《吡啶及其衍生物》PPT课件
吡啶材料介绍

吡啶及其衍生物是目前杂环类化合物中开发应用范围最多的品种之一,是重要的精细化工原料,主要广泛应用在农药、医药、染料、日用化工、香料、饲料添加剂、橡胶助剂等领域。
近几年全世界发展低毒农药迅速,吡啶及其衍生物在高效低毒杀虫剂、除草剂、杀菌剂等方面应用量迅速扩大,在医药和饲料业也有大量需求。
吡啶系列产品主要包括纯吡啶和合成产生的低碳烷基取代物3-甲基吡啶、2-甲基吡啶和4-甲基吡啶,主要用做下述三类衍生物的生产原料:百草枯、杀草快和敌草快等除草剂、烟酸、烟酰胺、农药中间体三氯代吡啶。
纯吡啶是重要的溶剂,可用于制造维生素、中枢神经兴奋剂、抗菌素以及一些高效农药和还原染料,其主要应用有:1)医药:为氟哌酸,维生素A、D2、D3,头孢4号、心脑血管用药、抗动脉硬化剂等40余种常用药的合成原料。
2)农药:用作高效除草剂百草枯、杀草快、敌草快、吡氟禾草灵,高效杀虫剂氯氟脲(定虫隆,兼有杀虫和不育功能,对人体无害)的合成原料。
3)染料:合成可溶性还原紫14R等10个品种及活性翠蓝KN-G、阳离子艳黄10GFF等。
3-甲基吡啶既是合成吡啶类香料的重要中间体,又是制备吡啶类农药的重要中间体,也可用来生产合成吡氟禾草灵(稳杀得)的关键中间体2-氯-5-三氟甲基吡啶。
稳杀得是用来防治稗科杂草的选择性芽后除草剂,适用于大豆、棉花、油菜等大田作物。
美国、日本等国已将它提升为除草剂的骨干品种;3-甲基吡啶还可作溶剂、酒精变性剂、染料和树脂中间体,用来生产橡胶硫化促进剂、防水剂和胶片感光剂添加物等。
在医药行业中, 3-甲基吡啶用于合成烟酸、烟酰胺、兰索拉唑、维生素B、尼可拉明和强心药等。
兰索拉唑主要用于食管炎和十二指肠溃疡的短期治疗,与奥美拉唑相比,兰索拉唑具有更好的疗效、较少的副作用和更强的稳定性。
我国幅员辽阔,拥有耕地面积近15亿亩,播种面积为23.4亿亩次,根据我国农业发展和农药行业现状,“十二五”期间我国农药行业发展的指导思想是:深入贯彻落实科学发展观,适应国内外形势新变化,以加快转变农药工业发展方式为主线,以满足国内农业生产需要为主要任务,着力提高农药科技创新能力,调整产品结构,提升质量和档次,优化产业布局,加快农药企业兼并重组,推动产业集聚和升级,切实保护生态环境,保障食品安全,促进农药行业长期平稳健康发展。
1[1].1万吨年吡啶及其衍生物
![1[1].1万吨年吡啶及其衍生物](https://img.taocdn.com/s3/m/de6af4c5b8f67c1cfad6b8aa.png)
安徽氯碱化工集团有限责任公司1.1万吨/年吡啶及其衍生物一、项目名称:1.1万吨/年吡啶及其衍生物二、建设单位:安徽氯碱化工集团有限责任公司三、法人代表:方立贵项目负责人:周杰联系电话:0551-传真:0551-四、企业概况:安徽氯碱化工集团有限责任公司是由合肥化工厂于1997年12月28日改制而成,2001年9月成功实现债转股,新公司注册资本金为3.683亿元人民币,现正积极运作上市,自2003年8月进入上市辅导期。
经过几十年的发展,该公司已成为安徽省最大的氯碱化工企业、全国化工百强企业、国家大型一档企业,银行信用等级AAA。
现有职工3026人,其中各类专业技术人员750人;企业占地面积77万平方米,建筑面积35万平方米。
公司主要产品及生产能力如下:1)烧碱10万吨/年,其中离子膜碱5万吨/年(含片碱2万吨/年),金属阳极隔膜碱5万吨/年;2)盐酸2万吨/年;3)液氯6万吨/年;4)聚氯乙烯 5万吨/年,其中糊状树脂3万吨/年,悬浮树脂2万吨/年;5)甲酸钠法保险粉4万吨/年;6)18%杀虫双水剂、杀虫单、虱螟单农药3万吨/年;7)草甘膦除草剂0.25万吨/年等。
公司共有16个产品,其中有11个产品分别获省、部级以上优质产品称号;公司于2002年通过ISO9001质量体系认证。
五、项目建设的必要性:吡啶及其衍生物是煤化工产品链的下游产品,主要产品有吡啶、3-甲基吡啶、其它吡啶混合物及烟酸/烟酰胺。
吡啶及其衍生物是农药、医药等领域的重要中间体或溶剂,其下游产品多,产品链长,辐射面大。
吡啶主要用于农药合成,如生产百草枯、敌草快、毒死蜱、甲基毒死蜱等。
多年来,我国吡啶类化合物一直依赖进口,价格较高,抑制了下游产品的研究开发,严重影响了我国农药、医药及其它精细化工产品的发展。
随着除草剂百草枯、敌草快、杀虫剂毒死蜱在中国市场的不断开发,预计吡啶的消费在中国将有明显的增长。
吡啶也是化学医药生产的重要原料,我国目前生产的二十一大类化学药中,有十二类与吡啶相关。
P122014410_王瑞苗_化学工程与工艺_吡啶及其衍生物稳定性的计算研究 (3)

毕业论文(设计)题目:吡啶及其衍生物稳定性的计算研究姓名:王瑞苗学号:P122014410学院:化工学院专业:化学工程与工艺班级:12级化学工程与工艺1班指导老师:张斌诚信声明此毕业论文是本人在导师的认真指导下,对我这大学四年学习的总结和检阅,此篇论文是本人独立进行实验的研究结果。
除文中已加标注引用的内容外,论文中不包括其他个人或集体已经发表或撰写过的研究成果,也不含其他人已经发表过或使用过的材料。
对本文的研究做出重要贡献的个人及相关导师,均在文中明确标明。
特此申明。
声明人:时间:年月日目录摘要 (1)1.前言 (3)1.1 绪论 (3)1.1.1 吡啶及其衍生物的研究意义 (3)1.1.2 吡啶及其衍生物的研究现状 (3)1.2 吡啶 (4)1.2.1 吡啶的物理性质 (4)1.2.2 吡啶的化学性质 (4)1.2.3 吡啶的存在与合成方法 (5)1.2.4 吡啶的用途 (5)1.3 2-氯吡啶 (5)1.3.1 2-氯吡啶的物理性质 (5)1.3.2 2-氯吡啶的制备方法 (6)1.3.3 2-氯吡啶的用途 (6)1.4 4-甲基吡啶 (7)1.4.1 4-甲基吡啶的物理性质 (7)1.4.2 4-甲基吡啶的化学性质 (7)1.4.3 4-甲基吡啶的制备方法及用途 (8)1.5 2-氨基吡啶 (8)1.5.1 2-氨基吡啶的物理性质 (8)1.5.2 2-氨基吡啶的制备方法及用途 (9)1.6 2-羟基吡啶 (9)1.6.1 2-羟基吡啶的物理性质 (9)1.6.2 2-羟基吡啶的化学性质 (10)1.6.3 2-羟基吡啶的制备方法及用途 (10)1.7 2-乙烯基吡啶 (10)1.7.1 2-乙烯基吡啶的物理性质 (10)1.7.2 2-乙烯基吡啶的化学性质 (11)1.7.3 2-乙烯基吡啶的制备方法及用途 (11)2. 实验方法与计算 (12)2.1 实验方法简介 (12)2.2 计算方法及概念简介 (12)3. 实验结果与讨论 (16)3.1 分子结构图 (16)3.2 电荷 (17)3.3 键长 (19)3.4 键角 (21)3.5 频率 (22)3.5.1 吡啶频率 (22)3.5.2 2-氯吡啶频率 (23)3.5.3 4-甲基吡啶频率 (24)3.5.4 2-氨基吡啶频率 (24)3.5.5 2-羟基吡啶频率 (25)3.5.6 2-乙烯基吡啶频率 (26)3.6 偶极距 (27)3.7 分子轨道 (27)3.8 静电势 (30)4. 结论 (33)4.1 实验结论 (33)参考文献 (34)答谢 (35)吡啶及其衍生物稳定性的计算研究专业:化学工程与工艺姓名:王瑞苗指导教授:张斌摘要从头算计算法是一种基本的量子化学计算方法。
吡啶衍生物的合成及其胶凝性能

2013年5月周家吉等.吡啶衍生物的合成及其胶凝性能45吡啶衍生物的合成及其胶凝性能周家吉,罗序中,王科军,柳辉金(江西省有机药物化学重点实验室,赣南师范学院,江西赣州341000)[摘要]以琥珀酸和2,6一二氨基吡啶为原料,设计合成了2,6一二[Ⅳ一(羧乙基羰基)氨基]吡啶(D A P),并研究了其胶凝性能。
结果表明,D A P 能在水溶液中自组装成水凝胶,其最小胶凝浓度为43m g /m L ;当稳定凝胶在温度升至8l ℃时,凝胶坍塌形成沉淀;溶液为碱性时,D A P 不能形成水凝胶。
x 射线衍射和扫描电镜检测结果显示,该水凝胶具有多晶态纤维网络结构;红外光谱表征结果表明,水凝胶通过分子间氢键作用形成。
[关键词]吡啶衍生物水凝胶胶凝性能超分子凝胶是由低相对分子质量的水凝胶因子通过分子间氢键、7r 一仃堆积、疏水相互作用、范德华力和电子转移等分子间非共价键相互作用,自组装形成线型、纤维状或带状结构,继而形成三维网络结构的聚集体,因其具有低毒、生物相容性、生物降解性以及容易制备等优点,在药物载体、组织工程、基因传递试剂、生物传感器和响应性材料等方面都有很好的应用价值,已成为研究的热点…。
M enger 等旧。
51深人研究了双电荷表面活性剂类胶凝情况,得到了可以形成凝胶的几个系列化合物。
Shi nkai 等M 。
7o 报道了利用巴比妥酸盐的衍生物和双胆固醇化合物、冠醚和二胺通过氢键互补作用形成双组分凝胶体系的研究成果。
唐黎明等旧1以2,6一二氨基吡啶为母体,将琥珀酸接于母体两头,合成了一种超分子水凝胶单体,实现了氢键结合其水凝胶的形成与结构调控,并对水凝胶的形成过程进行推测。
笔者以琥珀酸和2,6一二氨基吡啶为原料,设计合成同时含有羧基、吡啶基和酰胺基的化合物2,6一二[Ⅳ一(羧乙基羰基)氨基]吡啶(D A P)(反应原理见图l 旧1),探讨依靠分子间多个氢键的协同作用,以期制备稳定的水凝胶,并探究其胶凝性能和潜在应用价值。
吡啶及其衍生物的合成技术及在农药领域中的应用

Cl N
CH-NO2
Cl N
CH2NH2
+
CH2NH2
+
Cl N
SCH3 (CH3)2NC CHNO2
CH3OH
H N(CH3)2
CH2 N NH
Cl N
CH-NO2
O
CH3CH2CCH2NOH
CH2 N C2H5
Cl N
CH-NO2
Cl N
CH2NH2
+
(CH3S)2C=CHNO2
CH2NH2
Cl N
CH3COCF3
CS2 , CH3I
H
CH2 N SCH3
Cl N
CHCOCF3
H
(CH3)2NH
CH2 N N(CH3)2
Cl N
CHCOCF3
2-氯-5-氨甲基吡啶的应用
❖吡啶甲胺基噻唑基乙脒类杀虫剂
H
CH2NH2
+
Cl N
S OMe
N
N
CH3
EtOH
❖吡啶杂环基吡咯类杀虫剂
2,3,5,6-四氯吡啶的合成技术
五氯吡啶脱氯法
氯化法
分子闭和成环法
以3,3,5-三氯戊二酰 胺为原料
五氯吡啶脱氯法
❖ 五氯吡啶法是以五氯吡啶被锌粉等还原脱氯 生成2,3,5,6-四氯吡啶的方法
氯化法
❖ (1)液相催化制备2,3,5,6-四氯吡啶 ❖ (2)气相催化制备
3,3,5-三氯戊二酰胺为原料
吡啶及衍生物的合成技术 及在农药领域中的应用
肖国民 博士、教授、博导 张进 博士、副教授
吡啶及衍生物的合成技术 及在农药领域中的应用
---
吡啶及其衍生物.ppt

催化剂去再生塔再生后循环使用。 冷凝液经萃取、精馏(4个精馏塔)。
25
精馏塔制得产物: 2-甲基吡啶、 2-甲基-5-乙基吡啶。 另外一些副产物如4-甲基吡啶和二甲基吡
啶等。
2-甲基吡啶与2-甲基-5-乙基吡啶比例与反 应时间、压力、催化剂的浓度等有关。
17
乙醛 氨
常压、350-500℃,Al2O3为催化剂
气体
预热
催化反应
冷凝
工 艺
精制
分馏
过
程
2-甲基吡啶 4-甲基吡啶
含量:99.2% 收率40-60%
脱水
18
乙醛与氨气相反应与乙醛、甲醛和氨气相 反应所用的催化剂基本相同,
可以设计一套装置进行两种反应的生产, 意大利Montecation公司就有一套装置生产
26
⑤丙酮与丙烯腈反应
丙烯腈与过量的丙酮以伯胺及弱酸催化、在 180℃及2.2 MPa(表压)条件下进行液相反 应,主要得到单氰乙基产物。
后 经者氰在基的H2还流原下、经环过化含及钯脱催氢化,剂得的到气2相-甲反基应吡器, 啶。
O CH3CCH3 + CH2=CHCN
O CH3CCH2CH2CH2CN
N
CH3
27
三 3-甲基吡啶 3-Methylpyridine
(1)性质,无色油状液体,有不愉快的气 味,能与水,乙醇、乙醚混溶。
别名:β-甲基吡啶、3-皮考林。
分子式:C6H7N,
分子量:93.13,
CH3
结构式:
N
28
基本物性数据如下:
29
有毒,液体和蒸气刺激皮肤和粘膜,能使神经中枢 麻醉,可引起流泪、咳嗽、不适、眩晕、头痛、疲 劳、呼吸频繁、四肢震颤、麻醉和昏睡等症状。
第八章课件-1吡啶类药物的分析 药物分析(第二版)课件

第八章 杂环类药物分析
第一节 吡啶类药物的分析
四、检查
有关物质 本类药物在生产和贮藏过程中易引入有关杂质,因
其化学结构不明,故中国药典(2010年版)对异烟肼及
其片剂,尼克刹米及其注射液均采用高效液相色谱法检
返回
第八章 杂环类药物分析
第一节 吡啶类药物的分析
三、鉴别
吡啶开环反应
吡啶环在一定条件下开环,产生的醛类产物与羰基试 剂缩合,生成有色物质:当溴化氰与芳伯胺作用于吡啶环,
首先是在溴化氰作用下,吡啶环水解,形成戊烯二醛,后
者再与芳香第一胺缩合,生成有色的Schiff碱(聚甲炔染 料)沉淀,沉淀颜色随所用芳胺不同而异,如与苯胺缩合 形成黄至黄棕色,与联苯胺则形成淡红至红色。
第八章 杂环类药物分析
第一节 吡啶类药物的分析
二ห้องสมุดไป่ตู้性质
酰肼基的特性 异烟肼吡啶环γ位上被酰肼取代,酰肼基具有较强
的还原性,也可与某些含羰基的试剂发生缩合反应,可 用于定性鉴别、含量测定。
返回
第八章 杂环类药物分析
第一节 吡啶类药物的分析
二、性质
水解特性 尼可刹米分子中,吡啶环β位上被酰氨基取代,虽
第八章 杂环类药物分析
第一节 吡啶类药物的分析
三、鉴别
吡啶开环反应
反应式:
N
C3H
N
C
C3H
O
CNBr
NCBr
N
C3H 2 H 2 O
N
C
C3H
O
OCH CHOH
CH3
N
+ NH2CN + HBr
C
CH3
O
第八章 杂环类药物分析
天然产物课件第四章【2024版】

二、生物碱的分布
③少数单子叶植物如石蒜科,百部科 (Stemonaceae),百合科(Liliaceae)等植物中有分 布。在低等植物中,生物碱分布少,而且结构一般为 简单。生物碱在生物体中的存在部位和含量往往差别 很大,一般来说,含量在千分之一以上即为高含量。
三、生物碱的分类
按氮原子是否结合在环上可分为两大类: 有机胺类和氮杂环类:
• 酰胺型:P—π共轭,氮原子周围电子云密度下降, 碱性降低。
• 胍基型: 供电基和氮原子上未共享电子对共轭, 碱性增强(共轭酸的高度共振稳定性,使共轭酸稳 定,Ka小,则pKa大,碱性强)。
四、生物碱的性质
(4)、空间效应:阻碍质子靠近氮原子,使碱性降
低(莨菪碱和东莨菪碱)。
CH3 N
H CH2OH
羧基生物碱(槟榔次碱)NaHCO3 • 内酯型生物碱(喜树碱): 热NaOH
皂化)
+ (碱水解、
• 内酰胺生物碱(苦参碱): 碱水解
四、生物碱的性质
(3)生物碱的盐(离子型、极性大):
+
+
-
+
-
• 在水中的溶解度与酸有关: • 无机酸盐的水溶度大于有机酸盐的水溶度。 • 无机酸盐中,含氧酸盐的水溶度大于卤代酸盐。 • 卤代酸盐中,盐酸盐的水溶度最大,氢碘酸盐
四、生物碱的性质 影响碱性强弱的因素:
(1).氮原子的杂化方式: SP3 > SP2 > SP
NH RC N
N
四氢异喹啉(SP3 pKa9.5)
异喹啉(SP2 pKa5.4)
氰类(SP 中性 ) 电效应
四、生物碱的性质
(2)、诱导效应:
供电诱导效应(烷基):可使氮原子周围电子云密度 增加,碱性增强。 吸电诱导效应(含氧基团,双键,苯环):电子云密 度降低,碱性减弱。
吡啶及其衍生物(Pyridine_and_Pyridine_Derivates)
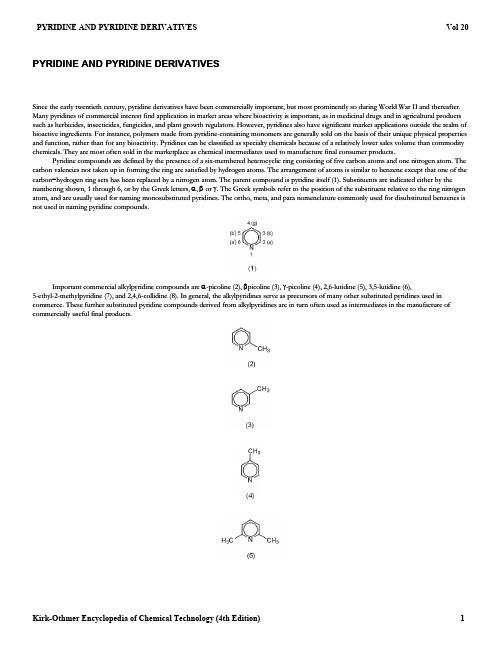
Since the early twentieth century, pyridine derivatives have been commercially important, but most prominently so during World War II and thereafter.Important commercial alkylpyridine compounds are PYRIDINE AND PYRIDINE DERIVATIVESMany pyridines of commercial interest find application in market areas where bioactivity is important, as in medicinal drugs and in agricultural products such as herbicides, insecticides, fungicides, and plant growth regulators. However, pyridines also have significant market applications outside the realm of bioactive ingredients. For instance, polymers made from pyridine-containing monomers are generally sold on the basis of their unique physical properties and function, rather than for any bioactivity. Pyridines can be classified as specialty chemicals because of a relatively lower sales volume than commodity chemicals. They are most often sold in the marketplace as chemical intermediates used to manufacture final consumer products.Pyridine compounds are defined by the presence of a six-membered heterocyclic ring consisting of five carbon atoms and one nitrogen atom. The carbon valencies not taken up in forming the ring are satisfied by hydrogen atoms. The arrangement of atoms is similar to benzene except that one of the carbon −hydrogen ring sets has been replaced by a nitrogen atom. The parent compound is pyridine itself (1). Substituents are indicated either by the numbering shown, 1 through 6, or by the Greek letters, α, β or γ. The Greek symbols refer to the position of the substituent relative to the ring nitrogen atom, and are usually used for naming monosubstituted pyridines. The ortho, meta, and para nomenclature commonly used for disubstituted benzenes isnot used in naming pyridine compounds.α-picoline (2), βpicoline (3), γ-picoline (4), 2,6-lutidine (5), 3,5-lutidine (6),5-ethyl-2-methylpyridine (7), and 2,4,6-collidine (8). In general, the alkylpyridines serve as precursors of many other substituted pyridines used in commerce. These further substituted pyridine compounds derived from alkylpyridines are in turn often used as intermediates in the manufacture ofcommercially useful final products.As is the case with most specialty organic compounds, pyridine sales are generally not publicized, and industrial processes for their manufacture The physical properties of pyridines are the consequence of a stable, cyclic, 6- Physical propertyValue 40.2are either retained as trade secrets or patented (see P ATENTS AND TRADE SECRETS ). Up to about 1950, most pyridines were isolated from coal-tar fractions;however, after 1950 synthetic manufacture began to take an ever-increasing share of products sold. By 1988, over 95% of all pyridines were produced by synthetic methods.Pyridine was first synthesized in 1876 (1) from acetylene and hydrogen cyanide. However, α-picoline (2) was the first pyridine compound reported to be isolated in pure form (2). Interestingly, it was the market need for (2) that motivated the development of synthetic processes for pyridines during the 1940s, in preference to their isolation from coal-tar sources. The basis for most commercial pyridine syntheses in use can be found in the early work of Chichibabin (3). There are few selective commercial processes for pyridine (1) and its derivatives, and almost all manufacturing processes produce (1)along with a series of alkylated pyridines in admixture. The chemistry of pyridines is significantly different from that of benzenoids. Pyridines undergo some types of reaction that only highly electron-deficient benzenoids undergo, and do not undergo some facile reactions of benzenoids, such as Friedel-Crafts alkylation and C-acylation, for example.Physical Propertiesπ-electron, π-deficient, aromatic structure containing a ring nitrogen atom.The ring nitrogen is more electronegative than the ring carbons, making the two-, four-, and six-ring carbons more electropositive than otherwise would be expected from a knowledge of benzenoid chemistries. The aromatic π-electron system does not require the participation of the lone pair of electrons on the nitrogen atom; hence the terms weakly basic and π-deficient used to describe pyridine compounds. The ring nitrogen of most pyridines undergoes reactions typical of weak, tertiary organic amines such as protonation, alkylation (qv), and acylation.Liquid pyridine and alkylpyridines are considered to be dipolar, aprotic solvents, similar to dimethylformamide or dimethyl sulfoxide. Mostpyridines form a significant azeotrope with water, allowing separation of mixtures of pyridines by steam distillation that could not be separated by simple distillation alone. The same azeotropic effect with water also allows rapid drying of wet pyridines by distillation of a small forecut of water azeotrope.Pyridine. Many physical properties of pyridine are unlike those of benzene, its homocyclic counterpart. For instance, pyridine has a boilingpoint 35.2°C higher than benzene (115.3 vs 80.1°C), and unlike benzene, it is miscible with water in all proportions at ambient temperatures. The much higher dipole moment of pyridine relative to benzene is responsible, in significant part, for the higher boiling point and water solubility. Benzene and pyridine are aromatic compounds having resonance energies of similar magnitude, and both are miscible with most other organic solvents. Pyridine is a weak organic base (p K a =5:22), being both an electron-pair donor and a proton acceptor, whereas benzene has little tendency to donate electron pairs or accept protons. Pyridine is less basic than aliphatic, tertiary amines. Table 1 lists some physical properties of pyridine, and Table 2 compares physical properties of pyridine to some alkyl- and alkenylpyridine bases.Table 1. Physical Properties of Pyridine enthalpy of fusion at ¡41:6±C , kJ/molenthalpy of vaporization, kJ/mol bat 25°C 115.26°C35.11Water azeotrope CAS Structure Freezing Boiling Density,Index of Water20[110-86-1](1)79.10¡115.30.9830 1.5102 5.2293.641.32-picoline ¡ 5.2(6)atom, such as critical temperature, °C346.8critical pressure, MPac5.63enthalpy of formation, gas at 25°C, kJ/molb140.37Gibbs free energy of formation, gas at 25°C, kJ/mol b190.48heat capacity, gas at 25°C, J/(K ¢mol)78.23ignition temperature, °C 550explosion limit, %1.7−10.6surface tension, liquid at 25°C, mN =m(=dyn =cm)36.6viscosity, liquid at 25°C, mPa(=cP)0.878dielectric constant, liquid at 25°C, ε13.5thermal conductivity, liquid at 25°C, W/(K ¢m)0.165aRef. 4.bTo convert kJ to cal, divide by 4.184.cTo convert MPa to atm, multiply by 9.87.Table 2. Physical Properties of Pyridine, Alkyl-, and Alkenylpyridine Derivatives aCompoundRegistry Number numberMol wtpoint, °Cpoint, °Cd 204refraction,n 204solubility bat 20°C,g/100 mLp K a at °C, inwater cBp d, °C Water, %pyridine41:6e f ,g[109-06-8](2)93.13¡66:7129.50.9462 1.5010e 5.9693.5483-picolineg[108-99-6](3)93.13¡18:3143.90.957 1.5043e 5.6396.7634-picoline f ,g[108-89-4](4)93.13 3.7144.90.9558 1.5058e5.9897.463.52,3-lutidine [83-61-9](58)107.1615:5161.50.9491 1.508513.36.572,4-lutidine [108-47-4]107.16¡64158.70.9325 1.5000e6.6371.567.42,5-lutidine [589-93-5]107.16¡15:71570.9331 1.500510.06.402,6-lutidine [108-48-5](5)107.16¡6:1143.70.923 1.4977e6.7293.351.53,4-lutidine [583-58-4]107.16¡10:6179.10.9534 1.5081 6.463,5-lutidine [591-22-0]107.16¡6:6172.70.944 1.5049 3.3 6.152,4,6-collidine [108-75-8](8)121.18¡44:5170.40.913 1.4981 3.67.435-ethyl-2-meth yl-pyridine[104-90-5](7)121.18¡70:9178.30.9208 1.4974 1.298.4722-vinylpyri-din e[100-69-6](23)105.14110h 0.9746 1.5509 2.75 4.9897.062.04-vinylpyri-din e [100-43-6]105.14121h0.9881.55252.915.6298.076.6aRef. 5, unless otherwise noted bRef. 6.cRef. 7.dRef. 8.eMisciblef Ignition temperature =535±C for (2) and 500°C for (4).gExplosion limit =1:48:6% for (2) and 1.3−8.7% for (3) and (4)hAt 20 kPa (150 mm Hg).Other Pyridine Bases. The nucleophilicity and basicity of pyridines can be reduced by large, sterically bulky groups around the nitrogentert -butyl in the 2- and 6-positions. Sterically undemanding groups like methyl tend to increase basicity relative to parent pyridine, asexpected. Electron-withdrawing substituents can also reduce pyridine basicity and nucleophilicity. 2,6-Dichloropyridine [2402-78-0] is sterically hindered and also contains strong electron-withdrawing substituents. As such, it cannot be titrated by acid, even by using extremely acidic media such as perchloricacid in acetic acid solvent.Generally, hydrophobic substituents on the pyridine ring reduce water solubility, polar ones capable of hydrogen bonding as acceptor or donor,increase it.Table 3 gives the corresponding physical properties of some commercially important substituted pyridines having halogen, carboxylic acid, ester,carboxamide, nitrile, carbinol, aminomethyl, amino, thiol, and hydroxyl substituents.Table 3. Physical Properties of Substituted Pyridines Water solubility 202-chloropyridine [109-09-1]113.5516887 1.627 2.10.9picolinic acid1.166 1.519 5.013.5 1.361.031.053 1.5431 1.431.065>8.3aRegistry Number number point, °Cpoint, °C d 204refraction,n 20D at 20°C, g/100 mLp K a at °C in water (17)¡46:5−170 1.205d1.5322.50.52,6-dichloropyridine [2402-78-0]147.99−89211ef2-bromopyridine[109-04-6]158.00192−194194.81.5714g[98-98-6]123.11134−136h1.4896 5.39nicotinic acid i [59-67-6](27)123.11236−239h 1.473 1.7 4.81isonicotinic acidj [55-55-1]123.11314−315h0.5 4.86methyl picolinate [2459-07-6]137.1418.792k 2.21ethyl nicotinate [614-18-6]151.178−10223−2241.107 1.50404.48picolinamide [1452-77-3]122.13110 dec 143l 1.3918 2.10nicotinamide [98-92-0](26)122.13128−131 1.401120 3.332-cyanopyridine [100-70-9](14)104.1126−28212−215 1.081 1.5290¡0:263-cyanopyridine [100-54-9](25)104.1150−52206 1.159 1.5252m4-cyanopyridine [100-48-1](15)104.1179195n4.01.903-pyridylcarbinol [100-55-0](30)109.13¡6:5266 1.124 1.5455o 4.904-pyridylcarbinol [586-95-8]109.1357−59146p o5.332-picolylamine [3731-51-9]108.1481203o 8.84-picolylamine [3731-53-1]108.14¡7:62291.0661.5493o4.392-mercaptopyridine [2637-34-5]111.17128−130¡1:074-mercaptopyridine [4556-23-4]111.17184−1862-pyridone [142-08-5](16)95.10107294>100 1.253-hydroxypyridine [109-00-2](40)95.101263.64.94-pyridone [626-64-2](38)95.10150181q >1003.232-aminopyridine [504-29-0](35)94.1258211100 6.93-aminopyridine [462-08-8](33)94.1262.2248 1.24>1005.984-aminopyridine [504-24-5](34)94.121592731.2509.24-dimethylamino-pyri dine [1122-58-3](24)122.17112162r 7.69.5aMeasured in the laboratories of Reilly Industries, Inc., unless otherwise noted.bRef. 6.cRef. 7.dRef. 9.eInsoluble.fCannot be protonated.g2-Carboxypyridine.hSublimes.i3-Carboxypyridine.j4-Carboxypyridine.kAt 4 Pa (0.03 mm Hg).lAt 2.7 kPa (20 mm Hg).mAt 50°C.nAt 80°C.oMiscible.At 1.3 kPa (10 mm Hg).knowledge of molecular structure, that seems to work well for pyridine compounds. Such a prediction can be used to estimate real physical properties of Chemical reactivity of pyridines is a function of ring aromaticity, presence of a basic ring nitrogen atom, Ring-atomic centers can undergo attack by electrophiles, easily at the ring nitrogen and less easily at ring carbons. Nucleophilic attack is also possible at Reactive halogen compounds, alkyl halides, and activated alkenes give quaternary pyridinium salts, such as (12). Oxidation with peracids gives p qAt 0.133 kPa (1 mm Hg).rAt 6.7 kPa (50 mm Hg).Quantitative Structure-Property Relationships. A useful way to predict physical property data has become available, based only on apyridines without having to synthesize and purify the substance, and then measure the physical property.The relationship between the structure of a molecule and its physical properties can be understood by finding a quantitative structure −property relation- ship (QSPR) (10). A basis set of similar compounds is used to derive an equation that relates the physical property, eg, melting point or boiling point, to structure. Each physical property requires its own unique QSPR equation. The compounds in the basis set used for QSPRs with pyridines have sometimes been quite widely divergent in respect to structural similarity or lack of it, yet the technique still seems to work well. The terms of the equation are composed of a coefficient and an independent variable called a descriptor. The descriptors can offer insight into the physical basis for changes in the physical property with changes in structure.The same strategy can be used to relate chemical reactivity, catalytic ability, and bioactivity (10) of pyridine compounds with their structure.Although such a prediction is still in its formative stage, early results have been encouraging.Chemical Propertiesπ-deficient character of the ring, large permanent dipole moment, easy polarizability of the π-electrons, activation of functional groups attached to the ring, and presence of electron-deficient carbon atom centers at the α- and γ-positions. Depending on the conditions of the chemical transformation, one or more of these factors can give rise to the observed chemistry. The chemistry of pyridines can be divided into two categories: reactions at the ring-atomic centers, and reactions at substituents attached to the ring-atomic centers.REACTIONS AT RING ATOMSring carbons or hydrogens.Electrophilic Attack at Nitrogen. The lone pair on pyridine (1) (p K a =5:22) reacts with electrophiles under mild conditions, withprotonic acids to give simple salts, with Lewis acids to form coordination compounds such as (9) [2903-67-5], and with transition metals to form complex ions, such as (10) [24444-58-4]. The complex ion pyridinium chlorochromate [67369-53-3] (11) is a mild oxidizing agent suitable for the conversion ofalcohols to carbonyl compounds.pyridine N -oxides, such as pyridine N -oxide itself [694-59-7] (13), which are useful for further synthetic transformations (11).electron-donating substituents are attached to the ring. Knowledge of this fact has resulted in the widespread use of pyridine as a solvent for reactions A modification of the Reissert-Henze reaction employing benzoyl chloride and (CH Pyridine and its methyl derivatives, (2), (3), and (4), undergo amination with sodium amide at the 2-position eg, 2-amino-3-methylpyridine Electrophilic Attack at Carbon. Electrophilic attack at a C-atom in pyridines is particularly difficult unless one or more stronginvolving electrophilic species. Pyridine undergoes nitration in low yield (15%) to give 3-nitropyridine [2530-26-9] (12) whereas pyridine N -oxide is nitrated at position 4 to give 4-nitropyridine N -oxide [1124-33-0] in high yield, and 2- and 4-aminopyridines may be dinitrated (13). The chlorination of pyridine and picolines (2), (3), and (4), on the other hand, is of great commercial importance (14).Nucleophilic Attack at Carbon or Hydrogen. Only the strongest of nucleophiles (eg, ¡NH 2) can replace a hydrogen in pyridine.However, N-oxides and quaternary salts rapidly undergo addition, followed by subsequent transformations (12).The Feely-Beavers procedure (eq. 1) provides a method for the introduction of a cyano group (9), principally at the 2-position, to give compoundssuch as (14).3)3SiCN gives good yields of 2-cyano pyridine (14) from pyridine N -oxide (13) (15).Methylpyridinium quaternary salts, such as (12), undergo oxidation in alkaline solution in the presence of potassium ferricyanide to give2-pyridones, eg, N -methyl-2-pyridone [694-85-9] (16). Frequently nucleophilic attack at position 2 by excess hydroxide leads to ring opening; this and synthetically useful recyclizations have been reviewed (17).Treatment of pyridine N-oxide (13) with acetic anhydride leads chiefly to 2-pyridone (16) formation (eq. 2) (11).[1603-40-3] and 2-amino-5-methylpyridine [1603-41-4] from (3) (eq. 3). This Chichibabin reaction is most important for introduction of a 2-aminosubstituent, which may be replaced readily by many other groups (18).The N-oxides readily undergo nucleophilic addition followed by elimination, which forms the basis of several useful syntheses of 2-substitutedPyridine undergoes 2- and 4-alkylation with Grignard reagents, depending on whether free metal is present (19). Free metal gives mixtures or Treatment of pyridine (1) with sodium metal in ethanol gives piperidine been, owing to low conversions and poor selectivity (21). However, some pyridinium salts have been found to undergo potentially useful regiospecific 4-Cyanopyridine (15) reacts with ketones not bearing an pyridines. Chlorination of (13) with POCl 3to give 2-chloropyridine (17) is a good example (eq. 4); some chlorination may occur also at C-4 (11).exclusive 4-alkylation. Substituent-directed metallation (eq. 5) has become an important approach to the synthesis of disubstituted pyridines (12). For example, 2-fluoro-pyridine [372-48-5] reacts with butyllithium and acetaldehyde to give a 93% yield of alcohol [79527-61-1].[1910-42-5] (18); however, dimerization to 4,4′-bipyridine [553-26-4] (19)is favored in aprotic solvents (12).The 4,4′-bipyridine (19) formed is a precursor of the important herbicide Paraquat [1910-42-5](20) (20).Free-Radical Attack at Carbon. Homolytic substitution of pyridines has not been as thoroughly studied as heterolytic processes havereactions (22). Protonated pyridines readily undergo acylation by acyl radicals, generated by abstracting a hydrogen atom from an aldehyde, eg,2-acetyl-4-cyanopyridine [37398-49-5]from protonated 4-cyanopyridine (eq. 6).α-hydrogen in the presence of sodium metal to afford a tertiary alcohol (a precursor of azacyclonol) in high yield, eg, the reaction of (15) and benzophenone yields the tertiary benzyl alcohol [1620-30-0](23):ring or those attached to a benzene ring. Carbanions can be formed readily at alkyl carbons attached at the 2- and 4-positions. This increased chemical An industrially important example is the condensation of 2-Vinylpyridine (23) came into prominence around 1950 as a component of latex. Butadiene and styrene monomers were used with (23) to make a Cyanopyridines are usually manufactured from the corresponding picoline by catalytic, vapor-phase ammoxidation (eq. 7) in a fixed- or fluid-bed Compound (27) may also be obtained directly by oxidation of REACTIONS OF SUBSTITUTED PYRIDINESCarbon Substituents. Alkyl groups at positions 2 and 4 of a pyridine ring are more reactive than either those at the 3-position of a pyridinereactivity has been used to form 2- and 4-(phenylpropyl)pyridines, eg, 4-(3-phenylpropyl)pyridine [2057-49-0](21) (24).α- (2) or γ-picoline (4) with aqueous formaldehyde to form the correspondingethanolpyridines, 2-ethanolpyridine [104-74-2] (22) and 4-ethanolpyridine [5344-27-4], respectively, followed by dehydration of the alcohols to give 2- (23)or 4-vinylpyridine.terpolymer that bonded fabric cords to the rubber matrix of automobile tires (25). More recently, the ability of (23) to act as a Michael acceptor has been exploited in a synthesis of 4-dimethylaminopyridine (DMAP) (24) (26). The sequence consists of a Michael addition of (23) to 4-cyanopyridine (15),replacement of the 4-cyano substituent by dimethylamine (taking advantage of the activation of the cyano group by quaternization of the pyridine ring),and base-catalyzed dequaternization (retro Michael addition). 4-Dimethylaminopyridine is one of the most effective acylation catalysts known (27).reactor (28). 3-Cyanopyridine (25) is the most important nitrile, as it undergoes partial or complete hydrolysis under basic conditions to give niacinamide [98-92-0](26) or niacin (nicotinic acid) (27), respectively (29).β-picoline (3) or by exhaustive oxidation of 5-ethyl-2-methylpyridine (7), followed by decarboxylation of the initially formed pyridine-2,5-dicarboxylic acid [100-26-5] (28) (eq. 8) (30).Hydrogenation of 3-cyanopyridine (25) in the presence of ammonia gives 3-picolylamine Treatment of cyanopyridines such as (25) with a Grignard reagent yields a ketone (32). A carboxylic ester is obtained by reaction of the nitrile (25)Isonicotinic hydrazide Nicotinamide which are insoluble in the media. On heating, these nitramines rearrange intermolecularly to nitroaminopyridines having the nitro group mainly adjacent [3731-52-0] (29); however, hydrogenation in the presenceof hydrogen chloride affords the corresponding 3-carbinol (30) (31).with sodium alkoxide, followed by hydrolysis (33).[54-83-3] (isoniazid) (31) is still an important tuberculostat (34). It may be obtained by reaction of isonicotinic esters such as ethyl isonicotinate [1570-45-2], or the 4-nitrile (15), with hydrazine.[98-92-0] (26) and isonicotinamide [1453-82-3] (32) undergo Hofmann rearrangements to form 3- (33) and 4-aminopyridine (34),respectively (35). This provides an important route for the manufacture of these amines.Nitrogen Substituents. 4-Aminopyridine (34) and 2-aminopyridine (35) react with cold nitric acid to give the corresponding nitramines,to the amino group (13). From (34) the products are the intermediate 4-nitraminopyridine [26482-55-3] and 4-amino-3-nitropyridine [1681-37-4] (eq. 9).The antibacterial agent nalidixic acid shown in equation 2, compound (16).Exclusive O-alkylation of (16) (eq. 10) may be achieved using ethyl iodide and silver carbonate (39); the product is 2-ethoxypyridine Reaction of 2-aminopyridine (35) with N -acetylsulfanilyl chloride, followed by hydrolysis, gives sulfapyridine [144-83-2](36), an antibacterial (36).[389-08-2] (37) is formed by reaction of 2-amino-6-methylpyridine [1824-81-3] with analkoxymethylenemalonic ester to form the 1,8-naphthyridine carboxylic ester followed by alkylation and ester hydrolysis (37).Oxygen Substituents. The presence of oxygen or sulfur attached to the ring can affect the chemistry of those compounds throughtautomerism. This phenomenon in the pyridine series has been well studied and reviewed (38). An example of 2-pyridone −2-pyridinol tautomerism was The compounds 2- (16) and 4-pyridone (38) undergo chlorination with phosphorus oxychloride; however, 3-pyridinol (39) is not chlorinated similarly. The product from (38) is 4-chloropyridine [626-61-9]. The 2- (16) and 4-oxo (38) isomers behave like the keto form of the keto −enoltautomers, whereas the 3-oxo (39) isomer is largely phenolic-like, and fails to be chlorinated (38).[14529-53-4].Heating 2- or 4-alkoxypyridines, with or without acid catalyst, induces intermolecular migration of the alkyl group on oxygen to the ring nitrogen to form N -alkyl-2- or N -alkyl-4-pyridones (39).The N-oxide function has proved useful for the activation of the pyridine ring, directed toward both nucleophilic and electrophilic attack (see [19829-29-9]Oxidation of a pyridinethione gives the corresponding sulfonic acid, eg, 6-carboxy-2-pyridinesulfonic acid Tetrachloropyridine-4-thiol A MINE OXIDES ). However, pyridine N-oxides have not been used widely in industrial practice, because reactions involving them almost invariably produce at least some isomeric by-products, adding to the cost of purification of the desired isomer. Frequently, attack takes place first at the O-substituent, with subsequent rearrangement into the ring. For example, 3-picoline N-oxide [1003-73-2] (40) reacts with acetic anhydride to give a mixture of pyridone products in equal amounts, 5-methyl-2-pyridone [1003-68-5] and 3-methyl-2-pyridone [1003-56-1](11).Alkylation at the oxygen atom of N-oxides is also a facile process, and the entire N-substituent may be removed with base (eq. 11).Treatment of N-oxides with phosphorus trichloride provides a good method for deoxygenation (eq. 12) to obtain the free base.Sulfur Substituents. Acetylation and alkylation of pyridinethiones usually take place on sulfur (39). An exception to this is 4-pyridinethionewhich is acetylated on nitrogen. Displacement of thioethers can be achieved with hydroxide or amines (eq. 13) (40). Thioether functionalgroups can also be removed by reduction (39).[18616-02-9] from 6-carboxy-2-pyridinethione [14716-87-1](eq. 14) (41).[10357-06-1] (41) reacts with chlorine in carbon tetrachloride to give a sulfenyl chloride (42), which is fairly stable. The sulfenyl chloride may be converted into a number of derivatives (39).at the 2-Chloropyridine Amines or ammonia replace activated halogens on the ring, but competing pyridyne The 4-chloro group can be removed selectively when pentachloropyridine Halogen Substituents. Halogen functional groups are readily replaced by nucleophiles, eg, hydroxide ion, especially when they are attachedα- or γ-position of the pyridine ring. This reaction has been exploited in the synthesis of the insecticide chlorpyrifos [2921-88-2] (43) (42), and the insecticide triclopyr [55335-06-3] (44) (14,43). 2,3,5,6-Tetrachloropyridine [2402-79-1] reacts with caustic to form the hydroxylated material [6515-38-4],which then can be used to form (44) and (43).N -oxide [20295-64-1] reacts with sodium hydrosulfide to give pyrithione [1121-31-9] (45), the zinc salt of which is used as anantifungal agent, most prominently in shampoos (44).[7129-66-0] (46) formation is observed for attack at 3- and 4-halo substituents, eg, in 3-bromopyridine [626-55-1] (39). The most acidic hydrogen in 3-halopyridines (except 3-fluoropyridine) has been shown to be the one in the 4-position. Hence, the 3,4-pyridyne is usually postulated to be an intermediate instead of a 2,3-pyridyne. Product distribution (40% (33) and20% (34)) tends to support the 3,4-pyridyne also.[2176-62-7] (47) is treated with zinc to form symmetrical 2,3,5,6-tetrachloropyridine (45).Organometallics. Pentachloropyridine (47) forms Grignard reagent (48) by the entrainment method (eq. 15) (46). Addition of CO 2 produces4-carboxy-2,3,5,6-tetrachloropyridine [19340-26-2].Metallation of 2-picoline N -oxide [931-19-1] with butyllithium followed by addition of the electrophilic cyclohexanone leads to two carbinolSubstituent-directed metallations are being used for the synthesis of disubstituted pyridines. A 2-substituent directs to the 3-position, and a Pyridine ring syntheses (48) can be classified into essentially two categories: ring synthesis from nonheterocyclic compounds, and synthesis from other Synthesis of 4,6-disubstituted-2-picolines and their corresponding nicotinamides has been developed using The formation of pyridine derivatives from products (47) (4% (49) [34277-46-8] and 20% (50) [34277-59-3]).3-substituent usually directs to the 4-position; however, in the presence of N,N,N ′N ′;-tetramethylethylenediamine (TMEDA), 2-metallation may be achieved (12).Synthesisring systems. The synthesis of pyridine derivatives by transformations on the pyridine ring atoms and side-chain atoms have been considered in the previous section.Ring Synthesis From Nonheterocyclic Compounds. These methods may be further classified based on the number of bonds formedduring the pyridine ring formation. Synthesis of α-picoline (2) from 5-oxohexanenitrile is a one-bond formation reaction (eq. 16) (49). The nitrile is obtained by reaction between acetone and acrylonitrile (50). If both reaction steps are considered together, the synthesis must be considered a two-bondforming one, ie, formation of (2) from acetone and acrylonitrile in a single step comes under the category of two-bond formation reaction.The formation of quinoline [91-22-5](51) from aniline and acrolein involves formation of two bonds during the ring synthesis.β-aminocrotonitrile (52) and α,β-unsaturated compounds, where R 1=R 2=aryl(51).α, β-unsaturated aldehydes and ammonia involves formation of three bonds during the ring synthesis.For example, with an α, β-unsaturated aldehyde, both 2,5-substituted as well as 3,4-substituted pyridines can be obtained, depending on whether a 1,2-(eq. 17) or 1,4-addition (eq. 18) occurs with ammonia. Reactions are performed in the vapor phase with catalysts.。
《吡啶及其衍生物》PPT课件

5
(3)吡啶生产工艺
①煤焦油分离法 焦炉煤气经硫酸洗涤、氨水中和得粗吡啶,
再经加热脱渣得水吡啶。 水吡啶用纯苯恒沸脱水,得无水吡啶; 蒸馏,截取110-120℃馏分,再精馏,即得
纯吡啶。
编辑ppt
6
焦炉煤气中吡啶及其同系物的含量约为 0.4-0.6g/m3。
合成吡啶工业化以后,由焦炉煤气回收 吡啶的量仅占很小的比重,
编辑ppt
50
方法一
配 料 比 : 2 - 甲 基 吡 啶 : 甲 醛 : NaOH 溶 液 (50%)=1:0.4:0.1。
2-甲基吡啶和甲醛混合均匀,经柱塞泵打 入管式反应器,控制流量使反应液停留时 间 为 8 min, 保 持 压 力 9 .08MPa, 温 度 250±2℃,进行加成反应,得2-羟乙基吡 啶溶液,收率30%。
啶基本相同。
分子式:C6H7N, 分子量:93.13,
结构式:
N
CH3
编辑ppt
13
基本物性数据如下:
编辑ppt
14
(2)用途
2-甲基吡啶可用于制取2-乙烯吡啶、长效磺 胺、抗矽肺病药、牲畜驱虫药、家禽用药、 有机磷解毒剂、局部麻醉剂、泻药等。
用于生产氮肥增效剂(N-Serve)、胶片感光 剂的添加物、染料中间体和橡胶促进剂等。
进料中甲醛乙醛的比例大大超过理进料中甲醛乙醛的比例大大超过理论量以防止论量以防止22甲基吡啶和甲基吡啶和44甲基吡啶的产生甲基吡啶的产生因为因为33甲基吡啶和甲基吡啶和44甲基吡啶的沸点仅相差甲基吡啶的沸点仅相差1144甲基吡啶的带入将使甲基吡啶的带入将使33甲基吡啶的精甲基吡啶的精制非常困难
2.2.2 吡啶(Pyridine)及其衍生物
CH3
吡啶及衍生物

2-氯-5-氨基甲基吡啶用途-1:
吡啶甲胺基硝基乙烯类杀虫剂
NO2 N H NH2 N H Cl N 壳牌公司 NO2 N Cl N Nitenpram (TI-304) NO2 N H Cl N NH N 日本武田公司 NH Cl N acetaniprid 吡虫清 1996 S Cl N N Cl N N CN 日本曹达公司
O N O N HN CO2H N NH O
Cl N Cl
4α-integrin抑制剂(抗肿瘤生长剂)
吡啶氯化物用途-4:
Cl Cl N
Cl Cl
Cl Cl N
Cl OH
Cl Cl N S
Cl O P
OC2H5 OC2H5
chlorpyrifos 毒死蜱 广谱、高效、低残留、低抗药性有机磷杀虫剂 世界上产量最大的农药品种之一
吡硫霉净
吡啶氯化物用途-2:
杀菌剂 吡扎地尔(pirozadil),降血脂、抗血小板聚集药物 Cl N Cl 麝香吡啶(香料)
吡啶氯化物用途-3:
N Cl HN Cl O
治疗认识混乱症药物 OMe
O N N H N N
Cl N N O Cl
N
Cl N
Cl
N Cl CN N 高效低毒杀虫剂
O
3,5-二氯吡啶 Protein Kinase阻碍剂
Cl S
Cl N F
CF3
除草剂
Cl N NC N 除草剂 N
CF3
其它吡啶衍生物-1
2-氨基-3-甲基吡啶是重要的有机中间体 ,可 用于合成抗哮喘药 、抗菌药 、止痛剂 、抗爱 滋病毒药 ;也是一氧化氮合成酶抑制剂
N
NH2
2-氨基-5-氟吡啶作为合成LBM415的中间体, LBM415即(2S)-N-(5-氟-1-氧-2-吡啶基)-1-[(2R)-2[(甲酸基羟氨酸)甲基] -1-己氧基]-2-氮杂戊环甲酰 胺的镁盐,对体外肽脱甲酰基酶(PDF)表现出极强 的抑制性,同时在肌体内表现出良好的抗菌活性, 能有效地阻止已对其他抗菌药物产生抗药性的致病 菌的应变,具有发展成为治疗多种细菌感染病的抗 菌药物的潜力.
生物碱ppt课件

• 3.苷类:一些生物碱以苷的形式存在 于植物中; • 4.酯类:多种吲哚类生物碱分子中的 羧基,常以甲酯形式存在。 • 5.N-氧化物:在植物体中已发现的氮 氧化物约一百余种。
4.2 生物碱的分类
可以 • 1) 按来源分类, • 2) 按化学骨架分类, • 3)按生源结合化学分类。 按3)法分类的生物碱可以分为氨 基酸途径生成的生物碱和萜类及甾体 来源的生物碱。
3
H
莨菪烷
喹啉衍生物Байду номын сангаас邻氨基苯甲酸)
O 5 6 7 8 N 1 4 3 2 N N O O O H
内 酯 结 构 碱 化 开 环
成 盐 溶 于 水
喹 啉
喜 树 碱 ( 治 白 血 病 和 直 肠 癌 )
• 喜树碱:来自于我国南方特产植物珙桐科喜 树中,其木部、根皮和种子中都含有生物碱, 并以喜树碱为主要成分。具有抗癌活性,对 白血病和直肠癌有一定临床疗效,但毒性很 大,其安全范围较小。
生物碱在植物体内主要存在的形式有
• 1.游离碱:由于部分生物碱的碱性极弱, 不易或不能与酸生成稳定的盐,因而以游 离碱的形式存在。 • 2.成盐:除少数极弱碱性生物碱(如:秋 水仙碱及吲哚类生物碱)外,大多生物碱, 在植物细胞中都是与酸类结合成盐的形式 存在。 常见的有机酸有:柠檬酸、酒石酸、苹果 酸、草酸、琥珀酸等。
一、有机胺类
• 结构特点:氮原子不结合在环内的一类生物碱。 如 O H
H C H C HC 3 O H N H C H 3
HC C HC H 3 N H C H 3
麻 黄 碱
( 1 R , 2 S )
伪 麻 黄 碱
( 1 S , 2 S )
麻黄碱和伪麻黄碱是属于芳烃仲胺类生物碱,有些性质 和生物碱类的通性不完全一样,例如:游离时可溶于 水,能与酸生成稳定的盐,有挥发性,不易与大多数 生物碱沉淀试剂反应生成沉淀。
含氮有机化合物ppt课件

CH3CH2CH2NH2 CH 3CH 2NHCH 2CH 3 CH3CH2-N-CH 2CH2CH3 CH 3
丙胺
二乙胺
甲乙丙胺
2.对于氮原子上连接有脂肪烃基的芳香仲胺和叔胺,常 在脂肪烃基之前冠以“N-”或“N,N-”字:
NH CH3
CH 3 N CH3
N-甲基苯胺
N,N-二甲基苯胺
3.对于比较复杂的胺,常以烃为母体,把氨基作为取代 基来命名。例如:
O
O
N+OH-
OCH3
OCH3
学 习 结 束 !
(一)尿素
尿素简称脲,白色结晶,熔点为133℃,易溶于水和乙醇。
O H2N C NH2
1.弱碱性
尿素分子中含有两个氨基,呈弱碱性,可与强酸生成盐。
硝酸脲
2.水解反应
脲在酸或碱的催化下,加热时发生水解;在脲酶作用下, 水解反应在常温下就能进行。
CO(NH2)2 + H2O 脲酶 CO2 + 2NH3
O
CH3
H3C C O CH2 CH2 N+ CH3 OH-
CH3
(四) 肾上腺素和去甲肾上腺素
肾上腺素和去甲肾上腺素是肾上腺髓质分泌的激素。人工 合成的肾上腺素为白色结晶性粉末,无臭,味苦,极微溶于 水。肾上腺素分子中有酚羟基和甲氨基,具酸碱两性;具有 邻苯二酚结构,遇光和空气易氧化变质。
HO HO
CO(NH2)2 + 2NaOH Na2CO3 + 2NH3
CO(NH2)2 + H2O + 2HCl CO2 + 2NH4Cl
3.与亚硝酸反应
脲能与亚硝酸反应,生成氮气、二氧化碳和水
CO(NH2)2 + 2HNO2
吡啶及其衍生物的合成技术及在农药领域中的应用共48页文档50页PPT

21、要知道对好事的称颂过于夸大,也会招来人们的反感轻蔑和嫉妒。——培根 22、业精于勤,荒于嬉;行成于思,毁于随。——韩愈
23、一切节省,归根到底都归结为时间的节省。——马克思 24、意志命运往往背道而驰,决心到最后会全部推倒。——莎士比亚
25、学习是劳动,是充满思想的劳动。——乌申斯基
谢谢!
吡啶及其衍生物的合成技术及在农药领 域中的应用共48页文档
56、极端的法规,就是极端的不公。 ——西 塞罗 57、法律一旦成为人们的需要,人们 就不再 配享受 自由了 。—— 毕达哥 拉斯 58、法律规定的惩罚不是为了私人的 利益, 而是为 了公共 的利益 ;一部 分靠有 害的强 制,一 部分靠 榜ห้องสมุดไป่ตู้的 效力。 ——格 老秀斯 59、假如没有法律他们会更快乐的话 ,那么 法律作 为一件 无用之 物自己 就会消 灭。— —洛克
第十六章--杂环化合物ppt课件

- 0 .0 4
- 0 .0 6
0
0
0
- 0 .0 3
O
+ 0 .1
S
+ 0 .2 0
- 0 .0 6
- 0 .1 0
N H
+ 0 .3 2
五元杂环具有芳香性,但其芳香性不
如苯环,因环上的π电子云密度比苯环大,
且分布不匀,它们在亲电取代反应中的速率
也比要苯快得多。
亲电取代反应的活性为:
吡咯 > 呋喃 > 噻吩 > 苯,主要进入α-位。
BuLi
O
Br(CH2)3Br
H3C O
(CH2)3Br
2.加氢反应
H 2 , Ni or Pd O
H 2 , Ni or Pd N H
H 2 , Ni
S
四 氢 呋 喃 ( THF ) O
四氢吡咯 N H
不 能 用 Pd 催 化
S
因 噻 吩 能 Pd 使 中 毒
3.呋喃、吡咯的特性反应 (1)呋喃易起D-A反应
3.糠醛的用途
O O CH C O
OH
糠醛是良好的溶剂,常用作精练石油的溶剂, 以溶解含硫物质及环烷烃等。可用于精制松香,脱 出色素,溶解硝酸纤维素等。糠醛广泛用于油漆及 树脂工业。
(二)吡咯的重要衍生物
最重要的吡咯衍生物是含有四个吡咯环和四 个次甲基(-CH= )交替相连组成的大环化合物。其取 代物称为卟啉族化合物。
2、 咪唑上有两个N,烷基化反应首先在吡啶N上发生 一 烷基化产物经互变异构又产生一个吡啶N,可进 一步产生二烷基化产物,因此咪唑烷基化时经常得 到一烷基化产物和二烷基化产物;
N C 3 I H N C 3 H N C 3 H -+ H N C 3
第一章 吡啶类衍生物的制备及对金属离子的识别作用 2

第一章联吡啶类衍生物的制备及对金属离子的识别作用镧系金属离子由于其化学性质相似,定量测量镧系金属离子成了一大难题。
现有的仪器测量方法如火焰光度检测法、原子吸收光谱法、电子显微镜分析法和中子活化分析法,大都存在测量成本高,样品需要量大并且不能连续检测的缺点。
光化学传感器是近年来发展起来的检测各种阴离子、阳离子和分子新方法。
具有简单、经济、能在比较大的浓度范围内光响应等优点[1]。
这种传感器的常需要有联接在识别基团载体上的发光基团[2]。
荧光激发的形状和位置以及载体识别的过程被转换为荧光团的荧光信号的变化,通常识别基团上给电子原子的拓扑、电荷、化学性质对传感器的选择性和键合效率起着至关重要的作用[3,4]。
三价铕离子(Eu3+)由于其荧光发射强并且寿命长,在生物医药领域有广泛的应用,特别是肿瘤成像的荧光标记和荧光分析。
但是,水溶液中Eu3+的选择性荧光传感器却鲜有报道[5,6]。
联吡啶(bpy)是最常用的能和金属离子配位形成带电化合物的配体, 通过改变环上杂原子的种类、数目和位置能精细调节联吡啶化合物的电子和光谱性质[7]。
本文获得了一种新型的非共轭化合物1-(N-甲基-吡咯)-2-(4,4`-二甲基-2,2`-联吡啶)-乙醇(dbpy)(路线.1),通过对其光化学性质的研究,表明其对Eu3+具有高度选择性。
2.1实验部分2.1.1仪器和试剂美国NICOLET NEXUS 470 FT-IR型傅立叶红外光谱仪,KBr压片,400~4000cm-1;意大利Perkin-Elemer 240 型元素分析仪;德国NETZSCH STA449C 型差热-热重分析仪,氮气气氛,10 ºC/min;日本岛津UV-2450型紫外可见分光光度仪,800-190 nm;德国BRUKERAC-P 400 型核磁共振仪,溶剂CDCl3,内标TMS;DHG-9140A型电热恒温鼓风干箱(上海-恒科技有限公司);DZF-6051型真空干燥箱(上海-恒科技有限公司);RE-52C型旋转蒸发器(巩义市予华仪器有限公司)。
矿产

矿产资源开发利用方案编写内容要求及审查大纲
矿产资源开发利用方案编写内容要求及《矿产资源开发利用方案》审查大纲一、概述
㈠矿区位置、隶属关系和企业性质。
如为改扩建矿山, 应说明矿山现状、
特点及存在的主要问题。
㈡编制依据
(1简述项目前期工作进展情况及与有关方面对项目的意向性协议情况。
(2 列出开发利用方案编制所依据的主要基础性资料的名称。
如经储量管理部门认定的矿区地质勘探报告、选矿试验报告、加工利用试验报告、工程地质初评资料、矿区水文资料和供水资料等。
对改、扩建矿山应有生产实际资料, 如矿山总平面现状图、矿床开拓系统图、采场现状图和主要采选设备清单等。
二、矿产品需求现状和预测
㈠该矿产在国内需求情况和市场供应情况
1、矿产品现状及加工利用趋向。
2、国内近、远期的需求量及主要销向预测。
㈡产品价格分析
1、国内矿产品价格现状。
2、矿产品价格稳定性及变化趋势。
三、矿产资源概况
㈠矿区总体概况
1、矿区总体规划情况。
2、矿区矿产资源概况。
3、该设计与矿区总体开发的关系。
㈡该设计项目的资源概况
1、矿床地质及构造特征。
2、矿床开采技术条件及水文地质条件。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
编辑ppt
5
(3)吡啶生产工艺
①煤焦油分离法 焦炉煤气经硫酸洗涤、氨水中和得粗吡啶,
再经加热脱渣得水吡啶。 水吡啶用纯苯恒沸脱水,得无水吡啶; 蒸馏,截取110-120℃馏分,再精馏,即得
纯吡啶。
编辑ppt
6
焦炉煤气中吡啶及其同系物的含量约为 0.4-0.6g/m3。
合成吡啶工业化以后,由焦炉煤气回收 吡啶的量仅占很小的比重,
反应温度约370℃,
反应后的气体经过萃取、精馏得到吡啶和 3-甲基吡啶,总收率约60%,
其中吡啶为40-50%、3-甲基吡啶为20-30%, 二者的比例取决于甲醛、乙醛的比率。
编辑ppt
9
反应可通过添加甲醇来提高产率。
注意:进料中甲醛、乙醛的比例大大超过理 论量,以防止2-甲基吡啶和4-甲基吡啶的产生, 因为3-甲基吡啶和4-甲基吡啶的沸点仅相差 1℃,4-甲基吡啶的带入将使3-甲基吡啶的精 制非常困难。
编辑ppt
19
③乙炔法
由乙炔和氨进行催化反应,主要产品为2-甲基 吡啶和4-甲基吡啶,同时有乙腈副产物生成。
编辑ppt
20
乙炔与乙腈在120-180℃、0.8-2.5MPa、钴 化合物为催化剂,反应得到2-甲基吡啶。
编辑ppt
21
④乙烯与氨反应
乙烯通入含二价钯盐的氨水中,生成2-甲基吡 啶和2-甲基-5-乙基吡啶的混合物。
2-甲基吡啶与2-甲基-5-乙基吡啶比例与反 应时间、压力、催化剂的浓度等有关。
编辑ppt
26
⑤丙酮与丙烯腈反应
丙烯腈与过量的丙酮以伯胺及弱酸催化、在 180℃及2.2 MPa(表压)条件下进行液相反 应,主要得到单氰乙基产物。
后 经者氰在基的H2还流原下、经环过化含及钯脱催氢化,剂得的到气2相-甲反基应吡器, 啶。
编辑ppt
3
有毒,能使神经中枢麻醉;
极臭,空气中含量达3×10-5时,大部分人便 不能忍受。吡啶的基本物性数据如下:
编辑ppt
4
(2)用途
医药工业用于制磺胺、合霉素、VA、可 的松以及驱虫药、局部麻醉药等;
印染的稳定剂、合成橡胶促进剂、涂料溶剂、 合成树脂的缩合剂; 重要的有机合成原料和溶剂; 制造乙烯吡啶、杀虫剂、除草剂等。
丙胺类抗组胺药,镇静作用弱。
用于皮肤粘膜、过敏性疾病,对眼部过 敏性疾病好。
编辑ppt
2
一 吡啶 Pyridine
(1)性质,又称杂氮苯,无色或淡黄色有吸 湿性的液体,有很苦的特殊臭味。
溶于水、醇、醚等多种有机溶剂。
易燃、易爆。
分子式:C5H5N, 分子量:79.101,
结构式:
N
啶基本相同。
分子式:C6H7N, 分子量:93.13,
结构式:
N
CH3
编辑ppt
13
基本物性数据如下:
编辑ppt
14Βιβλιοθήκη (2)用途2-甲基吡啶可用于制取2-乙烯吡啶、长效磺 胺、抗矽肺病药、牲畜驱虫药、家禽用药、 有机磷解毒剂、局部麻醉剂、泻药等。
用于生产氮肥增效剂(N-Serve)、胶片感光 剂的添加物、染料中间体和橡胶促进剂等。
编辑ppt
15
(2)合成
①煤焦油分离法 由煤焦油分离所得的粗吡啶、先脱渣得水吡
啶,再在填料塔内进行常压蒸馏,并用纯苯 与水共沸蒸馏脱去吡啶中的水;
截取100~120℃、120~160℃吡啶馏分,再将 120~160℃吡啶馏分进行精馏,截取126131℃馏分,即为2-甲基吡啶。
编辑ppt
16
②乙醛合成法 乙醛与氨进行气相反应而得,其反应式:
编辑ppt
10
用丙烯醛代替甲醛、乙醛则3-甲基吡啶 的收率可达40-50%。
编辑ppt
11
③以四氢化糠醇为原料 四氢化糠醇与氨反应,再脱氢。
在化学合成法中,采用醛(甲醛、乙醛) 生产吡啶是最重要的工业方法。
编辑ppt
12
二 2-甲基吡啶 2-Methylpyridine
(1)性质,别名α-皮考林,无色油状液体,有吡 啶臭味,易溶于水,能与醇、醚混溶。毒性与吡
在我国占一定比例。
编辑ppt
7
②由乙醛、甲醛和氨的气-固相催化反应
乙醛、甲醛和氨进行气-固相催化反应,可 得吡啶与3-甲基吡啶的混合物。
Al2O3为主催化剂、金属氧化物为助催化剂。
2C3 H CH+2 OHC+HNO 3 H
+
N
N
编辑ppt
8
工艺过程 乙醛、甲醛和氨进入装有催化剂的反应器,
24
乙烯与氨气在反应器反应后,进入气提 塔,通入N2气提,
气提气经冷却分出氨和N2,催化剂和冷 凝液。
催化剂去再生塔再生后循环使用。
冷凝液经萃取、精馏(4个精馏塔)。
编辑ppt
25
精馏塔制得产物: 2-甲基吡啶、 2-甲基-5-乙基吡啶。 另外一些副产物如4-甲基吡啶和二甲基吡
啶等。
编辑ppt
17
乙醛 氨
常压、350-500℃,Al2O3为催化剂
气体
预热
催化反应
冷凝
工 艺
精制
分馏
过
程
2-甲基吡啶 4-甲基吡啶
含量:99.2% 收率40-60%
编辑ppt
脱水
18
乙醛与氨气相反应与乙醛、甲醛和氨气相 反应所用的催化剂基本相同,
可以设计一套装置进行两种反应的生产,
意大利Montecation公司就有一套装置生产 4种产品。
2.2.2 吡啶(Pyridine)及其衍生物
吡啶及其衍生物是一类比哌嗪类应用更为广 泛的重要的医药中间体,
广泛应用于药物的合成,可作为甾族化合物、 磺胺类及抗组胺如非尼拉敏的合成原料。
编辑ppt
1
非尼拉敏Pheniramine
别名: 苯吡丙胺,抗感明,屈米通, Prophenpyridamine,Trimeton
O
O
C H 3 C C H 3+C H 2 = C H C N C H 3 C C H 2 C H 2 C H 2 C N
编辑ppt
N C H 3
27
三 3-甲基吡啶 3-Methylpyridine
(1)性质,无色油状液体,有不愉快的气 味,能与水,乙醇、乙醚混溶。
别名:β-甲基吡啶、3-皮考林。
编辑ppt
22
析出的金属钯,由于反应液中添加的二价铜 盐使其氧化成二价钯盐。
形成的亚铜复盐,经加氨后遇空气氧化成二 价铜的复盐。
编辑ppt
23
产物组成(1t产物) : 2-甲基吡啶0.8t, 2-甲基-5-乙基吡啶0.2t。
消耗定额: 乙烯1.5t、 氨0.6t 合理消耗的钯。
编辑ppt
