Thinning of CIGS solar cells Part II Cell characterizations
Energy Dispersive Spectrometry Analysis

Energy Dispersive Spectrometry Analysis of a CIGS Solar CellPat Camus, Ph.D., Thermo Fisher Scientific, Madison, WI, USAAbstractThe Thermo Scientific NORAN System 7 energy dispersivespectrometry (EDS) system was used to investigate theelemental structure of a thin-film CIGS solar cell. The samplewas investigated in both the planar and cross-sectional views.IntroductionCIGS solar cells are based on copper indium galliumdiselenide [Cu(In,Ga)Se2] thin films. These solar cells havedemonstrated excellent efficiencies and are potentialreplacements for silicon based solar cells, which are moreexpensive to produce and are substantially thicker.CIGS solar cells are formed by layering thin films ona substrate (historically glass, but currently polymers) asshown on Figure 1. The molybdenum layer and the zincoxide layer form the electrical contacts. The CIGS film actsas the sunlight absorber layer, with a thin CdS layer formingthe p-n junction. The most common manufacturing methods evaporate or sputter copper, indium, and gallium simultaneously or sequentially onto the substrate. Vaporized selenium is reacted with the film to establish the final film composition.The major challenge in producing these thin layer solar cells is to control film composition. Reproducibility of required layer structure in commercial volumes has proven to be problematic and this is critical as the electrical properties of the cell depend on the exact composition of the layers. EDS analyses can be used to determine the spatial distribution of the elements through the device.ExperimentalA commercially available CIGS solar cell was disassembled to extract a portion of a single cell. It was analyzed in both the planar and cross-sectional views. The cross-section view was prepared in an epoxy mount and polished.Examination took place using a Tungsten-filament SEM. Planar-view analyses were performed initially at 20 kV to determine the elemental constituents. Subsequent analyses of the cross section were performed at 5 kV to reduce the interaction volume. When it was discovered that a substantial portion of the substrate was polymer, care was taken to keep the induced charge to low levels. Even at these levels, a small amount of charging was present and drift compensation was required for mapping.NORAN System 7 was used to collect X-rays from a NanoTrace SiLi detector. Both Point and Shoot spectral mode and Spectral Imaging mapping mode were used to characterize elemental constituents of the sample.Figure 1: Scanning electron micrograph of a Cu(In,Ga)Se2solar cell(cross-section) and its mode of operation1Thermo Scientific NORAN System 7ResultsPlanar ViewDuring disassembly, some of the CIGS material delaminated from the substrate (Figure 2a). This provided a unique opportunity to measure (1) the top layers of the sample,(2) the underlying metallic substrate, and (3) the metallic surface electrical contact material. Figure 2b is a Point andShoot spectral analysis of these regions.O-KS-KCr-KFe-KCu-KZn-KGa-KSe-KMo-LAg-LCd-LIn-LArea 1(1)_pt117.38 4.590.82 2.4112.39 2.51 2.9022.93 2.317.4224.32Area 1(1)_pt217.60 4.530.69 2.4912.31 2.88 3.0121.63 2.097.7724.99Area 1(1)_pt3100.00Area 1(1)_pt417.6618.422.9261.00Figure 2a:Planar view of delaminated CIGS materialFigure 2b:EDS spectral analyses of the top surface of CIGS materialTable 1:Quantitative Elemental Composition Analysis of the Top Surface of CIGS MaterialLocations 1 and 2 on the film are the same materialconsisting of a majority of In and Se with a small amount of Cu and a small amount of Ga. Only small amounts of Zn, O, Cd and S are measured due to the thin nature of these layers.Location 3 on the metallic contact layer seems to be Ag paint.Location 4 where the film is removed shows the base Mo substrate that the layers are grown on.Polish X-section HV 5kvFigure 3:Electron image and net count elemental maps of CIGS cross-section at low magnificationPolish X-section HV 5kvFigure 4:Quantitative element maps of CIGS cross-section at highmagnificationPolish X-section HV 5kvRed = FeOrange = MoBlue = Cu-Ga-SeGreen = Cu-In-Ga-SeYellow = EpoxyPhase 2 - Epoxy Phase 3 - C + Absorber Phase 4 - Absorber Phase 5 - Molybdenum。
碲化镉薄膜太阳能电池及其溅射制备

3上海海事大学青年骨干教师培养项目(No.025063) 张榕:通信作者 Tel :021********* E 2mail :rongzhang @碲化镉薄膜太阳能电池及其溅射制备3张 榕1,周海平2,陈 红3(1 上海海事大学基础科学部,上海200135;2 四川师范大学物理与电子工程学院,成都610066;3 上海交通大学物理系凝聚态光谱与光电子物理实验室,上海200030) 摘要 简单综述了化合物半导体碲化镉太阳能电池的发展历史、基本结构和核心问题,在此基础上重点总结了用溅射法制备的多晶碲化镉薄膜太阳能电池的优缺点、面临问题、发展现状,展望了它的发展趋势,并讨论了用溅射法制备渐变带隙碲化镉薄膜太阳能电池以提高转化效率的可能性。
关键词 碲化镉 薄膜太阳能电池 溅射法中图分类号:TM914.42An Overvie w of CdT e Thin Film Solar Cells and R elevant Sputtering F abricationZHAN G Rong 1,ZHOU Haiping 2,C H EN Hong 3(1 Basic Science Department ,Shanghai Maritime University ,Shanghai 200135;2 Department of Physics and Electronic Engineering ,Sichuan Normal University ,Chengdu 610066;3 Laboratory of Condensed Matter Spectroscopy and Opto 2electronic Physics ,Department of Physics ,Shanghai Jiaotong University ,Shanghai 200030)Abstract This article firstly gives a brief overview to the development history ,basic structures and critical is 2sues of compound semiconductor Cd Te 2based solar cells ,then sheds light on the advatages and disadvantages ,current status ,and trend of development of the sputtered polycrystalline Cd Te thin film solar cells.Finally ,it also discusses the possibility to fabricate graded 2bandgap Cd Te solar cells by using the sputtering methodK ey w ords Cd Te ,thin film solar cells ,sputtering0 引言随着当今世界人口和经济的增长、能源资源的日益匮乏、环境的日益恶化以及人们对电能的需求量越来越大,太阳能的开发和利用已经在全球范围内掀起了热潮。
The effect of Ga-grading in CIGS thin film solar cells

The effect of Ga-grading in CIGS thin film solar cellsO.Lundberg *,M.Edoff,L.StoltUppsala University,A ˚ngstro ¨m Solar Center,P .O.Box 534,SE-75121Uppsala,SwedenAvailable online 2December 2004AbstractIn this paper the effect of an in depth variation of the Ga/(In+Ga)ratio in CIGS based thin film solar cells is presented.The conclusionsmade are based on a review of earlier publications,theoretical considerations and results from a large set of new devices.For standard devices with normally thick CIGS films (1.5–2A m)deposited at a relatively long deposition time (60min)an improved efficiency of around 0.4%units for the devices with an increased Ga/(In+Ga)ratio towards the back contact is observed.This improvement is due to a field assisted carrier collection resulting in an improved QE response at long wavelengths.When the CIGS thickness is reduced the importance of the increased Ga/(In+Ga)ratio towards the back contact is enhanced and at a CIGS thickness of 0.5A m a gain of 2.5%units is obtained.The gain is due to an improved V oc and FF.The main reason for the improvement is passivation of the back contact,which becomes increasingly detrimental for the device performance as the CIGS thickness is reduced.Also for pure CIS a significant improvement of the device performance is obtained by introducing an increased Ga concentration towards the back contact.This improvement is,however,more related to the introduction of Ga itself than the gradient of the Ga-concentration.From many simulations the largest gain is predicted for an increased Ga/(In+Ga)ratio towards the CIGS surface.However,neither in the literature nor from our own experiments we can find evidence for an improved device performance due to an increased Ga-concentration towards the CIGS surface.D 2004Elsevier B.V .All rights reserved.Keywords:Ga-grading;CIGS;Thin film solar cell1.IntroductionA special quality of the Cu(In,Ga)Se 2(CIGS)material is its variable band gap,which can be changed by varying the Ga/(In+Ga)ratio.This quality can be used,not only to optimize the general band gap level,but also to obtain different band gaps at different depths in the CIGS film,so called band gap profiling.In CIGS thin film solar cells an in-depth band gap variation due to changes in the Ga/(In+Ga)ratio is commonly referred to as Ga-grading .Band gap profiling is commonly classified in two categories,normal and double grading.Normal grading is an increase of the band gap towards the back contact,while the double grading profile has a minimum band gap some distance into the CIGS layer and an increased band gap both towards the back and front contact.Many CIGS based solar cells withboth normal and double Ga-grading have been fabricated in the last 15years,but the actual effect of such an in-depth variation of the band gap is still not clear.In this paper an attempt is made to clarify the effect of Ga-grading in CIGS thin film solar cells by combining theoretical considerations,literature review and new experimental results.2.Potential improvement by Ga-gradingThe variation of the Ga/(In+Ga)ratio,x ,will affect the band gap according toE g ½eV ¼1:02þ0:67b x þb b x x À1ðÞð1Þwhere values between 0.11and 0.24have been reported for the optical bowing coefficient,b [1].Wei and Zunger [1]have theoretically shown that a variation of the Ga/(In+Ga)ratio will mainly affect the level of the conduction band minima.In Fig.1,a band edge diagram for a CIGS layer with double grading profile is illustrated,i.e.an increased0040-6090/$-see front matter D 2004Elsevier B.V .All rights reserved.doi:10.1016/j.tsf.2004.11.080*Corresponding author.E-mail address:olle.lundberg@angstrom.uu.se (O.Lundberg).Thin Solid Films 480–481(2005)520–525/locate/tsfGa/(In+Ga)ratio both towards the back contact and in the space charge region (SCR).The locally increased band gap has two effects on the photo-generated electrons.First of all the recombination probability will be reduced in the regions with increased band gap since this probability is inversely proportional to the band gap [2].Secondly an additional electric field,n A ,is obtained and can be described by Eq.(2)[3].n A ¼d D E g d xð2Þwhere D E g is the change in band gap over the distance x due to the Ga-grading.How can these two effects,which always come together,be used to improve the solar cell device performance?In the following we have classified the potential improvements an in-depth variation the Ga/(In+Ga)ratio can have on the device performance into two main categories.2.1.Improved V oc by reduced impact of regions with high recombinationAt open circuit conditions the dominating part of the recombination is expected to occur in the SCR region [4,5].By increasing the Ga/(In+Ga)ratio here this recombination can be reduced,resulting in an improved V oc .But,as mentioned above,there will also be an additional electric field,which in this case will counter act the built in electric field (p–n junction)resulting in an overall weaker electric field in the SCR as illustrated in Fig.1.Such a reduction of the electric field will most likely have a negative effect on the device performance.An increased band gap in the front part will also reduce the absorption in this region.This can be compensated for by an increased absorption further into the CIGS layer,where the band gap not is increased.Photo-electrons generated deeper into the CIGS material will on the other hand have a lower collection probability.Whether an increased Ga/(In+Ga)ratio in the SCR will have a net beneficial effect or not is difficult to predict analytically.At the CIGS/Mo interface it would be desirable with an additional force keeping the photoelectrons away from this interface,which is expected to have a relatively high recombination velocity.By an increased conduction band minimum towards the back contact,we can keep the high conductivity for the majority holes and at the same time reject the minority electrons.An increased band gap will also here,further into the CIGS layer,lead to a reduced light absorption.But since the photo generation of carriers is expected to be rather small here anyway,this should only have a small effect on the resulting short circuit current.Whether a passivation of the CIGS back contact will have any significant beneficial effect or not,will depend on how detrimental this CIGS/Mo interface is for the device performance.By using the formulas in Ref.[6]describing how V oc is limited by the effective diffusion length and how this effective diffusion length is limited by back contact recombination an estimation can be made of the potential gain by a reduced back contact recombination,S b .Assuming a typical diffusion length of 1A m and a SCR width of 0.3A m,the gain in V oc by reducing S b from 106cm/s down to 104cm/s for a device with a standard CIGS thickness of 1.5A m,is only around 6mV .This clearly illustrates that the bulk diffusion length must be at least as long as the CIGS thickness if the back contact should have any significant influence on the device performance.For a device with a CIGS thickness of 0.5A m,the corresponding gain is 40mV.2.2.Improved J sc due to field assisted carrier collection Carrier collection of photoelectrons generated outside the SCR in homogeneous CIGS layers rely on diffusion.The collection probability,f c ,outside the SCR is given by [2]f c ¼e Àxdð3Þwhere x is the distance from the SCR and L d is the diffusion length.As illustrated in Fig.1an additional force acting on the electrons can be obtained by increasing the Ga/(In+Ga)ratio towards the back contact,potentially improving the carrier collection.In order to qualitatively determine how large influence a Ga-gradient can have on the carrier collection,we can estimate how far an electron can drift in the additional effective electric field during one minority carrier lifetime.Eq.(4)describes the additional length,L ,that an electron with mobility,l e can drift in the additional effective electric field n A ,during a lifetime,s e .L ¼l e b n A b s e ¼n A kT =qb L 2dc 1b 107b L 2d ½m ð4ÞFig.1.Band edge diagram of a CIGS thin film solar cell where the dotted line illustrates how the conduction band minimum (E c min )is changed for a CIGS layer with an increased Ga/(In+Ga)ratio towards the back contact and in the SCR.An additional electric field,n A ,is obtained due to the band gap variation.O.Lundberg et al./Thin Solid Films 480–481(2005)520–525521By the use of L d=(D e d s e)1/2and D e=l e d kT/q we come to the second expression in Eq.(4).Assuming a linear additional effective electric field of3d105V/m through the neutral bulk of the CIGS layer(corresponding to a conduction band minimum increase of0.3eV over1 A m)the last expression is obtained.For a typical diffusion length of1A m the additional length an electron in this electric field can move is in average10A m.This means that the carrier collection can be significantly improved with the additional field obtained from Ga-grading.From Eq.(4)we can also see that the length an electron can move in an electric field is proportional to L d2.This means that the additional length L becomes much longer in a material that has a long diffusion length already without Ga-grading.On the other hand,the carrier collection in such a material is expected to be high anyway,and the potential for improve-ment is smaller than in a material with shorter diffusion length.Ultimately,it will be a balance between an improved carrier collection and reduced absorption that decides whether there will be a net improvement of the short circuit current or not.3.Literature review3.1.Device simulationsThe complexity of how the device performance is affected by an in-depth variation of the band gap is well illustrated by the diverse results obtained by computer simulations on this topic.For the normal grading profile there exists some agreement on the results.Most of the simulations performed with a normal grading profile predict a small gain in J sc(around0.5mA/cm2)[7–10]. For a double grading profile the results become more diverse,but still a majority of these simulations predict an improved device performance,significantly larger than for a normal Ga-grading profile.The predicted gain for devices with such a profile is an improved V oc.In some simulations the improvement in V oc is accompanied with a reduction in J sc and FF resulting in an overall reduced device performance[8].3.2.Experimental resultsMany CIGS based solar cells with a Ga-graded CIGS layer have been fabricated during the last15years;see Lundberg[3]and references there in.In some processes,for example the selenization process,an increased concentration of Ga towards the back contact occurs spontaneously and in others it can be introduced intentionally.In the case when it is desirable to isolate the effect of the Ga-grading,the processes with a spontaneous Ga-grading formation are less suitable.The reason is that it becomes difficult to make reference devices,which are grown under similar conditions except for a homogeneous Ga/(In+Ga)ratio(no grading).The lack of good reference devices together with a low statistical significance(few devices)are the main reasons for the difficulty to draw conclusions concerning the effect of Ga-grading from the present results in the literature.A lot of devices with high efficiency have been fabricated with a Ga-graded CIGS layer,for example the present world record device[11].But this alone says little about the gain related to the Ga-grading.There is only one case in the literature in which a significant gain in solar cell performance clearly can be correlated to the use of a Ga-graded absorber layer.This is when the importance of the back contact is enlarged,either by reducing the CIGS thickness[12]or by introduction of a less good back contact material[13].In both these cases a significant increased performance is obtained when a Ga-graded CIGS layer is used.In Lundberg et al.[12]we observed that as the CIGS thickness is reduced the importance of the Ga-grading is increased and at an absorber thickness of0.5A m,the gain in efficiency when using a normal Ga-graded CIGS layer is more the2%units compared to a device with a homogenous CIGS layer.The gain for these devices is seen in the V oc and FF,which reaches the same values as for devices with CIGS layers of standard thickness.As illustrated analytically in Section2 the importance of the back contact is increased as the absorber thickness is reduced,if the diffusion length not is decreased correspondingly.These observations strongly indicate that the gain related to the Ga-grading for devices with thin absorber layers is due to a passivation of the back contact.For devices with absorber layers of pure CIS the incorporation of a Ga-gradient also leads to a significant improved device performance[14,15].However,here incorporation of Ga also means introduction of a fourth new element that alone is expected to have a number of beneficial effects on the device performance.For example can a more optimal band gap and an increased domain of the stable1:1:2compound in the phase diagram be obtained by introducing Ga[16].In order to explain the observed gain in V oc for these devices with a reduced back contact recombination a very long diffusion length(N4A m)needs to be assumed.Such a long diffusion length is not commonly observed and we thus think that the observed gain for CIS devices is more related to the incorporation of Ga itself than a true effect of Ga-grading.The largest predicted gain from a majority of the simulation was made for an increased Ga/(In+Ga)ratio in the front part of the CIGS layer.Many attempts have been made with such a profile,see for example[9,17,18]in which most observed an improved V oc but also a reduced J sc and FF resulting in an overall reduced performance.Of all attempts there is only one case in which a gain in efficiency can be related to an increased Ga/(In+Ga)ratio in the front part[9].However,the gain was in this case not due to an improved V oc as the simulations in the same paper predicted, but due to a higher FF and J sc.Thus,there exist noO.Lundberg et al./Thin Solid Films480–481(2005)520–525 522experimental evidence for that an increased Ga/(In+Ga)ratio in the SCR has a beneficial effect on the device performance.For standard devices with a normally thick absorber layer (1.5–2A m)the gain related to a normal grading profile is still unclear.Since the effect seems to be rather small a large set of devices with and without a Ga-grading needs to be compared in order to get a statistical significant result.In the following chapter such data will be presented.4.The effect of Ga-grading in standard devices In our lab we regularly fabricate both devices with a homogenous CIGS layer and CIGS layers with a normal Ga-grading profile.See Lundberg [3]for a detailed experimen-tal description of these processes.The Ga-gradient is obtained by starting the co-evaporation by evaporating a pure CGS layer with a thickness of 10–15%of the totalthickness.Due to diffusion of indium and gallium this CGS layer will result in a smooth gradient of the Ga concentration [19].Since the device performance is significantly affected by the Cu/(In+Ga)ratio [20]and the effect of Ga-grading is relatively small,it only makes sense to compare devices with a similar Cu/(In+Ga)ratio.In order to compare open circuit voltages (V oc )and short circuit currents (J sc )of devices with slightly different Ga/(In+Ga)ratios,we introduce the following b band gap normalized Q I –V para-meters:D V oc =V oc À(E g /q À0.6V )and J sc rel =J sc measured /J sc max.Here E g is the band gap of the CIGS layer,q is theelementary charge,J sc measuredis the J sc obtained from the QEmeasurement and J sc maxis the J sc that would be obtained in a device with the QE equal to 1between 360nm and the wavelength corresponding to the band gap,E g [3].E g was determined by finding the intercept of a straight line fitted to (E d QE)2,where E is the photon energy,and the energy axis.In Fig.2,the g ,D V oc ,J sc reland the FF are shown as a function of the Cu/(In+Ga)ratio for a large number ofTable 1The difference in the band gap normalized I –V parameters between Ga-graded and homogeneous CIGS layers in different Cu/(In+Ga)regions Cu/(In+Ga)ratio –0.750.76–0.850.86–0.950.96–Average g Graded –g Homo 0.270.340.770.160.4[%units]FF Graded –FF Homo À1.5À0.30.30.170.0[%units]J sc rel Graded –J sc rel Homo0.74 2.18 3.3 1.3 1.6[%units]D V oc_Graded –D V oc_Homo15À2À1À71.5[mV]The average is taken over the four intervals in thetable.Fig.2.The band gap normalized I –V parameters g ,FF,D V oc and J sc relvs.the Cu/(In+Ga)ratio for devices with and without a Ga-graded CIGS layer.The absorber thickness is 1.5–2A m.O.Lundberg et al./Thin Solid Films 480–481(2005)520–525523samples,grown at baseline conditions [3]both with and without a Ga-grading.Each point in Fig.2is an average over eight individual cells.The composition is measured by X-ray fluorescence.A slight overestimation of the Cu concentration close to stoicheometry leads to a unrealistic result of working devices for Cu/(In+Ga)ratios above 1.From Fig.2we can see that the effect of the Ga-grading is small,but there is a statistically significant beneficial effect.In order to obtain numbers that make sense to compare for these devices with such a large variation in the Cu/(In+Ga)ratio,we divided the CIGS layers after their Cu/(In+Ga)ratio into four different groups.Within each group the performance variation is relatively small.In Table 1the difference in the band-gap normalized I –V parameters between devices with Ga-graded and homogenous CIGS layers is shown for the four different Cu/(In+Ga)regions,where the last column shows the average over these four regions.In average the devices with a Ga-graded CIGS layer have 0.4%units higher efficiency than devices with a homoge-neous CIGS layer with a similar Cu/(In+Ga)ratio.The gain is largest for Cu/(In+Ga)ratios between 0.86and 0.95,where also the overall best device performance is obtained.All the gain in efficiency can be attributed to an improved J sc .No gain in V oc or FF due to the Ga-grading can be observed.In Fig.3,the QE as a function of wavelength is shown for two typical devices,with and without Ga-grading.The carrier collection for the device with the Ga-graded CIGS layer is improved for photoelectrons generated by light with a wavelength longer than 800nm.The improved carrier collection in Fig.3corresponds to a gain in J sc of 0.7mA/cm 2.These results are in good agreement with that the additional electric field obtained from the Ga-grading improves the carrier collection for photo generated electrons generated outside the SCR as discussed in Section 2.The size of the improvement in J sc is also in agreement with most computer simulations performed on this topic.5.ConclusionsConcerning the beneficial effect of Ga-grading we conclude:!For devices with a standard thick CIGS layer (1.5–2A m)with a Ga/(In+Ga)ratio between 0.25and 0.5and a Cu/(In+Ga)ratio between 0.7and 1the efficiency gain with a normal Ga-grading profile is in the order of 0.4%units.The main part of the gain is due to an improved carrier collection at long wavelengths due to the additional effective electric field obtained from the increased Ga/(In+Ga)ratio towards the back contact.No significant gain in V oc or FF related to the Ga-grading is obtained under these conditions.!By reducing the CIGS thickness,the beneficial effect of Ga-grading is increased.At an absorber thickness of 0.5A m the gain in efficiency is around 2.5%units due to an increased FF and V oc,which becomes comparable to those obtained for standard devices.The increased gain at thinner CIGS layers is due to a passivation of the CIGS back contact,which becomes increasingly detrimental for the device performance as the CIGS thickness is reduced.!No additional gain in performance due to an increased Ga/(In+Ga)ratio in the front part of the CIGS layer can be concluded.References[1]S.H.Wei,A.Zunger,J.Appl.Phys.78(1995)3846.[2]M.A.Green,Solar Cells;Operating Principles,Technology andSystem Applications,The University of New South Wales,Kensing-ton,1992,p.50(142).[3]O.Lundberg,Ph.D.thesis,Uppsala University,(2003),ISBN 91-544-5790-8,http://publications.uu.se/thesis/abstract.xsql?dbid=3757.[4]U.Rau,A.Jasenek,H.W.Schock,F.Engelhardt,T.Meyer,Thin SolidFilms 361–362(2000)298.[5]J.Malmstr f m,J.Wennerberg,M.Bodeg 3rd,L.Stolt,17thEuropean Photovoltaic Solar Energy Conference,P.Helm.,vol.II,p.1265.[6]U.Rau,H.W.Schock,Cu(In,Ga)Se2Solar Cells,1,Imperial CollegePress,London,2001,p.277.[7]J.L.Gray,Y .J.Lee,1st World Conference on Photovoltaic SolarEnergy Conversion,1994,p.123.[8]M.Topic,F.Smole,J.Furlan,J.Appl.Phys.79(1996)8537.[9]A.M.Gabor,J.R.Tuttle,M.H.Bode,A.Franz,A.L.Tennant,M.A.Contreras,R.Noufi,D.G.Jensen,A.M.Hermann,Sol.Energy Mater.Sol.Cells 41–42(1996)247.[10]A.Dhingra,A.Rothwarf,IEEE Trans.Electron Devices 43(1996)613.[11]K.Ramanathan,M.A.Contreras,C.L.Perkins,S.Asher,F.S.Hasoon,J.Keane,D.Young,M.Romero,W.Mtetzger,R.Noufi,J.Ward,A.Duda,Prog.Photovolt.11(2003)1.[12]O.Lundberg,M.Edoff,J.Malmstrom,L.Stolt,Prog.Photovolt.11(2003)77.[13]assa,H.W.Schock,J.H.Werner,Thin Solid Films 431–432(2003)387.[14]T.Dullweber,O.Lundberg,J.Malmstrom,M.Bodegard,L.Stolt,U.Rau,H.W.Schock,J.H.Werner,Thin Solid Films 387(2001)11.Fig.3.QE for devices with and without Ga-grading but similar Cu/(In+Ga)ratio.O.Lundberg et al./Thin Solid Films 480–481(2005)520–525524[15]C.Jensen,D.Tarrent,D.Ermer,G.Pollock,23rd IEEE PhotovoltaicSpecialists Conference,1993,p.577.[16]S.H.Wei,S.B.Zhang, A.Zunger,Appl.Phys.Lett.72(1998)3199.[17]R.Menner,T.Walter,H.W.Schock,Tenth E.C.Photovoltaic SolarEnergy Conference.Proceedings of the International Conference, 1991,p.787.[18]M.Bodegard,O.Lundberg,J.Malmstrom,L.Stolt,A.Rockett,Proceedings of28th IEEE Photovoltaic Specialists Conference,2000, p.430.[19]O.Lundberg,J.Lu,A.Rockett,M.Edoff,L.Stolt,J.Phys.Chem.Solids64(2003)1499.[20]O.Lundberg,M.Edoff,L.Stolt,to appear in Material research societysymposium proceedings,2003.O.Lundberg et al./Thin Solid Films480–481(2005)520–525525。
次世代薄膜太阳光电设备之发展机会 第三章 CIGS的可行制程分析1

第三章CIGS薄膜太陽能電池的製程分析第一節CIGS薄膜太陽能電池的技術概述薄膜太陽能電池(thin film solar cell)技術發展迄今已逾30年,可達到量產階段且有模組產品問世之薄膜太陽能技術有三種,即:矽(Si)薄膜太陽能電池、銅銦鎵硒(CuInGaSe2)薄膜太陽能電池、與碲化鎘(CdTe)薄膜太陽能電池。
目前,各種薄膜太陽能電池的光電轉換效率如表3-1所示,元件部分的光電轉換效率皆可達10%上,特別是銅銦鎵硒(CIGS)薄膜太陽能電池更可達20%,已接近單晶矽與多晶矽太陽能電池的效率表現;在模組部分,只有CIGS與CdTe 兩種薄膜太陽能電池在大模組(60 cm x 120 cm)的光電轉換效率可達到10%,矽薄膜部分並未達到此一水準,必須利用多層接面(multi-junction)的方式,如a-Si/nc-Si結構,才能將模組的光電轉換效率提升至10%以上。
表3-1 各種薄膜太陽能電池的光電轉換根據歐洲太陽能工業協會(EPIA)的預測,未來薄膜太陽能電池模組的技術發展大致依循現有趨勢,以CIGS薄膜太陽能電池模組的提升空間最大,預期2030年模組的光電轉換效率將可超越25%,矽薄膜與CdTe兩種薄膜太陽能電池模組則僅20%左右(詳見圖3-1),顯示出CIGS 薄膜太陽能電池模組具有較高的光電轉換效率。
因此,利用高模組效率以降低模組成本,使得CIGS薄膜太陽能電池模組有機會成為此類電池中成本最低者。
在開發高效率薄膜太陽能電池的前提下,勢必會投入更多的資源於CIGS薄膜太陽能電池部分,以加速整個CIGS 薄膜太陽能電池產業的發展,藉此置換高耗能與高成本的矽基太陽能電池。
圖3-1 2005-2035年各種薄膜太陽能電池模組的光電轉換效率一、CIGS薄膜太陽能電池元件的結構介紹CIGS薄膜太陽能電池的元件結構如圖3-2所示,一般採用鈉玻璃(soda-lime glass)、金屬、或Polyimide作為基板的材料,下電極部分則以鉬(Mo)金屬最為合適,此乃因Mo金屬容易與CIGS薄膜形成歐姆接觸(ohmic contact),有效達到電流傳導的目的,在高溫的加工環境下也不易被硒化,故成為最常使用的電極材料。
Solar cell--CIGS

Effect of Cu deficiency on the optical properties and electronic structure of CuInSe2 and CuIn0.8Ga0.2Se2 determined by spectroscopic ellipsometrySung-Ho Han, Allen M. Hermann, F. S. Hasoon, H. A. Al-Thani, and D. H. LeviCitation: Appl. Phys. Lett. 85, 576 (2004); doi: 10.1063/1.1776616View online: /10.1063/1.1776616View Table of Contents: /resource/1/APPLAB/v85/i4Published by the American Institute of Physics.Related ArticlesDilute-nitride GaInAsN/GaAs site-controlled pyramidal quantum dotsAppl. Phys. Lett. 99, 181113 (2011)Modifications in structural and electronic properties of TiO2 thin films using swift heavy ion irradiation J. Appl. Phys. 110, 083718 (2011)Point defects in gallium nitride: X-ray absorption measurements and multiple scattering simulations Appl. Phys. Lett. 99, 172107 (2011)Spontaneous polarization and band gap bowing in YxAlyGa1-x-yN alloys lattice-matched to GaNJ. Appl. Phys. 110, 074114 (2011)Band gap and electronic properties of wurtzite-structure ZnO co-doped with IIA and IIIAJ. Appl. Phys. 110, 063724 (2011)Additional information on Appl. Phys. Lett.Journal Homepage: /Journal Information: /about/about_the_journalTop downloads: /features/most_downloadedInformation for Authors: /authorsEffect of Cu deficiency on the optical properties and electronic structure of CuInSe2and CuIn0.8Ga0.2Se2determined by spectroscopic ellipsometry Sung-Ho Han a)and Allen M.HermannDepartment of Physics,University of Colorado,Boulder,Colorado80303-0390F.S.Hasoon,H.A.Al-Thani,and D.H.LeviNational Renewable Energy Laboratory,1617Cole Boulevard,Golden,Colorado80401-3393(Received29March2004;accepted4June2004)Spectroscopic ellipsometry measurements of CuInSe2(CIS)and CuIn0.8Ga0.2Se2(CIGS)reveal thatthere are important differences in electronic properties between stoichiometric CIS(CIGS)andCu-poor CIS(CIGS).Wefind a reduction in the absorption strength in the spectral region of1–3eV.This reduction can be explained in terms of the Cu3d density of states.Cu-poor CIS(CIGS)materials show an increase in band gap due to the reduction in repulsion between Cu3d andSe4p states.The experimental results have important implications for the function ofpolycrystalline optoelectronic devices.©2004American Institute of Physics.[DOI:10.1063/1.1776616]Polycrystalline thin-film chalcopyrite CuIn1−x Ga x Se2 (CIGS)is currently used as an absorber layer for high-efficiency photovoltaic(PV)solar cells.The efficiency of record laboratory polycrystalline thin-film solar cells based on CIGS has reached nearly20%,1while single-crystalline CIGS solar cells have just reached13%.2Electronic struc-tures of CuB III X2VI materials have been thoroughly studied.3–5 High-efficiency polycrystalline solar cells are always slightly Cu deficient,with about23.5–24.5at.%Cu.There have been studies of the optical properties of CIGS materials with different Ga compositions,6–8but considering the fact that high-efficiency PV solar cells use Cu-poor CIGS,it is crucial to study the effect of content on CIGS electronic properties. In this study,through the analysis of the dielectric function, we compare the electronic structure of Cu-poor ͑21.7at.%Cu͒CuInSe2(CIS)films with stoichemetric ͑25.1at.%Cu͒CISfilms.We also compare the electronic structure of slightly Cu-poor͑23.3at.%Cu͒CIGSfilms withstoichiometric͑24.8at.%Cu͒bulk polycrystalline CIGS.CIGS surfaces are inclined to have Cu vacancies.9,10In con-trast to zinc-blende semiconductors,where the nonpolar (110)surface is more stable than all polar surfaces,the chal-copyrite semiconductor CuInSe2has the lowest energy when the surface has the(112)-cation and͑1¯1¯2¯͒-anion polar facets through defect-induced reconstructions.9Previous work has studied electronic and geometric structures of nearly sto-ichiometric bulk and Cu-poor surfaces.4,10,11In contrast,we focus on Cu-poor CIS and CIGS samples where both the surface and bulk regions are Cu poor to probe the properties of Cu-deficient CIS(CIGS)materials.Spectroscopic ellipsometry(SE)is a powerful technique for determining the optical functions of bulk and thin-film materials.Alonso et al.have reported SE measurements of the pseudodielectric functions of single-crystalline CIS and CuGaSe2(CGS),6as well as bulk polycrystalline CIGS alloys.7In those studies,they used a two-phase model to analyze the ellipsometric data.12Such a treatment is not ap-propriate for the analysis of thin-film polycrystalline materi-als used for real-world CIGS solar cells.The polycrystalline thin-film CIS and CIGS samples were deposited onto molybdenum-coated soda-lime glass.The molybdenum thickness was about1.0m.CIS and CIGS layer thick-nesses were about1.2m and2.0m,respectively.These films were grown by the single-stage coevaporation tech-nique,where thefluxes of Cu,In,Ga,and Se were constant during deposition.To accurately determine the optical prop-erties of these multilayer thin-film samples,one must analyze the SE data using a full multilayer model including the ef-fects of the surface roughness and the underlying molybde-num layer.8We have applied these techniques to determine the dielectric functions for several polycrystalline thinfilms of CIS and CIGS alloys.The ellipsometer used to make the measurements in this study is a J.A.Woollam M2000variable-angle spectroscopic ellipsometer,which uses a rotating compensator design.For this work,ellipsometric spectra were measured at angles of incidence of65°,70°,75°,and80°to ensure an accurate determination of the dielectric function of the material,the thicknesses of the material layer,and surface roughness layer.Auger electron spectroscopy(AES)depth profiles showed that the materials have uniform compositions throughout the entire thickness of thefilms.Thicknesses measured by profilometer are in quantitative agreement with those determined by SE.Inductively coupled plasma(ICP) analysis measures the compositions of thin-film CIS and CIGS.Table I provides at.%Cu of thesefilms as determineda)Also with:National Renewable Energy Laboratory,Golden,CO80401; electronic mail:sung-ho.han@ TABLE I.at.%Cu and critical points analyzed by the CPPB model.All samples are polycrytalline.Stoichiometricthin-film CISCu-poorthin-film CISStoichiometricbulk CIGS aSlightlyCu-poorthin-film CIGS at.%Cu25.121.724.823.3E0͑A,B͒ 1.03 1.08 1.11 1.12E0͑C͒ 1.22 1.29 1.33 1.34a Ref.7.APPLIED PHYSICS LETTERS VOLUME85,NUMBER426JULY2004 0003-6951/2004/85(4)/576/3/$20.00©2004American Institute of Physics576by ICP.X-ray diffraction revealed that these films are single phase for stoichemetric thin-film CIS ͑25.1at.%Cu ͒and slightly Cu-poor ͑23.3at.%Cu ͒thin-film CIGS,and mixed phase for Cu-poor ͑21.7at.%Cu ͒thin-film CIS.The CuIn 0.8Ga 0.2Se 2material studied by Alonso et al.also showed uniform chalcopyrite structure with no secondary phases found.7More detailed discussion on the experimental conditions can be found in Ref.8.Figure 1(a )compares the absorption coefficient spectra of stoichemetric and Cu-poor CIS.Figure 1(b )extends this comparison to CIGS materials to generalize the effect of Cu on CIGS materials.Both Figs.1(a )and 1(b )show similar trends.Relative to the stoichiometric samples,absorption de-creases in E 0,E ͑⌫X ͒,and E 1͑A ͒transitions,but increases in the E ͑⌬X ͒transition for Cu-poor materials.The optical tran-sitions in the spectral range of 1–5eV,can be found else-where .6,7Depression of the absorption coefficient is found in Fig.1(a )between stoichiometric ͑24.8at.%Cu ͒thin-film CIS and Cu-poor ͑21.7at.%Cu ͒thin-film CIS in the spec-tral region,1–3eV.Although the band-gap energies are slightly different due to different Ga compositions,Fig.1(b )also shows the depression of absorption coefficient between stoichiometric bulk polycrystalline CuIn 0.8Ga 0.2Se 2and slightly Cu-poor thin-film CIGS with x ϵGa/͑In+Ga ͒=0.18.According to the theoretical calculations of the elec-tronic band structure and density of state (DOS )of the ter-nary chalcopyrite materials by Jaffe and Zunger,3the upper valence band,within 3–4eV of the valence-band maximum (VBM ),is composed primarily of the Cu 3d orbitals,hybrid-ized with the Se-4p orbitals.Several authors have calculated the band structure and DOS of extremely Cu-poor ␥-phase CIS ͑CuIn 5Se 8͒.5Both of these calculations show a reduction of the DOS within 3–4eV of the VBM.Reduction in the DOS of hole states near the VBM should produce a decrease in absorption coefficient near the band edge.This theoretical result is consistent with our experimental observations.Theoretical calculations of CIS band structure predict another effect of Cu deficiency.As stated above,in ternary chalcopyrite CuB III X 2VI ,the upper valence band is composed of Cu 3d and VI 4p state electrons.This was observed ex-perimentally using synchrotron radiation photoemission spectroscopy.13The repulsive p –d interaction pushes the an-tibonding p –d state that constitutes the VBM to higher en-ergies.In the case of Cu-poor CIS and CIGS,the p –d repul-sion is expected to be less than that of stoichiometric materials.The net effect of the decrease in this repulsive interaction would then be a lowering of the VBM.Hence,we expect an increase of the band gap for Cu-poor CIGS.14We analyze the band gap using the critical-point para-bolic band (CPPB )model.14The fitting procedure is done on the calculated second derivative of dielectric function d 2͑͒/d 2,using the method of smoothing polynomials 15to enhance the structure present in the spectra.The structure of the fundamental absorption edge of CuInSe 2is well known.3Considering crystal-field splitting and spin–orbit in-teraction,the three-fold degenerate ⌫15VBM splits into three transitions E 0͑A ͒,E 0͑B ͒,and E 0͑C ͒.The measured critical points are compiled in Table I.In the case of CIS and CuIn 0.8Ga 0.2Se 2,the separation between E 0͑A ͒and E 0͑B ͒cannot be measured because it is below our resolution.7Thus,the structure is composed of the two degenerate peaks,E 0͑A ,B ͒and E 0͑C ͒.E 0͑A ,B ͒and E 0͑C ͒of stoichemetric thin-film CIS 1.03eV and 1.22eV and those of Cu-poor thin-film CIS are 1.08eV and 1.29eV,respectively.We can see that the band gap increases by 0.05eV for Cu-poor CIS.CPs of CIGS materials show trends analogous to those of CIS materials.We compare the dielectric function of bulk polycrystalline stoichiometric ͑24.8at.%Cu ͒CuIn 0.8Ga 0.2Se 2from Alonso et al.7with the dielectric func-tion of slightly Cu-poor ͑23.3at.%Cu ͒thin-film CIGS with x =0.18.E 0͑A ,B ͒,and E 0͑C ͒of bulk stoichiometric CIGS are extracted from the equation with x =0.18in Alonso et al.7According to that calculation,E 0͑A ,B ͒,and E 0͑C ͒of sto-ichiometric bulk CIGS are 1.11eV and 1.33eV,whereas E 0͑A ,B ͒and E 0͑C ͒of slightly Cu-poor thin-film CIGS are 1.12eV and 1.34eV,respectively.We can see that the band gap increases by 0.01eV.This value is smaller than that of CIS due to the smaller difference in the quantities of at.%Cu.Considering our experimental results in the context of the theoretical calculations of the band structure of stoichio-metric and Cu-poor CIS (CIGS ),3–5we have shown that the reduction of the near band-edge absorption coefficient ob-served in Cu-poor CIS (CIGS )is related to a decrease in the density of states near the VBM.This result has important implications for the functioning of high-efficiencypolycrys-FIG.1.(a )Comparison of absorption coefficients between stoichiometric ͑25.1at.%Cu ͒thin-film CIS and Cu-poor thin-film CIS ͑21.7at.%Cu ͒(b ).Comparison of absorption coefficients between stoichiometric ͑24.8at.%Cu ͒bulk polycrystalline CIGS with x =0.2by Alonso et al.(Ref.7)and slightly Cu poor ͑23.3at.%Cu ͒with x =0.18.talline CIGS thin-film the highest.As stated previously,high-est efficiency CIGS PV devices are slightly Cu poor,with23.5–24.5at.%Cu.Theoretical calculations have shown thatthe most energetically favorable surfaces for CIS are the (112)-cation and͑1¯1¯2¯͒-anion polar facets with defect-induced reconstructions producing a layer of Cu vacancies atthe surface.9Numerous experimental measurements haveconfirmed that CIS(CIGS)surfaces are Cu poor.10Becausegrain boundaries(GBs)can be considered as interior sur-faces,it is reasonable to postulate that in the slightly Cu-poorCIGS material used in high-efficiency solar cells,the mate-rial near the GBs is Cu poor,while the grain interiors(GIs)are nearly stoichiometric.4As shown in Ref.4,the reductionof the DOS near the VBM at the GBs effectively produces acharge-neutral barrier to holes.This effectively passivatesthe GBs by only allowing minority-carrier electrons to pen-etrate the GB region.It is known that the GBs act to getterthe defects and impurities in these materials;hence,passiva-tion of the GBs is exceptionally effective in reducing nonra-diative recombination in CIS(CIGS)thin-film solar cells.The Cu-poor materials studied in this letter are significantly more Cu deficient than the materials used in solar cells.It is reasonable to assume that both GIs and GBs are Cu poor in thesefilms.Hence,our measurements of the optical proper-ties and electronic structure reveal the properties of the GB material in an actual solar cell.Hence,these experimental measurements serve as a confirmation of the theoretical cal-culations put forth in Ref.4.The authors thank H.Moutinho for assistance in atomic force microscopy measurements,R.Bhattacharya for assis-tance in ICP measurements,and J.Pankow for assistance in AES profile measurements.The authors acknowledge valu-able discussions with R.Noufiand C.Persson.This work was supported by the U.S.Department of Energy under Con-tract No.DE-AC36-99GO10337.1K.Ramanathan,M.A.Contreras,C.L.Perkins,S.Asher,F.S.Hasoon,J. Keane,D.Young,M.Romero,W.Metzger,R.Noufi,J.Ward,and A. Duda,Prog.Photovoltaics11,225(2003).2C.H.Champness,Proceedings of the29th IEEE Conference(IEEE,Pis-cataway,NJ,2002),p.732;L.S.Yip and I.Shih,Proceedings of the First World Conference on Photovoltaic Energy Conversion(IEEE,Piscataway, NJ,1994),p.210.3J.E.Jaffe and A.Zunger,Phys.Rev.B27,5176(1983);ibid.28,5822 (1983);ibid.29,1882(1984).4C.Persson and A.Zunger,Phys.Rev.Lett.91,266401(2003).5S.B.Zhang,S.-H.Wei,and A.Zunger,Phys.Rev.B57,9642(1998);C. Domain,ribi,S.Taunier,and J.F.Guillemoles,J.Phys.Chem.Solids 64,1657(2003).6M.I.Alonso,K.Wakita,J.Pascual,M.Garriga,and N.Yamamoto,Phys. Rev.B63,075203(2001).7M.I.Alonso,M.Garriga,C.A.Durante Rincön,E.Hernández,and M. León,Appl.Phys.A:Mater.Sci.Process.74,659(2002).8S.-H.Han,D.H.Levi,H.A.Al-Thani,F.S.Hasoon,R.N.Bhattacharya, and A.M.Hermann,Mater.Res.Soc.Symp.Proc.763,B1.8(2003).9J.E.Jaffe and A.Zunger,Phys.Rev.B64,241304(2001);S.B.Zhang and S.-H.Wei,ibid.65,081402(2002);J.E.Jaffe and A.Zunger,J.Phys. Chem.Solids64,1547(2003).10R.Herberholz,U.Rau,H.W.Schock,T.Haalboom,T.Gödecke,F.Ernst, C.Beilharz,K.W.Benz,and D.Cahen,Eur.Phys.J.:Appl.Phys.6,131 (1999);D.Liao and A.Rockett,Appl.Phys.Lett.82,2829(2003).11A.Meeder,L.Weinhardt,R.Stresing,D.Fuertes Marrón,R.Würz,S.M. Babu,T.Schedel-Niedring,M.C.Lux-Steiner,C.Heske,and E.Umbach, J.Phys.Chem.Solids64,1553(2003);I.M.Kötschau and H.W.Schock, ibid.64,1559(2003);J.M.Merino,M.Di Michiel,and M.León,ibid. 64,1649(2003).12R.M.A.Azzam and N.M.Bashara,Ellipsometry and Polarized Light (North-Holland,Amsterdam,1977).13M.Turowski,G.Magaritondo,M.K.Kelly,and R.D.Tomlinson,Phys. Rev.B31,1022(1985).utenschlager,M.Garriga,S.Logothetidis,and M.Cardona,Phys. Rev.B35,9174(1987).15A.Savitzky and M.J.E.Golay,Anal.Chem.36,1627(1964);J.Steinier, Y.Termonia,and J.Deltour,Anal.Chem.44,1906(1972).。
CIS以及CIGS太阳能电池板

✓ CIGS薄膜技术:单一相,结晶品质好
✓ 吸收层与金属有良好的欧姆接触,易制造
✓ CIGS足够的厚度,且厚度小于载子扩散长度
✓ CIGS为多晶结构,故要求缺陷少,降低再结合几率
✓ CIGS表面平整性好,促进良好接面状态
19
CIGS太阳电池结构—缓冲层
缓冲层:CdS(与p-CIGS形成p-n结) CdS直接能隙结构,2.4eV CdS与CIGS晶格匹配性好,随CIGS内Ga增加,匹配性变差 CdS制造:化学水域法(chemical bath deposition, CBD) ➢ 将CIGS浸入60-80化学溶液中 ➢ 溶液成分:氯化盐(CdCl2,CdSO4等)、氨水(NH3)、硫脲
(SC(NH2)2) ➢ 方程式:
C d ( N H 3 ) 4 2 S C ( N H 2 ) 2 2 O H C d S H 2 H C N 4 N H 3 2 H 2 O
20
CIGS太阳电池结构—缓冲层
水溶液对CIGS表面进行腐蚀清洗去除氧化层,特别是氨水 氧化层去除,促进CdS薄膜生长 研究发现:CdS-ZnS合金薄膜,能提高能隙宽度,提升电
吸收层CIGS(化学式CuInGase)是薄膜电池的 核心材料,属于正方晶系黄铜矿结构。作为直 接带隙半导体,其光吸收系数高达105量级(几 种薄膜太阳能材料中较高的)。禁带宽度在室 温时是1.04eV,电子迁移率和空穴迁移率很 高。
14
CIGS太阳电池结构 结构:玻璃基板,钼,CIGS,CdS,ZnO CIGS:晶粒大小与制造技术有关,~1微米 CIGS缺陷:位错,孪晶等
In/Ga比的调整可使CIGS材料的带隙范围覆盖 1.0一l.7eV,CIGS其带隙值随Ga含量x变化满 足下列公式其中,b值的大小为0.15一0.24eV
Thin solar cell
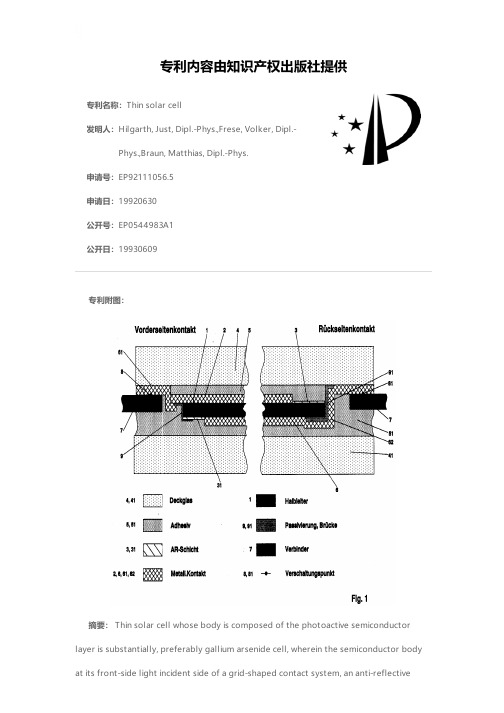
专利名称:Thin solar cell发明人:Hilgarth, Just, Dipl.-Phys.,Frese, Volker, Dipl.-Phys.,Braun, Matthias, Dipl.-Phys.申请号:EP92111056.5申请日:19920630公开号:EP0544983A1公开日:19930609专利内容由知识产权出版社提供专利附图:摘要: Thin solar cell whose body is composed of the photoactive semiconductor layer is substantially, preferably gallium arsenide cell, wherein the semiconductor body at its front-side light incident side of a grid-shaped contact system, an anti-reflectivelayer and a cover glass as well as having on its rear a rear contact. A proper and safe interconnection to large-scale and lightweight solar modules is made possible in that for the connection of a series and / or parallel connection of multiple solar cells enabling solar cell connectors 7 interconnection points 8, 81 are provided, one of which with the front side contact 2 and the other communicating with the back contact 6 in an electrically conductive connection, and in that the interconnection points 8, 81 are removed from the semiconductor body 1 on one side of the solar cell.申请人:Daimler-Benz Aerospace Aktiengesellschaft地址:D-81663 München DE国籍:DE代理机构:Frick, Gerhard, Dipl.-Ing.更多信息请下载全文后查看。
专业英语-太阳能电池

Solarcell 英文PPT

N-type silicon
After incorporation of phosphorus atoms, five phosphorus atoms because electrons, there will be a very active electronics, the formation of N (negative) type semiconductor. Yellow for the phosphorus nuclei, red for the extra electron.
Monocrystalline Silicon solar cell
Polycrystalline Silicon solar cell
Amorphous silicon/ Microcrystalline
Thin-film batteries
Copper indium gallium Selenium
P-N junction is a structure generated by the photovoltaic effect .
P-type silicon
Positive charge that silicon atom, negative charge that the four electronic around the silicon atom . Because only three the electron around boron atoms, so it will produce into the hole shown in blue, this hole become very unstable because there is no electrons , and easily absorb in electrons , take shape the P (positive) type semiconductors.
Low-cost CIGS solar cells by paste coating and selenization

Low-cost CIGS solar cells by paste coating and selenizationM.Kaelin a,*,D.Rudmann a ,F.Kurdesau a ,H.Zogg a ,T.Meyer b ,A.N.Tiwari caThin-Film Physics Group,Laboratory of Solid State Physics,Swiss Federal Institute of Technology Zurich,SwitzerlandbSolaronix SA,Rue de l’ouriette 129,1170Aubonne,SwitzerlandcCentre for Renewable Energy Systems and Technology,Department of Electronic and Electrical Engineering,Loughborough University,UKAvailable online 2December 2004AbstractA simple process for the deposition of Cu(In,Ga)Se 2(CIGS)absorber layers is described.A low-cost CIGS precursor paste deposited by simple and fast doctor blade technique is subsequently selenized under selenium vapour in a quartz tube at 10mbar (10min at 5508C).The precursor paste is prepared with metal chlorides and nitrates dissolved in alcohol.The solution is then mixed with a cellulose solution to adjust the viscosity for optimal deposition.The conversion of the precursor to the CIGS phase was confirmed by X-ray diffraction (XRD).Grain size and morphology were characterised with electron microscopy.A double-layer structure formed during selenization,with a CIGS layer on top of an amorphous carbon layer.Auger electron spectroscopy (AES)shows a decreasing Ga/In ratio from the carbon–CIGS interface towards the CIGS surface.The layer structure grown on Mo-coated glass substrates (conventional dc-sputtering)was processed to solar cells by depositing a CdS buffer layer (chemical bath deposition)and ZnO/ZnO:Al front contacts (conventional rf-sputtering).A maximum efficiency of 6.7%was achieved with approximately 0.5-A m-thick absorber layers.Quantum efficiency measurements reveal photon absorption losses for the longer wavelengths,which are attributed to the thin layers.D 2004Elsevier B.V .All rights reserved.Keywords:Cu(In,Ga)Se 2;Selenium vapour;Auger electron spectroscopy1.IntroductionChalcopyrite CuInSe 2(CIS)and its alloys with gallium (CIGS)and sulphur are used as absorber layers in thin film solar cells.Due to their high absorption coefficient,1–2-A m-thick layers are sufficient to absorb the useful part of the incident solar radiation.By changing the In/Ga and Se/S ratio,a wide range of band gap values (1.0–2.4eV)are obtained and graded band-gap structures enabling high efficiency can be realised.Solar cells based on polycrystal-line CIGS layers have shown efficiencies up to 19.2%[1].Industries have started pilot production based on high vacuum co-evaporation [2]or selenization processes [3].They have already reported average module level efficien-cies of 10–13%and aim for 13–15%efficiency with production costs that are lower than those of crystallinesilicon modules.These highly efficient CIGS modules are obtained with expensive vacuum technology requiring sophisticated process control and resulting in a loss in resource material of 20–50%.In order to reduce manufac-turing costs,alternative deposition methods using non-vacuum equipment have been proposed and investigated for absorber and buffer layer deposition,while ongoing R&D shows the potential of non-vacuum methods for front and metal back contacts.In general,these techniques should allow simple and fast thin-film deposition with material utilization efficiencies close to 100%.This is important since indium and gallium are expensive and not abundant.Besides the well known electrodeposition [4]and chemical spray pyrolysis methods (CSP)[5],the paste coating method attracted a lot of attention in the last 5years because high cell efficiencies exceeding 13%could be achieved with this method [6].The process involves the deposition of a nanosized oxide precursor paste,reduction in H 2and selenization using diluted H 2Se gas.In another process,metal organic compounds are dissolved in an0040-6090/$-see front matter D 2004Elsevier B.V .All rights reserved.doi:10.1016/j.tsf.2004.11.007*Corresponding author.Tel.:+4114451481;fax:+4114451499.E-mail address:Kaelin@phys.ethz.ch (M.Kaelin).Thin Solid Films 480–481(2005)486–490/locate/tsforganic solvent and spin-or dip-coated onto the substrate. After pyrolysis during a heat treatment in reducing or inert atmosphere,the alloyed metal layers are selenized and completed to solar cells with reported cell efficiencies of up to9%[7].This paper describes a paste coating process,which uses very low-cost precursor materials and allows non-hazardous selenization treatments with short reaction times.The advantage of this process can be seen in the simplicity of the used equipment and processing steps.While selenization of metal and metal-oxide precursors have been studied by many groups,selenization of metal-chlorides and nitrates have not been reported so far,although they are commonly used in CSP solutions together with an organic Se/S compound.The use of precursor solutions compared to the reported nano-powder precursors allows thinner films to be deposited(b1A m).Absorbers grown with this paste coating process and cells prepared from such absorbers are characterized.2.ExperimentalPrecursor pastes were prepared by dissolving appropriate quantities of Cu nitrate hemipentahydrate(99.99%),In chloride(99.99%)and Ga nitrate,hydrate(99.999%)in methanol.The precursor composition is adjusted to a metal ratio Cu/In/Ga of1/0.8/0.5with a concentration of1mmol copper atoms per gram methanol.In parallel,a higher viscosity cellulose paste is prepared:Ethylcellulose(30–50 mPa s)is dissolved in1-pentanol with a weight ratio of1/ 10.The pastes are mixed in a weight ratio of1/2to yield a precursor paste with suitable rheology for doctor blade coating.A1-mm-thick soda-lime glass substrate is coated with a 400-nm-thick molybdenum layer by conventional dc-sput-tering.The spacers defining the distance between the blade and the Mo-coated glass consisted of two stripes of scotch (approx.50A m thickness),applied on both sides of the substrate.In a one-pass movement,the precursor paste is evenly distributed on the substrate surface.The sample is then put on a hotplate and heated to2508C for a few minutes to evaporate the alcohol and burn the cellulose. When the colour of the layer turns from black to a metallic blue,the sample is removed from the hotplate and allowed to cool down.The sample is then placed in a tubular two-temperature-zone selenization reactor.After purging the reactor with nitrogen gas,the sample is selenized in nitrogen diluted selenium vapour.A continuous flux of nitrogen carries the evaporated selenium from the first temperature zone to the sample in the reaction zone.The pressure is maintained at 10mbar in this open reactor system.The sample temper-ature is ramped up to5608C held there for10min,whereas the selenium source is maintained at a temperature of~350 8C during selenization.The resulting CIGS layers are investigated by scanning electron microscopy(SEM),energy dispersive X-ray analysis(EDX),Auger electron spectroscopy(AES)and X-ray diffraction measurements(XRD).Solar cells were processed by applying a50nm CdS layer by conventional chemical bath deposition and an i-ZnO/ZnO:Al transparent front contact by rf-sputtering.The cells were investigated by measurements of the current–voltage characteristics under simulated AM1.5conditions and external quantum effi-ciency measurements.3.Results3.1.Precursor layerThe blade-deposited precursor layer has a copper content of10mmol/m2.After complete conversion without metal losses during preheating and selenization,this will yield a CIGS layer thickness of roughly600nm.Thicker CIGS films would require higher metal concentrations but due to adhesion problems encountered for thicker films,the metal concentration was kept low.The precursor layer after preheating,prior to selenization,is mainly in an amorphous state except for some peaks belonging to CuCl,as shown in the XRD pattern(Fig.1).Apparently,crystals of CuCl form during evaporation of the solvent.This is probably due to its low solubility and early precipitation in alcohol.The average size of these crystals of approx.55nm was determined with the Topas software(Bruker AXS,2000) based on the Rietvelt method.3.2.Selenized layerThe Cu/In ratio of selenized layers measured by EDX corresponds to the composition of the prepared paste whereas the Cu/Ga ratio is usually lower than the ratio in the paste.No chlorine residuals are detected within the accuracy of1at.%for completely selenized layers.Ascan Fig.1.The XRD pattern of the precursor layer shows CuCl nanocrystals (~55nm)in an amorphous matrix.M.Kaelin et al./Thin Solid Films480–481(2005)486–490487be seen from a cross-section micrograph of a selenized film (Fig.2),a thin polycrystalline layer is formed on top of an amorphous layer.AES depth profiles show that the amorphous layer contains mainly carbon (Fig.3).This indicates that the preheating does not evaporate all of the organic solvent and binder material.Since the formed CIGS layer thickness corresponds to the calculated layer thick-ness of 600nm,the metals must have diffused out of the carbon matrix to form the polycrystalline CIGS layer on its surface.Due to the large variations in grain size and layer thickness,the surface is rough and the carbon signal in the Auger spectrum appears already after a short sputtering time.The sputtering rate was not calibrated for CIGS and therefore it is not possible to relate the sputter-time to absolute depth levels.The Auger spectrum was taken from a selenized precursor layer with a paste composition of Cu/In/Ga 1/0.85/0.4.The Ga/In ratio increases towards the carbon layer and the Ga signal penetrates deeper into the carbon layer than the signals of the other metals and therefore the Ga concentration in the paste has to be compensated for this.Accumulation of Ga towards the back contact is a commonly encountered phenomenon in selenization processes and is due to lower reaction rates for CuGaSe 2compared to CuInSe 2[8].The incorporation of Ga into CIS was further investigated by comparing the XRD patterns of a CIS (Cu/In ratio of 1/1.1)and CIGS (Cu/In/Ga ratio of 1/0.8/0.6)layer (Fig.4).Addition of Ga broadens the peaks and results in a slight shift of the peak maxima,indicating CIGS phase formation with non-uni-form composition along the depth.XRD and AES data suggest that the layer has a Ga grading from a low Gacontent CIGS phase on the surface to a high Ga content CIGS phase at the back contact.Such a grading can lead to a back surface field which is favourable for thin absorber layers (b 1A m)as it reduces fill factor deterioration [9],but the low Ga content near the CdS interface would limit the V OC parameter.3.3.Cell characterizationTypical cell parameters obtained for solar cells obtained with this process are in the range of 4–5.5%efficiency (V OC :400mV ,I SC :25mA,FF:45%).However,using slightly different paste chemistry,cell efficiencies up to 6.7%have been obtained recently.The improvement originated form enhanced fill factors (50–60%)and slightly higher I SC .Fig.5shows the I –V curve of such a cell,having still a low V OC around 400mV .Quantum efficiency measurements of the same cell revealed significant losses in the long wavelength region,which again reflects the thin layer structure.Additionally,the minimum band gap for a cut-off wavelength of approximately 1200nm can be extracted,which belongs to the CIS compound.This explains the low V OC values obtained.4.DiscussionIn spite of the notable efficiencies,many aspects of the conversion reaction from the precursors to the CIGS compound remain unclear.For instance,nitrate precursors usually result in metal-oxides upon heating [10].A previous study [11]revealed difficulties in converting the stable compound In 2O 3,and probably also Ga 2O 3,into their selenide phase using selenium vapours.As seen in Fig.1,no oxide phases are detected upon preheating the precursor to 2508C in air.The organic matrix may prevent the formation of bulk oxides and thus have a beneficial effect by reducing the conversiontime.Fig. 2.Scanning electron microscopy cross-section micrograph of a selenized layer.The polycrystalline CIGS film forms on top of an amorphous carbonlayer.Fig.3.Auger electron spectroscopy data showing the elemental depth profiles of the selenized layer.A thin CIGS layer is formed on a carbon underlayer.Due to the rough surface,the carbon signal appears already after a short sputtering time.M.Kaelin et al./Thin Solid Films 480–481(2005)486–490488The role of the cellulose material in the precursor paste is manifold;it allows adjusting the paste rheology for optimal coating results,avoids formation of larger CuCl crystals upon evaporation of the solvent which would deteriorate local film stoichiometry,and prevents the precursor salts from evaporation during preheating and the first stages of selenization.Nevertheless,the formation of an amorphous carbon layer between the Mo and the CIGS layer had to be taken into account.The influence of this carbon layer on the cell parameters is still under investigation but the obtained cell efficiencies indicate that the carbon layer is not completely deteriorating the cell performance,although it is 1A m thick,porous,amorphous and probably increasing the series resistance of the cell.Thicker layers deposited by doctor blade resulted in poor adhesion of the CIGS–carbon structure at the Mo interface;further experiments are required to improve the adhesion.Carbon-free structures were prepared by spraying cellu-lose-free pastes in nitrogen ambient on substrates heated to 1008C and subsequently selenized as described above.Such CIGS layers were extremely rough and had irreproducible compositions.From the obtained results,it can be concluded that Ga is incorporated into the selenized layer but its distribution has to be improved to achieve higher V OC values,i.e.a higher Ga concentration near the CdS is required for higher V OC .Better results may be obtained with longer selenization times or an additional annealing step [8].CuCl 2and In(NO 3)3have also been used for paste formulations and were successfully converted to CIGS.Nevertheless,the obtained cell efficiencies are lower than for the described paste formulation.Other inorganic precursors and additives for paste rheology adjustment are under investigation.The influence of possible chlorine residuals in the CIGS layer was not investigated yet.EDX values for chlorine are below 1at.%,which is the accuracy of this method.A study using ion implantation of Cl into n-type CIS and subsequent annealing showed a donor like behaviour of Cl [12];therefore,a reduced p-type carrier concentration would probably result from Cl doping.The role of possible carbon and oxygen impurities has not yet been investigated (Fig.6).Fig.4.XRD patterns of selenized precursor layers comparing a Cu–In (Cu/In=1/1.1)and a Cu–In–Ga (Cu/In/Ga=1/0.8/0.6)precursorcomposition.Fig.5.Current–voltage characteristic of a 6.7%efficient solar cell under simulated AM1.5conditions.Fig.6.External quantum parison of a thin non-vacuum cell with a vacuum co-evaporated 1.7-A m-thick CIGS absorber cell.M.Kaelin et al./Thin Solid Films 480–481(2005)486–4904895.ConclusionA novel,simple,non-vacuum CIGS formation process consisting of the selenization of inorganic precursor materials embedded in an organic matrix is described.The precursor conversion to the CIGS compound is achieved in selenium vapour and no additional hazardous gases are necessary.The formation of thin layers allows very short conversion times of less than10min at5508C.Cell efficiencies up to6.7%were obtained.However,chemical and structural aspects of the conversion process remain unclear.The optimization should focus on reducing the carbon-and increasing the CIGS-layer thickness.Thus,the choice of chemicals needs further investigation.Additional annealing steps may homogenize the Ga distribution in the film to get a higher Ga concentration near the CdS interface, which is currently restricting the cells to low V OC parameters.The replacement of the vacuum processes for the contact layers remains a big challenge.This issue will be addressed in future work.AcknowledgementsThe authors gratefully acknowledge the National Science Academy in Minsk for the AES measurements and SEM pictures,and Markus Huber from the particle technology laboratory at the Swiss Federal Institute of Technology for the XRD measurements.This work was funded through the Top Nano21programme of the Swiss government under contract no.CTI5491.3TNS.References[1]K.Ramanathan,M.A.Contreras,C.L.Perkins,S.Asher,F.S.Hasoon,J.Keane,D.Young,M.Romero,W.Metzger,R.Noufi,J.Ward,A.Duda,Prog.Photovolt.11(2003)225.[2]M.Powalla,B.Dimmler,Proceedings of the3rd World Conferenceon Photovoltaic Energy Conversion,Osaka,Japan,May11–18,2003, p.566.[3]K.Kushiya,Proceedings of the3rd World Conference on PhotovoltaicEnergy Conversion,Osaka,Japan,May11–18,2003,p.319.[4]D.Guimard,N.Bodereau,J.Kurdi,J.F.Guillemoles,D.Lincot,P.P.Grand,M.BenFarrah,S.Taunier,O.Kerrec,P.Mogensen,Proceedings of the3rd World Conference on Photovoltaic Energy Conversion, Osaka,Japan,May11–18,2003,p.515.[5]S.Duchemin,J.Bougnot,A.El Ghzizal,K.Belghit,in:W.Palz,G.T.Wrixon,P.Helm(Eds.),Proceedings of the9th European Photo-voltaic Solar Energy Conference,Freiburg,Germany,Sept.25–29, 1989,p.476.[6]V.K.Kapur,A.Bansal,P.Le,O.Asensio,N.Shigeoka,Proceedingsof the3rd World Conference on Photovoltaic Energy Conversion, Osaka,Japan,May11–18,2003,p.465.[7]H.Ishihara,S.Nakagawa,N.Mochizuki,M.Ishida,U.S.Patent No.5T910T336,8June1999.[8]M.Marudachalam,R.W.Birkmire,H.Hichri,J.M.Schultz, A.Swartzlander,M.M.Al-Jassim,J.Appl.Phys.82(1997)2896. [9]O.Lundberg,M.Bodegard,J.Malmstr f m,L.Stolt,Prog.Photovolt.11(2003)77.[10]M.E.Beck,M.Cocivera,Thin Solid Films272(1996)71.[11]M.Kaelin,H.Zogg,A.N.Tiwari,O.Wilhelm,S.E.Pratsinis,T.Meyer,A.Meyer,Thin Solid Films457(2004)389.[12]T.Tanaka,T.Yamaguchi,T.Ohshima,H.Itoh,A.Wakahara,A.Yoshida,Sol.Energy Mater.Sol.Cells75(2003)109.M.Kaelin et al./Thin Solid Films480–481(2005)486–490 490。
太阳能电池英文综述Solar Cells An Overview

Dye Sensitized Solar Cells (DSSC)
Dye/QD Dye/QD TiO2 20 nm) TiO (~ (~ 20 nm)
2
e
-
Iodide/tri-iodide electrolyte
Prof. Michael Gratzel
LOAD LOAD
Excitation of dye molecule or Quantum Dot (QD) by incident sunlight Transfer of electron from dye/QD to TiO2 Regeneration of oxidized dye/QD using a hole carrying electrolyte Transport of electron through TiO2 and external load Regeneration of electrolyte at counter electrode
Cost Comparison of Various Photovoltaics
Nanotechnology: Towards low cost solar cells
Pre-requnt Conducting Oxide: Eg ≥ 3 eV e.g. ZnO, TiO2, SnO2 etc. • Molecular Levels: a) HOMO: Highest Occupied Molecular Orbital b) LUMO: Lowest Unoccupied Molecular Orbital
Solar Cell IV Measurement in Lab
Solar Simulator
0.0025 0.0020 0.0015 0.0010
Professional+English+Solar+Cells

High performance materials
The materials used in professional English single cells are of high quality and have excellent optical and electrical properties These materials help to ensure that the solar cells absorb more sunlight and convert it into electricity more effectively
• Polycystalline solar cells: Made from multiple crystals of silicon, these cells are less effective but also less expensive
• Thin film solar cells: Made from thin layers of materials such as cadmium telluride or copper indium gallium selenium, these cells are the least effective but also the least expensive
03
Solar cells can significantly reduce greenhouse gas emissions and help to lower utility bills, making it an attractive option for homeowners
Commercial electricity
Advanced cell structures
Thin Film Solar Cell(薄膜太阳能电池)

Emerging photovoltaics
Emerging photovoltaics, often called third generation photovoltaic cells, include:
Organic solar cell Polymer solar cell Quantum dot solar cell
Amorphous silicon
Amorphous silicon (aSi) is the non-crystalline allotropic form of silicon. It can be deposited in thin films at low temperatures onto a variety of substrates. It offers some unique capabilities for a variety of electronics.
Copper indium galliumFra bibliotekselenide
Copper indium gallium (di)selenide (CIGS) is a I-III-VI2 semiconductor material composed of copper, indium, gallium, and selenium. The material is a solid solution of copper indium selenide (often abbreviated "CIS") and copper gallium selenide. It has a chemical formula of CuInxGa(1-x)Se2 where the value of x can vary from 1 (pure copper indium selenide) to 0 (pure copper gallium selenide). CIGS is a tetrahedrally bonded semiconductor, with the chalcopyrite crystal structure, and a bandgap varying continuously with x from about 1.0 eV (for copper indium selenide) to about 1.7 eV (for copper gallium selenide).
CIGS-based Solar Cells for the Next Millennium

CIGS-based Solar Cells for the Next Millennium {Hans-Werner Schock 1and Rommel Noufi 2*1Universita Èt Stuttgart,Institut fu Èr Physikalische Elektronik,Pfa enwaldring 47,D-70569Stuttgart,Germany 2National Renewable Energy Laboratory,1617Cole Blvd.,Golden,CO 80401,USAThin-®lm photovoltaic (PV)solar cells based on Cu(In,Ga)Se 2(CIGS)have twokey distinctive features:highest performance of any true thin-®lm solar cell (18.8%e cient)and leading performance on the module level.There is no evidence of limitsto further improvement of the e ciency.Device stability is not curtailed by intrinsicmaterial properties.The obstacles to large-scale production and commercialization ofCu(In,Ga)Se 2-based modules are the complexity of the material and the manu-facturing processes.Published in 2000by John Wiley &Sons,Ltd.INTRODUCTIONIs copper indium gallium diselenide (CIGS)the PV material of the year 2000?Many features of CIGS solar cells promise a bright future because they combine very high e ciency and the advantages of thin-®lm technology.CIGS cells e ciency has approached 19%under standard test conditions.This means that the CIGS solar cell performs as well as the best polycrystalline silicon cells,and on the laboratory scale,it competes directly with crystalline-wafer-based technologies.The potential of this material has not yet been fully exploited.As a multinary compound,it behaves quite di erently than `conventional'semiconductors.From the very beginning of research,CIGS moved to the front of ®lm-®lm materials with respect to solar cell e ciencies (see Figure 1).However,manufacturability has always been an issue.Future development must show to what degree presently available thin-®lm technologies will make CIGS competitive in the large-scale production of photovoltaics.Despite its demonstrated accomplishments,this thin-®lm technology still faces hurdles that need to be overcome to be widely manufacturable.In this paper,we present basic material on device properties and on critical issues that must be addressed in di erent stages of research and development:fundamentals,applied,and potential for manufacturing.Of course,other issues may be identi®ed;however,we hope that here we will capture signi®cant topics to stimulate discussions and raise questions.FUNDAMENTAL ASPECTS OF THE MATERIALThe many elements in CIGS solar cells can form a great variety of compounds during cell processing;therefore,the CIGS system is very complicated.On the other hand,it is very tolerant to defects and impurities because the chemistry,as well as the structure,can adjust itself in many possible ways.1,2Fortunately,this adjustment generally occurs in a way that is favorable to photovoltaic properties.An amazingly high level of performance of the solar cells in the laboratory and in pilot production of modules has been achieved on an intuitive basis and by empirical optimization of the deposition process and device PROGRESS IN PHOTOVOLTAICS:RESEARCH AND APPLICATIONSProg.Photovolt.Res.Appl.8,151±160(2000)*Correspondence to:Rommel Nou®,National Renewable Energy Laboratory,1617Cole Blvd.,Golden,CO 80401,USA.{This article is a US Government work and is in the public domain in the USA.Contract/grant sponsor:Bundesministerium fu r Bilding,Wissenschaft und Technologie;contract/grant number:0328059D-F Contract/grant sponsor:DDE;contract/grant number:DE-AC36-98-G010337structure.The questions now are how far we will get with this development,how we can get closer to the theoretical limit,and whether there is an intrinsic mechanism that could limit the performance of solar cells fabricated with this material.An important example of the empirical development of the material is the deliberate incorporation of sodium into CIGS absorber ®lms.Known as a detrimental impurity in semiconductor technology,sodium has a positive in¯uence on the properties of CIGS ®lms.The challenge is to sort out how to in¯uence material properties to speci®cally tailor properties such as carrier concentration,di usion length,and lifetime and to apply optimization procedures that are known from single-crystal-based solar cells.We have to ask whether we have the appropriate tools for the design and manufacture of very high-e ciency devices.The knowledge of basic material properties has increased considerably in recent years.By combining chemistry/defect chemistry issues,solid-state physics,semi-conductor physics,and material science,a new view of this solar cell material has emerged.The speci®c issues to be addressed are the mechanisms of ®lm growth,the control of defects,role of grain boundaries,properties of the surface,and junction formation.Film growthThe most striking feature of CIGS is the tolerance of the parameters in manufacturing processes.It is important to note that ®lms for very high-e ciency devices can be realized along di erent routes.However,some basic rules must be followed to obtain device-quality ®lms.Recipes for the growth of ®lms generally include a Cu-rich growth step that leads to the formation of large grains.3However,the simple coevaporation of the elements or the adjusting of the composition by di usion Cu into In x Se y ®lms in the presence of Se vapor yield ®lms that could lead to devices with e ciencies exceeding 15%.The nucleation phase of the ®lms is important and should have controlled conditions so as not to create large,isolated grains and to obtain smooth,dense ®lms.One prerequisite for high-quality ®lms is the presence of Na during ®lm growth.The bene®t of Na incorporation has not yet been fully explained in terms of simple models.In view of the amount of Na (0.1at.%)necessary for optimum ®lm fabrication,arguments that consider the e ect on the ®lm growth slightly outweigh those dealing with the incorporation of Na into the completed ®lm.The explanations of the bene®cial e ect of Na on the properties of CIGS ®lms and solar cell performance are numerous,and the Na incorporation most likely has a variety of consequences.During ®lm growth,the incorporationof Figure 1.The best one-of-a-kind laboratory cell e ciencies for thin ®lm (standard conditions)152H.-W.SCHOCK AND R.NOUFICIGS-BASED SOLAR CELLS153 Na leads to the formation of NaSe2and/or NaSeO3compounds that slow the growth of CuInSe2and could simultaneously facilitate the Se incorporation into the®lm.4The addition of Na inhibits the growth of CuInSe2at temperatures below3808C,and the retarded phase formation is responsible for the better morphology of CuInSe2®lms formed from stacked elemental layers.5The presence of Na widens the range of the a-(CuInSe2)phase in the phase diagram and increases the tolerance to the deviation of the Cu/ (In Ga)ratio from stoichiometry of Na-containing thin-®lms.Control of bulk doping concentrationThe p-type carrier concentration in CIGS®lms cannot be deliberately chosen.Attempts to dope the®lms with extrinsic dopants have not been fruitful.However,the introduction of sodium changes the picture, even though it is not yet well understood.The higher p-type conductivity of Na-containing®lms could result from the diminished number of compensating selenium vacancy(V se)donors.The above explana-tions deal with the role of Na during growth.However,the amount of Na in device-quality Cu(In,Ga)Se2®lms is on the order of0.1at.%,i.e.,a concentration of1020cmÀ3,6far beyond the doping level of a conventional semiconductor.One may ask where these quantities of Na reside in the®nished absorber. The electronic e ectÐthe change of e ective doping that results from Na incorporationÐis on the order of1016cmÀ3,i.e.,four orders of magnitude below that of the absolute Na content.Earlier,it was thought that the main portion of Na was situated at the®lm surface and at the grain boundaries.7Corroborating evidence for this hypothesis was recently found through high spatial resolution Auger electron spectroscopy.8Another interpretation of the bene®cial e ect of Na is based on the incorporation of Na into the Cu(In,Ga)Se2lattice.Niles and co-workers identi®ed Na±Se bonds by means of X-ray photoelectron spectroscopy and concluded that the Na is built into the lattice,replacing In or Ga.The extrinsic defect Na In/Ga should then act as an acceptor and improve the p-type conductivity.The incorporation of Na into the Cu(In,Ga)Se2lattice is supported by X-ray di raction measurements,which indicate an increased volume of the unit cell.Na on a Cu site would prevent the formation of the deep double donor In Cu,which would also have a bene®cial e ect.9Schroeder and Rockett10found that Na driven into epitaxial Cu(In,Ga)Se2®lms at5508C decreased the degree of compensation by up to a factor of104.They attributed their®ndings to a Na-enhanced re-organization of the defects,which allows the defects to build electrically passive clusters.HETEROJUNCTION FORMATION AND THE ROLE OF THE BUFFER LAYEROne basic element of the superior and tolerant behavior of CIGS is the nature of the surface of this semiconductor.On one hand,p/n junctions are easily formed by simply depositing CdS at low temperature by chemical-bath deposition(CBD);on the other hand,grain boundaries do not have a signi®cant in¯uence on the electronic properties of the CIGS layers.Both of these®ndings are somewhat contradictory,because the high barrier at the surface,which is the prerequisite for an e cient buried p/n junction in the absorber layer,would considerably enhance grain±boundary recombination.Surface/interface chemistry and structure of the CIGS absorber layerWhat is the optimum surface chemistry and structure of the CIGS absorber that is compatible with the window materials at hand?And what de®nes the charge transport in highly e cient devices?There are con¯icting data in the literature about what impacts the current±voltage(I±V)characteristics and the barrier height.Is the crossover in the I±V induced by the bu er layer/CIGS absorber interface through some Fermi level pinning mechanism,or is it a property of the surface defect chalcopyrite layer?11The microstructure at the interface must be understood because the interfacial structure at the CdS/CIGS and at the defect chalcopyrite/chalcopyrite transition regions determines the electric®elds around the junction and also a ects the charge transport across the junction.A common element to the above issues is the surface Fermi energy.This energy can be a ected by the surface chemistry,whether de®ned by the process154H.-W.SCHOCK AND R.NOUFI composition(intrinsic)or as a result of extrinsic impurities(dopants)such as Cd or Zn.Oxidation of the surface also in¯uences the position of the surface Fermi energy.Surface probes such as the Kelvin probe to determine the surface Fermi energy(as a function of process conditions and composition)will give a good indication of the degree of surface inversion,if any.It has been shown that exposure of the free thin-®lm surface to ambient air immediately reduces the type inversion.From surface photovoltage(Kelvin probe)measurements performed on samples stored in air,it is routinely found that the surface band-bending is below50meV,i.e.,the Cu(In,Ga)Se2surface after a long exposure to air is nearly in¯at-band conditions.12Na promotes the oxygenation and passivation of grain boundaries.13This mechanism accounts for the observed enhancement of the net®lm doping by Na incorporation because of the diminished positive charge at the surface.It has been observed that the surfaces of Na-containing®lms tend to be more susceptible to oxygenation when compared to Na-free®lms.14Based on this phenomenon,the challenge is how to construct an inversion layer for a CuIn1Àx Ga x Se2 absorber with x40Á30.The expectation is to obtain a buried p/n junction similar to that with x50Á3,but with much higher built-in potential and,hence,a higher open-circuit voltage(V OC).The free surfaces of as-grown Cu(In,Ga)Se2®lms exhibit two prominent features:(i)The energy distance of the valence-band maximum to the Fermi level is about1.1eV for pureCuInSe2®lms.This energy is larger than the bandgap energy.This®nding was taken as an indication for a widening of bandgap at the surface of the®lm in Refs15and16.For Cu(In1Àx,Ga x)Se2,the energy distance was found to be0.8eV(nearly independent of the Ga content,if x40).17(ii)The surface composition of Cu-poor CuInSe2,as well as that of Cu(In,Ga)Se2®lms,corresponds to a surface composition of(Ga In)/(Ga In Cu)of about0.75for a range of bulk compositions of 0Á55 Ga In a Ga In Cu 50Á75.Both observations have led to the conclusion that a phase segregation of Cu(In,Ga)3Se5occurs at the surface of the®lms.Because this material exhibits n-type conductivity,it could yield the explanation for the type inversion.The existence of a separate phase on top of standard Cu(In,Ga)Se2®lm®lms has not yet,to our knowledge,been con®rmed by structural methods like X-ray di raction,high-resolution transmission electron microscopy,or electron di raction.Furthermore,if the surface phase would exhibit the weak n-type conductivity of bulk Cu(In,Ga)3Se5,this would not be su cient to achieve the type inversion,as can be concluded from simple charge neutrality estimations.Based on these arguments,the type inversion of the surface of Cu(In,Ga)Se2thin®lms and junction formation can be viewed as a result of Fermi-level pinning due to shallow surface acceptors.The positively charged surface acceptors are expected on theoretical grounds for the metal-terminated(112)surface of CuInSe2,because of the dangling bond to the missing Se.18±20Under this assumption,the Cu-poor surface composition is a consequence of the type inversion(rather than its origin),as the Cu is driven away from the surface by the built-in electrical®eld.On one hand,the simple picture of a buried p/n junctionÐas a consequence of a segregated surface phase of the Cu(In,Ga)Se2absorberÐsuggests that any change at the absorber surface (air exposure,bu er deposition)would have only minor impact on the electronic properties of the device. This is obviously not the case.On the other hand,electrical measurements indicate that the Cu-poor surface has di erent electronic properties compared to the bulk of the absorber.11A possible solution is to assume a defect-rich,disordered surface layer that can have an even larger bandgap than that of the absorber material.Because of the depletion of Cu(which contributes by its d-states to the valence band), the valence-band energy is lowered,and hence,forms an intrinsic barrier for holes to be emitted from the CIGS absorber into the window layer.The role of the CdS bu er layerOne of the most important technological steps that led to actual high-e ciency devices is the introduction of the chemical bath to deposit the CdS bu er layer.There are several bene®ts of the CdS layer: (i)CBD deposition of CdS provides complete coverage of the rough polycrystalline absorber surface,already at a®lm thickness of10nm.21CIGS-BASED SOLAR CELLS155 (ii)The layer protects against damages and chemical reactions resulting from the subsequent ZnO deposition process.(iii)The chemical bath removes the natural oxide from the®lm surface;22thus,it re-establishes positively charged surface states and,as a consequence,the natural type inversion at the CdS/Cu(In,Ga)Se2 interface.(iv)The Cd ions,reacting®rst with the absorber surface,remove elemental Se,possibly by the formation of CdSe.Cd also di uses to a certain extent into the Cu-poor surface layer of the absorber material,23,24where it possibly forms Cd Cu donors,thus providing additional positive charges that enhance the type inversion of the bu er/absorber interface.As discussed above,the open-circuit voltage limitations by interface recombination,also require a low surface recombination velocity,in addition to the type inversion of the absorber surface.Thus,one might conclude that interface states(except those shallow surface donors responsible for the type inversion)are also passivated by the chemical bath.WIDE-BANDGAP ALLOYSFor thin-®lm modules,it is important to further improve performance by increasing the bandgap to achieve individual cells with high voltage.The advantages are:(i)reduction in the number of scribes used for the monolithic integration of cells into a module; (ii)reduction in the top and bottom electrode thicknesses(Mo and conducting ZnO)because of reduced current density;and(iii)a lowering of the temperature coe cient at maximum power output.For the CuInSe2/CdS device,this coe cient is relatively high at d P max a d t$À5Á6(mW cmÀ28CÀ1). The desired bandgap of the absorber for high-e ciency modules is in the range of1.4±1.6eV.In this case, module technology could be simpli®ed,and better performance at high operation temperatures would be obtained.For the Ga alloy,this means a Ga/In Ga ratio of about0.5to0.75,and for the S incorpora-tion,this means a complete or almost complete substitution of S for Se.Earlier work on multinary chalcopyrite compounds alloying Ga and/or S with CuInSe2showed deterioration in the performance of the devices at high Ga or S concentrations.25For Ga incorporation, the decline in performance starts at a Ga/In Ga ratio of about0.3.For S incorporation,the decline threshold is not well established.What are the issues?The alloy Cu(In1Àx Ga x)Se2exhibits not only increasing bandgap with increasing x, but also,a change in material properties that is relevant to the device operation.First,a structural di erence exists between the bulk phase(chalcopyrite112phase)and the reconstructed surface(defect chalcopyrite135phase).At low Ga(x$0Á3),the lattice constants for the two phases are similar and then diverge for x40Á3.This lattice mismatch at high Ga results in an intrinsic defect density in the transition region.Second,the surface defect chalcopyrite layer has been shown to switch carrier type from n-to p-type as x increases above$0Á3.This inversion of the surface layer transforms a shallow buried`p/n' junction residing in the absorber away from the metallurgical junction to an abrupt heterojunction between the Cu(In1Àx Ga x)Se2absorber and the CdS window.Electrically,the I±V characteristics of the solar cell will change from being dominated by recombination in the space-charge region to recombination at the metallurgical hetero-interface.The experimentally measured rate of change of the open-circuit voltage as a function of x no longer increases at the same rate as the bandgap.Thirdly,the incorporation of Ga or S into CuInSe2causes a movement of the conduction-band minimum(CMB)and valence-band maximum(VBM)relative to the band positions of the pure CuInSe2.For Cu(In1Àx Ga x)Se2, the band movement is mostly in the CBM being closer to the vacuum level,and for CuIn(Se,S)2,the VBM moves downward.Similar band movements also occur for the surface defect layer.As a result,the band o set in the CBM between the absorber and the CdS window changes from a positive o set(spike)to anegative o set and hence to a favorable band alignment.Similar measured and calculated data26exist to compare the band alignment of the Cu(In,Ga)(Se,S)2absorber at di erent compositions with other window materials such as ZnSe,ZnS,and ZnO.Thus,the deterioration of device properties at x40Á3is the result of a combination of negative e ects.SOLAR CELL STABILITYStability appears to be no problem in CuInSe2.Long-term outdoor testing and tests at elevated temp-erature,as well as their operation in space,has proved that there is no intrinsic mechanism that a ects cell performance.To the contrary,cells often improve during actual operation.The stability issues inherent to the material system have been addressed recently.27A self-healing mechanism due to defect relaxation with the help of mobile copper makes this material unique.Therefore,an important prospective applica-tion for CIGS cells is in outer space,because the main power source in space is photovoltaics.Space satellites in the low orbits for communication systems require solar cells with high end-of-life e ciencies. CIS has proven superior radiation hardness,which could make this type of cell the material of the future for space applications.28The challenge for developing space cells is to reduce the weight by depositing the cells on foil substrates and at the same time to retain the performance achieved with devices on soda-lime glass.MANUFACTURING ISSUESCIS has the best potential of any thin®lm to reach more than15%module e ciency in the near future. Mini-modules,ranging in area from20to90cm2,that use the process sequence anticipated for use in a commercial module have reached e ciencies around14±15%.Recently,Siemens Solar Industries fabricated a1ftÂ4ft power module($44W)with an NREL-veri®ed e ciency of12.1%.29Using a totally di erent approach to the deposition of the(CIGS)absorber layer,ZSW fabricated a30cmÂ30cm module with a veri®ed e ciency of12.8%.30Even though a limited commercial product is beginning to emerge,CIGS technology has some hurdles to overcome before realizing®rst-time manufacturing founded on a credible and reliable fabrication process stream and on a de®nite understanding of the operation mechanism of the device(solar cell).However,the increase of basic knowledge in recent years provides more solid ground for the manufacturing of modules at a large scale.Module sizes up to60cmÂ120cm are planned so as to meet cost goals.The approaches range from sophisticated high-vacuum evaporation processes to non-vacuum techniques.Results of the di erent processes are summarized in Table I.On the high level demonstrated for laboratory cells,we can step back and think of process simpli®cations,Table I.Performance of CIGS cells and modulesProcess Status lab.cell(%)Module e .(%)/area(cm2)Laboratory/companyNon-vacuum processes12.4*8.0*/74ISET,Unisun31,32 Selenization of precursor metal®lms41612.1*/1Â4ft Siemens29 Coevaporation/sequential evaporation18.8*14.7{/18NREL3316.213.9{/90IPE3412.8/800ZSW309.6*/135EPV11.5*5.6/240Global Solar14.7{/19Angstroem Solar Center35 Independently con®rmed at*NREL,{ISE/Fraunhofer.156H.-W.SCHOCK AND R.NOUFICIGS-BASED SOLAR CELLS157e.g.,reduce the substrate temperature without losing too much cell performance.The future must show which direction to take.Regarding the availability of the material,it is advisable to choose the approach that uses the least amount of In with respect to the power produced by the module.Siemens used as much o -the-shelf equipment and processing as possible for fabricating CIGS®lms, whereas ZSW designed its own equipment for an in-line coevaporation process.Reducing absorber thicknessTo fabricate low-cost CIGS PV modules,it is desirable to increase production throughput and reduce the amount of materials used.Reducing the thickness of the CIGS absorber reduces the absorber processing time and the amount of each element used.And it is desirable to reduce the use of In,which is a relatively less abundant and expensive material.What is the necessary minimum CIGS absorber thickness before device performance begins to deteriorate?The high absorption coe cient of105cmÀ1for light energy greater than the bandgap ensures that all the light is absorbed in a®lm that is less than1m m thick.However,there is fundamental device physics,as well as practical experimental limitations,that may in¯uence device parameters.Assuming that all the light is absorbed by the minimum thickness,then minimizing the electronic losses will require the following:First,the distance between the space-charge edge and where the light is absorbed and electron-hole pairs are generated must be small compared to the minority-carrier di usion length.Second,the surface recombination velocity for the absorber needs to be minimized.This,for example,may be achieved with minority-carrier mirrors using wide-bandgap materials that have similar lattice constants to that of the absorber.Spitzer et al.36have calculated the expected device performance as a function of thickness for CIS-based devices using a particular combination of material parameters.They concluded that e ciencies exceeding 25%should be achievable for single-junction chalcopyrite-based devices using1m m absorber and assuming a surface recombination velocity approaching zero.This holds true for absorbers with bandgaps between1and1.5eV and no absorption in the window material.Currently,very few experiments are described in the literature that attempt to use the above guidelines.E ciencies in the range of13±15%are reported from the Central Research Laboratories in Japan37and from NREL(private communication)in the United States.We believe that the state-of-the-art schemes in material quality and fabrication are adequate to design a CIGS-based solar cell that follows the above guidelines,and that will approach the maximum device performance expected.New materials and processes for heterojunction formation and window layersWet-chemical processes for fabricating heterojunctions make process integration more di cult.Even though the chemically deposited CdS is the bu er layer that yields the best results,there are many important advantages to replacing it with a wide-bandgap material deposited in a physical vapor deposition(PVD)process:(i)A material with a wider bandgap for the bu er layer would lead to higher photocurrent.(ii)`Dry'PVD processes for heterojunction formation could be integrated into in-line fabrication.In principle,the intrinsic junction formation by the surface defect layer should make it easy to form a heterojunction by simply using a high conductive window layer.Currently,metal-organic chemical vapor deposition(MOCVD)or atomic layer chemical vapor deposition seem to be the most promising methods with respect to the properties of the bu er layer.These methods provide the most coherent growth of the bu er layers,i.e.,dense®lms with small thickness,and therefore,a wide process window.However,the chemical vapor deposition processes are generally batch processes that require extra handling of the substrate,which is not too easy if the areas approach square-meters.Recently,direct sputtering of ZnO onto the absorber layer yielded high-performance devices on small areas.33For manufacturing,a large enough process window is essential.158H.-W.SCHOCK AND R.NOUFIQuality control and in-situ diagnosticsQuality control of the base material will be an important issue in the future.Unlike in silicon-wafer-based technology,no de®nite criteria exist for establishing the quality of CIGS absorber®lms.The CIGS growth surface usually yields signi®cant compositional and structural information from the interrelationship of incident material¯uxes,surface reconstruction,strain,substrate temperature,and other factors.Still unknown are many of the intrinsic and extrinsic physical and chemical qualities that are characteristic of the growth surfaces.In-situ monitoring for diagnostics and control is required for high yield and high quality in the continuous processing of CIGS modules.Module performance is sensitive to variations in composition,structure,and the thicknesses of di erent semiconductor layers.Sensors and science-based models are needed to measure,interpret,and use the information obtained from the growth process and/or growth surface in real time.Numerous surface and bulk analytical techniques exist for determining surface properties and composition(e.g.,low-energy electron di raction,X-ray photoelectron spectroscopy,and X-ray¯uor-escence).However,the monitoring technique used in a manufacturing environment must be compatible with the®lm growth environment and the associated hardware.Each method of probing must take advantage of its physical principles of operating.An ideal in-situ analysis technique would be capable of operating at high data rates over long working distances,could be used for both smooth and rough surface morphology,and would be designed with simple geometry compatible with the growth environment.Three major science-based techniques may be considered,including traditional electron-based tech-niques,which require high vacuum but cannot be used with higher-pressure growth processes;optical methods,which may be used in transparent medium or in a re¯ection mode,may provide information integrated over some®nite depth and have the advantage of probing compositionally graded®lms(e.g., spectroellipsometry).Atomic absorption/emission may be used for real-time¯ux-rate monitoring.38 Optical re¯ection spectroscopy,scanning above and below the bandgap,may yield phase and composi-tional information.Temperature sensors using thermocouples or optical detectors may be used to measure temperature changes at the substrate that are induced by variation in emissivity of the thin-®lm layer during growth.39BEYOND20%Summarizing the features of CIGS,it is obvious that further improvements are very likely to be achieved if we attempt to better understand the material and develop appropriate deposition technologies.There are some obvious areas for improvement:(i)Increase the window transparency(e.g.,replace CdS by a window with wider bandgap,or eliminatethe need for a bu er layer).(ii)The carrier con®nement in the absorber layer needs to be investigated carefully for passivation at the interface and the back contact.But recombination is still dominated by defects in the space-charge region.Therefore,the quality of the material should be improved.Considering the purity of the starting materials generally used,there is much room for improvement.However,the methods used in optoelectronics for depositing high-quality ®lms,such as MOCVD and molecular-beam epitaxy(MBE),have not yet led to expected improvements. MOCVD-grown®lms su er from growth defects,and the quality of MBE®lms may be a ected by the low selenium activity.Therefore,the route for improving the material cannot be deduced simply from classical semiconductor manufacturing;rather,we need new ways to optimize materials.The present methods for fabricating the highest quality®lms include a recrystallization inherent to the growth process. Optimization guidelines can only be deduced from careful study of the material.。
THIN SOLAR CELLS

专利名称:THIN SOLAR CELLS 发明人:SPEAR, Reginald G.申请号:EP82902514.0申请日:19820716公开号:EP0084051A1公开日:19830727专利内容由知识产权出版社提供摘要: Solar cells and rows of solar cells are created in the form of thin films on insulating substrates. In one embodiment for example thin film conductors (4, 5) are deposited on a glass substrate (1) and semiconductor films (7, 8) are deposited above. The semiconductor films (7, 8) form a PN junction parallel to the substrate and may extend beyond at least some of the edges of the conductive films (7, 8) for insulation. Another conductive film (10) is deposited on the semiconductor film and is insulated from the first conductive film by an insulating strip (9). Contacts (2, 3) applied to the edges of the conductive films serve as output connections. In a row of such cells the edge of the second conductive film of a cell can overlap the edge of the first conductive film to an adjacent film to connect the cells in series. In one aspect of the present invention the films are thin enough to be transparent. In another aspect, a plurality of transparent films are stacked to absorberdes selective portions of the spectrum. In another aspect a stacking technique of thin films using the diagonal displacement of a mask makes it possible to obtain successive layers having exposed edges and covered for performing appropriate electrical connections.申请人:SPEAR, Reginald G.地址:1434 Park Place San Marino, CA 91108 US国籍:US代理机构:Plaisier, Aart 更多信息请下载全文后查看。
CIGS太阳能电池专利技术综述

CIGS太阳能电池专利技术综述张跃【摘要】铜铟镓硒(CIGS)是一种理想的用于制备薄膜太阳能电池的半导体材料,基于铜铟镓硒(CIGS)材料制作的薄膜太阳能电池是一种最有发展前景的薄膜太阳能电池,本文对涉及铜铟镓硒(CIGS)太阳能电池结构和制备方法的专利文献进行了全面检索,介绍了CIGS薄膜太阳能电池主要结构和制备方法,以及相关专利技术的发展情况,并对其发展前景进行了展望.【期刊名称】《科技视界》【年(卷),期】2018(000)023【总页数】2页(P38-39)【关键词】CIGS;太阳能电池;专利;综述【作者】张跃【作者单位】国家知识产权局专利局专利审查协作北京中心,中国北京 100160【正文语种】中文【中图分类】TM914.40 引言铜铟镓硒(CIGS)薄膜太阳电池,光吸收效率高、户外性能稳定,是目前国际上太阳电池的研究重点,转换效率高达 21.7%[1]。
铜铟镓硒(CIGS)是一种四元化合物半导体材料,是一种直接带隙半导体材料,其可见光波段的吸收系数高达105/cm量级,只需2微米左右厚度的CIGS薄膜就可以吸收几乎所有的入射太阳光[2],是一种理想的用于制备薄膜太阳能电池的半导体材料。
本文以国际分类号(IPC)H01L31/00在德温特世界专利数据库(DWPI)、世界专利文摘数据库(SIPOABS)和中国专利文摘数据库(CNABS)中检索。
文中数据截止到2018年3月1日,包括发明和实用新型专利。
1 CIGS太阳能电池的发展CIGS薄膜太阳能电池光吸收能力强,转换效率高,稳定性强,使其成为当前具有很强发展潜力的高效太阳能电池。
该技术在国外发展较早。
CIGS薄膜材料由三元CIS薄膜材料发展而来[1],1953年 Hahn等人首次合成CIS薄膜材料。
为了充分利用太阳光谱,自20世纪80年代末期开始人们在CuInSe2材料中掺入Ga和S元素,以提高禁带宽度,使之与太阳光谱更匹配,获得更高的光电转换效率。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Thinning of CIGS solar cells:Part II:Cell characterizationsZ.Jehl a ,F.Erfurth a ,N.Naghavi a ,⁎,L.Lombez a ,I.Gerard b ,M.Bouttemy b ,P.Tran-Van b ,A.Etcheberry b ,G.Voorwinden c ,B.Dimmler c ,W.Wischmann d ,M.Powalla d ,J.F.Guillemoles a ,D.Lincot aaInstitut de Recherche et Développement sur l'Energie Photovoltaïque (IRDEP-UMR 7174EDF-CNRS-ENSCP),6quai Watier,78401Chatou Cedex,France bILV-UMR 8180,Universitéde Versailles St Quentin,45Av.des Etats Unis,78035Versailles Cedex,France cWuerth Elektronik Research GmbH,Industriestr.4,70565Stuttgart,Germany dZentrum für Sonnenenergie-und Wasserstoff-Forschung (ZSW),Industriestr.6,70565Stuttgart,Germanya b s t r a c ta r t i c l e i n f o Available online 15January 2011Keywords:Chalcopyrite CIGSe Solar cellsUltra-thin films EtchingIn this paper,the in fluence of reducing the thickness of the CIGSe absorber layer by bromine etching from 2.5μm to 0.5μm on electrical and optical solar cell properties is addressed.We observe a decrease in ef ficiency which is mainly caused by a reduced short circuit current,whereas the fill factor and the open circuit voltage are stable.Even without deliberate light trapping or anti-re flection coating,an ef ficiency of 10.3%is obtained for a 0.5μm thick CIGSe absorber.A smoothing of the absorber surface is observed during the etching,its in fluence on the cell parameters will be discussed.©2011Elsevier B.V.All rights reserved.1.IntroductionDuring the last decade increased attention has been paid to the preparation of very thin absorber layers in solar cells based on Cu(In,Ga)(S,Se)2(CIGSSe)[1–4].The reduction of the absorber layer thickness has been identi fied as a key issue for a further improvement of the competitiveness of the CIGSSe technology,which will impact two aspects:firstly the reduction of the consumption of indium per Wp and thus the costs and availability for large production volumes;secondly the increase of the production speed for the CIGSSe deposition and thus the reduction of equipment costs.Representative results have already been obtained at Uppsala University [1]and at the University of Delaware and the National Renewable Energy laboratory (NREL)in USA [2–4].The approach presented in this paper is to start from the standard glass/Mo/CIGSe structure and to thin the absorber layer by etching from the top using a chemical bromine solution.From this it will be possible to engineer thinner CIGSe layers while keeping exactly the same back contact con figuration.The effect of thinner layers,down to 0.5μm obtained by this methodology on solar cell properties will be addressed,allowing to gain very important information on the thickness dependence of key device parameters (photovoltaic parameters,recombination processes,and optical losses)while keeping a standard front and back side process.The advantage of the thinning approach is to overcome the dif ficulties arising from roughness variations when dealing with the deposition of absorbers with different thicknesses.It also gives a guaranty that the back contact interface is the same in all cases which might not be the case when varying the deposition time.This method leads to flat and specular absorber surfaces with easily controlled layer thickness [5].This can permit a good quanti fication of optical losses in thin CIGSe solar cells.The effect of thinning the absorber layer down to 0.5μm on the electrical and optical solar cell properties is addressed.A good understanding of the thinning CIGSe properties will make it easier to design suitable front and back contacts for further cell improvement separately from the absorber formation.2.ExperimentalThe investigated Cu(In,Ga)Se 2absorbers (CIGSe)were deposited on Mo covered glass by coevaporation at Wurth Solar [6].The absorber layers with a standard thickness of 2.5μm were chemically etched using an aqueous solution of HBr/Br 2.This method is described in detail by Bouttemy et al.[7].By increasing the etching time stepwise from 4min to 15min we obtained layers with a nominal layer thickness of 2μm,1.5μm,1μm,700nm,and 500nm,respectively,estimated by atomic absorption spectroscopy during the etching process.The electrical properties of cells were characterized by current voltage measurements at 25°C under illumination (AM 1.5global spectrum).Individual cells of 0.1cm 2were delimited by mechanical scribing.Absolute spectral response measurements were made with a monochromator (Spectral Products CM 110)under chopped illumination and a lock-in technique.The surface morphology of the samples was investigated by scanning electron microscopy (SEM)using a Leo Supra 35field emission gun (FEG).The AFM images were obtained with a D3100microscope and Nanoscope IIIa controller,using contact mode with DNP-20tips.The optical re flectivity was measured in the range of 250to 2500nm using a Perkin-Elmer Lambda 900UV/VIS/NIR spectrometer.Photoluminescence measurements with an excitation energy of 514nm were applied using a homemade setup.Thin Solid Films 519(2011)7212–7215⁎Corresponding author.E-mail address:negar.naghavi@edf.fr (N.Naghavi).0040-6090/$–see front matter ©2011Elsevier B.V.All rights reserved.doi:10.1016/j.tsf.2010.12.224Contents lists available at ScienceDirectThin Solid Filmsj o u r n a l h o m e p a g e :w w w.e l s ev i e r.c o m /l o c a t e /ts f3.Results and discussionThe SEM pictures in Fig.1show the cross sections of the initial absorber surface and of two etched absorber layers with a nominal thickness of 2000nm and 500nm,respectively.In addition the cross sections of the same samples are shown after the deposition of the standard CdS/i-ZnO/ZnO:Al layer structure.In contrast to the rough surface of the as-deposited absorber,the surfaces of the etched samples are much smoother.A strong surface modi fication already occurs within the first minutes of etching,as can be seen for the 2000nm sample.With increasing etching time a further smoothing of the surface is observed.On top of all etched absorbers a very thin super ficial layer is observed whose origin is not clear.It seems to be caused by the etching process and might be related to the formation of Se 0-enrichment at the surface [7],but further experiments are needed to verify the nature of this thin layer.The smoothing effect of the HBr/Br 2etching on CIGSe absorbers is con firmed by AFM measurements.Fig.2shows the root mean scare (RMS)parameters of the surface roughness on a scanning area of 50×50μm 2applied on a supplemental sample series as a function of the absorber thickness.The RMS decreases from about 220nm for a non etched 2500nm thick CIGSe sample down to 75nm for a 500nm thick CIGSe absorber.This con firms that the roughness of the absorber surface is clearly reduced,especially within the first minutes of etching (down to 2μm thickness)as was also observed on SEM pictures (Fig.1).The strong decrease of the roughness at the beginning of the etching declines with increasing etching time.The impact of the surface smoothing on the re flectivity of the absorber samples is illustrated in Fig.3a,showing the total re flectivity of three absorber films with a thickness of 2500nm (non-etched),1500nm and 500nm,respectively.As the thickness is reduced interference fringes emerge in the IR range.In this wavelength range the light is not absorbed by the CIGSe material.The appearance of these fringes is caused by the smoothing of the absorber surface providing a more homogeneous thickness of the absorber film,which thus acts as interference layer.The as deposited reference CIGSe sample does not exhibit any interference fringes due to its high texturation.In the CIGSe absorption range (i.e.wavelength between 1100nm and 400nm),the total re flectivity increases with increasing etching time.The enhancement of the re flectivity for the 500nm sample represents a relative loss of light intensity penetrating the absorber of about 25%compared to the as-deposited non etched CIGSe sample.When cells are completed by deposition of the standard CdS/i-ZnO/ZnO:Al layer structure,the difference in re flectivity between standard and etched CIGSe is strongly reduced (Fig.3b).This pseudo anti-re flecting effect occurs due to the improved adaptation of the optical index at the interfaces air/ZnO/CIGSe compared to the interface air/CIGSe,as the optical index of ZnO is about 2and of CIGSe is about 2.9[8].The ZnO window layer causes additional interference fringes even in the CIGSe absorption range,which affect the effective light intensity penetrating the absorber bulk.In the non-absorption range these fringes superpose with the interference fringes originating from the absorber layer,giving a non-periodic fringe structure.To investigate the impact of the etching on the solar cell parameters,we performed J(V)measurement under illumination using standard conditions (AM 1.5,25°C).The obtained photovoltaic parameters are shown in Fig.4.The fill factor remains roughly constant for all samples,which indicates the good quality and homogeneity of the material throughout the thickness.The open circuit voltage slightly increases with increasing etching time,which can be attributed to the grading of the band-gap inside the absorber:The band-gap of the absorber surface is smaller than the bulk value.When the “original ”absorber surface is removed,the band-gap of the “new ”surface increases,approaching the bulk value with increasing etching time.This interpretation is con firmed by the results of photoluminescence (PL)measurements,which are shown in Fig.5.The peak position corresponds to the band gap energy within the investigated layer.For CIGSe the penetration depth of this method is about a few 100nm.Hence,the observed shift of the peak with increasing etching time to higher energies mainly indicates an increase of the band-gap within this surface region.The shift of a few 10meV between the non-etched absorber and the 500nm sample matches very well with the increase of V oc between both samples.The fact that V oc does not decrease with reduced thickness is a strong advantage of this etching technique.In comparison Ramanathan etal.Fig.1.SEM images (cross sections,65°tilt)of standard non etched (2500nm)and etched (2000nm and 500nm)CIGSe samples without and with CdS/ZnO/ZnO:Al windows layer.7213Z.Jehl et al./Thin Solid Films 519(2011)7212–7215showed that the absorber layer thickness has a strong impact on V oc for directly grown thin CIGSe films.A strong degradation in ef ficiency by about 50%for sub-micron CIGSe absorber films was reported [4].Thus even for cells with an absorber layer thickness of only 500nm we still achieve ef ficiencies of 10.3%(see Fig.4).The observed drop of the ef ficiency with decreasing layer thickness is mainly caused by the almost linear decrease of J sc from 28.1mA cm −2for the reference absorber (2500nm)to 20mA cm −2for the 500nm sample.This decrease can be attributed to both,the reduction of the CIGSe absorber thickness and the smoothing of the absorber surface.The latter enhances the surface re flectivity of the etched cells and thus diminishes the absorption of light.This correlation is nicely demonstrated in Fig.6,showing external quantum ef ficiency (EQE)measurements.The drop of the short circuit current with decreasing layer thickness is represented by the overall decrease of the quantum ef ficiency.First estimations indicate that this decrease even in the short wavelength range at about 550nm is mainly caused by reduced absorption due to the thinning of the absorber layer.In contrast to the results of Lundberg et al.[1]we do not consider possible recombination at the back contact to in fluence the spectralresponse of the solar cells discussed in this paper.Additionally,interference fringes can be observed for the etched CIGSe samples originating from the ZnO layer.The positions of the maxima correspond to the positions of the minima of the re flective curves (compare Fig.3b),and vice versa.Fig.6also shows EQE spectra which are corrected for the re flectivity losses (dotted lines)compared to the non-etched sample (see Fig.3b).Therefore the EQE data was multiplied by the adjusted ratio of the re flectivity data of the standard absorber R standard and the etched sample R etched (1−R standard )/(1−R etched ).These data show the direct impact of the absorber layer thinning on the EQE,the interference fringes of the ZnO are vanished.In the near infrared wavelength range (800nm –1000nm),the decrease in quantum ef ficiency between the non-etched and the 500nm sample is stronger than the decrease in the wavelength range of 500nm –700nm.This behavior cannot only be explained by the higher penetration depth of light for higher wavelengths.The loss in J sc only caused by the reduction of light absorption due to the absorber thinning between a 1500nm and 500nm thick absorber layer was estimated to be smaller than 10%,taking the (constant)absorption of the ZnO layer and a global AM 1.5solar spectrum into account.The observed decrease in J sc shown in Fig.4Fig.3.a)Total re flectivity for three different thick (2500nm,1500nm and 500nm)CIGSe absorbers.2500nm CIGSe absorber is non etched with natural surface texturation.1500nm and 500nm etched samples are smooth.b)Total re flectivity of complete solar cells for the same samples.(The kinky structure at about 900nm is an artefact caused by the re flectivity measurementsetup.)Fig.4.Photovoltaic parameters for prepared cells with different CIGSe thickness.The plotting direction is with decreasing absorber layerthickness.Fig. parison of normalized photoluminescence signal of CIGSe layers with thickness varying from 2500nm down to 500nm.Fig.2.Measured RMS versus CIGSe thickness after etching.Thinner absorber samples are smoother caused by the longer etching time.7214Z.Jehl et al./Thin Solid Films 519(2011)7212–7215is much larger,which cannot be explained by optical losses,since the re flectivity data of both samples is almost identical (see Fig.3b).Hence,the strong decrease of J sc indicates additional electronic losses,which could be caused by enhanced back contact recombinations.This interpretation is supported by the strong decrease of the EQE in the high wavelength range.Higher wavelengths create more electron hole pairs in the volume and near the back side of the absorber,which than can recombine at the back contact.The position of the onset of the graph at the low energy side indicates the band-gap of the absorber material.The shift to higher energies is thus caused by the already discussed increase of the surface band-gap with decreasing CIGSe film thickness.4.ConclusionWe succeeded in etching CIGSe absorber layers with a standard thickness of 2.5μm down to 500nm without altering the material quality.Ef ficiencies greater than 10%for an absorber layer thickness of 500nm have been achieved.The ef ficiency losses compared to the non-etched absorber can be assigned to the reduction of J sc ,which is caused by the thinning of the absorber material and by a slight enhancement of re flectivity.This enhancement is caused by the smoothing of the absorber surface during the etching,which was observed by SEM and AFM characterization.Furthermore we reported the increase of the surface band-gap induced by etching,which was observed in EQE as well as in photoluminescence measurements.The value of a few 10meV nicely corresponds to the enhancement of V oc for the etched absorber samples.AcknowledgementThis work is supported by the ANR's HABISOL program within the ULTRACIS project.References[1]O.Lundberg,M.Bodegard,J.Malmstrom,L.Stolt,Prog.Photovoltaics 11(2003)77.[2]W.N.Shafarman,R.X.S.Huang,S.H.Stephens,Conference Record of the 2006IEEE4th World Conference on Photovoltaic Energy Conversion,1–2(2006)420.[3]K.Ramanathan,J.C.Keane, B.To,R.G.Dhere,R.Nou fi,Proc.20th EuropeanPhotovoltaic Sol.Energy Conf..Barcelona.Spain.(2005)1695.[4]K.Ramanathan,R.Nou fi,B.To,D.L.Young,R.Bhattacharya,M.A.Contreras,R.G.Dhere,G.Teeter,4th World Conference on Photovoltaic Solar Energy Conversion,2006,p.380,Hawaii.[5] B.Canava,J.F.Guillemoles,J.Vigneron,D.Lincot,A.Etcheberry,J.Phys.Chem.Solids 64(2003)1791.[6]M.Powalla,M.Cemernjak,J.Eberhardt,F.Kessler,R.Kniese,H.D.Mohring,B.Dimmler,Sol.Energy Mater.Sol.Cells 18–19(2006)3158.[7]M.Bouttemy,P.Tran-Van ,I.Gerard,A.Causier,A.Etcheberry,J.L.Pelouard,Z.Jehl,N.Naghavi,G.Voorwinden,B.Dimmler,M.Powalla,J.F.Guillemoles,D.Lincot,Thin Solid Film (unpublished).[8] A.Campa,J.Krc,J.Malmström,M.Edoff,F.Smole,M.Topic,Thin Solid Films 515(2007)5968.parison of external quantum ef ficiency between non etched (2500nm thick)and etched (1500nm and 500nm thick)CIGSe based solar cells.The dashed lines show the EQE data after correction of the re flectivity losses caused by surface smoothing.(The kinky structure at about 900nm for the “1500nm ”sample is an artefact caused by the re flectivity measurement setup.)7215Z.Jehl et al./Thin Solid Films 519(2011)7212–7215。
