泉州某服装厂_沃尔玛FCCA质量验厂报告
沃尔玛验厂总结报告
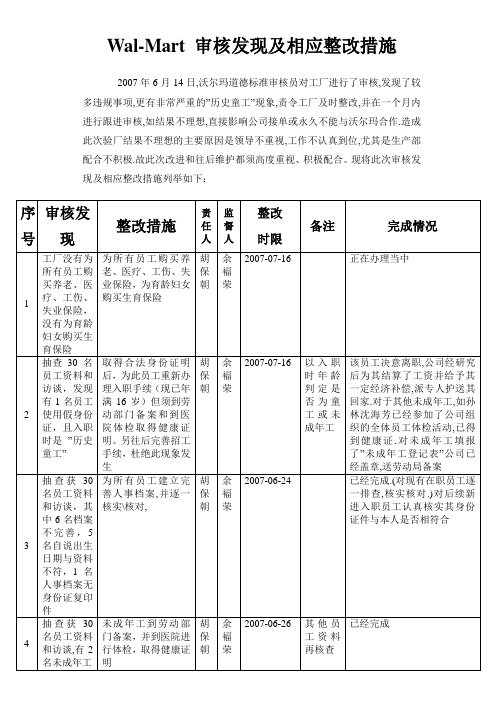
Wal-Mart 审核发现及相应整改措施
2007年6月14日,沃尔玛道德标准审核员对工厂进行了审核,发现了较多违规事项,更有非常严重的”历史童工”现象,责令工厂及时整改,并在一个月内进行跟进审核,如结果不理想,直接影响公司接单或永久不能与沃尔玛合作.造成此次验厂结果不理想的主要原因是领导不重视,工作不认真到位,尤其是生产部配合不积极.故此次改进和往后维护都须高度重视、积极配合。
现将此次审核发现及相应整改措施列举如下:
核准:整理:日期:。
walmart fcca报告模板

Walmart FCRA报告模板1. 前言Walmart作为全球最大的零售商之一,在其运营过程中积累了大量的财务与商业数据。
为了更好地管理这些数据,Walmart需要定期进行财务合规和风险管理检查。
在这一过程中,对于Walmart来说,FCRA(全称F本人r Credit Reporting Act,即《公平信用报告法案》)报告是至关重要的工具之一。
FCRA报告模板是Walmart进行财务合规检查的重要文档,本文旨在介绍Walmart FCRA报告模板的主要结构和内容。
2. 报告模板的结构2.1 报告封面- 报告名称:Walmart FCRA报告- 日期:报告提交日期- 编制部门:财务部门- 目的:财务合规及风险管理检查2.2 目录- 1. 前言- 2. 报告模板的结构- 3. 报告内容- 4. 结论与建议2.3 报告内容- 3.1 公司基本信息- 3.2 财务数据分析- 3.3 信用报告- 3.4 风险评估- 3.5 合规性检查- 3.6 建议与改进方案3. 报告内容3.1 公司基本信息在报告的开始部分,需要包括Walmart的基本信息,如公司名称、注册位置区域、营业范围等。
3.2 财务数据分析这一部分需要对Walmart最近一段时间的财务数据进行横向和纵向对比分析,包括营业额、利润、资产负债表等内容。
3.3 信用报告通过对Walmart的信用报告进行分析,了解公司的信用状况及与相关合作方的信用关系。
3.4 风险评估对Walmart所面临的各类财务和商业风险进行评估,包括市场风险、信用风险、供应链风险等。
3.5 合规性检查通过对Walmart的财务运作是否符合相关法规和标准进行检查,并提出意见。
3.6 建议与改进方案针对以上分析,提出Walmart的财务合规与风险管理方面的建议以及相应的改进方案。
4. 结论与建议在报告的最后部分,需要对全文进行总结,提出Walmart在财务合规与风险管理方面的一些关键问题,并给出相应的对策建议。
衣服抽样质检报告模板

衣服抽样质检报告模板
尊敬的质检部门领导:
经过我们公司对所生产的衣服进行抽样质检,以下是我们的质检报告:
1. 抽样方法和样本数量:
为了确保抽样结果的可靠性,我们采用了随机抽样的方法,并且按照国际质检标准,从生产批次中抽取了100件衣服作为样本。
2. 外观质量检验结果:
经过外观质量检验,样本中有8件衣服存在以下问题:
- 3件衣服的缝线不牢固,容易出现开线的情况;
- 2件衣服有明显的色差,与工艺图纸中的色彩不符;
- 2件衣服的纽扣存在松动的情况;
- 1件衣服的拉链拉扣有损坏,无法正常使用。
3. 尺寸质量检验结果:
样本中的衣服尺寸质量在总体上表现良好,符合我们公司的标准要求。
仅有2件衣服的尺寸存在偏差,超出了允许的误差范围。
4. 材料质量检验结果:
经过材料质量检验,样本中的衣服材料质量整体良好,无明显瑕疵。
5. 包装质量检验结果:
样本中的衣服包装质量符合我们公司的标准要求,无破损、破碎或混乱的情况发生。
综上所述,对于这批抽样的衣服,外观质量方面存在一定的问题,需要对生产工艺进行调整以提高质量。
尺寸、材料和包装质量方面表现良好,符合我们公司的标准要求。
我们将及时向生产部门反馈这些问题,并希望在下一次生产中能够得到改进。
同时,我们也建议在质检过程中加强对衣服外观质量的检测,以确保产品质量的稳定性。
感谢您对质检工作的支持与关注。
如有任何疑问或需要进一步的信息,请随时与我们联系。
最诚挚的问候,
[质检员姓名]
[质检员职位]
[公司名称]。
Walmart FCCA 质量体系审核验厂要求的详细解释及文件资料范例2020年11月9日更新
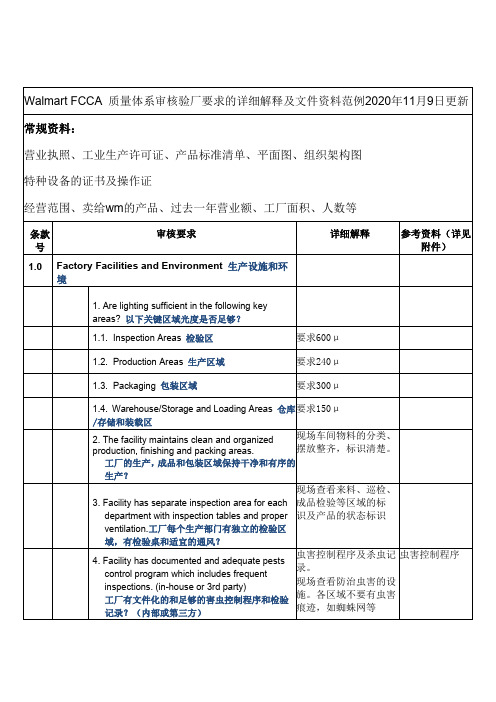
Walmart FCCA质量体系审核验厂要求的详细解释及文件资料范例2020年11月9日更新常规资料:营业执照、工业生产许可证、产品标准清单、平面图、组织架构图特种设备的证书及操作证经营范围、卖给wm的产品、过去一年营业额、工厂面积、人数等条款号审核要求详细解释参考资料(详见附件)1.0Factory Facilities and Environment生产设施和环境1.Are lighting sufficient in the following keyareas?以下关键区域光度是否足够?1.1.Inspection Areas检验区要求600μ1.2.Production Areas生产区域要求240μ1.3.Packaging包装区域要求300μ1.4.Warehouse/Storage and Loading Areas仓库/存储和装载区要求150μ2.The facility maintains clean and organized production,finishing and packing areas.工厂的生产,成品和包装区域保持干净和有序的生产?现场车间物料的分类、摆放整齐,标识清楚。
3.Facility has separate inspection area for eachdepartment with inspection tables and proper ventilation.工厂每个生产部门有独立的检验区域,有检验桌和适宜的通风?现场查看来料、巡检、成品检验等区域的标识及产品的状态标识4.Facility has documented and adequate pestscontrol program which includes frequentinspections.(in-house or3rd party)工厂有文件化的和足够的害虫控制程序和检验记录?(内部或第三方)虫害控制程序及杀虫记录。
FCCA验厂

FCCA验厂FCCA验厂是沃尔玛(Wal-mart)新推行的全称为:Factory Capability & Capacity Assessment,即工厂产量及能力评估,其目的是审核工厂的产量及生成能力是否符合沃尔玛的产能和质量要求,其主要内容包括以下几个方面:1、Factory Facilities and Environment工厂设施和环境2、Machine Calibration and Maintenance机器校准和维护3、Quality Management System质量管理体系4、Incoming Materials Control来料控制5、Process and Production Control过程和生产控制6、In-House Lab-Testing内部实验室测试7、Final inspection最终检验FCCA验厂文件和内容一、验厂文件的定义验厂文件是指工厂在验厂时为审核员提供工厂的一些基本文件资料及生产记录(包括:营业执照、员工手册、消防演习记录等),验厂文件主要是在人权、环境、安全、卫生和反恐及现场方面的文件资料,根据工厂的客户的要求可能侧重点有所不一样,有的重点是在人权,有的重点是在安全,这个要看工厂的客户是那一家。
专业的验厂文件是保证公司顺利通过验厂的护航使者根据自己的一些经历,总结的验厂文件清单请参考如下(并设有部分问卷):二、最新验厂文件清单文件资料1)品质手册和管理会议记录2)检验程序,检验标准及最近3个月的检验报告。
(来料,制程,包装,成品)3)主要机器设备清单(请准备中英文复印件各一份)4)机器设备保养计划和记录5)机器保养人员的专业证书6)产品规格书7)原料的来料和发料记录8)员工培训计划及培训记录9)产前会议程序及记录和质量会议记录10)供应商管理程序,记录,采购单及物料规格单11)实验室操作手册及测试报告12)缺陷统计报告及出货记录13)工厂组织架构图(请准备复印件一份)14)工厂营业执照(请准备复印件一份)15)当前的品质水准记录16)出货及时率的统计记录17)ISO证书及最近一次的审查报告(如有,请准备正本及复印件一份) 18)纠正预防措施的记录现场布设1)安全通道线2)安全出口、应急灯、火警铃3)化学药品摆放4)仓库特殊要点5)饮水区划分6)意见箱的设立及程序文件三、反恐重点C-TPAT证书保安制度装柜作业程序货柜检查程序及纪录封柜纪录封条管理程序则人权是随机抽查的FCCA验厂审核项目一、童工CL/未成年工U L二、非志愿劳工IL三、胁迫与骚扰CH四、不歧视DI五、最低工资WM六、加班工资OW七、加班时间OT八、社会保险SB九、福利OB十、监测与守法十一、卫生与安全HS&DO十二、宿舍DO十三、环保PE十四、分包Sub十五、其它要求OLFCCA质量验厂文件资料清单1. 营业执照 ;2. 质量体系认证证书 ;3. 组织架构图 ;4. 质量手册 ;5. 程序文件;6. 质量体系内审计划;7. 质量体系内审记录:1)内审员资格证书;2)首末次会议 ;3)检查表 ;4)不符合项报告;5)内审报告8. 质量体系管理评审计划9. 质量体系管理评审记录: 1)管理评审会议记录;2)管理评审报告;3)决议事项的跟进记录10. 主要生产设备清单;11. 设备保养计划 ;12. 设备保养记录;13. 仪器清单 ;14. 仪器校准计划 ;15. 仪器校准记录:1)外校报告 ;2)内校人员资格证书3)内校规程;4)内校报告16. 年度培训计划17. 培训记录 :1)签到表;2)测试卷18. 品管人员岗前资质认定资料(培训及测试记录;19. 新产品设计开发资料 :1)产品规格书;2) BOM表(BOM);3)安规认证证书;4)样品检测报告5)试产记录 ;6)试产评估报告 ;7)作业指导书 ;8)检验标准 ;9) FMEA分析资料 ;10)产品质量控制计划(QC工程图。
2009年沃尔玛给各供应商的验厂(ES、FCCA、GSV)新标准

2009年沃尔玛给各供应商的验厂(ES、FCCA、GSV)新标准尊敬各合作伙伴:沃尔玛人权标准在2009年有以下变化,请做好倡导及对策工作一.自2009年1月1日起所有WM验厂结果期限及补救措施时限延长1绿灯:2年后跟进评估(不变)2黄灯:每12个月跟进评估(由6个月改为12个月)3橙灯:每6个月跟进评估(由4个月改为6个月)二.所有由第三方进行评估的初审与追踪审核的费用由工厂负责---2009.4.1生效2.1以上适用于2009.4.1或之后按排/指定的所有审核2.2所有确认由Wal-Mart合作人完成的审核,工厂不用承担费用---2009.4.1生效三.. 追踪审核将是不通知的--2009.4.1生效3.1以上适用于2009.4.1或之后按排/指定的所有审核3.2第三方审核公司的审核可以提供4-6周的窗口给供货商/工厂,但具体的未宣布的审核日期将不会告知.四..对于A型GP供货商的资格预审----实时生效4.1如果工厂收到年龄违规(1~2个童工)的评估,工厂将不会通过资格预审的人权部分.4.2 如果追踪审核30天内确定补救发现的童工(1~2个童工),工厂可以通过Wal-mart验厂最新标准修正红灯-冻结的政策:从2009年5月1日起,从上次的审核日期算,两年内得到3个橙灯评估=红色-冻结一年。
今年2009年1月,橙灯的后续审计期间从120天改为180天,以便留出更多的时间给工厂改善和补救.让供应商发展的努力更加有效。
鉴于这种延长,红灯-拒绝进程GP 和非GP采购供应商的工厂也已修订。
此前是,2年内累计4个橙灯=红色-冻结一年。
•有2个橙灯的工厂:在过去两年内已经有2个橙灯,如果再增加一个橙灯,那么就等于等到一个红灯将导致他们被冻结。
•在第二个橙灯跟进审计,工厂将有6个月时间来替换工厂,在这6个月内,该工厂能继续生产和出货。
过了6个月的有效期后,工厂将会被变为“冻结”状态。
•第二个橙灯工厂的重新使用,必须获得相应的采购的批准.方可改为“正常”的状态•当一家工厂收到的第2次橙色评估的结果,今后的订单是无法保证的.取决与wal-mart,•在过去2年间有收到3个橙灯的工厂,最近一次审核的日期是2009年5月1日的工厂将仍然可以接收订单。
2014年6月最新WALMART质量验厂标准
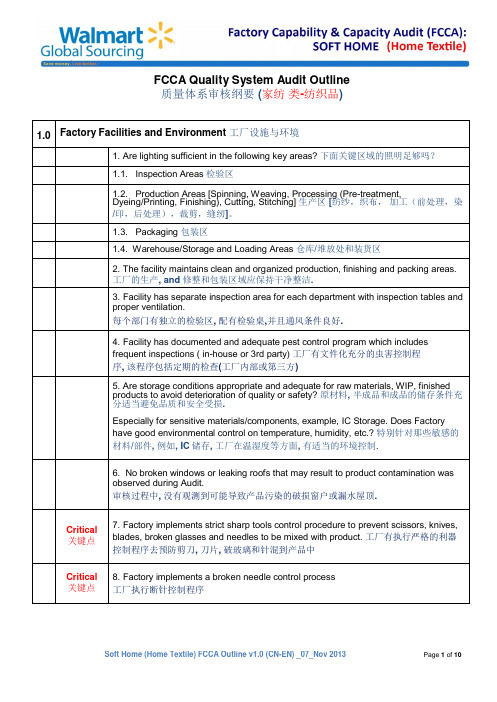
7.ProductionmanagementandQCteamdiscussandworktogetherinsolvingQualityissues/concerns.(Documented)生产管理层和品质部门一起工作讨论解决品质问题(文件化)
3.Facilityhasseparateinspectionareaforeachdepartmentwithinspectiontablesandproperventilation.
每个部门有独立的检验区,配有检验桌,并且通风条件良好.
4.Facilityhasdocumentedandadequatepestcontrolprogramwhichincludesfrequentinspections(in-houseor3rdparty)工厂有文件化充分的虫害控制程序,该程序包括定期的检查(工厂内部或第三方)
8.Factorymaintainseffectivetraceabilitysystemthatcandetermineimmediatesourcefromrawmaterialtofinishedproduct.工厂拥有有效的追溯机制,从原材料到成品能立即追根溯源.
9.Factoryhassystemsandproceduresinplacetocontroltherisk(RiskAssessment)ofphysicalorchemicalcontaminationthatmaydamage/affectrawmaterials,process,equipment&tools,productsandpersonnelaswell.
沃尔玛验厂申请及审核流程

沃尔玛一、沃尔玛定义沃尔玛公司(Wal-Mart Stores, Inc.)(NYSE:WMT)于1962年在阿肯色州成立,是一家美国的世界性连锁企业,以营业额计算为全球最大的公司,其控股人为沃尔顿家族。
总部位于美国阿肯色州的本顿维尔。
沃尔玛主要涉足零售业,是世界上雇员最多的企业,连续三年在美国《财富》杂志世界500强企业中居首位。
沃尔玛公司有9500家门店,分布于全球18个国家。
沃尔玛在美国50个州和波多黎各运营。
沃尔玛主要有沃尔玛购物广场、山姆会员店、沃尔玛商店、沃尔玛社区店等四种营业方式。
沃尔玛验厂分为三个部分:社会责任验厂(ES/RS验厂)、质量验厂或者也叫产能评估(FCCA)、反恐验厂(SCS验厂)。
二、沃尔玛验厂申请及审核流程:1、沃尔玛的供应商需向沃尔玛方面提交其所用工厂和所有分包商(必须是贸易商才可向沃尔玛申请,工厂不能直接赂沃尔玛申请审核;2、新的供应商或者工厂在申请的时候,系统会产生一个唯一的编号给到申请的工厂(8位数),同时会随机分配一个第三方的审核机构对这家申请的工厂进行审核;3、申请的工厂会收到一封关于道德采购审核程序的介绍信;4、供应商所提供的工厂和申请审核必须在出货前至少2个月完成,工厂的审核距离出货不能少于一个月,供应商要向沃尔玛方确保可以再规定的时间内完成工厂的审核。
5、审核前,审核机构会向申请的工厂发一份问卷,主要目的是了解工厂的一些基本情况,其中包括:生产、政策、环境、员工、工时、工资、福利、分包等;6、第三方审核公司会在收到沃尔玛通知后与工厂联系以及收取相关审核费用,但是具体的审核日期不会告知工厂(初审审核工厂或者重新激活工厂会通知审核日期);7、根本工厂不同人数,第三方审核公司安排不同人数的审核员到工厂进行审核;8、审核完后,审核员会出具现场审核报告,对于在审核过程中出现的问题,将会在报告中予以呈现;9、工厂高层签署报告,表示认同,审核结束;10、第三方审核公司会把审核当天的报告发送给沃尔玛,沃尔玛会15个工作日后把报告发至贸易商邮箱,FCCA审核不管是否有通过都需要做CAP把所有问题点关闭后方可进行下次年底跟进审核。
Walmart沃尔玛部分验厂标准变更已出台讲解

沃尔玛2015年部份验厂标准变更
将要踏入2015年,沃尔玛验厂的部份标准也有所修改,可以说有些标准比以前严格了,有些与以前相比,则有所放松,当然,对于那些没有更新的规定则继续沿用以前的标准。
最新的修改条款,具体如下:
1)、服装厂的生产车间、仓库和宿舍都要安装烟感报警器,某些工厂只要求仓库及宿舍安
装烟感报警器便可,要视乎工厂生产的产品而决定;
2)、每个车间至少要配备2名急救员,其中1人必须随时在岗;
3)、逃生走火门,一定要朝逃生出口方向打开;
思航验厂,高通过,零风险,保证100%通过。
4)、化学品存放仓库应与仓库、宿舍至少相距30米以上;
5)、需要建立ODS 清单;
6)、生产及生活污水排放,必须取得《环评报告》;
7)、所有消防引导的指示牌只接受灯光型的,如是荧光型的要换掉;
思航验厂,高通过,零风险,保证100%通过。
8)、厂房要有消防部门出具的合格“消防验收报告”;
9)、化学品仓库要有二级防漏,而且外面也要安装洗眼器,不能太简单,否则有些审核员
不认可;
10)、义务消防员需要进行专业培训,并取得证书;
11)、所有的《应急程序》、《事故处理》等文件,都要保存好计划、实施记录等。
Walmart FCCA 质量体系审核验厂要求的详细解释 (非电器类杂货)-2020年11月9日更新
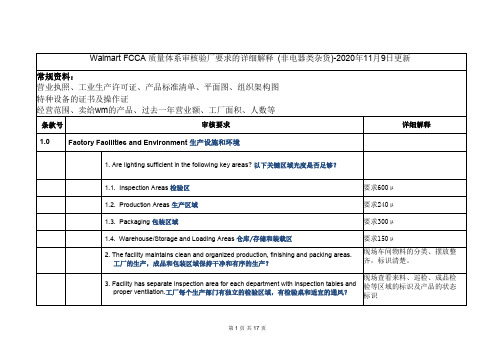
Walmart FCCA质量体系审核验厂要求的详细解释(非电器类杂货)-2020年11月9日更新常规资料:营业执照、工业生产许可证、产品标准清单、平面图、组织架构图特种设备的证书及操作证经营范围、卖给wm的产品、过去一年营业额、工厂面积、人数等条款号审核要求详细解释1.0Factory Facilities and Environment生产设施和环境1.Are lighting sufficient in the following key areas?以下关键区域光度是否足够?1.1.Inspection Areas检验区要求600μ1.2.Production Areas生产区域要求240μ1.3.Packaging包装区域要求300μ1.4.Warehouse/Storage and Loading Areas仓库/存储和装载区要求150μ2.The facility maintains clean and organized production,finishing and packing areas.工厂的生产,成品和包装区域保持干净和有序的生产?现场车间物料的分类、摆放整齐,标识清楚。
3.Facility has separate inspection area for each department with inspection tables andproper ventilation.工厂每个生产部门有独立的检验区域,有检验桌和适宜的通风?现场查看来料、巡检、成品检验等区域的标识及产品的状态标识4.Facility has documented and adequate pests control program which includesfrequent inspections.(in-house or3rd party)工厂有文件化的和足够的害虫控制程序和检验记录?(内部或第三方)虫害控制程序及杀虫记录。
walmart工厂生产能力评估fcca验厂要求

walmart工厂生产能力评估fcca验厂要求Wal-mart工厂生产能力评估(FCCA)----验厂要求·工厂质量体系1.0工厂的设备和环境1.0.1在生产,修整,成品,检验,包装和装载等车间的照明充足。
1.0.2在生产,成品和包装车间,工具设备要保持干净整齐,1.0.3工具设备等有单独的校验区域,配备校验工作台,并有适当的通风条件1.0.4要有书面的对设备工具的有害物/发霉和潮湿的管理计划,和经常进行检查的纪录。
1.0.5不能发现有窗户破损和屋漏等情况。
1.0.6必须有检针器1.0.7工厂必须严格执行尖锐物管理程序,防止剪刀,刀子,刀片,碎玻璃和断针等混在产品里面。
(非常重要!)1.0.8工厂有备用的电力供应设备:发电机等。
1.1机器的校准和维护1.1.1工厂的机器和设备适与生产沃尔玛的产品。
1.1.2工厂有定期清洁和修理机器设备的书面制度和程序1.1.3工厂的机器设备清洁,运行状况良好。
1.1.8工厂拥有具有一定的技术水准和设备的维修组,以对机器进行必要的维修和校准。
2.0质量管理体系2.0.1工厂已经建立起适合他们产品和程序的质量管理体系2.0.2.工人和检查员熟悉该质量政策和目标2.0.3工厂有书面的客户反馈体系和书面的召回程序。
2.0.4工厂的质检人员和生产活动是相分离的。
(非常重要!)2.0.5生产管理人员和质检小组一起工作并讨论解决质量问题。
2.0.6工厂要有适当的制度和程序控制物理上的,化学上的和生物学上的玷污和污染,以避免损坏产品,危及工作人员的健康。
2.0.7工厂要对化学品,原料和设备和工具的使用进行风险评估,确认其是否具有风险。
3.0来料控制3.0.1熟练的执行原料先进先出制度。
3.0.5.工厂有书面的程序和参考样品,以确保购入的原材料符合规格和说明。
3.0.6工厂要有对原料的隔离制度,以避免被不合格品意外玷污。
(非常重要!!)3.0.7.工厂需要将质量合格的产品同不合格的产品隔离,识别并替换不一致的产品或原料。
沃尔玛验厂需要准备的资料(FCCA)

45
QC应被授权当产品质量不符合规格时是否有权停止生产
品质工程/张福元
46
在每一个操作过程应由QC执行巡检(IPQC)
品质工程/张福元
47
抽查现行生产的产品质量是否可以接受?(检查8个已检验的完成品是否有主要缺陷)
品质工程/张福元
48
工厂QC检验应按照AQL抽样检验标准或按照工业标准,质检人员应能理解抽样表的使用
企管/杨国栋
17
工厂应建立顾客投诉体系及产品召回程序
企管/杨国栋
18
工厂QC团队应独立于生产部门
企管/杨国栋
19
应有书面记录显示生产管理和QC团队共同讨论、解决质量问题及其他相关的问题
品质工程/张福元
20
工厂应有系统和程序去控制那些可能会影响产品或对人造成伤害的物理、化学和微生物污染风险
品质工程/张福元
责任部门
1
在生产,修理,加工,检验,包装及装载的区域应有足够的照明
?工艺设备/刘志强
2
工厂应保持环境、设备清洁卫生,在生产,加工和包装区域应井然有序
生产/谢振昊、关德凯
3
工厂应有单独的检验区与检验台并且通风良好
品质工程/张福元
4
工厂应有害虫、霉菌和湿度的控制程序文件,应有经常巡查及记录(公司内部或第三方检查)
品质工程/张福元
29
工厂是否建立起适当的物料控制体系并实施,以隔离不合格的原材料及避免意外污染
品质工程/张福元
30
工厂应分离良品与不良材料,并标识所需更换的不良材料
品质工程/张福元
31
厂房的存储区域应有足够的照明、通风和清洁
工艺设备/刘志强
32
Walmart沃尔玛FCCA质量技术验厂标准审核条款清单对应的文件记录及现场要求

Walmart沃尔玛FCCA质量技术验厂标准审核条款清单对应的文件记录及现场要求更新日期:2020.08.18常规文件资料:营业执照、工业生产许可证、产品标准清单平面图、组织架构图特种设备的证书及操作证经营范围、卖给wm的产品、过去一年营业额、工厂面积、人数等FCCA生产能力评估清单书面程序记录现场巡视备注1.0工厂设施和环境1.在生产,修理,加工,检验,包装及装载的区域是否有足够的照明?►现场查看车间照明度:Wal-Mart的最低要求,生产区、成品区240lux,检测区域(IQC/FQC/OQC)600lux,包装区域360lux,原料仓150lux2.工厂是否保持清洁,在生产,加工和包装区域是否有秩序?►现场车间物料的分类、摆放整齐,标识清楚。
3.工厂是否有单独的检验区与检验台并且通风良好?►现场查看来料、巡检、成品检验等区域的标识及产品的状态标识4.工厂是否有害虫/霉菌和湿度的控制程序文件?是否有经常巡查(公司内部或第三方检查)?►灭虫/灭鼠控制程序►防潮/防霉菌控制程序►虫害/灭鼠巡查记录现场查看防治虫害的设施。
各区域不要有虫害痕迹,如蜘蛛网等5.在审核其间有没有发现窗户破损及房顶漏水可能导致产品污染。
玻璃破碎控制程序玻璃破碎巡查记录►现场查看窗户玻璃有无破损6.工厂是否实行严格的利器控制程序,以防止剪刀、小刀、刀片、碎玻►利器控制程序\利器、断针►利器收发记录、利器、断针►现场查看利器的管理璃及针等混入产品中。
Critical严重1.1机器校准和维护1.工厂是否有书面的文件系统和程序计划安排设备的清洁及维修。
►生产设施控制程序►机器设备保养记录►现场查看2.工厂的机器和设备是否清洁及运行良好。
►现场查看,设备的运行状态要做好标识如(运行、待机、停机、维修等)3.机器、设备和工具是否有最近的维护/校准日期及计划日期的标识。
►检测设备控制程序►检测设备清单►检测设备校准计划►校准报告►现场看检测设备的校准标识4.需要维修机器、设备和工具是否有维修标识以避免意外使用。
泉州某服装厂_沃尔玛FCCA质量验厂报告

initial Factory Capability & Capacity Audit (FCCA)QUALITY SYSTEM:Particulars Audit 3210N/A1.0Factory Facilities and Environment1. There is sufficient lighting on:Production, revising, finishing,inspection, packing and loading areas?X 2. The facility maintains clean andorganized production, finishing andpacking areas.X 3. Facility has separate inspection areawith inspection table and properventilation.X 4. Facility has documented pests/mildewand moisture control program whichincludes frequent inspections. (in-houseor 3rd party)X 5. No broken windows or leaking roofsthat may result to product contaminationwas observed during audit.X Critical 6. Factory implements strict sharp toolscontrol procedure to prevent scissors, knives, blades, broken glasses andneedles to be mixed with product.X 1.1 Machine Calibration and Maintenance3210N/A 1. Factory has documented system andprocedure for scheduled equipmentcleaning and repairs.X 2. Factory machines and equipmentsappear to be clean and in good runningcondition.X3. Machines, equipments and tools areXproperly labeled with date of lastmaintenance/calibration and schedule.4. Machines, equipments and tools thatneeds to be repaired are properlyXlabeled to avoid accidental use.5. Factory has proper, clean andorganized storage area of critical toolingX(i.e. injection moulds) with labeledshelves.6. Factory has proper documentationXand updated inventory of machines,tools, spare parts and equipments.7. Factory has maintenance team withsuitable skill level and equipments toXperform necessary repair and calibrationon machines.1.0 Total Possible Points Total Actual Points Total N/A Total Adjus10880010 REMARKS:The lighting was not sufficient on inspection area. Examples: inspection of final pro products:283lux. IQC workstation:200lux.1.0.4The factory did not establish humidity control and mildew control procedure and no1.0.5The roof for the finished goods packing and storage area was unclosed, and thereBlades using in Cutting workshop, Scissors using in sewing workshop were not cod1.1.3Factory did not have scheduled maintenance and calibration. Some of machines,labeled with date of last maintenance and calibration.2.0Quality Management System3210N/A1. Factory has established QualityXManagement System that is appropriateto their products and procedures.2. Workers & Supervisors are familiar toXthese quality policies and objectives.3. Factory has documented customer complaint system and documented recall program.XCritical 4. Factory QC team is independent from Production division.X5. Production management and QCteam discuss and work together insolving Quality issues/ concerns. (Documented)X6.Factory has systems and proceduresin place to control the risk of physical,chemical and biological contaminationthat may damage the product andpersonnel as well.X7.Factory conducts risk audits to identifyhazards from chemicals, raw materials,process equipments and tools.X8. Is factory accredited with anyinternational, national or customerquality standards association (e.g. ISO9001,etc.)?X2.0 Total Possible Points Total Actual Points Total N/A Total Adjus10881010 REMARKS:2.0.6Procedures and record of actions taken to control risks were available such as broken needprocedures to control these risks of other physical, chemical and biological contaminationFactory did not conduct risk audit and documents were not available to identify hazards from equipments and tools.The factory didn't accredit with any international, national or customer quality standards asso3.0Incoming Materials Control (Warehousing andStorage)3210N/A1. Has the factory taken adequatemeasures to assure raw materials conformance to required specificationsbefore use?X2. Proper first in-first out (FIFO) systemon materials are practiced.X3. Factory has procedures (instructions, guidelines and documented records) forquality inspection on incoming rawmaterials, accessories and components.X4. Is needed testing equipmentavailable, and maintained in goodcondition?X5. Are raw materials properly labeled,stored and traceable?X6. Factory has documented process and reference samples that ensure incomingraw materials conform to specifications.XCritical 7. Factory has proper system onmaterial segregation to avoid accidental contamination from rejected items.X8. Factory properly separate goodquality items from rejects and identifiesnon-conforming (rejects) materials for replacement.X9. Facility’s storage areas have sufficientlighting, well ventilated and cleansurrounding.X10. Materials, components andaccessories are properly stacked andidentified with tags / labels and off thefloor.XCritical 11. Chemicals and maintenancesubstances are properly marked andstored to prevent risk of contamination.X12. Does factory have a documentedsupplier selection and approval process?X13. Does factory track, evaluate anddocument material’s supplier reliability(performance)?X14. Does factory have an established,documented quality procedure and doesfactory evaluate, monitor sub-contractorquality performance and reliability?X3.0 Total Possible Points Total Actual Points Total N/A Total Adjus141106014 REMARKS:3.0.8In the Injection workshop, the factory didn't separate good quality items from rejects.3.0.11Chemicals such as alcohol, paint, and gas were not properly marked and about one thirdThe factory evaluated its material suppliers and kept evaluation records, but the evaluation per the procedure. For example, the evaluation score of one fabric supplier (Xingchangli)requirements of the procedure, it couldn't be used unless under urgent situation, and the fac Xingchangli was still the main suppliers of the factory.4.0Process and Production Control3210N/A1. Does factory PD study and applyproduct safety features, evaluatespatterns, moulds and samples duringproduct design and development?X2. Factory has documented Qualityprocedures (QP) at each stage ofoperation.X3. Does factory conduct Pre-productionmeeting prior to start of production?XCritical 4. Are critical quality and safety checksreviewed, identified, and actions forimprovement documented during Pre-production meeting?X5. Does factory conduct “pilot-run”,review product quality againstspecification sheet and documentresults with corrective actions prior toproduction?X6. Was in house lab-testing performedon current production? (Request for test copies)X7. Does factory QC compare first piece samples with approval sample and specification sheet?X8. Are there adequate approvedsamples, first piece samples, reference samples and work instructions to provide workers with proper guidelines?XCritical 9. Does Quality Control have authority tostop production if quality of products didnot meet specification?X10. In-line inspections (IPQC) areperformed by QC at every operationprocess.X11. Is quality of item acceptable oncurrent production? (Check 8 finishedproducts taken from factory finalinspected goods and check for majordefects on the item.)X12. Factory QC inspects per standardAQL or as per industry standards.X13. Factory performs 100% functionalitycheck on final products?X14. Does factory use corrective actionsand root cause analysis methods?(Please provide examples)x15. Does factory have guidelines inplace to ensure packaging is correct forproduct?X16. Does packing area have enoughspace to perform packing functionsproperly? Is it clean and organized?X17. Packed cartons are stored inenclosed area not exposed to sunshineand wet weather.X18. Does factory track and document on-time ship performance?X4.0 Total Possible Points Total Actual Points Total N/A Total Adjus180132018 REMARKS:4.0.4Parts of the pre-production meeting records were simple, and didn't state the improve meas 4.0.17The roof for the finished goods packing and storage area was unclosed and the packed cartweather.5.0In-House Lab-Testing 3210N/A1. Does factory perform in-house labtesting and are facilities appropriatelyXequipped? (Pls.refer to the FCCAattachment for in-house lab testingrequirements.)2. All gauges and test equipments haveXvalid calibrations.3. Testing manuals of various industryXstandards are available as reference.4. In-house Lab Technicians areXproperly trained to perform testingfunctions.5.0 Total Possible Points Total Actual Points Total N/A Total Adjus72510REMARKS:All gauges and test equipments were calibrated by unqualified person since 2008.The factory didn't have testing manual of any industry standard as reference.5.0.4The inspectors acted as In-house Lab Technicians, but they were not properly trained to per6.0Final Inspection 3210N/A1. Does factory have procedure andworking instruction for final QC?X2. Factory QC conducts final inspectionper standard AQL or as per industrystandards.X3. An approved sample or referencesample with packing list and shippingmarks are available as reference forfactory QC.X4. Are there formal written finalinspection reports? Are they properlyfiled and traceable to review quality ofproducts?X5. Does factory final QC perform internalmechanical tests to ensure the safety ofproduct?X6. Where appropriate, are inspectionand testing equipment used by theinspector in good condition andcalibrated?XCritical 7. Failed inspections are properly corrected prior to final inspection by customer.X8. Factory does not ship goods unless subjected to release procedures from customer.X6.0 Total Possible Points Total Actual Points Total N/A Total Adjus69576REMARKS:6.0.6No inspection and testing equipments were used during the final inspection process.7.0People Resources and Training3210N/ACritical 1. Factory conducts, documents andmaintains on-job training for allpersonnel or conducts pre-hire testing ofskilled workers prior to hiring.X2. Factory conducts and documentstechnical training programs for Electrical/ Mechanical Engineer, Machinist, QC andLab Test Technician.X3. Records of trainees and all regularpersonnel with correspondingXperformance records are kept andmaintained.7.0 Total Possible Points Total Actual Points Total N/A Total Adjus36200REMARKS:7.0.2Factory didn't conduct and maintain technical and quality training for machinist, lab test tech 7.0.3The records of trainees were incompletely.SummaryBasic Information:Capability:Capacity:Quality System:The quality management system was established according to principle of ISO9001:2000. ISO9001:2000 certificate. The latest internal audit date was on Feb. 11-14, 2009. The lates Feb. 16, 2009. The relevant records were reviewed during the audit. There were about 30 They were clearly divided to IQC, IPQC and QA. Incoming materials, semi-manufactured inspected by competent inspectors according to recognized standards GB/T2828.1-2003. Internal Lab was set up in the factory. Some tests were conducted by inspectors, and there reference.The operation procedure and related work instructions were established and all the docum During the factory audit, the operation was smooth and most of the operators were trained should make improvement in those areas that listed in the supplier CAP.Calculation of points:1.1 Per Categorya. Each question has a corresponding Audit Point and Weight. Multiply the marked “WTG” to get the score for that particular question.b. If the question is not relevant. Mark Not Applicable “N/A”. To get the score, multip points) to get the score.c. Once the category questions are completed and marked, total all scores by:- Relevant questions with corresponding points (Total Actual Points)- Not relevant questions marked N/A (Total N/A Points)d. Subtract the N/A points from the “Total Possible Points” to get the “Total Adjustede. Divide the "Total Actual Points" by the "Total Adjusted Points" to get the scoringf. A corresponding space is provided for all these scores in the report.g. If there are no N/A points, simply divide “Total Possible Points” by the “Total Actuh. Percentage score = Total Actual Points/ Total Adjusted Points(Total Adjusted Points = Total Possible Point – Total N/A Points)1.2 Report Total Scorea. Total all scores per category level:Total Possible PointsTotal Actual PointsTotal N/ATotal Adjusted Pointsb. Repeat the calculation steps on point 1.11.3 QA Point System GuideEvery question involving factory systems or process must be rated by QA baseda. 3 - Strongly Agreeb. 2 - Agreec. 1 - Needs Improvementd. 0 - Disagreee. N/A – Not Applicable. Question is not relevant to factory process.。
沃尔玛最新FCCA验厂标准[1]
![沃尔玛最新FCCA验厂标准[1]](https://img.taocdn.com/s3/m/6cd0e38883d049649b6658cb.png)
CAP Template
.Slight modification on the placement of WM Logo
Report Summary
Template
.FINAL AUDIT RESULT and MQE/TECHNICAL
COMMENTS have been added to Result Summary
7. Fine Jewelry
5. Apparel
8. Apparel
FCCA Product Category Revision
10
Bureau Veritas Presentation _ Date
. - Copyright Bureau Veritas
Bureau Veritas Presentation _ Date
. - Copyright Bureau Veritas
Old
FCCA Product Category
Revised
FCCA Product Category
1.Hardline
1. HL- E&E
. - Copyright Bureau Veritas
Seminar Objectives
1.To be familiar what has been changed on the scope of FCCA-
Soft Home- Home Textile audit checklist to be specific on home
. FCCA Enhancement Program
4
Bureau Veritas Presentation _ Date
Walmart FCCA质量技术验厂(非电器类杂货)要求清单updated_31-MAR-2016 v3.0
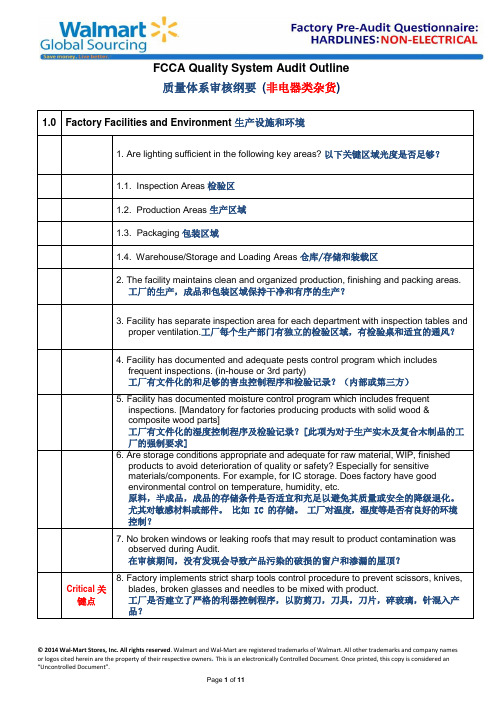
FCCA Quality System Audit Outline质量体系审核纲要(非电器类杂货)1.0 Factory Facilities and Environment 生产设施和环境1. Are lighting sufficient in the following key areas? 以下关键区域光度是否足够?1.1. Inspection Areas 检验区1.2. Production Areas 生产区域1.3. Packaging 包装区域1.4. Warehouse/Storage and Loading Areas 仓库/存储和装载区2. The facility maintains clean and organized production, finishing and packing areas.工厂的生产,成品和包装区域保持干净和有序的生产?3. Facility has separate inspection area for each department with inspection tables andproper ventilation.工厂每个生产部门有独立的检验区域,有检验桌和适宜的通风?4. Facility has documented and adequate pests control program which includesfrequent inspections. (in-house or 3rd party)工厂有文件化的和足够的害虫控制程序和检验记录?(内部或第三方)5. Facility has documented moisture control program which includes frequentinspections. [Mandatory for factories producing products with solid wood &composite wood parts]工厂有文件化的湿度控制程序及检验记录?[此项为对于生产实木及复合木制品的工厂的强制要求]6. Are storage conditions appropriate and adequate for raw material, WIP, finishedproducts to avoid deterioration of quality or safety? Especially for sensitivematerials/components. For example, for IC storage. Does factory have goodenvironmental control on temperature, humidity, etc.原料,半成品,成品的存储条件是否适宜和充足以避免其质量或安全的降级退化。
沃尔玛FCCA的评分标准

FCCA简介FCCA验厂全称为:Factory Capability & Capacity Assessment,即工厂产量及能力评估,沃尔玛(Wal-mart)新推行的工厂审核项目,其目的是审核工厂的产量及生成能力是否符合沃尔玛的产能和质量要求,其主要内容包括以下几个方面:1、Factory Facilities and Environment工厂设施和环境2、Machine Calibration and Maintenance机器校准和维护3、Quality Management System质量管理体系4、Incoming Materials Control来料控制5、Process and Production Control过程和生产控制6、In-House Lab-Testing内部实验室测试7、Final inspection最终检验FCCA验厂审核文件1.工厂简介2.营业执照3.质量手册4.操作规程5.组织结构6.岗位职责描述7.质量会议记录8.产前会议记录9.生产计划及生产进度表10. 供应商管理11.生产工序流程图12.文件控制程序13.来料检验规范及记录14.生产作业指导书15.客户投诉记录16.纠正预防措施17.生产设备清单18.保养维修记录19.计量器具校准记录20.测量仪器的检验书21.测试程序、记录22.不合格品的控制程序、记录23.首件样品评估确认程序24.员工培训记录(上岗、技能)25.外发产品检验报告26.产中检验报告27.出货检验报告FCCA vs. Pre-Qualification工厂产能产量审核与工厂预先资格评估的关系•FCCA is one audit of pre-qualification for new factory under A type supplier that include:工厂产能产量审核是工厂预先资格多种评估的其中一项,工厂预先资格评估包括:- Factory Capacity & Capability Audit (FCCA)工厂产能产量审核- Ethical standard audit (ES)社会道德标准审核- Supplier chain security (SCS) audit (GSV)供应链安全审核•Assessment Result 评估结果•Re-application for FCCA assessment will depend on correction of all non-compliances. CAP will be reviewed by third party and approved by GP Corp QA.如果初次评估不通过,工厂必须改善所有不符合项,提供的改进计划并通过第三方机构审核及WGP CORP QA的批准,才可申请再次评估•All new factories must achieve an FCCA rating no less than 60% with at least one score on critical check points由A类贸易商引进的新工厂必须取得FCCA分数不少于60%且所有关键评估点至少得1分•No order will be placed to new factories with rating under 60% or zero score on any critical check points.如果新工厂FCCA得分少于60%或任一关键评估点得0分,0分关键点未整改之前不得下定单给此工厂一、FCCA process新工厂FCCA流程备注: Factory Pre-qualification 新工厂预评估- Wal-Mart’s new factory under “A” type (Overseas) Supplier.FCCA 适用于所有A类贸易商引进的沃尔玛新工厂- Existing Factories under “A” type supplier have been inactive over 18 months 现有A类贸易商的工厂冻结超18个月,如重新启用,须通过FCCA1. New factory request is submitted to GP.贸易商向沃尔玛全球采办申请新工厂加入2. GP ES Coordinator sends supplier the third party contact instructions and notifies the third party of assessment request.采办GP的道德标准ES部门的联络员通知第三方机构此申请,并告知贸易商第三方机构的联络方式3. The third party send PI to supplier for audit fee claim, along with audit package that include: audit notice, pre-audit questionnaire, audit outline, etc 第三方机构收到审核申请后,直接提供形式发票给贸易商或工厂处理审核费用,同时将审核相关资料提供给贸易商或工厂4. Supplier makes payment, provide questionnaire to the third party and schedules assessment.贸易商支付评估费用,提供问卷调查表并与第三方机构协商评估排期5. The third party conducts assessment and reports findings to GP Corporate QA by a genericemail:********************第三方机构执行FCCA评估,然后将报告传给GP CORP QA6. Corporate QA notifies ES Coordinator of result.CORP QA审核完报告后,通知ES协调员FCCA最终的结果7. ES Coordinator notifies sourcing/merchandiser and supplier of overall result after completion of ES auditES协调员综合 ES及FCCA评估结果,给出新工厂预先资格评估的最终结果,将结果通知到沃尔玛全球采办业务部和贸易商二、FCCA process for existing factory现有在用工厂的FCCA流程Existing active factories备注:In line with GP’s continuous effort on our Quality Assurance programs, we will now initiate FCCA for all existing Toy Factories under type “A” Sup pliers from Sept. 2008.持续体现GP品管战略,从2008年9月起, 将对现有在用A类贸易商的工厂进行FCCA评估1. GP merchant provide existing factory list with supplier/factory’s contact information to Corp QA.GP业务员提供现有在用工厂名单及联络方式给GP Corp QA2. GP Corp QA send the factory list with sup plier/factory’s contact information to the third party for FCCA request.GP Corp QA提供现有在用工厂名单及联络方式给第三方机构做为FCCA的申请3. The third party contact with supplier/factory for payment settlement, and also send FCCA package to them for audit scheduling .第三方机构联络贸易商支付评估费用,并提供问卷调查表等资料给贸易商/工厂4. Supplier/factory settle payment,provide filled questionnaire to the third party, and confirm audit date within 4 working days贸易商/工厂4个工作日内付审核费用,填写问卷调查表回传给第三方机构, 并确认审核日期5. The third party conducts FCCA, communicate non-complies with supplier/factory. 第三方机构执行FCCA评估,与工厂沟通发现的不符合项6. Supplier/factory send CAP to the third party within 3 working days after FCCA audit工厂在评审结束后3个工作日提交改进计划7. The third party review and comment CAP第三方机构针对CAP进行评审并提出意见8. The third party send final audit repot with CAP to WGP Corp QA within 3 working days after receiving CAP.第三方机构在收到CAP的3个工作日内将最终的的FCCA 报告送交WGP Corp QA8. WGP Corp QA keep reports and resultWGP Corp QA保留报告和评估记录。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
initial Factory Capability & Capacity Audit (FCCA)QUALITY SYSTEM:Particulars Audit 3210N/A1.0Factory Facilities and Environment1. There is sufficient lighting on:Production, revising, finishing,inspection, packing and loading areas?X 2. The facility maintains clean andorganized production, finishing andpacking areas.X 3. Facility has separate inspection areawith inspection table and properventilation.X 4. Facility has documented pests/mildewand moisture control program whichincludes frequent inspections. (in-houseor 3rd party)X 5. No broken windows or leaking roofsthat may result to product contaminationwas observed during audit.X Critical 6. Factory implements strict sharp toolscontrol procedure to prevent scissors, knives, blades, broken glasses andneedles to be mixed with product.X 1.1 Machine Calibration and Maintenance3210N/A 1. Factory has documented system andprocedure for scheduled equipmentcleaning and repairs.X 2. Factory machines and equipmentsappear to be clean and in good runningcondition.X3. Machines, equipments and tools areXproperly labeled with date of lastmaintenance/calibration and schedule.4. Machines, equipments and tools thatneeds to be repaired are properlyXlabeled to avoid accidental use.5. Factory has proper, clean andorganized storage area of critical toolingX(i.e. injection moulds) with labeledshelves.6. Factory has proper documentationXand updated inventory of machines,tools, spare parts and equipments.7. Factory has maintenance team withsuitable skill level and equipments toXperform necessary repair and calibrationon machines.1.0 Total Possible Points Total Actual Points Total N/A Total Adjus10880010 REMARKS:The lighting was not sufficient on inspection area. Examples: inspection of final pro products:283lux. IQC workstation:200lux.1.0.4The factory did not establish humidity control and mildew control procedure and no1.0.5The roof for the finished goods packing and storage area was unclosed, and thereBlades using in Cutting workshop, Scissors using in sewing workshop were not cod1.1.3Factory did not have scheduled maintenance and calibration. Some of machines,labeled with date of last maintenance and calibration.2.0Quality Management System3210N/A1. Factory has established QualityXManagement System that is appropriateto their products and procedures.2. Workers & Supervisors are familiar toXthese quality policies and objectives.3. Factory has documented customer complaint system and documented recall program.XCritical 4. Factory QC team is independent from Production division.X5. Production management and QCteam discuss and work together insolving Quality issues/ concerns. (Documented)X6.Factory has systems and proceduresin place to control the risk of physical,chemical and biological contaminationthat may damage the product andpersonnel as well.X7.Factory conducts risk audits to identifyhazards from chemicals, raw materials,process equipments and tools.X8. Is factory accredited with anyinternational, national or customerquality standards association (e.g. ISO9001,etc.)?X2.0 Total Possible Points Total Actual Points Total N/A Total Adjus10881010 REMARKS:2.0.6Procedures and record of actions taken to control risks were available such as broken needprocedures to control these risks of other physical, chemical and biological contaminationFactory did not conduct risk audit and documents were not available to identify hazards from equipments and tools.The factory didn't accredit with any international, national or customer quality standards asso3.0Incoming Materials Control (Warehousing andStorage)3210N/A1. Has the factory taken adequatemeasures to assure raw materials conformance to required specificationsbefore use?X2. Proper first in-first out (FIFO) systemon materials are practiced.X3. Factory has procedures (instructions, guidelines and documented records) forquality inspection on incoming rawmaterials, accessories and components.X4. Is needed testing equipmentavailable, and maintained in goodcondition?X5. Are raw materials properly labeled,stored and traceable?X6. Factory has documented process and reference samples that ensure incomingraw materials conform to specifications.XCritical 7. Factory has proper system onmaterial segregation to avoid accidental contamination from rejected items.X8. Factory properly separate goodquality items from rejects and identifiesnon-conforming (rejects) materials for replacement.X9. Facility’s storage areas have sufficientlighting, well ventilated and cleansurrounding.X10. Materials, components andaccessories are properly stacked andidentified with tags / labels and off thefloor.XCritical 11. Chemicals and maintenancesubstances are properly marked andstored to prevent risk of contamination.X12. Does factory have a documentedsupplier selection and approval process?X13. Does factory track, evaluate anddocument material’s supplier reliability(performance)?X14. Does factory have an established,documented quality procedure and doesfactory evaluate, monitor sub-contractorquality performance and reliability?X3.0 Total Possible Points Total Actual Points Total N/A Total Adjus141106014 REMARKS:3.0.8In the Injection workshop, the factory didn't separate good quality items from rejects.3.0.11Chemicals such as alcohol, paint, and gas were not properly marked and about one thirdThe factory evaluated its material suppliers and kept evaluation records, but the evaluation per the procedure. For example, the evaluation score of one fabric supplier (Xingchangli)requirements of the procedure, it couldn't be used unless under urgent situation, and the fac Xingchangli was still the main suppliers of the factory.4.0Process and Production Control3210N/A1. Does factory PD study and applyproduct safety features, evaluatespatterns, moulds and samples duringproduct design and development?X2. Factory has documented Qualityprocedures (QP) at each stage ofoperation.X3. Does factory conduct Pre-productionmeeting prior to start of production?XCritical 4. Are critical quality and safety checksreviewed, identified, and actions forimprovement documented during Pre-production meeting?X5. Does factory conduct “pilot-run”,review product quality againstspecification sheet and documentresults with corrective actions prior toproduction?X6. Was in house lab-testing performedon current production? (Request for test copies)X7. Does factory QC compare first piece samples with approval sample and specification sheet?X8. Are there adequate approvedsamples, first piece samples, reference samples and work instructions to provide workers with proper guidelines?XCritical 9. Does Quality Control have authority tostop production if quality of products didnot meet specification?X10. In-line inspections (IPQC) areperformed by QC at every operationprocess.X11. Is quality of item acceptable oncurrent production? (Check 8 finishedproducts taken from factory finalinspected goods and check for majordefects on the item.)X12. Factory QC inspects per standardAQL or as per industry standards.X13. Factory performs 100% functionalitycheck on final products?X14. Does factory use corrective actionsand root cause analysis methods?(Please provide examples)x15. Does factory have guidelines inplace to ensure packaging is correct forproduct?X16. Does packing area have enoughspace to perform packing functionsproperly? Is it clean and organized?X17. Packed cartons are stored inenclosed area not exposed to sunshineand wet weather.X18. Does factory track and document on-time ship performance?X4.0 Total Possible Points Total Actual Points Total N/A Total Adjus180132018 REMARKS:4.0.4Parts of the pre-production meeting records were simple, and didn't state the improve meas 4.0.17The roof for the finished goods packing and storage area was unclosed and the packed cartweather.5.0In-House Lab-Testing 3210N/A1. Does factory perform in-house labtesting and are facilities appropriatelyXequipped? (Pls.refer to the FCCAattachment for in-house lab testingrequirements.)2. All gauges and test equipments haveXvalid calibrations.3. Testing manuals of various industryXstandards are available as reference.4. In-house Lab Technicians areXproperly trained to perform testingfunctions.5.0 Total Possible Points Total Actual Points Total N/A Total Adjus72510REMARKS:All gauges and test equipments were calibrated by unqualified person since 2008.The factory didn't have testing manual of any industry standard as reference.5.0.4The inspectors acted as In-house Lab Technicians, but they were not properly trained to per6.0Final Inspection 3210N/A1. Does factory have procedure andworking instruction for final QC?X2. Factory QC conducts final inspectionper standard AQL or as per industrystandards.X3. An approved sample or referencesample with packing list and shippingmarks are available as reference forfactory QC.X4. Are there formal written finalinspection reports? Are they properlyfiled and traceable to review quality ofproducts?X5. Does factory final QC perform internalmechanical tests to ensure the safety ofproduct?X6. Where appropriate, are inspectionand testing equipment used by theinspector in good condition andcalibrated?XCritical 7. Failed inspections are properly corrected prior to final inspection by customer.X8. Factory does not ship goods unless subjected to release procedures from customer.X6.0 Total Possible Points Total Actual Points Total N/A Total Adjus69576REMARKS:6.0.6No inspection and testing equipments were used during the final inspection process.7.0People Resources and Training3210N/ACritical 1. Factory conducts, documents andmaintains on-job training for allpersonnel or conducts pre-hire testing ofskilled workers prior to hiring.X2. Factory conducts and documentstechnical training programs for Electrical/ Mechanical Engineer, Machinist, QC andLab Test Technician.X3. Records of trainees and all regularpersonnel with correspondingXperformance records are kept andmaintained.7.0 Total Possible Points Total Actual Points Total N/A Total Adjus36200REMARKS:7.0.2Factory didn't conduct and maintain technical and quality training for machinist, lab test tech 7.0.3The records of trainees were incompletely.SummaryBasic Information:Capability:Capacity:Quality System:The quality management system was established according to principle of ISO9001:2000. ISO9001:2000 certificate. The latest internal audit date was on Feb. 11-14, 2009. The lates Feb. 16, 2009. The relevant records were reviewed during the audit. There were about 30 They were clearly divided to IQC, IPQC and QA. Incoming materials, semi-manufactured inspected by competent inspectors according to recognized standards GB/T2828.1-2003. Internal Lab was set up in the factory. Some tests were conducted by inspectors, and there reference.The operation procedure and related work instructions were established and all the docum During the factory audit, the operation was smooth and most of the operators were trained should make improvement in those areas that listed in the supplier CAP.Calculation of points:1.1 Per Categorya. Each question has a corresponding Audit Point and Weight. Multiply the marked “WTG” to get the score for that particular question.b. If the question is not relevant. Mark Not Applicable “N/A”. To get the score, multip points) to get the score.c. Once the category questions are completed and marked, total all scores by:- Relevant questions with corresponding points (Total Actual Points)- Not relevant questions marked N/A (Total N/A Points)d. Subtract the N/A points from the “Total Possible Points” to get the “Total Adjustede. Divide the "Total Actual Points" by the "Total Adjusted Points" to get the scoringf. A corresponding space is provided for all these scores in the report.g. If there are no N/A points, simply divide “Total Possible Points” by the “Total Actuh. Percentage score = Total Actual Points/ Total Adjusted Points(Total Adjusted Points = Total Possible Point – Total N/A Points)1.2 Report Total Scorea. Total all scores per category level:Total Possible PointsTotal Actual PointsTotal N/ATotal Adjusted Pointsb. Repeat the calculation steps on point 1.11.3 QA Point System GuideEvery question involving factory systems or process must be rated by QA baseda. 3 - Strongly Agreeb. 2 - Agreec. 1 - Needs Improvementd. 0 - Disagreee. N/A – Not Applicable. Question is not relevant to factory process.。
