Sodium-Valproate-5571[英国药典BP2009]
QIAGEN Endotoxin Removal Solution说明书

Endotoxin Removal Solution Catalog Number E4274Product DescriptionEndotoxins are lipopolysaccharides (LPS), a major component of the Gram-negative bacterial cell wall, and are commonly found as contaminants in plasmid DNA preparations from E. coli. Endotoxins are large, negatively charged molecules that co-purify with DNA on ion exchange and size exclusion columns and in CsCl banding. Endotoxins are extremely potent stimulators of the mammalian immune system and are toxic to primary cells and to animals. The endotoxin toxicity is an obstacle to in vitro and in vivo transfection experiments.Non-ionic detergents, traditionally used for separation of integral membrane proteins,1 can be utilized for removal of endotoxins from DNA solutions by phase separation.2The solubility behavior of a detergent in a dilute, aqueous solution at physiological salt and pH conditions is strongly dependent upon the temperature of the solution. At low temperatures, the detergent forms a clear, micellar solution, but above the cloud point temperature, the micelles form larger, turbid aggregates and ultimately fuse to form a separate phase. The lower phase is detergent-enriched and the detergent-depleted upper phase contains detergent at a concentration slightly above the critical micellar concentration (CMC). Amphiphilic and hydrophobic molecules associated with the micelles of the detergent will aggregate within the detergent-enriched phase, while the soluble, hydrophilic molecules will remain in the detergent-depleted upper phase.Extraction of endotoxin contaminated DNA solutions with the appropriate non-ionic detergent will separate the hydrophilic DNA from the amphiphilic endotoxin. The amphiphilic endotoxin will associate with the lower phase, while the DNA will remain in the upper, detergent-depleted phase.2Reagents and equipment required, but not provided • Water, Molecular Biology Reagent, Catalog Number W4502• E-TOXATE® Water, Catalog Number 2107, or Tris-EDTA (TE) buffer 100×, Catalog NumberT9285• DNA solution (0.5 ml), ~ 1 mg/ml in E-TOXATE®Water or TE buffer• 3 M sodium acetate solution, pH 7.5.• 2-Propanol, Catalog Number I9516, or Ethanol, 190 proof, Catalog Number E7148; 200 proof, CatalogNumber E7023• 70% Ethanol• E-TOXATE®reagents Kits, Catalog Numbers 210A1, 210B1 or 210C1• Ice bucket• Heat block or incubator at 37 °C• Microcentrifuge at room temperature• 1.5 or 2 ml sterile microcentrifuge tubes• Endotoxin-free pipet tips (40-200 µl, 200-1000 µl) Precautions and DisclaimerThis product is for R&D use only, not for drug, household, or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.StorageStore at room temperature.Note: Removal of endotoxins from DNA preparations can be performed either during the final stage of DNApreparation, or during an earlier stage.Procedures for Endotoxin RemovalDuring the final stage of DNA preparationNote: The procedure described below was performed on plasmid DNA produced in E. coli DH5α cells.• Losses of up to 50% of the DNA are expected. • Use of a DNA concentration above therecommended 1 mg/ml reduces the efficiency ofthe procedure.1. Pipette 500 µl of the DNA solution into a sterilemicrocentrifuge tube.2. Add 50 µl of the 3 M sodium acetate solution to theDNA sample.3. Incubate on ice for 5 minutes.4. Add 100 µl of cold Endotoxin Removal Solution.5. Mix thoroughly and incubate on ice for 10 minutes.The solution should be light blue and clear.6. Incubate the tube at 37 °C for 20 to 30 minutes oruntil the phases separate.7. Spin for 5 minutes at 3000 x g in themicrocentrifuge. The upper phase is colorless and clear, while the lower phase is blue.8. Carefully transfer the upper phase containing theDNA to a clean microcentrifuge tube.9. Repeat steps 4 through 8 twice.10. Add 0.6× volume of 2-propanol. Mix by inversion atroom temperature and centrifuge at 15,000 x g for30 minutes at 4 °C. Alternatively, add2.5× volumes of ethanol. Incubate overnight at –20°C or 20 minutes at –70 °C and centrifuge at15,000 x g for 30 minutes at 4 °C.11. Carefully remove the supernatant12. Wash the DNA pellet twice with cold 70% ethanol.Remove the supernatant.13. Air-dry the pellet.14. Suspend the DNA in 100 µl of endotoxin free wateror TE buffer.15. Determine DNA concentration and endotoxin levelsusing endotoxin assay reagents and compare tothe starting material. During an earlier stage of DNA preparationThis procedure is based on the alkaline lysis of E. coli DH5α cells.3 The endotoxins are removed immediately after alkaline cell lysis, neutralization, and a clarification step. The resulting high salt solution is suitable for the endotoxin removal step. It is performed under “endotoxin free” conditions. The plasticware used is either sterile and disposable, or NaOH-treated. The buffers are prepared with endotoxin free water.1. Add the Endotoxin Removal Solution (0.2× volume)to the cold, crude DNA solution.2. Incubate on ice and mix occasionally by inversionto obtain a homogenous, clear blue solution3. Incubate at 37 °C for 20 to 30 minutes until thephase separation is obvious.4. Spin for 5 minutes at low speed (3000 x g) at roomtemperature.5. Transfer the upper aqueous phase to an endotoxinfree container.6. Proceed with the DNA purification by any method.Use endotoxin-free buffers and containers. References1. Bordier, C., J. Biol. Chem., 256, 1604-1607, (1981).2. Cotten, M. et al., Gene Therapy, 1, 239-246,(1994).3. Sambrook et al., Molecular Cloning, a LaboratoryManual, 2nd Ed. p. 1.38RK,PHC 09/05-1Sigma brand products are sold through Sigma-Aldrich, Inc.Sigma-Aldrich, Inc. warrants that its products conform to the information contained in this and other Sigma-Aldrich publications. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see reverse side ofthe invoice or packing slip.。
Inosine-5'-monophosphate (sodium salt hydrate)_IMP脱氢酶(IMPDH)的底物_20813-76-7_Apexbio

Inosine-5'-monophosphate is a substrate of IMP dehydrogenase. Inosine-5'-monophosphate dehydrogenase (IMPDH) is an enzyme responsible to the biosynthesis of guanine nucleotides. IMPDH inhibitors have been approved for prevention of organ transplant rejection.
Soluble in DMSO
Store at -20°C
For obtaining a higher solubility , please warm the tube at 37°C and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months.
特别声明
产品仅用于研究,
不针对患者销售,望谅解。
每个产品具体的储存和使用信息显示在产品说明书中。ApexBio 产品在推荐的条件下是稳定 的。产品会根据不同的推荐温度进行运输。许多产品短期运输是稳定的,运输温度不同于长 期储存的温度。我们确保我们的产品是在保持试剂质量的条件下运输的。收到产品后,按照 产品说明书上的要求进行储存。
产品说明书
化学性质
产品名: Inosine-5'-monophosphate (sodium salt hydrate).: 分子量: 分子式: 别名: 化学名: SMILES:
溶解性: 储存条件: 一般建议:
索马鲁肽 质量标准

索马鲁肽(Semaglutide)是一种新型长效胰高血糖素样肽-1(GLP-1)受体激动剂,主要用于治疗2型糖尿病。
其通过促进胰岛素分泌和抑制胰高血糖素分泌来降低血糖,同时具有减少食物摄入和降低体重的效果。
索马鲁肽的质量标准通常包括以下几个方面:
1. 纯度:索马鲁肽的纯度要求通常非常高,常见的要求为95%或99%以上。
这保证了药品中活性成分的浓度和一致性,从而确保疗效的稳定。
2. 分子式与分子量:索马鲁肽的分子式为C187H291N45O59,分子量约为411
3.57754。
这些参数是化学表征的重要指标,用于确保产品的正确性。
3. 外观:索马鲁肽通常为白色粉末状,这与其化学性质和纯度有关。
4. 溶解性:索马鲁肽在水中的溶解性良好,通常可溶解至1 mg/ml。
这与其作为注射剂的给药形式相关。
5. 储存条件:索马鲁肽应在-20°C的条件下储存,以保持其稳定性和活性。
6. 质量检验:在生产过程中,索马鲁肽会经过严格的质量检验,包括使用高效液相色谱法(HPLC)等分析技术来确保其纯度和含量。
7. 生物活性:索马鲁肽的生物活性是通过与GLP-1受体结合的能力来评估的。
这一点对于确保其治疗效果至关重要。
8. 稳定性:索马鲁肽在储存和使用过程中的稳定性也是质量标准的一部分,这包括对温度、pH 值和光照等条件的稳定性评估。
总的来说,索马鲁肽的质量标准旨在确保其作为药物的安全性和有效性,这些标准通常由药品制造商和监管机构设定并执行。
英国药典BP标准品氯二甲苯酚盐酸扑尔敏头孢洛宁甲磺酸二氢麦角碱杂质A丙氧基苯甲酸阿莫西林杂质

英国药典于 1864年在英国成立,是英国制药标准的重要来源。
该药典由三卷本组成。
其中两卷为英国药典、一卷为英国兽药典(兽医药品部分)。
各条目均以药品名称字母顺序排列,内容包括药品性质、制法、血产品、免疫产品、电磁药品制法及外科材料等部分.公司一直在为药学方面提供权威的官方标准品,它在药品和保健品方面做出了重大贡献,英国药典不仅为我们提供了药用和成药配方标准以及公式配药标准,而且也向我们展示了许多明确分类并可参照的欧洲药典专著。
广州优瓦仪器有限公司是从事提供实验室分析领域内产品的专业公司;目前公司已是集研发、生产与销售为一体的综合性企业;在行业内具有良好的声誉;主要产品包括色谱产品、化学试剂、标准品、实验室用品、分析仪器配件及耗材等,总部位于香港。
我们代理的品牌有:USP、EP、BP、TLC、TRC、Molcan、LGC、Wellington、Chiron、Witega、NRC、ERM、Irmm、MBH、Fluorochem、TCI、Chemservice、Accstandard 、 GmbH 、CIL 、C/D/N ISOTopes 、InorganicVentures 、ULTRA Scientific、NSI 、KeyOrganics、LC-Laboratoies、Wibby 、APSC、SPEX CertiPrep 、NIST 、ACROS、Fluka、Matrix、Sigma、TCI、Strem、BP、Cerilliant 、Chromadex 、Frontier、Echelon、Serva 、MedicalIsotope 、ChemBridge 、AMRESCO、SGE Analytical Science、Brand BRAND、VITLAB、ISO 、Hamilton、ISOLAB我们专注进口标准品。
详情请与我司联系。
Code中文名称Product001萜烯苯酚的合成树脂Acepromazinemaleate904乙酰唑胺分析标准品Acetazolamide Assay Standard6814-乙酰 2-氟联苯4-Acetyl2-fluorobiphenyl907乙酰半胱氨酸分析标准品AcetylcysteineAssay Standard438阿昔洛韦分析标准品Aciclovir AssayStandard003肾上腺素酸式酒石酸盐分析标准品Adrenaline (epinephrine) acid tartrate Assay Standard763阿苯达唑分析标准品Albendazole AssayStandard436亚历山大番泻叶世果粉分析标准品Alexandrian senna fruit powder Assay Standard006阿法多龙三醋酸分析标准品Alfadoloneacetate Assay Standard007硫酸丁胺卡那霉素分析标准品AlfaxaloneAssay Standard825盐酸阿夫唑嗪分析标准品Alfuzosinhydrochloride Assay Standard415异丁嗪分析标准品Alimemazine tartrateAssay Standard831别嘌呤醇杂质AAllopurinol ImpurityA832别嘌呤醇杂质BAllopurinol ImpurityB843别嘌呤醇杂质CAllopurinol ImpurityC844别嘌呤醇杂质DAllopurinol ImpurityD845别嘌呤醇杂质EAllopurinol ImpurityE560溴苄铵托西酸盐9-Allyl-2-chlorothioxanthen-9-ol725枸橼酸阿尔维林杂质分析标准品Alverinecitrate Assay Standard724枸橼酸阿尔维林杂质标准品Alverine citrate impurity standard solution530盐酸金刚烷胺分析标准品Amantadinehydrochloride Assay Standard853硫酸丁胺卡那霉素分析标准品AmikacinSulphate Assay Standard008盐酸阿米洛利分析标准品Amiloridehydrochloride Assay Standard010头孢洛宁4-Amino-6-chlorobenzene-1-3-disulphonamide374头孢呋辛酯3-Amino-6-chloro-1-methyl-4-phenylquinolin-2-ol839苯丁酸氮芥3-amino-4-(2-chlorophenyl)-6-nitroquinolin-2(1H)-one813氯二甲苯酚2-Amino-5-chloropyridine012盐酸扑尔敏2-Amino-4,6-Dichlorophenolhydrochloride011顺铂7-Aminodesacetoxycephalosporanic acid602盐酸富马酸氯马斯汀(E)-4-Amino-2-ethylidenebutyricacid hydrochloride684氯美噻唑乙二磺酸盐分析标准品AminoglutethimideAssay Standard881甲磺酸二氢麦角碱杂质AAminoglutethimideimpurity A4172-Amino-1-(4-nitrophenyl)propane-1,3-diol2-Amino-1-(4-nitrophenyl)propane-1,3 -diol6013-Aminopent-4-ene-1,1-dicarboxylicacid3-Aminopent-4-ene-1,1-dicarboxylic acid5363-Amino-4-phenoxy-5-sulphamoylbenzoicacid3-Amino-4-phenoxy-5-sulphamoylbenzoicacid014丙氧基苯甲酸3-Amino-4-propoxybenzoicacid532盐酸丙氧基苯甲酸分析标准品Amiodaronehydrochloride Assay Standard785无水的氨苄西林分析标准品AnhydrousAmpicillin Assay Standard021氨苄青霉素Ampicillintrihydrate015双甲脒分析标准品Amitraz AssayStandard016盐酸阿米替林分析标准品Amitriptylinehydrochloride Assay Standard019阿莫西林三水物分析标准品AmoxicillinTrihydrate Assay Standard748阿莫西林杂质标准品Amoxicillinimpurity standard546阿泊拉霉素Apramycin694盐酸马来酸麦角新碱分析标准品Argininehydrochloride Assay Standard461酒石酸麦角胺Ascorbic acid617阿匹司林分析标准品Aspirin AssayStandard492阿替洛尔分析标准品Atenolol AssayStandard370阿替洛尔杂质标准品Atenolol impuritystandard023雌二醇半水合物分析标准品Atropinesulphate Assay Standard366依他尼酸1-(3-Azabicyclo[]oct-3-yl)-3-o-tolylsulphonylurea 024氮杂羟基嘌呤2-Azahypoxanthine025阿扎哌隆分析标准品Azaperone AssayStandard534阿扎丙宗分析标准品AAzapropazone AssayStandard515阿扎丙宗分析标准品BAzapropazoneimpurity A516阿扎丙宗分析标准品CAzapropazoneimpurity B517阿扎丙宗分析标准品Azapropazoneimpurity C527阿扎丙宗分析标准品Azapropazoneimpurity standard028氟比洛芬钠分析标准品Baclofen AssayStandard535膦甲酸钠Baclofen lactam030二丙酸倍氯米松分析标准品Beclometasonedipropionate Assay Standard760灰黄霉素Beclometasone17-propionate761胍乙啶Beclometasone21-propionate685盐酸氢溴酸后马托品分析标准品Benserazide036甲磺酸苯扎托品分析标准品Benzatropinemesilate Assay Standard610盐酸羟丙甲纤维素分析标准品Benzydaminehydrochloride Assay Standard426苯甲酸苄酯分析标准品Benzyl benzoateAssay Standard611单硝酸异山梨酯1-Benzyl-3-(3-diethylamino-propoxy)-1H-indazole037伊维菌素(1S,2R)-1-benzyl-3-dimethylamino-2-methyl-1-phenylpropyl acetate609左薄荷脑1-Benzyl-1-H-indazol-3-ol824盐酸左炔诺孕酮分析标准品Betahistinedihydrochloride Assay Standard575倍他米松分析标准品Betamethasone AssayStandard041倍他米松磷酸钠Betamethasone sodiumphosphate042倍他米松戊酸分析标准品Betamethasonevalerate Assay Standard043倍他米松Betamethasone21-valerate686盐酸倍他洛尔分析标准品Betaxololhydrochloride Assay Standard755苯扎贝特分析标准品Bezafibrate AssayStandard046莫美他松糠酸酯2-(Biphenyl-4-yl)propionicacid047比沙可啶分析标准品Bisacodyl AssayStandard612溴苄铵托西酸盐分析标准品Bretyliumtosilate Assay Standard613盐酸诺氟沙星2-Bromobenzyldimethylaminehydrochloride050甲磺酸溴隐亭分析标准品BromocriptineMesilate Assay Standard440盐酸芍药苷杂质标准品Buclizinehydrochloride impurity standard793布地奈德分析标准品Budesonide AssayStandard537布美他尼分析标准品Bumetanide AssayStandard479盐酸布比卡因定分析标准品Bupivacaine403白消安分析标准品Busulfan AssayStandard192强的松2-tert-Butylamino-1-(4-hydroxy-3-methylphenyl)ethanolsulphate531氢化泼尼松磷酸钠2-Butyl-3-(4-hydroxy-3,5-di-iodobenzoyl)benzofuran766咖啡因分析标准品Caffeine AssayStandard355降血钙素分析标准品Calcitonin (salmon)Assay Standard059硫酸卷曲霉素Capreomycinsulphate538卡托普利分析标准品Captopril AssayStandard500柳氮磺胺吡啶Captoprildisulphide477盐酸舒必利2-Carbamoyl-1-methyl-3-[2-(5-methylimidazol-4-yl-methylthio)ethyl]guanidine dihydrochloride523甲萘威分析标准品Carbaryl AssayStandard060卡比多巴分析标准品Carbidopa AssayStandard749卡比马唑分析标准品Carbimazole AssayStandard711卡帕分析标准品Carboplatin AssayStandard767羧甲基纤维素钠分析标准品Carmellosesodium Assay Standard567盐酸卡替洛尔分析标准品Carteololhydrochloride Assay Standard792头孢克洛分析标准品Cefaclor AssayStandard784头孢羟氨苄分析标准品Cefadroxil AssayStandard061头孢氨苄分析标准品Cefalexin AssayStandard562头孢洛宁分析标准品Cefalonium AssayStandard819头孢唑林钠Cefazolin Sodium820头孢西丁钠分析标准品Cefoxitin SodiumAssay Standard063头孢拉定分析标准品Cefradine AssayStandard976头孢曲松钠分析标准品Ceftriaxone sodiumAssay Standard977头孢曲松钠异构体Ceftriaxone sodiumE-isomer502头孢呋辛酯分析标准品Cefuroxime axetilAssay Standard925头孢呋辛钠分析标准品Cefuroxime sodiumAssay Standard757盐酸塞利洛尔分析标准品Celiprololhydrochloride Assay Standard926鹅去氧胆酸Chenodeoxycholicacid064苯丁酸氮芥分析标准品ChlorambucilAssay Standard854氯霉素分析标准品ChloramphenicolAssay Standard067盐酸氯环嗪Chlorcyclizinehydrochloride068醋酸氯己定分析标准品Chlorhexidineacetate Assay Standard4411,4-bis(4-Chlorobenzhydryl)piperazine1,4-bis(4-Chlorobenzhydryl)piperazine756N-p-chlorobenzoyltyramineN-p-chlorobenzoyltyramine6044-Chlorobenzylphthalazinone4-Chlorobenzylphthalazinone3766-氯-4-(2-氯苯基)喹啉-2-甲醛6-Chloro-4-(2-chlorophenyl)quinazoline-2-carboxaldehyde4426-Chloro-1,4-dihydro-1-methyl-4-phenylquinazolin-4-ol6-Chloro-1,4-dihydro-1-m ethyl-4-phenylquinazolin-4-ol4707-Chloro-1-5-dihydro-5-phenyl-1,5-benzodiazepine-2,4(3H)-dione7-Chloro-1-5-di hydro-5-phenyl-1,5-benzodiazepine-2,4(3H)-dione04917β,17'β-bis(3-[bis-(2-chloroethyl)carbamoyloxy]estra-1,3,5,(10)-trienyl)pyrophosphate17β,17'β-bis(3-[bis-(2-chloroethyl)carbamoyloxy]estra-1,3,5,(10)-trienyl)pyrophosphate4465-(2-Chloroethyl)-4-methyl-3-[2-(4-methylthiazol-5-yl)ethyl]thiazolium chloride5-(2-Chloroethyl)-4-methyl-3-[2-(4-methylthiazol-5-yl)ethyl]thiazolium chloride0714-[2-(5-Chloro-2-methoxybenzamido)ethyl]benzenesulphonamide4-[2-(5-Chloro-2-methoxybenzamido)ethyl]benzenesulphonamide9655-氯-1-甲基-4-硝基咪唑5-Chloro-1-methyl-4-nitroimidazole3785-Chloro-2-methylaminobenzophenone5-Chloro-2-methylaminobenzophenone4436-(2-Chlorophenyl)-2,4-dihydro-2-[(dimethylamino)methylene]8-nitroimidazo[1,2-a][1,4]benzodiazepin-1-one6-(2-Chlorophenyl)-2,4-dihydro-2-[( dimethylamino)methylene]8-nitroimidazo[1,2-a][1,4]benzodiazepin-1-one0744-Chloro-5-sulphamoylanthranilicacid4-Chloro-5-sulphamoylanthranilic acid075二苯甲酸2-(4-Chloro-3-sulphamoylbenzoyl)benzoicacid076氯噻嗪Chlorothiazide5592-氯噻吨酮2-Chlorothioxanthone0772-(6-Chlorothymoxy)ethyldimethylaminehydrochloride2-(6-Chlorothymoxy)ethyldimethylaminehydrochloride0792-Chlorotritanol2-Chlorotritanol080氯二甲苯酚分析标准品ChloroxylenolAssay Standard081扑尔敏分析标准品Chlorphenaminemaleate Assay Standard856盐酸氯丙嗪Chlorpromazinehydrochloride467氧氯丙嗪亚砜Chlorpromazinesulphoxide491氯塞酮分析标准品Chlortalidone AssayStandard783盐酸金霉素分析标准品Chlortetracyclinehydrochloride Assay Standard618胆酸分析标准品Cholic acid AssayStandard475西米替汀分析标准品Cimetidine AssayStandard809顺铂分析标准品Cisplatin AssayStandard833克拉霉素分析标准品ClarithromycinAssay Standard834克拉霉素杂质EClarithromycinimpurity E525富马酸氯马斯汀分析标准品Clemastinefumarate Assay Standard084氯碘羟喹分析标准品Clioquinol AssayStandard507氯巴占分析标准品受控药物Clobazam Assay Standard Controlled Substance522氯倍他索杂质AClobetasol impurityA521丙酸氯倍他索分析标准品Clobetasolpropionate Assay Standard482丁酸氯倍他松分析标准品Clobetasonebutyrate Assay Standard406氯可托龙己酸盐Clocortolonehexanoate663氯苯吩嗪分析标准品Clofazimine Assay Standard543氯美噻唑乙二磺酸盐分析标准品Clomethiazole edisilate Assay Standard980盐酸氯米帕明Clomipraminehydrochloride983氯丙咪嗪杂质FClomipramine ImpurityF981氯丙咪嗪杂质CClomipramine impurityC484氯安定分析标准品受控药物Clonazepam Assay Standard Controlled Substance085盐酸可乐定分析标准品Clonidinehydrochloride Assay Standard565二盐酸化物反式氯哌噻吨醋酸trans-Clopenthixol acetate dihydrochloride566二盐酸化物反氯哌噻吨癸酸酯trans-Clopenthixol decanoate dihydrochloride561盐酸反氯哌噻吨trans-Clopenthixol hydrochloride628氯前列烯醇钠分析标准品Cloprostenolsodium Assay Standard379克霉唑分析标准品Clotrimazole AssayStandard088苄星邻氯青霉素分析标准品Cloxacillin benzathine Assay Standard905氯氮平分析标准品Clozapine AssayStandard091甲磺酸二氢麦角碱分析标准品Co-dergocrine mesilate Assay Standard715盐酸薄片分析标准品受控药物Cocainehydrochloride Assay Standard Controlled Substance514盐酸可待因分析标准品受控药物Codeinehydrochloride Assay Standard Controlled Substance090磷酸可待因分析标准品受控药物CodeinePhosphate Assay Standard Controlled Substance787维生素D3分析标准品ColecalciferolAssay Standard614盐酸考来替泊Colestipolhydrochloride551考来烯胺分析标准品ColestyramineAssay Standard585醋酸可的松分析标准品Cortisone acetateAssay Standard094克罗米同分析标准品Crotamiton AssayStandard466维生素B12分析标准品CyanocobalaminAssay Standard4782-Cyano-1-methyl-3-[2-(5-methylimidazol-4-yl-methylsulphinyl)ethyl]guanidine2 -Cyano-1-methyl-3-[2-(5-methylimidazol-4-yl-methylsulphinyl)ethyl]guanidine096盐酸苯甲嗪分析标准品Cyclizinehydrochloride Assay Standard886盐酸环苯扎林Cyclobenzaprinehydrochloride098环戊噻嗪Cyclopenthiazide380盐酸环喷托酯分析标准品Cyclopentolatehydrochloride Assay Standard688醋酸环丙氯地孕酮分析标准品Cyproteroneacetate Assay Standard383阿糖胞苷分析标准品Cytarabine AssayStandard100达卡巴嗪Dacarbazine771丹曲林分析标准品Dantrolene AssayStandard428二羟蒽醌Dantron429二羟蒽醌杂质标准品Dantron impuritystandard102氨苯砜分析标准品Dapsone AssayStandard103硫酸异喹胍Debrisoquinesulphate104癸氧喹酯Decoquinate791Delta-3-CefaclorDelta-3-Cefaclor572DeltamedraneDeltamedrane631溴氰菊酯分析标准品Deltamethrin AssayStandard632溴氰菊酯杂质标准品Deltamethrinimpurity standard789盐酸去甲金霉素分析标准品Demeclocyclinehydrochloride Assay Standard889N-DemethylerythromycinAN-Demethylerythromycin A504去乙酰韦替洛尔Desacetylmetipranolol730N-DesalkylflurazepamN-Desalkylflurazepam987云铁胺甲磺酸酯Desferrioxaminemesilate931盐酸去郁敏Desipraminehydrochloride689盐酸去甲苯扎托品Desmethylbenzatropine hydrochloride568去氧孕烯分析标准品Desogestrel AssayStandard816去氧孕烯杂质DDesogestrel impurityD817去氧孕烯杂质EDesogestrel impurityE594DesogestrelΔ3-isomerDesogestrel Δ3-isomer578地塞米松分析标准品Dexamethasone AssayStandard108地塞米松磷酸钠Dexamethasone sodiumphosphate646磷酸地塞米松分析标准品Dexamethasonephosphate Assay Standard811地塞米松杂质标准品Dexamethasoneimpurity standard465右旋丙氧芬分析标准品受控药物DextropropoxypheneHCl Assay Standard Controlled Substance549荧光素二乙酸盐分析标准品DiacetylfluoresceinAssay Standard887 石斛 dongding;盐酸脉石中的矿石,填充料,物料分析标准品受控药物DiamorphineHydrochloride Assay Standard Controlled Substance111地西泮受控药物Diazepam ControlledSubstance1146,11-二氢二苯并[b,e]硫杂卓-11-酮Dibenzo[b,e]thiepin-11(6H)-one1243-(Dibenzo[b,e]thiepin-11(6H)-ylidene)-N,N-dimethylaminopropan-1-amine-S-oxid ehydrochloride3-(Dibenzo[b,e]thiepin-11(6H)-ylidene)-N,N-dimethylaminopropan-1-am ine-S-oxidehydrochloride85810,11-二氢二苯并[a,b]环庚烯-5-酮Dibenzosuberone6083-(1,5-Dibenzyl-1H-indazole-3-yl)oxypropyldimethylaminehydrochloride3-(1,5-Dibenzyl-1H-indazole-3-yl)oxypropyldimethylamine hydrochloride116双氯酚分析标准品Dichlorophen AssayStandard420二氯芬杂质分析标准品Dichlorophenimpurity standard Assay Standard7211-(2,5-Dichlorophenyl)-5-isopropylbiguanidehydrochloride1-(2,5-Dichlorophenyl)-5-isopropylbiguanidehydrochloride619双氯芬酸钠分析标准品Diclofenac sodiumAssay Standard598双氯芬酸二乙胺Diclofenacdiethylamine828双氯芬酸杂质ADiclofenac impurityA810双氯西林钠Dicloxacillinsodium118褐霉酸二乙醇胺分析标准品Diethanolaminefusidate Assay Standard3612-Diethylaminoethyl-3-(1-naphthyl)-2-(1-naphthylmethyl)propionateoxalate2-Diethylaminoethyl-3-(1-naphthyl)-2-(1-naphthylmethyl)propionate oxalate737枸橼酸二乙碳酰嗪Diethylcarbamazinecitrate119Diethyl-4-decyloxy-3-ethoxyanilinomethylenemalonateDiethyl-4-decyloxy-3-ethox yanilinomethylenemalonate859己烯雌酚分析标准品DiethylstilbestrolAssay Standard677DiethylstilbestrolmonophosphateDiethylstilbestrolmonophosphate401戊酸双氟可龙分析标准品Diflucortolonevalerate Assay Standard397戊酸双氟可龙杂质标准品Diflucortolonevalerate impurity standard855洋地黄毒甙分析标准品Digitoxin AssayStandard431酒石酸双氢可待因分析标准品受控药物DihydrocodeineTartrate Assay Standard Controlled Substance120甲磺酸双氢麦角汀Dihydroergocristinemesilate1222,3-二氢-6-苯基咪唑噻唑盐酸盐2,3-Dihydro-6-phenylimidazo[2,1-b] thiazole815bis-(1,7-dihydro-6H-purine)-6,6-disulphidebis-(1,7-dihydro-6H-purine)-6,6-dis ulphide726硫氮酮Diltiazem HCl899地尔硫卓杂质标准品Diltiazem ImpurityStandard5826,6-Dimethoxy-2,2'-binaphthyl6,6-Dimethoxy-2,2'-binaphthyl1954-Dimethylamino-3-methyl-1,2-diphenylbutan-2-olhydrochloride4-Dimethylamino-3-methyl-1,2-diphenylbutan-2-olhydrochloride6073-Dimethylaminopropyl 2-benzylaminobenzoatehydrochloride3-Dimethylaminopropyl2-benzylaminobenzoate hydrochloride4561,5-二甲基己胺1,5-Dimethylhexyl(methyl)amine865Dimethyl-2,6-dimethyl-4-(2-nitrophenyl)pyridine-3,5-dicarboxylateDimethyl-2,6 -dimethyl-4-(2-nitrophenyl)pyridine-3,5-dicarboxylate866Dimethyl-2,6-dimethyl-4-(2-nitrosophenyl)pyridine-3,5-dicarboxylateDimethyl-2 ,6-dimethyl-4-(2-nitrosophenyl)pyridine-3,5-dicarboxylate511Dimethyl{5-[2-(1-methylamino-2-nitrovinylamino)ethylsulphinylmethyl]furfuryl}amineDimethyl{5-[2-(1-methylamino-2-nitrovinylamin o)ethylsulphinylmethyl]furfuryl}amine3642,2-Dimethyl-5(2,4-xylyloxy)valericacid2,2-Dimethyl-5(2,4-xylyloxy)valericacid690敌匹硫磷分析标准品Dimpylate AssayStandard691Dimpylate forchromatographyDimpylate for chromatography902地诺前列酮分析标准品Dinoprostone AssayStandard896盐酸苯海拉明Diphenhydraminehydrochloride128盐酸地匹哌酮分析标准品受控药物Dipipanonehydrochloride Assay Standard Controlled Substance673盐酸地匹福林分析标准品Dipivefrinehydrochloride Assay Standard659地匹福林杂质标准品Dipivefrineimpurity standard589利巴韦林杂质标准品分析标准品DiprenorphineAssay Standard131双嘧达莫分析标准品Dipyridamole AssayStandard384N1,N2-Diquinoxalin-2-ylsulphanilamideN1,N2-Diquinoxalin-2-ylsulphanilamide615帕米膦酸钠分析标准品Disodiumpamidronate Assay Standard132二硫化四乙基秋兰姆分析标准品DisulfiramAssay Standard385蒽三酚分析标准品Dithranol AssayStandard674盐酸多巴酚丁胺分析标准品Dobutaminehydrochloride Assay Standard133多库酯钠分析标准品Docusate sodiumAssay Standard669马来酸多潘立酮分析标准品Domperidonemaleate Assay Standard468盐酸多巴胺分析标准品Dopaminehydrochloride Assay Standard134盐酸度琉平分析标准品Dosulepinhydrochloride Assay Standard135盐酸多沙普仑分析标准品Doxapramhydrochloride Assay Standard136盐酸多虑平分析标准品Doxepinhydrochloride Assay Standard823多塞平杂质标准品Doxepin ImpurityStandard990盐酸阿霉素分析标准品Doxorubicinhydrochloride Assay Standard780盐酸强力霉素分析标准品Doxycyclinehyclate Assay Standard675氟哌利多分析标准品Droperidol Assay Standard138去氢孕酮Dydrogesterone139硝酸益康唑分析标准品Econazole nitrate Assay Standard741马来酸依那普利Enalaprilmaleate742依那普利拉Enalaprilat743依那普利双酮Enalaprildiketopiperazine387盐酸麻黄碱分析标准品毒品Ephedrine hydrochloride Assay Standard Drug Precursor 992盐酸表阿霉素Epirubicinhydrochloride788alpha-骨化醇分析标准品Ergocalciferol Assay Standard405马来酸麦角新碱分析标准品Ergometrine maleate Assay Standard Drug Precursor141酒石酸麦角胺分析标准品Ergotamine tartrate Assay Standard Drug Precursor781红霉素Erythromycin794红霉素A分析标准品Erythromycin AAssay Standard795红霉素B分析标准品Erythromycin BAssay Standard796红霉素C分析标准品Erythromycin CAssay Standard790依托红霉素Erythromycinestolate798琥乙红霉素Erythromycin ethylsuccinate488硬脂酸红霉素分析标准品Erythromycin stearate Assay Standard396苯甲酸雌二醇分析标准品Estradiol benzoate Assay Standard729雌二醇半水合物分析标准品Estradiol hemihydrate Assay Standard142雌莫司汀Estramustine752雌三醇分析标准品Estriol AssayStandard753雌三醇杂质标准品Estriol impurity standard860雌酚酮Estrone616雌酮硫酸酯哌嗪分析标准品EstropipateAssay Standard143依他尼酸分析标准品Etacrynic acidAssay Standard421炔雌醇分析标准品EthinylestradiolAssay Standard145乙氧酰胺苯甲酯分析标准品Ethopabate Assay Standard146乙琥胺分析标准品Ethosuximide AssayStandard150Ethyldimethyl[2-(2-methylbenzhydryloxy)ethyl]ammonium chlorideEthyldimethyl[2-(2-methylbenzhydryloxy)ethyl]ammonium chloride 437N-EthylglucaminehydrochlorideN-Ethylglucamine hydrochloride149甲氯芬那酸Ethylmeclofenamate529依托度酸分析标准品Etodolac AssayStandard533依托度酸二聚体Etodolac aciddimer541依托度酸Etodolac 1-methylanalogue542依托度酸Etodolac 8-methylanalogue885依托泊苷分析标准品Etoposide AssayStandard653法莫替丁分析标准品Famotidine AssayStandard655法莫替丁降解杂质1Famotidinedegradation impurity 1656法莫替丁降解杂质2Famotidinedegradation impurity 2654法莫替丁杂质CFamotidine impurityC6204-联苯乙酸分析标准品Felbinac AssayStandard777非洛地平分析标准品Felodipine AssayStandard758非洛地平杂质标准品Felodipine impurity standard692苯硫咪唑Fenbendazole693达虫净杂质CFenbendazole impurityC {5-(phenylthio)-2-aminobenzimidazole}354联苯丁酮酸分析标准品Fenbufen Assay Standard661非诺特罗降解杂质AFenoteroldegradation impurity A660氢溴酸芬忒醇分析标准品Fenoterol hydrobromide Assay Standard665枸橼酸芬太尼分析标准品受控药物Fentanyl citrate Assay Standard Controlled Substance 666芬太尼杂质AFentanyl impurityA156倍硫磷分析标准品Fenthion AssayStandard740非那雄胺分析标准品Finasteride Assay Standard571盐酸黄酮哌酯分析标准品Flavoxate hydrochloride Assay Standard676醋酸氟卡胺分析标准品Flecainide acetate Assay Standard159醋酸氟氢可的松分析标准品Fludrocortisone acetate Assay Standard840氟硝西泮受控药物FlunitrazepamControlled Substance160醋酸肤轻松分析标准品Fluocinolone acetonide Assay Standard489醋酸氟轻松分析标准品Fluocinonide Assay Standard161氟可龙己酸分析标准品Fluocortolone hexanoate Assay Standard162氟可龙三甲基乙酸分析标准品Fluocortolone pivalate Assay Standard163氯苯丁酮4'-Fluoro-4-chlorobutyrophenone573氟米龙分析标准品FluorometholoneAssay Standard797盐酸氟西汀分析标准品Fluoxetine hydrochloride Assay Standard164氟甲睾酮Fluoxymesterone738氟哌噻吨cis-Flupentixol554二盐酸化物噻吨癸酸酯分析标准品Flupentixol decanoate dihydrochloride Assay Standard556盐酸反噻吨癸酸酯trans-Flupentixol decanoate dihydrochloride597盐酸顺式三氟噻吨丙酸cis-Flupenthixol propionate dihydrochloride167盐酸氟奋乃静分析标准品Fluphenazinehydrochloride Assay Standard574氟比洛芬钠分析标准品Flurbiprofensodium Assay Standard587丙酸氟替卡松分析标准品Fluticasonepropionate Assay Standard588氟及卡松丙酸酯杂质FluticasoneS-methyl impurity600马来酸氟伏沙明分析标准品Fluvoxaminemaleate Assay Standard671氟伏沙明杂质标准品Fluvoxamine maleateimpurity standard627甲酰基利福霉素 SV3-FormylrifamycinSV639Form-2',4'-xylidideForm-2',4'-xylidide623膦甲酸钠分析标准品Foscarnet sodiumAssay Standard678磷雌酚钠Fosfestrol sodium547呋噻米分析标准品Furosemide AssayStandard643加拉明杂质标准品Gallamine impuritystandard363吉非罗齐分析标准品Gemfibrozil AssayStandard303二甲苯氧庚酸杂质AGemfibrozilimpurity A 2,2-dimethyl-5-(4-propen-1-yl)-2,5-xylyloxy)valeric acid365吉非罗齐甲酯Gemfibrozil methylester174硫酸庆大霉素Gentamicinsulphate175格列本脲分析标准品Glibenclamide AssayStandard368格列齐特分析标准品Gliclazide AssayStandard580格列喹酮分析标准品Gliquidone AssayStandard581硫胺格列喹酮Gliquidonesulphonamide652硝酸甘油溶液分析标准品Glyceryltrinitrate solution Assay Standard814戈舍瑞林六胜肽GoserelinHexapeptide180灰黄霉素分析标准品Griseofulvin AssayStandard181胍乙啶分析标准品Guanethidinemonosulphate Assay Standard879鸟嘌呤Guanine407氟哌啶醇分析标准品Haloperidol AssayStandard185氢溴酸后马托品分析标准品Homatropinehydrobromide Assay Standard186双氢氯噻嗪分析标准品HydrochlorothiazideAssay Standard576氢化可的松分析标准品HydrocortisoneAssay Standard584醋酸氢化可的松分析标准品Hydrocortisoneacetate Assay Standard187氢化可的松半琥酯HydrocortisoneHydrogen Succinate188氢化可的松磷酸钠Hydrocortisonesodium phosphate190氢氟噻嗪Hydroflumethiazide770对羟基苯乙酮4'Hydroxyacetophenone197羟基脲分析标准品HydroxycarbamideAssay Standard526N-甲基-2-(2-羟乙基)吡咯烷2-(2-Hydroxyethyl)-1-methylpyrrolidine1965-[1-Hydroxy-2-(1-methyl-3-phenylpropylamino)ethyl]salicylicacidhydrochloride5-[1-Hydroxy-2-(1-methyl-3-phenylpropylamino)ethyl]salicylicacid hydrochloride829D-alpha-(4-hydroxyphenyl)glycineD-alpha-(4-hydroxyphenyl)glycine888(5RS)-3-(2-hydroxyphenyl)-5-phenylcyclohex-2-enone(5RS)-3-(2-hydroxyphenyl)-5 -phenylcyclohex-2-enone1942-(6-Hydroxythymoxy)ethyldimethylaminehydrochloride2-(6-Hydroxythymoxy)ethyldimethylaminehydrochloride198丁溴东莨菪碱分析标准品Hyoscinebutylbromide Assay Standard199东莨菪碱氢溴酸盐分析标准品HyoscineHydrobromide Assay Standard774羟丙甲纤维素分析标准品HypromelloseAssay Standard539布洛芬分析标准品Ibuprofen AssayStandard2025-碘-2'-脱氧尿苷分析标准品IdoxuridineAssay Standard759异环磷酰胺分析标准品Ifosfamide AssayStandard664IminophenazineIminophenazine932盐酸丙咪嗪Imipraminehydrochloride999吲达帕胺分析标准品Indapamide AssayStandard1000吲达帕胺杂质BIndapamide impurityB625异丙托溴铵分析标准品Ipratropiumbromide Assay Standard557异丁基苯乙酮4'-Isobutylacetophenone550硝酸异康唑分析标准品Isoconazolenitrate Assay Standard205盐酸异丙肾上腺素分析标准品Isoprenalinehydrochloride Assay Standard778盐酸异丙美沙嗪Isopromethazinehydrochloride206硝酸异山梨酯Isosorbidedinitrate799异脱二水山梨醇-2-硝酸酯Isosorbide2-nitrate800单硝酸异山梨酯分析标准品Isosorbidemononitrate Assay Standard4994'-(2-Isopropylaminoethyl)methanesulphonanilide hydrochloride4'-(2-Isopropylaminoethyl)methanesulphonanilide hydrochloride626苯酰甲硝唑8s-Isopropyl-3β-hydroxytropaniumbromide621萜烯苯酚的合成树脂分析标准品IsradipineAssay Standard622伊拉地平杂质BIsradipine impurityB624伊拉地平杂质DIsradipine impurityD864伊维菌素分析标准品Ivermectin AssayStandard861KanamycinmonosulpateKanamycin monosulpate736盐酸氯胺酮受控药物KetamineHydrochloride Controlled Substance6681-(2-甲氧苯基)哌嗪分析标准品Ketoprofen Assay Standard667酮基布洛芬乙酯Ketoprofen ethylester707甲基多巴分析标准品Lacidipine Assay Standard647拉西地平杂质标准品Lacidipineimpurity Standard802拉米夫定分析标准品Lamivudine Assay Standard872兰索拉唑分析标准品Lansoprazole Assay Standard873兰索拉唑杂质标准品Lansoprazoleimpurity standard212盐酸左旋咪唑Levamisolehydrochloride356盐酸甲基苯巴比妥分析标准品Levobunolol hydrochloride Assay Standard213甲基强的松龙分析标准品Levodopa Assay Standard772左薄荷脑分析标准品Levomenthol Assay Standard723甲基泼尼松龙醋酸酯分析标准品Levomepromazine maleate Assay Standard634左美丙嗪亚砜Levomepromazinesulphoxide501左炔诺孕酮分析标准品LevonorgestrelAssay Standard215盐酸林可霉素分析标准品Lincomycin hydrochloride Assay Standard727利多卡因分析标准品Lidocaine Assay Standard214盐酸利多卡因Lidocaine (Lignocaine) hydrochloride733亚麻油Linseed oil695赖诺普利分析标准品Lisinoprildihydrate Assay Standard696赖诺普利二酮哌嗪Lisinoprildiketopiperazine433乳酸锂分析标准品Lithium lactateAssay Standard927石胆酸Lithocholic acid697盐酸洛非帕明分析标准品Lofepramine hydrochloride Assay Standard635盐酸米赛林分析标准品Loperamide hydrochloride Assay Standard636洛派丁胺 N-氧化物LoperamideN-oxide874扑米酮分析标准品Loratadine Assay Standard875兰索拉唑杂质标准品LoratadineImpurity Standard447甲磺酸氯普唑仑受控药物Loprazolammesilate Controlled Substance434普鲁卡因青霉素分析标准品受控药物Lorazepam Assay Standard Controlled Substance528丙氯拉嗪分析标准品受控药物Lormetazepam Assay Standard Controlled Substance867环苯咯丙醇分析标准品Losartan Potassium Assay Standard218赖甲环素Lymecycline776利奈孕酮Lynestrenol1008甲苯咪唑Mebendazole220盐酸美克洛嗪片Meclozinehydrochloride221醋酸甲羟孕酮分析标准品Medroxyprogesterone acetate Assay Standard222甲地孕酮Megestrol223醋酸甲地孕酮分析标准品Megestrol acetate Assay Standard629美洛昔康分析标准品Meloxicam Assay Standard630美洛昔康杂质标准品Meloxicam impurity standard391马法兰分析标准品Melphalan AssayStandard699盐酸吡多素分析标准品Mepivacaine hydrochloride Assay Standard583盐酸酒石酸美托洛尔分析标准品Meptazinol hydrochloride Assay Standard7732-羟基丙酸单锂盐分析标准品Mercaptopurine Assay Standard762巯嘌呤杂质标准品Mercaptopurineimpurity standard496美索达嗪苯磺酸Mesoridazinebesilate226酒石酸美拉明Metaraminoltartrate227盐酸伊拉地平分析标准品Metforminhydrochloride Assay Standard552盐酸美沙酮分析标准品受控药物Methadonehydrochloride Assay Standard Controlled Substance232酮基布洛芬1-(2-Methoxyphenyl)piperazine644对乙酰氨基-邻甲氧基苯甲酸甲酯 Methyl4-acetamido-2-hydroxybenzoate6061-Methylazepan-4-onehydrochloride1-Methylazepan-4-onehydrochloride6051-Methyl-4-(2-benzoylhydrazino)azepanhydrochloride1-Methyl-4-(2-benzoylhydrazino)azepanhydrochloride2393-Methyl-2,2-diphenyl-4-piperidinobutyronitrile3-Methyl-2,2-diphenyl-4-piperi dinobutyronitrile240甲基多巴分析标准品Methyldopa AssayStandard241盐酸甲基多巴乙酯分析标准品Methyldopatehydrochloride Assay Standard569拉米夫定3-Methylflavone-8-carboxylicacid5703-Methylflavone-8-carboxylic acid ethylester3-Methylflavone-8-carboxylic acid ethylester4444-methyl-5-(2-hydroxyethyl)thiazole4-methyl-5-(2-hydroxyethyl)thiazole4572-Methyl-6-methylaminoheptan-2-ol2-Methyl-6-methylaminoheptan-2-ol4761-methyl-3-[2-(5-methylimidazol-4-yl-methylthio)ethyl]guanidine dihydrochloride1-methyl-3-[2-(5-methylimidazol-4-yl-methylthio)ethyl]guanidine dihydrochloride237MethylN-4[2-(5-chloro-2-methoxybenzamido)ethyl]benzenesulphonylcarbamateMethylN-4[2-(5-chloro-2-methoxybenzamido)ethyl]benzenesulphonylcarbamate244兰索拉唑2-Methyl-5-Nitroimidazole245(1-Methyl-5-nitroimidazol-2-yl)methanol(1-Methyl-5-nitroimidazol-2-yl)methano l641N-Methyl-N'-(2,4-xylyl)formamidinehydrochlorideN-Methyl-N'-(2,4-xylyl)formamidinehydrochloride662甲苯比妥分析标准品受控药物MethylphenobarbitalAssay Standard Controlled Substance248甲基强的松龙分析标准品MethylprednisoloneAssay Standard249左美丙嗪马来酸盐分析标准品Methylprednisoloneacetate Assay Standard846三盐酸化物左美丙嗪马来酸盐N-methyl-bis[beta-(2-pyridyl)ethyl]amine trihydrochloride4454-Methyl-5-vinylthiazoleedisilate4-Methyl-5-vinylthiazole edisilate409马来酸美西麦角分析标准品Methysergidemaleate Assay Standard642美替洛尔分析标准品Metipranolol AssayStandard357盐酸胃复安分析标准品Metoclopramidehydrochloride Assay Standard540酒石酸美托洛尔分析标准品Metoprololtartrate Assay Standard603甲硝唑分析标准品Metronidazole AssayStandard735苯酰甲硝唑Metronidazolebenzoate251美克西酮Mexenone252盐酸米安色林分析标准品Mianserinhydrochloride Assay Standard253硝酸咪康分析标准品Miconazole nitrateAssay Standard884咪康唑杂质AMiconazole impurityA722咪达唑仑分析标准品受控药物Midazolam AssayStandard Controlled Substance394盐酸米诺环素分析标准品Minocyclinehydrochloride Assay Standard637米诺地尔分析标准品Minoxidil AssayStandard842米氮平分析标准品Mirtazapine AssayStandard768莫美他松糠酸酯分析标准品Mometasone。
淫羊藿苷通过下调ATF6-DR5信号通路改善异丙肾上腺素所致小鼠左心室心肌细胞凋亡

-6674•中国药理学通报Chinsc PhmacoXgical Bulletin2022Doc;36(12):1674〜8网络出版时间:2220-16-315:45网络出版地址:htt p s://kns.cakh net/kcms/demil/34.1086.R.22221222.1516;418;html淫羊藿苷通过下调ATF6-DR5信号通路改善异丙肾上腺素所致小鼠左心室心肌细胞凋亡曾凡群,李晓彤,林小英,李叶丽,杨丹莉(遵义医科大学药学院,基础药理教育部重点实验室暨特色民族药教育部国际合作联合实验室,贵州遵义563099)doi:12.3969//Osn.1021-19782222.12.009文献标志码:A文章编号:1421-1978(2222)12-1674-25中国图书分类号:RA32;R284.1;R329.25;R329.411;R977.0摘要:目的以C57BL6(简称C57)小鼠为研究对象,并基于ATF6AR7信号通路研究淫羊藿苷(Oariin,ICA)对异丙肾上腺素(isoproterenW,ISO)诱导的左心室心肌细胞凋亡作用及机制。
方法34只6~7周龄的雄性C57小鼠连续14 d经颈背部皮下注射ISO(5my•ky-•d-)诱导心肌细胞凋亡,64只C57小鼠注射等体积生理盐水作为对照组。
注射ISO的小鼠随机分为3组(n=12):ISO组、、CAA组和ICAA组o ICA组分别灌胃给予ICA混悬液(15,64my•kg-•d-),连续14d o Control组和ISO组灌胃均给予等体积双蒸水。
末次给药结束后称全心重,分离左心室(含室间隔)称重,计算左心室质量指数。
TUNEL染色检测左心室心肌细胞凋亡情况;Western blot检测左心室GRP78、ATF6和DR7的蛋白水平。
结果与Control组相比,ISO组左心室质量指数明显升高(P<2.25);TUNEL染色结果发现,左心室心肌细胞凋亡率明显增加(P<2.45);Western blot结果发现,左心室GRP78、ATF6和DR7的蛋白水平明显升高(P< 2.25)°与ISO组相比,ICA高剂量组左心室质量指数明显降低(P<2.25);左心室心肌细胞凋亡率明显降低(P< 2.25);左心室GRP78、ATF6和DR7的蛋白水平明显降低(P <2.25)o结论ICA具有改善ISO所致的左心室心肌细胞凋亡作用,其机制可能与抑制ATF6AR7信号通路有关。
AMPICILLIN SODIUM 欧洲药典

Ampicillin sodium EUROPEAN PHARMACOPOEIA8.001/2008:0578corrected 6.0AMPICILLIN SODIUM AmpicillinumnatricumC 16H 18N 3NaO 4S M r 371.4[69-52-3]DEFINITIONSodium (2S ,5R ,6R )-6-[[(2R )-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate.Semi-synthetic product derived from a fermentation product.Content :91.0per cent to 102.0per cent (anhydrous substance).CHARACTERSAppearance :white or almost white powder,hygroscopic.Solubility :freely soluble in water,sparingly soluble in acetone,practically insoluble in fatty oils and in liquid paraffin.IDENTIFICATIONFirst identification:A,D.Second identification:B,C,D.A.Infrared absorption spectrophotometry (2.2.24).Preparation :dissolve 0.250g in 5mL of water R ,add 0.5mL of dilute acetic acid R ,swirl and allow to stand for 10min in iced water.Filter the crystals through a small sintered-glass filter (40)(2.1.2),applying suction,wash with 2-3mL of a mixture of 1volume of water R and 9volumes of acetone R ,then dry in an oven at 60°C for parison :ampicillin trihydrate CRS .B.Thin-layer chromatography (2.2.27).Test solution .Dissolve 25mg of the substance to be examined in 10mL of sodium hydrogen carbonate solution R .Reference solution (a).Dissolve 25mg of ampicillin trihydrate CRS in 10mL of sodium hydrogen carbonate solution R .Reference solution (b).Dissolve 25mg of amoxicillin trihydrate CRS and 25mg of ampicillin trihydrate CRS in 10mL of sodium hydrogen carbonate solution R .Plate :TLC silanised silica gel plate R .Mobile phase :mix 10volumes of acetone R and 90volumes of a 154g/L solution of ammonium acetate R previously adjusted to pH 5.0with glacial acetic acid R .Application :1μL.Development :over a path of 15cm.Drying :in air.Detection :expose to iodine vapour until the spots appear and examine in daylight.System suitability :reference solution (b):–the chromatogram shows 2clearly separated spots.Results :the principal spot in the chromatogram obtained with the test solution is similar in position,colour and size to the principal spot in the chromatogram obtained with reference solution (a).C.Place about 2mg in a test-tube about 150mm long and about 15mm in diameter.Moisten with 0.05mL of water R and add 2mL of sulfuric acid-formaldehyde reagent R .Mix the contents of the tube by swirling;the solution is practically colourless.Place the test-tube in a water-bath for 1min;a dark yellow colour develops.D.It gives reaction (a)of sodium (2.3.1).TESTSAppearance of solution .Solutions A and B are not more opalescent than reference suspension II (2.2.1)and the absorbance (2.2.25)of solution B at 430nm is not greater than 0.15.Place 1.0g in a conical flask and add slowly and with continuous swirling 10mL of 1M hydrochloric acid(solution A).Separately dissolve 1.0g in water R and dilute to 10.0mL with the same solvent (solution B).Examine immediately after dissolution.pH (2.2.3):8.0to 10.0.Dissolve 2.0g in carbon dioxide-free water R and dilute to 20mL with the same solvent.Measure 10min after dissolution.Specific optical rotation (2.2.7):+258to +287(anhydrous substance).Dissolve 62.5mg in a 4g/L solution of potassium hydrogen phthalate R and dilute to 25.0mL with the same solvent.Related substances .Liquid chromatography (2.2.29).Test solution (a).Dissolve 31.0mg of the substance to be examined in mobile phase A and dilute to 50.0mL with mobile phase A.Test solution (b).Dissolve 31.0mg of the substance to be examined in mobile phase A and dilute to 10.0mL with mobile phase A.Prepare immediately before use .Reference solution (a).Dissolve 27.0mg of anhydrousampicillin CRS in mobile phase A and dilute to 50.0mL with mobile phase A.Reference solution (b).Dissolve 2.0mg of cefradine CRS in mobile phase A and dilute to 50mL with mobile phase A.To 5.0mL of this solution add 5.0mL of reference solution (a).Reference solution (c).Dilute 1.0mL of reference solution (a)to 20.0mL with mobile phase A.Reference solution (d).To 0.20g of the substance to be examined add 1.0mL of water R .Heat the solution at 60°C for 1h.Dilute 0.5mL of this solution to 50.0mL with mobile phase A.Column :–size :l =0.25m,Ø=4.6mm;–stationary phase :octadecylsilyl silica gel for chromatography R (5μm).Mobile phase :–mobile phase A :mix 0.5mL of dilute acetic acid R ,50mL of 0.2M potassium dihydrogen phosphate R and 50mL of acetonitrile R ,then dilute to 1000mL with water R ;–mobile phase B :mix 0.5mL of dilute acetic acid R ,50mL of 0.2M potassium dihydrogen phosphate R and 400mL of acetonitrile R ,then dilute to 1000mL with water R ;Time (min)Mobile phase A (per cent V/V )Mobile phase B (per cent V/V )0-t R 8515t R -(t R +30)85→015→100(t R +30)-(t R +45)0100(t R +45)-(t R +60)8515t R =retention time of ampicillin determined with reference solution (c)If the mobile phase composition has been adjusted to achieve the required resolution,the adjusted composition will apply at time zero in the gradient and in the assay.Flow rate :1.0mL/min.Detection :spectrophotometer at 254nm.1564See the information section on general monographs (cover pages)EUROPEAN PHARMACOPOEIA 8.0AmpicillinsodiumInjection :50μL of reference solutions (b)and (c)withisocratic elution at the initial mobile phase composition and 50μL of test solution (b)and reference solution (d)according to the elution gradient described under Mobile phase;inject mobile phase A as a blank according to the elution gradient described under Mobile phase.Identification of peaks :use the chromatogram obtained with reference solution (d)to identify the peaks due to ampicillin and ampicillin dimer.Relative retention with reference to ampicillin:ampicillin dimer =about 2.8.System suitability :reference solution (b):–resolution :minimum 3.0between the peaks due toampicillin and cefradin;if necessary adjust the ratio A:B of the mobile phase.Limits :–ampicillin dimer :not more than 4.5times the area of the principal peak in the chromatogram obtained with reference solution (c)(4.5per cent);–any other impurity :for each impurity,not more than twice the area of the principal peak in the chromatogram obtained with reference solution (c)(2per cent).N,N -Dimethylaniline (2.4.26,Method B ):maximum 20ppm.2-Ethylhexanoic acid (2.4.28):maximum 0.8per cent m/m .Methylene chloride .Gas chromatography (2.2.28).Internal standard solution .Dissolve 1.0mL of ethylene chloride R in water R and dilute to 500.0mL with the same solvent.Test solution (a).Dissolve 1.0g of the substance to be examined in water R and dilute to 10.0mL with the same solvent.Test solution (b).Dissolve 1.0g of the substance to be examined in water R ,add 1.0mL of the internal standard solution and dilute to 10.0mL with water R .Reference solution.Dissolve 1.0mL of methylene chloride R in water R and dilute to 500.0mL with the same solvent.To 1.0mL of this solution add 1.0mL of the internal standard solution and dilute to 10.0mL with water R .Column :–material :glass;–size :l =1.5m,Ø=4mm;–stationary phase :diatomaceous earth for gaschromatography R impregnated with 10per cent m/m of macrogol 1000R .Carrier gas :nitrogen for chromatography R .Flow rate :40mL/min.Temperature :–column :60°C;–injection port :100°C;–detector :150°C.Detection :flame ionisation.Calculate the content of methylene chloride taking its density at 20°C to be 1.325g/mL.Limit :–methylene chloride :maximum 0.2per cent m/m .Heavy metals (2.4.8):maximum 20ppm.1.0g complies with test C.Prepare the reference solution using 2mL of lead standard solution (10ppm Pb)R .Water (2.5.12):maximum 2.0per cent,determined on 0.300g.Bacterial endotoxins (2.6.14):less than 0.15IU/mg,ifintended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins.ASSAYLiquid chromatography (2.2.29)as described in the test for related substances with the following modifications.Mobile phase :initial composition of the mixture of mobile phases A and B,adjusted where applicable.Injection :test solution (a)and reference solution (a).System suitability :reference solution (a):–repeatability :maximum relative standard deviation of 1.0per cent after 6injections.Calculate the percentage content of ampicillin sodium by multiplying the percentage content of ampicillin by 1.063.STORAGEIn an airtight container.If the substance is sterile,store in a sterile,airtight,tamper-proof container.IMPURITIESA.(2S ,5R ,6R )-6-amino-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (6-aminopenicillanicacid),B.(2S ,5R ,6R )-6-[[(2S )-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (L-ampicillin),C.(4S )-2-(3,6-dioxo-5-phenylpiperazin-2-yl)-5,5-dimethylthiazolidine-4-carboxylic acid (diketopiperazines ofampicillin),D.R =CO 2H:(4S )-2-[[[(2R )-2-amino-2-phenylacetyl]amino]-carboxymethyl]-5,5-dimethylthiazolidine-4-carboxylic acid (penicilloic acids of ampicillin),F.R =H:(2RS ,4S )-2-[[[(2R )-2-amino-2-phenylacetyl]amino]-methyl]-5,5-dimethylthiazolidine-4-carboxylic acid (penilloic acids ofampicillin),E.(2R )-2-[[[(2S ,5R ,6R )-6-[[(2R )-2-amino-2-phenylacetyl]-amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo-[3.2.0]hept-2-yl]carbonyl]amino]-2-phenylacetic acid (ampicillinyl-D -phenylglycine),General Notices (1)apply to all monographs and other texts1565Ampicillin trihydrate EUROPEAN PHARMACOPOEIA8.0G.(3R ,6R)-3,6-diphenylpiperazine-2,5-dione,H.3-phenylpyrazin-2-ol,I.(2S ,5R ,6R )-6-[[(2R )-2-[[(2R )-2-amino-2-phenylacetyl]-amino]-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid (D-phenylglycylampicillin),J.(2S ,5R ,6R )-6-[(2,2-dimethylpropanoyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylicacid,K.(2R )-2-[(2,2-dimethylpropanoyl)amino]-2-phenylaceticacid,L.(2R )-2-amino-2-phenylacetic acid (D-phenylglycine),M.co-oligomers of ampicillin and of penicilloic acids ofampicillin,N.oligomers of penicilloic acids of ampicillin.01/2008:0168corrected 6.0AMPICILLIN TRIHYDRATE AmpicillinumtrihydricumC 16H 19N 3O 4S,3H 2O M r 403.5[7177-48-2]DEFINITION(2S ,5R ,6R )-6-[[(2R )-2-Amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate.Semi-synthetic product derived from a fermentation product.Content :96.0per cent to 102.0per cent (anhydrous substance).CHARACTERSAppearance :white or almost white,crystalline powder.Solubility :slightly soluble in water,practically insoluble in ethanol (96per cent)and in fatty oils.It dissolves in dilute solutions of acids and of alkali hydroxides.IDENTIFICATIONFirst identification:A,D .Second identification:B,C,D .A.Infrared absorption spectrophotometry (2.2.24).Comparison :ampicillin trihydrate CRS .B.Thin-layer chromatography (2.2.27).Test solution .Dissolve 25mg of the substance to be examined in 10mL of sodium hydrogen carbonate solution R .Reference solution (a).Dissolve 25mg of ampicillin trihydrate CRS in 10mL of sodium hydrogen carbonate solution R .Reference solution (b).Dissolve 25mg of amoxicillin trihydrate CRS and 25mg of ampicillin trihydrate CRS in 10mL of sodium hydrogen carbonate solution R .Plate :TLC silanised silica gel plate R .Mobile phase :mix 10volumes of acetone R and 90volumes of a 154g/L solution of ammonium acetate R previously adjusted to pH 5.0with glacial acetic acid R .Application :1μL.Development :over a path of 15cm.Drying :in air.Detection :expose to iodine vapour until the spots appear and examine in daylight.System suitability :reference solution (b):–the chromatogram shows 2clearly separated spots.Results :the principal spot in the chromatogram obtained with the test solution is similar in position,colour and size to the principal spot in the chromatogram obtained with reference solution (a).1566See the information section on general monographs (cover pages)。
Potassium Phosphate Dibasic Trihydrate产品说明书

Potassium phosphate dibasic trihydrate Product Number P 5504Store at Room TemperatureProduct DescriptionMolecular Formula: K2HPO4• 3H2OMolecular Weight: 228.2CAS Number: 16788-57-1Synonyms: dipotassium phosphate, potassium biphosphate, sec-potassium phosphate, dipotassium hydrogen phosphate trihydratePotassium phosphate is a reagent with very high buffering capacity that is widely used in molecular biology, biochemistry, and chromatography. Potassium phosphate occurs in several forms: monobasic (KH2PO4), dibasic (K2HPO4), and tribasic (K3PO4). Most neutral potassium phosphate buffer solutions consist of mixtures of the monobasic and dibasic forms to varying degrees, depending on the desired pH. A table for preparation of 0.1 M potassium phosphate buffer at 25 °C using various proportions of potassium phosphate monobasic and potassium phosphate dibasic has been published.1,2 Some limitations of the usefulness of phosphate buffers include their precipitation of Ca2+ and Mg2+, their inhibition of restriction enzyme activity, and their interference in protocols related to DNA ligation and bacterial transformation.1 A study of the effect of freeze-thaw storage cycles on proteins in potassium phosphate and sodium phosphate buffer solutions has been reported.3The use of high concentrations of potassium phosphate in the immobilization of affinity ligands onto epoxide-activated stationary phases has been reviewed.4 A two-phase system of aqueous potassium phosphate and poly(ethylene glycol) for the isolation of E. coliβ-galactosidase and β-galactosidase fusion proteins has been published.5 The quantitation of nonionic surfactants in buffered solutions using strong cation and anion exchange HPLC guard columns and potassium phosphate solution has been investigated.6 Precautions and DisclaimerFor Laboratory Use Only. Not for drug, household or other uses.Preparation InstructionsThis product is soluble in water (100 mg/ml), yielding a clear, colorless solution.References1. Molecular Cloning: A Laboratory Manual, 3rd ed.,Sambrook, J. F., et al., Cold Spring HarborLaboratory Press (Cold Spring Harbor, NY: 2001), p. A1.5.2. Green, A. A., and Hughes, W. L., ProteinFractionation on the Basis of Solubility in Aqueous Solutions of Salts and Organic Solvents. Meth.Enzymol., 1, 67-90 (1955).3. Pikal-Cleland, K. A., et al., Protein denaturationduring freezing and thawing in phosphate buffersystems: monomeric and tetrameric beta-galactosidase. Arch. Biochem. Biophys., 384(2),398-406 (2000).4. Wheatley, J. B., and Schmidt, D. E. Jr., Salt-induced immobilization of affinity ligands ontoepoxide-activated supports. J. Chromatogr. A.,849(1), 1-12 (1999).5. Enfors, S. O., et al., Combined use of extractionand genetic engineering for protein purification:recovery of beta-galactosidase fused proteins.Bioseparation, 1(3-4), 305-310 (1990).6. Pardue, K., and Williams, D., Quantitativedetermination of non-ionic surfactants in proteinsamples, using ion-exchange guard columns.Biotechniques, 14(4), 580-583 (1993).GCY/RXR 1/03Sigma brand products are sold through Sigma-Aldrich, Inc.Sigma-Aldrich, Inc. warrants that its products conform to the information contained in this and other Sigma-Aldrich publications. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see reverse side ofthe invoice or packing slip.。
BP2015英国药典索引
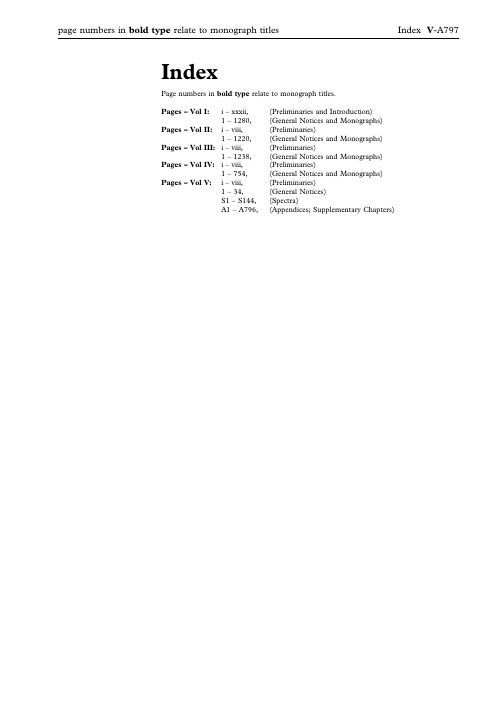
page numbers in bold type relate to monograph titles Index V-A797IndexPage numbers in bold type relate to monograph titles.Pages–Vol I:i–xxxii,(Preliminaries and Introduction)1–1280,(General Notices and Monographs)Pages–Vol II:i–viii,(Preliminaries)1–1220,(General Notices and Monographs)Pages–Vol III:i–viii,(Preliminaries)1–1238,(General Notices and Monographs)Pages–Vol IV:i–viii,(Preliminaries)1–754,(General Notices and Monographs)Pages–Vol V:i–viii,(Preliminaries)1–34,(General Notices)S1–S144,(Spectra)A1–A796,(Appendices;Supplementary Chapters)AAbacavir,V-S4Abacavir Oral Solution,III-85 Abacavir Sulfate,I-39Abacavir Tablets,III-86 Abbreviated,V-598Adjectives,V-598Anions,V-598Cations,V-598Preparations,V-598Titles of Monographs,V-598 Abbreviated Titles,Status of,I-7,II-7, III-7,IV-7,V-7Abbreviations and symbols,I-30,II-30, III-30,IV-30,V-30Abnormal Toxicity,Test for,V-409 About,definition of,I-5,II-5,III-5,IV-5,V-5Absence of Mycoplasmas,Test forV-487Absolute Ethanol,V-A61Absolute Ethanol R1,V-A62 Absorbent Cotton,IV-743Absorbent Viscose Wadding,IV-744 Absorption spectrophotometry,infrared, V-162Absorption Spectrophotometry, Ultraviolet and Visible,V-169 Acacia,I-41,V-A19Acacia Solution,V-A19Acacia Spray-dried,I-42 Acamprosate Calcium,I-43 Acanthopanax Bark,IV-49 Acarbose,I-44Accuracy,V-674Acebutolol Capsules,III-87 Acebutolol Hydrochloride,I-46,V-S5, V-A19Acebutolol Tablets,III-88 Aceclofenac,I-48Acemetacin,I-50Acenocoumarol,I-52,V-S5 Acenocoumarol Tablets,III-88 Acesulfame Potassium,I-52Acetal,V-A19Acetaldehyde,V-A19Acetaldehyde Ammonia Trimer Trihydrate,V-A20Acetaldehyde Standard Solution(100ppm C2H4O),V-A148 Acetaldehyde Standard Solution(100ppm C2H4O)R1,V-A148 Acetamide,V-A20Acetate Buffer pH2.8,V-A152 Acetate Buffer pH2.45,V-A152 Acetate Buffer pH3.4,V-A152 Acetate Buffer pH3.5,V-A152 Acetate Buffer pH3.7,V-A152 Acetate Buffer pH4.4,V-A152 Acetate Buffer pH4.6,V-A152 Acetate Buffer pH5.0,V-A152 Acetate Buffer pH6.0,V-A152 Acetate Buffer Solution pH4.7R1,V-A153Acetate Buffer Solution pH4.4,see Acetate Buffer pH4.4Acetate Buffer Solution pH4.6,see Acetate Buffer pH4.6Acetate Buffer Solution pH6.0,seeAcetate Buffer pH6.0Acetate Buffer Solution pH4.4,V-A152Acetate Buffer Solution pH4.5,V-A152Acetate Buffer Solution pH4.7,V-A152Acetate Buffer Solution pH5.0,V-A153Acetate Buffer Solution pH6.0,V-A153Acetate–edetate Buffer Solution pH5.5,V-A153Acetates,Reactions of,V-266Acetazolamide,I-54,V-S5Acetazolamide Oral Suspension,III-89Acetazolamide Tablets,III-90Acetic Acid,V-A20Acetic Acid(6per cent),I-56Acetic Acid(33per cent),I-56Acetic Acid,Anhydrous,V-A20Acetic Acid,Deuterated,V-A50Acetic Acid,Dilute,V-A20Acetic Acid,Dilute,see Acetic Acid(6per cent)Acetic Acid,Glacial,I-55,V-A20Acetic Acid in Synthetic Peptides,Determination of,V-299Acetic Acid VS,V-A142Acetic Acid,see Acetic Acid(33per cent)Acetic Anhydride,V-A20Acetic Anhydride Solution R1,V-A20Acetic Anhydride–Dioxan Solution,V-A20Acetic Anhydride–Sulfuric Acid Solution,V-A20Acetic Anhydride–Sulphuric AcidSolution,see Acetic Anhydride–SulfuricAcid SolutionAcetic Bromine Solution,V-A34Acetone,I-57,V-A20Acetone,Deuterated,V-A50Acetone Solution,Buffered,V-A153Acetone-dried Ox Brain,V-A98Acetonitrile,V-A20Acetonitrile for Chromatography,V-A20Acetonitrile R1,V-A20Acetoxyvalerenic Acid,V-A20Acetyl Chloride,V-A20Acetyl Groups,Reactions of,V-266Acetyl Salicylic Acid see AspirinAcetyl Value,Determination of,V-317Acetylacetamide,V-A20Acetylacetone,V-A20Acetylacetone Reagent R1,V-A20Acetylacetone Reagent R2,V-A204-Acetylbiphenyl,V-A20O-Acetyl Groups in PolysaccharideVaccines,V-467N-Acetyl-e-caprolactam,V-A20Acetylcholine Chloride,I-58,V-A20Acetylcysteine,I-59,V-S6Acetylcysteine Eye Drops,III-90Acetylcysteine Injection,III-91Acetyldigoxin,I-61b-Acetyldigoxin see AcetyldigoxinAcetyleugenol,V-A20N-Acetylglucosamine,V-A21Acetyl-11-keto-b-boswellic Acid,V-A21N-Acetyl-L-cysteine,V-A20N-Acetylneuraminic Acid,V-A21Acetylsalicylic Acid Tablets,see AspirinTabletsN-Acetyltryptophan,V-A21N-Acetyltryptophan see AcetyltryptophanAcetyltryptophan,I-63Acetyltyrosine,I-65N-Acetyltyrosine see AcetyltyrosineAcetyltyrosine Ethyl Ester,V-A21Acetyltyrosine Ethyl Ester,0.2M,V-A21Aciclovir,I-67Aciclovir Cream,III-93Aciclovir Eye Ointment,III-94Aciclovir Infusion,III-95Aciclovir Intravenous Infusion,seeAciclovir Infusion,Aciclovir Oral Suspension,III-97Aciclovir Sodium for Infusion,III-95Aciclovir Sodium for IntravenousInfusion,see Aciclovir Sodium forInfusion,Aciclovir Tablets,III-98Aciclovir Tablets,Dispersible,III-99Acid Blue92,V-A21Acid Blue92Solution,V-A21Acid Blue83,V-A21Acid Blue93Solution,V-A21Acid Blue90,V-A21Acid Gentian Mixture,IV-197Acid Gentian Oral Solution,IV-197Acid Potassium IodobismuthateSolution,V-A108Acid Value,V-317Acid/base Indicators,V-789Acid-base titrations,V-788Acidified Chloroform,V-A41Acidified Dichloromethane,V-A52Acidified Methanol,V-A85Acidified Methylene Chloride,seeAcidified DichloromethaneAcid-insoluble Ash,Determination of,V-336Acid-washed Diatomaceous Support,V-A51Acitretin,I-69Acitretin Capsules,III-100Acknowledgements,I-xxviiAcrylamide,V-A21Acrylamide/bisacrylamide(29:1)Solution,30per cent,V-A21Acrylamide/bisacrylamide(36.5:1)Solution,30per cent,V-A21Acrylic Acid,V-A21Actein,V-A21Acteoside,V-A21Action and Use Statement,Status of,I-17,II-17,III-17,IV-17,V-17Activated Acid Aluminium Oxide,V-A23Activated Attapulgite,I-220Activated Charcoal,I-496,V-A40Activated Zinc,V-A140Active Moiety,V-651Adamantane,V-A21Adapalene,I-71Adapalene Cream,III-101Adapalene Gel,III-103Additions,List of,I-xxviiiAdditions,List of Monographs,I-xxiiAdditives,Plastic,V-592Adenine,I-72,V-A21Adenosine,I-73,V-A21Adipic Acid,I-75,V-A21Adrenaline,V-A21Adrenaline/Epinephrine,I-76page numbers in bold type relate to monograph titles Index V-A799Adrenaline Acid Tartrate,V-A21 Adrenaline Acid Tartrate/Epinephrine Acid Tartrate,I-77Adrenaline and Cocaine Intranasal Solution,III-107Adrenaline(Epinephrine),V-S6 Adrenaline Eye Drops,Epinephrine Eye Drops,Neutral,III-104Adrenaline Eye Drops/Epinephrine Eye Drops,III-104Adrenaline Injection,Bupivacaine and, III-220Adrenaline Injection,Dilute(1in10,000),III-106Adrenaline Injection,Lidocaine and,III-751Adrenaline Injection/Epinephrine Injection,III-105Adrenaline Solution/Epinephrine Solution,III-106Adrenaline Tartrate see Adrenaline Acid TartrateAdrenaline TartrateInjection/Epinephrine Tartrate Injection,III-105Adrenaline TartrateSolution/Epinephrine Tartrate Solution,III-106Adrenalone Hydrochloride,V-A21 Adsorbed Diphtheria and Tetanus Vaccine,IV-537Adsorbed Diphtheria and Tetanus Vaccine for Adults and Adolescents, see Adsorbed Diphtheria and Tetanus Vaccine(adsorbed,Reduced Antigen(s) Content)Adsorbed Diphtheria,Tetanus and Pertussis(Acellular Component) Vaccine,IV-541Adsorbed Diphtheria,Tetanus,Pertussis (Acellular Component)and Haemophilus Type b Conjugate Vaccine,IV-545Adsorbed Diphtheria,Tetanus,Pertussis (Acellular Component)and Hepatitis B(rDNA)Vaccine,IV-547 Adsorbed Diphtheria,Tetanus,Pertussis (Acellular Component)and Inactivated Poliomyelitis Vaccine,IV-548Adsorbed Diphtheria Vaccine,IV-534 Adsorbed Diphtheria Vaccine for Adults and Adolescents,see Diphtheria Vaccine (Adsorbed,Reduced Antigen Content) Adsorbed Pertussis Vaccine(Acellular Component),IV-604Adsorbed Pertussis Vaccine(Acellular, Co-purified),IV-605Adsorbed Tetanus Vaccine,IV-633 Adsorbed Vaccines,Aluminium in,V-463Adsorbed Vaccines,Calcium in,V-464 Adsorption,Gas,Specific Surface Area By(2.9.26.)(5.8.),V-701Aescin,V-A22Aflatoxin B1,V-A22Aflatoxin B,in Herbal Drugs, Determination of,V-341Agar,I-79,V-A22Agarose for Chromatography,V-A22Agarose for Chromatography,Cross-linked,V-A22Agarose for Chromatography R1,Cross-linked,V-A22Agarose for Electrophoresis,V-A22Agarose/Cross-linked Polyacrylamide,V-A22Agarose-DEAE for Ion ExchangeChromatography,V-A22Agnus Castus Fruit,IV-50Agrimony,IV-52Air,Medical,I-78Air,Medicinal see Medical AirAir Permeability,Specific Surface Areaby,V-505Air,Synthetic,I-81Air,Synthetic Medicinal see Synthetic AirAlanine,I-83,V-A22ß-Alanine,see3-Aminopropionic AcidAlbendazole,I-84Albumin,Bovine,V-A22Albumin,Bovine R1,V-A22Albumin,Human,V-A22Albumin Solution,IV-467Albumin Solution,Human,V-A22Albumin Solution R1,Human,V-A22Alchemilla,IV-53Alcohol(20per cent),I-900Alcohol(25per cent),I-900Alcohol(45per cent),I-900Alcohol(50per cent),I-900Alcohol(60per cent),I-900Alcohol(70per cent),I-900Alcohol(80per cent),I-900Alcohol(90per cent),I-900Alcohol,Aldehyde-free,see Ethanol(96%),Aldehyde-freeAlcoholic Calcium Standard Solution(100ppm Ca),V-A149Alcoholic DimethylaminobenzaldehydeSolution,V-A55Alcoholic Hydroxylamine Solution,V-A74Alcoholic Iodine Solution,III-696,V-A75Alcoholic Potassium Hydroxide,2M,V-A108Alcoholic Potassium Hydroxide,seePotassium Hydroxide VS,EthanolicAlcoholic Potassium Hydroxide Solution,V-A108Alcoholic Potassium Hydroxide SolutionR1,V-A108Alcoholic Solution of Sulfuric Acid,V-A128Alcoholic Sulfuric Acid,0.25M,V-A128Alcoholimetric Tables,V-687Alcohol,see Ethanol(96%)Alcuronium Chloride,I-85Aldehyde Dehydrogenase,V-A22Aldehyde Dehydrogenase Solution,V-A22Aldehyde-free alcohol,see Ethanol(96%),Aldehyde-freeAldehyde-free Ethanol(96%),V-A62Aldehyde-free Methanol,V-A85Aldehydes,Determination of,V-321Aldrin,V-A22Alendronate Sodium Tablets,seeAlendronic Acid TabletsAlendronic Acid Tablets,III-109Aleuritic Acid,V-A22Alexandrian Senna Fruit,IV-362Alfacalcidol,I-87Alfadex,I-88Alfentanil Hydrochloride,I-89Alfuzosin,V-S7Alfuzosin Hydrochloride,I-91Alfuzosin Tablets,III-111Alfuzosin Tablets,Prolonged-release,III-112Alginate Antacid Oral Suspension,Compound,III-113Alginate Oral Suspension,Raft-forming,III-114Alginate Raft-forming Oral Suspension,III-114Alginic Acid,I-92Alimemazine,V-S7Alimemazine Oral Solution,Paediatric,III-115Alimemazine Tablets,III-116Alimemazine Tartrate,I-93Alizarin S,V-A22Alizarin S Solution,V-A22Alkaline Corallin Solution,V-A46Alkaline Eye Drops,see Hypromellose EyeDropsAlkaline Gentian Mixture,IV-198Alkaline Gentian Oral Solution,IV-198Alkaline Hydroxylamine Solution,V-A74Alkaline Hydroxylamine Solution R1,V-A74Alkaline Potassium Mercuri-iodideSolution,V-A109Alkaline Potassium TetraiodomercurateSolution,V-A109Alkaline Pyrogallol Solution,V-A111Alkaline Sodium Picrate Solution,V-A124Alkaline Tetrazolium Blue Solution,V-A132Alkali-washed Diatomaceous Support,V-A51Alkaloids,Complete Extraction of,V-335Alkaloids,Reactions of,V-266all--Alpha-Tocopherol,II-1051Allantoin,I-94,V-A23Allergen Products,I-95Allium Sativum for HomoeopathicPreparations,IV-427Allopurinol,I-98Allopurinol Oral Suspension,III-117Allopurinol Tablets,III-118all-rac-Alpha-Tocopheryl Acetate,II-1054all-rac-a-Tocopheryl see all-rac-Alpha-Tocopherylall-rac-Tocopheryl Acetate see all-rac-Alpha-Tocopheryl AcetateAlmagate,I-100Almond Oil Ear Drops,III-119Almond Oil,Refined,I-100Almond Oil,Virgin,I-99Almond Oil see Virgin Almond OilAloes,Barbados,IV-53Aloes,Cape,IV-54Alovudine,V-A23Alovudine(18F)Injection,IV-669V-A800IndexAloxiprin,I-102Aloxiprin Tablets,III-119Alpha Tocopheryl Acetate Concentrate (Powder Form),II-1057Alpha Tocopheryl Hydrogen Succinate, II-1058Alpha Tocopheryl Succinate Tablets,III-1164Alphacyclodextrin see Alfadex Alprazolam,I-103Alprenolol Hydrochloride,I-105 Alprostadil,I-107Alteplase for Injection,I-109 Alternative methods,I-20,II-20,III-20, IV-20,V-20Alternative Methods for Control of Microbiological Quality,V-745 Altizide,I-113Alum,I-114Aluminium,V-A23Aluminium Acetate Ear Drops,III-120 Aluminium Chloride,V-A23 Aluminium Chloride Hexahydrate,I-114 Aluminium Chloride Reagent,V-A23 Aluminium Chloride Solution,III-121, V-A23Aluminium Glycinate,I-115 Aluminium Hydroxide and Magnesium Trisilicate Tablets,Chewable,III-776 Aluminium Hydroxide,Dried,I-115 Aluminium Hydroxide Gel,V-A23 Aluminium Hydroxide,Hydrated for Adsorption,I-114Aluminium Hydroxide Oral Suspension, III-121Aluminium Hydroxide Oral Suspension, Magnesium Hydroxide and,III-401 Aluminium Hydroxide Tablets, Chewable,III-122Aluminium Hydroxide Tablets, Magnesium Hydroxide and,III-402 Aluminium Hydroxide Tablets see Chewable Aluminium Hydroxide Tablets,III-122Aluminium in Adsorbed Vaccines,V-463 Aluminium Magnesium Silicate,I-118 Aluminium Nitrate,V-A23Aluminium Oxide,Activated Acid,V-A23Aluminium Oxide,Anhydrous,V-A23 Aluminium Oxide,Basic,V-A23 Aluminium Oxide,Deactivated,V-A23 Aluminium Oxide G,V-A23 Aluminium Oxide,Neutral,V-A23 Aluminium Paste,Compound,III-120 Aluminium Phosphate Gel,I-120 Aluminium Phosphate,Hydrated see Dried Aluminium Phosphate Aluminium Potassium Sulfate,V-A23 Aluminium Potassium Sulphate,see Aluminium Potassium Sulfate Aluminium Powder,I-121Aluminium Salts,Reactions of,V-266 Aluminium Sodium Silicate,I-122 Aluminium Standard Solution(2ppm Al),V-A148Aluminium Standard Solution(10ppm Al),V-A148Aluminium Standard Solution(100ppm Al),V-A148Aluminium Standard Solution(200ppmAl),V-A148Aluminium Stearate,I-123Aluminium Sulfate,I-125,V-A23Aluminium Sulphate,see AluminiumSulfateAlverine Capsules,III-122Alverine Citrate,I-126,V-S7Amantadine Capsules,III-123Amantadine Hydrochloride,I-127Amantadine Oral Solution,III-124Amantidine,V-S8Amaranth S,V-A23Amaranth Solution,V-A23Ambroxol Hydrochloride,I-128Americium-243Spiking Solution,V-A23Amethocaine Eye Drops,see TetracaineEye DropsAmfetamine Sulfate,I-130Amfetamine Sulphote,see AmfetamineSulfate,I-130Amido Black10B Solution,V-A23Amidohexadecylsilyl Silica Gel forchromatography,V-A115Amidotrizoic Acid Dihydrate,I-130Amikacin,I-132Amikacin Injection,III-124Amikacin Sulfate,I-135Amiloride and Furosemide Tablets,seeCo-amilofruse TabletsAmiloride and Hydrochlorothiazide OralSolution,see Co-amilozide Oral SolutionAmiloride and HydrochlorothiazideTablets,see Co-amilozide TabletsAmiloride Hydrochloride,I-138Amiloride Tablets,III-125Amines,Primary Aromatic,Reactions of,V-266Amino Acid Analysis,V-221Amino Acid Analysis(2.2.56.)(5.8.),V-700Amino Acids,Use of Codes for,I-8,II-8,III-8,IV-8,V-8Aminoazobenzene,V-A23Aminobenzoic Acid,I-139,V-A23,V-A244-Aminobenzoic Acid Solution,V-A24(4-Aminobenzoyl)-L-glutamic Acid,V-A244-Aminobutanoic acid,see4-Amino-n-butyric Acid2-Aminobutan-1-ol,V-A24Aminocaproic Acid,I-1402-Amino-5-chlorobenzophenone,V-A24Aminochlorobenzophenone,see2-Amino-5-chlorbenzophenone4-Aminofolic Acid,V-A24Aminoglutethimide,I-141,V-S8Aminoglutethimide Tablets,III-126Aminohexadecylsilyl Silica Gel forChromatography,V-A1156-Aminohexanoic Acid,V-A24p-Aminohippuric Acid,V-A24Aminohippuric Acid Reagent,V-A244-Amino-3-hydroxynaphthalene-1-sulfonic Acid,V-A24Aminohydroxynaphthalenesulfonic AcidSolution,V-A24Aminohydroxynaphthalenesulfonic AcidSolution,Strong,V-A24AminohydroxynaphthalenesulphonicAcid Solution,Strong,seeAminohydroxynaphthalenesulfonic AcidSolution,StrongAminohydroxynaphthalenesulphonicAcid Solution,seeAminohydroxynaphthalenesulfonic AcidSolution4-Amino-3-hydroxynaphthalene-1-sulphonic Acid,see4-Amino-3-hydroxynaphthalene-1-sulfonic AcidAminohydroxynaphthalenesulphonic,Acid,see Aminonaphthalenesulfonic AcidSolution5-Aminoimidazole-4-carboxamideHydrochloride,V-A24cis-Aminoindanol,V-A24Aminomethylalizarindiacetic AcidReagent,V-A24Aminomethylalizarindiacetic AcidSolution,V-A253-Aminomethylalizarin-N,N-diaceticAcid,V-A244-Aminomethylbenzoic acid,V-A253-(Aminomethyl)pyridine,V-A258-Aminonaphthalene-2-sulfonic Acid,V-A25Aminonaphthalenesulfonic AcidSolution,V-A25Aminonaphthalenesulphonic AcidSolution,see AminonaphthalenesulfonicAcid Solution8-Aminonaphthalene-2-sulphonic Acid,see8-Aminonaphthalene-2-sulfonic Acid4-Amino-n-butyric Acid,V-A242-Amino-5-nitrobenzophenone,V-A25Aminonitrobenzophenone,see2-Amino-5-nitrobenzophenone4-Aminophenazone,V-A25Aminophenazone Solution,V-A253-Aminophenol,V-A254-Aminophenol-free Paracetamol,V-A99Aminophylline,I-143Aminophylline Hydrate,I-145Aminophylline Injection,III-128Aminophylline Tablets,III-128Aminophylline Tablets,Prolonged-release,III-129Aminopolyether,V-A253-Aminopropanol,V-A253-Aminopropionic Acid,V-A25Aminopropylmethylsilyl Silica Gel forChromatography,V-A115Aminopropylsilyl Silica Gel forChromatography,V-A115Aminopropylsilyl Silica Gel forChromatography R1,V-A115Aminopyrazolone,see4-AminophenazoneAminopyrazolone Solution,seeAminophenazone Solution3-Aminosalicylic Acid,V-A25Amiodarone,V-S8Amiodarone Concentrate,Sterile,III-130Amiodarone Hydrochloride,I-147Amiodarone Infusion,III-129Amiodarone Intravenous Infusion,seeAmiodarone Infusion,Amiodarone Oral Suspension,III-131Amiodarone Sterile Concentrate,III-130page numbers in bold type relate to monograph titles Index V-A801Amiodarone Tablets,III-132 Amisulpride,I-149,V-S9Amisulpride Oral Solution,III-133 Amisulpride Tablets,III-134 Amitriptyline Embonate,I-150 Amitriptyline Hydrochloride,I-151 Amitriptyline Tablets,III-135 Amlodipine Besilate,I-153 Ammonia,V-A25Ammonia(13N)Injection,IV-672 Ammonia Buffer pH9.5,see Ammonium Chloride Buffer Solution pH9.5 Ammonia Buffer pH10.9,V-A153 Ammonia Buffer pH10.9,Dilute,V-A153Ammonia Buffer pH10.0,V-A153 Ammonia,Chloride-free,V-A25 Ammonia,Concentrated,V-A25 Ammonia,Lead-free,V-A25 Ammonia,Methanolic,V-A25 Ammonia R1,Concentrated,V-A26 Ammonia R1,Dilute,V-A26 Ammonia R2,Dilute,V-A26 Ammonia R3,Dilute,V-A26 Ammonia Solution,Aromatic,III-136 Ammonia Solution,Concentrated see Strong Ammonia SolutionAmmonia Solution,Dilute,III-137 Ammonia Spirit,Aromatic,III-137 Ammoniacal Copper Oxide Solution,V-A46Ammoniacal Silver Nitrate Solution,V-A120Ammoniacal Solution of Copper Tetrammine,V-A46Ammonia-free Water,V-A139 Ammonio Methacrylate Copolymer (Type A),I-155Ammonio Methacrylate Copolymer (Type B),I-1560.5M Ammonium acetate buffer solution pH4.5,see Ammonium acetate buffer pH4.5,0.5M0.01M Ammonium and Cerium Nitrate, see Ammonium Cerium(IV)Nitrate VS 0.1M Ammonium and Cerium Sulfate, see Ammonium Cerium(IV)Sulfate VS Ammonium Acetate,V-A26 Ammonium acetate buffer pH4.5,0.5M, V-A153Ammonium Acetate Solution,V-A26 Ammonium Acetate Solution,Strong,III-137Ammonium and Cerium Nitrate,see Ammonium Cerium(IV)Nitrate Ammonium and Cerium Sulfate,see Ammonium Cerium(IV)Sulfate Ammonium and Cerium Sulphate,see Ammonium and Cerium Sulfate Ammonium Bicarbonate,I-157 Ammonium Bromide,I-158 Ammonium Carbamate,V-A26 Ammonium Carbonate,V-A26 Ammonium Carbonate Buffer Solution pH10.3,0.1M,V-A153Ammonium Carbonate Solution,V-A26 Ammonium Carbonate Solution,Dilute, V-A26Ammonium carbonate solution R1,V-A26Ammonium Cerium(IV)Nitrate,V-A26Ammonium Cerium(IV)Nitrate VS,V-A142Ammonium Cerium(IV)Sulfate,V-A26Ammonium Cerium(IV)Sulfate VS,V-A142Ammonium Cerium(IV)Sulphate VS,seeAmmonium Cerium(IV)Sulfate VSAmmonium Cerium(IV)Sulphate,seeAmmonium Cerium(iv)SulfateAmmonium Chloride,I-159,V-A26Ammonium Chloride Buffer SolutionpH10.0,see Ammonia Buffer pH10.0Ammonium Chloride Buffer SolutionpH10.4,V-A153Ammonium Chloride Buffer SolutionpH10.7,V-A153Ammonium Chloride Buffer SolutionpH9.5,V-A153Ammonium Chloride Buffer SolutionpH10.0,V-A153Ammonium Chloride Mixture,III-137Ammonium Chloride Oral Solution,III-137Ammonium Chloride Solution,V-A26Ammonium Citrate,V-A26Ammonium Citrate Solution,V-A26Ammonium CobaltothiocyanateSolution,V-A26Ammonium DihydrogenOrthophosphate,V-A26Ammonium Formate,V-A26Ammonium Glycyrrhizinate,I-160Ammonium Hexafluorogermanate,V-A26Ammonium Hydrogen Carbonate,V-A26Ammonium Hydrogen Carbonate seeAmmonium BicarbonateAmmonium Ichthosulphonate seeIchthammolAmmonium Iron(II)Sulfate,V-A26Ammonium Iron(II)Sulfate VS,V-A142Ammonium Iron(II)Sulphate VS,seeAmmonium Iron(II)Sulfate VSAmmonium Iron(II)Sulphate,seeAmmonium Iron(ii)SulfateAmmonium Iron(III)Citrate,V-A26Ammonium Iron(III)Sulfate,V-A26Ammonium Iron(III)Sulfate Solution R1,V-A26Ammonium Iron(III)Sulfate Solution R2,V-A26Ammonium Iron(III)Sulfate Solution R5,V-A26Ammonium Iron(III)Sulfate Solution R6,V-A27Ammonium Iron(III)Sulfate VS,V-A142Ammonium Iron(III)Sulphate SolutionR1,see Ammonium Iron(iii)SulfateSolution R1Ammonium Iron(III)Sulphate SolutionR2,see Ammonium Iron(iii)SulfateSolution R2Ammonium Iron(III)Sulphate SolutionR5,see Ammonium Iron(iii)SulfateSolution R5Ammonium Iron(III)Sulphate VS,seeAmmonium Iron(III)Sulfate VSAmmonium Iron(III)Sulphate,seeAmmonium Iron(iii)SulfateAmmonium Mercaptoacetate Solution,V-A27Ammonium Mercurithiocyanate Reagent,V-A27Ammonium Metavanadate,V-A27Ammonium Metavanadate Solution,V-A27Ammonium Molybdate,V-A27Ammonium Molybdate Reagent,V-A27Ammonium Molybdate Reagent R1,V-A27Ammonium Molybdate Reagent R2,V-A27Ammonium Molybdate Solution,V-A27Ammonium Molybdate Solution R2,V-A27Ammonium Molybdate Solution R3,V-A27Ammonium Molybdate Solution R4,V-A27Ammonium Molybdate Solution R5,V-A27Ammonium Molybdate Solution R6,V-A27Ammonium Molybdate-Sulfuric AcidSolution,V-A27Ammonium Molybdate-Sulphuric AcidSolution,see Ammonium Molybdate-Sulfuric Acid SolutionAmmonium Muriaticum,V-609Ammonium Nitrate,V-A27Ammonium Nitrate R1,V-A27Ammonium Oxalate,V-A27Ammonium Oxalate Solution,V-A27Ammonium Persulfate,V-A27Ammonium Persulphate,see AmmoniumPersulfateAmmonium Phosphate,see DiammoniumHydrogen OrthophosphateAmmonium Polysulfide Solution,V-A27Ammonium Polysulphide Solution,seeAmmonium Polysulfide SolutionAmmonium Pyrrolidinedithiocarbamate,V-A27Ammonium PyrrolidinedithiocarbamateSolution,V-A27Ammonium Reineckate,V-A27Ammonium Reineckate Solution,V-A28Ammonium Salts and Salts of VolatileBases,Reactions of,V-267Ammonium Salts Reactions of,V-266Ammonium Standard Solution(1ppmNH4),V-A148Ammonium Standard Solution(2.5ppmNH4),V-A148Ammonium Standard Solution(3ppmNH4),V-A148Ammonium Standard Solution(100ppmNH4),V-A148Ammonium Sulfamate,V-A28Ammonium Sulfate,V-A28Ammonium Sulfide Solution,V-A28Ammonium Sulphamate,see AmmoniumSulfamateAmmonium Sulphate,see AmmoniumSulfateAmmonium Sulphide Solution,seeAmmonium Sulfide SolutionV-A802IndexAmmonium Thiocyanate,V-A28 Ammonium Thiocyanate Solution,V-A28Ammonium Thiocyanate VS,V-A142 Ammonium Vanadate Solution,V-A28 Ammonium Vanadate,see Ammonium MetavanadateAmobarbital,I-161Amobarbital Sodium,I-162Amomum fruit,IV-56Amorphous Organosilica Polymer, Octadecylsilyl,V-A98Amoxicillin and Potassium Clavulanate Injection,see Co-amoxiclav Injection Amoxicillin and Potassium Clavulanate Oral Suspension,see Co-amoxiclav Oral SuspensionAmoxicillin and Potassium Clavulanate Tablets,Dispersible,see Dispersible Co-amoxiclav TabletsAmoxicillin and Potassium Clavulanate Tablets,see Co-amoxiclav Tablets Amoxicillin Capsules,III-138 Amoxicillin Injection,III-139 Amoxicillin Oral Suspension,III-141 Amoxicillin Sodium,I-163,V-S9 Amoxicillin Sodium for Injection,III-139Amoxicillin Trihydrate,I-165,V-S9,V-A28Ampere,Definition of,I-32,II-32,III-32,IV-32,V-32 Amperometric,Potentiometric and Voltametric Titrations,V-280 Amperometric Titration,V-280 Amphotericin,I-168Amphotericin B see Amphotericin Amphotericin for Infusion,III-142 Amphotericin Lozenges,I-xxix Amphotericin Oral Suspension,I-xxix Ampicillin,I-170Ampicillin Capsules,III-143Ampicillin Capsules,Flucloxacillin and, see Co-fluampicil CapsulesAmpicillin Injection,III-144Ampicillin Oral Suspension,III-146 Ampicillin Oral Suspension, Flucloxacillin and,see Co-fluampicil Oral SuspensionAmpicillin Sodium,I-172,V-S10 Ampicillin Sodium for Injection,III-144 Ampicillin Trihydrate,I-175,V-S10 Amyl Acetate,V-A28Amyl Alcohol,see Isoamyl Alcohola-Amylase,V-A28a-Amylase Solution,V-A28 Amylmetacresol,I-178,V-S10Amylose-derivative Silica Gel for Chromatography,V-A115b-Amyrin,V-A28Anacardium for Homoeopathic Preparations,IV-428Anaesthetic Ether,I-902Analytical Procedures,Validation of,V-673Analytical Sieving,Particle-size Distribution Estimation By,V-503 Anastrozole,I-179cis-Anethole,V-A28Anethum Graveolens L.Sowa Group,seeAnethum Graveolens Sowa Fruit,Anethum Graveolens Sowa Fruit,IV-58Angelica Archangelica Root,IV-59Angelica Dahurica Root,IV-60Angelica Pubescens Root,IV-62Angelica Sinensis Root,IV-63Angelica Sinensis Root,see ProcessedAngelica Sinensis RootAnhydrous Acetic Acid,V-A20Anhydrous Aluminium Oxide,V-A23Anhydrous Ampicilin see AmpicillinAnhydrous Azapropazone,V-S12Anhydrous Beclometasone Dipropionate,I-239Anhydrous Caffeine,see CaffeineAnhydrous Calcipotriol,I-353Anhydrous Calcium Acetate,see CalciumAcetateAnhydrous Calcium Chloride,V-A37Anhydrous Calcium Gluconate,I-372Anhydrous Calcium HydrogenPhosphate,I-377Anhydrous Calcium Lactate,I-378Anhydrous Chlorobutanol,I-518Anhydrous Citric Acid,I-569,V-A45Anhydrous Copper Sulfate,I-647Anhydrous Disodium HydrogenOrthophosphate,V-A59Anhydrous Disodium HydrogenPhosphate,I-788Anhydrous Docetaxel,I-796Anhydrous Ephedrine,I-849Anhydrous Formic Acid,V-A67Anhydrous Glucose,I-1083Anhydrous Iron(III)Chloride,V-A77Anhydrous Lactose,II-66Anhydrous Lithium Metaborate,V-A81Anhydrous Magnesium Citrate,II-166Anhydrous Methanol,V-A85Anhydrous Morphine,V-A91Anhydrous Nevirapine,II-358Anhydrous Niclosamide,II-362Anhydrous Paroxetine Hydrochloride,II-504Anhydrous Phloroglucinol,II-566Anhydrous Pyridine,V-A110Anhydrous Silica Gel,V-A114Anhydrous Silica,HydrophobicColloidal,II-807Anhydrous Sodium Acetate,V-A120Anhydrous Sodium Carbonate,II-830,V-A121,V-A141Anhydrous Sodium DihydrogenOrthophosphate,V-A122Anhydrous Sodium DihydrogenPhosphate,II-839,V-A122Anhydrous Sodium Sulfate,II-872,V-A124Anhydrous Sodium Sulfite,II-873,V-A124Anhydrous Sodium Sulphate seeAnhydrous Sodium SulfateAnhydrous Sodium Sulphite seeAnhydrous Sodium SulfiteAnhydrous Torasemide,II-1066Anhydrous Valaciclovir Hydrochloride,II-1134Aniline,V-A28Aniline Hydrochloride,V-A28Aniline Hydrochloride Solution,V-A28Animal Spongiform EncephalopathyAgents Via Human and VeterinaryMedicinal Products,Minimising theRisk of Transmitting,V-611Animals,Use of,I-15,II-15,III-15,IV-15,V-15Anion Exchange Resin,V-A28Anion Exchange Resin forChromatography,Strongly Basic,V-A28Anion Exchange Resin R1,V-A28Anion Exchange Resin R2,V-A28Anion exchange resin R3,V-A29Anion Exchange Resin,Strongly Basic,V-A28Anion Exchange Resin,Weak,V-A29Anion-exchange Resin forChromatography,Strongly Basic R1,V-A28Anionic Emulsifying Wax,see EmulsifyingWaxAnisaldehyde,V-A29Anisaldehyde Solution,V-A29Anisaldehyde Solution R1,V-A29Anise Ketone,V-A29Anise Oil,IV-71Anise Water,Concentrated,IV-73Aniseed,IV-66Aniseed Oil,see Anise Oilp-Anisidine,V-A29Anisidine Value,V-326Anolyte for Isoelectric Focusing pH3to5,V-A29Antazoline Hydrochloride,I-181Anthracene,V-A29Anthranilic Acid,see2-Aminobenzoic AcidAnthrax,see Anthrax Vaccine for HumanUse(Adsorbed,Prepared from CultureFiltrates)Anthrax Vaccine for Human Use(Adsorbed,Prepared from CultureFiltrates),IV-527Anthrone,V-A29Anthrone Reagent,V-A29Antibiotics,Microbiological Assay of,V-396,V-655Antibiotics,Potency of,I-14,II-14,III-14,IV-14,V-14Anticoagulant and Preservative Solutionsfor Blood,IV-461Anti-D Immunoglobulin for IntravenousUse,IV-497Anti-D(Rh0)Immunoglobulin,IV-496Antimicrobial Preservation,Efficacy of,V-494,V-653Antithrombin III ConcentrateAnticomplimentary activity ofimmunoglobulin,Test for V-427Anti-D immunoglobulin,human,Assayof V-429Anti-D antibodies in humanimmunoglobulin V-431Anti-A and anti-B haemogglutininsV-432Antimicrobial Preservatives,Definition ofSuitable,I-11,II-11,III-11,IV-11,V-11Antimony Compounds,Reactions of,V-267page numbers in bold type relate to monograph titles Index V-A803。
氯化钙对戊巴比妥钠致蟾蜍心力衰竭的拮抗作用

氯化钙对戊巴比妥钠致蟾蜍心力衰竭的拮抗作用赵小康,王蓓蓓,李清,胡代菊,马珺,赵晶晶[摘要] 目的:观察氯化钙对戊巴比妥钠诱发心衰的作用。
方法:斯氏法游离蟾蜍心脏,用含戊巴比妥钠(2mmol/L,1ml)的任氏液造成蟾蜍心衰模型(心肌收缩力降低一半时为心衰),然后加入氯化钙(90mmol/L,0.04ml),分别记录药前及药后心肌收缩力和心率。
用任氏液清洗至基本恢复正常后改用含戊巴比妥钠(2mmol/L,1ml)的任氏液和氯化钙(90mmol/L,0.04ml)的混合液灌流心脏,并记录心肌收缩力和心率变化。
结果:用含戊巴比妥钠的任氏液灌流心脏使心肌收缩力降低,心率变慢。
氯化钙可拮抗心肌收缩力的降低(P<0.05)。
结论:氯化钙可拮抗戊巴比妥钠诱发的心衰。
[关键词] 戊巴比妥钠;心力衰竭;蟾蜍;氯化钙Effects of chloride calcium on heart failure caused by pentobarbital sodium in toadsZHAO Xiao-kang,W ANG Bei-bei,LI Qing,HU Dai-ju,MA Jun,ZHAO Jing-jing[Abstract] Objective: To observe the effect of chloride calcium on heart failure in toads. Methods:The heart failure models were produced by perfusing Ringer’s solution containing pentobarbital sodium (2mmol/L,1ml) in isolated toad hearts. When the myocardiac contractile force decreased over 50 percent of the normal index, chloride calcium (90mmol/L,0.04ml) was added to antagonize it. The myocardiac contractile force and heart rate were recorded separately before and after giving drugs. After washing, the toad heart was perfused by the mixture of pentobarbital sodium (2mmol/L,1ml) and chloride calcium (90mmol/L,0.04ml). The parameters were taked down. Results:When adopting pentobarbital sodium to make a model of heart failure in toads, the myocardiac contractile force reduced and heart rate also lowered. The reduction of myocardiac contractile force can be antagonized by chloride calcium (P<0.05). Conclusion:Chloride calcium can antagonize the heart failure induced by pentobarbital sodium.[Key words]:pentobarbital sodium; heart failure; toad; chloride calcium戊巴比妥钠是镇静催眠药。
阿奇霉素BP2009翻译
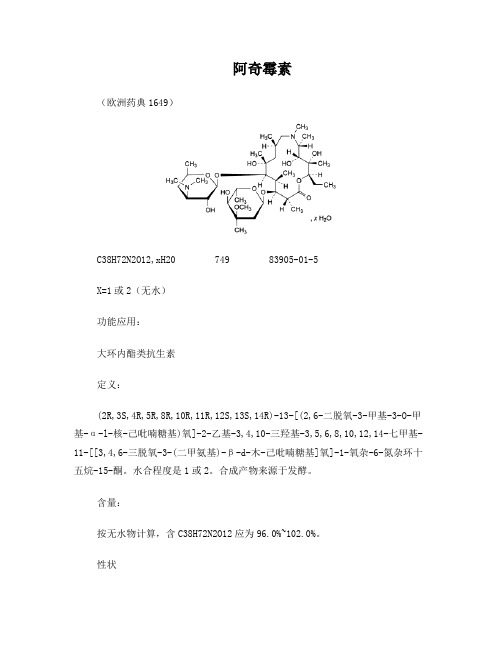
阿奇霉素(欧洲药典1649)C38H72N2O12,xH20 749 83905-01-5X=1或2(无水)功能应用:大环内酯类抗生素定义:(2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-二脱氧-3-甲基-3-O-甲基-α-l-核-己吡喃糖基)氧]-2-乙基-3,4,10-三羟基-3,5,6,8,10,12,14-七甲基-11-[[3,4,6-三脱氧-3-(二甲氨基)-β-d-木-己吡喃糖基]氧]-1-氧杂-6-氮杂环十五烷-15-酮。
水合程度是1或2。
合成产物来源于发酵。
含量:按无水物计算,含C38H72N2O12应为96.0%~102.0%。
性状外观本品为白色或类白色粉末。
溶解性在水中不溶,易溶于无水乙醇和二氯甲烷。
鉴别红外吸收光谱与阿奇霉素标准谱对比,如果固态得到光谱表现不同,将阿奇霉素90g/l溶解在二氯甲烷中测定。
测试溶液S:溶解0.500g阿奇霉素在无水乙醇中,用无水乙醇定容至50ml。
溶液外观:溶液S应为无色澄明状液体。
pH值 9.0-11.0,溶解0.100g阿奇霉素于25ml甲醇中,用脱气水定容至50ml测定。
旋光度 - 45 to - 49 (无水), 溶液S中检查。
有关物质液相色谱混合溶剂:配制1.73g/l磷酸二氢铵溶液,用氨水调节pH值至10.0。
取350ml磷酸二氢铵溶液至合适的容器,加入300ml乙腈和350ml甲醇,混匀。
供试溶液:称取0.200g样品至25.0ml容量瓶中,用混合溶剂稀释,定容。
对照溶液(a):取1.0ml的供试溶液定容至100ml。
对照溶液(b):用混合溶剂溶解阿奇霉素系统适用性标准品(含杂质F、H和J)于1.0ml小瓶中,超声5min。
对照溶液(c):用混合溶剂溶解8.0mg阿奇霉素峰识别CRS(含杂质A,B,C,E,F,G,I,J,L,M,N,O,P)在1.0ml小瓶中。
色谱柱:--型号:I=0.25m,? =4.6mm--固定相:end-capped octadecylsilyl amorphous organosilica polymer for mass spectrometry R (5 μm);--柱温:60°C流动相:--流动相A:1.80g/l的磷酸氢二钠溶液用稀氢氧化钠调节pH至8.9--流动相B:甲醇-乙腈(250:750)流速:1.0ml/min检测波长:210nm进样量:50ul阿奇霉素杂质相对保留时间(保留时间=45-50min)杂质L= 约0.29; 杂质M=约0.37; 杂质E=约0.43; 杂质F =约0.51; 杂质D=约0.54; 杂质J= 约0.54; 杂质I =约0.61; 杂质C=约0.73; 杂质N=约0.76; 杂质H =约0.79; 杂质A=约0.83; 杂质P=约0.92; 杂质O=约1.23; 杂质G=约1.26; 杂质B=约 1.31.杂质鉴别根据阿奇霉素标准图谱和对照溶液(c)的色谱峰来识别杂质峰A,C,E,F,G,I,J,L,M,N,O和P,根据对照溶液 (b) 的色谱峰来识别杂质峰H。
纯化水

源水预处理的常用手段
吸附: 在水处理过程中,利用多孔的固体材料, 是水中的污染物吸附在固体材料空隙内 的处理方法为吸附。使用吸附法可以去 除水中的有机物、胶体物质、微生物和 余氯等,吸附法还可以对水进行除臭、 脱色等。吸附过程常用的材料有活性炭 和大孔径的吸附树脂等。
源水预处理的常用手段
活性炭吸附:在工艺用水的制备过程中 活性炭过滤的主要功能有两个: 一是吸附水中残留余氯 二是吸附水中的部分(大约60%左右有 机物)这部分有机物多为无机和有机的 胶体、溶解性有机高分子杂质。
—
0 .2 μ g /m l 0 .1 μ g /m l 用于生产渗析液时控制 符合规定 0 .5 m g /l 4 .3 μ s /c m (2 0 ℃ ) 0 .2 5 E U /m l 符 合 规 定( 用 于 制 备 无 菌 制 剂时控制) 0 .5 m g /l 符合规定
微生物纠偏限度
1 0 0 个 /m l
概述 水是一切有机物和生命物质的源泉,是人类赖 以生存的宝贵资源。水也是许多人类生产生活 中不可缺少的重要原辅料,许多现代化生产中, 尤其是医药工业由于行业的特殊性对所需用水 有较高的质量要求,因此就派生出了许多不同 标准的处理水,象饮用水、纯化水、注射用水。
源水的选用
纯化水的质量不但和设备、工艺相关, 而且与所用原水有着紧密的联系。 现阶段纯化水的制备源水,一般采用自 来水厂提供的饮用水或深井地下水。 针对不同水源水质的不同,水处理的过 程也不相同。
工艺用水系统功能单元
离子交换系统 离子交换系统是水处理技术中最常用的 一种,离子交换器是利用阴阳交换树脂 对离子的选择性及平衡反应原理,去除 水中电解质离子的一种水处理装置,它 在水处理的应用方面最为广泛,是高纯 水制取的必备设备。
钨酸钠(二水)

产品用途用于制造金属钨、钨酸、钨酸盐、染料、油墨、催化剂等
钨酸钠(二水) - 性质
无色结晶或白色结晶性粉末。
熔点665℃;d 3.23。
能在干燥空气中风化。
溶于约1.1份水,溶液呈弱碱性,pH值8~9。
不溶于乙醇及酸。
100℃失去结晶水。
钨酸钠(二水) - 制法据
将过量钨酸加入氢氧化钠溶液加热,搅拌过滤,滤液浓缩至有结晶析出,冷却、抽滤出结晶,即得纯品钨酸钠。
钨酸钠(二水) - 用途
分析试剂,用于检验血清蛋白、无蛋白血清滤液、生物碱沉淀。
还用于金属钨、钨酸及钨酸盐类的制造。
也可用作媒染剂、颜料和催化剂、织物防火剂等。
钨酸钠(二水) - 安全性
兔肌肉注射LDso:105mg(钨酸盐)/kg;大鼠皮下注射LD5o:240mg(钨酸盐)/kg。
应贮存于阴凉、干燥库房中。
勿使铁桶破裂,保持包装完好。
注意防潮。
勿与酸类共贮混运,运输时要防雨淋、防日光曝晒。
warfarin sodium tablets 英文说明书

warfarin sodium tablets 英文说明书Warfarin Sodium Tablets: A Comprehensive GuideWarfarin sodium tablets are a commonly prescribed medication used to prevent and treat various thromboembolic disorders, such as deep vein thrombosis, pulmonary embolism, and atrial fibrillation. This anticoagulant medication works by interfering with the body's natural blood clotting process, reducing the risk of clot formation and subsequent complications.Understanding the Mechanism of ActionWarfarin sodium exerts its anticoagulant effect by inhibiting the synthesis of vitamin K-dependent clotting factors, including factors II (prothrombin), VII, IX, and X. This inhibition disrupts the normal clotting cascade, making it more difficult for the blood to form clots. The extent of the anticoagulant effect is measured by the prothrombin time (PT) and the international normalized ratio (INR), which are closely monitored during warfarin therapy to ensure the patient's blood remains within the desired therapeutic range.Dosing and AdministrationWarfarin sodium tablets are available in various strengths, typicallyranging from 1 mg to 10 mg. The initial dosage of warfarin is determined based on the patient's individual characteristics, such as age, weight, and underlying medical conditions. Healthcare providers often start with a lower dose and gradually adjust it based on the patient's response, as measured by the PT/INR. It is essential to take warfarin as prescribed, at the same time each day, and to avoid missing or doubling doses.Factors Affecting Warfarin EffectivenessThe anticoagulant effect of warfarin can be influenced by a variety of factors, including diet, concurrent medications, and individual patient characteristics. Patients taking warfarin are advised to maintain a consistent dietary intake of vitamin K-rich foods, as fluctuations in vitamin K levels can affect the INR. Additionally, certain medications, such as antibiotics, antifungals, and some over-the-counter drugs, can interact with warfarin, either increasing or decreasing its anticoagulant effect. Regular monitoring and close communication with healthcare providers are crucial to ensure the safe and effective use of warfarin.Monitoring and Dose AdjustmentsFrequent monitoring of the PT/INR is essential during warfarin therapy. Patients may need to undergo regular blood tests to assess their clotting time and adjust their warfarin dosage accordingly. The target INR range can vary depending on the patient's specificcondition, but it is typically maintained between 2.0 and 3.0 for most indications. Patients may need to undergo more frequent monitoring during periods of illness, changes in medication, or significant dietary changes.Adverse Effects and PrecautionsWarfarin therapy carries a risk of bleeding, which can range from minor bruising to life-threatening hemorrhage. Patients taking warfarin should be aware of the signs and symptoms of bleeding, such as nosebleeds, easy bruising, and unexplained bleeding or bruising. In the event of a suspected bleeding episode, patients should immediately contact their healthcare provider. Additionally, warfarin may interact with certain foods, beverages, and over-the-counter medications, so patients should consult with their healthcare provider before making any changes to their lifestyle or medication regimen.Warfarin Initiation and MonitoringThe initiation of warfarin therapy typically involves a loading dose to quickly achieve the desired anticoagulant effect, followed by a maintenance dose to maintain the therapeutic INR range. During the initial phase of treatment, patients may undergo more frequent INR testing and dose adjustments until a stable INR is achieved. Once the INR is within the target range, the frequency of INR monitoring may be reduced, but regular testing remains essential to ensure thecontinued safety and efficacy of the therapy.Transitioning Between AnticoagulantsIn some cases, patients may need to transition from one anticoagulant medication to another, such as from warfarin to a direct-acting oral anticoagulant (DOAC) or vice versa. This transition process requires careful coordination and monitoring to ensure a smooth transition and maintain the desired anticoagulant effect. Healthcare providers will typically provide specific instructions on how to manage the transition, including the appropriate timing and dosing of the new medication.Patient Education and AdherenceSuccessful warfarin therapy relies heavily on patient education and adherence to the prescribed regimen. Patients should be provided with comprehensive information about the purpose of the medication, the importance of regular monitoring, and the potential risks and management of adverse effects. Encouraging patients to actively participate in their own care, communicate any concerns or changes in their medical status, and maintain a consistent lifestyle can greatly improve the overall effectiveness and safety of warfarin therapy.ConclusionWarfarin sodium tablets are a crucial component of anticoagulationtherapy, offering a proven track record in the prevention and treatment of various thromboembolic disorders. However, the effective and safe use of warfarin requires a comprehensive understanding of its mechanism of action, dosing and administration, factors affecting its effectiveness, and the importance of regular monitoring and patient adherence. By working closely with healthcare providers and adhering to the prescribed treatment plan, patients can maximize the benefits of warfarin therapy and minimize the risk of adverse events.。
甲磺酸罗哌卡因氯化钠注射液

•73•中国药品标准Drug Standards of China2019,20(1)杂质uC17H26N2O274.4(2R)-N-(2,6-二甲基苯基)-1-丙基-2-哌碇甲酰胺国家药品监督管理局国家药品标准制订颁布件(简版)批件号:XGB2016-047实施日期:2017年04月10日颁布日期:2016年10月10日实施规定根据《药品管理法》及其有关规定,制定甲磺酸罗哌卡因氯化钠注射液国家药品标准。
本标准自实施之日起执行,实施日期前生产的药品可按原标准检验。
其他有关事宜参照国家食品药品监督管理局“关于实施《中国药典>2015年版有关事宜的公告(2015年第105号)”执行。
标准编号:WS.-XG-025-2016甲磺酸罗哌卡因氯化钠注射液Jiahuangsuan Luopaikayin Liihuana ZhusheyeRopivacaine Mesilate and Sodium Chloride Injection本品为甲磺酸罗哌卡因与氯化钠的灭菌水溶液。
含甲磺酸罗哌卡因(C17H26N2O•CH3SO3H)与氯化钠(NaCl)均应为标示量的95.0%~105.0%。
【性状】本品为无色的澄明液体。
【鉴别】(1)取本品适量(约相当于甲磺酸罗哌卡因50mg),小火蒸发至适量,加0.2g氢氧化钠混合溶解后,小火蒸干,再缓缓加热至熔融,继续加热数分钟,放冷,加水0.5mL与稍过量的稀盐酸,即产生二氧化硫气体的臭气。
(2)在含量测定项下记录的色谱图中,供试品溶液主峰的保留时间应与对照品溶液主峰的保留时间一致。
(3)本品显钠盐与氯化物鉴别(1)的反应(中国药典2015年版四部通则0301)o【检查1pH值应为4.0-6.0(中国药典2015年版四部通则0631)o有关物质精密量取本品适量,用流动相制成每1mL中约含甲磺酸罗哌卡因1.2mg的溶液作为供试品溶液;精密量取1mL,置100mL量瓶中,用流动相稀释至刻度,摇匀,作为对照溶液;另精密称取2,6-二甲基苯胺(杂质I)对照品适量,加流动相溶解并定量稀释制成每1mL中约含0.12|xg的溶液,作为对照品溶液。
BP2009氯化钾药典

BP2009氯化钾药典Potassium ChlorideSolution SDissolve 10.0 g in carbon dioxide-free water R prepared from distilled water R and dilute to 100 ml with the same solvent. Appearance of solutionSolution S is clear (2.2.1) and colourless (2.2.2, Method II).Acidity or alkalinityTo 50 ml of solution S add 0.1 ml of bromothymol blue solution R1. Not more than 0.5 ml of 0.01 M hydrochloric acid or 0.01 M sodium hydroxide is required to change the colour of the indicator.BromidesMaximum 0.1 per cent.Dilute 1.0 ml of solution S to 50 ml with water R. To 5.0 ml of the solution add 2.0 ml of phenol red solution R2 and 1.0 ml of chloramine solution R1 and mix immediately. After exactly 2 min add 0.15 ml of 0.1 M sodium thiosulphate, mix and dilute to 10.0 ml with water R. The absorbance (2.2.25) of the solution measured at 590 nm, using water R as the compensation liquid, is not greater than that of a standard prepared at the same time and in the same manner using 5 ml of a 3.0 mg/l solution of potassium bromide R.IodidesMoisten 5 g by the dropwise addition of a freshly prepared mixture of 0.15 ml of sodium nitrite solution R, 2 ml of 0.5 M sulphuric acid, 25 ml of iodide-free starch solution R and 25 ml of water R. After 5 min, examine in daylight. The substance shows no blue colour.Sulphates (2.4.13)Maximum 300 ppm.Dilute 5 ml of solution S to 15 ml with distilled water R.Aluminium (2.4.17)Maximum 1.0 ppm, if intended for use in the manufacture of haemodialysis solutions. Prescribed solution Dissolve 4 g in 100 ml of water R and add 10 ml of acetate buffer solution pH 6.0 R.Reference solution Mix 2 ml of aluminium standard solution (2 ppm Al) R, 10 ml of acetate buffer solution pH 6.0 R and 98 ml of water R.Blank solution Mix 10 ml of acetate buffer solution pH 6.0 R and 100 ml of water R.BariumTo 5 ml of solution S add 5 ml of distilled water R and 1 ml of dilute sulphuric acid R. After 15 min, any opalescence in the solution is not more intense than that in a mixture of 5 ml of solution S and 6 ml of distilled water R.Iron (2.4.9)Maximum 20 ppm.Dilute 5 ml of solution S to 10 ml with water R.Magnesium and alkaline-earth metals (2.4.7)Maximum 200 ppm, calculated as Ca, determined on 10.0 g using 0.15 g of mordant black 11 triturate R. The volume of 0.01M sodium edetate used does not exceed 5.0 ml.SodiumMaximum 0.10 per cent, if intended for use in the manufacture of parenteral preparations or haemodialysis solutions.Atomic emission spectrometry (2.2.22, Method I).Test solution Dissolve 1.00 g of the substance to be examined in water R and dilute to 100.0 ml with the same solvent.Reference solutions Prepare the reference solutions by diluting as required a solution containing 200 µg of Na per millilitre, prepared as follows: dissolve in water R 0.5084 g of sodium chloride R, previously dried at 100-105 °C for 3 h, and dilute to 1000.0 ml with the same solvent.Wavelength 589 nm.Heavy metals (2.4.8)Maximum 10 ppm.12 ml of solution S complies with test A. Prepare the reference solution using lead standard solution (1 ppm Pb) R.Loss on drying (2.2.32)Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C for 3 h. ASSAYDissolve 1.300 g in water R and dilute to 100.0 ml with the same solvent. To 10.0 ml of the solution add 50 ml of water R, 5 ml of dilute nitric acid R, 25.0 ml of 0.1 M silver nitrate and 2 ml of dibutyl phthalate R. Shake. Titrate with 0.1 M ammonium thiocyanate, using 2 ml of ferric ammonium sulphate solution R2 as indicator and shaking vigorously towards the end-point.1 ml of 0.1 M silver nitrate is equivalent to 7.46 mg of KCl.LABELLINGThe label states:— where applicable, that the substance is suitable for use in the manufacture of parenteral preparations;。
Pantoprazole-Sodium-中国药典标准翻译

Pantoprazole SodiumC16H14F2N3NaO4S·H2O 423.378 Pantoprazole Sodium is 5-difluoromethoxy-2-[[(3,4-dimethoxy-2-pyridinyl)methyl] sulfinyl]-1H-benzimidazole sodium, monohydrate. It contains not less than 98.0% and not more than 102.0% of C16H14F2N3NaO4S, calculated on anhydrous basis. [Description]A white or almost white crystalline powder.Freely soluble in water or methanol; practically insoluble in chloroform or diethyl ether.[Identification](1) Dissolve 10 mg in 20 ml of water to 2 ml of the resulting solution add 5 drops of dilute hydrochloric acid, then add 1 ml of silicotungstic acid TS dropwise, a white curdy precipitate is produced.(2) The light absorption of a solution of 15 μg per ml in ethanol exhibits a maximum at 292 nm, and minimum at 250 nm (Appendix Ⅳ A).(3) The infrared absorption spectrum is concordant with the reference spectrum of pantoprazole sodium (Appendix ⅩⅥ).(4) Yields the reaction characteristic of sodium salts (Appendix Ⅲ). [Inspection]AlkalinityAn aqueous solution of 20 mg per ml, pH 9.5-11.0 (Appendix Ⅵ H).Clarity and colour of solutionDissolve 0.20 g in 10 ml of water, the solution is clear and colourless; any colour produced is not more intense than that of reference solution Y2(Appendix ⅨA, method 1).Related substancesDissolve a quantity of the product in solvent [0.001 mol/L sodium hydroxide-acetonitrile (1:1)] to obtain a solution of 0.4 mg per ml as test solution. Pipet accurately 1 ml of the test solution, to a 100 ml volumetric flask, dilute tovolume with above solvent, mix well as reference solution. Carry out the method for high performance liquid chromatogrphy (Appendix Ⅴ D). The column is packed with octadecyl silane bonded silica gel, mobile phase A is 0.01 mol/L dipotassium hydrogen phosphate solution (adjust the pH to 7.0 by phosphoric acid), mobile phase B is acetonitrile, gradient elution chromatography is applied as the table below; detection wavelength is 289 nm; column temperature is 40℃. The number of the theoretical plates calculated from pantoprazole peak is not less than 2500. Inject 20 μl of reference solution, adjust detection sensitivity so that the peak height of the principle peak in the chromatogram is about 20% of full scale of the chart. Inject separately 20 μl of the test so lution and the reference solution and record the chromatogram. The area of individual impurity peak is not more than 0.3 times of the area (0.3%) of the principle peak in the chromatogram obtained with the reference solution., the sum of the areas of the impurity peaks is not more than 0.8 of the area (for injection) (0.8%) or not more than the area (for oral) (1.0%) of the principle peak in the chromatogram obtained with the reference solution. The area of any peak in the chromatogram of the test solution less than 0.05 times of principle peak area obtained with the reference solution is negligible.Time(min) Flowing phase A (%) Flowing phase B (%)0 90 1030 60 4045 15 85Residual solventstoluene and acetoneTransfer 0.2 g of the product, accurately weighted, to a headspace vial, dissolve with 2 ml of water and seal it up as test solution. Measure accurately a properquantity of tuluene and acetone, diluted quantitatively with water to obtain a mixed solution contain about 90 μg of toluene and 500 μg of acetone per ml(toluene is insoluble in water, dissolve it in DMF first and then disperse in the solvent), measure accurately 2ml of it into a headspace vial, and seal it up as reference solution. Carry out the method for Appendix ⅧP, method 2. The column is packed with 5% phenyl-95% polydimenthyl siloxane (or with similar polarity). Temperature program: initial temperature 40℃ for 4 minutes, then raise the temperature at a rate of 20℃ per min to 150℃ and hold for 3 minutes, the inlet temperature is 200℃, the detector temperature is 250℃; the head vial equilibration temperature is 60℃, equilibration time is 30 minutes. Inject separately the test preparation and the reference preparation and recordthe chromatogram. Calculate the quantity by peak area using external standard method, the content of tuluene and acetone shall meet the specification.WaterAccording to method of moisture test (Appendix ⅧM Method One), the moisture should be 4.0%~6.0%.Heavy metalsTransfer 0.5g of the product to a platinum crucible, then slowly Ignite the crucible until the product is the completely carbonized (about4 hours), cool the crucible in a desiccators, Moisten the sample with a small amount (usually 1.2~1.5mL) of sulfuric acid, then heat gently at a temperature as low as practicable until the steam of sulfuric acid is wiped off, add 0.5ml of nitric acid, keep heating until the fumes are no longer evolved, then ignite at 500~600℃ until the residue is completely incinerated, cool the crucible. The test is carried out according to Appendix Ⅷ H Method Two, starts from “add 2ml of hydrochloric acid”. The heavy metals should not be more than 20ppm. [Content Determination]Carry out the method for high performance liquid chromatography (Appendix Ⅴ D). Chromatographic conditions and system suitabilityThe column is packed with octadecyl silane bonded silica gel; The mobile phase is 0.01 mol/L dipotassium hydrogen phosphate solution (adjust pH to 7.0 by phosphoric acid) -acetonitrile (65:35). The detection wavelength is 288 nm. The number of the theoretical plate calculated from pantoprazole peak is not less than 2500.Procedure Dissolve a quantity of the product being examined, weigh accurately, in a mixture of 0.001 mol/L sodium hydroxide-acetonitrile (1:1) to obtain a solution of about 40 μg per ml. Inject 20 μl and record the chromatogram. Repeat the operation using Pantoprazole Sodium standard instead of the product being examined. Calculate the content of C16H14F2N3NaO4S with respect to the peak area obtained in the chromatogram by the external standard method.[Category] Digestive system medicine.[Storage] Preserve in tightly closed containers, stored in a cool place and dark place. [Preparation] Pantoprazole Sodium for Injection。
sodium valproate质量标准

Sodium Valproate质量标准目录1. 概述2. Sodium Valproate的药理作用3. Sodium Valproate的应用4. Sodium Valproate的质量标准4.1 外观4.2 理化性质4.3 含量测定4.4 溶解度4.5 不溶物4.6 酸度或碱度4.7 水分4.8 残留溶剂4.9 重金属5. 结论6. 参考文献1. 概述Sodium Valproate是一种常见的抗癫痫药物,也被用于治疗精神分裂症、双相情感障碍和其他神经系统疾病。
作为一种用于治疗神经系统疾病的药物,其质量标准至关重要。
本文将针对Sodium Valproate的质量标准进行详细介绍,以帮助读者更好地了解该药物的质量控制标准。
2. Sodium Valproate的药理作用Sodium Valproate是一种γ-氨基丁酸(GABA)转氨酶的抑制剂,通过增加GABA在中枢神经系统中的浓度,从而发挥抗癫痫和抗精神分裂作用。
Sodium Valproate还具有电压门控的钠通道阻滞作用,能够抑制神经元过度放电,从而减少癫痫发作的频率和强度。
3. Sodium Valproate的应用Sodium Valproate常用于治疗多种类型的癫痫,包括复杂部分性癫痫、全身性发作以及癫痫性惊厥。
Sodium Valproate还被广泛应用于双相情感障碍、精神分裂症和其他神经系统疾病的治疗中。
由于其疗效显著,临床应用范围十分广泛。
4. Sodium Valproate的质量标准Sodium Valproate的质量标准主要包括外观、理化性质、含量测定、溶解度、不溶物、酸度或碱度、水分、残留溶剂和重金属等指标。
4.1 外观Sodium Valproate应呈白色结晶性粉末,无明显杂质。
在光线充足的条件下,其颜色应一致,无结块或不均匀。
4.2 理化性质Sodium Valproate的理化性质包括熔点、比旋光度、红外光谱等指标。
USP氯化钠译文

Sodium Chloride氯化钠NaCl 58.44Sodium ChlorideSodium Chloride [7647-14-5].» Sodium Chloride contains not less than 99.0 percent and not more than 100.5 percent of NaCl, calculated on the dried basis.氯化钠按干燥品计算,含氯化钠应不少于99%不高于100.5%。
Packaging and storage— Preserve in well-closed containers.包装与贮藏:密封保存。
Labeling—Where Sodium Chloride is intended for use in the manufacture of injectable dosage forms, peritoneal dialysis solutions, hemodialysis solutions, or hemofiltration solutions, it is so labeled. Where Sodium Chloride must be subjected to further processing during the preparation of injectable dosage forms to ensure acceptable levels of Bacterial endotoxins, it is so labeled. Where Sodium Chloride is sterile, it is so labeled.以下情况均需注明:氯化钠应用于注射剂、腹膜透析溶液、血液透析液和血液过滤液的生产;氯化钠经过进一步加工应用于注射剂型时的细菌内毒素可接受水平;无菌情况。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Sodium Valproate General Notices(Ph Eur monograph 0678)IDENTIFICATIONA. Infrared absorption spectrophotometry (2.2.24).Comparison sodium valproate CRS.If the spectra obtained in the solid state show differences, record new spectra using discs prepared by placing 50 µl of a 100 g/l solution in methanol R on a disc of potassium bromide R and evaporating the solvent in vacuo. Examine immediately.B. Examine the chromatograms obtained in the test for related substances.Results The principal peak in the chromatogram obtained with test solution (b) is similar in retention time to the principal peak in the chromatogram obtained with reference solution (b).C. 2 ml of solution S (see Tests) gives reaction (a) of sodium (2.3.1) .TESTSSolution SDissolve 1.25 g in 20 ml of distilled water R in a separating funnel, add 5 ml of dilute nitric acid R and shake. Allow the mixture to stand for 12 h. Use the lower layer.Appearance of solutionThe solution is not more opalescent than reference suspension II (2.2.1) and not more intensely coloured than reference solution Y6(2.2.2, Method II).Dissolve 2.0 g in water R and dilute to 10 ml with the same solvent.Acidity or alkalinityDissolve 1.0 g in 10 ml of water R. Add 0.1 ml of phenolphthalein solution R . Not more than 0.75 ml of 0.1 M hydrochloric acid or 0.1 M sodium hydroxide is required to change the colour of the indicator.Related substancesGas chromatography (2.2.28).Internal standard solution Dissolve 10 mg of butyric acid R in heptane R and dilute to 200 ml with the same solvent.Test solution (a) Dissolve 0.500 g of the substance to be examined in 10 ml of water R . Add 5 ml of dilute sulphuric acid R and shake with 3 quantities, each of 20 ml, of heptane R. Add 10.0 ml of the internal standard solution to the combined upper layers, shake with anhydrous sodium sulphate R , filter and evaporate the filtrate, at a temperature not exceeding 30 °C, using a rotary evaporator. Take up the residue with heptane R and dilute to 10.0 ml with the same solvent. Dilute 1.0 ml of this solution to 10.0 ml with heptane R .Test solution (b) Dissolve 40 mg of the substance to be examined in 100 ml of water R . To 10 ml of this solution add 0.5 ml of dilute sulphuric acid R and shake with 3 quantities, each of 5 ml, of heptane R . Shake with anhydrous sodium sulphate R, filter and evaporate the filtrate, at a temperature not exceeding 30 °C, to a volume of about 10 ml, using a rotaryevaporator.Reference solution (a) Dissolve 20 mg of 2-(1-methylethyl)pentanoic acid CRS (impurity C) in 5.0 ml of test solution (b) and dilute to 10 ml with heptane R . Dilute 1 ml of this solution to 10 ml with heptane R.Reference solution (b) Prepare as prescribed for test solution (b), using sodium valproate CRS instead of the substance to be examined.Column:— material: wide-bore fused silica;— size: l = 30 m, Ø = 0.53 mm;— stationary phase: macrogol 20 000 2-nitroterephthalate R (film thickness 0.5 µm). Carrier gas helium for chromatography R.Flow rate 8 ml/min.Temperature:Detection Flame ionisation.Injection 1 µl.System suitability Reference solution (a):— resolution: minimum 3.0 between the peaks due to impurity C and valproic acid.Limits Test solution (a):— any impurity: for each impurity, not more than the area of the peak due to the internal standard (0.1 per cent);— total: not more than 3 times the area of the peak due to the internal standard (0.3 per cent);— disregard limit: 0.1 times the area of the peak due to the internal standard (0.01 per cent).Chlorides (2.4.4)Maximum 200 ppm.To 5 ml of solution S add 10 ml of water R.Sulphates (2.4.13)Maximum 200 ppm, determined on Solution S.Heavy metals (2.4.8)Maximum 20 ppm.1.0 g complies with test C. Prepare the reference solution using 2 ml of lead standard solution (10 ppm Pb) R.Loss on drying (2.2.32)Maximum 2.0 per cent, determined on 1.000 g by drying in an oven at 105 °CASSAYDissolve 0.1500 g in 25 ml of anhydrous acetic acid R . Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20) .1 ml of 0.1 M perchloric acid is equivalent to 16.62 mg of C8H15NaO2.STORAGEIn an airtight container.IMPURITIESA. R = R′ = H: pentanoic acid (valeric acid),B. R = H, R′ = CH2-CH3: (2RS)-2-ethylpentanoic acid,C. R = H, R′ = CH(CH3)2: (2RS)-2-(1-methylethyl)pentanoic acid,D. R = R′ = CH2-CH2-CH3: 2,2-dipropylpentanoic acid,E. R = R′ = H: pentanamide (valeramide),F. R = H, R′ = CH2-CH2-CH3: 2-propylpentanamide,G. R = R′ = CH2-CH2-CH3: 2,2-dipropylpentanamide,Soft SoapGeneral NoticesPreparationSoap SpiritDEFINITIONSoft Soap is soap made by the interaction of potassium hydroxide or sodium hydroxide with a suitable vegetable oil or oils or with fatty acids derived there from. It yields not less than44.0% of fatty acids. It may be coloured with chlorophyll or not more than 0.015% of a suitable green soap dye.CHARACTERISTICSA yellowish white to green or brown, unctuous substance.Soluble in water and in ethanol (96%).TESTSChlorides and other ethanol-insoluble substancesDissolve 5 g in 100 ml of hot ethanol (96%) previously neutralised to phenolphthalein solution R1, filter through a dried and tared filter, wash the residue thoroughly with hot neutralised ethanol (96%) and dry to constant weight at 105°. The residue weighs not more than 0.15 g. Free fatty acid or alkali hydroxideBoil 250 ml of ethanol (96%) to remove carbon dioxide, add 0.5 ml of phenolphthalein solution R1, allow to cool to 70° and neutralise, if necessary, with 0.1M sodium hydroxide VS or 0.05M sulphuric acid VS. To 100 ml of the neutral ethanol add 10 g of the substance being examined and dissolve it as quickly as possible by heating under a reflux condenser. Cool to 70° and, if the solution is not pink, titrate at 70° with 0.1M sodium hydroxide VS; not more than 0.2 ml is required. If the solution is pink add, in a thin stream, 5 ml of hot barium chloride solution previously neutralised to phenolphthalein solution R1, mix thoroughly and titrate with 0.1M hydrochloric acid VS until the pink colour disappears; not more than 1.0 ml is required.Total free alkaliTo 100 ml of the neutral ethanol prepared as described in the test for Free fatty acid or alkali hydroxide add 10 g of the substance being examined and dissolve it as quickly as possible by heating under a reflux condenser. Add immediately 3 ml of 0.5M sulphuric acid VS and boil under a reflux condenser on a water bath for at least 10 minutes. If the solution is not pink, cool to 70° and titrate with 1M sodium hydroxide VS until a pink colour is produced. The volume of 0.5M sulphuric acid VS neutralised by the substance being examined is not more than 1.0 ml.Unsaponifiable matter and unsaponified neutral fatDissolve 5 g in 80 ml of a mixture of 50 ml of ethanol (96%) and 100 ml of water, without。
