚合物和聚合物复合材料等。
在这些不同的组合中,活性自由基的形成可以由反应物的极性,热力学条件和其他因素来控制。
一旦形成活性自由基,就可以进行活性自由基反应,形成反应产物。
活性自由基聚合有许多优点。
首先,它是一种高选择性的反应方法,具有高效率,可以降低反应条件的复杂性。
,它的产物可以在一定的结构参数范围内有效地调控,以满足特定应用的要求。
最后,活性自由基聚合反应可以使试剂的使用量减少,从而更加环保。
由于活性自由基聚合有如此多的优势,它已经广泛应用于各种高分子材料的合成中。
例如,在塑料行业,活性自由基聚合可用于制备高性能聚合物,如聚酯和聚氨酯,以及复合材料材料,如复合橡胶,聚合物复合材料和复合塑料等。
此外,活性自由基聚合也可用于生物分子的合成,如蛋白质,脂质,糖类和抗原等。
活性自由基聚合可以用于调节生物分子的结构,从而增强其功能。
例如,在蛋白质合成中,可以通过活性自由基交联的方式来控制蛋白质的结构,从而使蛋白质具有更强的抗体活性。
因此,活性自由基聚合可以在许多不同的领域应用,有助于制备各种类型的有用材料和生物分子,改善生物分子的功能,以满足各种特殊的应用要求。
由于活性自由基聚合是一种高效、灵活、选择性高的反应方法,它最终会在不同领域取得更大的发展,特别是在医学,农业和化学工程领域,为各种特殊的应用提供更多的选择。
活性自由基聚合讲解

目前已发现很多可作为引发转移终止剂的化合物, 可分为热分解和光分解两种。
热引发转移终止剂:主要为是C-C键的对称六取 代乙烷类化合物。其中,又以1, 2-二取代的四苯基乙 烷衍生物居多,其通式如下图所示。主要品种包括四 苯基丁二腈TPSTN,五苯基乙烷PPE,四(对-甲氧 基)苯基丁二腈TMPSTN,l,1,2,2-四苯基-1,2-二苯氧 基乙烷TPPE和1,1,2,2-四苯基-l,2-二(三甲基硅氧基) 乙烷(TPSTE)等。
R R' + n M
R [ M ]n R'
16
根据以上反应机理,可将自由基聚合简单地视 为单体分子向引发剂分子中R-R’键的连续插入反 应,得到聚合产物的结构特征是两端带有引发剂碎 片。Otsu等由此得到启示,若能找到满足上述条件 的合适引发剂,则可通过自由基聚合很容易地合成 单官能或双官能聚合物,进而达到聚合物结构设计 之目的。由于该引发剂集引发、转移和终止等功能 于一体,故称之为引发转移终止剂(iniferter)。
C2H5 S
CH2 SCN C2H5 S C2H5
多官能度
C2H5
常用光引发转移终止剂结构式
NCS CH2
CH2 SCN C2H5
C2H5
H2
NCS
C
C2H5 S
C2H5
NCS
C
H2
C2H5
S
易断链
C2H5
H2
C
SCN
S
C2H5
C2H5
C
SCN
H2
S
C2H5
22
适用的单体
Iniferter技术不仅可以用于苯乙烯St和甲基丙烯酸
20
单官能度
活性自由基聚合

分子材料的性能和功能。
功能性化
通过活性自由基聚合,可以将功 能性单体引入高分子链中,制备 功能性高分子材料,如具有光敏、 热敏、导电、磁性等功能的高分
子材料。
高分子链结构调控
通过活性自由基聚合,可以精确 调控高分子链的微观结构和聚集 态结构,从而改善高分子材料的 力学性能、流变性能和加工性能
THANKS FOR WATCHING
感谢您的观看
特性
活性自由基聚合具有高分子量、窄分 子量分布、低副反应和易控制等特点 ,能够合成结构规整、性能优异的聚 合物材料。
历史与发展
历史
活性自由基聚合的概念最早由美 国科学家于20世纪50年代提出, 但直到20世纪80年代才得到实际 应用。
发展
随着对活性自由基聚合机理的深 入研究和新型聚合技术的开发, 活性自由基聚合已成为高分子合 成领域的重要研究方向之一。
压力
聚合过程中通常需要加压,以使单体更好地溶解和传递。
引发剂与抑制剂
选择适当的引发剂和抑制剂,以控制聚合反应的速度和产物的分 子量。
聚合产物的特性
高分子量
活性自由基聚合可制备高 分子量的聚合物,分子量 可达到数百万至数千万。
窄分子量分布
活性自由基聚合产物的分 子量分布较窄,有利于提 高聚合物材料的性能。
案例二:高分子改性研究
总结词
采用活性自由基聚合技术对现有高分子材料 进行改性,提高了其性能和应用范围。
详细描述
在案例二中,研究者采用活性自由基聚合方 法对现有高分子材料进行了改性。通过引入 功能性单体和共聚单体,成功改善了高分子 材料的亲水性、生物相容性和光敏性等性能。 此外,研究者还研究了改性后高分子材料的 流变性能和加工性能,为其在实际应用中的 加工和成型提供了理论支持。
活性自由基聚合TEMPO

1. NMP的发现及历史:
分子量与转化率间线性关系! TEMPO/BPO比例对聚合的影响!
2
1. NMP的发现及历史:
Many subsequent studies have confirmed Georges findings and have also shown that increasing the molar ratio of nitroxide to initiator result in slower reactions, lower PDIs, and lower molecular weight polymers.
As a comparison, Georges performed a suspension copolymerization of styrene and butadiene with (a) and without (b) TEMPO. The PDI of the polymer with TEMPO was 1.36 while the PDI of the polymer without TEMPO was 4.61.
双分子体系 BPO:TEMPO=1:1.3
Conditions: Initial heating at 95oC for 3.5h, followed by heating at
P1o2o3orClyfodre69fihn. ed nature of the initiating species
Results: Narrow molecular weight polystyrene with polydispersity
1. NMP的发现及历史:
2,2,4,4-四甲基派啶氮氧稳定自由基 (2,2,4,4-tetramethyl-1-piperidinyloxy, TEMPO)
可控 活性自由基聚合

反应方程式如下:
+
O
O
O PhC O
N O
-
O O CPh
PhC O O CPh + O N
TEMPO可以加速BPO的分解,活化能由 120kJ/mol降为40kJ/mol,大大提高了链引发 的速率。
SFRP方法在现实中的应用:
O C O CH2 CH n CH2 CH O N kL k-L
O C O CH2 CH n CH2 CH O N
以上四种方法都在进一步的进展中„„
Thank you!
BPO可以被TEMPO分解为初级自由基, 活化能为40kJ/mol,远低于BPO单独的分解 活化能(120kJ/mol)。初级自由基引发单 体聚合而增长。增长自由基迅速被TEMPO捕 捉,偶合成共价休眠种。在较高温度下,休 眠种均裂成链自由基,进一步与单体加成而 增长;均裂的另一个产物RNO· 又能与新的链 自由基结合为休眠种,如此反复下去,使分 子量不断增长,最终形成高分子化合物。
● ●
休眠种逆分解成增长自由基,继续与单 体加成而增长,如此反复,聚合度不断增加
• 13.4、原子转移自由基聚合(ATRP)法 • ATRP(Atom Transfer Radical Polymerization)聚合反应以过渡金属作为催 化剂,使卤原子实现可逆转移,包括卤原子从 烷基卤化物到过渡金属络合物(盐),再从过 渡金属络合物(盐)转移至自由基的反复循环 的原子转移过程,伴随着自由基活性(增长链 自由基)种和大分子有机卤化物休眠种之间的 可逆转换平衡反应,并抑制着自由基活性种在 较低的浓度,减少增长链自由基之间的不可逆 双基终止副反应,使聚合反应得到有效的控制。 ATRP的核心是引发剂卤代烷R-X与单体中C=C键 加成,加成物中C-X键断裂产生自由基引发聚 合。示意图如下:
“活性”可控自由基聚合

“活性”/可控自由基聚合熊鹏鹏2010214110 摘要: 自由基聚合是生产高分子量聚合物的重要方法, “活性”/ 可控自由基聚合综合了自由基聚合和离子聚合的优点, 使自由基聚合具有可控性。
本文对目前可以实现“活性”/ 可控自由基聚合的途径和各自机理进行介绍, 指出应该重视对“活性”/可控自由基聚合的研究。
关键词: “活性”/可控自由基聚合; 稳定自由基; 可逆加成-裂解链转移; 原子转移; 引发转移终止剂;退化转移。
自由基聚合是工业上和实验室中生产高分子量聚合物的重要方法, 该法具有可聚合的单体种类多、反应条件宽松、以水为介质、容易实现工业化生产等优点, 但也存在着缺陷, 如自由基聚合的本质( 慢引发, 快速链增长, 易发生链终止和链转移等) 决定了聚合反应的失控行为,其结果常常导致聚合产物呈现宽分布, 分子量和结构不可控, 有时甚至会发生支化、交联等,从而严重影响聚合物的性能, 此外, 传统的自由基聚合也不能用于合成指定结构的规整聚合物。
鉴于离子聚合和配位聚合可以很好地控制聚合物结构, 而能不能控制自由基聚合体系则成为当前的研究热点, 但近年来从离子聚合和可控有机自由基反应的研究进展来看, 答案是肯定的。
就聚合反应而言, 要合成具有确定结构的聚合物, 则要求所有的链应同时引发, 增长相似, 这就需要快速引发, 在聚合结束前增长链应保持活性, 链转移和链终止的效应可以忽略, 而自由基聚合的本质( 慢引发, 快终止) 与之正好相反。
所以实现可控自由基聚合要基于以下三个原则:1) 自由基体系中的增长反应应对自由基敏感, 终止反应对自由基浓度的敏感度次之。
这样, 在自由基浓度很低时, 链增长反应与终止反应的速率比才足够高, 才能合成出分子量很大的聚合物。
2) 增长链的浓度必须比初始游离自由基的浓度高得多, 在整个反应过程中所有的链均需保持活性, 且游离自由基与高浓度休眠链处于动态平衡之中, 这种持续自由基效应对任何控制自由基反应来说都是最重要的。
活性聚合

•
• • •
• ……
可控/“活性” 可控 “活性”自由基聚合 (CRP)
CRP成为当今高分子合成化学发展最迅速的领域 原因:大量可供聚合的单体,简单的反应装置,不苛刻的反应 条件对自由基的有效控制。 更重要的是,CRP产品具有巨大的市场潜力,不过要 充分发挥其潜力,在很多方面还需要研究。 今后的研究方向:开发新的引发/催化体系、 拓宽单体种类、合成结构清晰可控的新型 聚合物。更重要的是缩短工业化的进程。
三、对CRP的综合讨论与比较
所有可控自由基聚合具有一些共同的特征:链增长自由
基和各种休眠种达到动态平衡是所有可控自由基 聚合体系的关键。 聚合体系的关键。
四、CRP CRP的应用与前景 CRP
•
具有水溶性的双亲性嵌段共聚物已被成功用作表面活性剂,并且用于一 些高端产品,例如染料分散剂、添加剂、保健品和化妆品等。具有纳米形态 的嵌段共聚物可用作电子器件。接枝共聚物可用作聚合物共混增溶剂,并且 可以可以用到嵌段共聚物所能适用的许多领域。梯度共聚物非常有望用作表 面活性剂、噪音和振荡阻尼材料。 通过对支化度的调节,可以精确的控制聚合物加工过程中的熔融粘度。 这些聚合物(包括梳形和星形聚合物)可以用作黏度调节剂和润滑剂。大分 子拓扑结构控制的一个突出例子是大分子刷,这些聚合物经轻度交联可得到 超软弹性体。 CRP在链末端功能化方面也具有独特的优势 目前,结构规整的官能化聚合物与无机组分或者天然物质通过共价键结合成 的分子杂化材料受到了广泛关注,并且将会带来许多具有新功能的材料。( 分子纳米复合材料……) 潜在的应用包括微电子材料、软刻印刷技术、光电子元件、特种膜、传感器 和微流体组分
让我们坚强永不放弃 让我们勇敢面对困境 让我们对生活的爱和希望 燃烧在心里 付诸于行动 让我们微笑生活继续 让我们努力创造奇迹 让我们期待 这场属于我们的胜利
tempo捕获自由基原理

tempo捕获自由基原理Tempo捕获自由基原理是针对自由基反应机理的一种研究方法。
自由基是指具有不成对电子的分子或原子,其反应速度非常快,很容易引起化学反应。
这种反应过程对于环境、工业和生物化学等领域具有重要的意义。
为了更好地掌握自由基反应的机理以及控制其反应,科学家们对自由基的捕获和检测方法进行了研究。
Tempo就是其中的一种。
Tempo是2,2,6,6-四甲基哌啶氧化物的缩写。
它是一种稳定的有机自由基化合物,可以与其他自由基反应生成新的化合物。
Tempo可以被用作自由基陷阱,来捕获其他形成的自由基,并形成一个稳定的非自由基中间体。
这种过程被称为“Tempo捕获反应”。
Tempo捕获自由基原理的关键在于先将Tempo与自由基反应,然后测量反应产物。
由于Tempo具有明确的化学结构和已知的反应特征,可以通过测量Tempo反应产物的数量和种类,来推断原始自由基的性质和反应机理。
Tempo捕获自由基的反应过程可以用以下方程式表示:R• + Tempo → R-Tempo(Tempo捕获反应)R•代表新产生的自由基,Tempo捕获了这个自由基,并与之反应生成了一个过渡态产物R-Tempo。
通过对这个产物进行研究和分析,可以了解原始自由基的性质和反应机理。
Tempo捕获自由基原理是一种基于自由基反应机理研究的方法。
它利用Tempo与自由基反应的原理,将自由基阳离子化为稳定的非自由基中间体,通过研究产物的生成规律,来探究原始自由基的性质和反应机理。
这项研究不仅可以深化我们对自由基化学反应机理的认识,还为开发新型材料和减轻环境污染等问题的解决提供了新的思路和方法。
除了在化学研究中广泛应用外,Tempo捕获自由基原理还在其他领域具有重要的作用。
其中最重要的领域之一就是生物化学。
生物体内的自由基反应机理和环境中的自由基反应机理有许多不同之处。
在生物体内,自由基反应通常是由氧化还原反应引起的。
生物体内存在大量的氧分子,细胞内产生的活性氧物质或乃至于外部环境中的辐射都可能引起氧化反应。
TEMPO
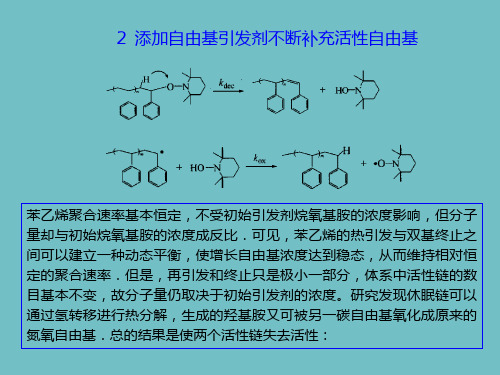
根据上面的讨论,TEMPO调控的自由基聚合速率慢的主要 原因是稳定自由基的积累,这是由双基终止和氢转移反应引 起的.实际上,自从此种体系诞生以来,提高其聚合速率一 直是人们关心的课题。从机理上讲,改变反应的平衡常数来 提高增长自由基的浓度,降低随反应时间而升高的TEMPO的 浓度,或适量补充增长自由基数目,都可以起到加速作用. 当然,上述方案的前提是要保证TEMPO调控的自由基聚合的 “活性”特征和窄的产物分子量分布。两种提高聚合速率的 方法:
氢转移反应对聚合动力学无疑具有重要影响,氢转移和热引发 对聚合结果的影响互相消长。当体系中没有热引发时,氢转移 反应使聚合速率明显降低.转化率达到约50%以后聚合基本停 止,同时多分散性指数升高,这是因为氢转移反应导致链终止 概率增加,终止链的含量大大增加,聚合结束时几乎全部为终 止链。由于丙烯酸酯类单体聚合时没有象苯乙烯那样的热引发 过程,并且氢转移反应可能更加显著,因此将没有热引发但有 氢转移的体系对应于丙烯酸酯在TEMPO存在下的自由基聚合 是很有意义的.迄今为止,用TEMPO调控丙烯酸酯类单体进 行自由基聚合不是很成功,聚合很难达到高转化率,只能得到 低聚物。
当体系中有热引发时,热引发对动力学的影响超过了氢转 移的影响,表现为聚合速率大大加快,但分子量分布指数 及失活链的含量皆保持在很低的水平.这是苯乙烯活性自 由基聚合可以顺利进行的原因之一。综上所述,在 TEMPO调控的自由基聚合中,由于双基终止不可避免, 反应后期游离的TEMPO量将大大过剩,增长自由基的浓 度急剧下降,从而聚合反应速率越来越馒.在苯乙烯聚合 中,单体自引发产生的自由基与双基终止可以达到动态平 衡,导致中前期的匀速聚合.氢转移反应破坏活性自由基 聚合反应的正常进行,降低聚合速率,限制产物分子量的 增长,并使多分散性指数上升。
temed促进剂 聚丙烯酸水凝胶

temed促进剂聚丙烯酸水凝胶
TEMPO(2,2,6,6-四甲基哌啶-1-氧基)是一种绿色氧化剂,常常被用于制备高分子水凝胶。
聚丙烯酸(PAA)是一种常用的可聚合单体,可以通过自由基聚合制备PAA水凝胶。
在制备PAA水凝胶时,可以通过添加TEMPO来促进氧化剂与丙烯酸单体的反应,从而加快聚合反应速率。
TEMPO可以通过提供活性自由基来加速聚合反应,从而提高PAA水凝胶的交联密度和机械性能。
需要注意的是,TEMPO促进剂的用量应该适当控制,因为过高的用量可能导致凝胶的机械性能下降。
此外,在制备PAA水凝胶时,还需要加入其他添加剂,如交联剂、引发剂、稳定剂等,以控制凝胶的交联程度和微观结构。
总之,TEMPO促进剂可以用于加速PAA水凝胶的聚合反应,从而提高凝胶的交联密度和机械性能。
在实际应用中,需要根据具体需求选择合适的TEMPO用量和其他添加剂,以获得具有优良性能的PAA水凝胶。
活性自由基聚合

32
CH3
CH3 C CH3 S2 C Z
双官能度
Z
S2 C
C CH3
CH2CS2Z
多官能度
ZS2CH2C
CH2CS2Z
ZS2CH2C
CH2CS2Z
ZS2CH2C
CH2CS2Z
ZS2CH2C CH2CS2Z
CH2CS2Z
33
可逆加成-断裂链转移自由基聚合的机理可用下 列反应式表示:
I 2 R Y R + n CH2 C X S Z R [ CH2 R1 Y C ]n S X C Z
36
ATRP的发现者
1995 年中国旅美博士王锦山博 士在卡内基梅隆大学做博士后研 究时首次发现了原子转移自由基 聚合(Atom Transfer Radical Polymerization , 简称ATRP) , 实现了真正意义上的活性自由基 聚合, 引起了世界各国高分子学 家的极大兴趣。这是聚合史上唯 一以中国人为主所发明的聚合方 法。
42
此外,含有弱S-Cl键的取代芳基磺酰氯是苯乙 烯和(甲基)丙烯酸酯类单体的有效引发剂,引发 效率大于卤代烷。近年的研究发现,分子结构中并 无共扼或诱导基团的卤代烷(如二氯甲烷、1, 2-二 氯乙烷)在FeCl2· 4H2O/PPh3的催化作用下,也可引 发甲基丙烯酸丁酯的可控聚合,从而拓宽了ATRP 的引发剂选择范围。
设: [M] = 1 mol/L )
Rt 104 ~ 105[ P] Rp
5
要降低链终止反应的影响
关键点: 控制恒定低的自由基 浓度 例: 自由基浓度为 10-8 mol/L 时,聚合速率 Rt 已很可观, 但这时 Rp 很小,(10-4 ~ 10-3),终止反应的影响很小 。
可控活性自由基聚合

3、原子转移自由基聚合(ATRP)
R-X + Cu(I) R . + XCu(II) M RM . Pn-X+ Cu(I) Pn . + XCu(II)
优点:适用单体多。聚合条件温和,分子设计能力强。 有待改进:提高聚合速率、降低聚合温度、进行溶液 或水溶液聚合、过渡金属的脱除等。
4、可逆加成-断裂转移法(RAFT)
3 2
BPO
R.
+nMPn .+RCNO .
Pn-ONR
H2C
NO .
H2C C(CH3)2
该方法的缺点是适用单体少、聚合温度高、聚合速率低
2、引发转移终止剂法(Iniferter),
C6H5-N=N-C(C6H5)3 C6H5 . + . C(C6H5)3 +N2
优点:可用单体多,缺点:分子量分布不够理想
三、活性聚合的特征 1、活性中心不消失,一直进行到单体消耗完全 2、当加入单体时,可进一步聚合,形成嵌段共聚物 3、聚合物的数均分子量与转化率呈线性 4、聚合物的分子数由引发剂数目确定,不依赖转化率 当引发过程很快时,所有增长链在瞬间形成,并具有相同长 时间增长寿命,从而使聚合产物具有很窄的分子量分布。 迄今为止,适合活性阴离子聚合的单体 1非极性单体:苯乙烯、甲基苯乙烯、共轭二烯等 2极性单体:甲基丙烯酸酯、2-丁酸酯等含有强吸电子基团 环状单体:环氧烷、环氧硅烷、内酯等
一、活性聚合:在链式聚合中有一种特殊的聚合反应, 其特征是活性种的寿命极长,甚至大于聚合反应时间,此时 增长链将一直存在不消失,称为活性聚合。
二、活性聚合的重要性:活性聚合大大提高了聚合反应和聚 合产物的结构可控性,为合成各种指定结构聚合物 提供了 传统聚合方法不能实现的手段: 1、通过控制单体与引发剂浓度之比,合成指定分子量的聚 合物。 2、按顺序加入不同单体,合成指定结构的嵌段共聚物
活性自由基聚合

配位剂 活化反应速率常数 ka kd
R-X + Mtn/L
卤代烷
R· + Mtn+1/L
+M
kp
氧化态过渡金属
还原态过渡金属 失活反应速率常数
增长反应速率常数
是以简单有机卤化物为引发剂、过渡金属络合物为卤原子 载体,通过氧化还原反应,在活性种与休眠种之间建立可逆动 态平衡,实现了对聚合反应的控制。其适用单体范围广、反应 条件温和、分子设计能力强。关于利用 ATRP法合成嵌段、接 枝共聚物、星形和超支化等各种带有分子设计的报道相继出现
增长反应
一级反应
矛盾
终止反应
二级反应
受活性阳离 子聚合启发
在聚合体系中活性种与暂时失 活的休眠种同时并存,通过在 两者之间建立快速交换反应, 成功地解决和协调上述矛盾
终止的可逆性
2. 3 实现设计思想的途径
三条途径 可逆增长 可逆终止
可逆转移
(1)增长自由基与稳定自由基可逆形成休眠共价化合物 失活反应速率常数
TEMPO价格昂贵
这些缺点限制了它的工业化应用
不过,由于TEMPO是首例活性自由基聚合,它的发 现为理论构想的实现提供了实际事例,对自由基活性聚合 的进一步研究来说,具有极其重要的指导和启发意义
3. 3 RAFT过程
可逆加成-断裂链转移自由基聚合 Reversible Addition-Fracture Transfer
利用它可以制备嵌段聚合物、接枝聚合物、不同官能团 封端的遥爪聚合物、星形聚合物、超支化聚合物等。它的核 心作用就是可以实现聚合物的分子设计
合成均一分子量的聚合物
tempo存在下聚合制备“模塑”多孔聚合物整体柱的多孔性能和表面化学调控

TEMPO 存在下聚合制备“模塑”多孔聚合物整体柱的多孔性能和表面化学调控作者:Eric C. Peters, Frantisek Svec, and Jean M. J. Fréchet (Department of Chemistry, University of California, Berkeley, California 94720-1460), Camilla Viklund and Knut Irgum (Department of Analytical Chemistry, University of Umeå, Umeå S-901 87, Sweden) 译者:罗翌阳(北京大学化学与分子工程学院 北京 100871)前言“活性”自由基聚合可以通过稳定的自由基反应,获得与传统活性聚合相类似的可调控组成和结构的聚合物,因此在近些年得到了广泛的关注。
文献报道了在乳浊液或分散体系中TEMPO 引发的活性自由基聚合。
我们曾报道过一种在封闭静置模具中制备的多孔聚合物整体柱,尽管这种材料已经有了广泛的应用,然而其孔隙度受到自由基聚合的动力学限制。
本文中我们介绍了一种直接通过“活性”自由基聚合的方法,通过乙烯和丁二烯在致孔溶剂中的共聚,直接制备多孔聚合物整体柱。
TEMPO 介导的聚合反应所带来的较慢的反应速率和较高的反应温度,使得聚合物获得了完全不同于传统方法的孔隙度曲线。
另外,聚合物中由TEMPO 封端的潜在自由基,可用于在其内部孔隙表面的链增长,这潜在地给予了这种材料一种额外的独特性能。
结果与讨论1多孔性能我们过去的工作中曾报道过,聚合温度的变化对材料的孔径分布曲线有很大的影响。
这是因为在引发剂浓度相同的情况下,其转化率和所产生的活性中心随温度而变化。
在我们之前的研究中,所应用的聚合温度都是传统的以AIBN 或BPO 引发的自由基聚合所接受的温度范围(55-80 °C )。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
2. NMP的聚合机理 2.1 平衡的建立: persistent radical effect
R·Y· , 活泼自由基,则产物有三种, 1:2:1。 R· 活泼自由基,Y· persistent radical, 产物只有一种
2. NMP的聚合机理 2.1 平衡的建立: persistent radical effect
the term living, controlled and step growth were used.
N O N O
polystyrene
CH 2
CH
polystyrene
CH 2
CH
Polymerization Systen: Monomer: Styrene 双分子体系 Initiator: BPO BPO:TEMPO=1:1.3 Additive: TEMPO Conditions: Initial heating at 95oC for 3.5h, followed by heating at 123oC for 69h. Poorly defined nature of the initiating species Results: Narrow molecular weight polystyrene with polydispersity of 1.26. The number-average molecular weight increased linearly
2. NMP的聚合机理
2.1 平衡的建立: persistent radical effect
Radicals (2) and (4) are present at very low concentrations (approx. 10-8 M) but the persistent radical (6) is at a concentration around 10-3 M. It is critical to note that the PRE is a very important concept, which is at the heart of both NMP and ATRP. it is possible to enhance the PRE by the addition of stable radicals at the beginning of the polymerization. The Persistent Radical Effect: A Principle for Selective Radical Reactions and Living Radical Polymerizations Hanns Fischer Chem. Rev. 2001, 101, 3581-3610
1. NMP的发现及历史:
2,2,4,4-四甲基派啶氮氧稳定自由基 (2,2,4,4-tetramethyl-1-piperidinyloxy, TEMPO)
H3C H3C N O
CH3 CH3
相对TD,其活性更低 ,不能引发聚合,且与 苯乙烯增长链末端结合 的键更弱。
自由基捕获剂、阻聚剂、抗老化剂、热降解抑制剂和热稳定剂等。
J. Am. Chem. Soc. 2000, 122, 5929-5939
2.4 副反应
苯乙烯中的 热引发:
说明双基终止 的存在?
热引发对苯乙烯的 聚合十分有利! 双基终止的程度:严 重影响速率,对分子 量和分布影响很小! 因自由基浓度:约为10-8M,大分子浓度为10-2M!
2.4 副反应
烷氧胺的分解或提氢副反应:
[P.]:利用转化率和时间的函数 Kd的测定: Macromolecules 1996, 29, 6393-6398
2.3 动力学参数
K=[P.][T.]/[PT]
Macromolecules 1996, 29, 6393-6398
N-tert-butyl-N-[1-diethylphosphono-(2,2dimethylpropyl)] nitroxide (DEPN).
2.2 关于自由基的性质:
does the radical pair exist as a caged pair or are they free to diffuse through the reaction?
2.2 关于自由基的性质:
radical: a caged pair or free to diffuse through the reaction?
2. NMP的聚合机理 2.1 平衡的建立: persistent radical effect
2.1 平衡的建立: persistent radical effect
The stable radical (6) is capable of reaction with either (2) or (4) but not itself. Due to the fact that irreversible termination will occur between active radicals the concentration of the stable radical present will increase. Due to the build up in concentration of (6), very little coupling products between active radicals (2)-(2), (2)-(4), or (4)-(4) are formed, as the active radicals react almost exclusively with the stable or persistent radical (6) .
J. Am. Chem. SOC. 1994,116, 11185-11186
SOLOMON八十年代的工作:
用稳定自由基封端研究St、 MMA关于引发和终止的结构
SOLOMON八十年代的工作:
SOLOMON八十年代的工作:
1. NMP的发现及历史:
Rizzardo等与TEMPO/SFRP失之交臂?温度低,分布宽
Steady-state
X• depends on the reversible reaction as well as the initiation/termination parameters.
Steady-state
Ri =0 Ri = 0
此式常用来测平衡常数
2.3 动力学参数
K=[P.][T.]/[PT] [T.]:ESR测定
Experimental results that quasi-equilibrium exists and the time needed to reach quasi-equilibrium is much less than 1 second, typically 1 – 100 ms. Ri > 0
1. NMP的发现及历史:
分子量与转化率间线性关系! TEMPO/BPO比例对聚合的影响!
1. NMP的发现及历史:
Many subsequent studies have confirmed Georges findings and have also shown that increasing the molar ratio of nitroxide to initiator result in slower reactions, lower PDIs, and lower molecular weight polymers. As a comparison, Georges performed a suspension copolymerization of styrene and butadiene with (a) and without (b) TEMPO. The PDI of the polymer with TEMPO was 1.36 while the PDI of the polymer without TEMPO was 4.61.
2.4 副反应
烷氧胺的分解或提氢副反应:
These results help to explain the differences in the polymerization of styrenics and methacrylates under the same NMP conditions. Due to the extra β hydrogen's on the methacrylates, there is a much greater amount of H transfer and therefore lack of control. This has been observed experimentally with significant amounts of “ene” terminated polymer chains detected by MALDI-MS and NMR and UV-vis studies. Acrylates : much greater amount of H transfer
单分子体系(Unimolecular) :
单分子体系可控效果 优于双分子体系!
2. NMP的聚合机理
Typically these propagating carbon centered radicals are transient species , tend to disappear by self-termination, which is typically a diffusion controlled process. This raises the question, why do we only see minor amounts of irreversible termination in NMP?
