Five-loop renormalization-group expansions for two-dimensional Euclidean lambda phi^4 theor
【F'A】环苯扎林d在美国的营销情况

环苯扎林盐酸环苯扎林(),国外的商品名主要有、、和,是一中枢性肌肉松驰剂,最早由公司研制并于年开发上专利药进行了仿制,被美国药典收载。
国内没有该药品生产及销售,属于化药.。
盐酸环苯扎林用于缓解肌肉痉挛及其伴随的骨骼肌剧烈疼痛的状态,例如疼痛、触痛、活动受限以及日常的作用机理,它能减轻骨骼肌痉挛状态,而不影响肌肉的功能,它对非中枢神经系统疾病造成的肌肉痉挛使用表明,该药起效快,解痉作用好,不良反应小,是该类病痛的临床首选药物,有关数据显示公司的环一千万张,连续几年均列入(按处方量统计美国前个高频使用药)中,显示出强劲的生命力。
痛性肌肉痉挛是常见病、多发病,病因很多,创伤、运动不当、受寒等引起的肌痉挛是当前医院门诊常见风湿及类风湿关节炎、肩周炎、颈腰部综合症、腰间盘突出、骨关节炎、手术、脑血管障碍、颈部脊椎病脱水、过度运动造成的低血钠症、出汗过多、腹泻、呕吐、或者是由于药物治疗和血液透析等均会引起肌然发生,持续数秒或数分钟。
孕妇、老人及有外周血管疾病的人更易在晚间发生原因不明的脚部、腿部骨疼痛,给病人造成很大痛苦。
目前国内治疗此类疾病的药物主要为氯唑沙宗及复方氯唑沙宗(氯唑沙宗与唑沙宗为国家医保目录品种,氯唑沙宗及其复方制剂销售良好,其它一些骨骼肌松弛剂由于不良反应都很扎林在美国的销售量远优于氯唑沙宗。
本品为中枢性肌肉肉松弛药,其作用部位可能在脑干而非脊髓,给药后再现镇静的骨骼肌松弛作用,本品用作治疗疼痛性局部肌肉痉挛的辅助用药。
较大剂量时对脑脊髓性痉挛无影响。
口服:成人,次,次天。
过周。
以下是国家局药品数据库中查询的结果在审品种目录浏览序号受理号药品名称药品类型申请类型注册类型承办日期盐酸环苯扎林片化药新药盐酸环苯扎林化药新药盐酸环苯扎林缓释胶囊化药新药盐酸环苯扎林化药新药盐酸环苯扎林缓释胶囊化药新药受理品种目录浏览共有条记录序号受理号药品名称药品类型申请类型注册类型承办日期盐酸环苯扎林片化药新药盐酸环苯扎林化药新药盐酸环苯扎林缓释胶囊化药新药盐酸环苯扎林化药新药盐酸环苯扎林缓释胶囊化药新药以下是在审评中心查询的结果:与陈经理相符。
迈克沃伊和法默的中国体外诊断分销指南说明书

McEvoy and Farmer's Complete Guide to IVD Distribution in Chinahttps:///r/W875106C98AEN.htmlDate: May 2009Pages: 240Price: US$ 3,995.00 (Single User License)ID: W875106C98AENAbstractsWho is Who in Clinical Diagnostics in China, produced jointly with the firm McEvoy & Farmer, is the product of on-the-ground primary research on Chinese labs conducted in fall and winter 2008. Included in this report:Market Size Estimate by Major Category (Chemistry, Critical Care Chemistry, Urine Chemistry, Hematology, Flow Cytometry, Coagulation, Immunochemistry, Molecular Testing, Other IVD)Country Industry OverviewChinese Hospital StatisticsProfiles of 42 International Diagnostic Companies with operations in ChinaProfiles of 130 Domestic Diagnostic CompaniesProfiles of 24 Local Distributors, indicating the companies they distribute forThe Chinese market represents a significant opportunity for IVD diagnostic companies. But actionable information about this emerging market is often difficult to get. Only with an exhaustive, on-the-ground research team can a company truly understand the chinese market. Now, a resource is available that can make on-the-ground research available to all companies at a fraction of the cost.Published jointly with trusted Asian IVD market experts, this Kalorama report is acomplete survey of the IVD market in China today. Market size for major categories of the IVD market, products on the market, important Chinese market trends and intense company profiles are part of this exhaustive study.This country of 1.3 billion people is now America’s sixth largest export market, and China’s economy, while showing some effects of the world recession, has been less impacted than other nations and is showing growth, although not as rapid as in recent years. A number of recent events and trends in the Chinese healthcare environment are making China an increasingly attractive market opportunity for in vitro diagnostics companies. The increasing numbers of private laboratories and expanded reference laboratories are expanding the market for tests of all kinds.Although there are a number of challenges for diagnostic manufacturers to understand and overcome, the market for clinical diagnostics in China (both reagents and instruments) remains one of the most promising emerging markets in the world.ContentsCOUNTRY SUMMARYMarket TotalsWhat is New in ChinaThe Healthcare System in ChinaMedical InsuranceA Brief Guide to the BureaucracyPrivate and Reference LaboratoriesPrivate Medical PracticeProduct RegistrationReimbursementTendersReagent RentalQuality ControlVacuum Tube ConversionSecond hand InstrumentsImported and Domestic Sales INTERNATIONAL MANUFACTURER PROFILESAbbott LaboratoriesABXAcon Biotech/InvernessAdaltisAffymetrixAgilent TechnologiesApplied BiosystemsArkrayAudit DiagnosticsBeckman CoulterBDBioneer TradeBio Rad LaboratoriesbioMérieuxDiaSorinDiaSys Diagnostic SystemsEppendorfEuroimmunFujirebio/CanAg DiagnosticsHitachi High Technologies CorporationHospitexHumanInverness MedicalJei Daniel (JD) BiotechJokohMedicine Devices Company (MDC) Melet Schloesing LaboratoriesMP BiomedicalsOrtho ClinicalDiagnosticsPerkinElmerPromegaQiagenRadiometerRandoxR BiopharmRocheSiemens Healthcare Diagnostics StagoSysmex CorporationThermoFisherVirionSerionYD DiagnosticsDOMESTIC MANUFACTURER PROFILES3V BioengineeringAccuBio TechAddcare Bio TechAi De Diagnostic (IND)AmpllyAntai DiagnosticsAudicom Medical InstrumentAutobio DiagnosticsAVE Science & TechnologyB & E Scientific InstrumentBasoDiagnosticsBeijing Genomics Institute (BGI Healthcare)Biocell InstituteBiocreateBiocup Biotech CompanyBioer TechnologyBiosino BiotechnologyBiote CompanyBioway BiotechnologyBlue Cross Bio MedicalBowlinman Sunshine Science & TechnologyCaihong Analytical InstrumentCaltech GroupCapitalBio CorporationCaretium Medical InstrumentsChang Chun Brother BiotechChangdao BiotechnologyChemClin Bio Tech / China Diagnostics Medical Chemtron BiotechChina Medical/Yuande Bio Medical EngineeringCondor Teco Medical TechnologyCornley Hi TechDa An GeneDecipher BioscienceDirui IndustrialDL Medical BiotechDoubleQ LabDragon MedicalElikan Biological TechnologyFenghua BioengineeringFengHui Medical Science & Technology First Sun ElectronicFosun DiagnosticsGenetel PharmaceuticalsGenius ElectronicsGoldsite DiagnosticsHai Tai BiologicalHaoyuan BiotechnologyHeal Force/Nison InstrumentHealthDigitHongcheng (HC) BiopharmaceuticalHongshi Medical TechnologyHope Industry and TradeHua Sin ScienceHua Tong Medical InstrumentHuaguan Biochip CompanyHuatai Biotechnology IndustrialHuayang Analysis InstrumentHybriBioInTec ProductsJian Ye Medical EquipmentinSangTe Medicine InstrumentKanghua Biotech CompanyKehua Bio EngineeringKinghawk TechnologyLabnovation TechnologiesLandwind International Medical Science LaoLa ElectronicLeadman Biochemistry TechnologyLengguang TechnologyLingyi Medical ScienceLivzon GroupLongx TechnologiesMaker Science TechnologyMaysun TechnologyMaxcom ElectronicMD Pacific TechnologyMeiyilin Electronics InstrumentMerit Choice BioengineeringMindray Medical ElectronicsModern Gold BiotechnologyNanfen Medical Biochemical InstrumentNeusoft Medical SystemsNew MoonNewScen Coast Bio PharmaceuticalOption Science & Technology Development Company Perlong GroupPG Biotech/QiagenPrecil InstrumentProcan ElectronicsRayto Life and Analytical SciencesRich Science IndustryRongsheng Biotech.Runbio Biotech .Sanco InstrumentSan Jose Medical Products..Sciarray BiotechSciendox Bio-Technology CompanySenlong Biotech..Share SunShensuo Medical DiagnosticsShenzhen New Industries Biomedical Engineering (SNIBE) Shining Sun TechnologySinnowa Medical Science & TechnologySTAC Medical Science & TechnologySteellex Scientific InstrumentStrong Biotechnologies...Success Technology DevelopmentSun BiotechSunostik Biomedical TechnologySym-Bio Lifescience.Tecom ScienceTechcompTellgen LifeTiangen Biotech...TianHai Medical Equipment (THME)Tianlong Science and TechnologyTigsun Biotinge Science & TechnologyTZD Technological.Urit Medical ElectronicWanCheng Bio-elect.Wantai BiologicalWasson An-Ze Bio-tech Company..WearmaxWeirikang Biological TechnologyW.H.P.M. Bioresearch & Technology/Hemosure Wondfo BiotechXun-Da Medical InstrumentYasen IndustrialYaxin Sheng WuZhong Tai BiotechZJ Bio-TechDISTRIBUTOR PROFILESAdvanced Clinical Laboratory Science (ACLS)Ailex Technology.....AusBio LaboratoriesBio-Asia DiagnosticsBiochem GroupBio-Star Technology DevelopmentChindex InternationalChinMax Medical SystemsDiamond BiotechnologyDong Hu Instrument/East LakeFu Li Tai (FLT) MeditecGene CompanyGiantech Medical Science & TechnologyGolden-Grand Medical Hongtex Bio-techLangkaNewtime TradingRainbow-Mega Scientific InstrumentScience International/Science LaboratoriesSunlionSuns-GroupTruth EnterpriseUnited Science InternationalVastec Medical..Zhi Cheng Biotech.Appendix: China’s Hospitals by Province.I would like to orderProduct name:McEvoy and Farmer's Complete Guide to IVD Distribution in ChinaProduct link:https:///r/W875106C98AEN.htmlPrice:US$ 3,995.00 (Single User License / Electronic Delivery)If you want to order Corporate License or Hard Copy, please, contact our CustomerService:*************************PaymentTo pay by Credit Card (Visa, MasterCard, American Express, PayPal), please, clickbutton on product page https:///r/W875106C98AEN.htmlTo pay by Wire Transfer, please, fill in your contact details in the form below:First name:Last name:Email:Company:Address:City:Zip code:Country:Tel:Fax:Your message:**All fields are requiredCustumer signature _______________________________________Please, note that by ordering from you are agreeing to our Terms& Conditions at https:///docs/terms.htmlTo place an order via fax simply print this form, fill in the information belowand fax the completed form to +44 20 7900 3970。
marked manuscript

Quality evaluation of Flos Lonicerae through a simultaneous determination of seven saponins by HPLC with ELSDXing-Yun Chai1, Song-Lin Li2, Ping Li1*1Key Laboratory of Modern Chinese Medicines and Department of Pharmacognosy, China Pharmaceutical University, Nanjing, 210009, People’s Republic of China2Institute of Nanjing Military Command for Drug Control, Nanjing, 210002, People’s Republic of China*Corresponding author: Ping LiKey Laboratory of Modern Chinese Medicines and Department of Pharmacognosy, China Pharmaceutical University, Nanjing 210009, People’s Republic of China.E-mail address: lipingli@Tel.: +86-25-8324-2299; 8539-1244; 135********Fax: +86-25-8532-2747AbstractA new HPLC coupled with evaporative light scattering detection (ELSD) method has been developed for the simultaneous quantitative determination of seven major saponins, namely macranthoidinB (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7)in Flos Lonicerae, a commonly used traditional Chinese medicine (TCM) herb.Simultaneous separation of these seven saponins was achieved on a C18 analytical column with a mixed mobile phase consisting of acetonitrile(A)-water(B)(29:71 v/v) acidified with 0.5% acetic acid. The elution was operated from keeping 29%A for 10min, then gradually to 54%B from 10 to 25 min on linear gradient, and then keep isocratic elution with 54%B from 25 to 30min.The drift tube temperature of ELSD was set at 106℃, and with the nitrogen flow-rate of 2.6 l/min. All calibration curves showed good linear regression (r2 0.9922) within test ranges. This method showed good reproducibility for the quantification of these seven saponins in Flos Lonicerae with intra- and inter-day variations of less than 3.0% and 6.0% respectively. The validated method was successfully applied to quantify seven saponins in five sources of Flos Lonicerae, which provides a new basis of overall assessment on quality of Flos Lonicerae.Keywords: HPLC-ELSD; Flos Lonicerae; Saponins; Quantification1. IntroductionFlos Lonicerae (Jinyinhua in Chinese), the dried buds of several species of the genus Lonicera (Caprifoliaceae), is a commonly used traditional Chinese medicine (TCM) herb. It has been used for centuries in TCM practice for the treatment of sores, carbuncles, furuncles, swelling and affections caused by exopathogenic wind-heat or epidemic febrile diseases at the early stage [1]. Though four species of Lonicera are documented as the sources of Flos Lonicerae in China Pharmacopeia (2000 edition), i.e. L. japonica, L. hypoglauca,L. daystyla and L. confusa, other species such as L. similes and L. macranthoides have also been used on the same purpose in some local areas in China [2]. So it is an important issue to comprehensively evaluate the different sources of Flos Lonicerae, so as to ensure the clinical efficacy of this Chinese herbal drug.Chemical and pharmacological investigations on Flos Lonicerae resulted in discovering several kinds of bioactive components, i.e. chlorogenic acid and its analogues, flavonoids, iridoid glucosides and triterpenoid saponins [3]. Previously, chlorogenic acid has been used as the chemical marker for the quality evaluation of Flos Lonicerae,owing to its antipyretic and antibiotic property as well as its high content in the herb. But this compound is not a characteristic component of Flos Lonicerae, as it has also been used as the chemical marker for other Chinese herbal drugs such as Flos Chrysanthemi and so on[4-5]. Moreover, chlorogenic acid alone could not be responsible for the overall pharmacological activities of Flos Lonicerae[6].On the other hand, many studies revealed that triterpenoidal saponins of Flos Lonicerae possess protection effects on hepatic injury caused by Acetaminophen, Cd, and CCl4, and conspicuous depressant effects on swelling of ear croton oil [7-11]. Therefore, saponins should also be considered as one of the markers for quality control of Flos Lonicerae. Consequently, determinations of all types of components such as chlorogenic acid, flavonoids, iridoid glucosides and triterpenoidal saponins in Flos Lonicerae could be a better strategy for the comprehensive quality evaluation of Flos Lonicerae.Recently an HPLC-ELSD method has been established in our laboratory for qualitative and quantitative determination of iridoid glucosides in Flos Lonicerae [12]. But no method was reported for the determination of triterpenoidal saponins in Flos Lonicera. As a series studies on the comprehensive evaluation of Flos Lonicera, we report here, for the first time, the development of an HPLC-ELSD method for simultaneous determination of seven triterpenoidal saponins in the Chinese herbal drug Flos Lonicerae, i.e.macranthoidin B (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7) (Fig. 1).2. Experimental2.1. Samples, chemicals and reagentsFive samples of Lonicera species,L. japonica from Mi county, HeNan province (LJ1999-07), L. hypoglauca from Jiujang county, JiangXi province (LH2001-06), L. similes from Fei county, ShanDong province (LS2001-07), L. confuse from Xupu county, HuNan province (LC2001-07), and L. macranthoides from Longhu county, HuNan province (LM2000-06) respectively, were collected in China. All samples were authenticated by Dr. Ping Li, professor of department of Pharmacognosy, China Pharmaceutical University, Nanjing, China. The voucher specimens were deposited in the department of Pharmacognosy, China Pharmaceutical University, Nanjing, China. Seven saponin reference compounds: macranthoidin B (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7) were isolated previously from the dried buds of L. confusa by repeated silica gel, sephadex LH-20 and Rp-18 silica gel column chromatography, their structures were elucidated by comparison of their spectral data (UV, IR, MS, 1H- NMR and 13C-NMR) with references [13-15]. The purity of these saponins were determined to be more than 98% by normalization of the peak areas detected by HPLC with ELSD, and showed very stable in methanol solution.HPLC-grade acetonitrile from Merck (Darmstadt, Germany), the deionized water from Robust (Guangzhou, China), were purchased. The other solvents, purchased from Nanjing Chemical Factory (Nanjing, China) were of analytical grade.2.2. Apparatus and chromatographic conditionsAglient1100 series HPLC apparatus was used. Chromatography was carried out on an Aglient Zorbax SB-C18 column(250 4.6mm, 5.0µm)at a column temperature of 25℃.A Rheodyne 7125i sampling valve (Cotati, USA) equipped with a sample loop of 20µl was used for sample injection. The analog signal from Alltech ELSD 2000 (Alltech, Deerfield, IL, USA)was transmitted to a HP Chemstation for processing through an Agilent 35900E (Agilent Technologies, USA).The optimum resolution was obtained by using a linear gradient elution. The mobile phase was composed of acetonitrile(A) and water(B) which acidified with 0.5% acetic acid. The elution was operated from keeping 29%A for 10min, then gradually to 54%B from 10 to 25 min in linear gradient, and back to the isocratic elution of 54%B from 25 to 30 min.The drift tube temperature for ELSD was set at 106℃and the nitrogen flow-rate was of 2.6 l/min. The chromatographic peaks were identified by comparing their retention time with that of each reference compound tried under the same chromatographic conditions with a series of mobile phases. In addition, spiking samples with the reference compounds further confirmed the identities of the peaks.2.3. Calibration curvesMethanol stock solutions containing seven analytes were prepared and diluted to appropriate concentration for the construction of calibration curves. Six concentrationof the seven analytes’ solution were injected in triplicate, and then the calibration curves were constructed by plotting the peak areas versus the concentration of each analyte. The results were demonstrated in Table1.2.4. Limits of detection and quantificationMethanol stock solution containing seven reference compounds were diluted to a series of appropriate concentrations with methanol, and an aliquot of the diluted solutions were injected into HPLC for analysis.The limits of detection (LOD) and quantification (LOQ) under the present chromatographic conditions were determined at a signal-to-noise ratio (S/N) of 3 and 10, respectively. LOD and LOQ for each compound were shown in Table1.2.5. Precision and accuracyIntra- and inter-day variations were chosen to determine the precision of the developed assay. Approximate 2.0g of the pulverized samples of L. macranthoides were weighted, extracted and analyzed as described in 2.6 Sample preparation section. For intra-day variability test, the samples were analyzed in triplicate for three times within one day, while for inter-day variability test, the samples were examined in triplicate for consecutive three days. Variations were expressed by the relative standard deviations. The results were given in Table 2.Recovery test was used to evaluate the accuracy of this method. Accurate amounts of seven saponins were added to approximate 1.0g of L. macranthoides,and then extracted and analyzed as described in 2.6 Sample preparation section. The average recoveries were counted by the formula: recovery (%) = (amount found –original amount)/ amount spiked ×100%, and RSD (%) = (SD/mean) ×100%. The results were given in Table 3.2.6. Sample preparationSamples of Flos Lonicerae were dried at 50℃until constant weight. Approximate 2.0g of the pulverized samples, accurately weighed, was extracted with 60% ethanol in a flask for 4h. The ethanol was evaporated to dryness with a rotary evaporator. Residue was dissolved in water, followed by defatting with 60ml of petroleum ether for 2 times, and then the water solution was evaporated, residue was dissolved with methanol into a 25ml flask. One ml of the methanol solution was drawn and transferred to a 5ml flask, diluted to the mark with methanol. The resultant solution was at last filtrated through a 0.45µm syringe filter (Type Millex-HA, Millipore, USA) and 20µl of the filtrate was injected to HPLC system. The contents of the analytes were determined from the corresponding calibration curves.3. Results and discussionsThe temperature of drift tube and the gas flow-rate are two most important adjustable parameters for ELSD, they play a prominent role to an analyte response. In ourprevious work [12], the temperature of drift tube was optimized at 90°C for the determination of iridoids. As the polarity of saponins are higher than that of iridoids, more water was used in the mobile phase for the separation of saponins, therefore the temperature for saponins determination was optimized systematically from 95°C to 110°C, the flow-rate from 2.2 to 3.0 l/min. Dipsacoside B was selected as the testing saponin for optimizing ELSD conditions, as it was contained in all samples. Eventually, the drift tube temperature of 106℃and a gas flow of 2.6 l/min were optimized to detect the analytes. And these two exact experimental parameters should be strictly controlled in the analytical procedure [16].All calibration curves showed good linear regression (r2 0.9922) within test ranges. Validation studies of this method proved that this assay has good reproducibility. As shown in Table 2, the overall intra- and inter-day variations are less than 6% for all seven analytes. As demonstrated in Table 3, the developed analytical method has good accuracy with the overall recovery of high than 96% for the analytes concerned. The limit of detection (S/N=3) and the limit of quantification (S/N=10) are less than 0.26μg and 0.88μg respectively (Table1), indicating that this HPLC-ELSD method is precise, accurate and se nsitive enough for the quantitative evaluation of major non- chromaphoric saponins in Flos Lonicerae.It has been reported that there are two major types of saponins in Flos Lonicerae, i.e. saponins with hederagenin as aglycone and saponins with oleanolic acid as the aglycone [17]. But hederagenin type saponins of the herb were reported to have distinct activities of liver protection and anti-inflammatory [7-11]. So we adoptedseven hederagenin type saponins as representative markers to establish a quality control method.The newly established HPLC-ELSD method was applied to analyze seven analytes in five plant sources of Flos Lonicerae, i.e. L. japonica,L. hypoglauca,L. confusa,L. similes and L. macranthoides(Table 4). It was found that there were remarkable differences of seven saponins contents between different plant sources of Flos Lonicerae. All seven saponins analyzed could be detected in L. confusa and L. hypoglauca, while only dipsacoside B was detected in L. japonica. Among all seven saponins interested, only dipsacoside B was found in all five plant species of Flos Lonicerae analyzed, and this compound was determined as the major saponin with content of 53.7 mg/g in L. hypoglauca. On the other hand, macranthoidin B was found to be the major saponin with the content higher than 41.0mg/g in L. macranthoides,L. confusa, and L. similis, while the contents of other analytes were much lower.In our previous study [12], overall HPLC profiles of iridoid glucosides was used to qualitatively and quantitatively distinguish different origins of Flos Lonicerae. As shown in Fig.2, the chromatogram profiles of L. confusa, L. japonica and L. similes seem to be similar, resulting in the difficulty of clarifying the origins of Flos Lonicerae solely by HPLC profiles of saponins, in addition to the clear difference of the HPLC profiles of saponins from L. macranthoides and L. hypoglauca.Therefore, in addition to the conventional morphological and histological identification methods, the contents and the HPLC profiles of saponins and iridoids could also be used as accessory chemical evidence toclarify the botanical origin and comprehensive quality evaluation of Flos Lonicerae.4. ConclusionsThis is the first report on validation of an analytical method for qualification and quantification of saponins in Flos Lonicerae. This newly established HPLC-ELSD method can be used to simultaneously quantify seven saponins, i.e. macranthoidin B, macranthoidin A, dipsacoside B, hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester, macranthoside B, macranthoside A, and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside in Flos Lonicerae. Together with the HPLC profiles of iridoids, the HPLC-ELSD profiles of saponins could also be used as an accessory chemical evidence to clarify the botanical origin and comprehensive quality evaluation of Flos Lonicerae.AcknowledgementsThis project is financially supported by Fund for Distinguished Chinese Young Scholars of the National Science Foundation of China (30325046) and the National High Tech Program(2003AA2Z2010).[1]Ministry of Public Health of the People’s Republic of China, Pharmacopoeia ofthe People’s Republic of China, V ol.1, 2000, p. 177.[2]W. Shi, R.B. Shi, Y.R. Lu, Chin. Pharm. J., 34(1999) 724.[3]J.B. Xing, P. Li, D.L. Wen, Chin. Med. Mater., 26(2001) 457.[4]Y.Q. Zhang, L.C. Xu, L.P. Wang, J. Chin. Med. Mater., 21(1996) 204.[5] D. Zhang, Z.W. Li, Y. Jiang, J. Pharm. Anal., 16(1996) 83.[6]T.Z. Wang, Y.M. Li, Huaxiyaoxue Zazhi, 15(2000) 292.[7]J.ZH. Shi, G.T. Liu. Acta Pharm. Sin., 30(1995) 311.[8]Y. P. Liu, J. Liu, X.SH. Jia, et al. Acta Pharmacol. Sin., 13 (1992) 209.[9]Y. P. Liu, J. Liu, X.SH. Jia, et al. Acta Pharmacol. Sin., 13 (1992) 213.[10]J.ZH. Shi, L. Wan, X.F. Chen.ZhongYao YaoLi Yu LinChuang, 6 (1990) 33.[11]J. Liu, L. Xia, X.F. Chen. Acta Pharmacol. Sin., 9 (1988) 395[12]H.J. Li, P. Li, W.C. Ye, J. Chromatogr. A 1008(2003) 167-72.[13]Q. Mao, D. Cao, X.SH. Jia. Acta Pharm. Sin., 28(1993) 273.[14]H. Kizu, S. Hirabayashi, M. Suzuki, et al. Chem. Pharm. Bull., 33(1985) 3473.[15]S. Saito, S. Sumita, N. Tamura, et al. Chem Pharm Bull., 38(1990) 411.[16]Alltech ELSD 2000 Operating Manual, Alltech, 2001, p. 16. In Chinese.[17]J.B. Xing, P. Li, Chin. Med. Mater., 22(1999) 366.Fig. 1 Chemical structures of seven saponins from Lonicera confusa macranthoidin B (1), macranthoidin A (2), dipsacoside B (3), hederagenin-28-O-β-D-glucopyranosyl(6→1)-O-β-D- glucopyranosyl ester (4), macranthoside B (5), macranthoside A (6), and hederagenin-3-O-α-L-arabinopyranosyl(2→1)-O-α-L-rhamnopyranoside (7)Fig. 2Representative HPLC chromatograms of mixed standards and methanol extracts of Flos Lonicerae.Column: Agilent Zorbax SB-C18 column(250 4.6mm, 5.0µm), temperature of 25℃; Detector: ELSD, drift tube temperature 106℃, nitrogen flow-rate 2.6 l/min.A: Mixed standards, B: L. confusa, C: L. japonica, D: L. macranthoides, E: L. hypoglauca, F: L. similes.Table 1 Calibration curves for seven saponinsAnalytes Calibration curve ar2Test range(μg)LOD(μg)LOQ(μg)1 y=6711.9x-377.6 0.9940 0.56–22.01 0.26 0.882 y=7812.6x-411.9 0.9922 0.54–21.63 0.26 0.843 y=6798.5x-299.0 0.9958 0.46–18.42 0.22 0.724 y=12805x-487.9 0.9961 0.38–15.66 0.10 0.345 y=4143.8x-88.62 0.9989 0.42–16.82 0.18 0.246 y=3946.8x-94.4 0.9977 0.40–16.02 0.16 0.207 y=4287.8x-95.2 0.9982 0.42–16.46 0.12 0.22a y: Peak area; x: concentration (mg/ml)Table 2 Reproducibility of the assayAnalyteIntra-day variability Inter-day variability Content (mg/g) Mean RSD (%) Content (mg/g) Mean RSD (%)1 46.1646.2846.2246.22 0.1346.2245.3647.4226.33 2.232 5.385.385.165.31 2.405.285.345.045.22 3.043 4.374.304.184.28 2.244.284.464.024.255.204 nd1)-- -- nd -- --5 1.761.801.821.79 1.701.801.681.841.77 4.706 1.281.241.221.252.451.241.341.201.26 5.727 tr2)-- -- tr -- -- 1): not detected; 2): trace. RSD (%) = (SD/Mean) ×100%Table 3 Recovery of the seven analytesAnalyteOriginal(mg) Spiked(mg)Found(mg)Recovery(%)Mean(%)RSD(%)1 23.0823.1423.1119.7122.8628.1042.7346.1351.0199.7100.699.399.8 0.722.692.672.582.082.913.164.735.515.7698.197.6100.698.8 1.632.172.152.091.732.182.623.884.404.6598.8103.297.799.9 2.94nd1)1.011.050.980.981.101.0297.0104.8104.1102.0 4.250.880.900.910.700.871.081.561.752.0197.197.7101.898.9 2.660.640.620.610.450.610.751.081.211.3397.796.796.096.8 0.97tr2)1.021.101.081.031.111.07100.9102.799.1100.9 1.81): not detected; 2): trace.a Recovery (%) = (Amount found –Original amount)/ Amount spiked ×100%, RSD (%) = (SD/Mean) ×100%Table 4 Contents of seven saponins in Lonicera spp.Content (mg/g)1 2 3 4 5 6 7 L. confusa45.65±0.32 5.13±0.08 4.45±0.11tr1) 2.04±0.04tr 1.81±0.03 L. japonica nd2)nd 3.44±0.09nd nd nd nd L. macranthoides46.22±0.06 5.31±0.13 4.28±0.10 tr 1.79±0.03 1.25±0.03 tr L. hypoglauca11.17±0.07 nq3)53.78±1.18nd 1.72±0.02 2.23±0.06 2.52±0.04 L. similes41.22±0.25 4.57±0.07 3.79±0.09nd 1.75±0.02tr nd 1): trace; 2): not detected.. 3) not quantified owing to the suspicious purity of the peak.。
现代分子生物学之转录剪切

A review about DNA/RNA-protein interaction
Functions of initiator protein:
Nucleotide Excision Repair:
Three-way junction
Step 2: The OH of the 5’ exon attacks the phosphoryl group at the 3’ splice site. --------- the 5’ and 3’ exons are joined and the intron is liberated in a “lariat”.
tract).
The intron is removed as a “Lariat” Two successive transesterification: Step 1: The OH of the A at the branch site attacks
the phosphoryl group of the G in the 5’ splice site. -- the 5’ exon is released and the 5’-end of the intron forms a three-way junction structure.
pre-initiation complex in transcription: Core promoter + regulatory elements + GTFs + Mediator complex +Transcriptional regulatory proteins + Nucleosome-modifying enzymes+RNAP
辉瑞大药模式营销并购201010

辉瑞大药模式:营销+并购201010营销+并购研发后进的现实选择以销售规模为维度,辉瑞制药是当之无愧的王者,2010年销售额将突破700亿美元。
辉瑞做大的关键在于专注制药主业后,加大研发和营销投入,通过“营销+并购”弥补研发相对弱势的商业策略奠定了江湖地位。
尽管与礼来、默克、安进等一流研发制药企业所走路径不同,但辉瑞不断通过并购提升研发实力的举措,也再次证明,要在全球制药领域确立竞争优势,研发仍然是战略核心。
时下,中国制药产业正面临做大做强的历史契机。
一方面,制药产业前景广阔,过去30年来,一直以两位数的增速快速扩容,且2009年总产值突破亿元大关。
但与美国相比,中国基数依然偏小,尤其是随着医改的推进和老龄化社会的到来,制药产业进一步扩容空间巨大。
另一方面,行业集中度低下,2009年中国制药百强产值3392.66亿元,不足辉瑞一家,市场集中度也不足35%。
这些问题越来越成为医改主症。
如何做大做强?中国药企在实践中探索了两条路径:以华润医药为代表的并购路径和以恒瑞医药为代表的内生增长路径。
从目前情况看,两条路径均不尽理想。
华润医药的并购成长虽有“短、平、快”特点,但仍停留在做大体量的阶段,尚未从构建核心竞争力角度开展战略布局;恒瑞虽然把握了制药业成长的真谛,但由于国内研发基础薄弱,仍局限在仿制阶段,离真正意义上的做大做强还有很长的路。
在这样一个大背景下研究辉瑞更显得意义重大。
辉瑞是极少数能够将相对落后的研发与一流营销成功结合的国际制药企业,其成功经验对于渴望做大做强但普遍缺乏研发能力的中国制药企业具有极大的借鉴价值。
成立于1849年的辉瑞制药是当今世界最大的制药企业,2009年销售额500亿美元,2010年将超过700亿美元。
上世纪90年代以后,依托强势营销不断弥补研发相对弱势的商业策略,辉瑞在制药界的江湖地位不断提升。
同时,为弥补研发的不足,近年来通过购买成熟技术与产品、收购有新药的公司等大资本运作手法,逐步提高了研发的短板,进而成就国际制药业的霸主地位。
美国市场营销案例:Silly_Bandz橡皮筋也疯狂

美国市场营销案例:Silly Bandz 橡皮筋也疯狂来源: Paypal外贸一站通社区 ()大奖征集"Silly Bandz"营销案例(最佳案例揭晓)https:///bbs/thread-33120-1-2.html活动主持人:Richard Zhang Paypal外贸一站通社区版主主持人空间:https:///bbs/space-uid-4683.html主持人网站:Silly Bandz 就是有造型的橡皮筋,没有任何科技含量,发明它的就是个小企业主,它起步也没有大笔的广告费用,就靠Twitter,Facebook和Youtube进行网络病毒营销 ……我们很多出口企业的物质条件要比Silly Bandz的发明企业好很多,应该从Silly Bandz 的创意,营销,供应链,产品升级过程中学到美国市场创新经营之道,因此,我们设立大奖征集Silly Bandz的营销案例,评选出以下优秀案例:1. Keeping Up: Kardashian Silly Bandz by icefable2010 (8楼)2. 橡皮筋狂潮背后的人 (10,11楼) 商业周刊中文转载,非参选作品,但对silly bandz创始人及其商业策略有精彩描述,绝对值得深读。
3. The Latest Kids' Craze: Rubber Bands (13楼) 奥美公关的市场部主任Rohit Bhargava 分析Silly Bandz产品成功的四个原因4. Silly Bandz: 橡皮筋造就的亿万富翁 (17楼) 这篇案例总结的比较全面,涵盖了BCP创造Silly Bandz的始末,包含不少关键资料,作者为此查阅了很多英文资料,心血之作呀,值得大家深入阅读******************************参与讨论— Silly Bandz的案例讨论:为什么橡皮筋也疯狂?https:///bbs/thread-32879-1-2.html>> 马上登陆Paypal 外贸社区参与Silly Bandz案例讨论https:///bbs/thread-32879-1-2.htmlCase 1:Silly Bandz 橡皮筋造就的亿万富翁*最佳原创案例*作者:张朋作者空间:https:///bbs/space-uid-9459.html作者网站:罗伯特是Silly Bandz的老板在Silly Bandz风靡美国之前,罗伯特(Robert Croak)经营着一家叫做BCP Imports的小型外贸公司,主要销售个性T恤衫,狗牌,杯子和硅胶手环。
无导线起搏与传统起搏对三尖瓣反流短期影响的对比研究

㊃心脏电生理学专题㊃无导线起搏与传统起搏对三尖瓣反流短期影响的对比研究郭雨龙㊀付明鹏㊀刘晨㊀乔宇㊀郭金锐㊀刘可㊀郭涛650102昆明,云南省阜外心血管病医院心律失常中心通信作者:郭雨龙,电子信箱:kktury8859@DOI:10.3969/j.issn.1007-5410.2023.04.004㊀㊀ʌ摘要ɔ㊀目的㊀比较无导线起搏与传统起搏患者的短期三尖瓣反流变化情况,并分析三尖瓣反流的相关因素㊂㊀方法㊀回顾性纳入2020年1月至2022年11月在云南省阜外心血管病医院就诊的新植入起搏器94例患者,分为无导线起搏组(47例)和传统起搏组(47例),通过经胸超声心动图评估术前㊁术后6月内的三尖瓣反流程度及恶化情况,比较两组三尖瓣反流恶化的发生率㊂Logistic回归分析发生三尖瓣反流的相关因素㊂㊀结果㊀发生三尖瓣反流恶化者共29例(30.9%),无导线起搏组和传统起搏组的发生风险相似(14例比15例,29.8%比31.9%),差异无统计学意义(χ2=0.050,P=0.823)㊂二元logistic回归分析结果显示,单腔起搏器是发生三尖瓣中㊁大量反流的独立影响因素(χ2=10.031,P=0.010)㊂㊀结论㊀与传统起搏器相比,无导线起搏器可能并不减少术后短期发生三尖瓣反流的风险㊂ʌ关键词ɔ㊀无导线起搏;㊀心脏起搏;㊀三尖瓣反流;㊀三尖瓣瓣下复合体基金项目:云南省临床医学中心项目;云南省卫生健康委员会医学后备人才培养计划(H-2018037);云南省阜外心血管病医院院级科研基金项目(2019YFKT-04)Comparison of the short-term effects of leadless pacing and traditional pacing on tricuspidregurgitation㊀Guo Yulong,Fu Mingpeng,Liu Chen,Qiao Yu,Guo Jinrui,Liu Ke,Guo TaoDepartment of Arrhythmia,Fuwai Yunnan Cardiovascular Hospital,Kunming650102,ChinaCorresponding author:Guo Yulong,Email:kktury8859@.ʌAbstractɔ㊀Objective㊀To compare the short-term risk of tricuspid regurgitation in patients treatedwith leadless pacemaker or traditional pacemaker,and to determine its related factors.㊀Methods㊀A totalof94patients who were newly implanted leadless or traditional pacemakers in Fuwai Yunnan CardiovascularHospital from January2020to November2022were retrospectively enrolled.All were divided into theleadless pacemaker group(n=47)and the tranditional pacemaker group(n=47).The transthoracic echocardiography was used to evaluate the severity and deterioration of tricuspid regurgitation before and6months after operation.Logistic regression analyses was used to assess its related factors.㊀Results㊀A totalof29(30.9%)patients had tricuspid regurgitation deterioration.There was no significant difference in therisk of tricuspid regurgitation deterioration between the leadless and tranditional pacemaker groups(14casesvs.15cases,29.8%vs.31.9%,χ2=0.050,P=0.823).Binary logistic regression analyses resultindicated that single-chamber pacemaker was an independent factor of moderate to massive tricuspid regurgitation(χ2=10.031,P=0.010).㊀Conclusions㊀Compared with traditional pacemaker,leadless pacemaker probably cannot reduce the short-term risk of tricuspid regurgitation after operation.ʌKey wordsɔ㊀Leadless pacemaker;㊀Cardiac pacing;㊀Tricuspid regurgitation;㊀Tricuspid subvalvular apparatusFund program:Yunnan Provincial Clinical Medical Center Project;Yunnan Provincial Health Commission Medical Reserve Talent Training Plan(H-2018037);Yunnan Fuwai Cardiovascular DiseaseHospital Hospital-Level Research Fund Project(2019YFKT-04)㊀㊀心脏起搏是严重心动过缓最有效的治疗方式㊂传统心脏起搏器由脉冲发生器及相连接的电极导线构成,电极导线一般通过上腔静脉途径植入心腔内,右心室起搏电极导线跨过三尖瓣进入右室内固定㊂但是在以机械机制为主的多种机制介导下,传统心脏起搏会有加重恶化三尖瓣反流风险㊂无导线起搏是近年来最新的心脏起搏技术,与传统起搏器不同,无导线起搏器体积仅有胶囊大小,可通过特殊的输送装置,经下腔静脉途径植入右心室内,植入成功后输送装置可完全撤除,具有创伤小㊁恢复快,以及避免了囊袋并发症等特点㊂其心腔内留存的无导线起搏器仅在右心室内,不会遗留跨三尖瓣的电极导线㊂但是,无导线起搏对患者三尖瓣反流影响的临床研究数据较少,且尚无直接比较无导线起搏与传统起搏的报道㊂因此,我们通过纳入无导线起搏与匹配的传统起搏患者,在术前与术后短期通过经胸超声心动图评估三尖瓣反流情况,比较两组之间三尖瓣反流恶化的发生率,以此评估无导线起搏对三尖瓣反流的影响,希望能为无导线起搏的进一步推广应用提供有价值的临床信息㊂1㊀对象和方法1.1㊀研究对象回顾性队列研究㊂纳入2020年1月至2022年11月在云南省阜外心血管病医院新植入起搏器患者,分为无导线起搏组和传统起搏组㊂纳入标准: (1)无导线起搏组符合无导线起搏适应证,且成功完成无导线起搏器植入手术者;(2)传统起搏组按照年龄㊁性别进行1ʒ1匹配,纳入同期就诊的符合传统起搏适应证且成功完成植入者㊂排除标准: (1)因传统起搏感染㊁电极故障或电池耗竭而转用无导线起搏者;(2)仅植入心房电极单腔起搏器者;(3)原有起搏器更换或升级者㊂本研究符合医学研究伦理学要求(编号:2022-94)㊂所有患者均知情同意㊂1.2㊀方法1.2.1㊀无导线起搏器植入手术㊀采用美敦力无导线起搏器(型号Micra TM MC1VR01或Micra TM AV MC1AVR1),所有操作均在导管室血管造影机透视指导下完成㊂穿刺右股静脉,置入导引钢丝,逐级扩张;若右股静脉穿刺或置入导丝不顺则换用左股静脉,沿导引钢丝置入输送装置,应用输送装置将无导线起搏器送至右心室内;多体位投照(至少三个体位:右前斜30ʎ㊁左前斜45ʎ及正位)确认跨过三尖瓣到达右心室中下间隔部,造影确认与心肌贴靠情况满意后,施加一定压力推送输送系统,并释放无导线起搏器,稍微回退输送鞘管,通过牵拉试验证实无导线起搏器头端勾挂满意,且测试阈值㊁阻抗㊁感知等参数满意后方可剪断拉绳;若不满意,则回收后重新定位释放㊂整个手术过程中,静脉推注3000U肝素,输送鞘管持续肝素盐水冲洗,最后撤除输送装置㊁缝合伤口,最后加压包扎㊂1.2.2㊀传统起搏器植入手术㊀采用传统起搏器,所有操作均在导管室血管造影机透视指导下完成㊂穿刺左锁骨下静脉或腋静脉,置入导引钢丝,若穿刺或置入导丝不顺则改为右侧植入,沿锁骨下做4~ 5cm皮肤切口,逐层分离至深筋膜层,制作合适大小的皮下囊袋,应用可撕开鞘管置入起搏电极,右房电极定位固定至右心耳,右室电极定位固定至右室间隔部或心尖部(均使用主动固定电极),测试阈值㊁阻抗㊁感知等参数满意后,拔除可撕开鞘管,电极尾端连接脉冲发生器并埋置于囊袋内,充分止血㊁冲洗后逐层缝合,最后加压包扎㊂1.3㊀观察指标和随访所有患者在植入术前1周内及术后6个月内完成经胸超声心动图对三尖瓣反流情况进行评估㊂使用GE或飞利浦超声探头,在彩色血流多普勒下测量三尖瓣反流束面积与右心房面积比和(或)缩流颈宽度综合评估三尖瓣反流情况的变化㊂其中,面积比<10%为微量反流,10%~20%为少量或轻度反流,21%~40%为中量或中度反流,>40%为大量或重度反流;缩流颈宽度<3mm为少量或轻度反流,缩流颈宽度3~7mm为中量或中度反流,缩流颈宽度>7mm为大量或重度反流㊂此外,三尖瓣反流恶化定义为与术前相比,术后面积比增加超过5%和(或)缩流颈宽度增加超过1mm㊂1.4㊀统计学方法应用SPSS20.0软件进行统计分析㊂符合正态分布的计量资料用 xʃs表示,组间比较采用独立样本t检验;计数资料用百分构成比表示,组间比较采用χ2检验㊂Logistic回归分析影响术后新增三尖瓣中㊁大量反流的相关因素㊂P<0.05为差异有统计学意义㊂2㊀结果2.1㊀两组的基线临床资料比较如表1所示,两组的诊断差异有统计学意义,表现为无导线起搏组诊断为ȡⅡ度房室传导阻滞的比例显著低于传统起搏组,而诊断为心房颤动伴RR长间歇的比例显著高于传统起搏组(P= 0.005)㊂其余基线临床资料相似,差异均无统计学意义(均为P>0.05)㊂2.2㊀两组的起搏器植入术后即刻右室电极参数比较无导线起搏组中,38例(80.9%)使用Micra TM表1㊀两组的基线临床资料比较项目总体(94例)无导线起搏组(47例)传统起搏组(47例)t/χ2值P值年龄( xʃs,岁)78.9ʃ9.979.3ʃ9.978.4ʃ10.00.4390.688男性[例(%)]56(59.6)28(59.6)28(59.6)0.000 1.000诊断[例(%)]10.7810.005㊀窦房结功能障碍38(40.4)19(40.4)19(40.4)㊀ȡⅡ度房室传导阻滞34(36.2)11(23.4)23(48.9)㊀心房颤动伴RR长间歇22(23.4)17(36.2)5(10.6)心功能指标( xʃs)㊀左室射血分数(%)62.0ʃ7.262.7ʃ6.261.2ʃ8.10.9900.325㊀左室舒张末期内径(mm)46.6ʃ6.646.4ʃ6.846.9ʃ6.50.2950.769表2㊀两组起搏器植入术后即刻右室电极参数比较( xʃs)项目总体(94例)无导线起搏组(47例)传统起搏组(47例)t值P值右室电极阻抗(Ω)797.8ʃ214.0819.6ʃ196.8775.9ʃ230.00.9880.326右室电极阈值(V@0.4ms)0.70ʃ0.420.70ʃ0.490.71ʃ0.340.1310.896 R波感知振幅(mV)9.96ʃ4.759.28ʃ4.4010.64ʃ5.02 1.3980.166表3㊀两组术前和术后的三尖瓣反流情况比较[例(%)]项目总体(94例)无导线起搏组(47例)传统起搏组(47例)χ2值P值术前三尖瓣反流14.7960.002㊀无或微量32(34.0)10(21.3)22(46.8) 6.8230.009㊀少量/轻度43(45.7)21(44.7)22(46.8)0.0430.836㊀中量/中度11(11.7)8(17.0)3(6.4) 2.5740.109㊀大量/重度8(8.5)8(17.0)0(0.0)8.7440.003术后三尖瓣反流14.1480.003㊀无或微量28(29.8)7(14.9)21(44.7)9.9700.002㊀少量/轻度35(37.2)18(38.3)17(36.2)0.0460.831㊀中量/中度18(19.1)11(23.4)7(14.9) 1.0990.294㊀大量/重度13(13.8)11(23.4)2(4.3)7.2310.007MC1VR01(起搏模式VVI),9例(19.1%)使用Micra TM AV MC1AVR1(起搏模式VDD),所有无导线起搏器均位于右室中低位间隔部㊂传统起搏组中,9例(19.1%)使用单腔起搏器(模式VVI),38例(80.9%)使用双腔起搏器(模式DDD);右室电极导线在心尖部3例(6.4%),在间隔部44例(93.6%)㊂如表2所示,两组起搏器植入术后右室电极的即刻参数均相似,差异无统计学意义(均为P>0.05)㊂2.3㊀两组术前和术后的三尖瓣反流情况比较如表3所示,两组术前的三尖瓣反流情况差异有统计学意义(P=0.002),表现为无导线起搏组的无或微量三尖瓣反流率明显低于传统起搏组(P= 0.009),而大量/重度三尖瓣反流率明显高于传统起搏组(P=0.003)㊂两组术后的三尖瓣反流情况差异也有统计学意义(P=0.003),表现为无导线起搏组的无或微量三尖瓣反流率明显低于传统起搏组(P=0.002),而大量/重度三尖瓣反流率明显高于传统起搏组(P=0.007)㊂与术前比较,术后新增的有临床意义的三尖瓣中大量反流有12例,其中无导线起搏组6例(12.8%),传统起搏组6例(12.8%),组间比较差异无统计学意义(χ2=0.000,P=1.000)(图1)㊂三尖瓣反流恶化者共29例(30.9%),其中无导线起搏组14例(29.8%),传统起搏组15例(31.9%),组间比较差异无统计学意义(χ2=0.050, P=0.823)(图1)㊂图1㊀两组发生三尖瓣反流情况比较2.4㊀术前三尖瓣反流程度对术后发生三尖瓣反流恶化的影响进一步研究显示,术前三尖瓣无或微少量反流者在术后出现反流恶化的比例为29.3%(22/75),而术前三尖瓣中㊁大量反流者在术后出现反流恶化的比例为36.8%(7/19),两者之间比较差异无统计学意义(χ2=0.401,P=0.527)㊂2.5㊀Logistic回归分析结果采用二元logistic回归分析(Wald后退法),分析术后新增三尖瓣中㊁大量反流的影响因素,无导线起搏组纳入因素为年龄㊁性别㊁起搏适应证诊断㊁术前左室射血分数㊁舒张末期内径及术前三尖瓣反流情况,传统起搏器纳入因素除上述外,增加右室电极位置及起搏器类型(单腔或双腔)㊂最终在无导线起搏组中未发现影响术后新增三尖瓣中㊁大量反流的有统计学意义相关因素(均为P>0.05);而在传统起搏患者中,发现起搏器类型[Exp(B)= 35.589,P=0.01]是独立影响因素,其中单腔起搏器患者术后出现新增三尖瓣中㊁大量反流的比例远高于双腔起搏器患者[4例比2例,44.4%(4/9)比5.3%(2/38),χ2=10.031,P=0.002]㊂3 讨论本研究发现,无导线起搏器与传统起搏器相比,术后短期发生三尖瓣反流恶化及新发中㊁大量三尖瓣反流的比例无统计学差异,故无导线起搏可能并不能够减少对三尖瓣反流的负面影响㊂三尖瓣反流是右心室起搏的常见并发症㊂国外研究报道术后三尖瓣反流的发生率7%~21%,三尖瓣反流恶化或加重的比例为10%~45%[1]㊂一般认为,导致或加重三尖瓣反流的机制以机械损伤为主,机械机制主要包括植入过程中起搏电极导线直接损伤瓣叶导致穿孔或撕裂㊁导线嵌顿于瓣叶之间㊁导线与瓣叶粘连或与腱索缠绕等㊂与普通电极导线相比,更粗㊁更硬的除颤电极导线导致三尖瓣反流的概率更高;此外,长期高比例右室非生理性起搏㊁慢性右心扩大及三尖瓣环扩张也是远期三尖瓣反流发生及加重的因素㊂其中,术中电极导管对三尖瓣的直接机械损伤是短期发生三尖瓣反流的主要机制㊂使用的电极越硬㊁越粗或暴力操作等均是潜在的危险因素,而慢性电极导线粘连㊁高比例右室非生理起搏及右心扩大瓣环扩张则是远期发生三尖瓣反流的主要机制[1-3]㊂国内关于三尖瓣反流的报道的例数较少且还有争议,赵波等[4]发现长期右室心尖部起搏仅导致轻微反流,引起有临床意义的三尖瓣反流恶化的比例更低㊂邹宝明等[5]发现无论右室心尖部还是间隔部起搏都不会在短期内明显加重三尖瓣反流㊂无导线起搏是最新的心脏起搏技术,与传统右室起搏不同,无导线起搏在植入后并不会长期遗留跨三尖瓣的电极导线,因此其对三尖瓣的影响及机制可能会不同㊂目前国外关于无导线起搏对三尖瓣反流作用的研究报道有限,而国内尚无相关报道㊂2019年Beurskens等[6]报道无导线起搏术后1年三尖瓣反流加重的比例为43%㊂2022年一项关于无导线起搏器的真实世界研究,纳入植入心房感知㊁心室起搏的无导线起搏器患者,发现中度以上的三尖瓣反流发生率为48.8%(21/43)[7]㊂因此,无导线起搏器导致或加重三尖瓣反流的风险依然存在,甚至可能比传统起搏器高㊂此项研究发现,在起搏器植入术后短期内,总体三尖瓣反流恶化发生概率为30.9%,无导线起搏组为29.8%,传统起搏组为31.9%,考虑到本研究中我们为了更早地发现短期影响效果,所定义的三尖瓣反流恶化的超声心动图指标较为敏感,远比临床症状更早出现变化,因此该比例应该会高于真实世界中有临床症状的三尖瓣反流发病率㊂我们发现两组间差异无统计学意义,可认为无导线起搏并不能够减少对三尖瓣的负面影响㊂表1中两组间的入院诊断有统计学差异,无导线起搏组的心房颤动伴RR长间歇患者比例更高,这是其最早及最强的植入适应证,很可能对结果造成一定影响㊂由表3中可看出,本研究无导线起搏组术前的中㊁大量三尖瓣反流者更多,这是由于我们在开展无导线起搏器植入术初期误以为其对三尖瓣反流的影响较小,因此在病人选择上有了偏差,而后续统计分析发现术前反流程度并未影响其术后恶化情况,故这种差异并不影响本文的主要研究结论㊂而经过本研究之后,我们对无导线起搏与三尖瓣的相互作用又有了更多认识,将进一步优化及改善今后对患者的处理决策,希望能更好改善预后㊂从表面上看,由于无导线起搏器并不会长期遗留跨瓣导线,从理论上来说慢性三尖瓣粘连及腱索缠绕的发生率可能会低于传统起搏器,似乎避免了传统起搏引发三尖瓣反流的一些机制㊂但是,无导线起搏器植入须使用更粗㊁更硬的输送装置,操作中对三尖瓣的损伤可能会大于传统起搏,发生瓣膜穿孔的风险是否增加尚无报道㊂另外,国外有学者发现,无导线起搏器植入后对三尖瓣瓣下复合体的干扰较大,瓣下复合体是个解剖概念,主要指与瓣膜㊁腱索连接的乳头肌,三尖瓣下一般有三组乳头肌,前组在右室游离壁,下组及间隔组分别在下壁及右室间隔面,下组及后组乳头肌可能表现不完全而腱索就直接连接到间隔面或心室壁[8]㊂固定于间隔面的无导线起搏器虽然无跨瓣导线,但可能会显著影响间隔侧的三尖瓣瓣下复合体功能,由此加重三尖瓣反流㊂然而,目前对于无导线起搏器与三尖瓣相互作用的认识有限,还需要进一步的研究及临床实践来明确,尤其需要心脏外科及心脏结构专业与起搏电生理专业的合作㊂本研究有一些局限性㊂本研究仅为临床观察性研究,例数较少,并非严密设计的随机对照研究,而且三尖瓣反流的可能影响因素较多,本研究也未能完全排除无导线/传统起搏选择以外的其他可能干扰因素,结论的说服力有限㊂总之,与传统起搏器相比,无导线起搏器可能并不减少术后短期发生三尖瓣反流的风险㊂利益冲突:无参㊀考㊀文㊀献[1]Addetia K,Harb SC,Hahn RT,et al.Cardiac ImplantableElectronic Device Lead-Induced Tricuspid Regurgitation[J].JACC Cardiovasc Imaging,2019,12(4):622-636.DOI:10.1016/j.jcmg.2018.09.028.[2]郑晓琳,张澍,陈珂萍.心内膜导线相关的三尖瓣反流[J].中国心脏起搏与心电生理杂志,2014,28(5):443-445.DOI:10.13333/ki.cjcpe.2014.05.018.㊀Zheng XL,Zhang S,Chen KP.Tricuspid valve regurgitationassociated with Endocardium leads[J].Chin J Card PacingElectrophysiol,2014,28(5):443-445.DOI:10.1333/ki.cjcpe.2014.05.018.[3]Kim JB,Spevack DM,Tunick PA,et al.The effect oftransvenous pacemaker and implantable cardioverter defibrillatorlead placement on tricuspid valve function:an observational study[J].J Am Soc Echocardiogr,2008,21(3):284-287.DOI:10.1016/j.echo.2007.05.022.[4]赵波,宋建平,邹操.长期右室心尖部起搏对三尖瓣反流的影响[J].中国心脏起搏与心电生理杂志,2012,26(4):315-318.DOI:10.13333/ki.cjcpe.2012.04.014.㊀Zhao B,Song JP,Zou C.Effects of long-term permanent rightventricular apical pacing on tricuspid regurgitation[J].Chin JCard Pacing Electrophysiol,2012,26(4):315-318.DOI:10.13333/ki.cjcpe.2012.04.014.[5]邹宝明,王景武,孙克陆,等.围术期右室流入道间隔部起搏对三尖瓣反流的影响[J].中华全科医学,2015,13(6):896-898.DOI:10.16766/ki.issn.1674-4152.2015.06.019.㊀Zou BM,Wang JW,Sun KL,et al.Influence of right ventricularinlet septum pacing on tricuspid regurgitation during perioperativeperiod[J].Chin J Gen Pract,2015,13(6):896-898.DOI:10.16766/ki.issn.1674-4152.2015.06.019. [6]Beurskens NEG,Tjong FVY,de Bruin-Bon RHA,et al.Impactof Leadless Pacemaker Therapy on Cardiac and AtrioventricularValve Function Through12Months of Follow-Up[J].CircArrhythm Electrophysiol,2019,12(5):e007124.DOI:10.1161/CIRCEP.118.007124.[7]Kowlgi GN,Tseng AS,Tempel ND,et al.A real-worldexperience of atrioventricular synchronous pacing with leadlessventricular pacemakers[J].J Cardiovasc Electrophysiol,2022,33(5):982-993.DOI:10.1111/jce.15430.[8]Tadic M.Multimodality Evaluation of the Right Ventricle:AnUpdated Review[J].Clin Cardiol,2015,38(12):770-776.DOI:10.1002/clc.22443.(收稿日期:2023-01-31)(本文编辑:李鹏)㊃读者㊃作者㊃编者㊃GB/T7713.2 2022‘学术论文编写规则“已于2023年7月1日实施㊀㊀2022年12月30日,国家市场监督管理总局和国家标准化管理委员会联合发布了GB/T7713.2 2022‘学术论文编写规则“,并已于2023年7月1日实施㊂无论是学术论文㊁学位论文还是科技报告,其撰写和编排都需要遵循一定的规范,以利于信息系统的收集㊁存储㊁处理㊁加工㊁检索㊁利用㊁交流㊁传播㊂GB/T7713 1987‘科学技术报告㊁学位论文和学术论文的编写格式“,对学术论文㊁学位论文和科技报告的撰写要求及编排格式作了统一规定㊂鉴于三者的使用对象及使用目的不尽相同,撰写要求及编排格式差异较大,后来修订GB/T7713时,将其分为3个部分分别进行修订㊂第1部分:学位论文编写规则㊂目的在于规定了学位论文的撰写格式和要求㊂第2部分:学术论文编写规则(简称 本规则 )㊂目的在于规定了学术论文的撰写要求和编排格式㊂第3部分:科技报告编写规则㊂目的在于规定了科技报告的编写㊁组织㊁编排等要求㊂本规则描述了撰写和编排学术论文的基本要求和格式规范㊂学术论文编写的标准化和规范化,是使其格式和体例规范化,语言㊁文字和符号规范化,技术和计量单位标准化,以便于学术论文的检索和传播,促进学术成果的交流和使用㊂本规则的适用范围,包括一切反映自然㊁社会和人文等的科学体系的学术论文㊂然而,由于学科门类㊁选定课题㊁研究工作方法㊁工作进行阶段㊁观测和调查等各方面的差异,采用本规则进行学术论文编写宜采取严肃性和灵活性相结合的原则㊂本规则对GB/T7713 1987中的学术论文编写内容进行了必要的检查㊁更新,进而形成单独的学术论文编写规则,代替GB/T7713 1987中的学术论文编写格式部分㊂现可登录国家标准化管理委员会网站或通过以下网址/bzgk/gb/showGb?type=online&hcno= 0B963916637B8F34B295FCF4A51A1BE5查询本规则全文㊂。
高露洁5P销售系统英文课件

Set prices based on the value the product offers to the customer, considering factors such as quality, features, and brand image.
Channel Strategy (Place)
The 5P model has since been widely used in the field of marketing and sales management.
The importance of 5P in sales
It provides a structured framework for sales planning and execution.
Continuous improvement action 3
Repeat evaluation and improvement cycle periodically.
BIG DATA EMPOWERS TO CREATE A NEW ERA
04
Case analysis of the Colgate 5P sales system
Product Development
Identify market trends and customer feedback to continuously innovate and improve products.
Price strategy
Cost-Based Pricing
Determine the price based on the cost of production, researching the market to ensure it is competitive.
Lorex N842安装指南说明书
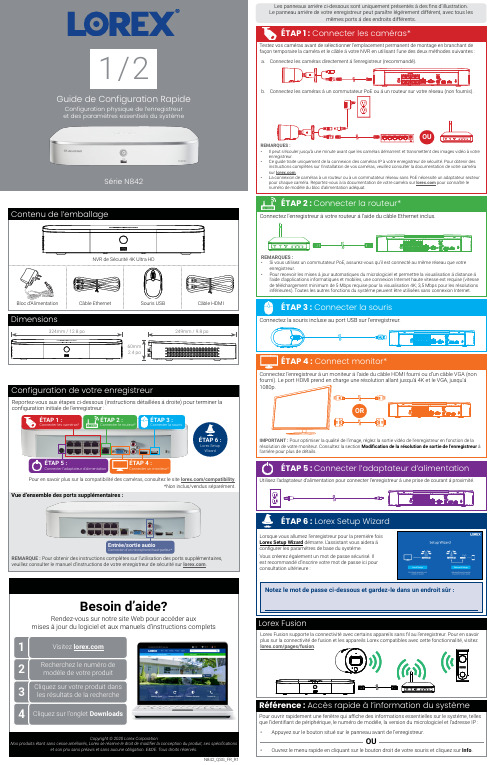
N842_QSG_FR_R1Série N8421 / 2Copyright © 2020 Lorex CorporationNos produits étant sans cesse améliorés, Lorex se réserve le droit de modifier la conception du produit, ses spécificationset son prix sans préavis et sans aucune obligation. E&OE. Tous droits réservés.Reportez-vous aux étapes ci-dessous (instructions détaillées à droite) pour terminer la configuration initiale de l’enregistreur :Lorex Fusion supporte la connectivité avec certains appareils sans fil au l’enregistreur. Pour en savoir plus sur la connectivité de fusion et les appareils Lorex compatibles avec cette fonctionnalité, visitez *Non inclus/vendus séparément.Pour en savoir plus sur la compatibilité des caméras, consultez le site /compatibility .Vue d’ensemble des ports supplémentaires :REMARQUE : Pour obtenir des instructions complètes sur l’utilisation des ports supplémentaires, veuillez consulter le manuel d’instructions de votre enregistreur de sécurité sur .Configuration de votre enregistreurLes panneaux arrière ci-dessous sont uniquement présentés à des fins d’illustration. Le panneau arrière de votre enregistreur peut paraître légèrement différent, avec tous lesmêmes ports à des endroits différents.ÉTAP 6 :Lorex Setup WizardNotez le mot de passe ci-dessous et gardez-le dans un endroit sûr :N842_QSG_FR_R1Ajouter des caméras à partir du LANSuivez les instructions ci-dessous pour ajouter des caméras qui ne sont pas directement connectées aux ports PoE à l’arrière de l’enregistreur.REMARQUE : Veuillez visiter Pour ajouter des caméras à partir du LAN :1. Connectez la caméra à un routeur ou à un commutateur branché sur le même réseau que l’enregistreur.2. Cliquez sur le bouton droit de la souris et sélectionnez l’aide du nom d’utilisateur du système (par défaut :3. Configurez les éléments suivants :a. Cliquez sur Camera Registration b. Cochez la/les caméra(s) à ajouter.c. Cliquez sur Add . L devient vert si la caméra est bien connectée.d. Les périphériques ajoutés apparaîtrontdans la liste Added Device le bouton droit de la souris pour quitter le Rechercher et lire des enregistrements vidéo depuis le disque dur.Pour rechercher et lire des enregistrements :Depuis le visionnement en direct, cliquez sur le bouton droit, puis sur Playback (lecture). Si vous y êtesinvité, connectez-vous à l’aide du nom d’utilisateur du système (par défaut : admin ) et votre nouveau Sauvegarder des enregistrements sur une clé USB (non fournie).Pour sauvegarder des enregistrements :Insérez une clé USB (non fournie) dans un port USB libre de l’enregistreur.Depuis le mode de visionnement en direct, cliquez avec le bouton droit de la souris, puis cliquez sur Main. Si vous y êtes invité, connectez-vous à l’aide du nom d’utilisateur du système (par défaut : admin ) et votre nouveau mot de passe sécurisé.Sélectionnezle canal d’unecaméra connectée avec détection de personnes et de Enable sous et/ou Vehicle . c. Cliquez sur Set à côté de Area pour définir des zones actives pour la détection despersonnes et/ou des véhicules. Consultez la Figure 1 ci-dessous pour plus de détails.d. Cliquez sur Set à côté de Schedule pour définir un calendrier hebdomadaire pour ladétection des personnes et/ou des véhicules. Consultez la Figure 2 ci-dessous pour plus de détails.e. Réglez les préférences pour la lumière d’avertissement et la sirène.f. Cliquez sur Apply .Pour déclencher les lumières d’avertissement et les sirènes de toutes les caméras de dissuasion connectées, appuyez sur le bouton du panneau avant et maintenez-le enfoncé pendant 3 secondes.Figure 2: CalendrierFigure 1: Zone de détection• Cliquez sur Add pour définir une zone de détection depersonnes ou de véhicules sur le canal sélectionné. Cliquez et faites glisser les coins pour redimensionner la zone.• Pour des résultats plus précis, définissez une zone où les objets d’intérêt se déplaceront à l’intérieur de la zone de délimitation ainsi qu’à l’entrée et à la sortie.• Cochez la Light à côté d’une règle pour faire clignoter la lumière d’avertissement de l’appareil lorsqu’un objet est détecté.• Consultez la documentation de votre caméra pour unpositionnement optimal de la caméra pour la détection des personnes et des véhicules.Option 1 : Caméras de détection avancée du mouvementOption 2 : Caméras de dissuasion active• L ’horaire par défaut, illustré à la Figure 2, est actif pendant la nuit, entre 17 h et 7 h. • Cliquez sur Set pour modifier l’horaire du jour de la semaine correspondant.• Cliquez sur OK lorsque vous avez terminé.Sélectionnez le canal d’une camérade dissuasion connectée.Enable .Set à côté de Area pourdéfinir des zones actives pour la détection des personnes et/ou des véhicules. Consultez la Figure 3 ci-dessous pour plus de détails.d. Cliquez sur Set à côté de Schedule pour définir un calendrierhebdomadaire pour la détection des personnes et/ou desvéhicules. Consultez la Figure 2 ci-dessous pour plus de détails.e. Réglez les préférences pour la lumière d’avertissement et la sirène.f.Réglez les niveaux de Sensitivity et de Threshold selon vos préférences.g. Cliquez sur Apply .• L ’image de la caméra apparaît avec une grillesuperposée. La zone verte est la zone active pour la dissuasion.• Cliquez ou cliquez et faites glisser pour ajouter/supprimer la zone de la grille rouge.• Dans la Figure 3, seul le mouvement autour de la porte déclenchera un voyant d’avertissement.• Cliquez à droite lorsque vous avez terminé.Figure 3: Zone de dissuasionModification de la résolution de sortie de l’enregistreurPour garantir la meilleure qualité d’image possible, réglez la résolution de sortie de l’enreg-istreur à la résolution la plus élevée prise en charge par votre moniteur.moniteur. Par exemple, sélectionnez Pour modifier la résolution de sortie de l’enregistreur :IMPORTANT : Si vous devez changer de moniteur, assurez-vous de régler l’enregistreur sur une résolution de sortie prise en charge par le nouveau moniteur avant de commuter.Pendant le visionnement en direct, passez le curseur de la souris au-dessus de l’écran pour ouvrir la barre de navigation. Déplacez le curseur de la souris en l’éloignant du dessus de l’écran pour fermer la barre de navigation.Lors du visionnement en direct :afin de faire un zoom avant et arrière.Utilisation du menu rapideCliquez avec le bouton droit n’importe où sur l’écran de visionnement en direct pour ouvrir le menu rapide.Ouvrir le menu principal.Rechercher et lire des enregistrements.Contrôle des caméras PTZ (nonabcabca b c defa c eb d fc d eb a gfab。
医疗保健-USANA五天健康重整套装 (

USANA五天健康重整套装(Reset Kit)2015-03-05 360高级营养师全新改良的五天健康重整套装,含有您喜爱的所有USANA®代餐食品。
健康重整USANA的RESET™健康重整体重管理计划简单、方便又有效。
只要每天用美味的USANA®代餐食品代替平常的餐食和点心,加上新鲜蔬果各一份。
在这个套装里,我们还提供您「健康套装™」小包装的顶级营养补充品。
我们鼓励您坚持该计划,每天喝64至80盎斯的水,并步行30分钟,即可获得最佳效果。
今天就加入#RESETNATION!五天健康重整套装15包营养餐™:巧克力味、香草味、及野草莓味5块美味乳脂软糖蛋白质点心3块迷人花生口味蛋白质点心2块巧克力脆饼蛋白质点心10小包健康套装计划指南激励性#RESETNATION手环健康要点:实行RESET,获得更佳效果。
五天最多可减重五磅†十二周,每周减重两磅†低升糖指数的成份,帮助控制对甜食的渴望*市面上最营养的减重计划之一为减重成功提供均衡的营养减肥机理:帮助机体在营养均衡的前提下进行热量控制,排除毒素,燃烧脂肪。
吃进的热量>消耗的热量,体重↑吃进的热量<消耗的热量,体重↓吃进的热量== 消耗的热量,体重不变普通一顿正餐含热量约800卡,一日三餐热量约800*3=2400卡,人体日消耗热量约2000-2400卡;1公斤的脂肪7700卡的热量1餐奶昔的热量:约200卡每日代3餐仅为200*3=600卡的热量,摄入热量差为:-1800卡,5天大约减3-4斤脂肪;每日代2餐仅为400+800=1200卡的热量,摄入热量差为:-1200卡,7天大约减2斤脂肪。
优莎娜营养餐的调制及使用方法:1、奶昔的调制:约300ml冷开水或温开水中加入2-3勺营养餐粉、用力摇匀,即可食用;2、奶昔的使用A. 营养代餐:每天代替1次正餐,保持体形,增加营养,使精力旺盛;B. 减肥:①.健康重整:第1—5天,每日用奶昔代替3次正餐,可配合适量蔬菜水果,喝大量水,5日减3-4斤脂肪。
盐酸米那普仑原料药联合申报

广州市桐晖药业有限公司
盐酸米那普仑原料药联合申报
产品名称:盐酸米那普仑
英文名:Milnacipran HCL
剂型规格:片:25mg
科室:精神科
家数原料:2A国+1I进制剂:1国,0进
原料来源:印度
备案状态:已备案
登记号:Y20200000968
USDMF状态已有USDMF, 已激活
注册分类5+4
可申报剂型:片剂
适应症治疗抑郁症
国内市场情况:医保乙类目录,仅一家国产制剂上市,竞争状况良好,国产原料供应受限,桐晖药业可供应已备案进口原料。
产品优势:盐酸米那普仑的开发公司为PierreFabre,最早于1996年在法国上市,2009年在美国获批新适应症纤维肌痛综合征,2010年在中国上市。
盐酸米那普仑是一种新型的抗抑郁药,作用于SERT和NET,是唯一一个NET作用大于SERT的选择性5-羟色胺再摄取抑制剂(SNRIs)药物,其抗抑郁药效较强。
与目前常用的三环类抗抑郁剂相比,盐酸米那普仑对α-肾上腺素受体、毒蕈碱受体或H1组胺受体均无亲和力,并不会像三环类抗抑郁剂那样引起体位性低血压及口干、便秘、视觉模糊等。
此外,盐酸米那普仑还具有耐受性好,不良反应率低的特点。
2013年-2018年我国抗抑郁药市场销售从41亿元增长至87亿元,2019年预计将超过95亿元。
目前,国内市场上盐酸米那普仑仅有一家仿制药企业生产并销售,2018年国内销售额为4263万元。
盖洛普员工敬业度调查课程讲解课件

股票增值
可持续发展
盖洛普路径
忠实客户
敬业员工
从此进入
优秀经理 发现优势
因才适用
THE GALLUP ORGANIZATION
“硬”数据与“软” 数据
企业财务指标
滞后指标
品牌 忠实度
不断增长的 忠实用户 员工
前导指标
THE GALLUP ORGANIZATION
17
THE GALLUP ORGANIZATION 股票增值
44
评估
实际利润增长
股票增值
可持续发展
盖洛普路径
忠实顾客 敬业员工
1 7
2 8
3 9
4 10
5 11
6 12
由此进入
优秀经理
识别优势
因才适用
45
THE GALLUP ORGANIZATION
经理的责任与Q12
实际利润增长 股票增值
可持续发展
忠实顾客 敬业员工
经理的目标是培养 “敬业”员工, Q12测量其成功程度
THE GALLUP ORGANIZATION
10
知识经济的挑战
“ 如果我们深入考察就会发现,财务报告的效能在下降 ,其对一家公司的真正价值的测量日趋片面。于是我们开 始减少对其重视程度,转而寻找其他的方法,来测量无形 指标,如研究与开发、顾客满意度、员工满意度等。” 史蒂夫 • 沃曼 前证交会主席 1997.4.7
THE GALLUP ORGANIZATION
2
THE GALLUP ORGANIZATION 盖洛普咨询有限公司
1935年由乔治 盖洛普博士创立 率先使用科学方法调查民意 在全球建立了40多个分公司 调查网覆盖世界60%的人口和 70%的产值 • 盖洛普博士被《生活》杂志评为100年来对美 国历史影响最大的人之一
5 Dysfunctions of a Team_

Inattention to Results Avoidance of Accountability Lack of Commitment Fear of Conflict Absence of Trust The Five Dysfunctions of a Team: A Leadership Fable. Patrick Lencioni.Overview of The Model2 Critical Truths:1. Genuine teamwork remains elusive in most organizations2. Organizations fail to achieve teamwork because they unknowingly fall prey to fivenatural pitfalls or dysfunctions.The 5 Dysfunctions can be addressed in isolation, but in reality they form an interrelated model.1. Absence of Trust – The unwillingness to bevulnerable within a group.❑ Team members who are not genuinely openwith one another about their mistakes and weaknesses make it impossible to build a foundation of trust.❑ The failure to build trust is damaging because it sets the tone for the second dysfunction.2. Fear of Conflict – Teams that lack trust are incapable of engaging in unfiltered and passionatedebate of ideas.❑ Instead, they resort to veiled discussions andguarded comments. ❑ A lack of healthy conflict is a problem becauseit ensures the third dysfunction of a team.3. Lack of Commitment – Without having aired their opinions in the course of passionate and open debate, team members rarely, if ever, buy in and commit to decisions, though the may feign agreement during meetings.❑ Because of this lack of real commitment and buy-in, team members develop the fourthdysfunction.4. Avoidance of Accountability – Without committing to a clear plan of action, even the most focused and driven people often hesitate to call their peers on actions and behaviors that seem counterproductive to the good of the team.❑ Failure to hold one another accountable creates an environment where the fifthdysfunction can thrive.5. Inattention to Results – Inattention to results occurs when team members put their individual needs (such as ego, career development, or recognition) or even the needs of their division(module team) above the collective goals of the team.Imagine how members of truly cohesive teams behave:❑ They trust one another.❑ They engage in unfiltered conflict around ideas.❑ They commit to decisions and plans of action.❑ They hold one another accountable for delivering against those plans.❑ They focus on the achievement of collective results.Understanding and Overcoming the Five DysfunctionsSummaryTeamwork ultimately comes down to practicing a small set of principles over a long period of time. Success is not a matter of mastering subtle, sophisticated theory, but rather of embracing common sense with uncommon levels of discipline and persistence.。
高露洁5P销售系统(英文版)

Hyperm arket
Anti-cavity Fresh Breath Natural Ingredients Multi-benefit Whitening Sensitive Others
No.of SKU 23 14 8 7 7 6 5
Value Share 35% 11% 14% 7% 11% 6% 2%
40g match Crest 40g 40g match Crest 40g 250g 100%
50g match Crest 40g
Weighted % distr. 120g 100% 100g 100% 120g 100% 105g 100% 105g 100% 180g 100%
165g 100% 150g 100% 175g 100%
Shopping Occasion
Product Strategy Prioritized Assortment Numeric % Distribution
Measurement: % of total category SKU % of SKUs on strategy Tracking
Hypermarket - Widest Assortment - High quality & premium product - Heavy purchase - Better shopping environment - More promotion
5P Tactics : Assortment - Fabric Softener
Shopping Occasion
Product Strategy
Prioritized Assortment Numeric % Distribution Measurement: % of total category SKU % of SKUs on strategy Tracking
出口和进口价格指数手册说明书

ForewordThis Export and Import Price Index (XMPI) Manual replaces the United Nations Strategies for Price and Quantity Measurement in External Trad e, Series M, No. 66 issued in 1981. The development of the XMPI Manual has been undertaken under the joint responsibility of five organizations—the International Labour Office (ILO), International Monetary Fund (IMF), Organisation for Economic Co-operation and Development (OECD), United Nations Economic Commission for Europe (UNECE), and World Bank—through the mechanism of an Inter-Secretariat Working Group on Price Statistics (IWGPS). It is published jointly by these organizations.The Manual contains detailed, comprehensive information and explanations for compiling XMPIs. It provides an overview of the conceptual and theoretical issues that statistical offices should consider when making decisions on how to deal with the various problems in the daily compilation of XMPIs, and it is intended for use by both developed and developing countries. The chapters cover many topics; they elaborate on the different practices currently in use, propose alternatives whenever possible, and discuss the advantages and disadvantages of each alternative. Given the comprehensive nature of the Manual, we expect it to satisfy the needs of many users.The main purpose of the Manual is to assist producers of XMPIs, particularly countries that are revising or setting up their XMPIs. The Manual draws on a wide range of experience and expertise in an attempt to describe practical and suitable measurement methods. It should also help countries to produce their XMPIs in a comparable way, so that statistical offices and international organizations can make meaningful international comparisons. Because it brings together a large body of knowledge on the subject, the Manual may be used for self-learning or as a teaching tool for training courses on XMPIs.Other XMPI users, such as businesses, policymakers, and researchers, make up another targeted audience of the Manual. The Manual will inform them not only about the different methods that are employed in collecting data and compiling such indices, but also about the limitations, so that the results may de interpreted correctly.The drafting and revision process has required many meetings over a three-year period, in which XMPI experts from national and international statistical offices, universities, and research organizations have participated. The Manual owes much to their collective advice and wisdom.The electronic version of the Manual is available on the Internet at . The IWGPS views the Manual as a “living document” that it will amend and update to address particular points in more detail. This is especially true for emerging discussions and recommendations made by international groups reviewing XMPIs, such as the International- 2 -Working Group on Price Indices (the Ottawa Group) and the International Working Group on Service Sector Statistics (the Voorburg Group).The IWGPS welcomes user’s comments on the manual, which should be sent to the IMF StatisticsDepartment(e-mail:**************).Theywillbetakenintoaccountinany future revisions.HeinrichYoungBrünggerA.SylvesterDirector DirectorStatisticsDivision StatisticsBureauofInternational Labour Office United Nations Economic Commissionfor EuropeShaidaBadieeKöhlerHorstDirectorDirectorManagingDataDevelopmentGroupFundInternationalMonetaryWorld BankEnrico GiovanniniChief Statistician and DirectorStatistical DirectorateOrganisation for Economic Co-operationand DevelopmentHervé CarréDirector Genera lStatistical Office of the European Communities。
The Five Dysfunctions Of A Team(团队的五个障碍)

The five dysfunctions of a Team
Dysfunction #1: Absence of trust
● The Fundamental attribution error;
We often attribute other people's negative behaviour to their character and our own negative behaviour to our
© David Barnholdt 2008
# 3 Teams that Lack commitment
● Create ambiguity among the team about direction and priorities
● Watch windows of opportunity close due to excessive analysis
Patrick Lencioni
”Teamwork is the most untapped competitive advantage in business”
The advantages of teamwork
● Groups that works as teams makes better and faster decisions than non-teams
● Mobile Access Group
© David Barnholdt 2008
TeamWork
TeamWork is not a virtue It's a choice !
© David Barnholdt 2008
美联英语:生命中5个球(上)

美联英语提供:美联英语:生命中的5个球(上)In a university commencement address several years ago, Brian Dyson, CEO of Coca Cola Ent-erprises, spoke of the relation of work to one’s other commitments:几年前,在一所大学的开幕典礼中,可口可乐的首席执行官布赖恩·戴森讲到工作与其他义务的关系:Imagine life as a game in which you are juggling some five balls in the air. You name t hem work, family, health, friends and spirit and you’re keeping all of these in the air. You will soon understand that work is a rubber ball. If you drop it, it will bounce back.想象生命是一场不停丢掷五个球于空中的游戏。
这五个球分别为工作、家庭、健康、朋友和心灵,而且你很努力地掷着这五个球,不让它们落地。
很快地你会了解工作是一个橡皮球。
如果你不幸失手落下它,它还是会弹回来。
But the other four balls family, health, friends and spirit are made of glass. If you drop one of these, they will be irrevocably scuffed, marked, nicked, damaged or even shattered. They will never be the same. You must understand that and strive for balance in your life. How?但是家庭、健康、朋友和心灵这四个球是用玻璃做成的。
梅特勒一托利多FIVE系列酸度计仪表新品上市通告

梅特勒一托利多FIVE系列酸度计仪表新品上市通告
佚名
【期刊名称】《中国医药工业杂志》
【年(卷),期】2007(38)10
【总页数】1页(P700-700)
【正文语种】中文
【中图分类】R
【相关文献】
1.梅特勒-托利多热分析超越系列新品上市!新技术/新仪器全国巡回推介活动 [J],
2.梅特勒-托利多热分析超越系列新品上市!新技术/新仪器全国巡回推介活动 [J],
3.梅特勒-托利多全新一代FiveEasy Plus^(TM)系列台式仪表于2012年3月隆重上市! [J],
4.梅特勒-托利多全新一代FiveEasy Plus^(TM)系列台式仪表于2012年3月隆重上市! [J],
5.无与伦比的多参数准确测量——SevenExcellence^(TM)系列仪表全新上市梅特勒-托利多公司 [J],
因版权原因,仅展示原文概要,查看原文内容请购买。
类药五规则——精选推荐

类药五原则(rule of five)也称为Lipinski规则,其内容如下:一个小分子药物中要具备以下性质
1.分子量小于500;
2.氢键给体数目小于5;
3.氢键受体数目小于10;
4.脂水分配系数小于5;
5.可旋转键的数量不超过10个。
类药五原则,是辉瑞公司资深药物化学家Christopher A. Lipinski在1997年提出的筛选类药分子的五条基本法则,符合Lipinski规则的化合物会有更好的药代动力学性质,在生物体内代谢过程中会有更高的生物利用度,因而也更有可能成为口服药物。
在药物研发领域,Lipinski规则被用于对化合物数据库的初筛,以期摒除那些不适合成为药物的分子,缩小筛选的范围并降低药物研发成本。
在长期的实践过程中,药物化学家们对Lipinski规则作出简化,形成“四规则”和“三规则”,但是四规则和三规则有时仍然被称作“五规则”,这里的五指的是各条规则的判别值均为5或500。
简化后的四规则去掉了关于可旋转键的数量限制;三规则进一步去掉了对氢键受体数量的限制。
这是根据口服药物总结出的经验型规律,是小分子药物设计的有效指导原则。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
(2)
Since the four-loop RG expansions at n = 1 are known [1] we are in a position to find corresponding series for arbitrary n and to calculate the five-loop terms. The results of our calculations are as follows: β (g ) g3 = −g + g 2 − 10.33501055n + 47.67505273 2 (n + 8)2 g4 + 5.000275928n2 + 149.1518586n + 524.3766023 (n + 8)3 g5 − 0.0888429n3 + 179.69759n2 + 2611.1548n + 7591.1087 (n + 8)4 g6 −0.00408n4 + 80.3096n3 + 5253.56n2 + 53218.6n + 133972 . + (n + 8)5 n+2 g2 γ =1− g+ (n + 2) 3.375628955 n+8 (n + 8)2 g3 − 4.661884772n2 + 34.41848329n + 50.18942749 (n + 8)3
estimates are usually extracted from the data obtained for the linear (χ) and non-linear (χ4 ) susceptibilities related to each another via g4 : ∂3M χ4 = = −χ2 m−2 g4 , ∂H 3 H =0
−1
(3)
2
+
g4 0.3189930n3 + 71.703302n2 + 429.42449n + 574.58772 (n + 8)4 g5 − 0.09381n4 + 85.4975n3 + 1812.19n2 + 8453.70n + 10341.1 . 5 (n + 8)
(4)
η=
g3 g2 ( n + 2) 0 . 9170859698 − (n + 2) 0.05460897758 (n + 8)2 (n + 8)2 g4 + −0.09268446n3 + 4.0564105n2 + 29.251167n + 41.535216 (n + 8)4 g5 0.07092n4 + 1.05240n3 + 57.7615n2 + 325.329n + 426.896 . − (n + 8)5
to clear up how effective is this machinery in two dimensions where i) the RG series are stronger divergent and ii) singular (non-analytic) contributions to RG functions are expected to be larger than for 3D systems. The Hamiltonian of the model describing the critical behaviour of various two-dimensional systems reads: H= d2 x 1 2 2 λ 2 (m0 ϕα + (∇ϕα )2 ) + (ϕ2 α) , 2 24 (1)
arXiv:hep-th/0301077v1 13 Jan 2003
Five-loop renormalization-group expansions for two-dimensional Euclidean λφ4 theory
E. V. Orlov, A. I. Sokolov Saint Petersburg Electrotechnical University, 197376, Saint Petersburg, Russia May 19,ical renormalization-group (RG) approach proved to be a powerful tool for calculating the critical exponents and other universal quantities of the basic three-dimensional (3D) models of phase transitions. Today, many-loop RG expansions for β -functions (six-loop), critical exponents (seven-loop), higher-order couplings (four-loop), etc. of the 3D O (n)symmetric and cubic models are known resulting in the high-precision numerical estimates for experimentally accessible quantities [1-7]. The main aim of this report is to elaborate further the field-theoretical RG technique, namely, 1
(5)
In more details, the calculations are described in Ref.[8]. Instead of the renormalized coupling constant g4 , a rescaled coupling g= n+8 g4 , 24π (6)
Abstract The renormalization-group functions of the two-dimensional nvector λφ4 model are calculated in the five-loop approximation. Perturbative series for the β -function and critical exponents are resummed by the Pade-Borel-Leroy techniques. An account for the five-loop term shifts the Wilson fixed point location only briefly, leaving it outside the segment formed by the results of the lattice calculations. This is argued to reflect the influence of the non-analytical contribution to the β -function. The evaluation of the critical exponents for n = 1, n = 0 and n = −1 in the five-loop approximation and comparison of the results with known exact values confirm the conclusion that non-analytical contributions are visible in two dimensions. For the 2D Ising model, the estimate ω = 1.31(3) for the correction-to-scaling exponent is found that is close to the values resulting from the hightemperature expansions.
∞ ∞
f (x) =
i=0
ci x =
0
i
e−t tb F (xt)dt,
F (y ) =
ci yi , ( i + b )! i=0
∞
(7)
is used. The analytical extension of the Borel transforms is performed by exploiting relevant Pad´ e approximants [L/M]. In particular, four subsequent diagonal and near-diagonal approximants [1/1], [2/1], [2/2], and [3/2] turn out to lead to numerical estimates for g ∗ which rapidly converge, via damped oscillations, to the asymptotic values; this is cleary seen from Table 1. These asymptotic values, i. e. the final five-loop RG estimates for g ∗ are presented in Table 2 for 0 ≤ n ≤ 8 (to avoid confusions, let us note that models with n ≥ 2 possessing no ordered phase are studied here only as polygons for testing the numerical power of the perturbative RG technique). As Table 2 demonstrates, the numbers obtained differ appreciably from numerical estimates for g ∗ given by the lattice and Monte Carlo calculations [9-15]; such 3
