胶体金免疫色谱试纸条及微芯片
胶体金试纸条原理

胶体金试纸条原理胶体金试纸条是一种常用于检测生物标志物的快速诊断工具,其原理基于胶体金纳米颗粒的特性和生物分子的相互作用。
胶体金纳米颗粒具有良好的生物相容性和生物亲和性,可以与生物分子(如蛋白质、核酸等)特异性结合,形成可见的颜色变化,从而实现生物分子的快速检测。
胶体金试纸条的原理主要包括两个方面,一是胶体金纳米颗粒的表面增强效应,二是生物分子的特异性识别。
首先,胶体金纳米颗粒具有表面增强效应,即在特定波长下,其表面等离子体共振会导致颜色的增强和变化。
当胶体金纳米颗粒与生物分子结合时,会引起表面等离子体共振的改变,从而导致胶体金纳米颗粒的颜色发生可见的变化。
其次,胶体金试纸条上的生物分子探针与待检测样品中的靶分子特异性结合,形成复合物,使胶体金纳米颗粒发生聚集或分散,进而导致颜色的变化。
通过比较试纸条上的颜色变化与标准颜色条,可以快速判断待测样品中的生物标志物的浓度或存在与否。
胶体金试纸条的原理具有以下特点,首先,具有高灵敏度和特异性。
胶体金纳米颗粒的表面增强效应和生物分子的特异性识别使得试纸条对生物标志物具有高度灵敏性和特异性,能够实现快速、准确的检测。
其次,具有快速反应和直观可见的特点。
胶体金试纸条的检测过程简单快速,无需复杂的仪器和操作,通过肉眼即可直接观察到颜色的变化,使得检测结果直观可见,便于临床诊断和现场应用。
再次,具有良好的可控性和稳定性。
胶体金纳米颗粒的制备工艺成熟,具有良好的可控性和稳定性,能够保证试纸条的稳定性和重复性,为实际应用提供了可靠的保障。
总之,胶体金试纸条基于胶体金纳米颗粒的表面增强效应和生物分子的特异性识别,实现了生物标志物的快速检测,具有高灵敏度、特异性、快速反应和直观可见的特点,逐渐成为临床诊断、食品安全和环境监测等领域的重要工具,对于提高诊断效率和保障公共健康具有重要意义。
免疫层析快速诊断技术简介胶体金

简便:不需其它任何仪器设备,操作也极其简单,无需专 灵敏度问题 业人员,携带方便,可随时随地进行。
廉价:单个测试条成本极低
可单份检测:对标本既能成批检测,又可单份检测,可立 刻拿到结果,不必等待。
稳定性好:金标试剂稳定,可长期保存。
检测标本种类多:在临床检测中,样品可能有尿、血液、 血清、唾液或其他体液。在非临床检测中,样本可能是由 土壤、粉尘、植物或者食品制备的微量溶液。
这种检测可以以独立的试纸条,也可以 以装在塑料盒中的形式进行。
总的来说,做此项检测至少需要50ul的 液体标本,可在5-30分钟完成。
胶体金纸条的不同种类
层析法技术优势:简单\现场\快速
试纸条组成结构
控制线(C线)
与常规诊断方法相比:
优点 快速:全部检测过程仅需 5-30分钟。
缺点 定量问题
AJQ3000: 0.5-5mm 最小喷量
BJQ3000:10nl/drop
AJQ3000:1ul/cm 划线精密度 BJQ3000:±1% AJQ3000: ±2%
切条机
仪器参数: 进料宽度:≤100 mm 进料长度:不限,可连续进料切割 切割宽度:≥1 mm, 宽度连续可调 切割宽度设定位数:0.01 mm 切割精度:±0.1 mm 切割速度:230次/分钟, 速度连续可调 切条计数:单次工作和总工作分别计数 刀片: 日本进口, 耐磨高碳钢材料 抗静电装置:建议选配 电源:220VAC 重量:20KG 尺寸:430(L)×330(W)×260(H) mm
胶体金结果判读方法:
1.色卡法
将反应好的试纸条T线颜色与制作的色卡比较,看颜色属于那一 个档次,则在数据记录时候填写该档次标识。最后做灵敏度比较。 2.图形扫描法
胶体金试纸条的制备

抗体与胶体金的偶联机制
抗体活性的保持
在抗体与胶体金结合过程中,应确保 抗体的活性不受影响,以保证检测的 灵敏度和特异性。
抗体与胶体金的结合是通过共价键或非共价 键实现的。共价键结合力较强,但不易解离 ;非共价键结合力较弱,但易于解离。
试纸条的制备工艺
基材选择与处理
选择合适的支持膜作为试纸条的基材,如硝酸纤维素膜、 聚酯膜等,并进行预处理,以提高其亲水性和结合力。
流感检测
在流感高发季节,胶体金试纸条可以快速检测流 感病毒抗原,为防控流感提供有力支持。
食品安全检测
农药残留检测
胶体金试纸条可用于检测果蔬等农产品中的农药残留,保障食品 安全。
兽药残留检测
通过胶体金试纸条检测肉类、禽蛋等食品中的兽药残留,有助于 监控食品安全,防止药物滥用。
重金属离子检测
用于检测食品中重金属离子的胶体金试纸条,能够快速、准确地 检测出食品中的重金属含量。
新材料的应用
高分子材料
研究新型高分子材料,提高试纸条的灵敏度、稳定性和耐用性。
纳米材料
利用纳米材料的特点,增强试纸条的信号放大效果和特异性。
生物材料
探索生物相容性更好的生物材料,降低试纸条对人体的潜在风险。
新技术的开发
免疫分析技术
研究更高效、特异的免疫分析技术,提高试纸条的检测准 确性。
微流控技术
环境监测
1 2
水质监测
胶体金试纸条可用于快速检测水体中的有害物质, 如铅、汞、砷等,确保水质安全。
大气污染监测
通过胶体金试纸条检测大气中的有害气体和颗粒 物,有助于及时发现和防控空气污染。
3
土壤污染监测
胶体金试纸条可用于检测土壤中的重金属和有机 污染物,为土壤污染治理提供依据。
胶体金试纸条的原理

胶体金试纸条的原理
胶体金试纸条是一种常见的检测方法,广泛应用于医学、环境监测和食品安全领域。
它的原理是基于胶体金的表面增强拉曼散射效应。
胶体金是一种纳米颗粒,其表面具有很高的比表面积和表面等离子共振效应,能够增强分子的振动信号,从而提高检测的灵敏度和准确性。
在胶体金试纸条中,胶体金纳米颗粒被固定在试纸条的表面上。
当待检样品中的目标分子与胶体金纳米颗粒表面的功能性分子结合时,就会发生表面增强拉曼散射效应。
通过激光照射样品,胶体金纳米颗粒表面的拉曼信号会被增强,形成特征峰,从而可以检测目标分子的存在和浓度。
胶体金试纸条的原理简单明了,操作方便快捷,且具有很高的灵敏度和特异性。
它在临床诊断中可以用于检测各种生物标志物,如蛋白质、DNA、RNA等,对各种疾病的早期诊断具有重要意义。
在环境监测中,胶体金试纸条可以用于检测水质中的重金属离子、有机污染物等,对保护环境和人类健康起着关键作用。
在食品安全领域,胶体金试纸条可以检测食品中的添加剂、农药残留、毒素等,保障食品安全。
总的来说,胶体金试纸条作为一种快速、灵敏、特异的检测方法,被广泛应用于各个领域。
它的原理是基于胶体金的表面增强拉曼散
射效应,通过检测胶体金纳米颗粒表面的拉曼信号来实现对目标分子的检测。
胶体金试纸条的发展为医学、环境监测和食品安全等领域的快速检测提供了重要工具,有望在未来发挥更大的作用。
胶体金免疫试纸条工艺流程

胶体金免疫试纸条工艺流程下载温馨提示:该文档是我店铺精心编制而成,希望大家下载以后,能够帮助大家解决实际的问题。
文档下载后可定制随意修改,请根据实际需要进行相应的调整和使用,谢谢!并且,本店铺为大家提供各种各样类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,如想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by theeditor. I hope that after you download them,they can help yousolve practical problems. The document can be customized andmodified after downloading,please adjust and use it according toactual needs, thank you!In addition, our shop provides you with various types ofpractical materials,such as educational essays, diaryappreciation,sentence excerpts,ancient poems,classic articles,topic composition,work summary,word parsing,copy excerpts,other materials and so on,want to know different data formats andwriting methods,please pay attention!胶体金免疫试纸条是一种快速检测病原微生物、蛋白质、细胞等生物标志物的诊断工具,其工艺流程如下:1. 试纸条原材料的选择与制备:- 选择合适的滤纸或硝酸纤维素膜作为试纸条的载体。
胶体金免疫色谱试验测定法

胶体金免疫色谱试验测定法
胶体金免疫色谱试验(Gold Immunochromatography Assay,简称GIA)是一种常用于检测生物样本中特定分子(如蛋白质、荷尔蒙、抗体等)的定性或定量分析方法。
一般的胶体金免疫色谱试验包括以下步骤:
1. 样品处理:将待检生物样品(如血液、尿液、唾液等)进行预处理,通常包括离心、稀释或某些特殊样品的处理方法。
2. 准备试纸条:将试纸条浸泡于样品中,使试纸条上的涂层带上样品中的目标分子。
3. 上样:将预处理后的样品滴入试纸条上的样品进样孔,让样品与试纸条上的特异抗体结合。
4. 试纸条扩散:待样品进入样品孔后,样品中的目标分子会与试纸条上固定的抗体结合,形成抗原-抗体复合物。
复合物会随着样品的扩散而向试纸条上移动。
5. 可视化结果:在试纸条上存在着特定检测线。
当复合物通过检测线时,会发生阳性反应,即出现颜色改变。
通过检测线的出现与否,可以判断样品中是否存在目标分子。
胶体金免疫色谱试验的优点包括操作简便、快速、便携、结果可视化等,因此被广泛应用于临床诊断、食品安全、环境监测等领域。
胶体金试纸条的制备

结合垫(Conjugate pad):
玻璃纤维、聚酯膜、纤维素滤纸、无纺布等多种材 质,多种规格,批间稳定。
结合垫的作用主要为:
- 吸附一定量的金标结合物颗粒; - 吸附并持续不断的将样品转移到NC膜上; - 保持金标结合物颗粒的稳定性; - 保证金标结合物颗粒定量完全释放等。
玻璃纤维膜/聚酯纤维膜
胶体金免疫层析试纸模式双抗体夹心模式疫病抗原检测竞争模式小分子物质检测spa蛋白g模式疫病抗体检测双抗体夹心模式判定检测线质控线阳性显色显色阴性不显色显色竞争模式判定检测线质控线阳性不显色显色阴性显色显色spag蛋白模式判定检测线质控线阳性显色显色阴性不显色显色在兽药残留检测中的应用zhang等用克伦特罗制备的单克隆抗体标记胶体金作为检测探针然后将偶联bsa的克伦特罗作为t线以竞争模式组装成试纸用于样品的克伦特罗残留检测为我国食品安全检测提供了有效的工具
4、5 号管颜色无变化与对照颜色一致,最低蛋白量即为4 号管的蛋白量。
最终结合(McAb)
• 取100ml金溶液,调整pH值至8.2 • 调整滤过和离心的抗体溶液(0.1μg/μl)至
pH8.2 • 当金溶液在快速搅拌时要逐滴加入定量的
抗体(蛋白最小浓度量加10%即蛋白稳定 量),大约5分钟加完 • 在金标溶液中加入10ml滤过的10%BSA至 终浓度为1%,并轻轻搅拌10分钟。
» 向沸腾的溶液中加入1.5ml的0.1%二合水柠檬酸三 钠,Na3C6H5O7.2H2O
» 当获得深红颜色的溶液时停止加热
胶体金的合成
胶体金 1%柠檬酸 粒径 三钠加入量 (nm) (ml)*
16
2.00
24.5 1.50
41
1.00
71.5 0.70
S100-β蛋白检测试纸(胶体金免疫层析法)产品技术要求taijieweiye
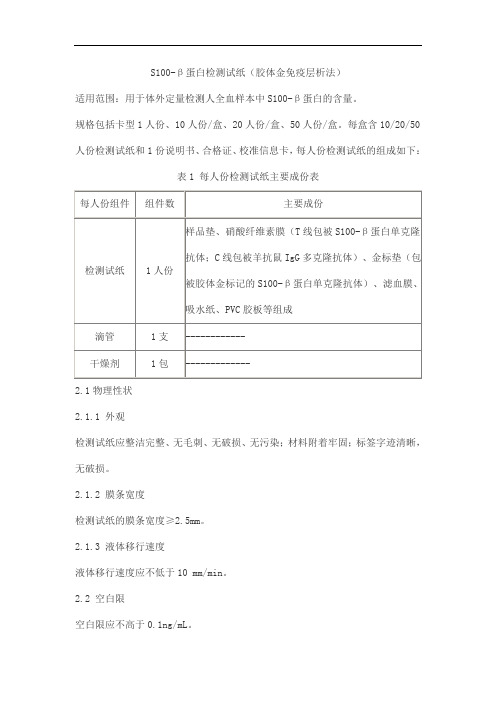
S100-β蛋白检测试纸(胶体金免疫层析法)
适用范围:用于体外定量检测人全血样本中S100-β蛋白的含量。
规格包括卡型1人份、10人份/盒、20人份/盒、50人份/盒。
每盒含10/20/50人份检测试纸和1份说明书、合格证、校准信息卡,每人份检测试纸的组成如下:
表1 每人份检测试纸主要成份表
2.1物理性状
2.1.1 外观
检测试纸应整洁完整、无毛刺、无破损、无污染;材料附着牢固;标签字迹清晰,无破损。
2.1.2 膜条宽度
检测试纸的膜条宽度≥2.5mm。
2.1.3 液体移行速度
液体移行速度应不低于10 mm/min。
2.2 空白限
空白限应不高于0.1ng/mL。
2.3 精密度
2.3.1重复性
CV 应不高于10.0%。
2.3.2 批间差
CV 应不高于15.0%。
2.4 剂量-反应曲线的线性
在 0.1ng/mL~10ng/mL的范围内,用线性拟合公式拟合,剂量-反应曲线相关系数r应不低于0.99。
2.5 准确度
检测S100-β蛋白纯品,样本回收率应在85%~115%范围内。
2.6特异性
检测浓度为1.0μg/mL的C反应蛋白样品液,检测试纸,检测结果小于0.1ng/mL。
2.7稳定性
将检测试纸在4℃~30℃的环境中放置18个月后,取样分别检测2.1、2.2、2.3.1、2.4、2.5、2.6项,结果应符合各项目的要求。
胶体金试纸条检测新冠原理

胶体金试纸条检测新冠原理胶体金试纸条检测新冠原理新冠肺炎是一种高传染性疾病,对全球人民的健康和生命安全造成了极大的威胁。
目前,新冠病毒的检测成为预防和控制疫情的重要手段。
胶体金试纸条检测技术是一种快速、简便、易操作的检测方法,被广泛应用于新冠病毒的检测领域。
本文将从胶体金试纸条的基本原理、新冠病毒检测的流程、胶体金试纸条检测方法的优点和市场应用等方面进行阐述。
一、胶体金试纸条的基本原理胶体金试纸条是一种基于胶体金技术的测定方法。
胶体金是一种物理性质稳定、化学性质活性的微型金颗粒,其表面特性对生物分子具有极强的亲和力。
在胶体金试纸条上,通过将对新冠病毒的抗体固定在纸条上,并与胶体金颗粒标记的抗体结合,打造出了一种快速、敏感、选择性的检测方法。
二、新冠病毒检测的流程新冠病毒检测通常包含样本采集、核酸提取、核酸扩增和检测结果读取四个步骤。
采样过程中,通常使用咽拭子或鼻咽拭子,利用提取试剂将提取到的病毒核酸分离并提取出来。
然后,通过核酸扩增技术,将样本中的新冠病毒的核酸扩增出来,并在PCR仪器中进行实时荧光检测。
最后,通过查看胶体金试纸条检测结果,比较检测线和质控线的颜色变化,以判断样本是否为新冠病毒阳性。
三、胶体金试纸条检测方法的优点胶体金试纸条检测方法具有许多优点。
首先,其检测速度快,通常只需要几十分钟,与传统的PCR检测技术相比,大大缩短了检测的周期。
其次,操作简单,无需特殊技术和专业设备,使检测可以向社区和家庭延伸,提高了社区和家庭的诊断能力。
此外,胶体金试纸条检测方法高度敏感,可以检测到少量的新冠病毒核酸,并且具有选择性,不会对其他病毒进行误判。
四、市场应用前景目前,胶体金试纸条检测技术已被广泛应用于新冠病毒的检测领域,并在全球范围内建立了大量的检测实验室和工厂。
随着社区、家庭和学校等场所新冠病毒检测需求的不断增加,胶体金试纸条检测技术有着广阔的应用前景。
此外,在未来的研究和发展过程中,未来确定可以进一步扩展其检测范围,包括其他病毒或疾病标志物的检测,为精确医学健康提供新的技术支持。
胶体金试纸条原理

胶体金试纸条原理胶体金试纸条(Colloidal Gold Test Strip)是一种简单、快速、准确的免疫学检测方法,广泛应用于生物医学研究、医学诊断、环境监测、食品安全等领域。
本文主要介绍胶体金试纸条的原理、制备及应用。
一、原理胶体金试纸条原理是基于金纳米颗粒与抗原或抗体的特异性结合作用。
金纳米颗粒在特定条件下呈现紫红色,具有很强的表面等离子体共振吸收波长。
当金纳米颗粒与特异抗原或抗体结合时,其颜色由紫红色变为红色或蓝色,颜色的变化与特异性结合反应形成的复合物的形态、数量有关,可以通过肉眼直接观察到。
二、制备制备胶体金试纸条需要先制备金纳米颗粒溶液,然后将其涂在滤纸或其他纸张上,并固定相应的抗原或抗体作为检测指针。
具体步骤如下:1. 制备金纳米颗粒溶液制备金纳米颗粒溶液的方法有多种,其中较为简单的方法是还原法。
将1 mL的氯金酸钠(10 mM)加入50 mL的去离子水中,搅拌均匀。
将10 mL的氢氟酸(1 mM)加入到氯金酸钠溶液中,并在室温下搅拌1分钟。
加入150 µL的氢氨水(10 mM),搅拌后黄色溶液转变为红紫色,表示金纳米颗粒合成成功。
2. 准备检测纸条将金纳米颗粒溶液用滴管或其他方法均匀地涂在滤纸或其他纸张上,并干燥20分钟。
随后,用荧光素偶联抗体或其他特异性指示剂涂抹到纸条上作为检测指针。
三、应用胶体金试纸条可以用于检测各种生物分子,例如细菌、病毒、抗体、癌细胞、DNA、RNA等,以及药物和毒性物质。
其应用领域包括:1. 生物医学研究胶体金试纸条可以用于研究基因检测、蛋白质检测、药物筛选等领域。
2. 医学诊断胶体金试纸条可以用于一些快速筛查、诊断疾病的场合,比如检测患者的生物样本中是否有某种病原菌,或者病人是否感染某种病毒等。
3. 环境监测胶体金试纸条可以用于监测环境中的各种有害物质,如重金属、农药、酸雨等。
4. 食品安全胶体金试纸条可以检测食品中添加的国家规定禁止使用的物质,如兽药、农药等。
一种新冠胶体金抗体检测试纸条及其制备方法

一种新冠胶体金抗体检测试纸条及其制备
方法
以下是一种新冠胶体金抗体检测试纸条及其制备方法的简要介绍:
该测试纸条主要包括纸基、检测线和负对照线,检测线和负对照线上分别固定有新冠病毒抗原和兔抗IgG抗体。
制备方法包括以下步骤:
1.准备纸基,将其涂上样品吸附剂。
2.在纸基上涂布含有新冠病毒抗原的检测线和含有兔抗IgG抗体
的负对照线。
3.干燥并切割纸条,制成测试纸条。
4.将制成的测试纸条包装好,存放于密封袋中。
使用时,将待检测样品滴到样品吸附剂处,样品会被纸基吸附并沿着纸条流动,如果样品中存在新冠病毒抗体,则会与检测线上的新冠病毒抗原结合,形成红色线条,从而显示检测结果为阳性;如果样品中不存在新冠病毒抗体,则只会显示负对照线,检测结果为阴性。
需要注意的是,该测试纸条仅供参考,具体操作时需要根据实际情况进行调整和验证。
胶体金免疫层析试纸条原理

胶体金免疫层析试纸条原理胶体金免疫层析试纸条(Colloidal Gold Immunochromatographic Strip)是一种通过胶体金纳米颗粒与抗体互作用检测特定生物标志物的生物分析方法。
胶体金免疫层析试纸条是一种基于抗体与抗原特异性结合的快速检测试剂,具有简单、快速、灵敏、特异性和便携等优点。
胶体金免疫层析试纸条的原理是利用胶体金纳米颗粒的特性与生物分子相互作用,完成快速检测分析。
试剂条通常由三个部分组成:采样孔、检测膜和吸收纸。
其中检测膜是由贴在聚丙烯酸纤维膜上的抗体分子组成。
检测过程中,样品通常在采样孔处进入试剂条,并沿着检测膜滤过。
样品中的目标分子与膜上的抗体分子结合,并形成膜上的免疫复合物。
在此过程中,胶体金纳米颗粒被添加进样品中。
这些胶体金纳米颗粒上载有反向包被(reverse electrophoretic coating),使胶体金纳米颗粒呈负电荷状态,防止颗粒之间发生不可逆聚集作用。
在测试区和控制区之间的吸收纸层包括了大量的胶体金纳米颗粒。
当测试完成时,样品和胶体金纳米颗粒将被移动到吸收纸层,并进一步与该层的各种化学试剂和物质反应。
控制区内的抗体分子能够与胶体金纳米颗粒发生特异性结合,从而产生红色的线条。
胶体金免疫层析试纸条是一种快速、可视化的生物分析方法,通过免疫反应,通过控制区和测试区之间可见免疫反应条带的形成,快速检测并定性或半定量特定生物标志物。
胶体金免疫层析试纸条的应用范围较广,主要应用于临床检测、环境监测和食品安全等领域。
在临床检测方面,胶体金免疫层析试纸条被广泛应用于诊断感染性疾病,如肺炎、流行性感冒、艾滋病和病毒性肝炎等。
胶体金免疫层析试纸条还可被用于检测肿瘤标志物。
在环境监测方面,胶体金免疫层析试纸条可被用于检测重金属、农药残留、水质监测和气体污染等。
在食品安全方面,胶体金免疫层析试纸条可被应用于检测食品中的防腐剂、色素、激素和有毒污染物等。
胶体金免疫层析试纸条具有多种优势。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ORIGINAL PAPERMicrochip based and immunochromatographic strip assays for the visual detection of interleukin-6and of tumor necrosis factorαusing gold nanoparticles as labelsYan Man&Xuefei Lv&Javed Iqbal&Guang Peng&Da Song&Congxiao Zhang&Yulin DengReceived:8June2014/Accepted:8September2014#Springer-Verlag Wien2014Abstract The enzyme-linked immunesorbent assay(ELISA) is most frequently used for the determination of interleukin-6 (IL-6)and tumor necrosis factor-α(TNF-α).However,this technique is time-consuming and requires a substantial sample volume.We have developed(a)a new kind of colloidal gold-based immunochromatographic strip,and(b)a colloidal gold-based immunochromatographic microchip for simultaneous, rapid,and visual detection of IL-6and TNF-α.The lowest limit of detection is62.5ng mL−1for IL-6,and30ng mL−1for TNF-α.Both the strip method and the microchip method are highly specific,user-friendly,and can be read out without the need for instrumentation.Keywords Immunochromatographic strip.IL-6.TNF-α. Immunochromatographic microchip.Simultaneous detection IntroductionInterleukin-6(IL-6)is a well know pleiotropic cytokine main-ly produced by the immune system cells,vascular endothelial cells and adipocytes.It has a wide range of biological activ-ities in T cells,B cells,hepatocytes,hematopoietic and neural cells[1,2].Its critical role in the immune response,the acute phase response,hematopoiesis,oncogenesis,and inflamma-tion is of utmost importance[3–7].Tumor necrosis factor-α(TNF-α)is another important inflammatory cytokine pro-duced by macrophages,lymphoid cells,neuronal tissue and many other tissues.Numerous studies have shown that the expression of IL-6and TNF-αwas associated with a number of age-related chronic diseases,i.e.,cardiovascular disease, diabetes,cancer,physical disabilities,and cognitive decline [8].So,it is necessary to establish a rapid,simple,sensitive, and specific detection method both for IL-6and TNF-α.The sensitivity is defined as the percentage of positive test re-sponses and specificity as percentage of negative tests.Presently,enzyme-linked immunosorbent assay(ELISA)is the conventional method used for the detection IL-6and TNF-α[9–13].However,this method is laborious,time-consuming and requires complicated cleanup steps,costly equipment and more sample quantity.All these limitations make ELISA unsuitable for the rapid diagnosis of plasma cytokines.The immunochromatographic strip technique has been de-veloped as a popular platform for rapid diagnosis since it was first introduced in the pared to ELISA,the immunochromatographic strip technique has a tendency to detect wide range of samples without pretreatment,low sam-ple volume requirement,long-term preservation,inexpensive and no need of high technology machinery.The critical role of the immunochromatographic strip technique is in point-of-care testing(POCT)[14]which is a test designed to be used at or near the site where the patient is located.This does not require permanent dedicated space and are performed outside the physical facilities of the clinical laboratories.In the immunochromatographic strip,colloidal gold nanoparticles are the most commonly used signal reporters due to their stability,controllable particles size,and good compatibility with biological molecules,i.e.,antibodies,antigens,DNA,and RNA[15].Chou[16]developed a manual self-assembled colloidal gold nanoparticle-immunochromatographicstrip Electronic supplementary material The online version of this article(doi:10.1007/s00604-014-1362-y)contains supplementary material,which is available to authorized users.Y.Man:X.Lv(*):J.Iqbal:G.Peng:D.Song:C.Zhang:Y.Deng(*)School of Life Science,Beijing Institute of Technology,Beijing100081,Chinae-mail:xuefeilv@e-mail:deng@Microchim ActaDOI10.1007/s00604-014-1362-yfor detection of interferon-γup to very low concentrations (ng mL−1level).This strip can be used for homecare and point-of-care testing in health evaluation and preventive medi-cine.Tripathi et al.[17]developed a rapid colloidal gold nano-particles based competitive immunochromatographic strip assay for detection of17-α-hydroxy progesterone(17-α-OH-P)in serum.The detection limit of the test strip was2.5ng mL−1by visual observation.The detection limit is the lowest quantity of a substance that can be distinguished from the absence of that substance(a blank value)within a stated confidence limit[18]. Therefore,this test could work better in screening congenital adrenal hyperplasia(CAH)patients with generally high17-α-OH-P values.In2009,Guo et al.[19]designed two formats of gold-labeled antibody lateral-flow strips for simultaneous detection of carbofuran and triazophos in water samples within a short time(8–10min)without any equipment.The results indicated that the detection limits for carbofuran and triazophos were32 and4ng mL−1using the preferable strip,respectively.For simultaneous detection of IL-6and TNF-α,Worsley et al.[20]prepared a lateral flow assay format utilizing fluo-rescent microspheres with8.5pg mL−1and55.5pg mL−1 limits of detection respectively in hydrogel samples and 7.15pg mL−1and10.7pg mL−1respectively in plasma.Abe et al.[21]recognized IL-6and TNF-αin the independent microchannels on a microchip by the sandwich ELISA with the detection limit of0.28pg mL−1and0.46pg mL−1,respec-tively.Above discussion shows that immunochromatographic strip provided better alternate of ELISA by reducing the problems related to ELISA and combined the advantages of microchip.This system is very quick and efficient that needs less sample volume and is applicable to POCT.Present study is specifically designed to develop a colloidal gold immunochromatographic strip for simultaneous,rapid, and visual detection of IL-6and TNF-α.Furthermore,com-bining the advantages of microchip,achieved the visual and simultaneous detection of IL-6and TNF-αon the fabricated immunochromatographic microchip.ExperimentalMaterials and chemicalsHuman IL-6was ordered from Cell Signaling Technology,Inc (Boston,USA,/).Human TNF-α, TNF-αmonoclonal antibodies(Anti-TNF-αmAbs),TNF-αpolyclonal antibodies(Anti-TNF-αpAbs),IL-6monoclonal antibodies(Anti-IL-6mAbs),IL-6polyclonal antibodies (Anti-IL-6pAbs),and Goat anti-mouse IgG were ordered from Abcam Inc.(Cambridge,USA,http://www.abcam. com/).Gold chloride(HAuCl4·3H2O)was purchased from Sigma-Aldrich(Shanghai,China,)and was directly used without further purification. Bovine serum albumin(BSA),sodium azide,sodium citrate, and Tween-20were ordered from AMRESCO(OH,USA, /).PEG20000was bought from Alfa Aesar(China)Chemical Co.,Ltd.(Shanghai,China, ).Skim milk from Becton, Dickinson and Company(BD,NJ,USA,http://www.bd. com/).Sucrose was ordered from Beijing Chemical Works (Beijing,China,/com-17148/). Water was purified and deionized with a Milli-Q system (Millipore,America).Norland optical adhesive81(NOA 81)was bought from Norland Products Inc.(Cranbury,NJ, USA).All of the chemicals used in current studies were of analytical grade.Conjugation of monoclonal antibodies with colloidal gold nanoparticlesColloidal gold nanoparticles were prepared according to the standard procedures in literatures[22,23]with little modification(See Electronic Supplementary Material, ESM).In order to achieve the optimal conjugation effi-ciency of monoclonal antibodies with colloidal gold nanoparticles,the conjugation conditions including the concentration of monoclonal antibodies and the pH val-ue of colloidal gold solution were determined prior to conjugation(See EMS).The conjugation of monoclonal antibodies with colloidal gold was conducted according to the method in the literature [24].Briefly,5mL of colloidal gold was mixed with40μg of monoclonal antibodies in50mL centrifugation tube with continuous magnetic stirring.This mixture was incubated for 4h at room temperature.Following incubation,the remaining binding sites of the colloidal gold were blocked by adding 10%(m/V)BSA(Tris–HCl,pH8.0)to the final concentration of0.5%(m/V)and continuous stirring for30min at room temperatures.The reaction was further stabilized with10% PEG20000(Tris–HCl,pH8.0),the final concentration of PEG20000was0.2%.The mixture was further incubated at room temperature for20min while stirring.After incu-bation,mixture was centrifuged at8,000rpm for30min to separate unbound monoclonal antibodies.Colorless super-natant was pumped out carefully with a syringe and was discarded.Subsequently,the precipitate of dark red gold-labeled antibody conjugate was resuspended in0.01M Tris–HCl(pH8.0)containing1%BSA and centrifuged at8,000rpm for30min.This procedure was repeated three times to ensure cleanup of the unlabeled monoclonal anti-bodies.Finally,the precipitate of the monoclonal antibodies -colloidal gold conjugate was resuspended in0.5mL of 20mM Tris–HCl buffer(pH8.0)containing1%BSA, 5%sucrose,0.05%PEG20,000,and0.1%Tween-20, and stored at4°C for further use.M.Yan et al.Preparation of immunochromatographic test stripsThree drops(0.5μL each)of polyclonal antibody solution (1mg mL−1)and anti-mouse IgG(1mg mL−1)were put on the nitrocellulose(NC)membrane as test and control dots,and dried at37°C for2h.An absorption pad,antibody-immobilized NC membrane,sample pad,and the conjugate pad were assembled as the test strip(50mm in length and 4mm in width),which was attached to polyvinyl chloride (P V C)s h e e t.A s c h e m a t i c d i a g r a m o f t h e immunochromatography test strip is shown in Fig.S1(See ESM).Firstly,the target proteins were captured by the gold nanoparticles immobilized with monoclonal antibody,and then these nanoparticles were captured by polyclonal antibody in the test dots.On the other hand,the monoclonal antibody immobilized gold nanoparticle without target proteins were captured by the anti-mouse IgG in the control dots.The positive test showed red color both in the test dots and control dots on the NC membrane.Weak positive showed light red color in the test dots and red color in the control dots.Negative test showed red color only in the control dots.The invalid test showed red only in the test dots or did not show red color both in the control dots and test dots.Chip fabricationThe microscope slides were taken,cleaned by alcohol follow-ed by deionized water,and then dried with nitrogen(N2).The structure of chip channel in the photomask was designed using the software of Adobe Illustrator CS5.Following method was adopted for the preparation of chip.First of all,0.4mL of NOA81was placed on the microscope slides,and limited in a given area by a batardeau made by photomask frame,and then the photomask with channel structure was placed on the NOA81layer.The thickness of this NOA81layer was con-trolled by the height of photomask frame.The microscope slides with NOA81were exposed to UV light of Mask Aligner (JKG3,Shanghai,China,/).The NOA81was cured by UV light except the chip channel.The NOA81in the channel can be washed off completely by the mixture of acetone and ethanol(V:V=8:2).In this way,the designed channel structure was fabricated on the microscope slides.In this way,the NOA81substrate with channel structure was bonded with microscope slides.Thirdly,the colloidal gold pad and NC membranes were placed gently in the chip channel by using tweezers.Then,a NOA81substrate with a fluid inlet and a fluid outlet was made on the glass wafers coated with a Cr layer(chrome plate)as described above.The NOA81substrate can be torn off from the chrome plate after UV curing.Finally,this NOA81substrate was bonded with the NOA81channel structure substrate prepared in the first step using the UV curing gun.Hence, an airtight microfluidic channel with a fluid inlet and a fluid outlet was formed,and the immunochromatographic chip was fabricated completely.Results and discussionCharacterization of the colloidal goldThe TEM image of the colloidal gold nanoparticles revealed that the colloidal gold nanoparticles were of nice round shape and homogeneous in size.Average diameter of the colloidal gold particles was24.42±1.78nm(n=100)(Fig.1a).The maximum absorbance of the colloidal gold solution was at 521nm(Fig.1b).From the absorbance spectrum,it can also be found that the colloidal gold solution was at a stable state, and the prepared reproducibility was good.Conjugation of monoclonal antibodies with colloidal gold nanoparticlesColloidal gold nanoparticles are negatively charged having electrical layers around them.As a result,its stable state is due to the repulsive interactions among the colloidal gold nano-particles.The conjugation of protein(monoclonal antibodies herein)with colloidal gold nanoparticles predominantly de-pends on electrostatic interactions.When the pH value of the colloidal gold solution is slightly lower than the pI value of the protein,the protein is partially positively charged,which leads to the formation of the ionic bonds with the colloidal gold nanoparticles.When the colloidal gold particles are tremen-dously coated with the protein,NaCl is unable to induce the aggregation of colloidal gold nanoparticles.So,the optimal pH value and concentration of monoclonal antibodies concen-tration is crucial for the conjugation of monoclonal antibodies with colloidal gold.Optimal concentration of monoclonal antibodiesfor conjugation with colloidal goldThe concentration of monoclonal antibodies for conjugation with colloidal gold particles was optimized under the constant volume of colloidal gold particles and by increasing mono-clonal antibodies concentrations.The results are shown in Table S3and Fig.S2(See ESM).It was found that the color of colloidal gold was blue-black in the absence of monoclonal antibodies.By increasing the concentration of monoclonal antibodies from0.5to2μg,the color of mixture gradually changed from blue-black to blue-violet.When the monoclonal antibodies concentration was4μg,the color of mixture changed to purple red,however,when the concentration of monoclonal antibodies increased from6to12μg,the color of the mixture changed to red,and after that it remained almostGold nanoparticles-labeled immunochromatographic microchipunchanged.So,6μg of monoclonal antibodies per 1mL of colloidal gold was selected as the minimal amount.The opti-mal concentration of monoclonal antibodies was 7.8μg mL −1colloidal gold,i.e.,20%more than the minimal amount.Optimal pH value for conjugation of monoclonal antibodies with colloidal gold particlesThe pH value for conjugation of monoclonal antibodies with colloidal gold was optimized under the constant volume of colloidal gold and the constant amount of monoclonal anti-bodies.The results are shown in Table S4and Fig.S3(see ESM)indicated that when pH value increased from 7.0to 8.0,the color of mixture turns gradually into brilliant red,and did not change further.However,the color of mixture changed from brilliant red to purplish red when pH value increased from 8.5to 9.5.As is clear,the optimal pH value for conju-gation of monoclonal antibodies with colloidal gold is the highest pH value for keeping the colloidal gold at a stable state.So,pH value of 8.0was selected as the optimal value for conjugation of monoclonal antibodies with colloidal gold particles.Characterization of colloidal gold and monoclonal antibodies conjugateThe UV-visible absorption spectrum of colloidal gold parti-cles,colloidal gold -IL-6mAbs conjugate and colloidal gold particles -TNF-αmAbs conjugate was scanned by UV-visible spectrophotometer at a wavelength of 400to 600nm.As shown in Fig.S4(See ESM),the uncoated gold nanoparticles has a UV absorption wavelength at 521nm.When it was covered by the protein (antibody),the UV absorption wave-length will be changed.So,the results indicated that themonoclonal antibodies were successfully conjugated with colloidal gold particles.Determination of human IL-6with immunochromatographic stripStandard solutions of IL-6with concentrations of 2,000,1,000,250,125,62.5and 0ng mL −1were prepared by diluting IL-6with PB buffer.120μL of different concentra-t i o n s o f I L -6s a m p l e s w e r e u s e d i n t h e immunochromatographic strip detection assay.After 30min,various shades of red color on the test dots could be visually observed by naked eyes,the intensity of red color increased with the increase of IL-6concentration (Fig.2).When the concentration of IL-6was 62.5ng mL −1,the red color on the test dots became almost invisible.The results indicated that the detection limit for IL-6is 62.5ng mL −1.Determination of TNF-αwith immunochromatographic strip Standard solutions of TNF-αat concentrations of 500,300,100,50,25,10,0ng mL −1were prepared by diluting TNF-αwith PB buffer.120μL of different concentrations o f T N F -αs a m p l e s w e r e u s e d i n t h e immunochromatographic strip detection.As shown in Fig.3a ,the intensity of red color was elevated with the increase of concentration of TNF-α.It can be observed that with 50–100ng mL −1concentration of TNF-αis,the test dots still showed clear red color.So,another group of TNF-αstandard solution at concentrations of 100,60,40,30,and 15ng mL −1were prepared and detected by the strip.The results shown in Fig.3b demonstrated that when the concentrations of TNF-αis 30ng mL −1,the red color on the test dots is almost invisible.Moreover,belowthisFig.1TEM image of colloidal gold nanoparticles (a )and UV-visible spectrum of the colloidal gold solution (b ).Three lines in (b)represented that the colloidal gold nanoparticles were repeatedly prepared three timesM.Yan et al.concentration,no red color was observed on the test dots.So,the detection limit of TNF-αfor the test strip was found to be 30ng mL −1.The whole analysis process using immunochromatographic strip test for determination of TNF-αwas completed in 10min.Similar results were also found for some macromolecular proteins detection using the same typical method.For exam-ple,the detection limits of 30,50,and 50ng mL −1were obtained for troponin I [25],ricin [26],and human IgG (HIgG)[27],respectively with the nakedeyes.Fig.2Determination of human IL-6with different concentrations using the prepared IL-6colloidal gold immunochromatographicstripFig.3Determination of human TNF-αwith differentconcentrations using the prepared TNF-αcolloidal goldimmunochromatographic stripGold nanoparticles-labeled immunochromatographic microchipIn addition,in order to study the stability of antibody-gold conjugates in a complex media such as serum and if the prepared strip can be appropriate for detecting TNF-αin serum,the TNF-αconcentrations of 160,80,and 0ng mL −1were prepared by diluting TNF-αwith human serum,then the different concentrations of TNF-αwere detected using the prepared strips.As shown in Fig.S5(See ESM),the colorof testing dots was in accordance with the Fig.3.So the serum sample can be detected by the prepared strip and the serum has no influence on the stability of antibody-gold conjugates.Simultaneous detection of IL-6and TNF-αusing the immunochromatographic stripFor the simultaneous detection of IL-6and TNF-α,IL-6test dots,TNF-αtest dots and control dots were prepared on one NC membrane simultaneously.The antibody concentration of the dots is 1mg mL −1.Rests of the steps are the same as mentioned above.The concentrations of IL-6and TNF-αsamples used in the prepared strip can be seen in Table S5(See ESM),and test results are shown in Fig.4.Strip No.1showed red color both at IL-6and TNF-αtest dots;strip No.2showed only red color at IL-6test dots,and strip No.3showed red color only at TNF-αtest pared to strip No.1,strip No.2and strip No.3can be selected as a control test.From the control test,it was found that there is no false positive in the test strip.Simultaneous detection of IL-6and TNF-αusing the prepared colloidal gold immunochromatographic microchip by visual observationT h e 3D s t r u c t u r a l d i a g r a m o f t h e p r e p a r e d immunochromatographic chip is shown in Fig.5a .A tube (1mm O.D.×0.5mm I.D.)was inserted in the inlet and connected to the peristaltic pump.The sample flowed into the chip under the drive of peristaltic pump,and reached the conju-gate pad first.The colloidal gold-monoclonal antibody ontheFig.4Simultaneous detection of IL-6and TNF-αusing the prepared colloidal gold immunochromatographicstripFig.5Simultaneous detection of IL-6and TNF-αusing the prepared colloidal gold immunochromatographicmicrochip.a shows 3D structural diagram of the preparedimmunochromatographic chip.The sample containing 1,000ng mL −1of IL-6and 500ng mL −1of TNF-αwas detected in (b ).The sample only containing 500ng mL −1of TNF-αwas detected in cM.Yan et al.conjugate pad will capture the target in the sample. Then the colloidal gold-monoclonal antibody-target complex flowed into the channel,and would be cap-tured by the polyclonal antibody on the NC membrane. The unbound colloidal gold nanoparticles will be cap-tured by the second antibody.So,when the sample contains the target,both the test dots and control dots will show red color.On contrary,when the sample does not contain target,only control dots will show the red color.As for the colloidal gold immunochromatographic microchip for simultaneous detection of IL-6and TNF-α,the concentration of the polyclonal antibody and the second antibody concentration of IL-6and TNF-αwas1mg mL−1each on the NC membrane. The samples containing1,000ng mL−1of IL-6and 500ng mL−1of TNF-αwere injected in the chip as shown in Fig.5b using a peristaltic pump(TS-2A/ L0107-2A,China,/)with the flow rate of15μL min−1.At the same time,the sample containing only500ng mL−1of TNF-αwas injected in the chip as shown in Fig.5c.As shown in Fig.5b,two test dots and one control dot altogether showed red color due to injection of the sample containing IL-6and TNF-α,while only the TNF-αtest dot and control dot showed red color in Fig.5c as a result of injection of the sample only containing TNF-α.It can be concluded that simultaneous detection of IL-6and TNF-αw a s p e r f o r m e d b y u s i n g t h e p r e p a r e d immunochromatographic chip based on the colloidal gold particles.ConclusionsSimultaneous and visual detection of IL-6and TNF-αwas successfully achieved by using the immunochromatographic strip.Moreover,combining the colloidal gold immunochromatographic strip with microchip,IL-6and TNF-αwere simultaneously and visually detected on micro-chip as well.The developed immunochromatographic strip and microchip offered considerable advantages,i.e.,high specificity,required no pretreatment,less sample amount,easy to operate,rapid determination,no cross-reaction,and long-term preservation.With further development,the immunochromatographic strip and microchip might be cho-sen as a potential analytical tool in real time detection. Acknowledgments This work was supported by the National Nature Science Foundation of China(21275019),the National Key Technology R&D Program of China(2009BAK59B01)and National Key Scientific Instrument and Equipment Development Projects of China (2012YQ040140).References1.Zhang XG,Klein B,Bataille R(1989)Interleukin-6is a potentmyeloma-cell growth factor in patients with aggressive multiple myeloma.Blood74:11–132.Kishimoto T(1989)The biology of interleukin-6.Blood74:1–103.Snick JV(1990)Interleukin-6:an overview.Annu Rev Immunol8:253–2784.Ishihara K,Hirano T(2002)IL-6in autoimmune disease and chronicinflammatory proliferative disease.Cytokine Growth Factor Rev13: 357–3685.Kishimoto T(2010)IL-6:from its discovery to clinical applications.Int Immunol22:347–3526.Rincon M,Irvin CG(2012)Role of IL-6in asthma and otherinflammatory pulmonary diseases.Int J Biol Sci8:1281–12907.Naugler WE,Karin M(2008)The wolf in sheep’s clothing:the roleof interleukin-6in immunity,inflammation and cancer.Trends Mol Med14:109–1198.Singh T,Newman AB(2011)Inflammatory markers in populationstudies of aging.Ageing Res Rev10:319–3299.Helle M,Boeije L,Groot E,Vos A,Aarden L(1991)Sensitive ELISA for interleukin-6.Detection of IL-6in bio-logical fluids:synovial fluids and sera.J Immunol Methods 138:47–5610.Nuntaprasert A,Mori Y,Tsukiyama-Kohara K,Kai C(2005)Establishment of swine interleukin-6sandwich p Immunol Microbiol28:121–13011.Ida N,Sakurai S,Hosaka T,Hosoi K,Kunitomo T,Matsuura Y,Kohase M(1990)An enzyme-linked immunosorbent assay for the measurement of human interleukin-6.J Immunol Methods133:279–28412.Yücel A,Aybay C(2005)Development of ELISA systems formeasurement of human tumor necrosis factor-alpha(TNF-a).Turk J Med Sci35:385–39313.Petyovka N,Lyach L,V oitenok NN(1995)Homologous ELISA fordetection of oligomeric human TNF:properties of the assay.J Immunol Methods186:161–17014.Yang H,Li D,He R,Guo Q,Wang K,Zhang X,Huang P,Cui D(2010)A novel quantum dots–based point of care test for syphilis.Nanoscale Res Lett5:875–88115.Hou SY,Chen HK,Cheng HC,Huang CY(2007)Development of zeptomole and attomolar detection sensitivity of biotin-peptide using a dot-blot gold nanoparticle immuno-assay.Anal Chem79:980–98516.Chou SF(2013)Development of a manual self-assembled colloidalgold nanoparticle-immunochromatographic strip for rapid determi-nation of human interferon-γ.Analyst138:2620–262317.Tripathi V,Nara S,Singh K,Singh H,Shrivastav TG(2012)Acompetitive immunochromatographic strip assay for17-α-hydroxy progesterone using colloidal gold nanoparticles.Clin Chim Acta413: 262–26818.MacDougall D,Crummett WB(1980)Guidelines for data acquisitionand data quality evaluation in environmental chemistry.Anal Chem 52:2242–224919.G u o Y R,L i u S Y,G u i W J,Z h u G N(2009)G o l dimmunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples.Anal Biochem389: 32–3920.Worsley GJ,Attree SL,Noble JE,Horgan AM(2012)Rapid dupleximmunoassay for wound biomarkers at the point-of-care.Biosens Bioelectron34:215–22021.Abe K,Hashimoto Y,Yatsushiro S,Yamamura S,Bando M,Hiroshima Y,Kido J,Tanaka M,Shinohara Y,Ooie T,Baba Y, Kataoka M(2013)Simultaneous immunoassay analysis of plasma IL-6and TNF-a on a microchip.Plos One8:e53620Gold nanoparticles-labeled immunochromatographic microchip22.Frens G(1973)Controlled nucleation for the regulation of the particlesize in monodisperse gold suspensions.Nat Phys241:20–2223.He Y,Zhang S,Zhang X,Baloda M,Gurung A,Xu H,Zhang X,LiuG(2011)Ultrasensitive nucleic acid biosensor based on enzyme-gold nanoparticle dual label and lateral flow strip biosensor.Biosens Bioelectron26:2018–202424.Dungchai W,Siangproh W,Chaicumpa W,Tongtawe P,Chailapakul O(2008)Salmonella typhi determination using voltammetric amplification of nanoparticles:a highly sensitive strategy for metalloimmunoassay based on a copper-enhanced gold label.Talanta77:727–73225.Choi DH,Lee SK,Oh YK,Bae BW,Lee SD,Kim S,Shin YB,KimMG(2010)A dual gold nanoparticle conjugate-based lateral flow assay(LFA)method for the analysis of troponin I.Biosens Bioelectron25:1999–200226.Shyu RH,Shyu HF,Liu HW,Tang SS(2002)Colloidal gold-basedimmunochromatographic assay for detection of ricin.Toxicon40: 255–25827.Parolo C,Muñiz AE,Merkoçi A(2013)Enhanced lateral flowimmunoassay using gold nanoparticles loaded with enzymes.Biosens Bioelectron40:412–416M.Yan et al.。
