Human neural stem cell transplants to address multiple pathologies associated with traumat
干细胞与再生医学

需要重点看的概念1 embryonic stem cells, ES 胚胎干细胞2 Stem cells 干细胞3 hematopoietic stem cell 造血干细胞4 Neural stem cells (NSCs) 神经干细胞are initially present in a single layer of pseudostratified epithelium spanning the entire distance from the central canal to the external limiting membrane. NSCs continue to proliferate, and are patterned over several days in vivo to generate mature neurons, oligodendrocytes, and astrocytes. 神经干细胞起先呈现为单层假复层上皮,覆盖于整个中央管到外部的限制性膜。
神经干细胞能增殖,并在数天内产生成熟的神经元、星形胶质细胞和少突胶质细胞。
5 plasticity 可塑性一种成体干细胞具有生成另一个组织的特化细胞的能力,即成体干细胞具有一定跨系、甚至跨胚层分化的特性,称其为干细胞的可塑性,也称为成体干细胞的横向分化。
Transdifferentiation (plasticity of stem cell): means the adult stem cell from one embryonic layer can differentiate into cells derives from other layer.6 Human mesenchymal stem cells 人间充质干细胞7.fate mapping 干细胞命运图:在正常环境下受各种稳态因素调节的分化趋势。
这些趋势包括干细胞对机体正常发育活动的参与,以及干细胞对各种生物学危险诸如组织损伤、器官衰老以及疾病的反应。
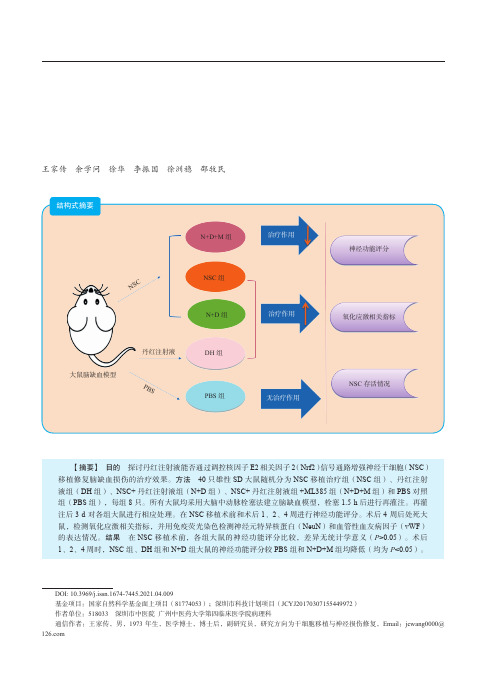
丹红注射液改善神经干细胞移植治疗脑缺血损伤效果的机制研究
N+D+M 组
治疗作用
ห้องสมุดไป่ตู้
NSC 组 N+D 组
治疗作用
神经功能评分 氧化应激相关指标
大鼠脑缺血模型
丹红注射液 PBS
DH 组 PBS 组
无治疗作用
NSC 存活情况
【摘要】 目的 探讨丹红注射液能否通过调控核因子 E2 相关因子 2(Nrf2)信号通路增强神经干细胞(NSC) 移植修复脑缺血损伤的治疗效果。方法 40 只雄性 SD 大鼠随机分为 NSC 移植治疗组(NSC 组)、丹红注射 液组(DH 组)、NSC+ 丹红注射液组(N+D 组)、NSC+ 丹红注射液组 +ML385 组(N+D+M 组)和 PBS 对照 组(PBS 组),每组 8 只。所有大鼠均采用大脑中动脉栓塞法建立脑缺血模型,栓塞 1.5 h 后进行再灌注。再灌 注后 3 d 对各组大鼠进行相应处理。在 NSC 移植术前和术后 1、2、4 周进行神经功能评分。术后 4 周后处死大 鼠,检测氧化应激相关指标,并用免疫荧光染色检测神经元特异核蛋白(NeuN)和血管性血友病因子(vWF) 的表达情况。结果 在 NSC 移植术前,各组大鼠的神经功能评分比较,差异无统计学意义(P>0.05)。术后 1、2、4 周时,NSC 组、DH 组和 N+D 组大鼠的神经功能评分较 PBS 组和 N+D+M 组均降低(均为 P<0.05)。
丹红注射液是一种广泛用于脑梗死、冠状动脉粥 样硬化性心脏病、肺源性心脏病、糖尿病周围神经病 变治疗的中药制剂 [12-16],具有改善血液循环、保护血 管内皮、抗炎、抗氧化应激损伤、抗神经元凋亡、保 护神经、促进神经元突触重塑、降低血清同型半胱氨 酸水平等作用。为了确定丹红注射液是否能通过调控 Nrf2 信号通路改变移植微环境,增强干细胞移植治疗 脑缺血损伤的效果,本研究建立大鼠脑缺血模型,探 讨丹红注射液协同干细胞移植治疗在脑缺血损伤中的 治疗作用。
人类神经干细胞的长期培养和传代_cropped

〃神经干细胞基础研究〃人类神经干细胞的长期培养和传代杨立业 刘相名 惠国桢 赵文娟 费俭 郭礼和【摘要】 目的 探讨人类神经干细胞的体外培养条件及其传代的方法 。
方法 采用机械方法从胎脑中分离神经细胞 , 应用 N2 培养基进行培养 , bFG F 和 EG F 刺激细胞扩增 ; 传统方法和对神经球切 割的方法进行传代培养 ; 应用免疫组织化学染色对培养的细胞及其分化的细胞进行鉴定 。
结果 从胎脑当中成功培养出人类的神经干细胞 , 培养条件下呈悬浮状态生长 , 形成神经球 , 绝大多数的细胞表 达波形蛋白和 Musashil 两种神经干细胞的标志物 ; 这种细胞可分化为神经元和星型胶质细胞 , 早期的 培养有少量的少突胶质细胞 ; 在这种培养条件下 , 神经干细胞生长速度较慢 , 而采用切割神经球的方 法保持了细胞间的联系 , 神经干细胞可获得较大的扩增速度 。
结论 在体外的培养条件下 , 可从胎脑 组织中培养出神经干细胞 , 它可做为中枢神经系统疾病移植治疗的潜在细胞来源 。
【关键词】 神经干细胞 人类 培养T he long 2term culture and p a ssa ging of human neural stem cells Y ANG Liye , L I U Xiangming , HUI G uozhen , et al . Department o f Neurosurgery , the First H ospital o f suihow Univ ersity , S u zhou 215007 , C hina【Abstract 】 O b jectiv e T o explore the cu lture cond itions for hu man neural stem cells and to investig ate th epassag ing method. M ethods The cells from the embry onic hu man cortex w ere mechanically dissociated. N2 med i 2 u m w as ad apted to cu lture the cells , bFG F and EG F w ere added to expand the cells , The cells w ere id entified b y im 2 munocytochemistry. R esults Neural stem cells from embry onic hu mans have b een su ccessf u lly cu ltured. Th ey formed typical neurosp heres in suspension , and the m ajorities of the cells expressed vimentin and Musashil , w hich w ere the markers for neural stem cells. The cu ltured cell cou ld d ifferentiated into neurons and astrocy tes. The neural stem cells mu ltiplied very slowly und er the cu lture cond itions , how ever the best expansion w as achieved w hen the neu 2 rosp heres w ere d isected into several parts and the cell link w as conserved when passag ing. Conclusio ns Hu man neural stem cells cou ld b e cu ltured from embry onic brains. They cou ld form the ty pical neurosp heres in suspension in vitr o , w hich may b e potential source for transplan tation in treating C NS disord ers in hu man s.【 K ey w ords 】 Neural stem cell Hu man Cu ltureco ΠBR L ) ; bFG F (碱性成纤维细胞生长因子) 、EG F(表 皮 生 长 因 子) 、多 聚 鸟 氨 酸 、层 粘 连 蛋 白 、肝素 、胰岛素 、腐胺 、转铁蛋白 、硒化钠和孕激素 ;抗波形蛋白单克隆抗体 (B D Bi osciences , 1 ∶100) ; 抗 Musashil 抗 体 ( 由 日 本 大 阪 大 学 Okano 教 授 惠 赠 , 1 ∶200 ) ; 抗 G FAP ( Dako , 1 ∶100 ) ; 抗 NF神经干细胞在体外有持续的增殖能力 , 是将来 移植细胞较为理想的来源 。
cell tracing techniques in

Cell Tracing Techniques in Stem Cell Transplantation Li Yan&Ying Han&Yuanlong He&Huahong Xie&Jingmei Liu&Lina Zhao&Jingbo Wang&Liuchun Gao&Daiming FanPublished online:8November2007#Humana Press Inc.2007Abstract Pluripotent stem cells have shown great thera-peutic promise because of their natural capacity to regen-erate damaged tissue.Likewise,autologous stem cells or genetically modified stem cells have already been suc-cessfully applied in animal or clinical experimental studies including cardiopathy,diabetic disease,system lupus ery-thema,pancreatic disease,and liver disease.In these studies regarding stem cell transplants in different diseases,iden-tifying the location of implanted cells and distinguishing them from endogenous cells is the first and most important step.Moreover,different tracing techniques were applied in different studies for their different sensitivity,dynamic range,convenience and reliability of their assays.There-fore,we will here review different tracing techniques and their applications in stem cell transplants,including both experiment studies and preclinical trials.Keywords Stem cells.Transplants.Tracing techniques Bone marrow(BM)derived stem cells have been known to possess the unique capacity for self-renewal and differen-tiation into hematopoietic and mesenchymal cell lineages [1].That this plasticity extended to nonhematopoietic line-ages including hepatic oval cells,hepatocytes,cholangiocytes [2,3],neurons[4],skeletal muscle cells[5],and epithelial cells[6]is a relatively new observation[7].Although all of these indicated that stem cells could be used for the regen-eration and reconstitution of damaged organs or tissues,to identify whether the transplanted stem cells could be located in the damaged organ or tissue or not is a preliminary step for stem cell based therapies.Currently,a series of stem cell labeling techniques are applied in stem cell transplants fields,which include the BrdU,fluorescent dye,green fluorescent protein,magnetic, and isotope labeling techniques,and others.Moreover, different tracing techniques applied in stem cell transplants were accorded with the different aims of the experiments and the characteristics of the stem cells.BrdU and fluorescent dye were firstly used as stem cell tracers for their convenience.However,their labeling in-tense will be gradually decreased with the prolonged time, and they can’t detect the labeled stem cell in vivo.In recent years,GFP is widely used for stem cell tracing for its stable expression,high specificity,and easily tracing in vivo,but it is limited by the fact that high levels of GFP have certain cell toxicity.MRI and isotope labeling tech-niques were attempted to trace the transplanted stem cells in vivo for their non-invasive tracking currently.However, MRI labeling technique can cause false positive results and isotope labeling was limited by the specific stem cell markers.Therefore,we here review different cell tracing techniques in stem cell transplants based on some com-mon characteristics.BrdUBromodeoxyuridine(5-bromo-2-deoxyuridine,BrdU)is a synthetic nucleoside,which is commonly used in the detec-Stem Cell Rev(2007)3:265–269DOI10.1007/s12015-007-9004-yLi Yan,Ying Han and Yuanlong He contributed equally to this work. L.Yan:Y.Han:Y.He:H.Xie:J.Liu:L.Zhao:J.Wang:L.Gao:D.Fan(*)Department of Gastroenterology,Xijing Hospital,Fourth Military Medical University,17Changle Western Road,Xi’an,Shaanxi Province710032,Chinae-mail:fandaim@L.YanDepartment of Gastroenterology,401Hospital of PLA,Qingdao,Shandong province266071,Chination of proliferating cells in living tissues.It can be incor-porated into the newly synthesized DNA of replicating cells during the S phase of the cell cycle,and antibodies specific to BrdU can be used to detect the incorporated chemical. Stem cells are proposed to segregate chromosomes asym-metrically during self-renewing divisions so that older (‘immortal’)DNA strands are retained in daughter stem cells whereas newly synthesized strands segregate to differentiating cells.Stem cells are also proposed to retain DNA labels,such as5-bromo-2-deoxyuridine(BrdU), either because they segregate chromosomes asymmetrically or because they divide slowly.Kopeal group once used BrdU to label bone marrow stem cells(BMSCs)and then transplanted them into the lateral cerebral ventricle of the new born mice.Twelve days later,they observed BrdU labeled bone marrow stem cells in the procerebrum and cerebellum of the recipient mice, and some transplanted BMSCs had differentiated into ma-ture astrocytes[8].BrdU was also applied to label BMSCs but were transplanted into the cerebral ischemic model,and the results showed that BrdU labeled stem cell migrated to the ischemic focus and transformed into neuron cells and gliocytes[9].However,in a current study,investigators administered BrdU to the newborn mice,which were treated with cyclophosphamide and granulocyte colony-stimulating factor,and normal adult mice for4–10days, followed by70days without BrdU.In each case,less than 6%of HSCs retained BrdU and less than0.5%of all BrdU-retaining haematopoietic cells were HSCs.Their results revealed that BrdU has poor specificity and sensitivity as an HSC marker,and HSCs cannot be identified on the basis of BrdU-label retention[10].Although BrdU was already used for cell tracing in stem cell transplant fields including myo-cardial,skin,neuron and liver disease,the purity of stem cells among BrdU-label-retaining cells has not been docu-mented in any tissue,and the‘immortal strand hypothesis’has not been tested in a system with definitive stem cell markers[11–14].Fluorescent DyeThere is a series of fluorescent dyes that can be used for cell tracing,which includes CM-DiI,CFSE,hochst33342, DAPI and PKH26.Among them,CM-DiI had a toxicity level of45–70%,and only3–4%of proliferating cells were stained on day35,whereas CFSE revealed clear cytoplas-mic coloring in proliferating cells with5–6%stained cells and a toxicity from55to90%dead cells[15].DNA-binding dye Hoechst33342can be used as a tracking dye for short-term(up to3day)cell migration experiments[16]. DAPI is a simple nuclear labeled dye with high labeling efficiency and without cell toxicity.However,it will cause false positive results after DAPI is released from dead cells. Therefore,it can only be used for short-term cell tracing.PKH26is a fluorescent marker that stains the cell membrane with the lowest level of toxicity,and can’t be transferred between labeled and non-labeled cells,or signif-icantly impact the function of cell proliferation and adhe-sion.Moreover,fluorescent dye PKH26can be detected even60days after transplants in vivo[17].Szilvassy group used fluorescent PKH26dye to label the progenitor cells to demonstrate the homing and engraftment properties of hematopoietic progenitor cells from murine bone marrow, mobilized peripheral blood,and fetal liver,and their re-sults demonstrated that PKH26(+)cells could be reisolated from the BM and spleen by fluorescence-activated cell sorting and assayed for in vitro3h later[18].A recent study reported that PKH26labeled hematopoietic stem cells (HSC)can differentiate into hepatocytes in the normal liver and in some pathologic environments.Moreover,they found PKH26labeled HSC could express hepatocyte-specific markers Alb and CK8,but did not express alpha-SMA in liver fibrosis[19].In addition,PKH26was also used in the stem cell transplants including retinal disease, myocardial disease,skeletal regeneration,and cerebral dis-ease in recent years[20–23].Taken together,PKH26is an ideal tool for both tracking and describing the biology of stem cells in vivo,and allows investigators to further study the biology of short-term and long-term repopulating cells. However,its fluorescent intensity will decrease gradually after it is distributed to daughter cells.Therefore,the main drawback of PKH26is the limited time for cell tracing[24]. Green Fluorescent ProteinGFP is a27-kDa monomeric protein isolated from jellyfish, and it has become popular as a reporter system in fixed and liver tissue since the cloning of its gene[25].GFP absorbs blue light and emits green fluorescence without exogenous substrates or cofactors and provides a convenient and effi-cient way to identify labeled cells.After the GFP transgenic mice were successfully established,GFP was found to be expressed in all the tissues of transgenic mice without any toxicity.Furthermore,whenever the bone marrow stem cells from transgenic mice are in differentiation or prolif-eration state,GFP is still expressed.However,the estab-lishment of transgenic mice is very complicated and highly costly.Therefore,vector mediated transfection was widely adopted for GFP labeling now.Recently,Yoo reported that a fluorescence of bacterial cells expressing a variant(GFPm)of the green fluorescent protein(GFP)was reduced to background levels by global replacement of the leucine residues of GFPm by5,5,5-trifluoroleucine,and the median fluorescence of cells express-ing the fluorinated protein was improved approximately 650-fold in comparison to that of cells expressing fluorinated GFPm[26].In current studies,Beaudry group reported that wild type and green GFP-bone marrow could repair the liver injury after transplanted into wild-type mice,and they detected the GFP expression in the transplanted cells [27].Zhang group constructed plasmid pEGFP-C2-TH (tyrosine hydroxylase)and then transfected it into8-day-cultured neuronal stem cells derived from bone marrow stem cells(NdSCs-D-BMSCs)to investigated the therapeutic effects of TH-transfected NdSCs-D-BMSCs on Parkinson's disease through different transplantation protocols.Their re-sults showed5days after plasmid pEGFP-C2-TH transfec-tion into NdSCs-D-BMSCs,GFP was expressed in62.1% of the cells and the rate of co-expression with TH was83.5% [28].In addition,GFP or GFPm was widely applied in car-diomyocytes regeneration[29,30],retinal pigment epi-thelium cell repair[31],and skin repair[32].Although GFP labeled stem cells or stem cells from GFP-transgenic mice are widely used in the stem cell tracing field,limi-tations in its application lie in:its difficulty in obtaining continuous,high performance,stable expressions of GFP clone;when the GFP is especially highly expressed and the growth of cells decreased,which indicates that high levels of GFP have a certain cell toxicity;Signals of GFP can’t be amplified,thus low expressions of stem cells can’t be detected,and quantitative analysis can’t be made.Lac-Z Reporter and Y Chromosome MarkerThe Lac-Z is one lac operon gene from Escherichia coli, which can catalyze lactose hydrolysis,cause the substrate to decompose,and then form a blue chemical compound.It is commonly used as a histochemical reporter to track trans-planted cells in vivo by cell transfection for its stabilized expression,easy detection,and no toxicity.In2003,Jeong group established experimental intracerebral hemorrhage (ICH)by intrastriatal administration of bacterial collagenase in adult rats.One day after surgery,the rats were randomly divided into2groups to receive intravenously either immor-talized Lac z-positive human NSCs(5×106cells in500μl, n=12)or the same amount of saline(n=13).Transplanted NSCs were detected by X-gal histochemistry orβ-gal immunohistochemistry with double labeling of GFAP, NeuN,neurofilament,or CNPase.Their results suggested that intravenously transplanted NSCs migrated selectively to the perihematomal areas and differentiated into neurons (≈10%ofβ-gal+cells)and astrocytes(≈75%)[33].Lac-Z reporter was also transfected into the adipose-derived stem cells(ADSCs)to repair the full-thickness cartilage in an ani-mal model.Autologous ADSCs were isolated and induced with growth medium and placed in a fibrin glue scaffold and into3×4-mm full-thickness chondral defects in rabbits with negative controls.Specimens were evaluated for early healing using transfection with the Lac Z,and Lac-Z gene products were identified in12of12experimental specimens,which exhibited a collagen type II:I protein ratio similar to that of normal rabbit cartilage[34].In a recent report,replication-defective adenoviral vector,AxCALacZ,which encodes the enzyme Escherichia coli beta-galactosidase,was applied to mouse olfactory epithelium by intranasal instillation,and 90days later,the Lac-Z gene product was expressed not only in the olfactory receptor neurons and their axons,but also in the olfactory bulbs[35].Y Chromosome MarkerThe Y chromosome marker was used for cell tracing, because the location of the implanted cells in recipients can be detected by FISH analysis after cells derived from male donors were transplanted into the female -pared with gene expressions of GFP and lac-Z,the Y chro-mosome tracing technique is a very simple process with a higher labeling efficiency.In2005,Crain used FISH to examine paraffin sections from female patients who had received bone marrow transplants from male donors and the results showed that Y-chromosomes labeled with Neurons and astrocytes were identified in the neocortex,hippocam-pus,striatum,and cerebellum[36].Theise group reported that hepatocytes could be derived from human bone mar-row cells by sex matched experiment.Biopsy and autopsy liver specimens from human recipients of therapeutic bone marrow or liver transplants,in which there was gender discordance between donor and recipient,were analyzed for marrow-derived hepatocytes and cholangiocytes using fluorescence in situ hybridization(FISH)for the Y chro-mosome to indicate cells that were engrafted from bone marrow-derived population of cells[2].In addition,the Y chromosome marker was widely used in stem cell trans-plants for cardiac disease,intestine disease,and skin injury to identify the implanted stem cells in recent years[37–39]. Although the Y chromosome marker shows a great stability and specificity for stem cell tracing in some sex-matched experiments,problems still lie in the Y chromosome marker: it can not be used in the autologous stem cell transplants field for it is limited only to sex-matched studies.Magnetic Resonance Contrast Media and Isotope LabelingMagnetic resonance imaging(MRI)may provide a unique tool for non-invasive tracking of transplanted cells.Gado-linium and ferric oxide are two common contrast mediaused for cell labeling in MRI.Gadolinium was first used for frog stem cell labeling and was clearly detectable by MRI. In2002,Modo reported that the distribution of modified GRID-labeled neuron stem cells identified by MRI and their results demonstrated that GRID-enhanced MRI can reliably identify transplanted stem cells and their migration in the brain[40].Currently,ferric oxide was widely used for contrast media,including Standard Superparamagnetic Iron Oxide(SSPIO)and superparamagnetic iron oxide (USPIO).Recently,there were several studies using MRI to trace the transplanted stem cells,mainly in heart or brain disease models[41–43].Their results showed that the transplanted stem cells could be readily detected in the heart or brain in vivo using non-invasive MRI techniques.Their results also showed that MRI can not only accurately identify the location of the transplanted stem cells but also can be used for quantitative analysis with relaxation rate.Although MRI provides a promising method for stem cell tracing,there are also some limitations for MRI tracing:false positive results can be caused after the contrast media is released from dead cells;the contrast media can not be distributed to the divided cells and thus the proliferated and differentiated cells can’t be traced by MRI.For stem cell therapy,one of the necessary steps before it can be pushed on into clinics is finding a non-invasive method to track stem cells implanted inside the human body.SPECT(single photon emission computed tomogra-phy)is a special type of emission computed tomography (ECT)scan that can be used to make detailed images that are highly sensitive to the location of the radioactive mate-rials inside the body.Currently,metabolism imaging,anti-body imagining,receptor imagining,and gene expression imagining are the four common labeling methods,and18F, 99Tcm,131I,125I,123I,and111In were the common isotopes applied in SPECT tracing.Although there were a few reports about SPECT being applied for stem cell tracing, especially in cardiac diseases[44,45],it is still limited by the specific stem cell markers and the imperfection of each technique.ConclusionIn conclusion,every cell tracing technique has its own advantages and disadvantages.For stem cell therapy,one of the necessary steps before it can be pushed on into clinics is to find a non-invasive method to track stem cells implanted inside human body.Therefore,a possible and feasible stem cell tracing technique established by the kinds of bone marrow stem cells,experiment aims,and current experi-ment conditions is essential.In the near future,with the development of cell tracing techniques,the application of stem cells derived from bone marrow will show an even wider prospective.Acknowledgements We would like to thank the members of the Tissue Engineering Lab(Jieshi Hu,Faming Chen,and Yan Jin)for helpful discussions concerning the topics presented in this review.The work about stem cell research was supported by Chinese National Foundation of National Sciences(30470788)and Natural Science Foundation of Shanxi Province(2006K09-G8).References1.Petersen,B.E.,Bowen,W.C.,Patrene,K.D.,Mars,W.M.,Sullivan,A.K.,Murase,N.,et al.(1999).Bone marrow as a potential source of hepatic oval cells.Science,284,1168–1170.2.Theise,N.D.,Nimmakayalu,M.,Gardner,R.,Illei,P.B.,Morgan,G.,Teperman,L.,et al.(2000).Liver from bone marrow in humans.Hepatology,32,11–16.3.Alison,M.R.,Poulsom,R.,Jeffery,R.,Dhillon,A.P.,Quaglia,A.,Jacob,J.,et al.(2000).Hepatocytes from non-hepatic adultstem cells.Nature,406,257.4.Li,Y.,Chen,J.,Wang,L.,Lu,M.,&Chopp,M.(2001).Treatment of stroke in rat with intracarotid administration of marrow stromal cells.Neurology,56,1666–1672.5.Ferrari,G.,Cusella-De,Angelis,G.,Coletta,M.,Paolucci,E.,Stornaiuolo,A.,Cossu,G.,et al.(1998).Muscle regeneration by bone marrow-derived myogenic progenitors.Science,279,1528–1530.6.Korbling,M.,Katz,R.L.,Khanna,A.,Ruifrok,A.C.,Rondon,G.,Albitar,M.,et al.(2002).Hepatocytes and epithelial cells ofdonor origin in recipients of peripheral-blood stem cells.New England Journal of Medicine,346,738–746.7.Dalakas,E.,Newsome,P.N.,Harrison,D.J.,&Plevris,J.N.(2005).Hematopoietic stem cell trafficking in liver injury.F ASEB Journal,19,1225–1231.8.Kopen,G.C.,Preckop,D.J.,&Phinney,D.G.(1999).Marrowstromal cells migrate throughout forebrain and cerebellum,and they differentiate into astrocytes after injection into neonatal mouse brains.Proceedings of the National Academy of Sciences of the United States of America,96,10711–10716.9.Chen,J.,Li,Y.,&Chopp,M.(2000).Intracerebral transplantationof bone marrow with BDNF after MCAo in rat.Neurepharmacol-ogy,39,711.10.Kiel,M.J.,He,S.,Ashkenazi,R.,Gentry,S.N.,Teta,M.,Kushner,J.A.,et al.(2007).Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU.Nature, 449,238–242.11.Hou,M.,Yang,K.M.,Zhang,H.,Zhu,W.Q.,Duan,F.J.,Wang,H.,et al.(2007).Transplantation of mesenchymal stem cells fromhuman bone marrow improves damaged heart function in rats.International Journal of Cardiology,115,220–228.12.Fu,X.B.,Fang,L.J.,Wang,Y.X.,Sun,T.Z.,&Cheng,B.(2004).Enhancing the repair quality of skin injury on porcine after autografting with the bone marrow mesenchymal stem cells.Chinese Journal of Medicine,84,920–924.13.Munoz,J.R.,Stoutenger,B.R.,Robinson,A.P.,Spees,J.L.,&Prockop,D.J.(2005).Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice.Proceedings of the National Academy of Sciences of the United States of America,102,18171–18176.14.Best,D.H.,&Coleman,W.B.(2007).Cells of origin of smallhepatocyte-like progenitor cells in the retrorsine model of rat liver injury and regeneration.Journal of Hepatology,46,1055–1063.15.Hemmrich,K.,Meersch,M.,von Heimburg,D.,&Pallua,N.(2006).Applicability of the dyes CFSE,CM-DiI and PKH26for tracking of human preadipocytes to evaluate adipose tissue engineering.Cells Tissues Organs,184,117–127.16.Hendrikx,P.J.,Martens,C.M.,Hagenbeek,A.,Keij,J.F.,&Visser,J.W.(1996).Homing of fluorescently labeled murine hematopoietic stem cells.Experimental Hematology,24,129–140.17.Ford,J.W.,Welling,T.H.,Stanley,J.C.,&Messina,L.M.(1996).PKH26and1251-PKH95:characterization and efficacy as labels for in vitro and in vivo endothelial cell localization and tracking.Journal of Surgical Research,62,23–28.18.Svilvassy,S.J.,Meyerrose,T.E.,Ragland,P.L.,&Grimes,B.(2001).Differential homing and engraftment properties of hematopoietic progenitor cells from murine bone marrow,mobi-lized peripheral blood,and fetal liver.Blood,98,2108–2115. 19.Zhan,Y.,Wang,Y.,Wei,L.,Chen,H.,Cong,X.,Fei,R.,et al.(2006).Differentiation of hematopoietic stem cells into hepato-cytes in liver fibrosis in rats.Transplantation Proceedings,38, 3082–3085.20.Canola,K.,Angenieux,B.,Tekaya,M.,Quiambao,A.,Naash,M.I.,Munier,F.L.,et al.(2007).Retinal stem cells transplanted intomodels of late stages of retinitis pigmentosa preferentially adopt a glial or a retinal ganglion cell fate.Investigative Ophthalmology and Visual Science,48,446–454.21.Boomsma,R.A,Swaminathan,P.D.,&Geenen,D.L.(2007).Intravenously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size.International Journal of Cardiology,122,17–28.22.Brzóska,E.,Grabowska,I.,Hoser,G.,Stremińska,W.,Wasilewska,D.,Machaj,E.K.,et al.(2006).Participation of stem cells fromhuman cord blood in skeletal muscle regeneration of SCID mice.Experimental Hematology,34,1262–1270.23.Chu,K.,Park,K.I.,Lee,S.T.,Jung,K.H.,Ko,S.Y.,Kang,L.,etal.(2005).Combined treatment of vascular endothelial growth factor and human neural stem cells in experimental focal cerebral ischemia.Neuroscience Research,53,384–390.24.Parish,C.R.(1999).Fluorescent dyes for lymphocyte migrationand proliferation studies.Immunology and Cell Biology,77,499–508.25.Shimomura,O.,Johnson,F.H.,&Saiga,Y.(1962).Extraction,purification and properties of aequorin,a bioluminescent protein from the luminous hydromedusan,Aequorea.Journal of Cellular and Comparative Physiology,59,223–239.26.Yoo,T.H.,Link,A.J.,&Tirrell,D.A.(2007).Evolution of afluorinated green fluorescent protein.Proceedings of the National Academy of Sciences of the United States of America,104, 13887–13890.27.Beaudry,P.,Hida,Y.,Udagawa,T.,Alwayn,I.P.,Greene,A.K.,Arsenault, D.,et al.(2007).Evolution of a fluorinated green fluorescent protein.Journal of Pediatric Surgery,42,1190–1198.28.Zhang,S.,Zou,Z.,Jiang,X.,Xu,R.,Zhang,W.,Zhou,Y.,et-al(2007).The therapeutic effects of tyrosine hydroxylase gene transfected hematopoetic stem cells in a rat model of Parkinson’s disease.Cellular and Molecular Neurobiology(in press),Aug23.29.Dai,W.,Field,L.J.,Rubart,M.,Reuter,S.,Hale,S.L.,Zweigerdt,R.,et al.(2007).Survival and maturation of human embryonic stem cell-derived cardiomyocytes in rat hearts.Journal of Molecular and Cellular Cardiology,43,504–516.30.Fujita,J.,Mori,M.,Kawada,H.,Ieda,Y.,Tsuma,M.,Matsuzaki,Y.,et-al.(2007).Administration of granulocyte colony-stimulating factor after myocardial infarction enhances the recruitment of hematopoietic stem cell-derived myofibroblasts and contributes to cardiac repair.Stem Cells(in press),Aug9.31.Li,Y.,Atmaca-Sonmez,P.,Schanie,C.L.,Ildstad,S.T.,Kaplan,H.J.,&Enzmann,V.(2007).Endogenous bone marrow derivedcells express retinal pigment epithelium cell markers and migrate to focal areas of RPE damage.Investigative Ophthalmology and Visual Science,48,4321–4327.32.Falanga,V.,Iwamoto,S.,Chartier,M.,Yufit,T.,Butmarc,J.,Kouttab,N.,et al.(2007).Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds.Tissue Engineering,13,1299–1312.33.Jeong,S.W.,Chu,K.,Jung,K.H.,Kim,S.U.,Kim,M.,&Roh,J.K.(2003).Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage.Stroke,34,2258–2263.34.Dragoo,J.L.,Carlson,G.,McCormick,F.,Khan-Farooqi,H.,Zhu,M.,Zuk,P.A.,et al.(2007).Healing full-thickness cartilage defects using adipose-derived stem cells.Tissue Engineering,13,1615–1621.35.Doi,K.,Nibu,K.,Ishida,H.,Okado,H.,&Terashima,T.(2005).Adenovirus-mediated gene transfer in olfactory epithelium and olfactory bulb:A long-term study.Annals of Otology,Rhinology &Laryngology,114,629–633.36.Crain,B.J.,Tran,S.D.,&Mezey,E.(2005).Transplanted humanbone marrow cells generate new brain cells.Journal of the Neurological Sciences,233,121–123.37.Jiang,W.,Ma,A.,Wang,T.,Han,K.,Liu,Y.,Zhang,Y.,et al.(2006).Intravenous transplantation of mesenchymal stem cells improves cardiac performance after acute myocardial ischemia in female rats.Transplant International,19,570–580.38.Kudo,K.,Abe,Y.,Hu,D.L.,Kijima,H.,&Nakane,A.(2007).Colonization and differentiation of transplanted embryonic stem cells in the irradiated intestine of mice.Tohoku Journal of Experimental Medicine,212,143–150.39.Deng,W.,Han,Q.,Liao,L.,Li,C.,Ge,W.,Zhao,Z.,et al.(2005).Engrafted bone marrow-derived flk-(1+)mesenchymal stem cells regenerate skin tissue.Tissue Engineering,11,110–119.40.Modo,M.,Cash,D.,Mellodew,K.,Williams,S.C.,Fraser,S.E.,Meade,T.J.,et al.(2002).Tracking transplanted stem cell migration using bifunctional,contrast agent-enhanced,magnetic resonance imaging.Neuroimage,17,803–811.41.Amsalem,Y.,Mardor,Y.,Feinberg,M.S.,Landa,N.,Miller,L.,Daniels,D.,et al.(2007).Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium.Circulation,116,138–145.42.Ebert,S.N.,Taylor,D.G.,Nguyen,H.L.,Kodack,D.P.,Beyers,R.J.,Xu,Y.,et-al.(2007).Non-invasive tracking of cardiac embryonic stem cells in vivo using magnetic resonance imaging techniques.Stem Cells(in press),Aug9.43.Rice,H.E.,Hsu,E.W.,Sheng,H.,Evenson,D.A.,Freemerman,A.J.,Safford,K.M.,et al.(2007).Superparamagnetic iron oxidelabeling and transplantation of adipose-derived stem cells in middle cerebral artery occlusion-injured mice.American Journal of Roentgenology,188,1101–1108.44.Tran,N.,Franken,P.R.,Maskali,F.,Nloga,J.,Maureira,P.,Poussier,S.,et al.(2007).Intramyocardial Implantation of bone marrow-derived stem cells enhances perfusion in chronic myo-cardial infarction:Dependency on initial perfusion depth and follow-up assessed by gated pinhole SPECT.Journal of Nuclear Medicine,48,405–412.45.Caveliers,V.,De Keulenaer,G.,Everaert,H.,Van Riet,I.,VanCamp,G.,Verheye,S.,et al.(2007).In vivo visualization of111In labeled CD133+peripheral blood stem cells after intracoronary administration in patients with chronic ischemic heart disease.Quarterly Journal of Nuclear Medicine and Molecular Imaging, 51,61–66.。
《自然》子刊:突破性进展!复旦团队首次将星形胶质细胞重编程成脊髓类器官,并修复脊髓损伤

《自然》子刊:突破性进展!复旦团队首次将星形胶质细胞重编程成脊髓类器官,并修复脊髓损伤让脊髓损伤的瘫痪患者重新站起来,一直是医学界最想要解决的难题之一。
目前,解决这一问题较为成熟的方法是移植各种具有神经修复功能的细胞到脊髓损伤处,如少突胶质细胞前体细胞[1]、神经干细胞[2]和人多能干细胞(hPSC)[3]等。
虽然这些方法对于改善脊髓损伤小鼠的运动功能具有一定的效果,但是或多或少都存在一定缺陷,如原始细胞获取困难、需要进行移植手术以及移植后的排斥反应等[4]。
近年来,科学家提出了一种新治疗策略来解决以上的问题,那就是直接在体内重编程神经胶质细胞,使其成为具有治疗脊髓损伤的能力[5]。
已有研究表明,通过基因编辑联合小分子化合物,人类和小鼠的星形胶质细胞可以在体外、体内有效地转化为不同种类、不同功能的神经元,但这些神经元无法增殖并形成类似神经系统结构的神经类器官,因而治疗潜力有限[6, 7]。
近日,来自复旦大学的邵志成团队在《自然·生物医学工程》杂志发表重磅研究成果,他们开发了将星形胶质细胞诱导成为神经类器官的方法(称为Op53-CSBRY),即在人星形胶质细胞中过表达OCT4(O)、抑制p53,以及添加小分子化合物CHIR99021(C)、SB431542(SB)、RepSox(R)和Y27632(Y)[8]。
更为重要的是,通过Op53-CSBRY方法生成的类器官,在添加bFGF、SAG和BMP激活脊髓发育相关信号通路后,可形成具有脊髓背侧和腹侧神经元功能的脊髓类器官。
在将脊髓类器官移植给脊髓损伤的小鼠后,类器官可存活并分化为脊髓神经元,并与宿主神经元形成突触,改善小鼠的运动功能。
该研究使得人星形胶质细胞在体内直接重编程为神经类器官,以修复神经系统损伤的设想往前迈出了一大步。
论文首页截图为了使神经胶质细胞形成神经类器官,研究人员首先在人星形胶质细胞中过表达OCT4(维持细胞多潜能性,为诱导产生多能干细胞的必要因子[9])的同时敲低p53(可促进细胞增殖[10])[以下称为Op53],但他们发现仅有少数星形胶质细胞可以重编程为MAP2+(神经元标志物)细胞。
银杏达莫注射液联合骨髓间充质干细胞移植改善脑梗死后的神经功能

银杏达莫注射液联合骨髓间充质干细胞移植改善脑梗死后的神经功能杨朝阳【摘要】背景:通过细胞移植重建损伤脑组织成为治疗脑梗死的新途径,骨髓间充质干细胞成为近年来细胞移植治疗领域的研究热点。
<br> 目的:探讨银杏达莫注射液联合骨髓间充质干细胞移植对脑梗死大鼠神经功能的改善作用及相关机制。
<br> 方法:利用线栓法制作大鼠大脑中动脉闭塞模型,建模成功后60只SD大鼠随机分为对照组、细胞移植组及联合组。
对照组尾静脉注射PBS、细胞移植组尾静脉注射2.5×109 L-1的骨髓间充质干细胞悬液、联合组尾静脉注射2.5×109 L-1的骨髓间充质干细胞悬液和银杏达莫2 mL/kg,1次/d,连续注射5 d。
于移植后的1,3 d及1,2周进行mNSS行为学评分,以观察大鼠神经功能缺损状况。
移植后2周RT-PCR检测脑组织中脑源性神经生长因子、生长相关蛋白43基因表达变化,TUNEL法检测细胞凋亡情况,免疫组化法检测BrdU阳性细胞数。
<br> 结果与结论:移植后的1,3 d各组大鼠神经功能缺损评分差异无显著性意义(P >0.05),在移植后1,2周,联合组神经功能缺损评分低于细胞移植组及对照组(P <0.05);移植后2周,联合组脑源性神经生长因子、生长相关蛋白43 mRNA表达明显高于细胞移植组及对照组(P<0.05),联合组凋亡细胞数目明显少于细胞移植组及对照组(P <0.05),联合组BrdU阳性细胞数量明显多于细胞移植组及对照组(P <0.05)。
结果表明骨髓间充质干细胞联合银杏达莫干预能促进脑梗死组织脑源性神经生长因子、生长相关蛋白43 mRNA的表达,抑制细胞凋亡,改善大鼠神经功能。
%BACKGROUND:Reconstruction of damaged brain tissue through cel transplantation has become a new way to treat cerebral infarction. In recent years, bone marrow mesenchymal stem celshave become the new darling in cel transplantation therapy. <br> OBJECTIVE:To investigate the effect of ginkgo-damole injection combined with bone marrow mesenchymal stem cel transplantation to improve the neurological function of acute cerebral infarction rats and its mechanism. <br> METHODS:Animal models of middle cerebral artery occlusion were made in rats using suture method, and then 60 rat models were randomly divided into control group, cel transplantation group and combination group. The control group was given intravenous injection of PBSvia the tail vein; the cel transplantation group was given intravenous injection of bone marrow mesenchymal stem cel suspension (2.5×109/L) via the tail vein; the combination group was given intravenous injection of bone marrow mesenchymal stem cel suspension (2.5×109 /L) and ginkgo-damole injection (2 mL/kg, once a day, totaly 5 days)via the tail vein. Modified neurological severity scores were recorded at 1, 3 days and 1, 2 weeks after transplantation. At 2 weeks after transplantation, expressions of brain-derived neurotrophic factor and growth associated protein 43 in the brain were detected using RT-PCR; cel apoptosis detected using MTT assay; BrdU positive cels counted using <br> immunohistochemistry method.<br> RESULTS AND CONCLUSION:There were no differences in the modified neurologic severity scores among the three groups at 1, 3 days after transplantation (P > 0.05), but the modified neurological severity scores in the combination group were lower than those in the cel transplantation group and control group at 1, 2 weeks after transplantation (P < 0.05). The expressions of brain-derived neurotrophicfactor and growth associated protein 43 in the brain were significantly higher in the combination group than the other two groups at 2 weeks after transplantation (P < 0.05); compared with the other two groups, the number of apoptotic cels was less but the number of BrdU positive cels was higher in the combination group (P < 0.05). These findings indicate that the combination of ginkgo-damole injection and bone marrow mesenchymal stem cel transplantation can increase the expressions of brain-derived neurotrophic factor and growth associated protein 43 in the brain, inhibit cel apoptosis and improve neurological function in rats with cerebral infarction.【期刊名称】《中国组织工程研究》【年(卷),期】2015(000)050【总页数】6页(P8108-8113)【关键词】干细胞;移植;银杏达莫注射液;骨髓间充质干细胞;干细胞移植;脑源性神经生长因子;GAP-43;脑梗死【作者】杨朝阳【作者单位】济源市人民医院普内科,河南省济源市 454000【正文语种】中文【中图分类】R394.2文章亮点:1“干细胞循环”理论与中医理论中的“活血化瘀”法与有相通之处,银杏达莫注射液是从中药银杏叶中提取的复方制剂,能清除自由基、改善血液循环。
造血干细胞捐献志愿者的入库流程

造血干细胞捐献志愿者的入库流程英文回答:The process of stem cell donation for the purpose of storage involves several steps. I will walk you through the process in detail.Step 1: Registration.The first step to become a volunteer stem cell donor is to register with a stem cell donor registry. In my case, I registered with the local registry called the National Marrow Donor Program (NMDP). This can be done online or by visiting a registration center. During the registration process, I provided my personal information, medical history, and consent for the collection and storage of my stem cells.Step 2: Health Screening.Once registered, I was contacted by the registry to schedule a health screening. This screening is necessary to ensure that I am in good health and eligible to donate stem cells. The screening involved a comprehensive medical questionnaire, physical examination, and blood tests. The blood tests checked for infectious diseases, tissue typing, and other factors that could affect the compatibility of my stem cells with potential recipients.Step 3: Tissue Typing.After the health screening, my blood sample was sent to a laboratory for tissue typing. Tissue typing, also known as human leukocyte antigen (HLA) typing, is a process of identifying the unique genetic markers on the surface of cells. This information is crucial for matching potential donors with recipients. The laboratory analyzed my blood sample to determine my HLA type and entered the information into the registry's database.Step 4: Waiting.Once my HLA type was determined, I became part of the registry's database, and my information was made available to transplant centers worldwide. I had to wait until a patient in need of a stem cell transplant matched my HLA type. The waiting period could range from a few weeks to several years, depending on the rarity of my HLA type and the demand for stem cell donors.Step 5: Matching and Compatibility Testing.When a potential match was found, I was contacted by the registry and asked to undergo further compatibility testing. This involved additional blood tests and sometimes a cheek swab to collect DNA samples. The tests were performed to confirm the compatibility between my stemcells and the recipient's immune system. If the compatibility tests were successful, the donation process would proceed.Step 6: Donation.There are two main methods of stem cell donation:peripheral blood stem cell (PBSC) donation and bone marrow donation. PBSC donation is the most common method and involves the collection of stem cells from the bloodstream. Before the donation, I received injections of a medication called filgrastim to increase the number of stem cells in my bloodstream. During the donation, my blood was drawn through a needle in one arm, passed through a machine that separated the stem cells, and then returned to my body through a needle in the other arm. The process took several hours and was done on an outpatient basis.Bone marrow donation, on the other hand, is a surgical procedure that involves the extraction of stem cells directly from the bone marrow. This method is less common and usually reserved for cases where PBSC donation is not feasible or preferred.Step 7: Follow-up.After the donation, I received follow-up care to monitor my recovery and ensure my well-being. The registry provided support and guidance throughout the process, and Iwas encouraged to stay in touch and update my contact information in case further testing or donations were needed in the future.中文回答:造血干细胞捐献志愿者的入库流程包括以下几个步骤。
感染相关英文词汇-17曲霉感染

感染相关英文词汇小结真菌感染(15.7曲霉感染)1.Aspergillosis曲霉病(由曲霉属真菌引起的传染病,多见于鸟类)2.collective term集合名词3.any one of -35 pathogenic and allergenic species of Aspergillus由曲霉菌属中约35个致病、致敏的菌种引起4.grow at 37°C can cause invasive infection 在37°C生长的曲霉可造成侵袭性感染5. A. fumigatus烟曲霉6.chronic aspergillosis慢性曲霉菌病7. A. flavus黄曲霉8.cutaneous infections and keratitis皮肤感染及角膜炎9. A. niger黑曲霉10.colonizes the respiratory tract and causes external otitis定植于呼吸道,还可造成外耳炎11. A. terreus土曲霉12.with a poor prognosis预后不良1 13. A. nidulans构巢曲霉14.chronic granulomatous disease慢性肉芽肿病15.decomposing plant materials腐败的植物post堆肥17.hyaline (nonpigmented)透明的(无色素的)18.septate有隔膜的19.branching mold分枝霉菌20.conidia (spores)抱子21.mycelial growth菌丝生长22.indoor and outdoor air室内外空间均有23.Daily exposures vary from a few to many millions of conidia每日接触的抱子可有数个至百万不等24.hay barns谷仓25.dusty environments肮脏的环境26.The required size of the infecting inoculum is uncertain;致病所需菌种数量不明确27.intense exposures (e.g., during construction work, handling of moldy bark or hay, or composting)大量摄入(如:在建筑工地,接触并操作长霉木材干柴,堆肥)28.healthy immunocompetent individuals 免疫功能正常的健康人29.Allergic syndromes may be exacerbated by continuous antigenic exposure arising from sinus or airway colonization or from nail infection由窦道、呼吸道定植或指甲接触导致的长期抗原接触可加重过敏症状30.High-efficiency particulate air (HEPA)filtration高效微粒空气过滤31.protective against infection 防止感染32.monitored for efficiency 随时监测(HEPA)的效率33.The incubation period of invasive aspergillosis after exposure is highly variable, extending in documented cases from 2 to 90 days 致病菌大量暴露后,侵袭性曲霉病的潜伏期长短不一,从已有记录的病例来看,最短2 天,最长90天34.Thus community-acquired acquisition of an infecting strain frequently manifests as invasive infection during hospitalization, although nosocomial acquisition is also common.因此,社区获得性感染菌可能是早期入院期间获得的感染,虽然社会获得的可能性也存在35. a contaminated air source 污染的空气36.The primary risk factors 最主要的风险因素37.profound neutropenia and glucocorticoid use 严重的粒细胞缺乏或糖皮质激素使用38.risk increases with longer duration of these conditions随着上述情况时间的延长,风险升高39.neutrophil and/or phagocyte dysfunction 中性粒细胞和/或巨噬细胞功能不全40.relapsed leukemia 复发的白血病41.temporary abrogation of protective responses as a result of glucocorticoid use or a general anti-inflammatory state is a significant risk factor由于使用糖皮质激素或处于抗炎状态,使得机体保护反应暂时缺失,是(曲霉病)的危险因素42.prior pulmonary disease 既往肺部疾病43.sinusitis 鼻窦炎44.Anti-tumor necrosis factor therapy alsocarries an increased risk of infection 抗肿瘤坏死因子的治疗也回提升感染的风险45.underlying pulmonary disease 基础肺部疾病46.tuberculosis 结核47.sarcoidosis 结节病48.Glucocorticoids accelerate disease progression 糖皮质激素加速病程进展49.Allergic bronchopulmonary aspergillosis (ABPA) 变应性支气管肺曲菌病50.Invasive pulmonary aspergillosis 侵袭性肺曲霉病51.Invasive aspergillosis is arbitrarily divided into acute and subacute forms that have courses of W 1 month and 1 - 3 months, respectively.侵袭性曲霉病主要分为急性型(病程<1 个月)和亚急性型(病程1-3月)。
人类胚胎干细胞体外诱导分化为神经上皮细胞及多巴胺能神经元的初步研究的开题报告

人类胚胎干细胞体外诱导分化为神经上皮细胞及多巴胺能
神经元的初步研究的开题报告
1. 研究目的
人类胚胎干细胞 (human embryonic stem cells, hESCs) 可以分化成各种不同类型的细胞,是研究细胞发生和分化的重要模型。
本研究旨在通过体外诱导分化的方法,将人类胚胎干细胞分化成神经上皮细胞和多巴胺能神经元,为神经疾病的治疗提供新的治疗策略。
2. 研究方法
2.1 细胞培养
使用已知的方法将人类胚胎干细胞 (hESCs) 培养至适当的培养状态。
2.2 神经上皮细胞的诱导分化
将 hESCs 用化学试剂诱导分化成神经上皮细胞,包括添加 BMP 、FGF 和 Noggin 等生长因子。
2.3 多巴胺能神经元的诱导分化
使用已知的方法将神经上皮细胞诱导分化成多巴胺能神经元,包括添加 Sonic hedgehog (SHH),FGF8 和 Follicle stimulating hormone (FSH) 等因子。
2.4 细胞鉴定
使用流式细胞术、免疫印迹等方法对分化后的神经上皮细胞和多巴胺能神经元进行鉴定。
3. 预期结果
预期通过上述方法,成功分化出稳定的神经上皮细胞和多巴胺能神经元群体,初步明确其在某些神经疾病治疗中的潜在应用价值。
4. 意义和价值
本研究将有助于进一步探究人类胚胎干细胞的分化规律和分化诱导方法,同时将为神经疾病的治疗提供新的治疗策略,具有重要的理论和应用价值。
人体器官在生科技英语作文

人体器官在生科技英语作文**Title: The Marvels of Biotechnology in Human Organ Regeneration**In the realm of biotechnology, the prospect of human organ regeneration stands as a beacon of hope, revolutionizing medical treatments and offering unprecedented avenues for healing. This cutting-edge field combines advances in genetics, tissue engineering, and stem cell research to challenge the traditional limitations imposed by organ transplantation and donor shortages. By harnessing the body's innate regenerative capabilities, scientists aim to regenerate damaged or lost organs, thereby alleviating suffering and enhancing the quality of life for millions worldwide.Stem cells, often referred to as the building blocks of life, play a pivotal role in this endeavor. These versatile cells can differentiate into any type of cell in the body, presenting a limitless resource for tissue repair and organ reconstruction. Through careful manipulation, researchers induce these cells to form three-dimensional organoids—miniature, functional versions of organs—that mimic the structure and function of their full-sized counterparts.Tissue engineering further propels this progress by creating scaffolds that guide cell growth into the precise architecture of the targeted organ. Using biomaterials, these scaffolds provide the necessary support for cells to grow and organize, gradually being absorbed as the new tissue matures. Genetic editing tools, like CRISPR-Cas9, also contribute by correcting genetic defects responsible for organ failure, ensuring the regenerated organs function optimally.The successful implementation of such technologies promises not only to alleviate the burden of organ waiting lists but also to reduce the risk of organ rejection, as the regenerated organs would be derived from the patient's own cells. This personalized medicine approach ushers in a new era of healthcare, where treatment is tailored to the individual's unique genetic makeup, significantly improving therapeutic outcomes.Moreover, the potential economic impact is substantial. The high costs associated with organ transplants andlifelong immunosuppressant therapies could be dramatically reduced. By fostering self-healing, biotechnology in organ regeneration contributes to a more sustainable and cost-effective healthcare system.In conclusion, the fusion of biotechnology with human organ regeneration is reshaping the landscape of medical science. As research progresses, the dream of regenerating complex organs becomes increasingly attainable, marking a monumental leap towards enhancing human longevity and well-being. The future of medicine lies in these advancements, offering a testament to humanity's unwavering pursuit of healing and the boundless potential of scientific innovation.---**人体器官再生中的生物技术奇迹**在生物技术领域,人类器官再生的前景如同希望的灯塔,革新了医疗手段,为治疗开辟了前所未有的途径。
淋巴瘤 移植标准

淋巴瘤移植标准Non-Hodgkin lymphoma is a type of cancer that affects the lymphatic system, which is a crucial part of the body's immune system. It can develop in various parts of the body, including the lymph nodes, bone marrow, spleen, and other organs. For patients with advanced-stage non-Hodgkin lymphoma who have failed conventional treatments, a stem cell transplant may be considered as a treatment option.非霍奇金淋巴瘤是一种影响淋巴系统的癌症类型,淋巴系统是人体免疫系统的重要组成部分。
它可以在体内的各个部位发展,包括淋巴结、骨髓、脾脏和其他器官。
对于晚期非霍奇金淋巴瘤且经过传统治疗失败的患者来说,干细胞移植可能被考虑作为一种治疗选择。
Stem cell transplant, also known as a bone marrow transplant, involves replacing the patients' damaged or diseased bone marrow with healthy stem cells. These stem cells can come from the patient themselves (autologous transplant) or from a donor (allogeneic transplant). The goal of the transplant is to help the patient'simmune system recover after high-dose chemotherapy and/or radiation therapy, which is used to kill cancer cells.干细胞移植,也称为骨髓移植,涉及将患者受损或患病的骨髓替换为健康的干细胞。
《人诱导多能干细胞》团体标准

《人诱导多能干细胞》团体标准人诱导多能干细胞是一种重要的细胞工程技术,它可以将成体细胞重新编程为多能干细胞,具有巨大的临床应用前景。
本文将从人诱导多能干细胞的定义、发现历程、应用前景以及团体标准等方面进行详细介绍。
人诱导多能干细胞(induced pluripotent stem cells,iPSCs)是指通过基因转导等手段,将成体细胞重新编程为类似于胚胎干细胞的多能干细胞。
与传统的胚胎干细胞相比,人诱导多能干细胞无需侵入性手术获取,避免了伦理道德问题,具有更广泛的来源和更好的应用前景。
人诱导多能干细胞的发现历程可以追溯到2006年,当时日本科学家山中伦也等人通过转导4种基因,成功将小鼠成纤维细胞转化为多能干细胞,并命名为iPS细胞。
这一突破性发现引起了全球科学界的广泛关注和研究热潮。
随后,研究人员又成功将这一技术应用于人类细胞,并在2007年取得了重要突破。
人诱导多能干细胞具有广泛的临床应用前景。
首先,它可以解决传统胚胎干细胞获取过程中的伦理道德问题,为干细胞研究提供了新的方向。
其次,人诱导多能干细胞可以作为疾病模型进行研究,帮助科学家深入了解疾病发生机制,并开发新的治疗方法。
此外,它还可以用于药物筛选、组织工程和再生医学等领域,为临床医学带来革命性的变革。
为了规范和推动人诱导多能干细胞的研究和应用,国际科学界制定了一系列团体标准。
首先,对于iPSCs的制备过程,要求严格遵循操作规范和实验室安全要求,确保实验结果的准确性和可重复性。
其次,对于iPSCs的鉴定和鉴别,要求使用标准化的检测方法,确保其真实性和稳定性。
此外,在iPSCs的应用过程中,还要遵循伦理原则和法律法规,保护受试者的权益和安全。
此外,为了促进国内外学术界和产业界在人诱导多能干细胞领域的交流与合作,各国科学家还建立了多个国际合作组织和学术会议。
这些组织和会议不仅提供了一个交流平台,还推动了技术的进一步创新和应用。
总之,人诱导多能干细胞作为一种重要的细胞工程技术,在医学和生物科学领域具有巨大的潜力。
9(单选题2分)Timothy Ray Brown, thH

9(单选题2分)Timothy Ray Brown, thH 单选题(2分)
Timothy Ray Brown, the first man cured of HIV, initially opted against the stem cell transplant that ______ history.
A.could have later made。
B.should have made later。
C.might make later。
D.would later make。
翻译:一个人,首个治愈HIV的人。
起初选择反对“造血干细胞移植”that能够改变历史。
AB都是「虚拟态」,均「未发生的事情」,但用法上,有区别,A.could have done,「与过去事实相反」,或者「对过去事情的推测」。
B.should have done,理应做某事但实际上却没做某事。
翻译过后,能get到语境——治疗HIV与“造血干细胞”有关,而进行相关的实验研究,有助于人类攻克人体免疫缺陷疾病。
选AB 挺离谱的,我靠语感感觉的,提高效率,不多说了。
如果有小伙伴,且会有小伙伴,通过语境而写这个句子时,能用到“A/B”的话,非要去死磕“改变历史”就是「与过去事实相反」/「对过去事情的推测」/「理应做某事但实际上却没做某事」,感觉没有错呀,进而死结、无解。
可以交流交流。
或者补补语法知识,补补相关时态的用法习惯。
C.D放在一起,就要讲「difference」,而不是「similarity」。
浅谈干细胞移植的护理方法

浅谈干细胞移植的护理方法发表时间:2013-07-30T16:15:13.200Z 来源:《中外健康文摘》2013年第21期供稿作者:孙晓彤[导读] “知情同意”是现代医学伦理学中的一项基本原则,亦是医疗实践中一个重要的法律概念。
孙晓彤(辽宁省大连大学附属中山医院VIP1病房辽宁大连 116001)【中图分类号】R473.6 【文献标识码】B【文章编号】1672-5085(2013)21-0316-01 【摘要】干细胞是一类具有自我复制能力的多潜能细胞。
生物医学服务中心A57部资料显示干细胞是一种未充分分化,尚不成熟的细胞,具有再生各种组织,器官和人体的潜在功能,医学界称为“万用细胞”。
所以干细胞移植对治愈人类许多疾病及延年益寿有重要的意义。
【关键词】干细胞移植护理【Abstract】 The stem cell is one kind has the self-duplication ability multi-potential cell. The biomedicine service center A57 material demonstration stem cell is one kind of not full differentiation, still not the mature cell, had regenerates each kind of organization organ and human body's latent function, the medical arena is called “the multi-purpose cell”. Therefore the stem cell transplant to cures human many illness to get sick and to prolong the life has the vital significance. 【key word】Stem cell Transplant Nurses采用干细胞治疗有着多种优势:低毒性(或无毒性),即使不完全了解疾病发病的确切机理,治疗也可达到较好的治疗效果。
肝衰竭治疗新策略——间充质干细胞移植

肝衰竭治疗新策略——间充质干细胞移植朱传龙;张永婷【摘要】肝衰竭是一种严重的临床病症,目前尚缺乏有效治疗,亟待寻找一种新的有效的治疗手段.间充质干细胞,尤其是脐带间充质干细胞,对急性肝功能衰竭具有治疗作用,然而移植后细胞的归巢将影响其治疗效果.文章通过回顾近年来间充质干细胞治疗肝衰竭基础与临床研究成果,提出今后本领域的发展方向,以干细胞归巢为切入点,提高间充质干细胞移植治疗肝衰竭疗效.%Liver failure is a severe clinical syndrome and hitherto lack of effective treatment. Large numbers of studies have shown that mesenchymal stem cells (MSCs),especially umbilical cord MSCs,have a therapeutic effect on acute liver failure. Yet,the homing of MSCs in vivo affects the effectiveness of engraftment. The author presents an overview of the results of recent basic and clini-cal studies on the treatment of liver failure with MSCs and proposes a direction of development in this field,hoping to give some enlight-enment to the postgraduates and clinicians of hepatology.【期刊名称】《医学研究生学报》【年(卷),期】2018(031)006【总页数】4页(P561-564)【关键词】肝衰竭;间充质干细胞;归巢【作者】朱传龙;张永婷【作者单位】210029 南京,南京医科大学第一附属医院感染内科;210029 南京,南京医科大学第一附属医院感染内科【正文语种】中文【中图分类】R5750 引言肝衰竭是多种因素引起的严重肝损伤,伴有严重肝功能功能障碍或失代偿,从而导致凝血机制障碍、黄疸、肝性脑病、腹水等的临床症候群[1]。
人体器官在生科技英语作文

人体器官在生科技英语作文The Human Body: A Remarkable Machine in the Age of BiotechnologyThe human body is a marvel of nature, a complex system of interconnected organs and systems that work in harmony to sustain life. In the age of biotechnology, our understanding of the human body has reached new heights, allowing us to explore the intricacies of its structure and function like never before. From the intricate workings of the cardiovascular system to the remarkable adaptability of the nervous system, the human body continues to captivate scientists and researchers around the world.One of the most fascinating aspects of the human body is the cardiovascular system. This intricate network of blood vessels, including arteries, veins, and capillaries, is responsible for transporting oxygen, nutrients, and waste throughout the body. The heart, the central organ of this system, is a remarkable pump that beats approximately 100,000 times per day, ensuring a constant supply of blood to every cell in the body. Advances in biotechnology have allowed us to better understand the underlying mechanisms of the cardiovascular system, leading to the development of innovativetreatments and therapies for a wide range of heart-related conditions.Another remarkable system within the human body is the respiratory system. This system is responsible for the exchange of gases, allowing us to take in oxygen and expel carbon dioxide. The lungs, the primary organs of the respiratory system, are incredibly efficient at this task, with millions of tiny air sacs called alveoli that facilitate the exchange of gases. Biotechnology has played a crucial role in understanding the mechanisms of the respiratory system, leading to the development of advanced respiratory therapies and devices, such as artificial lungs and ventilators.The nervous system is another remarkable component of the human body, responsible for coordinating and regulating the various functions of the body. This complex network of neurons and nerve fibers is capable of transmitting electrical signals at lightning-fast speeds, allowing us to perceive and respond to our environment. Advances in biotechnology have led to a deeper understanding of the nervous system, paving the way for the development of cutting-edge technologies, such as brain-computer interfaces and neural prosthetics.The endocrine system is another crucial component of the human body, responsible for the production and regulation of hormones.These chemical messengers play a vital role in a wide range of bodily functions, from growth and development to metabolism and reproduction. Biotechnology has enabled researchers to better understand the intricate workings of the endocrine system, leading to the development of new treatments and therapies for hormone-related disorders.The immune system is another remarkable aspect of the human body, serving as our primary defense against a wide range of pathogens and diseases. This complex system of cells, tissues, and organs works tirelessly to identify and neutralize threats to our health, from bacteria and viruses to cancer cells. Biotechnology has played a crucial role in our understanding of the immune system, leading to the development of groundbreaking treatments, such as immunotherapies and vaccines.The digestive system is another critical component of the human body, responsible for the breakdown and absorption of the nutrients we consume. This system includes a diverse array of organs, from the mouth and esophagus to the stomach and intestines, all working in harmony to ensure that our bodies receive the necessary fuel and resources. Biotechnology has enabled researchers to better understand the complex mechanisms of the digestive system, leading to the development of new treatments and therapies for a wide range of gastrointestinal disorders.The urinary system is another essential component of the human body, responsible for the filtration and elimination of waste products from the body. This system includes the kidneys, ureters, bladder, and urethra, all working together to ensure that our bodies remain in a state of balance. Biotechnology has played a crucial role in our understanding of the urinary system, leading to the development of advanced diagnostic tools and treatments for kidney and bladder-related conditions.The reproductive system is another remarkable aspect of the human body, responsible for the production and maturation of reproductive cells, as well as the development and maintenance of secondary sexual characteristics. This system includes a diverse array of organs, from the gonads to the external genitalia, all working in harmony to ensure the continuation of the human species. Biotechnology has played a crucial role in our understanding of the reproductive system, leading to the development of advanced fertility treatments and reproductive technologies.In conclusion, the human body is a remarkable machine, a complex and interconnected system of organs and systems that work together to sustain life. In the age of biotechnology, our understanding of the human body has reached new heights, enabling us to develop innovative treatments and therapies for awide range of health conditions. From the intricate workings of the cardiovascular system to the remarkable adaptability of the nervous system, the human body continues to captivate scientists and researchers around the world, inspiring us to push the boundaries of what is possible in the field of biotechnology.。
Differentiation of Human Pluripotent Stem Cells into Retinal Cells

87M.A. Hayat (ed.), Stem Cells and Cancer Stem Cells, Volume 6,DOI 10.1007/978-94-007-2993-3_9, © Springer Science+Business Media B.V . 20129A bstractRetinal and macular degeneration disorders are characterized by a progressive loss of photoreceptors, which causes visual impairment and blindness. In some cases, the visual loss is caused by dysfunction, degen-eration and loss of underlying retinal pigment epithelial (RPE) cells and the subsequent death of photoreceptors. The grim reality is that there is no successful treatment for most of these blindness disorders. Cell therapy aimed at replenishing the degenerating cells is considered a potential ther-apeutic approach that may delay, halt or perhaps even reverse degenera-tion, as well as improve retinal function and prevent blindness in the aforementioned conditions. Human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSCs) may serve as an unlimited donor source of photoreceptors and RPE cells for transplantation into degenerat-ing retinas and for retinal disease modeling.I ntroductionThe vertebrate eyes form as bilateral evaginations of the forebrain, called optic vesicles (Martínez-Morales et al. 2004 ; Fig. 9.1a ). During develop-ment, the optic vesicles begin to invaginate to form a cup-shaped structure, the optic cup. The inner, thicker neural layer of the optic cup differ-entiates into the neural retina, and the outer, thin-ner pigmented layer forms the retinal pigmentepithelium (RPE). At the early developmental stages, the neuroepithelial cells that compose the optic vesicle are morphologically and molecu-larly identical and are all able to give rise to neu-ral retina and RPE. Exogenous signals coming from the adjacent tissues, including factors from the fi broblast growth factor (FGF) and transform-ing growth factor beta (TGF b ) families, dictate the fate of these cells. The mature vertebrate ret-ina is comprised of six types of neurons and one type of glia (the Müller glia). These seven cell types constitute three nuclear layers: retinal gan-glion cells in the ganglion cell layer (GCL); the horizontal, bipolar and amacrine interneurons, and Müller glial cells in the inner nuclear layer (INL); and rod and cone photoreceptors in the outer nuclear layer (ONL; Harada et al. 2007;M . I delson • B . R eubinoff (*)T he Hadassah Human Embryonic Stem Cell Research Center, The Goldyne Savad Institute of Gene Therapy & The Department of Obstetrics and Gynecology , H adassah University Medical Center ,E in Kerem 12000 ,J erusalem 91120 ,I srael e -mail: b enjaminr@ekmd.huji.ac.il D ifferentiation of HumanPluripotent Stem Cells into Retinal Cells Masha Idelson and Benjamin Reubinoff88M. Idelson and B. ReubinoffFig. 9.1b ). The photoreceptor cells capture lightphotons and transform their energy into electrical signals by a mechanism called phototransduction. The visual pigment which is utilized in this process is located on membranal discs in the outer seg-ments of photoreceptors. The outer segments are continuously renewed: the old discs are shed and new disks form. When the photoreceptors absorb light, they send the signal through the retinal interneurons to the ganglion cells which transmit the electrical impulse to the brain by their axons forming the optic nerve. Rods are responsible for night vision, whereas cones are responsible for color vision and detecting fi ne details. The macula is a small part of the retina which is rich in cones and responsible for detailed central vision.R PE cells that compose the outer layer of the optic cup are pigmented cuboidal cells which lie between the neural retina and the choriocapil-laris, which include the blood vessels supplying the retina. The multiple villi on their apical side are in direct contact with the outer segments ofextraocular mesenchymeabneural retinalensoptic nerveoptic cupsurface ectodermRPEFGFoptic vesiclechoroidBM RPE cone ONLINL GCLlightHC BC MC ACONrod F ig. 9.1 D evelopment and structural arrangement of the retina. ( a ) Schematic representation of retinal development including the transition from optic vesicle to optic cup and retinal patterning. ( b ) Schematic diagram of retinal cells arrangement and connections. A bbreviations :A C amacrinecell, B C bipolar cell, B M Bruch’s membrane, G CL gan-glion cell layer, H C horizontal cell, I NL inner nuclear layer, M C Müller cell, O N optic nerve, O NL outer nuclear layer89 9 Differentiation of Human Pluripotent Stem Cells into Retinal Cellsthe photoreceptor cells; on their basal side, the RPE is in contact with the underlying basal mem-brane, termed Bruch’s membrane that separates the RPE from the choroid. These cells play cru-cial roles in the maintenance and function of the retina and its photoreceptors. As a layer of pig-mented cells, the RPE absorbs the stray light that was not absorbed by the photoreceptors. The RPE cells form a blood–retinal barrier due to decreased permeability of their junctions. The RPE cells transport ions, water, and metabolic end products from the retina to the bloodstream. They are involved in supplying the neural retina with nutrients from the bloodstream, such as glu-cose, retinol, and fatty acids. Another important function of the RPE is the phagocytosis of shed photoreceptor outer segments. After the outer segments are digested, essential substances such as retinal are recycled. Retinal is also recycled and returned to photoreceptors by the process known as the visual cycle. The precise functioning of the RPE is essential for visual performance. Failure of one of these functions can lead to degeneration of the retinal photoreceptors, vision impairment and blindness.T here are many inherited and age-related eye disorders that cause degeneration of the retina as a consequence of loss of photoreceptor cells. Retinal and macular degeneration disorders can be divided into two main groups. The fi rst group primarily affects the photoreceptors and involves the majority of cases of retinitis pigmentosa. In the second group, the primary damage is to the adjacent RPE cells, and as a consequence of this damage, the photoreceptors degenerate. This group includes age-related macular degeneration, Stargardt’s macular dystrophy, a subtype of Leber’s congenital amaurosis in which RPE65 is mutated, Best’s disease and some cases of retini-tis pigmentosa, as well.W ith regard to retinitis pigmentosa (RP), it is a group of inherited retinal degeneration diseases that are caused, as mentioned above, by a primary progressive loss of rod and cone photoreceptors, followed by a subsequent degeneration of RPE (Hartong et al. 2006). The disease affects approxi-mately 1.5 million patients worldwide and is the most common cause of blindness in people under 70 years of age in the western world. The disease can be characterized by retinal pigment deposits visible on the fundus examination. In most cases, the disease primarily affects rods. At later stages of the disease, the degeneration of cones takes place. As a consequence of disease progression, the patients’ night vision is reduced. Patients initially lose peripheral vision while retaining central vision (a visual status termed “tunnel vision”). In advanced cases, central vision is also lost, commonly at about 60 years of age. The disease affects about 1 in 4,000. The inheritance can be autosomal-recessive, autosomal-dominant or X-linked (in ~50–60%, 30–40%, and 5–15% of cases, respectively). Mutations in more than 140 genes have been iden-tifi ed as causing RP (Hartong et al. 2006).Among these genes are those involved in phototransduc-tion, like rhodopsin, the a- and b- subunits of phos-phodiesterase, the a- and b- subunits of Rod cGMP gated channel and arrestin. The additional muta-tions were found in genes encoding structural pro-teins, like peripherin, rod outer segment protein and fascin. They were also found in transcription factors involved in photoreceptors’ development such as Crx and Nrl, and in other genes, whose products are involved in signaling, cell-cell interac-tion and trafficking of intracellular proteins. Currently, there is no effective cure for RP. Treatment with vitamin A palmitate, omega-3 fatty acids and other nutrients may somewhat slow the rate of the disease progression in many cases. Reduction in exposure to light was also shown to decrease the rate of retinal degeneration.A mong the group of retinal degenerations that are caused by primary loss of RPE cells or their function, age-related macular degeneration (AMD) is the most frequent condition and the leading cause of visual disability in the western world (Cook et al. 2008).Among people over 75 years of age, 25–30% are affected by AMD, with progressive central visual loss that leads to blindness in 6–8%. The retinal degeneration pri-marily involves the macula. The dry form of AMD is initiated by hyperplasia of the RPE and formation of drusen deposits, consisting of meta-bolic end products underneath the RPE or within the Bruch’s membrane. It may gradually progress into the advanced stage of geographic atrophy90M. Idelson and B. Reubinoff with degeneration of RPE and photoreceptorsover large areas of the macula causing central visual loss. Ten percent of dry AMD patients will progress to neovascular (wet) AMD, with blood vessels sprouting through the Bruch’s membrane with subsequent intraocular leakage and/or bleed-ing, accelerating the loss of central vision. While the complicating neovascularization can be treated with anti-VEGF agents, currently there is no effective treatment to halt RPE and photore-ceptor degeneration and the grim reality is that many patients eventually lose their sight (Cook et al. 2008).S targardt’s macular dystrophy (SMD) is the most common form of inherited macular dystro-phy affecting children (Walia and Fishman 2009). The disease is symptomatically similar to AMD. The prevalence of SMD is about 1 in 10,000 chil-dren. The disease involves progressive central visual loss and atrophy of the RPE beneath the macula following accumulation of lipofuscin in RPE cells, which is suggested to consist of non-degradable material, derived from ingested pho-toreceptor outer segments. The inheritance is predominantly autosomal recessive, although an autosomal dominant form has also been described. The mutation in the ABCA4 gene was found to be a most common cause of SMD. The product of the ABCA4 gene is involved in energy transport to and from photoreceptors. The mutated protein cannot perform its transport function and, as a result, photoreceptor cells degenerate and vision is impaired. Currently, there is no effective treat-ment for SMD.C ell therapy to replenish the degenerating cells appears as a promising therapeutic modality that may potentially halt disease progression in the various retinal and macular degeneration dis-orders caused by loss and dysfunction of RPE cells and photoreceptors (da Cruz et al. 2007).I n this chapter we will discuss the potential of human pluripotent cells which includes human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSCs), to gen-erate various types of retinal cells that could be used for transplantation therapy of retinal degen-eration disorders and disease modeling for drug discovery. C ell Therapy of Retinal and Macular DegenerationsT he eye is an attractive organ for cell therapy as it is easily accessible for transplantation and for simple monitoring of graft survival and potential complications by direct fundoscopic visualiza-tion. Anatomically, it is a relatively confi ned organ limiting the potential of unwanted extra-ocular ectopic cell distribution, and a low number of cells are required to replenish the damaged cells. The eye is also one of the immune privi-leged sites of the body.T he concept of replacing dysfunctional or degenerated retina by transplantation has been developing ever since the fi rst retina-to-retina transplant in 1986 (Turner and Blair 1986).In most studies, primary retinal immature (fetal) tissue has been used as donor material. It was demonstrated that such transplants can survive, differentiate, and even establish connections with the host retina to a limited degree (Ghosh et al. 1999). The subretinal transplantation of healthy RPE has some advantages over neural retinal transplantation, as it concerns only one cell type that is not involved in neural networking. Transplantation of RPE has been studied exten-sively in animal models (Lund et al. 2001).The most commonly used animal model of retinal degeneration is the Royal College of Surgeons (RCS) rat model, in which primary dysfunction of the RPE occurs as a result of a mutation in the receptor tyrosine kinase gene M ertk(D’Cruz et al. 2000). This leads to impaired phagocytosis of shed photoreceptor outer segments, with sec-ondary degeneration and progressive loss of pho-toreceptors within the fi rst months of life. It was reported that rat and human RPE cells rescued photoreceptor cells from degeneration when transplanted into the subretinal space of RCS rats (Li and Turner 1988; Coffey et al. 2002).The ability of transplanted RPE cells to restore retinal structure and function has been demonstrated in clinical trials. In humans, autologous transplanta-tions of peripheral RPE as well as macular trans-locations onto more peripheral RPE provide a proof that positioning the macula above relatively91 9 Differentiation of Human Pluripotent Stem Cells into Retinal Cellshealthier RPE cells can improve visual functionin AMD patients (Binder et al. 2004; da Cruz et al. 2007). Nevertheless, the surgical procedures for autologous grafting are challenging and are often accompanied by signifi cant complications. In addition, autologous RPE transplants may carry the same genetic background, environmen-tal toxic and aging-related effects that may have led to macular RPE failure and the development of AMD in the patient. It is also problematic to use autologous cells when all the RPE cells are damaged. Cell sources that can be used for such therapy include allogeneic fetal and adult RPE (Weisz et al. 1999; Binder et al. 2004; da Cruz et al. 2007). However, the use of fetal or adult retinal tissues for transplantation is severely lim-ited by ethical considerations and practical prob-lems in obtaining sufficient tissue supply. The search for a cell source to replace autologous RPE such as immortalized cell lines, umbilical cord-derived cells as well as bone marrow-derived stem cells continues.T he derivation of hESCs more than a decade ago has raised immense interest in the potential clinical use of the cells for regeneration (Thomson et al. 1998; Reubinoff et al. 2000).Along the years, signifi cant progress has been made towards the use of hESCs in clinical trials.T he other promising source of cells for transplantation therapy is iPSCs that are simi-lar to hESCs in their stemness characteristics and pluripotency. These cells could be gener-ated from different human somatic cells by transduction of four defi ned transcription fac-tors: Oct3/4, Sox2, Klf4, and c-Myc (Takahashi et al. 2007).G eneration of RPE and neural retina from hESCs and iPSC has numerous advantages, as it can be done from pathogen-free cell lines under good manufacturing practice (GMP) conditions with minimal variation among batches. Such cells can be characterized extensively prior to preclinical studies or for clinical applications, and an unlimited numbers of donor cells can be generated from them. In the following para-graphs, strategies for induction of differentiation of hESCs and iPSCs towards RPE and neural retina fate are reviewed. D ifferentiation into Retinal Pigment EpitheliumI t was reported for the fi rst time in mice and pri-mates that the differentiation of ES cells into RPE could be induced by co-culture with PA6 stromal cells (Kawasaki et al. 2002; Haruta et al. 2004). The resulting cells had polygonal epithelial mor-phology and extensive pigmentation. The cells expressed the markers that are characteristic of RPE. They developed typical ultrastructures and exhibited some functions of RPE. The differenti-ation of hESC into RPE was first reported by Klimanskaya et al. (2004).According to their protocol, hESCs underwent spontaneous differ-entiation by overgrowth on mouse embryonic fibroblasts (MEF), in feeder-free conditions or, alternatively, as embryoid bodies (EBs) in com-bination with withdrawal of bFGF from the medium. The yield of the formation of RPE cells after 4–8 weeks of spontaneous differentiation was relatively low; for example,<1% of EBs con-tained pigmented cells at this stage. However, after 6–9 months in culture, all the EBs contained pigmented cells. The areas of pigmented cells could be further isolated mechanically and prop-agated by passaging as RPE lines. Klimanskaya and colleges characterized the hESC-derived RPE cells by transcriptomics and demonstrated their higher similarity to primary RPE tissue than to human RPE lines D407 and ARPE-19. The low yield of spontaneously differentiating RPE cells was improved by induction of differentia-tion with Wnt and Nodal antagonists, Dkk1 and LeftyA, respectively, the factors that are sug-gested to promote retinal differentiation. This treatment gave rise to pigmented cells within 38% of the hESC colonies after 8 weeks (Osakada et al. 2008). Immunostaining with the ZO-1 anti-body showed that by day 120, hESC-derived pig-mented cells formed tight junctions (about 35% of total cells). We showed that differentiation toward the neural and further toward the RPE fate could be augmented by vitamin B3 (nicotin-amide; Idelson et al. 2009).We further showed that Activin A, in the presence of nicotinamide, effi ciently induces and augments differentiation92M. Idelson and B. Reubinoffinto RPE cells. This is in line with the presumed role of Activin A in RPE development i n vivo .In the embryo, extraocular mesenchyme-secreted members of the TGF b superfamily are thought to direct the differentiation of the optic vesicle into RPE (Fuhrmann et al. 2000).Under our culture conditions, when the cells were grown in suspen-sion as free-fl oating clusters, within 4 weeks of differentiation, 51% of the clusters contained pigmented areas and about 10% of the cells within the clusters were pigmented. When we modifi ed the differentiation conditions to includea stage of monolayer culture growth, the yield of the RPE-like pigmented cells was signifi cantly improved and 33% of the cells were pigmented after 6 weeks of differentiation. The derivation of RPE from hESCs and iPSCs without any external factor supplementation was also demonstrated by other groups (Vugler et al. 2008 ; Meyer et al. 2009 ; Buchholz et al. 2009).T he hESC-derived RPE cells were extensively characterized, including demonstration, both at the mRNA and the protein levels, of the expres-sion of RPE-specifi c markers, such as RPE65, CRALBP, Bestrophin, Tyrosinase, PEDF, PMEL17, LRAT, isoforms of MiTF abundant in RPE, and others. The cells expressed markers of tight junctions that join the adjacent RPE cells: ZO-1, occludin and claudin-1 (Vugler et al. 2008 ) . Electron microscopic analysis revealed that the hESC-derived RPE cells showed features characteristic of RPE. The cells were highly polarized with the nuclei located more basally, and the cytoplasm with the mitochondria and melanin granules of different maturity more api-cally. A formation of basal membrane was observed on the basal surface of the RPE cell. Similar to putative RPE, the hESC-derived RPE basal membrane was shown to be composed of extracellular matrix proteins, collagen IV , lami-nin and fi bronectin (Vugler et al.2008).The appearance of apical microvilli was demonstrated at the apical surface of the RPE. The presence of tight and gap junctions on the apical borders of the RPE cells was also confi rmed by electron microscopy. O ne of the most important functions of RPE cells i n vivo is phagocytosis of shed photoreceptor outer segments, as part of the continuous renewal process of rods and cones. The hESC-derived RPE cells demonstrated the ability to phagocyto-size latex beads or purifi ed photoreceptor outer segments, confi rming that these cells are func-tionali n vitro . It may be concluded from all these studies that human pluripotent stem cells have a potential to give rise to pigmented cells exhibiting the morphology, marker expression and functionof authentic RPE.D ifferentiation into Retinal Progenitors and Photoreceptors O ur group showed, for the fi rst time, the potential of highly enriched cultures of hESC-derived neu-ral precursors (NPs) to differentiate towards the neural retina fate (Banin et al. 2006).We demon-strated that the NPs expressed transcripts of key regulatory genes of anterior brain and retinal development. After spontaneous differentiation i n vitro , the NPs gave rise to progeny expressing markers of retinal progenitors and photoreceptor development, though this was uncommon and cells expressing markers of mature photorecep-tors were not observed. We showed that after transplantation into rat eyes, differentiation into cells expressing specifi c markers of mature photoreceptors occurred only after subretinal transplantation (between the host RPE and pho-toreceptor layer) suggesting that this specifi c microenvironment provided signals, yet unde-fi ned, that were required to support differentia-tion into the photoreceptoral lineage.P rogress towards controlling and inducing the differentiation of hESCs into retinal progenitors and neurons i n vitro was reported in the study of Lamba et al. ( 2006).They treated hESC-derived EBs for 3 days with a combination of factors,including Noggin, an inhibitor of BMP signaling, Dkk1, a secreted antagonist of the Wnt signaling pathway and insulin-like growth factor 1 (IGF-1), which is known to promote retinal progenitor dif-ferentiation. The cultivation of EBs with these factors was followed by differentiation on Matrigel or laminin for an additional 3 weeks in the presence of the combination of the three93 9 Differentiation of Human Pluripotent Stem Cells into Retinal Cellsfactors together with bFGF. Under these culture conditions, the majority of the cells developed the characteristics of retinal progenitors and expressed the specifi c markers Pax6 and Chx10 (82% and 86% of the cells, respectively). The authors showed that after further differentiation, the cells expressed markers of photoreceptor development Crx and Nrl (12% and 5.75%, respectively). About 12% of the cells expressed also HuC/D, the marker of amacrine and ganglion cells. The expression of markers of the other sub-types of retinal neurons was demonstrated, as well. However, only very few cells (<0.01%) expressed markers of mature photoreceptors, blue opsin and rhodopsin. The abundance of cells expressing markers of photoreceptors could be accelerated by co-culture with retinal explants, especially when the explants originated from mice bearing a mutation that causes retinal degeneration.T o better characterize the phenotype of retinal cells obtained with this differentiation protocol, a microarray-based analysis comparing human retina to the hESC-derived retinal cells was per-formed (Lamba and Reh 2011).It was demon-strated that gene expression in hESC-derived retinal cells was highly correlated to that in the human fetal retina. In addition, 1% of the genes that were highly expressed in the hESC-derived cultures could be attributed to RPE and ciliary epithelium differentiation.A n alternative protocol for the derivation of retinal progenitors and photoreceptors was pro-posed by Osakada et al. (2008).Similar to the protocol for the derivation of RPE cells, they used serum-free fl oating cultures in combination with the Dkk1 and LeftyA. After 20 days of cul-ture in suspension, the cells were replated on poly-D-lysine/laminin/fi bronectin-coated slides. Osakada and co-authors demonstrated that on day 35 in culture, about 16% of colonies were positive for retinal progenitor markers Rx and Pax6. Differentiation towards photoreceptor fate was augmented in the presence of N2 by treat-ment with retinoic acid and taurine, which are known inducers of rod fate differentiation. Under these conditions, after an extended culture period of 170 days, about 20% of total cells were positive for Crx, an early photoreceptor marker. On day 200, about 8.5% of the cells expressed the mature rod photoreceptor marker, rhodopsin, as well as cone photoreceptor markers, red/green and blue opsins (8.9% and 9.4%, respectively).A n alternative approach was proposed by the same group based on the use of small molecules. In this method, the chemical inhibitors CKI-7 and SB-431542 that inhibit Wnt and Activin A signaling, respectively, and Y-27632, the Rho-associated kinase inhibitor, which prevents disso-ciation-induced cell death, were used. These molecules were shown to mimic the effects of Dkk1 and LeftyA (Osakada et al. 2009).This strategy, which doesn’t involve the use of recom-binant proteins which are produced in animal or E scherichia coli cells, is more favorable for the gen-eration of cells for future transplantation therapy.I n another study that was published by Meyer et al .(2009), after initial differentiation in sus-pension for 6 days, the aggregates were allowed to attach to laminin–coated culture dishes. After further differentiation as adherent cultures, neu-roepithelial rosettes were formed, which were mechanically isolated and subsequently culti-vated as neurospheres. The authors didn’t use any soluble factors; moreover, they showed that under these conditions, the cells expressed endogenous Dkk1 and Noggin. They also demonstrated that in concordance with the role of bFGF in retinal specifi cation, the inhibition of endogenous FGF-signaling abolished retinal differentiation. Under their differentiation protocol, by day 16, more than 95% of the cells expressed the retinal pro-genitor markers, Pax6 and Rx. The authors dem-onstrated that by day 80 of differentiation, about 19% of all neurospheres contained Crx+ cells and within these Crx+ neurospheres, 63% of all cells express Crx and 46.4% of the cells expressed mature markers, such as recoverin and cone opsin.I n all of the above studies, differentiated cells expressing the retinal markers were obtained; however, the cells were not organized in a three-dimensional retinal structure. In a paper recently published by Eiraku et al. (2011),the authors cul-tured free-fl oating aggregates of mouse ES cells in serum-free medium in the presence of base-ment membrane matrix, Matrigel, that could also94M. Idelson and B. Reubinoffbe substituted with a combination of laminin, entactine and Nodal. Using a mouse reporter ES cell line, in which green fl uorescent protein (GFP) is knocked in at the Rx locus, the authors showed that Rx-GFP+ epithelial vesicles were evaginated from the aggregates after 7 days of differentiation under these conditions. On days 8–10, the Rx-GFP+ vesicles changed their shape and formed optic cup-like structures. The inner layer of these structures expressed markers of the neural retina whereas the outer layer expressed markers of RPE. The authors demonstrated that differen-tiation into RPE required the presence of the adjacent neuroectodermal epithelium as a source of diffusible inducing factors. In contrast, the differentiation into neural retina did not require tissue interactions, possibly because of the intrinsic inhibition of the Wnt-signaling pathway. Eiraku and colleagues showed that the retinal architecture, which was formed within the optic vesicle-like structures, was comparable to the native developing neural retina.R ecently, optic vesicle-like structures were also derived from hESCs and iPSCs using the protocol described above, which is based on iso-lating the neural rosette-containing colonies and culturing them in suspension (Meyer et al. 2011). The cells within the structures expressed the markers of retinal progenitors, and after differen-tiation gave rise to different retinal cell types. It was shown that the ability of optic vesicle-like structures to adopt RPE fate could be modulated by Activin A supplementation. The production of these three-dimensional retinal structures opens new avenues for studying retinal development in normal and pathological conditions.T ransplantation of Pluripotent Stem Cell-Derived Retinal CellsA key step towards future clinical transplanta-tions of hESC-derived RPE and neural retina is to show proof of their therapeutic potential i n vivo. Various animal models of retinal degeneration have been used to evaluate the therapeutic effect of transplanted retinal cells. Human ESC-derived RPE cells were transplanted subretinally to the degenerated eyes of RCS rats. Transplantation of the hESC-derived RPE cells between the RPE and the photoreceptor layer rescued retinal struc-ture and function (Lund et al. 2006; Vugler et al. 2008; Idelson et al. 2009; Lu et al. 2009).The subretinally engrafted hESC-derived RPE cells salvaged photoreceptors in proximity to the grafts as was shown by the measurement of the thick-ness of the ONL, the layer of photoreceptor nuclei, which is an important monitor of photore-ceptor cell survival. The ONL thickness was significantly increased in transplanted eyes in comparison to the degenerated non-treated eyes.I n order to evaluate the functional effect of transplanted cells i n vivo, the electroretinography (ERG) that directly measures the electrical activ-ity of the outer (a-wave) and inner (b-wave) retina in response to light stimulation was used. It was demonstrated that after transplantation of hESC-derived RPE, ERG recordings revealed a signifi -cant preservation of retinal function in the treated eyes as compared to control untreated eyes (Lund et al. 2006; Idelson et al. 2009).The visual func-tion of the animals was also estimated by an optomotor test, which monitors the animal’s refl exive head movements in response to a rotat-ing drum with fi xed stripes. Animals transplanted with hESC-derived RPE showed signifi cantly better visual performance in comparison to con-trol animals (Lund et al. 2006; Lu et al. 2009). The presence of rhodopsin, a major component of photoreceptor outer segments, within the sub-retinaly transplanted pigmented cells suggested that they could perform phagocytosis i n vivo (Vugler et al. 2008; Idelson et al. 2009).B ridging the gap between basic research and initial clinical trials requires immense resources to ensure safety and efficacy. Human ESC-derived RPE cell lines were generated using a current Good Manufacturing Practices (cGMP)-compliant cellular manufacturing process (Lu et al. 2009). Long-term studies analyzing safety and efficacy of transplantation of these GMP-compliant hESC-derived RPE cells revealed that the subretinally transplanted cells survived for a period of up to 220 days and provided prolonged functional improvement for up to 70 days after transplantation. The potential of the hESC-derived。
FDA Warns About Stem Cell Claims中英对照

FDA Warns About Stem Cell ClaimsFDA提醒有关干细胞的研究Stem cell therapies offer the potential to treat diseases or conditions for which few treatments exist.干细胞疗法提供了潜在去治疗疾病或条件的方法存在一些Stem cells, sometimes called the body’s “master cells,” are the precursor cells that develop into blood, brain, bones and all of your organs. Their promise in medical treatments is that they have the potential to repair, restore, replace and regenerate cells that could then be used to treat many medical conditions and diseases.干细胞,有时被称为人体的“主人细胞”是最先的细胞发展成血液,大脑,骨头和所有的人体器官。
它们在医学治疗方面的保证是它们有潜在的能力去修复,恢复,代替和再生能治疗医学方面的情况和疾病的细胞。
But the Food and Drug Administration (FDA) is concerned that the hope that patients have for cures not yet available may leave them vulnerable to unscrupulous providers of stem cell treatments that are illegal and potentially harmful.但是食物和药品管理部门(FDA)担心病人对于治好病的希望不会实现,这可能会让他们容易受到不合法的也是有伤害的干细胞治疗。
异基因造血干细胞移植后出血性膀胱炎及BK、JC病毒感染

CHINESE COMMUNITY DOCTORS中国社区医师2021年第37卷第2期出血性膀胱炎(HC)是异基因造血干细胞移植术后常见并发症之一,按发病时间可分为早发型出血性膀胱炎(EOHC)和迟发型出血性膀胱炎(LOHC)。
与移植前预处理相关的EOHC,由于美司钠的使用以及水化,其发生率较前降低。
然而,LOHC 发生率依然较高,病毒相关性HC 比例可达87.5%[1],以BK、JC 病毒感染为主。
对于单倍体造血干细胞移植,由于其治疗过程中使用较多免疫抑制剂,使得免疫力恢复慢,从而使得病毒感染风险增加。
本文对我中心2019年行异基因造血干细胞移植且发生出血性膀胱炎的患者以及BK与JC病毒感染情况分析两者的相关性,从而更好地了解LOHC的发病原因。
资料与方法选取2019年1-12月我中心进行异基因造血干细胞移值患者54例,男29例,女25例;年龄4~61岁,平均37.5岁;病种包括急性髓系白血病(AML)30例,其中高危25例,中危5例;急性淋巴细胞白血病(ALL)13例,其中高危12例,中危1例;重型再生障碍性贫血(AA)6例;骨髓增生异常综合征(MDS)2例,均为RAEB-Ⅱ;慢性粒细胞白血病(CML)2例,均T315i 突变,黏多糖贮积病1例。
其中HLA 配型全相合12例,半相合42例。
以上患者随访日期截至2020年5月31日,54例患者中,发生出血性膀胱炎17例(31.5%)。
预处理方案与HC 预防:⑴年龄<50岁,全相合使用Ara-C+Bu/Cy 方案预处理,不全相合使用Ara-C+Bu/Cy+ATG 方案预处理。
年龄>50岁,行Flu+Ara-C+Bu+ATG 方案预处理。
再生障碍性贫血患者,全相合行FBu +Cy +ATG 方案预处理,半相合行Bu+Cy+ATG 方案预处理。
⑵HC 预防:①大剂量补液,每日总液量按100~120mL/(kg ·d)计算,24h 持续均匀静脉滴注;②碱化尿液:使用碳酸氢钠碱化尿液,其用了占总体补液量的0.5%。
造血干细胞移植治疗自身免疫病

造血干细胞移植治疗自身免疫病孙凌云欧阳健张杏书自身免疫病是机体免疫系统平衡失调后对自身抗原发生免疫反应造成的组织损伤。
不论对自身免疫病(AD)的发病机制和治疗的研究进展如何,迄今为止,对它们发病的确切机制仍了解不多,一些病情严重的患者仍得不到令人满意的疗效。
无容置疑,糖皮质激素和细胞毒药物是治疗绝大部分AD的主要武器,但对部分疾病如重症红斑狼疮(SLE)、系统硬化症(SSc)、难治性类风湿关节炎(RA)等治疗依然束手无策。
免疫系统中所有的细胞都来源于造血干细胞,AD患者免疫细胞异常是由于干细胞受损还是子代细胞受损造成的仍不清楚。
动物实验证实部分病变用健康同种异体或自体造血干细胞移植(HSCT)可治愈。
近年来HSCT治疗AD愈演愈烈,并初步收到了一定的疗效,成为探讨治疗多种风湿病的新方法。
1 HSCT的分类和预处理根据造血干细胞来源不同的HSCT可分为:①骨髓干细胞移植(BMT);②外周血干细胞移植(PBSCT);③脐带血干细胞移植;④胎肝干细胞移植。
BMT和PBSCT是常用的方法,以供者来源不同又可分为同种异体(allogeneic)和自体(autologous)移植,同种异体移植又分为同基因(identical twin)和异基因(sibling)HSCT。
同基因移植最理想,但来源毕竟太少;异基因移植病死率较高(15%~35%),但只要供者和受者HLA抗原相容都可移植。
由于随年龄的增加,移植相关病变的病死率也增高,因此,大于55岁的患者行同种异体移植时要慎重〔1〕。
自体移植因相对安全,病死率也低(3%~5%),且年龄放宽到65岁而广为应用。
理论上BMT和PBSCT都能选用。
PBSCT能使患者较快地康复,在自体移植中较BMT优先采用,但外周血中混含成熟自身反应的T细胞较骨髓多,这些T 细胞介导了异常的免疫反应,在T细胞去除不充分时,会引起PBSCT的失败和疾病的复发。
HSCT的主要步骤如下:①用环磷酰胺和G-CSF动员HSC;②用细胞分离仪采集HSC;③HSC冻存;④HSC复苏回输。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
PeRsPeCTIVeHuman neural stem cell transplantsto address multiple pathologiesassociated with traumatic brain injuryTraumatic brain injury (TBI), an unmet need: TBI is analteration in brain function caused by an external force withevidence of brain pathology. It could be from a bump, blow,blast or jolt to the head including penetrating the cranium.TBI is a public health concern worldwide due to its econom-ic impact. Most TBIs are survivable, do not need hospitaliza-tion but may influence productivity. A smaller percentage ofTBI due to falls or penetrating TBI (PTBI) needs hospitaliza-tion and accounts for largest fraction of TBI care costs. PTBIespecially that involving firearm injury is an increasinglyserious issue. In the United States, PTBI is an issue both inthe military and civilian context costing more than $70–75billion annually. PTBI has become increasingly survivableincluding previously lethal midline crossing of projectiledue to brain trauma foundation guidelines as well as timelyneurosurgical intervention. The extent of recovery is propor-tional to initial damage; injuries limited to single hemispherestabilize earlier than those crossing the midline do. However,currently the consequence of surviving a PTBI is most likelyto be permanent disability. Rate of disability has not changedover the past 5 decades. Almost 3.2 million Americans livewith neurobehavioral disability i.e., chronic cognitive andfunctional impairment requiring support from their familiesand the State, with lifetime costs of millions of dollars perpatient. The TBI lesion is dynamic with continued brain at-rophy, which correlates with persistent neurological deficitand overall social outcome. Observations of post-TBI tissueloss by pathologists were confirmed by longitudinal imagingstudies in living TBI survivors. Progressive volume loss wascoincident with persistent neuroinflammation thought tobe due to chronic microglial activation (Smith, 2013; Lee etal., 2018). In a study of veterans living with TBI spanning4 decades, loss of tissue following PTBI was approximately56 mL. Magnification of the primary injury via secondarymechanism underlies such volume loss and consequent dis-ability. The PTBI penumbra (tissue surrounding the PTBIinjury core) is render vulnerable by pyroptosis of chronicallyactivated microglia. Continued pyroptosis of microglia andadjacent cells facilitate the lesion expansion into otherwiseintact remote regions (Figure 1A & B). Spontaneous recov-ery in TBI generally takes place within the first 3 monthsafter injury and is mainly due to neural plasticity but notendogenous reparative neurogenesis. Success in restoringan injured brain with current therapies is limited by inabil-ity of central nervous system to regenerate spontaneously.Hence, current medical treatments albeit unsuccessfully,have sought to (i) prevent neuroinflammation driven dete-rioration and (ii) replace lost cells. The historical failure ofacute neuroprotective trials has led to alternate approachesi.e., recruit endogenous neural stem cells (NSCs) or replacevia transplantation of exogenous NSCs with a goal to rebuildcircuitry. B oth preclinical and clinical attempts to boostendogenous NSCs have failed to repair injured brains. Inaddition, the concept of recruiting endogenous NSCs torepair injured brain developed in rodents may have limitedscope in humans given the differences in neurogenesis rateand recruitment distances (Kassi et al., 2018). Could precisestereotactic placement of NSCs help address the unmet needand repair an injured brain?Human NsC (hNsC) transplantation studies in preclin-ical TBI: Transplantation of exogenous rodent NSCs notonly replaces lost neurons but also boosts endogenous neu-rogenesis, angiogenesis ameliorating ischemia/TBI inducedcognitive deficits (Ryu et al., 2016; Kassi et al., 2018). Lackof robust durable engraftment of human NSC in rodent TBImodels failed to provide rational for clinical trials aimed atassessing cell therapy potential in human TBI. Recently ame-lioration of cognitive deficits via sub-acute transplantation ofclinical trial grade hNSCs in immunosuppressed rats as wellas athymic rats has been reported (Haus et al., 2016; Spur-Figure 1 schematic of an intact gyrencephalic brain.The picture shows the gyri, sulci, six layers of cortical neurons (red triangles with yellow nuclei) in gray matter (GM) and neuronal density depen-dent projections into white matter (WM) with normal astrocytes (red stars), highly ramified resting (filled blue arrow) and primed microglia (open blue arrow) in (A). After penetrating traumatic brain injury (PTBI), represented by a cortical tissue penetration the penumbra surrounding the injury core is replete with proinflammatory-activated microglia (orange arrow). Activated microglial pyroptosis (secondary damage) in penumbra causing cortical thinning (red arrow) and manifest as ventriculomegaly (VM). Persistent axonal debris and chronic microglial activation mediate toxic inflammation fueling tissue loss years after injury (B). Stereotaxic transplantation of human neural stem cells (green shapes) in penumbra would provide a two-in-one solution, a source of cells to replace lost cell types, resolve chronic microglial activation preventing further tissue loss (C).A B C1699Clervius H, Baig M, Mahavadi A, Gajavelli S (2019) Human neural stem cell transplants to address multiple pathologies associated with traumatic brain injury. Neural Regen Res 14(10):1699-1700. doi:10.4103/1673-5374.255620lock et al., 2017). In other TBI and central nervous system disease models, NSC transplants mediated increased phago-cytosis, decreased gliosis, and disrupted toxic inflammatory signaling (Kassi et al., 2018). Another secondary mechanism known to exacerbate TBI i.e., posttraumatic epilepsy, hNSC transplantation mitigated it too. In a temporal lobe epilepsy model 6 months post-transplantation, hNSC derived neu-rons conferred both neuroprotection and antiepileptogenic effects via synaptic connections with host (Upadhya et al., 2019). hNSCs transplant studies in other central nervous system regions with longer survival periods (9–18 months) in primates and rodents replaced lost neurons, oligodendro-cytes and astrocytes depending on transplant location reca-pitulating neural development (Lu et al., 2012; Rosenzweig et al., 2018; Lien et al., 2019).hNsCs in TBI: Autologous NSC transplantation in human TBI provides the evidence for feasibility but not sufficient to launch a clinical trial. The absence of NSC-TBI clinical trial in the 50 ongoing clinical trials listed by California Institute for Regenerative Medicine is a glaring reminder of the gap in knowledge (Kassi et al., 2018). The only human cell therapies for TBI in clinical trials are NCT02416492, NCT02525432, both mesenchymal stem cells (MSC). In rodents, the mech-anism of action for MSCs is anti-apoptotic (Zhang et al., 2019) and recruitment of endogenous stem cells/immature neurons to TBI lesion ‘biobridge’. MSCs cannot function as agents of cell replacement as they do not differentiate into neural cell types nor engraft. Therapeutic role of ‘MSC trans-plant induced’ endogenous cell recruitment to TBI lesion and its relevance to humans remains to be determined. Hu-man neural stem cell based therapy not yet available for TBI has precedent in stroke. An immortalized hNSC line derived CTX0E03 from first trimester fetal brain tissue (of unspeci-fied age) has progressed to multiple stroke clinical trials with encouraging results despite low engraftment and persistence of cells only up to 5 weeks post-transplantation (Sinden et al., 2017).Conclusions: The preclinical studies support the notion that hNSC therapy can address multiple TBI pathologies. Transplanted hNSCs could (i) be an enduring source of cells to replace lost neural cells, recapitulating neural develop-ment in transplantation site dependent manner, (ii) confer neuroprotection via immunomodulation in sub-acute phase (Figure 1C), (iii) mitigate vulnerability to injury induced posttraumatic epilepsy. Prior to use of the approach in clinic, mechanistic insights with gyrencephalic TBI models and a safety trial in TBI patients will be necessary.Helene Clervius, Mirza Baig, Anil Mahavadi, shyam Gajavelli* University of Miami Miller School of Medicine, Miami, FL, USA*Correspondence to: Shyam Gajavelli, PhD, sgajavel@. orcid: 0000-0002-5947-6973 (Shyam Gajavelli)Received:February 11, 2019Accepted:March 20, 2019doi: 10.4103/1673-5374.255620Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.Plagiarism check: Checked twice by iThenticate.Peer review: Externally peer reviewed.Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms. ReferencesHaus DL, Lopez-Velazquez L, Gold EM, Cunningham KM, Perez H, Anderson AJ, Cummings BJ (2016) Transplantation of human neural stem cells restores cognition in an immunodeficient ro-dent model of traumatic brain injury. Exp Neurol 281:1-16. Kassi AAY, Mahavadi AK, Clavijo A, Caliz D, Lee SW, Ahmed AI, Yokobori S, Hu Z, Spurlock MS, Wasserman JM, Rivera KN, Nodal S, Powell HR, Di L, Torres R, Leung LY, Rubiano AM, Bullock RM, Gajavelli S (2018) Enduring neuroprotective effect of subacute neural stem cell transplantation after penetrating TBI. Front Neurol 9:1097.Lee SW, Gajavelli S, Spurlock MS, Andreoni C, de Rivero Vaccari JP, Bullock MR, Keane RW, Dietrich WD (2018) Microglial in-flammasome activation in penetrating ballistic-like brain injury. J Neurotrauma 35:1681-1693.Lien BV, Tuszynski MH, Lu P (2019) Astrocytes migrate from hu-man neural stem cell grafts and functionally integrate into the injured rat spinal cord. Exp Neurol 314:46-57.Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH (2012) Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150:1264-1273.Rosenzweig ES, Brock JH, Lu P, Kumamaru H, Salegio EA, Kadoya K, Weber JL, Liang JJ, Moseanko R, Hawbecker S, Huie JR, Havton LA, Nout-Lomas YS, Ferguson AR, Beattie MS, Bresnah-an JC, Tuszynski MH (2018) Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med 24:484-490. Ryu S, Lee SH, Kim SU, Yoon BW (2016) Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen Res 11:298-304.Sinden JD, Hicks C, Stroemer P, Vishnubhatla I, Corteling R (2017) Human neural stem cell therapy for chronic ischemic stroke: charting progress from laboratory to patients. Stem Cells Dev 26:933-947.Smith C (2013) Review: the long-term consequences of microglial activation following acute traumatic brain injury. Neuropathol Appl Neurobiol 39:35-44.Spurlock MS, Ahmed AI, Rivera KN, Y okobori S, Lee SW, Sam PN, Shear DA, Hefferan MP, Hazel TG, Johe KK, Gajavelli S, Tortella FC, Bullock RM (2017) Amelioration of penetrating ballistic-like brain injury induced cognitive deficits after neuronal differen-tiation of transplanted human neural stem cells. J Neurotrauma 34:1981-1995.Upadhya D, Hattiangady B, Castro OW, Shuai B, Kodali M, Attaluri S, Bates A, Dong Y, Zhang SC, Prockop DJ, Shetty AK (2019) Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbid-ities via synaptic integration. Proc Natl Acad Sci U S A 116:287-296.Zhang Y, Yu S, Tuazon JP, Lee JY, Corey S, Kvederis L, Kingsbury C, Kaneko Y, Borlongan CV (2019) Neuroprotective effects of human bone marrow mesenchymal stem cells against cerebral ischemia are mediated in part by an anti-apoptotic mechanism. Neural Regen Res 14:597-604.C-Editors: Zhao M, Li JY; T-Editor: Jia Y1700。
