The next-generation sphingosine-1 receptor modulator BAF312(siponimod) improves cortical n
《2024年丁苯酞预处理对脑缺血再灌注大鼠皮质神经元超微结构和白介素-1β的影响》范文

《丁苯酞预处理对脑缺血再灌注大鼠皮质神经元超微结构和白介素-1β的影响》篇一一、引言脑缺血是一种常见的神经系统疾病,其病理过程涉及神经元损伤和炎症反应的复杂交互。
近年来,丁苯酞作为一种神经保护剂在脑缺血治疗中引起了广泛关注。
本实验旨在研究丁苯酞预处理对脑缺血再灌注大鼠皮质神经元超微结构和白介素-1β(IL-1β)的影响,以期为临床治疗提供理论依据。
二、材料与方法1. 实验动物与分组本实验选用健康成年SD大鼠,随机分为四组:正常对照组、缺血再灌注组、丁苯酞预处理组及假手术组。
2. 模型建立与药物处理采用线栓法建立大鼠脑缺血再灌注模型,丁苯酞预处理组在缺血前进行丁苯酞药物注射。
3. 观察指标通过透射电镜观察皮质神经元的超微结构变化,同时检测IL-1β的表达水平。
三、实验结果1. 神经元超微结构观察透射电镜结果显示,与正常对照组相比,缺血再灌注组大鼠皮质神经元出现明显的超微结构损伤,如线粒体肿胀、内质网扩张等。
而丁苯酞预处理组的大鼠皮质神经元超微结构损伤程度明显减轻。
2. IL-1β表达水平检测与正常对照组相比,缺血再灌注组大鼠IL-1β表达水平显著升高。
而丁苯酞预处理组大鼠IL-1β表达水平较缺血再灌注组明显降低。
四、讨论本实验结果表明,丁苯酞预处理能够减轻脑缺血再灌注大鼠皮质神经元的超微结构损伤,同时降低IL-1β的表达水平。
这可能与丁苯酞的抗炎和神经保护作用有关。
在脑缺血过程中,炎症反应是导致神经元损伤的重要机制之一,而IL-1β是一种重要的炎症介质。
丁苯酞可能通过抑制炎症反应,减轻神经元损伤,从而发挥神经保护作用。
此外,丁苯酞还可能通过改善线粒体功能、调节内质网应激等途径,进一步保护神经元超微结构。
五、结论本实验结果表明,丁苯酞预处理能够减轻脑缺血再灌注大鼠的神经元超微结构损伤和炎症反应,降低IL-1β的表达水平。
这为丁苯酞在脑缺血治疗中的应用提供了理论依据,为临床治疗脑缺血提供了新的思路和方法。
新型抗过敏药英文版

development of pharmacology, antihistamines have been developed to six categories nearly hundred kinds of varieties.
The first generation of antiallergic drug
7.Indications:
Oral treatment: Allergic rhinitis, skin irritations, pleomorphic exudative erythema and
urticaria
Eye drops:Allergic conjunctivitis Dosage form: Tablets:5 mg/tablet Eye drops:1% eye drops
References:
[1] 薛建英. 新型抗过敏药盐酸奥洛他定的合成工艺研究[M]
Ophthalmol CHN,2008(17):166-170
[2] 王金龙,陈孝治. H1受体拮抗剂及其研究的新进展[J],中国药房,1999,10(3):140 [3] 李莹,张潇等. 过敏性结膜炎的流行病学及奥洛他定滴眼液开放性 多中心治疗的初步效果[J] [4] 博恩. 如何选用抗过敏药[J] 中国中医药报 2010(5) [5] Kyosuke Satake, JunichiIkeda et. Olopatadine hydrochloride suppresses hot flashes induced by topical treatment with tacrolimus ointment in rats[J] European Journal of Pharmacology, Volume 765, 2015(10): 402-405 [6]B. Mortemousque, T. Bourcier, M. Khairallah et. Comparison of preservative-free ketotifen fumarate and preserved olopatadine hydrochloride eye drops in the treatment of moderate to severe seasonal allergic conjunctivitis[J] Journal Franç ais d'Ophtalmologie, 2014(1): 1-8 [7]Saki Matsui, Hiroyuki Murota, Emi Ono et. Olopatadine hydrochloride restores histamine-induced impaired sweating[J] Journal of Dermatological Science, 2014(6): 260-261
CD4_T淋巴细胞亚群研究进展_杨雪

·综述·在免疫应答期间,外周血T淋巴细胞发育为功能不同的亚群,并通过分泌特殊的细胞因子,影响细胞免疫,调节体液免疫应答。
T淋巴细胞按表型不同可分为CD4+和CD8+两大亚群,它们多数表达T细胞受体,但识别的抗原和所受的主要组织相容性复合体(major histocompatibility complex,MHC)的限制性不同。
现将近年来关于CD4+T淋巴细胞亚群的研究进展综述如下。
一、CD4+T淋巴细胞分类CD4+T淋巴细胞具有高度异质性。
根据其表面标志和功能特征可分为若干亚群,各亚群之间相互调节,共同发挥其免疫学功能。
其中最主要的是CD4+辅助性T细胞(help T cell,Th)亚群,根据其功能和分泌的细胞因子不同,可分为Th1、Th2和Th3亚群。
初始CD4+T淋巴细胞接受抗原刺激后首先分化为Th0细胞,Th细胞继续分化为Th1、Th2和Th33个细胞亚群。
Th1细胞分泌白细胞介素(白介素)-2、干扰素(IFN)-α、白介素-12和IFN-γ等细胞因子;Th2细胞则分泌白介素-4、白介素-5、白介素-10和白介素-13等细胞因子;Th3细胞分泌大量的转化生长因子-β(transforming growth factorβ,TGF-β)。
一般情况下,Th1细胞与迟发性过敏反应(delayed type hy-persensitivity,DTH)、细胞毒性T淋巴细胞(cytotoxic T lymphocyte,CTL)的成熟以及自然杀伤细胞(na-ture killer,NK)的激活等有关;Th2细胞则与抗体的生成、B细胞的增殖和抗寄生虫感染等有关;Th3细胞分泌的TGF-β主要效应功能是抑制Th1介导的免疫应答和炎症反应[1]。
根据所处的活化阶段,T细胞可分为初始T细胞、效应T细胞和记忆T细胞。
记忆T细胞又可分为中心记忆细胞(central memory T cell,TCM)和效应记忆细胞(effect memory cell,TEM)[2]。
活化蛋白_1在硒拮抗砷致细胞周期改变中的作用_王艺陪

C ARCI NO G EN ES I S,TE RATO G E NE SI S&M UT A G E NE SIS收稿日期:2008-12-12;修订日期:2009-02-03基金项目:国家自然科学基金资助项目(30471500)作者简介:王艺陪(1982-),女,汉族,河北省晋州市人,硕士研究生,研究方向:遗传毒理学。
*Correspond en ce to:LU Wen-qin g,E-mail:luwq@mails.tjmu.ed The Ant a g o nis m o f S e le nium o n Ars e nic-ind uc e d Ce ll C y c le Cha n g e s b y Ac t iva t o r P ro t e in-1Re g ula t io nW ANG Yi-pei1,2,LI Xiao-nuan1,LAI Rui-ping1,YU Ri-an3,LU Wen-qing1,* (1.De p artm ent o f Occu p ational and Environm ental Health,MOE Ke y Lab o f Environm ent and Health,School o f Public Health, Ton gj i Medical Colle g e,Huazhon g U niversit y o f Science andTechnolo gy,Wuhan430030,Hubei,China)活化蛋白-1在硒拮抗砷致细胞周期改变中的作用王艺陪1,2/李晓暖1/来瑞平1/余日安3/鲁文清1,*(1.华中科技大学同济医学院公共卫生学院劳动卫生与环境卫生学系,教育部环境与健康重点实验室,湖北武汉430030;2.上海大学环境与化学工程学院污染与健康研究所,上海200444; 3.广东药学院公共卫生学院劳动卫生与环境卫生学系,广东广州510310)=摘要>背景与目的:研究激活蛋白1(activator p rotein-1,AP-1)在硒拮抗砷致细胞周期改变中的作用。
Sphingosine Kinase assay(SPHK活性检测)
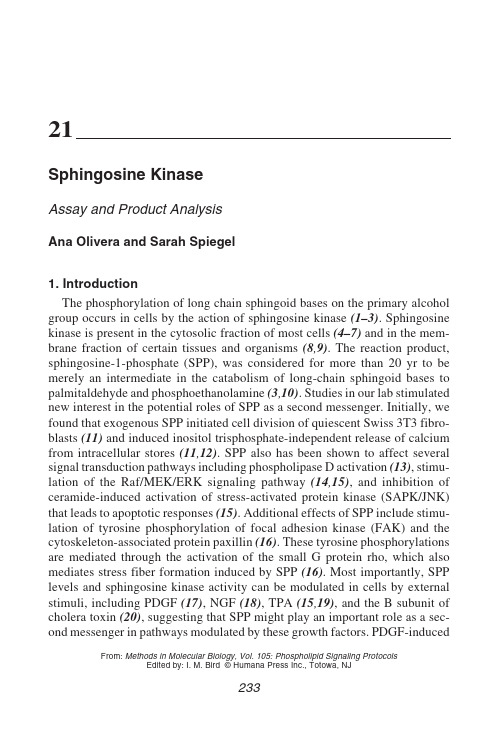
mitogenesis and cell survival in Swiss 3T3 fibroblasts is mediated, at least in part, by the formation of SPP (17). Similarly, in PC12 cells, activation of sph-ingosine kinase and generation of SPP is involved in the neurotrophic actions of NGF (18). Moreover, antigen clustering of IgE receptors on mast cells stimu-lates SPP formation, but not inositol trisphosphate, leading to calcium mobili-zation(21). Further support for the notion that SPP functions as a second messenger emerged from the use of inhibitors of sphingosine kinase. For example, the competitive inhibitors, dihydrosphingosine and N,N-dimethyl sphingosine, not only inhibit cell growth (17)and survival responses (15), but also block MAPK activation, phosphorylation of the SH2/SH3 adaptor protein Crk, and inhibit stimulation of AP-1 DNA-binding activity and cyclin-depen-dent kinase activation (cdc2 and cdk2) induced by PDGF (22,23).1.1. Sphingosine Kinase AssayIn view of the importance of SPP in cell growth and survival and the respon-siveness of sphingosine kinase to external mediators, we developed a reliable and sensitive method to measure sphingosine-kinase activity. Previously, sev-eral types of assays have been used to determine sphingosine-kinase activity utilizing either [32P]ATP or [3H]sphingosine as a tracer. A major deficiency of these methods has been the separation of the product from the substrate, since SPP is soluble, at least to some extent, in both polar and nonpolar solvents. Complex isolation procedures were developed when [3H]sphingosine was used as substrate (2,5,7), in which either the [3H]SPP product was separated on a Dowex 1 (OH-form) ion exchange column in several large-volume fractions from [3H]sphingosine(7), or separated using TCA precipitation, acetone extraction, basic extraction, saponification, acidification, and finally extrac-tion with chloroform to eliminate most of the contaminating [3H]sphingosine (2). Perhaps the simplest method of separating [3H]sphingosine from [3H]SPP is chloroform:methanol (2:1) extraction in alkaline conditions, in which most of the [3H]SPP has been reported to partition in the aqueous phase (5). How-ever, for samples that do not contain high sphingosine kinase activity, we have found that this assay with [3H]sphingosine is not reliable. Moreover, synthesis of [3H]sphingosine is not a simple task and the commercially available mate-rial is very costly.A quantitative sphingosine kinase assay using [32P]ATP has been described by Buehrer and Bell (9), in which the product is acylated to form N-caproyl-SPP. After alkaline hydrolysis to eliminate glycerophospholipids and excess caproic anhydride, N-caproyl-SPP was extracted with a biphasic system and the radiolabeled N-caproyl-SPP in the organic phase was resolved by thin-layer chromatography (TLC). We have developed a different method that is simple, sensitive, reproducible, and relatively rapid to measure sphingosine kinaseFig. 1. Autoradiogram from a representative experiment demonstrating increased formation of SPP with increasing amounts of BSA complex added. Arrow indicates the location of standard SPP visualized with ninhydrin spray.activity using [32P]ATP and sphingosine as substrates. The protocol, described below in detail, includes a description of the preparation of sphingosine kinase from Swiss 3T3 fibroblasts, which can be used for the measurement of sphin-gosine levels in cells (17). A similar protocol can also be used for preparation of sphingosine kinase from other cell types or tissues. In brief, the samples to be assayed are incubated with sphingosine and [32P]-labeled ATP at 37°C. As specified in the Subheading 4., attention should be paid to the form of delivery of sphingosine and the presence in the reaction mixture of Mg2+, the only ion required for activity. After the incubation period, [32P]-labeled lipids are extracted with a solvent in acidic conditions, in which 70–80% of the labeled SPP partitions in the organic phase, separated from the [32P]ATP. The extracted phospholipids are then separated by thin layer chromatography, visualized by autoradiography, and radioactive spots corresponding to authentic SPP are scraped from the plates and counted in a scintillation counter for quantitation. In some instances, the only radioactive spots detected had the same rela-tive migration as standard SPP (see Fig. 1). In such cases, TLC separa-tion is not necessary.Determination of sphingosine-kinase activity in crude enzyme preparations by assays described above have demonstrated a high degree of substrate ste-reospecificity. The naturally occurring D(+)-erythro-isomer of sphingosine is the most favored substrate (9,24,25). The D(+)-threo(9,24)and L(-)-threo forms (9,25)have been found to inhibit sphingosine kinase activity, whereas theL(-)-erythro isomer has been reported to be either a poor substrate (9)or an inhibitor(24).1.2. Identification and Characterization of the ProductAlthough comigration with authentic SPP on TLC and the absence of a radioactive spot corresponding to SPP when sphingosine is not included in assays are important routine controls to identify [32P]-SPP, it is important when measuring sphingosine kinase activity from different sources to ini-tially characterize the product unequivocally. Various solvent system com-binations can be used in TLC to determine whether the putative radioactive SPP comigrates with standard SPP. Apropriate solvent systems other than that described below include:choloroform:ethanol:water (65:35:8, v/v) (SPP R f= 0.31); choloroform:methanol:ammonium hydroxide (13:7:1, v/v) (R f= 0.0), in which most phospholipids, but not SPP, migrate from the ori-gin; choloroform:methanol:acetic acid (30:30:2:5, v/v) (R f= 0.35) (11); and choloroform:methanol:ammonium hydroxide (4:1:0.1, v/v).In addition, the resistance of SPP to alkaline hydrolysis can be used to fur-ther identify the product. Thus, the organic phase containing standard SPP together with the radiolabeled lipid is evaporated to near dryness, resuspended in methanolic KOH (0.1 M), and incubated at 37˚C for 1 h. After neutraliza-tion, lipids are extracted with chloroform/water and the lipids in the organic phase resolved by TLC. SPP can also be cleaved by periodate oxidation fol-lowed by borohydride reduction to yield [32P]-ethylene glycol monophosphate, which can then be separated and identified by paper chromatography (6).2. Materials2.1. Preparation of Sphingosine Kinase from Swiss 3T3 Fibroblasts 1.Culture media for Swiss 3T3 fibroblasts: cells are subcultured at a density of 1.5×104cells/cm2in DMEM supplemented with 2 m M glutamine, penicillin (100 U/mL), streptomycin (100 μg/mL), and 10% calf serum.2.Phosphate-buffered saline (PBS).3.Ice tray.4.Aspiration system.5.Sphingosine kinase buffer: 20 m M Tris-HCl (pH 7.4) (or 0.1 M potassium phos-phate, pH 7.4) containing 20% glycerol, 1 m M mercaptoethanol, 1 m M EDTA, 1 m M sodium orthovanadate, 15 m M NaF, 10 μg/mL leupeptin and aprotinin, 1 m M PMSF, 0.5 m M 4-deoxypyridoxine, and 40 m Mβ-glycerophosphate.6.Ice bucket with liquid nitrogen.7.Microfuge tubes and floating rack.8.Ultracentrifuge and 10-mL ultracentrifuge tubes.2.2. Substrate Solutions2.2.1. Sphingosine1.D-erythro-sphingosine: dissolve in ethanol at 50 m M, in a screw-capped glasstube, and store at –70°C. This solution is stable for months.2.Bovine serum albumin (BSA), tissue culture grade (4 mg/mL in PBS), or 5%Triton X-100.3.Vortex mixer.4.Bath sonicator.2.2.2. [32P]ATP/Mg2+ mixture1.ATP: 20 m M, freshly prepared in a solution containing 200 m M MgCl2.2.γ[32P]ATP (10 mCi/mL).2.3. Reaction Mixture and Incubation1.Ice bucket.2.Sphingosine kinase buffer (see Subheading2.1.).3.15-mL Conical glass centrifuge tubes with screw caps.4.Test tube rack.5.Vortex mixer.6.Water bath at 37˚C.2.4. Separation and Analysis of Sphingosine-1-Phosphate1. 1 N HCl.2.Choloform:methanol:HCl (100:200:1, v/v).3.Chloroform.4. 2 N KCl.5.Vortex mixer.6.Centrifuge.7.Aspiration system.8.TLC glass chamber containing butanol:ethanol:acetic acid:water (80:20:10:20, v/v).9.TLC silica gel G60 plates (20 × 20 or 10 × 20 cm).10.Ruler and pencil.11.Standard SPP solution (1 m M in 4 mg/mL BSA).12.Hamilton syringe or 100- to 200-μL pipet.13.Hair dryer.14.Autoradiography film.15.0.2% (w/v) Ninhydrin spray solution in ethanol.16.Silica gel scraper.17.Scintillation tubes and scintillation fluid.18.Scintillation counter set to count [32P].3. Methods3.1. Preparation of Cell Lysates Containing Sphingosine Kinase 1.Culture Swiss 3T3 fibroblasts to confluency in 100-mm Petri dishes in DMEMcontaining 10% calf serum, then serum-starve them overnight if the effect of various growth factors on sphingosine kinase is to be investigated.2.Wash cells twice on ice with 10 mL cold PBS, aspirate, and add 500 μL of sphin-gosine kinase buffer.3.Scrape cells, transfer to 1.5-mL tubes on ice, and immediately freeze in liquidnitrogen.4.Disrupt cells by freeze-thawing. This step is performed by succesively placingcells in liquid nitrogen, then thawing in 37˚C bath. Repeate six times.5.Transfer cell lysates to prechilled ultracentrifuge tubes and spin at 105,000g for90 min at 4˚C.6.Measure protein concentration of the supernatants, which correspond to cytoso-lic fractions. Supernatants can be stored at –70˚C and sphingosine kinase activity is stable for several months. However, when cells have been treated with differ-ent stimuli to determine their effect on sphingosine kinase activity, it is better to perform the assay as soon as possible.3.2. Preparation of Substrates3.2.1. Preparation of Sphingosine1.To prepare 1 m M sphingosine complexed with BSA, pipet 1 mL of the BSAsolution in a glass tube and vortex while adding 20 μL of 50 m M sphingosine drop by drop. Vortex for an additional few seconds. Sonicate the solution in a bath sonicator for 1–2 min. This solution may be slightly cloudy, but no particu-late matter should be present.2.To prepare 1 m M sphingosine-Triton X-100 micelles, pipet 1 mL of 5% Triton-X100 into a glass tube. Add 20 μL of 50 m M sphingosine, vortex, and sonicate fora few seconds. A clear solution should result.3.Both preparations of sphingosine are stable at –20˚C for a few months.3.2.2. Preparation of Radiolabeled ATP/Mg2+ Mixture1.Calculate the volume of ATP/Mg2+ mixture required by multiplying the numberof samples times 10 (10 μL of ATP mixture/sample).2.Immediatly before starting the reaction, mix 9 parts of unlabeled ATP- MgCl2and 1 part [γ32P]ATP (approx 10 μCi/sample), and vortex. Keep on ice.3.3. Reaction Mixture1.Place glass conical tubes in rack in ice-water tray.2.Add cell extracts. The protein concentration suitable for assays ranges from40–120μg for Swiss 3T3 fibroblast extracts, but this must be determined inde-pendently for each type of cell to be analyzed.3.Add sphingosine kinase buffer to 180 μL.4.Add 10 μL of 1 m M sphingosine (the final concentration, 50 μM, is saturating) deliv-ered either as sphingosine-BSA complex or sphingosine-Triton X-100 micelles.5.Vortex tubes gently.6.Start reactions by addition of 10 μL of [γ32P]ATP (10 μCi, 20 m M/ MgCl2, 200m M) and vortex gently.7.Place rack in water bath and incubate for 30 min at 37˚C (linearity with time ofincubation must be established for each cell type).8.Pipet an aliquot (1 μL) of the [γ32P]ATP into a scintillation vial and count todetemine total radioactivity added.3.4. Separation and Analysis of Sphingosine-1-Phosphate: Quantitation of Sphingosine Kinase Activity1.After the incubation period, place the rack in iced water.2.Terminate reactions by addition of 20 μL of 1 N HCl followed by 0.8 mL ofchloroform:methanol:HCl (100:200:1, v/v).3.Vortex vigorously and let stand at room temperature for 5–10 min.4.Add 240 μL of chloroform and 240 μL of 2 N KCl to separate phases.5.Vortex vigorously and let stand for 5–10 min.6.Centrifuge for 5–10 min at 400g.7.Aspirate the aqueous (upper) phase and cap tubes.8.With a pencil and a ruler, mark the origin on a TLC plate where samples will beapplied 2 cm from the bottom of the plate and 0.5 cm apart. Apply standard SPP in lanes at the end of each plate.9.Spot samples of the organic phase (50–100 μL) drop by drop onto the TLC plates,with either a Hamilton syringe or 200-μL pipet with a gel loading tip. For more rapid application of the sample, dry each spot with a hair dryer or heat the TLC plate on a warm hot plate.10.After the sample spots are completely dry, place the TLC plate in a TLC chambercontaining 1-butanol:methanol:acetic acid:water (80:20:10:20, v/v).11.When the solvent front reaches the top of the plate, remove the plate from thechamber and allow it to air dry in a fume hood.12.Expose the plate to autoradiography film for 5–16 h and then develop the film.13.As shown in Fig. 1, the only major radiolabeled phospholipid detected has thesame R f as standard SPP. However, when sphingosine kinase activity is mea-sured using extracts from other types of cells, additional spots may be detected.14.To determine the R f of standard SPP, spray the end of the plate where SPP stan-dard was spotted with ninhidryn solution, while covering up the rest of the plate.Warm the sprayed area with a hair dryer. After a few seconds, the band corre-sponding to standard SPP will be stained pink.15.Mark the areas on the TLC plate that correspond to the radioactive spots andauthentic SPP.16.Spray these areas lightly with water to wet the plates and scrape the marked areasonto a piece of weighing paper. Transfer to scintillation vials.17.Add scintillation fluid, shake, and count in a scintillation counter.18.To determine specific activity, expressed as pmol of SPP formed per minute permg protein (U), it is assumed that the ratio of radiolabeled ATP to unlabeled ATP is the same as the ratio of radiolabeled SPP to unlabeled SPP generated. First, calcu-late the specific activity of ATP as cpm/pmol by dividing cpm of [γ32P]ATP added per tube (from the total counts) by 180 nmol of unlabled ATP. From the specific activity of [γ32P]ATP, convert the cpm of the SPP spots into pmol of SPP.4. Notes1.When sphingosine is added as Triton X-100 micelles (final concentration of Tri-ton X-100 in the assay, 0.25%), the apparent activity of the enzyme is two to threefold higher than when sphingosine is added as a BSA complex. In sphin-gosine-kinase preparations from Swiss 3T3 fibroblasts and some other cell types and tissues, the stimulatory effect of Triton X-100 is detectable at con-centrations as low as 0.1%, and is maintained up to 0.5%. However, the effect of Triton X-100 in other cell types should be examined independently.2.The conditions for the sphingosine-kinase assay described (concentration of pro-tein, time of incubation, saturating concentration of sphingosine) have been opti-mized for Swiss 3T3 cells. In these experiments, the K m for sphingosine was found to be 9 μM and V max was reached at concentrations of 30–50 μM(26). 3.The specific activity of ATP can be modified, depending on the application. Ifthe sphingosine kinase activity is very low, ATP specific activity can be increased for greater sensitivity.AcknowledgmentsThis work was supported by Research Grants RO1 CA61774 and R01CA61774 from the National Institutes of Health and BE-275 from The American Cancer Society.References1.Stoffel, W., Sticht, G., and LeKim, D. (1968) Degradation in vitro ofdihydrosphingosine and dihydrosphingosine phosphate to palmitaldehyde and ethanolamine phosphate. Hoppe-Seyler’s Z. Physiol. Chem.349, 1745–1748.2.Keenan, R. W. and Haegelin, B. (1969) The enzymatic phosphorylation ofsphinganine.Biochem.Biophys. Res. Commun.37, 888–894.3.Stoffel, W., Assmann, G., and Binczek, E. (1970) Metabolism of sphingosine bases.XIII. Enzymatic synthesis of l-phosphate esters of 4t-sphingenine (sphingosine), sphinganine (dihydrosphingosine), 4-hydroxysphineanine (phytosphingosine) and 3-dehydrosphingosine by erythrocytes. Hoppe-Seyler’s Z. Physiol. Chem.351, 635–642.4.Olivera, A. and Spiegel, S. (1993) Sphingosine-l-phosphate as second messengerin cell proliferation induced by PDGF and FCS mitogens. Nature365. 557–560.5.Louie, D. D., Kisic, A., and Schroepfer, G. J. (1976) Sphingolipid base metabo-lism. Partial purification and properties of sphinganine kinase of brain. J. Biol.Chem.52, 4557–4564.6.Hirschberg, C. B., Kisic, A., and Schroepfer, G. J. (1970) Enzymatic formationof dihydrosphingosine 1-phosphate. J. Biol. Chem.245, 3084–3090.7.Stoffel, W., Heimann, G., and Hellenbroich, B. (1973) Sphingosine kinase inblood platelets. Hoppe-Seyler’s Z. Physiol. Chem.354, 562–566.8.Keenan, R. W. (1972) Sphingolipid base phosphorylation by cell-free prepara-tions from Tetrahymena pyriformis. Biochim. Biophys. Acta270, 383–396.9.Buehrer, B. M. and Bell, R. M. (1992) Inhibition of sphingosine kinase in vitroand in platelets. Implications for signal transduction pathways. J. Biol. Chem.267, 3154–3159.10.Stoffel, W., Sticht, G., and LeKim, D. (1968) Synthesis and degradation of sph-ingosine bases in Hansenula ciferrii.Hoppe-Seyler’s Z. P hysiol. Chem.349, 1149–1156.11.Zhang, H., Desai, N. N., Olivera, A., Seki, T., Brooker, G., and Spiegel, S. (1991)Sphingosine-1- phosphate, a novel lipid, involved in cellular proliferation. J. Cell Biol.114, 155–167.12.Mattie, M. E., Brooker, G., and Spiegel, S. (1994) Sphingosine-1-phosphate, aputative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J. Biol. Chem.269,3181–3188.13.Desai, N. N., Zhang, H., Olivera. A., Mattie, M. E., and Spiegel. S. (1992) Sphin-gosine-1-phosphate, a metabolite of sphingosine, increases phosphatidic acid lev-els by phospholipase D activation. J. Biol. Chem.267, 23,122–23,128.14.Wu, J., Spiegel, S., and Sturgill, T. W. (1995) Sphingosine-l-phosphate rapidlyactivates the MAP kinase pathway by a G-protein dependent mechanism. J. Biol.Chem.270, 11,484–11,488.15.Cuvillier, O., Pirianov, G., Kleuser, B., Vanek, P. G., Coso, O. A., Gutkind, J. S.,and Spiegel, S. (1996) Suppression of ceramide-mediated progammed cell death by sphingosine- 1 - phosphate. Nature381, 800–803.16.Wang, F., Nobes, C. D., Hall, A., and Spiegel, S. (1997) Sphingosine-l-phosphatestimulates rho-mediated tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 fibroblasts. Biochem. J.324, 481–488.17.Olivera, A., Rosenthal, J., and Spiegel, S. (1994) Sphingosine kinase from Swiss3T3 fibroblasts: a convenient assay for the measurement of intracellular levels of free sphingoid bases. Anal. Biochem. 233, 306–312.18.Edsall, L. C., Pirianov, G., and Spiegel, S. (1997) Involvement of sphingosine1-phosphate in nerve growth factor-mediated neuronal survival and differen-tiation.J. Neurosci.17, 6952–6960.19.Mazurek, N., Megidish, T., Hakomori, S., and Igarashi, Y. (1994) Regulatoryeffect ot phorbol esters on sphingosine kinase in BALB/C 3T3 fibroblasts (vari-ant A31): demonstration of cell type specific response–a preliminary note.Biochem. Biophys. Res. Commun.198, 1–9.20.Wang, F., Buckley, N. E., Olivera, A., Goodemote, K. A., Su, Y., and Spiegel, S.(1996) Involvement of sphingolipids metabolites in cellular proliferation modu-lated by ganglioside GM1. Glycoconjugate J.13, 937–945.21.Choi, O. H., Kim, J. H., and Kinet, J. P. (1996) Calcium mobilization by sphin-gosine kinase in signalling by the FceRI antigen receptor. Nature380,634–636.22.Rani, C. S., Wang, F., Fuior, E., Berger, A., Wu, J., Sturgil, T. W., Beitner-Johnson,D., LeRoith, D., Vartikovski, L., and Spiegel, S. (1997) Divergence in signal trans-duction pathways of platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) receptors. Involvement ol sphingosine-1-phosphate in PDGF but not EGF signaling. J.Biol. Chem.272, 10,777–10,783.23.Su, Y., Rosenthal, D., Smulson, M., and Spiegel, S. (1994) Sphingosine-l-phosphate,a novel signaling molecule, stimulates DNA binding activity of AP-1 in quiescentSwiss 3T3 fibroblasts. J. Biol. Chem.269, 16,512–16,517.24.Stoffel, W., Hellenbroich, B., and Heimann, G. (1973) Properties and specifici-ties of sphingosine kinase from blood platelets. Hoppe-Seyler’s Z. Physiol. Chem.354, 1311–1316.25.Olivera, A., Zhang, H., Robert, O. C., Mattie, M. E., Schmidt, R. R., and Spiegel,S. (1994) Stereospecificity of sphingosine-induced intracellular calcium mobili-zation and cellular proliferation. J. Biol. Chem.269, 17,924–17,930.26.Olivera, A., Rosenthal, J., and Spiegel, S. (1996) Effect of acidic phospholipidson sphingosine kinase. J. Cell. Biochem.60, 529–537.。
T细胞怎样致力于阿尔茨海默病时海马神经元的再生

T细胞怎样致力于阿尔茨海默病时海马神经元的再生阿尔茨海默病的发生与脑内神经元新生机制障碍有关,免疫学机制对神经元再生的影响成为阿尔茨海默病研究领域的关注点,T细胞是否有助于海马区神经元的再生目前还没有直接证据。
中国南方医科大学刘靖所在课题组进行的一项实验研究结果证实,T细胞有助于阿尔茨海默病时海马神经元再生,而T细胞缺陷时海马神经元再生受限,其机制可能与外周T细胞和中枢小胶质细胞产生的细胞因子表达增加有关。
实验为揭示T细胞在阿尔茨海默病病理中对神经元的作用提供了实验依据。
文章发表在《中国神经再生研究(英文版)》杂志2014年8月第16期。
免疫组织化学染色结果表明,注射Aβ1-42肽的BALB/c-nude小鼠海马神经元数量较少。
Article: "T cells promote the regeneration of neural precursor cells in the hippocampus of Alzheimer’s disease mice," by Jing Liu1, 2, Yuxin Ma2, Sumin Tian2, Li Zhang2, Mengmeng Zhao2, Yaqiong Zhang2, Dachuan Xu1 (1 Department of Human Anatomy, School of Basic Medical Sciences, Southern Medical University, Guangzhou, Guangdong Province, China;2 Department of Human Anatomy, School of Basic Courses, Guangdong Pharmaceutical University, Guangzhou, Guangdong Province, China)Liu J, Ma YX, Tian SM, Zhang L, Zhao MM, Zhang YQ, Xu DC. T cells promote the regenera tion of neural precursor cells in the hippocampus of Alzheimer’s disease mice. Neural Regen Res. 2014;9(16):1541-1547.欲获更多资讯:Neural Regen ResT cells promote the neural regeneration in the hippocampus of Alzheimer’s disease miceAlzheimer’s disease is closely associated with disorders of neurogen esis in the brain, and growing evidence supports the involvement of immunological mechanisms in the development of the disease. However, at present, the role of T cells in neuronal regeneration in the brain is unknown. Jing Liu and co-workers from Southern Medical University in China discovered that T cells promote hippocampal neurogenesis in Alzheimer’s disease and T-cell immunodeficiency restricts neuronal regeneration in the hippocampus. The mechanism underlying the promotion of neuronal regeneration by T cells is mediated by an increased expression of peripheral T cells and central microglial cytokines in Alzheimer’s disease mice. Their findings which have been reported in the Neural Regeneration Research (Vol. 9, No. 16, 2014) provide an experimental basis for understanding the role of T cells in Alzheimer’s disease.The number of neurons in the hippocampus decreased after hippocampal injection of Aβ1–42 (immunohistochemistry staining).Article: " T cells promote the regeneration of neural precursor cells in the hippocampus o f Alzheimer’s disease mice," by Jing Liu1, 2, Yuxin Ma2, Sumin Tian2, Li Zhang2, Mengmeng Zhao2, Yaqiong Zhang2, Dachuan Xu1 (1 Department of Human Anatomy, School of Basic Medical Sciences, Southern Medical University, Guangzhou, Guangdong Province, China;2 Department of Human Anatomy, School of Basic Courses, Guangdong Pharmaceutical University, Guangzhou, Guangdong Province, China)Liu J, Ma YX, Tian SM, Zhang L, Zhao MM, Zhang YQ, Xu DC. T cells promote the regeneration of neural precursor cells in the hippocampus of Alzheimer’s disease mice. Neural Regen Res. 2014;9(16):1541-1547.。
国内外异种器官移植的现状及进展

· 综述·国内外异种器官移植的现状及进展张小燕 王国辉 韩士超 戚若晨 刘克普 魏迪 杨晓剑 马帅军 窦科峰 秦卫军【摘要】 器官短缺已成为阻碍器官移植发展的主要难题,异种移植是解决全球器官匮乏最有价值的方法之一。
近年来,基因工程技术的发展和新型免疫抑制药的研发为异种移植提供了新的理论基础。
国外陆续开展基因修饰猪-非人灵长类动物或脑死亡受者的相关异种移植研究,并取得一些实质性的进展,但大部分的研究仍处于临床前阶段,距离投入临床跨越巨大。
因此,本文结合目前国内外最新的临床前实验研究进展,对异种移植的历史、基因修饰技术发展、异种移植排斥反应及免疫抑制方案等问题进行综述,以期为异种移植的进一步研究提供参考,促进异种移植临床应用,造福更多终末期疾病患者。
【关键词】 异种移植;基因修饰猪;免疫抑制药;非人灵长类动物;排斥反应;炎症反应;凝血功能障碍;感染【中图分类号】 R617,Q78 【文献标志码】 A 【文章编号】 1674-7445(2024)02-0017-06Present situation and progress of xenotransplantation at home and abroad Zhang Xiaoyan, Wang Guohui, Han Shichao,Qi Ruochen, Liu Kepu, Wei Di, Yang Xiaojian, Ma Shuaijun, Dou Kefeng, Qin Weijun. Department of Urology , Xijing Hospital of Air Force Medical University , Xi’an 710032, ChinaCorrespondingauthors:DouKefeng,Email:***************.cnQinWeijun,Email:**************.cn【Abstract 】 Organ shortage has become one of the major challenges hindering the development of organ transplantation. Xenotransplantation is one of the most valuable methods to resolve global organ shortage. In recent years,the development of genetic engineering technique and research and development of new immunosuppressant have provided novel theoretical basis for xenotransplantation. International scholars have successively carried out researches on xenotransplantation in genetically modified pigs to non-human primates or brain death recipients, making certain substantial progresses. However, most of the researches are still in the preclinical stage, far from clinical application.Therefore, according to the latest preclinical experimental research progress at home and abroad, the history of xenotransplantation, the development of gene modification technology, xenotransplantation rejection and immunosuppression regimens were reviewed, aiming to provide reference for subsequent research of xenotransplantation,promote clinical application of xenotransplantation and bring benefits to more patients with end-stage diseases.【Key words 】 Xenotransplantation; Genetically modified pig; Immunosuppressant; Non-human primate; Rejection;Inflammation; Coagulation disorder; InfectionDOI: 10.3969/j.issn.1674-7445.2023193基金项目:国家自然科学基金(82101322、82200845)作者单位: 710032 西安,空军军医大学西京医院泌尿外科作者简介:张小燕(ORCID 0000-0002-1199-988X ),硕士,住院医师,研究方向为肾移植与肾纤维化,Email :156****6095@通信作者:窦科峰(ORCID 0000-0003-1708-8048),主任医师,中国科学院院士,研究方向为异种移植与肝胆疾病,Email:***************.cn ;秦卫军(ORCID 0000-0001-5202-642X ),博士,主任医师,研究方向为肾移植与泌尿系肿瘤,Email:**************.cn第 15 卷 第 2 期器官移植Vol. 15 No.2 2024 年 3 月Organ Transplantation Mar. 2024 我国慢性肾病的发病率高达10.8%[1]。
Role of alkaline ceramidases in the generation ofsphingosine and its phosphate in erythrocytes

The FASEB Journal•Research CommunicationRole of alkaline ceramidases in the generation of sphingosine and its phosphate in erythrocytes Ruijuan Xu,*Wei Sun,*Junfei Jin,*Lina M.Obeid,*,†,‡and Cungui Mao*,†,1¶*Department of Medicine,†Department of Biochemistry and Molecular Biology,and‡Ralph H.Johnson Veterans Administration Hospital at the Medical University of South Carolina,Charleston, South Carolina,USAABSTRACT Plasma sphingosine-1-phosphate(S1P) has been suggested to mainly originate from erythro-cytes;however,within the erythrocyte,how sphingosine (SPH)generation—the precursor to S1P—is controlled is unknown.SPH is only generated from the hydrolysis of ceramides via ceramidases.Five human ceramidases have been identified:1acid,1neutral,and3alkaline ceramidases(ACER1,ACER2,and ACER3).Here,we demonstrate that only alkaline ceramidase activity is expressed in erythrocytes and that it is instrumental for SPH generation.Erythrocytes have alkaline but not acid or neutral ceramidase activity on D-e-C18:1-ceramide,a common substrate of ceramidases.Not only alkaline ceramidase activity but also the generation of SPH and S1P are increased during erythroid differentiation in K562erythroleukemic cells.Such SPH and S1P in-creases were inhibited by the alkaline ceramidase inhib-itor D-e-MAPP,suggesting that alkaline ceramidases have a role in the generation of SPH and S1P in erythroid cells.Alkaline ceramidase activity is highly expressed in mouse erythrocytes,and intravenous ad-ministration of D-e-MAPP decreased both SPH and S1P in erythrocytes and plasma.Collectively,these results suggest that alkaline ceramidase activity is important for the generation of SPH,the S1P precursor in erythrocytes.—Xu,R.,Sun,W.,Jin,J.,Obeid,L.M., Mao,C.Role of alkaline ceramidases in the generation of sphingosine and its phosphate in erythrocytes. FASEB J.24,2507–2515(2010)Key Words:ceramide⅐erythroid cells⅐S1P⅐and S1P lyaseSphingosine-1-phosphate(S1P),a sphingolipid me-tabolite,is a ligand of the5G-protein-coupled recep-tors,S1P1-5,which mediate cell proliferation,survival, adhesion,and migration,in addition to various biolog-ical processes,including cardiovascular development (1),angiogenesis(2),and immunity(3).The metabolic pathway of S1P in mammalian cells has been delineated.Sphingomyelin is hydrolyzed by sphin-gomyelinases to generate ceramide(4–6),which is fur-ther hydrolyzed via ceramidases(7)(8–11)to generate sphingosine(SPH),which,in turn is phosphorylated to form S1P by sphingosine kinases(12,13),SPHK1and SPHK2.Subsequently,S1P is irreversibly cleaved by S1P lyase(SPL)(14,15)to produce ethanolamine phosphate and hexadecenal,which are incorporated into phosphati-dylethanolamine and glycerolipids,respectively.S1P can also be dephosphorylated to yield SPH by S1P-specific phosphatases(16,17),SPP1and SPP2,or lipid phospha-tases with broad specificity,LPP1–3(18).S1P is abundant in plasma(19–21),and plasma S1P has been suggested to mainly originate from erythrocytes(21, 22).However,how the generation of the S1P precursor, SPH,is regulated in erythrocytes remains unclear.As stated earlier,SPH is only generated from cer-amide by ceramidases.To date,5human ceramidase genes have been identified:1acid ceramidase gene (AC/ASAH1)(7),1neutral ceramidase gene(NC/ ASAH2)(9),and3alkaline ceramidase genes,ACER1/ ASAH3(23),ACER2/ASAH3L(11),and ACER3/APHC/ PHCA(24).These ceramidases have different cellular localizations and substrate specificities.AC/ASAH1is a lysosomal ceramidase that has an optimal pH value of 4.5for its in vitro activity.It catalyzes the hydrolysis of ceramides with saturated medium acyl chains(C10-14) or unsaturated long acyl chains(C18:1or C18:2)(25). ASAH2is localized to the plasma membrane or se-creted from cells(26).It has an optimal pH value of ϳ7.0and uses various ceramides as substrates(27). ACER1is localized to the endoplasmic reticulum(ER) and has an optimal pH value ofϳ9.0.It only catalyzes the hydrolysis of ceramides with unsaturated long acyl chains(C18:1and C20:1)(unpublished data)or a very long saturated(C24:0)or unsaturated(C24:1)acyl chain (23).ACER2is a Golgi ceramidase with an optimal pH value ofϳ9.0.It uses various ceramides as substrates (11,28).ACER3is localized to both the ER and Golgi complex,and it has an optimal pH ofϳ9.0.We initially demonstrated that ACER3preferentially catalyzes the hydrolysis of phytoceramide,an analog of ceramide (24).Our recent studies showed that ACER3preferen-tially catalyzes the hydrolysis of ceramides carrying unsaturated long acyl chains(C18:1and C20:1)(29).In this study,we investigate the role of ceramidases in controlling the generation of SPH and S1P in erythro-cytes.We demonstrate that erythrocytes express alka-line ceramidase activity but not acid or neutral cerami-dase.Alkaline ceramidase activity,SPH,and S1P are1Correspondence:Division of General Internal Medicine, Department of Medicine,114Doughty St.,Rm.646,STB, P.O.Box250779,Charleston,SC29425,USA.E-mail: maoc@doi:10.1096/fj.09-15363525070892-6638/10/0024-2507©FASEBupregulated during erythroid differentiation of K562 cells,an erythroblastic leukemia cell line.Inhibiting the activity of ACERs with the alkaline ceramidase inhibitor D-e-MAPP blocks the SPH and S1P increases in re-sponse to erythroid differentiation,and intravenous administration of D-e-MAPP also decreases SPH and S1P in erythrocytes and plasma in mice.This suggests that ACERs have an important role in controlling the generation of SPH and S1P in erythrocytes and plasma.MATERIALS AND METHODSReagentsAnti-S1P lyase antibody was a kind gift from Drs.Akio Kihara and Yasuyuki Igarashi(Hokkaido University,Sapporo,Japan).MEM and RPMI1640medium,fetal bovine serum(FBS),trypsin-EDTA,PBS,penicillin/streptomycin solution,blasticidin,and zeocin were purchased from Invitrogen(Carlsbad,CA,USA). d-erythro(e)-C18:1-ceramide,N-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]lauroyl]-phytosphingosine(D-ribo-C12-NBD-phytocer-amide),and(1S,2R)-d-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol(D-e-MAPP)were synthesized in the Lipidomics Core Facility at Medical University of South Carolina.D-e-C16-ceramide, D-e-C24:1-ceramide,SPH,S1P,and other sphingolipids were from Avanti Polar Lipids(Alabaster,AL,USA).Other unstated reagents were from Sigma(St.Louis,MO,USA).AnimalsFVB/N mice(4to6wk old)were purchased from Charles River(Wilmington,MA,USA).All animal procedures were carried out in accordance with the Medical University of South Carolina animal care committee guidelines.Cell lines and culture conditionsHuman leukemia K562cells(American Type Culture Collec-tion,Manassas,VA,USA)were cultured in RPMI1640 medium supplemented with10%FBS and1%penicillin/ streptomycin.Wild-type T-REx HeLa cells(Invitrogen)were cultured in MEM supplemented with10%FBS,1%penicil-lin/streptomycin solution,and10g/ml blasticidin.T-REx HeLa derivatives AC-TET-ON,ACER1-TET-ON,ACER2-TET-ON,and ACER3-TET-ON cells were cultured in the same medium supplemented with50M/ml zeocin.AC-TET-ON, ACER1-TET-ON,and ACER2-TET-ON cell lines that stably overexpress AC,ACER1,and ACER2,respectively,under the control of the tetracycline-inducible promoter system,CMV-TET-ON,were constructed in our previous studies(11,30). The same procedures were used to create the ACER3-TET-ON cell line in this study.The ACER3coding sequence was cloned inframe with the FLAG coding sequence in pcDNA4-FLAG,as described previously(11),and the result-ing expression construct pcDAN4-ACER3was transfected into T-REx HeLa cells.Transfected cells were selected in medium containing300g/ml zeocin,and zeocin-resistant clones were confirmed to express the FLAG-tagged ACER3strictly under the control of the CMV-TET-ON promoter system.Erythroid differentiation of K562cellsTo induce erythroid differentiation,K562cells were treated for72h with1--d-arabinofuranosylcytosine(Ara-C,5ϫ10Ϫ7 M),as described previously(31).Erythroid differentiation was determined by staining with benzidine as described previously(32).Blood cell preparationMouse blood was drawn into heparinized syringes from anesthetized mice via cardiac puncture and immediately put into15-ml tubes with heparin solution(50U/ml blood)on ice.Blood cells were separated from plasma by centrifugation at1200g for5min at4°C,and washed twice with ice-cold PBS. Erythrocytes were separated from other cell types by Ficoll gradient(GE Healthcare,Piscataway,NJ,USA),according to the manufacturer’s instructions and the method of Hanel et al.(22).Human erythrocytes were purchased from Innovative Research(Novi,MI,USA),and further purified by the Ficoll gradient procedure as described above.siRNA transfectionControl siRNA(siCON)[r(UUCUCCGAACGUGUCAC-GU)d(TT)(sense)and r(ACGUGACACGUUCGGAG-AA)d(TT)(antisense)]and a SPL-specific siRNA(siSPL) [r(GUGCCCAUGCUGCAUUUAA)d(TT)(sense)and r(UUAAAUGCAGCAUGGGCAC)d(TT)(antisense)]were chem-ically synthesized by Dharmacon Inc.(Lafayette,CO,USA);siRNAs at5nM were transfected into T-REx HeLa cells and derivatives using Oligofectamine(Invitrogen),according to the manufactur-er’s instructions.Lentiviral shRNA transductionK562cells were transduced with Mission lentiviral transduc-tion particles(Sigma)containing a control shRNA(shCON) targeting none of the human genes or expressing a set of shRNAs that specifically target ACER1(shACER1),ACER2 (shACER2),ACER3(shACER3),or SPL(shSPL)(Sigma) according to the manufacturer’s instructions.Quantitative PCR analysisTotal RNA was isolated from K562cells using an RNeasy mini kit(Qiagen,Valencia,CA,USA)according to the manufac-turer’s instructions.RNA was reversely transcribed into cDNA and subjected to real-time PCR analysis,which was performed on an iCycler system(Bio-Rad,Hercules,CA,USA).Standard reaction volume was25l,including10l of iQ SYBR Green Supermix(Bio-Rad),10l of cDNA template,and2.5l of a primer mixture.The initial PCR step was3min at95°C, followed by40cycles of a10-s melting at95°C and a45-s annealing/extension at60°C.Thefinal step was1-min incu-bation at60°C.All reactions were performed in triplicate. Real-time RT-PCR results were analyzed using Q-Gene soft-ware(),which expresses data as mean normalized expression(MNE)(33).MNE is directly propor-tional to the amount of mRNA of a target gene relative to the amount of mRNA of the reference gene(-actin).Primers used in this study were5Ј-TGATGCTTGACAAGGCACCA-3Јand5Ј-GGCAATTTTTCATCCACCACC-3Јfor ACER1;5Ј-AGTGTCCTGTCTGCGGTTACG-3Јand5Ј-TGTTGTTGATG-GCAGGCTTGAC-3Јfor ACER2;5Ј-CAATGTTCGGTGCAAT-TCAGAG-3Јand5Ј-GGATCCCATTCCTACCACTGTG-3Јfor ACER3;and5Ј-CAATGTTCGGTGCAATTCAGAG-3Јand5Ј-GGATCCCATTCCTACCACTGTG-3Јfor-actin.Protein concentration determinationProtein concentrations were determined with BSA as a stan-dard using a BCA protein determination kit(Pierce,Rock-ford,IL,USA)according to the manufacturer’s instructions.2508Vol.24July2010XU ET AL.The FASEB Journal⅐Western blot analysisProteins were resolved by SDS-PAGE,and then subjected to Western blot analysis using various antibodies,as described previously(11).Ceramidase assaysCeramidase activity was determined by the release of SPH from the hydrolysis of various ceramides,according to the method we previously developed(11).Acid ceramidase activ-ity and neutral ceramidase activity in total cell lysates were determined using D-e-C18:1-ceramide at pH 5.0and7.0, respectively.Alkaline ceramidase activity was determined in total membranes at pH9.4using various ceramides as sub-strates.The substrate concentration was150M.SPL activity assaysSPL activity assays were performed essentially as described by Bandhuvula et al.(34)using thefluorescent analog NBD-S1P as a substrate.Electrospray ionization/dual mass spectroscopy(ESI/MS/MS)lipid analysisTotal lipids were extracted from cell pellets or conditioned medium and sphingolipids were subjected to ESI/MS/MS analysis that was performed on a Thermo Finnigan TSQ7000 triple quadrupole mass spectrometer(Thermo Finnegan,San Jose,CA,USA),operating in a multiple reaction monitoring (MRM)positive ionization mode,as described previously (35).Sphingolipid contents were normalized to total phos-phate(P)in lipid extracts.Statistic analysisStudent’s t test was applied for statistical analysis using the software GraphPad Prism(GraphPad,San Diego,CA,USA). Values of PϽ0.05were considered significant.Data repre-sent meanϮsd values ofՆ3independent experiments.RESULTSAlkaline but not acid or neutral ceramidase activity is expressed in erythrocytesTo determine how SPH generation is controlled in erythrocytes,wefirst determined which ceramidase activity is expressed in these anucleated cells.We measured ceramidase activity using D-e-C18:1-cer-amide,a common substrate for all known cerami-dases in the presence of Ca2ϩ,which is required for alkaline ceramidase activity.We demonstrated that erythrocytes had high alkaline ceramidase activity on D-e-C18:1-ceramide in the presence of1mM Ca2ϩ, with undetectable acid or neutral ceramidase activity (Fig.1A),suggesting that erythrocytes express alka-line ceramidase activity but not acid or neutral ceramidase activity.To differentiate the activity of individual alkaline cer-amidases,we measured erythrocyte alkaline ceramidase activity on D-e-C16-ceramide(a substrate specific for ACER2),D-e-C24:1-ceramide(a substrate for both ACER1 and ACER2but not for ACER3),and D-ribo-C12-NBD-phytoceramide(a substrate specific for ACER3),in the presence of Ca2ϩ.As shown in Fig.1B,erythrocytes have high alkaline ceramidase activity on D-ribo-C12-NBD-phy-toceramide and moderate alkaline ceramidase activity on either D-e-C16-ceramide or D-e-C24:1-ceramide,suggesting that erythrocyte alkaline ceramidase activity is mainly encoded by ACER3.Alkaline ceramidases are upregulated in erythroid cellsBecause erythrocytes are anucleated cells,it is impossi-ble to genetically manipulate protein expression in vitro.Therefore,it is difficult to define the role of the alkaline ceramidases in regulating SPH and S1P di-rectly in these anucleated cells.To circumvent this Figure1.Alkaline but not acid or neutral ceramidase is expressed in human erythrocytes.A)Total membranes were prepared from human erythrocytes and subjected to cerami-dase activity assays using D-e-C18:1-ceramide as a substrate at pH5.0or7.0in the absence of Ca2ϩor at pH9.4in the presence of1mM CaCl2.B)Total membranes of human erythrocytes were assayed for alkaline ceramidase activity on D-e-C12-NBD-ceramide,D-e-C16-ceramide,or D-e-C24:1-cer-amide at pH9.4and in the presence of1mM Ca2ϩ.Data represent meansϮsd of3experiments performed in dupli-cate.2509ALKALINE CERAMIDASES IN PLASMA S1P GENERATIONproblem,we investigated the role of alkaline cerami-dases in controlling the generation of SPH and S1P in an in vitro erythroid differentiation model.It has been shown that on treatment with a chemo-therapeutic agent Ara-C,K562cells,a pluripotent erythroleukemic cell line,differentiate into erythroid cells(31).As thefirst step to define the role of alkaline ceramidase activity in regulating the generation of SPH and S1P in erythroid cells,we determined whether alkaline ceramidase activity and SPH and S1P levels are coregulated during erythroid differentiation.We showed that treatment of K562cells with Ara-C markedly increased the number of cells stained pos-itive with benzidine,erythroid cells(Fig.2A),con-firming the erythroid differentiation of K562cells. Similar to erythrocytes,K562cells have higher alka-line ceramidase activity on D-ribo-C12-NBD-phytocer-amide or C18:1-ceramide than alkaline ceramidase activity on C16-ceramide or D-e-C24:1-ceramide,and these activities were increased by treatment with Ara-C(Fig.2B),suggesting that erythroid cells differ-entiated from K562cells have a similar expression pattern of alkaline ceramidases as erythrocytes and that alkaline ceramidase activity is up-regulated dur-ing erythroid differentiation.qPCR analyses revealed that treatment with Ara-C increased mRNA of ACER2 and ACER3,but not ACER1(Fig.2C),suggesting that both ACER2and ACER3are up-regulated in ery-throid cells.ESI/MS/MS analysis demonstrated that treatment with Ara-C caused a3-fold and5-fold increase in SPH and S1P,respectively,in K562cells (Fig.2D),suggesting that the generation of SPH and S1P is also increased during erythroid differentia-tion.Collectively,these results suggest that alkaline ceramidase activity and the generation of SPH and S1P are up-regulated simultaneously during ery-throid differentiation of K562cells.Alkaline ceramidases have a compensatory role in controlling SPH and S1P generation in erythroid cells To determine whether alkaline ceramidase activity is responsible for the Ara-C-induced generation of both SPH and S1P in erythroid cells,we knocked down the expression of ACER1,ACER2,or ACER3in K562cells using their specific short hairpin RNAs(shRNAs)de-livered by a lentiviral vector system.Interestingly,we found that compared to transduction with lentiviral particles expressing a control shRNA(shCON),trans-duction with lentiviral particles expressing an ACER3-specific shRNA(shACER3)decreased ACER3mRNA but increased ACER2mRNA in K562cells;and trans-duction with lentiviral particles expressing ACER2-specific shRNA(shACER2)decreased ACER2mRNA but increased ACER1mRNA and vice versa(Fig.3A), suggesting that knocking down the expression of one alkaline ceramidase up-regulates another.Because of this complementary effect,we attempted to knock down the expression of ACER1,ACER2,and ACER3 simultaneously in K562cells.However,cotransduction of K562cells with shACER1,shACER2,and shACER3 lentiviral particles knocked down none of the alkaline ceramidases(data not shown).To block all alkaline ceramidase activity,we used the alkaline ceramidase inhibitor,D-e-MAPP,that potently and specifically inhibits alkaline ceramidase activity(32).To confirm that D-e-MAPP indeed in-hibits ACER1,ACER2,and ACER3activity in cells,we tested whether treatment with D-e-MAPP inhibited the SPH generation catalyzed by ACER1,ACER2,or ACER3in cells.We previously established the T-REx-HeLa-based stable cell lines,ACER1-TET-ON and ACER2-TET-ON cells that overexpress ACER1and ACER2,respectively,under the control of a tetracy-cline-inducible promoter system,CMV-TET-ON(30). In these stable cell lines,the overexpression ofFigure2.Both alkaline ceramidase activity and SPH and S1P levels are increasedduring erythroid differentiation.A)K562cells were treated with Ara-C or H2O(vehicle control)for72h before erythroid differentiation was determined bybenzidine staining as described in Materials and Methods.Benzidine-positive cellswere counted using a microscope.B)Microsomes were isolated from K562cellstreated with Ara-C as in A,and alkaline ceramidase activity on different substrates wasmeasured as in Fig.1B.C)Total RNA was isolated from K562cells treated with Ara-Cor H2O and subjected to qPCR analysis to measure ACER1,ACER2,or ACER3mRNA.D)K562cells treated with Ara-C or H2O were harvested by centrifugation,washed with50mM Tris-HCl(pH7.4)containing150mM NaCl,and subjected toESI/MS/MS for SPH and S1P measurement.Data represent meansϮsd of3experiments performed in duplicate.*PϽ0.05.2510Vol.24July2010XU ET AL.The FASEB Journal⅐ACER1or ACER2was induced by the addition of tetracycline (TET)to medium but not by the addi-tion of ethanol (ET),the vehicle control,resulting in the increased generation of SPH in cells (30).ACER1-TET-ON or ACER2-TET-ON cells grown in the presence of ET or TET were treated with D-e-MAPP or DMSO,the vehicle control.HPLC analyses showed that treatment with D-e-MAPP at 10M completely inhibited the TET-induced increase in SPH generation in either ACER1-TET-ON or ACER2-TET-ON cells (Fig.3B ,C ),suggesting that D-e-MAPP indeed inhibits cellular ACER1and ACER2activity.To determine whether D-e-MAPP also inhibited cel-lular ACER3activity,we generated the cell line ACER3-TET-ON,a T-REx HeLa derivative that stably overexpresses ACER3under the control of the TET-ON promoter system.Western blot confirmed that the overexpression of ACER3was induced by TET but not ET in ACER3-TET-ON cells (Fig.3D ).ACER3overexpression increased SPH,and this was inhibited by D-e-MAPP (Fig.3E ),suggesting thatD-e-MAPP also inhibits ACER3cellular activity.We previously demonstrated that overexpression of AC also increases the generation of SPH in T-REx HeLa cells (30).To confirm that D-e-MAPP specifically inhibits alkaline ceramidase activity but not AC,we investigated whether D-e-MAPP inhibits the AC-catalyzed generation of SPH in cells.We previously generated the AC-TET-ON cell line that overex-presses AC under the control of the CMV-TET-ON system,and TET-induced overexpression of AC in-creased the generation of cellular SPH (30).AC-TET-ON cells grown in the presence of ET or TET were treated with D-e-MAPP or DMSO.HPLC analysis revealed that AC overexpression increased SPH to a similar extent in AC-TET-ON cells treated with DMSO and those treated with D-e-MAPP (Fig.3F ),confirming that D-e-MAPP does not inhibit AC activ-ity in cells.Subsequently,we tested whether treatment with D-e-MAPP inhibited the generation of SPH and S1P in erythroid cells.K562cells were treated with Ara-CorFigure 3.Inhibition of alkaline ceramidase activity suppresses SPH and S1P increases in response to erythroid differentiation.A )K562cells were transduced with Mission lentiviral particles expressing a control nontargeting shRNA (shCON)or a shRNA specific for ACER1(shACER1),ACER2(shACER2),or ACER3(shACER3)at a MOI of 5.At 72h after lentiviral transduction,ACER1or ACER2mRNA levels were determined by qPCR analysis as described in Materials and Methods.B,C )ACER1-TET-ON cells (B )or ACER2-TET-ON cells (C )were grown in the presence of TET (10ng/ml)or ET (vehicle control)for 24h before being treated with D-e-MAPP (10M)or DMSO (vehicle control)for 4h.SPH in these cells was measured by HPLC.D )ACER3-TET-ON cells,created as described in Materials and Methods,were grown in the presence of ET and TET for 48h before expression ofthe FLAG-tagged ACER3(FLAG-ACER3)was analyzed by Western blot with anti-FLAG antibody.E )ACER3-TET-ON cells were grown in the presence of ET or TET for 24h before being treated with D-e-MAPP or DMSO for 4h.SPH was measured by HPL.F )AC-TET-ON cells were grown in the presence of ET or TET for 24h before being treated with D-e-MAPP or DMSO for 4h.SPH was measured by HPLC.G )K562cells were treated with Ara-C or H 2O in the presence of D-e-MAPP or DMSO for 72h before SPH and S1P were measured by ESI/MS/MS.Data represent means Ϯsd of 3experiments performed in duplicate.*P Ͻ0.05.2511ALKALINE CERAMIDASES IN PLASMA S1P GENERATIONH2O(the vehicle control)in the presence of D-e-MAPP and its vehicle,DMSO.Treatment with Ara-C increased both SPH and S1P in K562cells treated with DMSO but failed to do so in K562cells treated with D-e-MAPP(Fig. 3G),suggesting that alkaline ceramidase activity is responsible for the Ara-C-induced generation of SPH and S1P in erythroid cells.Inhibition of alkaline ceramidase activity blocks SPL knockdown-induced accumulation of S1P in erythroid cellsAlthough erythroid cells differentiated from K562cells have more S1P than undifferentiated K562cells,they produce less S1P than erythrocytes.It has been sug-gested that anucleated erythrocytes accumulate S1P due to a lack of SPL activity(20).This prompted us to investigate whether inhibiting SPL would also lead to S1P accumulation in erythroid cells,and if so,whether blocking alkaline ceramidase activity inhibits S1P accu-mulation.We thus knocked down SPL expression in K562cells by RNA interference(RNAi).K562cells were transduced with lentiviral particles expressing a SPL-specific shRNA(shSPL)or shCON lentiviral particles. Compared to transduction with shCON,transduction with shSPL markedly decreased SPL protein in K562 cells(Fig.4A).The decrease in SPL protein led to a marked decrease in SPL activity(Fig.4B).ESI/MS/MS analyses demonstrated that SPL knockdown or Ara-C treatment alone caused a7-to8-fold increase in S1P in K562cells,whereas the combination of SPL knockdown and Ara-C treatment caused a40-fold increase in S1P (Fig.4C)and that the SPL knockdown-induced accu-mulation of S1P was inhibited by treatment with D-e-MAPP(Fig.4D),suggesting that alkaline ceramidase activity is required for S1P accumulation in erythroid cells,in which SPL activity is inhibited.Alkaline ceramidase overexpression along with SPL knockdown results in S1P accumulation in cellsTo further confirm that high alkaline ceramidase activ-ity is sufficient for S1P accumulation in cells in which SPL is inhibited,we determined whether SPL knock-down along with ACER1,ACER2,or ACER3overex-pression also results in S1P accumulation in T-REx HeLa cells.ACER1-TET-ON,ACER2-TET-ON cells,or ACER3-TET-ON cells were transfected with siCON or siSPL for24h before the overexpression of ACER1, ACER2,or ACER3was induced by treatment with TET or not induced by treatment with pared to transfection with siCON,transfection with a SPL-spe-cific siRNA(siSPL)markedly decreased SPL activity in ACER1-TET-ON,ACER2-TET-ON,or ACER3-TET-ON cells(Fig.5A,C,E).ESI/MS/MS analyses revealed that knocking down the expression of SPL caused a several-fold increase in S1P in ACER1-TET-ON and ACER2-TET-ON cells grown in the presence of ET,but greater than a20-fold increase in S1P in either ACER1-TET-ON or ACER2-TET-ON cells grown in the presence of TET (Fig.5B,D).SPL knockdown caused a2-fold and3-fold increase in S1P in ACER3-TET-ON cells grown in the presence of ET and TET,respectively(Fig.5F).These results suggest that high alkaline ceramidase activity, especially encoded by ACER1or ACER2,is sufficient for S1P accumulation in cells in which SPL is inhibited. Interestingly,we showed that SPL knockdown along with ACER1or ACER2overexpression also markedly increased DHS1P in cells(Fig.5B,D),suggesting that ACER1and ACER2also play an important role in controlling DHS1P levels by catalyzing the hydrolysis of dihydroceramides into DHS in cells.Alkaline ceramidase activity is important for the generation of SPH and S1P in mouse erythrocytes and plasmaOur in vitro studies suggest that alkaline ceramidase activity is important for the generation of both SPH and S1P in human erythroid cells.To determine whether mouse alkaline ceramidase activity is impor-tant for the generation of S1P in mouse erythrocytes and plasma,we investigated whether inhibiting alka-line ceramidase activity in vivo inhibits the genera-tion of S1P in mouse erythrocyte plasma.First,we determined whether mouse erythrocytes alsoexpressFigure4.Inhibition of alkaline ceramidase activity blocks S1P accumulation in response to knockdown of SPL in erythroid cells. A,B)K562cells were transduced with Mission lentiviral particles expressing a SPL-specific shRNA(shSPL)or the control siRNA (shCON)for72h before being analyzed by Western blot with anti-SPL antibody(A)or by in vitro activity assays(B).C)K562 cells were transduced with shCON or shSPL Mission lentiviral particles in the presence of Ara-C or H2O for72h before S1P was measured by ESI/MS/MS.D)K562cells transduced with shSPL lentiviruses were treated with Ara-C or H2O in the presence of D-e-MAPP or DMSO for72h before S1P was measured.Data represent meansϮsd of3experiments performed in duplicate. *PϽ0.05.2512Vol.24July2010XU ET AL.The FASEB Journal⅐alkaline ceramidase activity.We detected high alka-line ceramidase activity on D-e-C 18:1-ceramide in membranes isolated from mouse erythrocytes (Fig.6A ).To inhibit alkaline ceramidase activity in mouse erythrocytes in vivo ,mice were administered D-e-MAPP (2.5nmol/g iv)or vehicle control DMSO for 6h before the measurement of SPH and S1P.ESI/MS/MS analyses revealed that compared to those animals treated with DMSO,mice given D-e-MAPP had less SPH (Fig.6B )and S1P (Fig.6C )in erythro-cytes or plasma (Fig.6B ),suggesting that alkaline ceramidase activity is important for the generation of SPH and S1P in mouse erythrocytes.DISCUSSIONErythrocytes were found to be the major source of plasma S1P (21).However,how erythrocytes generate SPH,the S1P precursor,remains unclear.In this study,we provide strong evidence that alkaline ceramidase activity plays an important role in controlling the generation of SPH in erythrocytes,and thereby S1P in plasma.The study by Ito et al.(20)suggested that erythro-cytes have no ceramidase activity.In contrast,our results indicate that erythrocytes have alkaline cerami-dase activity—a discrepancy brought about bydiffer-Figure 5.Increasing alkaline ceramidase activity results in S1P accumulation in cells with SPL knockdown.A ,B )ACER1-TET-ON cells grown in the presence of ET or TET were transfected with a SPL-specific siRNA (siSPL)or a control siRNA (siCON)for 72h before SPL activity (A )and S1P levels (B )were measured.C ,D )ACER2-TET-ON cells grown in the presence of ET or TET were transfected with siSPL or siCON for 72h before SPL activity (C ),and the levels of S1P (D )were determined.E ,F )ACER3-TET-ON cells grown in the presence of ET or TET were transfected with siSPL or siCON for 72h before SPL activity (E ),and the levels of S1P (F )were determined.Data represent means Ϯsd of 3experiments performed in duplicate.*P Ͻ0.05.Figure 6.Inhibition of alkaline ceramidase activity decreases mouse plasma S1P and DHS1P.A )Blood was drawn from mice,and blood cells were separated from plasma.Erythrocytes were separated from other blood cells by Ficoll gradient and subjected to alkaline ceramidase activity assays with indicated substrates.B ,C )D-e-MAPP (2.5or 5.0nmol/g)or vehicle control DMSO was injected into mice through tail veins.At 12h postinjection,blood was drawn from mice,and erythrocytes and plasma were subjected to ESI/MS/MS to measure SPH (B )and S1P (C ).Data represent means Ϯsd in 3mice.*P Ͻ0.05.2513ALKALINE CERAMIDASES IN PLASMA S1P GENERATION。
RIC1在拟南芥根的生长发育过程中正调控生长素信号负调控ABA信号

Arabidopsis ROP-interactive CRIB motif-containing protein 1(RIC1)positively regulates auxin signalling and negatively regulates abscisic acid (ABA)signalling during root developmentYUNJUNG CHOI 1,YUREE LEE 1,SOO YOUNG KIM 3,YOUNGSOOK LEE 1,2&JAE-UNG HWANG 11POSTECH-UZH Global Research Laboratory,Division of Molecular Life Sciences,Pohang University of Science andTechnology (POSTECH),Pohang 790-784,Korea,2Division of Integrative Bioscience and Biotechnology,POSTECH,Pohang 790-784,Korea and 3Department of Molecular Biotechnology &Kumho Life Science Laboratory,College of Agriculture and Life Sciences,Chonnam National University,Gwangju 500-757,KoreaABSTRACTAuxin and abscisic acid (ABA)modulate numerous aspects of plant development together,mostly in opposite directions,suggesting that extensive crosstalk occurs between the signal-ling pathways of the two hormones.However,little is known about the nature of this crosstalk.We demonstrate that ROP-interactive CRIB motif-containing protein 1(RIC1)is involved in the interaction between auxin-and ABA-regulated root growth and lateral root formation.RIC1expression is highly induced by both hormones,and expressed in the roots of young seedlings.Whereas auxin-responsive gene induction and the effect of auxin on root growth and lateral root formation were suppressed in the ric1knockout,ABA-responsive gene induction and the effect of ABA on seed germination,root growth and lateral root for-mation were potentiated.Thus,RIC1positively regulates auxin responses,but negatively regulates ABA responses.Together,our results suggest that RIC1is a component of the intricate signalling network that underlies auxin and ABA crosstalk.Key-words :hormone crosstalk;lateral root;RIC protein;root growth;ROP GTPase.INTRODUCTIONAuxin and abscisic acid (ABA)are two major plant growth regulators.In general,auxin promotes the growth of vegeta-tive tissues,whereas ABA suppresses proliferation and confers stress resistance.For example,auxin promotes lateral root initiation,whereas ABA inhibits it.Auxin opens stomata,whereas ABA closes them.Such antagonistic effects of two hormones have been reported to regulate numerous stress responses and developmental and physiological pro-cesses in the plant (Gehring,Irving &Parish 1990;Casimiro et al .2003;Tanaka et al .2006).The interaction between auxin and ABA seems to be more complex during early seedlingdevelopment and primary root elongation than later on.Although both auxin and ABA are necessary for early seed-ling development,exogenously applied ABA,which presum-ably is applied at a significantly greater concentration than the endogenous hormone,inhibits growth.Primary root elon-gation is promoted by nanomolar amounts of both auxin and ABA (Gaither,Lutz &Forrence 1975;Mulkey,Kuzmanoff &Evans 1982),but is inhibited by higher concentrations of these hormones (Pilet &Chanson 1981;Mulkey et al .1982;Eliasson,Bertell &Bolander 1989).The observation that the two hormones function together to regulate many responses indicates that the signalling pathways that transduce the primary hormonal signals to downstream responses may intersect at specific points and/or involve common players.Indeed,the expression of ABA INSENSITIVE 3(ABI3)is activated by both auxin and ABA,and ABI3functions as a positive regulator of ABA-mediated inhibition of seed ger-mination and as a negative regulator of auxin-mediated lateral root formation and ABA-mediated inhibition of primary root growth (Brady et al .2003;Zhang,Garreton &Chua 2005).Given the vast array of responses of plants to auxin and ABA,one would expect that many such points of crosstalk exist;however,this aspect of auxin and ABA signal transduction remains largely unexplored.Rho family GTPases act as molecular switches that mediate diverse cellular responses to multiple extracellular signal including hormones (Bos 2000).ROP (Rho of plants;also called RAC)GTPases represent the sole Rho family of Ras-related G proteins in plants (Yang 2002),and the model plant Arabidopsis contains 11ROP GTPases in its genome (Bischoff et al .1999;Winge et al .2000;Zheng &Yang 2000).Several studies have reported that ROP GTPases play impor-tant roles in auxin-and ABA-related responses (Lemichez et al .2001;Tao,Cheung &Wu 2002;Zheng et al .2002;Bloch et al .2005;Tao et al .2005).Auxin treatment increases the amount of activated ROP GTPase in tobacco (Tao et al .2002)and Arabidopsis (Xu et al .2010;Lin et al .2012)seed-lings.Overexpression of wild-type or constitutively active forms of ROP GTPases stimulates auxin-related phenotypes and auxin-responsive gene expression in Arabidopsis and tobacco (Li et al .2001;Tao et al .2002,2005).Activated ROP GTPases promote the 26S proteasome-dependentCorrespondence:Y.Lee.Fax:+82542792199;e-mail:ylee@postech.ac.kr;J-U.Hwang.Fax:+82542792199;e-mail:thecute@postech.ac.krY.L.and J-U.H.contributed equally to the manuscript.Plant,Cell and Environment (2013)36,945–955doi:10.1111/pce.12028©2012Blackwell Publishing Ltd945degradation of auxin/indole-3-acetic acid(AUX/IAA)pro-teins in tobacco and Arabidopsis(Tao et al.2005).ROP GTPase mutations cause defects in auxin-dependent cell expansion(Fu et al.2005,2009;Xu et al.2010).In contrast to the positive role of ROP GTPases in the auxin response, ROP GTPases appear to be negative regulators of ABA responses.ABA treatment reduces the amount of activated ROP GTPase in Arabidopsis suspension cells and seedlings (Lemichez et al.2001).Expression of constitutively active forms of Arabidopsis ROP2and ROP6reduces sensitivity to ABA during seed germination(Li et al.2001)and stomatal closing(Lemichez et al.2001;Hwang et al.2011).The obser-vation that an Arabidopsis mutant that lacks ROP10expres-sion is hypersensitive to ABA,and that ROP10expression is suppressed by ABA,suggests the existence of an interesting feedback regulation loop in the ABA signalling pathway (Zheng et al.2002).RICs(ROP-interactive CRIB motif-containing proteins) are a unique group of interacting partners of activated ROP GTPases.RIC proteins interact with multiple ROP GTPases via their conserved CRIB motif,and link ROP proteins to diverse target molecules that bind to their variable domains (Yang2002).The11RIC genes present in Arabidopsis are categorized into four phylogenetic groups(Wu et al.2001;Gu et al.2005).However,knowledge on RIC functions is limited; RIC3and RIC4have been shown to regulate[Ca2+]cyt and F-actin dynamics during the polar growth of pollen tubes (Wu et al.2001;Gu et al.2005).RIC7is reported to interact with active ROP2in stomatal guard cells and to suppress light-induced stomatal opening(Jeon et al.2008).In epider-mal cells of the leaf and hypocotyl,RIC1suppresses aniso-tropic cell expansion by regulating microtubule(MT) dynamics(Fu et al.2005,2009;Xu et al.2010).RIC1is expressed in a broad range of tissues(Wu et al. 2001).However,the function of RIC1has been analysed mostly in the development of leaf pavement cells(Fu et al. 2005).In this cell type,RIC1is associated with MTs and regulates their assembly.In the lobe-forming regions of pave-ment cells,RIC1is inactivated by active ROP2,which sup-presses MT assembly,but promotesfine F-actin assembly and thereby induces outgrowth of the region.In contrast,in the neck-forming regions of pavement cells,RIC1is activated by active ROP6and then promotes the assembly of MTs,which limits the expansion of the region and results in the forma-tion of a narrow neck.The cortical MTs in the leaf pavement cells of ric1mutants are randomly organized,resulting in pavement cells with wider necks.This ROP6-RIC1-MT sig-nalling pathway seems to function in both hypocotyl elonga-tion and leaf epidermal cell development(Fu et al.2005, 2009).In pollen tubes,however,RIC1is localized to the apical plasma membrane,where MTs are absent,and over-expression of RIC1suppresses the depolarized tube growth induced by ROP1overexpression(Wu et al.2001).Given its broad expression pattern,RIC1may mediate diverse pro-cesses in the growth and development of plants,which have yet to be elucidated.In this work,we established that RIC1positively regulates the auxin effect and negatively regulates the ABA effect during root growth and lateral root development.These results will advance our current limited understanding on the mode of action of RIC1protein during regulation of plant development by auxin and ABA.MATERIALS AND METHODSPlant materials and growth conditionsSeeds of wild-type,ric1,and ric1/RIC1p:GFP:RIC1Arabi-dopsis thaliana plants(ecotype Ws)were surface sterilized, placed at4°C in the dark for2d,and then sown in half-strength Murashige and Skoog(MS)agar medium.Arabi-dopsis seedlings were grown in a growth chamber with a16h light/8h dark cycle at22°C.Isolation of the RIC1knockout mutantsSeeds of T-DNA insertion mutant for RIC1(ric1; FLAG_075E05)were obtained from Institut National de la Recherche Agronomique(INRA)-Versailles Genomic Resource Center(http://www-ijpb.versailles.inra.fr/en/cra/ cra_accueil.htm).Reverse transcriptase(RT)-PCR analysis using gene-specific primers confirmed that this is a null mutant.Primer information used for RT-PCR is available in Supporting Information Table S1.Complementation of ric1with RIC1p:GFP:RIC1For the ric1complementation assay,the RIC1promoter region(~2kb)and the RIC1open reading frame were indi-vidually obtained by PCR amplification.These genomic DNA fragments and GFP coding sequence were sequentially cloned into a pCR®8/GW/TOPO®vector(Invitrogen,Carls-bad,CA,USA),and then transferred into a pMDC100 gateway vector(Curtis&Grossniklaus2003).The RIC1p:GFP:RIC1construct was transformed into ric1plants by the Agrobacterium-mediatedfloral dipping method (Clough&Bent1998).The phenotypes of the T3seedlings of homozygous ric1/RIC1p:GFP:RIC1lines were observed. RIC1p:GUS expression assayThe genomic DNA fragment containing the promoter region (~2kb)andfirst exon of RIC1was amplified by PCR and fused to the GUS-coding region of the pMDC164vector (RIC1p:GUS).The RIC1p:GUS construct was transformed into wild-type Arabidopsis plants using the Agrobacterium-mediatedfloral dipping method(Clough&Bent1998). RIC1p:GUS expression was observed in T3plants from six independently transformed lines.Briefly,the seedlings of RIC1p:GUS were incubated in GUS staining buffer[100m m Na2HPO4(pH7.2),3m m potassium ferricyanide,3m m potas-sium ferrocyanide,10m m ethylenediaminetetraacetic acid (EDTA),0.1%Triton X-100,and2m m5-bromo-4-chloro-3-indolyl-b-D-glucuronide(Duchefa,Haarlem,The Nether-lands)]at37°C for12h.Chlorophyll was extracted in70% ethanol solution.946Y.Choi et al.©2012Blackwell Publishing Ltd,Plant,Cell and Environment,36,945–955Observation of the cellular localizationof GFP:RIC1Wild-type Arabidopsis plants were stably transformed with a GFP:RIC1construct under the control of CaMV35S pro-moter.In multiple independently transformed lines of Arabidopsis,the subcellular localization of GFP:RIC1was observed by using a Zeiss LSM510Meta Laser scanning microscope(Zeiss,/).To investigate the effects of auxin and ABA on the cellular localization of RIC1,7-day-old seedlings that stably express GFP:RIC1 were incubated in half-strength MS medium containing1m m auxin[naphthalene-1-acetic acid(NAA)]or10m m ABA for1h.Quantification of RIC and ABA-orauxin-responsive gene transcript levelsQuantitative real-time RT-PCR(Q-PCR)was used to quan-tify transcript levels of RIC genes,ABA-responsive genes and auxin-responsive genes.Total RNA was extracted from each sample and then reverse transcribed into cDNA. Q-PCR was carried out using a Takara TP800thermal cycler and Takara SYBR RT-PCR Kit(Takara Bio,Kyoto,Japan), following the manufacturer’s instructions.Transcript levels of RIC s,ABA-responsive genes and auxin-responsive genes were normalized against that of tubulin8. Measurement of lateral root formation and primary root growthTo examine the effects of ABA or auxin on lateral root formation and primary root growth,Arabidopsis seedlings were grown for4d under a16h photoperiod and then trans-ferred to fresh half-strength MS agar plates supplemented with the indicated concentrations of ABA or auxin.After an additional5–7d,the net elongation of primary roots was measured and the number of lateral roots was counted using a stereo microscope(Olympus SZX12,Tokyo,Japan). Seed germination assayFor germination assays,the seeds were placed in the dark at 4°C for2d and then sown on MS medium agar plates con-taining1%sucrose in the presence or absence of0–1.5m m concentrations of ABA.The seeds were incubated under a 16h photoperiod at22°C.Germinated seeds(as determined by cotyledon greening or radicle emergence)were scored every12h for6d.Germination ratio refers to the number of germinated seeds as a proportion of the total number of seeds tested.RESULTSRIC1expression is induced by both auxinand ABAMembers of the ROP small GTPase family are reported to mediate ABA and auxin responses(Li et al.2001;Zheng et al.2002;Tao et al.2005).We hypothesized that RIC proteins might serve an important intermediary in the ROP-mediated ABA and auxin signalling pathways.If this hypothesis was true,then the level of the RIC proteins may be substantially regulated by ABA and auxin.Thus,we tested the effect of auxin and ABA on the expression of10out of the11Arabi-dopsis RIC genes(Fig.1).Arabidopsis seedlings were grown on half-strength MS medium for7d and then treated with 1m m NAA or10m m ABA for1h.Total RNA was isolated from these seedlings and RIC gene transcript levels were examined using quantitative real-time PCR(Q-PCR).Upon NAA and ABA treatment,the transcript level of many RIC s (RIC2,3,4,5,7,9and11)increased,but the increase in RIC1 was the highest.Therefore,we chose to focus our analysis on RIC1.Loss of RIC1expression alters gene induction by auxin and ABAAuxin and ABA induce the expression of sets of genes, which are known as auxin-responsive and ABA-responsive genes,respectively(Brady et al.2003;Zhang et al.2005;Li et al.2009).To examine the involvement of RIC1in auxin and ABA signal transduction,we evaluated the effect of RIC1knockout(ric1)on the expression levels of typical auxin-and ABA-responsive genes.ric1,a T-DNA insertion mutant(Stock No.FLAG_075E05),was obtained from INRA-Versailles Genomic Resource Center(http://www-ijpb.versailles.inra.fr/en/cra/cra_accueil.htm).RT-PCR analy-sis confirmed that the T-DNA insertion into the fourth exon completely blocked the expression of RIC1in ric1(Fig.2a). The small auxin-up RNAs(SAURs)encode short tran-scripts that accumulate rapidly upon auxin treatment(Li et al.2009).IAA6and IAA19are members of INDOLE-3-ACETIC ACID/AUXIN(IAA/AUX)genes andtheir Figure1.Expression of RIC genes were induced by auxin and abscisic acid(ABA).Seven-day-old Arabidopsis seedlings were treated without or with1m m auxin[naphthalene-1-acetic acid (NAA)]or10m m ABA for1h,and total RNA was isolated from seedlings for Q-PCR analysis.The transcript levels of RIC genes were normalized against the transcript level of Tubulin8,which served as the internal control,and are presented as values relative to the untreated control.Data are meansϮSEM of three to eight biological replicates.Asterisks indicate values that are statistically significantly different from the untreated control(***P<0.005;**P<0.001;*P<0.05).RIC1regulates root development947©2012Blackwell Publishing Ltd,Plant,Cell and Environment,36,945–955948Y.Choi et al.©2012Blackwell Publishing Ltd,Plant,Cell and Environment,36,945–955expressions are induced by auxin(Abel,Nguyen&Theologis 1995;Tatematsu et al.2004).Using Q-PCR analysis,we com-pared the transcript levels of SAUR and IAA genes in ric1 seedlings with those in wild-type seedlings(Fig.2b).Under control conditions without NAA treatment,the transcript levels of these genes in ric1seedlings were similar to or slightly higher than those in wild-type seedlings(Fig.2b).In 7-day-old wild-type seedlings,treatment with1m m NAA for 1h induced a two-to fourfold increase in SAUR gene expres-sion(Fig.2b).Interestingly,however,the induction of SAUR genes by1m m NAA was suppressed in ric1seedlings (Fig.2b);SAUR9transcript level increased3.3Ϯ0.1-fold in the wild type,but1.7Ϯ0.1-fold in ric1(t-test,P<0.005); SAUR15transcript level increased3.9Ϯ0.6-fold in the wild type,but1.8Ϯ0.2-fold in ric1(t-test,P<0.01);SAUR23 transcript level increased3.0Ϯ0.3-fold in the wild type,but 1.4Ϯ0.2-fold in ric1(t-test,P<0.005);SAUR62transcript level increased2.5Ϯ0.2-fold in the wild type but1.4Ϯ0.2-fold in ric1(t-test,P<0.001);and SAUR66transcript level increased3.9Ϯ0.4-fold in the wild type,but1.7Ϯ0.2-fold in ric1(t-test,P<0.001).Similarly,induction of IAA6and IAA19upon NAA treatment was suppressed by ric1(Fig.2b bottom panel),whereas the transcript level of IAA6 increased21.9Ϯ1.3-fold in the wild type,but only11.3Ϯ0.2-fold in ric1(P<0.05),and the transcript level of IAA19 increased27.1Ϯ3.3-fold in the wild type,but only13.1Ϯ1.9-fold in ric1(P<0.05).These results indicate that RIC1is involved in the control of the auxin signalling pathway.ABI3,ABI5,responsive to ABA18(RAB18),and respon-sive to dehydration29A(RD29A)and29B(RD29B)are well-characterized ABA-responsive genes that play critical roles in ABA signalling(Parcy et al.1994;Finkelstein& Lynch2000;Lopez-Molina&Chua2000;Hoth et al.2002; Kang et al.2010).To analyse the involvement of RIC1in ABA signalling,we gauged the effects of ABA on expres-sions of these genes in the roots of wild-type and ric1seed-lings(Fig.2c).Seven-day-old seedlings were incubated in half-strength liquid MS medium in the presence or absence of0.5m m ABA for1h.Under control conditions(i.e.in the absence of ABA),transcript levels of ABI3,ABI5,RD29A, RD29B and RAB18were slightly higher in ric1seedlings than in wild-type seedlings,and this difference was further increased after ABA treatment(Fig.2c).Upon ABA treat-ment,ric1seedlings exhibited much higher transcript levels of thosefive ABA-responsive genes,compared with wild-type seedlings(Fig.2c);ABI3transcript level increased 2.4Ϯ0.5-fold in the wild type,but 5.5Ϯ0.9-fold in ric1 (P<0.01);ABI5transcript level increased5.9Ϯ0.4-fold in the wild type,but12.9Ϯ1.9in ric1(P<0.005);RD29A tran-script level increased12.3Ϯ3.9-fold in the wild type,but 17.4Ϯ6.6-fold in ric1(P<0.06);RD29B transcript level increased5.5Ϯ1.2-fold in the wild type,but10.9Ϯ0.8-fold in ric1(P<0.05);and RAB18transcript level increased 4.6Ϯ1.1-fold in the wild type,but8.0Ϯ1.3-fold in ric1 (P<0.01).In summary,RIC1knockout suppressed the induction of auxin-responsive genes by auxin,but promoted the induction of ABA-responsive genes by ABA.These results suggest that RIC1exerts opposite regulatory functions in the auxin and ABA signalling pathways.RIC1is expressed in the roots ofyoung seedlingsTo identify which auxin-and ABA-mediated processes are regulated by RIC1,wefirst determined the tissue-specific and developmental stage-specific expression of RIC1.The genomic DNA region containing the RIC1promoter(~2kb) and thefirst exon was fused to the GUS-coding region (RIC1p:GUS),and introduced into wild-type plants. RIC1p:GUS expression was observed in T3seeds and seed-lings from six independently transformed Arabidopsis lines (Fig.3a,b).In germinating seeds and young seedlings,the RIC1p:GUS signal was evident in roots.In germinating seeds,RIC1p:GUS signal was limited to the embryonic root tip(Fig.3a,left),and in seedlings at1–3d after sowing,RIC1p:GUS extended the expression to other parts of root including differentiation zone,root hairs and root–shoot junction(Fig.3a,right).In the roots of2-week-old plants,RIC1p:GUS signal was detected in root tips and also in maturation zone,where lateral roots grow out(Fig.3b).RIC1p:GUS signal was strongly detected in columella cells from the root tip, (Fig.3b-d).In maturation zone of root,cells surrounding emerged lateral root(Fig.3b-b)and epidermal cells at the base of lateral roots(Fig.3b-c)showed clear RIC1p:GUS signals.These RIC1expression patterns indicate that RIC1is likely to be involved in the regulation of seed germination, early seedling development and root development.In addition to being expressed in the roots,RIC1p:GUS was also expressed in the hypocotyls,petioles,and weakly in the leaves of young seedlings(Fig.3a,b).In Arabidopsis plants of later development stages,expression of RIC1p:GUS was weak except inflowers,where RIC1expression was pre-viously reported(Wu et al.2001;Fu et al.2005,2009;Xu et al.Figure2.ric1knockout mutation altered induction of auxin-and abscisic acid(ABA)-responsive genes.(a)Schematic structure of theRIC1gene(left).The triangle indicates the T-DNA insertion site in ric1.Exons are represented as boxes and introns as lines.RT-PCR analysis using a RIC1-specific primer set(RT-F and RT-R)shows that ric1is a true null mutant(right).Tubulin8was used as an internal control.(b)Expression of SAUR9,SAUR15,SAUR23,SAUR62,SAUR66,IAA6and IAA19in plants treated or not with1m m auxin [naphthalene-1-acetic acid(NAA)].(c)Expression of ABI3,ABI5,RD29A,RD29B and RAB18in plants treated or not with0.5m m ABA.Q-PCR analyses of transcripts of auxin-and ABA-responsive genes were performed using total RNA isolated from the roots of8-day-old seedlings after1h of treatment without or with auxin or ABA.Data were normalized using Tubulin8as an internal control,and are presented as values relative to the untreated wild type(WT).Data are meansϮSEM of four independent experiments.Asterisks indicate values that are significantly different from those of the WT(***P<0.005;**P<0.01;*P<0.05;#P<0.06).RIC1regulates root development949©2012Blackwell Publishing Ltd,Plant,Cell and Environment,36,945–9552010);RIC1p:GUS signal was strongly observed in the anthers and mature pollen grains (Supporting Information Fig.S1).RIC1knockout suppresses the effect of auxin on lateral root formation and primary root elongationAs RIC1is expressed in root (Fig.3)and RIC1expression is up-regulated by auxin (Fig.1),we examined whether auxin-dependent root growth and lateral root formation were affected in the ric1mutant (Fig.4).Arabidopsis seedlings were grown on half-strength MS medium for 4d and then transferred to fresh half-strength MS medium supplemented with various concentrations (0–100n m )of NAA.After 5d,the number of lateral roots (including newly emerged ones)was counted.Auxin promoted the formation of lateral roots in different genotypes,including the wild type,ric1,and ric1/RIC1p:GFP:RIC1(complementation lines,C1and C2;Fig.4a);however,this effect was significantly less in ric1than in the wild type and complementation lines (Fig.4b).InFigure 3.RIC1expression in Arabidopsis plants.(a)One dayafter sowing (DAS),an embryo exhibited RIC1p::GUS signal at the root tip (left,indicated by arrow).Seed coat was removed after GUS staining for observation.A young seedling that had justgerminated but had not yet undergone cotyledon expansion (right;1–3DAS)exhibited relatively stronger RIC1p::GUS signal in the root tip and differentiation zone of the root including the root hairs.Bar =300m m.(b)A 2-week-old seedling displayed RIC1p::GUS signal in the root tip and maturation zone withemerged lateral roots,and,weakly,in the shoot.(b-a )Bar =1cm.(b-b ),(b-c )and (b-d )are enlarged images of maturation zone with an emerged lateral root,a lateral root with GUS staining at the base,and a root tip with GUS staining in columellacells.Figure 4.Auxin responses were reduced in ric1plants.Arabidopsis seedlings were grown vertically on half-strength Murashige and Skoog (MS)medium for 4d,transferred to fresh half-strength MS medium supplemented or not with 0–100n m auxin [naphthalene-1-acetic acid (NAA)],and grown for anadditional 5–7d.(a)Levels of RIC1transcript in wild-type (WT),ric1,and ric1/RIC1p:GFP:RIC1(lines C1and C2)plants.RIC1expression in seedlings was quantified using Q-PCR and presented values relative to that of WT.Data are means ϮSEM of four independent experiments.(b)Number of lateral roots formed in the absence or presence of NAA (means ϮSEM of 28seedlings from four independent experiments),measured 5d after transfer to medium supplemented with or without NAA.Asterisks indicate values that are significantly different from those of the WT atP <0.05.(c)Relative values of lateral root number (mean ϮSEM,n =28).Values in (b)were normalized to the values of non-treated controls.Asterisks indicate values that are significantly different from those of the WT (***P <0.005).(d)Primary root elongation in the absence or presence of NAA (means ϮSEM of 28seedlings from four independent experiments).The net primary root growth was measured 7d after transfer to medium supplemented with or without NAA.Asterisks indicate values that are significantly different from those of the WT (**P <0.05;*P <0.1).(e)Relative values of net primary root elongation (mean ϮSEM,n =28).Values in (d)were normalized to the values ofnon-treated controls.Asterisks indicate values that are significantly different from those of the WT (***P <0.005;*P <0.05).950Y.Choi et al .©2012Blackwell Publishing Ltd,Plant,Cell and Environment,36,945–955the absence of exogenous auxin(0n m NAA),ric1plants produced more lateral roots than did the wild type and ric1 complementation lines(n=28,N=4,P<0.05;Fig.4b). However,in the presence of auxin,lateral root number was less in ric1plants than in the wild type(Fig.4b,P<0.05). Whereas80and100n m NAA increased the number of lateral roots per unit length(cm)of primary root in wild-type seedlings to734and920%of non-treated control values, respectively,the same concentration of NAA increased this number in ric1to466and613%of control values,respec-tively(Fig.4c).This alteration in lateral root formation in ric1 plants was completely reversed by the expression of RIC1 driven by its native promoter(ric1/RIC1p:GFP:RIC1).In two independent ric1/RIC1p:GFP:RIC1lines(C1and C2), lateral root formation was recovered to wild-type levels both in the absence and presence of NAA(Fig.4b,c).The effect of RIC1knockout on primary root elongation was also examined(Fig.4d,e).Seven days after transfer to half-strength MS medium supplemented or not with NAA, the net elongation of primary roots was measured.In medium lacking NAA,ric1mutants had reduced primary root elongation compared with wild-type plants(n=28, N=4,P<0.05);the net primary root elongation of wild-type seedlings was4.9Ϯ0.16cm,while that of ric1seedlings was 4.3Ϯ0.22cm(Fig.4d).NAA(50–100n m)inhibited primary root elongation in both ric1and wild-type seedlings (Fig.4d,e).However,ric1seedlings were less sensitive than the wild type to NAA(n=28,N=4,P<0.1);whereas100n m NAA reduced primary root elongation in wild-type seedlings by34.4%(to3.2Ϯ0.16cm),it inhibited that in ric1seedlings by only19.4%(to3.5Ϯ0.13cm).Primary root elongation in the complementation lines(C1and C2)was similar to that in the wild type,in both the absence and presence of NAA (Fig.4d,e).These results suggest that RIC1participates in auxin-regulated lateral root development and primary root elongation.RIC1knockout enhances the effect of ABA on seed germination,lateral root formation and primary root elongationWe then tested whether RIC1knockout also altered the plant’s response to ABA by comparing seed germination and root development in ric1and wild-type plants treated with ABA(Figs5&6).Seeds were sown on half-strength MS medium after2d of stratification.In the presence of0.5m m ABA,seed germination,as gauged by cotyledon greening, was delayed to a greater extent in ric1than in wild-type seeds (Fig.5a,b);whereas only25%of ric1seedlings exhibited cotyledon greening84h after sowing,66%of wild-type seed-lings exhibited greening(Fig.5b).Similar enhanced sensitiv-ity to ABA in inhibition of seed germination was observed when germination rate was analysed based on radicle emer-gence(Supporting Information Fig.S2).Seed germination rates in the ric1/RIC1p:GFP:RIC1lines were restored to wild-type levels in the presence of0.5m m ABA,confirming that loss of RIC1expression was responsible for the enhanced suppression of seed germination by ABA(Fig.5b).The delayed germination of ric1seeds in the presence of ABA was not due to a defect in seed development,because the germination rate of ric1seeds in the absence of ABA was not significantly different from that of wild type(Fig.5c and Supporting Information Fig.S2b).ABA was reported to inhibit root elongation and lateral root development(Pilet&Chanson1981).We compared primary root elongation and lateral root number in ric1and wild-type plants in the presence and absence ofexogenous Figure5.Inhibition of seed germination by abscisic acid(ABA) was enhanced in the ric1mutant.(a)Representative photographs showing young seedlings of wild type(WT),ric1,and the tworic1/RIC1p:GFP:RIC1lines(C1and C2)in the presence of0.5m m ABA(taken96h after sowing).(b)Seed germination rate in the presence of0.5m m ABA,measured as a percentage of seedlings with green cotyledons at the indicated time points after sowing on half-strength Murashige and Skoog(MS)medium supplemented with ABA.Data are meansϮSEM of three independent experiments.(c)Seed germination rate in the absence of ABA. There was no significant difference between genotypes.RIC1regulates root development951©2012Blackwell Publishing Ltd,Plant,Cell and Environment,36,945–955。
泛连接蛋白1参与炎症调控及细胞焦亡的研究进展

泛连接蛋白1参与炎症调控及细胞焦亡的研究进展*马源1, 段倩雯1, 董旭鹏1, 刘澈1, 马玉清2△(1兰州大学第一临床医学院,甘肃 兰州 730000;2兰州大学第一医院麻醉科,甘肃 兰州 730000)Progress in pannexin 1 involved in inflammation regulation and pyroptosisMA Yuan 1, DUAN Qianwen 1, DONG Xupeng 1, LIU Che 1, MA Yuqing 2△(1The First School of Clinical Medicine of Lanzhou University , Lanzhou 730000, China ; 2Department of Anesthesiology ,The First Hospital of Lanzhou University , Lanzhou 730000, China. E -mail : myq 2392466@ )[ABSTRACT ] Pannexin 1 (Panx1), a member of the ubiquitin family , is widely expressed in mammalian tis⁃sues. When the body is in an inflammatory state , Panx1 channel is activated and opened by high concentration of ion stimulation , caspase shearing , tyrosine phosphorylation and mechanical stretching pathway , which allows intracellular ATP to be released outside the cell and aggravates inflammatory response. Panx1 is also involved in the occurrence of py⁃roptosis in inflammatory response , and activates and releases a large number of interleukin -1-related inflammatory factors. Inflammatory response is the body's defense response to infection , but overexpression of Panx1 leads to uncontrolled in⁃flammatory response. Therefore , Panx1, as a new intervention target of inflammation , has certain research value and ap⁃plication prospect.[关键词] 泛连接蛋白1;炎症;细胞焦亡;胱天蛋白酶;白细胞介素1β[KEY WORDS ] pannexin 1; inflammation ; pyroptosis ; caspase ; interleukin -1β[中图分类号] R363; R329.2+8 [文献标志码] Adoi : 10.3969/j.issn.1000-4718.2023.07.0201 泛连接蛋白1(pannexin 1, Panx1)通道及其调控1.1 Panx1概况 Panx1作为泛连接蛋白家族成员之一,激活后可在细胞膜上形成通道,释放10 kD 以内的物质于细胞外,如腺苷三磷酸(adenosine triphos⁃phate , ATP )、尿苷三磷酸(uridine triphosphate , UTP )、K +和Ca 2+等。
真核翻译延伸因子eEF1A功能研究进展

真核翻译延伸因子eEF1A 功能研究进展*高爽,查笑君,潘建伟【摘要】摘要:总结了近年来对于真核翻译延伸因子eEFlA 生物学功能和作用机制研究的一些新进展,具体包括:eEFlA 参与介导受损或错误折叠的蛋白质的降解;对微丝、微管骨架系统的组织与调控;参与调控细胞凋亡;参与细胞核输出氨酰-tRNA;与病毒基因组和RNA 依赖性RNA 聚合酶类(RNA-dependent RNA polymerase,RdRP)互作,参与病毒繁殖.阐述了对eEFlA 进行研究的意义和价值,并提供了新的见解和展望.【期刊名称】浙江师范大学学报(自然科学版)【年(卷),期】2013(036)004【总页数】6【关键词】关键词:真核生物;翻译延伸因子1A(eEF1A);功能;作用机制蛋白质翻译延伸因子(translation elongation factor,EF)最初从大肠杆菌(Escherichia coli)细胞中分离获得,具有三磷酸鸟苷(GTP)或鸟苷二磷酸(GDP)亲和性,参与肽链的延伸过程.在原核细胞中,有3 类延伸因子,分别被命名为EF-Tu(elongation factor thermo unstable),EF-Ts(elongation factor thermo stable)和EF-G(elongation factor G);而在真核细胞中,相对应的分别为eEF1A(eukaryotic translation elongation factor 1A),eEF1B(eukaryotic translation elongation factor 1B)和eEF2(eukaryotic translation elongation factor 2).蛋白质生物合成过程大致可分为3 个阶段:起始、延伸和终止.在肽链延伸阶段,EF1A 与GTP 结合产生EF1A·GTP 复合体,此复合体再与特异的氨酰-tRNA 结合并将其运送到核糖体A 位点,并伴随着GTP 的水解,最后EF1A·GDP 从核糖体释放出来.EF1A·GDP 经EF1B 催化又重新形成EF1A·GTP.在核糖体肽酰转移酶作用下,位于核糖体P 位点的多肽被转移到A 位点,与新进入的氨酰-tRNA 形成新的肽键,再由EF2 催化肽基-tRNA·mRNA 复合物从核糖体A 位点转移至P位点,空出的A 位点将接纳下一个新的氨酰-tRNA.重复此过程,蛋白多肽链将最终被合成[1-2].eEF1A 胞内含量很高,仅次于肌动蛋白,其基因及表达调控十分保守.eEF1A 由一个多基因家族编码,不同的物种具有不同数量的eEF1A 同源基因,如:酵母中有2 个eEF1A 同源基因;拟南芥和水稻中分别有4 个eEF1A 同源基因;玉米中有10~15 个eEF1A 同源基因;人类有多于18 个eEF1A 同源基因.蛋白结构分析表明,eEF1A 具有3 个构象不同的功能结构域,结构域Ⅰ与GTP 结合,结构域Ⅱ与氨酰-tRNA 结合,结构域Ⅰ和Ⅱ还与e EF1Bα(组成eEF1B 的亚基之一)互作,结构域Ⅱ和Ⅲ共同参与和肌动蛋白的互作[3].过去20 年的研究表明,eEF1A 除参与蛋白质翻译外,还具有多种生物学功能.本文主要就真核细胞eEF1A 在蛋白质降解、细胞骨架组织调控、细胞凋亡、核物质输出和病毒繁殖等过程中的生物学功能作一扼要综述.1 eEF1A 与蛋白降解泛素(ubiquitin)介导的蛋白降解是蛋白质代谢的主要机制之一,在动植物生长发育过程中具有重要的调控作用.最初发现eEF1A 作为一个必需因子参与泛素介导的N-α-蛋白降解过程[4].eEF1A 的原核同源蛋白EF-Tu 被证实有类似分子伴侣的活性[5].随后的研究表明,eEF1A 既能与合成中的新生肽链互作,也能与翻译后折叠错误的蛋白质结合[6].进一步的证据表明:eEF1A能有效缓解参与蛋白降解的RAD23 和RPN10 功能缺失后所引起的细胞生长缓慢等表型[7];eEF1A 能与蛋白酶体的19S 调节亚基RPT1 直接互作,RPT1 的功能缺失可降低eEF1A 与蛋白酶体的互作,同时胞内出现受损蛋白的代谢缺陷[7];刀豆氨酸(canavanine)能诱导蛋白折叠错误而使后者进入泛素化降解途径,但当eEF1A 的GTP 结合域第156 位天冬氨酸(Asp)突变为天冬酰胺(Asn)后,细胞对刀豆氨酸表现出较高的抗性[7].这些研究结果充分暗示,eEF1A 参与介导受损或错误折叠的蛋白从核糖体到蛋白酶体的过程.2 eEF1A 与细胞骨架细胞骨架是细胞内错综复杂的动态纤维状网络结构,除具有维持细胞形态和调控细胞增殖外,对蛋白质翻译的组织与调控也具有重要的生物学意义[8].许多蛋白质翻译系统的组分,如氨酰-tRNA合成酶、真核起始因子eIF(eukaryotic initiation factor)和翻译延伸因子EF 直接或间接地与细胞骨架相连.来自哺乳动物细胞和酿酒酵母的证据表明,微丝骨架系统的任何缺陷均会影响肽链合成的正常进行[9].尽管eEF1A 最初被鉴定为翻译系统的重要作用因子,但后续的研究表明eEF1A 是一类进化上保守的肌动蛋白结合蛋白(actin-binding protein),具有调控肌动蛋白组装微丝的功能[10].eEF1A 通过抑制微丝纤维末端肌动蛋白单体的加聚和解聚,从而调控微丝纤维的组装,最终影响与微丝相关的货物运输、定位及mRNA 的翻译[11].eEF1A 与肌动蛋白纤维或氨酰-tRNA 的结合受胞内pH 调控:当pH 值逐渐增大时,促进eEF1A 与肌动蛋白的解离,利于eEF1A 与氨酰-tRNA 的结合和肽链合成;而pH 值逐渐减小时,促进eEF1A与肌动蛋白的结合.而且竞争性结合实验进一步证实,这两种结合是相互排斥的[12].在海胆的受精过程中,胞内pH 值的增加作为信号刺激蛋白质合成[13].eEF1A 能与微丝骨架调节蛋白Rho1p的下游靶蛋白Bni1p 互作[14].这些研究结果表明,细胞通过pH 的变化调控eEF1A 介导的肽链延伸和微丝骨架组织之间的切换.为进一步提供eEF1A 在微丝骨架组织中的遗传学证据,超表达eEF1A 的酿酒酵母在没有显著影响蛋白质合成的前提下引起了微丝骨架的组织紊乱,细胞生长缓慢[15].酿酒酵母eEF1A 遗传突变筛选获得2 类突变体:一类使蛋白质合成功能维持正常,但存在微丝骨架组织缺陷,其eEF1A与肌动蛋白结合的功能维持正常,但将肌动蛋白组装成束的功能下降[16];另一类表现为更严重的微丝骨架组织紊乱,蛋白质翻译起始缺陷,细胞生长缓慢[9].最近的体外实验发现,eEF1Bα 能抑制eEF1A 对肌动蛋白组装成束的生物学功能[17].皮肤性人乳头瘤病毒HPV38 的E7 蛋白能与eEF1A结构域Ⅲ的C 末端区域结合,从而抑制后者对微丝骨架的组织功能[18].eEF1A 除了参与微丝骨架的组织外,也参与微管蛋白的组装.在海胆卵中,首次发现eEF1A作为有丝分裂活动的重要组分[19].体外实验表明,胡萝卜eEF1A 以一种Ca2+/钙调蛋白依赖的方式结合并促进微管组装成束,稳定微管骨架[20-21].非洲爪蟾和哺乳动物eEF1A 均被鉴定具有切割微管的活性[22].然而,eEF1A 参与微管组织的分子调控机制至今仍不清楚.3 eEF1A 与细胞凋亡早期的研究发现,体外培养的鼠成纤维细胞内eEF1A 表达水平与去除血清后诱导的细胞凋亡率呈正相关[23].在过氧化氢诱导的细胞凋亡前,胞内eEF1A 表达水平迅速上升[24].这些结果暗示eEF1A 能促进细胞凋亡.而另一研究中筛选细胞凋亡抑制因子时,分离得到了eEF1A[25].这似乎与之前的研究结果互相矛盾,但后续的研究为其作出了解释.哺乳动物中存在功能差异的2种eEF1A 亚型,即eEF1A1 和eEF1A2,分别由不同的基因编码,氨基酸序列同源性约为92%[26].尽管两者在多肽延伸过程中作用相似,但它们的表达模式却具有不同的时空特异性,eEF1A1 在各组织中广泛表达,而eEF1A2 似乎只在骨骼肌、心肌和脑细胞中表达[26-27].在成肌细胞分化过程中,发现eEF1A1 具有促进细胞凋亡的作用,而eEF1A2 的作用则相反[28].在研究脂毒性细胞凋亡机制时,也发现抑制eEF1A1 的表达可阻碍细胞凋亡[29].这些研究结果表明,eEF1A1 和eEF1A2 表达水平的差异参与决定细胞的命运[27-28,30].最近的研究表明,胁迫刺激如病毒感染等诱导的干扰素诱导蛋白IFIT1(interferon-induced protein with tetratricopeptide repeats-1)通过与eEF1A1 互作,从而促进细胞凋亡[31].在人巨噬细胞内,艾滋病毒HIV-1 Nef 蛋白与eEF1A1 结合,通过eEF1A1 和tRNA 的核-质重定位从而抑制由内质网应激介导的细胞凋亡[32].另有研究表明,eEF1A2 通过与抗氧化蛋白peroxiredoxinⅠ的互作,从而抑制由氧化胁迫诱导的细胞凋亡[33].这些研究结果说明,eEF1A 通过与功能不同的靶蛋白互作,行使不同的功能.上述研究所涉及的都是依赖于半胱天冬酶的细胞凋亡.然而,越来越多的证据说明,细胞还有不依赖于半胱天冬酶的凋亡途径[34-35].在四倍体细胞中发现了一种不依赖于半胱天冬酶的细胞凋亡,这种凋亡由eEF1A1 的表达下调引起,能帮助除去异常四倍体细胞和抑制肿瘤发生[36].4 eEF1A 与核物质输出有实验证据表明eEF1A 参与细胞核物质的输出过程.在酿酒酵母中,表达突变的eEF1AE286K或E291K(tRNA 结合位点突变)的菌株表现为核输出氨酰-tRNA 障碍而累积于核中[37-38].tRNA 氨酰化是eEF1A 与tRNA 有效结合的前提[39],也是tRNA 被运输到核外的前提[37].Exportin-5 属于细胞核质转运受体importinβ 家族.在哺乳动物中,eEF1A 通过氨酰-tRNA 与Exportin-5 结合形成输出复合物,随后eEF1A 与氨酰-tRNA 一同被输出到核外[40-41].eEF1A 不仅参与核输出氨酰-tRNA,在哺乳动物细胞核输出蛋白质的过程中也起到重要的作用.转录依赖的核输出序列TD-NEM(transcription-dependent nuclear export motif)是一种新发现的核输出信号序列[42],eEF1A 与TD-NEM 互作,参与介导了含有此信号序列的蛋白向核外输出的过程[43].自从发现eEF1A 参与核物质输出以来,其在细胞核与质之间的穿梭成为了讨论的热点.由于正常条件下eEF1A 定位于核外,而且Exportin-5对eEF1A 的输出也确保了其在核外,所以人们推测其在核物质输出过程中所起的作用都是在核膜的胞质侧完成的.然而,在一个核物质输出受体Msn5 突变的酿酒酵母菌株的细胞核中能检测到eEF1A 的存在,暗示了其进入细胞核内参与核物质输出的可能性[44].因此,eEF1A 参与核物质输出的具体作用位置仍有待进一步验证.5 eEF1A 与病毒繁殖eEF1A 作为细胞内最丰富的蛋白质之一,也参与了病毒生活史的循环.所报道的eEF1A 参与病毒复制大都来自于正链RNA 病毒,如登革热病毒(DV)[45]、黄萝卜花叶病病毒(TYMV)[46]、烟草花叶病毒(TMV)[47]、西尼罗河病毒(WNV)[48]和芜菁皱缩病毒(TCV)[49];但也有报道表明其参与负链RNA病毒如水疱性口炎病毒(VSV)的复制[50].eEF1A 能与这些病毒基因组3′非编码区的类tRNA 二级结构结合,并与它们各自编码的RNA 依赖性RNA 聚合酶类(RdRP)互作.eEF1A能刺激TCV 的RdRP 活性及负链RNA 的合成[49].WNV 基因组的eEF1A 结合位点突变,引起eEF1A 结合障碍,表现为负链RNA 合成降低,病毒复制受阻[51].然而,对于TYMV,eEF1A 对其基因组的结合似乎强烈抑制了负链RNA 的合成[46].这可能是因为:病毒侵染早期,eEF1A 结合其基因组,阻碍了RdRP 以正链RNA 为模板的复制行为,但正链RNA 指导的翻译活动正常进行;当RdRP 等病毒蛋白质合成达到一定量时,RdRP与eEF1A 竞争并结合到基因组的3′端,合成负链RNA.在本氏烟中,eEF1A 表达下调抑制TMV 的复制和传播[52].eEF1A 可能作为番茄丛矮病毒(TBSV)复制酶复合体的一个组分,通过提高复制辅助因子p33 的稳定性来促进病毒复制.从酿酒酵母中分离出的一个突变体eEF1AT22S,表现为p33 的半衰期缩减,病毒复制受阻[53].以上研究结果表明,eEF1A 对病毒复制具有重要作用.病毒的复制由一系列程序化事件组成,eEF1A 可能帮助维持了这种程序.6 展望综上所述,eEF1A 是一类具有多种生物学功能的重要调控蛋白.对于eEF1A 生物学功能的研究具有重要的应用价值.由于eEF1A 参与调控细胞凋亡,为肿瘤疾病的防治提供了新的策略和靶点,经工程改良的eEF1A 很可能成为肿瘤治疗的重要药物.同时,eEF1A 也参与心血管系统的调节,为相关疾病的防治提出了新的思路[54].eEF1A 作为细胞内含量第2 高的蛋白质,其基因的表达必定由强启动子启动,该启动子可用来提高外源基因的表达和一些蛋白质的工业生产[55].另外,eEF1A 还可作为玉米、大麦和高粱胚乳赖氨酸含量的指示物,谷物籽粒的赖氨酸含量是判断其营养价值的重要指标[56].目前,有关eEF1A 生物学功能的证据主要来自于酵母和哺乳动物,对于植物eEF1A 的生物学功能和作用机制知之甚少.因此,对于植物eEF1A的研究还有待进一步深入.参考文献:[1]Dever T E,Green R.The elongation,termination,and recycling phases of translation in eukaryotes[J].Cold Spring Harb Perspect Biol,2012,4(7):a013706.[2]Kavaliauskas D,Nissen P,Knudsen C R.The busiest of all ribosomal assistants:elongation factor Tu[J].Biochemistry,2012,51(13):2642 2651.[3]Sasikumar A N,Perez W B,Kinzy T G.The many roles of the eukaryotic elongation factor 1 complex[J].Wiley Interdiscip Rev RNA,2012,3(4):543-555.[4]Gonen H,Smith C E,Siegel N R,et al.Protein synthesis elongation factor EF-1 alpha is essential for ubiquitin-dependent degradation of certain N alpha-acetylated proteins and may be substituted for by the bacterial elongation factor EF-Tu[J].Proc Natl Acad Sci USA,1994,91(16):7648-7652.[5]Caldas T D,Yaagoubi A El,Richarme G.Chaperone properties of bacterial elongation factor EF-Tu[J].J Biol Chem,1998,273(19):11478 11482.[6]Hotokezaka Y,Tobben U,Hotokezaka H,et al.Interaction of the eukaryotic elongation factor 1A with newly synthesized polypeptides [J].J Bio Chem,2002,277(21):18545-18551.[7]Chuang S M,Li Chen,Lambertson D,et al.Proteasome-mediated degradation of cotranslationally damaged proteins involves translation elonga tion factor 1A[J].Mol Cell Biol,2005,25(1):403-413.[8]Kim S,Coulombe P A.Emerging role for the cytoskeleton as an organizer and regulator of translation[J].Nat Rev Mol Cell Biol,2010,11(1):75-81.[9]Gross S R,Kinzy T G.Improper organization of the actin cytoskeleton affects protein synthesis at initiation[J].Mol Cell Biol,2007,27(5):1974-1989.[10]Fan Yang,Demma M,Warren V,et al.Identification of an actin-binding protein from Dictyostelium as elongation factor 1a[J].Nature,1990,347(6292):494-496.[11]Murray J W,Edmonds B T,Liu Gang,et al.Bundling of actin filaments by elongation factor 1 alpha inhibits polymerization at filament end[J].J Cell Biol,1996,135(5):1309-1321.[12]Liu Gang,Tang Jianzhong,Edmonds B T,et al.F-actin sequesters elongation factor 1 alpha from interaction with aminoacyl-tRNA in a pH-de pendent reaction[J].J Cell Biol,1996,135(4):953-963.[13]Winkler M,Steinhardt R,Grainger J,et al.Dual ionic controls for the activation of protein synthesis at fertilization[J].Nature,1980,287(5782):558-560.[14]Umikawa M,Tanaka K,Kamei T,et al.Interaction of Rho1p target Bni1p with F-actin-binding elongation factor 1alpha:implication in Rho1p regulated reorganization of the actin cytoskeleton in Saccharomyces cerevisiae[J].Oncogene,1998,16(15):2011-2016.[15]Munshi R,Kandl K A,Carr-Schmid A,et al.Overexpression of translation elongation factor 1A affects the organization and function of the actin cytoskeleton in yeast[J].Genetics,2001,157(4):1425-1436.[16]Gross S R,Kinzy T G.Translation elongation factor 1A is essential for regulation of the actin cytoskeleton and cell morphology[J].Nat Struc Mol Biol,2005,12(9):772-778.[17]Pittman Y R,Kandl K,Lewis M,et al.Coordination of eukaryotic translation elongation factor 1A (eEF1A)function in actin organization and translation elongation by the guanine nucleotide exchange factor eEF1Bα[J].J Biol Chem,2009,284(7):4739-4747.[18]Yue J,Shukla R,Accardi R,et al.Cutaneous human papillomavirus type 38 E7 regulates actin cytoskeleton structure for increasing cell prolif eration through CK2 and the eukaryotic elongationfactor 1A[J].J Virol,2011,85(17):8477-8494.[19]Ohta K,Toriyama M,Miyazaki M,et al.The mitotic apparatus-associated 51-kDa protein from sea urchin eggs is a GTP-binding protein and i immunologically related to yeast polypeptide elongation factor 1 alpha[J].J Biol Chem,1990,265(6):3240-3247.[20]Durso N A,Cyr R J.A calmodulin-sensitive interaction between microtubules and a higher plant homolog of elongation factor-1 alpha [J].Plan Cell,1994,6(6):893-905.[21]Moore R C,Durso N A,Cyr R J.Elongation factor-1a stabilizes microtubules in a calcium/calmodulin-dependent manner[J].Cell Motil Cy toskelet,1998,41(2):168-180.[22]Shiina N,Gotoh Y,Kubomura N,et al.Microtubule severing by elongation factor 1 alpha[J].Science,1994,266(5183):282-285.[23]Duttaroy A,Bourbeau D,Wang Xiaoling,et al.Apoptosis rate can be accelerated or decelerated by overexpression or reduction of the level of e longation factor-1alpha[J].Exp Cell Res,1998,238(1):168-176.[24]Edwin C,Proestou G,Bourbeau D,et al.Rapid up-regulation of peptide elongation factor EF-1alpha protein levels is an immediate early even during oxidative stress-induced apoptosis[J].Exp Cell Res,2000,259(1):140-148.[25]Talapatra S,Wagner J D O,Thompson C B.Elongation factor-1alpha is a selective regulator of growth factor withdrawal and ER stress-induced apoptosis[J].Cell Death Differ,2002,9(8):856-861.[26]Kahns S,Knudsen C,Clark B,et al.The elongation factor 1 A-2 isoform from rabbit:cloning of the cDNA and characterization of the protein[J].Nucleic Acids Res,1998,26(8):1884-1890.[27]Lee S,Francoeur A M,Liu S,et al.Tissue-specific expression in mammalian brain,heart,and muscle of S1,a member of the elongation factor 1 alpha gene family[J].J Biol Chem,1992,267(33):24064-24068.[28]Ruest L B,Marcotte R,Eugenia W.Peptide elongation factor eEF1A-2/S1 expression in cultured differentiated myotubes and its protective effect against caspase-3-mediated apoptosis[J].J Biol Chem,2002,277(7):5418-5425.[29]Borradaile N M,Buhman K K,Listenberger L L,et al.A critical role for eukaryotic elongation factor 1A-1 in lipotoxic cell death [J].Mol Bio Cell,2006,17(2):770-778.[30]Lee S,Wolfraim L A,Wang E.Differential expression of S1 and elongation factor-1 alpha during rat development[J].J Biol Chem,1993,268(32):24453-24459.[31]Li Hongtao,Su Yongping,Cheng Tianmin,et al.The interaction between interferon-induced protein with tetratricopeptide repeats-1 and eukary otic elongation factor-1A[J].Mol Cell Biochem,2010,337(1/2):101-110.[32]Abbas W,Khan K A,Tripathy M K,et al.Inhibition of ER stress-mediated apoptosis in macrophages by nuclear-cytoplasmic relocalization o eEF1A by the HIV-1 Nef protein[J].Cell Death Dis,2012,3(4):e292.[33]Chang Ruying,Eugenia W.Mouse translation elongation factor eEF1A-2 interacts with Prdx-I to protect cells against apoptotic death induced by oxidative stress[J].J Cell Biochem,2007,100(2):267-278.[34]Chipuk J E,Green D R.Do inducers of apoptosis trigger caspase-independent cell death?[J].Nat Rev Mol Cell Biol,2005,6(3):268-275.[35]Okada H,Mak T W.Pathways of apoptotic and non-apoptotic death in tumour cells[J].Nat Rev Cancer,2004,4(8):592-603.[36]Kobayashi Y,Yonehara S.Novel cell death by downregulation of eEF1A1 expression in tetraploids[J].Cell Death Differ,2008,16(1):139 150.[37]Grosshans H,Hurt E,Simos G.An aminoacylation-dependent nuclear tRNA export pathway in yeast[J].Genes Dev,2000,14(7):830-840.[38]Grosshans H,Simos G,Hurt E.Review:transport of tRNA out of the nucleus-direct channeling to the ribosome?[J].J Struct Biol,2000,129(2/3):288-294.[39]Dreher T W,Uhlenbeck O C,Browning K S.Quantitative assessment of EF-1a·GTP Binding to aminoacyl-tRNAs,aminoacyl-viral RNA,and tRNA shows close correspondence to the RNA binding properties of EF-Tu[J].J Biol Chem,1999,274(2):666-672.[40]Bohnsack M T,Regener K,Schwappach B,et al.Exp5 exports eEF1A via tRNA from nuclei and synergizes with other transport pathways to confine translation to the cytoplasm[J].EMBO J,2002,21(22):6205-6215.[41]Calado A,Treichel N,Müller E C,et al.Exportin-5-mediated nuclear export of eukaryotic elongation factor 1A and tRNA[J].EMBO J,2002,21(22):6216-6224.[42]Khacho M,Mekhail K,Pilon-Larose K,et al.Cancer-causing mutations in a novel transcription-dependent nuclear export motif of VHL abrogate oxygen-dependent degradation of hypoxia-inducible factor[J].Mol Cell Biol,2008,28(1):302-314.[43]Khacho M,Mekhail K,Pilon-Larose K,et al.eEF1A is a novel component of the mammalian nuclear protein export machinery [J].Mol Cel Biol,2008,19(12):5296-5308.[44]Murthi A,Shaheen H H,Huang H Y,et al.Regulation of tRNA bidirectional nuclear-cytoplasmic trafficking in Saccharomyces cerevisiae[J].Mol Biol Cell,2010,21(4):639-649.[45]Nova-Ocampo M De,Villegas-Sepulveda N,del Angel RM.Translation elongation factor-1alpha,La,and PTB interact with the 3′ untranslated region of dengue 4 virus RNA[J].Virology,2002,295(2):337-347.[46]Matsuda D,Yoshinari S,Dreher T W.eEF1A binding to aminoacylated viral RNA represses minus strand synthesis by TYMV RNA-dependen RNA polymerase[J].Virology,2004,321(1):47-56.[47]Yamaji Y,Kobayashi T,Hamada K,et al.In vivo interaction between Tobacco mosaic virus RNA-dependent RNA polymerase and host transla tion elongation factor 1A[J].Virology,2006,347(1):100-108.[48]Blackwell J L,Brinton M A.Translation elongation factor-1 alpha interacts with the 3′stem-loop region of West Nile virus genomic RNA [J].J Virol,1997,71(9):6433-6444.[49]Li Zhenghe,Pogany J,Tupman S,et al.Translation elongation factor 1A facilitates the assembly of the tombusvirus replicase and stimulates mi nus-strand synthesis[J].PLoS Pathog,2010,6(11):e1001175.[50]Das T,Mathur M,Gupta A K,et al.RNA polymerase of vesicular stomatitis virus specifically associates with translation elongation factor-1 αβγ for its activity[J].Proc Natl Acad Sci USA,1998,95(4):1449-1454.[51]Davis W G,Blackwell J L,Shi Peiyong,et al.Interaction betweenthe cellular protein eEF1A and the 3′-terminal stem-loop of West Nile viru genomic RNA facilitates viral minus-strand RNA synthesis[J].J Virol,2007,81(18):10172-10187.[52]Yamaji Y,Sakurai K,Hamada K,et al.Significance of eukaryotic translation elongation factor 1A in tobacco mosaic virus infection [J].Arch Virol,2010,155(2):263-268.[53]Li Zhenghe,Pogany J,Panavas T,et al.Translation elongation factor 1A is a component of the tombusvirus replicase complex and affects the stability of the p33 replication co-factor[J].Virology,2009,385(1):245-260.[54]程君,芮耀诚.真核延长因子eEF1A 功能研究进展[J].药学实践杂志,2012,30(2):89-92.[55]Ahn J,Hong J,Lee H,et al.Translation elongation factor 1-alpha gene from Pichia pastoris:molecular cloning,sequence,and use of its promot er[J].Appl Microbiol Biotechnol,2007,74(3):601-608[56]Habben J E,Moro G L,Hunter B G,et al.Elongation factor 1 alpha concentration is highly correlated with the lysine content of maize endo sperm[J].Proc Natl Acad Sci USA,1995,92(19):8640-8644.* 收文日期:2013-04-23;基金项目:国家自然科学基金资助项目(31 000741;31171520)。
Scince人类基因组计划翻译

全基因组霰弹枪测序法产生了291亿碱基对(bp)人基因组的全基因组序列。
在9个月的时间里,通过从5个个体的DNA中提取的27271,853个高质量序列(5.11倍的基因组覆盖)产生了148亿个bp DNA序列。
两个装配策略——一个全基因组组装和一个区域染色体组合——都被使用,每个都结合了Celera的序列数据和公共资助的基因组计划。
这些公共数据被分解成550个bp的片段,以创造一个2.9倍的覆盖范围排序,不包括由公共资助的小组所使用的克隆和装配程序固有的偏见。
这使大会的有效覆盖范围扩大到八倍,减少了最终大会上的差距的数目和差距,以5.11倍的覆盖范围获得。
这两种组装策略产生了非常相似的结果,基本上同意独立的映射数据。
这些程序集有效地覆盖了人类染色体的全染色质区域。
超过90%的基因组在10万bp以上的支架组装中,25%的基因组在1000万bp或更大的支架内。
对基因组序列的分析显示,有26588个蛋白质编码转录本,有很强的确证证据和额外的;12000个计算派生的基因与鼠标匹配或其他薄弱的支持证据。
虽然基因密集的簇是明显的,但几乎一半的基因分散在低G1C序列中,由大量明显的非编码序列分隔。
只有1.1%的基因组是由外显子进行的,而24%的基因组是内含子,其中75%的基因组是基因间的DNA。
基因片段的复制,大小不等到染色体长度,在整个基因组中很丰富,揭示了复杂的进化史。
比较基因组分析显示与神经元功能有关的脊椎动物扩张,与组织特异性发育调节,以及与止血和免疫系统有关。
序列和公共资助的基因组数据之间的DNA序列比较提供了210万单核苷酸多态性的位置。
一个随机成对的人类单倍体基因组在平均每1250个基点上有差异,但在基因组的多态性水平上有明显的异质性。
不到1%的snp导致了蛋白质的变异,但是决定哪些snp具有功能性的任务仍然是一个公开的挑战。
DNA测序的现代历史始于1977年,当时桑格报告了他的方法,用链终止核苷酸类似物来确定DNA核苷酸的顺序。
哺乳动物雷帕霉素靶蛋白复合物1诱导体内多巴胺能神经元的神经营养因子表达

哺乳动物雷帕霉素靶蛋白复合物1诱导体内多巴胺能神经元的神经营养因子表达韩国庆北国立大学生命科学院脑科学及工程学研究所助理教授以往研究表明哺乳动物雷帕霉素靶蛋白复合物1(mTORC1)激活由腺相关病毒[hRheb(S16H)]转导有编码人的ras基因的组成型活性形式的基因在脑(hRheb)同源富含丝氨酸的突变成组氨酸,可诱导神经营养作用,保护和修复帕金森病大鼠模型受损的多巴胺神经元,但修复的机制并不清楚。
作者最新研究发现,通过病毒载体hRheb(S16H)可以极大的诱导成年多巴胺神经元体内胶质细胞源性神经营养因子和脑源性神经营养因子的表达,但此作用可被雷帕霉素,一个特定的mTORC1抑制剂显著的减弱。
除了神经营养因子的诱导,作者的研究表明,使用针对的胶质细胞源性神经营养因子和脑源性神经营养因子的中和抗体,hRheb(S16H)诱导营养因子通过在PD的MPP+处理过的大鼠模型中的协同机制起到黑质纹状体多巴胺突起的保护作用。
这些结果表明,特定的基因递送到多巴胺神经元中黑质,从而可以提高的mTORC1的激活,导致持续的生成神经营养因子,维持和保护成人大脑中黑质纹状体多巴胺系统。
此观点发表在《中国神经再生研究(英文版)》2014年23期杂志上。
Article: "Mammalian target of rapamycin complex 1 as an inducer of neurotrophic factors in dopaminergic neurons" by Sang Ryong Kim (School of Life Sciences, BK21 plus KNU Creative BioResearch Group, Institute of Life Science & Biotechnology, Kyungpook National University, Daegu 702-701, Korea; Brain Science and Engineering Institute, Kyungpook National University, Daegu 700-842, Korea)Kim SR. Mammalian target of rapamycin complex 1 as an inducer of neurotrophic factors in dopaminergic neurons. Neural Regen Res. 2014;9(23):2036-2037.mTORC1 as an inducer of neurotrophic factors in dopaminergic neurons Mammalian target of rapamycin complex 1 (mTOR) kinase exists in two complexes, mTOR complex 1 (mTORC1) and mTORC2, which play central roles in the integration of cell growth in response to environmental conditions, including growth factors, amino acids, energy substrates, and oxygen.The researchers from Kyungpook National University have previously reported that the activation of mTORC1 by adeno-associated virus 1 (AAV1) transduction with a gene encoding the constitutively active form of human ras homolog enriched in brain (hRheb) with a mutation of serine to histidine at position 16 [hRheb(S16H)] could induce trophic effects. This resulted in the protection and restoration of DA neurons in the 6-hydroxydopamine (6-OHDA)-treated model ofPD. In addition to the induction of neurotrophic factors, our results using neutralizing antibodies against GDNF and BDNF have shown that Rheb(S16H)-induced trophic factors contribute to the protection of nigrostriatal DA projections via a synergetic mechanism in the MPP+-treated rat model of PD (Nam et al., 2014). These results suggest that a specific gene delivery to DA neurons in the SN, which can enhance the activation of mTORC1, results in the sustained production of diverse neurotrophic factors that are involved in the maintenance and protection of the nigrostriatal DA system in the adult brain. In conclusion, hRheb(S16H) has robust trophic and protective effects on nigrostriatal DA projections in the adult brain. The sustained production of GDNF and BDNF via an mTORC1-dependent pathway contributes to neuroprotection in animal models of PD (Nam et al., 2014). Similar to these effects, naringin can induce the production of GDNF in adult DA neurons, highlighting it as a therapeutic agent against neurodegeneration through mTORC1 activation (Leem et al., 2014). Although more research is necessary to support these results and to optimize treatment methods, our observations suggest that activation of mTORC1 by specific gene delivery or treatment with specific compounds can induce the production of neurotrophic factors, such as GDNF and BDNF, in DA neurons. This may be a useful strategy to protect and maintain the nigrostriatal DA system in the adult brain.Article: "Mammalian target of rapamycin complex 1 as an inducer of neurotrophic factors in dopaminergic neurons" by Sang Ryong Kim (School of Life Sciences, BK21 plus KNU Creative BioResearch Group, Institute of Life Science & Biotechnology, Kyungpook National University, Daegu 702-701, Korea; Brain Science and Engineering Institute, Kyungpook National University, Daegu 700-842, Korea)Kim SR. Mammalian target of rapamycin complex 1 as an inducer of neurotrophic factors in dopaminergic neurons. Neural Regen Res. 2014;9(23):2036-2037.。
鱼腥草雾化吸入的作用与功效

鱼腥草雾化吸入的作用与功效鱼腥草(Artemisia annua)是一种常见的中药植物,历来被用于治疗感冒、疟疾等疾病。
最近几年,鱼腥草的雾化吸入疗法作为一种新的治疗方式逐渐受到关注。
本文将详细介绍鱼腥草雾化吸入的作用与功效。
1. 鱼腥草的雾化吸入原理鱼腥草雾化吸入是将鱼腥草制成草本粉末后,通过雾化器将粉末转化为微细颗粒,然后通过呼吸道吸入进入人体。
这种吸入方式可以使药物直接进入呼吸道的上部和下部,并趋于周围肺组织,提高治疗效果。
2. 鱼腥草雾化吸入的作用2.1 抗炎作用研究发现,鱼腥草中含有一种称为青蒿素的活性成分,具有明显的抗炎作用。
青蒿素通过抑制炎症介质的产生,调节免疫反应,减轻炎症反应及相关症状。
雾化吸入可以使青蒿素直接作用于呼吸道黏膜,发挥更好的抗炎作用。
2.2 改善气道通畅鱼腥草雾化吸入可以有效改善气道通畅,减轻哮喘、慢性阻塞性肺病等疾病引起的呼吸困难。
鱼腥草中的活性成分能够扩张气道,减少支气管痉挛,缓解气道狭窄等症状。
2.3 抗菌作用鱼腥草中的活性成分具有显著的抗菌作用,可以抑制多种细菌、病毒和真菌的生长。
雾化吸入可以使药物直接作用于感染病灶,起到更好的抗菌效果。
尤其对于呼吸道感染引起的咳嗽、喉咙痛等症状,鱼腥草雾化吸入具有较好的治疗效果。
2.4 免疫调节作用鱼腥草中的一些活性成分可以调节人体免疫系统的功能,提高机体的抵抗力。
雾化吸入可以使这些活性成分直接作用于呼吸道黏膜和肺部组织,增强呼吸系统的免疫功能,减少感染的发生。
2.5 抗癌作用鱼腥草中的活性成分青蒿素被广泛应用于治疗疟疾,同时也具有一定的抗癌作用。
雾化吸入可以使青蒿素直接作用于肺部组织,对肺癌等呼吸道肿瘤有一定的治疗效果。
3. 鱼腥草雾化吸入的功效3.1 缓解呼吸系统疾病症状鱼腥草雾化吸入可以减轻咳嗽、喉咙痛、气短、胸闷等呼吸系统疾病引起的症状,改善呼吸困难,提高患者的生活质量。
3.2 防止感染和复发鱼腥草雾化吸入具有较好的抗菌作用,可以预防呼吸道感染的发生,同时对已经感染的疾病也能起到治疗作用。
Differentiation of Human Pluripotent Stem Cells into Retinal Cells

87M.A. Hayat (ed.), Stem Cells and Cancer Stem Cells, Volume 6,DOI 10.1007/978-94-007-2993-3_9, © Springer Science+Business Media B.V . 20129A bstractRetinal and macular degeneration disorders are characterized by a progressive loss of photoreceptors, which causes visual impairment and blindness. In some cases, the visual loss is caused by dysfunction, degen-eration and loss of underlying retinal pigment epithelial (RPE) cells and the subsequent death of photoreceptors. The grim reality is that there is no successful treatment for most of these blindness disorders. Cell therapy aimed at replenishing the degenerating cells is considered a potential ther-apeutic approach that may delay, halt or perhaps even reverse degenera-tion, as well as improve retinal function and prevent blindness in the aforementioned conditions. Human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSCs) may serve as an unlimited donor source of photoreceptors and RPE cells for transplantation into degenerat-ing retinas and for retinal disease modeling.I ntroductionThe vertebrate eyes form as bilateral evaginations of the forebrain, called optic vesicles (Martínez-Morales et al. 2004 ; Fig. 9.1a ). During develop-ment, the optic vesicles begin to invaginate to form a cup-shaped structure, the optic cup. The inner, thicker neural layer of the optic cup differ-entiates into the neural retina, and the outer, thin-ner pigmented layer forms the retinal pigmentepithelium (RPE). At the early developmental stages, the neuroepithelial cells that compose the optic vesicle are morphologically and molecu-larly identical and are all able to give rise to neu-ral retina and RPE. Exogenous signals coming from the adjacent tissues, including factors from the fi broblast growth factor (FGF) and transform-ing growth factor beta (TGF b ) families, dictate the fate of these cells. The mature vertebrate ret-ina is comprised of six types of neurons and one type of glia (the Müller glia). These seven cell types constitute three nuclear layers: retinal gan-glion cells in the ganglion cell layer (GCL); the horizontal, bipolar and amacrine interneurons, and Müller glial cells in the inner nuclear layer (INL); and rod and cone photoreceptors in the outer nuclear layer (ONL; Harada et al. 2007;M . I delson • B . R eubinoff (*)T he Hadassah Human Embryonic Stem Cell Research Center, The Goldyne Savad Institute of Gene Therapy & The Department of Obstetrics and Gynecology , H adassah University Medical Center ,E in Kerem 12000 ,J erusalem 91120 ,I srael e -mail: b enjaminr@ekmd.huji.ac.il D ifferentiation of HumanPluripotent Stem Cells into Retinal Cells Masha Idelson and Benjamin Reubinoff88M. Idelson and B. ReubinoffFig. 9.1b ). The photoreceptor cells capture lightphotons and transform their energy into electrical signals by a mechanism called phototransduction. The visual pigment which is utilized in this process is located on membranal discs in the outer seg-ments of photoreceptors. The outer segments are continuously renewed: the old discs are shed and new disks form. When the photoreceptors absorb light, they send the signal through the retinal interneurons to the ganglion cells which transmit the electrical impulse to the brain by their axons forming the optic nerve. Rods are responsible for night vision, whereas cones are responsible for color vision and detecting fi ne details. The macula is a small part of the retina which is rich in cones and responsible for detailed central vision.R PE cells that compose the outer layer of the optic cup are pigmented cuboidal cells which lie between the neural retina and the choriocapil-laris, which include the blood vessels supplying the retina. The multiple villi on their apical side are in direct contact with the outer segments ofextraocular mesenchymeabneural retinalensoptic nerveoptic cupsurface ectodermRPEFGFoptic vesiclechoroidBM RPE cone ONLINL GCLlightHC BC MC ACONrod F ig. 9.1 D evelopment and structural arrangement of the retina. ( a ) Schematic representation of retinal development including the transition from optic vesicle to optic cup and retinal patterning. ( b ) Schematic diagram of retinal cells arrangement and connections. A bbreviations :A C amacrinecell, B C bipolar cell, B M Bruch’s membrane, G CL gan-glion cell layer, H C horizontal cell, I NL inner nuclear layer, M C Müller cell, O N optic nerve, O NL outer nuclear layer89 9 Differentiation of Human Pluripotent Stem Cells into Retinal Cellsthe photoreceptor cells; on their basal side, the RPE is in contact with the underlying basal mem-brane, termed Bruch’s membrane that separates the RPE from the choroid. These cells play cru-cial roles in the maintenance and function of the retina and its photoreceptors. As a layer of pig-mented cells, the RPE absorbs the stray light that was not absorbed by the photoreceptors. The RPE cells form a blood–retinal barrier due to decreased permeability of their junctions. The RPE cells transport ions, water, and metabolic end products from the retina to the bloodstream. They are involved in supplying the neural retina with nutrients from the bloodstream, such as glu-cose, retinol, and fatty acids. Another important function of the RPE is the phagocytosis of shed photoreceptor outer segments. After the outer segments are digested, essential substances such as retinal are recycled. Retinal is also recycled and returned to photoreceptors by the process known as the visual cycle. The precise functioning of the RPE is essential for visual performance. Failure of one of these functions can lead to degeneration of the retinal photoreceptors, vision impairment and blindness.T here are many inherited and age-related eye disorders that cause degeneration of the retina as a consequence of loss of photoreceptor cells. Retinal and macular degeneration disorders can be divided into two main groups. The fi rst group primarily affects the photoreceptors and involves the majority of cases of retinitis pigmentosa. In the second group, the primary damage is to the adjacent RPE cells, and as a consequence of this damage, the photoreceptors degenerate. This group includes age-related macular degeneration, Stargardt’s macular dystrophy, a subtype of Leber’s congenital amaurosis in which RPE65 is mutated, Best’s disease and some cases of retini-tis pigmentosa, as well.W ith regard to retinitis pigmentosa (RP), it is a group of inherited retinal degeneration diseases that are caused, as mentioned above, by a primary progressive loss of rod and cone photoreceptors, followed by a subsequent degeneration of RPE (Hartong et al. 2006). The disease affects approxi-mately 1.5 million patients worldwide and is the most common cause of blindness in people under 70 years of age in the western world. The disease can be characterized by retinal pigment deposits visible on the fundus examination. In most cases, the disease primarily affects rods. At later stages of the disease, the degeneration of cones takes place. As a consequence of disease progression, the patients’ night vision is reduced. Patients initially lose peripheral vision while retaining central vision (a visual status termed “tunnel vision”). In advanced cases, central vision is also lost, commonly at about 60 years of age. The disease affects about 1 in 4,000. The inheritance can be autosomal-recessive, autosomal-dominant or X-linked (in ~50–60%, 30–40%, and 5–15% of cases, respectively). Mutations in more than 140 genes have been iden-tifi ed as causing RP (Hartong et al. 2006).Among these genes are those involved in phototransduc-tion, like rhodopsin, the a- and b- subunits of phos-phodiesterase, the a- and b- subunits of Rod cGMP gated channel and arrestin. The additional muta-tions were found in genes encoding structural pro-teins, like peripherin, rod outer segment protein and fascin. They were also found in transcription factors involved in photoreceptors’ development such as Crx and Nrl, and in other genes, whose products are involved in signaling, cell-cell interac-tion and trafficking of intracellular proteins. Currently, there is no effective cure for RP. Treatment with vitamin A palmitate, omega-3 fatty acids and other nutrients may somewhat slow the rate of the disease progression in many cases. Reduction in exposure to light was also shown to decrease the rate of retinal degeneration.A mong the group of retinal degenerations that are caused by primary loss of RPE cells or their function, age-related macular degeneration (AMD) is the most frequent condition and the leading cause of visual disability in the western world (Cook et al. 2008).Among people over 75 years of age, 25–30% are affected by AMD, with progressive central visual loss that leads to blindness in 6–8%. The retinal degeneration pri-marily involves the macula. The dry form of AMD is initiated by hyperplasia of the RPE and formation of drusen deposits, consisting of meta-bolic end products underneath the RPE or within the Bruch’s membrane. It may gradually progress into the advanced stage of geographic atrophy90M. Idelson and B. Reubinoff with degeneration of RPE and photoreceptorsover large areas of the macula causing central visual loss. Ten percent of dry AMD patients will progress to neovascular (wet) AMD, with blood vessels sprouting through the Bruch’s membrane with subsequent intraocular leakage and/or bleed-ing, accelerating the loss of central vision. While the complicating neovascularization can be treated with anti-VEGF agents, currently there is no effective treatment to halt RPE and photore-ceptor degeneration and the grim reality is that many patients eventually lose their sight (Cook et al. 2008).S targardt’s macular dystrophy (SMD) is the most common form of inherited macular dystro-phy affecting children (Walia and Fishman 2009). The disease is symptomatically similar to AMD. The prevalence of SMD is about 1 in 10,000 chil-dren. The disease involves progressive central visual loss and atrophy of the RPE beneath the macula following accumulation of lipofuscin in RPE cells, which is suggested to consist of non-degradable material, derived from ingested pho-toreceptor outer segments. The inheritance is predominantly autosomal recessive, although an autosomal dominant form has also been described. The mutation in the ABCA4 gene was found to be a most common cause of SMD. The product of the ABCA4 gene is involved in energy transport to and from photoreceptors. The mutated protein cannot perform its transport function and, as a result, photoreceptor cells degenerate and vision is impaired. Currently, there is no effective treat-ment for SMD.C ell therapy to replenish the degenerating cells appears as a promising therapeutic modality that may potentially halt disease progression in the various retinal and macular degeneration dis-orders caused by loss and dysfunction of RPE cells and photoreceptors (da Cruz et al. 2007).I n this chapter we will discuss the potential of human pluripotent cells which includes human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSCs), to gen-erate various types of retinal cells that could be used for transplantation therapy of retinal degen-eration disorders and disease modeling for drug discovery. C ell Therapy of Retinal and Macular DegenerationsT he eye is an attractive organ for cell therapy as it is easily accessible for transplantation and for simple monitoring of graft survival and potential complications by direct fundoscopic visualiza-tion. Anatomically, it is a relatively confi ned organ limiting the potential of unwanted extra-ocular ectopic cell distribution, and a low number of cells are required to replenish the damaged cells. The eye is also one of the immune privi-leged sites of the body.T he concept of replacing dysfunctional or degenerated retina by transplantation has been developing ever since the fi rst retina-to-retina transplant in 1986 (Turner and Blair 1986).In most studies, primary retinal immature (fetal) tissue has been used as donor material. It was demonstrated that such transplants can survive, differentiate, and even establish connections with the host retina to a limited degree (Ghosh et al. 1999). The subretinal transplantation of healthy RPE has some advantages over neural retinal transplantation, as it concerns only one cell type that is not involved in neural networking. Transplantation of RPE has been studied exten-sively in animal models (Lund et al. 2001).The most commonly used animal model of retinal degeneration is the Royal College of Surgeons (RCS) rat model, in which primary dysfunction of the RPE occurs as a result of a mutation in the receptor tyrosine kinase gene M ertk(D’Cruz et al. 2000). This leads to impaired phagocytosis of shed photoreceptor outer segments, with sec-ondary degeneration and progressive loss of pho-toreceptors within the fi rst months of life. It was reported that rat and human RPE cells rescued photoreceptor cells from degeneration when transplanted into the subretinal space of RCS rats (Li and Turner 1988; Coffey et al. 2002).The ability of transplanted RPE cells to restore retinal structure and function has been demonstrated in clinical trials. In humans, autologous transplanta-tions of peripheral RPE as well as macular trans-locations onto more peripheral RPE provide a proof that positioning the macula above relatively91 9 Differentiation of Human Pluripotent Stem Cells into Retinal Cellshealthier RPE cells can improve visual functionin AMD patients (Binder et al. 2004; da Cruz et al. 2007). Nevertheless, the surgical procedures for autologous grafting are challenging and are often accompanied by signifi cant complications. In addition, autologous RPE transplants may carry the same genetic background, environmen-tal toxic and aging-related effects that may have led to macular RPE failure and the development of AMD in the patient. It is also problematic to use autologous cells when all the RPE cells are damaged. Cell sources that can be used for such therapy include allogeneic fetal and adult RPE (Weisz et al. 1999; Binder et al. 2004; da Cruz et al. 2007). However, the use of fetal or adult retinal tissues for transplantation is severely lim-ited by ethical considerations and practical prob-lems in obtaining sufficient tissue supply. The search for a cell source to replace autologous RPE such as immortalized cell lines, umbilical cord-derived cells as well as bone marrow-derived stem cells continues.T he derivation of hESCs more than a decade ago has raised immense interest in the potential clinical use of the cells for regeneration (Thomson et al. 1998; Reubinoff et al. 2000).Along the years, signifi cant progress has been made towards the use of hESCs in clinical trials.T he other promising source of cells for transplantation therapy is iPSCs that are simi-lar to hESCs in their stemness characteristics and pluripotency. These cells could be gener-ated from different human somatic cells by transduction of four defi ned transcription fac-tors: Oct3/4, Sox2, Klf4, and c-Myc (Takahashi et al. 2007).G eneration of RPE and neural retina from hESCs and iPSC has numerous advantages, as it can be done from pathogen-free cell lines under good manufacturing practice (GMP) conditions with minimal variation among batches. Such cells can be characterized extensively prior to preclinical studies or for clinical applications, and an unlimited numbers of donor cells can be generated from them. In the following para-graphs, strategies for induction of differentiation of hESCs and iPSCs towards RPE and neural retina fate are reviewed. D ifferentiation into Retinal Pigment EpitheliumI t was reported for the fi rst time in mice and pri-mates that the differentiation of ES cells into RPE could be induced by co-culture with PA6 stromal cells (Kawasaki et al. 2002; Haruta et al. 2004). The resulting cells had polygonal epithelial mor-phology and extensive pigmentation. The cells expressed the markers that are characteristic of RPE. They developed typical ultrastructures and exhibited some functions of RPE. The differenti-ation of hESC into RPE was first reported by Klimanskaya et al. (2004).According to their protocol, hESCs underwent spontaneous differ-entiation by overgrowth on mouse embryonic fibroblasts (MEF), in feeder-free conditions or, alternatively, as embryoid bodies (EBs) in com-bination with withdrawal of bFGF from the medium. The yield of the formation of RPE cells after 4–8 weeks of spontaneous differentiation was relatively low; for example,<1% of EBs con-tained pigmented cells at this stage. However, after 6–9 months in culture, all the EBs contained pigmented cells. The areas of pigmented cells could be further isolated mechanically and prop-agated by passaging as RPE lines. Klimanskaya and colleges characterized the hESC-derived RPE cells by transcriptomics and demonstrated their higher similarity to primary RPE tissue than to human RPE lines D407 and ARPE-19. The low yield of spontaneously differentiating RPE cells was improved by induction of differentia-tion with Wnt and Nodal antagonists, Dkk1 and LeftyA, respectively, the factors that are sug-gested to promote retinal differentiation. This treatment gave rise to pigmented cells within 38% of the hESC colonies after 8 weeks (Osakada et al. 2008). Immunostaining with the ZO-1 anti-body showed that by day 120, hESC-derived pig-mented cells formed tight junctions (about 35% of total cells). We showed that differentiation toward the neural and further toward the RPE fate could be augmented by vitamin B3 (nicotin-amide; Idelson et al. 2009).We further showed that Activin A, in the presence of nicotinamide, effi ciently induces and augments differentiation92M. Idelson and B. Reubinoffinto RPE cells. This is in line with the presumed role of Activin A in RPE development i n vivo .In the embryo, extraocular mesenchyme-secreted members of the TGF b superfamily are thought to direct the differentiation of the optic vesicle into RPE (Fuhrmann et al. 2000).Under our culture conditions, when the cells were grown in suspen-sion as free-fl oating clusters, within 4 weeks of differentiation, 51% of the clusters contained pigmented areas and about 10% of the cells within the clusters were pigmented. When we modifi ed the differentiation conditions to includea stage of monolayer culture growth, the yield of the RPE-like pigmented cells was signifi cantly improved and 33% of the cells were pigmented after 6 weeks of differentiation. The derivation of RPE from hESCs and iPSCs without any external factor supplementation was also demonstrated by other groups (Vugler et al. 2008 ; Meyer et al. 2009 ; Buchholz et al. 2009).T he hESC-derived RPE cells were extensively characterized, including demonstration, both at the mRNA and the protein levels, of the expres-sion of RPE-specifi c markers, such as RPE65, CRALBP, Bestrophin, Tyrosinase, PEDF, PMEL17, LRAT, isoforms of MiTF abundant in RPE, and others. The cells expressed markers of tight junctions that join the adjacent RPE cells: ZO-1, occludin and claudin-1 (Vugler et al. 2008 ) . Electron microscopic analysis revealed that the hESC-derived RPE cells showed features characteristic of RPE. The cells were highly polarized with the nuclei located more basally, and the cytoplasm with the mitochondria and melanin granules of different maturity more api-cally. A formation of basal membrane was observed on the basal surface of the RPE cell. Similar to putative RPE, the hESC-derived RPE basal membrane was shown to be composed of extracellular matrix proteins, collagen IV , lami-nin and fi bronectin (Vugler et al.2008).The appearance of apical microvilli was demonstrated at the apical surface of the RPE. The presence of tight and gap junctions on the apical borders of the RPE cells was also confi rmed by electron microscopy. O ne of the most important functions of RPE cells i n vivo is phagocytosis of shed photoreceptor outer segments, as part of the continuous renewal process of rods and cones. The hESC-derived RPE cells demonstrated the ability to phagocyto-size latex beads or purifi ed photoreceptor outer segments, confi rming that these cells are func-tionali n vitro . It may be concluded from all these studies that human pluripotent stem cells have a potential to give rise to pigmented cells exhibiting the morphology, marker expression and functionof authentic RPE.D ifferentiation into Retinal Progenitors and Photoreceptors O ur group showed, for the fi rst time, the potential of highly enriched cultures of hESC-derived neu-ral precursors (NPs) to differentiate towards the neural retina fate (Banin et al. 2006).We demon-strated that the NPs expressed transcripts of key regulatory genes of anterior brain and retinal development. After spontaneous differentiation i n vitro , the NPs gave rise to progeny expressing markers of retinal progenitors and photoreceptor development, though this was uncommon and cells expressing markers of mature photorecep-tors were not observed. We showed that after transplantation into rat eyes, differentiation into cells expressing specifi c markers of mature photoreceptors occurred only after subretinal transplantation (between the host RPE and pho-toreceptor layer) suggesting that this specifi c microenvironment provided signals, yet unde-fi ned, that were required to support differentia-tion into the photoreceptoral lineage.P rogress towards controlling and inducing the differentiation of hESCs into retinal progenitors and neurons i n vitro was reported in the study of Lamba et al. ( 2006).They treated hESC-derived EBs for 3 days with a combination of factors,including Noggin, an inhibitor of BMP signaling, Dkk1, a secreted antagonist of the Wnt signaling pathway and insulin-like growth factor 1 (IGF-1), which is known to promote retinal progenitor dif-ferentiation. The cultivation of EBs with these factors was followed by differentiation on Matrigel or laminin for an additional 3 weeks in the presence of the combination of the three93 9 Differentiation of Human Pluripotent Stem Cells into Retinal Cellsfactors together with bFGF. Under these culture conditions, the majority of the cells developed the characteristics of retinal progenitors and expressed the specifi c markers Pax6 and Chx10 (82% and 86% of the cells, respectively). The authors showed that after further differentiation, the cells expressed markers of photoreceptor development Crx and Nrl (12% and 5.75%, respectively). About 12% of the cells expressed also HuC/D, the marker of amacrine and ganglion cells. The expression of markers of the other sub-types of retinal neurons was demonstrated, as well. However, only very few cells (<0.01%) expressed markers of mature photoreceptors, blue opsin and rhodopsin. The abundance of cells expressing markers of photoreceptors could be accelerated by co-culture with retinal explants, especially when the explants originated from mice bearing a mutation that causes retinal degeneration.T o better characterize the phenotype of retinal cells obtained with this differentiation protocol, a microarray-based analysis comparing human retina to the hESC-derived retinal cells was per-formed (Lamba and Reh 2011).It was demon-strated that gene expression in hESC-derived retinal cells was highly correlated to that in the human fetal retina. In addition, 1% of the genes that were highly expressed in the hESC-derived cultures could be attributed to RPE and ciliary epithelium differentiation.A n alternative protocol for the derivation of retinal progenitors and photoreceptors was pro-posed by Osakada et al. (2008).Similar to the protocol for the derivation of RPE cells, they used serum-free fl oating cultures in combination with the Dkk1 and LeftyA. After 20 days of cul-ture in suspension, the cells were replated on poly-D-lysine/laminin/fi bronectin-coated slides. Osakada and co-authors demonstrated that on day 35 in culture, about 16% of colonies were positive for retinal progenitor markers Rx and Pax6. Differentiation towards photoreceptor fate was augmented in the presence of N2 by treat-ment with retinoic acid and taurine, which are known inducers of rod fate differentiation. Under these conditions, after an extended culture period of 170 days, about 20% of total cells were positive for Crx, an early photoreceptor marker. On day 200, about 8.5% of the cells expressed the mature rod photoreceptor marker, rhodopsin, as well as cone photoreceptor markers, red/green and blue opsins (8.9% and 9.4%, respectively).A n alternative approach was proposed by the same group based on the use of small molecules. In this method, the chemical inhibitors CKI-7 and SB-431542 that inhibit Wnt and Activin A signaling, respectively, and Y-27632, the Rho-associated kinase inhibitor, which prevents disso-ciation-induced cell death, were used. These molecules were shown to mimic the effects of Dkk1 and LeftyA (Osakada et al. 2009).This strategy, which doesn’t involve the use of recom-binant proteins which are produced in animal or E scherichia coli cells, is more favorable for the gen-eration of cells for future transplantation therapy.I n another study that was published by Meyer et al .(2009), after initial differentiation in sus-pension for 6 days, the aggregates were allowed to attach to laminin–coated culture dishes. After further differentiation as adherent cultures, neu-roepithelial rosettes were formed, which were mechanically isolated and subsequently culti-vated as neurospheres. The authors didn’t use any soluble factors; moreover, they showed that under these conditions, the cells expressed endogenous Dkk1 and Noggin. They also demonstrated that in concordance with the role of bFGF in retinal specifi cation, the inhibition of endogenous FGF-signaling abolished retinal differentiation. Under their differentiation protocol, by day 16, more than 95% of the cells expressed the retinal pro-genitor markers, Pax6 and Rx. The authors dem-onstrated that by day 80 of differentiation, about 19% of all neurospheres contained Crx+ cells and within these Crx+ neurospheres, 63% of all cells express Crx and 46.4% of the cells expressed mature markers, such as recoverin and cone opsin.I n all of the above studies, differentiated cells expressing the retinal markers were obtained; however, the cells were not organized in a three-dimensional retinal structure. In a paper recently published by Eiraku et al. (2011),the authors cul-tured free-fl oating aggregates of mouse ES cells in serum-free medium in the presence of base-ment membrane matrix, Matrigel, that could also94M. Idelson and B. Reubinoffbe substituted with a combination of laminin, entactine and Nodal. Using a mouse reporter ES cell line, in which green fl uorescent protein (GFP) is knocked in at the Rx locus, the authors showed that Rx-GFP+ epithelial vesicles were evaginated from the aggregates after 7 days of differentiation under these conditions. On days 8–10, the Rx-GFP+ vesicles changed their shape and formed optic cup-like structures. The inner layer of these structures expressed markers of the neural retina whereas the outer layer expressed markers of RPE. The authors demonstrated that differen-tiation into RPE required the presence of the adjacent neuroectodermal epithelium as a source of diffusible inducing factors. In contrast, the differentiation into neural retina did not require tissue interactions, possibly because of the intrinsic inhibition of the Wnt-signaling pathway. Eiraku and colleagues showed that the retinal architecture, which was formed within the optic vesicle-like structures, was comparable to the native developing neural retina.R ecently, optic vesicle-like structures were also derived from hESCs and iPSCs using the protocol described above, which is based on iso-lating the neural rosette-containing colonies and culturing them in suspension (Meyer et al. 2011). The cells within the structures expressed the markers of retinal progenitors, and after differen-tiation gave rise to different retinal cell types. It was shown that the ability of optic vesicle-like structures to adopt RPE fate could be modulated by Activin A supplementation. The production of these three-dimensional retinal structures opens new avenues for studying retinal development in normal and pathological conditions.T ransplantation of Pluripotent Stem Cell-Derived Retinal CellsA key step towards future clinical transplanta-tions of hESC-derived RPE and neural retina is to show proof of their therapeutic potential i n vivo. Various animal models of retinal degeneration have been used to evaluate the therapeutic effect of transplanted retinal cells. Human ESC-derived RPE cells were transplanted subretinally to the degenerated eyes of RCS rats. Transplantation of the hESC-derived RPE cells between the RPE and the photoreceptor layer rescued retinal struc-ture and function (Lund et al. 2006; Vugler et al. 2008; Idelson et al. 2009; Lu et al. 2009).The subretinally engrafted hESC-derived RPE cells salvaged photoreceptors in proximity to the grafts as was shown by the measurement of the thick-ness of the ONL, the layer of photoreceptor nuclei, which is an important monitor of photore-ceptor cell survival. The ONL thickness was significantly increased in transplanted eyes in comparison to the degenerated non-treated eyes.I n order to evaluate the functional effect of transplanted cells i n vivo, the electroretinography (ERG) that directly measures the electrical activ-ity of the outer (a-wave) and inner (b-wave) retina in response to light stimulation was used. It was demonstrated that after transplantation of hESC-derived RPE, ERG recordings revealed a signifi -cant preservation of retinal function in the treated eyes as compared to control untreated eyes (Lund et al. 2006; Idelson et al. 2009).The visual func-tion of the animals was also estimated by an optomotor test, which monitors the animal’s refl exive head movements in response to a rotat-ing drum with fi xed stripes. Animals transplanted with hESC-derived RPE showed signifi cantly better visual performance in comparison to con-trol animals (Lund et al. 2006; Lu et al. 2009). The presence of rhodopsin, a major component of photoreceptor outer segments, within the sub-retinaly transplanted pigmented cells suggested that they could perform phagocytosis i n vivo (Vugler et al. 2008; Idelson et al. 2009).B ridging the gap between basic research and initial clinical trials requires immense resources to ensure safety and efficacy. Human ESC-derived RPE cell lines were generated using a current Good Manufacturing Practices (cGMP)-compliant cellular manufacturing process (Lu et al. 2009). Long-term studies analyzing safety and efficacy of transplantation of these GMP-compliant hESC-derived RPE cells revealed that the subretinally transplanted cells survived for a period of up to 220 days and provided prolonged functional improvement for up to 70 days after transplantation. The potential of the hESC-derived。
【文献翻译】Hallmarks of Cancer:The Next Generation/肿瘤的新十大特征

Hallmarks of Cancer: The Next Generation摘要肿瘤的标志包括在人类肿瘤多步发展过程中所获得的6种生物学功能。
这些标志构成了一种系统化的规律,使得肿瘤疾病的复杂性变得合理化,它们包括:1.持续增殖的信号2.逃避生长抑制因子3.抵抗细胞死亡4.使其能够无限复制5.诱导血管生成6.激活肿瘤侵袭性和转移性。
在这些标志下隐藏着的是基因的不稳定性,基因的不稳定性造成了基因多样性并加快了它们的习得性和炎症表现,这种不稳定性也培养出多种标志功能。
在过去的十年里,概念上的进步在于:在这个名单里增加了两种新兴的的具有潜在普遍性的特征——能量代谢的重新编程和逃避免疫破坏。
除了癌细胞以外,肿瘤还表现出另一种复杂的维度:他们控制一群被招募的、表面看上去正常的细胞,通过建立一个“肿瘤微环境”来有利于这些标志特性的获得。
认识这些概念上的广泛适用性会日益影响人类肿瘤治疗新方法的发展过程。
简介为了了解肿瘤疾病的显著差异性,我们提出了6种癌症的标志,并将它们组织在一起形成系统性的规律,从而提供了一种符合逻辑的框架结构。
在我们的讨论中隐含着这样的一个信息:随着正常细胞逐渐发展为癌症状态,它们会逐渐获得这一系列标志功能。
初期的癌症细胞会需要获得这些特征,从而使其致癌甚至最终恶性,人类肿瘤病例特征的多级发展正是通过这一需求而表现地合理化。
我们注意到,作为一个辅助命题,肿瘤不仅仅是大量孤立增殖的癌细胞,正相反,他们是彼此之间相互参与异型性作用的由多个不同类型细胞所构成的复合组织。
我们描述出这些被招募的正常细胞(它们形成了肿瘤相关基因)在肿瘤发生过程中是积极的参与者而不只是消极的旁观者;如此一来,这些间质细胞有助于某些标志功能的发展和表现。
在随后的十年里,这一概念被固化并延伸,揭示出:肿瘤的生物学不再可以被仅仅理解为“列举肿瘤细胞的特征”,相反,必须通盘考虑“肿瘤微环境”为肿瘤的发生所做出的贡献。
在此后发表的癌症研究显著进展的过程中,新的观察研究被提供出来,得以阐明和修正这些标志功能的原始构想。
新一代测序技术的原理

SOLiD的特点
• 通量大 • 成本低 • 序列短
– Ligase的方式虽然能一定程度避免phasing/prephasing,但增加的复杂度也降低了效率
• 灵活性差,对小数据量测序不适用
• Two-base coding
Next-generation sequencing/Deep sequencing technology main platforms
DNA打断 末端修复 加A 加接头 纯化
模板杂交 桥式PCR扩增 测序引物准备测序
3 测序
测序 生成 base calls
4 数据分析
拍照图片
Intensities Read种类:
Single-Read Sequencing
单向测序
H H
OH
OH(H) O O
OH(H)
P
O
OH
CH2
O
N
H H
OH
H H
OH (H)
核苷酸形成3,5-磷酸二酯键示意图
“双脱氧末端终止”的含 义
终止物标记方法
Template
Primer
Terminator
A
G
C
T
dNTP
Polymerase
因为颜色不同,4种终止物可以在一起进行反应。
3730xl Sequence Map
Invention of Nanopore single
molecular sequencing
(Oxford Nanopore corporation)
1950 1960
Chemical degradation method by
Whitfield (1954)
Application of next-generation sequencing technolo

Clinical Oncology
Xuchao Zhang12, Zhiyong Liang3, Shengyue Wang4, Shun Lu5, Yong Song6, Ying Cheng7, Jianming Ying8, Weiping Liu9, Yingyong Hou10, Yangqiu Li11, Yi Liu12, Jun Hou13, Xiufeng Liu14, Jianyong Shao15, Yanhong Tai16, Zheng Wang17, Li Fu18, Hui Li7, Xiaojun Zhou19, Hua Bai20, Mengzhao Wang21, You Lu22, Jinji Yang23, Wenzhao Zhong23, Qing Zhou23, Xuening Yang23, Jie Wang24, Cheng Huang25, Xiaoqing Liu26, Xiaoyan Zhou27, Shirong Zhang28, Hongxia Tian1-2, Yu Che门陀 Ruibao Ren29, Ning Liao30, Chunyan Wu31r Zhongzheng Zhu32, Hongming Pan33, Yanhong Gu34, Liwei Wang35, Yunpeng Liu36, Suzhan Zhang3?, Tianshu Liu38, Gong Chen39, Zhimin Shao40, Binghe Xu24, Qingyuan Zhang41, Ruihua Xu42, Lin Shen43, Yilong Wu1-2, On behalf of Chinese Society of Clinical Oncology (CSCO) Tumor Biomarker Committee
鞘氨醇激酶1抑制剂的研究进展

Journal of China Pharmaceutical University2021,52(6):759-768学报鞘氨醇激酶1抑制剂的研究进展杨倩1,汪小涧2*(1国家知识产权局专利局专利审查协作北京中心,北京100160;2中国医学科学院、北京协和医学院药物研究所,天然药物活性物质与功能国家重点实验室,北京100050)摘要鞘氨醇激酶1(SphK1)是调控细胞膜脂质微环境的重要蛋白,在神经酰胺,鞘氨醇和鞘氨醇-1-磷酸的动态平衡中发挥重要作用。
SphK1的过度表达与肿瘤的发生,发展和迁移过程以及产生耐药性有着密切的联系。
SphK1抑制剂可以诱导多种肿瘤细胞凋亡,并且可以逆转肿瘤耐药性,具有良好的药物开发前景。
本文综述了SphK1的结构生物学以及SphK1抑制剂的结构类型和构效关系研究进展。
关键词鞘氨醇激酶-1;抑制剂;抗肿瘤;结构类型;构效关系;进展中图分类号R979.1文献标志码A文章编号1000-5048(2021)06-0759-10doi:10.11665/j.issn.1000-5048.20210615引用本文杨倩,汪小涧.鞘氨醇激酶1抑制剂的研究进展[J].中国药科大学学报,2021,52(6):759–768.Cite this article as:YANG Qian,WANG Xiaojian.Research progress of sphingosine kinase1inhibitors[J].J China Pharm Univ,2021,52(6):759–768.Research progress of sphingosine kinase1inhibitorsYANG Qian1,WANG Xiaojian2*1Patent Examination Cooperation(Beijing)Center of the Patent Office,China National Intellectual Property Administration,Beijing 100160;2State Key Laboratory of Bioactive Substances and Functions of Natural Medicines,Institute of Materia Medica,Peking Union Medical College and Chinese Academy of Medical Sciences,Beijing100050,ChinaAbstract Sphingosine kinase1(SphK1)is an important protein that regulates the lipid microenvironment of cell membranes,and plays an important role in the dynamic equilibrium of ceramide,sphingosine and sphingo‐sine-1-phosphate.The overexpression of SphK1is closely related to the occurrence,development and migration of tumors as well as the generation of drug resistance.SphK1inhibitors can induce apoptosis of various tumor cells and reverse drug resistance,which has a good prospect for drug development.In this article,the structural biology of SphK1,the structural types and structure-activity relationships of SphK1inhibitors are reviewed. Key words sphingosine kinase1(SphK1);inhibitors;anti-tumor;structural types;SAR;advances鞘脂(sphingolipids)是生物膜的重要组成部分,具有两性结构,两端分别是长链脂肪酸和极性醇[1]。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1950ReSeARCH ARTICLeThe next-generation sphingosine-1 receptor modulator BAF312 (siponimod) improves cortical network functionality in focal autoimmune encephalomyelitis*Correspondence to:Petra Hundehege, PhD,petra.hundehege@ukmuenster.de.#These authors contributed equally to this work.orcid:0000-0001-9562-0285 (Petra Hundehege)doi: 10.4103/1673-5374.259622 Received: February 9, 2019Accepted: May 7, 2019Petra Hundehege 1, *, #, Manuela Cerina 1, #, Susann Eichler 1, #, Christian Thomas 1, Alexander M. Herrmann 1, Kerstin Göbel 1, Thomas Müntefering 1, Juncal Fernandez-Orth 1, Stefanie Bock 1, Venu Narayanan 1, Thomas Budde 2, Erwin-Josef Speckmann 2, Heinz Wiendl 1, Anna Schubart 3, Tobias Ruck 1, Sven G. Meuth 11 Department of Neurology with Institute of Translational Neurology, Westfälische Wilhelms-Universität, Münster, Germany2 Institute of Physiology I, Westfälische Wilhelms-Universität, Münster, Germany3 Novartis Institutes of Biomedical Research, Basel, SwitzerlandFunding: This study was supported by the Novartis Institutes of Biomedical Research, Basel, Switzerland (to SGM).AbstractAutoimmune diseases of the central nervous system (CNS) like multiple sclerosis (MS) are characterized by inflammation and demyelinated lesions in white and grey matter regions. While inflammation is present at all stages of MS, it is more pronounced in the relapsing forms of the disease, whereas progressive MS (PMS) shows significant neuroaxonal damage and grey and white matter atrophy. Hence, disease-modifying treatments beneficial in patients with relapsing MS have limited success in PMS. BAF312 (siponimod) is a novel sphingosine-1-phosphate receptor modulator shown to delay progression in PMS. Besides reducing inflammation by sequestering lymphocytes in lymphoid tissues, BAF312 crosses the blood-brain barrier and binds its receptors on neurons, astrocytes and oligodendrocytes. To evaluate potential direct neuropro-tective effects, BAF312 was systemically or locally administered in the CNS of experimental autoimmune encephalomyelitis mice with distinct grey- and white-matter lesions (focal experimental autoimmune en-cephalomyelitis using an osmotic mini-pump). Ex-vivo flow cytometry revealed that systemic but not local BAF312 administration lowered immune cell infiltration in animals with both grey and white matter le-sions. Ex-vivo voltage-sensitive dye imaging of acute brain slices revealed an altered spatio-temporal pattern of activation in the lesioned cortex compared to controls in response to electrical stimulation of incoming white-matter fiber tracts. Here, BAF312 administration showed partial restore of cortical neuronal circuit function. The data suggest that BAF312 exerts a neuroprotective effect after crossing the blood-brain bar-rier independently of peripheral effects on immune cells. Experiments were carried out in accordance with German and EU animal protection law and approved by local authorities (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen; 87-51.04.2010.A331) on December 28, 2010.Key Words: multiple sclerosis; focal experimental autoimmune encephalomyelitis; cortical grey matter; white matter; BAF312; neuroaxonal damage; neuroprotection Chinese Library Classification No. R453; R363; R741IntroductionMultiple sclerosis (MS) is an autoimmune central nervous system (CNS) disease characterized by demyelination and neurodegeneration. The most common disease course (re-lapsing MS, RMS), affects approximately 85% of patients and manifests with recurring focal neurological deficits followed by total or partial recovery. Progressive forms of MS (PMS) are defined by the accumulation of neurological disability occurring independently of relapses (Lublin et al., 2014). Focal demyelinated lesions with variable degrees of inflam-mation (Kuhlmann et al., 2017) are found in white matter regions, cerebral cortex (Kidd et al., 1999; Bø et al., 2003b) and in deep brain structures like the thalamus (Deppe et al., 2016). While cortical damage and grey matter lesions can already be found in early stages of the disease (Haider et al., 2014; Mandolesi, 2015), cortical demyelination is the most pronounced hallmark in PMS (Haider et al., 2014). In patients with RMS, MS lesions contain a high number ofmacrophages, microglia and perivascular T cells (Kuhlmann et al., 2017). In contrast, lesions of patients with PMS, are mainly characterized by neuroaxonal damage and a small number of inflammatory cells (Frischer et al., 2015). In line with these findings, disease-modifying treatments altering or suppressing the immune system were beneficial in RMS pa-tients, but most of these drugs failed to ameliorate disability progression in PMS (Montalban et al., 2017). Hence, suitable drugs that promote the latter process are urgently needed. Siponimod (BAF312), a novel sphingosine-1 receptor (S1P-R) modulator selectively targeting S1P1-R and S1P5-R (Pan et al., 2013), showed to ameliorate disability progres-sion in patients with PMS, including ones who reached a non-relapsing stage in one recent large clinical trial (EX-PAND (Kappos et al., 2018)). Mechanistically, beside reduc-ing inflammation by sequestering lymphocytes in lymphoid tissues, BAF312 crosses the blood-brain barrier and binds its receptors on neurons, astrocytes and oligodendrocytes influ-Hundehege P, Cerina M, Eichler S, Thomas C, Herrmann AM, Göbel K, Müntefering T, Fernandez-Orth J, Bock S, Narayanan V, Budde T, Speckmann EJ, Wiendl H, Schubart A, Ruck T, Meuth SG (2019) The next-generation sphingosine-1 receptor modulator BAF312 (siponimod) improves cortical network functionality in focal autoimmune encephalomyelitis. Neural Regen Res 14(11):1950-1960. doi:10.4103/1673-5374.259622encing regulation of astrogliosis (Choi et al., 2011), modu-lating oligodendrocyte processes and cell survival (Jaillard et al., 2005). Recently, evidence of a potential neuroprotective effect of BAF312 administration were shown in an animal model of experimental autoimmune encephalomyelitis (EAE) with beneficial effect on clinical scores, significant re-duction of astro-microgliosis and improvement of GABAer-gic transmission in the striatum (Gentile et al., 2016). However, while EAE is the most frequently used active im-munization model in which animals are immunized with the myelin oligodendrocyte glycoprotein 35–55 (MOG35–55) pep-tide (Mendel et al., 1995; Bittner et al., 2014), inflammatory lesions are typically confined to the spinal cord and occur in the brain in randomly distributed locations (Pomeroy et al., 2005; Storch et al., 2006). However, studying neurodegener-ative aspects in a reproducible localization of brain lesions is achieved by performing focal grey matter lesions induced in MOG35–55 immunized mice through injection of interferon-γ (IFN-γ) and tumor necrosis factor α (TNF-α), thus allowing the study of cortical lesion pathophysiology and treatment effects (Chaudhary et al., 2015; Lagumersindez-Denis et al., 2017).This study aimed to dissect the peripheral immunomodu-latory effects of BAF312 from potential direct neuroprotec-tive effects in the CNS. For this purpose, we took advantage of a multidimensional approach by using a focal EAE model with distinct grey or white matter lesions and administrating BAF312 either systemically or intracerebrally using osmotic mini-pumps. The effects of BAF312 on the inflammatory response in focal cortical grey and white matter lesions were analyzed by using multicolor flow cytometry. In addition, the integrity of the thalamocortical neuronal network cir-cuits was assessed using a voltage sensitive dye technique. Material and MethodsFocal eAe ModelExperiments were carried out in accordance with German and EU animal protection law and approved by local author-ities (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen; 87-51.04.2010.A331) on December 28, 2010. EAE was induced as described previously (Bittner et al., 2014; Göbel et al., 2016b). Briefly, 10 days prior to focal EAE induction, C57BL/6J mice (female, ~10 weeks, Envigo, Rossford, Germany) were subcutaneously immunized with 200 µg of murine MOG35–55 peptide (Charité, Berlin, Germa-ny) emulsified in 200 µL complete Freund’s adjuvant (Merck KGaA, Darmstadt, Germany; former name Sigma-Aldrich Chemie GmbH, Steinheim, Germany) containing 200 µg Mycobacterium tuberculosis (strain H37 Ra; Becton, Dick-inson and Company (BD), Sparks, MD, USA). Pertussis tox-in (400 ng in 200 µL phosphate-buffered saline (PBS); Enzo Life Sciences, Farmingdale, NY, USA) was injected intraperi-toneally (i.p.) on the day of immunization (day 0) and 2 days later. The clinical course of EAE was monitored daily by two blinded investigators using the following scoring system: grade 0, no abnormality; grade 1, limp tail tip; grade 2, limp tail; grade 3, moderate hindlimb weakness; grade 4, com-plete hindlimb weakness; grade 5, mild paraparesis; grade 6, paraparesis; grade 7, heavy paraparesis or paraplegia; grade 8, tetraparesis; grade 9, quadriplegia or premoribund state; or grade 10, death. Animals with a score of 8 were killed and the last score observed was included in the analysis until the end of the experiment. Ten days after MOG immunization (10 d.p.i.), mice were anesthetized and mounted on a stereo-tactic device. A hole was drilled through the skull using the following coordinates: anteroposterior, −2.18 mm; lateral, 4.2 mm from bregma; and dorsoventral, 1 mm from the brain surface for the auditory cortex (AC), and anteroposterior, −0.94 mm; lateral, 2.10 mm; dorsoventral, 2.5 mm for the internal capsule (IC) (Paxinos and Franklin, 2001). Two μL of pro-inflammatory cytokine solution containing TNF-α (150 U; Merck KGaA) and 800 U of IFN-γ (Merck KGaA) dissolved in PBS was slowly injected either into AC or the IC of the left hemisphere. The contralateral hemisphere (right side) was used as control. Mice were sacrificed 2 days post injection (day 12; Figure 1a and d). An additional group of mice used for histopathological evaluation only was sacri-ficed 5 days post-injection (Figure 3).Oral and intracerebral BAF312 treatmentFor systemic application, BAF312 (3 mg/kg; Novartis Phar-ma AG, Basel, Switzerland) was administered daily via oral gavage in 1% aqueous carboxy-methylcellulose. For intrace-rebral application, BAF312 was dissolved in a solution con-taining 10% Solutol/Kolliphor HS15 (BASF Pharma Solu-tions, Ludwigshafen am Rhein, Germany) with a final pH range between 6 and 7 at a final concentration of 2 mg/mL. This preparation allowed stability of the drug for up to 6 weeks at 37°C. At the day of focal EAE induction, mice were implanted with subcutaneous osmotic mini pumps (Alzet Osmotic Pumps, Cupertino, CA, USA) allowing continuous intracerebral (i.c.) infusion of either vehicle or BAF312 (0.3 and 1 µg/d) to the focal lesion site.Flow cytometryMulticolor flow cytometric analyses of murine peripheral leukocytes were performed as previously described (Göbel et al., 2016a). Prior to staining, EDTA-blood was treated with red blood cell (RBC) lysis buffer according to the manufac-turer’s protocol (BioLegend, London, UK). For purification of brain-infiltrating leukocytes, brains from animals were removed after transcardial perfusion with PBS in order to efficiently remove circulating blood. Brain slices of lesioned vs. non-lesioned hemispheres were prepared and mechan-ically homogenized in PBS, layered on a 30–50% Percoll (Merck KGaA) gradient and continuously centrifuged for 30 minutes at around 1300 × g. Mononuclear cells were isolated at the interphase. After isolation, leukocytes were stained for 30 minutes at 4°C with the appropriate combination of in-dicated fluorescence-labeled monoclonal antibodies in PBS containing 0.1% NaN3 (Merck KGaA) and 0.1% bovine se-rum albumin (BSA) (Merck KGaA). Corresponding isotype controls were used for the staining. For blocking of Fc recep-tors, cells were preincubated with purified anti-CD16/CD321951Hundehege P, Cerina M, Eichler S, Thomas C, Herrmann AM, Göbel K, Müntefering T, Fernandez-Orth J, Bock S, Narayanan V, Budde T, Speckmann EJ, Wiendl H, Schubart A, Ruck T, Meuth SG (2019) Thenext-generation sphingosine-1 receptor modulator BAF312 (siponimod) improves cortical network functionality in focal autoimmune encephalomyelitis. Neural Regen Res 14(11):1950-1960. doi:10.4103/1673-5374.259622antibody (BioLegend, 1.0 µg per 106 cells in 100 µL volume) for 5 minutes on ice prior to immunostaining. The following mAbs were used for the detection of cell surface markers: CD3 (clone 17A2, BioLegend), CD4 (clone RM4-5, BioLeg-end), CD8a (clone 53-6.7, BioLegend), CD11b (clone M1/70, eBioscience), CD45 (clone 30-F11, BioLegend) and CD45R (also known as B220; clone RA3-6B2, BD). Concentrations of mAbs were carefully titrated prior to experiments. Stained cells were assayed on a Gallios flow cytometer (Beckman Coulter, Krefeld, Germany) using Kaluza software (Beckman Coulter).Immunofluorescence stainingIn order to verify the injection site, evaluate myeloid cells infiltration from the periphery and neuronal survival brains were used for histopathological evaluation. Briefly, mice were deeply anesthetized using ketamine/xylazine and tran-scardially perfused using phosphate-buffered saline (PBS), in order to efficiently remove circulating blood, as described before (Cerina et al., 2017). Afterwards, brains were quickly removed, embedded in cryoprotective compound (TissuTeK, Science Service GmbH, Munich, Germany) and frozen using liquid nitrogen. Coronal cryo-sections (10 µm thick-ness) were cut using a cryotome (Leica, Wetzlar, Germany), positioned on glass slides (two per slide) and conserved at −20°C. Slices were fixed in a solution containing 4% parafor-maldehyde (PFA) for 10 minutes and then washed with PBS. In order to avoid false-positive results, slices were incubated overnight at 4°C with a blocking solution containing PBS, 0.03% Triton X-100, 10% goat serum and 10% BSA. After blocking, slices were incubated with the primary antibody CD11b to identify myeloid cells (1:200, rat anti mouse; Bio-Rad Laboratories GmbH, Hercules, CA, USA; former name Serotec, Puchheim, Germany). The antibody was diluted in a cold solution containing 1% donkey serum, 10% BSA and PBS. Overnight incubation followed. Slides were then*************Figure 1 Oral, but not intracerebral treatment with BAF312 ameliorates the disease course in the focal eAe model with grey and white matter lesions. (a, d) Experimental scheme. 10 days after immunization (day 0) with MOG35–55 pep-tide in C57BL/6J mice, focal EAE lesions were induced by stereotactical injection of proinflammatory cytokines (interfer-on-γ and tumor necrosis factor α) into the auditory cortex or the internal capsule to induce cortical grey or white matter lesions, respectively. There were two experimental groups: Group 1 received BAF312 (a sphin-gosine-1-phosphate receptor modulator) via oral gavage (3 mg/kg) versus vehicle starting 2 days before immunization, and group 2 re-ceived continuous intracerebral injection of BAF312 (1 µg/d) versus vehicle using an os-motic mini pump from the day of focal EAE induction. (b) Clinical courses of focal EAE mice with cortical grey matter lesions (b1) or white matter lesions (b2) that received oral BAF312 application (3 mg/kg). The vertical black arrows indicate the time point corresponding to induction of focal EAE (10 days post induction). (c) Bar graph showing the percentage of peripheral blood lympho-cyte counts two days after focal EAE induc-tion (upper panel). The panel below shows a representative scatter plot for CD4+ and CD8+ T lymphocytes. (e) Clinical courses of focal EAE mice with cortical grey matter lesions (e1) or the white matter lesion group (e2) that received continuous intracerebral injection of BAF312 (1 µg/d). (f) Bar graph showing the percentage of peripheral blood lymphocytes two days after pump implanta-tion. The panel below shows a representative scatter plot for CD4+ and B (CD45R(B220)+) lymphocytes. *P < 0.05 and ****P < 0.0001 (two-way analysis of variance with Bonfer-roni post-hoc test). The right panel shows a representative scatter plot for CD4+ and CD45R(B220) lympho c ytes. EAE: Exper-imental autoimmune encephalomyelitis; Siponimod (BAF312): novel sphingosine-1 receptor modulator.1952Hundehege P, Cerina M, Eichler S, Thomas C, Herrmann AM, Göbel K, Müntefering T, Fernandez-Orth J, Bock S, Narayanan V, Budde T, Speckmann EJ, Wiendl H, Schubart A, Ruck T, Meuth SG (2019) The next-generation sphingosine-1 receptor modulator BAF312 (siponimod) improves cortical network functionality in focal autoimmune encephalomyelitis. Neural Regen Res 14(11):1950-1960. doi:10.4103/1673-5374.259622incubated for 1 hour with fluorophore-conjugated second-ary antibody: Cyanine Cy3 (1:300, donkey anti-rat; Jackson ImmunoResearch Inc., Cambridgeshire, UK). Finally, the mounting medium Fluoromount-G containing DAPI (Ther-mo Fisher Scientific, Darmstadt, Germany; former name Invitrogen, San Diego, CA, USA) was applied as marker for cell nuclei. For evaluating apoptosis, we performed TUNEL/ NeuN staining using an in situ cell death detection kit with fluorescein according to manufacturer’s instructions (In Situ Cell Death Detention Kit with Fluorescein, Roche via Merck KGaA).Immunohistochemistry analysisImages were acquired using a Zeiss Examiner microscope (Zeiss, Göttingen, Germany). Images of slices containing the AC were collected from both hemispheres. All image analy-ses were performed in a blinded manner using ImageJ (open source image processing software (https:///ij/ index.html); Schneider et al., 2012). Images were acquired using 10- and 20-fold objectives and analyzed by counting the number of CD11b+ cells per mm2. For TUNEL/NeuN im-ages were acquired using a 20-fold objective and analyzed by counting the number of fluorescence positive cells per mm2. voltage-sensitive dye technique and analysisEAE and focal EAE were induced as described above. Two days after cytokine injection for inducing focal EAE, the animals were deeply anesthetized and the brains quickly removed. Brains were glued onto an agar block with a 25° angle in order to cut (vibratome from Leica) sagittal slices (500 µm of thickness) containing the full-functioning au-ditory thalamocortical (TC) system (Broicher et al., 2010; Ghaffarian et al., 2016). Cutting was performed in ice-cold artificial-cerebrospinal fluid (ACSF) solution containing the following in mM: sucrose, 200; glucose, 10; PIPES, 20; KCl, 2.5; MgSO4, 10; CaCl2, 0.5; pH 7.35 with NaOH. Slices were then incubated with the voltage-sensitive dye RH-795 (12 µg/mL, Thermo Fisher Scientific, Waltham, MA, USA) in oxygenated ACSF for 60 minutes at 30°C. Afterwards, slices were transferred to an incubation chamber and experiments started after 60 minutes, the time necessary to wash-out the additional dye. Optical signal recordings were performed at 30°C in carbonated ACSF in a submerged chamber on an in-verted microscope (Zeiss, Göttingen, Germany). Every slice was tested twice: in the absence (as control) and in the pres-ence of BAF312 (10 µM). Optical recordings were performed in the primary AC as described previously (Broicher et al., 2010) and governed by the software Neuroplex (Redshirt Imaging, Decatur, GA, USA). Fluorescence changes were detected using a hexagonal photodiode array consisting of 464 elements through a 20× objective covering the whole AC in a given slice (Figure 4a). The sampling interval was 1.274 ms and the maximal length of the recording was 1.305 ms. The excitation wavelength of RH-795 was bandpass filtered at 546 ± 20 nm, and after passing a dichroic mirror, emitted light was highpass filtered at 590 nm with transmission and emission maxima being 530 and 712 nm, respectively. Opti-cal signals were detected as fractional changes of the fluores-cence from the resting light intensity (I rest – I recording/I rest; dI/ I in the text). Color-coded activity maps were constructed using the spatiotemporal cortical inputs recorded as fluo-rescence signals. Scales were calculated for each recording based on the maximal calculated amplitude and therefore are different in every experimental condition. Statistical analysisFor each type of experiment, group sizes are given in the fig-ure legends. Statistical analyses and graphs were prepared us-ing Prism 5.04 (Graph Pad, San Diego, CA, USA). Data were presented as the mean ± SEM. The significance level was set to P < 0.05. Statistical analysis was performed using Student’s t-test for comparisons between groups and non-parametric Mann-Whitney U test, where needed. Multiple comparisons were analyzed by Kruskal-Wallis test followed by Dunn’s post-hoc test and by two-way analysis of variance (ANOV A) with Bonferroni post-hoc test for independent measures. ResultsOral, but not intracerebral treatment with BAF312 ameliorates the disease course in a focal eAe mouse model with grey and white matter lesionsTo investigate effects of BAF312 treatment on cortical grey and subcortical white matter inflammatory lesions in a focal EAE model, C57BL/6J mice were immunized with MOG35–55 peptide 10 days prior to stereotactical injection of proinflam-matory cytokines (IFN-γ and TNF-α) in either the AC or the internal capsule (IC) to induce cortical grey or white matter lesions, respectively (day 0; Figure 1a and d). Furthermore, we differentiated between oral administration of BAF312 from 2 days prior to immunization (group 1, AC: vehicle [n = 19], BAF312 [n = 20] and IC: vehicle [n = 17], BAF312 [n = 22]; Figure 1a) and direct delivery of the drug into the brain using an osmotic mini pump with continuous intra-cerebral infusion starting at the day of focal EAE induction (10 d.p.i.; group 2, AC: vehicle and BAF312 [n = 30] and IC: vehicle [n = 30] and BAF312 [n = 29]; Figure 1d). Independent of the induced lesion site, the effect of system-ic BAF312 treatment (3 mg/kg) led to significantly reduced disease severity throughout the whole observation period of 15 days compared to the vehicle-treated group (Figure 1b). In line with the previous report (Gergely et al., 2012), animals orally treated with BAF312 showed only minimal clinical symptoms during the whole observation period. As expected, flow cytometry analysis of peripheral blood indi-cated a significant lymphopenia of orally treated mice (n = 23) compared to those treated with vehicle (n = 23; open and black bars, respectively; CD4+ T lymphocytes: 2.83 ± 0.25% vs. 8.70 ± 0.72%, respectively; CD8+ T lymphocytes: 1.44 ± 0.09% vs. 5.20 ± 0.62%, respectively; B cells: 3.61 ± 0.27% vs. 11.19 ± 1.07%, respectively; two-way ANOVA, F(2,132) = 133.9; P < 0.0001, Bonferroni post-hoc test: all controls vs. BAF312-treated groups, P < 0.001; Figure 1c). To distin-guish between systemic and direct effects on the brain, we performed local administration of BAF312 using an osmotic1953Hundehege P, Cerina M, Eichler S, Thomas C, Herrmann AM, Göbel K, Müntefering T, Fernandez-Orth J, Bock S, Narayanan V, Budde T, Speckmann EJ, Wiendl H, Schubart A, Ruck T, Meuth SG (2019) Thenext-generation sphingosine-1 receptor modulator BAF312 (siponimod) improves cortical network functionality in focal autoimmune encephalomyelitis. Neural Regen Res 14(11):1950-1960. doi:10.4103/1673-5374.259622minipump (Figure 1d). Given that in the literature the in-tra-cerebroventricular application of 0.45 µg/d was reported to induce lymphopenia (Gentile et al., 2016), we performed a dose-response screening using a higher and lower concen-tration of BAF312, namely 1 µg/d and 0.3 µg/d, respectively, via intracerebral application in order to target only confined brain regions and evaluate if local treatment will induce pe-ripheral effects (Additional Figure 1). The effects induced by application of both concentrations did not differ concerning the total number of CD4+ T lymphocytes, CD8+ T lympho-cytes and B cells obtained from peripheral blood (control n = 4, 0.3 µg/d BAF312, n = 4; Additional Figure 1a) and lymph nodes (Additional Figure 1b). Therefore, we assume that any biological effect after intracerebral treatment is central and decided to use the BAF312 concentration of 1 µg/d for all the following experiments. On a clinical level, intracere-bral application of 1 µg/d BAF312 did not affect the motor disability of EAE mice throughout the observation period of 15 days compared to non-treated controls (Figure 1e). This was irrespective of the lesion site (grey matter vs. white matter) and, therefore, region of drug infusion. Unlike in the orally treated animals, two days after starting continuous intracerebral administration of BAF312 (n = 14), we could not detect a decrease of the total number of CD4+ and CD8+ T lymphocytes in the peripheral blood in comparison to controls (n = 19; CD4+ lymphocytes: 8.70 ± 1.02% vs. 11.90 ± 0.57%, respectively; CD8+ lymphocytes: 4.50 ± 0.91% vs. 6.44 ± 0.64%, respectively; Figure 1f). However, a small but signif-icant difference between BAF312- and vehicle-treated mice was observed by evaluating the percentage of B cells (10.89 ± 1.56% vs. 15.47 ± 1.52%, respectively; two-way ANOVA, F(2,93) = 12.85, P = 0.0005; Bonferroni post-hoc test: control vs. BAF312-treated group, P < 0.05; Figure 1f). Intracerebral BAF312 treatment does not reduce harmful immune cell infiltration into the brain parenchyma compared to non-treated controlsAs active EAE lesions are characterized by hypercellular-ity and prominent infiltration of T lymphocytes, we next examined the composition of immune cells as assessed by ex vivo brain slices that were retrieved two days after focal lesion induction. The slices were split into the lesioned and the non-lesioned hemisphere and subsequently analyzed by multicolor flow cytometry. In mice with focal EAE orally treated with BAF312, total leukocyte counts (CD45+ cells) were increased on the lesioned hemisphere compared to the contralateral hemisphere (two way ANOV A, effect of the le-sion site: F(1,16) = 27.52, P < 0.0001) independent of a grey or white matter lesion (Figure 2a). Oral BAF312 (B) treatment (Figure 2b) resulted in a notably lower number of leukocytes within the lesioned hemisphere compared to vehicle-inject-ed (V) controls (AC lesioned, V: 1 ± 0.22 vs. B: 0.40 ± 0.14 and IC lesioned: V: 1.22 ± 0.21 vs. B: 0.22 ± 0.03; two-way ANOV A, effect of BAF312 treatment: F(3,16) = 10.44, P = 0.001; Bonferroni post-hoc test in lesioned hemisphere: grey-mat-ter control vs. grey-matter BAF312, P < 0.05 and white-mat-ter control vs. white-matter BAF312, P < 0.001; Figure 2a). Analyzing lymphocyte subpopulations, CD4+ T lymphocyte infiltration was found to be reduced in both the cortical grey matter (V: 1 ± 0.18 vs. B: 0.09 ± 0.07, Student’s t-test: t = 4.64, df = 4, P = 0.009; Figure 2b) and subcortical white matter lesioned hemisphere of orally treated animals in comparison to vehicle-treated animals (V: 1 ± 0.22 vs. B: 0.04 ± 0.007, Student’s t-test: t = 4.31, df = 4, P = 0.012; Figure 2b). Counts of CD8+ T lymphocytes were found to be nominally and significantly lower in cortical grey and white matter lesions, respectively (white matter lesion, Student’s t-test: t = 8.02, df = 4, P = 0.0013; Figure 2c). In comparison, continuous in-tracerebral application of BAF312 through an osmotic mini pump neither resulted in any notable changes of total CD45+ leukocytes (two-way ANOVA, effect of BAF312 treatment, F(3,30) = 1.46, P = 0.24; Figure 2d) nor CD4+ (grey matter, P = 0.99, and white matter: P = 0.11; Figure 2e) or CD8+ lym-phocyte counts in the lesioned hemispheres (grey matter, P = 0.06 and white matter: P = 0.18; Figure 2f).These results were corroborated by immunofluorescence staining performed to identify infiltrating peripheral my-eloid cells, including macrophages in the AC. Counting the cells revealed a tendency to decreased number of CD11b+ cells 2 days after cytokine injection in orally treated mice in comparison to the ones that received vehicle (253.7 ± 63.3 cells/mm2 and 743.3 ± 125.7 cells/mm2, respectively, Figure 3a). In the immunofluorescence analysis, the values reached significance threshold 5 days after focal cytokine injection when the oral treatment with BAF312 decreased the number of CD11b+ cells/mm2 from 4197 ± 778 to 326 ± 61 (Krus-kall-Wallis test: 10.60, P = 0.0141, Dunn’s post-hoc test: P = 0.0204; Figure 3a). Local application of BAF312 showed a similar trend but values did not reach significance thresh-old at any of the analyzed time points (Figure 3a). Further analysis to assess neuroprotective effects of intracerebral administration of BAF312 was performed by investigating the effects of the drug on neuronal apoptosis. Immuno-fluorescence staining with the specific marker TUNEL/ NeuN showed a tendency to a reduced apoptosis in the BAF312-treated group 2 days after focal cytokine injection (Figure 3b).BAF312 positively affects increased neuronal network activity in the AC of a focal eAe mouse modelNext, we sought to assess the effect of BAF312 administra-tion at the neuronal network level in the primary AC. We therefore prepared acute brain slices containing AC and IC obtained from MOG35–55 immunized mice two days af-ter cytokine injections, and incubated them with a voltage sensitive-dye. Changes in cortical neuronal activity, evoked by electrical stimulation in the IC, could be detected as fractional changes of fluorescence signals. The usage of a photodiode array (red hexagonal structure in Figure 4a) ensured detection of the fluorescence changes in a very pre-cise spatio-temporal manner. The latter consists of 464 small photo-diodes which anatomically overlap with AC, thereby allowing analysis of the activity of the different layers of the cortex over a time period of 1.3 seconds. Taken together, the1954。
